- 1Shandong Key Laboratory of Animal Disease Control and Breeding, Institute of Animal Science and Veterinary Medicine, Shandong Academy of Agricultural Sciences, Jinan, China
- 2Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, Tai’an, China
Newcastle disease virus (NDV) infection leads to disproportion of intestinal tract microbiol population in chickens. Whether vertical infection of NDV affects the formation of a healthy and diverse intestinal community in newly hatched chicks, which might further perturb the establishment of a normal intestinal mucosal immunity, is unclear. This study examined the effects of NDV infection of chick embryos on the formation of the intestinal microbiome of chicks at hatch using 16S rRNA genes pyrosequencing. Eleven-day-old specific-pathogen-free chicken eggs were inoculated via intra-allantoic way with Class I NDV strain. At hatch, chicks were randomly selected and their duodenal and cecal contents were extracted and examined for the composition of gut microflora by Illumina sequencing of the V3+V4 region of the 16S rRNA genes. The results showed that the duodenal flora possesses a greater sample richness and higher microbial diversity as compared with the ceca flora in newly hatched chicks. In addition, there is a clear association with loss of important bacterial population in concert with an enrichment of potentially pathogenic population and NDV infections, both in the duodenum and ceca. It is also increasingly observed that the NDV infection may be associated with the dysbiosis of gut flora. This study presented a profile of the early intestinal microbiota in specific-pathogen-free chicks at hatch and strongly indicates that NDV infection interferes with the formation of intestinal microbiome in newly hatched chicks.
Introduction
The microflora in the gastrointestinal tract (GIT) of chickens play an important role in nutrition, detoxification of certain compounds, growth performance, and protection against pathogenic bacteria (Kelly et al., 2005; Roto et al., 2016; Varmuzova et al., 2016). Successions occurred from a transient community to one of increasing complexity as the birds aged (Lu et al., 2003). In ovo inoculation of chicken embryos with probiotic bacteria led to the establishment of a healthy and diverse community of microorganisms to colonize the developing GIT (de Oliveira et al., 2014; Roto et al., 2016). However, insights into the interaction occurring between host and bacteria, as well as the influence of environmental factors especially pathogens on the formation of microbial communities in the GIT of chickens are still lacking.
The intestinal microflora is relatively stable under common circumstances, but is easily influenced by various diseases (Barnes, 1979; Gong et al., 2007; Ma et al., 2017). Since bacteria may play a role in the disease process due to the modification of intestinal innate immunity by dysbiosis and translocation of bacteria, changes of normal microflora in the digestive tract are important sites in the susceptibility of chickens to bacterial infection (Mead and Adams, 1975; Barnes, 1979; Stanley et al., 2014). Simian immunodeficiency virus (SIV) infection of chimpanzees led to the disorder of gut communities marked by temporally stable microflora structure and followed by resultant microbial translocation with accelerated rate of change in gut microbiota composition (Moeller et al., 2013). Newcastle disease virus (NDV) is the causative agent of Newcastle disease (ND), which is one of the most highly contagious diseases in chickens, and result in severe economic losses to the poultry industry worldwide (Alexander, 1989; Turmagambetova et al., 2017). It has been reported that NDV infection of chicks induced disproportion of GIT microbiol population and the influence of NDV became more serious with time going on by ERIC-PCR based fingerprints of total DNA from fecal samples (Li, 2007).
Polymerase chain reaction (PCR)-based culture-independent methods have been employed and the diversity and complexity of the intestinal microbiota were much higher than had been reported previously by culture-based studies (Handelsman, 2004). To better characterize the interaction between NDV and host from the perspective of intestinal flora, we performed Illumina high-throughput sequencing of the V3+V4 region of the 16S rRNA genes to examine and analyze the composition of duodenal and cecal microflora in the newly hatched chicks under NDV infection of chick embryo. The profile of early intestinal microbiota in duodenum and ceca of chicks at hatch, and the dysbiosis of bacteria associated with NDV infection were shown in our study.
Materials and Methods
Virus and Cultivation
The plaque-purified NDV isolate (Class I) SD21/13 was originally isolated in 2013 and propagated using SPF chicken eggs (Huang et al., 2015). The mean death time (MDT) value of strain SD21/13 in embryonated chicken eggs was >120 h, and the intracerebral pathogenicity index (ICPI) in 1-day-old chickens was 0.18. The virus is effectively proliferated in the chick intestine and induced shed of the epithelial cells from the duodenal intestinal mucosa with lymphocyte infiltration in the intrinsic layer. The purified viruses were tested negative by RT-PCR and excluded the existence of avian influenza virus (AIV), infectious bronchitis virus (IBV), and infectious bursal disease virus (IBDV). The virus-containing allantoic fluids harvested was titrated as described elsewhere (King, 1999) and stored at -80°C until use.
Experiment Design
SPF chickens were used to avoid the infection of other vertical pathogens. Prepared NDV culture was inoculated into 20 11-day-old SPF chicken eggs via intra-allantoic way with 1 × 106 50%-egg-infectious doses (EID50) per egg. Ten other SPF chicken eggs were inoculated with sterile PBS and used as un-infected controls. All eggs were incubated at 37.5°C (standard temperature) with 65% humidity. Eggs were candled daily and early death embryos in the first 24 h were removed. Once the eggs have hatched, three birds were taken from the NDV infected and un-infected group. Day-old birds were killed by cervical displacement, and in a laminar flow cabinet, the GIT was exposed. Using sterile surgical knife and forceps, the duodenum and ceca of chicks were collected into microtubes and properly stored at -80°C.
Extraction of Total DNA
DNA was extracted from intestinal samples including the contents and the mucosal wall of the duodenum and ceca from the each chicken according to the protocol described by the E.Z.N.A Soil DNA Kit (OMEGA). Acquired DNAs were measured for their quantities and integrity by Nanodrop ND-1000 spectrophotometer (Thermo Scientific) and 1% agarose gel electrophoresis, respectively. The range of DNA size was measured using the standard low DNA mass ladder and then was stored at -20°C until further processing.
16S rRNA Amplification and MiSeq Sequencing
A gene region about 415 nt, covering the V3–V4 region of the 16S rRNA gene was selected to construct community library through tag pyrosequencing. The target gene was firstly amplified using broadly-conserved primers Amplicon PCR Primer F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTACGGRAGGCAGCAG-3′) and Amplicon PCR Primer R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGAGGGTATCTAATCCT-3′). The primer pairs contain transposase sequences as indicated above in italicized letters. PCR were carried out in a 25 μl reaction containing 2× KAPA HiFi HotStart ReadyMix, Amplicon PCR Primer F (1 μM), Amplicon PCR Primer R (1 μM), and 5 ng DNA template. The PCR condition were initial denaturation at 95°C for 3 min, followed by 25 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s, with a final extension phase at 72°C for 10 min. PCR products were purified with 0.8 times of AMPure XP Beads. The second round of the PCR was carried out using the index primer pairs Index 1 Primer (5′-AATGATACGGCGACCACCGAGATCTACAC[i5]TCGTCGGCAGCGTC-3′) and Index 2 Primer (5′-CAAGCAGAAGACGGCATACGAGAT[i7]GTCTCGTGGGCTCGG-3′) containing P5/P7 adaptors and 8 nt of index sequences. PCR were then carried out in a 50 μl reaction. Illumina MiSeq was performed on a PE300 instrument commercially at Shanghai Hanyu Bio-Tech, on a MiSeq Sequencer platform.
Bioinformatic Analysis of Sequencing Data
PE reads get from MiSeq sequencing were spliced according to overlap relationships. Quality of the sequence was evaluated with filtration and quality control to obtain high-quality sequences. All of the sequences from all of the samples were clustered into operational taxonomic units (OTUs) based on a 97% identity threshold. The most abundant sequence of each OTU (97% similarity) was BLAST searched against the Greengene/sliva databases to determine the phylogeny of the OTU. A variety of diversity index analyses were carried out on the basis of OUT and OUT clustering results. Metastats analyses of community structure at the genus level were carried out on the basis of taxonomist analysis using mothur v.1.32.1 (Schloss et al., 2009).
Statistical Analyses
Differences between populations had been analyzed using parametric (ANOVA) and non-parametric statistical methods. All results were presented as the mean value (± SE). Differences between groups were declared significant at p < 0.05.
Results
Description of the Sequencing Data
Samples were obtained from the duodenum and ceca of chickens in control group and chickens infected with NDV. After two separate runs on a PE300 instrument and quality-filtering as described in the methods, 123, 730 total sequences with an average of 415 bp in length were available and used for analysis. The sequences were rarified to the minimum number of high quality sequences in all samples and normalized by total count for the alpha and beta diversity analyses as conducted below.
Establishment of Duodenal and Cecal Flora in the Newly Hatched Chicks
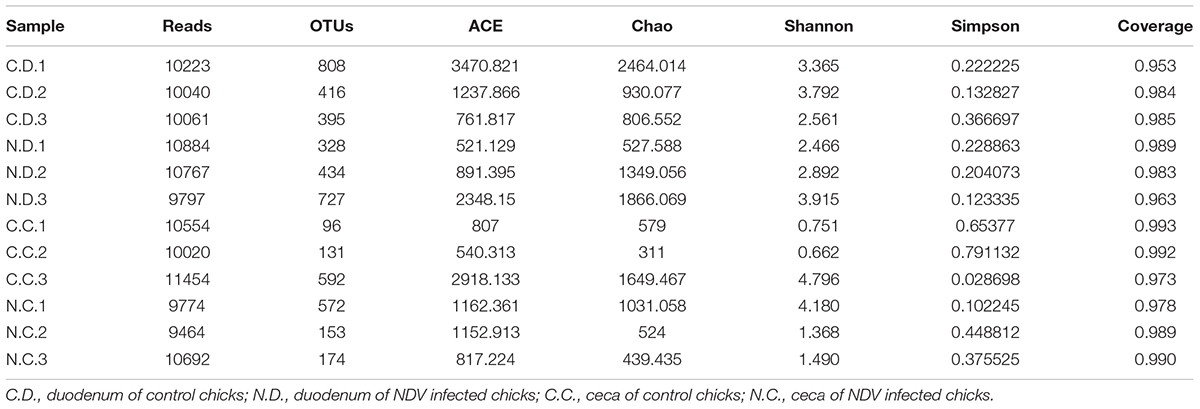
The sample richness was assessed by OTU counts in each individual sample as shown in Table 1. The number of observed OTUs in the duodenum and ceca samples were 540 and 273, respectively, in the newly hatched chicks. Consistent with that, duodenal flora also showed a higher sample richness as reflected by the abundance index Ace and Chao and greater bacterial diversity as assessed by both the Shannon and Simpson analyses.
The overall microbial composition for each group at the phylum level was shown in Figure 1A. Where bacteria in normal duodenum of 1-day-old chick were identifiable to the phylum level, they belonged predominantly to the Proteobacteria (79.22%), Bacteroidetes (9.59%), Firmicutes (4.16%), and Actinobacteria (2.06%), with Acidobacteria, Chloroflexi, Cyanobacteria, Verrucomicrobia, Nitrospirae, Gemmatimonadetes also identified. When analyzed from the genus level as shown in Figure 2A, flora of chick duodenum comprised of Serratia (51.97%), Escherichia (6.66%), Chitinophagaceae unclassified (3.26%), Saprospiraceae unclassified (2.65%), Lactobacillus (2.26%), etc. The duodenum showed relatively homogeneous flora among individuals.
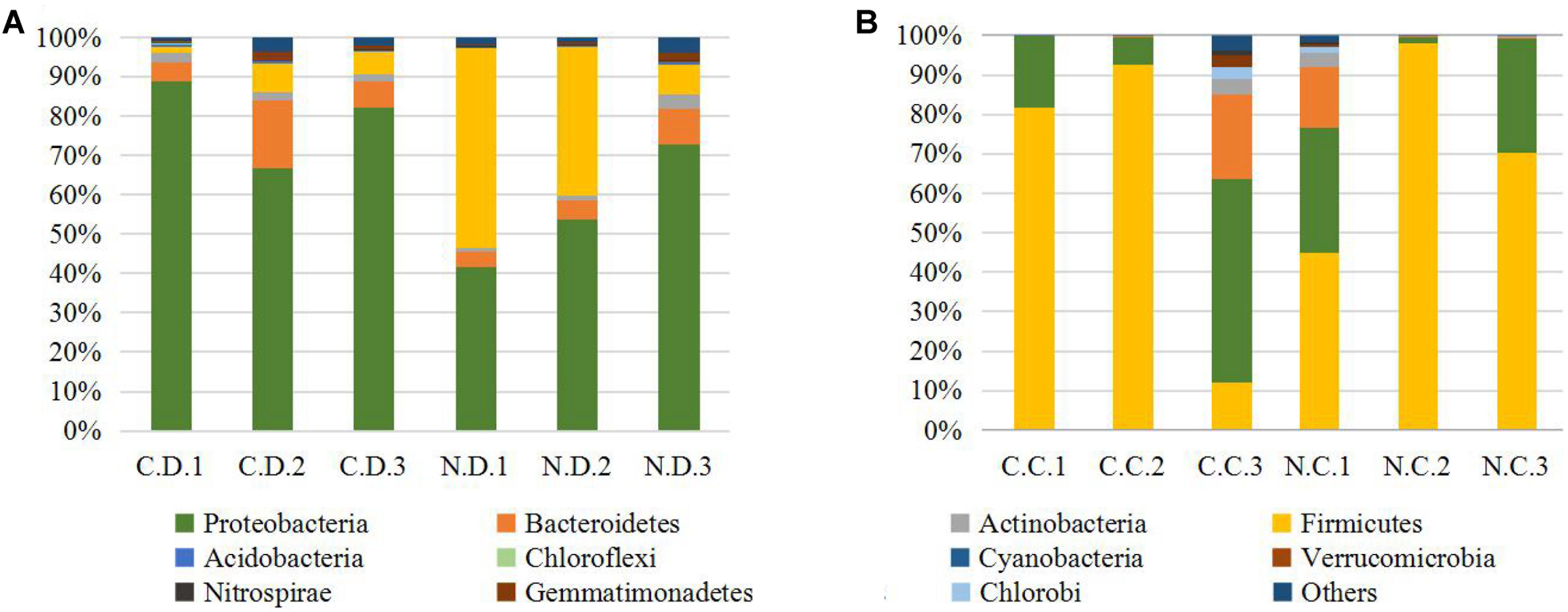
FIGURE 1. Histogram combination analysis at the bacterial phyla level. (A) Duodenum of control chicks (C.D.) and NDV infected chicks (N.D.); (B) ceca of control chicks (C.C.) and NDV infected chicks (N.C.).
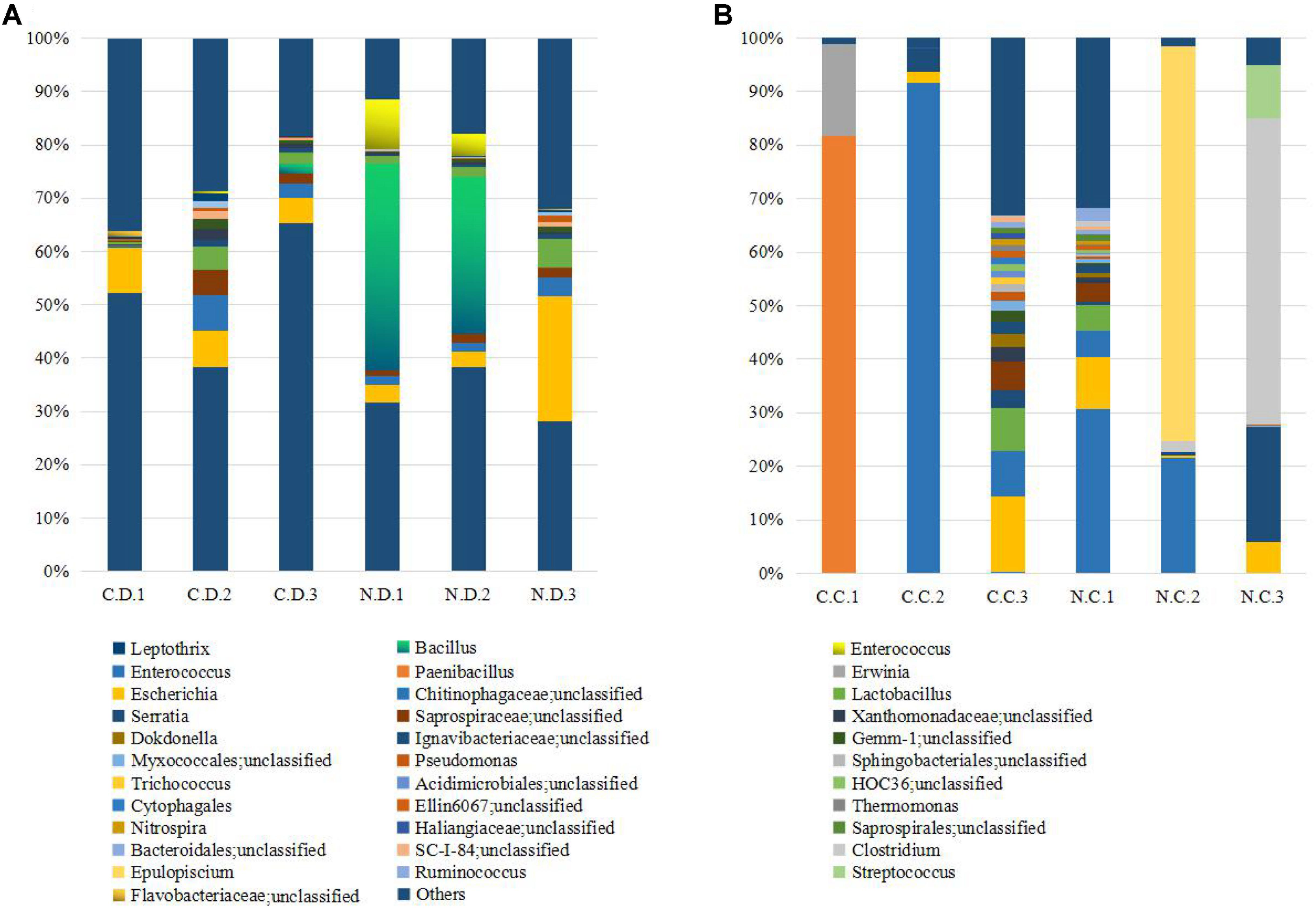
FIGURE 2. Histogram combination analysis at the bacterial genus level. (A) Duodenum of control chicks (C.D.) and NDV infected chicks (N.D.); (B) ceca of control chicks (C.C.) and NDV infected chicks (N.C.).
Bacteria in normal ceca of 1-day-old chick were dominated by Firmicutes (87.22%) and Proteobacteria (12.56%), and Bacteroidetes, Actinobacteria, Chlorobi, Gemmatimonadetes, and Nitrospirae were also identified (Figure 1B). When analyzed from the genus level, Enterococcus (28.78%) Paenibacillus (26.91%), Erwinia (5.66%), Escherichia (5.66%), Lactobacillus (2.88%) and Serratia (2.76%) accounted for the large part of the cecal flora (Figure 2B). The cecal microflora composition varied in different individuals as compared with the duodenal ones.
Infection of NDV Caused Differential Microbial Diversity in the Duodenal and Cecal Flora
The number of observed OTUs in the duodenum samples of NDV-infected chicks was less than that of the uninfected controls, while those of the ceca samples were almost the same (Table 1). NDV infection also showed lower sample richness in duodenal flora as reflected by the abundance index Ace and Chao. Within sample bacterial diversity was assessed by both the Shannon and Simpson analyses, which further demonstrated decreased overall diversity among the duodenal samples from NDV infected chickens as compared with the uninfected controls. The richness and diversity of cecal microflora varied in different individuals as compared with the duodenal ones, and no differences was found between NDV infected and uninfected chicks. Collectively, this data pointed toward a less diverse bacterial population in the duodenal flora from NDV infected chicks.
The Microbial Composition Was Significantly Altered in NDV Infected Chicks
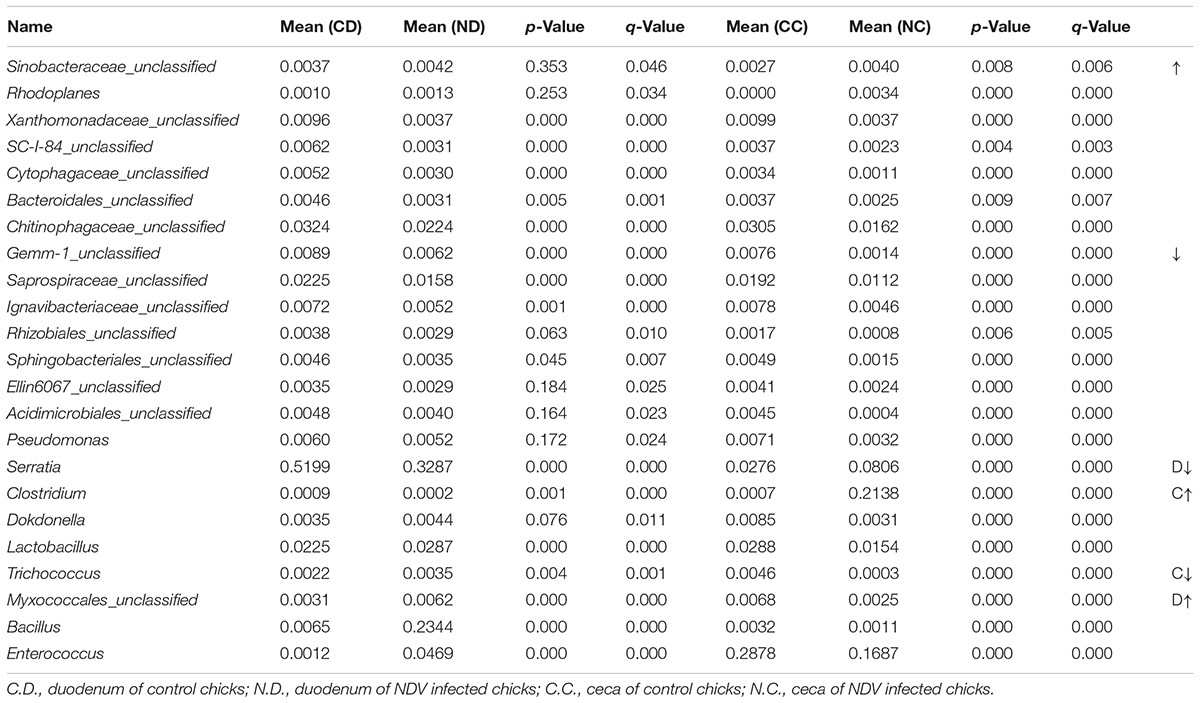
In order to examine the overall differences in bacterial composition between the NDV infected chicks and the controls, statistical analysis of the community structure at the genus level was performed on the relative abundance of OTUs in each birds. Members of Sinobacteraceae and Rhodoplanes were uniformly increased in both the duodenum and ceca samples of NDV infected chicks. On the contrary, NDV infection led to the loss of vast of genera, including members of Xanthomonadaceae, SC-I-84, Cytophagaceae, Bacteroidales, Chitinophagaceae, Gemm-1, Saprospiraceae, Ignavibacteriaceae, Rhizobiales, Sphingobacteriales, Ellin6067, Acidimicrobiales, and Pseudomonas, in both the duodenum and ceca samples. Of particular note, the relative abundance of members of Xanthomonadaceae and Cytophagaceae were sharply decreased. Therefore, a uniform of enrichment and depletion of microflora in the duodenum and ceca samples of NDV-infected chicks were characterized.
NDV Infection Induced Differential Microbial Structures in Duodenum and Ceca of Chicks
Since the microbiota of individuals were significantly enriched or depleted in specific taxa, we examined the differential microbial structures between duodenum and ceca at the genus level after NDV infection in chicks (Table 2). The relative abundance of Serratia and Clostridium were significantly decreased in duodenum, but greatly enhanced in the ceca, suggesting that Serratia and Clostridium may be translocated from the duodenum to the ceca. On the contrary, we demonstrated a subset of bacteria that possibly translocated from the ceca to the duodenum, including Dokdonella, Lactobacillus, Trichococcus, Bacillus, Enterococcus, and members of Myxococcales, etc.
Discussion
The intestinal immune system in Gallus species must rapidly adapt to colonization by commensal bacteria, as well as the possible entry of pathogenic bacteria. Very low numbers of total bacteria could be counted from the ceca of broiler chickens at hatch as colony-forming units on agar plates (Barnes, 1979; van der Wielen et al., 2002). In contrast, using the cultured-independent 16S rRNA genes Illumina sequencing method, our study showed that much more complex microflora in both the duodenum and the ceca in newly hatched chicks than those in previous studies.
Although it has been generally assumed that the alimentary tract of a chick is devoid of microorganisms, birds examined within only a few hours of hatching frequently contain appreciable numbers of bacteria. It is possible that microorganisms present in the breeder house and incubator are able to transpose the eggshell barrier, reach the albumen, and then become established in the bird intestinal tract during the late stages of embryogenesis (Mead, 1989; Pedroso et al., 2005). Other authors indicate that the acquisition of bacteria occurred immediately after birth from the environment at the hatchery (Lev and Briggs, 1956; van der Wielen et al., 2002). Based on our preliminary analysis of the richness and diversity indexes, the duodenal flora possesses a greater sample richness and higher microbial diversity as compared with that of the ceca flora. Previous studies reported that the bacterial community in the ceca displayed the most diverse in adult chickens (Zhu et al., 2002). Combined with our results, we suggested that the commensal bacteria may enter the digestive tract through the mouth and then implanted in the duodenum at first and enriched in the ceca afterward. The relative homogeneously established microbiota of duodenum among individuals as compared with that of the ceca further supported that the microbiota of duodenum formed earlier. Collectively, the results reported here have confirmed that the bacteria were obtained from the commensal environment immediately at the hatchery.
It has been reported that NDV infection of chickens induced disproportion of GIT microbiome (Li, 2007). This study clearly indicates that NDV infection interferes with the formation of GIT microbiol population in newly hatched chicks. As shown in our study, loss of a subset of bacteria along with decreased richness and diversity were observed in GIT of NDV infected newly hatched chicks. Similar phenomena are also found in many other important poultry diseases like Eimeria tenella (Zhou et al., 2017), Marek’s disease virus (Perumbakkam et al., 2014), avian leukosis virus (Ma et al., 2017), etc. The normal microbiota of the GIT of chickens play an important role in inhibiting the establishment of intestinal pathogens (Gomez de Agüero et al., 2016; Günther et al., 2016). Indeed, some important pathogens like Rhodoplanes were found to be enriched in both the duodenum and the ceca of the NDV infected chicks. It is resumed that Rhodoplanes sp. might be an emerging human pathogen involved in unknown febrile conditions and could cause local infection of any tissues or organs (Zhang et al., 2011). In addition, the duodenum showed relatively homogeneous flora among individuals, but NDV infection made a preference for implantation of known conditioned-pathogens. Similarly, the ceca of normal chicks were dominated by Paenibacillus or Enterococcus at hatch. Several Paenibacillus species produce antimicrobial substances that affect a wide spectrum of micro-organisms such as fungi, soil bacteria, plant pathogenic bacteria, and even important anaerobic pathogens such as Clostridium botulinum and Paenibacillus pasadenensis (Girardin et al., 2002; Passera et al., 2017). In line with this, more Epulopiscium and Clostridium established accompanied by the complete loss of Paenibacillus after the infection of NDV in newly hatched individuals. Clostridium contains around 100 species that include common free-living bacteria, as well as important pathogens. Previous study conferred that bacterial infections could also be enhanced by NDV in a mice model (Hugh et al., 1971), which is consistent with our results. Therefore, these observations as well as previously published studies strongly indicated that NDV infection increased the chance of secondary infection since the intestinal gut might be a great source of conditioned-pathogens.
Bacterial dysbiosis has been linked to altered immune function and/or persistent inflammation. Understanding the roles of microbiota in the intestinal muscosal immunity should offer novel insight into gastrointestinal disease pathophysiology and deliver new immunotherapy strategies (Chang and Lin, 2016). The microbial dysbiosis and translocation is associated with systemic immune activation in HIV and SIV infections, which in turn helps the increase of virus load in the host (Hunt et al., 2011; Marchetti et al., 2011). It is increasingly observed that the NDV infection may be associated with the dysbiosis of gut flora. NDV infection is associated with the mucosal damage in chickens. Thus, it is imperative to better understand the interplay between intestinal microbiota changes and the pathogenesis o of NDV infection.
Overall, the 16S rRNA genes Illumina sequencing technique in this study presented a profile of the early intestinal microbiota in chicks at hatch, and the dysbiosis of bacteria associated with NDV infection. The data will be useful for future studies related to the pathophysiology of NDV in chickens and for experiments evaluating the interactions of NDV and bacteria, and other mixed infections in poultry.
Ethics Statement
The study protocol and all animal studies were approved by the Shandong Agricultural University Animal Care and Use Committee (SACUC Permission No. AVM140301-19).
Author Contributions
NC, XH, and ZK collection and assembly of the data, manuscript writing, and data analysis. NC, YH, QH, SY, and LZ discussion, manuscript revision. CX, XZ, and YC concept and design, data analysis, manuscript revision, and final approval of the manuscript.
Funding
The study was supported by the earmarked fund for Modern Agro-industry Technology Research System and Chinese Special Fund for Agro-scientific Research in the Public Interest (CARS-41-Z10).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexander, D. J. (1989). “Newcastle disease,” in A Laboratory Manual for the Isolation and Identification of Avian Pathogens, 3rd Edn, eds H. G. Eds, L. H. Arp, C. H. Domermuth, and J. E. Pearson (Kennett Square, PA: American Association for Avian Pathologists, Inc), 114–120.
Barnes, E. M. (1979). The intestinal microflora of poultry and game birds during life and after storage. J. Appl. Bacteriol. 46, 407–419. doi: 10.1111/j.1365-2672.1979.tb00838.x
Chang, C., and Lin, H. (2016). Dysbiosis in gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 30, 3–15. doi: 10.1016/j.bpg.2016.02.001
de Oliveira, J. E., van der Hoeven-Hangoor, E., van de Linde, I. B., Montijn, R. C., and van der Vossen, J. M. (2014). In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 93, 818–829. doi: 10.3382/ps.2013-03409
Girardin, H., Albagnac, C., Dargaignaratz, C., Nguyen-The, C., and Carlin, F. (2002). Antimicrobial activity of foodborne Paenibacillus and Bacillus spp. against Clostridium botulinum. J. Food Prot. 65, 806–813. doi: 10.4315/0362-028X-65.5.806
Gomez de Agüero, M., Ganal-Vonarburg, S. C., Fuhrer, T., Rupp, S., Uchimura, Y., Li, H., et al. (2016). The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302. doi: 10.1126/science.aad2571
Gong, J., Si, W., Forster, R. J., Huang, R., Yu, H., Yin, Y., et al. (2007). 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken. FEMS Microbiol. Ecol. 59, 147–157. doi: 10.1111/j.1574-6941.2006.00193.x
Günther, C., Josenhans, C., and Wehkamp, J. (2016). Crosstalk between microbiota, pathogens and the innate immune responses. Int. J. Med. Microbiol. 306, 257–265. doi: 10.1016/j.ijmm.2016.03.003
Handelsman, J. (2004). Metagenomics: application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 68, 669–685. doi: 10.1128/MMBR.68.4.669-685.2004
Huang, Y., Yang, S., Hu, B., Xu, C., Gao, D., Zhu, M., et al. (2015). Genetic, pathogenic and antigenic diversity of Newcastle disease viruses in Shandong Province, China. Vet. Microbiol. 180, 237–244. doi: 10.1016/j.vetmic.2015.09.002
Hugh, R., Huang, K. Y., and Elliott, T. B. (1971). Enhancement of bacterial infections in mice by Newcastle disease virus. Infect. Immun. 3, 488–493.
Hunt, P. W., Landay, A. L., Sinclair, E., Martinson, J. A., Hatano, H., Emu, B., et al. (2011). A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One 31:e15924. doi: 10.1371/journal.pone.0015924
Kelly, D., Conway, S., and Aminov, R. (2005). Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 26, 326–333. doi: 10.1016/j.it.2005.04.008
King, D. J. (1999). A comparison of the onset of protection induced by Newcastle disease virus strain B1 and a fowl poxvirus recombinant Newcastle disease vaccine to a viscerotropic velogenic Newcastle disease virus challenge. Avian Dis. 43, 745–755. doi: 10.2307/1592743
Lev, M., and Briggs, C. A. E. (1956). The gut flora of the chick. 1. The flora of newly hatched chicks. J. Appl. Bact. 19, 36–38. doi: 10.1111/j.1365-2672.1956.tb00041.x
Li, Y. P. (2007). ERIC-PCR Based Fingerprint to Analyze the Structural Features of Intestinal Microbial Communities of Chicks Infected with Salmonella gallinarum, Newcastle Disease Virus and the Healthy Chicks. Ph.D. thesis, Sichuan Agricultural University, Sichuan.
Lu, J., Idris, U., Harmon, B., Hofacre, C., Maurer, J. J., and Lee, M. D. (2003). Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 69, 6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003
Ma, X. X., Wang, Q., Li, H. M., Xu, C. T., Cui, N., and Zhao, X. M. (2017). 16S rRNA genes Illumina sequencing revealed differential cecal microbiome in specific pathogen free chickens infected with different subgroup of avian leukosis viruses. Vet. Microbiol. 207, 195–204. doi: 10.1016/j.vetmic.2017.05.016
Marchetti, G., Cozzi-Lepri, A., Merlini, E., Bellistrì, G. M., Castagna, A., Galli, M., et al. (2011). Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS 25, 1385–1394. doi: 10.1097/QAD.0b013e3283471d10
Mead, G. C. (1989). Microbes of the avian cecum-types present and substrates utilized. J. Exp. Zool. 3, 48–54. doi: 10.1002/jez.1402520508
Mead, G. C., and Adams, B. W. (1975). Some observation on the caecal microflora of the chick during the first two weeks of life. Br. Poult. Sci. 16, 169–176. doi: 10.1080/00071667508416174
Moeller, A. H., Shilts, M., Li, Y., Rudicell, R. S., Lonsdorf, E. V., Pusey, A. E., et al. (2013). SIV-induced instability of the chimpanzee gut microbiome. Cell Host Microbe 14, 340–345. doi: 10.1016/j.chom.2013.08.005
Stanley, D., Wu, S. B., Rodgers, N., Swick, R. A., and Moore, R. J. (2014). Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One 9:e104739. doi: 10.1371/journal.pone.0104739
Passera, A., Venturini, G., Battelli, G., Casati, P., Penaca, F., Quaglino, F., et al. (2017). Competition assays revealed Paenibacillus pasadenensis strain R16 as a novel antifungal agent. Microbiol. Res. 198, 16–26. doi: 10.1016/j.micres.2017.02.001
Pedroso, A. A., Menten, J. F. M., and Lambais, M. R. (2005). The structure of bacterial community in the intestines of newly hatched chicks. J. Appl. Poult. Res. 14, 232–237. doi: 10.1093/japr/14.2.232
Perumbakkam, S., Hunt, H. D., and Cheng, H. H. (2014). Marek’s disease virus influences the core gut microbiome of the chicken during the early and late phases of viral replication. FEMS Microbiol. Ecol. 90, 300–312. doi: 10.1111/1574-6941.12392
Roto, S. M., Kwon, Y. M., and Ricke, S. C. (2016). Applications of In Ovo Technique for the Optimal Development of the Gastrointestinal Tract and the Potential Influence on the Establishment of Its Microbiome in Poultry. Front. Vet. Sci. 3:63. doi: 10.3389/fvets.2016.00063
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Turmagambetova, A. S., Alexyuk, M. S., Bogoyavlenskiy, A. P., Linster, M., Alexyuk, P. G., Zaitceva, I. A., et al. (2017). Monitoring of Newcastle disease virus in environmental samples. Arch. Virol. 162, 2843–2846. doi: 10.1007/s00705-017-3433-y
van der Wielen, P. W., Keuzenkamp, D. A., Lipman, L. J., van Knapen, F., and Biesterveld, S. (2002). Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb. Ecol. 44, 286–293. doi: 10.1007/s00248-002-2015-y
Varmuzova, K., Kubasova, T., Davidova-Gerzova, L., Sisak, F., Havlickova, H., Sebkova, A., et al. (2016). Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella enteritidis infection. Front. Microbiol. 7:957. doi: 10.3389/fmicb.2016.00957
Zhang, L. J., Wang, G. Q., Yu, H. L., Wang, J., Wang, S. W., Jia, Y., et al. (2011). First report of human infection by Rhodoplanes sp. Alphaproteobacteria in China. Asian Pac. J. Trop. Med. 4, 248–250. doi: 10.1016/S1995-7645(11)60079-5
Zhou, Z., Nie, K., Huang, Q., Li, K., Sun, Y., Zhou, R., et al. (2017). Changes of cecal microflora in chickens following Eimeria tenella challenge and regulating effect of coated sodium butyrate. Exp. Parasitol. 177, 73–81. doi: 10.1016/j.exppara.2017.04.007
Keywords: Newcastle disease virus, 16S rRNA sequencing, duodenal microflora, cecal microflora, dysbiosis
Citation: Cui N, Huang X, Kong Z, Huang Y, Huang Q, Yang S, Zhang L, Xu C, Zhang X and Cui Y (2018) Newcastle Disease Virus Infection Interferes With the Formation of Intestinal Microflora in Newly Hatched Specific-Pathogen-Free Chicks. Front. Microbiol. 9:900. doi: 10.3389/fmicb.2018.00900
Received: 29 July 2017; Accepted: 18 April 2018;
Published: 07 May 2018.
Edited by:
Andrea Guido Oreste Manetti, GlaxoSmithKline (United Kingdom), United KingdomReviewed by:
Haider Abdul-Lateef Mousa, University of Basrah, IraqMichael Kogut, Agricultural Research Service (USDA), United States
Copyright © 2018 Cui, Huang, Kong, Huang, Huang, Yang, Zhang, Xu, Zhang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuantian Xu, eGN0dGFpYW4yMDAyQDE2My5jb20= Xiumei Zhang, enhtODIwNDEwQDE2My5jb20= Yanshun Cui, eXNjdWlAc2RhdS5lZHUuY24=
†These authors have contributed equally to this work.
 Ning Cui
Ning Cui Xiaoying Huang1,2†
Xiaoying Huang1,2†