- 1Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
- 2Wuhan Botanical Garden, University of Chinese Academy of Sciences (UCAS), Beijing, China
- 3The Institute for Advanced Study in Coastal Ecology, Ludong University, Yantai, China
Cadmium (Cd) pollution is becoming increasingly prevalent, posing a global environmental hazard due to its negative effects on plants growth and human health. Phytoremediation is a green technology that involves uptake of Cd from the soil by a combination of plants and associated microbes. The objective of this study was to investigate the role of Aspergillus aculeatus in perennial ryegrass Cd tolerance. This fungus produced indole-3-acetic acid, siderophores, and 1-aminocyclopropane-1-carboxylate deaminase. Physiological traits including growth rate, turf quality and chlorophyll content were measured to evaluate the physiological responses of perennial ryegrass to Cd stress. These physiological traits were improved after inoculated with A. aculeatus. Inoculation of A. aculeatus actively reduced DTPA-Cd concentration in the soil and Cd translocation to plant shoots. Chlorophyll a fluorescence transient and the C/N ratio in shoots were elevated by A. aculeatus, which implied that the fungus could protect the photosystem II against Cd stress and increase the photosynthetic efficiency. These results suggested that A. aculeatus is beneficial in improving Cd tolerance of perennial ryegrass and reducing Cd-induced injuries, thus, it has promising potential for application of phytostabilization in Cd contaminated soil.
Introduction
Cadmium (Cd) is a notorious toxic heavy metal pollutant, which is introduced into the soil mainly from the applications of P-fertilizers, atmospheric deposition, sewage sludge, and smelting of metals (Smolders and Mertens, 2013). Cd with high mobility can be assimilated by plants and then translocated into edible parts and eventually enter the food chain to undermines animals and human health (Wagner, 1993). Moreover, increased accumulation of Cd in human body can lead to bone disease (van de Mortel et al., 2008). Hence, finding an effective remediation for Cd-contaminated environments is an ecological priority.
Phytoremediation is a relatively new technique, utilizing plants to remediate polluted soils (Glick, 2003). Categorically, it has been classified into several approaches such as phytoextraction, phytostabilization, and phytostimulation. However, there are some limiting factors to this technology. For example, there need for a longer treatment time to reach a satisfying effect because the plants with poor adaptability grow slowly and absorb subtle amounts of heavy metal from the contaminated soil (Cunningham et al., 1995).
Many microbial communities are sensitive to heavy metals. It has been reported that various soil microorganisms can dispose toxic chemical compounds in the soil by degradation, hence such microbes are commonly applied in bioremediation (Hallberg and Johnson, 2005; Kao et al., 2006; Umrania, 2006). Bioremediation approaches based on microbial-plant combinations might contribute to the success of phytoremediation and mitigating the heavy metal-polluted soil.
Plant growth-promoting microbes have attracted remarkable attention because they can greatly accelerate the plants bioremediation process by stimulating growth through various mechanisms. Aspergillus aculeatus (A. aculeatus), which was isolated from a Cd contaminated site, showed tolerance to Cd and was identified as a kind of Cd-resistant fungus strain (Xie et al., 2014b). Previous research has also confirmed that A. aculeatus inoculation on bermudagrass could improve turf quality and assuage the Cd toxicity for plants grown in Cd polluted soil (Xie et al., 2014b). Furthermore, this fungus has the characteristics of absorbing Cd and therefore has been widely used to remove Cd from industrial wastewater (Pandey and Banerjee, 2012). Our preliminary study has shown that the fungus enters roots outer epidermic cells of ryegrass grown in A. aculeatus-inoculated substrate (data not shown). Consequently, the fungus has become a suitable plant growth-promoting microbe in Cd-polluted soil and has a high potential to improve the efficiency of phytoremediation.
Perennial ryegrass (Lolium perenne L.) is an important grass which is widely used in many countries. It can enrich Cd in the soil (Xiong et al., 2007; Luo et al., 2011). Previous investigation has indicated that perennial ryegrass can grow normally on Cd-polluted soil which is attributed to its higher tolerance to Cd (Arienzo et al., 2004). Therefore, the perennial ryegrass with high biomass yields is an appropriate species to apply in the remediation of heavy metals contaminated soil.
The effects of A. aculeatus on turf quality, chlorophyll content and photochemical efficiency on bermudagrass exposed to Cd stress have been well addressed, but little is known about the influence on perennial ryegrass in response to Cd stress. Thus, for illuminating the potential function of A. aculeatus in phytoremediation, it is necessary to study on the physiological traits of perennial ryegrass exposed to Cd stress after inoculated with this fungus. The toxicity of Cd on plants can be evaluated by measuring some associated physiological traits of plants under the Cd stress. It has been confirmed that Cd can tamper with many biochemical and physiological processes in plants such as photosynthesis, respiration and nitrogen metabolism (Gupta et al., 2009; Zhang et al., 2009; Piotrowska et al., 2010). In addition, Cd accumulation in plants causes stunted plant growth, chlorosis in plant tissue, lower uptake of water and nutrients by roots and higher electrolyte leakage, which could be assessed as membrane damage by measuring the ion leakage of the cell (Garg and Aggarwal, 2012).
In this study, we designed a pot experiment to measure these physiological traits including the growth rate, chlorophyll content, chlorophyll a fluorescence transient, water content, electrolyte leakage, some critical ions content and C/N ratio in the plants. The study was designed to investigate perennial ryegrass physiological responses to Cd stress after inoculation with A. aculeatus and what influence the A. aculeatus has on the capacity of perennial ryegrass to resist Cd stress.
Materials and Methods
Preparation of Growth Matrix
A. aculeatus preserved in Martin medium at the −80°C was activated by streaking the culture fresh Martin medium, and incubation for 48 h at 30°C. After a 48 h of propagation phase, the A. aculeatus were transferred into flasks containing 150 mL of liquid Martin medium (Xie et al., 2014b). All flasks inoculated with A. aculeatus were placed in the Orbital Shaker Incubator at 180 rpm cultured for 72 h at 30°C.
The substrate was made by mixing sand with sawdust and stirring thoroughly at a ratio of 2:1 (v/v) then the mixture was divided into 30 groups, of 500 g each. All substrates were sterilized before use and half of them were inoculated with the same amount of A. aculeatus which was filtered with multilayer gauze from the liquid medium after 72 h of establishment and washed with the sterile water in advance. Then all the substrates were cultured at 30°C for 48 h before moved into container (15 cm in diameter and 20 cm tall). The substrate prepared previous were considered as the “soil” in the following context.
Plant Materials and Growth Conditions
Seeds of the perennial ryegrass (cv. ‘lark’) were selected based on their uniformity and sown in plastic pots (13 cm in diameter and 15 cm deep) filled with solid growth substances (nutrient soils: sand = 2:1, v/v). All the pots were maintained in a plant growth chamber for 45 days with a daily temperature regime of 26/20°C (day/night), 14 h daylight, photosynthetic active radiation (PAR) levels of 720 μmol m−2s−1 and irrigated with 200 mL half-strength Hoagland's solution (Hogland and Arnon, 1950) every week.
After a 45-days period of growth, the roots were taken out from the pots and rinsed with tap water until all the growth substances adhered to the root surface were removed. An equal proportion of plants with similar growth vigor were selected and transplanted into the soil above-mentioned. Before the Cd treatments, all the plants were placed in plant growth chamber for 2 weeks with the same conditions as before.
Experimental Treatments
After 2-weeks of adaptation period, perennial ryegrass was subjected to three Cd concentration treatments: 0 (half-strength Hoagland nutrition as the control), 200 and 400 mg kg−1 Cd (CdSO4·8/3 H2O dissolved in the half-strength Hoagland nutrition). The 30 pots containing grass were distributed into 6 groups in a randomized complete block design and were used to perform different treatments, including control (0 Cd, 0 Cd + A. aculeatus), Cd treatment only (200 Cd, 400 Cd), Cd treatment coupled with A. aculeatus (200 Cd+A. aculeatus, 400 Cd+A. aculeatus). The plants were trimmed to equal height (10 cm tall) in order to maintain consistency. The fungus-inoculated pots were supplemented with 15 mL of fungal suspension at the rhizosphere of plants before the Cd treatment.
The final Cd concentration was reached by irrigating soil with 100 mL of half-strength Hoagland solution containing set volume of 10 mM CdSO4 on the first, 5th, 10th, and 15th day after the treatment was initiated. On the other hand, the control groups were also irrigated with the same volume of half-strength Hoagland solution without CdSO4. There was a saucer under each pot to avoid fungus leaching and make sure all of the irrigation solution was reabsorbed. The whole treatment process was conducted in plant growth chamber with the above-mentioned conditions and lasted for 18 days.
Measurements
IAA, Siderophore and ACC Deaminase Production
The iodole-3-acetic acid (IAA) concentration in culture was detected with the presence of L-tryptophan according to the procedures mentioned in previous study (Gordon and Weber, 1951). The absorbance values of pink color formed after 25 min incubation at 530 nm were read and calibrated with calibration curve of pure IAA to calculate the IAA concentration in culture. The siderophore secreted by the fungus was detected following the procedure in previous study (Schwyn and Neilands, 1987) with the supplement of dye Chrome azurol S (CAS) in blue agar plates. The siderophore excretion generated by the fungus developed into orange halos around the colonies on blue agar. Otherwise, the amount of α-ketobutyrate (α KB) generated by the enzymatic hydrolysis of 1-aminocyclopropane-1-carboxylate (ACC) were monitored to determine the ACC deaminase activity (Saleh and Glick, 2001).
Relative Growth Rate
Relative growth rate (RGR) was calculated by Equation (1). The RGR of the shoot was calculated by measuring the vertical average height of grass recorded as H0 before Cd treatment; after the treatment the height recorded as Ht. The interval time between two measurements were recorded as Δt. In order to determine the root RGR, the final average root length was measured when the roots harvested, the initial length of root was set at 7 cm.
Turf Quality
Turf quality (TQ) was evaluated visually by three different researchers and was based on the degree of shoot wilting and leaf senescence, turf grass color and plant density using a regime of nine points where 9 means the grass is uniform, dense and green; 0 represents senesced, yellow and dead grass; 6 is minimum acceptable level of quality (Turgeon, 2002).
Chlorophyll Content
Chlorophyll (Chl) content was measured according to the previous method (Hiscox and Israelstam, 1979) and the 3rd fully expanded blades of the grass were collected and cut into pieces.
The Fluorescence Transient of Chlorophyll
Chlorophyll a fluorescence transient (OJIP transient) were measured using a pulse-amplitude modulation fluorometer (PAM 2500, Heinz Walz GmbH. Effeltrich, Germany), and the time resolution was set at 10 μs. The 3rd fully expanded leaves of the ryegrass were selected for subsequent measurement of fluorescence transient with 8 replicates of each treatment. Before the measurement, a dark adaptation of leaves was conducted for 25 min. Chlorophyll a fluorescence transient containing many fluorescence parameters and crucial information about photosynthesis can be calculated by the JIP-Test according to previous studies (Han et al., 2009; Chen et al., 2013).
The Shoot Water Content
At the end of the assay, samples were separated into two parts: shoots and roots. The shoots of ryegrass were weighted and recorded as the fresh weight (FW). Subsequently, the shoots were oven killed for 30 min at 105°C, and then dried for 3 days at 80°C until a constant weight was attained. The samples were recorded as the dry weight (DW). Finally, the shoot water content was calculated by the Equation (2):
The Electrolyte Leakage
The 3rd fully expanded leaves of the ryegrass per pot were collected to determine the electrolyte leakage (EL). The samples were washed several times using deionized water and then dried out with filter paper. After washing, the leaves were cut into small pieces and 0.2 g was weighed and added into 50-mL tubes containing 10 mL deionized water. The tubes were shaken on the table concentrator at 25°C for 24 h then the first electric conductivity was measured using the conductivity meter (JENCO-3173, JENCO Instruments, Inc., San Diego, CA, USA) and recorded as A. Subsequently, the tubes were autoclaved at 100°C for 15 min. When the tubes were cooled to room temperature and the second electric conductivity value was determined recorded as B. The EL of leaves was calculated by the Equation (3):
Ion Concentration and Soil pH
The shoots of perennial ryegrass were harvested individually after 18-days of treatments and then washed using sterilized ultra-purified water for three times. The leaves were dried for 3 days at 80°C and then ground into fine powder using multichannel tissue ball milling instrument (Scientz-192, Scientz biotechnology, GmbH, Ning Bo, China). The powder of shoot was weighed (0.1 g) separately and digested with a mixture of concentrated HNO3, HCl and HF (5:2:2, v/v/v). After digesting, the mixture was placed in heating plate at 135°C for 30 min to eliminate all acids. The residual solution was transferred into a 50-mL volumetric flask and rinsed with 1% HNO3 solution to 50 mL and evenly mixed. The purified solution was filtered through a 0.45 μm-millipore membrane and collected into a 15-mL tubes which was used to determine the Cd concentration by ICP-MS (X Series 2, Perkin Elmer, USA).
The soil DTPA-Cd concentrations extracted by diethylene-triaminepentaacetic acid (DTPA) and the other ions concentration (P, Cu, Ca, Mg, and Mn) were determined using ICP-OES (OPTIMA 8000DV, Perkin Elmer, USA). Each treatment had five replicates. The soil extracted by 10 mM CaCl2 solution was utilized for determining the pH of the soil under different treatments.
The Content of Total Organic C and N
The total organic C (TOC) and total N (NC) concentration in shoots and roots were measured by a stable isotope mass spectrometer (Delta V Advantage, Thermo Finnigan, Germany), running in continuous flow mode. Subsamples were oven-dried and grounded into fine powder of shoot samples (0.3–0.4 mg), root samples (0.5–0.55 mg), and urea (0.3–0.4 mg) and regarded as reference materials. The materials were put into tin capsules separately and sealed, and then placed into different holes of the automatic sampler in proper order before analysis. Finally, the content of TOC, TN, and the C/N ratio in shoot and root were determined by this apparatus.
Statistical Analyses
Each treatment had five replicates and all the values of this experiment were expressed as mean ± SD (standard deviation). The significant difference of means was determined based on Student-Newman-Keuls test (SNK) at 5% probability level. The data were analyzed by one-way analysis of variance (One-Way ANOVA) using SPSS statistical software package (version 20.0; SPSS Inc., Chicago, IL, USA).The graphs were created using SigmaPlot 12.3 (Systat Software, Richmond, CA) and Origin 9.0 (Origin Lab Inc., Hampton, USA) for OJIP transient.
Results
Characteristics of the Strains
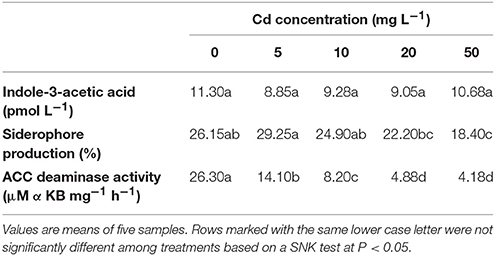
As showed in Table 1, the A. aculeatus strains were capable of producing siderophore, IAA with the presence of L-tryptophan in the medium and enhancing the ACC deaminase activity in culture. Furthermore, the Siderophore production and ACC deaminase activity significantly decreased with the Cd concentration increase, whereas the concentration of IAA was slightly decreased under Cd stress.
A. aculeatus Improved the Growth of Ryegrass Under Cd Stress
The non-inoculated plants showed stunted growth compared with inoculated under the Cd treatment. The relative growth rate of shoots and roots were both decreased when subjected to 200 mg kg−1 and 400 mg kg−1 Cd compared to the plants without Cd stress, to a sharper decline for the shoots between the plants without Cd stress and Cd-treated (Figures 1A,B). Moreover, the results indicated that the A. aculeatus could also cause a greater increase in root RGR under Cd stress (Figure 1A). Moreover, the RGR of the ryegrass shoots or roots inoculated with A. aculeatus was higher than those non-inoculated plants under the same level of Cd treatment.
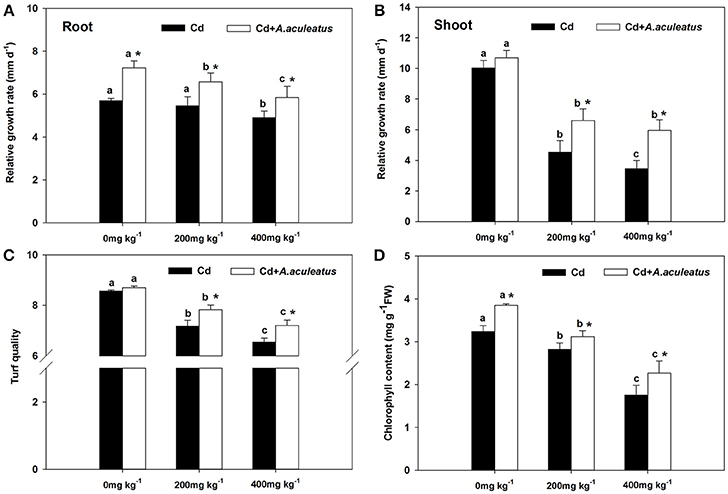
Figure 1. Effects of A. aculeatus on the relative growth rate in the root (A) or shoot (B) and the turf quality (C), chlorophyll content (D) of ryegrass grown with or without A. aculeatus under 0, 200 and 400 mg kg−1 Cd stress. Error bars, SD. Bars marked with same lower-case letter for a given treatment (i.e., A. aculeatus-inoculated or non-inoculated) were not significantly different for the comparison of Cd concentrations based on a SNK test at P < 0.05. Columns labeled with the * represent significant difference for the comparison between different inoculation treatments at the same level of Cd stress at P < 0.05 (SNK test).
Cd stress had the negative effect on turf quality and chlorophyll content which were both declined gradually with the increase of Cd (Figures 1C,D). Furthermore, A. aculeatus-inoculated plants had a greater level of turf quality and chlorophyll content than non-inoculated plants regardless of Cd concentrations. In addition, there were significant differences in chlorophyll content between the inoculated and non-inoculated plants (P < 0.05).
A. aculeatus Reduces Electrolyte Leakage in Cd-Treated Ryegrass
The water content of shoot and the electrolyte leakage of leaf responded differently to increasing Cd concentration (Figure 2). The former declined but the latter was improved by Cd stress throughout the assay. Although there was no obvious difference in water content between the inoculated and non-inoculated plants, the water content of A. aculeatus-inoculated regimes was slightly higher than non-inoculated plants under the same Cd exposure (Figure 2A). The electrolyte leakage of leaf samples was greatly enhanced with the rising Cd concentration (Figure 2B).The electrolyte leakage in leaves of inoculated plants was markedly decreased in contrast with non-inoculated plants when subjected to same Cd stress.
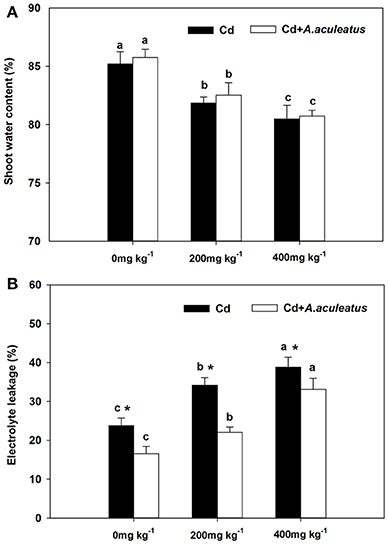
Figure 2. Effects of A. aculeatus on the water content of the shoot (A) and the electrolyte leakage (B) for perennial ryegrass subjected to different levels of Cd concentrations. Error bars, SD. Bars marked with same lower-case letter for a given treatment (i.e., A. aculeatus-inoculated or non-inoculated) were not significantly different for the comparison of Cd concentrations based on a SNK test at P < 0.05. Columns labeled with the * represent significant difference for the comparison between different inoculation treatments at the same level of Cd stress at P < 0.05 (SNK test).
A. aculeatus Enhances Photosynthetic Efficiency of Cd-Treated Ryegrass
OJIP fluorescence transient curves of the plants under different treatments were measured and plotted in Figure 3. There were different responses in OJIP fluorescence transient of ryegrass under Cd stress in the presence and absence of A. aculeatus. No significant difference between A. aculeatus-inoculated and non-inoculated plants was found in the condition without Cd stress (Figure 3A). However, when subjected to 200 mg kg−1 Cd stress, the plants without inoculation dropped dramatically compared to the inoculated plants (Figure 3B). Furthermore, the results showed that the ryegrass inoculated with A. aculeateus appeared a higher OJIP fluorescence transient than non-inoculated ryegrass under 200 and 400 mg kg−1 Cd stress (Figures 3B,C).
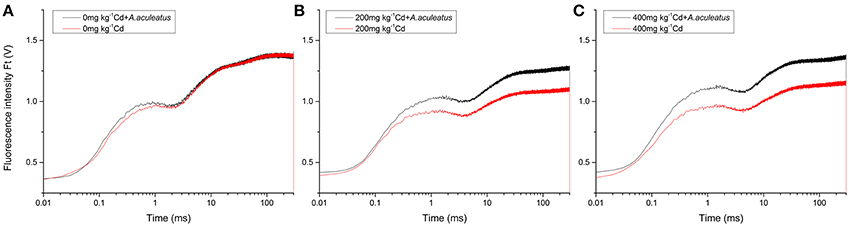
Figure 3. Effects of A. aculeatus on OJIP fluorescence transient for perennial ryegrass subjected to different levels of Cd concentrations. Plants were treated with 0 (A), 200 (B) and 400 mg kg−1 (C) Cd treatments.
The OJIP transient curve of the inoculated plants were not significantly affected by Cd stress compared with the plants without Cd treatment. Consequently, the OJIP transients of ryegrass leaves were significantly improved by A. aculeatus under Cd stress. Interestingly, both of the A. aculeatus-inoculated and non-inoculated plants had a slight increase in the OJIP transient curve when exposed to 400 mg kg−1 Cd treatment than 200 mg kg−1 Cd treatment respectively. Moreover, the J-step and I-step in OJIP fluorescence transient of plants under Cd stress were postponed contrasted with the control. The Cd stress significantly decreased OJIP fluorescence transient of ryegrass leaves, to a greater decline in the non-inoculated plants. In order to further explore the function of A. aculeatus on PSII in ryegrass under Cd stress, the JIP-test was analyzed based on the OJIP transient curves and the fluorescence parameters were listed in Table 2.
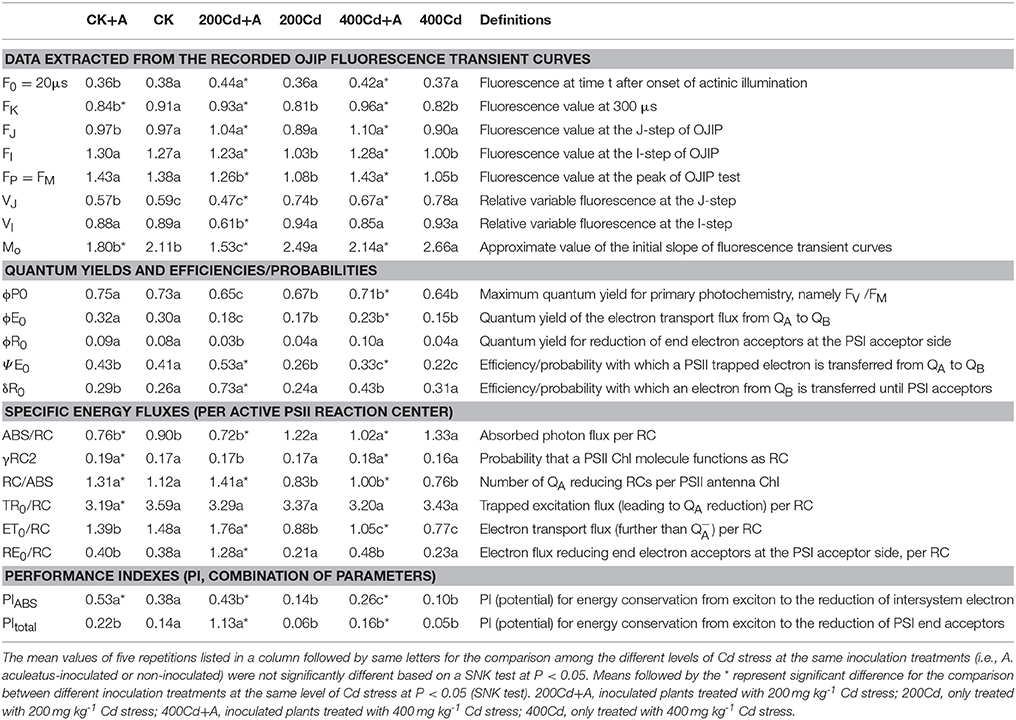
Table 2. Photosynthetic parameters calculated by analyzing the JIP-test of OJIP fluorescence transients.
The basic fluorescence parameters values extracted directly from the OJIP fluorescence transient curves displayed notable differences after treatments, such as F0, FK, FJ, FI, and FP = FM.. Five basic fluorescence parameters of inoculated ryegrass were significantly higher than the non-inoculated ryegrass when subjected to the same level of Cd stress (Table 2). Moreover, the plants treated with 400 mg kg−1 Cd and inoculated with A. aculeatus had a set of higher basic fluorescence parameters compared to the control groups, except for FI and FP. The inoculated plants under 200 mg kg−1 Cd treatment had higher F0, FK, FJ but a lower FP than control groups, overall, there was no significant difference in FI. The treatment with Cd stress only resulted in a significant increase in VJ, VI, and M0 which were calculated based on basic fluorescence parameters, however, after inoculation, the parameters were decreased remarkably than those Cd only treated.
The Cd stress significantly declined the ϕP0, ϕE0, ϕR0, and ΨE0 values which reflected Quantum yields and efficiencies. Furthermore, after inoculation with A. aculeatus, some of the parameters such as ϕE0, ΨE0, and δR0 were significantly improved caused by A. aculeatus. Particularly under the 400Cd+A treatment, ϕP0, ϕR0, and ΨE0 were also enhanced compared to the non-inoculated at the same levels of Cd treatment. The parameters of specific energy fluxes which were referred to ABS/RC, γRC2, RC/ABS, TR0/RC, RE0/RC, and ET0/RC also changed remarkably under different treatments. There was a greater decline in RC/ABS, ET0/RC and RE0/RC which were induced by Cd stress. Meanwhile, a lager rise in the ABS/RC of the leaves was observed after the Cd stress compared to the control, especially under the Cd only treatment regime. Inoculation with A. aculeatus significantly enhanced the parameters of specific energy fluxes except for the ABS/RC and TR0/RC compared to non-inoculated plants under the same levels of Cd stress. Both of the PIABS and PItotal were crucial indices, which play important roles in describing the overall activity of PSII. As shown in Table 2, there was a greater decline in both the PIABS and PItotal after Cd exposure. However, inoculation with A. aculeatus could significantly improve the values of the two indices. Interestingly, inoculated plants even under the Cd stress also had a higher PItotal values than control groups without Cd treatment.
Changes in Cd Uptake and Ionic Homeostasis
Cd concentration in the plants was strongly improved compared with the plants without Cd treatment after the 18-days Cd stress, to a larger extent in the non-inoculated plants. The significant decline of Cd concentration in shoot were observed in inoculated plants when exposed to 200 and 400 mg kg−1 Cd stress (Figure 4).
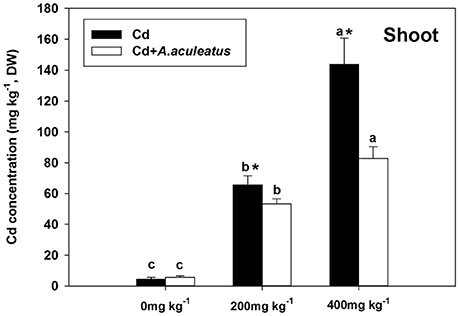
Figure 4. Effects of A. aculeatus on plant Cd concentration accumulated in shoot for perennial ryegrass subjected to different levels of Cd concentrations. Error bars, SD. Bars marked with same lower-case letter for a given treatment (i.e., A. aculeatus-inoculated or non-inoculated) were not significantly different for the comparison of Cd concentrations based on a SNK test at P < 0.05. Columns labeled with the * represent significant difference for the comparison between different inoculation treatments at the same level of Cd stress at P < 0.05 (SNK test).
Different ions contents in shoot and root tissues responded differently to Cd stress (Table 3). Ca2+ concentrations in shoot declined profoundly with the increasing Cd levels, however, the results shown in root were adverse. There was no difference in Ca2+ concentration between A. aculeatus-inoculated plants and non-inoculated regime shoots. A. aculeatus inoculation significantly improved the Ca2+ concentrations in root compared with the Cd only treated under the same Cd stress. The variation tendency of Cu2+ concentrations in the plant was similar with the Ca2+. Cd had no significant influence on Cu2+ concentrations in shoot but A. aculeatus improved the Cu2+ concentrations in root notably after exposed to 400 mg kg−1 Cd stress. When plants exposed to 400 mg kg−1 Cd stress, Mg2+ in shoot was significantly improved but in root were decreased pronouncedly. Inoculation with A. aculeatus caused a slight increase of Mg2+ in root when plants subjected to the 400 mg kg−1 Cd stress. Furthermore, a significant increase in Mg2+ content in inoculated root was observed compared to non-inoculated counterpart when subjected to 200 mg kg−1 Cd stress.
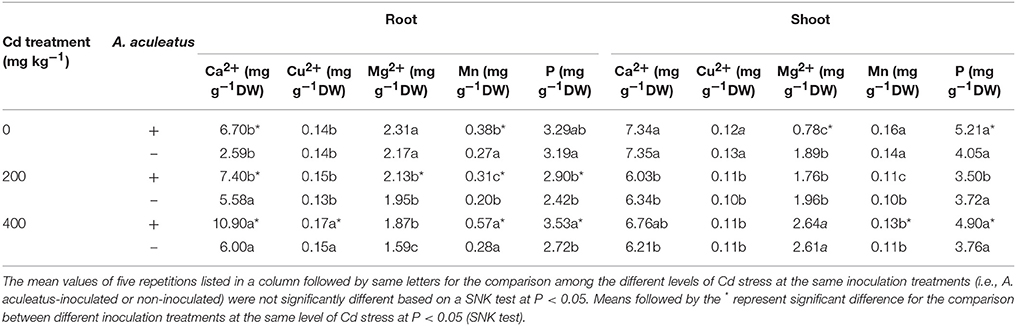
Table 3. Effects of A. aculeatus on calcium (Ca), cuprum (Cu), magnesium (Mg), manganese (Mn), and phosphorus (P) concentrations of perennial ryegrass subjected to different levels of Cd concentrations.
On the other hand, the accumulation of Mn in shoot and root were all reduced after 200 mg kg−1 Cd treatment, in contrast to the control groups without Cd treatment. Interestingly, when plants exposed to 400 mg kg−1 of Cd stress, there was a slight rise in Mn concentration both in shoots and roots compared to 200 mg kg−1 Cd level. By contrast, inoculation of A. aculeatus significantly increased the content of Mn in root (P < 0.05). The P concentrations in the plant displayed similar tendency with Mn. In shoot, the P concentrations were dramatically improved by A. aculeatus except at 200 mg kg−1 Cd exposure. Furthermore, A. aculeatus resulted in a significant increase in P concentration in root contrasted with uninfected plants under the same Cd treatment.
Changes in TOC and TN
The shoot TN content declined and showed a notable difference with the increasing Cd concentration (P < 0.05) from the Table 4. In addition, the ryegrass inoculated with A. aculeatus had a higher level of TN in shoot in contrast to uninfected plants when subjected to the same Cd stress. However, in root, TN content was gradually enhanced under different Cd stress, and there was no significant difference between infected and uninfected plants. TOC content in the shoot was not affected significantly by Cd treatment, but a notable decline was observed in root after the Cd stress. The results showed that A. aculeatus had no evident influence on the content of TOC compared to uninfected plants.
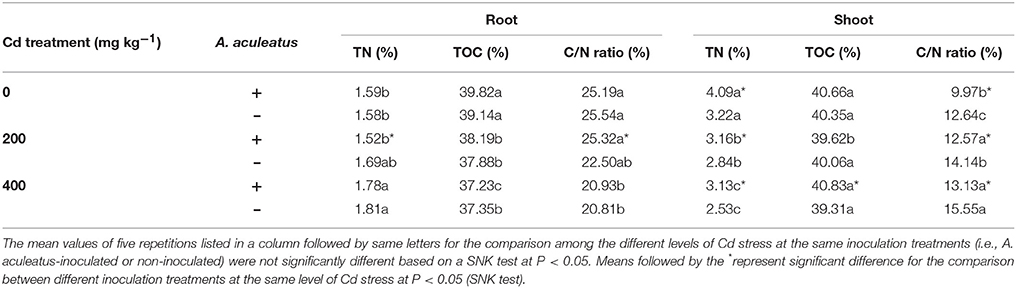
Table 4. Effects of A. aculeatus on the content of total organic C and total N in perennial ryegrass subjected to different levels of Cd concentrations.
The C/N ratio in the shoot were markedly increased after Cd treatment (P < 0.05). Furthermore, the ratio of C/N in the shoot was decreased remarkably by inoculation with A. aculeatus compared to uninfected plants. Nevertheless, in root, C/N ratio was declined significantly with the increasing levels of Cd stress. The ratio of C/N in root under the same Cd treatment was not sensitive to A. aculeatus, and no significant difference was observed in C/N ratio between inoculated and non-inoculated plants in root.
DTPA-Cd Concentration and pH Value in the Soil Affected by A. aculeatus
DTPA-Cd concentration was significantly improved with increasing level of Cd treatment (Figure 5). However, when subjected to 400 mg kg−1 Cd stress, a significant decrease in the DTPA-Cd concentration was detected in the A. aculeatus-inoculated soil compared with non-inoculated soil (Figure 5A).
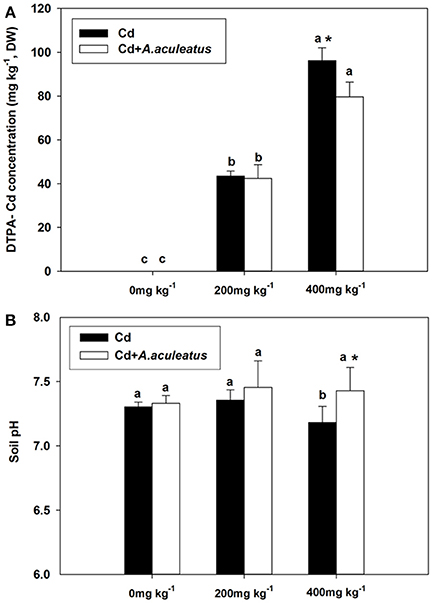
Figure 5. Effects of A. aculeatus on the DTPA-Cd concentration (A) and pH (B) in the soil for perennial ryegrass subjected to different levels of Cd concentrations. Error bars, SD. Bars marked with same lower-case letter for a given treatment (i.e.,A. aculeatus-inoculated or non-inoculated) were not significantly different for the comparison of Cd concentrations based on a SNK test at P < 0.05. Columns labeled with the * represent significant difference for the comparison between different inoculation treatments at the same level of Cd stress at P < 0.05 (SNK test).
The pH in the soil slightly increased under the 200 mg kg−1 Cd stress compared with the control without Cd stress (Figure 5B). Moreover, there was slight increase in the soil pH for inoculated soil than non-inoculated regime when subjected to three levels of Cd treatment. We found that 400 mg kg−1 Cd stress caused a significant decline in the pH of non-inoculated soil. A. aculeatus-inoculated soil had a significant rise in the soil pH compared with non-inoculated soil subjected to 400 mg kg−1 Cd exposure, the lowest pH in the soil was detected in the non-inoculated under 400 mg kg−1 Cd stress (Figure 5B).
Discussion
The Bacteria producing IAA, siderophores and ACC deaminase can contribute to plant growth (Glick et al., 1995), which was consistent with our results that A. aculeatus was able to produce IAA, siderophores and ACC deaminase to promote plant growth. In addition, Aspergillus aculeatus has rock phosphate solubilizing ability reported in previous research (Narsian and Patel, 2000). Previous observations showed that the strains PsA4 and Ba32 can protect the plants against the inhibitory effects of chromium, probably through solubilization of phosphate and IAA, siderophores (Rajkumar et al., 2006). These findings support previous study which has demonstrated that root colonizing microorganisms reduce deleterious effects of stresses probably through producing phytohormones (Frankenberger and Arshad, 1995). AMF has been reported to enhance IAA in leaves and roots and ABA in leaves of Lycium barbarum L. grown on saline soils (Liu et al., 2016) and it restored plant biomass which grown on Cu and Zn polluted site although higher Cu and Zn accumulation in roots (Cicatelli et al., 2010). This research suggested that Cd stress caused toxicity to perennial ryegrass. Previous studies have reported that Cd stress has a negative effect on the growth rate of bermudagrass (Xie et al., 2014c). Otherwise, the results not only indicated that Cd stress caused toxicity to perennial ryegrass, but also suggested A. aculeatus had a positive effect on the growth rate of perennial ryegrass. All substrate were sterilized by autoclaving before use to guarantee that it is the A. aculeatus strain that offered this tolerance rather than the total microbial community. Hassan et al. have reported that arbuscular mycorrhizal fungi inoculated on the sunflower could facilitate the growth of plant and alleviate the damage of Cd stress (Hassan et al., 2013). In addition, endophytic bacteria can also promote the growth of rape grown in Pb contaminated soil (Sheng et al., 2008) which supports our result that A. aculeatus-inoculated plants had a significant increase in RGR than non-inoculated regime when exposed to the same levels of Cd treatments (Figure 1).
The turf quality of plants decreased accompanied with the lower RGR, reduced chlorophyll content and the higher the electrolyte leakage under Cd stress. Fortunately, from the results we can deduce that the A. aculeatus could help improve the turf quality and chlorophyll content in leaves under Cd stress. It has also been demonstrated that endophytic fungus Piriformospora indica colonization increases chlorophyll content, plant biomass and lateral roots density of Arabidopsis under salt stress conditions (Abdelaziz et al., 2017). The degree of cellular damage can be determined according to the cell membrane stability (Saneoka et al., 2004). Hence, the electrolyte leakage which represents the degree of cellular damage was measured to evaluate the toxicity of Cd stress for perennial ryegrass. Remarkably, the results showed that A. aculeatus-inoculated plants had a lower electrolyte leakage than Cd only treated regime when exposed to the same Cd treatment, which indicated that A. aculeatus can alleviate the toxicity of Cd, such as chlorosis (Das et al., 1997; Zhou and Qiu, 2005). Previous investigation has also demonstrated that Cd stress can lead to chlorosis in plants and even death, while high Cd concentration reduce the chlorophyll content of perennial ryegrass (Milone et al., 2003), which supports the present results. The efficiency of photosynthesis has been reduced for the degradation of chlorophyll (Singh and Dubey, 1995; Luo et al., 2011). It has also documented that Cd could inhibit protochlorophyllide reductase and water-splitting enzyme which presents in the oxidizing site of photosystem II. Eventually, the chlorophyll biosynthesis was affected and the photosynthetic electron transport was also halted (Van and Clijsters, 1990). Chlorophyll a fluorescence is a good indicator to describe the degree of impairment which is usually used to study the photosystem of plants exposed to abiotic stress conditions (Chen et al., 2013; Roopin et al., 2013). Here we addressed the question on whether A. aculeatus plays any role in perennial ryegrass Cd tolerance by applying chlorophyll a fluorescence analysis (OJIP fluorescence transient and JIP-test). The plants treated with Cd showed severe decline of OJIP fluorescence transient than those untreated plants, however, the OJIP fluorescence transient were significantly enhanced in those inoculated plants compared with non-inoculated plants under the corresponding Cd treatments. This discovery implied that A. aculeatus had a positive effect on improving the resistance to Cd stress in the perennial ryegrass.
The fluorescence parameters were determined based on the OJIP fluorescence by JIP-test so that the energy flux through PSII could be quantified at the level of reaction center (RC) (Strasser and Strasser, 1995). On the one hand, we discovered that inoculation with A. aculeatus remarkably increased the basic parameters including FM, FJ, and FI, when the plants were subjected to the same Cd treatments. On the other hand, the M0, VI, and VJ values were all decreased notably in A. aculeatus-inoculated plants vs. non-inoculated. The significant difference in these parameters implied that A. aculeatus was actively responsible for Cd resistance of perennial ryegrass and can alleviate Cd toxicity on PSII system. The performance index PItotal is an important sensitive parameter to assess photochemical activities of the plant under the stress situation. It incorporates several independent parameters such as RC/ABS, ϕP0 and ΨE0 calculated from fluorescence transient OJIP (Clark et al., 2000; Yusuf, 2010). In our study, PItotal and PIABS were both greatly enhanced after inoculation with A. aculeatus under the Cd treatments, which indicated the protective role of A. aculeatus in Cd resistance. Furthermore, ϕP0, ϕR0, and ϕE0 which reflected Quantum yields and efficiencies were strongly improved by A. aculeatus when exposed to Cd stress. These findings suggested that A. aculeatus could affect the quantum yield of donor and acceptor located at different sides of PSII. In addition, grasses inoculated with A. aculeatus had a less decrease in ABS/RC and TR0/RC in contrast with those that were non-inoculated. These results further demonstrated that A. aculeatus could alleviate the damage in PSII RC caused by Cd toxicity.
A. aculeatus not only improved the efficiency of photosynthesis but also affected the content of TOC and TN in plants. In this study, the remarkable decline in shoot TN and root TOC under Cd stress implied that Cd stress might inhibit the transportation of C from leaves to roots and the N from root to shoot. Previous findings showed that Cd treatment caused an accumulation of NO3− in the root and changed the translocation of NO3− to the shoot (Hernandez et al., 1996). However, the greater accumulation of N in root resulted in the lower C/N ratio under the Cd treatments which indicated that Cd stress disturbed the balance between carbon metabolism and nitrogen metabolism in plants. Previous investigation had reported that the C/N in seagrass was decreased after exposure to diuron (a kind of herbicide) for 11 weeks (Negri et al., 2015). We hypothesized that Cd stress reduced the C/N ratio by declining photosynthetic C assimilation. Conversely, C/N ratios were enhanced in the shoots in the presence of Cd, which might be attributed to the lower accumulation of N. It has been demonstrated that Cd stress can inhibit NO3− assimilation in the shoot by declining relevant nitrate reductase (NR) activity (Hernandez et al., 1996). In addition, the plants inoculated with A. aculeatus significantly enhanced the C/N ratios by increasing the accumulation of N in shoot. This result further confirmed that A. aculeatus could improve the resistance to Cd stress in perennial ryegrass and relieve the damage to plant metabolism caused by Cd stress.
Additionally, the C/N ratios, the ionic homeostasis can also be regulated by A. aculeatus. It has been demonstrated that Cd can alter the concentration of mineral nutrition such as Mn in pea seedlings (Hernandez et al., 1996). It is more likely that P. indica colonization regulated ion homeostasis of Na+/K+ to promote Arabidopsis growth under salt stress (Abdelaziz et al., 2017). Different ions in different tissue of plant showed different ionic response to Cd stress. In this study, Mg concentration decreased in root but increased in shoot with the increasing Cd stress, which indicated the Cd stress might facilitate the transport of Mg from root to shoot. On the other hand, the Ca, P, and Mn concentrations in the root were severely affected among several ionic nutrients studied (i.e. Ca, Cu, Mg, Mn, or P), which were significantly increased by the inoculation of A. aculeatus compared with non-inoculated plants. Previous studies have reported that the hyphae of mycorrhizal fungus could efficiently absorb P (Li et al., 1991; Rufyikiri et al., 2004). Arbuscular mycorrhizal (AM) fungus and the root of host plants formed a symbiosis, in which nutrients could be transported in both directions (Smith and Read, 1997). Mycorrhizal inoculation improved cork oak forest resistance capacity to deal with coming climate change by forming symbiotic relationship and improve root capacity to take up nutrients (Sebastiana et al., 2017). Hence, we assumed that these significant differences were attributed to the interaction mechanism between the fungus and the root of perennial ryegrass which can help alleviate the Cd toxicity. A further investigation is required to clarify the integrated mechanism between fungus and perennial ryegrass under the Cd stress.
Generally, this study deduced that inoculation with A. aculeatus significantly decreased the available Cd in the soil. Previously, DTPA, which is a kind of chelate, was commonly used for extracting metals from soils (Misra et al., 1990; Erickson et al., 1991; Hughes and Noble, 1991) especially for heavy metals such as Cd, Zn, Pb (Ma et al., 1997). Recently, DTPA-Cd, which is recognized as a high-bioavailability chemical form of Cd has become a more available indicator to estimate the amount of Cd absorbed by plants and their toxicity effects for plants. Previous reports have confirmed that DTPA-extractable concentrations are more available to plants due to their higher mobility (Lindsay and Norvell, 1978; Petruzzelli, 1989; Ma et al., 1997; Zufiaurre et al., 1998). The DTPA-Cd concentration in the soil was significantly enhanced with the increasing level of Cd treatment. Meanwhile, A. aculeatus resulted in a remarkable decrease of DTPA-Cd when exposed to 400 mg kg−1 Cd stress. Interestingly, the highest concentration of DTPA-Cd was found in the soils which were non-inoculated and with lower soil pH. This was more likely to have resulted from the low soil pH values. Similar observations have been made in previous study using carambola trees (Li et al., 2006). The pH and organic matter were confirmed to be two critical factors affecting the heavy metal from soil absorbed by plants (Mclaughlin et al., 1999; Evangelou et al., 2004). Moreover, it has been demonstrated that soil pH is closely related to heavy metals bioavailability (Li et al., 2006). In addition, Cd existed mainly as free ions forms in the acidic pH soil solutions (Ge et al., 2000) hence we inferred that Cd has a higher bioavailability in the relative lower pH. However, the present results showed that soil pH was higher in the A. aculeatus-inoculated soil compared with uninfected soil. Therefore, we presumed that the A. aculeatus could help to reduce Cd availability in the soil by improving the soil pH.
Furthermore, we found that the Cd concentration in shoots were lower in A. aculeatus-inoculated plants than Cd only treated regime when objected to the same Cd treatments due to the lower available Cd in the soil caused by A. aculeatus. Otherwise, a previous investigation has also reported that A. aculeatus-inoculated bermudagrass have a lower Cd concentration in leaves (Xie et al., 2014b) which is consistent with our results. The present study suggested that inoculation with A. aculeatus inhibited the Cd transportation to shoots and similar results have been reported in the sunflowers inoculated with F. mosseae strain (Hassan et al., 2013). Moreover, another previous study indicated that the Cd-tolerant genotype WB242 of bermudagrass accumulated less Cd in the shoot when subjected to Cd stress (Xie et al., 2014a), which also supports our results. The A. aculeatus decreased available Cd in the soil and accumulated much Cd in their mycelia which led to the lower Cd level in plant so that it can filter out Cd and prevent absorption by roots.
Conclusions
The A. aculeatus strains colonizing the roots outer epidermal interiors might promote the plant growth and alleviate the toxic effects of high concentrations of Cd by secreting IAA, siderophore or ACC deaminase. In summary, A. aculeatus could alleviate Cd toxicity in their host plants and strengthen the Cd-resistant in perennial ryegrass by improving soil pH to reduce available Cd in soil, inhibiting the uptake of Cd by plants. Through these mechanisms, A. aculeatus facilitated the growth rate of perennial ryegrass under Cd stress, remarkably improved turf quality, chlorophyll content and photosynthetic efficiency and significantly decreased the electrolyte leakage. Moreover, the nitrogen and carbon metabolism and ionic homeostasis were all regulated by A. aculeatus to defense against Cd damage (Figure 6). All these evidences had confirmed that A. aculeatus is a potential candidate to be applied into the phytostabilization of Cd contaminated soil. However, there might be other unknown mechanisms underlying this phenomenon and thus further research on relationship between the plant and this fungus is essential.
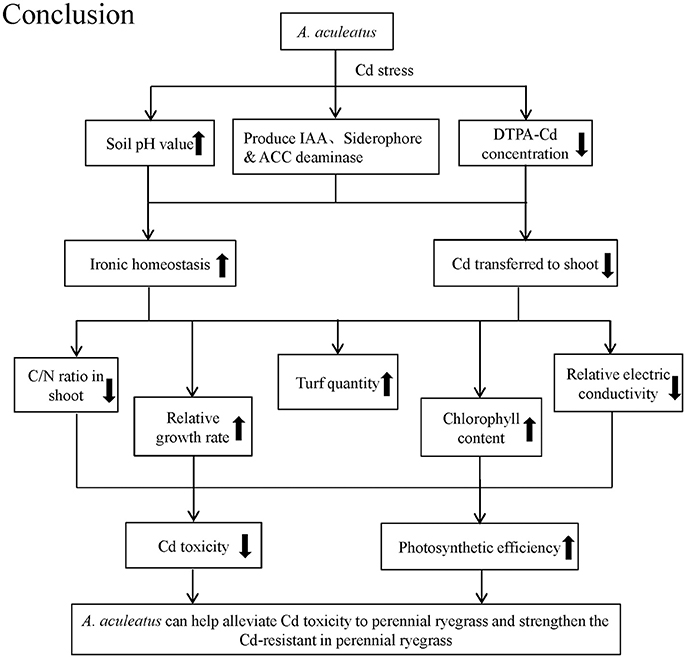
Figure 6. The conclusions about how A. aculeatus affected perennial ryegrass when exposed to different levels of Cd concentrations.
Author Contributions
JF and YX designed the experiments; SH and XL performed the experiments, analyzed the data and wrote the manuscript; EA, YX, and JF revised the manuscript. All authors approved the final manuscript and declare no competing financial interests.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31401915).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdelaziz, M. E., Kim, D., Ali, S., Fedoroff, N. V., and Al-Babili, S. (2017). The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 263, 107–115. doi: 10.1016/j.plantsci.2017.07.006
Arienzo, M., Adamo, P., and Cozzolino, V. (2004). The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 319, 13–25. doi: 10.1016/S0048-9697(03)00435-2
Chen, K., Chen, L., Fan, J., and Fu, J. (2013). Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res. 116, 21–31. doi: 10.1007/s11120-013-9883-5
Cicatelli, A., Lingua, G., Todeschini, V., Biondi, S., Torrigiani, P., and Castiglione, S. (2010). Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann. Bot. 106, 791–802. doi: 10.1093/aob/mcq170
Clark, A. J., Landolt, W., Bucher, J. B., and Strasser, R. J. (2000). Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 109, 501–507. doi: 10.1016/S0269-7491(00)00053-1
Cunningham, S. D., Berti, W. R., and Huang, J. W. (1995). Phytoremediation of contaminated soils. Trends Biotechnol. 13, 393–397. doi: 10.1016/S0167-7799(00)88987-8
Das, P., Samantaray, S., and Rout, G. R. (1997). Studies on cadmium toxicity in plants: a review. Environ. Pollut. 98, 29–36 doi: 10.1016/S0269-7491(97)00110-3
Erickson, D. C., White, E., and Loehr, R. C. (1991). Comparison of extraction fluids used with contaminated soils. Hazard. Waste Hazard. Mater. 8, 185–194 doi: 10.1089/hwm.1991.8.185
Evangelou, M. W., Daghan, H., and Schaeffer, A. (2004). The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 57, 207–213. doi: 10.1016/j.chemosphere.2004.06.017
Frankenberger, W. Jr, and Arshad, M. (1995). “Microbial Production and Function,” Phytohormones in Soils (New York, NY: Marcel Dekker Inc.).
Garg, N., and Aggarwal, N. (2012). Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. genotypes grown in cadmium and lead contaminated soils. Plant Growth Regul. 66, 9–26. doi: 10.1007/s10725-011-9624-8
Ge, Y., Murray, P., and Hendershot, W. H. (2000). Trace metal speciation and bioavailability in urban soils. Environ Pollut. 107, 137–144. doi: 10.1016/S0269-7491(99)00119-0
Glick, B. R. (2003). Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 21, 383–393. doi: 10.1016/S0734-9750(03)00055-7
Glick, B. R., Karaturovíc, D. M., and Newell, P. C. (1995). A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can. J. Microbiol. 41, 533–536. doi: 10.1139/m95-070
Gordon, S. A., and Weber, R. P. (1951). Colorimetric estimation of indoleacetic acid. Plant Physiol. 26:192. doi: 10.1104/pp.26.1.192
Gupta, D. K., Nicoloso, F. T., Schetinger, M. R., Rossato, L. V., Pereira, L. B., Castro, G. Y., et al. (2009). Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 172, 479–484. doi: 10.1016/j.jhazmat.2009.06.141
Hallberg, K. B., and Johnson, D. B. (2005). Microbiology of a wetland ecosystem constructed to remediate mine drainage from a heavy metal mine. Sci. Total Environ. 338, 53–66. doi: 10.1016/j.scitotenv.2004.09.005
Han, S., Tang, N., Jiang, H. X., Yang, L. T., Li, Y., and Chen, L. S. (2009). CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci. 176, 143–153. doi: 10.1016/j.plantsci.2008.10.004
Hassan, S. E., Hijri, M., and St-Arnaud, M. (2013). Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. N. Biotechnol. 30, 780–787. doi: 10.1016/j.nbt.2013.07.002
Hernandez, L. E., CarpenaRuiz, R., and Garate, A. (1996). Alterations in the mineral nutrition of pea seedlings exposed to cadmium. J. Plant Nutr. 19, 1581–1598. doi: 10.1080/01904169609365223
Hiscox, J. D., and Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Can.J. Bot. 57, 1332–1334 doi: 10.1139/b79-163
Hogland, C., and Arnon, D. (1950). The solution-culture method for growing plants without soil. Calif. Agric. Exp. Circ. 247.
Hughes, J. C., and Noble, A. D. (1991). Extraction of chromium, nickel and iron and the availability of chromium and nickel to plants from some serpentinite-derived soils from the eastern Transvaal as revealed by various single and sequential extraction techniques. Commun. Soil Sci. Plant Anal. 22, 1753–1766. doi: 10.1080/00103629109368533
Kao, P. H., Huang, C. C., and Hseu, Z. Y. (2006). Response of microbial activities to heavy metals in a neutral loamy soil treated with biosolid. Chemosphere 64, 63–70. doi: 10.1016/j.chemosphere.2005.11.039
Li, J. T., Qiu, J. W., Wang, X. W., Zhong, Y., Lan, C. Y., and Shu, W. S. (2006). Cadmium contamination in orchard soils and fruit trees and its potential health risk in Guangzhou, China. Environ. Pollut. 143, 159–165. doi: 10.1016/j.envpol.2005.10.016
Li, X. L., George, E., and Marschner, H. (1991). Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 136, 41–48. doi: 10.1007/BF02465218
Lindsay, W. L., and Norvell, W. A. (1978). Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 42, 421–428. doi: 10.2136/sssaj1978.03615995004200030009x
Liu, H., Wang, Y., Hart, M., Chen, H., and Tang, M. (2016). Arbuscular mycorrhizal symbiosis regulates hormone and osmotic equilibrium of Lycium barbarum L. Under salt stress. Mycosphere 7, 828–843. doi: 10.5943/mycosphere/7/6/14
Luo, H., Li, H., Zhang, X., and Fu, J. (2011). Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 20, 770–778. doi: 10.1007/s10646-011-0628-y
Ma, L. Q., Choate, A. L., and Rao, G. N. (1997). Effects of Incubation and Phosphate rock on lead extractability and speciation in contaminated soils. J. Environ. Qual. 26, 801–807. doi: 10.2134/jeq1997.00472425002600030030x
Mclaughlin, M. J., Parker, D. R., and Clarke, J. M. (1999). Metals and micronutrients – food safety issues. Field Crops Res. 60, 143–163. doi: 10.1016/S0378-4290(98)00137-3
Milone, M. T., Sgherri, C., Clijsters, H., and Navari-Izzo, F. (2003). Antioxidative responses of wheat treated with realistic concentration of cadmium. Environ.Exp. Bot. 50, 265–276. doi: 10.1016/S0098-8472(03)00037-6
Misra, A. K., Sarkunan, V., Das, M., and Nayar, P. K. (1990). Transformation of added heavy metals in soils under flooded condition. J. Indian Soc. Soil Sci. 38, 416–418.
Narsian, V., and Patel, H. H. (2000). Aspergillus aculeatus as a rock phosphate solubilizer. Soil Biol. Biochem. 32, 559–565. doi: 10.1016/S0038-0717(99)00184-4
Negri, A. P., Flores, F., Mercurio, P., Mueller, J. F., and Collier, C. J. (2015). Lethal and sub-lethal chronic effects of the herbicide diuron on seagrass. Aquat. Toxicol. 165, 73–83. doi: 10.1016/j.aquatox.2015.05.007
Pandey, A., and Banerjee, D. (2012). Biosorption of cadmium (II) using discarded biomass of Aspergillus aculeatus DBF9. Terrestrial Aquat. Environ. Toxicol. 6, 8–13.
Petruzzelli, G. (1989). Recycling wastes in agriculture: heavy metal bioavailability. Agric. Ecosyst. Environ. 27, 493–503. doi: 10.1016/0167-8809(89)90110-2
Piotrowska, A., Bajguz, A., Godlewskazyłkiewicz, B., and Zambrzycka, E. (2010). Changes in growth, biochemical components, and antioxidant activity in aquatic plant Wolffia arrhiza (Lemnaceae) exposed to cadmium and lead. Arch. Environ. Contam. Toxicol. 58, 594–604. doi: 10.1007/s00244-009-9408-6
Rajkumar, M., Nagendran, R., Lee, K. J., Lee, W. H., and Kim, S. Z. (2006). Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62, 741–748. doi: 10.1016/j.chemosphere.2005.04.117
Roopin, M., Yacobi, Y. Z., and Levy, O. (2013). Occurrence, diel patterns, and the influence of melatonin on the photosynthetic performance of cultured Symbiodinium. J. Pineal Res. 55, 89–100. doi: 10.1111/jpi.12046
Rufyikiri, G., Declerck, S., and Thiry, Y. (2004). Comparison of 233U and 33P uptake and translocation by the arbuscular mycorrhizal fungus Glomus intraradices in root organ culture conditions. Mycorrhiza 14, 203–207. doi: 10.1007/s00572-003-0258-1
Saleh, S. S., and Glick, B. R. (2001). Involvement of gacS and rpoS in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and UW4. Can. J. Microbiol. 47:698. doi: 10.1139/w01-072
Saneoka, H., Moghaieb, R. E. A., Premachandra, G. S., and Fujita, K. (2004). Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ. Exp. Bot. 52, 131–138. doi: 10.1016/j.envexpbot.2004.01.011
Schwyn, B., and Neilands, J. (1987). Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. doi: 10.1016/0003-2697(87)90612-9
Sebastiana, M., Martins, J., Figueiredo, A., Monteiro, F., Sardans, J., Peñuelas, J., et al. (2017). Oak protein profile alterations upon root colonization by an ectomycorrhizal fungus. Mycorrhiza 27, 109–128. doi: 10.1007/s00572-016-0734-z
Sheng, X. F., Xia, J. J., Jiang, C. Y., He, L. Y., and Qian, M. (2008). Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 156, 1164–1170. doi: 10.1016/j.envpol.2008.04.007
Singh, A. K., and Dubey, R. S. (1995). Changes in chlorophyll a and b contents and activities of photosystems I and II in rice seedlings induced by NaCl. Photosynthetica 31, 489–499.
Smolders, E., and Mertens, J. (2013). Cadmium. Environ. Pollut. 22, 283–311. doi: 10.1007/978-94-007-4470-7_10
Strasser, B. J., and Strasser, R. J. (1995). “Measuring fast fluorescence transients to address environmental questions: the JIP-test,” in Photosynthesis: from Light to Biosphere, Vol. 5, (Dordrecht: Kluwer Academic Publishers)
Umrania, V. V. (2006). Bioremediation of toxic heavy metals using acidothermophilic autotrophes. Bioresour. Technol. 97, 1237–1242. doi: 10.1016/j.biortech.2005.04.048
Van, A. F., and Clijsters, H. (1990). A biological test system for the evaluation of the phytotoxicity of metal-contaminated soils. Environ. Pollut. 66, 157–172. doi: 10.1016/0269-7491(90)90118-V
van de Mortel, J. E., Schat, H., Moerland, P. D., Ver Loren van Themaat, E., van der Ent, S., Blankestijn, H., et al. (2008). Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 31, 301–324. doi: 10.1111/j.1365-3040.2007.01764.x
Wagner, G. J. (1993). Accumulation of cadmium in crop plants and its consequences to human health. Adv. Agron. 51, 173–212. doi: 10.1016/S0065-2113(08)60593-3
Xie, Y., Hu, L., Du, Z., Sun, X., Erick, A., Fan, J., et al. (2014a). Effects of cadmium exposure on growth and metabolic profile of bermudagrass [Cynodon dactylon(L.) Pers.]. PLoS ONE 9:e115279. doi: 10.1371/journal.pone.0115279
Xie, Y., Luo, H., Du, Z., Hu, L., and Fu, J. (2014b). Identification of cadmium-resistant fungi related to Cd transportation in bermudagrass [Cynodon dactylon (L.) Pers.]. Chemosphere 117, 786–792. doi: 10.1016/j.chemosphere.2014.10.037
Xie, Y., Luo, H., Hu, L., Sun, X., Lou, Y., and Fu, J. (2014c). Classification of genetic variation for cadmium tolerance in bermudagrass [Cynodon dactylon (L.) Pers.] using physiological traits and molecular markers. Ecotoxicology 23, 1030–1043. doi: 10.1007/s10646-014-1247-1
Xiong, Y., Fei, S. Z., Arora, R., Brummer, E. C., Barker, R. E., Jung, G., et al. (2007). Identification of quantitative trait loci controlling winter hardiness in an annual × perennial ryegrass interspecific hybrid population. Mol. Breed. 19, 125–136. doi: 10.1007/s11032-006-9050-1
Yusuf, M. A., Kumar, D., Rajwanshi, R., Strasser, R. J., Tsimilli-Michael, M., Govindjee, et al. (2010). Overexpression of γ-tocopherol methyl transferase gene in transgenic brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochimica et Biophysica Acta 1797, 1428–1438. doi: 10.1016/j.bbabio.2010.02.002
Zhang, F., Zhang, H., Wang, G., Xu, L., and Shen, Z. (2009). Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Hazard. Mater. 168, 76–84. doi: 10.1016/j.jhazmat.2009.02.002
Zhou, W., and Qiu, B. (2005). Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae). Plant Sci. 169, 737–745. doi: 10.1016/j.plantsci.2005.05.030
Keywords: Aspergillus aculeatus, Lolium perenne L., Cd stress, physiological trait, chlorophyll a fluorescence transient, Cd tolerance
Citation: Han S, Li X, Amombo E, Fu J and Xie Y (2018) Cadmium Tolerance of Perennial Ryegrass Induced by Aspergillus aculeatus. Front. Microbiol. 9:1579. doi: 10.3389/fmicb.2018.01579
Received: 27 March 2018; Accepted: 25 June 2018;
Published: 18 July 2018.
Edited by:
Brigitte Mauch-Mani, University of Neuchâtel, SwitzerlandReviewed by:
M. Sudhakara Reddy, Thapar University, IndiaNaresh Singhal, University of Auckland, New Zealand
Copyright © 2018 Han, Li, Amombo, Fu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinmin Fu, jfu@wbgcas.cn
Yan Xie, xieyan60b@126.com
†These authors have contributed equally to this work.
 Shijuan Han
Shijuan Han Xiaoning Li
Xiaoning Li Erick Amombo
Erick Amombo Jinmin Fu
Jinmin Fu Yan Xie
Yan Xie