- 1Laboratory of Medical Microbiology, Molecular Diagnostics Unit, Maasstad Hospital, Rotterdam, Netherlands
- 2Erasmus University Medical Center, Rotterdam, Netherlands
- 3BaseClear, Leiden, Netherlands
- 4Section Molecular Microbiology, Department of Biology, Faculty of Science, Utrecht University, Utrecht, Netherlands
The emergence of carbapenem-resistant Pseudomonas aeruginosa represents a worldwide problem. To understand the carbapenem-resistance mechanisms and their spreading among P. aeruginosa strains, whole genome sequences were determined of two extensively drug-resistant strains that are endemic in Dutch hospitals. Strain Carb01 63 is of O-antigen serotype O12 and of sequence type ST111, whilst S04 90 is a serotype O11 strain of ST446. Both strains carry a gene for metallo-β-lactamase VIM-2 flanked by two aacA29 genes encoding aminoglycoside acetyltransferases on a class 1 integron. The integron is located on the chromosome in strain Carb01 63 and on a plasmid in strain S04 90. The backbone of the 159-kb plasmid, designated pS04 90, is similar to a previously described plasmid, pND6-2, from Pseudomonas putida. Analysis of the context of the integron showed that it is present in both strains on a ∼30-kb mosaic DNA segment composed of four different transposons that can presumably act together as a novel, active, composite transposon. Apart from the presence of a 1237-bp insertion sequence element in the composite transposon on pS04 90, these transposons show > 99% sequence identity indicating that transposition between plasmid and chromosome could have occurred only very recently. The pS04 90 plasmid could be transferred by conjugation to a susceptible P. aeruginosa strain. A second class 1 integron containing a gene for a CARB-2 β-lactamase flanked by an aacA4′-8 and an aadA2 gene, encoding an aminoglycoside acetyltransferase and adenylyltransferase, respectively, was present only in strain Carb01 63. This integron is located also on a composite transposon that is inserted in an integrative and conjugative element on the chromosome. Additionally, this strain contains a frameshift mutation in the oprD gene encoding a porin involved in the transport of carbapenems across the outer membrane. Together, the results demonstrate that integron-encoded carbapenem and carbapenicillin resistance can easily be disseminated by transposition and conjugation among Pseudomonas aeruginosa strains.
Introduction
Pseudomonas aeruginosa is an opportunistic gram-negative pathogen causing acute and chronic infections in hospitalized and immune-compromised patients (Kerr and Snelling, 2009). Pseudomonas species are known to have evolved from a wide variety of environments and are highly adaptable (Silby et al., 2011). In recent years, extensively drug-resistant P. aeruginosa present a globally increasing problem in hospital environments (Edelstein et al., 2013; Wright et al., 2015). P. aeruginosa can rapidly become resistant to antibiotics due to various mechanisms (Oliver et al., 2015), including chromosomal mutations leading to inducible hyper-production of chromosomal AmpC β-lactamase, overexpression of efflux pumps, and/or reduced membrane permeability. Such mutations together are referred to as the mutational resistome (Lopéz-Causapé et al., 2018). Additionally, horizontal transfer of mobile genetic elements, such as integrons, transposons, or plasmids, can confer resistance mechanisms. The increasing prevalence of metallo-β-lactamases (MBLs), such as VIM or IMP, can be caused by horizontal acquisition of integrons, which are often found to contain also genes encoding aminoglycoside resistance. As a result of frequent acquisition of mobile DNA elements, the genome of Pseudomonas can be divided into a core genome and an accessory genome (Battle et al., 2009; Oliver et al., 2015). The population structure therefore was termed non-clonal epidemic, with a high recombination frequency between isolates (Kidd et al., 2012).
Carbapenemase-producing P. aeruginosa are often only susceptible to colistin (Edelstein et al., 2013; Kos et al., 2015), thus limiting therapeutic options to treat infected patients. Worldwide, carbapenemase-producing P. aeruginosa were assigned to successful clonal complexes (CCs) by multi-locus sequence typing (MLST) and O-antigen serotyping (Thrane et al., 2015). CCs 111 and 235 are considered responsible for the worldwide dissemination of extensively drug-resistant lineages which are of the serotypes O12 and O11, respectively (Thrane et al., 2015). The prevalence of ST111 in the Netherlands was previously described (Van der Bij et al., 2012), and this sequence type was also involved in outbreaks in the United Kingdom (Breathnach et al., 2012). ST235/O11 is more prevalent in Eastern European countries, including Russia (Oliver et al., 2015).
For several years, multidrug-resistant, VIM-producing P. aeruginosa were isolated in several hospitals in the Rotterdam area, The Netherlands. In the Erasmus University Medical Centre, two different genotypes predominate (Van der Bij et al., 2011), one of which, an ST111 clone, also dominates in the Maasstad Hospital as well as in many other hospitals in the Netherlands (Van der Bij et al., 2012). Both strains persist and spread through the hospitals via the sinks and drains in spite of hypochlorite treatment. Here, we analyzed representative isolates of both genotypes, which were subjected to whole genome sequencing to study their genetic background. To gain insight into the mobilization of integrons, we analyzed the context of the integrons in detail.
Materials and Methods
Ethics Statement
P. aeruginosa strains were selected under designated names and were not related to patients. According to the Dutch regulation for research with human subjects, no medical or ethical approval was required to conduct this study. The regional medical ethics committee (Toetsingscommissie Wetenschappelijk Onderzoek Rotterdam e.o.) waived the need for informed consent and approved the study (L201586), in agreement with national law by the Federation of Dutch Medical Scientific Societies1.
Strains
P. aeruginosa strain Carb01 63 is a representative of the dominant ST111 genotype isolated from drains and sinks of the Intensive Care Unit at Maasstad Hospital, Rotterdam. Strain S04 90 is a representative of a distinct genotype, i.e., ST446, and was isolated from a patient in Erasmus University Medical Center. Strain PAO1 (Holloway, 1955) was used in conjugation experiments.
Whole Genome Sequencing
Genomic DNA libraries for the Illumina and PacBio platforms were generated and sequenced at BaseClear B.V. (Leiden, Netherlands). For Illumina sequencing, high-molecular-weight genomic DNA was used as input for library preparation using the Illumina TruSeq library preparation kit. Briefly, the genomic DNA was fragmented by nebulization and subjected to end repair, A-tailing, ligation of adaptors including sample-specific barcodes, and size selection. After PCR enrichment, the resultant library was checked on a Bioanalyzer (Agilent) and quantified. The libraries were multiplexed, clustered, and sequenced on an Illumina HiSeq 2000 instrument with paired-end protocol. For PacBio sequencing, high-molecular-weight genomic DNA was sheared to fragments of about 10 kb in length using G-tubes (Covaris) and further processed into a PacBio sequencing library using the standard protocols (Pacific Biosciences). The resulting PacBio library was checked on a BioAnalyzer (Agilent), quantified and sequenced on a PacBio RSII instrument.
Illumina FASTQ sequence files were generated using the Illumina Casava pipeline version 1.8.3. Initial quality assessment was based on data passing the Illumina Chastity filtering. Subsequently, reads containing adapters and/or PhiX control signal were removed using an in-house filtering protocol. The second quality assessment was based on the remaining reads using the FASTQC quality control tool version 0.10.0.
The long read data collected from the PacBio RS instrument were processed and filtered using the SMRT Analysis software suite. The Continuous Long Read (CLR) data were filtered by Read length (>50), Sub read length (>50), and Read quality (>0.75).
The quality of the Illumina FASTQ sequences was enhanced by trimming off low-quality bases using the “Trim sequences” option of the CLC Genomics Workbench version 7.5.1. The quality-filtered sequence reads were puzzled into a number of contig sequences using the “De novo assembly” option of the CLC Genomics Workbench version 7.5.1. The optimal k-mer size was automatically determined using KmerGenie (Chikhi and Medvedev, 2014). The contigs were linked and placed into super-scaffolds based on the alignment of the PacBio CLR reads. Alignment was performed with BLASR (Boetzer and Pirovano, 2014). From the alignment, the orientation, order and distance between the contigs were estimated using the SSPACE-LongRead scaffolder version 1.0 (Boetzer and Pirovano, 2014). Final adjustments were manually made based on the assembly graph. The gapped regions within the super-scaffolds were (partially) closed in an automated manner using GapFiller version 1.10 (Boetzer and Pirovano, 2012). The method takes advantage of the insert size between the Illumina paired-end reads.
Genome Analysis
All sequences were automatically annotated using the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP)2 followed by the Rapid Annotations using Subsystems Technology (RAST) server (Aziz et al., 2008). Prophages were assigned by Phage Search Tool (PHAST) (Zhou et al., 2011). The CRISPRFinder (Grissa et al., 2007) was used to search the genomes for possible CRISPR fragments. Restriction-modification systems were analyzed by submission of PacBio data in REBASE (Roberts et al., 2015). Multi-locus sequence types were assigned3 for both strains. O-antigen types of both strains were determined on the BLAST server of NCBI, where the whole genome sequences were aligned against the different O-antigen sequences of O1-O20. IS finder4 was used to identify IS elements (Siguier et al., 2006).
Conjugation
Strain PAO1 was consecutively exposed to increasing concentrations of fosfomycin (FOS) to select for mutants with minimum inhibitory concentration (MIC) > 256 μg ml-1, needed for counter selection. After overnight growth, 5 μl of FOS-resistant PAO1 was mixed with 5 μl of strain S04 90. Subsequently, the suspension was plated on MacConkey agar plates containing FOS (150 μg ml-1), and five disks each containing 10 μg meropenem (MER) were placed on the plates. The selected colonies resistant to FOS and MER were investigated for the presence of the VIM gene by PCR (Van der Zee et al., 2014). VIM-positive colonies were typed by amplified fragment length polymorphism (AFLP) to verify if they were PAO1 derivatives. VITEK2 (Biomérieux, Marcy l’Etoile, France) analysis was used to determine antibiotic resistance in donor, recipient and transconjugant strains.
AFLP Typing
Amplified fragment length polymorphism typing was performed essentially as described previously (Van der Zee et al., 2003), except that primers were labeled at the 5′ end with Yakima Yellow. Fragments were analyzed by capillary electrophoresis in an ABI3500 instrument and compared to GeneScanTM 600 LIZ® Size Standard v2.0 (Life Technologies, Bleiswijk, Netherlands). Peak patterns were converted to banding patterns using Bionumerics v7.6 (Applied Maths, St Martens Latem, Belgium). Cluster analysis of the fingerprints was performed by Unweighted Pair Group Method with Arithmetic mean (UPGMA).
Nucleotide Sequence Accession Numbers
The nucleotide sequences of the Carb01 63 chromosome, the S04 90 chromosome, and the pS04 90 plasmid were deposited in Genbank under accession numbers CP011317.1, CP011369.1, and CP011370.1, respectively. Complete assemblies of Carb01 63 and S04 90 are filed under assembly numbers ASM98182v1 and ASM98850v1, respectively.
Results
Bacterial Isolates
Genotyping by multi-locus variable-number tandem-repeat analysis (MLVA) revealed two separate clusters of strains among the VIM-2 MBL-producing P. aeruginosa in the hospitals in the Rotterdam area (Van der Bij et al., 2011). MLST revealed that the main cluster consisted of ST111 strains, whilst a minor cluster contains strains of ST446 (Van der Bij et al., 2012). A representative of each of these clusters was elected for genome sequence analysis, i.e., strains Carb01 63 and S04 90 of ST111 and ST446, respectively. VITEK2 analysis showed that both strains are resistant to most antibiotics commonly used to treat P. aeruginosa infections but are sensitive for colistin (Table 1).
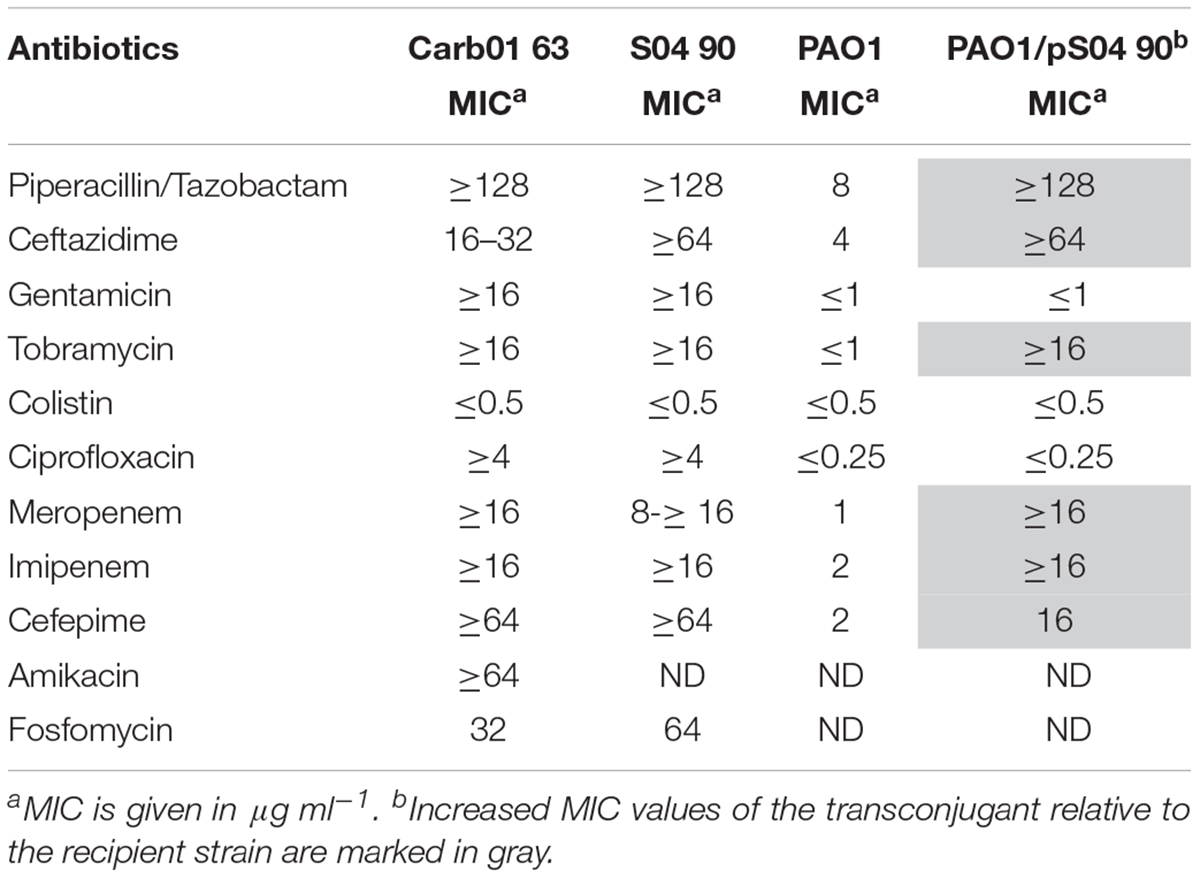
TABLE 1. Antibiograms of strains Carb01 63, S04 90, PAO1, and a transconjugant of PAO1 carrying pS04 90.
Genome Analysis
The complete nucleotide sequence of the Carb01 63 genome was recovered by de novo hybrid assembly. To overcome sequencing difficulties as a result of large repeats in the genome of S04 90, the sequences were aligned with that of UCBB-PA14 (Lee et al., 2006) which resulted in a single sequence of the chromosome with five gaps of in total 152 bp. This strain was found to contain a large plasmid of 159,187 bp, designated pS04 90. The plasmid has a GC content of 57.7% (Table 2), which is substantially lower than the average chromosomal GC content of P. aeruginosa. The strains carry different O-antigens, i.e., O12 in Carb01 63 and O11 in S04 90 (Table 2). Bacterial defense systems against the uptake of foreign DNA are CRISPR/Cas and restriction-modification systems. No genes for functional CRISPR/Cas systems were observed in the chromosome of either isolate, but the plasmid pS04 90 encodes a putative CRISPR (Table 2). Carb01 63 contains type I, II, and III restriction-modification systems, and two unique type I target recognition domains could be identified. S04 90 contains type I and II restriction-modification systems. Plasmid pS04 90 also contains a type II system. Bacteriophage searches revealed the presence of nine prophages, of which four questionable, in Carb01 63 and of 11 prophages, of which four questionable, in S04 90 (Table 2). These high numbers of prophages might be related to the absence of CRISPR/CAS systems on the chromosomes of the strains.
Genes for all major virulence factors were found in the genomes of Carb01 63 and S04 90, including alkaline protease AprA, which is a substrate for the type I protein secretion system (T1SS), and elastase LasB, exotoxin A, and the haemolytic and non-haemolytic phospholipases C PlcH and PlcN, which are substrates of the T2SS (Bleves et al., 2010). The T3SS substrates ExoS and ExoU are mutually exclusive and predominantly found in invasive and cytotoxic P. aeruginosa strains, respectively (Bleves et al., 2010). Strain Carb01 63 contains an exoS gene (locus tag YQ19_07370), whilst an exoU gene (locus tag YH69_22740) was found in strain S04 90.
Comparison of the nucleotide sequences of the chromosomes of Carb01 63 and S04 90 revealed 99% identity with query coverage Carb01 63/S04 90 of 88% and S04 90/Carb01 63 of 92% and only minor differences in size and GC content (Table 2). Based on the dendrogram generated by genomic BLAST5, these sequences are quite distinct (Supplementary Figure S1). Carb01 63 belongs to a large clade of 152 leaves which is represented by P17_North_West_14_VIM_2_03_10, whilst S04 90 belongs to a clade of 34 leaves which is represented by the lineage of 468_PAER. Both clades contain related strains of medical origin mostly from the United Kingdom and France. Schematic representations of both genomes and of some well-described P. aeruginosa genomes were made by progressive Mauve (Darling et al., 2010) to indicate the similarities and differences (Supplementary Figure S2).
Characteristics of the Integrons in Strains Carb01 63 and S04 90
Outbreak strains in the Rotterdam area were reported to contain a blaV IM-2 gene on an integron (Van der Bij et al., 2011). Consistently, blaV IM-2-containing class 1 integrons were found in the genome sequences of strains Carb01 63 and S04 90 and they were designated In1163 and In1025, respectively (Figure 1). In Carb01 63, the integron was found on the chromosome, whilst it was present on the plasmid in S04 90. The sequences of integrons In1025 and ln1163 are closely related (Figure 1) and very similar but not identical to that of In59 (Poirel et al., 2001). Small differences were observed in the gene cassette promoter Pc and in the aacA genes flanking blaV IM-2 and conferring aminoglycoside resistance (Figure 1). Differences in the Pc promoter affect promoter strength and, because the Pc promoter is located within the intI1 coding sequence, also the integron-excision activity of the encoded integrase (Jové et al., 2010). Whilst In1025 contains two aacA29b genes, these genes are replaced by aacA29e genes in In1163 (Figure 1). With respect to the aacA29 genes, both strains are also different from other analyzed outbreak strains in the Rotterdam area, which were all (n = 25) reported to contain aacA29a and aacA29b genes upstream and downstream of the blaV IM-2 gene, respectively (Van der Bij et al., 2012). The aacA29e genes in In1163 are different from previously described aacA genes and their products differ from those of the aacA29b genes by a single F41L amino-acid substitution. Although the function of the newly found aacA29e genes on the integron of Carb01 63 was not determined, aacA29a and -29b are known to cause decreased susceptibility to amikacin and to tobramycin, but not to gentamicin (Poirel et al., 2001).
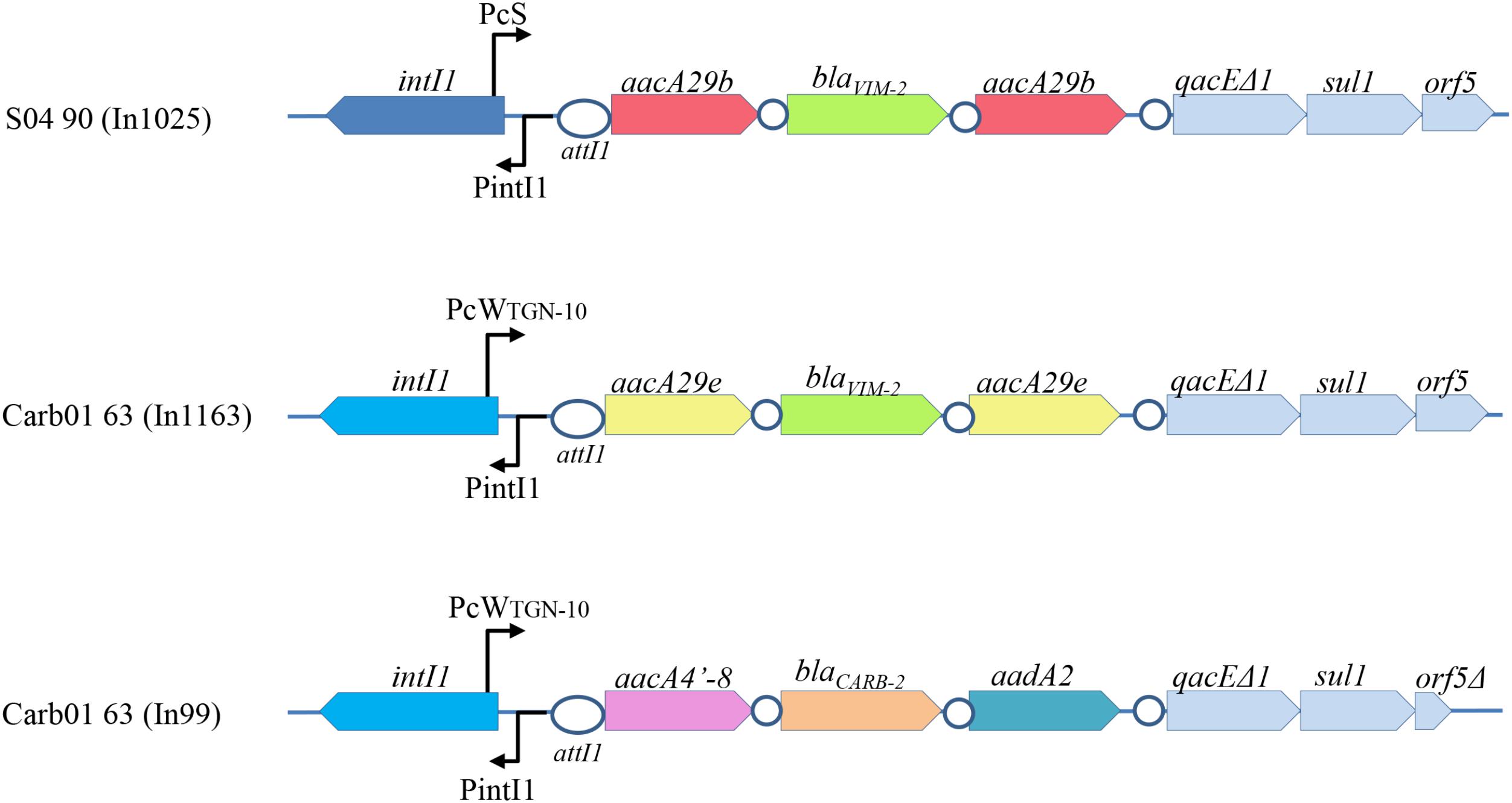
FIGURE 1. Schematic representation of the integrons of Carb01 63 and S04 90. The gene cassette promoters (Pc) are different. PcS represents a strong promoter, whereas PcWTGN-10 represents a promoter that is considerably weaker due to nucleotide substitutions in the –10 and –35 regions, which are, however, partially compensated by a C to G substitution upstream of the –10 region, resulting in an extended –10 motif (Jové et al., 2010). These differences also affect the primary structures of the integrases encoded by the intI1 genes (dark and bright blue). Cassettes with the same nucleotide sequences are indicated with the same color. No differences were observed in the 3′ conserved sequences, except that orf5 in In99 is truncated. The integration sites attI1 and attC are indicated by ovals and circles, respectively.
Besides the blaV IM-2-containing integron, Carb01 63 contains a second class 1 integron, designated In99, containing a blaCARB-2 gene (Figure 1). The blaCARB-2 gene is flanked by an aacA4′-8 gene [a.k.a. aac(6′)-Ib] encoding an aminoglycoside 6′-N-acetyltransferase and an aadA2 gene encoding an aminoglycoside-3′′-adenylyltransferase, which is associated with resistance to streptomycin and spectinomycin. Class 1 integrons with such cassette composition were previously described in P. aeruginosa isolates from Portugal (Caetano et al., 2007).
Genetic Context of the blaV IM-2 Containing Integrons
In spite of the different genomic location of the blaV IM-2-containing integrons in Carb01 63 and S04 90, i.e., on the chromosome and on a plasmid, respectively, they are both located on a ∼30-kb DNA fragment with very high sequence similarity between the strains. On the 159,187-bp plasmid pS04 90, this segment covers nucleotides 129,245–159,187 plus 1–20 (Figure 2). The integron is contained in a Tn402 transposon (Gillings, 2017) that is bounded by 25-nt inverted repeats (IR) (Figure 2A). This transposon is immobilized as the tni transposition module is incomplete with tniQ and tniC genes being absent. The Tn402 transposon is inserted into a Tn21-like transposon between two open reading frames, designated tnpM (locus tag YH69_34320) and a truncated urf2 (locus tag YH69_34365). Presumably, these two open reading frames are derived from a single gene, designated urf2M that was split by the Tn402 insertion (Liebert et al., 1999). Insertion resulted in a 5-nt (5′-TCCAT-3′) duplication of the target site. The Tn21-like transposon contains several genes involved in conferring mercury resistance but the locus is incomplete as the essential merP, merT, and merR genes are deleted (Figure 2A). This deletion also covers the IRR of the Tn21 transposon, which is, therefore, immobilized. The Tn21-like transposon is contained within a severely disrupted Tn4661 transposon the remnants of which are an intact 47-nt IRL, the 5′ end of the tnpA gene encoding the transposase, and an incomplete IRR that covers only 20 of the 47 nt of a complete IRR (Figure 2A). Also inserted in this transposon is a complete Tn4661 (Figure 2A), which has >99% sequence identity with Tn4661 of P. aeruginosa plasmid RMS148 (Yano et al., 2013). Tn4661 is often found inserted in chromosomes of P. aeruginosa, e.g., one copy is found on the chromosome of S04 90, whilst strain S86968 (Genbank accession number CP008865.2) contains two copies. In plasmid pS04 90, this transposon contains an IS222 insertion element of 1237 bp, containing two overlapping ORFs encoding InsE and the transposase InsM that is generated by translational frameshifting at an (A)6G site (Kropinski et al., 1994) (Figure 2A). This element is inserted with a 3-nt target site duplication (5′-TAC-3′) into codon 11 of the tnpA transposase gene of the Tn4661 transposon and, probably, prevents expression of this gene. The inserted complete Tn4661 is separated from the merA gene of Tn21 by a 332-bp element that is bounded by 29-nt perfect inverted repeats (5′-GTTGTGGGATGCAAATAAAGTTTCATCCT-3′). Since three copies of this element are found at different positions in the chromosome of strain Carb01 63, it might be a replicative transposable element, but it does not contain a discernible transposase gene. Probably, it represents a hitherto undescribed miniature inverted-repeat transposable element (MITE), which are non-autonomous mobile elements found in both eukaryotes and prokaryotes (Delihas, 2008). The entire 30-kb composite transposon extending from the IRL to the incomplete IRR of the disrupted Tn4661 transposon is inserted into a plasmid with a core that shows high similarity with the 117-kb plasmid pND6-2 from Pseudomonas putida (Li et al., 2013) with a query coverage and sequence identity of 78 and 95%, respectively (Figure 2B). The transposon is inserted into a gene corresponding to orf042 of the pND6-2 plasmid encoding a large hypothetical protein (Li et al., 2013). Consequently, this gene is split into two pseudogenes with locus tags YH69_34300 and YH69_ 33605 on pS04 90, and the insertion resulted in a target site duplication of five nucleotides (5′-TGTTC-3′).
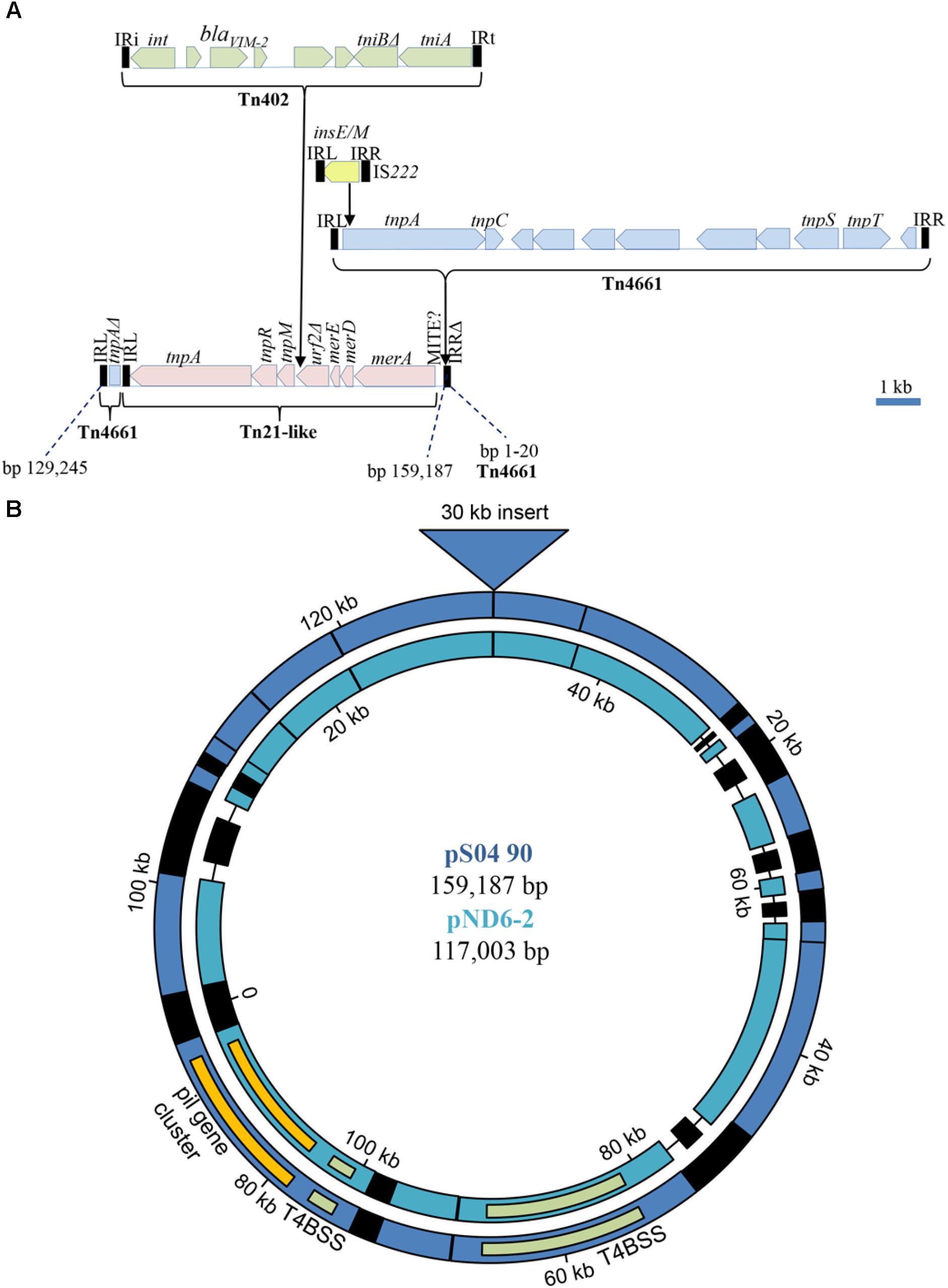
FIGURE 2. Genetic context of the blaV IM-2 containing integron on pS04 90. (A) Composition of a mosaic 30 kb transposon containing the integron with the blaV IM-2 gene. Open reading frames of different transposable elements are indicated by different colors. MITE?, 332-bp inserted element possibly representing a MITE. (B) Comparison of pS04 90 (blue) with pND6-2 (sea-green). Regions of homology are indicated by color. Non-homologous regions are black and deletions are indicated by a line. The positions of the pil gene cluster (yellow) and the genes involved in the type IVB secretion system (T4BSS) (green) are indicated in both plasmids. The large insertion, detailed in (A), is indicated by a triangle at the top. The dissimilar regions (black) around 20, 90, and 105 kb in pS04 90, encode, amongst others, a toxin/antitoxin addiction module, an O-antigen transacetylase OafA, and CRISPR-related proteins, respectively. Most of the proteins putatively encoded by the dissimilar regions are hypothetical proteins and several transposases/integrases.
In strain Carb01 63, the complete 30-kb fragment described above, except for the inserted IS222 element and the incomplete IRR of the disrupted Tn4661, is present on the chromosome (nt 3,707,795–3,736,497). The IS222 element is found on six other chromosomal sites in the Carb01 63 chromosome. As the tnpA gene of the complete Tn4661 is not disrupted, an active transposase can be produced and the entire 30-kb element may constitute an active composite transposon extending from the IRL of the disrupted Tn4661 to the IRR of the complete Tn4661. Insertion of this composite transposon into the Carb01 63 chromosome, has split a gene putatively encoding a MOSC domain-containing molybdenum cofactor sulfurase into two pseudogenes, one of which (the 5′ end) was not annotated and the other (the 3′ end) was annotated with locus tag YQ19_17550. Insertion resulted in a 5-nt (5′-ATGGA-3′) duplication of the target site. Thus, it can be inferred that the complete composite transposon, including the integron with blaV IM-2, was acquired by transposition.
pS04 90 Is a Conjugative Plasmid
pND6-2 is a conjugative plasmid that was reported to mobilize a co-resident plasmid from P. putida to Escherichia coli (Li et al., 2013). Like pND6-2, pS04 90 carries genes encoding an icm/dot type IVB secretion system and type IV pili, suggesting it might also be a conjugative plasmid (Figure 2B). To investigate whether indeed pS04 90 is transferrable, a conjugation experiment was performed using a FOS-resistant derivative of strain PAO1 as the recipient. After conjugation, seven colonies that were resistant to both MER and FOS were further analyzed. These seven strains, which were all positive for blaV IM-2 in PCR analysis, were genotyped by AFLP. Two strains showed a similar AFLP profile as PAO1 (see Supplementary Figure S3 for an example) and are therefore regarded as transconjugants. The other five strains resembled the AFLP profile of S04 90 (data not shown) and are, therefore, presumably spontaneous FOS-resistant mutants of this isolate. FOS-resistant strain PAO1 and both transconjugants were analyzed by VITEK, which confirmed the susceptibility of the PAO1 strain to most antibiotics tested and multidrug resistance of the transconjugants (Table 1). Repeated attempts to transfer the plasmid to E. coli, either by conjugation or by electroporation, failed indicating that it has a narrow host range.
Genetic Context of the blaCARB-2-Containing Integron
The In99 integron with the blaCARB-2 gene (Figure 1) is contained in a severely disrupted Tn402 transposon that is bounded by 25-nt inverted repeats (Figure 3A). This transposon lacks the entire tni transposition module. The orf5 gene of the integron is disrupted by the insertion of an IS6100 element (Figure 3A). The defective Tn402 is inserted in a Tn5051-like transposon, which, apart from the insertion of the Tn402 and of the IS1071 element described below, shows high sequence similarity with other Tn5051-like transposons, such as TnAO22 from Achromobacter sp. AO22 (Ng et al., 2009) with only 15 single-nucleotide polymorphisms (SNPs) over an 8230-bp sequence. Like in the Tn21 transposon described above, the Tn402 is inserted in the urf2M gene but more toward the 3′ end of the gene, i.e., at a position identical to a previously reported integron-insertion site in Tn5051 (Toleman et al., 2003). Insertion resulted in a 5-nt (5′-GAGTC-3′) duplication of the target site. The genes that are essential for mercury resistance, i.e., merR, merT, merP, and merA, are complete in the Tn5051 transposon, and they have indeed been shown to confer mercury resistance in the case of TnAO22 (Ng et al., 2009). The tnpA gene of the Tn5051 transposon is truncated by the insertion of an IS1071 element (Figure 3A), which probably renders the Tn5051 TnpA inactive. The Tn5051 is bounded by 38-nt inverted repeats and inserted into a gene encoding a hypothetical protein with a DUF4158 domain that is thereby split into two pseudogenes with locus tags YQ19_26565 and YQ19_26485. Insertion resulted in a 5-nt (5′-CTCAA-3′) target site duplication. This disrupted gene is situated within an integrative and conjugative element (ICE), which is integrated in a tRNA-gly gene (locus tag YQ19_26215). This 89,494-bp ICE is flanked by two 20-bp direct repeats, corresponding to the 3′ end of the tRNA-gly gene and representing the attL and attR sites (Figure 3B). With only very few SNPs, the core composition of the ICE is almost identical to that of the P. aeruginosa genomic island PAGI-16 of strain KMU11, but the cassette composition of the integron of this Korean ST235 isolate is different (Hong et al., 2016).
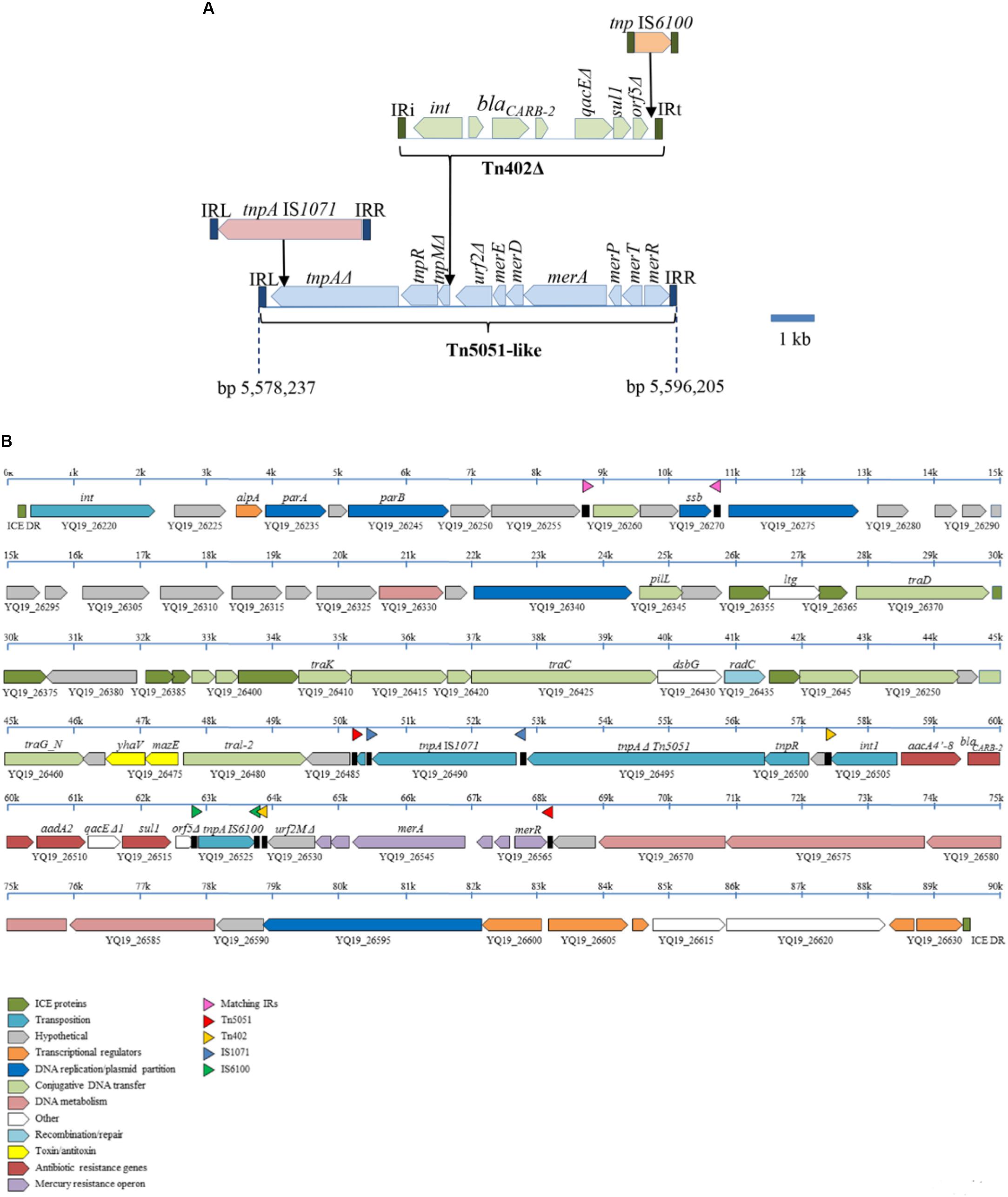
FIGURE 3. Genetic context of the blaCARB-2-containing integron in Carb01 63. (A) Composition of a mosaic 18-kb transposon containing the integron with the blaCARB-2 gene. Open reading frames of different transposable elements are indicated by different colors. (B) Composition of the ICE into which the composite transposon depicted in (A) is inserted. The ICE is bounded by 20-bp direct repeats (ICE DR). ORFs encoding proteins of different functional classes are indicated by different colors as outlined in the inset at the bottom. Inverted repeats are indicated by black boxes in between the ORFs with a colored triangle above.
Mutational Resistome
Besides by the acquisition of genes by horizontal gene transfer, antibiotic susceptibility can be decreased by mutations in the core genome, together constituting the mutational resistome (Lopéz-Causapé et al., 2018). Several mutations are present in the core genome of strains Carb01 63 and S04 90 that likely contribute to the observed resistance phenotypes. The porin OprD mediates the diffusion of carbapenems across the outer membrane, and disruption of the oprD gene or downregulation of its expression represents an important carbapenem-resistance mechanism (Pirnay et al., 2002; Lister et al., 2009; Ruiz-Martínez et al., 2011; Cabot et al., 2016). In strain Carb01 63, the oprD gene (locus tag YQ19_24920) is disrupted by an 11-bp deletion leading to a frame-shift. This mutation likely contributes to the high level of resistance of the strain to carbapenems. In strain S04 90, the oprD gene contains nine mutations leading to amino-acid substitutions relative to OprD of strain PAO1. This variant is identical to variant T1-IV described previously (Ocampo-Sosa et al., 2012), which is not associated with resistance to carbapenems. In strain S04 90, but not in Carb01 63, the mexZ gene (locus tag YH69_17355) is disrupted by a frameshift mutation. Inactivation of MexZ leads to overproduction of the MexXY components of the MexXY-OprM efflux pump and is associated with increased resistance to aminoglycosides, fluoroquinolones, and zwitterionic cephalosporins, such as cefepime, amongst others (Guénard et al., 2014). Both strains carry missense mutations leading to a T83I amino-acid substitution in the GyrA protein and an S87L substitution in ParC, which are associated with resistance to fluoroquinolones (Kos et al., 2015). These mutations explain the observed resistance of the strains to ciprofloxacin (Table 1).
Discussion
The two P. aeruginosa strains analyzed here belong to two MBL-producing clones that are endemic in Dutch hospitals (Van der Bij et al., 2011). Carb01 63 is a representative of the most prevalent clone. The spread of this ST111 clone among various hospitals in The Netherlands has been demonstrated (Van der Bij et al., 2012), and this type caused the first outbreak (Van der Bij et al., 2011). Carb01 63, which was isolated from drains and sinks in the Maasstad hospital, is closely related to the recently sequenced strain RIVM-EMC2982 (accession number CP016955.1), a patient isolate from the Erasmus University Medical Center in Rotterdam. Carb01 63 is also closely related to previously described outbreak strains of the same ST111 and O12 antigen, reported in hospitals in the United Kingdom (Breathnach et al., 2012; Witney et al., 2014; Turton et al., 2015). The most closely related neighbors of strain S04 90 are two clinical ST446 isolates from France, strains AZPAE15043 (Kos et al., 2015) and WH-SGI-V-07172 (van Belkum et al., 2015), which are susceptible to carbapenems.
Both strains are resistant to many antibiotics, and the presence of blaV IM-2 and other resistance genes was demonstrated. The presence of blaV IM-2 genes in P. aeruginosa isolates has been repeatedly described, but how these genes spread among strains is usually not clear because, apart from the cassette composition of the class 1 integrons in which they are located, their genetic context is often not described. In P. aeruginosa, blaV IM-2-containing integrons are usually associated with the chromosome as was found in strain Carb01 63, but it is present on a plasmid in strain S04 90. Although plasmid-associated blaV IM-2 genes have occasionally been reported before (Poirel et al., 2000; Edelstein et al., 2013; Wright et al., 2015), only in a single case the gene was shown to be present on a conjugative plasmid (Botelho et al., 2017). We have experimentally demonstrated that also pS04 90 is a conjugative plasmid. The plasmid shows high similarity to pND6-2, a previously described plasmid from P. putida strain ND6 that was suggested to belong to a new plasmid incompatibility (Inc) group (Li et al., 2013). Nucleotide BLAST searches showed that pS04 90 has high sequence similarity to pND6-2 also in the DNA fragment containing the oriV and the repB gene, suggesting that pS04 90 belongs to this same new Inc group. DNA segments with high sequence similarity to the plasmid backbone were also found on contigs of several other incomplete P. aeruginosa genome sequences (see Supplementary Figure S4 for an example), indicating that the plasmid is more commonly found in this species. As compared with pND6-2, pS04 90 has acquired a large DNA fragment of ∼30 kb that contains the integron with the blaV IM-2 gene. Interestingly, the blaV IM-2-containing integron is located on a very similar DNA fragment on the chromosome of strain Carb01 63 that, apart from the absence of the IS222 element and the incomplete IRR of the severely disrupted Tn4661, deviates from the DNA fragment of pS04 90 in the presence of only 42 SNPs over the entire 29,963 nt sequence. Also the closely related strain RIVM-EMC2982 contains this DNA fragment on the chromosome in the same position as in Carb01 63, and it is even more closely related to that on pS04 90 with only 5 SNPs besides the absence of the IS222 element and the incomplete IRR. Thus, it appears that the entire ∼30-kb DNA fragment can move position between plasmid and chromosome as a novel, composite transposon, and, in view of the very high sequence identity of these transposons, this could have occurred only very recently. Since pS04 90 is a conjugative plasmid, this illustrates how MBL-encoding integrons can be mobilized and transferred between strains. Interestingly, the entire composite transposon also appears to be present in a P. aeruginosa isolate of Czech origin, i.e., strain Pae-31448cz (Papagiannitsis et al., 2017). However, the severely disrupted Tn4661, although present in the available nucleotide sequence (Genbank accession number KY860571.1), was not noticed and, therefore, the possibility that entire fragment could function as a composite mobile element was not considered.
Besides the blaV IM-2-containing integron, strain Carb01 63 contains an additional class 1 integron containing aacA4, blaCARB-2, and aadA2 gene cassettes. The integron is located on a composite transposon that is integrated into an ICE. Apart from the cassette composition of the integron, this ICE is very similar to PAGI-16 in the Korean isolate KMU11 (Hong et al., 2016), probably reflecting a common origin of these genomic islands. The presence of the island in strains of different sequence types, i.e., ST111 and ST235 for Carb01 63 and KMU11, respectively, suggests that it may be transferred between strains. Interestingly, in strain RIVM-EMC2982, which is highly related to Carb01 63, the ICE is split into two parts located at different chromosomal positions and each associated with an IS6100 element. Similarly, PAGI-16 has been reported to be split into two parts in Korean strain BP14 by a large chromosomal inversion resulting from duplication and insertion of the IS6100 element (Hong et al., 2016). A similar recombination event apparently occurred in strain RIVM-EMC2982, where it disrupted the oprD gene, which encodes a porin mediating transport of carbapenems across the outer membrane.
In our hospitals, MBL-producing clones of ST111 are persistent in sinks and drains despite treatment with 10% hypochlorite, and they are spreading to different departments. These unpublished findings oppose previous results that showed successful reduction of extensively drug-resistant strains from these systems (Witney et al., 2014). Further studies into the resistance of the strains to disinfectants will be facilitated by the available genome sequences. The P. aeruginosa strains studied are susceptible only to colistin treatment. Recent discovery of widespread colistin resistance in bacteria urges to keep alert of possible colistin resistance of already extensively drug-resistant Pseudomonas, which is a major concern for hospitals, since no option is available for adequate disinfection of drains and sinks, while colonization, infection and outbreaks via these routes are difficult to control.
Conclusion
The genome sequences of two multidrug-resistant P. aeruginosa strains endemic in Dutch hospitals revealed the presence of a novel, large, composite transposon that carries a class 1 integron with a blaV IM-2 gene and aminoglycoside-resistance genes. This transposon can apparently move position between chromosome and a conjugative plasmid, with which it can be transferred to other P. aeruginosa strains. Besides, the genome sequences revealed other mobile resistance genes and mutations in the core genome that contribute to the multidrug-resistance phenotype.
Author Contributions
AZ, JO, and WG initiated the study. WK and AB performed the experimental work. WP performed the whole genome sequencing and assembly. WK, AZ, and JT performed the bioinformatic analysis and drafted the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ria van Boxtel for assistance in some of the experiments. Strain PAO1 was kindly provided by Gilles Vergnaud and Christine Pourcel, Université Paris-Sud, Orsay, France.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02057/full#supplementary-material
Footnotes
- ^http://www.federa.org
- ^http://www.ncbi.nlm.nih.gov/
- ^http://pubmlst.org/paeruginosa/
- ^http://www-is.biotoul.fr
- ^http://www.ncbi.nlm.nih.gov/genome/187
References
Aziz, R. K., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75
Battle, S. E., Rello, J., and Hauser, A. R. (2009). Genomic islands of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 290, 70–78. doi: 10.1111/j.1574-6968.2008.01406.x
Bleves, S., Viarre, V., Salacha, R., Michel, G. P. F., Filloux, A., and Voulhoux, R. (2010). Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int. J. Med. Microbiol. 300, 534–543. doi: 10.1016/j.ijmm.2010.08.005
Boetzer, M., and Pirovano, W. (2012). Toward almost closed genomes with GapFiller. Genome Biol. 13:R56. doi: 10.1186/gb-2012-13-6-r56
Boetzer, M., and Pirovano, W. (2014). SSPACE-LongRead: scaffolding bacterial draft genomes using long read sequence information. BMC Bioinformatics 15:211. doi: 10.1186/1471-2105-15-211
Botelho, J., Grosso, F., Quinteira, S., Mabrouk, A., and Peixe, L. (2017). The complete nucleotide sequence of an IncP-2 megaplasmid unveils a mosaic architecture comprising a putative novel blaVIM-2-harbouring transposon in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 72, 2225–2229. doi: 10.1093/jac/dkx143
Breathnach, A. S., Cubbon, M. D., Karunaharan, R. N., Pope, C. F., and Planche, T. D. (2012). Multidrug-resistant Pseudomonas aeruginosa outbreaks in two hospitals: association with contaminated hospital waste-water systems. J. Hosp. Infect. 82, 19–24. doi: 10.1016/j.jhin.2012.06.007
Cabot, G., López-Causapé, C., Ocampo-Sosa, A. A., Sommer, L. M., Angeles Domínguez, M., Zamorano, L., et al. (2016). Deciphering the resistome of the widespread Pseudomonas aeruginosa sequence type 175 international high-risk clone through whole-genome sequencing. Antimicrob. Agents Chemother. 60, 7415–7423. doi: 10.1128/AAC.01720-16
Caetano, T., Ferreira, S., Mondego, A. P., Correia, A., and Mendo, S. (2007). In99, an In100-related integron, its occurrence and prevalence in clinical Pseudomonas aeruginosa strains from a central region of Portugal. Epidemiol. Infect. 135, 502–504. doi: 10.1017/S095026880600700X
Chikhi, R., and Medvedev, P. (2014). Informed and automated k-mer size selection for genome assembly. Bioinformatics 30, 31–37. doi: 10.1093/bioinformatics/btt310
Darling, A. E., Mau, B., and Perna, N. T. (2010). progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147
Delihas, N. (2008). Small mobile sequences in bacteria display diverse structure/function motifs. Mol. Microbiol. 67, 475–481. doi: 10.1111/j.1365-2958.2007.06068.x
Edelstein, M. V., Skleenova, E. N., Shevchenko, O. V., D’souza, J. W., Tapalski, D. V., Azizov, I. S., et al. (2013). Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect. Dis. 13, 867–876. doi: 10.1016/S1473-3099(13)70168-3
Gillings, M. R. (2017). Class 1 integrons as invasive species. Curr. Opin. Microbiol. 38, 10–15. doi: 10.1016/j.mib.2017.03.002
Grissa, I., Vergnaud, G., and Pourcel, C. (2007). CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 35, W52–W57. doi: 10.1093/nar/gkm360
Guénard, S., Muller, C., Monlezun, L., Benas, P., Broutin, I., Jeannot, K., et al. (2014). Multiple mutations lead to MexXY-OprM-dependent aminoglycoside resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 58, 221–228. doi: 10.1128/AAC.01252-13
Holloway, B. W. (1955). Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13, 572–581. doi: 10.1099/00221287-13-3-572
Hong, J. S., Yoon, E. J., Lee, H., Jeong, S. H., and Lee, K. (2016). Clonal dissemination of Pseudomonas aeruginosa sequence type 235 isolates carrying blaIMP-6 and emergence of blaGES-24 and blaIMP-10 on novel genomic islands PAGI-15 and PAGI-16 in South Korea. Antimicrob. Agents Chemother. 60, 7216–7223. doi: 10.1128/AAC.01601-16
Jové, T., Da Re, S., Denis, F., Mazel, D., and Ploy, M. C. (2010). Inverse correlation between promoter strength and excision activity in class 1 integrons. PLoS Genet. 6:e1000793. doi: 10.1371/journal.pgen.1000793
Kerr, K. G., and Snelling, A. M. (2009). Pseudomonas aeruginosa: a formidable and ever-present adversary. J. Hosp. Infect. 73, 338–344. doi: 10.1016/j.jhin.2009.04.020
Kidd, T. J., Ritchie, S. R., Ramsay, K. A., Grimwood, K., Bell, S. C., and Rainey, P. B. (2012). Pseudomonas aeruginosa exhibits frequent recombination, but only a limited association between genotype and ecological setting. PLoS One 7:e44199. doi: 10.1371/journal.pone.0044199
Kos, V. N., Déraspe, M., McLaughlin, R. E., Whiteaker, J. D., Roy, P. H., Alm, R. A., et al. (2015). The resistome of Pseudomonas aeruginosa in relationship to phenotypic susceptibility. Antimicrob. Agents Chemother. 59, 427–436. doi: 10.1128/AAC.03954-14
Kropinski, A. M., Farinha, M. A., and Jansons, I. (1994). Nucleotide sequence of the Pseudomonas aeruginosa insertion sequence IS222: another member of the IS3 family. Plasmid 31, 222–228. doi: 10.1006/plas.1994.1024
Lee, D. G., Urbach, J. M., Wu, G., Liberati, N. T., Feinbaum, R. L., Miyata, S., et al. (2006). Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 7:R90. doi: 10.1186/gb-2006-7-10-r90
Li, S., Zhao, H., Li, Y., Niu, S., and Cai, B. (2013). Complete nucleotide sequence of plasmid pND6-2 from Pseudomonas putida ND6 and characterization of conjugative genes. Gene 512, 148–156. doi: 10.1016/j.gene.2012.09.065
Liebert, C. A., Hall, R. M., and Summers, A. O. (1999). Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63,507–522.
Lister, P. D., Wolter, D. J., and Hanson, N. D. (2009). Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22, 582–610. doi: 10.1128/CMR.00040-09
Lopéz-Causapé, C., Cabot, G., del Barrio-Tofiño, E., and Oliver, A. (2018). The versatile mutational resistome of Pseudomonas aeruginosa. Front. Microbiol. 9:685. doi: 10.3389/fmicb.2018.00685
Ng, S. P., Davis, B., Palombo, E. A., and Bhave, M. (2009). A Tn5051-like mer-containing transposon identified in a heavy metal tolerant strain Achromobacter sp. AO22. BMC Res. Notes 2:38. doi: 10.1186/1756-0500-2-38
Ocampo-Sosa, A. A., Cabot, G., Rodríguez, C., Roman, E., Tubau, F., Macia, M. D., et al. (2012). Alterations of OprD in carbapenem-intermediate and -susceptible strains of Pseudomonas aeruginosa isolated from patients with bacteremia in a Spanish multicenter study. Antimicrob. Agents Chemother. 56, 1703–1713. doi: 10.1128/AAC.05451-11
Oliver, A., Mulet, X., López-Causapé, C., and Juan, C. (2015). The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updat. 2, 41–59. doi: 10.1016/j.drup.2015.08.002
Papagiannitsis, C. C., Medvecky, M., Chudejova, K., Skalova, A., Rotova, V., Spanelova, P., et al. (2017). Molecular characterization of carbapenemase-producing Pseudomonas aeruginosa of Czech origin and evidence for clonal spread of extensively resistant sequence type 357 expressing IMP-7 metallo-β-lactamase. Antimicrob. Agents Chemother. 61, e1811–e1817. doi: 10.1128/AAC.01811-17
Pirnay, J. P., De Vos, D., Mossialos, D., Vanderkelen, A., Cornelis, P., and Zizi, M. (2002). Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ. Microbiol. 4, 872–882. doi: 10.1046/j.1462-2920.2002.00281.x
Poirel, L., Lambert, T., Türkoglü, S., Ronco, E., Gaillard, J., and Nordmann, P. (2001). Characterization of class 1 integrons from Pseudomonas aeruginosa that contain the blaVIM-2 carbapenem-hydrolyzing β-lactamase gene and of two novel aminoglycoside resistance gene cassettes. Antimicrob. Agents Chemother. 45, 546–552. doi: 10.1128/AAC.45.2.546-552.2001
Poirel, L., Naas, T., Nicolas, D., Collet, L., Bellais, S., Cavallo, J. D., et al. (2000). Characterization of VIM-2, a carbepenem-hydrolyzing metallo-ß-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44, 891–897. doi: 10.1128/AAC.44.4.891-897.2000
Roberts, R. J., Vincze, T., Posfai, J., and Macelis, D. (2015). REBASE–a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res. 43, D298–D299. doi: 10.1093/nar/gku1046
Ruiz-Martínez, L., Lopéz-Jiménez, L., d’Ostuni, V., Fusté, E., Vinuesa, T., and Viñas, M. (2011). A mechanism of carbapenem resistance due to a new insertion element (ISPa133) in Pseudomonas aeruginosa. Int. Microbiol. 14, 51–58. doi: 10.2436/20.1501.01.135
Siguier, P., Perochon, J., Lestrade, L., Mahillon, J., and Chandler, M. (2006). ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36. doi: 10.1093/nar/gkj014
Silby, M. W., Winstanley, C., Godfrey, S. A. C., Levy, S. B., and Jackson, R. W. (2011). Pseudomonas genomes: diverse and adaptable. FEMS Microbiol. Rev. 35, 652–680. doi: 10.1111/j.1574-6976.2011.00269.x
Thrane, S. W., Taylor, V. L., Freschi, L., Kukavica-Ibrulj, I., Boyle, B., Laroche, J., et al. (2015). The widespread multidrug-resistant serotype O12 Pseudomonas aeruginosa clone emerged through concomitant horizontal transfer of serotype antigen and antibiotic resistance gene clusters. mBio 6:e01396-15. doi: 10.1128/mBio.01396-15
Toleman, M. A., Biedenbach, D., Bennett, D., Jones, R. N., and Walsh, T. R. (2003). Genetic characterization of a novel metallo-β-lactamase gene, blaIMP13, harboured by a novel Tn5051-type transposon disseminating carbapenemase genes in Europe: report from the SENTRY worldwide antimicrobial surveillance programme. J. Antimicrob. Chemother. 52, 583–590. doi: 10.1093/jac/dkg410
Turton, J. F., Wright, L., Underwood, A., Witney, A. A., Chan, Y. T., Al-Shahib, A., et al. (2015). High-resolution analysis by whole-genome sequencing of an international lineage (sequence type 111) of Pseudomonas aeruginosa associated with metallo-carbapenemases in the United Kingdom. J. Clin. Microbiol. 53, 2622–2631. doi: 10.1128/JCM.00505-15
van Belkum, A., Soriaga, L. B., LaFave, M. C., Akella, S., Veyrieras, J. B., Barbu, E. M., et al. (2015). Phylogenetic distribution of CRISPR-Cas systems in antibiotic-resistant Pseudomonas aeruginosa. mBio 6:e01796-15. doi: 10.1128/mBio.01796-15
Van der Bij, A. K., Van der Zwan, D., Peirano, G., Severin, J. A., Pitout, J. D. D., Van Westreenen, M., et al. (2012). Metallo-β-lactamase-producing Pseudomonas aeruginosa in the Netherlands: the nationwide emergence of a single sequence type. Clin. Microbiol. Infect. 18, E369–E372. doi: 10.1111/j.1469-0691.2012.03969.x
Van der Bij, A. K., Van Mansfeld, R., Peirano, G., Goessens, W. H. F., Severin, J. A., Pitout, J. D., et al. (2011). First outbreak of VIM-2 metallo-β-lactamase-producing Pseudomonas aeruginosa in The Netherlands: microbiology, epidemiology and clinical outcomes. Int. J. Antimicrob. Agents 37, 513–518. doi: 10.1016/j.ijantimicag.2011.02.010
Van der Zee, A., Roorda, L., Bosman, G., and Ossewaarde, J. M. (2014). Screening rectal swabs for carbapenemase genes. J. Clin. Microbiol. 52, 4401–4403. doi: 10.1128/JCM.02256-14
Van der Zee, A., Steer, N., Thijssen, E., Nelson, J., van’t Veen, A., and Buiting, A. (2003). Use of multienzyme multiplex PCR amplified fragment length polymorphism typing in analysis of outbreaks of multiresistant Klebsiella pneumoniae in an intensive care unit. J. Clin. Microb. 41, 798–802. doi: 10.1128/JCM.41.2.798-802.2003
Witney, A. A., Gould, K. A., Pope, C. F., Bolt, F., Stoker, N. G., Cubbon, M. D., et al. (2014). Genome sequencing and characterization of an extensively drug-resistant sequence type 111 serotype O12 hospital outbreak strain of Pseudomonas aeruginosa. Clin. Microbiol. Infect. 20, O609–O618. doi: 10.1111/1469-0691.12528
Wright, L. L., Turton, J. F., Livermore, D. M., Hopkins, K. L., and Woodford, N. (2015). Dominance of international ‘high-risk clones’ among metallo-β-lactamase-producing Pseudomonas aeruginosa in the UK. J. Antimicrob. Chemother. 70, 103–110. doi: 10.1093/jac/dku339
Yano, H., Genka, H., Ohtsubo, Y., Nagata, Y., Top, E. M., and Tsuda, M. (2013). Cointegrate-resolution of toluene-catabolic transposon Tn4651: determination of crossover site and the segment required for full resolution activity. Plasmid 69, 24–35. doi: 10.1016/j.plasmid.2012.07.004
Keywords: Pseudomonas aeruginosa, genome sequence, carbapenem resistance, VIM-2, integron, transposon, conjugation, integrative and conjugative element
Citation: van der Zee A, Kraak WB, Burggraaf A, Goessens WHF, Pirovano W, Ossewaarde JM and Tommassen J (2018) Spread of Carbapenem Resistance by Transposition and Conjugation Among Pseudomonas aeruginosa. Front. Microbiol. 9:2057. doi: 10.3389/fmicb.2018.02057
Received: 17 June 2018; Accepted: 13 August 2018;
Published: 05 September 2018.
Edited by:
Katy Jeannot, UMR6249 Chrono Environment, FranceReviewed by:
Antonio Oliver, Hospital Universitario Son Dureta, SpainJosep M. Sierra, University of Barcelona, Spain
Copyright © 2018 van der Zee, Kraak, Burggraaf, Goessens, Pirovano, Ossewaarde and Tommassen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Tommassen, Si5QLk0uVG9tbWFzc2VuQHV1Lm5s
 Anneke van der Zee1
Anneke van der Zee1 Walter Pirovano
Walter Pirovano Jan Tommassen
Jan Tommassen