
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 01 June 2018
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.01147
Vegetables harboring bacteria resistant to antibiotics are a growing food safety issue. However, data concerning carbapenem-resistant Enterobacteriaceae (CRE) in ready-to-eat fresh vegetables is still rare. In this study, 411 vegetable samples from 36 supermarkets or farmer's markets in 18 cities in China, were analyzed for CRE. Carbapenemase-encoding genes and other resistance genes were analyzed among the CRE isolates. Plasmids carrying carbapenemase genes were studied by conjugation, replicon typing, S1-PFGE southern blot, restriction fragment length polymorphism (RFLP), and sequencing. CRE isolates were also analyzed by pulsed-field gel electrophoresis (PFGE). Ten vegetable samples yielded one or more CRE isolates. The highest detection rate of CRE (14.3%, 4/28) was found in curly endive. Twelve CRE isolates were obtained and all showed multidrug resistance: Escherichia coli, 5; Citrobacter freundii, 5; and Klebsiella pneumoniae, 2. All E. coli and C. freundii carried blaNDM, while K. pneumoniae harbored blaKPC−2. Notably, E. coli with blaNDM and ST23 hypervirulent Klebsiella pneumoniae (hvKP) carrying blaKPC−2 were found in the same cucumber sample and clonal spread of E. coli, C. freundii, and K. pneumoniae isolates were all observed between vegetable types and/or cities. IncX3 plasmids carrying blaNDM from E. coli and C. freundii showed identical or highly similar RFLP patterns, and the sequenced IncX3 plasmid from cucumber was also identical or highly similar (99%) to the IncX3 plasmids from clinical patients reported in other countries, while blaKPC−2 in K. pneumoniae was mediated by similar F35:A-:B1 plasmids. Our results suggest that both clonal expansion and horizontal transmission of IncX3- or F35:A-:B1-type plasmids may mediate the spread of CRE in ready-to-eat vegetables in China. The presence of CRE in ready-to-eat vegetables is alarming and constitutes a food safety issue. To our knowledge, this is the first report of either the C. freundii carrying blaNDM, or K. pneumoniae harboring blaKPC−2 in vegetables. This is also the first report of ST23 carbapenem-resistant hvKP strain in vegetables.
The food chain has attracted public attention not only because the contamination of pathogens but also it can serve as a reservoir for resistance genes. Several studies have investigated the prevalence of antibiotic-resistant bacteria in the food chain around the world, especially in retail meat (Leverstein-van Hall et al., 2011; Belmar Campos et al., 2014; Petternel et al., 2014; Wu et al., 2015; Xie et al., 2016). Fresh vegetables can be also a threat to public health because outbreaks of foodborne diseases linking with contaminated vegetables have been increasing in recent years (Jung et al., 2014). Besides zoonotic pathogens, the commensal and environmental bacteria in contaminated vegetables can even serve as a reservoir for antibiotic resistance genes, prompting fresh vegetables to be a growing food safety issue (Zurfluh et al., 2015a). The contamination of bacteria can occur not only through animal manure fertilization, soil, and irrigation water, but also by washing, handling, and processing vegetables during post-harvest period (Berger et al., 2010; Seo and Matthews, 2014). In fresh vegetables, commensal Enterobacteriaceae such as E. coli are the biggest issue because of the antimicrobial-resistance among them, and some even caused outbreaks of foodborne diseases (Friesema et al., 2008; Edelstein et al., 2014), including the contaminated Shiga toxin-producing Escherichia coli (STEC) producing extended-spectrum β-lactamase (ESBL) in sprouts causing the outbreak in Germany in 2011 (Buchholz et al., 2011). Thereafter, ESBL-producing Enterobacteriaceae in vegetables were reported in several countries (Veldman et al., 2014; Zurfluh et al., 2015a; Luo et al., 2017; Mesbah Zekar et al., 2017; Randall et al., 2017).
The emergence of carbapenem-resistant Enterobacteriaceae (CRE) is of great concern to public health, and CRE isolates have been found in samples of different origins around the world in recent years, including humans (Singh-Moodley and Perovic, 2016; Lodise et al., 2017; Zhang et al., 2017), hospital wastewater (Lamba et al., 2017), animals (Liu et al., 2017), seafood products (Morrison and Rubin, 2015), and retail meat (Wang et al., 2017). In fresh vegetables, one Klebsiella variicola producing OXA-181 and three Klebsiella pneumoniae producing OXA-48 were found in Switzerland (Zurfluh et al., 2015b) and Algeria (Touati et al., 2017), respectively. Notably, only one E. coli co-producing NDM-1 and KPC-2 carbapenemases was recently reported in lettuce in Guangzhou, China (Wang et al., 2018). However, studies focusing on carbapenemase-producers in contaminated fresh vegetables were still few. Considering the high occurrence of CRE in different origins in China including humans (Zhang et al., 2017), animals (Liu et al., 2017), and retail meat (Wang et al., 2017), there is an urgent need to investigate the prevalence of CRE in vegetables, especially ready-to-eat fresh vegetables. There is also a need to investigate the CRE besides K. variicola, K. pneumonia, and E. coli in vegetables in China.
In this study, we conducted a surveillance of the prevalence of CRE in fresh vegetables in China and investigated the molecular epidemiological features of these strains. Findings of this work shall provide essential insight into development of effective strategies for control of CRE in food and reducing untreatable infections in clinical settings.
Between May and Nov 2017, 17 different types of fresh vegetables were purchased from 36 supermarkets or farmer's markets in 18 cities or districts of 7 provinces (Beijing, Tianjin, Shanghai, Shandong, Henan, Jiangsu, and Heilongjiang) in China (Table S1). In total, 411 samples from fresh vegetables were collected for analysis. The 411 vegetable samples included cucumber (Cucumis sativus L., n = 74), tomato (Lycopersicon esculentum Mill., n = 67), romaine lettuce (Lactuca sativa L., n = 35), green pepper (n = 34), leaf rape (Brassica napus L., n = 33), curly endive (Cichorium endivia L., n = 28), chili pepper (Capsicum annuum L., n = 22), spinach (Spinacia oleracea L., n = 22), mungbean sprouts (Vigna radiata L., n = 21), coriander (Coriandum sativum L., n = 19), leaf lettuce (Lactuca sativa var longifoliaf. Lam, n = 1 6), pakchoi (Brassica chinensis L., n = 10), carrot (Daucus carota L., n = 9), soybean sprouts (Glycine max L. Merr., n = 8), garland chrysanthemum (Chrysanthemum coronarium L., n = 5), eggplant (Solanum melongena L., n = 4), and green shallots (Allium ascalonicum L., n = 4). All samples were collected in sterile containers, stored under refrigeration, and processed within 24 h.
Of each unwashed sample, 10 g was placed aseptically in a sterile flask containing 90 ml of trypticase soy broth (Becton Dickinson, Breda, the Netherlands) supplemented with meropenem (1.0 mg/L) and vancomycin (8 mg/L), and then was shaken vigorously (incubation overnight at 37°C). Vancomycin was added to the broth to ensure inhibition of the growth of Gram-positive bacteria. Thereafter, 50 mL of the sample suspension with survived bacteria was centrifuged at 6,000 × g for 10 min at 4°C. The obtained pellets were weighted (200 mg per sample) and extraction of the total DNA from each pellet was performed using a MoBio Powersoil DNA isolation kit (MoBio) following the manufacturer's instructions. The remaining broth with survived bacteria was diluted in series of 1:10 and an aliquot (100 μL) of appropriate dilution was spread onto CHROMagar KPC plates (CHROMagar, Paris, France) which were prepared according to the manufacturer's instructions, followed by incubation for 18 h at 37°C. As different colonies might exist in the same sample, 3 colonies with the same Enterobacteriaceae appearance on one CHROMagar KPC plate were selected for PFGE subtyping. The CRE isolates obtained were identified by the typical appearances on plate and confirmed by 16S rRNA sequence and rpoB sequence analysis (Mollet et al., 1997). The total DNA of sample-processed broth and DNA of confirmed CRE isolates were screened for carbapenemase-encoding genes blaIMP, blaVIM, blaNDM, blaSPM, blaAIM, blaDIM, blaGIM, blaSIM, blaKPC, blaBIC, and blaOXA−48 using primers previously described (Poirel et al., 2011). The presence of these genes was confirmed by sequencing obtained amplicons. To identify the subtypes of blaNDM, the complete coding sequence was amplified and sequenced using reported primers (Zong and Zhang, 2013).
The minimal inhibitory concentrations (MICs) of 20 antibiotics, namely cefotaxime, ceftiofur, ceftazidime, ertapenem, imipenem, meropenem, ampicillin, enrofloxacin, ciprofloxacin, levofloxacin, nalidixic acid, amikacin, gentamicin, kanamycin, streptomycin, doxycycline, tetracycline, tigecycline, fosfomycin, and florfenicol were assayed by the agar dilution method, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2015b). The breakpoints for each antimicrobial drug except tigecycline were recommended by the CLSI (CLSI, 2015a,b). The MIC method for colistin and resistant breakpoints for colistin and tigecycline were recommended by the 2017 EUCAST (available at http://www.eucast.org/clinical_breakpoints/). E. coli ATCC 25922 was used as the control strain. Multidrug resistance was defined as non-susceptibility to at least one agent in three or more antimicrobial categories (Magiorakos et al., 2012).
The CRE isolates were screened for the presence of PMQR (qnrA, qnrB, qnrS, qnrC, qnrD, qepA, oqxA, and oqxB), blaCTX-M and fosA3 genes by PCR (Briñas et al., 2003; Weill et al., 2004; Liu et al., 2007, 2011; Yue et al., 2008; Cavaco et al., 2009; Wang et al., 2009). 16S rRNA methyltransferase genes (armA, rmtA, rmtB, rmtC, rmtD, npmA, and rmtE) among the CRE isolates were detected by PCR as previously described (Doi and Arakawa, 2007; Wachino et al., 2007; Davis et al., 2010). The presence of transferable colistin resistance genes (mcr-1 to mcr-7) was also determined using primers previously described (Liu et al., 2016; AbuOun et al., 2017; Rebelo et al., 2018; Yang et al., 2018).
Multilocus sequence typing (MLST) of the E. coli isolates was performed as previously described (Wirth et al., 2006). Sequences were imported into the E. coli MLST database website (http://mlst.ucc.ie/mlst/dbs/Ecoli) to determine MLST types. MLST of the K. pneumoniae isolates was determined according to the previously described method (Diancourt et al., 2005). Sequence types (STs) were determined according to the MLST database website (http://bigsdb.pasteur.fr/klebsiella/submission_mlst.html). Clonal relationships of all CRE isolates were also investigated by PFGE of XbaI-digested genomic DNAs as previously described (Gautom, 1997). The XbaI-digested DNA of Salmonella Braenderup strain H9812 was used as a molecular weight marker. The PFGE patterns were analyzed with BioNumerics software version 2.5 (Applied Maths) to describe the relationships of the test strains.
Conjugation experiments were performed using the broth-mating method as previously described (Chen et al., 2007). E. coli C600 (streptomycin resistant) was used as the recipient. Transconjugants were selected on MacConkey agar plates containing streptomycin (2000 mg/L) and meropenem (1.0 mg/L). The transconjugants were confirmed by PCRs mentioned above and Enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) previously described (Versalovic et al., 1991). The transconjugants should show the same ERIC-PCR patterns with the recipient.
Among the transconjugants, Incompatibility (Inc) groups were assigned by the PCR-based replicon typing method (Carattoli et al., 2005). The IncX and IncI2 replicons were detected according to previous methods (Johnson et al., 2012; Chen et al., 2013). To analyze the location of the carbapenemase-encoding genes of transconjugants, S1 nuclease-PFGE was performed twice as previously described (Barton et al., 1995). Subsequently, Southern blot hybridization was performed repeatedly with DNA probes specific for blaNDM or blaKPC−2 which were prepared using the purified products of obtained PCR amplicons and were non-radioactively labeled with a DIG High Prime DNA labeling and detection kit (Roche Diagnostics, Mannheim, Germany). E. coli isolate harboring plasmids without blaNDM was used to prove the specific for blaNDM. Plasmid DNA extraction was performed using a QIAGEN Plasmid Midi kit (QIAGEN, Germany). Plasmids of transconjugants were digested with the endonuclease EcoRI (TaKaRa Biotechnology, Dalian, China) to analyze the RFLP profiles.
To investigate the genetic characteristic of prevalent plasmids harboring blaNDM, representative plasmid was selected for sequencing. Briefly, the total genomic DNA from transconjugants was extracted using the Wizard Genomic DNA Purification kit (Promega), and then sequenced using both the Illumina Hiseq platform and the Pacbio RS platform. After assembling the sequence reads and filtering the data of C600 chromosomal DNA, contigs of the plasmid were obtained. The RAST annotation pipeline was chosen to perform rapid annotation of the plasmid (Overbeek et al., 2014). Comparison of the plasmid against the highly homologous plasmids in the NCBI database was performed by BRIG (Alikhan et al., 2011).
In total, ten (2.4%) of the 411 vegetable samples were found to carry carbapenemase-encoding genes when screening the total DNA of broth, including 4 samples with blaNDM−5, 5 with blaNDM−1, and 2 with blaKPC−2. Notably, one cucumber sample was found to carry blaNDM−5 and blaKPC−2, simultaneously. Twelve CRE isolates were retrieved from the 10 samples using the CHROMagar KPC plates and all produced carbapenemases (Table 1). VS1 and VS2 with identical PFGE pattern were from the same romaine lettuce sample, however, oqxAB was found in VS2. Analysis of 16S rRNA sequences and rpoB sequences of these CRE isolates showed that the number of E. coli, Citrobacter freundii, and Klebsiella pneumoniae was 5 (35.7%), 5 (35.7%), and 2 (14.3%), respectively (Table 1). All 12 CRE isolates carried one carbapenemase gene and no difference was found in carbapenemase genes content between the direct analysis of the total DNA of broth and the analysis of CRE isolates grown on the selective plates. blaNDM was the dominant type and found in 10 isolates (5 E. coli isolates carrying blaNDM−5 and 5 C. freundii isolates with blaNDM−1), whereas blaKPC−2 was only found in the 2 K. pneumoniae isolates (Table 1). The “string test” was also performed on the 2 K. pneumoniae isolates and K. pneumoniae strains with a positive string test (a viscous string longer than 5 mm could be generated by touching and pulling a single colony upwards with a standard inoculation loop) were designated hvKP (Liu et al., 2014). K1, K2, K5, K20, K54, and K57 serotypes were also analyzed as previously described (Fang et al., 2007). Both two KPC-2-producing K. pneumoniae isolates in this study were hvKP and also positive for K1 serotype. Of note, two CRE isolates with different morphologies (E. coli VH1 with blaNDM−5 and hvKP VH1-2 harboring blaKPC−2) were obtained from the cucumber sample carrying both blaNDM−5 and blaKPC−2. No other carbapenemase gene was found in this study.
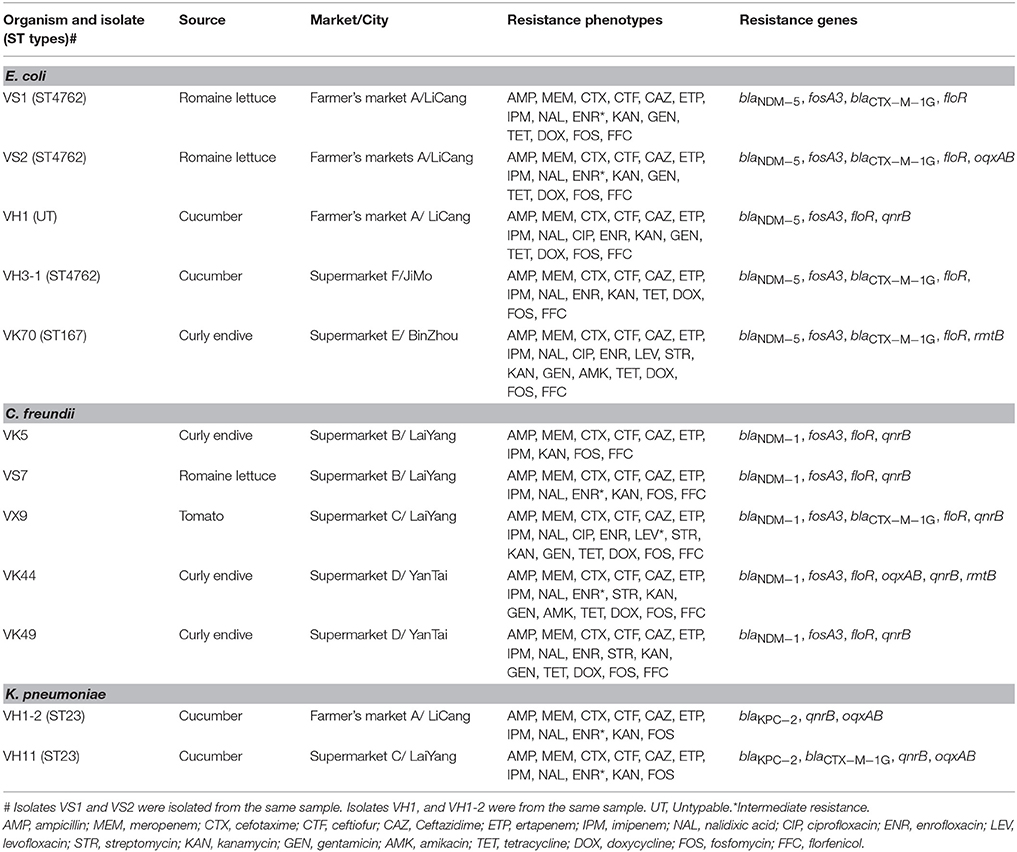
Table 1. Characteristics of Carbapenem-resistant Enterobacteriaceae isolates from vegetables in China.
The 10 samples with CRE belonged to 4 types of fresh vegetables and were all from Shandong province (Table 1, Table S1). The highest detection rate of CRE was found in curly endive (4/28, 14.3%) and the 4 samples were from 3 supermarkets in 3 cities (Binzhou, Laiyang, and Yantai) (Table 1, Table S1). City Laiyang was near to Yantai, while Binzhou was far away from both the two cities. Of the 35 romaine lettuce samples, 2 (5.7%) harbored CRE isolates, and they were from a farmer's market in City LiCang and a supermarket in City LaiYang (Table 1, Table S1). Among the 74 cucumber samples, 3 (4.1%) harbored CRE isolates and they were recovered from 2 supermarkets and 1 farmer's market in 3 cities. One of the 67 tomato samples (1.5%) carried CRE isolate. Of the 18 cities included in this study, the highest detection rate of CRE was found in Yantai (18.2%, 2/11), followed by Laiyang (12.9%, 4/31), Licang (10%, 2/20), Jimo (7.1%, 1/14), and Binzhou (6.7%, 1/15). None of the fresh samples in other 6 provinces carried CRE isolates in this study.
Among the 5 carbapenem-resistant E. coli isolates, 3 different PFGE patterns were obtained (Figure 1). Notably, isolates VH3-1 and VS1 from cucumber and romaine lettuce samples in different cities, respectively, shared identical PFGE pattern and ST type (Figure 1, Table 1). Isolate VK70 from curly endive belonged to ST167.
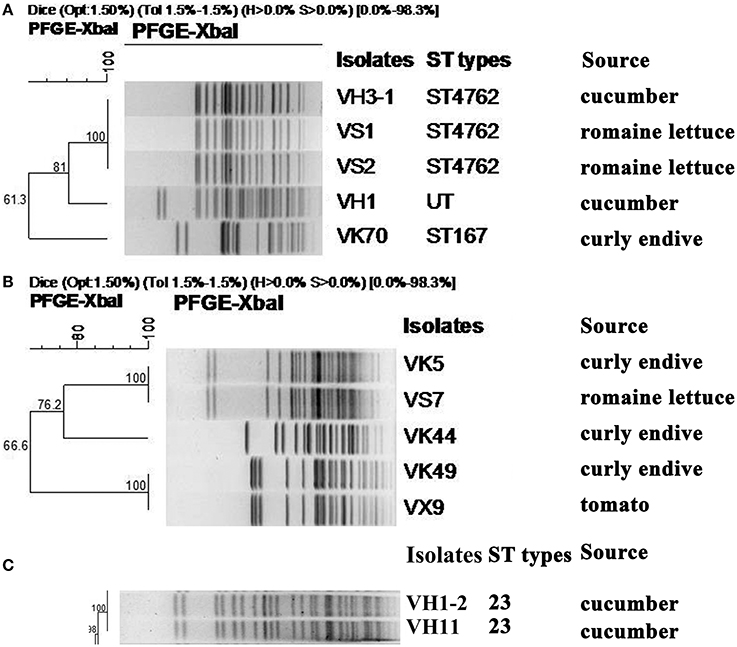
Figure 1. XbaI-PFGE patterns and sources of CRE isolates recovered from ready-to-eat fresh vegetables. (A) MLST and PFGE-based dendrogram of 5 E. coli isolates carrying blaNDM. UT, Untypable. (B) PFGE-based dendrogram of 5 C. freundii isolates carrying blaNDM. (C) MLST and PFGE-based dendrogram of 2 K. pneumoniae isolates carrying blaKPC−2.
In the 5 carbapenem-resistant C. freundii isolates, 3 different PFGE patterns were obtained (Figure 1). VK5 and VS7 from curly endive and romaine lettuce in the same supermarket, respectively, shared the same PFGE pattern. Of note, VK49 and VX9 from curly endive and tomato samples in City Yantai and Laiyang, respectively, had the same PFGE pattern (Figure 1, Table 1). The two hvKP isolates VH11 and VH1-2 also shared identical PFGE pattern and belonged to ST23, although they were from different cities.
As shown in Table 1, the 12 CRE isolates were resistant to all β-lactam antibiotics tested. All E. coli and C. freundii isolates were resistant to fosfomycin and the veterinary drug, florfenicol. Three isolates, VH1, VK70, and VX9 were resistant to ciprofloxacin, and 2 isolates, VK70 and VK44 showed resistance to amikacin. Notably, all 12 isolates showed multidrug resistance. No isolate was resistant to tigecycline and colistin in this study.
For E. coli isolates VS1 and VH3-1 with the same PFGE pattern, different resistance phenotypes were found (Table 1). Although identical PFGE pattern was exist in some of the C. freundii isolates, the 5 isolates from different markets or vegetable types showed different resistance phenotypes. The 2 ST23 hvKP isolates with identical PFGE pattern had the same resistance phenotypes, although they were from different cities.
All E. coli and C. freundii isolates harbored floR and fosA3 genes, responsible for the resistance to florfenicol and fosfomycin, respectively. Both VK70 and VK44 resistant to amikacin, harbored rmtB, and no other 16S rRNA methylase gene was found in this study. qnrB found in 8 isolates was the most prevalent PMQR gene and all the C. freundii and K. pneumoniae isolates harbored qnrB (Table 1). oqxAB was found in 4 CRE isolates, including the 2 K. pneumoniae isolates. No mcr genes were found in the 12 CRE isolates.
Notably, the 2 E. coli isolates VS1 and VS2 with identical PFGE pattern in the same romaine lettuce sample had different genotypes and additional oqxAB was present in VS2 (Table 1, Figure 1). Isolates VH3-1 and VS1 sharing the same PFGE pattern and ST type, had the same genotypes. As shown in Table 1, VK5 and VS7 sharing the same PFGE pattern also had the same genotypes. Of note, C. freundii isolates VK49 and VX9 from curly endive and tomato samples in City Yantai and Laiyang, respectively, showed different genotypes, although the same PFGE patterns were found between the two isolates (Figure 1, Table 1). The same phenomenon was also observed in the 2 hvKP isolates VH11 and VH1-2.
Nine transconjugants were successfully obtained from the 12 CRE isolates (VK5, VS7, and VK44 failed) by conjugation experiments. Seven transconjugants carried blaNDM and 2 harbored blaKPC−2, resulting in that all transconjugants were resistant to meropenem, ertapenem, imipenem, cefotaxime, ceftiofur ceftazidime, and ampicillin (Table 2). The results of S1 nuclease-PFGE and Southern blot hybridization revealed that all the 7 transconjugants harboring blaNDM carried one plasmid (~40 kb) hybridized with blaNDM (Figure 2). Interestingly, there were additional bigger bands in all 7 transconjugants in the S1-PFGE and these bands could be also hybridized with the blaNDM probe. These bigger bands were the portion of the blaNDM-carrying plasmids not exposed to S1 nuclease in the S1-PFGE experiment. In both the blaKPC−2-positive transconjugants, only one plasmid of ~130 kb in size was detected and blaKPC−2 was confirmed to be on this plasmid as shown in Figure 2. IncX3 replicon type was detected in the 7 plasmids with blaNDM, while both the blaKPC−2-positive plasmids belonged to F35:A-:B1 replicon type (Table 2). In the 7 blaNDM-positive plasmids, co-transfer of resistance to kanamycin and fosfomycin was observed in 1 plasmid, respectively, and fosA3 was also found in VX9T from C. freundii in tomato (Table 2). Co-transfer of resistance to kanamycin was found in both the blaKPC−2-positive plasmids.
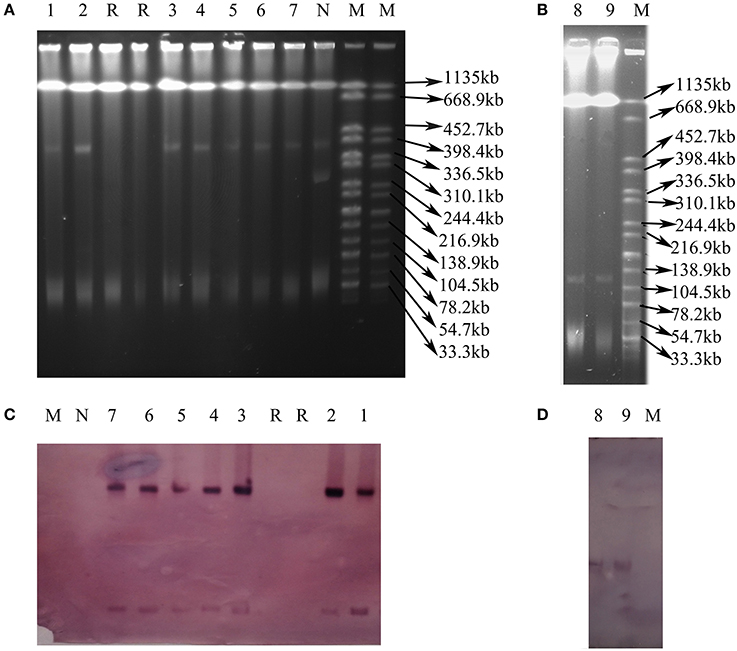
Figure 2. Plasmid analysis of transconjugants carrying blaNDM or blaKPC−2. (A) S1 nuclease-PFGE of transconjugants carrying blaNDM. (B) S1 nuclease-PFGE of transconjugants carrying blaKPC−2. (C) Southern blot hybridization with the blaNDM probe. (D) Southern blot hybridization with the blaKPC−2 probe. Lane 1–9: VS2T, VS1T, VX9T, VK70T, VH1T, VK49T, VH3-1T, VH1-2T, VH11T. Lane M: H9812; Lane R: Receipt E. coli C600; Lane N: E. coli isolate without blaNDM.
As shown in Figure 3, the 7 IncX3 plasmids shared highly similar RFLP profiles. The IncX3 plasmids in C. freundii VX9 and VK49 had the same EcoRI digestion patterns, although the two isolates were from tomato and curly endive in different cities, respectively (Figure 3, Table 2). The two F35:A-:B1 type blaKPC−2-positive plasmids from cucumbers in two different cities also shared the same RFLP profiles (Figure 3, Table 2). Notably, the plasmids of 3 E. coli isolates (VS2, VH1, and VH3-1) from different vegetable types or cities had identical RFLP profiles (Figure 3, Table 2), and isolates VS2 and VH1 from different vegetables in the same farmer's market had different PFGE patterns.
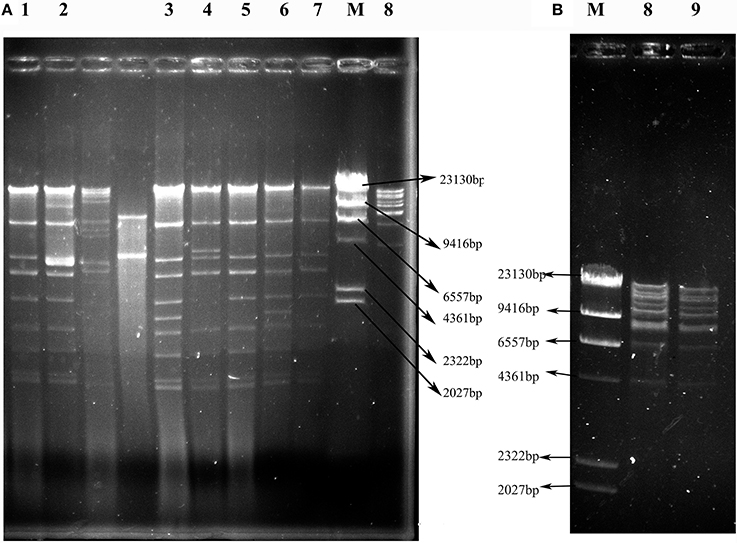
Figure 3. EcoRI restriction digestion profiles of plasmids harboring blaNDM or blaKPC−2 genes from transconjugants containing only one plasmid. (A) Profiles of IncX3 plasmids. (B) Profiles of F35:A-:B1 plasmids. Lanes 1–9: VS2T, VS1T, VX9T, VK70T, VH1T, VK49T, VH3-1T, VH1-2T, and VH11T; Lane M: λ-HindIII marker.
The IncX3 plasmid pVH1 in VH1T was selected for sequencing in this study, and a single contig that was manually closed to a circle was obtained. pVH1 (accession number CP028705) was 46, 161 bp in length with an average G+C content of 46.7%, and encoded 39 open reading frames (ORF). Beyond blaNDM−5, the plasmid did not carry any other antibiotic resistance gene and this could account for the phenotypes of transconjugants VH1T. The full-plasmid comparison revealed that pVH1 was closely related to the other IncX3 plasmids in GenBank (Figure 4). Notably, pVH1 from cucumber in Shandong province in this study was identical to plasmids pCREC-591_4 (GenBank accession number CP024825) from E. coli of clinical Peritoneal fluid in South Korea and pCRCB-101_1 (CP024820) from C. freundii of clinical Open pus in South Korea (Figure 4). Additionally, the three IncX3 plasmids mentioned above were highly similar (99%) to the two blaNDM−7-bearing plasmids pKW53T-NDM (KX214669) and tig00000221 (CP021534) from clinical E. coli isolates in Kuwait and the USA, respectively.
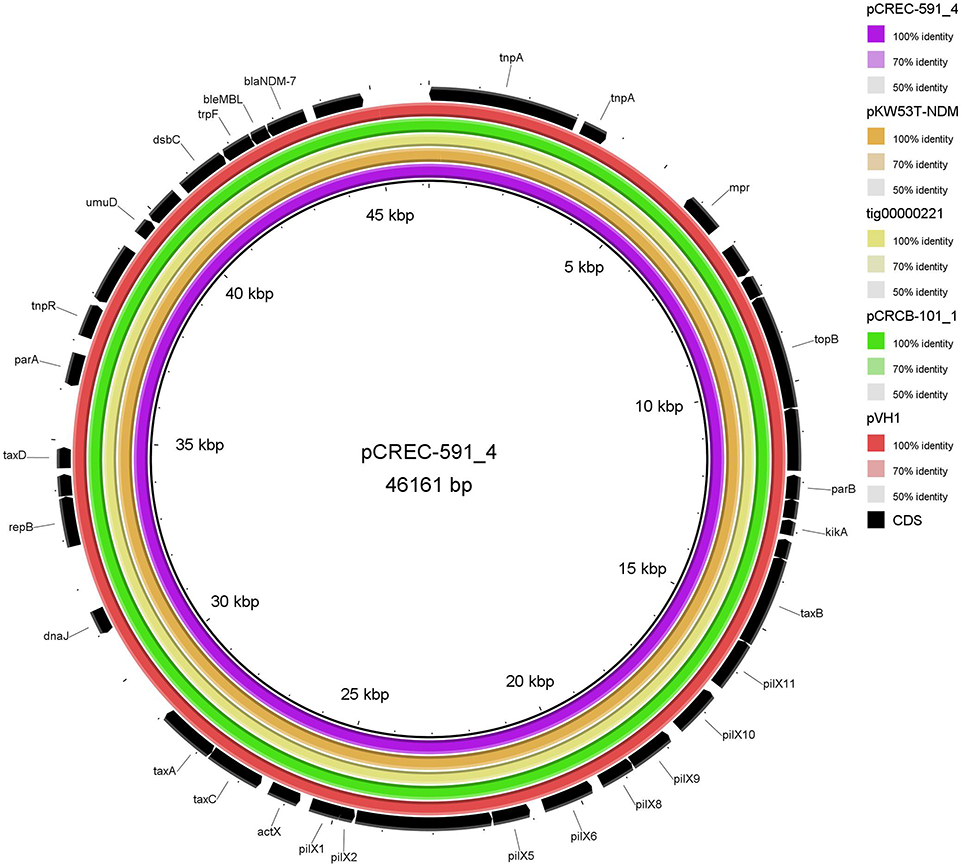
Figure 4. BLASTn-based comparison using BRIG to map complete sequences of plasmids pVH1, pCRCB-101_1, pKW53T-NDM, and tig00000221 against plasmid pCREC-591_4. The innermost ring represents the reference plasmid pCREC-591_4 (GenBank accession number CP024825) from E. coli of clinical Peritoneal fluid in South Korea. The orange, yellow, green, and dark red ring represents pKW53T-NDM (KX214669) from E. coli of clinical urine in Kuwait, tig00000221 (CP021534) from E. coli in the USA, pCRCB-101_1 (CP024820) from Citrobacter freundii of clinical Open pus in South Korea and pVH1 (CP028705) from E. coli of cucumber in China (this study), respectively. The outermost ring shows annotations of the plasmid pKW53T-NDM.
The presence of antibiotic-resistant bacteria in food is a threat to public health, and particular focus has been given to CRE in the food chain. Although K. variicola producing OXA-181 and K. pneumoniae producing OXA-48 was respectively found in fresh vegetables (Zurfluh et al., 2015b; Touati et al., 2017), both the numbers and locations of the vegetable samples in the two previous reports were relatively few. Recently, only one E. coli co-producing NDM-1 and KPC-2 carbapenemases from lettuce was reported in Guangzhou, China. In view of this, we performed a large study to seek CRE isolates from ready-to-eat vegetables purchased at supermarkets and farmer's markets. In the current study, 411 vegetable samples from 18 cities in China were included, and 10 samples, including curly endive, romaine lettuce, cucumber, and tomato were found to harbor NDM and/or KPC-2-producing Enterobacteriaceae, implying that these types of vegetables in China may be a source of carbapenemases genes for human microflora and be a threat to human, as previous studies identifying vegetables as a possible route for the dissemination of resistance genes in the community (Zurfluh et al., 2015a,b; Randall et al., 2017). This also confirmed previous finding that blaKPC−2 and blaNDM were the key genes mediating the development of carbapenem resistance phenotypes in CRE in clinical settings in China (Zhang et al., 2017). The highest detection rate of CRE was found in curly endive (14.3%) in this study, different from the three previous reports about CRE in vegetables, indicating that ready-to-eat curly endive should attract more attention because this type of vegetable is often consumed as salads (Francis and O'beirne, 2006). Besides the carbapenemase-producing K. pneumoniae and E. coli isolates in vegetables reported previously (Touati et al., 2017; Wang et al., 2018), we found K. pneumoniae with blaKPC−2, E. coli with blaNDM, and C. freundii with blaNDM, simultaneously in this study, suggesting various genera of bacteria should be monitored in the future.
Notably, the 2 NDM-producing E. coli isolates (VS1 and VS2) found in the same romaine lettuce sample from a farmer's market shared the same PFGE profiles but different genotypes, implying that E. coli isolates in vegetables have complex evolutionary process. E. coli VH1 with blaNDM−5 and K. pneumoniae VH1-2 with blaKPC−2 occurred in a single cucumber sample in the same farmer's market as VS1, indicating the environment in farmer's market might further facilitate the spread of CRE isolates. These results also prove that the conventional isolation method that one isolate was selected from each sample will underestimate the detection rate of resistant bacteria, and similar finding was also found in Chinese poultry production (Wang et al., 2017). Of note, E. coli VH3-1 and VS1 sharing the same PFGE pattern and ST type were from different vegetable origins and different cities (Table 1, Figure 1), indicating clonal spread exists between different vegetable types or cities in China. The result was confirmed by the 2 C. freundii isolates (VK49 and VX9) and the 2 blaKPC−2-positive K. pneumoniae isolates in this study (Figure 1). The reason for this phenomenon was unknown. The contamination of bacteria may occur through irrigation water or animal manure fertilization. Moreover, presence of carbapenem-resistant bacteria could be related to human contamination during manipulation and conservation of vegetables. One E. coli isolate VK70 harboring blaNDM−5 from a curly endive sample belonged to ST167, which has become an internationally disseminated pathogen among human clinical ESBL-producing E. coli (Sánchez-Benito et al., 2017) and NDM-producing isolates including NDM-1 (Zhang et al., 2017), NDM-5 (Huang et al., 2016), and NDM-7 (Cuzon et al., 2013). The NDM-5-producing ST167 isolate in vegetable in this study further proved the tight association of ST167 and blaNDM−5 in China, and is of particular concern. Notably, both the two blaKPC−2-positive K. pneumoniae isolates from cucumbers in this study belonged to ST23 carbapenem-resistant K1 hvKP, which was first reported in patients in China in 2015 (Zhang et al., 2015), and hvKP was more likely to cause liver abscess, sepsis, and invasive infections than classic K. pneumoniae strain (Liu et al., 2014; Zhang et al., 2016). Thus, the emergence of ST23 carbapenem-resistant K1 hvKP in fresh vegetables will give rise to further concern for consumer health.
fosA3, conferring resistance to fosfomycin, a therapeutic agent effective against common uropathogens in many countries (Falagas et al., 2010), was found in 10 CRE isolates in vegetables, proving the close relationship between fosA3 and blaNDM (Liu et al., 2017). rmtB was found in the two CRE isolates resistant to amikacin, a clinically important aminoglycosides drug, proving that 16S rRNA methylase confers high-level resistance to aminoglycosides especially amikacin (Doi and Arakawa, 2007), and rmtB is the most prevalent 16S rRNA methylase gene in vegetables, similar to that in clinical isolates in China (Yu et al., 2010). Nine plasmids of the 12 CRE isolates in this study were transferable, representing a threat to human health. The current study showed that the dissemination of blaNDM among CRE in ready-to-eat vegetables was mainly mediated by IncX3 conjugative plasmids, consistent with that among clinical CRE strains in China (Yang et al., 2015; Zhang et al., 2017). The bigger bands in the 7 transconjugants (Figure 2) were the portion of the blaNDM-carrying plasmids not exposed to S1 nuclease in the S1-PFGE experiment according to the findings of a previous study (Barton et al., 1995). So all the 7 transconjugants with blaNDM carried only one IncX3 plasmid. The 7 IncX3 plasmids from different genera of bacteria or types of vegetables in this study shared highly similar EcoRI digestion patterns and some even had identical patterns, suggesting that horizontal transfer of such mobile elements is also the major mechanism responsible for emergence and transmission of blaNDM in vegetables in China, even among various genera of bacteria. The full-plasmid comparison showed that pVH1 with blaNDM−5 was identical or highly similar (99%) to the other IncX3 plasmids from clinical patients in various countries (Figure 4), suggesting pVH1-like IncX3 plasmids have disseminated around the world and can also spread between clinical isolates and isolates from food. Compared with plasmids harboring blaNDM, the plasmids carrying blaKPC−2 were more divergent in clinical settings in China (Zhang et al., 2017), however the two F35:A-:B1 blaKPC−2-bearing plasmids in K. pneumoniae from different cities in our study shared identical backbone structure. This suggests that this type of plasmid might have disseminated among K. pneumoniae in vegetables in China and further surveillance of this plasmid should be performed.
In summary, this study reported a high occurrence of CRE in ready-to-eat vegetables in China. blaNDM and blaKPC−2 were the major carbapenemase genes, with blaNDM being mediated by highly similar IncX3 plasmids in E. coli and C. freundii, and blaKPC−2 being mediated by similar F35:A-:B1 plasmids in K. pneumoniae. The sequenced prevalent IncX3 plasmid from cucumber was identical or highly similar (99%) to the other IncX3 plasmids from clinical patients reported in other countries. E. coli with blaNDM and ST23 type K1 hvKP carrying blaKPC−2 were observed in a single vegetable sample. Clonal spread also existed between different vegetable types and cities in China. The presence of CRE-producing organisms in the ready-to-eat vegetables is alarming and constitutes a food safety issue. Measures need to be taken to ensure the ready-to-eat vegetables consumer health and further studies are required for monitoring the prevalence of CRE in vegetables in China and other countries. To our knowledge, this is the first report of the high occurrence of CRE in vegetable samples. This is also the first report of either the C. freundii carrying blaNDM, or hvKP harboring blaKPC−2 in vegetables.
B-TL and F-JS conceived and designed the experiments. F-JS, X-YZ, and S-WW performed the experiments. F-JS, B-TL, J-JH, and R-DJ analyzed the data. B-TL and F-JS wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by the National Natural Science Foundation of China (grant no. 31502122), the Scientific and Technological Projects of Qingdao (grant no. 16-5-1-49-jch), the Natural Science Foundation of Shandong Province of China (grant no. BS2015NY005), the Advanced Talents Foundation of Qingdao Agricultural University (grant no. 663/1115014), and the Priority Academic Talent Team Cultivation Program of Shandong Colleges and Universities.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01147/full#supplementary-material
AbuOun, M., Stubberfield, E. J., Duggett, N. A., Kirchner, M., Dormer, L., Nunez-Garcia, J., et al. (2017). mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 72, 2745–2749. doi: 10.1093/jac/dkx286
Alikhan, N. F., Petty, N. K., Ben Zakour, N. L., and Beatson, S. A. (2011). BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402
Barton, B. M., Harding, G. P., and Zuccarelli, A. J. (1995). A general method for detecting and sizing large plasmids. Anal. Biochem. 226, 235–240. doi: 10.1006/abio.1995.1220
Belmar Campos, C., Fenner, I., Wiese, N., Lensing, C., Christner, M., Rohde, H., et al. (2014). Prevalence and genotypes of extended spectrum beta-lactamases in Enterobacteriaceae isolated from human stool and chicken meat in Hamburg, Germany. Int. J. Med. Microbiol. 304, 678–684. doi: 10.1016/j.ijmm.2014.04.012
Berger, C. N., Sodha, S. V., Shaw, R. K., Griffin, P. M., Pink, D., Hand, P., et al. (2010). Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 12, 2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x
Briñas, L., Moreno, M. A., Zarazaga, M., Porrero, C., Saenz, Y., Garcia, M., et al. (2003). Detection of CMY-2, CTX-M-14, and SHV-12 beta-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob. Agents Chemother. 47, 2056–2058. doi: 10.1128/AAC.47.6.2056-2058.2003
Buchholz, U., Bernard, H., Werber, D., Bohmer, M. M., Remschmidt, C., Wilking, H., et al. (2011). German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365, 1763–1770. doi: 10.1056/NEJMoa1106482
Carattoli, A., Bertini, A., Villa, L., Falbo, V., Hopkins, K. L., and Threlfall, E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228. doi: 10.1016/j.mimet.2005.03.018
Cavaco, L. M., Hasman, H., Xia, S., and Aarestrup, F. M. (2009). qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 53, 603–608. doi: 10.1128/AAC.00997-08
Chen, L., Chavda, K. D., Al Laham, N., Melano, R. G., Jacobs, M. R., Bonomo, R. A., et al. (2013). Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 57, 5019–5025. doi: 10.1128/AAC.01397-13
Chen, L., Chen, Z. L., Liu, J. H., Zeng, Z. L., Ma, J. Y., and Jiang, H. X. (2007). Emergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in China. J. Antimicrob. Chemother. 59, 880–885. doi: 10.1093/jac/dkm065
CLSI (2015a). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacterial Isolated from Animals; Approved Standard-Fourth Edition and Supplement, VET01A4E and VET01S3E. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2015b). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI document M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute.
Cuzon, G., Bonnin, R. A., and Nordmann, P. (2013). First identification of novel NDM carbapenemase, NDM-7, in Escherichia coli in France. PLoS ONE 8:e61322. doi: 10.1371/journal.pone.0061322
Davis, M. A., Baker, K. N., Orfe, L. H., Shah, D. H., Besser, T. E., and Call, D. R. (2010). Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob. Agents Chemother. 54, 2666–2669. doi: 10.1128/AAC.01743-09
Diancourt, L., Passet, V., Verhoef, J., Grimont, P. A., and Brisse, S. (2005). Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43, 4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005
Doi, Y., and Arakawa, Y. (2007). 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45, 88–94. doi: 10.1086/518605
Edelstein, M., Sundborger, C., Hergens, M. P., Ivarsson, S., Dryselius, R., Insulander, M., et al. (2014). Barriers to trace-back in a salad-associated EHEC Outbreak, Sweden, June 2013. PLoS Curr. 6. doi: 10.1371/currents.outbreaks.80bbab3af3232be0372ea0e904dcd1fe
Falagas, M. E., Kastoris, A. C., Kapaskelis, A. M., and Karageorgopoulos, D. E. (2010). Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect. Dis. 10, 43–50. doi: 10.1016/S1473-3099(09)70325-1
Fang, C. T., Lai, S. Y., Yi, W. C., Hsueh, P. R., Liu, K. L., and Chang, S. C. (2007). Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis. 45, 284–293. doi: 10.1086/519262
Francis, G. A., and O'beirne, D. (2006). Isolation and pulsed-field gel electrophoresis typing of Listeria monocytogenes from modified atmosphere packaged fresh-cut vegetables collected in Ireland. J. Food Prot. 69, 2524–2528. doi: 10.4315/0362-028X-69.10.2524
Friesema, I., Sigmundsdottir, G., Van Der Zwaluw, K., Heuvelink, A., Schimmer, B., De Jager, C., et al. (2008). An international outbreak of Shiga toxin-producing Escherichia coli O157 infection due to lettuce, September–October 2007. Euro Surveill. 13:19065. doi: 10.2807/ese.13.50.19065-en
Gautom, R. K. (1997). Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35, 2977–2980.
Huang, Y., Yu, X., Xie, M., Wang, X., Liao, K., Xue, W., et al. (2016). Widespread dissemination of carbapenem-resistant Escherichia coli sequence type 167 strains harboring blaNDM-5 in clinical settings in China. Antimicrob. Agents Chemother. 60, 4364–4368. doi: 10.1128/AAC.00859-16
Johnson, T. J., Bielak, E. M., Fortini, D., Hansen, L. H., Hasman, H., Debroy, C., et al. (2012). Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 68, 43–50. doi: 10.1016/j.plasmid.2012.03.001
Jung, Y., Jang, H., and Matthews, K. R. (2014). Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb. Biotechnol. 7, 517–527. doi: 10.1111/1751-7915.12178
Lamba, M., Graham, D. W., and Ahammad, S. Z. (2017). Hospital wastewater releases of carbapenem-resistance pathogens and genes in urban India. Environ. Sci. Technol. 51, 13906–13912. doi: 10.1021/acs.est.7b03380
Leverstein-van Hall, M. A., Dierikx, C. M., Cohen Stuart, J., Voets, G. M., Van Den Munckhof, M. P., Van Essen-Zandbergen, A., et al. (2011). Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 17, 873–880. doi: 10.1111/j.1469-0691.2011.03497.x
Liu, B. T., Song, F. J., Zou, M., Zhang, Q. D., and Shan, H. (2017). High incidence of Escherichia coli strains coharboring mcr-1 and blaNDM from chickens. Antimicrob. Agents Chemother. 61:e02347-16. doi: 10.1128/AAC.02347-16
Liu, B. T., Wang, X. M., Liao, X. P., Sun, J., Zhu, H. Q., Chen, X. Y., et al. (2011). Plasmid-mediated quinolone resistance determinants oqxAB and aac(6')-Ib-cr and extended-spectrum beta-lactamase gene blaCTX-M-24 co-located on the same plasmid in one Escherichia coli strain from China. J. Antimicrob. Chemother. 66, 1638–1639. doi: 10.1093/jac/dkr172
Liu, J. H., Wei, S. Y., Ma, J. Y., Zeng, Z. L., Lu, D. H., Yang, G. X., et al. (2007). Detection and characterisation of CTX-M and CMY-2 beta-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int. J. Antimicrob. Agents 29, 576–581. doi: 10.1016/j.ijantimicag.2006.12.015
Liu, Y. M., Li, B. B., Zhang, Y. Y., Zhang, W., Shen, H., Li, H., et al. (2014). Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob. Agents Chemother. 58, 5379–5385. doi: 10.1128/AAC.02523-14
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Lodise, T., Ye, M. J., and Zhao, Q. (2017). Prevalence of invasive infections due to carbapenem-resistant Enterobacteriaceae among adult patients in U.S. Hospitals. Antimicrob. Agents Chemother. 61:e00228-17. doi: 10.1128/AAC.00228-17
Luo, J., Yao, X., Lv, L., Doi, Y., Huang, X., Huang, S., et al. (2017). Emergence of mcr-1 in Raoultella ornithinolytica and Escherichia coli isolates from retail vegetables in China. Antimicrob. Agents Chemother. 61:e01139-17. doi: 10.1128/AAC.01139-17
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Mesbah Zekar, F., Granier, S. A., Marault, M., Yaici, L., Gassilloud, B., Manceau, C., et al. (2017). From farms to markets: gram-negative bacteria resistant to third-generation cephalosporins in fruits and vegetables in a region of north africa. Front. Microbiol. 8:1569. doi: 10.3389/fmicb.2017.01569
Mollet, C., Drancourt, M., and Raoult, D. (1997). rpoB sequence analysis as a novel basis for bacterial identification. Mol. Microbiol. 26, 1005–1011. doi: 10.1046/j.1365-2958.1997.6382009.x
Morrison, B. J., and Rubin, J. E. (2015). Carbapenemase producing bacteria in the food supply escaping detection. PLoS ONE 10:e0126717. doi: 10.1371/journal.pone.0126717
Overbeek, R., Olson, R., Pusch, G. D., Olsen, G. J., Davis, J. J., Disz, T., et al. (2014). The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42, D206–D214. doi: 10.1093/nar/gkt1226
Petternel, C., Galler, H., Zarfel, G., Luxner, J., Haas, D., Grisold, A. J., et al. (2014). Isolation and characterization of multidrug-resistant bacteria from minced meat in Austria. Food Microbiol. 44, 41–46. doi: 10.1016/j.fm.2014.04.013
Poirel, L., Walsh, T. R., Cuvillier, V., and Nordmann, P. (2011). Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70, 119–123. doi: 10.1016/j.diagmicrobio.2010.12.002
Randall, L. P., Lodge, M. P., Elviss, N. C., Lemma, F. L., Hopkins, K. L., Teale, C. J., et al. (2017). Evaluation of meat, fruit and vegetables from retail stores in five United Kingdom regions as sources of extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Escherichia coli. Int. J. Food Microbiol. 241, 283–290. doi: 10.1016/j.ijfoodmicro.2016.10.036
Rebelo, A. R., Bortolaia, V., Kjeldgaard, J. S., Pedersen, S. K., Leekitcharoenphon, P., Hansen, I. M., et al. (2018). Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 23:17-00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672
Sánchez-Benito, R., Iglesias, M. R., Quijada, N. M., Campos, M. J., Ugarte-Ruiz, M., Hernandez, M., et al. (2017). Escherichia coli ST167 carrying plasmid mobilisable mcr-1 and blaCTX-M-15 resistance determinants isolated from a human respiratory infection. Int. J. Antimicrob. Agents. 50, 285–286. doi: 10.1016/j.ijantimicag.2017.05.005
Seo, S., and Matthews, K. R. (2014). Exposure of Escherichia coli O157:H7 to soil, manure, or water influences its survival on plants and initiation of plant defense response. Food Microbiol. 38, 87–92. doi: 10.1016/j.fm.2013.08.015
Singh-Moodley, A., and Perovic, O. (2016). Antimicrobial susceptibility testing in predicting the presence of carbapenemase genes in Enterobacteriaceae in South Africa. BMC Infect. Dis. 16:536. doi: 10.1186/s12879-016-1858-7
Touati, A., Mairi, A., Baloul, Y., Lalaoui, R., Bakour, S., Thighilt, L., et al. (2017). First detection of Klebsiella pneumoniae producing OXA-48 in fresh vegetables from Bejaia city, Algeria. J. Glob. Antimicrob. Resist. 9, 17–18. doi: 10.1016/j.jgar.2017.02.006
Veldman, K., Kant, A., Dierikx, C., Van Essen-Zandbergen, A., Wit, B., and Mevius, D. (2014). Enterobacteriaceae resistant to third-generation cephalosporins and quinolones in fresh culinary herbs imported from Southeast Asia. Int. J. Food Microbiol. 177, 72–77. doi: 10.1016/j.ijfoodmicro.2014.02.014
Versalovic, J., Koeuth, T., and Lupski, J. R. (1991). Distribution of repetitive DNA-sequences in Eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19, 6823–6831. doi: 10.1093/nar/19.24.6823
Wachino, J., Shibayama, K., Kurokawa, H., Kimura, K., Yamane, K., Suzuki, S., et al. (2007). Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob. Agents Chemother. 51, 4401–4409. doi: 10.1128/AAC.00926-07
Wang, J., Yao, X., Luo, J., Lv, L., Zeng, Z., and Liu, J. H. (2018). Emergence of Escherichia coli co-producing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J. Antimicrob. Chemother. 73, 252–254. doi: 10.1093/jac/dkx335
Wang, M., Guo, Q., Xu, X., Wang, X., Ye, X., Wu, S., et al. (2009). New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 53, 1892–1897. doi: 10.1128/AAC.01400-08
Wang, Y., Zhang, R., Li, J., Wu, Z., Yin, W., Schwarz, S., et al. (2017). Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2:16260. doi: 10.1038/nmicrobiol.2016.260
Weill, F. X., Lailler, R., Praud, K., Kerouanton, A., Fabre, L., Brisabois, A., et al. (2004). Emergence of extended-spectrum-beta-lactamase (CTX-M-9)-producing multiresistant strains of Salmonella enterica serotype Virchow in poultry and humans in France. J. Clin. Microbiol. 42, 5767–5773. doi: 10.1128/JCM.42.12.5767-5773.2004
Wirth, T., Falush, D., Lan, R., Colles, F., Mensa, P., Wieler, L. H., et al. (2006). Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60, 1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x
Wu, H., Wang, Y., Wu, Y., Qiao, J., Li, H., Zheng, S., et al. (2015). Emergence of beta-lactamases and extended-spectrum beta-lactamases (ESBLs) producing Salmonella in retail raw chicken in China. Foodborne Pathog. Dis. 12, 228–234. doi: 10.1089/fpd.2014.1859
Xie, M., Lin, D., Chen, K., Chan, E. W., Yao, W., and Chen, S. (2016). Molecular characterization of Escherichia coli strains isolated from retail meat that harbor blaCTX-M and fosA3 Genes. Antimicrob. Agents Chemother. 60, 2450–2455. doi: 10.1128/AAC.03101-15
Yang, Q., Fang, L., Fu, Y., Du, X., Shen, Y., and Yu, Y. (2015). Dissemination of NDM-1-producing Enterobacteriaceae mediated by the IncX3-type plasmid. PLoS ONE 10:e0129454. doi: 10.1371/journal.pone.0129454
Yang, Y.-Q., Li, Y.-X., Lei, C.-W., Zhang, A.-Y., and Wang, H.-N. (2018). Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. doi: 10.1093/jac/dky111. [Epub ahead of print].
Yue, L., Jiang, H. X., Liao, X. P., Liu, J. H., Li, S. J., Chen, X. Y., et al. (2008). Prevalence of plasmid-mediated quinolone resistance qnr genes in poultry and swine clinical isolates of Escherichia coli. Vet. Microbiol. 132, 414–420. doi: 10.1016/j.vetmic.2008.05.009
Yu, F. Y., Yao, D., Pan, J. Y., Chen, C., Qin, Z. Q., Parsons, C., et al. (2010). High prevalence of plasmid-mediated 16S rRNA methylase gene rmtB among Escherichia coli clinical isolates from a Chinese teaching hospital. BMC Infect. Dis. 10:184. doi: 10.1186/1471-2334-10-184
Zhang, R., Lin, D., Chan, E. W., Gu, D., Chen, G. X., and Chen, S. (2015). Emergence of Carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob. Agents Chemother. 60, 709–711. doi: 10.1128/AAC.02173-15
Zhang, R., Liu, L., Zhou, H., Chan, E. W., Li, J., Fang, Y., et al. (2017). Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19, 98–106. doi: 10.1016/j.ebiom.2017.04.032
Zhang, Y., Zhao, C., Wang, Q., Wang, X., Chen, H., Li, H., et al. (2016). High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob. Agents Chemother. 60, 6115–6120. doi: 10.1128/AAC.01127-16
Zong, Z., and Zhang, X. (2013). blaNDM-1-carrying Acinetobacter johnsonii detected in hospital sewage. J. Antimicrob. Chemother. 68, 1007–1010. doi: 10.1093/jac/dks505
Zurfluh, K., Nuesch-Inderbinen, M., Morach, M., Zihler Berner, A., Hachler, H., and Stephan, R. (2015a). Extended-spectrum-beta-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl. Environ. Microbiol. 81, 3115–3120. doi: 10.1128/AEM.00258-15
Keywords: characteristics, Enterobacteriaceae, carbapenemase, plasmids, vegetables
Citation: Liu B-T, Zhang X-Y, Wan S-W, Hao J-J, Jiang R-D and Song F-J (2018) Characteristics of Carbapenem-Resistant Enterobacteriaceae in Ready-to-Eat Vegetables in China. Front. Microbiol. 9:1147. doi: 10.3389/fmicb.2018.01147
Received: 02 February 2018; Accepted: 14 May 2018;
Published: 01 June 2018.
Edited by:
Miklos Fuzi, Semmelweis University, HungaryReviewed by:
Tommaso Giani, Università degli Studi di Firenze, ItalyCopyright © 2018 Liu, Zhang, Wan, Hao, Jiang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bao-Tao Liu, bGl1YmFvdGFvLTE5ODZAMTYzLmNvbQ==
Feng-Jing Song, bGNzZmoxMTMwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.