- 1CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 2Tropical Marine Biological Research Station in Hainan, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Sanya, China
- 3Department of Microbiology and Plant Biology, Institute for Environmental Genomics, University of Oklahoma, Norman, OK, USA
The coral holobiont often resides in oligotrophic waters; both coral cells and their symbiotic dinoflagellates possess ammonium assimilation enzymes and potentially benefit from the nitrogen fixation of coral-associated diazotrophs. However, the seasonal dynamics of coral-associated diazotrophs are not well characterized. Here, the seasonal variations of diazotrophic communities associated with three corals, Galaxea astreata, Pavona decussata, and Porites lutea, were studied using nifH gene amplicon pyrosequencing techniques. Our results revealed a great diversity of coral-associated diazotrophs. nifH sequences related to Alphaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria were ubiquitous and dominant in all corals in two seasons. In contrast with the coral P. decussata, both G. astreata and P. lutea showed significant seasonal changes in the diazotrophic communities and nifH gene abundance. Variable diazotroph groups accounted for a range from 11 to 49% within individual coral samples. Most of the variable diazotrophic groups from P. decussata were species-specific, however, the majority of overlapping variable groups in G. astreata and P. lutea showed the same seasonal variation characteristics. Rhodopseudomonas palustris- and Gluconacetobacter diazotrophicus-affiliated sequences were relatively abundant in the summer, whereas a nifH sequence related to Halorhodospira halophila was relatively abundant in spring G. astreata and P. lutea. The seasonal variations of all diazotrophic communities were significantly correlated with the seasonal shifts of ammonium and nitrate, suggesting that diazotrophs play an important role in the nitrogen cycle of the coral holobiont.
Introduction
The coral holobiont is a multi-partner symbiotic system that forms associations with both external and internal microbiota (Mouchka et al., 2010). There is increasing evidence that coral-associated microbial communities are crucial for biogeochemistry and control the health and resilience of coral reef ecosystems (Mouchka et al., 2010; Thompson et al., 2014). Due to their relative size and high per cell activity, a small change in microbial biomass may signal a large reallocation of available energy in the ecosystem (McDole et al., 2012; Haas et al., 2016). Corals often reside in oligotrophic waters. Nitrogen fixation, one of the new fixed nitrogen input into the ecosystem, has long been thought to be an important linchpin to sustain biological productivity in coral reef areas (Wiebe et al., 1975; Capone et al., 1977; Shashar et al., 1994b; Cardini et al., 2014). Nitrogen fixation activity within the coral holobiont has been detected using acetylene reduction and isotopic assays in several coral species (Williams et al., 1987; Shashar et al., 1994a; Lesser et al., 2007; Chimetto et al., 2008; Grover et al., 2014; Benavides et al., 2016). Coral-associated nitrogen-fixing bacteria, capable of fixing and converting gaseous nitrogen (N2) to biologically available nitrogen forms, contribute an important source of nitrogen to the coral holobiont (Lesser et al., 2007; Cardini et al., 2014). Both coral cells and their symbiotic dinoflagellates (Symbiodinium) have the capacity to assimilate ammonium (Pernice et al., 2012) and benefit from nitrogen-fixing bacteria (Lesser et al., 2007; Cardini et al., 2014; Santos et al., 2014). Additionally, a close relationship was found between the abundance of coral-associated diazotrophs and symbiotic dinoflagellates (Lesser et al., 2007; Olson et al., 2009; Santos et al., 2014), and between coral-associated diazotrophs and coral productivity (Cardini et al., 2014).
Accumulating evidence shows that diazotrophic organisms are ubiquitous members of coral-associated microbial communities and form species-specific associations with their hosts (Lema et al., 2012, 2014b). However, the degree to which nitrogen-fixing bacterial communities are specific to their coral hosts can vary, as both species-specific (Lema et al., 2012) and site-specific (Lema et al., 2014b) nitrogen-fixing microbial communities have been reported. Nitrogen fixation activity in corals is also highly dynamic and can be rapidly affected by changes in environmental conditions (Lesser et al., 2007; Rädecker et al., 2014). It is suggested that coral holobionts harbor both a core microbiome determined by holobiont macroorganisms, and a variable microbiome to adapt to local conditions (Kelly et al., 2014; Ainsworth et al., 2015). As the disturbance of microbial nitrogen-cycling may be tightly linked to coral bleaching and disease (Rädecker et al., 2015), knowledge of the variable diazotrophic groups would help to further evaluate the importance of these communities to the coral host; however, such knowledge is currently lacking.
The nifH gene encodes a conserved subunit of the dinitrogenase iron protein responsible for nitrogen fixation, and is conserved in all known diazotrophs (Zehr et al., 2003). As the agreement with 16S rRNA gene-based phylogeny, nifH is an ideal molecular target and is widely used for gene-based phylogenetic characterization of diazotrophs (Gaby and Buckley, 2012). In this study, high-throughput Illumina sequencing was used to investigate seasonal and species-specific patterns in diazotrophic communities associated with the corals Galaxea astreata, Pavona decussata, and Porites lutea. The core and variable diazotrophic microbiome were tested through comparative analysis. The aims were to: (i) investigate the diversity and abundance of diazotrophic communities associated with three coral species; (ii) determine the seasonal shifts of diazotrophic communities and the variable diazotrophic species associated with different coral species; (iii) explore the possible relationship between the coral-associated seasonal-variable diazotroph groups and environmental variables, given the important role of diazotrophic microbes in driving biogeochemical cycles.
Materials and Methods
Study Site and Sampling Collection
Coral samples were collected from Luhuitou fringing reef (18°12’19”N, 109°28’27”E) located in Sanya Bay of South China Sea, which is affected by cold-water upwelling during the summer. Cold-water upwelling affects the distribution of a variety of dissolved and particulate forms of nitrogen in Sanya Bay (Huang et al., 2003; Zhang et al., 2010; Wu et al., 2012). Furthermore, in summer, tropical cyclones and monsoonal rainfall may also carry high nutrient loads. Differences in nutrient loads between spring and summer may influence overall diversity of coral-associated diazotrophic communities.
G. astreata, P. decussata, and P. lutea are important scleractinian species and are all a natural occurrence in the Luhuitou fringing reef region. All coral samples were collected in April and June 2013 using a punch and hammer. Three healthy coral colonies from each coral species were collected at a depth of 5–10 m. Triplicate coral fragments (∼2 cm2) for each coral colony were placed in sealed plastic bags, rinsed thoroughly with sterile seawater at the surface, placed on ice and transported to the laboratory (Tropical Marine Biological Research station in Hainan). Samples were cryopreserved at -20°C.
Seawater samples within 20 cm of the coral colonies (n = 3) for temperature and salinity were measured using a YSI 6600V2 water quality sonde. Dissolved oxygen (DO) was determined by DO meter, pH was measured using a standard hydrogen electrode and reference electrode, and chemical oxygen demand (COD) was determined by alkaline potassium permanganate method. Inorganic nutrients including nitrate, ammonium, nitrite, and phosphate were measured using standard methods as described previously (Huang et al., 2003).
DNA Extraction, Amplification, Sequencing, and Data Processing
The coral fragments were suspended in TE buffer and homogenized in a sterilized mortar and pestle with liquid nitrogen. The homogenized solution was transferred to a clean tube and the total community DNA was extracted using an E.Z.N.A.® Soil DNA Kit (Omega Biotek). The DNA was then purified with a Promega Wizard DNA clean-up system (Madison, WI, USA). DNA concentration was measured by Pico Green using a FLUOstar OPTIMA fluorescence plate reader (BMG Labtech, Jena, Germany). The primer sets used to amplify the nifH gene were PolF and PolR (Poly et al., 2001) combined with Illumina adapter sequences and barcode sequences (Caporaso et al., 2012). Sample libraries were generated from purified PCR products. The Miseq 300 cycles kit was used for 2x 150 bp paired-end sequencing on Miseq machine (Illumina, San Diego, CA, USA).
Raw nifH gene sequences were separated to samples using barcodes and permission of up to one mismatch. Quality trimming was done using Btrim (Kong, 2011). Forward and reverse reads were merged into full-length sequences by FLASH (Magoč and Salzberg, 2011). Sequences were removed if they were too short or contained ambiguous bases. Random re-sampling was performed with 10,000 sequences per sample. The operational taxonomic units (OTUs) were classified using UCLUST at the 90% similarity level, and singletons were removed. The frameshift errors caused by insertions and deletions in DNA sequences were checked and corrected using RDP FrameBot. After processing, the valid nifH sequences (310–330 bp) were translated into protein sequences and taxonomic assignment was performed using the RDP FrameBot tool (Wang et al., 2013). The significantly seasonally changed diazotrophic OTUs identified in this study have been deposited in the GenBank database under nucleotide accession numbers KX078090 to KX078212 for nifH gene sequences.
Quantification of nifH Gene Copy Number
To quantify the number of copies of the nifH gene, the primers PolF and PolR were used. Absolute quantification was carried out on the Lightcycler 480 System (Roche). Standard curves were developed by serially diluting plasmid containing a nifH gene to final concentrations from 103 to 108 copies/μl. The qPCR efficiency (E) was 1.90. The R2 of standards was higher than 0.99. Triple qPCRs were performed for all samples and standard curve concentrations. The specificity of the amplification products was confirmed by melting-curve analysis, and the amplified fragments were checked by electrophoresis in 2% agarose gel to confirm the expected sizes of amplicon. The nifH copy number was ultimately expressed as per μg coral colony dry weight.
Statistical Analysis
All analyses were performed using the R vegan package (R Foundation for Statistical Computing, Vienna, Austria), our R-based pipeline1, and the software package CANOCO 4.5 for Windows. Diazotrophic bacteria richness and diversities were calculated using Chao1, Shannon–Wiener’s (H’) and evenness. Principal coordinates analysis (PCoA) was used to visualize the changes of overall diazotrophic community structure. Dissimilarity tests by permutational multivariate analysis of variance (PERMANOVA) were performed with Euclidean, Manhattan, Bray–Curtis, and Jaccard for comparing seasonal variation of diazotrophic communities. Significance tests based on unpaired Student’s t-test were applied to identify seasonal variation of nifH gene diversities and nifH copy number abundance. Response ratio analysis was conducted to detect significantly seasonally changed OTU (Deng et al., 2012). Redundancy analysis (RDA) was performed to determine the relationship between variable diazotroph groups and environmental parameters.
Results
Environmental Characteristics, Diazotrophic Composition, Community Structure, and nifH Gene Abundance
The temperature of ambient seawater was 24.63 ± 0.32°C in the spring, and 25.83 ± 0.45°C in the summer. The average water salinity was 33.46‰ in the spring and 35.01‰ in the summer. The nutrient concentrations of ammonium and phosphorus were higher in the spring. However, the concentration of nitrate was higher in the summer. Higher values of COD and DO were detected in the summer. The concentration of Chlorophyll a was higher in the spring (Supplementary Table S1).
After processing, 426,535 high quality nifH sequences (310-330 bp) were retrieved from the fragments of coral G. astreata, Pavona decussata and Porites lutea. Samples were rarefied to 10,000 sequences per sample. All sequences obtained could be assigned to 2,146 OTUs (90% similarity level). The diazotrophic communities were highly diverse (Supplementary Table S2). Shannon–Wiener’s (H’) index ranged from 3.24 to 4.43 and Simpson evenness (E) ranged from 0.07 to 0.14. Student’s t-test was used to describe the difference between spring and summer samples. The results showed that season did not significantly influence the diazotrophic richness and diversities (t-test, P > 0.05; Supplementary Table S2).
The nifH sequences retrieved from the three coral species belong to a wide range of bacterial types (Figure 1). Although seasonal variations were detected in diazotrophic communities, diazotrophic sequences related to Alphaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria were ubiquitous and dominant groups that constituted 76.78% of all nifH sequences (Figure 1). A dissimilarity test based on the Euclidean, Manhattan and Bray–Curtis matrix showed that the diazotrophic communities associated with coral G. astreata and Porites lutea in spring were significantly different from those in summer (PERMANOVA, P = 0.001; Table 1), indicating that for these two coral species the diazotrophic communities significantly changed according to the season. However, no significant changes were detected in coral Pavona decussata. PCoA also showed the significant seasonal variation in the diazotrophic communities associated with coral G. astreata and Porites lutea as revealed by the plot. However, Pavona decussata -associated diazotrophic communities did not vary in a seasonal manner (Figure 2).
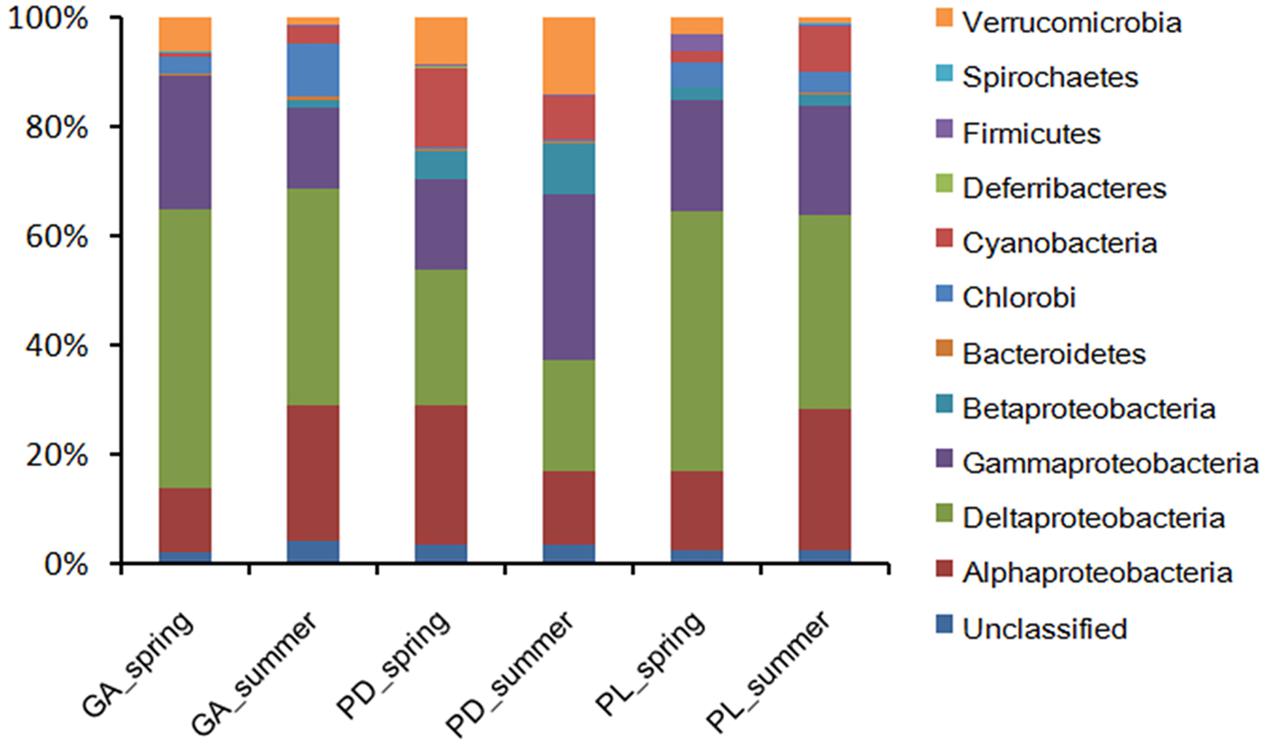
FIGURE 1. Diazotrophic composition profiles. Diazotrophic taxa were categorized at the phylum level except for the Proteobacteria, which were categorized by class. GA, G. astreata. PD, P. decussata. PL, P. lutea.
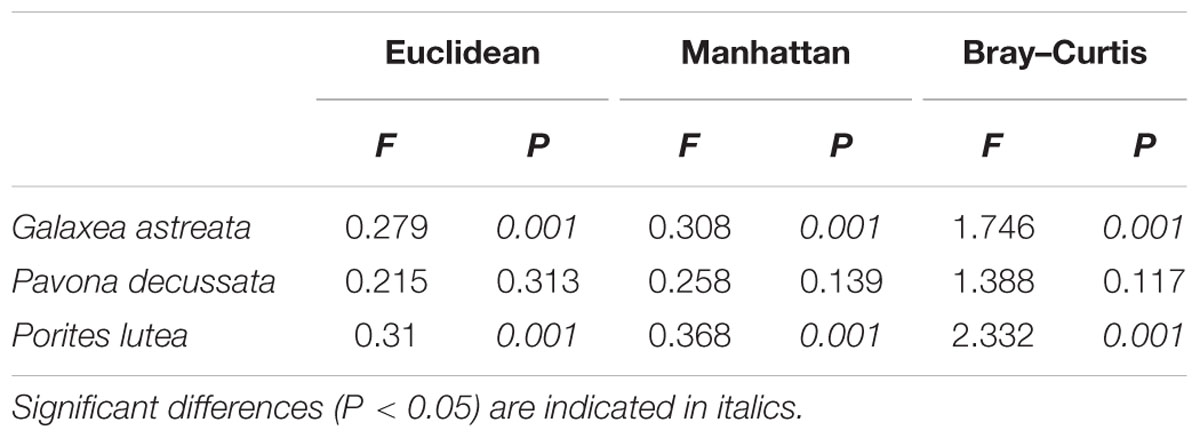
TABLE 1. Dissimilarity tests of diazotrophic communities’ dissimilarity between spring and summer by ADONIS.
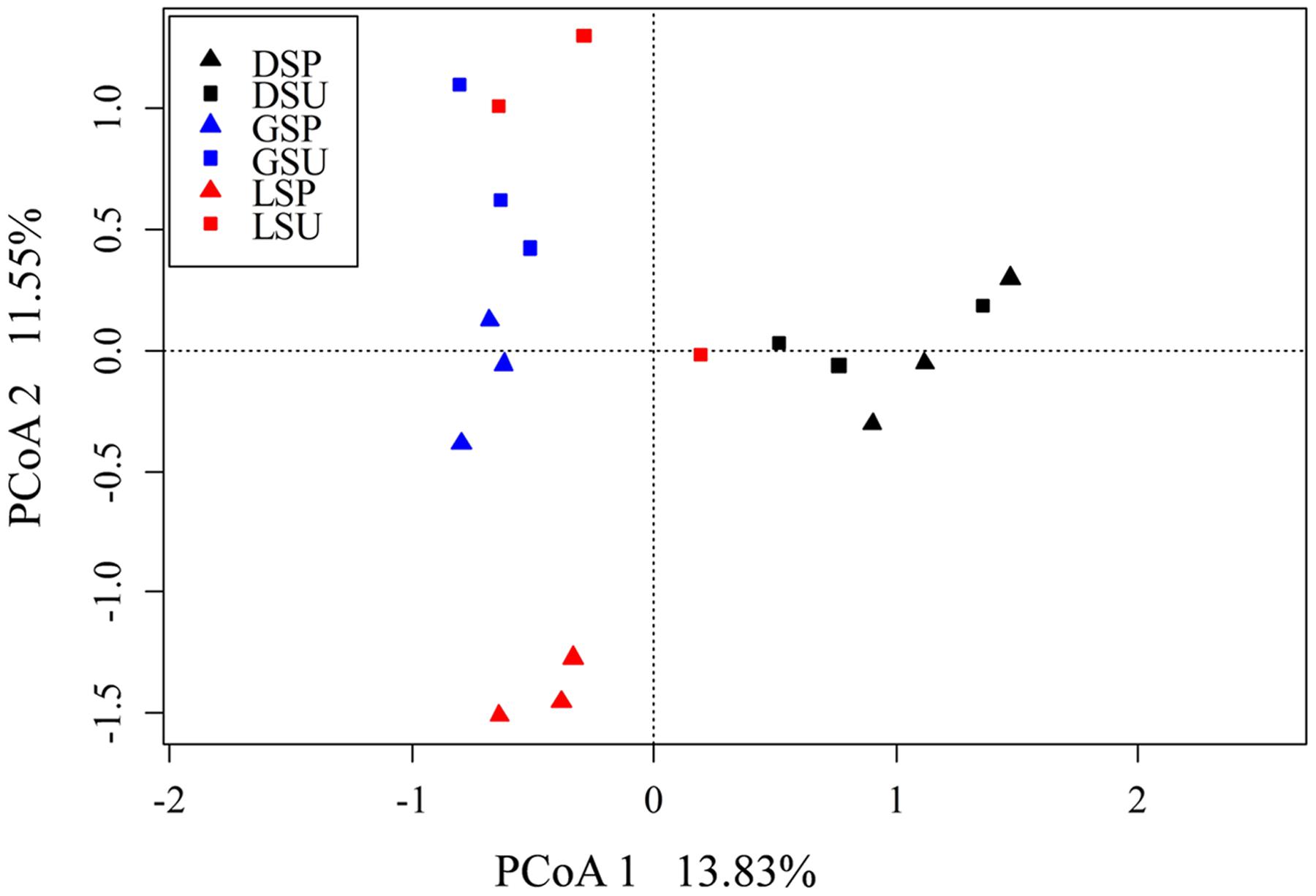
FIGURE 2. Principal coordinates analysis (PCoA) of diazotrophic community based on high-throughput nifH sequencing data. The percentage of variation explained by each axis is shown. DSP, spring P. decussata. DSU, summer P. decussata. GSP, spring G. astreata. GSU, summer G. astreata. LSP, spring P. lutea. LSU, summer P. lutea.
The nifH copy number was determined by quantitative PCR with the absolute quantification method. Significant seasonal variations were detected in both G. astreata and Porites lutea coral colonies (Table 2). The nifH gene copies in spring G. astreata colonies were significantly higher than those in summer (t-test, P < 0.001). However, the nifH gene copies in summer Porites lutea colonies were much higher than those collected in spring (t-test, P < 0.001). No statistical seasonal difference was detected in abundance of nifH gene copies from Pavona decussata colonies (t-test, P = 0.841; Table 2).
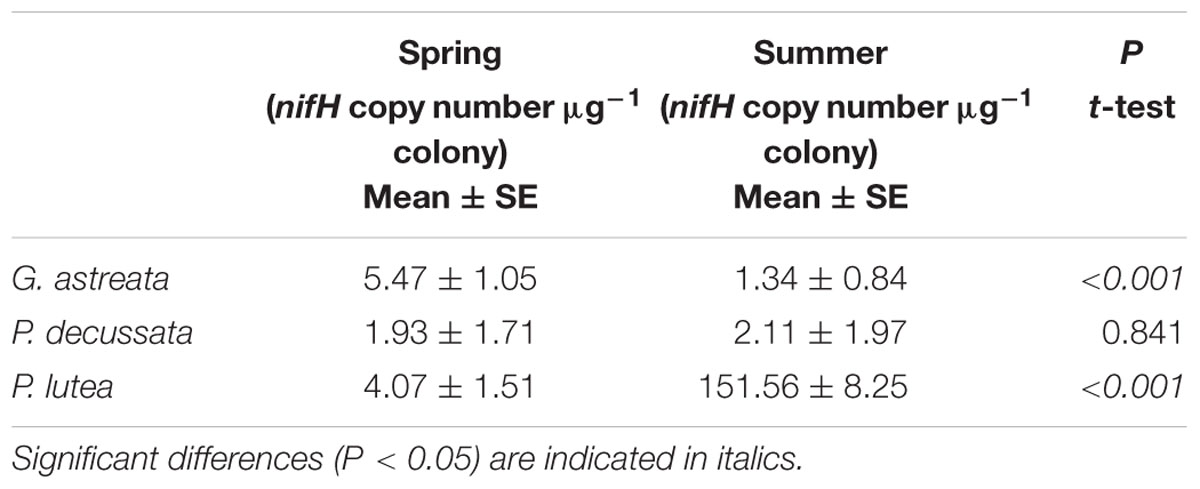
TABLE 2. Abundance of nifH copy number from three coral species in spring and summer (expressed as mean value and standard error, SE).
Seasonal Variations of Diazotrophic Communities
Seasonal variable diazotrophic communities were detected at 95% confidence interval. The numbers of variable OTUs were 45, 44, and 48 in coral G. astreata, Pavona decussata, and Porites lutea, respectively (Figure 3). These variable OTUs accounted for a range from 13.92 ± 1.56 to 38.45 ± 5.12% of the total sequences within individual coral samples (Supplementary Table S3). For all coral samples, the majority of variable nifH sequences fell within Alphaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria, and a few within Cyanobacteria, Betaproteobacteria, Chlorobi, Firmicutes, and Verrucomicrobia (Figure 3).
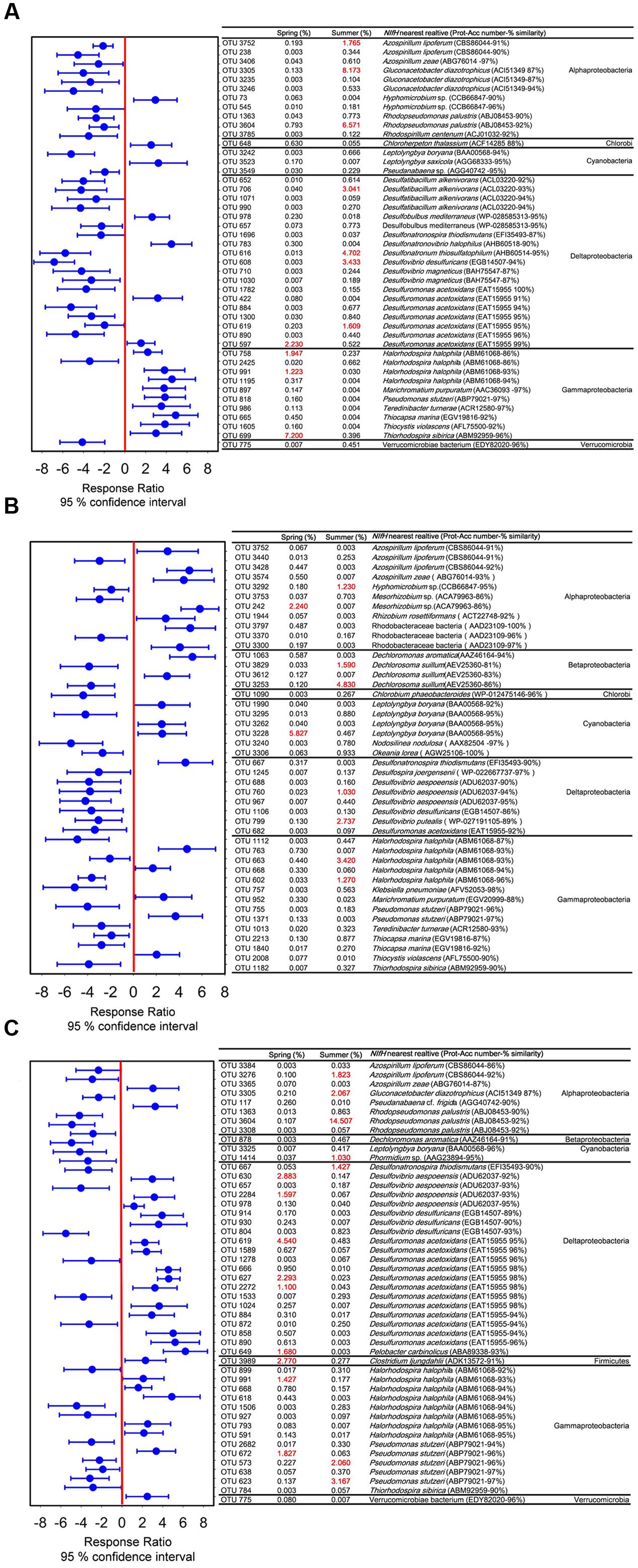
FIGURE 3. Significantly seasonal variable OTUs between spring and summer in G. astreata (A), P. decussata (B), and P. lutea (C) samples determined using the response ratio method at a 95% confidence interval based on the relative abundances. The variable OTUs are represented with their closest sequence match determined from GenBank BLAST, with corresponding accession number and taxonomic affiliation.
The dominant variable nifH sequences retrieved from spring G. astreata samples belonged to Gammaproteobacteria, however, those dominant in summer G. astreata samples belonged to Alphaproteobacteria and Deltaproteobacteria (Figure 3A). Within the Gammaproteobacteria, three dominant OTUs were identified (OTU669, OTU991, and OTU758) and constituted 10.37% of the total sequences recovered from spring G. astreata samples. The dominant group (OTU669) was affiliated with Thiorhodospira sibirica (96% similarity). Both OTU991 and OTU758 were affiliated with Halorhodospira halophila (93 and 86% similarity). More than 74% of total summer G. astreata variable nifH sequences (29.29% of 39.57%) belonged to seven OTUs (OTU3305, OTU3604, OTU3752, OTU706, OTU608, OTU616, and OTU619). The nifH sequences within the Alphaproteobacteria were relatively abundant in summer. The dominant groups (OTU3305, OTU3604, and OTU3752) comprising up to 16.51% of sequences recovered from summer G. astreata samples, were affiliated with Gluconacetobacter diazotrophicus (87% similarity), Rhodopseudomonas palustris (92% similarity) and Azospirillum lipoferum (91% similarity), respectively. The variable OTUs within the Deltaproteobacteria were all affiliated with anaerobic sulfate-reducing bacteria of which the dominant members OTU616, OTU608, OTU706 and OTU619 were affiliated with Desulfonatronum thiosulfatophilum (95% similarity), Desulfovibrio desulfuricans (94% similarity), Desulfatibacillum alkenivorans (93% similarity) and Desulfuromonas acetoxidans (95% similarity) respectively.
In contrast with G. astreata samples, diazotrophic communities associated with Pavona decussata samples showed different seasonal variation patterns (Figure 3B). OTU242 and OTU3228 dominated the variable nifH sequences derived from spring Pavona decussata samples. OTU3228 was closely related to a nifH sequence from cyanobacterium Leptolyngbya boryana (95% similarity). OTU242 demonstrated 86% identity with Alphaproteobacteria Mesorhizobium sp. Seven OTUs (OTU799, OTU760, OTU663, OTU602, OTU3292, OTU3253, and OTU3829) constituting more than 65% of total summer Pavona decussata variable nifH sequences (16.11 of 24.66%) fell within Alphaproteobacteria, Betaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria. The dominant groups (OTU3253 and OTU3829) represented 6.42% of the total sequences recovered from summer Pavona decussata samples affiliated with nifH sequences from Betaproteobacteria Dechlorosoma suillum. OTU799 and OTU760 were members of the Deltaproteobacteria class, affiliated with anaerobic sulfate-reducing bacterial genus Desulfovibrio. Within the Gammaproteobacteria class, the dominant groups (OTU663 and OTU602) which represented 4.69% of the total sequences recovered from summer Pavona decussata samples were both closest to Halorhodospira halophila. Furthermore, OTU3292 was affiliated with nifH sequences from Alphaproteobacteria, Hyphomicrobium sp. (95% similarity).
For coral Porites lutea, Deltaproteobacteria dominated the variable nifH sequences retrieved from spring samples, and the Alphaproteobacteria dominated those from summer ones (Figure 3C). Within the Deltaproteobacteria, the majority of these sequences were affiliated with the genera Desulfovibrio and Desulfuromonas, with six dominant ribotypes (OTU619, OTU627, OTU2272, OTU630, OTU2284, and OTU649) comprising up to 14.09% of sequences recovered from spring Porites lutea samples. All were affiliated with anaerobic sulfate-reducing bacteria Desulfovibrio aespoeensis, Desulfuromonas acetoxidans, and Pelobacter carbinolicus. With one exception in Deltaproteobacteria, the dominant variable (OTU667) affiliated with Desulfonatronospira thiodismutans (90% similarity) was relatively abundant in spring Porites lutea samples. Three dominant ribotypes (OTU3604, OTU3305, and OTU3276) falling within the Alphaproteobacteria, constituted 18.39% of total summer Porites lutea nifH sequences. The most dominant group (OTU3604), which represented 14.51% of the total sequences recovered from summer Porites lutea samples, affiliated with purple non-sulfur bacterium Rhodopseudomonas palustris (92% similarity). Two other Alphaproteobacteria groups (OTU3305 and OTU3276) affiliated with Gluconacetobacter diazotrophicus (87% similarity) and Azospirillum lipoferum (92% similarity), respectively. There were four dominant variable ribotypes recovered from Porites lutea samples falling within the Gammaproteobacteria. Two ribotypes (OTU991 and OTU672) were relatively abundant in spring samples affiliated with Halorhodospira halophila (93% similarity) and Pseudomonas stutzeri (96% similarity), respectively. Other groups (OTU623 and OTU573) were significantly abundant in summer samples, and both affiliated with Pseudomonas stutzeri (96–97% similarity). Within the Cyanobacteria, the dominant group (OTU1414), affiliated with Phormidium sp. (95% similarity), was relatively abundant in summer Porites lutea samples. In addition, the dominant ribotype (OTU3989) affiliated with Firmicutes, Clostridium arbusti (93% similarity), was relatively abundant in spring Porites lutea samples.
The majority of variable nifH groups from coral Pavona decussata were species-specific. Only one overlap variable OTU was found between the coral G. astreata and Pavona decussata samples and two overlap OTUs were detected between the coral Pavona decussata and Porites lutea colonies (Supplementary Table S4). These overlap OTUs were generally present in relatively low abundance in all Pavona decussata colonies. Ten overlap OTUs were recovered from both G. astreata and Pavona decussata, of which the majority of dominant groups showed the same seasonal variation (Supplementary Table S4). Two Alphaproteobacteria OTUs (OTU3604 and OTU3305) were relatively abundant, representing 14.74 and 16.57% of the total sequences recovered from summer G. astreata and Porites lutea, respectively. The Gammaproteobacteria variable group (OTU991) was dominant in both G. astreata and Porites lutea spring colonies. One exception to this pattern is the Deltaproteobacteria variable (OTU619), which dominated summer G. astreata samples and spring Porites lutea samples.
The Relationship between Seasonal Variable Diazotrophic Community and the Surrounding Seawater Environmental Factors
To explore the possible relationship between the seasonal variable diazotrophic microbial community and environmental variables, RDA was performed. The results showed that all seasonal variable diazotrophic communities of three coral species were significantly correlated with ammonium and nitrate (P < 0.05, Table 3). In addition, the seasonal variable diazotrophic communities of coral G. astreata and Pavona decussata were significantly correlated with phosphorus (P < 0.05, Table 3). Axis 1 and 2 of the RDA biplot together were shown to contribute 84.8, 87.6, and 93.5% to the overall pattern of three coral species, respectively (Figure 4). Most of the dominant variable diazotrophic OTUs of spring samples were highly positively correlated with ammonium and phosphate (Figure 4). In contrast, those from summer samples were negatively correlated with ammonium and phosphate, but positively correlated with nitrate, salinity and temperature (Figure 4).
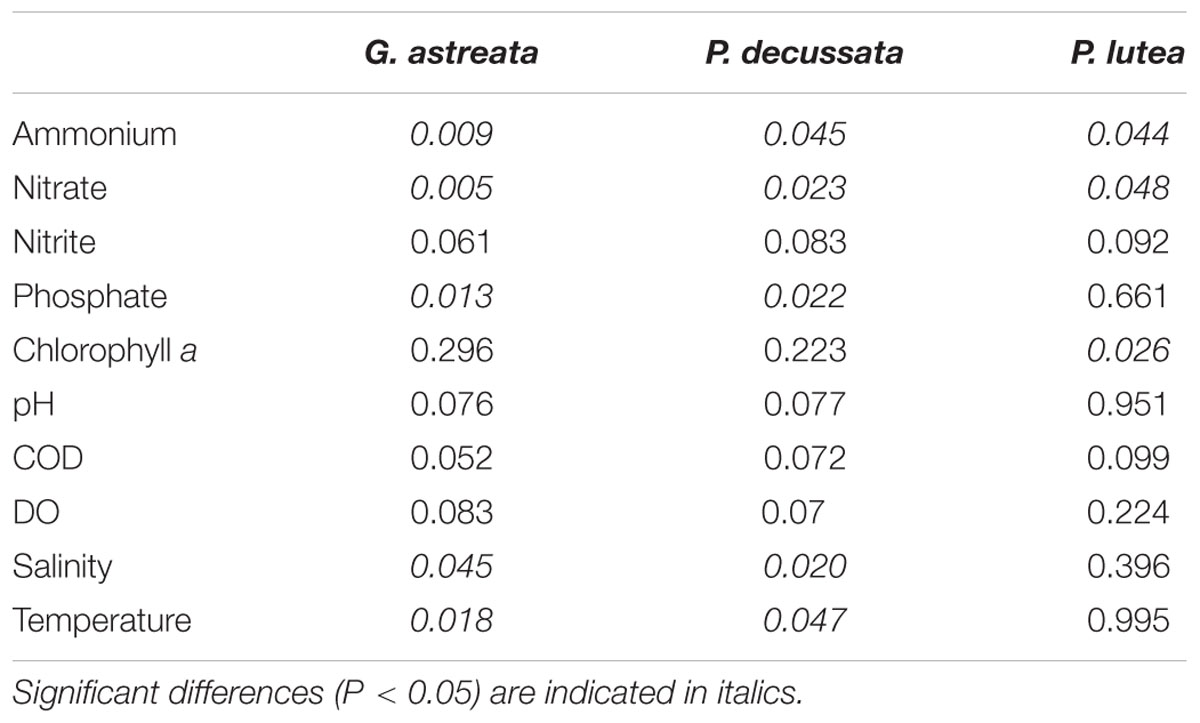
TABLE 3. Monte Carlo permutation test of environmental attributes with nifH high-throughput sequencing data.
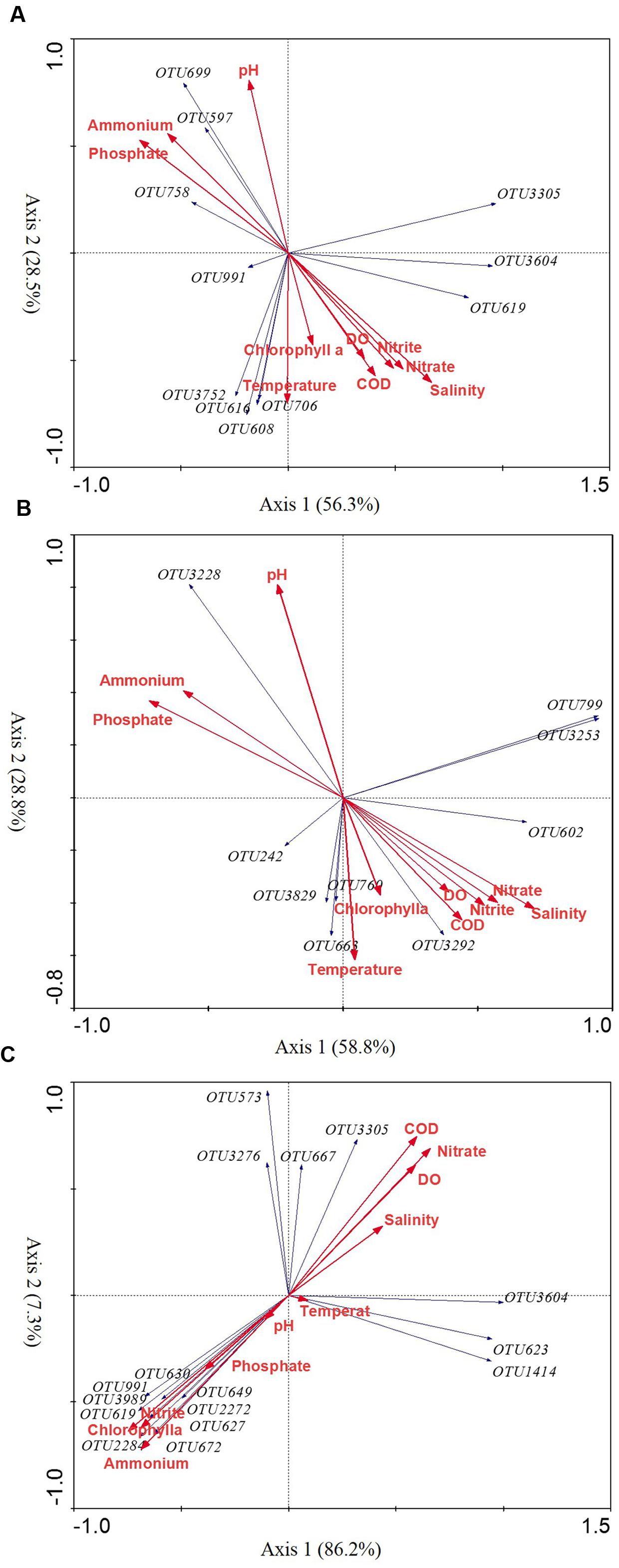
FIGURE 4. The redundancy analysis (RDA) ordination biplot showing the relationship between environmental variables and significantly seasonal variable OTUs in G. astreata (A), P. decussata (B), and P. lutea (C) samples. Only abundant variable OTUs (1%) are shown in the biplot.
Discussion
Although high-throughput sequencing approaches are now commonly applied to investigate coral-associated microbial communities (Ceh et al., 2011; Chen et al., 2011; Lee et al., 2012; Mckew et al., 2012; Sunagawa et al., 2009), it has not been widely used to target nitrogen-fixing functional genes (nifH) to explore the coral-associated diazotrophic communities (Lema et al., 2014b). Compared with previous high-throughput sequencing studies on diazotrophic communities associated with the common Great Barrier Reef (GBR) coral Acropora millepora (Lema et al., 2014b), a much greater diversity of bacteria having the potential to fix nitrogen (possess the nifH gene) associated with G. astreata, Pavona decussata, and Porites lutea from Luhuitou fringing reef, South China Sea (Table 1), and might be attributed to the differences in coral species. Alphaproteobacteria was reported as the dominant diazotrophic group associated with the common GBR coral Acropora (i.e., A. millepora and A. muricata) (Lema et al., 2012), while Gammaproteobacteria was the most commonly found class in the Hawaiian Montipora corals (M. capitata and M. flabellata) (Olson et al., 2009). In the present study, nifH sequences related to Alphaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria were the ubiquitous and dominant groups of three coral species in two seasons. These three groups constitute from 46.65 to 91.1% of the sequences within individual coral samples (Figure 1), suggesting that those groups play an important functional role in the coral holobiont. The coral holobiont is a complex habitat, and microenvironmental variability can strongly influence the abundance of associated microbial communities (Rohwer et al., 2002; Ainsworth et al., 2010). Previous results reported that the coral-associated bacterial community showed rapid seasonal changes for several coral species distributed throughout different regions (Ceh et al., 2011; Chen et al., 2011). However, season did not influence the diazotrophic communities associated with common GBR coral A. millepora (Lema et al., 2014b). Significant differences were detected in the diazotrophic abundance of G. astreata and Porites lutea, indicating that for these two coral species the diazotrophic communities significantly changed according to the season.
Understanding the seasonal dynamics of this association is important, as they have ecological implications. In this study, seasonal variable diazotrophic sequences were detected based on the relative abundances at 95% confidence interval. The analyses showed that three corals have a low diversity group of seasonal variable species (range from 44 to 48 OTUs) whose abundance varies widely across individuals (Supplementary Table S3). These results suggested that there are core diazotrophic microbiomes associated with corals and these cores are complemented with seasonal variable diazotrophic microbiomes. Most of the variable nifH groups from Pavona decussata were species-specific. Only a few variable OTUs overlap with two other coral species. This may be an indicator of a specific association between coral and diazotrophic microorganisms. The majority of overlapping OTUs in G. astreata and Porites lutea showed the same seasonal variation suggesting that except host species, the variation of diazotrophic communities might be related to the biogeochemical cycling processes within the holobiont, given the important role of diazotrophs in driving biogeochemical cycles (Rädecker et al., 2014).
Alphaproteobacteria affiliated with the order Rhizobiales were reported as the continuous and dominant diazotrophic assemblages associated with the common GBR Acropora corals (Lema et al., 2012, 2014a,b). Additionally, the rhizobia found within pure mucus samples of New Caledonia reefs (predominantly composed of Acropora sp.) was much higher than in the surrounding seawater in the summer period and 400-fold higher in the winter (Camps et al., 2016). In the present study, the variable Alphaproteobacteria affiliated with Rhizobiales and Rhodospirillales. One rhizobial group (OTU3604) was most closely affiliated with purple non-sulfur phototrophic bacterium Rhodopseudomonas palustris. This group dominated all spring G. astreata and Porites lutea colonies, representing up to 6.57 and 14.51% of the total nifH sequences in spring G. astreata and Porites lutea samples, respectively (Figure 3). The Rhodospirillales groups closely related to Gluconacetobacter diazotrophicus and Azospirillum lipoferum were also the dominant variable groups found in all G. astreata and Porites lutea colonies. It is notable that the most dominant variable group of G. astreata was closely related to Gluconacetobacter diazotrophicus. Here Rhodopseudomonas palustris- and Gluconacetobacter diazotrophicus-affiliated OTUs were not correlated with investigated environmental factors, which suggested the seasonal variations of these diazotrophs are not affected by environmental factors. Rhodopseudomonas palustris is notable for its ability to flexibly switch between four different modes of metabolism: photoautotrophic, photoheterotrophic, chemoautotrophic, and chemoheterotrophic (Larimer et al., 2004), which might protect it from high concentrations of oxygen arising from dinoflagellate photosynthesis in coral tissues (Kuhl et al., 1995). The most common physiological characteristics of Gluconacetobacter diazotrophicus are its growth and nitrogen fixation at low pH and that nitrogen fixation is not affected by high concentrations of nitrate nitrogen (Pedraza, 2008; Eskin et al., 2014). Additionally, it is capable of undergoing nitrogen fixation with low amounts of ammonium-based nitrogen (Stephan et al., 1991; Fisher and Newton, 2005). In contrast with G. astreata and Porites lutea, the dominant Alphaproteobacteria variable groups in Pavona decussata showed different patterns. One rhizobial group affiliated with Hyphomicrobium sp. was the dominant group in spring colonies while another rhizobial group affiliated with Mesorhizobium sp. was the dominant group in summer colonies.
The second most seasonal variable group of diazotrophs detected by our nifH-based gene assay were all affiliated with anaerobic sulfate-reducing Deltaproteobacteria. Anaerobic sulfate-reducing bacteria were commonly detected in both 16S rRNA and nifH gene surveys of presumably healthy corals (Olson et al., 2009; Sunagawa et al., 2010 Lema et al., 2012, 2014b; Fernando et al., 2015). Although oxygenic photosynthesis renders most of the coral interior oxic during the day, coral microhabitats may host diazotrophic communities under oxygen-depleted conditions within the gastrodermis (Hoegh-Guldberg and Williamson, 1999) and microaerophilic regions in the gastrovascular cavities of coral polyps (Agostini et al., 2012). Additionally, coral tissues compromised by contact with stagnant water or sediment and coral surfaces (mucus layer) and skeletons enable anaerobic forms of bacterial respiration and fermentation to occur within the holobiont (Thompson et al., 2014). The present study results showed that the seasonal variations of Deltaproteobacteria diazotrophs differed among coral species. The Deltaproteobacteria groups were relatively abundant in summer G. astreata and Pavona decussata samples and spring Porites lutea samples. The dominant variable Deltaproteobacteria groups of G. astreata and Pavona decussata were positively correlated with nitrate and salinity; however, the dominant variable Deltaproteobacteria groups of Porites lutea were negatively correlated with nitrate, but positively correlated with ammonium (Figure 4). Additionally, the dominant variable Deltaproteobacteria groups also varied among coral host species, indicating that both host species and environmental factors cause seasonal shifts of Deltaproteobacteria diazotrophs.
Gammaproteobacteria genus Vibrio was reported as consistent members of diazotrophic assemblages associated with Hawaiian corals in the genus Montipora (Olson et al., 2009) and common GBR coral A. millepora (Lema et al., 2014b) based on nifH sequencing. Here, genus Vibrio were also consistent members of diazotrophic assemblages associated with three coral species, and no significant seasonal variation was detected based on nifH sequence. However, genus Vibrio is a variable bacterial group associated with coral G. astreata and Porites lutea and Vibrio-affiliated bacterial sequences represented 0–0.84% of the 16S rRNA gene sequences recovered. The dominant seasonal variable Gammaproteobacteria affiliated with Halorhodospira halophila, Thiorhodospira sibirica, and Pseudomonas stutzeri. Halorhodospira halophila, an obligately photosynthetic and extremely halophilic purple sulfur bacterium (Tsuihiji et al., 2006), showed relative abundance in spring coral G. astreata and Porites lutea, but was dominant in summer Pavona decussata samples. One group affiliated with Thiorhodospira sibirica, one of the alkaliphilic purple sulfur bacteria (Bryantseva et al., 1999), represented up to 7.2% of the total nifH sequences in spring G. astreata samples and positively correlated with ammonium and phosphate. Additionally, Pseudomonas stutzeri, a nitrogen-fixing and denitrifying bacterium (Lalucat et al., 2006), was relatively abundant in summer Porites lutea samples.
The most dominant variable group of Pavona decussata, closely related to Cyanobacteria Leptolyngbya boryana, represented up to 5.83% of the total nifH sequences in spring Pavona decussata samples, positively correlated with ammonium and phosphate. Another Cyanobacteria group closely related to Phormidium sp. was relatively abundant in summer Porites lutea samples. Both genera Leptolyngbya and Phormidium have previously been reported in a number of different coral species black band disease (BBD) samples where they have been hypothesized to play roles as pathogens (Frias-Lopez et al., 2003; Richardson and Kuta, 2003; Myers et al., 2007). The nifH phylotypes affiliated with Firmicutes Clostridium ljungdahlii were only detected showing significant seasonal variation in Porites lutea samples, comprising 2.77% of nifH phylotypes derived from spring Porites lutea and positively correlated with ammonium and phosphate.
The seasonal variations in coral-associated bacterial and diazotrophic communities may be influenced by the nutrient loads between spring and summer. The sample collection location Sanya Bay is affected by cold-water upwelling during the summer (Huang et al., 2003; Zhang et al., 2010; Wu et al., 2012). Cold-water upwelling affects the distribution of a variety of dissolved and particulate forms of nitrogen in Sanya Bay (Huang et al., 2003). Ammonium was the predominant dissolved nitrogen in spring, while nitrate was the predominant dissolved nitrogen in summer (Supplementary Table S1). In our previous study, the functional gene composition of the microbial community was significantly correlated with the concentrations of inorganic nitrogen and phosphate (Zhang et al., 2015). Here, all diazotrophic communities of the three coral species were significantly correlated with ammonium and nitrate (Table 3). In addition, the diazotrophic communities of coral G. astreata and Pavona decussata were significantly correlated with phosphorus (Table 3). This suggested that the apparent seasonal change in diazotrophic communities of corals could be linked to the seasonal shifts of nutrients. A priority for future studies should be to identify environmental variables contributing to these shifts in coral bacterial communities and to determine how they influence the health of the coral host.
Conclusion
This study revealed a much greater diversity of diazotrophs associated with G. astreata, Pavona decussata, and Porites lutea. Alphaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria were the ubiquitous and dominant groups in all corals in two seasons. Seasonal factors did not cause shifts in diazotrophic richness and diversities of the three coral species; even no shifts in diazotrophic communities and abundance were observed in coral Pavona decussata. In contrast, the diazotrophic communities and nifH gene abundance of both G. astreata and Porites lutea showed significant seasonal changes. Most of the variable nifH groups from Pavona decussata were species-specific. The dominant overlap OTUs in G. astreata and Porites lutea showed the same seasonal variation. The seasonal variations of diazotrophic communities were significantly correlated with the seasonal shifts of nutrients. Variable diazotroph groups are widely distributed in the environment and may be of relevance to diverse metabolic potential, such as carbon fixation and sulfate reduction. This suggests that their potential to provide additional sources of fixed nitrogen to the coral holobiont may be functionally important. However, almost all metabolic potential of these diazotrophs was referred from crops and land plants. The physiological roles of these nitrogen-fixing symbionts in the nitrogen budget and cycling within corals need to be investigated in detail.
Author Contributions
YZ and JD conceived the research. YZ and QY performed the experiments. YZ wrote the manuscript. JVN and JZ edited the manuscript. QY and ZS contributed sampling or data analysis pipeline. All authors reviewed and accepted the manuscript.
Funding
The research was supported by the National Natural Science Foundation of China (41276114, 41276113, 41676107), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA13020300), the Sanya Station Database and the Information System of CERN, and the China Scholarship Council (201308440117).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Gail Welch for helpful edits. We thank all of the members of the tropical Marine Biological Research station in Hainan for their work in the field samples collection.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01870/full#supplementary-material
Footnotes
References
Agostini, S., Suzuki, Y., Higuchi, T., Casareto, B. E., Yoshinaga, K., Nakano, Y., et al. (2012). Biological and chemical characteristics of the coral gastric cavity. Coral Reefs 31, 147–156. doi: 10.1007/s00338-011-0831-6
Ainsworth, T. D., Krause, L., Bridge, T., Torda, G., Raina, J. B., Zakrzewski, M., et al. (2015). The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 9, 2261–2274. doi: 10.1038/ismej.2015.39
Ainsworth, T. D., Thurber, R. V., and Gates, R. D. (2010). The future of coral reefs: a microbial perspective. Trends Ecol. Evol. 25, 233–233. doi: 10.1016/j.tree.2009.11.001
Benavides, M., Moisander, P. H., Daley, M. C., Bode, A., and Arístegui, J. (2016). Longitudinal variability of diazotroph abundances in the subtropical North Atlantic Ocean. J. Plankton Res. 38, 662–672. doi: 10.1093/plankt/fbv121
Bryantseva, I., Gorlenko, V. M., Kompantseva, E. I., Imhoff, J. F., Süling, J., Mityushina, L., et al. (1999). Thiorhodospira sibirica gen. nov., sp. nov., a new alkaliphilic purple sulfur bacterium from a Siberian soda lake. Int. J. Syst. Evol. Microbiol. 49, 697–703.
Camps, M., Benavides, M., Lema, K. A., and Bonnet, S. (2016). Released coral mucus does not enhance planktonic N2 fixation rates. Aquat. Microb. Ecol. 77, 51–63. doi: 10.3354/ame01787
Capone, D. G., Taylor, D. L., and Taylor, B. F. (1977). Nitrogen fixation (acetylene reduction) associated with macroalgae in a coral-reef community in the Bahamas. Mar. Biol. 40, 29–32. doi: 10.1007/BF00390624
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Cardini, U., Bednarz, V. N., Foster, R. A., and Wild, C. (2014). Benthic N2 fixation in coral reefs and the potential effects of human-induced environmental change. Ecol. Evol. 4, 1706–1727. doi: 10.1002/ece3.1050
Ceh, J., Keulen, M. V., and Bourne, D. G. (2011). Coral-associated bacterial communities on Ningaloo Reef, Western Australia. FEMS Microbiol. Ecol. 75, 134–144. doi: 10.1111/j.1574-6941.2010.00986.x
Chen, C., Tseng, C., Chen, C. A., and Tang, S. (2011). The dynamics of microbial partnerships in the coral Isopora palifera. ISME J. 5, 728–740. doi: 10.1038/ismej.2010.151
Chimetto, L. A., Brocchi, M., Thompson, C. C., Martins, R. C. R., Ramos, H. R., and Thompson, F. L. (2008). Vibrios dominate as culturable nitrogen-fixing bacteria of the Brazilian coral Mussismilia hispida. Syst. Appl. Microbiol. 31, 312–319. doi: 10.1016/j.syapm.2008.06.001
Deng, Y., He, Z., Xu, M., Qin, Y., Van Nostrand, J. D., Wu, L., et al. (2012). Elevated carbon dioxide alters the structure of soil microbial communities. Appl. Environ. Microbiol. 78, 2991–2995. doi: 10.1128/AEM.06924-11
Eskin, N., Vessey, K., and Tian, L. (2014). Research progress and perspectives of nitrogen fixing bacterium, Gluconacetobacter diazotrophicus, in monocot plants. Int. J. Agron. 2014, 1–13. doi: 10.1155/2014/208383
Fernando, S., Wang, J., Sparling, K., Garcia, G., Francini-Filho, R., de Moura, R., et al. (2015). Microbiota of the major South Atlantic reef building coral Mussismilia. Microb. Ecol. 69, 267–280. doi: 10.1007/s00248-014-0474-6
Fisher, K., and Newton, W. E. (2005). Nitrogenase proteins from Gluconacetobacter diazotrophicus, a sugarcane-colonizing bacterium. Biochim. Biophys. Acta 1750, 154–165. doi: 10.1016/j.bbapap.2005.04.010
Frias-Lopez, J., Bonheyo, G. T., Jin, Q., and Fouke, B. W. (2003). Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Appl. Environ. Microbiol. 69, 2409–2413. doi: 10.1128/AEM.69.4.2409-2413.2003
Gaby, J. C., and Buckley, D. H. (2012). A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS ONE 7:e42149. doi: 10.1371/journal.pone.0042149
Grover, R., Ferrier-Pagès, C., Maguer, J. F., Ezzat, L., and Fine, M. (2014). Nitrogen fixation in the mucus of Red Sea corals. J. Exp. Biol. 217, 3962–3963. doi: 10.1242/jeb.111591
Haas, A. F., Fairoz, M. F., Kelly, L. W., Nelson, C. E., Dinsdale, E. A., Edwards, R. A., et al. (2016). Global microbialization of coral reefs. Nature Microbiol. 1:16042. doi: 10.1038/nmicrobiol.2016.42
Hoegh-Guldberg, O., and Williamson, J. (1999). Availability of two forms of dissolved nitrogen to the coral Pocillopora damicornis and its symbiotic zooxanthellae. Mar. Biol. 133, 561–570. doi: 10.1007/s002270050496
Huang, L., Tan, Y., Song, X., Huang, X., Wang, H., Zhang, S., et al. (2003). The status of the ecological environment and a proposed protection strategy in Sanya Bay, Hainan Island, China. Mar. Pollut. Bull. 47, 180–186. doi: 10.1016/S0025-326X(03)00070-5
Kelly, L. W., Williams, G. J., Barott, K. L., Carlson, C. A., Dinsdale, E. A., Edwards, R. A., et al. (2014). Local genomic adaptation of coral reef-associated microbiomes to gradients of natural variability and anthropogenic stressors. Proc. Natl. Acad. Sci. U.S.A. 111, 10227–10232. doi: 10.1073/pnas.1403319111
Kong, Y. (2011). Btrim: a fast, light weight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98, 152–153. doi: 10.1016/j.ygeno.2011.05.009
Kuhl, M., Cohen, Y., Dalsgaard, T., and Revsbech, N. P. (1995). Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 117, 159–172. doi: 10.3354/meps117159
Lalucat, J., Bennasar, A., Bosch, R., García-Valdés, E., and Palleroni, N. J. (2006). Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 70, 510–547. doi: 10.1128/MMBR.00047-05
Larimer, F. W., Chain, P., Hauser, L., Lamerdin, J., Malfatti, S., Do, L., et al. (2004). Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 22, 55–61. doi: 10.1038/nbt923
Lee, O. O., Yang, J., Bougouffa, S., Wang, Y., Batang, Z., Tian, R., et al. (2012). Spatial and species variations in bacterial communities associated with corals from the Red Sea as revealed by pyrosequencing. Appl. Environ. Microbiol. 78, 7173–7184. doi: 10.1128/AEM.01111-12
Lema, K. A., Bourne, D. G., and Willis, B. L. (2014a). Onset and establishment of diazotrophs and other bacterial associates in the early life history stages of the coral Acropora millepora. Mol. Ecol. 23, 4682–4695. doi: 10.1111/mec.12899
Lema, K. A., Willis, B. L., and Bourne, D. G. (2012). Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Appl. Environ. Microbiol. 78, 3136–3144. doi: 10.1128/AEM.07800-11
Lema, K. A., Willis, B. L., and Bourne, D. G. (2014b). Amplicon pyrosequencing reveals spatial and temporal consistency in diazotroph assemblages of the Acropora millepora microbiome. Environ. Microbiol. 16, 3345–3359. doi: 10.1111/1462-2920.12366
Lesser, M. P., Falcon, L. I., Rodriguez-Roman, A., Enriquez, S., Hoegh-Guldberg, O., and Iglesias-Prieto, R. (2007). Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar. Ecol. Prog. Ser. 346, 143–152. doi: 10.3354/meps07008
Magoč, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
McDole, T., Nulton, J., Barott, K. L., Felts, B., Hand, C., Hatay, M., et al. (2012). Assessing coral reefs on a Pacific-wide scale using the microbialization score. PLoS ONE 7:e43233. doi: 10.1371/journal.pone.0043233
Mckew, B. A., Dumbrell, A. J., Daud, S. D., Hepburn, L., Thorpe, E., Mogensen, L., et al. (2012). Characterization of geographically distinct bacterial communities associated with coral mucus produced by Acropora spp. and Porites spp. Appl. Environ. Microbiol. 78, 7173–7184. doi: 10.1128/AEM.07764-11
Mouchka, M. E., Hewson, I., and Harvell, C. D. (2010). Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integr. Comp. Biol. 50, 662–674. doi: 10.1093/icb/icq061
Myers, J. L., Sekar, R., and Richardson, L. L. (2007). Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 73, 5173–5182. doi: 10.1128/AEM.00900-07
Olson, N. D., Ainsworth, T. D., Gates, R. D., and Takabayashi, M. (2009). Diazotrophic bacteria associated with Hawaiian Montipora corals: diversity and abundance in correlation with symbiotic dinoflagellates. J. Exp. Mar. Biol. Ecol. 371, 140–146. doi: 10.1016/j.jembe.2009.01.012
Pedraza, R. O. (2008). Recent advances in nitrogen-fixing acetic acid bacteria. Int. J. Food Microbiol. 125, 25–35. doi: 10.1016/j.ijfoodmicro.2007.11.079
Pernice, M., Meibom, A., Van Den Heuvel, A., Kopp, C., Domart-Coulon, I., Hoegh-Guldberg, O., et al. (2012). A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis. ISME J. 6, 1314–1324. doi: 10.1038/ismej.2011.196
Poly, F., Monrozier, L. J., and Bally, R. (2001). Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 152, 95–103. doi: 10.1016/S0923-2508(00)01172-4
Rädecker, N., Cardini, U., Wild, C., Meyer, F. W., and Bednarz, V. N. (2014). Ocean acidification rapidly reduces dinitrogen fixation associated with the hermatypic coral Seriatopora hystrix. Mar. Ecol. Prog. Ser. 511, 297–302. doi: 10.3354/meps10912
Rädecker, N., Pogoreutz, C., Voolstra, C. R., Wiedenmann, J., and Wild, C. (2015). Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol. 23, 490–497. doi: 10.1016/j.tim.2015.03.008
Richardson, L. L., and Kuta, K. G. (2003). Ecological physiology of the black band disease cyanobacterium Phormidium corallyticum. FEMS Microbiol. Ecol. 43, 287–298. doi: 10.1016/S0168-6496(03)00025-4
Rohwer, F., Seguritan, V., Azam, F., and Knowlton, N. (2002). Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 243, 1–10. doi: 10.3354/meps243001
Santos, H. F., Carmo, F. L., Duarte, G., Dini-Andreote, F., Castro, C. B., Rosado, A. S., et al. (2014). Climate change affects key nitrogen-fixing bacterial populations on coral reefs. ISME J. 8, 2272–2279. doi: 10.1038/ismej.2014.70
Shashar, N., Cohen, Y., Loya, Y., and Sar, N. (1994a). Nitrogen fixation (acetylene reduction) in stony corals: evidence for coral- bacteria interactions. Mar. Ecol. Prog. Ser. 111, 259–264. doi: 10.3354/meps111259
Shashar, N., Feldstein, T., Cohen, Y., and Loya, Y. (1994b). Nitrogen fixation (acetylene reduction) on a coral reef. Coral Reefs 13, 171–174. doi: 10.1007/BF00301195
Stephan, M. P., Oliveira, M., Teixeira, K. R. S., Martinez-Drets, G., and Döbereiner, J. (1991). Physiology and dinitrogen fixation of Acetobacter diazotrophicus. FEMS Microbiol. Lett. 77, 67–72. doi: 10.1111/j.1574-6968.1991.tb04323.x
Sunagawa, S., DeSantis, T. Z., Piceno, Y. M., Brodie, E. L., DeSalvo, M. K., Voolstra, C. R., et al. (2009). Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 3, 512–521. doi: 10.1038/ismej.2008.131
Sunagawa, S., Woodley, C. M., and Medina, M. (2010). Threatened corals provide underexplored microbial habitats. PLoS ONE 5:e9554. doi: 10.1371/journal.pone.0009554
Thompson, J. R., Rivera, H. E., Closek, C. J., and Medina, M. (2014). Microbes in the coral holobiont: partners through evolution, development, and ecological interactions. Front. Cell Infect. Microbiol. 4:176. doi: 10.3389/fcimb.2014.00176
Tsuihiji, H., Yamazaki, Y., Kamikubo, H., Imamoto, Y., and Kataoka, M. (2006). Cloning and characterization of nif structural and regulatory genes in the purple sulfur bacterium, Halorhodospira halophila. J. Biosci. Bioeng. 101, 263–270. doi: 10.1263/jbb.101.263
Wang, Q., Quensen, J. F., Fish, J. A., Kwon Lee, T., Sun, Y., Tiedje, J. M., et al. (2013). Ecological patterns of nifH genes in four terrestrial climatic zones explored with targeted metagenomics using FrameBot, a new informatics tool. mBio 4:e592-13. doi: 10.1128/mBio.00592-13
Wiebe, W. J., Johannes, R. E., and Webb, K. L. (1975). Nitrogen fixation in a coral reef community. Science 188, 257–259. doi: 10.1126/science.188.4185.257
Williams, W. M., Viner, A. B., and Broughton, W. J. (1987). Nitrogen fixation (acetylene reduction) associated with the living coral Acropora variabilis. Mar. Biol. 94, 531–535. doi: 10.1007/BF00431399
Wu, M., Ling, J., Long, L., Zhang, S., Zhang, Y., Wang, Y., et al. (2012). Influence of human activity and monsoon dynamics on spatial and temporal hydrochemistry in tropical coastal waters (Sanya Bay, South China Sea). Chem. Ecol. 28, 1–16.
Zehr, J. P., Jenkins, B. D., Short, S. M., and Steward, G. F. (2003). Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5, 539–554. doi: 10.1046/j.1462-2920.2003.00451.x
Zhang, Y., Dong, J., Ling, J., Wang, Y., and Zhang, S. (2010). Phytoplankton distribution and their relationship to environmental variables in Sanya Bay, South China Sea. Sci. Mar. 74, 783–792.
Keywords: diazotrophs, diversity, nifH, pyrosequencing, coral reef
Citation: Zhang Y, Yang Q, Ling J, Van Nostrand JD, Shi Z, Zhou J and Dong J (2016) The Shifts of Diazotrophic Communities in Spring and Summer Associated with Coral Galaxea astreata, Pavona decussata, and Porites lutea. Front. Microbiol. 7:1870. doi: 10.3389/fmicb.2016.01870
Received: 08 July 2016; Accepted: 07 November 2016;
Published: 22 November 2016.
Edited by:
Baolei Jia, Chung-Ang University, South KoreaReviewed by:
Mar Benavides, Mediterranean Institute of Oceanography, New CaledoniaXiaoshou Liu, Ocean University of China, China
M. F. M. Fairoz Mohamed Farook, Ocean University of Sri Lanka, Sri Lanka
Copyright © 2016 Zhang, Yang, Ling, Van Nostrand, Shi, Zhou and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junde Dong, ZG9uZ2pkQHNjc2lvLmFjLmNu
 Yanying Zhang
Yanying Zhang Qingsong Yang1
Qingsong Yang1 Joy D. Van Nostrand
Joy D. Van Nostrand Jizhong Zhou
Jizhong Zhou