
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 11 April 2018
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.00720
This article is part of the Research TopicAntibiotic Alternatives and Combinational Therapies for Bacterial InfectionsView all 21 articles
 Mian L. Ooi1
Mian L. Ooi1 Katharina Richter1,2
Katharina Richter1,2 Catherine Bennett1
Catherine Bennett1 Luis Macias-Valle1,3
Luis Macias-Valle1,3 Sarah Vreugde1
Sarah Vreugde1 Alkis J. Psaltis1
Alkis J. Psaltis1 Peter-John Wormald1*
Peter-John Wormald1*Background: The management of recalcitrant chronic rhinosinusitis (CRS) is challenged by difficult-to-treat polymicrobial biofilms and multidrug resistant bacteria. This has led to the search for broad-spectrum non-antibiotic antimicrobial therapies. Colloidal silver (CS) has significant antibiofilm activity in vitro and in vivo against S. aureus, MRSA, and P. aeruginosa. However, due to the lack of scientific efficacy, it is only currently used as an alternative medicine. This is the first study looking at the safety and efficacy of CS in recalcitrant CRS.
Methods: Patients were included when they had previously undergone endoscopic sinus surgery and presented with signs and symptoms of sinus infection with positive bacterial cultures. Twenty-two patients completed the study. Patients were allocated to 10–14 days of culture directed oral antibiotics with twice daily saline rinses (n = 11) or 10 days of twice daily 0.015 mg/mL CS rinses (n = 11). Safety observations included pre- and post-treatment serum silver levels, University of Pennsylvania Smell Identification Test (UPSIT) and adverse event (AE) reporting. Efficacy was assessed comparing microbiology results, Lund Kennedy Scores (LKS) and symptom scores using Visual Analog Scale (VAS) and Sino-Nasal Outcome Test (SNOT-22).
Results: CS demonstrated good safety profile with no major adverse events, no changes in UPSIT and transient serum silver level changes in 4 patients. CS patients had 1/11 (9.09%) negative cultures, compared to 2/11 (18.18%) in the control group upon completion of the study. Whilst not statistically significant, both groups showed similar improvement in symptoms and endoscopic scores.
Conclusion: This study concludes that twice daily CS (0.015 mg/mL) sinonasal rinses for 10 days is safe but not superior to culture-directed oral antibiotics. Further studies including more patients and looking at longer treatment or improving the tonicity of the solution for better tolerability should be explored.
The management of recalcitrant chronic rhinosinusitis (CRS) is increasingly challenged by difficult-to-treat polymicrobial biofilms and multidrug resistant bacteria which antibiotics often cannot effectively eradicate. For recalcitrant patients, antibiotics often alleviate symptoms in acute exacerbations but fail to eradicate the biofilm nidus which periodically sheds planktonic organisms resulting in a relapsing and remitting course of disease (Foreman et al., 2011). This has fuelled a continuous search for broad-spectrum topical non-antibiotic anti-biofilm therapies. Topical agents allow increased concentration, localized action, less systemic side effects and lessen the risk of antibiotic resistance.
To date, numerous topical agents have been tested and although some have shown anti-biofilm activity (Chiu et al., 2008; Le et al., 2008; Alandejani et al., 2009; Jardeleza et al., 2011; Jervis-Bardy et al., 2012; Paramasivan et al., 2014; Richter et al., 2016, 2017a,b), none have been widely accepted as a treatment option in recalcitrant CRS. Recent evidence suggests that colloidal silver (CS) may be effective against bacterial biofilms. We have previously shown that CS showed significant anti-biofilm activity in vitro and in vivo against S. aureus (Goggin et al., 2014; Rajiv et al., 2015), and against methicillin-resistant S. aureus (MRSA) and P. aeruginosa biofilms. Spherical nanoparticles were also shown to be non-toxic in human cell culture (THP-1, Nuli-1) (Richter et al., 2017c) and safe in a sheep sinusitis model (Rajiv et al., 2015). Moreover, they were physically stable for over 6 months in storage with no observed loss in anti-biofilm activity (Richter et al., 2017c).
However, due to the lack of evidence for their efficacy, it is only currently used as an alternative medicine. This is the first study investigating the safety and efficacy of CS in recalcitrant CRS patients.
This was a prospective, open-label, single-blinded, pilot study looking at the safety and efficacy of CS sinonasal rinses in patients with recalcitrant CRS between December 2016 to July 2017. Ethics approval was granted by the Central Northern Adelaide Health Service, Ethics of Human Research Committee (TQEH/LMH/MH HREC) to conduct the trial within its network of teaching hospitals in Adelaide, Australia. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
A total of 22 patients were enrolled in the study (8 females, 14 males, aged 27–86). Patients were allocated to either the colloidal silver arm (CS) (n = 11) or control arm (CON) (n = 11) depending on availability of silver stock and patient's adverse reaction to culture-sensitive oral antibiotics (Figure 1). Full inclusion and exclusion criterias are outlined in Table 1. Baseline demographic and clinical characteristic are demonstrated in Table 2.
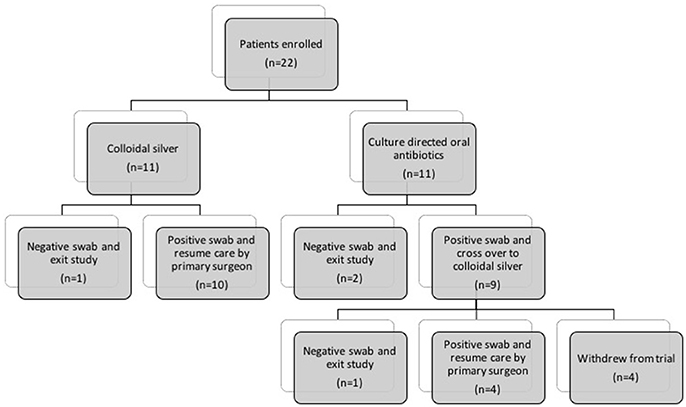
Figure 1. Flow diagram describing patients allocated to (1) Culture-directed oral antibiotics with twice daily saline flush (CON) and (2) Colloidal silver (CS) with twice-daily saline flush. CON, Control; CS, Colloidal silver.
CS patients were provided with 20 sealed bottles of pre-filled 120 mL CS solution in standard nasal irrigation squeeze bottles. Patients were instructed to store these bottles away from light and in the refrigerator. Prior to use, patients were asked to warm the solution to room temperature, fill the rinse bottle to 240 mL with cooled boiled water, then perform the rinses twice daily for 10 days. Patients are to apply gentle pressure onto squeeze bottles which delivers the solution through the inner tube and out of the tip of the bottle into the nostril. CS patients were specifically instructed not to add the usual proprietary buffered salts sachets to avoid chemical interaction with the CS nanoparticles. All squeeze bottles were provided by NeilMed Pharmaceuticals (Santa Rosa, CA). If there were signs of persistent infection on endoscopic examination and a positive culture swab post-treatment, CS patients exited the study and resumed treatment based on clinical grounds.
CON patients received a 10 to 14-day course of culture-directed oral antibiotics and were instructed to perform twice daily saline rinses similar to the delivery of CS. If the patient had persistent infection on endoscopic examination and a positive culture swab at the end of treatment, they received CS.
Those taking INCs on enrolment were instructed to continue throughout the duration of the study.
Spherical silver nanoparticles were prepared as previously described (Richter et al., 2017c). Briefly, a mixture of 6.25 mL water, 1.25 mL sodium citrate (1% wt.), 1.25 mL silver nitrate (1% wt.) and 50 μl potassium iodide (300 μM) was prepared under stirring at room temperature and incubated for 5 min. This mixture was added to 237.5 mL of boiling water that included 250 μl ascorbic acid (0.1M). The colorless solution changed to yellow and finally slightly orange, indicating particle formation. The silver nanoparticles were further boiled for 1 h under reflux and stirring at 1,500 rpm. After cooling, the silver nanoparticles were characterized by UV-Vis spectrometry and transmission electron microscopy (quality control). This confirmed a spherical particle shape and size of approximately 40 nm. Silver nanoparticles were stored in amber glass flasks under dark condition at 4°C prior to utilization as a nasal rinse.
Endoscopic guided sinonasal swabs were taken at every scheduled visit for microbiological evaluation. All patients completed symptoms score questionnaire at every visit, using Sino-Nasal Outcome Test-22 (SNOT-22) (Kennedy et al., 2013) (22 items, each scored from 0 to 5; total score range 0 to 110) and Visual Analog Scale (VAS) (Walker and White, 2000) (average of 6 items and an overall symptom score; each scored from 0 to 100, total score range 0 to 100). At each visit, all patients had entry and exit endoscopic videos recorded and scored by a blinded surgeon using the Lund Kennedy Score (LKS) (Lund and Kennedy, 1995; Kennedy et al., 2013) (score range, 0–20).
All patients on CS treatment were required to have pre- and post-treatment serum silver levels and completed the University of Pennsylvania Smell Identification Test (UPSIT). If serum silver level post-treatment was above normal limits, a repeat serum silver level was performed 7 days later to confirm return to baseline. Patients were advised to report any adverse outcomes while on the study.
Statistical power was calculated for the primary end-point of culture negativity post-treatment. Power analysis estimates determined a sample size of 11 patients per group would be required to achieve statistical significance (80%, p < 0.05) based on response rates of 25 and 90% in the control and silver groups, respectively.
All results were statistically analyzed at the completion of the study using 2-way analysis of variance (ANOVA) and student's t-test, with a significance value set at p < 0.05.
2/11 (18.18%) patients in CON group had negative swabs while 1/11 (9.09%) CS patients had negative swabs upon completion of treatment. List of pathogens treated in both cohorts are described in Table 3.
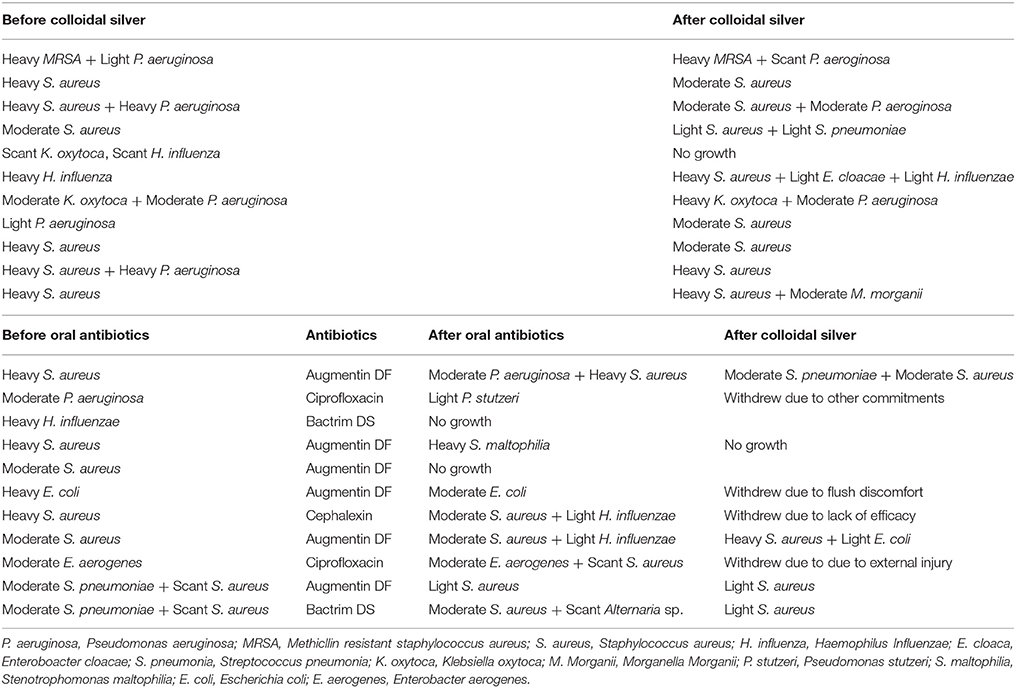
Table 3. Standard semi-quantitative analysis of bacterial load reported as scant, light, moderate or heavy (equivalent to 1+, 2+, 3+, or 4+) by laboratory.
VAS scores in both CON and CS groups showed a similar trend of improvement post-treatment, but both were not statistically significant (CON 1.728 [95% CI −7.785 to 11.24] vs. CS 3.536 [95% CI −5.977 to 13.05]) (Figure 2).
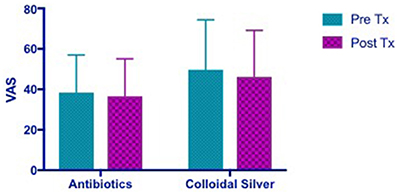
Figure 2. Bar graph showing no significant difference in VAS scores between CON and CS treated groups. VAS, Visual Analog Scale; CON, Control; CS, Colloidal silver.
Patients in the CON group showed no change in SNOT-22 scores post- treatment while CS group showed a trend toward an improvement in SNOT-22 scores, but it was not statistically significant (CON −0.6364 [95% CI −6.673 to 5.4] vs. CS 5.818 [95% CI −0.2183 to 11.85]) (Figure 3).
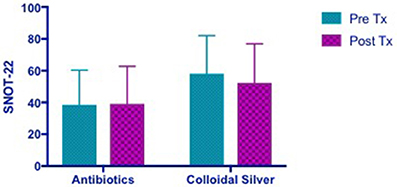
Figure 3. Bar graph showing no change in SNOT-22 scores in CON group, while CS group showed a trend of improved SNOT-22 scores, but not statistically significant. SNOT-22, Sino-Nasal Outcome Test-22; CON, Control; CS, Colloidal silver.
Both CON and CS group showed trends of similar improvements in Lund Kennedy Scores but this was not statistically significant (CON 1.818 [95% CI −1.373 to 5.009] vs. CS 2.167 [95% CI −2.154 to 6.488]) (Figure 4).
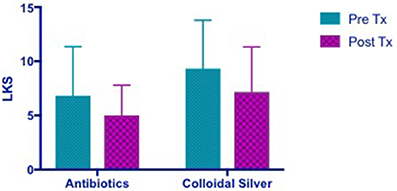
Figure 4. Bar graph showing similar improvements in LKS in both CON and CS groups, but not statistically significant. LKS, Lund Kennedy Scores; CON, Control; CS, Colloidal silver.
Five patients completed the crossover CS arm after failing oral antibiotics. Subgroup analyses were performed comparing VAS, SNOT-22, and LKS scores of patients while on either treatment. The mean score difference post antibiotic treatment vs. post CS treatment were compared using Wilcoxon matched-pairs signed rank tests. However, due to the small sample size of our subgroup analyses, data presented is focused on describing observed trends.
1/5 patient had successful infection eradication from CS treatment after failing culture-sensitive oral antibiotics.
There were slight improvements in VAS scores after culture sensitive oral antibiotics and CS treatment. There was a trend of greater improvement in VAS while on CS compared to when patients were treated with culture sensitive oral antibiotics. It is also observed that patients' VAS scores appeared to return to baseline after completing course of oral antibiotics and before commencing CS which is consistent with what is observed in clinical practice (Figure 5). Mean difference in VAS scores when patients were on culture sensitive oral antibiotics 4.546 [95% CI −8.156 to 17.25] vs. CS treatment 5.94 [95% CI −3.347 to 15.23], p = 0.4750.
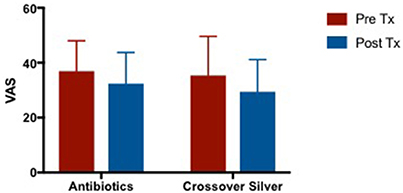
Figure 5. Bar graph comparing VAS scores of patients following failed culture sensitive oral antibiotics and crossed-over to CS treatment. VAS, Visual Analog Scale; CS. Colloidal silver.
There were no changes in SNOT-22 scores after culture sensitive oral antibiotics treatment but showed trends of improvement when patients were crossed over to CS treatment (Figure 6). Mean difference in SNOT-22 scores when patients were on culture sensitive oral antibiotics 0.2 [95% CI −2.021 to 2.421] vs. CS treatment −13 [95% CI −22.42 to −3.585], p = 0.06.
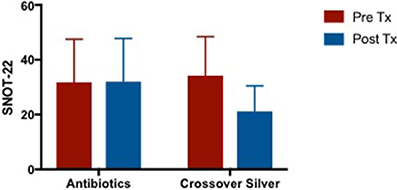
Figure 6. Bar graph comparing SNOT-22 scores of patients following failed culture sensitive oral antibiotics and crossed-over to CS treatment. SNOT-22, Sino-Nasal Outcome Test-22; CS, Colloidal silver.
Patients demonstrated an improvement in LKS post antibiotic treatment and further improvements were observed after completion of CS treatment (Figure 7). Mean difference in LKS scores when patients were on culture sensitive oral antibiotics −2.8 [95% CI −7.311 to 1.711] vs. CS treatment −1.4 [95% CI −4.259 to 1.459], p = 0.50.
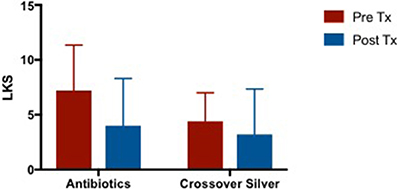
Figure 7. Bar graph comparing LKS scores of patients following failed culture sensitive oral antibiotics and crossed-over to CS treatment. LKS, Lund Kennedy Scores; CS, Colloidal silver.
Four patients who had received CS had serum silver levels that were above normal limits measured within 24 h after receiving final silver dose. 3 patients had a repeat test 10 days after study exit which saw serum silver levels had returned to normal parameters. One patient had serum silver levels which were above normal ranges pre-treatment and on repeat test had returned to baseline. Our laboratory reference indicates that argyria can be present at serum silver levels of approximately 100 nmol/L, the highest level of serum silver level recorded in our study was 57.3 nmol/L.
There were no significant changes in smell pre- and post-treatment between both groups measured using the University of Pennsylvania Smell Identification Test (UPSIT).
There were no serious adverse events reported.
In this study, looking at the primary end-point of culture negativity post-treatment, CS has not been shown to be superior to culture-directed oral antibiotics. Although interesting to note, CS patients had more severe baseline disease when compared to CON, but demonstrated comparable improvement in subjective symptoms and objective endoscopic scores suggesting it may be more than just a placebo effect. It is possible that CS treatment over 10 days is sufficient to demonstrate symptomatic and endoscopic improvement but insufficient time to achieve bacterial eradication. Indeed, when compared with topical mupirocin rinses which have been one of the more successful topical treatments for recalcitrant patients (Solares et al., 2006; Uren et al., 2008; Jervis-Bardy and Wormald, 2012; Jervis-Bardy et al., 2012; Seiberling et al., 2013), mupirocin has been used as a twice-daily rinse over 3–4 weeks. We believe that this reflects the duration of CS treatment needs to be further optimized. A longer study period including a larger number of study participants would be needed to assess the safety and efficacy of CS topical application in these patients.
The spherical CS nanoparticles used in this study has been shown to have substantial anti-biofilm activity in vitro with 96, 97, and 98% biofilm reduction of S. aureus, MRSA, and P. aeruginosa respectively (Richter et al., 2017c). It has been postulated that CS exerts its antimicrobial properties via multiple mechanisms. It can act on bacterial cell membranes by disrupting phosphate (Schreurs and Rosenberg, 1982) and sodium channels (Semeykina and Skulachev, 1990), inhibits mitochondrial ATPase (Chappell and Greville, 1954) and interacts with bacterial DNA to form dissociable complexes (Rosenkranz and Rosenkranz, 1972; Modak and Fox Jr., 1973).
Some immunomodulatory functions of CS have also been observed in the literature. It has the ability to inhibit matrix metalloproteinases (MMPs) which is pro-inflammatory (Wright et al., 2002) and metallothionein (Wright et al., 2002) (MT) which promotes resistance to immune-mediated apoptosis (Dutsch-Wicherek et al., 2006). Both MTs and MMPs have been found at increased levels in patient with CRS with nasal polyps (CRSwNP) (Wicherek et al., 2007; Eisenberg et al., 2008; Sauter et al., 2008). CS has also been shown to induce inflammatory cells apoptosis by TNF-α and IL-12 suppression (Bhol and Schechter, 2005). An improved host response might be able to account for the efficacy observed in the CS cohort even though there was no eradication of bacteria.
However, one of the limitations of this study is the time-consuming process of manufacturing CS rinses using small scale equipment. Currently, to prepare sufficient CS for a 10-day treatment course a full-time laboratory personnel requires over 10–15 h. If production cannot be upscaled, CS could be evaluated as an adjunct to oral antibiotics.
In the literature, silver has been described to exhibit low toxicity with minimal risks expected from clinical exposure. Silver is absorbed into the systemic circulation as a protein complex and eliminated by the liver and kidneys (Lansdown, 2006). Prolonged silver exposure commonly associated with occupational and/or systemic administration can lead to deposition of silver particles in skin (argyria), eye (argyrosis), and other organs (Tomi et al., 2004). Argyria is a cosmetic concern with irreversible blue-gray skin discoloration in sun-exposed areas, but not life-threatening.
Reported cases of silver toxicity are limited. In the literature, very little data exists correlating serum silver levels with symptomatic presentation of argyria and at present there are no medical guidelines available regarding its use. The World Health Organisation reported that a person can have a total lifetime oral intake of approximately 10 g of silver with no observed adverse effects (World Health Organisation, 1996). The United States Environmental Protection Agency's has reported that a maximum acceptable oral dose of silver to be 0.005 mg/kg/day or about 0.35 mg for a 70 kg person a day, every day during their lifetime (Fung and Bowen, 1996). In this study patients will be exposed to a total of 72 mg of topical CS rinses, which is well under the total lifetime amount of 10 g and to an equivalent of 7.2 mg/day of topical silver treatment for 10 days. Our laboratory reference of serum silver levels indicates argyria could be present when serum silver levels exceed 100 nmol/L. The serum silver levels were well below this concentration and no symptoms of argyria were observed in any patient of this study.
Although this study has shown that CS is safe based on serum silver levels and smell tests, the discomfort of using CS rinses have been noted. This discomfort is likely due to the tonicity and temperature of the rinses and possible stinging properties from silver. To improve the tonicity of the rinse solution for better tolerability, we are currently looking at mixing CS with 5% dextrose isotonic solution.
This study concludes that twice daily CS (0.015 mg/mL) sinonasal rinses for 10 days is safe but not superior to culture-directed oral antibiotics. Future studies looking at optimizing the tolerability, duration of treatment and investigating the role of CS as an adjunct treatment to oral antibiotics should be explored and evaluated in a randomized, double-blinded, placebo-controlled trial.
MO: project design, data collection and analysis, manuscript preparation; KR: project design, product manufacture and quality control, manuscript preparation; CB: product manufacture and quality control; LM-V: data analysis; AP project design, manuscript preparation; SV: project design, manuscript preparation; P-JW: project design, manuscript preparation.
The University of Adelaide, School of Medicine, Department of Otolaryngology Head and Neck Surgery, Adelaide, SA, Australia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CRS, chronic rhinosinusitis; INC, intranasal corticosteroid; CON, control; CS, Colloidal Silver; VAS, Visual Analog Scale; SNOT-22, Sino-Nasal Outcome Test-22; LKS, Lund Kennedy Scores; UPSIT, University of Pennsylvania Smell Identification Test; AE, Adverse Event.
World Health Organisation (1996). Silver in Drinking Water: Background Document for the Development of WHO Guidelines for Drinking Water Quality. Geneva: WHO.
Alandejani, T., Marsan, J., Ferris, W., Slinger, R., and Chan, F. (2009). Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 141, 114–118. doi: 10.1016/j.otohns.2009.01.005
Bhol, K. C., and Schechter, P. J. (2005). Topical nanocrystalline silver cream suppresses inflammatory cytokines and induces apoptosis of inflammatory cells in a murine model of allergic contact dermatitis. Br. J. Dermatol. 152, 1235–1242. doi: 10.1111/j.1365-2133.2005.06575.x
Chappell, J. B., and Greville, G. D. (1954). Effect of silver ions on mitochondrial adenosine triphosphatase. Nature 174, 930–931. doi: 10.1038/174930b0
Chiu, A. G., Palmer, J. N., Woodworth, B. A., Doghramji, L., Cohen, M. B., Prince, A., et al. (2008). Baby shampoo nasal irrigations for the symptomatic post-functional endoscopic sinus surgery patient. Am. J. Rhinol. 22, 34–37. doi: 10.2500/ajr.2008.22.3122
Dutsch-Wicherek, M., Tomaszewska, R., Strek, P., Wicherek, L., and Skladzien, J. (2006). The analysis of RCAS1 and DFF-45 expression in nasal polyps with respect to immune cells infiltration. BMC Immunol. 7:4. doi: 10.1186/1471-2172-7-4
Eisenberg, G., Pradillo, J., Plaza, G., Lizasoain, I., and Moro, M. A. (2008). [Increased expression and activity of MMP-9 in chronic rhinosinusitis with nasal polyposis]. Acta Otorrinolaringol. Esp. 59, 444–447. doi: 10.1016/S0001-6519(08)75116-1
Foreman, A., Jervis-Bardy, J., and Wormald, P.-J. (2011). Do biofilms contribute to the initiation and recalcitrance of chronic rhinosinusitis? Laryngoscope 121, 1085–1091. doi: 10.1002/lary.21438
Fung, M. C., and Bowen, D. L. (1996). Silver products for medical indications: risk-benefit assessment. J. Toxicol. Clin. Toxicol. 34, 119–126. doi: 10.3109/15563659609020246
Goggin, R., Jardeleza, C., Wormald, P. J., and Vreugde, S. (2014). Colloidal silver: a novel treatment for Staphylococcus aureus biofilms? Int. Forum Allergy Rhinol. 4, 171–175. doi: 10.1002/alr.21259
Jardeleza, C., Foreman, A., Baker, L., Paramasivan, S., Field, J., Tan, L. W., and Wormald, P. J. (2011). The effects of nitric oxide on Staphylococcus aureus biofilm growth and its implications in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 1, 438–444. doi: 10.1002/alr.20083
Jervis-Bardy, J., Boase, S., Psaltis, A., Foreman, A., and Wormald, P. J. (2012). A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope 122, 2148–2153. doi: 10.1002/lary.23486
Jervis-Bardy, J., and Wormald, P. J. (2012). Microbiological outcomes following mupirocin nasal washes for symptomatic, Staphylococcus aureus-positive chronic rhinosinusitis following endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2, 111–115. doi: 10.1002/alr.20106
Kennedy, J. L., Hubbard, M. A., Huyett, P., Patrie, J. T., Borish, L., and Payne, S. C. (2013). Sino-nasal outcome test (SNOT-22): a predictor of post-surgical improvement in patients with chronic sinusitis. Ann. Allergy Asthma Immunol. 111, 246–251.e2. doi: 10.1016/j.anai.2013.06.033
Lansdown, A. B. (2006). Silver in health care: antimicrobial effects and safety in use. Curr. Probl. Dermatol. 33, 17–34. doi: 10.1159/000093928
Le, T., Psaltis, A., Tan, L. W., and Wormald, P. J. (2008). The efficacy of topical antibiofilm agents in a sheep model of rhinosinusitis. Am. J. Rhinol. 22, 560–567. doi: 10.2500/ajr.2008.22.3232
Lund, V. J., and Kennedy, D. W. (1995). Quantification for staging sinusitis. The Staging and Therapy Group. Ann. Otol. Rhinol. Laryngol. Suppl. 167, 17–21.
Modak, S. M., and Fox, C. L., Jr. (1973). Binding of silver sulfadiazine to the cellular components of Pseudomonas aeruginosa. Biochem. Pharmacol. 22, 2391–2404. doi: 10.1016/0006-2952(73)90341-9
Paramasivan, S., Drilling, A. J., Jardeleza, C., Jervis-Bardy, J., Vreugde, S., and Wormald, P. J. (2014). Methylglyoxal-augmented manuka honey as a topical anti-Staphylococcus aureus biofilm agent: safety and efficacy in an in vivo model. Int. Forum Allergy Rhinol. 4, 187–195. doi: 10.1002/alr.21264
Rajiv, S., Drilling, A., Bassiouni, A., James, C., Vreugde, S., and Wormald, P. J. (2015). Topical colloidal silver as an anti-biofilm agent in a Staphylococcus aureus chronic rhinosinusitis sheep model. Int. Forum Allergy Rhinol. 5, 283–288. doi: 10.1002/alr.21459
Richter, K., Facal, P., Thomas, N., Vandecandelaere, I., Ramezanpour, M., Cooksley, C., et al. (2017c). Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl. Mater. Interfaces 9, 21631–21638. doi: 10.1021/acsami.7b03672
Richter, K., Ramezanpour, M., Thomas, N., Prestidge, C. A., Wormald, P. J., and Vreugde, S. (2016). Mind “De GaPP”: in vitro efficacy of deferiprone and gallium-protoporphyrin against Staphylococcus aureus biofilms. Int. Forum Allergy Rhinol. 6, 737–743. doi: 10.1002/alr.21735
Richter, K., Thomas, N., Claeys, J., McGuane, J., Prestidge, C. A., Coenye, T., et al. (2017b). A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrob. Agents Chemother. 61:e00481-17. doi: 10.1128/AAC.00481-17
Richter, K., Van den Driessche, F., and Coenye, T. (2017a). Innovative approaches to treat Staphylococcus aureus biofilm-related infections. Essays Biochem. 61, 61–70. doi: 10.1042/EBC20160056
Rosenkranz, H. S., and Rosenkranz, S. (1972). Silver sulfadiazine: interaction with isolated deoxyribonucleic acid. Antimicrob. Agents Chemother. 2, 373–383. doi: 10.1128/AAC.2.5.373
Sauter, A., Stern-Straeter, J., Sodha, S., Hormann, K., and Naim, R. (2008). Regulation of matrix metalloproteinases (MMP)-2/-9 expression in eosinophilic chronic rhinosinusitis cell culture by interleukin-5 and−13? In Vivo 22, 415–421.
Schreurs, W. J., and Rosenberg, H. (1982). Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 152, 7–13.
Seiberling, K. A., Aruni, W., Kim, S., Scapa, V. I., Fletcher, H., and Church, C. A. (2013). The effect of intraoperative mupirocin irrigation on Staphylococcus aureus within the maxillary sinus. Int. Forum Allergy Rhinol. 3, 94–98. doi: 10.1002/alr.21076
Semeykina, A. L., and Skulachev, V. P. (1990). Submicromolar Ag+ increases passive Na+ permeability and inhibits the respiration-supported formation of Na+ gradient in Bacillus FTU vesicles. FEBS Lett. 269, 69–72. doi: 10.1016/0014-5793(90)81120-D
Solares, C. A., Batra, P. S., Hall, G. S., and Citardi, M. J. (2006). Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant Staphylococcus aureus with mupirocin irrigations. Am. J. Otolaryngol. 27, 161–165. doi: 10.1016/j.amjoto.2005.09.006
Tomi, N. S., Kränke, B., and Aberer, W. (2004). A silver man. Lancet 363:532. doi: 10.1016/S0140-6736(04)15540-2
Uren, B., Psaltis, A., and Wormald, P.-J. (2008). Nasal lavage with mupirocin for the treatment of surgically recalcitrant chronic rhinosinusitis. Laryngoscope 118, 1677–1680. doi: 10.1097/MLG.0b013e31817aec47
Walker, F. D., and White, P. S. (2000). Sinus symptom scores: what is the range in healthy individuals? Clin. Otolaryngol. Allied Sci. 25, 482–484. doi: 10.1046/j.1365-2273.2000.00349.x
Wicherek, L., Galazka, K., and Lazar, A. (2007). Analysis of metallothionein, RCAS1 immunoreactivity regarding immune cell concentration in the endometrium and tubal mucosa in ectopic pregnancy during the course of tubal rupture. Gynecol. Obstet. Invest. 65, 52–61. doi: 10.1159/000107649
Wright, J. B., Lam, K., Buret, A. G., Olson, M. E., and Burrell, R. E. (2002). Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 10, 141–151. doi: 10.1046/j.1524-475X.2002.10308.x
Keywords: chronic rhinosinusitis, recalcitrant, infection, antimicrobial, topical agent, safety, efficacy
Citation: Ooi ML, Richter K, Bennett C, Macias-Valle L, Vreugde S, Psaltis AJ and Wormald P-J (2018) Topical Colloidal Silver for the Treatment of Recalcitrant Chronic Rhinosinusitis. Front. Microbiol. 9:720. doi: 10.3389/fmicb.2018.00720
Received: 03 December 2017; Accepted: 27 March 2018;
Published: 11 April 2018.
Edited by:
Maria Olivia Pereira, University of Minho, PortugalReviewed by:
Debora Barros Barbosa, São Paulo State University-UNESP, BrazilCopyright © 2018 Ooi, Richter, Bennett, Macias-Valle, Vreugde, Psaltis and Wormald. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter-John Wormald, cGV0ZXJqLndvcm1hbGRAYWRlbGFpZGUuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.