
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 05 April 2017
Sec. Systems Microbiology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.00597
This article is part of the Research Topic Inter-kingdom communication mechanisms mediated by non-coding RNAs View all 13 articles
Environmental and lifestyle factors, including diet and nutritional habits have been strongly linked to colorectal cancer (CRC). Of note, unhealthy dietary habits leading to adiposity represent a main risk factor for CRC and are associated with a chronic low-grade inflammatory status. Inflammation is a hallmark of almost every type of cancer and can be modulated by several food compounds exhibiting either protective or promoting effects. However, in spite of an extensive research, the underlying mechanisms by which dietary patterns or bioactive food components may influence tumor onset and outcome have not been fully clarified yet. Growing evidence indicates that diet, combining beneficial substances and potentially harmful ingredients, has an impact on the expression of key regulators of gene expression such as the non-coding RNA (ncRNA). Since the expression of these molecules is deranged in chronic inflammation and cancer, modulating their expression may strongly influence the cancer phenotype and outcomes. In addition, the recently acquired knowledge on the existence of intricate inter-kingdom communication networks, is opening new avenues for a deeper understanding of the intimate relationships linking diet to CRC. In this novel scenario, diet-modulated ncRNA may represent key actors in the interaction between plant and animal kingdoms, capable of influencing disease onset and outcome. In this review, we will summarize the studies demonstrating a link between bioactive food components, including food-derived, microbiota-processed, secondary metabolites, and host ncRNA. We will focus on microRNA, highlighting how this plant/animal inter-kingdom cross-talk may have an impact on CRC establishment and progression.
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in women and the third in men worldwide (Ferlay et al., 2015) that presents one of the highest rates of morbidity and mortality worldwide (Siegel et al., 2016). The number of new cases of cancer including CRC, has been increasing in the last decades (Torre et al., 2016), and such increased incidence has been attributed to environmental factors, i.e., adoption of Western diets and lifestyles (Haggar and Boushey, 2009). Chronic intestinal inflammation (Ekbom et al., 1990; Feagins et al., 2009) and obesity (Pietrzyk et al., 2015) represent additional risk factors associated with increased CRC incidence. Obesity has become a major threat to public health because of its high global prevalence and association with an increased risk of developing chronic diseases (Abdoullaye et al., 2010). Obesity affects over half a billion adults worldwide, with approximately 3.5 million attributable deaths each year (WHO, 2015). Similarly to gender, race, dietary habits or smoking history, obesity not only represents a risk factor for several tumors including CRC (Park et al., 2014; Parkin et al., 2014), but contributes to 3–20% of cancer deaths in Western populations (Renehan et al., 2008; Beddy et al., 2010). Abdominal rather than total adiposity is associated with a 1.5- to 3.5-fold increased risk of developing CRC as compared to lean individuals (Bardou et al., 2013).
Notably, dietary components influence inflammatory processes, and many types of cancer, including CRC, can be prevented/delayed through healthy life styles (Turati et al., 2012). The most extensive review of the existing evidence connecting diet and cancer is the 2007 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR; Lozcano-Ponce, 2009) report and its subsequent update (Wiseman, 2008; Martinez-Gonzalez et al., 2015; Norat et al., 2015).
CRC initiation/progression results from the accumulation over-time of genetic changes in oncogenic/oncosuppressor genes in colonic epithelium, with epigenetic alterations recognized as significant contributors to cancer development. CRC “epigenome” assessment revealed that virtually all CRC have aberrantly methylated genes and altered microRNA (miR) expression (Okugawa et al., 2015). Dysregulation of miR and their mRNA targets contributes to the initiation/progression of colon carcinogenesis as well as to invasion, angiogenesis, and metastasis (Ramalingam et al., 2015; Ress et al., 2015). Interestingly, bioactive food ingredients exert not only direct effects on carcinogenesis but likely influence cancer development indirectly by affecting gut microbiota composition/metabolism, and by epigenetically regulating gene expression. Complex interactions among food components and histone modifications, chromatin remodeling, DNA methylation and non-coding RNA (ncRNA) expression lead to a dynamic regulation of gene expression controlling cellular phenotype (Milagro et al., 2013). Dietary changes also affect gut microbiota in terms of relative abundance of microbial species. In turn, microbiota influences the conversion of food components and fibers into metabolites acting as epigenetic regulators in cancer, as well as nutrient uptake and epithelial resilience (Ha et al., 2014; O’Keefe et al., 2015; Bultman, 2016). Additionally, the presence of cancer-associated circulating miR (Schou et al., 2016) and the growing attention to xeno-miR, absorbed with food ingestion (Fabris and Calin, 2016), further highlight the complexity of inter-kingdom communication and its potential role in the balance between homeostasis and disease.
In this review, we focus on diet-induced modulation of host miR and discuss their potential contribution to CRC. In the following paragraphs we will examine different outcomes of this plant/animal inter-kingdom cross-talk including both preventing and pro-tumorigenic effects.
It has long been known that modifications of diet can prevent, slow, and even reverse some disease-associated events, and growing evidence suggests that one of the mechanisms by which nutrients and bioactive compounds affect metabolic traits is epigenetics. Various diets and dietary interventions, including high-fat diets (HFD) and caloric restriction (CR), as well as bioactive nutrients and plant derivatives, have been associated with epigenetic changes that alter cellular signaling (Hardy and Tollefsbol, 2011; Garcia-Segura et al., 2013) and may have an impact on CRC development (Bultman, 2016; Lee et al., 2016). There is currently overwhelming evidence that consumption of red and processed meat as well as animal fat, typical of Western style diets, increases CRC risk (Bernstein et al., 2015). Conversely, bioactive dietary molecules, such as ω3 polyunsaturated fatty acids (PUFA), curcumin, fermentable fibers (basic components of the Mediterranean diet), folate, calcium, vitamin D, and physical activity exert chemoprotective effects (Hou et al., 2016). Although the mechanisms underlying the role of food in preventing or favoring CRC are not fully elucidated, growing evidence indicates that at least some of them involve miR (Gavrilas et al., 2016; Hou et al., 2016).
A summary of the main results achieved in both in vitro and in vivo models is shown in Table 1.
Dietary patterns and some bioactive food components including polyphenols, ω3 PUFA, and short chain fatty acids (SCFA) exhibit a chemopreventive role against CRC. Among phytochemicals, polyphenols are ubiquitous secondary metabolites found in fruits and vegetables, whole grain cereals, and beverages (e.g., tea, coffee, and wines). Initial studies showed that resveratrol (RES), a stilbenoid found in dried fruits, berries, peanuts and especially in grapes, modulates the levels of miR targeting both oncogenes and tumor suppressor genes. In particular, RES increases the levels of miR-663, a tumor suppressor miR targeting TGFβ1 transcripts (Tili et al., 2010). Likewise, α-mangostin (α-M), a xanthone from mangosteen pericarps, exhibits anti-proliferative/pro-apoptotic effects by targeting ERK5/c-Myc via miR-143 (Nakagawa et al., 2007). Subsequent studies investigated the effects of phytochemical combinations, including epigallocatechin-3-gallate (EGCG), RES, quercetin, and α-M, or phytochemical association with anti-cancer drug 5-fluorouracil (5-FU). In this regard, it was demonstrated that the combination of substances naturally occurring in the colonic lumen after ingestion of polyphenol-containing food, such as RES and quercetin, has a pro-apoptotic effect on CRC cells (Del Follo-Martinez et al., 2013). The interplay of RES and quercetin with the miR-27a-ZBTB10 axis, repressing Sp-1 activity, was identified as one possible underlying mechanism. Combinations of RES with EGCG or α-M acted as chemo-sensitizer through up-regulation of miR-34a and down-modulation of its target genes E2F3 and Sirt1, leading to apoptosis induction (Kumazaki et al., 2013). Lastly, α-M and 5-FU exerted a synergistic effect on growth inhibition (Nakagawa et al., 2007). Additional in vitro evidence showed that the flavonol-rich fractions from botanical extracts, as well as red wine polyphenolics, inhibited the generation of reactive oxygen species (ROS) and NF-κB activation in colon cells by inducing miR-126 and miR-146a (Noratto et al., 2011; Angel-Morales et al., 2012; Ojwang et al., 2015).
The therapeutic potential of pomegranate (PO), of its main polyphenolic compounds (ellagic acid, and ellagitannins) as well as of their gut microbiota-derived metabolites (urolithins), has been reported in vitro and in vivo CRC models. Gonzalez-Sarrias et al. (2016) found relevant changes in cancer markers and identified the induction of p21waf1/Cip1 (CDKN1A) as a common step underlying urolithin anticancer properties. Interestingly, miR-224 down-regulation or miR-215 up-regulation was associated with CDKN1A induction (Gonzalez-Sarrias et al., 2016).
Among in vivo tested compounds, RES and proanthocyanidin-rich extracts prevented tumorigenesis in sporadic CRC models by suppressing Kras activity (Saud et al., 2014) and inflammatory pathways (Derry et al., 2013), through miR modulation. Likewise, PO polyphenols exerted cytotoxic and anti-inflammatory effects in experimentally induced colon carcinogenesis in rat and in CRC cells. Interaction of PO with miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR axes were identified as mechanisms that at least in part mediate the anti-inflammatory/anti-proliferative activities of these compounds (Banerjee et al., 2013). Interestingly, in a controlled human trial, Nunez-Sanchez et al. (2015) demonstrated that PO consumption affected specific colon miR other than miR-126. Lastly, canolol, an anti-oxidant from canola oil, inhibited gastric tumor by blocking COX-2/PGE2/EP2 signaling pathway in mouse models. Interestingly, COX-2 is a functional target of miR-7, a tumor suppressor miR reactivated after canolol administration (Cao et al., 2015).
Another compound relevant to CRC prevention is curcumin, a bioactive ingredient of turmeric, with anti-inflammatory, antioxidant, and anti-carcinogenic properties (Patel et al., 2010). Similarly to other polyphenols, modulation of miR expression by curcumin in cell lines has been reported as a mechanism underlying the effects of this compound (Reuter et al., 2011). Several studies highlighted miR-21, an onco-miR overexpressed in many tumors, as an important target of curcumin activity. Curcumin inhibited miR-21 expression, tumor growth, invasion and in vivo metastasis, and stabilized its tumor suppressor target Pdcd4 in CRC cells (Mudduluru et al., 2011). Likewise, curcumin-difluorinated (CDF), a curcumin analog with a greater bioavailability, down-regulated miR-21 expression in chemo-resistant CRC cell lines by restoring PTEN levels and reducing Akt phosphorylation (Roy et al., 2013). Lastly, miR-21 suppression in CRC cells induced differentiation and increased their susceptibility to conventional (5-FU/oxaliplatin) or non-conventional (CDF) therapeutic regimens as well as their combination (Yu et al., 2013). In addition to miR-21, curcumin and CDF rescued the expression of miR-34 family members, lost in CRC, partly through demethylation of the respective promoters (Roy et al., 2012). Curcumin-mediated chemosensitization to 5-FU also occurred by up-regulation of epithelial–mesenchymal transition (EMT)-suppressive miR, including miR-34, further highlighting its potential therapeutic usefulness as an adjunct in patients with chemoresistant advanced CRC (Toden et al., 2015b). Likewise, key molecular mechanisms were identified when curcumin or boswellic acid (AKBA) were administered individually or in combination. These compounds synergized to affect specific miR and target genes involved in cell cycle regulation in CRC cell lines, including up-modulation of miR-34 and down-regulation of miR-27 ultimately leading to apoptosis induction, cell-cycle arrest and suppression of proliferation (Toden et al., 2015a). In line with these findings, curcumin or its most active synthetic analog RL197, inhibited CRC cell growth by ROS induction and reduction of Sp transcription factors and their regulators (i.e., ZBTB10 and ZBTB4) through miR-27a, miR-20a, and miR-17 (Gandhy et al., 2012), similarly to RES (Del Follo-Martinez et al., 2013). This regulation has important implications because Sp transcription factors regulate genes involved in cell death and angiogenesis and are often overexpressed in tumors. Moreover, curcumin is known to modulate DNA methylation in CRC cells, potentially exerting its anti-cancer effect by affecting other epigenetic mechanisms (Link et al., 2013).
ω3 PUFA found in walnuts, fish-oil, soybeans, green leafy vegetables, and seed oils are among dietary factors known to have an impact on miR involved in various stages of carcinogenesis, with a documented protective role in cancer, including CRC (Garcia-Segura et al., 2013). Conversely, ω6 PUFA (linoleic acid and arachidonic acid) found in vegetable oils and red meat, favor CRC onset (Abel et al., 2014). The protective effect of ω3 PUFA [docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)] rich diets against CRC relies on their ability to modify gene expression and signaling pathways (Hou et al., 2016). In gastric cancer models, DHA and EPA have been reported to modulate apoptotic pathways. In fact ω3 PUFA treatment increased miR-15b and miR-16 and decreased miR-21, resulting in Bcl-2 down-regulation (Sun et al., 2013) and TNF-α up-regulation (Fluckiger et al., 2016), respectively. DHA also modulated the expression of specific miR (e.g., miR-30c and miR-192) in enterocytes, targeting genes related to lipid metabolism and cancer biology (Gil-Zamorano et al., 2014). In preclinical models, administration of fish oil- or walnut-enriched diets at early stages of carcinogenesis, modulated carcinogen-directed miR expression, as well as that of miR associated with inflammation, proliferation and apoptosis (Davidson et al., 2009; Tsoukas et al., 2015). Furthermore, combination of dietary fish oil and fermentable fiber pectin led to up-regulation of several miR, including miR-19b, miR-26b, and miR-203, whose validated targets (PDE4B, PTK2B, TCF4, IGF1R, and BACE1) promote tumorigenesis, as compared to control corn oil diet. Surprisingly, miR-21 was increased by the combination diet as compared to the control diet (Shah et al., 2011, 2016).
SCFA, such as acetate, butyrate, and propionate, represent additional protective metabolites produced by gut microbiota following fermentation of dietary fibers. Butyrate, a putative chemoprotective agent, acts as a histone deacetylase inhibitor (HDI) capable of decreasing proliferation and increasing apoptosis in CRC cells (Bultman, 2016). Studies have demonstrated that these effects are mediated in part through induction of CDKN1A expression (Crim et al., 2008) and by modulation of miR implicated in intestinal homeostasis and malignant transformation. Humphreys et al. (2013) explored the effects of butyrate and several other HDI on miR expression in human CRC cell lines. They reported that these HDI decrease miR-17∼92 cluster, while their target genes (e.g., PTEN, BCL2L11, CDKN1A) increase. Furthermore, butyrate induced expression of CDKN1A by suppressing members of the miR-106b family (Hu et al., 2011). Likewise, butyrate reduced the levels of pri-miR17-92a, precursor and mature miR-92a, as well as c-Myc, a main inducer of miR-17-92a promoter activity. This led to enhanced expression of CDKN1C (p57KIP2; Hu et al., 2015), one of the cyclin-dependent kinase inhibitors found dysregulated in cancer (Kavanagh and Joseph, 2011). As mentioned above, SCFA and fish oil ω3 PUFA worked coordinately in vivo to protect against colon tumorigenesis by modulating miR (Davidson et al., 2009; Shah et al., 2011, 2016). Collectively, these findings uncovered a novel mechanism whereby butyrate suppresses onco-miR biogenesis, promotes apoptosis and diminishes CRC cell proliferation.
Although most bioactive nutrients exert a chemoprotective role in CRC, tumor-promoting effects have also been reported. Countries with high rates of overweight/obesity and consumption of red and processed meat and high fat intake show the highest CRC incidence (Bernstein et al., 2015). Conversely, CR is inversely associated with CRC risk and progression. The molecular mechanisms underlying these effects are being elucidated and include miR regulation of gene expression.
The effects of HFD and CR on miR expression were compared in a mouse CRC model (Olivo-Marston et al., 2014). Together with increased body weight and tumor numbers, HFD modulated miR expression in colonic mucosa, up-regulating onco-miR (e.g., miR-196 and miR-155) and concomitantly decreasing those involved in apoptosis regulation (e.g., miR-150). Interestingly, an opposite effect on tumor growth and miR expression was induced by CR diet (Olivo-Marston et al., 2014). Furthermore, in sporadic and inflammation-associated CRC models, HFD promoted weight gain and cancer development through EGFR-mediated induction of c-Myc and Kras (Dougherty et al., 2009). miR-143 and miR-145, negatively targeting these proto-oncogenes, were down-regulated by HFD in both tumor models only in EGFR-expressing mice, indicating that epigenetic changes associated with diet-induced colon tumorigenesis require EGFR signaling (Zhu et al., 2011). In a recent randomized dietary intervention study, the impact of high red meat (HRM) diet on rectal mucosa miR expression was examined (Humphreys et al., 2014). Short duration HRM consumption increased the levels of miR-17-92 cluster as well as miR-21, highly expressed in CRC and associated with poor survival. The enhanced miR-17-92 expression was associated with decreased levels of the target gene CDKN1A, and increased colonic cell proliferation. Supplementation with resistant starch, yielding high butyrate/propionate production when fermented, was able to reverse HRM effects on both miR-17-92 expression and cell proliferation. This study reported the first evidence in humans that HRM diet and resistant starch have opposite effects on rectal mucosa miR expression, supporting increased dietary fiber consumption as a mean for maintaining intestinal health and reducing HRM diet-associated CRC risk (Thompson, 2014). Likewise, dietary spinach had a protective effect when administered to rats together with heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), a widely consumed carcinogen from cooked meat (Parasramka et al., 2012). This study demonstrated that a dietary carcinogen induces colon tumors with a signature loss of miR-215 and miR-let7 family members, whose targets (i.e., cyclin D1, HMGA2, β-catenin, c-Myc, p53) are known to promote EMT or regulate cell cycle. Once again, PhIP-induced down-modulation of miR expression, and concomitant increase in tumor incidence, were reversed after dietary spinach treatment, pointing to an important role of these latter in CRC chemoprevention.
Dietary components involved in one-carbon metabolism (folate, vitamin B12, cysteine, homocysteine) modulate miR expression and influence cancer risk by regulating DNA methylation pathways. Although a miR-mediated tumor protective role for folic acid has been reported in several cancers (Davis and Ross, 2008), in a recent study a pro-oncogenic effect was instead observed in human CRC (Beckett et al., 2015). Circulating folate levels directly correlated with serum miR-21 expression and with adenomatous polyps occurrence in females. Moreover, following stimulation of different CRC cell lines with excess folic acid, a significant increase in miR-21 release was found, suggesting a direct role for folate in driving tumor growth (Beckett et al., 2015). As serum miR-21 has been proposed as a CRC biomarker, these data suggest that dietary components/nutritional status may not only affect cancer development/progression via ncRNA modulation but also need to be considered when assessing the value of these molecules as possible biomarkers.
ncRNA are recognized epigenetic regulators with a well-established role in cancer (Di Leva et al., 2014). The potential cross-interaction among plant or microbial and human miR and their mRNA targets has been hypothesized by several authors and has been suggested to play a role in disease onset. The diet-mediated inter-kingdom signaling between plants and animals, object of this review, can be considered as part of a general vision of inter-kingdom communications mediated by ncRNA. As schematically depicted in Figure 1, plants and food, in addition to represent a source of xeno-miR, epigenetically influence host gene expression and potentially CRC development via miR deregulation. This supports the evidence that consumption of certain types of food is relevant for disease pathogenesis. Furthermore, diet indirectly affects the composition and metabolism of gut microbiota. In turn, microbiota influences nutrient uptake and epithelial resilience and drives the conversion of food components and fibers into metabolites that epigenetically regulate host gene expression. Of note, a cross-talk between human and gut microbiota through fecal miR has been recently highlighted (Celluzzi and Masotti, 2016). Overall we can envisage that each individual is placed in a complex inter-kingdom communication network that contributes to maintain homeostasis. Disruption of this equilibrium could set the basis for pathological states, for instance intestinal dysbiosis, inflammation and CRC development. Understanding the effects of dietary- and microbial-derived factors on ncRNA regulation will likely represent an important undertaken in human disease management. If successful, it may provide insights for the developing novel prevention strategies to reduce CRC burden.
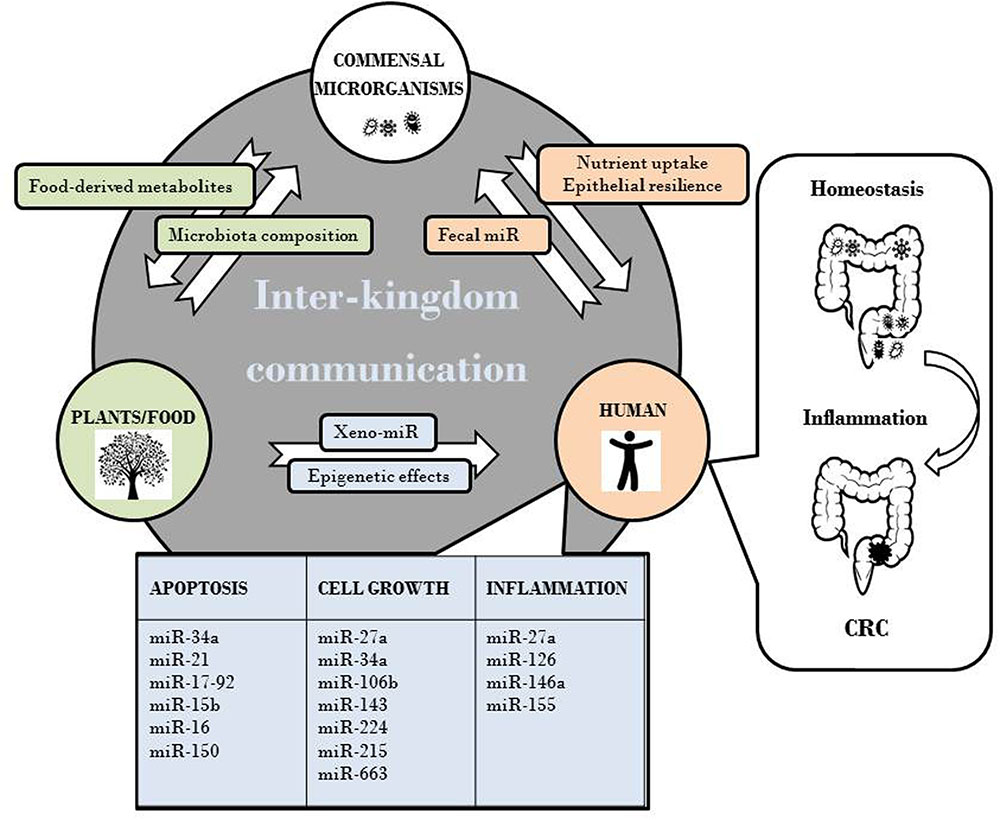
FIGURE 1. Effects of plant-animal inter-kingdom communication on CRC development via miR deregulation.
MD, GD, LC, and SG contributed to the conception, writing, and editing of this manuscript. All authors read and approved the final manuscript.
This study was supported by a grant of the Italian Association for Cancer Research (AIRC) project (IG2013 N.14185) to SG.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdoullaye, D., Acevedo, I., Adebayo, A. A., Behrmann-Godel, J., Benjamin, R. C., Bock, D. G., et al. (2010). Permanent genetic resources added to molecular ecology resources database 1 august 2009-30 september 2009. Mol. Ecol. Resour. 10, 232–236. doi: 10.1111/j.1755-0998.2009.02796.x
Abel, S., Riedel, S., and Gelderblom, W. C. (2014). Dietary PUFA and cancer. Proc. Nutr. Soc. 73, 361–367. doi: 10.1017/S0029665114000585
Angel-Morales, G., Noratto, G., and Mertens-Talcott, S. (2012). Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: potential role of microRNA-126. Food Funct. 3, 745–752. doi: 10.1039/c2fo10271d
Banerjee, N., Kim, H., Talcott, S., and Mertens-Talcott, S. (2013). Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci and inflammation: possible role of miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR. Carcinogenesis 34, 2814–2822. doi: 10.1093/carcin/bgt295
Bardou, M., Barkun, A. N., and Martel, M. (2013). Obesity and colorectal cancer. Gut 62, 933–947. doi: 10.1136/gutjnl-2013-304701
Beckett, E. L., Martin, C., Choi, J. H., King, K., Niblett, S., Boyd, L., et al. (2015). Folate status, folate-related genes and serum miR-21 expression: Implications for miR-21 as a biomarker. BBA Clin. 4, 45–51. doi: 10.1016/j.bbacli.2015.06.006
Beddy, P., Howard, J., McMahon, C., Knox, M., de Blacam, C., Ravi, N., et al. (2010). Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br. J. Surg. 97, 1028–1034. doi: 10.1002/bjs.7100
Bernstein, A. M., Song, M., Zhang, X., Pan, A., Wang, M., Fuchs, C. S., et al. (2015). Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLoS ONE 10:e0135959. doi: 10.1371/journal.pone.0135959
Bultman, S. J. (2016). Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 61, 1–2. doi: 10.1002/mnfr.201500902
Cao, D., Jiang, J., Tsukamoto, T., Liu, R., Ma, L., Jia, Z., et al. (2015). Canolol inhibits gastric tumors initiation and progression through COX-2/PGE2 pathway in K19-C2mE transgenic mice. PLoS ONE 10:e0120938. doi: 10.1371/journal.pone.0120938
Celluzzi, A., and Masotti, A. (2016). How our other genome controls our epi-genome. Trends Microbiol. 24, 777–787. doi: 10.1016/j.tim.2016.05.005
Crim, K. C., Sanders, L. M., Hong, M. Y., Taddeo, S. S., Turner, N. D., Chapkin, R. S., et al. (2008). Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis 29, 1415–1420. doi: 10.1093/carcin/bgn144
Davidson, L. A., Wang, N., Shah, M. S., Lupton, J. R., Ivanov, I., and Chapkin, R. S. (2009). n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 30, 2077–2084. doi: 10.1093/carcin/bgp245
Davis, C. D., and Ross, S. A. (2008). Evidence for dietary regulation of microRNA expression in cancer cells. Nutr. Rev. 66, 477–482. doi: 10.1111/j.1753-4887.2008.00080.x
Del Follo-Martinez, A., Banerjee, N., Li, X., Safe, S., and Mertens-Talcott, S. (2013). Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer 65, 494–504. doi: 10.1080/01635581.2012.725194
Derry, M. M., Raina, K., Balaiya, V., Jain, A. K., Shrotriya, S., Huber, K. M., et al. (2013). Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prev. Res. 6, 625–633. doi: 10.1158/1940-6207.CAPR-13-0053
Di Leva, G., Garofalo, M., and Croce, C. M. (2014). MicroRNAs in cancer. Annu. Rev. Pathol. 9, 287–314. doi: 10.1146/annurev-pathol-012513-104715
Dougherty, U., Cerasi, D., Taylor, I., Kocherginsky, M., Tekin, U., Badal, S., et al. (2009). Epidermal growth factor receptor is required for colonic tumor promotion by dietary fat in the azoxymethane/dextran sulfate sodium model: roles of transforming growth factor-{alpha} and PTGS2. Clin. Cancer Res. 15, 6780–6789. doi: 10.1158/1078-0432.CCR-09-1678
Ekbom, A., Helmick, C., Zack, M., and Adami, H. O. (1990). Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 323, 1228–1233. doi: 10.1056/NEJM199011013231802
Fabris, L., and Calin, G. A. (2016). Circulating free xeno-microRNAs - The new kids on the block. Mol. Oncol. 10, 503–508. doi: 10.1016/j.molonc.2016.01.005
Feagins, L. A., Souza, R. F., and Spechler, S. J. (2009). Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 6, 297–305. doi: 10.1038/nrgastro.2009.44
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. doi: 10.1002/ijc.29210
Fluckiger, A., Dumont, A., Derangere, V., Rebe, C., de Rosny, C., Causse, S., et al. (2016). Inhibition of colon cancer growth by docosahexaenoic acid involves autocrine production of TNFalpha. Oncogene 35, 4611–4622. doi: 10.1038/onc.2015.523
Gandhy, S. U., Kim, K., Larsen, L., Rosengren, R. J., and Safe, S. (2012). Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer 12:564. doi: 10.1186/1471-2407-12-564
Garcia-Segura, L., Perez-Andrade, M., and Miranda-Rios, J. (2013). The emerging role of MicroRNAs in the regulation of gene expression by nutrients. J. Nutrigenet. Nutrigenomics 6, 16–31. doi: 10.1159/000345826
Gavrilas, L. I., Ionescu, C., Tudoran, O., Lisencu, C., Balacescu, O., and Miere, D. (2016). The role of bioactive dietary components in modulating miRNA expression in colorectal cancer. Nutrients 8:E590. doi: 10.3390/nu8100590
Gil-Zamorano, J., Martin, R., Daimiel, L., Richardson, K., Giordano, E., Nicod, N., et al. (2014). Docosahexaenoic acid modulates the enterocyte Caco-2 cell expression of microRNAs involved in lipid metabolism. J. Nutr. 144, 575–585. doi: 10.3945/jn.113.189050
Gonzalez-Sarrias, A., Nunez-Sanchez, M. A., Tome-Carneiro, J., Tomas-Barberan, F. A., Garcia-Conesa, M. T., and Espin, J. C. (2016). Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancer and key-associated molecular hallmarks: microRNA cell specific induction of CDKN1A (p21) as a common mechanism involved. Mol. Nutr. Food Res. 60, 701–716. doi: 10.1002/mnfr.201500780
Ha, C. W., Lam, Y. Y., and Holmes, A. J. (2014). Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 20, 16498–16517. doi: 10.3748/wjg.v20.i44.16498
Haggar, F. A., and Boushey, R. P. (2009). Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 22, 191–197. doi: 10.1055/s-0029-1242458
Hardy, T. M., and Tollefsbol, T. O. (2011). Epigenetic diet: impact on the epigenome and cancer. Epigenomics 3, 503–518. doi: 10.2217/epi.11.71
Hou, T. Y., Davidson, L. A., Kim, E., Fan, Y. Y., Fuentes, N. R., Triff, K., et al. (2016). Nutrient-gene interaction in colon cancer, from the membrane to cellular physiology. Annu. Rev. Nutr. 36, 543–570. doi: 10.1146/annurev-nutr-071715-051039
Hu, S., Dong, T. S., Dalal, S. R., Wu, F., Bissonnette, M., Kwon, J. H., et al. (2011). The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS ONE 6:e16221. doi: 10.1371/journal.pone.0016221
Hu, S., Liu, L., Chang, E. B., Wang, J. Y., and Raufman, J. P. (2015). Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol. Cancer 14, 180. doi: 10.1186/s12943-015-0450-x
Humphreys, K. J., Cobiac, L., Le Leu, R. K., Van der Hoek, M. B., and Michael, M. Z. (2013). Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol. Carcinog. 52, 459–474. doi: 10.1002/mc.21879
Humphreys, K. J., Conlon, M. A., Young, G. P., Topping, D. L., Hu, Y., Winter, J. M., et al. (2014). Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: a randomized trial. Cancer Prev. Res. 7, 786–795. doi: 10.1158/1940-6207.CAPR-14-0053
Kavanagh, E., and Joseph, B. (2011). The hallmarks of CDKN1C (p57, KIP2) in cancer. Biochim. Biophys. Acta 1816, 50–56. doi: 10.1016/j.bbcan.2011.03.002
Kumazaki, M., Noguchi, S., Yasui, Y., Iwasaki, J., Shinohara, H., Yamada, N., et al. (2013). Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 24, 1849–1858. doi: 10.1016/j.jnutbio.2013.04.006
Lee, D. H., Keum, N., and Giovannucci, E. L. (2016). Colorectal cancer epidemiology in the nurses’ health study. Am. J. Public Health 106, 1599–1607. doi: 10.2105/AJPH.2016.303320
Link, A., Balaguer, F., Shen, Y., Lozano, J. J., Leung, H. C., Boland, C. R., et al. (2013). Curcumin modulates DNA methylation in colorectal cancer cells. PLoS ONE 8:e57709. doi: 10.1371/journal.pone.0057709
Lozcano-Ponce, E. (2009). Second expert report, food, nutrition, physical activity and the prevention of cancer: a global perspective. Salud Publica Mexico 51, S678–S680. doi: 10.1590/S0036-36342009001000024
Martinez-Gonzalez, M. A., Salas-Salvado, J., Estruch, R., Corella, D. D., Fito, M., and Ros, E. (2015). Benefits of the mediterranean diet: insights from the PREDIMED study. Prog. Cardiovasc. Dis. 58, 50–60. doi: 10.1016/j.pcad.2015.04.003
Milagro, F. I., Mansego, M. L., De Miguel, C., and Martinez, J. A. (2013). Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol. Aspects Med. 34, 782–812. doi: 10.1016/j.mam.2012.06.010
Mudduluru, G., George-William, J. N., Muppala, S., Asangani, I. A., Kumarswamy, R., Nelson, L. D., et al. (2011). Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 31, 185–197. doi: 10.1042/BSR20100065
Nakagawa, Y., Iinuma, M., Naoe, T., Nozawa, Y., and Akao, Y. (2007). Characterized mechanism of alpha-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem. 15, 5620–5628. doi: 10.1016/j.bmc.2007.04.071
Norat, T., Scoccianti, C., Boutron-Ruault, M. C., Anderson, A., Berrino, F., Cecchini, M., et al. (2015). European code against cancer 4th edition: diet and cancer. Cancer Epidemiol. 39(Suppl. 1), S56–S66. doi: 10.1016/j.canep.2014.12.016
Noratto, G. D., Kim, Y., Talcott, S. T., and Mertens-Talcott, S. U. (2011). Flavonol-rich fractions of yaupon holly leaves (Ilex vomitoria, Aquifoliaceae) induce microRNA-146a and have anti-inflammatory and chemopreventive effects in intestinal myofibroblast CCD-18Co cells. Fitoterapia 82, 557–569. doi: 10.1016/j.fitote.2011.01.013
Nunez-Sanchez, M. A., Davalos, A., Gonzalez-Sarrias, A., Casas-Agustench, P., Visioli, F., Monedero-Saiz, T., et al. (2015). MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: critical issues to discern between modulatory effects and potential artefacts. Mol. Nutr. Food Res. 59, 1973–1986. doi: 10.1002/mnfr.201500357
Ojwang, L. O., Banerjee, N., Noratto, G. D., Angel-Morales, G., Hachibamba, T., Awika, J. M., et al. (2015). Polyphenolic extracts from cowpea (Vigna unguiculata) protect colonic myofibroblasts (CCD18Co cells) from lipopolysaccharide (LPS)-induced inflammation–modulation of microRNA 126. Food Funct. 6, 146–154. doi: 10.1039/c4fo00459k
O’Keefe, S. J., Li, J. V., Lahti, L., Ou, J., Carbonero, F., Mohammed, K., et al. (2015). Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6:6342. doi: 10.1038/ncomms7342
Okugawa, Y., Grady, W. M., and Goel, A. (2015). Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology 149, 1204–1225.e12. doi: 10.1053/j.gastro.2015.07.011
Olivo-Marston, S. E., Hursting, S. D., Perkins, S. N., Schetter, A., Khan, M., Croce, C., et al. (2014). Effects of calorie restriction and diet-induced obesity on murine colon carcinogenesis, growth and inflammatory factors, and microRNA expression. PLoS ONE 9:e94765. doi: 10.1371/journal.pone.0094765
Parasramka, M. A., Dashwood, W. M., Wang, R., Abdelli, A., Bailey, G. S., Williams, D. E., et al. (2012). MicroRNA profiling of carcinogen-induced rat colon tumors and the influence of dietary spinach. Mol. Nutr. Food Res. 56, 1259–1269. doi: 10.1002/mnfr.201200117
Park, J., Morley, T. S., Kim, M., Clegg, D. J., and Scherer, P. E. (2014). Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 10, 455–465. doi: 10.1038/nrendo.2014.94
Parkin, E., O’Reilly, D. A., Sherlock, D. J., Manoharan, P., and Renehan, A. G. (2014). Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes. Rev. 15, 434–451. doi: 10.1111/obr.12140
Patel, V. B., Misra, S., Patel, B. B., and Majumdar, A. P. (2010). Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr. Cancer 62, 958–967. doi: 10.1080/01635581.2010.510259
Pietrzyk, L., Torres, A., Maciejewski, R., and Torres, K. (2015). Obesity and obese-related chronic low-grade inflammation in promotion of colorectal cancer development. Asian Pac. J. Cancer Prev. 16, 4161–4168. doi: 10.7314/APJCP.2015.16.10.4161
Ramalingam, S., Subramaniam, D., and Anant, S. (2015). Manipulating miRNA expression: a novel approach for colon cancer prevention and chemotherapy. Curr. Pharmacol. Rep. 1, 141–153. doi: 10.1007/s40495-015-0020-3
Renehan, A. G., Roberts, D. L., and Dive, C. (2008). Obesity and cancer: pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 114, 71–83. doi: 10.1080/13813450801954303
Ress, A. L., Perakis, S., and Pichler, M. (2015). microRNAs and colorectal cancer. Adv. Exp. Med. Biol. 889, 89–103. doi: 10.1007/978-3-319-23730-5_6
Reuter, S., Gupta, S. C., Park, B., Goel, A., and Aggarwal, B. B. (2011). Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 6, 93–108. doi: 10.1007/s12263-011-0222-1
Roy, S., Levi, E., Majumdar, A. P., and Sarkar, F. H. (2012). Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J. Hematol. Oncol. 5:58. doi: 10.1186/1756-8722-5-58
Roy, S., Yu, Y., Padhye, S. B., Sarkar, F. H., and Majumdar, A. P. (2013). Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS ONE 8:e68543. doi: 10.1371/journal.pone.0068543
Saud, S. M., Li, W., Morris, N. L., Matter, M. S., Colburn, N. H., Kim, Y. S., et al. (2014). Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 35, 2778–2786. doi: 10.1093/carcin/bgu209
Schou, J. V., Johansen, J., Nielsen, D., and Rossi, S. (2016). Circulating microRNAs as prognostic and predictive biomarkers in patients with colerectal cancer. Noncoding RNA 2:5. doi: 10.3390/ncrna2020005
Shah, M. S., Kim, E., Davidson, L. A., Knight, J. M., Zoh, R. S., Goldsby, J. S., et al. (2016). Comparative effects of diet and carcinogen on microRNA expression in the stem cell niche of the mouse colonic crypt. Biochim. Biophys. Acta 1862, 121–134. doi: 10.1016/j.bbadis.2015.10.012
Shah, M. S., Schwartz, S. L., Zhao, C., Davidson, L. A., Zhou, B., Lupton, J. R., et al. (2011). Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: effect of a chemo-protective diet. Physiol. Genomics 43, 640–654. doi: 10.1152/physiolgenomics.00213.2010
Siegel, R. L., Miller, K. D., and Jemal, A. (2016). Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30. doi: 10.3322/caac.21332
Sun, H., Meng, X., Han, J., Zhang, Z., Wang, B., Bai, X., et al. (2013). Anti-cancer activity of DHA on gastric cancer–an in vitro and in vivo study. Tumour. Biol. 34, 3791–3800. doi: 10.1007/s13277-013-0963-0
Thompson, P. A. (2014). Navigating the maize between red meat and oncomirs. Cancer Prev. Res. 7, 777–780. doi: 10.1158/1940-6207.CAPR-14-0196
Tili, E., Michaille, J. J., Alder, H., Volinia, S., Delmas, D., Latruffe, N., et al. (2010). Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem. Pharmacol. 80, 2057–2065. doi: 10.1016/j.bcp.2010.07.003
Toden, S., Okugawa, Y., Buhrmann, C., Nattamai, D., Anguiano, E., Baldwin, N., et al. (2015a). Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev. Res. 8, 431–443. doi: 10.1158/1940-6207.CAPR-14-0354
Toden, S., Okugawa, Y., Jascur, T., Wodarz, D., Komarova, N. L., Buhrmann, C., et al. (2015b). Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 36, 355–367. doi: 10.1093/carcin/bgv006
Torre, L. A., Siegel, R. L., Ward, E. M., and Jemal, A. (2016). Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol. Biomarkers. Prev. 25, 16–27. doi: 10.1158/1055-9965.EPI-15-0578
Tsoukas, M. A., Ko, B. J., Witte, T. R., Dincer, F., Hardman, W. E., and Mantzoros, C. S. (2015). Dietary walnut suppression of colorectal cancer in mice: mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 26, 776–783. doi: 10.1016/j.jnutbio.2015.02.009
Turati, F., Edefonti, V., Bravi, F., Ferraroni, M., Talamini, R., Giacosa, A., et al. (2012). Adherence to the European food safety authority’s dietary recommendations and colorectal cancer risk. Eur. J. Clin. Nutr. 66, 517–522. doi: 10.1038/ejcn.2011.217
WHO (2015). Obesity and Overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
Wiseman, M. (2008). The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc. Nutr. Soc. 67, 253–256. doi: 10.1017/S002966510800712X
Yu, Y., Sarkar, F. H., and Majumdar, A. P. (2013). Down-regulation of miR-21 induces differentiation of chemoresistant colon cancer cells and enhances susceptibility to therapeutic regimens. Transl. Oncol. 6, 180–186. doi: 10.1593/tlo.12397
Keywords: microRNA, colorectal cancer, inter kingdom communication, diet, bioactive food components, epigenetic mechanisms
Citation: Del Cornò M, Donninelli G, Conti L and Gessani S (2017) Linking Diet to Colorectal Cancer: The Emerging Role of MicroRNA in the Communication between Plant and Animal Kingdoms. Front. Microbiol. 8:597. doi: 10.3389/fmicb.2017.00597
Received: 12 October 2016; Accepted: 23 March 2017;
Published: 05 April 2017.
Edited by:
Wolfgang R. Streit, University of Hamburg, GermanyReviewed by:
Milad Bastami, Tabriz University of Medical Sciences, IranCopyright © 2017 Del Cornò, Donninelli, Conti and Gessani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandra Gessani, c2FuZHJhLmdlc3NhbmlAaXNzLml0 Lucia Conti, bHVjaWEuY29udGlAaXNzLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.