
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 02 March 2017
Sec. Microbial Immunology
Volume 8 - 2017 | https://doi.org/10.3389/fmicb.2017.00328
This article is part of the Research Topic Mechanisms of Activation and Evasion of The Complement System by Pathogens View all 13 articles
Lyme disease and relapsing fever are caused by various Borrelia species. Lyme disease borreliae, the most common vector-borne pathogens in both the U.S. and Europe, are transmitted by Ixodes ticks and disseminate from the site of tick bites to tissues leading to erythema migrans skin rash, arthritis, carditis, and neuroborreliosis. Relapsing fever borreliae, carried by ticks and lice, trigger reoccurring fever episodes. Following transmission, spirochetes survive in the blood to induce bacteremia at the early stages of infection, which is thought to promote evasion of the host complement system. The complement system acts as an important innate immune defense mechanism in humans and vertebrates. Upon activation, the cleaved complement components form complexes on the pathogen surface to eventually promote bacteriolysis. The complement system is negatively modulated by a number of functionally diverse regulators to avoid tissue damage. To evade and inhibit the complement system, spirochetes are capable of binding complement components and regulators. Complement inhibition results in bacterial survival in serum (serum resistance) and is thought to promote bloodstream survival, which facilitates spirochete dissemination and disease manifestations. In this review, we discuss current methodologies to elucidate the mechanisms of Borrelia spp. that promote serum resistance and bloodstream survival, as well as novel methods to study factors responsible for bloodstream survival of Lyme disease borreliae that can be applied to relapsing fever borreliae. Understanding the mechanisms these pathogens utilize to evade the complement system will ultimately aid in the development of novel therapeutic strategies and disease prevention to improve human health.
The spirochete Borrelia is the bacterial agent causing both Lyme disease (LD) and relapsing fever (RF) (Steere et al., 2004; Radolf et al., 2012). LD, the most common vector-borne illness in the U.S. and Europe, is caused by the Borrelia burgdorferi sensu lato complex, consisting of 20 species of which 6 cause illness in humans (Rudenko et al., 2011). B. burgdorferi sensu stricto (B. burgdorferi) causes most infections in the U.S., whereas this species as well as B. garinii and B. afzelii cause most infections in Europe (Baranton et al., 1992; Canica et al., 1993; Steere et al., 2004). LD borreliae are transmitted by Ixodes ticks to reservoir animals and humans (Steere et al., 2004). After a tick bite, the bacteria infect the skin at the feeding site, often accompanied with the development of an erythema migrans skin rash (Steere et al., 2004). If left untreated, LD borreliae are capable of disseminating to tissues and organs to cause diverse manifestations including arthritis, carditis, and neuroborreliosis (Steere et al., 2004). Human RF infections are transmitted by ticks or lice, resulting in tick-borne relapsing fever (TBRF), or louse-borne relapsing fever (LBRF; Cutler, 2015). At least 10 species of TBRF borreliae, including Borrelia hermsii, Borrelia parkeri, and B. duttonii, are transmitted through bites by various Ornithodoros ticks whereas LBRF B. recurrentis is solely transmitted by the clothing louse P. humanus via crushed lice or feces contacting irritated human skin. Upon transmission, RF borreliae cause bacteremia, and alternating febrile/afebrile episodes corresponding with antigenic variation (Cutler, 2015). The spirochetes then disseminate to the central nervous system and may lead to complications in the brain, lungs, kidneys, and spleen (Dworkin et al., 2008; Cutler, 2015).
Survival in the bloodstream is thought to be essential for LD and RF borreliae to cause systemic disease. The complement system is an innate immune defense mechanism in the bloodstream of humans and other vertebrate animals against pathogens (Zipfel and Skerka, 2009). The complement system can be activated via three pathways: classical, lectin, and alternative, all of which result in the formation of C3 convertases (Figure 1). The classical pathway is initiated by the active form of C1 complex (C1qr2s2) binding to antibody-bacterial antigen complexes. The lectin pathway is initiated by binding of lectins [mannan-binding lectin (MBL) or ficolins] to an MBL serine protease (MASP) and microbial carbohydrate. Activation of these pathways leads to the generation of the C3 convertase C4b2a. The alternative pathway is initiated by interaction of C3b with the microbial surface and generates the C3 convertase C3bBb. Both C3 convertases recruit C3b to form C5 convertases, which further promotes the formation of C5b-9 membrane attack complex (MAC) and pathogen lysis. The activation of complement also promotes the release of proinflammatory peptides (C3a and C5a) and deposition of opsonic C3b molecules on the microbial surface to enhance phagocytic clearance (Figure 1). To avoid potential self-damage due to complement activation, vertebrate animals produce a number of diverse complement regulators to negatively regulate the complement system (Figure 1). Examples include C1 inhibitor (C1-INH), which binds to inactive C1rs and/or MASP to block the initiation of the classical and/or lectin pathways. Factor H (FH) and FHL-1 (a truncated form of FH) both bind to and promote the cleavage of C3b via recruiting the protease factor I (FI) to prevent the formation of C3 convertase C3bBb. C4b-binding protein (C4BP) binds to and triggers the degradation of C4b via recruiting FI to inhibit the formation of the C3 convertase C4b2a. Lastly, CD59 binds to C8 and C9 to block the formation of the MAC to avoid lysis of host cells.
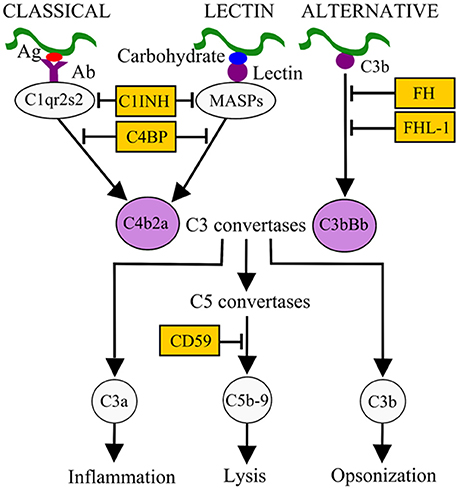
Figure 1. Activation and control mechanisms of the human complement system. The classical pathway, initiated by antibody (Ab)-pathogen antigen (Ag) complexes, and the lectin pathway, initiated by lectin-microbial carbohydrate complexes, generate the C3 convertase C4b2a. The alternative pathway, initiated by interaction of C3b with the microbial surface, generates the C3 convertase C3bBb. These C3 convertases, by recruiting other complement components, generate C5 convertases (C4b2a3b and C3bBb3b), which in turn result in the release of pro-inflammatory peptides (C5a), deposition of opsonins (C3b) on the microbial surface to enhance phagocytic clearance, and generation of the membrane attack complex (MAC or C5b-9). Different complement regulators exists to modulate complement activation. For example, C1 inhibitor (C1-INH) binds to C1r/C1s or MASPs and inhibits their proteolytic activity, thus inactivating the classical and lectin pathways. C4BP binds to C4b, factor H (FH), and factor H-like protein 1 (FHL-1) bind to C3b on C3 convertase. These interactions recruit factor I (FI) to inactivate C3b and subsequent activation steps.
Bacterial pathogens, including LD borreliae, produce outer surface proteins that bind and recruit complement regulators on the cell surface to inhibit complement activation and prevent killing (Table 1 for references; Kraiczy, 2016). B. burgdorferi and B. garinii produce the C4BP-binding protein p43, which may recruit C4BP to the bacterial surface to promote C4b degradation and eventually inhibit both classical and lectin pathways. Except B. bavariensis, all other serum-resistant LD borreliae produce up to five Complement Regulator-Acquiring Surface Proteins (CRASPs): CRASP-1 (CspA), CRASP-2 (CspZ), CRASP-3 (ErpP), CRASP-4 (ErpC), and CRASP-5 (ErpA). CspA and CspZ bind FH (and/or FHL-1). These proteins simultaneously bind C3b and then promote C3b degradation on spirochete surface to downregulate the alternative pathway (Meri et al., 2013). ErpP, ErpC, and ErpA facilitate serum resistance of LD borreliae and bind to FH, but the biological significance of these interactions is unclear.
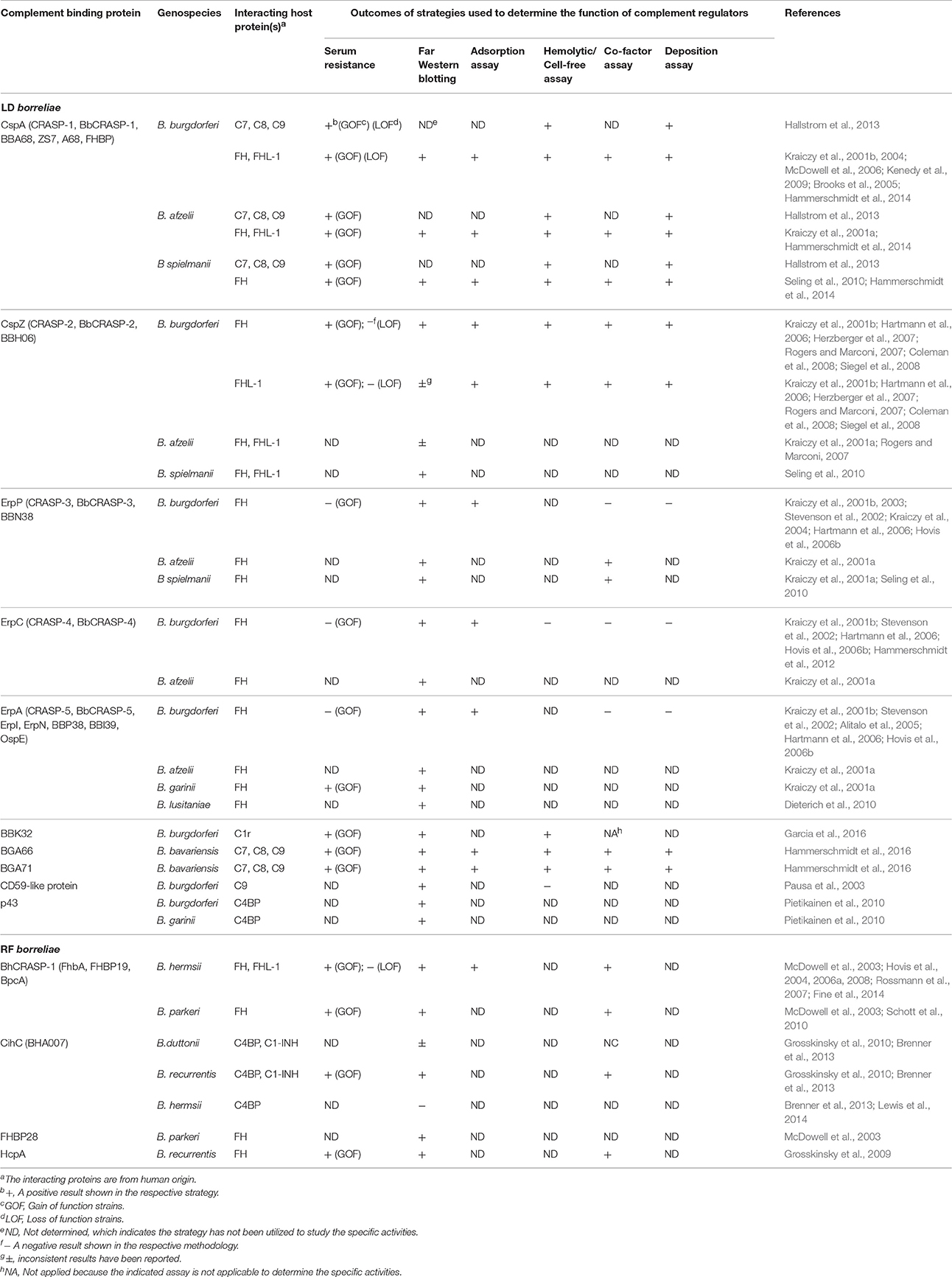
Table 1. LD and RF borreliae complement binding proteins and the outcomes of the strategies utilized to demonstrate their functions.
Similar to LD borreliae, RF borreliae produce complement regulator-binding proteins on their surface [Table 1 for references; Embers and SpringerLink, (Online service), 2012]. BHA007 in B. hermsii and its homolog CihC in both B. recurrentis and B. duttonii bind C4BP. CihC also binds C1-INH. The association of these proteins with C1-INH and C4BP on the surface of spirochetes prevents the formation of C1 and MASP complexes and induces the cleavage of C4b, respectively, to presumably inhibit the classical and lectin pathways. BhCRASP-1 and FhbA in B. hermsii, BpcA in B. parkeri, and HcpA in B. recurrentis bind FH (and/or FHL-1), which promotes C3b cleavage on bacterial surface and inhibits the alternative pathway.
LD borreliae also produce other outer surface proteins that interact with complement components to inhibit the formation of complement complexes and negatively modulate the complement system (Table 1 for references; Kraiczy, 2016). BBK32 of B. burgdorferi, known for both fibronectin (Probert and Johnson, 1998) and glycosaminoglycan binding (Fischer et al., 2006), was recently reported as a C1r-binding protein. By binding to the inactive form of C1r, BBK32 blocks the formation of the active C1 complex and inhibits the classical pathway. CspA of B. burgdorferi, B. afzelii, and B. spielmanii, and BGA66 and BGA71 of B. bavariensis, bind C7, C8, and C9. An unknown CD59-like protein of B. burgdorferi binds C9. These interactions result in the inhibition of MAC, thereby preventing bacteriolysis.
Investigating the role of spirochete proteins in interfering complement pathways allows us to elucidate the mechanisms of bacterial bloodstream survival. Because complement components and regulators are present in the blood, serum resistance assays (also known as bactericidal, growth inhibition, and serum susceptibility assays) are frequently utilized to determine the ability of spirochetes to survive in the serum in vitro, which is likely correlated with their ability to survive in the bloodstream in vivo. Bacterial survival can be determined by (i) counting viable cells using dark field microscopy, (ii) measuring the color change of the culture media (bacterial growth leads to the acidification of the media, resulting in color change), (iii) staining the DNA of live and dead bacteria, or (iv) plating bacteria on semi-solid agar plates (Table 1 for references). To test the role of a specific protein for serum resistance of LD and RF borreliae, spirochetes in the infectious background are genetically engineered to be deficient of these proteins (loss-of-function strains), and these strains are expected to be susceptible to complement-mediated killing (Brooks et al., 2005; Kenedy et al., 2009). However, loss-of-function strains currently can only be generated in B. burgdorferi. In addition, any redundant functions provided by other proteins involved in serum resistance in such a strain background may make the defect of a single gene undetectable (Coleman et al., 2008; Fine et al., 2014). Therefore, the alternative strategy is to ectopically produce these factors on the surface of the serum-susceptible spirochetes (gain-of-function strains). Frequently used gain-of-function strains include B. burgdorferi strains B313 and B314, and B. garinii strain G1. Note, B313 and B314 are non-infectious and only harbor six of the 21 plasmids due to repeated in vitro passaging (Sadziene et al., 1993). Gain-of-function strains allow us to study a serum resistance factor without complications from redundant serum resistance proteins.
The concentration of serum used in these assays is important. Although 10–40% serum has been used, only concentrations above 40% effectively eliminate serum-sensitive spirochetes (Breitner-Ruddock et al., 1997; van Dam et al., 1997; Kurtenbach et al., 1998; Kraiczy et al., 2000; Hartmann et al., 2006; Meri et al., 2006; Grosskinsky et al., 2009; Kenedy et al., 2009; van Burgel et al., 2010; Hammerschmidt et al., 2012, 2014; Hallstrom et al., 2013; Garcia et al., 2016). Interestingly, bactericidal activity is not consistently observed by serum from laboratory mouse strains (e.g., C3H/HeN, BALB/c, and C57B/6 strains), likely due to instability of mouse complement in vitro (Kurtenbach et al., 1998; Ristow et al., 2012; Caine and Coburn, 2015). The serum from white-footed mouse (Peromyscus leucopus), the natural reservoir host of LD spirochetes, invariably displayed ability in serum-sensitive bacterial killing, suggesting the serum from this species may be an alternative for rodent serum resistance assays (Rynkiewicz et al., 2013).
To explain the molecular mechanism of serum resistance by LD and RF borreliae, Far western blotting (also known as ligand affinity blotting) and adsorption assays have been utilized to determine if complement proteins or regulators bind to the outer surface proteins of spirochetes [Table 1 for the references of specific proteins; Embers and SpringerLink, (Online service), 2012]. In Far western blotting, borrelial proteins from lysed cells are separated on a blot and incubated with either a complement component, regulator, or serum, and then treated with antibodies for detection of the bound complement components or regulators. Reverse ligand blotting, a modified version of Far western blotting, separates serum proteins by size on a blot. The blot is incubated with a purified complement component- or regulator-binding protein and treated with antibodies to detect the complement component- or regulator-binding protein. However, as the binding of these components or regulators to borrelial proteins occurs on the spirochete surface, lysing the cells prior to incubation may change the structure of borrelial proteins and prevent binding. This may explain some inconsistent results when analyzing the complement regulator-binding activity of borreliae using this method (Table 1; Hartmann et al., 2006; McDowell et al., 2006; Rogers and Marconi, 2007; Bhide et al., 2009; Grosskinsky et al., 2010; Brenner et al., 2013).
Unlike Far western blotting, serum adsorption assays immobilize whole bacterial cells. After incubating the cells with either complement components, regulators, or serum, bound cells are lysed, separated by SDS-PAGE, and detected by antibodies. This is a more biologically-relevant approach because binding of complement components or regulators occurs under physiological settings on the spirochete surface. Both techniques, however, rely on antibodies for binding detection. As some complement components or regulators (e.g., FH) are polymorphic between animal species (Blom et al., 2004), antibodies against complement components or regulators from one species may not effectively recognize that from another species (McDowell et al., 2006; Rogers and Marconi, 2007), making research in infrequently studied animals inconvenient.
Hemolytic assays have been utilized to quantitatively determine the ability of LD or RF borreliae to negatively modulate each complement pathway via complement-component or -regulator-binding proteins. These assays incubate human serum with foreign erythrocytes and borrelial proteins, and measure the level of erythrocyte lysis (Table 1 for references of specific proteins; Dodds and Sim, 1997; Morgan, 2000). These proteins recruit complement components (e.g., C3b, C4b, C7, or C9) by either directly binding to these components or to complement regulators that simultaneously associate with these complement components. This binding reduces the concentration of said complement components in the serum and ultimately inhibits erythrocyte lysis. To maximize hemolysis triggered by the classical pathway or the MAC, erythrocytes are sensitized by pre-incubating with antibodies and the C5b-6 complex, respectively, prior to adding serum. Note, erythrocytes do not need to be incubated with any additional activators prior to adding serum to measure the hemolytic activity induced by the alternative pathway. A lower concentration of serum (1%) can be used to measure the erythrocyte lysis from classical pathways or MAC formation, whereas a higher concentration of serum (above 2.5%) permits detection of hemolysis caused by the alternative pathway (Dodds and Sim, 1997; Morgan, 2000; Hallstrom et al., 2013; Hammerschmidt et al., 2016). Thus, both the serum concentration and the activators used to sensitize erythrocytes are critical to differentiate the pathway-specific hemolysis. In addition, serum deficient in one or more complement components or regulators essential to activation of each pathway can be used to determine which pathways the complement component- or regulator-binding proteins inhibit.
WIESLAB® recently developed a cell-free assay (Wielisa) to quantitatively measure the activation of different complement pathways, which has been used to study spirochete complement component- or regulator-binding proteins (Garcia et al., 2016; Hammerschmidt et al., 2016). Serum incubated with spirochete complement component- or regulator-binding proteins is added to microtiter plates that have been coated with immobilized immunoglobulin (classical pathway), mannan (lectin pathway), or lipopolysaccharides (alternative pathway). The ability of these bacterial proteins to inhibit complement activation is determined by detecting the level of MAC formed on the surface of microtiter plates.
Cofactor assays determine if complement regulators bound by spirochete proteins facilitate the cleavage of the target complement components [Table 1 for references of specific proteins; Embers and SpringerLink, (Online service), 2012]. For example, following the binding of complement regulators to the immobilized protein or spirochete surface, the ability of FH (or FHL-1) to promote C3b degradation in the presence of FI can be detected by identifying cleaved C3b using Western blotting. The ability of spirochete C4BP-binding protein to promote C4b degradation by binding to C4BP and FI can also be performed in a similar fashion. Although the concentrations of the complement regulator-binding proteins used in this assay are generally higher than what is likely physiologically relevant, this technique allows us to demonstrate a molecular mechanism of these proteins in inactivating complement system by binding to respective regulators.
Complement complexes form on the surface of spirochetes during complement activation (Table 1 for references of specific proteins). Therefore, detecting C3b (a component of C3 and C5 convertases), and C6 and C5b-9 (the components of MAC) allows us to measure the level of complement activation on the surface of LD or RF borreliae. Deposition assays utilize immunofluorescence staining or ELISA to measure the levels of the aforementioned complement components bound on the bacterial surface after spirochetes strains are incubated with serum. LD and RF borreliae that bind complement components or regulators from serum should have reduced or no deposition of C3b, C6, and C5b-9. Note, serum concentrations used range from 10 to 25% because serum concentrations >40% eliminate Borrelia, which prevents observation of complement deposition (Kurtenbach et al., 1998; Kenedy et al., 2009; Hammerschmidt et al., 2014).
In the natural transmission of LD or RF borreliae from ticks to vertebrate animals, the spirochetes first colonize the skin at the tick feeding site prior to disseminating into the bloodstream and migrating into the surrounding tissues (Radolf et al., 2012; Coburn et al., 2013). In traditional models, mice are inoculated subcutaneously or intradermally, or by bite from a tick infected with LD or RF borreliae to study the contribution of spirochete factors during infection (Barthold et al., 1990; Simon et al., 1991). However, since a failure at either initial skin colonization or bloodstream survival would lead to low or undetectable bacterial burdens in the animal, it can be difficult to distinguish the roles of spirochete factors in promoting survival within the mammalian host using traditional models.
A short-term murine model has recently been developed using the LD spirochete B. burgdorferi to investigate the roles of bacterial outer surface proteins in mammalian bloodstream survival (Caine and Coburn, 2015). This model intravenously inoculates mice with a high number of spirochetes for up to 1 h. The ability of the spirochetes to survive in the bloodstream can be detected by measuring bacteremia (Caine and Coburn, 2015). Intravenous inoculation bypasses the initial step of skin colonization allowing the study of non-infectious mutant spirochetes. Therefore, this strategy teases apart the contributions of Borrelia factors with multiple functions in bloodstream survival, protein adhesion, and tissue attachment. For example, B. burgdorferi outer surface protein BBK32 contributes to colonization of the inoculation site of skin (Seshu et al., 2006; Hyde et al., 2011; Lin et al., 2015). Whether this protein contributes to mammalian bloodstream survival during Lyme infection is difficult to assess by subcutaneous needle or tick infection. Using short-term intravenous inoculation in a murine model, BBK32 ectopically-produced on a non-infectious, serum-sensitive B. burgdorferi strain promotes spirochete survival in the bloodstream (Caine and Coburn, 2015). This strategy has also been applied to identify the contribution of other B. burgdorferi factors in promoting bloodstream survival (Caine and Coburn, 2015). As RF borreliae are also blood-borne pathogens that disseminate into host tissues, this short-term model could be employed to further characterize serum resistance and disease progression in RF borreliae. Though some complement components or regulator are polymorphic among vertebrate animals (Lu et al., 2008), which raises a concern that the in vivo murine models may not be relevant to humans, recent developed humanized mouse strains may be utilized as a solution of this issue (Beernink et al., 2012).
Bloodstream survival of LD or RF borreliae is thought to be essential for spirochetes to survive in humans, and ultimately cause LD or RF disease manifestations. Serum resistance, adsorption, hemolytic, cofactor, and deposition assays, as well as a recently established short term intravenous inoculation murine model are all used to elucidate the mechanism of LD and RF borreliae evasion of the complement system and survival in the bloodstream. The data reviewed here are mainly on borrelial interactions with humans, but these assays can also be applied to the interactions with other vertebrate hosts, which will elucidate the role of the borrelial complement evasion in the enzootic cycle. Understanding these mechanisms in both humans and other vertebrate hosts will aid in the development of novel therapeutic strategies and disease prevention by targeting these complement component- or regulator-binding proteins to ultimately improve human health.
AM, PK, and YL wrote the manuscript; and AM and YL prepared the figure and table.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Jennifer Caine for critical reading of the manuscript. This work was supported by the New York State Department of Health Wadsworth Center Start-Up Grant (to YL and AM) and NIH R01AI121401 (to PK).
Ab, Antibody; Ag, Antigen; B313, B. burgdorferi strain B313; B314, B. burgdorferi strain B314; C4BP, C4b-binding protein; CRASP, Complement Regulator-Acquiring Surface Protein; FH, Factor H; FHL-1, Factor H-like protein 1; FI, Factor I; G1, B. garinii strain G1; LBRF, louse-borne relapsing fever; LD, Lyme disease; MAC, membrane attack complex; MASP, Mannan-binding lectin serine protease; MBL, Mannan-binding lectin; RF, relapsing fever; TBRF, tick-borne relapsing fever.
Alitalo, A., Meri, T., Comstedt, P., Jeffery, L., Tornberg, J., Strandin, T., et al. (2005). Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 35, 3043–3053. doi: 10.1002/eji.200526354
Baranton, G., Postic, D., Saint Girons, I., Boerlin, P., Piffaretti, J. C., Assous, M., et al. (1992). Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42, 378–383. doi: 10.1099/00207713-42-3-378
Barthold, S. W., Beck, D. S., Hansen, G. M., Terwilliger, G. A., and Moody, K. D. (1990). Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162, 133–138. doi: 10.1093/infdis/162.1.133
Beernink, P. T., Shaughnessy, J., Pajon, R., Braga, E. M., Ram, S., and Granoff, D. M. (2012). The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 8:e1002688. doi: 10.1371/journal.ppat.1002688
Bhide, M. R., Escudero, R., Camafeita, E., Gil, H., Jado, I., and Anda, P. (2009). Complement factor H binding by different Lyme disease and relapsing fever Borrelia in animals and human. BMC Res. Notes 2:134. doi: 10.1186/1756-0500-2-134
Blom, A. M., Villoutreix, B. O., and Dahlback, B. (2004). Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol. Immunol. 40, 1333–1346. doi: 10.1016/j.molimm.2003.12.002
Breitner-Ruddock, S., Wurzner, R., Schulze, J., and Brade, V. (1997). Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 185, 253–260. doi: 10.1007/s004300050038
Brenner, C., Bomans, K., Habicht, J., Simon, M. M., and Wallich, R. (2013). Mapping the ligand-binding region of Borrelia hermsii fibronectin-binding protein. PLoS ONE 8:e63437. doi: 10.1371/journal.pone.0063437
Brooks, C. S., Vuppala, S. R., Jett, A. M., Alitalo, A., Meri, S., and Akins, D. R. (2005). Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175, 3299–3308. doi: 10.4049/jimmunol.175.5.3299
Caine, J. A., and Coburn, J. (2015). A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect. Immun. 83, 3184–3194. doi: 10.1128/IAI.00349-15
Canica, M. M., Nato, F., du Merle, L., Mazie, J. C., Baranton, G., and Postic, D. (1993). Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25, 441–448. doi: 10.3109/00365549309008525
Coburn, J., Leong, J., and Chaconas, G. (2013). Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol. 21, 372–379. doi: 10.1016/j.tim.2013.06.005
Coleman, A. S., Yang, X., Kumar, M., Zhang, X., Promnares, K., Shroder, D., et al. (2008). Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE 3:e3010. doi: 10.1371/journal.pone.0003010
Cutler, S. J. (2015). Relapsing fever Borreliae: a global review. Clin. Lab. Med. 35, 847–865. doi: 10.1016/j.cll.2015.07.001
Dieterich, R., Hammerschmidt, C., Richter, D., Skerka, C., Wallich, R., Matuschka, F. R., et al. (2010). Inadequate binding of immune regulator factor H is associated with sensitivity of Borrelia lusitaniae to human complement. Infect. Immun. 78, 4467–4476. doi: 10.1128/IAI.00138-10
Dworkin, M. S., Schwan, T. G., Anderson, D. E. Jr., and Borchardt, S. M. (2008). Tick-borne relapsing fever. Infect. Dis. Clin. North Am. 22, 449–468. doi: 10.1016/j.idc.2008.03.006
Embers, M. E., and SpringerLink (Online service). (2012). “The pathogenic spirochetes: strategies for evasion of host immunity and persistence,” in The Pathogenic Spirochetes: Strategies for Evasion of Host Immunity and Persistence, ed M. E. Embers (New York, NY: Springer), 63–88.
Fine, L. M., Miller, D. P., Mallory, K. L., Tegels, B. K., Earnhart, C. G., and Marconi, R. T. (2014). The Borrelia hermsii factor H binding protein FhbA is not required for infectivity in mice or for resistance to human complement in vitro. Infect. Immun. 82, 3324–3332. doi: 10.1128/IAI.01892-14
Fischer, J. R., LeBlanc, K. T., and Leong, J. M. (2006). Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74, 435–441. doi: 10.1128/IAI.74.1.435-441.2006
Garcia, B. L., Zhi, H., Wager, B., Hook, M., and Skare, J. T. (2016). Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking Activation of the C1 complement complex. PLoS Pathog. 12:e1005404. doi: 10.1371/journal.ppat.1005404
Grosskinsky, S., Schott, M., Brenner, C., Cutler, S. J., Kraiczy, P., Zipfel, P. F., et al. (2009). Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS ONE 4:e4858. doi: 10.1371/journal.pone.0004858
Grosskinsky, S., Schott, M., Brenner, C., Cutler, S. J., Simon, M. M., and Wallich, R. (2010). Human complement regulators C4b-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLoS Negl. Trop. Dis. 4:e698. doi: 10.1371/journal.pntd.0000698
Hallstrom, T., Siegel, C., Morgelin, M., Kraiczy, P., Skerka, C., and Zipfel, P. F. (2013). CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio 4, e00481-13. doi: 10.1128/mBio.00481-13
Hammerschmidt, C., Hallstrom, T., Skerka, C., Wallich, R., Stevenson, B., Zipfel, P. F., et al. (2012). Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin. Dev. Immunol. 2012:349657. doi: 10.1155/2012/349657
Hammerschmidt, C., Klevenhaus, Y., Koenigs, A., Hallstrom, T., Fingerle, V., Skerka, C., et al. (2016). BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol. Microbiol. 99, 407–424. doi: 10.1111/mmi.13239
Hammerschmidt, C., Koenigs, A., Siegel, C., Hallstrom, T., Skerka, C., Wallich, R., et al. (2014). Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect. Immun. 82, 380–392. doi: 10.1128/IAI.01094-13
Hartmann, K., Corvey, C., Skerka, C., Kirschfink, M., Karas, M., Brade, V., et al. (2006). Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61, 1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x
Herzberger, P., Siegel, C., Skerka, C., Fingerle, V., Schulte-Spechtel, U., van Dam, A., et al. (2007). Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1. Infect. Immun. 75, 4817–4825. doi: 10.1128/IAI.00532-07
Hovis, K. M., Freedman, J. C., Zhang, H., Forbes, J. L., and Marconi, R. T. (2008). Identification of an antiparallel coiled-coil/loop domain required for ligand binding by the Borrelia hermsii FhbA protein: additional evidence for the role of FhbA in the host-pathogen interaction. Infect. Immun. 76, 2113–2122. doi: 10.1128/IAI.01266-07
Hovis, K. M., Jones, J. P., Sadlon, T., Raval, G., Gordon, D. L., and Marconi, R. T. (2006a). Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 74, 2007–2014. doi: 10.1128/IAI.74.4.2007-2014.2006
Hovis, K. M., McDowell, J. V., Griffin, L., and Marconi, R. T. (2004). Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186, 2612–2618. doi: 10.1128/JB.186.9.2612-2618.2004
Hovis, K. M., Tran, E., Sundy, C. M., Buckles, E., McDowell, J. V., and Marconi, R. T. (2006b). Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 74, 1967–1972. doi: 10.1128/IAI.74.3.1967-1972.2006
Hyde, J. A., Weening, E. H., Chang, M., Trzeciakowski, J. P., Hook, M., Cirillo, J. D., et al. (2011). Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol. Microbiol. 82, 99–113. doi: 10.1111/j.1365-2958.2011.07801.x
Kenedy, M. R., Vuppala, S. R., Siegel, C., Kraiczy, P., and Akins, D. R. (2009). CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immun. 77, 2773–2782. doi: 10.1128/IAI.00318-09
Kraiczy, P. (2016). Hide and Seek: how Lyme disease spirochetes overcome complement attack. Front. Immunol. 7:385. doi: 10.3389/fimmu.2016.00385
Kraiczy, P., Hellwage, J., Skerka, C., Becker, H., Kirschfink, M., Simon, M. M., et al. (2004). Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279, 2421–2429. doi: 10.1074/jbc.M308343200
Kraiczy, P., Hellwage, J., Skerka, C., Kirschfink, M., Brade, V., Zipfel, P. F., et al. (2003). Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33, 697–707. doi: 10.1002/eji.200323571
Kraiczy, P., Hunfeld, K. P., Breitner-Ruddock, S., Wurzner, R., Acker, G., and Brade, V. (2000). Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology 201, 406–419. doi: 10.1016/S0171-2985(00)80094-7
Kraiczy, P., Skerka, C., Brade, V., and Zipfel, P. F. (2001a). Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69, 7800–7809. doi: 10.1128/IAI.69.12.7800-7809.2001
Kraiczy, P., Skerka, C., Kirschfink, M., Brade, V., and Zipfel, P. F. (2001b). Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31, 1674–1684. doi: 10.1002/1521-4141(200106)31:6lt;1674::AID-IMMU1674gt;3.0.CO;2-2
Kurtenbach, K., Sewell, H. S., Ogden, N. H., Randolph, S. E., and Nuttall, P. A. (1998). Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66, 1248–1251.
Lewis, E. R., Marcsisin, R. A., Campeau Miller, S. A., Hue, F., Phillips, A., Aucoin, D. P., et al. (2014). Fibronectin-binding protein of Borrelia hermsii expressed in the blood of mice with relapsing fever. Infect. Immun. 82, 2520–2531. doi: 10.1128/IAI.01582-14
Lin, Y. P., Chen, Q., Ritchie, J. A., Dufour, N. P., Fischer, J. R., Coburn, J., et al. (2015). Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell. Microbiol. 17, 860–875. doi: 10.1111/cmi.12407
Lu, L., Ma, Z., Jokiranta, T. S., Whitney, A. R., DeLeo, F. R., and Zhang, J. R. (2008). Species-specific interaction of Streptococcus pneumoniae with human complement factor H. J. Immunol. 181, 7138–7146. doi: 10.4049/jimmunol.181.10.7138
McDowell, J. V., Hovis, K. M., Zhang, H., Tran, E., Lankford, J., and Marconi, R. T. (2006). Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 74, 3030–3034. doi: 10.1128/IAI.74.5.3030-3034.2006
McDowell, J. V., Tran, E., Hamilton, D., Wolfgang, J., Miller, K., and Marconi, R. T. (2003). Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave c3b. J. Clin. Microbiol. 41, 3905–3910. doi: 10.1128/JCM.41.8.3905-3910.2003
Meri, T., Amdahl, H., Lehtinen, M. J., Hyvarinen, S., McDowell, J. V., Bhattacharjee, A., et al. (2013). Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 9:e1003308. doi: 10.1371/annotation/41169409-3260-4295-baf4-a1a4621a8e48
Meri, T., Cutler, S. J., Blom, A. M., Meri, S., and Jokiranta, T. S. (2006). Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect. Immun. 74, 4157–4163. doi: 10.1128/IAI.00007-06
Pausa, M., Pellis, V., Cinco, M., Giulianini, P. G., Presani, G., Perticarari, S., et al. (2003). Serum-resistant strains of Borrelia burgdorferi evade complement-mediated killing by expressing a CD59-like complement inhibitory molecule. J. Immunol. 170, 3214–3222. doi: 10.4049/jimmunol.170.6.3214
Pietikainen, J., Meri, T., Blom, A. M., and Meri, S. (2010). Binding of the complement inhibitor C4b-binding protein to Lyme disease Borreliae. Mol. Immunol. 47, 1299–1305. doi: 10.1016/j.molimm.2009.11.028
Probert, W. S., and Johnson, B. J. (1998). Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30, 1003–1015.
Radolf, J. D., Caimano, M. J., Stevenson, B., and Hu, L. T. (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99. doi: 10.1038/nrmicro2714
Ristow, L. C., Miller, H. E., Padmore, L. J., Chettri, R., Salzman, N., Caimano, M. J., et al. (2012). The β3-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol. Microbiol. 85, 1105–1118. doi: 10.1111/j.1365-2958.2012.08160.x
Rogers, E. A., and Marconi, R. T. (2007). Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect. Immun. 75, 5272–5281. doi: 10.1128/IAI.00850-07
Rossmann, E., Kraiczy, P., Herzberger, P., Skerka, C., Kirschfink, M., Simon, M. M., et al. (2007). Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 178, 7292–7301. doi: 10.4049/jimmunol.178.11.7292
Rudenko, N., Golovchenko, M., Grubhoffer, L., and Oliver, J. H. Jr. (2011). Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2, 123–128. doi: 10.1016/j.ttbdis.2011.04.002
Rynkiewicz, E. C., Hawlena, H., Durden, L. A., Hastriter, M. W., Demas, G. E., and Clay, K. (2013). Associations between innate immune function and ectoparasites in wild rodent hosts. Parasitol. Res. 112, 1763–1770. doi: 10.1007/s00436-013-3335-1
Sadziene, A., Wilske, B., Ferdows, M. S., and Barbour, A. G. (1993). The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect. Immun. 61, 2192–2195.
Schott, M., Grosskinsky, S., Brenner, C., Kraiczy, P., and Wallich, R. (2010). Molecular characterization of the interaction of Borrelia parkeri and Borrelia turicatae with human complement regulators. Infect. Immun. 78, 2199–2208. doi: 10.1128/IAI.00089-10
Seling, A., Siegel, C., Fingerle, V., Jutras, B. L., Brissette, C. A., Skerka, C., et al. (2010). Functional characterization of Borrelia spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen. Infect. Immun. 78, 39–48. doi: 10.1128/IAI.00691-09
Seshu, J., Esteve-Gassent, M. D., Labandeira-Rey, M., Kim, J. H., Trzeciakowski, J. P., Hook, M., et al. (2006). Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59, 1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x
Siegel, C., Schreiber, J., Haupt, K., Skerka, C., Brade, V., Simon, M. M., et al. (2008). Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J. Biol. Chem. 283, 34855–34863. doi: 10.1074/jbc.M805844200
Simon, M. M., Schaible, U. E., Wallich, R., and Kramer, M. D. (1991). A mouse model for Borrelia burgdorferi infection: approach to a vaccine against Lyme disease. Immunol. Today 12, 11–16. doi: 10.1016/0167-5699(91)90106-4
Steere, A. C., Coburn, J., and Glickstein, L. (2004). The emergence of Lyme disease. J. Clin. Invest. 113, 1093–1101. doi: 10.1172/JCI21681
Stevenson, B., El-Hage, N., Hines, M. A., Miller, J. C., and Babb, K. (2002). Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70, 491–497. doi: 10.1128/IAI.70.2.491-497.2002
van Burgel, N. D., Kraiczy, P., Schuijt, T. J., Zipfel, P. F., and van Dam, A. P. (2010). Identification and functional characterisation of complement regulator acquiring surface protein-1 of serum resistant Borrelia garinii OspA serotype 4. BMC Microbiol. 10:43. doi: 10.1186/1471-2180-10-43
van Dam, A. P., Oei, A., Jaspars, R., Fijen, C., Wilske, B., Spanjaard, L., et al. (1997). Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65, 1228–1236.
Keywords: Lyme disease, relapsing fever, spirochetes, borrelia, innate immunity, complement system, blood stream survival
Citation: Marcinkiewicz AL, Kraiczy P and Lin YP (2017) There Is a Method to the Madness: Strategies to Study Host Complement Evasion by Lyme Disease and Relapsing Fever Spirochetes. Front. Microbiol. 8:328. doi: 10.3389/fmicb.2017.00328
Received: 19 December 2016; Accepted: 16 February 2017;
Published: 02 March 2017.
Edited by:
Angela Silva Barbosa, Instituto Butantan, BrazilReviewed by:
Taru Meri, University of Helsinki, FinlandCopyright © 2017 Marcinkiewicz, Kraiczy and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Pin Lin, eWktcGluLmxpbkBoZWFsdGgubnkuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.