- 1Department of Environmental Microbiology, Babasaheb Bhimrao Ambedkar University, Lucknow, India
- 2Molecular Glyco-biotechnology Group, Discipline of Biochemistry, School of Natural Sciences, National University of Ireland Galway, Galway, Ireland
Methane (CH4), a potent greenhouse gas, contributes about one third to the global green house gas emissions. CH4-assimilating microbes (mostly methanotrophs) in upland soils play very crucial role in mitigating the CH4 release into the atmosphere. Agricultural, environmental, and climatic shifts can alter CH4 sink profiles of soils, likely through shifts in CH4-assimilating microbial community structure and function. Landuse change, as forest and grassland ecosystems altered to agro-ecosystems, has already attenuated the soil CH4 sink potential, and are expected to be continued in the future. We hypothesized that variations in CH4 uptake rates in soils under different landuse practices could be an indicative of alterations in the abundance and/or type of methanotrophic communities in such soils. However, only a few studies have addressed to number and methanotrophs diversity and their correlation with the CH4 sink potential in soils of rehabilitated/restored lands. We focus on landuse practices that can potentially mitigate CH4 gas emissions, the most prominent of which are improved cropland, grazing land management, use of bio-fertilizers, and restoration of degraded lands. In this perspective paper, it is proposed that restoration of degraded lands can contribute considerably to improved soil CH4 sink strength by retrieving/conserving abundance and assortment of efficient methanotrophic communities. We believe that this report can assist in identifying future experimental directions to the relationships between landuse changes, methane-assimilating microbial communities and soil CH4 sinks. The exploitation of microbial communities other than methanotrophs can contribute significantly to the global CH4 sink potential and can add value in mitigating the CH4 problems.
An Overview of Current Atmospheric CH4 Status
Methane (CH4) is one of the most important greenhouse gases, accounting for ∼ 15–25% of the global warming (Zhou et al., 2013). CH4 sources are variable but their figure and enormity appear to be on the increase, while CH4 sinks are more unpredictable (Aronson et al., 2013). The chief global CH4 sources are natural and flooded soils, which contribute around one third of annual emissions (IPCC, 2007). Anthropogenic sources, including paddy agriculture, domestic cattle, landfills, fossil fuel burning, as well as biomass use for energy and agriculture, has been estimated to be >60% of total emissions (Wang et al., 2004). Moreover, it seems that the overall contribution of CH4 to the global emissions has been underestimated, since some studies demonstrated that CH4 can be readily formed in situ by terrestrial plants (Keppler et al., 2006). This newly identified source may have important implications in the global CH4 budget, and may call for a reconsideration of the role of natural CH4 sources in the ongoing climate change. These new findings are likely to increase the importance of CH4 sinks in the mitigation of its atmospheric concentrations.
It is well accepted that anthropogenic activities (landuse changes and use of chemicals in agriculture) are contributing to the global declining soil CH4 sink potential (Zheng et al., 2010). Landuse type is one of the major causes of soil characteristics variations and consequently the CH4 sink activity. It may be argued that a higher rate of CH4 consumption in forest soils, compared to other ecosystems could be attributed to the greater viable population size of methanotrophs (Figure 1). However, the experimental evidences for such arguments are still to be investigated. During the last few decades, the atmospheric concentration of CH4 has increased dramatically because of the imbalance between the overall sources and sinks (Singh, 2011). Anthropogenic nitrogen enrichment, grazing, deforestation, and alterations in water availability and temperature have received particular attention in terms of their effects on soil CH4 oxidation strength (Smith et al., 2000; Dai et al., 2013). The recent reports indicate that the problems of excess atmospheric load of this potent GHG can be mitigated either by reducing CH4 emissions or enhancing its consumption (Singh, 2011). It may be argued that ∼10% uplifting in the soil CH4 consumption could stabilize the current problem of atmospheric CH4 buildup (Singh, 2011). Consequently, it is imperative to adopt a more viable and eco-friendly approaches that could significantly contribute to augmentation of soil CH4 sink potential.
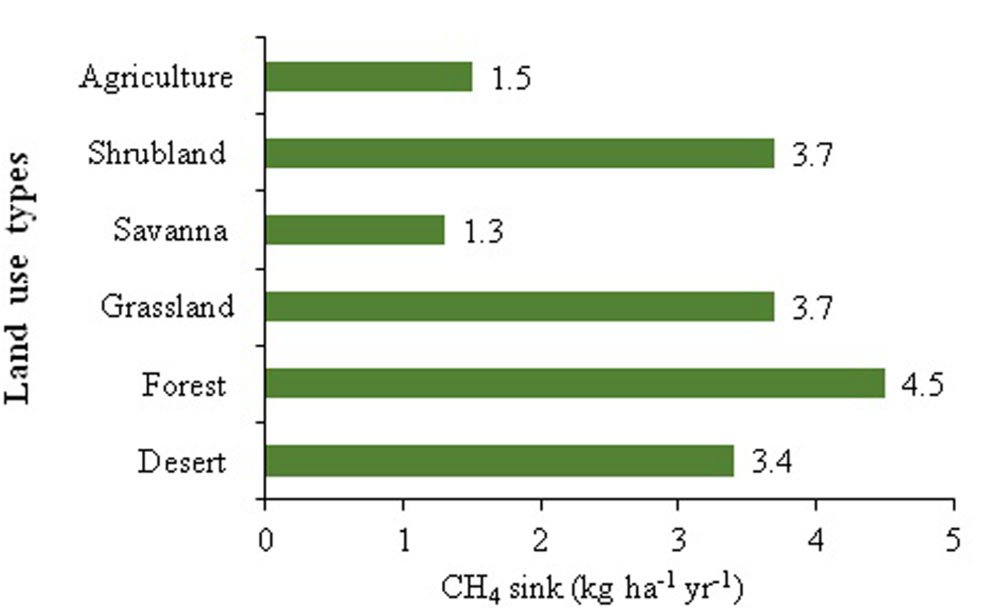
FIGURE 1. Methane sink activity across different land uses (Developed from Levine et al., 2011).
In environments the high CH4 content (freshwater wetlands, rice paddies, landfills, etc.) release to the atmosphere is controlled by methanotrophs via “low-capacity oxidation”. According to current concepts, the phylogenetically diverse uncultivable “high-affinity methanotrophs” are dominants in undisturbed soils and believed to be responsible for significant amount of atmospheric CH4 destruction. Landuse changes (conversion of natural forest and grassland ecosystems to agricultural lands) are the major consequences to the reduced soil CH4 oxidation rate, and are likely to persist more extensive due to anthropogenic activities. Even when restored back to its original form, previously cultivated land may have continuous lower CH4 sink activity than undisturbed lands. Variations in CH4 uptake strength in soils under different landuse practices could be due to the variation in methanotrophic community types. Based on the above arguments, it may be hypothesized that restoration of soil CH4 sink strength in restored degraded land could be correlated to the variation in restored methanotrophic bacterial community composition. However, only a few studies addressed to methanotrophic activity and diversity in restored lands (Knief et al., 2005; Dorr et al., 2010; Levine et al., 2011; Zhou et al., 2013). The methanotrophs distribution and their CH4 sink potential under prevailing influential environmental factors in resorted soils are still enigmatic. In addition, investigations on the influence of plant species on restored soil methanotrophs distribution are still very limited (Degelmann et al., 2010; Dorr et al., 2010), and the variation of methanotrophic communities in rehabilitated land with different plantation ways (natural and managed) remains unresolved (Dai et al., 2015).
The previous investigations have typically assessed either CH4 flux or the microbial methanotrophic community at similar ecosystems, but hardly ever has the role mediated by the efficient methanotrophic population size/community been studied concomitantly across a land-use gradient. My proposal is not to untangle the origin causes of difference in either methanotrophic community structure or CH4 flux, but to explain a relationship that, if directional, possibly will suggest insights into long-term management strategies for the methanotrophic community and CH4 flux. To our knowledge, there is no study to correlate methanotroph and bacterial diversity to both the stability and magnitude of the associated in situ CH4 sink across a landuse gradient, and to exploit the results to put on insights into management practices for abatement of atmospheric CH4 gas increases. Therefore, there is urgent need to investigate the methanotrophs abundance and diversity variations correlated with the atmospheric CH4 sink potential in restored soils under varied environmental regimes and dominant/selected vegetation cover.
Methane-Assimilating Mircobes and CH4 Sink
Among the microbes, CH4-consuming bacteria (primarily methanotrophs), ammonium-oxidizers (a type of nitrifiers) and sulfate-reducing bacteria (SRBs) are the key microbial groups that consume CH4 (Strong et al., 2015). SRBs reduce sulfate into sulfide using CH4 as a source of energy (Knittel and Boetius, 2009). Verrucomicrobia, a recently discovered group of CH4-utilizing microbes that consists of thermophiles, has also been identified to the group of known CH4-assimilating bacteria (Kalyuzhnaya et al., 2015). In addition to the more common methanotrophs, a group of anaerobic CH4-oxidizing Archaea has been also reported to be involved in denitrification coupled with anaerobic CH4 oxidation (Raghoebarsing et al., 2006). However, it seems that microbes such as nitrifiers, SRBs, Archaea, etc. involved in CH4 assimilation other than methanotrophs cannot grow as usual as aerobic CH4-oxidizing methanotrophs (Jones and Morita, 1983). In hypothesis, microbes should be capable of using inorganic nitrogen to assimilate CH4 anaerobically, but such microorganisms have neither been assessed in nature nor isolated in the laboratory (Raghoebarsing et al., 2006). Therefore, the potential contribution of such microbes in global CH4 sink phenomenon is largely unexplored and needs urgent attention (Singh, 2015).
Due to the key role of methanotrophs in the biogeochemical carbon cycle and in global climate change, the influence of landuse on methanotrophs diversity has attracted ample consideration (Robertson et al., 2000). A number of studies have been performed to evaluate the influence of different landuse changes on the CH4 uptake capacity with contrasting results (Prieme and Christensen, 1999; Suwanwaree and Robertson, 2005; Singh et al., 2008). For instance, Menyailo et al. (2008) confirmed that landuse patterns suppressed the soil CH4 uptake without affecting the diversity of methanotrophs. Similarly, tree species affected atmospheric CH4 oxidation in grassland soil exclusive of altering methanotrophic community (Menyailo et al., 2010). The lower population size of methanotrophs in degraded land soil could be due to the disturbances in soil conditions and micro-ecological niches of the bacteria (Figure 2). It may be argued that variations in CH4 oxidation and methanotrophs diversity and abundance may largely result from, and soils analyzed across a wide variety of habitats, however, the exact explanations about these arguments have not been fully addressed.
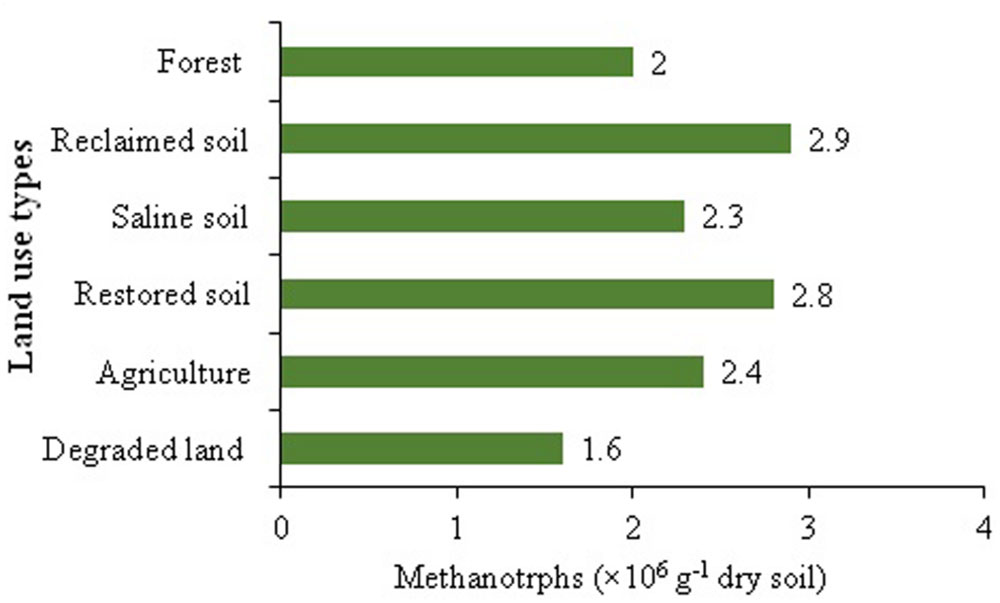
FIGURE 2. Methanotrophs abundance across different land uses (Developed from Tiwari et al., 2015).
Methanotrophs are the only known potential biological sink for CH4 in terrestrial/upland soils (Singh, 2011; Pandey et al., 2014). It has been suggested that deforestation and land management practices alter the soil characteristics, which in turn, adversely affect the viable soil methanotrophic community and their CH4 sink activity (King and Nanba, 2008; Dorr et al., 2010). However, reforestation may be the promising strategy to promote terrestrial CH4 uptake by soils owing to recovery of methanotrophs diversity. The afforestation of pastures experienced a shift in the methanotrophs community in soils from three different rainforest sites (Singh et al., 2007). Similarly, the conversion of natural forest to agricultural land also lowered the CH4 consumption by soils; nevertheless, it could be restored through revegetation (Livesley et al., 2009; Zhang et al., 2014). A recent field study from natural ecosystem suggests that soil restoration, even if performed within a rather limited time, may have the positive effect on CH4 consumption by terrestrial soils (Kizilova et al., 2013). It is suggested that the bio-fertilizer can be applied to minimize CH4 emission from flooded paddy soils and also holds promise as the efficient device for controlling the potent greenhouse gas CH4.
Many studies claimed that long-term N fertilization in paddy soils alters the methanotrophic composition, resulting in inhibited CH4 oxidation (Bodelier et al., 2000; Bodelier and Laanbroek, 2004; Mohanty et al., 2006; Noll et al., 2008; Banger et al., 2012; Zheng et al., 2013). Several management practices, including the organic amendments and residue managements have been proposed to restore the microbial diversity and methanotrophs numbers in deteriorated agricultural lands (Singh et al., 2011; Singh and Pandey, 2013; Carlson et al., 2015). It has been proposed that direct introduction of beneficial bio-filmed bio-fertilizers may significantly contribute to the revival of microbial diversity in degraded agro-ecosystems (Singh et al., 2011). Bio-fertilizer applications, particularly the nitrogen-fixing bio-agents, such as cyanobacteria, free-living diazotrophs, and Azolla may augment methanotrophs diversity and CH4 oxidation while reducing the amount of N fertilizer applied (Singh and Strong, 2016; Singh et al., 2016). Cyanobacteria are the exceptional model systems that can offer the biotechnologist with novel genes and stress tolerance capability having diverse uses in environmental sustainability and also; hold promise as the effective tool for harnessing CH4 (Prasanna et al., 2002). It may be argued that applications of above-described microbial bio-fertilizers in place of their chemical counterparts may mitigate the onset of global CH4 emission by conserving the viable methanotrophs diversity in disturbed agriculture soils (Pingak et al., 2014). The application of these bio-agents in paddy fields may be considered as the innovative tool for promoting the methanotrophs community composition. It is suggested that the application of bio-fertilizers as the nitrogen fertilizer replacement would be cost-effective, eco-friendly, and the safer means for degraded land restoration, and also to conserve the methanotrophic diversity and CH4 consumption in the long term.
Conclusions and Future Prospects
It is comprehensible from the above findings that ecological distribution, diversity, and CH4 sink potential of methanotrophs are largely affected by landuse changes. However, pertaining to the growth and activity of the methanotrophic community with CH4 sinks and the physico-chemical soil conditions remains an unresolved to understand the underlying mechanisms driving the methanotrophs–CH4 sink phenomenon. As postulated, a drastic change in soil chemical properties in a given ecosystem owing to landuse changes could be a potential threat to adversely affect both the CH4 sink activity and community composition of methanotrophs. However, in restored degraded ecosystems the improved and favorable soil conditions could help to establish and recovery of methanotrophs diversity. The use of bio-fertilizers, in place of inorganic nitrogen fertilizers, can enhance the optimum nitrogen availability to plants and CH4 assimilation efficiency of methanotrophs in the agriculture soils. It is possible that spore-forming methanotrophs, lying dormant in the disturbed soils owing to unfavorable environmental conditions, might be instrumental in restoring methanotrophic bacterial diversity once the ecological conditions were favorable (Jasper, 2007). The restoration of the ecological niche of methanotrophs in reforested and recovered soils would possibly favor the diverse methanotrophs community establishment, and to perform optimally (Singh and Singh, 2013). However, information on CH4-consuming bacterial diversity under different landuse types is still in a very incipient state. It is also not known whether different disturbed ecosystems following restoration have similar or different CH4-consuming bacterial community compositions. Further, there is no knowledge on viability of different methanotrophic community compositions (Type I or II) in a given re-established ecosystem. Even when rehabilitated back to its natural state, previously cultivated land continues to have a lower CH4 oxidation rate than undamaged areas. This apparent irreversibility of human impact on the CH4 oxidation rates has important implications for the future of land management strategies. It is expected that only future research investigations would possibly provide answer to some of the questions raised so far. Further, an understanding of the microbial agents other than CH4-consuming bacteria and its physiological behavior in the reclaimed/restored degraded soils is important to verify the significant contribution of such microbes in soil CH4 sink event. Therefore, the increased abundance and community composition of viable methanotrophs in soils of restored ecosystems could be the innovative approach to notably enhance the strength of CH4 uptake. Herein a list of few directions for future research on the ecology of methanotrophs in restored soils is anticipated:
(1) Since the soils of restored lands will have heterogeneous complex substrates and may be affected by many influential environmental factors including abnormal climatic conditions, the unique bacteria living in the soils will vary greatly by space and time. A short time-frame study on this aspect might bias our results and understanding; long-term monitoring of methanotrophs biodiversity and abundance in restored soils will enhance our understanding of their viability, stability, and CH4 sink potential.
(2) Would global climate change have any direct effect on abundance and methanrophs species in the soil of transformed lands? How do these soil microbes adapt or acclimate to global climate change?
(3) It assumed that in a restored or derived ecosystems the newly exotic microbial flora such as nematodes may influence the potential of CH4 oxidation indirectly through exerting their effect on efficiency and number/diversity of methanotrophs.
Author Contributions
JSS contributed the about role of land restoration in retrieving soil methanotrophs diversity and methane sink potential. VKG discussed about various arguments related to the methanotrophs and their roles in methane consumption under different lands use.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aronson, E. L., Allison, S. D., and Helliker, B. R. (2013). Environmental impacts on the diversity of methane-cycling microbes and their resultant function. Front. Microbiol. 4:225. doi: 10.3389/fmicb.2013.00225
Banger, K., Tian, H., and Lu, C. (2012). Do nitrogen fertilizers stimulate or inhibit methane emissions from rice fields? Glob. Change Biol. 18, 3259–3267. doi: 10.1111/j.1365-2486.2012.02762.x
Bodelier, P. L. E., and Laanbroek, H. J. (2004). Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 47, 265–277. doi: 10.1016/S0168-6496(03)00304-0
Bodelier, P. L. E., Roslev, P., Henckel, T., and Frenzel, P. (2000). Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 403, 421–424. doi: 10.1038/35000193
Carlson, J., Saxena, J., Basta, N., Hundal, L., Busalacchi, D., and Dick, R. P. (2015). Application of organic amendments to restore degraded soil: effects on soil microbial properties. Environ. Monit. Assess. 187, 109. doi: 10.1007/s10661-015-4293-0
Dai, Y., Di, H. J., Cameron, K. C., and He, J. Z. (2013). Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on methanotroph abundance and methane uptake in a grazed pasture soil. Environ. Sci. Pollut. Res. 20, 8680–8689. doi: 10.1007/s11356-013-1825-4
Dai, Y., Wu, Z., Xie, S., and Liu, Y. (2015). Methanotrophic community abundance and composition in plateau soils with different plant species and plantation ways. Appl. Microbiol. Biotechnol. 99, 9237–9244. doi: 10.1007/s002530156782z
Degelmann, D. M., Borken, W., Drake, H. L., and Kolb, S. (2010). Atmospheric methane-oxidizing communities differ in European Beech and Norway Spruce soils. Appl. Environ. Microbiol. 76, 3228–3235. doi: 10.1128/AEM.02730-09
Dorr, N., Glaser, B., and Kolb, S. (2010). Methanotrophic communities in Brazilian ferralsols from naturally forested, afforested, and agricultural sites. Appl. Environ. Microbiol. 76, 1307–1310. doi: 10.1128/AEM.02282-09
IPCC (2007). “The physical science basis,” in Contribution of Working Group to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, et al. (Cambridge: Cambridge University Press).
Jasper, D. A. (2007). Beneficial soil microorganisms of the Jarrah forest and their recovery in bauxite mine restoration in south-western Australia. Restor. Ecol. 15, 74–84. doi: 10.1111/j.1526-100X.2007.00295.x
Jones, R. D., and Morita, R. Y. (1983). Methane oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. App. Environ. Microbiol. 45, 401–410.
Kalyuzhnaya, M. G., Puri, A. W., and Lidstrom, M. E. (2015). Metabolic engineering in me-thanotrophic bacteria. Metab. Eng. 29,142–152. doi: 10.1016/j.ymben.2015.03.010
Keppler, F., Hamilton, J. T. G., Brass, M., and Rockmann, T. (2006). Methane emissions from terrestrial plants under aerobic conditions. Nature 439, 187–191. doi: 10.1038/nature04420
King, G. M., and Nanba, K. (2008). Distribution of atmospheric methane oxidation and methanotrophic communities on Hawaiian volcanic deposits and soils. Microbes Environ. 23, 326–330. doi: 10.1264/jsme2.ME08529
Kizilova, A., Yurkov, A., and Kravchenko, I. (2013). Aerobic methanotrophs in natural and agricultural soils of European Russia. Diversity 5, 541–556. doi: 10.3390/d5030541
Knief, C., Vanitchung, S., Harvey, N. W., Conrad, R., Dunfield, P. F., and Chidthaisong, A. (2005). Diversity of methanotrophic bacteria in tropical upland soils under different land uses. Appl. Environ. Microbiol. 71, 3826–3831. doi: 10.1128/AEM.71.7.3826-3831.2005
Knittel, K., and Boetius, A. (2009). Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63, 311–334. doi: 10.1146/annurev.micro.61.080706.093130
Levine, U. Y., Teal, T. K., Robertson, G. P., and Schmidt, T. M. (2011). Agriculture’s impact on microbial diversity and associated fluxes of carbon dioxide and methane. ISME J. 5, 1683–1691. doi: 10.1038/ismej.2011.40
Livesley, S. J., Kiese, R., Miehle, P., Weston, C. J., Butterbach-Bahl, K., and Arndt, S. K. (2009). Soil–atmosphere exchange of greenhouse gases in a Eucalyptus marginata woodland, a clover-grass pasture, and Pinus radiata and Eucalyptus globules plantations. Glob. Change Biol. 15, 425–440. doi: 10.1111/j.1365-2486.2008.01759.x
Menyailo, O. V., Abraham, W. R., and Conrad, R. (2010). Tree species affect atmospheric CH4 oxidation without altering community composition of soil methanotrophs. Soil Biol. Biochem. 42, 101–107. doi: 10.1016/j.soilbio.2009.10.005
Menyailo, O. V., Hungate, B. A., Abraham, W.-R., and Conrad, R. (2008). Changing land use reduces soil CH4 uptake by altering biomass and activity but not composition of high-affinity methanotrophs. Glob. Change Biol. 14, 2405–2419. doi: 10.1111/j.1365-2486.2008.01648.x
Mohanty, S. R., Bodelier, P. L. E., Floris, V., and Conrad, R. (2006). Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest Soils. Appl. Environ. Microbiol. 72, 1346–1354. doi: 10.1128/AEM.72.2.1346-1354.2006
Noll, M., Frenzel, P., and Conrad, R. (2008). Selective stimulation of type I methanotrophs in a rice paddy soil by urea fertilization revealed by RNA-based stable isotope probing. FEMS Microbiol. Ecol. 65, 125–132. doi: 10.1111/j.1574-6941.2008.00497.x
Pandey, V. C., Singh, J. S., Singh, D. P., and Singh, R. P. (2014). Methanotrophs: promising bacteria for environmental remediation. Int. J. Environ. Sci. Technol. 11, 241–250. doi: 10.2134/jeq2011.0179
Pingak, G. M. F., Sutanto, H., Akhdiya, A., and Rusmana, I. (2014). Effectivity of methanotrophic bacteria and Ochrobactrum anthropi as bio-fertilizer and emission reducer of CH4 and N2O in inorganic paddy fields. J. Med. Bioeng. 3, 217–221.
Prasanna, R., Kumar, V., Kumar, S., Yadav, A. K., Tripathi, U., Singh, A. K., et al. (2002). Methane production in rice soils is inhibited by cyanobacteria. J. Microbiol. Res. 157, 1–6. doi: 10.1078/0944-5013-00124
Prieme, A., and Christensen, S., (1999). Methane uptake by a selection of soils in Ghana with different land use. J. Geophy. Res. 104, 23617–23622. doi: 10.1029/1999JD900427
Raghoebarsing, A. A., Pol, A., van de Pas-Schoonen, K. T., Smolders, A. J. P., Ettwig, K. F., Rijpstra, W., et al. (2006). A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440, 918–921. doi: 10.1038/nature04617
Robertson, G. P., Paul, E. A., and Harwood, R. R. (2000). Greenhouse gases in intensive agriculture: contributions of individual gases to the radiative forcing of the atmosphere. Science 289, 1922–1925. doi: 10.1126/science.289.5486.1922
Singh, B. K., Tate, K. R., Kolipaka, G., Hedley, C. B., Macdonald, C. A., Millard, P., et al. (2007). Effect of afforestation and reforestation of pastures on the activity and population dynamics of methanotrophic bacteria. Appl. Environ. Microbiol. 73, 5153–5161. doi: 10.1128/AEM.00620-07
Singh, J. S. (2011). Methanotrophs: the potential biological sink to mitigate the global methane load. Curr. Sci. 100, 29–30.
Singh, J. S. (2015). Microbes: the chief ecological engineers in reinstating equilibrium in degraded ecosystems. Agric. Ecosyst. Environ. 203, 80–82. doi: 10.1016/j.agee.2015.01.026
Singh, J. S., Kumar, A., Rai, A. N., and Singh, D. P. (2016). Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 7:529. doi: 10.3389/fmicb.2016.00529
Singh, J. S., and Pandey, V. C. (2013). Fly ash application in nutrient poor agriculture soils: impact on methanotrophs population dynamics and paddy yields. Ecotoxicol. Environ. Safe 89, 43–51. doi: 10.1016/j.ecoenv.2012.11.011
Singh, J. S., Pandey, V. C., and Singh, D. P. (2011). Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 140, 339–353. doi: 10.1016/j.agee.2011.01.017
Singh, J. S., and Singh, D. P. (2013). Impact of anthropogenic disturbances on methanotrophs abundance in dry tropical forest ecosystems, India. Expert Opin. Environ. Biol. 2, 1–3. doi: 10.4172/2325-9655.1000106
Singh, J. S., and Strong, P. J. (2016). Biologically derived fertilizer: a multifaceted bio-tool in methane mitigation. Ecotoxicol. Environ. Saf. 124, 267–276. doi: 10.1016/j.ecoenv.2015.10.018
Singh, S., Kashyap, A. K., and Singh, J. S. (2008). Methane flux in relation to growth and phenology of a high yielding rice variety as affected by fertilization. Plant Soil 201, 157–164. doi: 10.1023/A:1004318727672
Smith, K. A., Dobbie, K. E., Ball, B. C., Bakken, L. R., Sitaula, B. K., and Hansen, S. et al. (2000). Oxidation of atmospheric methane in Northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob. Change Biol. 6, 791–803. doi: 10.1046/j.1365-2486.2000.00356.x
Strong, P. J., Xie, S., and Clarke, W. P. (2015). Methane as a resource: can the methanotrophs add value? Environ. Sci. Technol. 49, 4001–4018. doi: 10.1021/es504242n
Suwanwaree, P., and Robertson, G. P. (2005). Methane oxidation in forest, successional, and no-till agricultural ecosystems: effects of nitrogen and soil disturbance. Soil Sci. Am. J. 69, 1722–1779. doi: 10.2136/sssaj2004.0223
Tiwari, S., Singh, J. S., and Singh, D. P. (2015). Methanotrophs and CH4 sink: effect of human activity and ecological perturbations. Clim. Change Environ. Sustain. 3, 35–50. doi: 10.5958/2320-642X.2015.00004.6
Wang, J. S., Logan, J. A., McElroy, M. B., Duncan, B. N., Megretskaia, I. A., and Yantosca, R. M. (2004). A 3-D model analysis of the slow down and inter annual vari- ability in the methane growth rate from1988 to1997. Glob. Biogeochem. Cycles 18:GB3011. doi: 10.1029/2003GB002180
Zhang, W., Wang, K., Luo, Y., Fang, Y., Yan, J., Zhang, T., et al. (2014). Methane uptake in forest soils along an urban-to-rural gradient in Pearl River Delta, South China. Sci. Rep. 4, 5120. doi: 10.1038/srep05120
Zheng, Y., Liu, X., Zhang, L., Zhou, Z., and He, J. (2010). Do land utilization patterns affect methanotrophic communities in a Chinese upland red soil? J. Environ. Sci. 22, 1936–1943. doi: 10.1016/S1001-0742(09)60342-9
Zheng, Y., Zhang, L.-M., and He, J.-Z. (2013). Immediate effects of nitrogen, phosphorus, and potassium amendments on the methanotrophic activity and abundance in a Chinese paddy soil under short-term incubation experiment. J. Soils Sediments 13, 189–196. doi: 10.1007/s11368-012-0601-2
Keywords: agriculture, degraded lands, forests, GHGs, methanotrophs
Citation: Singh JS and Gupta VK (2016) Degraded Land Restoration in Reinstating CH4 Sink. Front. Microbiol. 7:923. doi: 10.3389/fmicb.2016.00923
Received: 23 January 2016; Accepted: 31 May 2016;
Published: 14 June 2016.
Edited by:
Rajesh K. Sani, South Dakota School of Mines and Technology, USAReviewed by:
Harvinder Singh Saini, Guru Nanak Dev University, IndiaRajeeva Gaur, Dr. Ram Manohar Lohia Avadh University, India
Sudhir Syal, Jaypee University of Information Technology, India
Copyright © 2016 Singh and Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jay Shankar Singh, amF5c2hhbmthcl8xQHlhaG9vLmNvLmlu
 Jay Shankar Singh
Jay Shankar Singh Vijai K. Gupta
Vijai K. Gupta