- 1Department of Rheumatology and Clinical Immunology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece
- 2Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, University of Patras, Patras, Greece
Rheumatoid arthritis (RA) is associated with HLA-DRB1 shared epitope (HLA-DRB1SE) and anti-citrullinated protein autoantibodies (ACPAs). ACPAs precedes the onset of clinical and subclinical RA. There are strong data for three infectious agents as autoimmunity triggers in RA, namely Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans causes of periodontal disease (PD), and Epstein-Barr virus (EBV). P. gingivalis expresses arginine gingipains, that cleave proteins at the arginine residues, and peptidyl arginine deiminase (PPAD), which citrullinates arginine residues of proteins, thus forming neoantigens that lead to ACPA production. Peripheral blood plasmablasts from ACPA+RA patients produce ACPAs the majority of which react against P. gingivalis. A. actinocycetemcomitans produces leukotoxin A, a toxin that forms pores in the neutrophil membranes and leads to citrullination and release of citrullinated autoantigens in the gums. EBV can infect B cells and epithelial cells and resides as latent infection in resting B cells. Abs against citrullinated peptides derived from EBV nuclear antigen appear years before RA and cross-react with human citrullinated fibrin. Citrullinated proteins are potential arthritogenic autoantigens in RA. The conversion of arginine to citrulline increases the peptide binding affinity to HLA-DRB1SE. Also, citrullinated fibrinogen induces arthritis in HLA-DRB1*0401 transgenic mice, and transfer of their splenic T cells causes arthritis to recipient mice.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease mainly manifested with peripheral polyarthritis. The aetiopathogenesis of the disease is incompletely understood. Risk factors for RA include HLA-DR genes, periodontal disease (PD), and smoking (Bartold et al., 2005; Scher et al., 2012; Mikuls et al., 2014; Kharlamova et al., 2016). The early HLA-DR4 association of RA classified RA by many investigators as an immune-mediated disease and suggested that T cells recognized an antigen presented on HLA-DR4 molecules. The discovery of HLA-DRB1 shared epitope (SE, HLA-DRB1SE), a hypervariable DRβ chain sequence shared by all alleles associated with RA, reinforced this concept (Gregersen et al., 1987; Wordsworth et al., 1989). The discovery of autoantibodies against citrullinated antigens (ACPAs) greatly advanced our understanding of the pathogenetic mechanisms in this disease. ACPAs appear years before clinical onset of RA (Nielen et al., 2004), predict subsequent development of the disease, occur in 50–67% of RA patients, are associated with severe disease, and are highly specific for the disease (van Gaalen et al., 2004; van der Helm-van Mil et al., 2005; Alexiou et al., 2007; Barouta et al., 2017; Hensvold et al., 2017).
Citrullination is a post-translational modification of proteins in which arginine residues are converted to citrulline by the action of enzyme peptidylarginine deiminase (PAD). There are five PAD isoforms (PAD1-4, PAD6), and PAD2 and PAD4 have been implicated in RA. The production of ACPAs means break of tolerance. Tolerance is no immune response to unmodified self. Many proteins are extensively post-translationally modified that including citrullination. In this context, citrullination is a physiological process in many tissues and only in specific circumstances this leads to immune response. Thus, citrullination could create particular neoantigens that would activate T cells, which in turn will provide antigen-specific help to B cells to produce ACPA. Indeed, citrullination increases the affinity of citrullinated antigen to HLA-DRB1SE allele (Hill et al., 2003; Scally et al., 2013). ACPAs in RA recognize many citrullinated autoantigens (Table 1) and are associated with HLA-DRB1SE (Snir et al., 2009), and HLA-DRB1SE appears to be a risk factor for ACPA production in RA rather than an independent risk factor for RA development (van der Helm-van Mil et al., 2006). These findings and the fact that ACPAs are of IgG and IgA class suggest that T cells provide help to B cells for the subsequent ACPA production.

Table 1. Examples of citrullinated peptides which are targeted by immune responses against self and non-self immune responses in patients with rheumatoid arthritis.
Although smoking is a risk factor for RA (van der Helm-van Mil et al., 2007; Lundberg et al., 2013; Hensvold et al., 2015), and increases citrullination in bronchial tissues (Makrygiannakis et al., 2008), other environmental factors, in addition to smoking, appear to play a predominant role in the development of ACPA+RA (Lee et al., 2007; Hensvold et al., 2015) and infections are likely candidates (Bogdanos and Sakkas, 2017).
Infections as Generators of ACPA in RA
ACPAs precede the subclinical joint inflammation in pre-RA patients (van de Sande et al., 2011) and can be detected in joints and epithelial sites, such as periodontium in PD (Nesse et al., 2012) and bronchial tissues in early RA (Reynisdottir et al., 2014). Identical citrullinated peptides were found in pulmonary bronchial tissue and synovial membrane and were found to be targets of ACPAs in RA thus providing a link between lungs and joints in ACPA+RA (Ytterberg et al., 2015). A monoclonal ACPA derived from RA patients cross-reacted with many viral, bacterial fungal and plant proteins (Tsuda et al., 2015). The most widely studied infection has been with P. gingivalis and Ebstein-Barr virus. The mechanisms by which these infectious agents could trigger RA are illustrated in Figure 1.
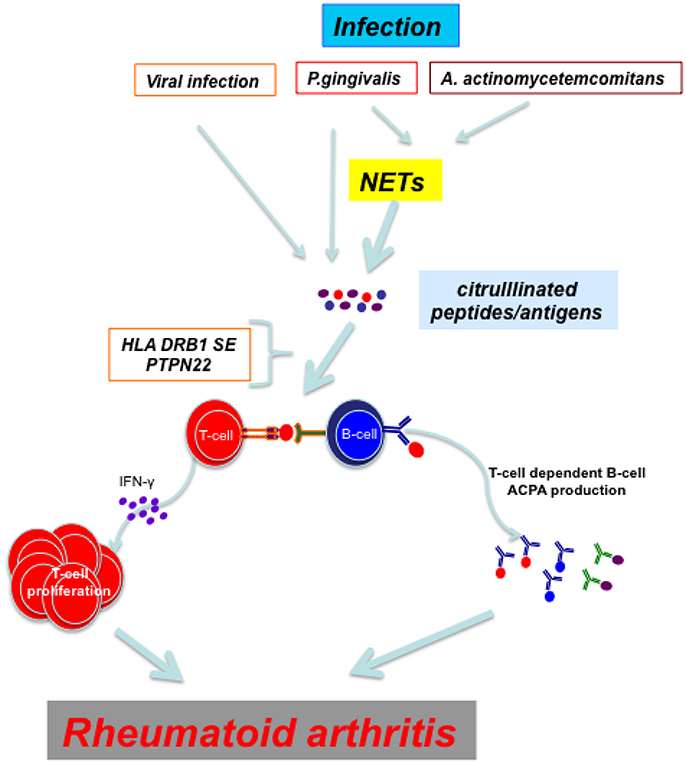
Figure 1. Viral infections and periodontal disease caused by P. gingivalis and A. actinocycetemcomitans induce directly or through NETs citrullination of proteins/peptides. In an individual with proper genetic background (HLA DRB1 SE and PTPN22 risk allele R620W) T cells recognize citrullinated peptides and mount an immune response which culminates in the development of rheumatoid arthritis.
Porphyromonas Gingivalis
Chronic PD is very common affecting nearly 30% of adult population (Brown and Loe, 1993) and is caused by various microbes including Porphyromonas gingivalis (P. gingivalis).
P. gingivalis infection, detected by abs against P. gingivalis components, have been associated with ACPA in HLA-DRB1SE+RA patients. Anti-P. gingivalis abs, detected as abs against RgpB, potent virulent factors of P. gingivalis (Haffajee and Socransky, 1994; Kadowaki et al., 1998), showed stronger association with ACPA+RA (Kharlamova et al., 2016). Furthermore, there was additive interaction between these two factors. Anti-RgpB abs also showed more than additive interaction with HLA-DRB1SE in ACPA+RA (Kharlamova et al., 2016). Using anti-P. gingivalis lipopolysaccharide abs, one study reported association of anti-P. gingivalis abs with ACPA in HLA-DRB1SE+ RA patients and their relatives (Hitchon et al., 2010) whereas another study did not find an association with RA or ACPA status (Seror et al., 2015).
P. gingivalis has two unique enzymes, peptidylarginine deiminase (PPAD) and arginine ginpains (Rgps) which are expressed on the bacterial outer membrane and can also be secreted (Potempa et al., 1995; McGraw et al., 1999). Rgps are proteases that cleave proteins at arginine residues, and PPAD citrullinates both bacterial and human proteins (Wegner et al., 2010). P. gingivalis PAD citrullinates carboxy-terminal arginine of human proteins following proteolytic cleavage by P. gingivalis arginine-gingipains (Wegner et al., 2010). Crystal structure of PPAD and the use of synthetic peptides also revealed that PPAD exhibits a definitive specificity for C-terminal arginine residue created by Rgps, whereas PAD2 and PAD4 preferentially citrullinate internal arginine residues (Goulas et al., 2015; Montgomery et al., 2016). Thus P. gingivalis creates neoantigens, not formed by PAD2 and PAD4 and this may explain its pathogenic potential.
It is reasonable to assume that neoantigens, created by Rpgs in conjunction with PPAD in the periodontium of PD, can lead to loss of tolerance and ACPA production. In PD, increased concentrations of anti-CCP and anti-α-enolase autoAbs are detected (Lappin et al., 2013). A peptide 1 of human citrullinated α-enolase (CEP1), an immunodominant epitope, shares 92% homology with P. gingivalis α-enolase and cross-reacts with it (Lundberg et al., 2008). This links periodontitis with RA and suggests that periodontal infection can be the inciting agent that breaks immune tolerance in ACPA+RA, although other studies did not find association of PD with RA (Arkema et al., 2010; Eriksson et al., 2016). Using a single-cell ab cloning method, Li et al showed that peripheral blood plasmablasts in ACPA+RA patients produce ACPAs the majority of which cross-react with outer membrane antigens and/or citrullinated a-enolase from P. gingivalis (Li et al., 2016).
In addition, P. gingivalis can induce neutrophil extracellullar trap (NET) formation (Delbosc et al., 2011), another source of citrullinated autoantigens. NETs are externalized chromatin fibers containing DNA and histones, and decorated with cytoplasmic granular peptides, such as myeloperoxidase, proteinase 3, neutrophil elastase, cathepsin G, LL37, and others, in a process of programmed neutrophil death called NETosis (Yang et al., 2016). PAD4-induced citrullination is an important step in NETosis during which citrullinated histones, vimentin, α-enolase and others are externalized and recognized by ACPAs (Li et al., 2010; Pratesi et al., 2014). PAD4 is also essential for the antibacterial neutrophil immunity (Li et al., 2010). NETosis is enhanced in RA peripheral blood and synovial fluid neutrophils (Khandpur et al., 2013). A positive feedback loop between NETosis and ACPA has been proposed. ACPAs induce NETosis and NETosis provides citullinated autoantigens for ACPA production, as NET components are recognized by RA autoantibodies (Khandpur et al., 2013). Also, B cells from synovial ectopic lymphoid structures (ELS), recognize citrullinated histones of NETs. For instance, monoclonal abs generated from synovial ELS single B cell cloning from patients with ACPA+RA, recognized citrullinated histones from NETs (Corsiero et al., 2016). Neutrophils provide citrullinated autoantigens to the attention of the immune system also via immune-mediated membranolytic pathways (Romero et al., 2013), and this has led to introduction of another pathogen of PD as candidate trigger of autoimmunity in RA, namely Aggregatibacter actinomycetemcomitans.
Aggregatibacter Actinomycetemcomitans
Aggregatibacter actinomycetemcomitans (A.actinomycetemcomitans) a periodontal pathogen associated with aggressive PD (Haubek and Johansson, 2014) can cause citullination of a broad range of proteins by a completely different mechanism. A.actinomycetemcomitans produces leukotoxin A (LtxA), which forms pores on the cell membrane of neutrophils at the crevicular fluid of PD. This leads to neutrophil PAD activation and citrullination of a broad range of proteins, which are released from neutrophils (Konig et al., 2016a). In addition, 47% of RA patients show evidence of A.actinimycetemcomitans infection that is associated with ACPA presence. More interestingly, in patients with RA HLA-DRB1SE is associated with ACPA only in patients exposed to A.actinomycetemcomitans (Konig et al., 2016a). Thus A.actinomycetemcomitans is identified as a strong bacterial candidate triggering autoimmunity in RA.
Epstein-Barr Virus
Epstein-Barr virus (EBV) is a herpes virus infecting most of the adult population. EBV can infect B cells and epithelial cells and cause primary infection usually asymptomatic in childhood and then a life-long latent infection in resting memory B cells (Kalla and Hammerschmidt, 2012). High titers of anti-EBV abs are detected in RA patients (Alspaugh et al., 1981), and the EBV DNA load was found to be increased 7–10-fold in PBMCs from RA patients compared to healthy EBV carriers (Balandraud et al., 2003; Lunemann et al., 2008). Furthermore, substantial expansions of CD8+Tcells specific for EBV antigens were detected in PB (Lunemann et al., 2008) and expansions of CD8+T cells reactive against key transactivators of EBV lytic infection were also detected in RA joints (Scotet et al., 1996). Latent membrane protein (LMP) 2A through its immunoreceptor tyrosine activation motif (ITAM) phosphorylates (activates) downstream proteins of B cell receptor thus positively regulating B cell survival and activation (Swanson-Mungerson and Longnecker, 2007).
Abs against citrullinated peptide corresponding to EBV nuclear antigen (EBNA)1 (viral citrullinated peptide 1, VCP1) were detected in RA. Furthermore, affinity-purified anti-VCP1 abs reacted with citrullinated fibrinogen (Pratesi et al., 2006). More importantly, Abs against citrullinated peptides derived from EBNA2 (VCP2) along with abs against histone-4-derived citrullinated peptides appear years before the onset of clinical RA and predict subsequent development of RA (Johansson et al., 2016). These abs were associated with HLA-DRB1SE (Johansson et al., 2016). Abs against VCP1 and VCP2 cross-react with human citrullinated peptides (Pratesi et al., 2011). In particular, competition assays showed that abs to citrullinated peptide EBNA (VCP1) strongly cross-reacted with the citrullinated peptide β60-74 which bears the immunodominant epitope of citrullinated fibrin in RA (Cornillet et al., 2014, 2015). EBV latent transcripts and EBV latent and lytic proteins were detected in germinal center-like ectopic lymphoid structures (ELS) of RA synovial membrane (Croia et al., 2013). Also ACPA producing plasma cells (anti-citrullinated fibrinogen abs) at the periphery of ELS were infected with EBV (expressed lytic proteins). Furthermore, ELS-containing RA synovia transplanted onto severe combined immunodeficiency (SCID) mice produced abs against citrullinated EBV proteins (VCP1 and VCP2) (Croia et al., 2013). These findings provide strong circumstantial evidence that EBV may initiate an immune response which subsequently may be re-directed against self antigens by way of cross-reactivity and epitope spreading.
Citrullinated Proteins as Arthritogenic Autoantigens
ACPAs are associated with severe disease and are strong predictors of joint erosions in RA (Alexiou et al., 2007; Jilani and Mackworth-Young, 2015). Although association does not prove causation, several lines of evidence suggest that citrullinated proteins are likely to be arthritogenic autoantigens in RA (Sakkas et al., 2014). This means that citrullinated peptides are recognized by and activate T cells, which in turn (a) produce pro-inflammatory mediators and talk to other cells causing joint damage, and (b) provide help to B cells for ACPA production, which by themselves may be pathogenetic.
As already mentioned, the conversion of arginine to citrulline increases the affinity of citrullinated antigen binding to HLA-DRB1SE alleles (Hill et al., 2003; Scally et al., 2013). This has been confirmed in a study by Scally et al who showed that citrulline but no arginine is accommodated within the electropositive P4 pocket of RA-susceptible HLA-DRB1*0401/04 alleles (Scally et al., 2013). Furthermore, using HLA-DR4 tetramers, the authors found that citrullinated vimentin- and citrullinated aggrecan-specific T cells were present in peripheral blood of RA patients and their numbers were correlated with disease activity (Scally et al., 2013). Also, a T cell line recognizing citrullinated fibrinogen, abundant in RA joints, induced proinflammatory cytokines, and transfer of this T cell line to mice with CIA exacerbated arthritis (Cordova et al., 2013). Immunization with human fibrinogen (containing citrullinated peptides) in complete Freud adjuvant enhanced arthritis and T cells from these mice were fibrinogen-reactive and produced high levels of IL-6, IFNγ and IL-17 (Ho et al., 2010). Furthermore, adoptive transfer of plasma or T cells caused arthritis in naïve mice (Ho et al., 2010). Citrullinated fibrinogen but not unmodified fibrinogen induced arthritis in HLA-DRB1*04:01-IE transgenic mice but not in wild-type C57BL/6 mice (Hill et al., 2008). Furthermore, transfer of splenocytes from these transgenic arthritic mice caused arthritis to recipient mice, indicating that activated citrullinated fibrinogen-specific T cells are crucial for arthritis development (Yue et al., 2010). Also, a pan-PAD inhibitor (Cl-amidine) decreased the clinical severity of collagen-induced arthritis (CIA) and joint and serum protein citrullination (Willis et al., 2011). These studies show that citrullinated peptides in conjuction with HLA-DRB1SE activate T cells which become arthritogenic.
Infection with P. ginvivalis further contributes to joint inflammation that is dependent on citrullination. For instance, infection with P. gingivalis caused exacerbation of collagen-induced arthritis that was dependent on P. gingivalis PAD (PPAD) (Maresz et al., 2013). High levels of citrullinated proteins at the site of infection with P. gingivalis were detected as well as ACPAs (Maresz et al., 2013). Also, CIA was much less severe in the presence of PAD-deficient P. gingivalis (Gully et al., 2014). On the other hand, P. gingivalis components may cause arthritis through molecular mimicry. For instance, immunization of HLA-DR4-IE-transgenic mice with P. gingivalis α-enolase either citrullinated or noncitrullinated caused arthritis and abs reactive with human α-enolase (Kinloch et al., 2011). As already mentioned, P. gingivalis α-enolase shares sequence similarity with human α-enolase.
ACPAs contribute to joint inflammation and damage since they induce secretion of inflammatory cytokines and differentiation of osteoclasts. ACPA-containing immune complexes induced TNFα secretion by peripheral blood-derived macrophages (Clavel et al., 2008; Laurent et al., 2011; Sokolove et al., 2011), via toll-like receptor 4 (TLR4) and FcγR (Sokolove et al., 2011), whereas citrullinated fibrinogen stimulated TNFα production through TLR4 (England et al., 2017). Also, citrullinated histones increase macrophage TNFα production via TLR4, and immune complexes containing citrullinated histones activate macrophage production of TNFα via TLR4 and FcγR and neutrophils. Over 90% of RA patients have abs against neutrophil-derived citrullinated histones (citrullinated H2B).
P. gingivalis may also contribute to arthritis through inflammatory cytokine release. For instance, periodontal disease induced by P. gingivalis (Marchesan et al., 2013) or Prevotella nigrescens (de Aquino et al., 2014) exacerbated collagen-induced arthritis and promoted Th17 responses.
The effect of citrullinated proteins and ACPAs in wild-type animals (not transgenic animals) in the induction or exacerbation of arthritis is not certain. For instance, immunization of mice with citrullinated histone did not cause arthritis but exacerbated collagen-induced arthritis (Sohn et al., 2015). Similarly, some studies show exacerbating effect of ACPAs on collagen-induced arthritis and others suppressing effects (Kuhn et al., 2006; Shoda et al., 2011; Cantaert et al., 2013). For instance, it has been reported that co-administration of anti-citrullinated fibrinogen abs with anticollagen II abs enhanced CIA (Kuhn et al., 2006).
NETs, apart from providing targets for ACPAs, contribute to inflammatory process in RA. The nuclear and cytoplasmic molecules in NETs have antimicrobial properties, and stimulate adaptive and innate immune responses. LL37 NETs increase fibroblast-like synoviocyte IL-6 and IL-8 production (Khandpur et al., 2013). Also, LL37 can form complexes with DNA and RNA and stimulate innate TLRs (Lande et al., 2007; Ganguly et al., 2009).
ACPAs also contribute to joint erosions. ACPAs and monoclonal ACPAs derived from RA synovial fluid (SF) single B cells enhanced differentiation of osteoclasts through PAD-dependent IL-8 production. Furthermore, transfer of monoclonal ACPAs into mice induced IL-8-mediated bone loss (Krishnamurthy et al., 2016). Also, affinity-purified abs against mutated citrullinated vimentin (MCV) bind to osteoclast surface and induce osteoclastogenesis, whereas adoptive transfer of anti-MCV abs into mice causes osteopenia (Harre et al., 2012).
Relevance to Treatment
Although there may be few disagreements (Konig et al., 2016b), the research data outlined above imply that citrullination, in conjunction with genetic factors, such as HLA-DRB1SE, protein tyrosine phosphatase nonreceptor type 22 (PTPN22) risk allele (Joshua et al., 2016) encoding an R620W amino acid change that allows survival of autoreactive B cells (Menard et al., 2011), is the key element in breaking tolerance. Thus citrullinated peptides may offer new therapeutic strategies for RA. For instance, CTLA-4Ig blocked the development of arthritis induced by citrullinated fibrinogen in HLA-DRB1*0401 transgenic mice (Yue et al., 2010). This concept is re-enforced by the study of Gertel et al. who used citrullinate multiepitope peptide derived from prevalent citrullinated autoantigens in RA to reduce disease severity in adjuvant-induced arthritis in rats (Gertel et al., 2015). Also, citrullinated peptide autologous dendritic cells immunotherapy administered to once reduced effector T cells and increased regulatory T cells at 1 month (Benham et al., 2015).
Another strategy could be inhibition of TLR4. TLR4 is an innate immunity receptor for various ligands, including immune complexes containing ACPAs, mainly against citrullinated fibrinogen. Inhibition of TLR4 has been shown to decrease inflammatory arthritis in mouse models (Abdollahi-Roodsaz et al., 2007; Pierer et al., 2011). More importantly, the presence of ACPAs against citrullinated peptides from α-chains and β-chains of fibrinogen and histone 2A in RA patients predicts the anti-inflammatory response of TLR4 inhibition by a therapeutic ab (NI-0101) in an ex vivo model of RA (Hatterer et al., 2016). Therefore, it is likely that these new therapeutic strategies will be fruitful in human RA in the near future.
Author Contributions
LS, DD, SL, and DB substantially contributed on drafting the work and revising the manuscript, and approved the final version to be published. LS, DD, SL, and DB agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LS had the original idea of drafting the manuscript and overall supervision of manuscript's shaping.
Funding
This work was supported by the Research Committee of the University of Thessaly (Grant No. 4052).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdollahi-Roodsaz, S., Joosten, L. A., Roelofs, M. F., Radstake, T. R., Matera, G., Popa, C., et al. (2007). Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967. doi: 10.1002/art.22848
Alexiou, I., Germenis, A., Ziogas, A., Theodoridou, K., and Sakkas, L. I. (2007). Diagnostic value of anti-cyclic citrullinated peptide antibodies in Greek patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 8:37. doi: 10.1186/1471-2474-8-37
Alspaugh, M. A., Henle, G., Lennette, E. T., and Henle, W. (1981). Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J. Clin. Invest. 67, 1134–1140. doi: 10.1172/JCI110127
Arkema, E. V., Karlson, E. W., and Costenbader, K. H. (2010). A prospective study of periodontal disease and risk of rheumatoid arthritis. J. Rheumatol. 37, 1800–1804. doi: 10.3899/jrheum.091398
Balandraud, N., Meynard, J. B., Auger, I., Sovran, H., Mugnier, B., Reviron, D., et al. (2003). Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction. Arthritis Rheum. 48, 1223–1228. doi: 10.1002/art.10933
Barouta, G., Katsiari, C. G., Alexiou, I., Liaskos, C., Varna, A., Bogdanos, D. P., et al. (2017). Anti-MCV antibodies predict radiographic progression in Greek patients with very early (<3 months duration) rheumatoid arthritis. Clin. Rheumatol. 36, 885–894. doi: 10.1007/s10067-016-3494-4
Bartold, P. M., Marshall, R. I., and Haynes, D. R. (2005). Periodontitis and rheumatoid arthritis: a review. J. Periodontol. 76, 2066–2074. doi: 10.1902/jop.2005.76.11-S.2066
Benham, H., Nel, H. J., Law, S. C., Mehdi, A. M., Street, S., Ramnoruth, N., et al. (2015). Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl. Med. 7:290ra87. doi: 10.1126/scitranslmed.aaa9301
Bogdanos, D. P., and Sakkas, L. I. (2017). From microbiome to infectome in autoimmunity. Curr. Opin. Rheumatol. 29, 369–373. doi: 10.1097/BOR.0000000000000394
Brown, L. J., and Loe, H. (1993). Prevalence, extent, severity and progression of periodontal disease. Periodontology 2000 2, 57–71. doi: 10.1111/j.1600-0757.1993.tb00220.x
Burkhardt, H., Sehnert, B., Bockermann, R., Engstrom, A., Kalden, J. R., and Holmdahl, R. (2005). Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur. J. Immunol. 35, 1643–1652. doi: 10.1002/eji.200526000
Cantaert, T., Teitsma, C., Tak, P. P., and Baeten, D. (2013). Presence and role of anti-citrullinated protein antibodies in experimental arthritis models. Arthritis Rheum. 65, 939–948. doi: 10.1002/art.37839
Clavel, C., Nogueira, L., Laurent, L., Iobagiu, C., Vincent, C., Sebbag, M., et al. (2008). Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 58, 678–688. doi: 10.1002/art.23284
Cordova, K. N., Willis, V. C., Haskins, K., and Holers, V. M. (2013). A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J. Immunol. 190, 1457–1465. doi: 10.4049/jimmunol.1201517
Cornillet, M., Sebbag, M., Verrouil, E., Magyar, A., Babos, F., Ruyssen-Witrand, A., et al. (2014). The fibrin-derived citrullinated peptide beta60-74Cit(6)(0),(7)(2),(7)(4) bears the major ACPA epitope recognised by the rheumatoid arthritis-specific anticitrullinated fibrinogen autoantibodies and anti-CCP2 antibodies. Ann. Rheum. Dis. 73, 1246–1252. doi: 10.1136/annrheumdis-2012-202868
Cornillet, M., Verrouil, E., Cantagrel, A., Serre, G., and Nogueira, L. (2015). In ACPA-positive RA patients, antibodies to EBNA35-58Cit, a citrullinated peptide from the Epstein-Barr nuclear antigen-1, strongly cross-react with the peptide beta60-74Cit which bears the immunodominant epitope of citrullinated fibrin. Immunol. Res. 61, 117–125. doi: 10.1007/s12026-014-8584-2
Corsiero, E., Bombardieri, M., Carlotti, E., Pratesi, F., Robinson, W., Migliorini, P., et al. (2016). Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 75, 1866–1875. doi: 10.1136/annrheumdis-2015-208356
Croia, C., Serafini, B., Bombardieri, M., Kelly, S., Humby, F., Severa, M., et al. (2013). Epstein-Barr virus persistence and infection of autoreactive plasma cells in synovial lymphoid structures in rheumatoid arthritis. Ann. Rheum. Dis. 72, 1559–1568. doi: 10.1136/annrheumdis-2012-202352
de Aquino, S. G., Abdollahi-Roodsaz, S., Koenders, M. I., van de Loo, F. A., Pruijn, G. J., Marijnissen, R. J., et al. (2014). Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J. Immunol. 192, 4103–4111. doi: 10.4049/jimmunol.1301970
Delbosc, S., Alsac, J. M., Journe, C., Louedec, L., Castier, Y., Bonnaure-Mallet, M., et al. (2011). Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS ONE 6:e18679. doi: 10.1371/journal.pone.0018679
England, B. R., Thiele, G. M., and Mikuls, T. R. (2017). Anticitrullinated protein antibodies: origin and role in the pathogenesis of rheumatoid arthritis. Curr. Opin. Rheumatol. 29, 57–64. doi: 10.1097/BOR.0000000000000356
Eriksson, K., Nise, L., Kats, A., Luttropp, E., Catrina, A. I., Askling, J., et al. (2016). Prevalence of periodontitis in patients with established rheumatoid arthritis: a swedish population based case-control study. PLoS ONE 11:e0155956. doi: 10.1371/journal.pone.0155956
Ganguly, D., Chamilos, G., Lande, R., Gregorio, J., Meller, S., Facchinetti, V., et al. (2009). Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206, 1983–1994. doi: 10.1084/jem.20090480
Gertel, S., Serre, G., Shoenfeld, Y., and Amital, H. (2015). Immune tolerance induction with multiepitope peptide derived from citrullinated autoantigens attenuates arthritis manifestations in adjuvant arthritis rats. J. Immunol. 194, 5674–5680. doi: 10.4049/jimmunol.1402457
Goulas, T., Mizgalska, D., Garcia-Ferrer, I., Kantyka, T., Guevara, T., Szmigielski, B., et al. (2015). Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci. Rep. 5:11969. doi: 10.1038/srep11969
Gregersen, P. K., Silver, J., and Winchester, R. J. (1987). The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213. doi: 10.1002/art.1780301102
Gully, N., Bright, R., Marino, V., Marchant, C., Cantley, M., Haynes, D., et al. (2014). Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS ONE 9:e100838. doi: 10.1371/journal.pone.0100838
Haffajee, A. D., and Socransky, S. S. (1994). Microbial etiological agents of destructive periodontal diseases. Periodontology 2000 5, 78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x
Harre, U., Georgess, D., Bang, H., Bozec, A., Axmann, R., Ossipova, E., et al. (2012). Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802. doi: 10.1172/JCI60975
Hatterer, E., Shang, L., Simonet, P., Herren, S., Daubeuf, B., Teixeira, S., et al. (2016). A specific anti-citrullinated protein antibody profile identifies a group of rheumatoid arthritis patients with a toll-like receptor 4-mediated disease. Arthritis Res. Ther. 18:224. doi: 10.1186/s13075-016-1128-5
Haubek, D., and Johansson, A. (2014). Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J. Oral Microbiol. 6. doi: 10.3402/jom.v6.23980
Hensvold, A. H., Frisell, T., Magnusson, P. K., Holmdahl, R., Askling, J., and Catrina, A. I. (2017). How well do ACPA discriminate and predict RA in the general population: a study based on 12 590 population-representative Swedish twins. Ann. Rheum. Dis. 76, 119–125. doi: 10.1136/annrheumdis-2015-208980
Hensvold, A. H., Magnusson, P. K., Joshua, V., Hansson, M., Israelsson, L., Ferreira, R., et al. (2015). Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann. Rheum. Dis. 74, 375–380. doi: 10.1136/annrheumdis-2013-203947
Hill, J. A., Bell, D. A., Brintnell, W., Yue, D., Wehrli, B., Jevnikar, A. M., et al. (2008). Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J. Exp. Med. 205, 967–979. doi: 10.1084/jem.20072051
Hill, J. A., Southwood, S., Sette, A., Jevnikar, A. M., Bell, D. A., and Cairns, E. (2003). Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 171, 538–541. doi: 10.4049/jimmunol.171.2.538
Hitchon, C. A., Chandad, F., Ferucci, E. D., Willemze, A., Ioan-Facsinay, A., van der Woude, D., et al. (2010). Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J. Rheumatol. 37, 1105–1112. doi: 10.3899/jrheum.091323
Ho, P. P., Lee, L. Y., Zhao, X., Tomooka, B. H., Paniagua, R. T., Sharpe, O., et al. (2010). Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J. Immunol. 184, 379–390. doi: 10.4049/jimmunol.0901639
Jilani, A. A., and Mackworth-Young, C. G. (2015). The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int. J. Rheumatol. 2015:728610. doi: 10.1155/2015/728610
Johansson, L., Pratesi, F., Brink, M., Arlestig, L., D'Amato, C., Bartaloni, D., et al. (2016). Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res. Ther. 18:127. doi: 10.1186/s13075-016-1031-0
Joshua, V., Schobers, L., Titcombe, P. J., Israelsson, L., Ronnelid, J., Hansson, M., et al. (2016). Antibody responses to de novo identified citrullinated fibrinogen peptides in rheumatoid arthritis and visualization of the corresponding B cells. Arthritis Res. Ther. 18:284. doi: 10.1186/s13075-016-1181-0
Kadowaki, T., Nakayama, K., Yoshimura, F., Okamoto, K., Abe, N., and Yamamoto, K. (1998). Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J. Biol. Chem. 273, 29072–29076. doi: 10.1074/jbc.273.44.29072
Kalla, M., and Hammerschmidt, W. (2012). Human B cells on their route to latent infection–early but transient expression of lytic genes of Epstein-Barr virus. Eur. J. Cell Biol. 91, 65–69. doi: 10.1016/j.ejcb.2011.01.014
Khandpur, R., Carmona-Rivera, C., Vivekanandan-Giri, A., Gizinski, A., Yalavarthi, S., Knight, J. S., et al. (2013). NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5:178ra40. doi: 10.1126/scitranslmed.3005580
Kharlamova, N., Jiang, X., Sherina, N., Potempa, B., Israelsson, L., Quirke, A. M., et al. (2016). Antibodies to Porphyromonas gingivalis indicate interaction between oral infection, smoking, and risk genes in rheumatoid arthritis etiology. Arthritis Rheumatol. 68, 604–613. doi: 10.1002/art.39491
Kinloch, A. J., Alzabin, S., Brintnell, W., Wilson, E., Barra, L., Wegner, N., et al. (2011). Immunization with Porphyromonas gingivalis enolase induces autoimmunity to mammalian alpha-enolase and arthritis in DR4-IE-transgenic mice. Arthritis Rheum. 63, 3818–3823. doi: 10.1002/art.30639
Konig, M. F., Abusleme, L., Reinholdt, J., Palmer, R. J., Teles, R. P., Sampson, K., et al. (2016a). Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 8:369ra176. doi: 10.1126/scitranslmed.aaj1921
Konig, M. F., Giles, J. T., Nigrovic, P. A., and Andrade, F. (2016b). Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 75, 2022–2028. doi: 10.1136/annrheumdis-2015-208529
Krishnamurthy, A., Joshua, V., Haj Hensvold, A., Jin, T., Sun, M., Vivar, N., et al. (2016). Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 75, 721–729. doi: 10.1136/annrheumdis-2015-208093
Kuhn, K. A., Kulik, L., Tomooka, B., Braschler, K. J., Arend, W. P., Robinson, W. H., et al. (2006). Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J. Clin. Invest. 116, 961–973. doi: 10.1172/JCI25422
Lande, R., Gregorio, J., Facchinetti, V., Chatterjee, B., Wang, Y. H., Homey, B., et al. (2007). Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569. doi: 10.1038/nature06116
Lappin, D. F., Apatzidou, D., Quirke, A. M., Oliver-Bell, J., Butcher, J. P., Kinane, D. F., et al. (2013). Influence of periodontal disease, Porphyromonas gingivalis and cigarette smoking on systemic anti-citrullinated peptide antibody titres. J. Clin. Periodontol. 40, 907–915. doi: 10.1111/jcpe.12138
Laurent, L., Clavel, C., Lemaire, O., Anquetil, F., Cornillet, M., Zabraniecki, L., et al. (2011). Fcgamma receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann. Rheum. Dis. 70, 1052–1059. doi: 10.1136/ard.2010.142091
Lee, H. S., Irigoyen, P., Kern, M., Lee, A., Batliwalla, F., Khalili, H., et al. (2007). Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 56, 1745–1753. doi: 10.1002/art.22703
Li, P., Li, M., Lindberg, M. R., Kennett, M. J., Xiong, N., and Wang, Y. (2010). PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862. doi: 10.1084/jem.20100239
Li, S., Yu, Y., Yue, Y., Liao, H., Xie, W., Thai, J., et al. (2016). Autoantibodies from single circulating plasmablasts react with citrullinated antigens and Porphyromonas gingivalis in rheumatoid arthritis. Arthritis Rheumatol. 68, 614–626. doi: 10.1002/art.39455
Lundberg, K., Bengtsson, C., Kharlamova, N., Reed, E., Jiang, X., Kallberg, H., et al. (2013). Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann. Rheum. Dis. 72, 652–658. doi: 10.1136/annrheumdis-2012-201484
Lundberg, K., Kinloch, A., Fisher, B. A., Wegner, N., Wait, R., Charles, P., et al. (2008). Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 58, 3009–3019. doi: 10.1002/art.23936
Lunemann, J. D., Frey, O., Eidner, T., Baier, M., Roberts, S., Sashihara, J., et al. (2008). Increased frequency of EBV-specific effector memory CD8+T cells correlates with higher viral load in rheumatoid arthritis. J. Immunol. 181, 991–1000. doi: 10.4049/jimmunol.181.2.991
Makrygiannakis, D., Hermansson, M., Ulfgren, A. K., Nicholas, A. P., Zendman, A. J., Eklund, A., et al. (2008). Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 67, 1488–1492. doi: 10.1136/ard.2007.075192
Marchesan, J. T., Gerow, E. A., Schaff, R., Taut, A. D., Shin, S. Y., Sugai, J., et al. (2013). Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Res. Ther. 15:R186. doi: 10.1186/ar4376
Maresz, K. J., Hellvard, A., Sroka, A., Adamowicz, K., Bielecka, E., Koziel, J., et al. (2013). Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9:e1003627. doi: 10.1371/journal.ppat.1003627
McGraw, W. T., Potempa, J., Farley, D., and Travis, J. (1999). Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect. Immun. 67, 3248–3256.
Menard, L., Saadoun, D., Isnardi, I., Ng, Y. S., Meyers, G., Massad, C., et al. (2011). The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 121, 3635–3644. doi: 10.1172/JCI45790
Mikuls, T. R., Payne, J. B., Yu, F., Thiele, G. M., Reynolds, R. J., Cannon, G. W., et al. (2014). Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 1090–1100. doi: 10.1002/art.38348
Montgomery, A. B., Kopec, J., Shrestha, L., Thezenas, M. L., Burgess-Brown, N. A., Fischer, R., et al. (2016). Crystal structure of Porphyromonas gingivalis peptidylarginine deiminase: implications for autoimmunity in rheumatoid arthritis. Ann. Rheum. Dis. 75, 1255–1261. doi: 10.1136/annrheumdis-2015-207656
Nesse, W., Westra, J., van der Wal, J. E., Abbas, F., Nicholas, A. P., Vissink, A., et al. (2012). The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J. Clin. Periodontol. 39, 599–607. doi: 10.1111/j.1600-051X.2012.01885.x
Nielen, M. M., van Schaardenburg, D., Reesink, H. W., van de Stadt, R. J., van der Horst-Bruinsma, I. E., de Koning, M. H., et al. (2004). Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50, 380–386. doi: 10.1002/art.20018
Pierer, M., Wagner, U., Rossol, M., and Ibrahim, S. (2011). Toll-like receptor 4 is involved in inflammatory and joint destructive pathways in collagen-induced arthritis in DBA1J mice. PLoS ONE 6:e23539. doi: 10.1371/journal.pone.0023539
Potempa, J., Pike, R., and Travis, J. (1995). The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect. Immun. 63, 1176–1182.
Pratesi, F., Dioni, I., Tommasi, C., Alcaro, M. C., Paolini, I., Barbetti, F., et al. (2014). Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 73, 1414–1422. doi: 10.1136/annrheumdis-2012-202765
Pratesi, F., Tommasi, C., Anzilotti, C., Chimenti, D., and Migliorini, P. (2006). Deiminated Epstein-Barr virus nuclear antigen 1 is a target of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 54, 733–741. doi: 10.1002/art.21629
Pratesi, F., Tommasi, C., Anzilotti, C., Puxeddu, I., Sardano, E., Di Colo, G., et al. (2011). Antibodies to a new viral citrullinated peptide, VCP2: fine specificity and correlation with anti-cyclic citrullinated peptide (CCP) and anti-VCP1 antibodies. Clin. Exp. Immunol. 164, 337–345. doi: 10.1111/j.1365-2249.2011.04378.x
Reynisdottir, G., Karimi, R., Joshua, V., Olsen, H., Hensvold, A. H., Harju, A., et al. (2014). Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 66, 31–39. doi: 10.1002/art.38201
Romero, V., Fert-Bober, J., Nigrovic, P. A., Darrah, E., Haque, U. J., Lee, D. M., et al. (2013). Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med. 5:209ra150. doi: 10.1126/scitranslmed.3006869
Sakkas, L. I., Bogdanos, D. P., Katsiari, C., and Platsoucas, C. D. (2014). Anti-citrullinated peptides as autoantigens in rheumatoid arthritis-relevance to treatment. Autoimmun. Rev. 13, 1114–1120. doi: 10.1016/j.autrev.2014.08.012
Scally, S. W., Petersen, J., Law, S. C., Dudek, N. L., Nel, H. J., Loh, K. L., et al. (2013). A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J. Exp. Med. 210, 2569–2582. doi: 10.1084/jem.20131241
Scher, J. U., Ubeda, C., Equinda, M., Khanin, R., Buischi, Y., Viale, A., et al. (2012). Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 64, 3083–3094. doi: 10.1002/art.34539
Scotet, E., David-Ameline, J., Peyrat, M. A., Moreau-Aubry, A., Pinczon, D., Lim, A., et al. (1996). T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J. Exp. Med. 184, 1791–1800. doi: 10.1084/jem.184.5.1791
Sebbag, M., Moinard, N., Auger, I., Clavel, C., Arnaud, J., Nogueira, L., et al. (2006). Epitopes of human fibrin recognized by the rheumatoid arthritis specific autoantibodies to citrullinated proteins. Eur. J. Immunol. 36, 2250–2263. doi: 10.1002/eji.200535790
Seror, R., Le Gall-David, S., Bonnaure-Mallet, M., Schaeverbeke, T., Cantagrel, A., Minet, J., et al. (2015). Association of Anti-Porphyromonas gingivalis antibody titers with nonsmoking status in early rheumatoid arthritis: results from the prospective French cohort of patients with early rheumatoid arthritis. Arthritis Rheumatol. 67, 1729–1737. doi: 10.1002/art.39118
Shoda, H., Fujio, K., Shibuya, M., Okamura, T., Sumitomo, S., Okamoto, A., et al. (2011). Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res. Ther. 13:R191. doi: 10.1186/ar3520
Snir, O., Widhe, M., von Spee, C., Lindberg, J., Padyukov, L., Lundberg, K., et al. (2009). Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann. Rheum. Dis. 68, 736–743. doi: 10.1136/ard.2008.091355
Sohn, D. H., Rhodes, C., Onuma, K., Zhao, X., Sharpe, O., Gazitt, T., et al. (2015). Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol. 67, 2877–2887. doi: 10.1002/art.39283
Sokolove, J., Zhao, X., Chandra, P. E., and Robinson, W. H. (2011). Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 63, 53–62. doi: 10.1002/art.30081
Swanson-Mungerson, M., and Longnecker, R. (2007). Epstein-Barr virus latent membrane protein 2A and autoimmunity. Trends Immunol. 28, 213–218. doi: 10.1016/j.it.2007.03.002
Tsuda, R., Ozawa, T., Kobayashi, E., Hamana, H., Taki, H., Tobe, K., et al. (2015). Monoclonal antibody against citrullinated peptides obtained from rheumatoid arthritis patients reacts with numerous citrullinated microbial and food proteins. Arthritis Rheumatol. 67, 2020–2031. doi: 10.1002/art.39161
van der Helm-van Mil, A. H., Verpoort, K. N., Breedveld, F. C., Huizinga, T. W., Toes, R. E., and de Vries, R. R. (2006). The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 54, 1117–1121. doi: 10.1002/art.21739
van der Helm-van Mil, A. H., Verpoort, K. N., Breedveld, F. C., Toes, R. E., and Huizinga, T. W. (2005). Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 7, R949–R958. doi: 10.1186/ar1767
van der Helm-van Mil, A. H., Verpoort, K. N., le Cessie, S., Huizinga, T. W., de Vries, R. R., and Toes, R. E. (2007). The HLA-DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum. 56, 425–432. doi: 10.1002/art.22373
van de Sande, M. G., de Hair, M. J., van der Leij, C., Klarenbeek, P. L., Bos, W. H., Smith, M. D., et al. (2011). Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann. Rheum. Dis. 70, 772–777. doi: 10.1136/ard.2010.139527
van Gaalen, F. A., Linn-Rasker, S. P., van Venrooij, W. J., de Jong, B. A., Breedveld, F. C., Verweij, C. L., et al. (2004). Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 50, 709–715. doi: 10.1002/art.20044
Verpoort, K. N., Cheung, K., Ioan-Facsinay, A., van der Helm-van Mil, A. H., de Vries-Bouwstra, J. K., Allaart, C. F., et al. (2007). Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 56, 3949–3952. doi: 10.1002/art.23127
Wegner, N., Wait, R., Sroka, A., Eick, S., Nguyen, K. A., Lundberg, K., et al. (2010). Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62, 2662–2672. doi: 10.1002/art.27552
Willis, V. C., Gizinski, A. M., Banda, N. K., Causey, C. P., Knuckley, B., Cordova, K. N., et al. (2011). N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J. Immunol. 186, 4396–4404. doi: 10.4049/jimmunol.1001620
Wordsworth, B. P., Lanchbury, J. S., Sakkas, L. I., Welsh, K. I., Panayi, G. S., and Bell, J. I. (1989). HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc. Natl. Acad. Sci. U.S.A. 86, 10049–10053. doi: 10.1073/pnas.86.24.10049
Yang, H., Biermann, M. H., Brauner, J. M., Liu, Y., Zhao, Y., and Herrmann, M. (2016). New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front. Immunol. 7:302. doi: 10.3389/fimmu.2016.00302
Ytterberg, A. J., Joshua, V., Reynisdottir, G., Tarasova, N. K., Rutishauser, D., Ossipova, E., et al. (2015). Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann. Rheum. Dis. 74, 1772–1777. doi: 10.1136/annrheumdis-2013-204912
Keywords: anti-citrullinated protein antibodies, arthritis, Ebstein-Barr virus, HLA-DRB1 shared epitope, Porphyromonas gingivalis
Citation: Sakkas LI, Daoussis D, Liossis S-N and Bogdanos DP (2017) The Infectious Basis of ACPA-Positive Rheumatoid Arthritis. Front. Microbiol. 8:1853. doi: 10.3389/fmicb.2017.01853
Received: 03 July 2017; Accepted: 11 September 2017;
Published: 27 September 2017.
Edited by:
Marina I. Arleevskaya, Kazan State Medical Academy, RussiaReviewed by:
Jan Potempa, University of Louisville, United StatesAngelo A. Manfredi, Vita-Salute San Raffaele University, Italy
Copyright © 2017 Sakkas, Daoussis, Liossis and Bogdanos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lazaros I. Sakkas, bHNha2thc0BtZWQudXRoLmdy
 Lazaros I. Sakkas
Lazaros I. Sakkas Dimitrios Daoussis
Dimitrios Daoussis Stamatis-Nick Liossis
Stamatis-Nick Liossis Dimitrios P. Bogdanos1
Dimitrios P. Bogdanos1