- Department of Microbiology, Panjab University, Chandigarh, India
Colorectal cancer is closely associated with environment, diet and lifestyle. Normally it is treated with surgery, radiotherapy or chemotherapy but increasing systemic toxicity, resistance and recurrence is prompting scientists to devise new potent and safer alternate prophylactic or therapeutic strategies. Among these, probiotics, prebiotics, synbiotics, and metabiotics are being considered as the promising candidates. Metabiotics or probiotic derived factors can optimize various physiological functions of the host and offer an additional advantage to be utilized even in immunosuppressed individuals. Interestingly, anti colon cancer potential of probiotic strains has been attributable to metabiotics that have epigenetic, antimutagenic, immunomodulatory, apoptotic, and antimetastatic effects. Thus, it’s time to move one step further to utilize metabiotics more smartly by avoiding the risks associated with probiotics even in certain normal/or immuno compromised host. Here, an attempt is made to provide insight into the adverse effects associated with probiotics and beneficial aspects of metabiotics with main emphasis on the modulatory mechanisms involved in colon cancer.
Introduction
Cancer refers to a heterogeneous collection of neoplastic cells evolving in microenvironments with complex ecologies (Aktipis and Nesse, 2013). Colorectal cancer (CRC) is the third most common cancer, affecting mainly colon and rectum in both men and women and is the second leading cause of cancer-associated mortality (Cancer Facts and Figures, 2016). CRC affects more than 1 million people annually, accounting for about 500,000 deaths worldwide and its incidence is increasing at an alarming rate. Further, CRC has been predicted to affect at least half of the western population by the age of 70 due to consumption of red or processed meat, alcoholic beverages, less fruits and vegetables as well as lack of physical activity (Jemal et al., 2010). Recently, World Health Organization has predicted about 27 million new cases of cancer, 17 million deaths and about 75 million people will be affected with CRC by 2030 (Kumar et al., 2015).
The etiology of CRC is complex comprising of a well-defined series of histological changes paralleled with mutational activation of oncogenes and inactivation of tumor suppressor genes regulated by a multifactorial interplay between diet, environment, carcinogenic chemicals, and mutagens (Sankpal et al., 2012; Raman et al., 2013). It has been proposed that sporadic cancer arises due to mutant low-penetrance genes which interact extensively with environmental factors (Birt and Phillips, 2014). The most common driving mutations of colon cancer affect adenomatous polyposis coli (APC), rat sarcoma family of genes (ras), phosphatidyl inositol-3-kinase (PI3K), and transforming growth factor (TGFβ) (Vogelstein et al., 2013).
CRC can be treated either by surgery, radiotherapy or chemotherapy predominantly with 5-fluorouracil and oxaliplatin depending on the progressional stage but due to systemic toxicity, resistance to chemotherapy and recurrence of cancer, alternate interventions are needed. Therefore, life style and dietary interventions such as exercise, fiber rich foods and fermented food products have been gaining a lot of interest by scientists round the globe for preventing colon cancer with main focus on probiotics. Probiotics are “Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” as per International Scientific Association for Probiotics and Prebiotics (Colin et al., 2014).
It is widely acknowledged that susceptibility as well as progression of colon cancer is determined by gene-environment interactions and microbial communities constituting micro environment of the gut. In 400 BC Hippocrates stated ‘Death sits in the bowel,’ implying the importance of gut microbiome in human health and Elie Metchnikoff too linked the longevity in Bulgarian peasants with consumption of fermented dairy products. The gut microbiome is metabolically so active that it is regarded as a complete organ which dynamically participates in various physiological functions and has 10 times more cells and 100 times more genes than human body (Nicholson et al., 2012). Colon cancer risk is influenced to a great degree by the balance between microbial production of health-promoting metabolites such as lactic acid, short chain fatty acids (SCFAs), linoleic acid, some glycoproteins/peptides and potentially carcinogenic metabolites such as amines and secondary bile acids. All these beneficial bioactive substances associated with probiotics are referred as either metabiotics, postbiotics, biogenics, or simply metabolites/CFS (Cell free supernatants). The term “metabiotics” refers to the structural components of probiotic microorganisms and/or their metabolites and/or signaling molecules with a determined chemical structure that can optimize host-specific physiological functions, regulatory, metabolic and/or behavior reactions connected with the activity of host indigenous microbiota (Shenderov, 2013). Among these, SCFAs are the most studied being a source of energy for colonocytes and modulator of various metabolic activities.
No doubt probiotic bacteria with antimutagenic and/or antigenotoxic activities, have been found to exert generalized prophylactic effect against colon cancer but SCFAs have been thought to be more selective in targeting cancer cells and regulating the expression of oncogenes and tumor suppressor genes by epigenetic mechanisms (Cousin et al., 2012; Verma and Shukla, 2014, 2015; Papadimitriou et al., 2015). Besides these, it has been reported that some of the gut bacteria, e.g., Streptococcus gallolyticus, Bacteroides fragilis, Fusobacterium have been implicated in the genesis of CRC. More specifically, the translocation of Streptococcus gallolyticus to blood stream has been linked to development of inflammation and colon tumor as well as toxin produced by Bacteroides fragilis has been shown to cause genetic alterations due to upregulation of myc and NFκB expression leading to CRC (Sears, 2009; Wu et al., 2009; Boleij and Tjalsma, 2013). Furthermore, Ahn et al. (2013) and Kostic et al. (2013) have also reported Fusobacterium to be the more dominant bacteria in colon adenomas. Although, it is very well-evident that probiotic microorganisms have health benefits, yet may cause opportunistic infections, increase the incidence of allergic sensitization and autoimmune disorders, alter the micro ecological balance, modify gene expression, transfer antibiotic resistance and virulence genes, lead to disorders in epigenome and genome integrity, induce chromosomal DNA damage and activate signaling pathways associated with cancer and other chronic diseases in certain healthy or in immunocompromised and high risk individuals, thereby limiting their use (Snydman, 2008; Gratz et al., 2010). To overcome such adverse effects associated with probiotics, metabiotics are preferred due to their known chemical structure, effective dose, safety assurance and longer shelf-life (Shenderov, 2013).
Implications of Probiotics
Probiotics may be highly beneficial to the host as it has been described that they can maintain epithelial integrity, compete for adhesion and nutrition with pathogens, stimulate cell mediated immunity, IgA production and gut associated lymphoid tissue. Additionally, probiotics detoxify carcinogens, reduce serum cholesterol level, alleviate lactose intolerance, produce active metabolites including organic acids, bacteriocin, H2O2 and enhance the production of vitamins (Kechagia et al., 2013). Moreover, probiotics are also being used either as prophylactic or therapeutic agent for various diseases such as antibiotic induced or infectious diarrhea, ulcerative colitis, Crohn’s disease and irritable bowel syndrome (Verna and Lucak, 2010). Despite having a history of safe use, introducing live microbes in the human body could be a potential threat especially to immunocompromised and genetically predisposed individuals and is a matter of concern (Pan et al., 2014). Scientists have reported that an infant and a child without any underlying GIT disease or immunocompromised status suffered from bacteremia upon lactobacilli supplementation (Cabana et al., 2006). In addition, some medical reports have also highlighted that lactic acid bacteria and even bifidobacteria have been associated with human opportunistic infections such as infective endocarditis, sepsis, bacteremia, pneumonia, abdominal abscesses, peritonitis, meningitis, urological infections, rheumatic vascular diseases in immune compromised individuals and patients with allergic sensitization and autoimmune disorders (Ohishi et al., 2010; Berer et al., 2011). Though incidence of such cases may be lower, yet depends very much on probiotic species and strain specificity. Probiotic strains have also been found to increase platelet aggregation and aggravate hemolytic uremic syndrome; (Yazdankhah et al., 2009; Van Reenen and Dicks, 2011) and are potential source of toxic metabolites like biogenic amines in certain individuals (Bourdichon et al., 2012). It should not be ignored that some silent genes of probiotic bacteria may be induced by host cell signals during passage through intestinal tract, leading to undesirable effects (Di Caro et al., 2005). Probiotics have also been reported to transfer genetic information including antibiotic resistance to commensals or pathogens due to the presence of antibiotic resistance plasmids leading to genomic and epigenomic alterations (Shenderov, 2011).
Enterococcus faecium, having a long history of probiotic use in preventing antibiotic-associated diarrhea, may act as opportunistic pathogen owing to the presence of potential reservoir of antibiotic resistance and virulence genes (Dirienzo, 2013). A recurrent septicemia and central nervous system deterioration has also been observed in an immunocompromised patient with spores of the probiotic strains of Bacillus subtilis EG-RN and EG-CM (Fijan, 2014). Some studies have also revealed increased bacterial translocation leading to mortality upon Lactobacillus delbrueckii UFV-H2b20 and Bifidobacterium lactis Bb12 supplementation in mice with 1,2-dimethyl hydrazine (DMH)-induced injuries (Liboredo et al., 2010). Additionally, some genotoxic effects have also been linked to probiotic strain Escherichia coli Nissle 1917 possessing a set of genes (pks island) responsible for the induction of double-strand breaks in host cell DNA (Cuevas-Ramos et al., 2010).
It’s ironical that live microbes could potentially be risky in immuno compromised patients where probiotic applications are often considered, thereby emphasizing the careful examination and application of probiotics which in turn depends upon species and strains of probiotics and host status. Moreover, traditional way of administering probiotics may not be able to produce required concentration of the metabolites to produce desired effect at the target sites in vivo. Therefore, to enhance in situ production of beneficial metabolites, the administration of either probiotic or specific prebiotic may be useful or these multifunctional metabolites may be given instead of probiotics as such but needs to be thoroughly investigated before implementation.
Physiological Effects of Metabiotics
Probiotics colonize, multiply and produce variety of bioactive substances “metabiotics,” accounting for their beneficial effects in gastrointestinal tract (GIT) diseases. These metabolites produced by probiotics help in maintaining homeostasis in the gut and enhance the growth of friendly bacteria that inhibit the conversion of procarcinogens into carcinogens by decreasing harmful enzyme levels such as nitroreductase, β-glucuronidase and β-glucosidase (Verma and Shukla, 2013; Figure 1 ‘1’). SCFAs also induce chemopreventive enzymes glutathione S transferase and Glutathione transferase pi (Scharlau et al., 2009; Johnson et al., 2012) and impart genetic stability to the colon cells (Clarke et al., 2012). Butyrate produced by fermentation of high amylose starch was reported to reduce the overall oxidative stress in gut and may also activate different procarcinogen metabolizing enzymes to aid in colon cancer prevention (Treptow-van et al., 1999). Butyrate acts as the preferred source of energy for colonocytes and has anti inflammatory and anticancerous properties, acetate percolates to peripheral tissues and could be metabolized in systemic areas (muscle) and generates ATP while propionate is transported to the liver via portal circulation (Hijova and Chmelarova, 2007). Acetate, a multifunctional SCFA plays significant role in regulating normal epithelial cell division, ileal motility, and colonic blood circulation (Hong et al., 2005). It was notable that cancer cell state was shifted from apoptosis to necrosis in an acidic extracellular pH created by exposure to SCFAs produced by probiotic Propionibacteria freudenreichii (Lan et al., 2007). Liong (2008) have shown that Bifidobacterium produced metabolites might interfere with the conversion of azoxymethane to its active carcinogenic form and subsequently lowered colon cancer risk. Interestingly, proteins derived from probiotic LGG, namely p75 and p40 have been reported to maintain gut homeostasis by regulating signaling mechanisms (Yan et al., 2007).
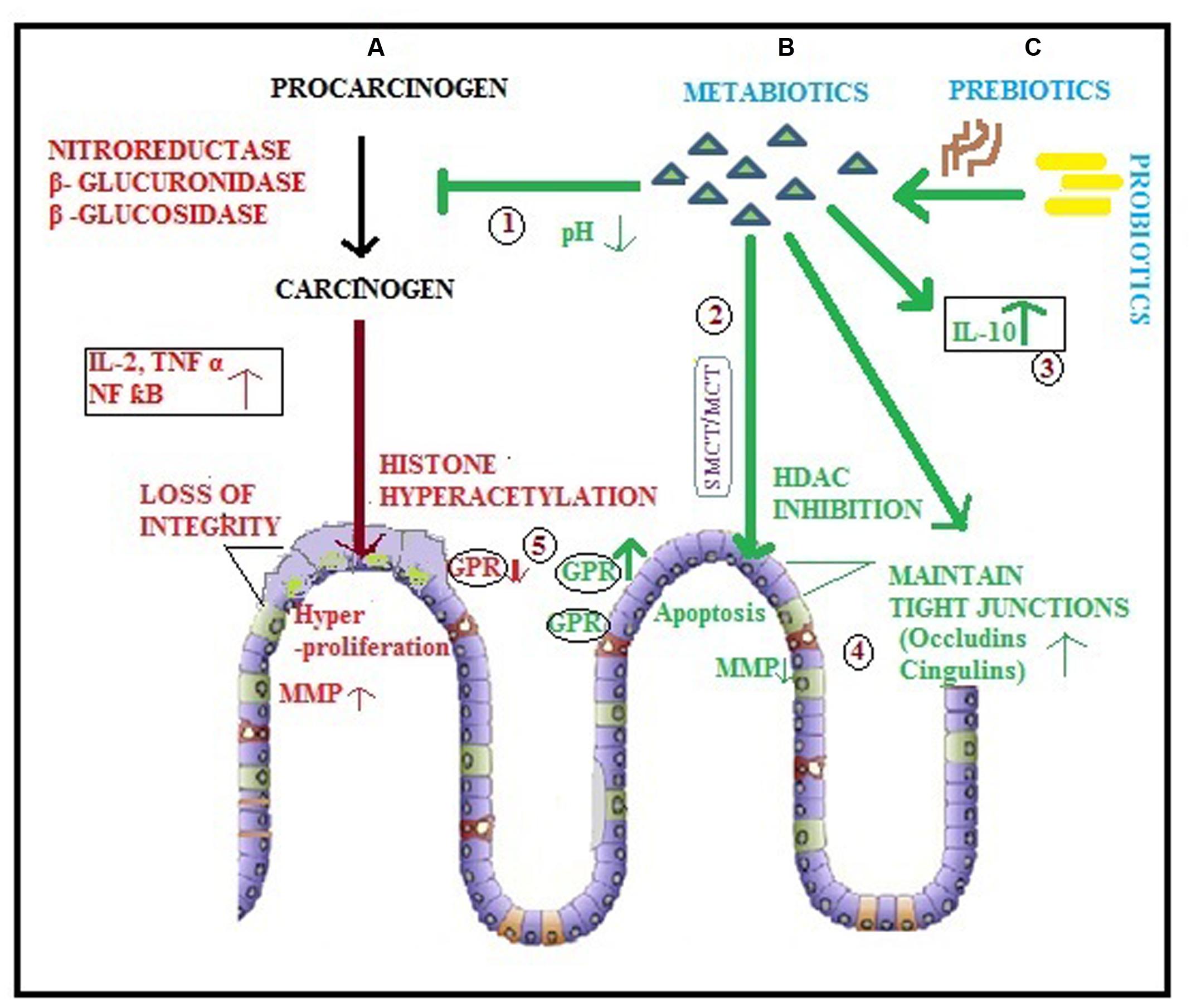
FIGURE 1. Diagrammatic representation of colorectal cancer and various modulatory potentials of metabiotics. (A) Procarcinogens are converted to carcinogens by enzymes leading to initiation of CRC which is accelerated by various factors such as inflammatory cytokines, genetic and epigenetic alterations (histone hyperacetylation) resulting into hyper proliferation of colonocytes that leads to metastasis due to lack of intestinal integrity and MMP production; (B) Metabiotics ( ) derived directly by probiotics (
) derived directly by probiotics ( ) or (C) produced as a result of prebiotic (
) or (C) produced as a result of prebiotic ( ) utilization by probiotic (
) utilization by probiotic ( ) may: (1) inhibit the conversion of procarcinogen to carcinogen by creating a low pH environment in colonic lumen and hindering the synthesis as well as activity of harmful enzymes; (2) act as HDAC inhibitors via SMCT/MCT transporters leading to enhanced apoptosis of cancerous cells; (3) modulate inflammation by augmenting the level of anti-inflammatory cytokines (IL-10); (4) maintain intestinal integrity by ameliorating the expression of tight junction proteins (occludins, cingulins) and inhibit MMP to impede metastasis of cancer cells; (5) enhance the expression of GPRs, the cell surface receptors.
) may: (1) inhibit the conversion of procarcinogen to carcinogen by creating a low pH environment in colonic lumen and hindering the synthesis as well as activity of harmful enzymes; (2) act as HDAC inhibitors via SMCT/MCT transporters leading to enhanced apoptosis of cancerous cells; (3) modulate inflammation by augmenting the level of anti-inflammatory cytokines (IL-10); (4) maintain intestinal integrity by ameliorating the expression of tight junction proteins (occludins, cingulins) and inhibit MMP to impede metastasis of cancer cells; (5) enhance the expression of GPRs, the cell surface receptors.
Short chain fatty acids stimulate overall health of the gut by improving mineral absorption, increasing mucosal weight, blood circulation and gut motility, thus preventing colitis which may gradually lead to cancer. Low pH environment created by SCFAs inhibits 7-α-Dehydroxylase and also renders free bile acid less soluble, hindering the generation of secondary bile acids (Lupton, 2000). Toxic metabolites produced in the human gut such as nitrates and oxides in response to inflammation have also been known to influence microbial populations and hence their metabolites formation (Bultman, 2014). Since, intestinal tract is constantly exposed to potentially harmful substances and toxic metabolites produced by them, it requires balancing by protective metabolites produced by the probiotics suggesting that an optimum balance between various metabolites ensures a healthy gut.
Metabolites Lead to Epigenetic Alterations
The term “epigenetics” implies modification of the expression of certain genes induced by environmental factors without changing their DNA sequences, such as histone modifications (methylation, phosphorylation, deacetylation), DNA methylation, chromatin remodeling as well as mechanisms mediated by non-coding RNA molecules. Both initiation and progression of colon cancer include breach in epigenetic regulation of cell growth rate, differentiation and apoptosis which are influenced to a great degree by environmental factors (Coppedè, 2014). The epigenetic alterations paralleled with genetic mutations may lead to transformation of normal colonic mucosa to colorectal carcinoma. Histone deacetylases, a class of critical enzymes cause post-translational modifications of histone proteins leading to altered chromatin structure, influencing the binding of transcription factors and silencing tumor suppressor genes (Dokmanovic and Marks, 2005; Bolden et al., 2006). Further, it has been documented that overexpression of histone deacetylase is linked with severity of various cancers such as 62% expression in adenomas and 82% in colorectal carcinomas compared with 53% in normal human tissue (Ashktorab et al., 2009). Interestingly, these epigenetic changes are potentially reversible paving way for the discovery of a new class of drugs called “epigenetic drugs” or histone deacetylase inhibitors (HDACi) such as SCFAs (butyrate, acetate, propionate, and valerate) produced by good bacteria, the Probiotics (Licciardi et al., 2010; Kim et al., 2014; Figure 1 ‘2’). These HDACi relax the chromatin giving access to transcription factors to activate the key target genes involved in anti cancerous effect as well as modulates the immune response by generating T regulatory cells (Arpaia et al., 2013; Park et al., 2015). The supernatant of human fecal slurry and pectin has been reported to have potent HDAC in inhibitory effects on colon cancer, due to production of butyrate (Waldecker et al., 2008) but the epigenetic effect of butyrate varies with type of dietary lipid intake and more pronounced effect was observed with fish oil compared with corn oil (Crim et al., 2008). Louis and Flint (2009) have reported Faecalibacterium prausnitzii and Eubacterium rectal to be the major butyrate producers in the gut. Furthermore, butyrate has been found to have potential to differentiate between cancer cells and normal cells in order to exert epigenetic effects and inhibits growth of colon cancer cells mainly by de-repressing epigenetically silenced genes such as p21 and the anti-apoptotic protein Bcl-2 but also promotes the growth of normal cells by regulating these genes (Zhong et al., 2014). Butyrate, the most studied SCFA, has been attributed in induction of apoptosis in colon cancer cells due to its ability to convert procaspase 3 to active caspase 3 (Medina et al., 1997). Furthermore, it has been found to induce both cell cycle arrest as well as terminal differentiation in HT-29 cells owing to the down regulation of cell cycle regulator CB1 mRNA expression and induction of cell cycle inhibitor p21 (Archer et al., 1998; Hinnebusch et al., 2002). To conclude, SCFAs offer a new strategy to fight colon cancer by acting as HDACi in order to modify all major functions such as induction of apoptosis, cell cycle arrest and differentiation. Since aberrant gene transcription due to epigenetic alterations is often the underlying cause of the onset of chronic inflammation and cancer, thus the epigenetic regulation by metabolites produced by probiotic microorganisms (Kumar et al., 2013) emerges as an explanation to the various anticancerous effects and suggests metabiotics as an alternative strategy for both cancer prevention and therapy or butyrate producers in the gut could be the target for enhancing in situ production of butyrate and its epigenetic benefits.
Antimutagenic Properties Associated with Metabiotics
Antimutagenic potential of probiotics has primarily been attributed to binding of live bacteria with mutagens but now there is increasing evidence that even cell free supernatants might either scavenge the reactive carcinogen intermediates or influence the ability of carcinogen activating/deactivating enzymes (Treptow-van et al., 1999; Wollowski et al., 2001). Supernatants of probiotic cultures supplemented with prebiotics were reported to substantially reduce the genotoxicity of human fecal slurry (Burns and Rowland, 2004). Similarly, metabolites produced in soymilk fermented by mixed culture of Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium infantis, Bifidobacterium longum have also been found to exhibit high antimutagenicity against mutagen 3, 2-dimethyl-4-amino-biphenyl (Hsieh and Chou, 2006). Further, it was observed that colon cells treated with supernatant of inulin fermentation by lactic acid bacteria elevated Glutathione S-transferase-pi [(GST)- pi] activity, a chemopreventive enzyme against mutagens (Scharlau et al., 2009). The enhanced antimutagenic activity of cell free supernatant of Lactobacillus plantarum KLAB21 was attributed to three secretory glycoproteins 16, 11, and 14 kD (Rhee and Park, 2001). In addition, exopolysaccharides (EPSs) produced by Lactobacillus plantarum 301102 have also been shown to inactivate tryptophan pyrolysate-1, another known mutagen (Tsuda et al., 2008). Nadathur et al. (1995) prepared acetone extracts of yogurt and showed that active metabolites responsible for antigenotoxic activity in the extract vary with type of carcinogen as were found to be different for methylnitronitrosoguanidine (MNNG) and 3,2-dimethyl-4 aminobiphenyl (DMAB). In another interesting study, acetone extracts prepared from either non-fermented milk/fermented milk or L. acidophilus grown in MRS broth have been found to exhibit variable antigenotoxic activity as these metabolites prevented DNA damage in colonocytes to different degrees after MNNG treatment. These metabolites have been found to exert more profound antigenotoxic effect compared with cellular components of LAB like peptidoglycan or cytoplasmic fractions (Wollowski, 1998). However, mutagen binding potential of probiotics (lactobacilli and bifidobacteria) has been found to be associated with cellular components such as peptidoglycans and polysaccharides but the antimutagenic activity very much depends upon the growth phase, cell number of bacterial strain and mutagen type (Raman et al., 2013). Butyrate binds irreversibly to mutagen and is responsible for the antimutagenic potentials exhibited by probiotic microorganisms, lactobacilli and bifidobacteria (Stein et al., 1996; Lankaputhra and Shah, 1998). Further, butyrate enhances the production of (GST) – pi in colon cancer cells and has also been found to inhibit the genotoxic activity of nitrosamides and hydrogen peroxide production in human colon cells as well as in experimental model (Wollowski et al., 2001; Clarke et al., 2012). In addition to butyrate; acetate, another SCFA has also been documented to have antimutagenic properties (Christophersen et al., 2013). Taken together, antimutagenic potential of metabiotics has been linked with butyrate, acetate, some glycoproteins, peptidoglycans, and polysaccharides. It can be suggested that the metabiotics may exhibit antimutagenicity by various mechanisms such as irreversible binding to mutagens, inhibiting carcinogen activating enzymes, inducing chemopreventive enzymes, preventing DNA damage and offers them unique opportunity to be nominated as effective prophylactic agents against colorectal cancer.
Immunomodulatory Potential
Inflammation and cancer are complementary to each other and the correlation between these was first observed by Virchow more than a century ago by observing the presence of leukocytes in neoplastic tissues (Francescone et al., 2014). About 20–25% of all cancers occur as a result of chronic inflammation due to its involvement both in tumor initiation and progression, e.g., inflammatory bowel disease leads to colon cancer, hepatitis to hepatocellular carcinoma and H. pylori-induced gastritis to gastric cancer (Grivennikov et al., 2010). Chronic and uncontrolled inflammation facilitates cancer development either due to epigenetic alterations, genomic instability, enhanced proliferation, invasiveness or resistance to apoptosis (Kundu and Surh, 2012). In fact, tumor itself can recruit pro-inflammatory cytokines, immune cells and growth factors in its microenvironment to enhance cancer development and create metastatic niche for secondary growth resulting into “Tumor elicited inflammation” (Coussens et al., 2013). Interestingly, tumor expression of oncogene k-ras upregulates the pro-inflammatory cytokine IL-8, resulting in immune cell infiltration, enlarged tumor and enhanced angiogenesis in colon cancer (Sparmann and Bar-Sagi, 2004). Moreover, damaged or leaky epithelial junctions in colon cancer too encourage vigorous inflammatory response that in turn accelerates tumor initiation and progression (Grivennikov et al., 2012).
Notably, degree of mucosal inflammation has been correlated with levels, type and proportion of various SCFAs. These metabiotics play important role in optimizing immune response generated by intestinal epithelial cells in order to prevent chronic inflammation. SCFAs maintain a balance by inhibiting inflammatory cytokines such as IL-2, IL-6, and TNF-α as well as stimulating the anti inflammatory cytokine IL-10 (Zhong et al., 2014; Figure 1 ‘3’). SCFAs are known to regulate immune function possibly by monitoring neutrophils, antigen presenting cells, T-cell differentiation and histone deacetylase inhibition. In addition, SCFAs may also interact with G-protein-coupled receptors in the gut and affect inflammatory signaling by regulating chemotaxis of neutrophils and other immune cells such as dendritic cells and macrophages (Kimura et al., 2011; Vinolo et al., 2011).
In addition to SCFAs, various bio active factors derived from probiotics are EPSs, peptidoglycan, single layer proteins, lipoteichoic acids, conjugated linoleic acids and peptides that have also been found to regulate immune system (Matsuguchi et al., 2003; Lahtinen, 2012). Zakostelska et al. (2011) emphasized that even heat killed probiotics have equally effective anti inflammatory properties as live probiotics and suggested to adopt former as the safer option for treating chronic gastrointestinal inflammation. In nutshell, it can be stated that the efficacy of various metabiotics in modulating inflammation has been established using cell lines, murine models and even human trials mainly by upregulating the synthesis of antiinflammatory cytokines and down regulating the inflammatory mediators (Table 1).
Antiproliferative and Apoptotic effects
Both in vitro and in vivo studies have demonstrated the significant role of bioactive components in cell free supernatants of probiotic strains, e.g., SCFAs (mainly butyrate) in direct inhibition of colon cancer cell growth by various mechanisms (Table 2). The most striking feature of butyrate is that it can differentiate phenotypically between normal and cancer cells as it induces differentiation, apoptosis and inhibits angiogenesis in cancerous cells but regulates the proliferation of normal cells (Davis and Milner, 2009).
In addition to butyrate other SCFAs, propionate and acetate have also been reported to enhance apoptosis by affecting the mitochondrial trans-membrane potential, generating ROS, caspase-3-processing and causing condensation of nuclear chromatin in colon cancer cells (Jan et al., 2002; Lan et al., 2007; Hosseini et al., 2011). These SCFAs (propionate and acetate) are however required in higher concentrations to induce apoptosis and show different metabolic effects compared with butyrate (Topping and Clifton, 2001). It has been suggested that propionate also inhibits cancer other than in the gut as its being easily transported to liver via portal circulation (Bindels et al., 2012). Surprisingly, butyrate and propionate have also been found to induce autophagy rather than apoptosis in colon cancer cells due to some mitochondrial defects, revealing the reason behind mixed results observed with SCFA treatment. Further, this could result into the development of resistance in colon cancer cells suggesting the use of autophagic inhibitors to enhance the apoptotic effect of SCFAs (Tang et al., 2011a; Adom and Nie, 2013).
Apart from SCFAs, medium chain fatty acids such as capric, caprylic, and caproic acids have also been reported to be cytotoxic against colon cancer cell lines (Narayanan et al., 2015). Additionaly; long chain fatty acids (CLA) have been found to possess potent anticancerous effects. A commercially available probiotic mixture VSL#3 and few strains of Bifidobacterium have been shown to produce CLA, a potent inducer of apoptosis, COX-2 inhibitor and activator of peroxisome proliferator-activated receptor gamma (PPAR γ) in colon cancer and have also been able to mitigate intestinal tumorigenesis in Apc-/+ mice (Davis and Milner, 2009; Urbanska et al., 2009; Uccello et al., 2012).
Kim et al. (2008) reported that CFS of Bifidobacterium adolescentis SPM0212 was more potent in inhibiting growth of colon cancer cells than whole cells or heat killed cells. CFS of Enterococcus lactis IW5 was also found to induce apoptosis in various cancer cell lines including Caco-2 and HT-29 (Nami et al., 2015). Similarly, CFS of probiotics (Lactobacillus plantarum A7 and LGG) were more effective in inhibiting cancer cell growth mainly due to organic acids produced by them compared with E. coli supernatant (Sadeghi-Aliabadi et al., 2014). In addition to organic acids, EPS (released EPSs, cell bound EPSs) of Lactobacillus rhamnosus ATCC 9595 also showed antiproliferative effect against HT-29 cells (Kim et al., 2006). Recently, it was highlighted that the upregulation of early apoptosis gene markers cfos and cjun constitutes the major mechanism involved in cytotoxicity of cell free supernatants of probiotic cultures toward colon cancer HT-29 and HCT116 cells (Shyu et al., 2014). Further, it has been documented that supernatants of Lactobacillus delbrueckii were able to induce apoptosis through intrinsic pathway and downregulation of Bcl-2 along with arrest of colon cancer cells in G1 phase (Wan et al., 2014). Conditioned medium of Bacillus polyfermenticus (BPCM) effectively suppressed the growth of colon cancer cells (HT-29, DLD-1, and Caco-2) and reduced their colony formation on soft agar due to decreased cyclin D1, ErbB2, and ErbB3 [epidermal growth factor receptors (EGFRs)] expression and inhibited tumor growth in mice because of heat stable bacterial proteins (Ma et al., 2010). LGG homogenate and cytoplasm extracts have been found to reduce the percentage of colon cancer cell viability to almost 55% in DLD-1 colon cancer cell lines; while heat killed LGG reduced the viability of HT-29 and Caco-2 to 62.7 and 73% respectively (Choi et al., 2006; Orlando et al., 2009). Very recently, it was reported that metabolites produced by new potential probiotic strains (Pediococcus pentosaceus and Weissella confusa) were cytotoxic against Caco-2 cell lines (Sevda et al., 2015). Based on these inferences, it is reasonable to postulate that metabolites produced by probiotics possess antiproliferative activity due to their ability to induce differentiation, arrest cell cycle and upregulate the proapoptotic mechanisms in cancer cells which in turn depends upon the phenotypic state of cells, probiotic strain and the active component involved.
Intestinal Integrity and Metastasis Inhibition
Leaky gut syndrome (LGS) refers to increase in intestinal permeability and occurs either due to irregular expression of certain tight junction proteins or epithelial barrier dysfunction. LGS plays important role in promoting inflammation and cancer development by encouraging tissue invasion and metastasis. It could be both cause and effect of gastrointestinal ailments like inflammatory bowel disease and even colon cancer (Wang et al., 2005). Probiotics contribute to intestinal homeostasis due to the production of metabiotics (such as SCFAs and polyphosphates) which exert protective gut barrier effects by regulating induction, maintenance and assembly of tight junction proteins, production of mucin and/or gastrointestinal peptides employed in barrier function (Rao and Samak, 2013).
Butyrate, the major probiotic metabolite in CRC not only acts as HDAC inhibitor and regulator of apoptosis but also promotes intestinal epithelial barrier integrity by regulating the expression of various tight junction proteins such as occludins and cingulin (Bordin et al., 2004; Peng et al., 2009; Figure 1 ‘4’). Caco-2 cells when treated with cell-free supernatant of B. lactis-420 showed improved integrity of tight junctions (Ewaschuk et al., 2008; Putaala et al., 2008). A decrease in colonic permeability was also recorded by oral administration of bioactive factors from B. infantis in murine colitis model (Segawa et al., 2011). Metabolites produced by B. infantis Y1 increased ZO-1 and occludin expression in T cells (Rao and Samak, 2013). It has been observed that surface components of probiotic microorganisms such as pilus, lipoteichoic acids, and mucus binding proteins interact directly with both epithelial and immune cells of the host to maintain gut barrier homeostasis. In addition, Patel et al. (2012) reported an interesting observation that even heat-killed LGG regulated intestinal barrier in mice with abnormal gut. Recently p40, a soluble protein produced by LGG has been found to enhance the chemical barrier by activating EGFR which in turn stimulates goblet cells to produce mucin (Wang et al., 2014). Both p40 and p75 protect not only the epithelial tight junctions against oxidative stress through MAP kinase signaling pathways but also inhibit the redistribution of tight junction proteins induced by hydrogen peroxide (Seth et al., 2008). Cell-free conditioned media of LGG has also been observed to induce the production of cytoprotective heat shock proteins (hsp25, hsp72) by intestinal epithelial cells which promote tight junctions (Thomas and Versalovic, 2010). LGG supernatant is capable of reversing the epithelial barrier dysfunction induced by alcohol, both in vitro as well as in vivo and have been found to regulate levels of claudin-1, ITF, P-gp and cathelin-related antimicrobial peptide to re- establish intestinal barrier functions (Wang et al., 2011, 2012). Competence and sporulation factor (CSF), a quorum-sensing peptide produced by Bacillus subtilis JH642 has the potential to induce hsp25, hsp27, and hsp72 in intestinal epithelial cells (Fujiya et al., 2007). It has also been observed that proteins derived from Clostridium butyricum CGMCC0313-1 regulate the intestinal barrier function by enhancing the expression of protein A20 in HT-29 cells (Song et al., 2012).
Metastasis is the major cause of mortality in cancer patients. Metastasis or excessive proliferation and invasion of cancerous cells occurs due to degradation of extracellular matrix by enzyme matrix metalloproteinases (MMPs) and MMP-9 levels have been significantly correlated with the stage of colon cancer (Baker and Leaper, 2002; Leeman et al., 2003; Mook et al., 2004). Colorectal cancer metastasis occurs due to loss of tight junction proteins such as occludin, ZO-1 and clodulin-4 (Kaihara et al., 2003; Tobioka et al., 2004; Ohtani et al., 2009). Escamilla et al. (2012) have demonstrated that metabolites produced by L. casei and LGG potentially interfere with metastatic process in colorectal cancer by decreasing MMP-9 activity and increasing the levels of ZO-1 in HCT-116 cells. Butyrate hindered the activity of MMPs due to activation of tissue inhibitor matrix metalloproteinase (TIM) 1- and 2 (Adom and Nie, 2013). Another study observed that supernatants of Lactobacillus delbrueckii ameliorated cell invasion in colon cancer cells by decreasing MMP-9 activity (Wan et al., 2014). Further, it was revealed that fermented products of synbiotic (inulin and oligofructose with LGG and/or Bifidobacterium lactis BB12) inhibited cell invasion both in vitro and in experimentally induced colon carcinogenesis in rats (Pool-Zobel, 2005; Pool-Zobel and Sauer, 2007). In nutshell, it can be stated that metabolites produced by probiotics offer a safe and effective tool for combating colon cancer by their ability to maintain the intestinal integrity; regulating tight junctions and to inhibit metastasis by decreasing MMP activity.
Receptors and Transporters Associated with Biological Actions of Metabiotics
Continuous extensive research on the interaction of bacterial metabolites especially SCFAs with host has advanced toward the molecular approach of butyrate and other SCFAs toward their modulatory mechanisms in colon, both extracellularly (via activating specific receptors) and intracellularly (via transportation mediated by specialized transporters) (Ganapathy et al., 2013). SCFAs synthesized by friendly bacteria in the lumen of colon could be absorbed passively or carried via transporters like SMCT1/SLC5a8 and MCT1/SLC16a1 into colonocytes. SMCT1 and MCT1 are sodium-coupled monocarboxylate transporter and H+-coupled transporter respectively. Despite the presence of SMCT1 along entire length of the large intestine, MCT1 found in apical membrane of colonocytes accounts for the major transport of SCFAs and could be transported either by apical or basolateral membrane of colonocytes. MCT1 is also expressed on the apical membrane of dendritic cells, kidney cells, and even brain cells and is downregulated during early adenoma formation accompanied with enhanced expression of the high affinity glucose transporter, GLUT1 and reduced expression of the low affinity glucose transporter GLUT2 (Lambert et al., 2002).
Undissociated SCFAs can passively diffuse across the apical membrane of epithelial cells lining but due to acidic intestinal pH; most of the SCFAs are dissociated and have to be transported actively via transporters (Sellin, 1999). Scientists have proposed three fundamental mechanisms for the active transport of SCFAs including SCFA-HCO3- exchange, electroneutral SCFA anion cotransport with cations mediated by MCT1 and electrogenic sodium-dependent transport carried out by SMCT1 (Besten et al., 2013). Gonçalves et al. (2011) have reported that butyrate is transported faster from apical membrane of colonocytes via SMCT-1 compared with acetate and propionate and essential for histone deacetylase inhibition (Li et al., 2003; Gopal et al., 2004; Miyauchi et al., 2004). SCFAs not only enter colonocytes but also the immune cells via SMCT1 thereby hindering the inflammatory signal by blocking the generation of dendritic cells from bone marrow stem cells (Singh et al., 2010).
Short chain fatty acids are not fully consumed by colonocytes but have to be transported actively across their basolateral membrane via cation-SCFA anion symport or SCFA -HCO3- antiport and is proposed to be mediated by MCT4 (SLC16A3) and MCT5 (SLC16A4) (Gill et al., 2005). SCFAs can also enter other organs such as liver and muscle via portal circulation. The transporters for butyrate and propionate across sinusoidal membrane of liver cells have been identified as the organic anion transporters OAT7 and OAT2 respectively (Shin et al., 2007; Islam et al., 2008).
Receptors present on different cells play important role in pathophysiology of various diseases. GPR 109A and GPR43/free fatty acid receptor (FFA2) are cell- surface G-protein-coupled receptors expressed on the colonic epithelium, adipose tissue, and immune cells which are activated by SCFAs (Blad et al., 2012; Figure 1 ‘5’). GPR41/free fatty acid receptor 3 (FFA3) is another receptor found on colonocytes, adipocytes, spleen, lymph nodes, bone marrow, renal smooth muscle cells, enteric neuronal cells, and pancreatic cells (Xiong et al., 2004; Kim et al., 2014). Butyrate and niacin (Vitamin B3) are the known potential agonists for GPR109A while acetate and propionate are for GPR 43 receptors (Bindels et al., 2013). FFA2 has higher affinity for both acetate and propionate and is present primarily in immune cells whereas FFA3 has greater affinity for butyrate and propionate but has highest expression in adipose tissue (Brown et al., 2003; Le Poul et al., 2003; Nilsson et al., 2003). However, FFA2 suppresses inflammation in healthy colon as reduced expression of GPR43 has been found both at the primary site of tumor as well as the metastatic sites in CRC. Further, cancer cells with increased expression of GPR43 were found to be more susceptible for apoptosis and cell cycle arrest caused by SCFAs (Tang et al., 2011b). Acetate, has been able to impart protection to germ-free mice from dextran sulfate sodium (DSS) induced colitis by acting as a GPR43 Singh et al. (2014). The expression of neuropilin-1 (receptor for VEGF) has been inversely correlated with levels of SCFAs in colon and has been shown to be reduced by butyrate in colon cancer cell lines (Yu Danny et al., 2011).
GPR109A and SLC5A8 (SMCT1) both aid significantly in enhancing apoptosis induction potential of butyrate which implies both extracellular as well as intracellular regulation (Andoh et al., 2003; Thangaraju et al., 2008). GPR109A is responsible for enabling macrophages and dendritic cells to differentiate T regulatory cells and induce production of anti- inflammatory cytokines as evident by the ability of induction of expression of IL-18 in colonic epithelium by butyrate and niacin in WT mice (possessing GPR109A) but inability to do so in case of Niacr1-/- mice (lacking GPR109A) (Singh et al., 2014). This study has shown that animals deficient in GPR109A are more susceptible to both inflammation-induced and mutated Apc driven colon carcinogenesis. Cresci et al. (2010) reported that the levels of SLC5A8 mRNA and GPR109A mRNA in colon were markedly reduced in germ-free mice but restored with recolonization of the gut microflora by maintaining conventional conditions for 3–4 weeks as gut bacteria play important role in the expression of both receptors and transporters of SCFAs. Moreover, optimal dietary fiber intake increases the concentration of SCFAs in the gut lumen leading to entry of butyrate and other HDAC inhibitors into the cell even without SLC5A8 via direct diffusion or by other low affinity monocarboxylate transporters such as SLC16A1. But SLC5A8 becomes indispensable in case of low dietary fiber intake, as SCFAs in low concentrations will not be able to diffuse or enter the cell via low affinity transporters. However, GPR109A and GPR43 are essential for extracellular functions of SCFAs in the presence of both, optimal as well as low dietary fiber intake.
The immune modulating activity of either probiotic or metabiotic is thought to be due to binding of SCFAs to GPRs, that helps in recruitment of immune cells, activation of effector T cells and production of cytokines and chemokines (Sina et al., 2009; Smith et al., 2013). SCFAs have been extensively involved in the modulation of the expression of both receptors and transporters associated with various cells which hold paramount importance in pathogenesis of CRC. Thus, it can be stated that the interactions between SCFAs and these receptors/transporters need to be explored to reveal the molecular mechanism of action of metabiotics.
Future Directions
Although, it may take a while to unravel and utilize the remarkable attributes associated with metabiotics against colon cancer but it seems quite possible due to increasingly overwhelming studies emerging in this direction such as commercialized metabiotics, ‘Hylak Forte’ (Lactic acid and SCFAs) and ‘Zakofalk’ (Inulin and butyrate) derived from multiple probiotics to promote general health and to treat moderate inflammatory conditions respectively (Belousova et al., 2005; Roda et al., 2007). Moreover, probiotic strains should be screened for specific potential metabiotics in their cell free supernatants as there is much ground to be covered yet. However, delivery of metabiotics to the right place (i.e., distal colon) however remains a challenge in CRC which could be met by devising new strategies of targeted delivery. The fact that metabiotics affect gene expression even at translational level by altering miRNAs has added another dimension to their mechanistic study but warrants detailed investigation. Though considerable amount of in vitro work regarding the undeniable role of metabiotics in colon cancer has been accomplished but intensive in vivo studies followed by placebo controlled, double blind clinical trials need to be performed.
Conclusion
Probiotics are known to exert various health benefits such as immunomodulation, inactivation of carcinogens and maintenance of gut integrity but the present review argues the notion that live probiotic strains are essential for the beneficial effects associated with them in colon cancer. Moreover, adverse effects associated with probiotics even in certain normal/or immunosupressed individuals limit their use. Thus, attempts are being made to have alternative safer and effective biointerventions such as metabiotics and to enhance their in situ production by selectively optimizing the growth conditions for beneficial probiotics. Moreover, metabiotics, the multifunctional metabolites produced by probiotics have been found to possess remarkable antimutagenic, antiinflammatory, antiproliferative and even antimetastatic potentials attributed to their epigenetic effects in one or other way, and may target CRC at different stages. This information suggests that the administration of metabiotics may attenuate both the adverse effects as well as worry of maintaining the viability of probiotics. Thus, metabiotics independently or in conjunction with other approaches could be considered as a potent prophylactic/or therapeutic modulator for colon cancer or other diseases in the post-antibiotic era.
Author Contributions
Collected and compiled information: MS; wrote the paper: MS, GS; edited: GS.
Funding
Financial assistance provided by Indian Council of Medical Research is highly acknowledged.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers FT and MM and handling Editor declared their shared affiliation and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
References
Adom, D., and Nie, D. (2013). “Regulation of autophagy by short chain fatty acids in colon cancer cells,” in Autophagy - A Double-Edged Sword - Cell Survival or Death?, ed. Y. Bailly (Rijeka: INTECH). doi: 10.5772/54999
Ahn, J., Sinha, R., Pei, Z., Dominianni, C., Wu, J., Shi, J., et al. (2013). Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 105, 1907–1911. doi: 10.1093/jnci/djt300
Aktipis, C. A., and Nesse, R. M. (2013). Evolutionary foundations for cancer biology. Evol. Appl. 6, 144–159. doi: 10.1111/eva.12034
Andoh, A., Tsujikawa, T., and Fujiyama, Y. (2003). Role of dietary fiber and short-chain fatty acids in the colon. Curr. Pharm. Des. 9, 347–358. doi: 10.2174/1381612033391973
Archer, S., Meng, S., Shei, A., and Hodin, R. A. (1998). p21(WAF1) is required for butyrate mediated growth inhibition of human colon cancer cells. Proc. Natl. Acad. Sci. U.S.A. 95, 6791–6796. doi: 10.1073/pnas.95.12.6791
Arpaia, N., Campbell, C., Fan, X., Dikiy, S., van der Veeken, J., deRoos, P., et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. doi: 10.1038/nature12726
Ashktorab, H., Belgrave, K., Hosseinkhah, F., Brim, H., Nouraie, M., Takkikto, M., et al. (2009). Global histone H4 acetylation and HDAC2 expression in colon adenoma and carcinoma. Dig. Dis. Sci. 54, 2109–2117. doi: 10.1007/s10620-008-0601-7
Baker, E. A., and Leaper, D. J. (2002). Measuring gelatinase activity in colorectal cancer. Eur. J. Surg. Oncol. 28, 24–29. doi: 10.1053/ejso.2001.1179
Bassaganya-Riera, J., Viladomiu, M., and Pedragosa, M. (2012a). Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS ONE 7:e31238. doi: 10.1371/journal.pone.0031238
Bassaganya-Riera, J., Viladomiu, M., Pedragosa, M., Simone, C. D., and Hontecilla, R. (2012b). Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS ONE 7:e34676. doi: 10.1371/journal.pone.0034676
Belousova, E. F., Nikitina, Y. V., Mishurovskaya, N. C., and Zlatkina, A. R. (2005). Possibilities of microbial metabolite preparations for intestinal microbiota restoration. Consilium Medicum. 7, 9–13.
Berer, K., Mues, M., Koutrolos, M., Rasbi, Z. A., Boziki, M., Johner, C., et al. (2011). Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. doi: 10.1038/nature10554
Bertkova, I., Hijova, E., Chmelarova, A., Mojzisova, G., Petrasova, D., Strojny, L., et al. (2010). The effect of probiotic microorganisms and bioactive compounds on chemically induced carcinogenesis in rats. Neoplasma 57, 422–428. doi: 10.4149/neo_2010_05_422
Besten, G. D., Eunen, K. V., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Bindels, L. B., Dewulf, E. M., and Delzenne, N. M. (2013). GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 34, 226–232. doi: 10.1016/j.tips.2013.02.002
Bindels, L. B., Porporato, P., Dewulf, E. M., Verrax, J., Neyrinck, A. M., Martin, J. C., et al. (2012). Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 107, 1337–1344. doi: 10.1038/bjc.2012.409
Birt, D. F., and Phillips, G. J. (2014). Diet, genes, and microbes: complexities of colon cancer prevention. Toxicol. Pathol. 42, 182–188. doi: 10.1177/0192623313506791
Blad, C. C., Tang, C., and Offermanns, S. (2012). G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 11, 603–619. doi: 10.1038/nrd3777
Bolden, J. E., Peart, M. J., and Johnstone, R. W. (2006). Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5, 769–784. doi: 10.1038/nrd2133
Boleij, A., and Tjalsma, H. (2013). The itinerary of Streptococcus gallolyticus infection in patients with colonic malignant disease. Lancet Infect. Dis. 13, 719–724. doi: 10.1016/S1473-3099(13)70107-5
Bordin, M., D’Atri, F., Guillemot, L., and Citi, S. (2004). Histone deacetylase inhibitors upregulate the expression of tight junction proteins. Mol. Cancer Res. 2, 692–701.
Bourdichon, F., Berger, B., Casaregola, S., Frisvad, J. C., Gerds, M. L., Hammes, W. P., et al. (2012). Safety demonstration of microbial food cultures in fermented food products. Bull. Int. Dairy Fed. 455, 7–8.
Breuer, R. I., Buto, S. K., Christ, M. L., Bean, J., Vernia, P., Paoluzi, P., et al. (1991). Rectal irrigation with short-chain fatty acids for distal ulcerative colitis. Preliminary report. Dig. Dis. Sci. 36, 185–187. doi: 10.1007/BF01300754
Brown, A. J., Goldsworthy, S. M., Barnes, A. A., Eilert, M. M., Tcheang, L., Daniels, D., et al. (2003). The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319. doi: 10.1074/jbc.M211609200
Bultman, S. J. (2014). Emerging roles of the microbiome in cancer. Carcinogenesis 35, 249–255. doi: 10.1093/carcin/bgt392
Burns, A. J., and Rowland, I. R. (2004). Antigenotoxicity of probiotics and prebiotics on faecal water-induced DNA damage in human colon adenocarcinoma cells. Mutat. Res. 13, 233–243. doi: 10.1016/j.mrfmmm.2004.03.010
Cabana, M. D., Shane, A. L., Chao, C., and Oliva-Hemker, M. (2006). Probiotics in primary care pediatrics. Clin. Pediatr. (Phila). 45, 405–410. doi: 10.1177/0009922806289614
Cancer Facts and Figures (2016). Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society.
Choi, S. S., Kim, Y., Han, K. S., You, S., Oh, S., and Kim, S. H. (2006). Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 42, 452–458. doi: 10.1111/j.1472-765X.2006.01913.x
Christophersen, C. T., Petersen, A., Licht, T. R., and Conlon, M. A. (2013). Xylo-oligosaccharides and inulin affect genotoxicity and bacterial populations differently in a human colonic simulator challenged with soy protein. Nutrients 5, 3740–3756.
Clarke, J. M., Young, G. P., Topping, D. L., Bird, A. R., Cobiac, L., Scherer, B. L., et al. (2012). Butyrate delivered by butyrylated starch increases distal colonic epithelial apoptosis in carcinogen-treated rats. Carcinogenesis 33, 197–202. doi: 10.1093/carcin/bgr254
Colin, H., Francisco, G., Gregor, R., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Coppedè, F. (2014). Epigenetic biomarkers of colorectal cancer: focus on DNA methylation. Cancer Lett. 342, 238–247. doi: 10.1016/j.canlet.2011.12.030
Cousin, F. J., Jouan-Lanhouet, S., Dimanche-Boitrel, M. T., Corcos, L., and Jan, G. (2012). Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1human gastric cancer cells. PLoS ONE 7:e31892. doi: 10.1371/journal.pone.0031892
Coussens, L. M., Zitvogel, L., and Palucka, A. K. (2013). Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339, 286–291. doi: 10.1126/science.1232227
Cresci, G. A., Thangaraju, M., Mellinger, J. D., Liu, K., and Ganapathy, V. (2010). Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 14, 449–461. doi: 10.1007/s11605-009-1045-x
Crim, K. C., Sanders, L. M., Hong, M. Y., Taddeo, S. S., Turner, N. D., Chapkinet, R. S., et al. (2008). Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenes 29, 1415–1420. doi: 10.1093/carcin/bgn144
Cuevas-Ramos, G., Petit, C. R., Marcq, I., Boury, M., Oswald, E., and Nougayrede, J. P. (2010). Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 107, 11537–11542. doi: 10.1073/pnas.1001261107
Davis, C. D., and Milner, J. A. (2009). Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem. 20, 743–752. doi: 10.1016/j.jnutbio.2009.06.001
Di Caro, S., Tao, H., Grillo, A., Elia, C., Gasbarrini, G., Sepulveda, A. R., et al. (2005). Effects of Lactobacillus GG on gene expression pattern in small bowel mucosa. Dig. Liver Dis. 37, 320–329. doi: 10.1016/j.dld.2004.12.008
Dirienzo, D. B. (2013). Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutr. Rev. 72, 18–29. doi: 10.1111/nure.12084
Dokmanovic, M., and Marks, P. A. (2005). Prospects: histone deacetylase inhibitors. J. Cell. Biochem. 96, 293–304. doi: 10.1002/jcb.20532
Escamilla, J., Lane, M. A., and Maitin, V. (2012). Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr. Cancer 64, 871–878. doi: 10.1080/01635581.2012.700758
Ewaschuk, J. B., Diaz, H., Meddings, L., Diederichs, B., Dmytrash, A., Backer, J., et al. (2008). Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1025–G1034. doi: 10.1152/ajpgi.90227.2008
Ewaschuk, J. B., Walker, J. W., Diaz, H., and Madsen, K. L. (2006). Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. 136, 1483–1487.
Fijan, S. (2014). Microorganisms with claimed probiotic properties: an overview of recent literature. Int. J. Environ. Res. Public Health 11, 4745–4767. doi: 10.3390/ijerph110504745
Fotiadis, C. I., Stoidis, C. N., Spyropoulos, B. G., and Zografos, E. D. (2008). Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J. Gastroenterol. 14, 6453–6457. doi: 10.3748/wjg.14.6453
Francescone, R., Hou, V., and Grivennikov, S. I. (2014). Microbiome, inflammation, and cancer. Cancer J. 20, 181–189. doi: 10.1097/PPO.0000000000000048
Fujiya, M., Musch, M. W., Nakagawa, Y., Hu, S., Alverdy, J., Kohgo, Y., et al. (2007). The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1, 299–308. doi: 10.1016/j.chom.2007.05.004
Furusawa, Y., Obata, Y., Fukuda, S., Endo, T. A., Nakato, G., Takahashi, D., et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. doi: 10.1038/nature12721
Ganapathy, V., Thangaraju, M., Prasad, P. D., Martin, P. M., and Singh, N. (2013). Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol. 13, 869–874. doi: 10.1016/j.coph.2013.08.006
Gill, R. K., Saksena, S., Alrefai, W. A., Sarwar, Z., Goldstein, J. L., Carroll, R. E., et al. (2005). Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol Cell Physiol. 289, C846–C852. doi: 10.1152/ajpcell.00112.2005
Gonçalves, P., Araújo, J. R., and Martel, F. (2011). Characterization of butyrate uptake by non transformed intestinal epithelial cell lines. J. Membr. Biol. 240, 35–46. doi: 10.1007/s00232-011-9340-3
Gopal, E., Fei, Y. J., Sugawara, M., Miyauchi, S., Zhuang, L., Martin, P., et al. (2004). Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J. Biol. Chem. 279, 44522–44532. doi: 10.1074/jbc.M405365200
Gratz, S. W., Mykkanen, H., and El-Nezami, H. S. (2010). Probiotics and gut health: a special focus on liver diseases. World J. Gastroenterol. 16, 403–410. doi: 10.3748/wjg.v16.i4.403
Grivennikov, S. I., Greten, F. R., and Karin, M. (2010). Immunity, inflammation, and cancer. Cell 140, 883–899. doi: 10.1016/j.cell.2010.01.025
Grivennikov, S. I., Wang, K., Mucida, D., Stewart, C. A., Schnabl, B., Jauch, D., et al. (2012). Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258.
Hijova, E., and Chmelarova, A. (2007). Short chain fatty acids and colonic health. Bratisl. Med. J. 108, 354–358.
Hinnebusch, B. F., Meng, S., Wu, J. T., Archer, S. Y., and Hodin, R. A. (2002). The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 132, 1012–1017.
Hong, Y. H., Nishimura, Y., Hishikawa, D., Tsuzuki, H., Miyahara, H., Gotoh, C., et al. (2005). Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099. doi: 10.1210/en.2005-0545
Hosseini, E., Grootaert, C., Verstraete, W., and Van de Wiele, T. (2011). Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 69, 245–258. doi: 10.1111/j.1753-4887.2011.00388.x
Hsieh, M. L., and Chou, C. C. (2006). Mutagenicity and antimutagenic effect of soymilk fermented with lactic acid bacteria and bifidobacteria. Int. J. Food Microbiol. 111, 43–47. doi: 10.1016/j.ijfoodmicro.2006.04.034
Hu, S., Dong, T. S., Dalal, S. R., Wu, F., Bissonnette, M., Kwon, J. H., et al. (2011). The microbe-derived short chain fatty acid butyrate targets mirna-dependent p21 gene expression in human colon cancer. PLoS ONE 6:e16221. doi: 10.1371/journal.pone.0016221
Islam, R., Anzai, N., Ahmed, N., Ellapan, B., Jin, C. J., Srivastava, S., et al. (2008). Mouse organic anion transporter 2 (mOat2) mediates the transport of short chain fatty acid propionate. J. Pharmacol. Sci. 106, 525–528. doi: 10.1254/jphs.SC0070291
Iyer, C., Kosters, A., Sethi, G., Kunnumakkara, A. B., Aggarwal, B. B., and Versalovic, J. (2008). Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NFkB and MAPK signalling. Cell. Microbiol. 10, 1442–1452.
Jacobi, C. A., Grundler, S., Hsieh, C.-J., Frick, J. S., Adam, P., Lamprecht, G., et al. (2012). Quorum sensing in the probiotic bacterium Escherichia coli Nissle 1917 (Mutaflor) – evidence that furanosyl borate diester (AI-2) is influencing the cytokine expression in the DSS colitis mouse model. Gut Pathog. 4:8. doi: 10.1186/1757-4749-4-8
Jan, G., Belzacq, A. S., Haouzi, D., Rouault, A., Métivier, D., Kroemer, G., et al. (2002). Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death. Differ. 9, 179–188. doi: 10.1038/sj.cdd.4400935
Jemal, A., Siegel, R., Xu, J., and Ward, E. (2010). Cancer statistics. CA Cancer J. Clin. 60, 277–300. doi: 10.3322/caac.20073
Johnson, C. H., Patterson, A. D., Idle, J. R., and Gonzalez, F. J. (2012). Xenobiotic metabolomics: major impact on the metabolome. Annu. Rev. Pharmacol. Toxicol. 52, 37–56. doi: 10.1146/annurev-pharmtox-010611-134748
Kaihara, T., Kawamata, H., Imura, J., Fujii, S., Kitajima, K., Omotehara, F., et al. (2003). Redifferentiation and ZO-1 reexpression in liver-metastasized colorectal cancer: possible association with epidermal growth factor receptor-induced tyrosine phosphorylation of ZO-1. Cancer Sci. 94, 166–172. doi: 10.1111/j.1349-7006.2003.tb01414.x
Kazemi sefat, N. A., Mohammadi, M. M., Hadjati, J., Talebi, S., Ajami, M., and Daneshvar, H. (2015). The cytotoxicity of a short chain fatty acid histone deacetylase inhibitor on HCT116 human colorectal carcinoma cell line. MO J. Immunol. 2:00034.
Kechagia, M., Basoulis, D., Konstantopoulou, S., Dimitriadi, D., Gyftopoulou, K., Skarmoutsou, N., et al. (2013). Health benefits of probiotics: a review. ISRN Nutr. 2013:481651. doi: 10.5402/2013/481651
Kim, C. H., Park, J., and Kim, M. (2014). Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 14, 277–288. doi: 10.4110/in.2014.14.6.277
Kim, J. U., Kim, Y., Han, K. S., Oh, S. J., Wang, K. Y., Kim, J. N., et al. (2006). Function of cell bound and released exopolysaccharides produced by Lactobacillus rhamnosus ATCC 9595. J. Microbiol. Biotechnol. 16, 939–945.
Kim, Y., Lee, D., Kim, D., Cho, J., Yang, J., Chung, M., et al. (2008). Inhibition of proliferation in colon cancer cell lines and harm ful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch. Pharm. Res. 1, 468–473. doi: 10.1007/s12272-001-1180-y
Kimura, I., Inoue, D., Maeda, T., Hara, T., Ichimura, A., Miyauchi, S., et al. (2011). Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. U.S.A. 108, 8030–8035. doi: 10.1073/pnas.1016088108
Kostic, A. D., Chun, E., Robertson, L., Glickman, J. N., Gallini, C. A., Michaud, M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215. doi: 10.1016/j.chom.2013.07.007
Kumar, K. S., Sastry, N., Polaki, H., and Mishra, V. (2015). Colon cancer prevention through probiotics: an overview. J. Cancer Sci. Ther. 7, 081–092.
Kumar, M., Nagpal, R., Verma, V., Kumar, A., Kaur, N., Hemalatha, R., et al. (2013). Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 7, 23–34. doi: 10.1111/j.1753-4887.2012.00542.x
Kundu, J. K., and Surh, Y. L. (2012). Emerging avenues linking inflammation and cancer. Free Radic. Biol. Med. 52, 2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035
Lambert, D. W., Wood, I. S., Ellis, A., and Shirazi-Beechey, S. P. (2002). Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br. J. Cancer 86, 1262–1269. doi: 10.1038/sj.bjc.6600264
Lan, A., Lagadic-Gossmann, D., Lemaire, C., Brenner, C., and Jan, G. (2007). Acidic extracellular pH shifts colorectal cancer cell death from apoptosis to necrosis upon exposure to propionate and acetate, major end-products of the human probiotic propionibacteria. Apoptosis 12, 573–591. doi: 10.1007/s10495-006-0010-3
Lankaputhra, W. E. V., and Shah, N. P. (1998). Antimutagenic properties of probiotic bacteria and of organic acids. Mutat. Res. 397, 169–182. doi: 10.1016/S0027-5107(97)00208-X
Le Marechal, C., Peton, V., Ple, C., Vroland, C., Jardin, J., Briard-Bion, V., et al. (2014). Surface proteins of Propionibacterium freudenreichii are involved in its anti-inflammatory properties. J. Proteomics 113, 447–461. doi: 10.1016/j.jprot.2014.07.018
Le Poul, E., Loison, C., Struyf, S., Springael, J. Y., Lannoy, V., Decobecq, M. E., et al. (2003). Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489. doi: 10.1074/jbc.M301403200
Lee, D. K., Jang, S., Kim, M. J., Kim, J. H., Chung, M. J., Kim, K. J., et al. (2008). Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer 8:310. doi: 10.1186/1471-2407-8-310
Leeman, M. F., Curran, S., and Murray, G. I. (2003). Newinsights into the roles of matrix metalloproteinases in colorectal cancer development and progression. J. Pathol. 201, 528–534. doi: 10.1002/path.1466
Li, H., Myeroff, L., Smiraglia, D., Romero, M. F., Pretlow, T. P., Kasturi, L., et al. (2003). SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl. Acad. Sci. U.S.A. 100, 8412–8417. doi: 10.1073/pnas.1430846100
Liboredo, J. C., Anastacio, L. R., Mattos, L. V., Nicoli, J. R., and Correia, M. I. (2010). Impact of probiotic supplementation on mortality of induced 1, 2-dimethylhydrazine carcinogenesis in a mouse model. Nutrition 26, 779–783. doi: 10.1016/j.nut.2010.01.008
Licciardi, P. V., Wong, S. S., Tang, M. L. K., and Karagiannis, T. C. (2010). Epigenome targeting by probiotic metabolites. Gut Pathog. 2:24. doi: 10.1186/1757-4749-2-24
Liong, M. T. (2008). Roles of probiotics and prebiotics in colon cancer prevention: postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 9, 854–863. doi: 10.3390/ijms9050854
Louis, P., and Flint, H. J. (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294, 1–8. doi: 10.1111/j.1574-6968.2009.01514.x
Lupton, J. R. (2000). Is fiber protective against colon cancer? Where the research is leading us. Nutrition 16, 558–561. doi: 10.1016/S0899-9007(00)00350-6
Ma, E. L., Choi, Y. J., Choi, J., Pothoulakis, C., Rhee, S. H., and Im, E. (2010). The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer 127, 780–790.
Maier, S., Daroqui, M. C., Scherer, S., Roepcke, S., Velcich, A., Shenoy, S. M., et al. (2009). Butyrate and vitamin D3 induce transcriptional attenuation at the cyclin D1 locus in colonic carcinoma cells. J. Cell. Physiol. 218, 638–642. doi: 10.1002/jcp.21642
Matsuguchi, T., Takagi, A., Matsuzaki, T., Nagaoka, M., Ishikawa, K., Yokokura, T., et al. (2003). Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor a-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 10, 259–266.
Matsumoto, S., Hara, T., Nagaoka, M., Mike, A., Mitsuyama, K., Sako, T., et al. (2008). A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology 128(1 Suppl.), e170–e180. doi: 10.1111/j.1365-2567.2008.02942.x
Medina, V., Young, G. P., Edmonds, B., James, R., Appleton, S., and Zalewski, P. D. (1997). Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin a (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome C-dependent pathway. Cancer Res. 57, 3697–3707.
Menard, S., Candalh, C., Bambou, J. C., Terpend, K., Cerf-Bensussan, N., and Heyman, M. (2004). Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53, 821–828. doi: 10.1136/gut.2003.026252
Mitsuma, T., Odajima, H., Momiyama, Z., Watanabe, K., Masuguchi, M., Sekine, T., et al. (2008). Enhancement of gene expression by a peptide (CHWPR) produced by Bifidobacterium lactis BB-12. Microbiol. Immunol. 52, 144–155. doi: 10.1111/j.1348-0421.2008.00022.x
Miyauchi, S., Gopal, E., Fei, Y. J., and Ganapathy, V. (2004). Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J. Biol. Chem. 279, 13293–13296. doi: 10.1074/jbc.C400059200
Mook, O. R., Frederiks, W. M., and Van Noorden, C. J. (2004). The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 1705, 69–89.
Nadathur, S. R., Gould, S. J., and Bakalinsky, A. T. (1995). Antimutagenicity of an acetone extract of yogurt. Mutat. Res. 334, 213–224. doi: 10.1016/0165-1161(95)90014-4
Nami, Y., Haghshenas, B., Haghshenas, M., Abdullah, N., and Yari, K. A. (2015). The prophylactic effect of probiotic Enterococcus lactis IW5 against different human cancer cells. Front. Microbiol. 6:1317. doi: 10.3389/fmicb.2015.01317
Narayanan, A., Baskaran, S. A., Amalaradjou, M. A., and Venkitanarayanan, K. (2015). Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int. J. Mol. Sci. 16, 5014–5027. doi: 10.3390/ijms16035014
Nicholson, J. K., Holmes, E., Kinross, J., Burcelin, R., Gibson, G., Jia, W., et al. (2012). Host-gut microbiota metabolic interactions. Science 336, 1262–1267. doi: 10.1126/science.1223813
Nilsson, N. E., Kotarsky, K., Owman, C., and Olde, B. (2003). Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 303, 1047–1052. doi: 10.1016/S0006-291X(03)00488-1
Ohishi, A., Takahashi, S., Ito, Y., Tsukamoto, K., Nanba, Y., Ito, N., et al. (2010). Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatrics 156, 679–681. doi: 10.1016/j.jpeds.2009.11.041
Ohtani, S., Terashima, M., Satoh, J., Soeta, N., Saze, Z., Kashimura, S., et al. (2009). Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer 12, 43–51. doi: 10.1007/s10120-008-0497-0
Orlando, A., Messa, C., Linsalata, M., Cavallini, A., and Russo, F. (2009). Effects of Lactobacillus rhamnosus GG on proliferation and polyamine metabolism in HGC-27 human gastric and DLD-1 colonic cancer cell lines. Immunopharmacol. Immunotoxicol. 31, 108–116. doi: 10.1080/08923970802443631
Pan, X., Yang, Y., and Zhang, J. R. (2014). Molecular basis of host specificity in human pathogenic bacteria. Emerg. Microbes Infect. 3:e23. doi: 10.1038/emi.2014.23
Papadimitriou, K., Zoumpopoulou, G., Foligné, B., Alexandraki, V., Kazou, M., Pot, B., et al. (2015). Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front. Microbiol. 6:58. doi: 10.3389/fmicb.2015.00058
Park, J., Kim, M., Kang, S. G., Jannasch, A. H., Cooper, B., Patterson, J., et al. (2015). Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93. doi: 10.1038/mi.2014.44
Patel, R. M., Myers, L. S., Kurundkar, A. R., Maheshwari, A., Nusrat, A., and Lin, P. W. (2012). Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 180, 626–635. doi: 10.1016/j.ajpath.2011.10.025
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R., and Lin, J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625. doi: 10.3945/jn.109.104638
Pool-Zobel, B. L. (2005). Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data. Br. J. Nutr. 93, S73–S90. doi: 10.1079/BJN20041349
Pool-Zobel, B. L., and Sauer, J. (2007). Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J. Nutr. 137, 2580S–2584S.
Putaala, H., Salusjärvi, T., Nordström, M., Saarinen, M., Ouwehand, A. C., Bech Hansen, E., et al. (2008). Effect of four probiotic strains and Escherichia coli O157:H7 on tight junction integrity and cyclo-oxygenase expression. Res. Microbiol. 159, 692–698. doi: 10.1016/j.resmic.2008.08.002
Raman, M., Ambalam, P., Kondepudi, K. K., Pithva, S., Kothari, C., Patel, A. T., et al. (2013). Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes 4, 181–192. doi: 10.4161/gmic.23919
Rao, R. K., and Samak, G. (2013). Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr. Nutr. Food Sci. 9, 99–107. doi: 10.2174/1573401311309020004
Rhee, C. H., and Park, H. D. (2001). Three glycoproteins with antimutagenic activity identified in Lactobacillus plantarum KLAB21. Appl. Environ. Microbiol. 67, 3445–3449. doi: 10.1128/AEM.67.8.3445-3449.2001
Roda, A., Simoni, P., Magliulo, M., Nanni, P., Baraldini, M., Roda, G., et al. (2007). A new oral formulation for the release of sodium butyrate in the ileocecal region and colon. World J. Gastroenterol. 13, 1079–1084. doi: 10.3748/wjg.v13.i7.1079
Round, J. L., and Mazmanian, S. K. (2010). Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209. doi: 10.1073/pnas.0909122107
Ruemmele, F. M., Schwartz, S., Seidman, E. G., Dionne, S., Levy, E., and Lentze, M. J. (2003). Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway. Gut 52, 94–100. doi: 10.1136/gut.52.1.94
Sadeghi-Aliabadi, H., Mohammadi, F., Fazeli, H., and Mirlohi, M. (2014). Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran J. Basic Med. Sci. 17, 815–819.
Saldanha, S. N., Kalaa, R., and Tollefsbol, T. O. (2014). Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 324, 40–53. doi: 10.1016/j.yexcr.2014.01.024
Sankpal, U. T., Pius, H., Khan, M., Shukoor, M. I., Maliakal, P., Lee, C. M., et al. (2012). Environmental factors in causing human cancers: emphasis on tumorigenesis. Tumour Biol. 33, 1265–1274. doi: 10.1007/s13277-012-0413-4
Scharlau, D., Borowicki, A., and Habermann, N. (2009). Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 682, 39–53. doi: 10.1016/j.mrrev.2009.04.001
Sears, C. L. (2009). Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 22, 349–369. doi: 10.1128/CMR.00053-08
Segawa, S., Fujiya, M., Konishi, H., Ueno, N., Kobayashi, N., Shigyo, T., et al. (2011). Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS ONE 6:e23278. doi: 10.1371/journal.pone.0023278
Sellin, J. H. (1999). SCFAs: the enigma of weak electrolyte transport in the colon. News Physiol. Sci. 14, 58–64.
Seth, A., Yan, F., Polk, D. B., and Rao, R. K. (2008). Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1060–G1069. doi: 10.1152/ajpgi.00202.2007
Sevda, E. R., Koparal, A. T., and Kivan, M. (2015). Cytotoxic effects of various lactic acid bacteria on Caco-2 cells. Turk. J. Biol. 39, 23–30. doi: 10.3906/biy-1402-62
Shenderov, B. A. (2011). Probiotic (symbiotic) bacterial languages. Anaerobe 17, 490–495. doi: 10.1016/j.anaerobe.2011.05.009
Shenderov, B. A. (2013). Metabiotics: novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 24:20399.
Shin, H. J., Anzai, N., Enomoto, A., He, X., Kim, D. K., Endou, H., et al. (2007). Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 45, 1046–1055. doi: 10.1002/hep.21596
Shyu, P. T., Oyong, G. G., and Cabrera, E. C. (2014). Cytotoxicity of probiotics from philippine commercial dairy products on cancer cells and the effect on expression of cfos and cjun early apoptotic-promoting genes and interleukin-1β and tumor necrosis factor-α proinflammatory cytokine genes. BioMed Res. Int. 9:491740.
Sina, C. O., Gavrilova, M., Forster, M., Till, A., Derer, S., Hildebrand, F., et al. (2009). G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 183, 7514–7522. doi: 10.4049/jimmunol.0900063
Singh, N., Gurav, A., Sivaprakasam, S., Brady, E., Padia, R., Shi, H., et al. (2014). Activation of the receptor (Gpr109a) for niacin and the commensal metabolite butyrate suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139. doi: 10.1016/j.immuni.2013.12.007
Singh, N., Thangaraju, M., Prasad, P. D., Martin, P. M., Lambert, N. A., Boettger, T., et al. (2010). Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J. Biol. Chem. 285, 27601–27608. doi: 10.1074/jbc.M110.102947
Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly, Y. M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Snydman, D. R. (2008). The safety of probiotics. Clin. Infect. Dis. 46, S104–S111. doi: 10.1086/523331
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn’s disease patients. Proc. Natl. Acad. Sci. U.S.A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Song, C. H., Liu, Z. Q., Huang, S., Zheng, P. Y., and Yang, P. C. (2012). Probiotics promote endocytic allergen degradation in gut epithelial cells. Biochem. Biophys. Res. Commun. 426, 135–140. doi: 10.1016/j.bbrc.2012.08.051
Sougioultzis, S., Simeonidis, S., Bhaskar, K. R., Chen, X., Anton, P. M., Keates, S., et al. (2006). Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NFkB-mediated IL-8 gene expression. Biochem. Biophys. Res. Commun. 343, 69–76.
Sparmann, A., and Bar-Sagi, D. (2004). Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6, 447–458. doi: 10.1016/j.ccr.2004.09.028
Stein, J., Schroder, O., Bonk, M., Oremek, G., Lorenz, M., and Caspary, W. F. (1996). Induction of glutathione-S-transferase-pi by short-chain fatty acids in the intestinal cell line Caco-2. Eur. J. Clin. Invest. 26, 84–87. doi: 10.1046/j.1365-2362.1996.113252.x
Switzer, C. H., Glynn, S. A., Ridnour, L. A., Cheng, R. Y., Vitek, M. P., Ambs, S., et al. (2011). Nitric oxide and protein phosphatase 2A provide novel therapeutic opportunities in ER-negative breast cancer. Trends Pharmacol. Sci. 32, 644–651. doi: 10.1016/j.tips.2011.07.001
Tang, Y., Chen, Y., Jiang, H., and Nie, D. (2011a). The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy 7, 235–237. doi: 10.4161/auto.7.2.14277
Tang, Y., Chen, Y., Jiang, H., Robbins, G. T., and Nie, D. (2011b). G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 128, 847–856. doi: 10.1002/ijc.25638
Thangaraju, M., Cresci, G., Itagaki, S., Mellinger, J., Browning, D. D., Berger, F. G., et al. (2008). Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J. Gastrointest. Surg. 12, 1773–1781. doi: 10.1007/s11605-008-0573-0
Thomas, C. M., and Versalovic, J. (2010). Probiotics-host communication; Modulation of signaling pathways in the intestine. Gut Microbes. 1, 148–163. doi: 10.4161/gmic.1.3.11712
Tobioka, H., Isomura, H., Kokai, Y., Tokunaga, Y., Yamaguchi, J., Sawada, N., et al. (2004). Occludin expression decreases with the progression of human endometrial carcinoma. Hum. Pathol. 35, 159–164. doi: 10.1016/j.humpath.2003.09.013
Topping, D. L., and Clifton, P. M. (2001). Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064.
Treptow-van, L. S., Rechkemmer, G., Rowland, I., Dolara, P., and Pool-Zobel, B. L. (1999). The carbohydrate crystalean and colonic microflora modulate expression of glutathione S-transferase subunits in colon of rats. Eur. J. Nutr. 38, 76–83. doi: 10.1007/s003940050047
Tsuda, H., Hara, K., and Miyamoto, T. (2008). Binding of mutagens to exopolysaccharide produced by Lactobacillus plantarum mutant strain 301102S. J. Dairy Sci. 91, 2960–2966. doi: 10.3168/jds.2007-0538
Uccello, M., Malaguarnera, G., Basile, F., D’agata, V., Malaguarnera, M., Bertino, G., et al. (2012). Potential role of probiotics on colorectal cancer prevention. BMC Surg. 12(Suppl. 1):S35. doi: 10.1186/1471-2482-12-S1-S35
Urbanska, A. M., Bhathena, J., Martoni, C., and Prakash, S. (2009). Estimation of the potential antitumor activity of microencapsulated Lactobacillus acidophilus yogurt formulation in the attenuation of tumorigenesis in Apc(Min/+) mice. Dig. Dis. Sci. 54, 264–273. doi: 10.1007/s10620-008-0363-2
Van Reenen, C. A., and Dicks, L. M. T. (2011). Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? A review. Arch. Microbiol. 193, 157–168. doi: 10.1007/s00203-010-0668-3
Verma, A., and Shukla, G. (2013). Administration of prebiotic inulin suppresses 1, 2 dimethylhydrazine dihydrochloride induced procarcinogenic biomarkers fecal enzymes and preneoplastic lesions in early colon carcinogenesis in Sprague Dawley rats. J. Funct. Foods 5, 991–996. doi: 10.1016/j.jff.2013.02.006
Verma, A., and Shukla, G. (2014). Synbiotic (Lactobacillus rhamnosus+Lactobacillus acidophilus+inulin) attenuates oxidative stress and colonic damage in 1, 2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague–Dawley rats: a long-term study. Eur. J. Cancer Prev. 23, 550–559. doi: 10.1097/CEJ.0000000000000054
Verma, A., and Shukla, G. (2015). Modulation of apoptosis and immune response by synbiotic in experimental colorectal cancer. Int. J. Pharm. Biol. Sci. 6, 529–543.
Verna, E. C., and Lucak, S. (2010). Use of probiotics in gastrointestinal disorders: what to recommend? Therap. Adv. Gastroenterol. 3, 307–319. doi: 10.1177/1756283X10373814
Vernia, P., Cittadini, M., Caprilli, R., and Torsoli, A. (1995). Topical treatment of refractory distal ulcerative colitis with 5-ASA and sodium butyrate. Dig. Dis. Sci. 40, 305–307. doi: 10.1007/BF02065414
Vieira, E. L., Leonel, A. J., Sad, A. P., Beltrão, N. R., Costa, T. F., Ferreira, T. M., et al. (2012). Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 23, 430–436. doi: 10.1016/j.jnutbio.2011.01.007
Vinolo, M. A., Rodrigues, H. G., Hatanaka, E., Sato, F. T., Sampaio, S. C., and Curi, R. (2011). Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 22, 849–855. doi: 10.1016/j.jnutbio.2010.07.009
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., and Kinzler, K. W. (2013). Cancer genome landscapes. Science 339, 1546–1558. doi: 10.1126/science.1235122
Waldecker, M., Kautenburger, T., Daumann, H., Busch, C., and Schrenk, D. (2008). Inhibition of histone-deacetylase activity by short chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 19, 587–593. doi: 10.1016/j.jnutbio.2007.08.002
Wan, Y., Xin, Y., Zhang, C., Wu, D., Ding, D., Tang, L., et al. (2014). Fermentation supernatants of Lactobacillus delbrueckii inhibit growth of human colon cancer cells and induce apoptosis through a caspase 3-dependent pathway. Oncol. Lett. 7, 1738–1742.
Wang, F., Graham, W. V., Wang, Y., Witkowski, E. D., Schwarz, B. T., and Turner, J. R. (2005). Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166, 409–419. doi: 10.1016/S0002-9440(10)62264-X
Wang, L., Cao, H., Liu, L., Wang, B., Walker, W. A., Acra, S. A., et al. (2014). Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J. Biol. Chem. 289, 20234–20244. doi: 10.1074/jbc.M114.553800
Wang, Y., Kirpich, I., Liu, Y., Ma, Z., Barve, S., Mc Clain, C. J., et al. (2011). Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 79, 2866–2875. doi: 10.1016/j.ajpath.2011.08.039
Wang, Y., Liu, Y., Sidhu, A., Ma, Z., Mc Clain, C., and Feng, W. (2012). Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G32–G41. doi: 10.1152/ajpgi.00024.2012
Wollowski, I. (1998). Studies on the Protective Effects of Bacterial Metabolites on Molecular Processes of Colon Carcinogenesis. Giessen: Fachverlag Kohler.
Wollowski, I., Rechkemmer, G., and Pool-Zobel, B. L. (2001). Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutr. 73, 451S–455S.
Wu, G. D., Huang, N., Wen, X., Keilbaugh, S. A., and Yang, H. (1999). High-level expression of IkB-bin the surface epithelium of the colon: in vitro evidence for an immunomodulatory role. J. Leukoc. Biol. 66, 1049–1056.
Wu, S., Rhee, K. J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H. R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022. doi: 10.1038/nm.2015
Xiong, Y., Miyamoto, N., Shibata, K., Valasek, M. A., Motoike, T., Kedzierski, R. M., et al. (2004). Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. U.S.A. 101, 1045–1050. doi: 10.1073/pnas.2637002100
Yan, F., Cao, H., Cover, T. L., Washington, M. K., Shi, Y., Liu, L., et al. (2011). Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 121, 2242–2253. doi: 10.1172/JCI44031
Yan, F., Cao, H., Cover, T. L., Whitehead, R., Washington, M. K., and Polk, D. B. (2007). Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132, 562–575. doi: 10.1053/j.gastro.2006.11.022
Yazdankhah, S. P., Midtvedt, T., Narvhus, J. A., Berstad, A., Lassen, J., and Halvorsen, R. (2009). The use of probiotics for critically ill patients in hospitals. Microb. Ecol. Health Dis. 21, 114–121. doi: 10.3109/08910600903495046
Yu, D. C., Waby, J. S., Chirakkal, H., Staton, C. A., and Corfe, B. M. (2010). Butyrate suppresses expression of neuropilin I in colorectal cell lines through inhibition of Sp1 transactivation. Mol. Cancer. 9:276. doi: 10.1186/1476-4598-9-276
Yu Danny, C., Bury, J. P., Tiernan, J., Waby, J. S., Staton, C. A., and Corfe, B. M. (2011). Short-chain fatty acid level and field cancerization show opposing associations with enteroendocrine cell number and neuropilin expression in patients with colorectal adenoma. Mol. Cancer. 10:27. doi: 10.1186/1476-4598-10-27
Zakostelska, Z., Kverka, M., Klimesova, K., Rossmann, P., Mrazek, J., Kopecny, J., et al. (2011). Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 6:e27961. doi: 10.1371/journal.pone.0027961
Zeng, H., Lazarova, D. L., and Bordonaro, M. (2014). Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol. 6, 41–51. doi: 10.4251/wjgo.v6.i2.41
Keywords: metabiotics, metabolites, short chain fatty acids, probiotics, colorectal cancer
Citation: Sharma M and Shukla G (2016) Metabiotics: One Step ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer. Front. Microbiol. 7:1940. doi: 10.3389/fmicb.2016.01940
Received: 19 June 2016; Accepted: 18 November 2016;
Published: 02 December 2016.
Edited by:
Andrea Gomez-Zavaglia, CONICET, ArgentinaReviewed by:
Luca Pastorelli, University of Milan, ItalyMicaela Medrano, CONICET, Argentina
Fernando Miguel Trejo, CONICET, Argentina
Michele Barone, University of Bari, Italy
Copyright © 2016 Sharma and Shukla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geeta Shukla, Z2VldGFfc2h1a2xhQHB1LmFjLmlu
 Mridul Sharma
Mridul Sharma Geeta Shukla
Geeta Shukla