
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 06 October 2016
Sec. Microbial Physiology and Metabolism
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.01560
This article is part of the Research Topic Multicellularity, cell-cell interactions and social behavior in bacterial populations View all 33 articles
In addition to its role in DNA damage repair and recombination, the RecA protein, through its interaction with CheW, is involved in swarming motility, a form of flagella-dependent movement across surfaces. In order to better understand how SOS response modulates swarming, in this work the location of RecA and CheW proteins within the swarming cells has been studied by using super-resolution microscopy. Further, and after in silico docking studies, the specific RecA and CheW regions associated with the RecA-CheW interaction have also been confirmed by site-directed mutagenesis and immunoprecipitation techniques. Our results point out that the CheW distribution changes, from the cell poles to foci distributed in a helical pattern along the cell axis when SOS response is activated or RecA protein is overexpressed. In this situation, the CheW presents the same subcellular location as that of RecA, pointing out that the previously described RecA storage structures may be modulators of swarming motility. Data reported herein not only confirmed that the RecA-CheW pair is essential for swarming motility but it is directly involved in the CheW distribution change associated to SOS response activation. A model explaining not only the mechanism by which DNA damage modulates swarming but also how both the lack and the excess of RecA protein impair this motility is proposed.
RecA is a multifunctional protein present in almost all members of the Bacteria domain (Eisen, 1995). In the presence of single-stranded DNA (ssDNA), generated, for instance, by direct or indirect DNA damage, RecA becomes activated (RecA∗) (Sassanfar and Roberts, 1990; Michel, 2005) acquiring co-protease activity that prompts auto-cleavage of the LexA repressor which governs the SOS response (Little, 1991). LexA cleavage triggers not only the expression of recA itself but also that of other SOS genes, mostly those involved in DNA recombination and repair (Courcelle et al., 2001). Further, it has been described that RecA is associated with the cell membrane forming foci often located at the cell poles that are redistributed along the cell in response to DNA damage (Renzette et al., 2005; Lesterlin et al., 2014; Rajendram et al., 2015). RecA, however, aside from its role in DNA damage repair and as a DNA damage sensor, has been directly related to swarming motility (Gómez-Gómez et al., 2007; Medina-Ruiz et al., 2010), through its interaction with the CheW protein (Mayola et al., 2014; Irazoki et al., 2016).
Swarming motility is the rapid, organized multicellular translocation of bacteria across a moist surface. It is powered by rotating flagella (Henrichsen, 1972) and is widely distributed through the Bacteria Domain (Harshey, 1994). Swarming is associated with elevated resistance to multiple antibiotics (Ottemann and Miller, 1997; Kim and Surette, 2003; Kim et al., 2003; Wang et al., 2004; Overhage et al., 2008; Katribe et al., 2009) and is essential for bacterial colonization of host surfaces (Nakajima et al., 2008; Barak et al., 2009; Katribe et al., 2009). Like other components of the chemotaxis pathway, the CheW protein plays a key role in swarming ability (Burkart et al., 1998; Mariconda et al., 2006). As the chemoreceptor adaptor, CheW couples the transmembrane methyl-accepting chemoreceptor protein trimers of dimers (MCPs) to CheA, a histidine kinase that transfers the signal to the CheY response regulator, which acts on the flagellar motor by switching flagellar rotation according to the stimuli detected by the MCPs (Boukhvalova et al., 2002; Sourjik and Wingreen, 2012). To avoid saturation of the sensory system, the chemoreceptor signal is reset by the activity of a methyltransferase (CheR) and a methylesterase (CheB), both of which are located in the vicinity of the chemoreceptors and which restore pre-stimulus activity through reversible covalent modification of the MCPs (Sourjik and Wingreen, 2012).
These signaling complexes pack together to form large chemoreceptor arrays, ranging from a few to 1000s of proteins and located at the cell poles. By acting as antennae, they amplify the signal generated in response to slight changes in the concentrations of attractants or repellents detected by MCPs (Briegel et al., 2012, 2014a; Sourjik and Wingreen, 2012; Cassidy et al., 2015). The chemoreceptor array assembly has been the focus of several studies (Shiomi et al., 2006; Thiem and Sourjik, 2008; Jones and Armitage, 2015). The newly synthesized signaling complexes are distributed in a helical fashion at the cell membrane via their association with cytoskeletal proteins such as MreB or the Sec secretion system (Shiomi et al., 2006; Oh et al., 2014). Then, by stochastic self-assembly (Greenfield et al., 2009) or by an active process (Shiomi et al., 2006), these complexes form large clusters by joining existing arrays or by the formation of new nucleation centers. Stabilization of these clusters is a function of both the cell membrane curvature in the polar region (Oh et al., 2014) and the presence of CheA and CheW (Shiomi et al., 2005). These proteins are directly involved in the stabilization of these clusters, as they interact to form structural core linkers [CheW-CheA2-CheW] across the cytoplasmic domain of MCPs, thereby clustering the chemoreceptors into hexagonal rings. The assembled array may thus contain dozens to 100s of hexagons (Briegel et al., 2014b; Cassidy et al., 2015). Within the hexagons is a CheW ring that couples neighboring chemoreceptors and strengthens the stability of the chemosensory array (Cassidy et al., 2015).
The presence of polar chemoreceptor arrays is essential for swarming motility in soft swarmers, such as Escherichia coli and Salmonella enterica (Cardozo et al., 2010; Santos et al., 2014). In the latter bacterium, an alteration in the balance of RecA/CheW impairs chemoreceptor cluster assembly and thus modulates bacterial swarming motility (Cardozo et al., 2010; Medina-Ruiz et al., 2010; Irazoki et al., 2016). The overexpression of RecA, without its activation, is sufficient to abolish swarming (Irazoki et al., 2016). Thus, by using RecA as a sensor mechanism, S. enterica cells can adapt their surface motility in response to the presence of direct or indirect DNA-damaging agents, by sensing these compounds through SOS system induction (Irazoki et al., 2016).
Although, RecA is known to interact with CheW (Mayola et al., 2014), where the interaction occurs within the cell and its nature are poorly understood. In an attempt to answer these questions and to better understand how the SOS response modulates swarming, in this work we have determined the regions involved in RecA and CheW interaction and the location of these proteins within SOS response induced-S. enterica swarming cells as well as RecA-CheW interaction relationship with swarming motility.
All bacterial strains and vectors used in this work are indicated in Table 1. Except when indicated, all strains were grown at 37°C in Luria-Bertani (LB) broth or on LB plates, supplemented, when necessary, with ampicillin (100 μg/mL), chloramphenicol (34 μg/mL), and/or kanamycin (10 μg/mL).
Fluorescent immunolabeling was carried out as described (Buddelmeijer et al., 2013), with a few modifications. All samples were obtained from the edge of the corresponding swarming plates as previously described (Kim and Surette, 2005). The cells were grown, as described below for swarming assays, on LB-swarming plates [1% tryptone, 0.5% yeast extract, 0.5% NaCl, 0.5% D-(+)-glucose, and 0.5% agar] supplemented when needed with 0.08 mitomycin C/mL or 30 μM of IPTG. After 14 h of incubation at 37°C, the cells were suspended in 1 mL of ice-cold tethering buffer (10 mM potassium-phosphate pH 7, 67 mM NaCl, 10 mM Na-lactate, 0.1 mM EDTA, and 0.001 mM L-methionine) by gently tilting the plates back and forth and harvested by 15 min of low-speed centrifugation (5000 g). With this method, migrating cells were easily lifted off the surface, whereas the vast majority of cells in the middle of the plates remained intact on the surface. Non-swarming colonies were recovered using the same method but with 0.5 mL of cold tethering buffer.
Then, the cells were permeabilized by two subsequent treatments with 0.1% Triton X-100 and freshly prepared PBS-lysozyme-EDTA buffer (1× PBS, 100 μg lysozyme/mL and 5 mM EDTA), each for 1 h at room temperature. Then they were incubated in 0.5% blocking reagent (Sigma-Aldrich) at 37°C for 30 min with shaking. After, cells were then centrifuged at 4,500 g for 5 min, re-suspended in 100 μL of the appropriate primary antibody (diluted 1:20), and incubated overnight at 37°C. After three washes in wash buffer (1× PBS, 0.05% Tween20), the cells were recovered by centrifugation at 4,500 g, re-suspended in 100 μL of the secondary antibody (diluted 1:100), and incubated at 37°C for 2 h without shaking. Finally, after three washing steps with wash buffer, the labeled cells were resuspended in 1× PBS and placed on 35-mm poly-L-lysine pre-coated coverslips using Mowiol-DABCO mounting medium (1× PBS, 2.5% DABCO, 25% Mowiol, and 1× glycerol). The samples were air-dried and then examined under an AxioImager M2 microscope (Carl Zeiss Microscopy) equipped with the appropriate filter set (for the green channel the GFP (Zeiss filter set 38) and for the red Rhod (Zeiss filter set 20) to ensure that at least 90% of cells were correctly permeabilized and immunolabeled. Afterward, at least 300 double marked cells were visually inspected in each sample and the presence and type of clusters as well as RecA distribution were analyzed. Each experiment was performed in triplicate using independent cultures; a minimum of 900 cells from each studied S. enterica strain or condition were therefore examined. In all cases, at least the 70% of cells present the same RecA and CheW distribution profile.
Afterward, super-resolution images of the previously analyzed samples were taken on a Leica TCS SP8 STED3X microscope (Leica Microsystems) using a highly corrected 1.4 NA 100x Plan Apo objective specified for STED imaging. Imaging was done using the lateral resolution improvement lightpath (z-STED set to zero). The fluorophore labels were emission depleted using a 660 nm continuous wave (CW) laser for the stimulated emission effect and time-gating (rejection of early emission events) to further increase the resolution. Data were acquired in the form of two channel z-stacks for subsequent deconvolution and rendering.
For deconvolution of the z-stacks the STED module of the Huygens software package (Scientific Volume Imaging, SVI) was used. Images were analyzed using ImageJ software (National Institutes of Health). In all cases, images of 10 different randomly chosen cells were obtained for each sample. As each experiment was performed in triplicate, a total of 30 cells from each studied strain or condition were therefore examined. The images presented in Figures 1, 2, and 7 are representative of the entire image set. All images shown in the Figures present the same contrast settings.
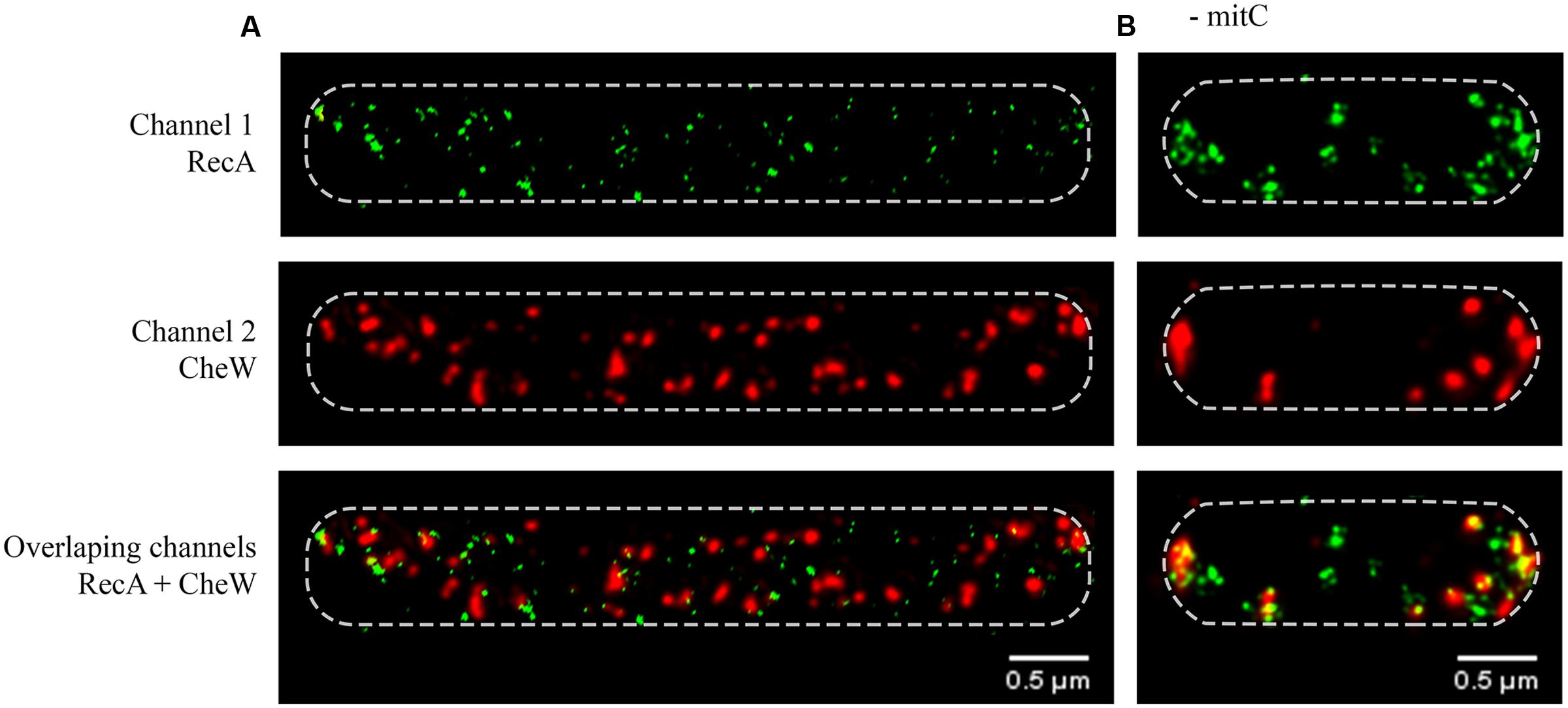
FIGURE 1. Representative STED (Stimulated Emission Depletion) images of the subcellular locations of RecA and CheW in Salmonella enterica wild-type swarming cells (A) in the presence of mitomycin C (0.08 μg/mL) or (B) in the absence of this SOS inducer. The RecA and CheW proteins were labeled with Alexa Fluor® A488 (channel 1) and Alexa Fluor® A568 (channel 2), respectively. For all images, each channel is shown both individually and overlapped. In all cases, the maximum intensity projection images of the obtained z-stacks are shown.

FIGURE 2. Representative STED images of the subcellular locations of RecA and CheW in the S. enterica ΔrecA overexpressing strain. (A) The effect of basal expression of wild-type RecA in the absence of IPTG. (B) Addition of the inducer (30 μM of IPTG) yielded the overexpression of wild-type RecA and the change of the CheW distribution within the cell. It is worth noting that the basal expression of wild-type RecA recovers swarming ability. RecA and CheW proteins were labeled with Alexa Fluor® A488 (channel 1) and Alexa Fluor® A568 (channel 2), respectively. In the images, each channel is shown individually and overlapped. In all cases, the maximum intensity projection images of the obtained z-stacks are shown.
Simple protein–protein docking was conducted using the ClusPro server (Comeau et al., 2004a) to generate an in silico model for the RecA-CheW protein complex. The available resolved structures of the E. coli RecA (PDB: 2REB) and CheW (PDB: 2HO9) proteins were used to run the analyses. The resultant model was presumed to be reliable also for S. enterica as the reported BLAST identity between E. coli K-12 and S. enterica sv. Typhimurium ATCC14028 proteins is 97 and 92% for RecA and CheW, respectively.
The protein–protein docking assay was carried out in duplicate, selecting RecA as the receptor and CheW as the ligand, and vice versa. The protein structures and the obtained in silico models were visualized and analyzed using PyMOL software (Schrödinger, 2010).
The cell lysates were obtained as previously described (Irazoki et al., 2016). Cultures of S. enterica ΔrecA ΔcheW harboring the plasmids encoding the corresponding tagged proteins were used, and the gene overexpression was induced by the addition of 1 mM IPTG. As a control, cell lysates of S. enterica ΔrecA ΔcheW containing the pUA1108 overexpression vector (Mayola et al., 2014) were processed according to the same procedure.
The immunoprecipitation assays were performed using Pure Proteome Protein A magnetic beads (Millipore) coated with either mouse anti-FLAG IgG (Sigma-Aldrich) or mouse anti-HA IgG (Sigma-Aldrich) monoclonal primary antibodies, following the manufacturer’s instructions. Cell lysates were mixed at a molecular ratio of 1:1 and incubated at 30°C for 1 h without shaking to allow protein–protein interaction.
As a final step, the samples were separated by SDS-PAGE on a 15% polyacrylamide gel and analyzed by Western blotting using a horseradish-peroxidase (HRP)-coupled anti-mouse antibody (Acris). The membranes were developed using a HRP chemoluminiscent substrate (Luminata ForteTM Western HRP substrate, Millipore) following the manufacturer’s instructions. The membranes were imaged using a ChemiDocTM XRS+ system (Bio-Rad).
CheW::FLAG- and RecA::HA-carrying plasmids were constructed by PCR-amplifying the recA and cheW genes using the appropriate oligonucleotide pairs (Supplementary Table S1). In both cases, the corresponding tag sequence was included at the 3′ end of the gene, such that the tag was placed at the C-terminus of the protein. A 3×Gly linker between the tag and the gene sequence was also added. The same strategy was used to obtain the recA and cheW tagged mutant derivatives. In this case, the oligonucleotides included the suitable point mutation (Supplementary Table S1).
All PCR products were digested and cloned into pUA1108 overexpression vector (Mayola et al., 2014) under the control of the IPTG-inducible Ptac promoter. These plasmids were transformed into E. coli DH5α and confirmed by sequencing. When needed, the plasmids containing the tagged proteins were transformed into the corresponding S. enterica strains with the appropriate genetic backgrounds. The selected transformants were confirmed again by PCR and sequencing.
The cheW::FLAG recA::HA double-tagged strain was constructed as described previously (Irazoki et al., 2016), using the pKO3 plasmid (Latasa et al., 2012). The recA::HA construct was obtained by PCR overlap-extension (which added the epitope YPYDVPDYA to the RecA protein), cloned into the pKO3 vector, and introduced into the previously constructed S. enterica cheW::FLAG strain (Irazoki et al., 2016). The S. enterica ΔrecA cheW::FLAG strain was constructed by one-step PCR gene replacement as described previously (Datsenko and Wanner, 2000; Irazoki et al., 2016) using the S. enterica cheW::FLAG strain as a recipient strain. In all cases, it was confirmed that neither the FLAG nor the HA tag insertion affected the surface motility of the tagged strains.
Swarming assays were carried out as described previously (Gómez-Gómez et al., 2007; Mayola et al., 2014; Irazoki et al., 2016). In short, a single colony was picked from bacterial strains grown on LB plates at 37°C and inoculated in the center of a freshly prepared LB swarming plate [1% tryptone, 0.5% yeast extract, 0.5% NaCl, 0.5% D-(+)-glucose, and 0.5% agar] using a sterile toothpick and avoiding medium penetration. The plates were supplemented with IPTG (10 or 30 μM) or mitomycin C (0.08 μg/mL) as needed, incubated overnight at 37°C, and then imaged using a ChemiDocTM XRS+ system (Bio-Rad).
The phenotypic assays for biofilm formation were performed as previously described (Latasa et al., 2012). After 96 h of incubation at 25°C without agitation, the biofilm formed in standing LB broth was visualized as a floating pellicle at the air–broth interface that totally blocked the surface of the culture and could not be dispersed by shaking.
To determine the recombination efficiency of the S. enterica strains carrying overexpression vectors containing the recA tagged mutant derivatives, the P22 transduction frequency of the strain was compared with that of the same strain but carrying an overexpression vector with wild-type tagged recA; the method was described previously (McGrew and Knight, 2003). The transduction experiments were performed as described elsewhere (Campoy et al., 2002). The recombination efficiency was calculated as the number of transductants relative to the initial recipient cell concentration. The relative recombination frequency was the recombination efficiency (%) of each overexpressing strain with respect to the strain overexpressing wild-type recA.
RecA and CheW quantification was performed by ELISA as described before (Irazoki et al., 2016). All samples recovered from the colony edge were resuspended in sonication buffer (PBS 1×, cOmplete mini EDTA-free tablets, pH 7.3) and sonicated (2x 30-s pulses and 20% amplitude, Digital sonifierR 450, Branson) obtaining the whole cell lysates. After centrifugation (12000 g for 10 min), the supernatants were recovered and the total protein concentration of each sample was quantified according to the Bradford method using the protein reagent DyeR (BioRad) and a bovine serum albumin standard curve (range: 1.5–200 μg/mL).
The RecA and CheW::FLAG proteins used in the standard quantification curves were obtained as previously described (Irazoki et al., 2016). The RecA::HA and CheW::FLAG proteins were quantified by ELISA as described (Mayola et al., 2014) using anti-RecA (monoclonal antibody to ARM193 RecA clone, MBL) and anti-FLAG (monoclonal antibody to DYKDDDDK epitope Tag, Acris) mouse IgG. The secondary antibody was an anti-mouse-IgG horseradish-peroxidase-conjugated antibody [polyclonal antibody to mouse IgG (HEL)-HRP, Acris]. The BD OptEIA TMB substrate reagent set (BD Biosciences), prepared following the manufacturer’s instructions, was used as the developing solution. Plate measurements were made at 650 nm using a multiplate reader (Sunrise, Tecan).
The location of RecA and CheW proteins within SOS response activated-S. enterica swarming cells was analyzed by using 3D-stimulated emission depletion microscopy (3D-STED), a super-resolution fluorescence imaging technique that increases axial resolution by up to 20–40 nm in biological samples (Han and Ha, 2015). Thus, a S. enterica cheW::FLAG recA::HA strain was constructed and the appropriate antibodies were used to locate these proteins inside the swarming cells in the presence of a SOS response inducer.
As it is shown in Figure 1, besides the expected cell filamentation due to the induction of the SOS response by mitomycin C, the SOS inducer treatment gives rise to a dramatic change in the subcellular location of both RecA and CheW within cells cultured on swarming plates containing sub-lethal concentration of mitomycin C (Figure 1A). In agreement with E. coli cells grown in liquid medium under non-DNA-damaging conditions (Greenfield et al., 2009), in the non-mitomycin C-treated S. enterica swarming cells, the CheW protein was majorly located at the cell poles (Figure 1B). This CheW location is the same than that previously described for chemoreceptor polar arrays and accordingly more than 70% of cells presented chemoreceptor polar clusters in these conditions (Kentner et al., 2006; Greenfield et al., 2009; Cardozo et al., 2010; Mayola et al., 2014; Santos et al., 2014).
Nevertheless, the SOS response induction prompts a change in the CheW distribution, which instead of being at the cell poles was indeed organized in smaller foci distributed in a spiral-like configuration along the cell membrane (Figure 1A). Further, and in agreement with previous data about cluster assembly under SOS induction (Irazoki et al., 2016), this CheW distribution was present in more than 70% of analyzed cells. Likewise, the CheW foci resembled the distribution and organization of RecA (Figure 1A). Under this DNA-damaging conditions, the SOS system induction gives rise not only an increase in the RecA concentration (Irazoki et al., 2016) but also to a higher amount of cellular RecA aggregates (Figure 1A). After SOS induction, the RecA foci seemed to be smaller and were distributed not only at the cell poles but also along the filamented cell axis, assuming a helical configuration just underneath the bacterial wall (Figure 1A). These observations were in agreement with the previously reported changes in the location of RecA in liquid cultures of E. coli cells growing in DNA-damaging conditions and the described reduction of RecA storage structures at cell poles (Renzette et al., 2005; Lesterlin et al., 2014; Rajendram et al., 2015). Nevertheless, in our experimental settings, using sub-lethal concentration of mitomycin C, RecA was not distributed forming bundles as those described for E. coli (Lesterlin et al., 2014), that were only observed when S. enterica was grown in liquid cultures adding higher amount of the SOS inducer (data not shown).
To rule out an indirect effect of either DNA damage or SOS-dependent filamentation on the CheW distribution and to determine whether RecA activation plays a significant role in the distribution of its partner protein, recA was overexpressed under non-DNA-damaging conditions and the locations of the CheW and RecA tagged proteins were examined.
In this experiment, S. enterica ΔrecA cheW::FLAG strain carrying the pUA1135 vector, the pUA1108 overexpression vector containing the recA::HA gene under the control of an IPTG-inducible promoter was used (Table 1). Induction was achieved by adding IPTG to the swarming plates. The basal expression of the wild-type recA carried in this plasmid is enough to recover both swarming ability and the polar-clustered CheW arrangement (Figure 2A). In agreement, more than 70% of cells present polar chemoreceptor arrays. Following the addition of IPTG to the swarming plates, the increase in RecA was accompanied by a helical distribution of CheW along the cell axis (Figure 2B), as occurs following activation of the SOS response (Figure 1B). And, as expected, about 70% of cells did not present polar chemoreceptor clusters.
These findings indicate that neither the cell filamentation, the activated RecA protein nor DNA damage is required to modify the subcellular location of CheW.
To further determine how does the RecA-CheW interaction occurs, the RecA and CheW residues associated with this interaction were identified by using an in silico model for RecA-CheW complex-formation in which simple protein–protein docking was conducted using the resolved ternary structures E. coli RecA (PDB: 2REB) and CheW (PDB: 2HO9).
RecA has three major functional domains. The amino domain contains a large α-helix and short β-strand that are implicated in the formation of the RecA polymer. The central domain (consisting primarily of a twisted β-sheet with eight β-strands bound by eight α-helices) is involved in DNA and ATP binding. The carboxyl domain is made up of three α-helices and three β-strands that facilitate interfilamentous associations (Story et al., 1992). On the other hand, the folded CheW has a SH3-like regulatory domain and two intertwined five-stranded β-barrels, designated subdomains 1 and 2 (Li et al., 2013).
As little is known about the forces guiding protein-complex formation, balanced-coefficient docking models were considered to be the most accurate for the analysis of the RecA-CheW interaction (Comeau et al., 2004b). Ten of the highest-scoring models were analyzed individually for each combination of RecA receptor protein and CheW ligand, and vice versa. Although, the spatial arrangement was not exactly the same in each paired combination, the results allowed the putative interacting regions of each protein to be identified, as they were those that were repeated in all models.
Figure 3 shows the residues of the folded RecA that putatively participate in the interaction with CheW. These were predicted to be located in the amino-terminal and central domains (in α1, α10, α11, β8, and β9) whereas the presumed CheW regions were located in both subdomains, specifically, in the β1 and β4 (subdomain 1) and the T4, β8, and B6 regions (subdomain 2).
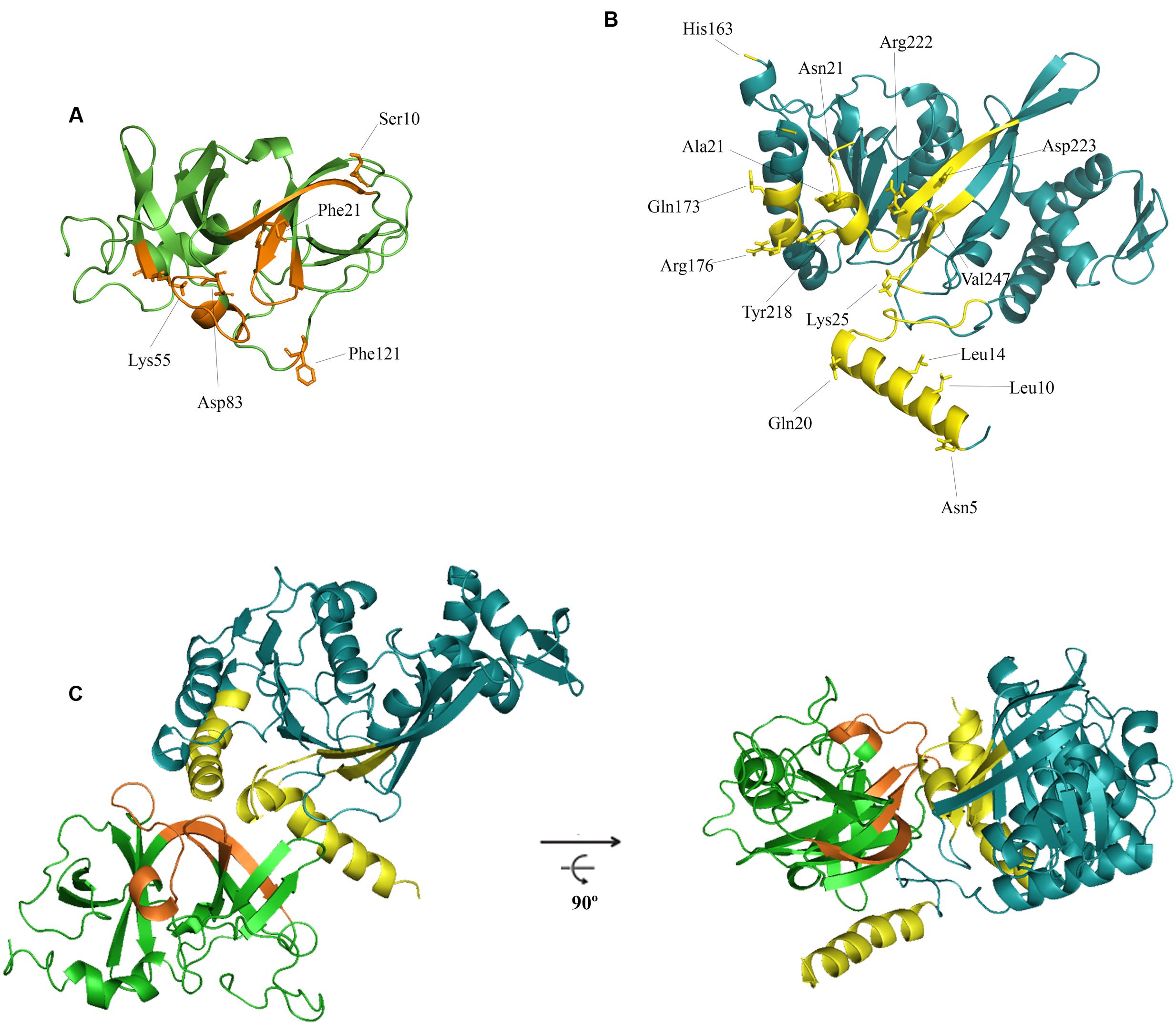
FIGURE 3. In silico model for the interaction of RecA and CheW proteins. The predicted ternary structures of S. enterica CheW (A) and RecA (B) proteins are shown. The putative interface of RecA and CheW involved in the reciprocal interaction of the two proteins is highlighted in yellow and orange, respectively. The residues selected for site-directed mutagenesis and their locations are also indicated. (C) Ribbon diagrams of one of the highest-scoring models generated for RecA-CheW pair formation analyzed in this study. The two views of the interaction are rotated 90° about the x-axis.
To corroborate the interaction interfaces identified in silico, site-directed mutagenesis was used to construct several mutant derivatives for each protein in which the relevant residues were affected. In all cases, the corresponding recA and cheW gene mutants constructed in vitro were HA- and FLAG-tagged, respectively, and cloned into the overexpression vector (pUA1108) under the control of an IPTG-inducible promoter.
Fourteen RecA and five CheW residues were selected based on their potential roles in RecA-CheW pair formation (Tables 2 and 3) as well as their reactivity and exposure on the corresponding protein surface (Figure 3). With the exception of the A214V RecA mutant, in which the Ala residue was changed to a Val, all other selected residues were converted to an Ala (Tables 2 and 3), as this aliphatic amino acid is considered to be non-reactive (Cunningham and Wells, 1989). The effect of each substitution on the RecA-CheW interaction was determined in vitro and in vivo by co-immunoprecipitation and swarming inhibition assays, respectively.
For the in vitro co-immunoprecipitation assays, each RecA::HA mutant protein was mixed with wild-type CheW::FLAG; anti-HA-antibody coated beads were used to hijack the proteins. The CheW::FLAG mutated derivatives were mixed with the RecA::HA wild-type protein and hijacked using anti-FLAG-antibody coated beads. Previous assays confirmed the ability of RecA::HA to pull down CheW::FLAG and vice versa (Mayola et al., 2014). It was therefore expected that if the mutated residue altered the RecA-CheW interaction, the antibody would pull down only the corresponding tagged mutant protein and would not co-immunoprecipitate both tagged proteins (Figure 4). The results showed that, among the mutants tested, only four RecA (Q20A, R222A, K250A, and R176A) and four CheW (F21A, D83A, K55A, and F121A) mutants impaired the RecA-CheW interaction (Tables 2 and 3). These results corroborated the in silico docking predictions and pointed out that some residues from α1, α10, and β8 regions of RecA and from the β1, β4, T6, and B6 regions of CheW participate in the interaction between the two proteins.
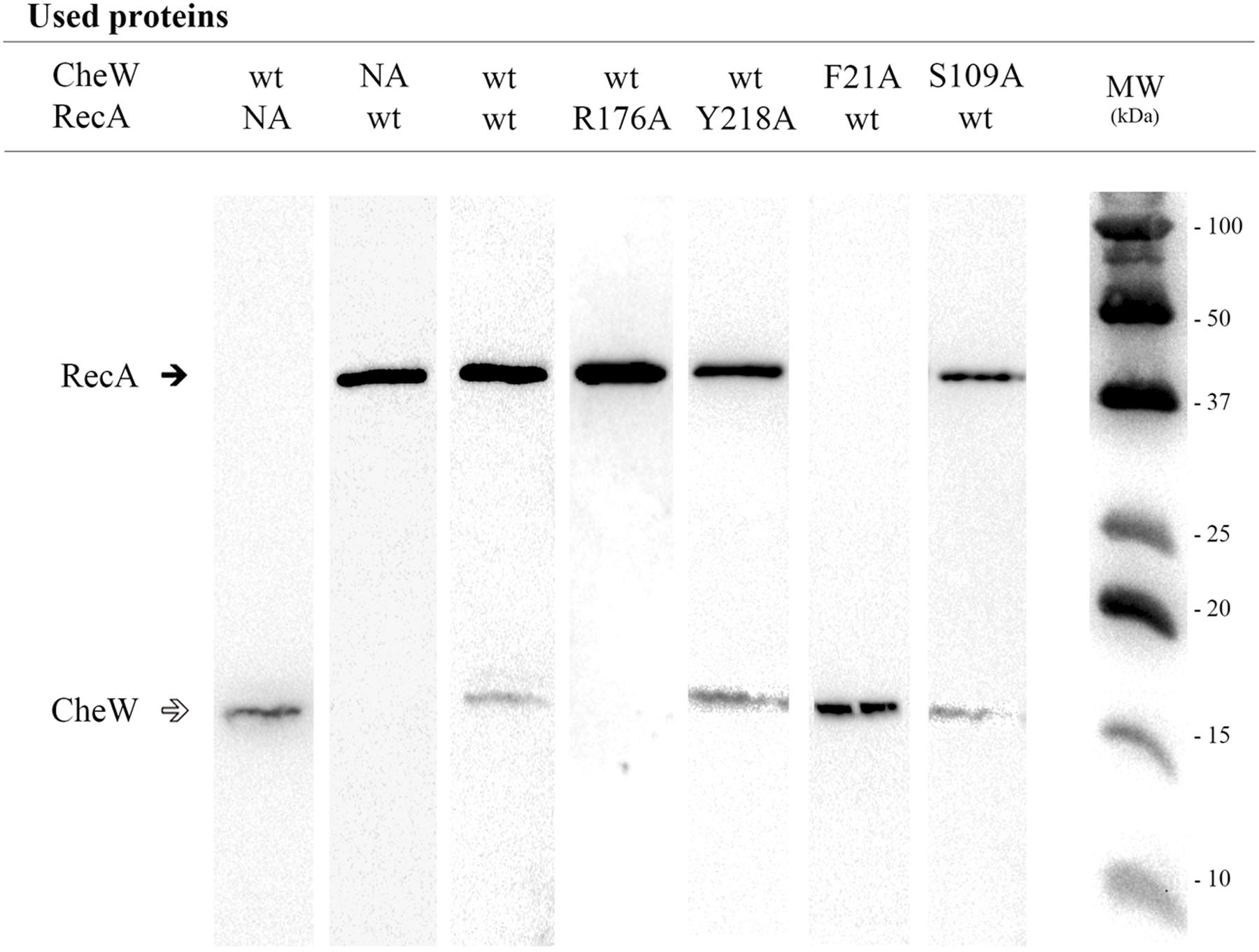
FIGURE 4. Co-immunoprecipitation assays of RecA and CheW S. enterica mutant derivatives. Representative images of the co-immunoprecipitation of mutant derivatives that allow (Y218A RecA::HA or S109A CheW::FLAG) or impair (R176A RecA::HA or F21A CheW::FLAG) the interaction of RecA-CheW are shown. Each lane contains a mixture of cell lysates prepared from the overexpressed mutant derivative and the wild-type (wt) tagged protein. As controls, mixtures containing either wild-type (wt) RecA or CheW or both were included. All experiments were done at least in triplicate; in all cases, the results for all mutant derivatives were exactly the same as those shown in the figure. Black and white arrows indicate RecA and CheW protein bands, respectively. NA, non-added; MW, molecular mass marker.
To determine the contribution of these eight residues to swarming motility, in vivo swarming assays were carried out using the constructed mutants. As it has been previously mentioned, the overexpression of either RecA or CheW inhibits swarming (Cardozo et al., 2010; Medina-Ruiz et al., 2010; Irazoki et al., 2016; Figure 5A). In these swarming assays the effect on swarming of RecA and CheW mutant derivative overexpression in wild-type cells was determined. For this reason, all the vectors overexpressing the RecA and CheW mutants were transformed to S. enterica wild-type cells, and cultured on swarming plates containing 30 μM of IPTG. In all cases, it was confirmed by ELISA that the RecA and CheW concentration increases were at least more than 20-fold for RecA and 100-fold for CheW after IPTG induction. Representative images of in vivo swarming assays of S. enterica wild-type strains overexpressing RecA and CheW mutant derivatives that allow or impair RecA-CheW interaction are shown in Figure 5B. The results for all mutant derivatives are summarized in Tables 2 and 3. In agreement with the data obtained in the in vitro co-immunoprecipitation assays, only the mutant derivatives unable to interact with the corresponding wild-type protein do not inhibit swarming when overexpressed. These results confirm the importance of these residues in RecA-CheW in vivo interaction. Further, it is worth noting that none of the non-interacting RecA or CheW mutant derivatives are able to recover the swarming ability of the either S. enterica ΔrecA or ΔcheW strains (data not shown).
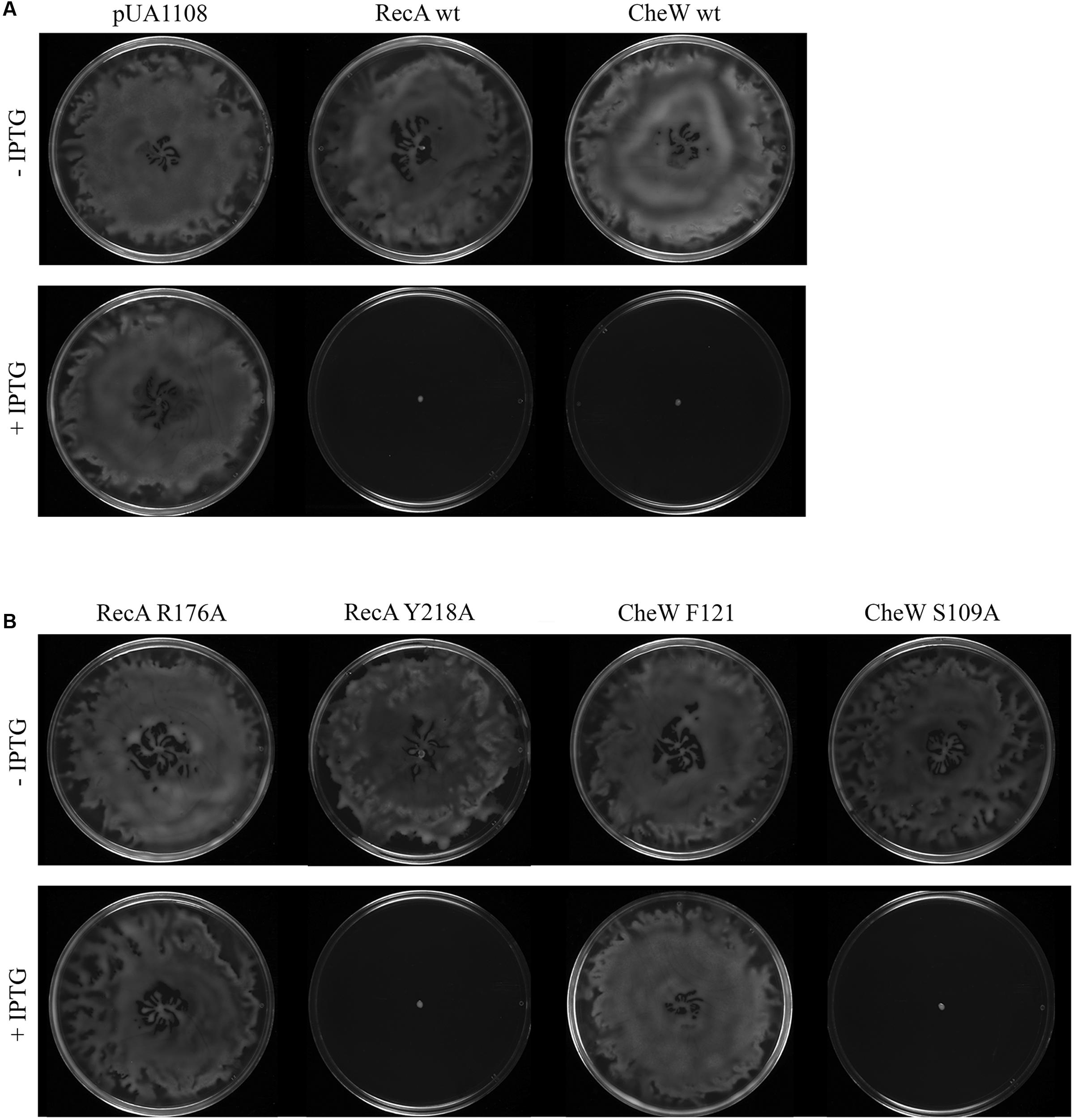
FIGURE 5. (A) Representative images of the in vivo swarming ability of the S. enterica wild-type (wt) strain overexpressing either RecA wt or CheW wt proteins or containing only the overexpression vector (pUA1108). (B) In vivo swarming assay of RecA and CheW mutant derivatives. Representative images of the swarming ability of S. enterica wt strain containing the overexpression vectors encoding RecA or CheW mutant derivatives that allow or impair RecA-CheW coupling are presented. The cells were cultured in the presence (+) or absence (-) of 30 μM IPTG. Swarming was inhibited in the presence of IPTG only in mutants that maintained the RecA-CheW interaction (RecA Y218A and CheW S109A). When the interaction of the two proteins was abolished (using RecA R176A and CheW F21A mutant strains), swarming was not affected when IPTG was added. Each experiment was performed at least in triplicate. The same results were observed for all of the mutant derivatives tested.
The three domains of RecA exhibit functional overlap (Takahashi et al., 1996; McGrew and Knight, 2003; Adikesavan et al., 2011). For example, in E. coli, Arg176 and Lys250 RecA residues, identified in this work as essential for the RecA-CheW interaction, are involved in recombination activity (Chen et al., 2008; Adikesavan et al., 2011). To determine whether the interaction interfaces associated with RecA-CheW coupling also have other overlapping functions, the recombination ability of the obtained RecA derivatives was determined. The results showed that all of the RecA mutants causing impaired RecA-CheW coupling had also lost their recombination ability (Figure 6). Further, some other residues that are not involved in RecA-CheW pair formation (H163A, A214V, K216A, and D224A) also present a clear decrease in their recombinase activity (Figure 6). These data are not surprising since as stated above, their location matches with regions previously described to be associated with recombination (Chen et al., 2008; Adikesavan et al., 2011).
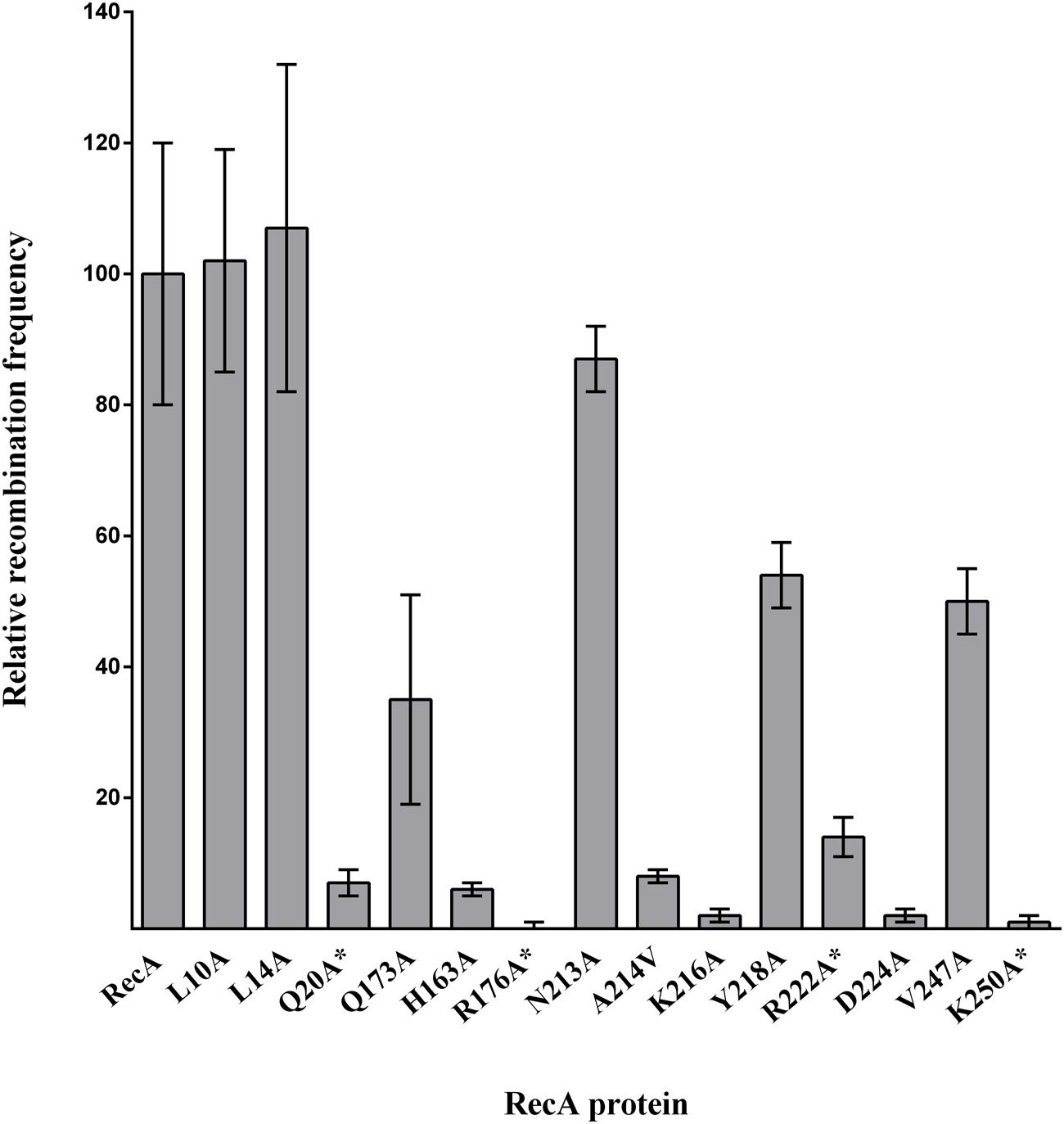
FIGURE 6. In vivo recombination activity of the RecA mutant derivatives. The efficiency of strains, containing the overexpression vector carrying the corresponding RecA derivative, to recombine the selectable genetic marker transduced by bacteriophage P22intH7 was tested. The relative recombination frequency was calculated as the recombination efficiency of each mutant derivative with respect to that of the strain overexpressing wt RecA, and the recombination efficiency of each strain as the number of transductants compared to the initial recipient cell concentration. The RecA mutant derivatives unable to interact with CheW are indicated by an asterisk (∗). The relative recombination frequencies were calculated as the mean of three independent experiments. Error bars indicate the standard deviation.
Once identified the RecA and CheW residues associated with interaction and to unequivocally associate the RecA-CheW pair formation with CheW location, the behavior of the non-CheW-interacting RecA R176A mutant was analyzed. In this case, the recA mutant derivative (R176A) was overexpressed in a S. enterica ΔrecA strain under non-DNA-damaging conditions and the locations of the CheW and RecA tagged proteins were examined.
As it is seen in Figure 7, the overexpression of the RecA R176A mutant by IPTG addition does not prompt any change in CheW distribution, as it happens when wild-type RecA is overexpressed (Figure 2). In the presence of RecA R176A mutant protein the CheW was never located at cell poles regardless the RecA concentration (Figure 7).
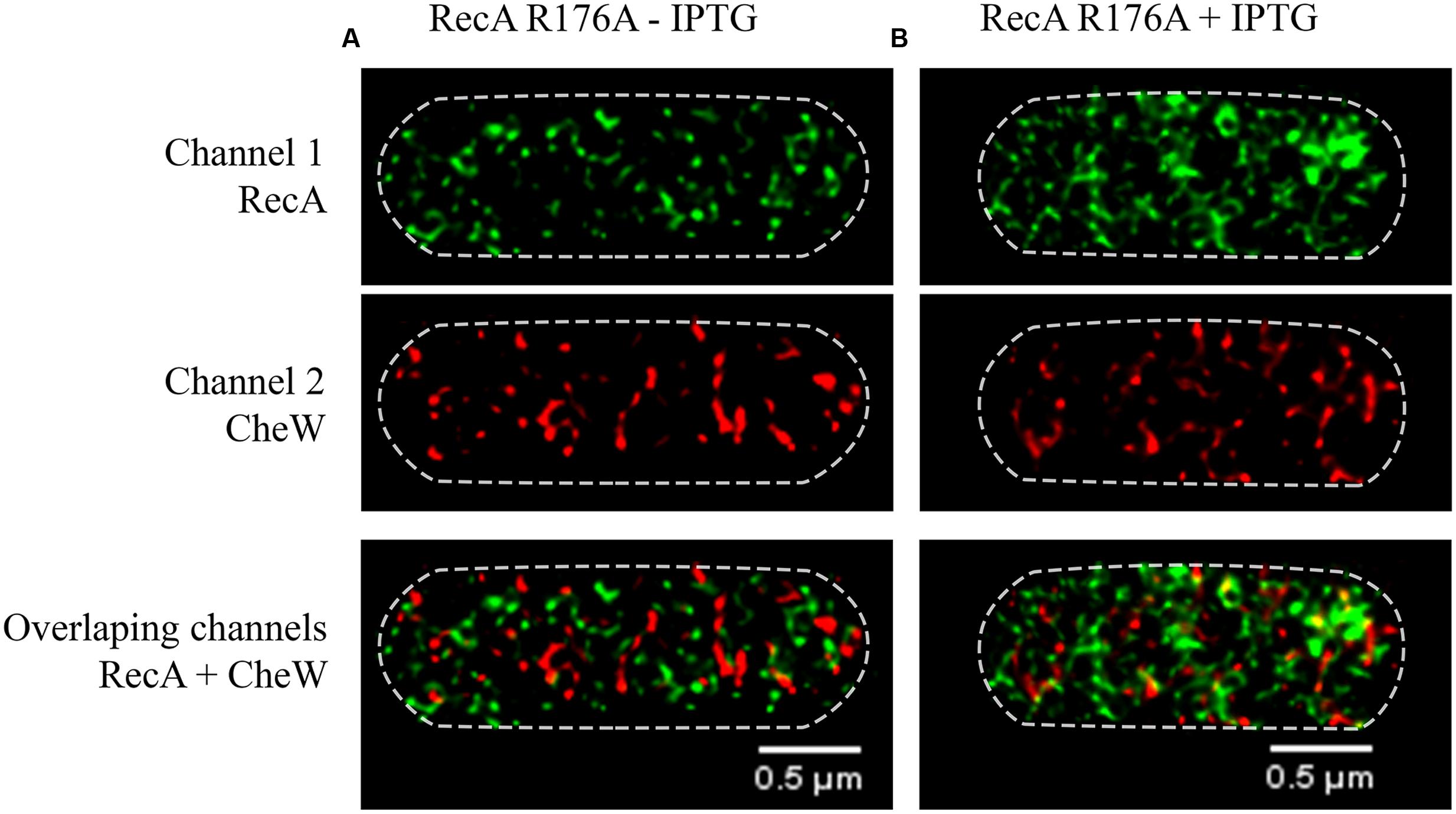
FIGURE 7. Representative STED images of the subcellular locations of RecA and CheW in the S. enterica ΔrecA strain overexpressing the non-CheW-binding RecA R176A mutant. (A) The effect of basal expression, in the absence of IPTG, of RecA R176A mutant on RecA-CheW distribution is shown. (B) Addition of the inducer (30 μM of IPTG) yielded the overexpression of the non-CheW-binding RecA R176A mutant. RecA and CheW proteins were labeled with Alexa Fluor® A488 (channel 1) and Alexa Fluor® A568 (channel 2), respectively. In the images, each channel is shown individually and overlapped. In all cases, the maximum intensity projection images of the obtained z-stacks are shown.
These results indicate that not only the concentration of RecA but also the ability of the protein to interact with CheW is required for CheW distribution and thus for chemoreceptor clustering at the cell poles, a sine qua non condition for bacterial colony swarming.
The experiments performed herein have identified the protein interfaces involved in the interaction between RecA and CheW in S. enterica. The regions of CheW specifically associated with RecA are β1, β4, T6, and B6 (Figure 1; Table 3), which are not those that interact with CheA, CheW, or MPCs (Bilwes et al., 1999; Underbakke et al., 2011; Cassidy et al., 2015). Accordingly, the interaction of RecA and CheW should not interfere with any of the three CheW-binding targets identified thus far (CheA, CheW, and MCPs). The interaction interfaces of RecA are located within the N-terminal and central domains, thus involving the α1, α10, and β8 regions of the protein (Figure 3; Table 2). These are the same regions previously reported to be involved in RecA polymer formation, ATP hydrolysis, and ssDNA and LexA interactions (Story et al., 1992; Campbell and Davis, 1999; Chen et al., 2008; Adikesavan et al., 2011), such that none of the non-CheW interacting RecA derivatives here described were able to carry out recombination (Figure 6). These results suggest that when a molecule of RecA is interacting with CheW, it cannot participate in DNA recombination and repair. Nevertheless, only part of the total RecA amount present in a SOS-induced cell will be associated to CheW hijack, ensuring that DNA repair and recombination take place in the DNA-damaged cells.
By using super-resolution 3D-STED, we were able to show that following SOS induction the increase in the concentration of RecA, but not the activation of other SOS response associated functions, is enough to induce the redistribution of CheW from aggregates at the cell poles to foci with a helicoid configuration along the cell axis, showing the same subcellular location than RecA (Figures 1B and 2B). This finding is consistent with the impairment of the chemoreceptor array assembly that occurs when the SOS response is activated (Irazoki et al., 2016). By contrast, in cells carrying a non-CheW-interacting mutant RecA, CheW is unable to cluster at the cell poles (Figure 7). Thus pointing out that the interaction between RecA and CheW is essential for both swarming modulation (Figure 5) and CheW clustering at the cell poles (Figure 7), and confirming the previously described for S. enterica RecA-defective strains, in which chemoreceptor array assembly was inhibited (Mayola et al., 2014).
Taken together, these results suggest two different scenarios to explain the role of RecA in chemoreceptor polar cluster formation and swarming modulation. Thus, RecA may be a component of the chemoreceptor array, since either its absence or overexpression interferes directly with chemoreceptor assembly. However, this is unlikely since polar array clusters have been characterized in detail (Li et al., 2013; Briegel et al., 2014b; Cassidy et al., 2015; Eismann and Endres, 2015) and their well-organized structure does not seem to allow for RecA attachment. Alternatively, RecA could prompt the titration of CheW, thus preventing chemoreceptor assembly and therefore also polar cluster array formation during activation of the SOS response (Figure 8). A similar control strategy has been described for other interacting proteins whose regulatory function relies on the availability of the protein with which they interact (Liu and Richardson, 1993; Plumbridge, 2002; Hill et al., 2013; Hernández-Rocamora et al., 2015; Paget, 2015).
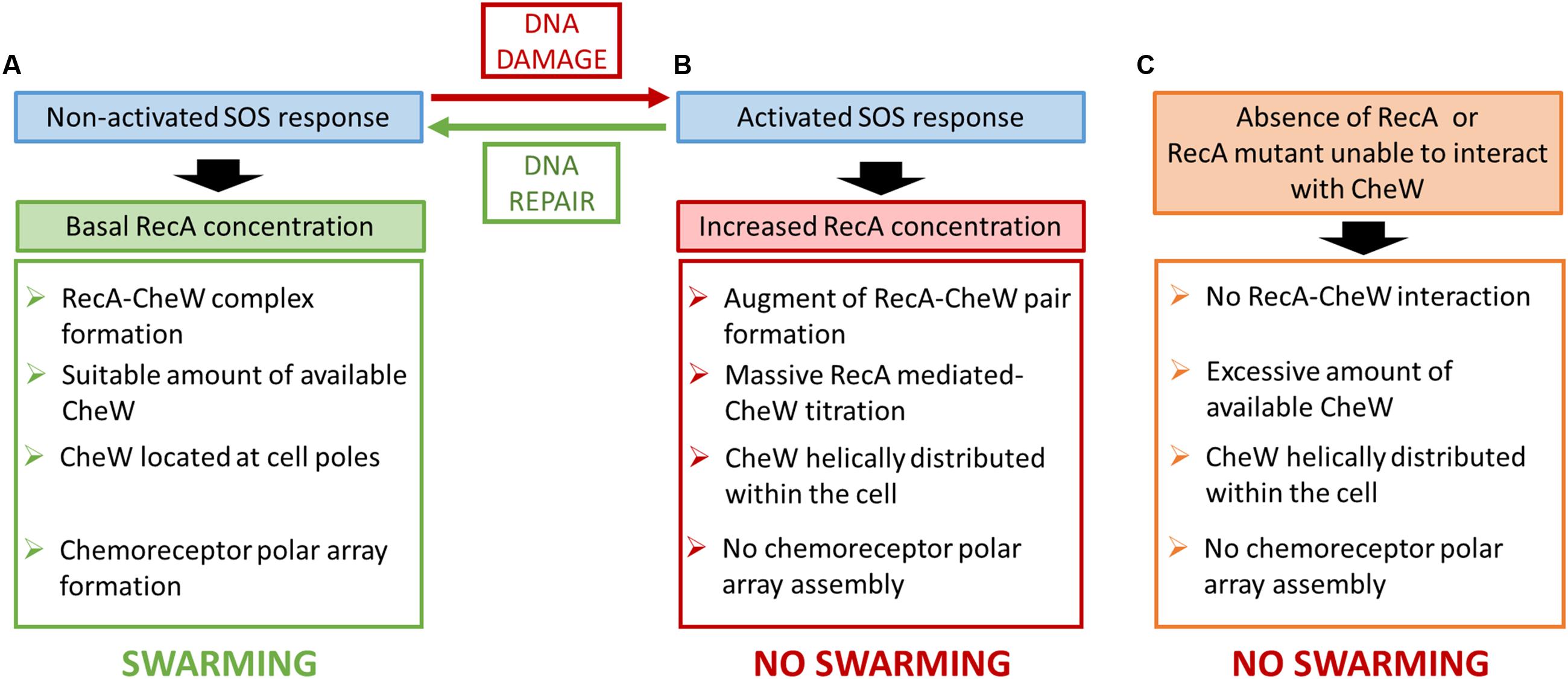
FIGURE 8. Schematic diagram of the putative mechanism for the control of S. enterica swarming motility by RecA and SOS response. The effect of non-activated SOS response (A), the induction of the SOS system (B) and the absence or the presence of a non-interacting RecA mutant (C) is proposed.
The concentration of CheW is essential for chemoreceptor cluster formation and the absence or overexpression of this protein inhibits array assembly (Avram Sanders et al., 1989; Cardozo et al., 2010). In addition, a recent study showed that in these arrays, CheW not only serves as an adaptor protein anchoring MCPs to CheA but that, via ring formation, it is also responsible for chemoreceptor array stability (Cassidy et al., 2015). Therefore, in the absence of DNA damage, RecA is able to bind CheW adjusting the availability of this protein needed to allow chemosensory system assembly and thus swarming ability (Figure 2A).
Since activation of the SOS response increases the concentration of RecA but not of CheW (Irazoki et al., 2016), then during SOS activation the amount of RecA-CheW complex formation will be stimulated but the CheW availability will thereby be reduced (Figure 1A), which will affect the stability of the hexagonal receptor signaling array (Cassidy et al., 2015). Following DNA damage repair, the recA expression returns to its basal level restoring chemoreceptor array assembly and thus swarming ability (Irazoki et al., 2016) returning the cell the non-DNA damage condition (Figure 1B). This explains why only CheW overexpression can reestablish polar cluster assembly in a RecA-overexpressing strain (Irazoki et al., 2016), i.e., the increased availability of CheW restores chemosensory array assembly. Moreover, the absence of RecA (Mayola et al., 2014) or an inability of the protein to interact with CheW (Figure 7) will increase the available amount of CheW (Figure 7A), thus interfering with chemoreceptor ring structuring and cluster formation (Cardozo et al., 2010).
Besides CheW is a key protein in the S. enterica chemoreceptor pathway (Baker et al., 2006) and RecA seem to play a role in chemotaxis (Mayola et al., 2014), chemoreceptor arrays are not essential for chemotaxis response. It has been previously reported that despite the absence of structuration of polar clusters, the association of chemoreceptors with the chemotaxis pathway is still functional (Maki et al., 2000; Briegel et al., 2014b). Furthermore, chemotaxis and swimming are not affected when E. coli is treated with cephalexin (Maki et al., 2000), a beta-lactam antibiotic that induce SOS response (Bano et al., 2014). Moreover, biofilm formation is affected neither by the absence or the overexpression of RecA (data not shown).
It is also worth noting that although the absence or overexpression of RecA inhibits cluster assembly, the presence of chemoreceptor clusters is not completely abolished in either ΔrecA or RecA overexpressing strains. It has been widely reported that chemoreceptor arrays are highly stable structures. Several models have been proposed to describe its assembly and stabilization and not only CheW but also CheA and even the cell membrane curvature seem to be involved (Shiomi et al., 2005; Thiem and Sourjik, 2008; Greenfield et al., 2009; Jones and Armitage, 2015). Then, the alteration in CheW availability would clearly affect chemoreceptor cluster assembly but not completely abolish it. Accordingly, once the SOS response is activated, more than the 70% of the cells presented CheW distributed along the cell instead of being at cell poles due to the RecA mediated – CheW titration.
As mentioned in Section “Introduction” RecA is associated to the inner-membrane anionic phospholipids (Rajendram et al., 2015). This interaction is necessary for RecA activity during DNA damage repair. However, the RecA residues that interact with anionic phospholipids do not overlap with those interacting with the CheW interface (Table 2). RecA proteins also form foci that may or may not be associated with DNA (Renzette et al., 2005). The DNA-less proteins, referred to as RecA storage structures, are often located at the cell poles and are redistributed along the cell in response to DNA damage (Renzette et al., 2005; Lesterlin et al., 2014; Rajendram et al., 2015). Interestingly, an E. coli R28A RecA mutant with an amino acid substitution in the α1 RecA region, shown in this study to be associated with RecA-CheW pair formation in S. enterica (Table 2), is unable to generate DNA-less RecA foci (Renzette and Sandler, 2008). Thus, our results suggest that in addition to RecA storage, DNA-less RecA foci participate in the modulation of swarming motility.
Our data therefore shed light on a new role of RecA, the titration effect on CheW protein, which based on protein–protein interaction strategy, modulates the CheW distribution within the cell thus controlling the swarming ability.
OI, JA, and SC performed the in silico analyses and site-directed mutagenesis. OI and SC analyzed swarming phenotypes and chemoreceptor polar cluster assembly. OI, SC, and TZ performed the fluorescent immunolabeling and STED imaging. OI, SC, and JB conceived the experiment, coordinated the research, discussed the findings and interpreted the results.
The authors acknowledge financial support from the Ministerio de Economía y Competitividad (BIO2016-77011-R) and Generalitat de Catalunya (2014SGR 572). The funders had no role in the design of the study, in data collection and analysis, in the decision to publish, or in the preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Joan Ruiz, Susana Escribano, and Andrés Magán for their technical support during some of the experimental procedures. Also Pablo Loza and Jordi Andilla at the Super-resolution light microscopy and nanoscopy (SLN) group of Institute of Photonic Sciences (ICFO) for their help and guidance on super-resolution microscopy experiments.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01560
Adikesavan, A. K., Katsonis, P., Marciano, D. C., Lua, R., Herman, C., and Lichtarge, O. (2011). Separation of recombination and SOS response in Escherichia coli RecA suggests LexA interaction sites. PLoS Genet. 7:e1002244. doi: 10.1371/journal.pgen.1002244
Avram Sanders, D., Mendez, B., and Koshland, D. E. (1989). Role of the CheW protein in bacterial chemotaxis: overexpression is equivalent to absence. J. Bacteriol. 171, 6271–6278.
Baker, M. D., Wolanin, P. M., and Stock, J. B. (2006). Signal transduction in bacterial chemotaxis. Bioessays 28, 9–22. doi: 10.1002/bies.20343
Bano, S., Vankemmelbeke, M., Penfold, C. N., and James, R. (2014). Detection of induced synthesis of colicin E9 using ColE9p::gfpmut2 based reporter system. World J. Microbiol. Biotechnol. 30, 2091–2099. doi: 10.1007/s11274-014-1635-y
Barak, J. D., Gorski, L., Liang, A. S., and Narm, K.-E. (2009). Previously uncharacterized Salmonella enterica genes required for swarming play a role in seedling colonization. Microbiology 155, 3701–3709. doi: 10.1099/mic.0.032029-0
Bilwes, A. M., Alex, L. A., Crane, B. R., and Simon, M. I. (1999). Structure of CheA, a signal transducing histidine kinase. Cell 96, 131–141. doi: 10.1016/S0092-8674(00)80966-6
Boukhvalova, M. S., Dahlquist, F. W., and Stewart, R. C. (2002). CheW binding interactions with CheA and Tar. Importance for chemotaxis signaling in Escherichia coli. J. Biol. Chem. 277, 22251–22259. doi: 10.1074/jbc.M110908200
Briegel, A., Ladinsky, M. S., Oikonomou, C., Jones, C. W., Harris, M. J., Fowler, D. J., et al. (2014a). Structure of bacterial cytoplasmic chemoreceptor arrays and implications for chemotactic signaling. Elife 2014, 1–16. doi: 10.7554/eLife.02151
Briegel, A., Li, X., Bilwes, A. M., Hughes, K. T., Jensen, G. J., and Crane, B. R. (2012). Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. Proc. Natl. Acad. Sci. U.S.A 109, 3766–3771. doi: 10.1073/pnas.1115719109
Briegel, A., Wong, M. L., Hodges, H. L., Oikonomou, C. M., Piasta, K. N., Harris, M. J., et al. (2014b). New insights into bacterial chemoreceptor array structure and assembly from electron cryotomography. Biochemistry 53, 1575–1585. doi: 10.1021/bi5000614
Buddelmeijer, N., Aarsman, M. E. G., and den Blaauwen, T. (2013). Immunolabeling of proteins in situ in Escherichia coli K12 strains protocols. Bio Protoc. 3, 2–6.
Burkart, M., Toguchi, A., and Harshey, R. M. (1998). The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 95, 2568–2573. doi: 10.1073/pnas.95.5.2568
Campbell, M. J., and Davis, R. W. (1999). Toxic mutations in the recA gene of E. coli prevent proper chromosome segregation. J. Mol. Biol. 286, 417–435. doi: 10.1006/jmbi.1998.2456
Campoy, S., Jara, M., Busquets, N., de Rozas, A. M. P., Badiola, I., and Barbé, J. (2002). Intracellular cyclic AMP concentration is decreased in Salmonella typhimurium fur mutants. Microbiology 148, 1039–1048. doi: 10.1099/00221287-148-4-1039
Cardozo, M. J., Massazza, D. A., Parkinson, J. S., and Studdert, C. A. (2010). Disruption of chemoreceptor signalling arrays by high levels of CheW, the receptor-kinase coupling protein. Mol. Microbiol. 75, 1171–1181. doi: 10.1111/j.1365-2958.2009.07032.x
Cassidy, C. K., Himes, B. A., Alvarez, F. J., Ma, J., Zhao, G., Perilla, J. R., et al. (2015). CryoEM and computer simulations reveal a novel kinase conformational switch in bacterial chemotaxis signaling. Elife 4:e08419. doi: 10.7554/eLife.08419
Chaveroche, M. K., Ghigo, J. M., and d’Enfert, C. (2000). A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 28:E97. doi: 10.1093/nar/28.22.e97
Chen, Z., Yang, H., and Pavletich, N. P. (2008). Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature 453, 489–494. doi: 10.1038/nature06971
Comeau, S. R., Gatchell, D. W., Vajda, S., and Camacho, C. J. (2004a). ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 32, W96–W99. doi: 10.1093/nar/gkh354
Comeau, S. R., Gatchell, D. W., Vajda, S., and Camacho, C. J. (2004b). ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20, 45–50. doi: 10.1093/bioinformatics/btg371
Courcelle, J., Khodursky, A., Peter, B., Brown, P. O., and Hanawalt, P. C. (2001). Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158, 41–64.
Cunningham, B., and Wells, J. (1989). High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science 244, 1081–1085. doi: 10.1126/science.2471267
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
Eisen, J. A. (1995). The RecA protein as a model molecule for molecular systematic studies of bacteria: comparison of trees of RecAs and 16S rRNAs from the same species. J. Mol. Evol. 41, 1105–1123. doi: 10.1007/BF00173192
Eismann, S., and Endres, R. G. (2015). Protein connectivity in chemotaxis receptor complexes. PLoS Comput. Biol. 11:e1004650. doi: 10.1371/journal.pcbi.1004650
Gómez-Gómez, J.-M., Manfredi, C., Alonso, J.-C., and Blázquez, J. (2007). A novel role for RecA under non-stress: promotion of swarming motility in Escherichia coli K-12. BMC Biol. 5:14. doi: 10.1186/1741-7007-5-14
Greenfield, D., McEvoy, A. L., Shroff, H., Crooks, G. E., Wingreen, N. S., Betzig, E., et al. (2009). Self-organization of the Escherichia coli chemotaxis network imaged with super-resolution light microscopy. PLoS Biol. 7:e1000137. doi: 10.1371/journal.pbio.1000137
Han, K. Y., and Ha, T. (2015). Dual-color three-dimensional STED microscopy with a single high-repetition-rate laser. Opt. Lett. 40, 2653–2656. doi: 10.1364/OL.40.002653
Harshey, R. M. (1994). Bees aren’t the only ones: swarming in gram-negative bacteria. Mol. Microbiol. 13, 389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x
Henrichsen, J. (1972). Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36, 478–503.
Hernández-Rocamora, V. M., Alfonso, C., Margolin, W., Zorrilla, S., and Rivas, G. (2015). Evidence that bacteriophage /g/g/g kil peptide inhibits bacterial cell division by disrupting FtsZ protofilaments and sequestering protein subunits. J. Biol. Chem. 290, 20325–20335. doi: 10.1074/jbc.M115.653329
Hill, N. S., Buske, P. J., Shi, Y., and Levin, P. A. (2013). A moonlighting enzyme links Escherichia coli cell size with central metabolism. PLoS Genet. 9:e1003663. doi: 10.1371/journal.pgen.1003663
Irazoki, O., Mayola, A., Campoy, S., and Barbé, J. (2016). SOS system induction inhibits the assembly of chemoreceptor signaling clusters in Salmonella enterica. PLoS ONE 11:e0146685. doi: 10.1371/journal.pone.0146685
Jones, C. W., and Armitage, J. P. (2015). Positioning of bacterial chemoreceptors. Trends Microbiol. 23, 247–256. doi: 10.1016/j.tim.2015.03.004
Katribe, E., Bogomolnaya, L. M., Wingert, H., and Andrews-Polymenis, H. (2009). Subspecies Ilia and Illb salmonellae are defective for colonization of murine models of salmonellosis compared to Salmonella enterica subsp. i serovar typhimurium. J. Bacteriol. 191, 2843–2850. doi: 10.1128/JB.01223-08
Kentner, D., Thiem, S., Hildenbeutel, M., and Sourjik, V. (2006). Determinants of chemoreceptor cluster formation in Escherichia coli. Mol. Microbiol. 61, 407–417. doi: 10.1111/j.1365-2958.2006.05250.x
Kim, W., Killam, T., Sood, V., and Surette, M. G. (2003). Swarm-cell differentiation in Salmonella enterica serovar typhimurium results in elevated resistance to multiple antibiotics. J. Bacteriol. 185, 3111–3117. doi: 10.1128/JB.185.10.3111-3117.2003
Kim, W., and Surette, M. G. (2003). Swarming populations of Salmonella represent a unique physiological state coupled to multiple mechanisms of antibiotic resistance. Biol. Proced. Online 5, 189–196. doi: 10.1251/bpo61
Kim, W., and Surette, M. G. (2005). Prevalence of surface swarming behavior in Salmonella. J. Bacteriol. 187, 6580–6583. doi: 10.1128/JB.187.18.6580-6583.2005
Latasa, C., García, B., Echeverz, M., Toledo-Arana, A., Valle, J., Campoy, S., et al. (2012). Salmonella biofilm development depends on the phosphorylation status of RcsB. J. Bacteriol. 194, 3708–3722. doi: 10.1128/JB.00361-12
Lesterlin, C., Ball, G., Schermelleh, L., and Sherratt, D. J. (2014). RecA bundles mediate homology pairing between distant sisters during DNA break repair. Nature 506, 249–253. doi: 10.1038/nature12868
Li, X., Fleetwood, A. D., Bayas, C., Bilwes, A. M., Ortega, D. R., Falke, J. J., et al. (2013). The 3.2 Å resolution structure of a receptor: CheA:CheW signaling complex defines overlapping binding sites and key residue interactions within bacterial chemosensory arrays. Biochemistry 52, 3852–3865. doi: 10.1021/bi400383e
Link, A. J., and Phillips, D. (1997). Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179, 6228–6237.
Little, J. W. (1991). Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie 73, 411–421. doi: 10.1016/0300-9084(91)90108-D
Liu, Q., and Richardson, C. C. (1993). Gene 5.5 protein of bacteriophage T7 inhibits the nucleoid protein H-NS of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 90, 1761–1765. doi: 10.1073/pnas.90.5.1761
Maki, N., Gestwicki, J. E., Lake, E. M., Laura, L., Adler, J., and Kiessling, L. L. (2000). Motility and chemotaxis of filamentous cells of Escherichia coli. J Bacteriol. 182, 4337–4342. doi: 10.1128/JB.182.15.4337-4342.2000
Mariconda, S., Wang, Q., and Harshey, R. M. (2006). A mechanical role for the chemotaxis system in swarming motility. Mol. Microbiol. 60, 1590–1602. doi: 10.1111/j.1365-2958.2006.05208.x
Mayola, A., Irazoki, O., Martínez, I. A., Petrov, D., Menolascina, F., Stocker, R., et al. (2014). RecA protein plays a role in the chemotactic response and chemoreceptor clustering of Salmonella enterica. PLoS ONE 9:e105578. doi: 10.1371/journal.pone.0105578
McGrew, D. A., and Knight, K. L. (2003). Molecular design and functional organization of the RecA protein. Crit. Rev. Biochem. Mol. Biol. 38, 385–432. doi: 10.1080/10409230390242489
Medina-Ruiz, L., Campoy, S., Latasa, C., Cardenas, P., Alonso, J. C., and Barbé, J. (2010). Overexpression of the recA gene decreases oral but not intraperitoneal fitness of Salmonella enterica. Infect. Immun. 78, 3217–3225. doi: 10.1128/IAI.01321-09
Michel, B. (2005). After 30 years of study, the bacterial SOS response still surprises us. PLoS Biol. 3:e255. doi: 10.1371/journal.pbio.0030255
Nakajima, K., Inatsu, S., Mizote, T., Nagata, Y., Aoyama, K., Fukuda, Y., et al. (2008). Possible involvement of putA gene in Helicobacter pylori colonization in the stomach and motility. Biomed. Res. 29, 9–18. doi: 10.2220/biomedres.29.9
Oh, D., Yu, Y., Lee, H., Wanner, B. L., and Ritchie, K. (2014). Dynamics of the serine chemoreceptor in the Escherichia coli inner membrane: a high-speed single-molecule tracking study. Biophys. J. 106, 145–153. doi: 10.1016/j.bpj.2013.09.059
Ottemann, K. M., and Miller, J. F. (1997). Roles for motility in bacterial–host interactions. Mol. Microbiol. 24, 1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x
Overhage, J., Bains, M., Brazas, M. D., and Hancock, R. E. W. (2008). Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 190, 2671–2679. doi: 10.1128/JB.01659-07
Paget, M. S. (2015). Bacterial sigma factors and anti-sigma factors: structure, function and distribution. Biomolecules 5, 1245–1265. doi: 10.3390/biom5031245
Plumbridge, J. (2002). Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5, 187–193. doi: 10.1016/S1369-5274(02)00296-5
Rajendram, M., Zhang, L., Reynolds, B. J., Auer, G. K., Tuson, H. H., Ngo, K. V., et al. (2015). Anionic phospholipids stabilize RecA filament bundles in Escherichia coli. Mol. Cell 60, 374–384. doi: 10.1016/j.molcel.2015.09.009
Renzette, N., Gumlaw, N., Nordman, J. T., Krieger, M., Yeh, S.-P., Long, E., et al. (2005). Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol. Microbiol. 57, 1074–1085. doi: 10.1111/j.1365-2958.2005.04755.x
Renzette, N., and Sandler, S. J. (2008). Requirements for ATP binding and hydrolysis in RecA function in Escherichia coli. Mol. Microbiol. 67, 1347–1359. doi: 10.1111/j.1365-2958.2008.06130.x
Santos, T. M. A., Lin, T. Y., Rajendran, M., Anderson, S. M., and Weibel, D. B. (2014). Polar localization of Escherichia coli chemoreceptors requires an intact Tol-Pal complex. Mol. Microbiol. 92, 985–1004. doi: 10.1111/mmi.12609
Sassanfar, M., and Roberts, J. W. (1990). Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J. Mol. Biol. 212, 79–96. doi: 10.1016/0022-2836(90)90306-7
Schrödinger, L. L. C. (2010). The PyMOL Molecular Graphics System, Version 1.3r1. Available at: http://www.pymol.org
Shiomi, D., Banno, S., Homma, M., and Kawagishi, I. (2005). Stabilization of polar localization of a chemoreceptor via its covalent modifications and its communication with a different chemoreceptor. J. Bacteriol. 187, 7647–7654. doi: 10.1128/JB.187.22.7647-7654.2005
Shiomi, D., Yoshimoto, M., Homma, M., and Kawagishi, I. (2006). Helical distribution of the bacterial chemoreceptor via colocalization with the Sec protein translocation machinery. Mol. Microbiol. 60, 894–906. doi: 10.1111/j.1365-2958.2006.05145.x
Sourjik, V., and Wingreen, N. S. (2012). Responding to chemical gradients: bacterial chemotaxis. Curr. Opin. Cell Biol. 24, 262–268. doi: 10.1016/j.ceb.2011.11.008
Story, R. M., Weber, I. T., and Steitz, T. A. (1992). The structure of the E. coli recA protein monomer and polymer. Nature 355, 318–325. doi: 10.1038/355318a0
Takahashi, M., Maraboeuf, F., and Norden, B. (1996). Locations of functional domains in the RecA protein. overlap of domains and regulation of activities. Eur. J. Biochem. 242, 20–28. doi: 10.1111/j.1432-1033.1996.0020r.x
Thiem, S., and Sourjik, V. (2008). Stochastic assembly of chemoreceptor clusters in Escherichia coli. Mol. Microbiol. 68, 1228–1236. doi: 10.1111/j.1365-2958.2008.06227.x
Underbakke, E. S., Zhu, Y., and Kiessling, L. L. (2011). Protein footprinting in a complex milieu: identifying the interaction surfaces of the chemotaxis adaptor protein CheW. J. Mol. Biol. 409, 483–495. doi: 10.1016/j.jmb.2011.03.040
Keywords: SOS response, swarming, chemoreceptor polar arrays, chemosensory cluster assembly, RecA, CheW, 3D-STED
Citation: Irazoki O, Aranda J, Zimmermann T, Campoy S and Barbé J (2016) Molecular Interaction and Cellular Location of RecA and CheW Proteins in Salmonella enterica during SOS Response and Their Implication in Swarming. Front. Microbiol. 7:1560. doi: 10.3389/fmicb.2016.01560
Received: 29 July 2016; Accepted: 20 September 2016;
Published: 06 October 2016.
Edited by:
Akos T. Kovacs, University of Jena, GermanyReviewed by:
Pamela Gamba, Newcastle University, UKCopyright © 2016 Irazoki, Aranda, Zimmermann, Campoy and Barbé. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susana Campoy, c3VzYW5hLmNhbXBveUB1YWIuY2F0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.