
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol., 17 August 2018
Sec. Fungi and Their Interactions
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.01810
This article is part of the Research TopicDiagnostic Approaches for Aspergillus InfectionsView all 20 articles
Purpose: The diagnosis of chronic pulmonary aspergillosis (CPA) is occasionally complicated due to poor sensitivity of mycological culture and colonization of Aspergillus species in the airway. Several diagnostic methods have been developed for the diagnosis of invasive pulmonary aspergillosis; however, their interpretation and significance are different in CPA. This study aimed to review the recent advances in diagnostic methods and their characteristics in the diagnosis of CPA.
Recent findings: Radiological findings of lung, histopathology, and culture are the gold standard of CPA diagnosis. Serodiagnosis methods involving the use of galactomannan and β-D-glucan have low sensitivity and specificity. An Aspergillus-specific IgG antibody assay showed good performance and had better sensitivity and reproducibility than conventional precipitant antibody assays. Currently, it is the most reliable method for diagnosing CPA caused by Aspergillus fumigatus, but evidence on its effectiveness in diagnosing CPA caused by non-fumigatus Aspergillus is lacking. Newly developed lateral flow device Aspergillus and detection of volatile organic compounds in breath have potential, but evidence on its effectiveness in diagnosing CPA is lacking. The increasing prevalence of azole-resistant A. fumigatus strains has become a threat to public health. Some of the azole-resistant-related genes can be detected directly from clinical samples using a commercially available kit. However, its clinical efficacy for routine use remains unclear, since resistance-related genes greatly differ among regions and countries.
Conclusion: Several issues surrounding the diagnosis of CPA remain unclear. Hence, further investigations and clinical studies are needed to improve the accuracy and efficiency of CPA diagnosis.
Aspergillus species are environmental molds that produce airborne spores, and the average human is estimated to inhale hundreds of Aspergillus conidia daily (Hospenthal et al., 1998). Host immunity and the underlying pulmonary diseases are critical factors in determining the outcome of this daily exposure. Patients with defects in cell-mediated immunity, including those with neutropenia due to cytotoxic chemotherapy, or T-cell dysfunction due to corticosteroid or other immunosuppressive therapy are at risk of developing invasive pulmonary aspergillosis (IPA) characterized by hyphal invasion of lung tissues and dissemination to other organs (Baddley, 2011; Patterson et al., 2016). However, patients with underlying chronic respiratory disorders, such as chronic obstructive pulmonary disease, post-pulmonary tuberculosis, non-tuberculosis mycobacteriosis (NTM), cystic fibrosis (CF), bronchiectasis, or allergic bronchopulmonary aspergillosis could develop saprophytic Aspergillus colonization and infection, namely, chronic pulmonary aspergillosis (CPA) (Saraceno et al., 1997; Takeda et al., 2016; Lowes et al., 2017). CPA is a slowly progressive pulmonary disease caused by Aspergillus spp. (Saraceno et al., 1997) and its prognosis is poor; the 5-year mortality rate of CPA patients is approximately 50–85% (Lowes et al., 2017). CPA is categorized into five disease entities based on the recent guidelines of the European Respiratory Society: Aspergillus nodule, simple pulmonary aspergilloma, chronic cavitary pulmonary aspergillosis (CCPA), chronic fibrosing pulmonary aspergillosis (CFPA), and subacute invasive pulmonary aspergillosis (SAIA) (Denning et al., 2016).
The diagnosis of CPA is occasionally complicated, as there are several disease entities in CPA, which are described in the following section, and some patients with underlying pulmonary diseases develop Aspergillus airway colonization. Diagnostic methods used for CPA are similar with those of IPA, but their interpretation and significance are different. Clinicians need various clinical information such as patients' background, radiological images, clinical courses, cultural tests, and other supportive diagnostic methods to diagnose CPA. The present review describes the currently available diagnostic methods and discusses new approaches for diagnosing CPA and their future directions.
Simple pulmonary aspergilloma is defined as single pulmonary cavity containing a fungal ball in a non-immunocompromised patient with minor or no symptoms and no radiological progression over at least 3 months of observation. Aspergillus nodule is characterized by the presence of one or more nodules without cavitation caused by Aspergillus spp. (Denning et al., 2016; Muldoon et al., 2016).
On the contrary, CCPA and SAIA are characterized by one or more cavities with or without fungal ball and its radiological progression such as expanding thick-walled cavities and pericavitary infiltration (Denning et al., 2016). The crucial difference between them is that SAIA involves hyphal invasion into the lung parenchyma (Yousem, 1997; Hope et al., 2005); however, it is not occasionally easy and practical to obtain sufficient histopathological samples to confirm the diagnosis. Therefore, clinical information such as time course of radiological progression (CCPA >3 months; SAIA 1–3 months) and process of cavity formation are indispensable for clinical diagnosis; CCPA usually occurs in pre-existing cavities, whereas in SAIA, cavities can be subsequently formed by the necrotic change of nodules or infiltration lesion due to Aspergillus species. infection (Izumikawa et al., 2014). However, it is hard to distinguish them if the serial radiography films are not available. Particularly, the patients with NTM infection are difficult to diagnose due to their similarity in radiological findings such as nodular shadows and cavity formation (Kobashi et al., 2006). CFPA is defined as severe fibrotic destruction of at least two lung lobes complicating CCPA leading to a major loss of lung function and generally the end result of untreated CCPA (Denning et al., 2003, 2016). Thus, these three clinical entities are vague and overlapping in some cases; however, it is essential to distinguish them in order to estimate their prognoses. Although triazole antifungals are recommended in these entities, their efficacy was better in patients with SAIA than in those with CCPA, as reported in a prospective study in France (Cadranel et al., 2012). Recently, “scab-like sign” observed inside the cavitary lesion in CT was proposed as a high-risk sign of hemoptysis in CPA patients, this could be useful when following the CPA patients (Sato et al., 2018).
Mycological culture is the basic methods for diagnosing CPA, although it has several limitations. The culture positivity rates of Aspergillus species from respiratory specimens in CPA vary widely, ranging from 11.8 to 81.0% depending on reports (Kitasato et al., 2009; Kohno et al., 2010; Nam et al., 2010; Shin et al., 2014). Uffredi et al. reported that 48 (63%) individuals were colonized patients among 76 non-granulocytopenic patients whose respiratory specimens yielded Aspergillus fumigatus (Uffredi et al., 2003). In our previous study, only 11 (16.4%) of 67 individuals were colonized patients among those with culture positive for A. fumigatus. By contrast, 58 (65.9%) of 88 individuals were colonized patients whose cultures yielded non-fumigatus Aspergillus strains (Tashiro et al., 2011). These reports imply that the clinicians need to be careful when interpreting the results of fungal cultures from respiratory specimens, as Aspergillus species are ubiquitous organism that is present in the air, and some of them are saprophytic fungus and cannot be the target of treatment. The most important way to distinguish the colonization from infection is to confirm clinical information, such as the transitional change of radiological findings; however, films are not always available. Therefore, we need a biomarker that reflects the invasiveness of Aspergillus infection.
It is not always easy to obtain the histopathological specimen, as some patients are not tolerable for invasive diagnostic procedure such as transbronchial lung biopsy due to their general conditions; therefore, serodiagnosis is indispensable for the diagnosis of CPA. Galactomannan (GM) antigen assays in serum and bronchial alveolar lavage (BAL) fluid have high sensitivity and specificity for the diagnosis of IPA, with cutoff values of 0.5 and 1.0, respectively (Maertens et al., 2007, 2009). However, the GM serum assay has lower sensitivity and specificity for CPA, with a cutoff value of 0.5 (Kitasato et al., 2009; Shin et al., 2014), than for IPA. GM antigen in BALF showed relatively higher sensitivity (77.2%) and specificity (77.0%), with a cutoff value of 0.4, than that in serum (Izumikawa et al., 2012).
Although the β-D-glucan (BDG) assay has high sensitivity for the screening of a wide range of invasive fungal infections such as candidemia, pneumocystis pneumonia, and IPA, its specificity is limited (Karageorgopoulos et al., 2011; Onishi et al., 2012). Furthermore, its sensitivity is very low (about 20%) in CPA patients (Kitasato et al., 2009; Kohno et al., 2010). Urabe et al. recently reported that the combination of GM and BDG assays in BALF had a higher diagnostic accuracy compared with other single or combinations of diagnostic methods including PCR (Urabe et al., 2017).
Detection of the Aspergillus-specific antibody plays an important role in the diagnosis of CPA and Allergic bronchopulmonary aspergillosis and this method has been widely used. The precipitating Aspergillus IgG antibody has better sensitivity (80–90%) than GM and BDG assays (Kitasato et al., 2009; Kohno et al., 2010) At the moment, commercial Aspergillus-specific IgG plate ELISA tests are currently produced by Serion (Germany), IBL (Germany/USA), Dynamiker/Bio-Enoche (China), Bio-Rad (France), Bordier (Switzerland), and Omega/Genesis (UK) (Page et al., 2015). Siemens (Germany) supplies an automated Aspergillus-specific IgG ELISA system (Immunolite), while Thermo Fisher Scientific/Phadia (multinational) supplies an automated Aspergillus-specific IgG fluoroenzyme immunoassay system (ImmunoCAP), which is an ELISA variant (Page et al., 2015). The Phadia ImmunoCAP IgG assay and Bio-Rad Platelia Aspergillus IgG method have been reported to possess better sensitivity and reproducibility compared with the method involving the use of the conventional precipitant antibody (Baxter et al., 2013). These detection kits have excellent performance in the diagnosis of CPA and ABPA (Baxter et al., 2013; Dumollard et al., 2016; Fujiuchi et al., 2016; Page et al., 2016, 2018). However, all these tests use purified antibodies to culture extracts or recombinant antigens of A. fumigatus, and were originally designed to detect A. fumigatus. As non-fumigatus strains account for 40% (30 of 74) of CPA patients in Japan (Tashiro et al., 2011) and 38% in India (Shahid et al., 2001), these assays might have limitations in diagnosing CPA caused by non-fumigatus strains in some areas.
Polymerase chain reaction (PCR) for the diagnosis of IPA has been used for over 2 decades, though is not included in the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) definitions of invasive fungal disease (White et al., 2015). Aspergillus PCR from blood sample has similar sensitivity and specificity for the diagnosis of IPA (White et al., 2015), but failed to detect Aspergillus DNA in patients with SPA and CPA (Imbert et al., 2016), conversely, this implies that PCR could be useful to eliminate disseminated infection from CPA. In BALF sample, PCR showed tolerable sensitivity (66.7–86.7%) and specificity (84.2–94.2%) compared to GM or BDG (Urabe et al., 2017). RT-PCR has advantages, (1) its quantitative aspect offers the possibility to establish precise cutoff values that could distinguish colonization from active infections, (2) since RT-PCR detects RNA, which is an indicator of the living fungal cells.
Aspergillus-specific lateral flow device (LFD) was newly developed. It uses the mouse monoclonal antibody JF5, which binds to a protein epitope present on an extracellular glycoprotein antigen secreted constitutively during the active growth of A. fumigatus. This method can detect Aspergillus antigens in human serum within 15 min. An early clinical trial showed that LFD is comparable to GM in serum in terms of diagnosing IPA, with a sensitivity and specificity of 81.8 and 98%, respectively (White et al., 2013). In a single center prospective study, LFD test using BALF specimen also showed tolerable sensitivity (77%) and specificity (92%) for proven/probable IPA (Prattes et al., 2014). However, recently, a single center study reported that LFD showed low sensitivity of 38% for IPA (Castillo et al., 2018). The evidence of LFD's utility in CPA diagnosis is quite limited to date, clinical studies on the diagnosis of CPA are needed to better understand the clinical use of LFD.
Volatile organic compounds (VOCs) are known to be detected from the breath of an infected individual. Initially, 2-pentylfuran was reported as the potential diagnostic VOC in IPA patients (Syhre et al., 2008; Chambers et al., 2009). A recent proof-of-principle study was conducted using electronic noses to detect the characteristic VOC pattern of IPA and showed high sensitivity of 100% and a specificity of 83.3% (de Heer et al., 2013). Other researchers used thermal desorption-gas chromatography/mass spectrometry to detect the specific VOCs pattern of IPA and also showed high sensitivity of 94% and specificity of 93% (Koo et al., 2014). Moreover, Heer et al. applied the same methods to detect A. fumigatus colonization in CF patients and showed sensitivity of 78% and specificity of 94% (de Heer et al., 2016). These methods can be useful screening tests, as they are noninvasive diagnostic procedures; however, there might be an issue in distinguishing CPA from Aspergillus-colonized patients.
Galactosaminogalactan (GAG) is a newly discovered extracellular polysaccharide of Aspergillus species, composed of α-1-4-linked galactose and α-1-4-linked N-acetylgalactosamine. It was observed only in hyphae form (Fontaine et al., 2011). GAG is particularly abundant in A. fumigatus, which is the most pathogenic specie among hundreds of Aspergillus species (Lee et al., 2015). Furthermore, GAG is required for its virulence (Gravelat et al., 2013). Therefore, this component could be a potential biomarker to estimate the invasiveness of Aspergillus infection.
In recent years, the global increase of azole-resistant A. fumigatus became an emerging concern for public health, despite the fact that the rates of resistant strains vary among regions, countries, or continents, and rates of resistant strains are especially high in European countries (van der Linden et al., 2015; Meis et al., 2016; Rivero-Menendez et al., 2016). Azole antifungals are the mainstay of treatments for pulmonary aspergillosis. The mortality rates in IPA patients infected with azole-resistant strains were higher than those infected with azole-sensitive ones (88% vs. 30–50%) (van der Linden et al., 2011). Lowes et al reported that the 10-year survival of CPA patients in United Kingdom with isolates fully susceptible to azoles was 68%, in contrast to 46% in patients with an isolate with reduced susceptibility to azoles, though there was not a significant difference (Lowes et al., 2017). However, it is still unclear how patients acquired azole-resistant strain infection affects the clinical course or mortality in CPA patients, because some azole-resistant strains obtained from aspergillosis patients treated with azoles showed poor condition and attenuated growth activity in in vitro condition (Ballard et al., 2018).
CPA patients need at least 6 months of oral azole treatment (Denning et al., 2016); detecting the azole-resistant strain earlier could provide them benefit by changing the treatment regimen. However, it is difficult to diagnose azole-resistant A. fumigatus infection in the clinical setting, as in vitro antifungal susceptibility testing of Aspergillus species is not routinely done in most clinical laboratories due to its cost and technical problems. The screening test with azole containing (itraconazole, 4 mg/L; voriconazole, 1 mg/L; posaconazole, 0.5 mg/L; and no antifungal) 4-well agar plate showed a sensitivity of 99% and a specificity of 99%, to screen the azole-resistant mutants (Arendrup et al., 2017); this could be useful and practical for routine test in clinical laboratories in countries where azole-resistance rate is high.
Azole-resistant A. fumigatus strains are mainly categorized into “environmental route” and “patient-acquired route” by means of resistance acquisition. The former was estimated to be generated by the agricultural fungicides used for crop protection and carries the tandem repeats (TR) of 34, 46, and 53 base pairs upstream in the promoter region of CYP51A with a single point mutation of CYP51A gene. By contrast, the latter were generated by the long-term use of medical azoles and carries various single point mutations of CYP51A gene (Meis et al., 2016). The environmentally obtained azole-resistant strains seemed to originate in Europe and have already spread into other regions worldwide (Meis et al., 2016).
The most commonly used method is simple polymerase chain reaction (PCR) amplification of the entire coding and promoter region with sequence analysis of the PCR products; however, this method is not practical for clinical use as it is time consuming. Restriction fragment length polymorphism by AluI is valuable as it can detect TR34 and L89H mutations from DNA samples faster than sequencing (Ahmad et al., 2014). The commercially available AsperGenius® (PathoNostics) can detect L98H, T289A, Y121F, and TR34 mutations as well as A. fumigatus gene directly from BALF specimen by multiplex real time PCR. In a multicenter clinical study, it showed good diagnostic performance on BAL and could detect A. fumigatus with resistance-associated mutations, including in culture-negative BALF samples, and detection of mutations was associated with azole treatment failure (Chong et al., 2016). However, the efficacy of this detection kit for CPA patients is unclear, as these mutations are relatively rare among patient-acquired azole-resistant strains obtained worldwide (Meis et al., 2016; Chowdhary et al., 2017); on the contrary, 27 (93.1%) of 29 of CPA patients from Europe had an L98H mutation from BALF samples and 16 (55.2%) had a TR34 mutation (Denning et al., 2011).
Needless to say, the gold standard of CPA diagnosis is the radiological findings of the lungs, its histopathology, and culture from the focus of infection. The definitive diagnosis by histopathology and culture is not always easy to perform; thereby, other diagnostic tools are also dispensable and biomarkers to reflect the disease status are needed. Diagnostic methods for CPA described in this review are summarized in Table 1. Currently, the Aspergillus-specific IgG antibody is the most promising tool for diagnosing CPA caused by A. fumigatus. We propose the algorithm for the diagnosis and treatment of CPA (Figure 1). When the patient is suspected of chronic aspergillus infection, it is important to rule out the mycobacterium infection first. Indication of bronchoscopy examination should be considered depending on the result of Aspergillus IgG antibody test. If it is negative, bronchoscopy examination is strongly recommended, as non-fumigatus Aspergillus infection can be the causative organism. If it is positive, bronchoscopy examination is however, optional, to determine which antifungal agents to be used, or collect more precise epidemiological information.
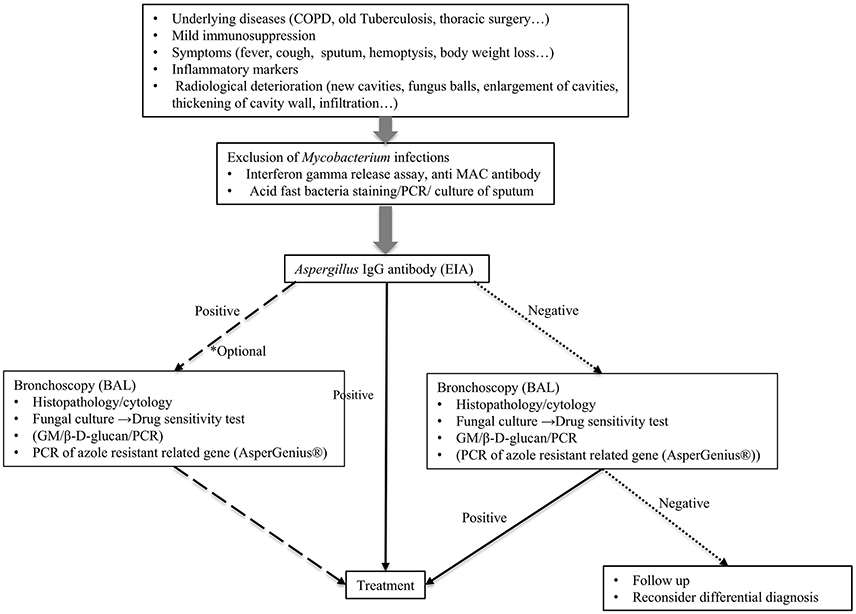
Figure 1. Proposed algorithm for the diagnosis of chronic pulmonary aspergillosis. BAL, bronchoalveolar lavage.
Since the emergence of azole-resistant A. fumigatus strains is a serious concern, convenient detection methods are required to detect these directly from clinical samples; however, further investigation is required. In addition, we need to investigate how these azole mutants are produced inside the lungs and how they affect CPA patients to discover other methods to decrease their prevalence.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CCPA, chronic cavitary pulmonary aspergillosis; CFPA, chronic fibrosing pulmonary aspergilllosis; CT, Computed tomography; ABPA, Allergic bronchopulmonary aspergillosis; CPA, chronic pulmonary aspergillosis; BAL, bronchial alveolar lavage; BDG, β-D-glucan; GAG, galactosaminogalactan; GM, galactomannan; IPA, invasive pulmonary aspergillosis; LFD, lateral flow device; PCR, polymerase chain reaction; RT-PCR, Reverse transcription-polymerase chain reaction; SAIA, subacute invasive pulmonary aspergillosis; TR, tandem repeats.
Ahmad, S., Khan, Z., Hagen, F., and Meis, J. F. (2014). Simple, low-cost molecular assays for TR34/L98H mutations in the cyp51A gene for rapid detection of triazole-resistant Aspergillus fumigatus isolates. J. Clin. Microbiol. 52, 2223–2227. doi: 10.1128/JCM.00408-14
Arendrup, M. C., Verweij, P. E., Mouton, J. W., Lagrou, K., and Meletiadis, J. (2017). Multicentre validation of 4-well azole agar plates as a screening method for detection of clinically relevant azole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 72, 3325–3333. doi: 10.1093/jac/dkx319
Baddley, J. W. (2011). Clinical risk factors for invasive aspergillosis. Med. Mycol. 49(Suppl. 1), S7–S12. doi: 10.3109/13693786.2010.505204
Ballard, E., Melchers, W. J. G., Zoll, J., Brown, A. J. P., Verweij, P. E., and Warris, A. (2018). In-host microevolution of Aspergillus fumigatus: a phenotypic and genotypic analysis. Fungal. Genet. Biol. 113, 1–13. doi: 10.1016/j.fgb.2018.02.003
Baxter, C. G., Denning, D. W., Jones, A. M., Todd, A., Moore, C. B., and Richardson, M. D. (2013). Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Clin. Microbiol. Infect. 19, E197–E204. doi: 10.1111/1469-0691.12133
Cadranel, J., Philippe, B., Hennequin, C., Bergeron, A., Bergot, E., Bourdin, A., et al. (2012). Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur. J. Clin. Microbiol. Infect. Dis. 31, 3231–3239. doi: 10.1007/s10096-012-1690-y
Castillo, C. G., Kauffman, C. A., Zhai, J., Jiang, H., Agozino, S. M., and Miceli, M. H. (2018). Testing the performance of a prototype lateral flow device using bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in high-risk patients. Mycoses 61, 4–10. doi: 10.1111/myc.12694
Chambers, S. T., Syhre, M., Murdoch, D. R., McCartin, F., and Epton, M. J. (2009). Detection of 2-pentylfuran in the breath of patients with Aspergillus fumigatus. Med. Mycol. 47, 468–476. doi: 10.1080/13693780802475212
Chong, G. M., van der Beek, M. T., von dem Borne, P. A., Boelens, J., Steel, E., Kampinga, G. A., et al. (2016). PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bronchoalveolar lavage: a multicentre validation of the AsperGenius assay(R) in 201 patients with haematological disease suspected for invasive aspergillosis. J. Antimicrob. Chemother. 71, 3528–3535. doi: 10.1093/jac/dkw323
Chowdhary, A., Sharma, C., and Meis, J. F. (2017). Azole-Resistant Aspergillosis: epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 216(Suppl. 3), S436–S444. doi: 10.1093/infdis/jix210
de Heer, K., Kok, M. G., Fens, N., Weersink, E. J., Zwinderman, A. H., van der Schee, M. P., et al. (2016). Detection of airway colonization by Aspergillus fumigatus by use of electronic nose technology in patients with cystic fibrosis. J. Clin. Microbiol. 54, 569–575. doi: 10.1128/JCM.02214-15
de Heer, K., van der Schee, M. P., Zwinderman, K., van den Berk, I. A., Visser, C. E., van Oers, R., et al. (2013). Electronic nose technology for detection of invasive pulmonary aspergillosis in prolonged chemotherapy-induced neutropenia: a proof-of-principle study. J. Clin. Microbiol. 51, 1490–1495. doi: 10.1128/JCM.02838-12
Denning, D. W., Cadranel, J., Beigelman-Aubry, C., Ader, F., Chakrabarti, A., Blot, S., et al. (2016). Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 47, 45–68. doi: 10.1183/13993003.00583-2015
Denning, D. W., Park, S., Lass-Florl, C., Fraczek, M. G., Kirwan, M., Gore, R., et al. (2011). High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 52, 1123–1129. doi: 10.1093/cid/cir179
Denning, D. W., Riniotis, K., Dobrashian, R., and Sambatakou, H. (2003). Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin. Infect. Dis. 37(Suppl 3), S265–S280. doi: 10.1086/376526
Dumollard, C., Bailly, S., Perriot, S., Brenier-Pinchart, M. P., Saint-Raymond, C., Camara, B., et al. (2016). Prospective evaluation of a new Aspergillus IgG enzyme immunoassay kit for diagnosis of chronic and allergic pulmonary aspergillosis. J. Clin. Microbiol. 54, 1236–1242. doi: 10.1128/JCM.03261-15
Fontaine, T., Delangle, A., Simenel, C., Coddeville, B., van Vliet, S. J., van Kooyk, Y., et al. (2011). Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 7:e1002372. doi: 10.1371/journal.ppat.1002372
Fujiuchi, S., Fujita, Y., Suzuki, H., Doushita, K., Kuroda, H., Takahashi, M., et al. (2016). Evaluation of a quantitative serological assay for diagnosing chronic pulmonary aspergillosis. J. Clin. Microbiol. 54, 1496–1499. doi: 10.1128/JCM.01475-15
Gravelat, F. N., Beauvais, A., Liu, H., Lee, M. J., Snarr, B. D., Chen, D., et al. (2013). Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS Pathog. 9:e1003575. doi: 10.1371/journal.ppat.1003575
Hope, W. W., Walsh, T. J., and Denning, D. W. (2005). The invasive and saprophytic syndromes due to Aspergillus spp. Med. Mycol. 43(Suppl. 1), S207–238. doi: 10.1080/13693780400025179
Hospenthal, D. R., Kwon-Chung, K. J., and Bennett, J. E. (1998). Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med. Mycol. 36, 165–168. doi: 10.1080/02681219880000241
Imbert, S., Gauthier, L., Joly, I., Brossas, J. Y., Uzunov, M., Touafek, F., et al. (2016). Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin. Microbiol. Infect. 22. e561–e568. doi: 10.1016/j.cmi.2016.01.027
Izumikawa, K., Tashiro, T., Tashiro, M., Takazono, T., Kosai, K., Morinaga, Y., et al. (2014). Pathogenesis and clinical features of chronic pulmonary aspergillosis - is it possible to distinguish CNPA and CCPA clinically? J. Infect. Chemother. 20, 208–212. doi: 10.1016/j.jiac.2013.10.016
Izumikawa, K., Yamamoto, Y., Mihara, T., Takazono, T., Morinaga, Y., Kurihara, S., et al. (2012). Bronchoalveolar lavage galactomannan for the diagnosis of chronic pulmonary aspergillosis. Med. Mycol. 50, 811–817. doi: 10.3109/13693786.2012.682228
Karageorgopoulos, D. E., Vouloumanou, E. K., Ntziora, F., Michalopoulos, A., Rafailidis, P. I., and Falagas, M. E. (2011). beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin. Infect. Dis. 52, 750–770. doi: 10.1093/cid/ciq206
Kitasato, Y., Tao, Y., Hoshino, T., Tachibana, K., Inoshima, N., Yoshida, M., et al. (2009). Comparison of Aspergillus galactomannan antigen testing with a new cut-off index and Aspergillus precipitating antibody testing for the diagnosis of chronic pulmonary aspergillosis. Respirology 14, 701–708. doi: 10.1111/j.1440-1843.2009.01548.x
Kobashi, Y., Fukuda, M., Yoshida, K., Miyashita, N., Niki, Y., and Oka, M. (2006). Chronic necrotizing pulmonary aspergillosis as a complication of pulmonary Mycobacterium avium complex disease. Respirology 11, 809–813. doi: 10.1111/j.1440-1843.2006.00952.x
Kohno, S., Izumikawa, K., Ogawa, K., Kurashima, A., Okimoto, N., Amitani, R., et al. (2010). Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: a multicenter trial in Japan. J. Infect. 61, 410–418. doi: 10.1016/j.jinf.2010.08.005
Koo, S., Thomas, H. R., Daniels, S. D., Lynch, R. C., Fortier, S. M., Shea, M. M., et al. (2014). A breath fungal secondary metabolite signature to diagnose invasive aspergillosis. Clin. Infect. Dis. 59, 1733–1740. doi: 10.1093/cid/ciu725
Lee, M. J., Liu, H., Barker, B. M., Snarr, B. D., Gravelat, F. N., Al Abdallah, Q., et al. (2015). The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog. 11:e1005187. doi: 10.1371/journal.ppat.1005187
Lowes, D., Al-Shair, K., Newton, P. J., Morris, J., Harris, C., Rautemaa-Richardson, R., et al. (2017). Predictors of mortality in chronic pulmonary aspergillosis. Eur. Respir. J. 49:1601062. doi: 10.1183/13993003.01062-2016
Maertens, J., Maertens, V., Theunissen, K., Meersseman, W., Meersseman, P., Meers, S., et al. (2009). Bronchoalveolar lavage fluid galactomannan for the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Clin. Infect. Dis. 49, 1688–1693. doi: 10.1086/647935
Maertens, J. A., Klont, R., Masson, C., Theunissen, K., Meersseman, W., Lagrou, K., et al. (2007). Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin. Infect. Dis. 44, 1329–1336. doi: 10.1086/514349
Meis, J. F., Chowdhary, A., Rhodes, J. L., Fisher, M. C., and Verweij, P. E. (2016). Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 371:20150460. doi: 10.1098/rstb.2015.0460
Muldoon, E. G., Sharman, A., Page, I., Bishop, P., and Denning, D. W. (2016). Aspergillus nodules; another presentation of chronic pulmonary aspergillosis. BMC Pulm. Med. 16:123. doi: 10.1186/s12890-016-0276-3
Nam, H. S., Jeon, K., Um, S. W., Suh, G. Y., Chung, M. P., Kim, H., et al. (2010). Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. Int. J. Infect. Dis. 14, e479–e482. doi: 10.1016/j.ijid.2009.07.011
Onishi, A., Sugiyama, D., Kogata, Y., Saegusa, J., Sugimoto, T., Kawano, S., et al. (2012). Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J. Clin. Microbiol. 50, 7–15. doi: 10.1128/JCM.05267-11
Page, I. D., Baxter, C., Hennequin, C., Richardson, M. D., van Hoeyveld, E., van Toorenenbergen, A. W., et al. (2018). Receiver operating characteristic curve analysis of four Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis. Diagn. Microbiol. Infect. Dis. 91, 47–51 doi: 10.1016/j.diagmicrobio.2018.01.001
Page, I. D., Richardson, M., and Denning, D. W. (2015). Antibody testing in aspergillosis–quo vadis? Med. Mycol. 53, 417–439. doi: 10.1093/mmy/myv020
Page, I. D., Richardson, M. D., and Denning, D. W. (2016). Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J. Infect. 72, 240–249. doi: 10.1016/j.jinf.2015.11.003
Patterson, T. F., Thompson, G. R. III., Denning, D. W., Fishman, J. A., Hadley, S., Herbrecht, R., et al. (2016). Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 63, e1–e60. doi: 10.1093/cid/ciw444
Prattes, J., Flick, H., Pruller, F., Koidl, C., Raggam, R. B., Palfner, M., et al. (2014). Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 190, 922–929. doi: 10.1164/rccm.201407-1275OC
Rivero-Menendez, O., Alastruey-Izquierdo, A., Mellado, E., and Cuenca-Estrella, M. (2016). Triazole resistance in aspergillus spp.: a worldwide problem? J. Fungi (Basel) 2:21. doi: 10.3390/jof2030021
Saraceno, J. L., Phelps, D. T., Ferro, T. J., Futerfas, R., and Schwartz, D. B. (1997). Chronic necrotizing pulmonary aspergillosis: approach to management. Chest 112, 541–548. doi: 10.1378/chest.112.2.541
Sato, H., Okada, F., Matsumoto, S., Mori, H., Kashiwagi, J., Komatsu, E., et al. (2018). The scab-like sign: a CT finding indicative of haemoptysis in patients with chronic pulmonary aspergillosis? Eur. Radiol. doi: 10.1007/s00330-018-5434-y. [Epub ahead of print].
Shahid, M., Malik, A., and Bhargava, R. (2001). Prevalence of aspergillosis in chronic lung diseases. Indian J. Med. Microbiol. 19, 201–205.
Shin, B., Koh, W. J., Jeong, B. H., Yoo, H., Park, H. Y., Suh, G. Y., et al. (2014). Serum galactomannan antigen test for the diagnosis of chronic pulmonary aspergillosis. J. Infect. 68, 494–499. doi: 10.1016/j.jinf.2014.01.005
Syhre, M., Scotter, J. M., and Chambers, S. T. (2008). Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 46, 209–215. doi: 10.1080/13693780701753800
Takeda, K., Imamura, Y., Takazono, T., Yoshida, M., Ide, S., Hirano, K., et al. (2016). The risk factors for developing of chronic pulmonary aspergillosis in nontuberculous mycobacteria patients and clinical characteristics and outcomes in chronic pulmonary aspergillosis patients coinfected with nontuberculous mycobacteria. Med. Mycol. 54, 120–127. doi: 10.1093/mmy/myv093
Tashiro, T., Izumikawa, K., Tashiro, M., Takazono, T., Morinaga, Y., Yamamoto, K., et al. (2011). Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med. Mycol. 49, 581–587. doi: 10.3109/13693786.2010.548084
Uffredi, M. L., Mangiapan, G., Cadranel, J., and Kac, G. (2003). Significance of Aspergillus fumigatus isolation from respiratory specimens of nongranulocytopenic patients. Eur. J. Clin. Microbiol. Infect. Dis. 22, 457–462. doi: 10.1007/s10096-003-0970-y
Urabe, N., Sakamoto, S., Sano, G., Suzuki, J., Hebisawa, A., Nakamura, Y., et al. (2017). Usefulness of two Aspergillus PCR assays and Aspergillus galactomannan and beta-d-glucan testing of bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. J. Clin. Microbiol. 55, 1738–1746. doi: 10.1128/JCM.02497-16
van der Linden, J. W., Arendrup, M. C., Warris, A., Lagrou, K., Pelloux, H., Hauser, P. M., et al. (2015). Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 21, 1041–1044. doi: 10.3201/eid2106.140717
van der Linden, J. W., Snelders, E., Kampinga, G. A., Rijnders, B. J., Mattsson, E., Debets-Ossenkopp, Y. J., et al. (2011). Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg. Infect. Dis. 17, 1846–1854. doi: 10.3201/eid1710.110226
White, P. L., Parr, C., Thornton, C., and Barnes, R. A. (2013). Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 51, 1510–1516. doi: 10.1128/JCM.03189-12
White, P. L., Wingard, J. R., Bretagne, S., Löffler, J., Patterson, T. F., Slavin, M. A., et al. (2015). Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin. Infect. Dis. 61, 1293–1303. doi: 10.1093/cid/civ507
Keywords: aspergillosis, Aspergillus, galactomannan, Aspergillus IgG antibody, azole resistance
Citation: Takazono T and Izumikawa K (2018) Recent Advances in Diagnosing Chronic Pulmonary Aspergillosis. Front. Microbiol. 9:1810. doi: 10.3389/fmicb.2018.01810
Received: 23 April 2018; Accepted: 18 July 2018;
Published: 17 August 2018.
Edited by:
Helmut J. F. Salzer, Forschungszentrum Borstel (LG), GermanyReviewed by:
Ritesh Agarwal, Post Graduate Institute of Medical Education and Research, IndiaCopyright © 2018 Takazono and Izumikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takahiro Takazono, dGFrYWhpcm8tdGFrYXpvbm9AbmFnYXNha2ktdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.