
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol., 17 May 2016
Sec. Infectious Agents and Disease
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.00683
Aspergillus fumigatus is the main etiologic agent of invasive aspergillosis (IA). Other Aspergillus species belonging to the section Fumigati (A. fumigatus complex) may occasionally be the cause of IA. These strains are often misidentified, as they cannot be distinguished from A. fumigatus by conventional morphological analysis and sequencing methods. This lack of recognition may have important consequences as these A. fumigatus-related species often display some level of intrinsic resistance to azoles and other antifungal drugs. A. lentulus, A. udagawae, A. viridinutans, and A. thermomutatus (Neosartorya pseudofischeri) have been associated with refractory cases of IA. Microbiologists should be able to suspect the presence of these cryptic species behind a putative A. fumigatus isolate on the basis of some simple characteristics, such as defect in sporulation and/or unusual antifungal susceptibility profile. However, definitive species identification requires specific sequencing analyses of the beta-tubulin or calmodulin genes, which are not available in most laboratories. Multiplex PCR assays or matrix-assisted laser desorption ionization – time-of-flight mass spectrometry (MALDI-TOF MS) gave promising results for rapid and accurate distinction between A. fumigatus and other Aspergillus spp. of the section Fumigati in clinical practice. Improved diagnostic procedures and antifungal susceptibility testing may be helpful for the early detection and management of these particular IA cases.
Aspergillus fumigatus is the most important pathogenic filamentous fungus in humans causing a spectrum of diseases including allergic bronchopulmonary aspergillosis, chronic pulmonary aspergillosis and invasive aspergillosis. Among the growing population of patients with depressed immune defenses, A. fumigatus represents one of the major infectious cause of death. Other important pathogenic Aspergillus spp. include A. flavus, A. niger, A. terreus, A. versicolor, A. calidoustus, and A. nidulans (Steinbach et al., 2012). These designations actually represent sections (or complexes) of closely related species (also referred as cryptic species) that cannot be clearly distinguished morphologically. While A. fumigatus sensu stricto represents by far the leading human pathogen, some other species belonging to the A. fumigatus sensu latu complex (i.e., section Fumigati) have been recognized as occasional causes of invasive aspergillosis in 3 to 6% of cases (Balajee et al., 2009b; Alastruey-Izquierdo et al., 2013, 2014; Escribano et al., 2013; Table 1). Their actual prevalence may be underestimated because of their lack of recognition by conventional diagnostic approaches. Moreover, the diagnosis of invasive aspergillosis often relies on suggestive radiological findings and/or positive fungal biomarkers (galactomannan, beta-1,3-D-glucan; De Pauw et al., 2008), in the absence of microbiological documentation and the actual contribution of Aspergillus spp. other than A. fumigatus in this setting is unknown.
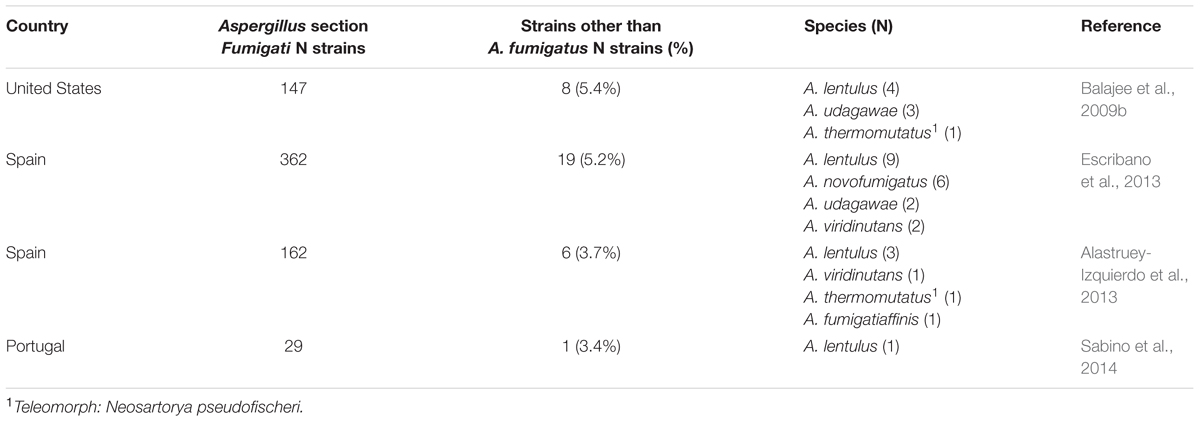
TABLE 1. Prevalence of Aspergillus spp. of section Fumigati other than Aspergillus fumigatus in clinical specimens.
Apart from A. fumigatus, the species of the section Fumigati that are most frequently recovered in clinical specimens and associated with invasive fungal diseases are A. lentulus, A. udagawae, A. viridinutans, A. thermomutatus (Neosartorya pseudofischeri), A. novofumigatus, and A. hiratsukae (Balajee et al., 2006, 2009b; Alcazar-Fuoli et al., 2008; Alastruey-Izquierdo et al., 2013; Escribano et al., 2013). Their limited pathogenic role compared to A. fumigatus may be explained by a lower thermotolerance and different profiles of secondary metabolites with decreased production of mycotoxins, such as gliotoxin (Frisvad and Larsen, 2015; Tamiya et al., 2015). However, while resistance to triazoles is rare among A. fumigatus sensu stricto, these sibling or cryptic species commonly exhibit decreased susceptibility to azoles and other antifungal agents (Alcazar-Fuoli et al., 2008; Escribano et al., 2013; Alastruey-Izquierdo et al., 2014). A recent multicentre prospective survey reported a rate of azole resistance of 3.2% among A. fumigatus isolates (van der Linden et al., 2015). Among these resistant strains, 78% were A. fumigatus sensu stricto harboring mutations of the Cyp51A gene, while the remaining 22% were actually sibling species (A. lentulus, A. thermomutatus, and A. udagawae). Microbiologists and clinicians thus must be aware of the existence of these sibling species that may be the cause of refractory cases of invasive aspergillosis. The aim of this review is to provide an overview of the characteristics of the most clinically relevant Aspergillus spp. of section Fumigati (other than A. fumigatus).
The genus Aspergillus is divided in subgenera and sections (Peterson, 2008). A. fumigatus belongs to the section Fumigati, in which 63 species have now been described, although some of them are doubtful because of possible synonymy with other species (Samson et al., 2007; Frisvad and Larsen, 2015). Species delimitation within section Fumigati relies on a polyphasic approach including phenotypic characteristics, rate of growth at variable temperatures, extrolite patterns, and genotyping characterization by random amplification of polymorphic DNA-polymerase chain reaction (RAPD-PCR) and multilocus sequence typing (Hong et al., 2005; Samson et al., 2007). Based on this approach, Yaguchi et al. (2007) have proposed a subdivision of the section Fumigati into five clades: (I) A. fumigatus, (II) species including A. lentulus and A. fumisynnematus, (III) species including A. fumigatiaffinis and A. novofumigatus, (IV) A. viridinutans, A. udagawae, and other atypical strains, and (V) species including A. hiratsukae, A. brevipes, A. duricaulis, and A. unilateralis.
Teleomorphic homothallic species producing ascomata (i.e., sexual stage of reproduction) were initially classified under the genus name Neosartorya. However, many species thought to be strictly anamorphic further demonstrated the ability to reproduce sexually with an opposite mating type (e.g., A. fumigatus, and A. lentulus; O’Gorman et al., 2009; Swilaiman et al., 2013). From 2012, taxonomists recommend to apply a single-name nomenclature keeping the name Aspergillus for all species of this genus (Samson et al., 2014). This system will be used in this review with reference to the old teleomorphic name in brackets when applicable.
Morphological examination of macroscopic and microscopic features remains the standard method for identification of filamentous fungi. While phenotypic characteristics allow distinguishing Aspergillus spp. at the section level, accurate species identification is usually not possible. Compared to A. fumigatus sensu stricto, other Aspergillus spp. of the section Fumigati may exhibit distinct morphological features, such as loss of pigmentation, poor sporulation, presence of ascomata, or variable growth at different temperature (Balajee et al., 2005a; Hong et al., 2005). These distinctive features, summarized in Table 2, are non-specific and variable, according to the growth media and conditions. Molecular methods are increasingly used as an adjunctive diagnostic tool in medical mycology. However, sequencing methods usually targeting fungal ribosomal DNA [internal transcribed spacer region (ITS), 18S rDNA, 26S/28S rDNA] are reliable for distinction at the section level, but not at the species level for Aspergillus spp. (Balajee et al., 2007). Phylogenetic studies integrate data of partial DNA sequences of various genes, such as beta-tubulin (benA), calmodulin, actin and hydrophobin (rodA), to distinguish the different species within the section Fumigati (Hong et al., 2005; Samson et al., 2007; Yaguchi et al., 2007). This approach is fastidious and not convenient for rapid identification in clinical practice. Currently, experts recommend comparative sequence analyses of the ribosomal ITS region (ITS1 and ITS2 flanking regions of the 5.8S rDNA) for intersection identification of Aspergillus spp. and of the beta-tubulin or calmodulin genes for intrasection identification at the species level (Balajee et al., 2007, 2009a; Samson et al., 2014). Multiplex PCR assays targeting microsatellite markers or specific gene sequences (ITS2 region, benA, rodA) have been developed for rapid detection and discrimination of Aspergillus species within the section Fumigati in clinical samples (Serrano et al., 2011; Araujo et al., 2012; Fernandez-Molina et al., 2014). Other techniques have also been described, such as PCR-restriction fragment length polymorphism (PCR-RFLP) of the benA gene (Staab et al., 2009) and microsphere-based Luminex assay (Etienne et al., 2009).
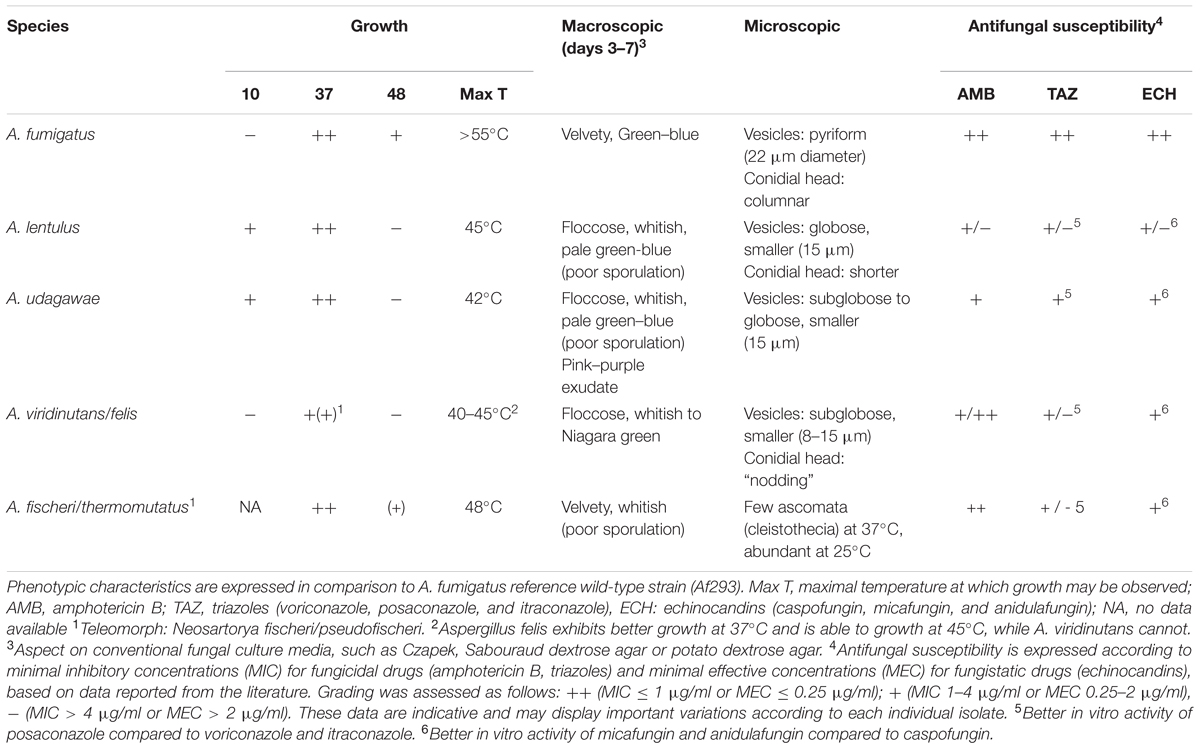
TABLE 2. Phenotypic characteristics of the most clinically relevant Aspergillus spp. of section Fumigati.
Matrix-assisted laser desorption ionization – time-of-flight mass spectrometry (MALDI-TOF MS) also gave promising results for the distinction between A. fumigatus and A. lentulus in clinical isolates (Verwer et al., 2014). This approach demonstrated accuracy for identification of Aspergillus spp. at the species level (Sanguinetti and Posteraro, 2014). Mass spectra of A. lentulus and other cryptic Aspergillus spp. have been included in different commercial or in-house libraries. However, protein extraction procedures for filamentous fungi are not standardized and further analyses of mass spectra of these fungal species, performed under the same experimental conditions, are warranted in order to obtain a complete reference database.
Various cases of fungal infections involving Aspergillus spp. of section Fumigati other than A. fumigatus have been reported in the medical literature (Table 3). Clinical presentation is often similar to that of other invasive aspergillosis and positive fungal biomarkers (galactomannan, beta-1,3-D-glucan) may also be observed. A. lentulus is the most frequent species recovered in clinical specimens and mixed infections with concomitant A. fumigatus are also reported. Invasive fungal infections due to A. lentulus or other A. fumigatus-related species are associated with a particularly high mortality rate (about 60%). In addition, these cryptic species have also been associated with allergic fungal diseases and chronic colonization in patients with cystic fibrosis (Shivaprakash et al., 2009; Symoens et al., 2010). Some microbiological and clinical features of the most clinically important species will be discussed in this chapter.
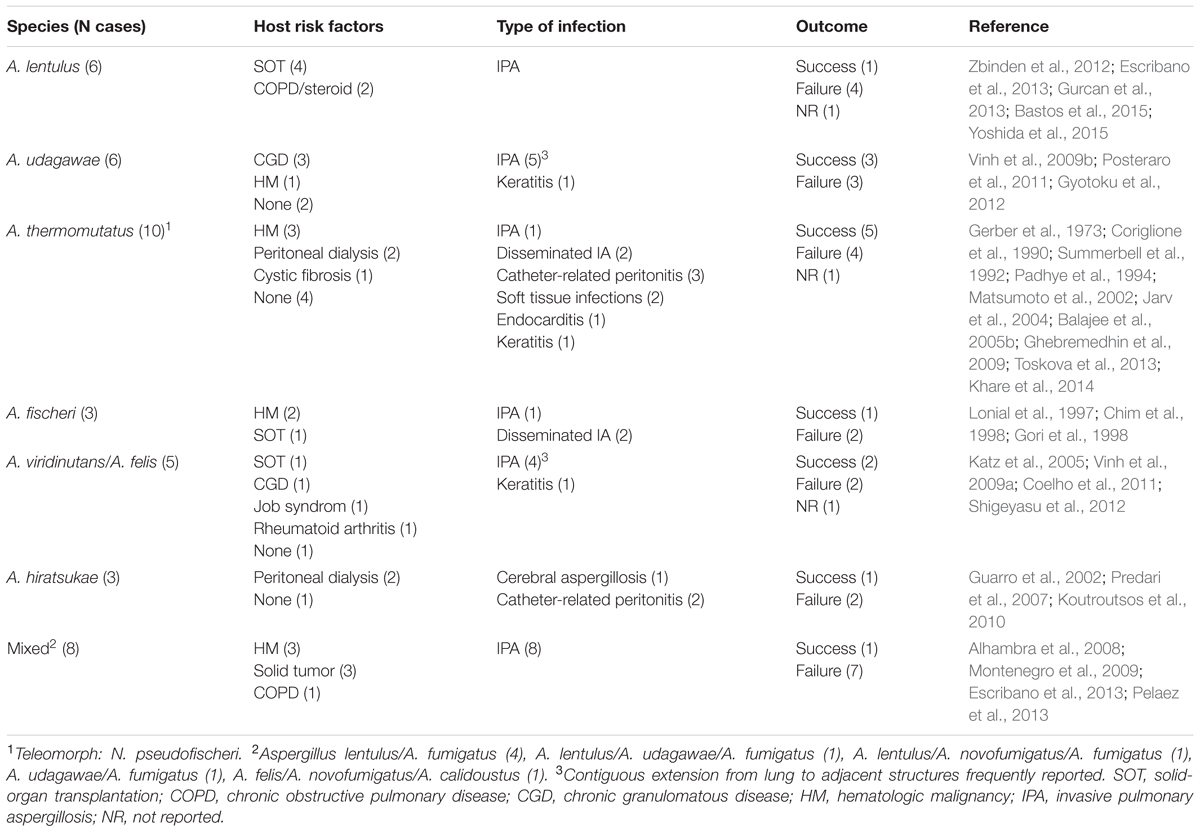
TABLE 3. Case reports of invasive aspergillosis attributed to Aspergillus spp. of section Fumigati other than A. fumigatus.
Aspergillus lentulus was first identified in 2004. While retrospectively screening for itraconazole resistance in a collection of 128 A. fumigatus isolates obtained from hematopoietic stem cell transplant recipients between 1991 and 2000 in Seattle, Balajee et al. (2004) described an A. fumigatus variant exhibiting poor sporulation and decreased susceptibility to azoles, amphotericin B and echinocandins. These atypical isolates were found to be the cause of invasive fungal infections in 4 (5%) of these patients. On the basis of phylogenetic analyses, these isolates were assigned to a new species within the section Fumigati under the name of A. lentulus (Balajee et al., 2005a). Phenotypic characteristics of A. lentulus compared to A. fumigatus are poor sporulation, smaller conidial heads and inability to grow at temperature above 48°C. Mass spectrometry methods (MALDI-TOF, SELDI-TOF) showed promising results for rapid discrimination between A. lentulus and A. fumigatus (Pinel et al., 2011; Verwer et al., 2014).
Aspergillus lentulus seems to represent only a small proportion (<3%) of all Aspergillus spp. of the section Fumigati isolated in clinical specimens (Balajee et al., 2009b; Alastruey-Izquierdo et al., 2013; Escribano et al., 2013). However, it may account for a substantial proportion (10–30%) of azole-resistant isolates that are morphologically classified as A. fumigatus (Mortensen et al., 2010; Kidd et al., 2015; van der Linden et al., 2015). Clinical reports of A. lentulus infections mainly consist of invasive pulmonary aspergillosis in patients with hematologic malignancies or solid-organ transplant recipients (Balajee et al., 2004, 2009b; Zbinden et al., 2012; Escribano et al., 2013; Gurcan et al., 2013; Bastos et al., 2015; Yoshida et al., 2015). Interestingly, A. lentulus is often found in association with A. fumigatus in mixed infections with poor outcomes (Alhambra et al., 2008; Montenegro et al., 2009; Escribano et al., 2013). A. lentulus was pathogenic in murine models of invasive aspergillosis, but was associated with a decreased mortality and a delayed course of infection compared to A. fumigatus (Mellado et al., 2011). A. lentulus was also pathogenic in larvae of Galleria mellonella who did not respond to voriconazole therapy (Alcazar-Fuoli et al., 2015).
Decreased susceptibility to all three antifungal classes (azoles, polyenes and echinocandins) are reported in most clinical isolates (Alcazar-Fuoli et al., 2008; Alastruey-Izquierdo et al., 2013, 2014; Escribano et al., 2013). The level of resistance of A. lentulus to triazoles is variable, with higher MICs observed for voriconazole and itraconazole compared to posaconazole. The new triazole isavuconazole displays good in vitro activity against A. lentulus (Datta et al., 2013). Intrinsic azole resistance in A. lentulus is dependant of the Cyp51A gene (AlCyp51A), which shares 92% homology with its A. fumigatus ortholog (AfCyp51A). Substitution of AfCyp51A by AlCyp51A induced the same phenotype of azole resistance in A. fumigatus (Mellado et al., 2011). Molecular dynamics modeling suggested that some differences in the BC-loop between AfCyp51A and AlCyp51A may affect the closed form of the protein upon voriconazole binding (Alcazar-Fuoli et al., 2011). Among echinocandins, decreased susceptibility of A. lentulus is mainly observed for caspofungin (Alcazar-Fuoli et al., 2008; Alastruey-Izquierdo et al., 2014). However, there was no polymorphisms in the known hot spot regions of the A. lentulus fks gene (encoding for the β-1,3-glucan synthase target of echinocandins), which shares 98% homology with A. fumigatus fks (Staab et al., 2010).
Aspergillus udagawae was first isolated in 1995 from the soil of a sugar cane plantation in Brazil (Horie et al., 1995). Morphological features that may help distinguishing A. udagawae from A. fumigatus are the decreased spore production and the secretion of a pink-purple pigment on agar plates. A. udagawae also exhibits a different range of thermotolerance, being able to grow at 10°C, but not at ≥42°C, with an optimal growth at 30–35°C (Sugui et al., 2010). Decreased rates of germination and increased susceptibility to neutrophils and hydrogen peroxide may explain the lower virulence of A. udagawae compared to A. fumigatus in murine models (Sugui et al., 2010; Gyotoku et al., 2012).
Molecular analyses suggest that A. udagawae may account for a small proportion of the Aspergillus spp. of section Fumigati isolated from clinical specimens (Balajee et al., 2006; Vinh et al., 2009b; Escribano et al., 2013). Few clinical cases have been reported in the literature. A. udagawae may cause a chronic form of pulmonary aspergillosis with a propensity for contiguous spread to adjacent structures, which may be refractory to antifungal therapy (Vinh et al., 2009b). Cases of endobronchial infection and post-traumatic keratitis have also been reported in immunocompetent patients (Posteraro et al., 2011; Gyotoku et al., 2012). A. udagawae isolates usually exhibit higher MIC (1 to 3 dilutions) to amphotericin B and voriconazole compared to A. fumigatus sensu stricto (Balajee et al., 2006; Vinh et al., 2009b). The new triazole isavuconazole demonstrated increased activity (MIC ranging 0.031–0.25 μg/ml; Datta et al., 2013).
Aspergillus viridinutans was first isolated from a rabbit dung in Australia and subsequently documented in some human clinical isolates (Alcazar-Fuoli et al., 2008; Vinh et al., 2009a; Coelho et al., 2011; Escribano et al., 2013; Pelaez et al., 2013). Recently, some specimens previously identified as A. viridinutans have been reassigned to the novel closely related species A. pseudoviridinutans, A. felis, A. pseudofelis, and A. parafelis (Katz et al., 2005; Barrs et al., 2013; Alvarez-Perez et al., 2014; Sugui et al., 2014). These species sporulate weakly and produce a typical “nodding” conidial head (Barrs et al., 2013). Contrarily to A. viridinutans, A. felis is able to grow at 45°C. A. felis is a common cause of sino-orbital aspergillosis in cats (Barrs and Talbot, 2014). Similar to A. udagawae, A. viridinutans, and A. felis cause human pulmonary aspergillosis characterized by a chronic evolution and spread across contiguous anatomic planes (Vinh et al., 2009a; Coelho et al., 2011). A case of fungal keratitis was also described in human (Shigeyasu et al., 2012). Most clinical isolates showed high MICs for voriconazole and itraconazole (usually ≥4 μg/ml), but lower values for posaconazole and amphotericin B (Alcazar-Fuoli et al., 2008; Vinh et al., 2009a; Coelho et al., 2011; Shigeyasu et al., 2012; Escribano et al., 2013; Pelaez et al., 2013).
Aspergillus fischeri (teleomorph: N. fischeri) is a ubiquitous fungus producing thermotolerant ascospores, which is responsible of food spoilage of fruit juices and other heat-processed fruit products. A. thermomutatus (teleomorph: N. pseudofischeri) can only be differentiate from A. fischeri by electron microscopic analysis of the ascospores (Peterson, 1992). These homothallic fungi produce whitish fast-growing colonies with few sporulation at 37°C and can tolerate higher temperatures (48°C). Ascomata of the sexual form are usually abundant, but may be scarce or appear at later stages, especially at 37°C.
A. fischeri and A. thermomutatus have been identified as the cause of human mycoses in only few case reports with a broad spectrum of disease’s presentation including extrapulmonary manifestations in most cases, such as endocarditis, osteomyelitis, peritonitis, gastro-intestinal tract infection, keratitis, and disseminated infections (Gerber et al., 1973; Coriglione et al., 1990; Summerbell et al., 1992; Padhye et al., 1994; Lonial et al., 1997; Chim et al., 1998; Gori et al., 1998; Matsumoto et al., 2002; Jarv et al., 2004; Balajee et al., 2005b; Ghebremedhin et al., 2009; Toskova et al., 2013; Khare et al., 2014). Susceptibility testing of these isolates usually shows higher MICs for triazoles compared to A. fumigatus, but similar values for amphotericin B and echinocandins (Balajee et al., 2005b; Alcazar-Fuoli et al., 2008).
Aspergillus (Neosartorya) hiratsukae has been identified in some clinical specimens (Alcazar-Fuoli et al., 2008) and has been associated with rare and essentially extrapulmonary fungal diseases, such as cerebral aspergillosis and peritonitis in patients under peritoneal dialysis (Guarro et al., 2002; Predari et al., 2007; Koutroutsos et al., 2010). A. novofumigatus, A. fumigatiaffinis, and A. fumisynnematus have also been isolated in clinical samples, but their pathogenic role remained unclear (Alcazar-Fuoli et al., 2008; Escribano et al., 2013; Pelaez et al., 2013).
Recent advances in phylogenetic analyses and molecular methods have revealed the important diversity of Aspergillus species within the section Fumigati. Some clinical isolates may actually be misidentified as A. fumigatus by conventional diagnostic methods, which may result in inappropriate antifungal therapy because of the decreased susceptibility of these cryptic species to many antifungal agents. Azole resistance among A. fumigatus isolates is an emerging problem, which has been highlighted by multiple recent reports throughout the world, but its prevalence is still low. Actually, in case of refractory invasive aspergillosis, clinicians should suspect that a sibling species of A. fumigatus may be the causal agent. Slow or poor sporulation is usually the first hint that should alert the microbiologist. Sequencing of the ITS region, followed by targeted sequencing of the beta-tubulin or calmodulin genes seems to date the most appropriate method for reliable species identification. However, these procedures are not available in most institutions. MALDI-TOF MS may be a very convenient alternative for the rapid detection of A. lentulus and other A. fumigatus-related species, but further investigations for standardized sample treatment procedures of filamentous fungi and spectra characterization are required. Antifungal susceptibility testing may be useful because of the unpredictable susceptibility profile of these species. However, results should be interpreted with caution because of the lack of clinical breakpoints and the absence of data correlating MICs with clinical outcomes. Recommendations for the antifungal management of such cases cannot be clearly defined on the basis of limited published data consisting in case reports of refractory invasive aspergillosis with often fatal outcomes despite the use of multiple antifungal agents. Decreased susceptibility to azoles is common and higher MICs to other antifungal drugs (amphotericin B, caspofungin) may also be observed, especially for A. lentulus. Among triazoles, posaconazole displays better in vitro antifungal activity compared to voriconazole and itraconazole, but is not yet approved as first-line therapy of invasive aspergillosis. In addition, the new extended-spectrum isavuconazole, which has recently been approved for the treatment of invasive aspergillosis (Miceli and Kauffman, 2015), may become an interesting alternative as both A. lentulus and A. udagawae displayed low MICs comparable to that of A. fumigatus (Datta et al., 2013). Combination therapies may be considered in refractory cases, although data are lacking to demonstrate a benefit in this setting.
FL: Design and redaction of the manuscript.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alastruey-Izquierdo, A., Alcazar-Fuoli, L., and Cuenca-Estrella, M. (2014). Antifungal susceptibility profile of cryptic species of Aspergillus. Mycopathologia 178, 427–433. doi: 10.1007/s11046-014-9775-z
Alastruey-Izquierdo, A., Mellado, E., Pelaez, T., Peman, J., Zapico, S., Alvarez, M., et al. (2013). Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP Study). Antimicrob. Agents Chemother. 57, k3380–3387. doi: 10.1128/AAC.00383-13
Alcazar-Fuoli, L., Buitrago, M., Gomez-Lopez, A., and Mellado, E. (2015). An alternative host model of a mixed fungal infection by azole susceptible and resistant Aspergillus spp strains. Virulence 6, 376–384. doi: 10.1080/21505594.2015.1025192
Alcazar-Fuoli, L., Cuesta, I., Rodriguez-Tudela, J. L., Cuenca-Estrella, M., Sanglard, D., and Mellado, E. (2011). Three-dimensional models of 14alpha-sterol demethylase (Cyp51A) from Aspergillus lentulus and Aspergillus fumigatus: an insight into differences in voriconazole interaction. Int. J. Antimicrob. Agents 38, 426–434. doi: 10.1016/j.ijantimicag.2011.06.005
Alcazar-Fuoli, L., Mellado, E., Alastruey-Izquierdo, A., Cuenca-Estrella, M., and Rodriguez-Tudela, J. L. (2008). Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52, 1244–1251. doi: 10.1128/AAC.00942-07
Alhambra, A., Catalan, M., Moragues, M. D., Brena, S., Ponton, J., Montejo, J. C., et al. (2008). Isolation of Aspergillus lentulus in Spain from a critically ill patient with chronic obstructive pulmonary disease. Rev. Iberoam. Micol 25, 246–249. doi: 10.1016/S1130-1406(08)70058-5
Alvarez-Perez, S., Mellado, E., Serrano, D., Blanco, J. L., Garcia, M. E., Kwon, M., et al. (2014). Polyphasic characterization of fungal isolates from a published case of invasive aspergillosis reveals misidentification of Aspergillus felis as Aspergillus viridinutans. J. Med. Microbiol. 63, 617–619. doi: 10.1099/jmm.0.068502-0
Araujo, R., Amorim, A., and Gusmao, L. (2012). Diversity and specificity of microsatellites within Aspergillus section Fumigati. BMC Microbiol. 12:154. doi: 10.1186/1471-2180-12-154
Balajee, S. A., Borman, A. M., Brandt, M. E., Cano, J., Cuenca-Estrella, M., Dannaoui, E., et al. (2009a). Sequence-based identification of Aspergillus, fusarium, and mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47, 877–884. doi: 10.1128/JCM.01685-08
Balajee, S. A., Gribskov, J., Brandt, M., Ito, J., Fothergill, A., and Marr, K. A. (2005a). Mistaken identity: neosartorya pseudofischeri and its anamorph masquerading as Aspergillus fumigatus. J. Clin. Microbiol. 43, 5996–5999. doi: 10.1128/JCM.43.12.5996-5999.2005
Balajee, S. A., Gribskov, J. L., Hanley, E., Nickle, D., and Marr, K. A. (2005b). Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 4, 625–632. doi: 10.1128/EC.4.3.625-632.2005
Balajee, S. A., Houbraken, J., Verweij, P. E., Hong, S. B., Yaghuchi, T., Varga, J., et al. (2007). Aspergillus species identification in the clinical setting. Stud. Mycol. 59, 39–46. doi: 10.3114/sim.2007.59.05
Balajee, S. A., Kano, R., Baddley, J. W., Moser, S. A., Marr, K. A., Alexander, B. D., et al. (2009b). Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J. Clin. Microbiol. 47, 3138–3141. doi: 10.1128/JCM.01070-09
Balajee, S. A., Nickle, D., Varga, J., and Marr, K. A. (2006). Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryot. Cell 5, 1705–1712. doi: 10.1128/EC.00162-06
Balajee, S. A., Weaver, M., Imhof, A., Gribskov, J., and Marr, K. A. (2004). Aspergillus fumigatus variant with decreased susceptibility to multiple antifungals. Antimicrob. Agents Chemother. 48, 1197–1203. doi: 10.1128/AAC.48.4.1197-1203.2004
Barrs, V. R., and Talbot, J. J. (2014). Feline aspergillosis. Vet. Clin. North Am. Small Anim. Pract. 44, 51–73. doi: 10.1016/j.cvsm.2013.08.001
Barrs, V. R., van Doorn, T. M., Houbraken, J., Kidd, S. E., Martin, P., Pinheiro, M. D., et al. (2013). Aspergillus felis sp. nov., an emerging agent of invasive aspergillosis in humans, cats, and dogs. PLoS ONE 8:e64871. doi: 10.1371/journal.pone.0064871
Bastos, V. R., Santos, D. W., Padovan, A. C., Melo, A. S., Mazzolin Mde, A., Camargo, L. F., et al. (2015). Early invasive pulmonary aspergillosis in a kidney transplant recipient caused by Aspergillus lentulus: first Brazilian report. Mycopathologia 179, 299–305. doi: 10.1007/s11046-014-9840-7
Chim, C. S., Ho, P. L., and Yuen, K. Y. (1998). Simultaneous Aspergillus fischeri and Herpes simplex pneumonia in a patient with multiple myeloma. Scand. J. Infect. Dis. 30, 190–191. doi: 10.1080/003655498750003627
Coelho, D., Silva, S., Vale-Silva, L., Gomes, H., Pinto, E., Sarmento, A., et al. (2011). Aspergillus viridinutans: an agent of adult chronic invasive aspergillosis. Med. Mycol. 49, 755–759. doi: 10.3109/13693786.2011.556672
Coriglione, G., Stella, G., Gafa, L., Spata, G., Oliveri, S., Padhye, A. A., et al. (1990). Neosartorya fischeri var fischeri (Wehmer) Malloch and Cain 1972 (anamorph: Aspergillus fischerianus Samson and Gams 1985) as a cause of mycotic keratitis. Eur. J. Epidemiol. 6, 382–385. doi: 10.1007/BF00151712
Datta, K., Rhee, P., Byrnes, E. III, Garcia-Effron, G., Perlin, D. S., Staab, J. F., et al. (2013). Isavuconazole activity against Aspergillus lentulus, Neosartorya udagawae, and Cryptococcus gattii, emerging fungal pathogens with reduced azole susceptibility. J. Clin. Microbiol. 51, 3090–3093. doi: 10.1128/JCM.01190-13
De Pauw, B., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., et al. (2008). Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 46, 1813–1821. doi: 10.1086/588660
Escribano, P., Pelaez, T., Munoz, P., Bouza, E., and Guinea, J. (2013). Is azole resistance in Aspergillus fumigatus a problem in Spain? Antimicrob. Agents Chemother. 57, 2815–2820. doi: 10.1128/AAC.02487-12
Etienne, K. A., Gade, L., Lockhart, S. R., Diekema, D. J., Messer, S. A., Pfaller, M. A., et al. (2009). Screening of a large global Aspergillus fumigatus species complex collection by using a species-specific microsphere-based Luminex assay. J. Clin. Microbiol. 47, 4171–4172. doi: 10.1128/JCM.01415-09
Fernandez-Molina, J. V., Abad-Diaz-de-Cerio, A., Sueiro-Olivares, M., Pellon, A., Ramirez-Garcia, A., Garaizar, J., et al. (2014). Rapid and specific detection of section Fumigati and Aspergillus fumigatus in human samples using a new multiplex real-time PCR. Diagn. Microbiol. Infect. Dis. 80, 111–118. doi: 10.1016/j.diagmicrobio.2014.06.003
Frisvad, J. C., and Larsen, T. O. (2015). Extrolites of Aspergillus fumigatus and other pathogenic species in Aspergillus section fumigati. Front. Microbiol. 6:1485. doi: 10.3389/fmicb.2015.01485
Gerber, J., Chomicki, J., Brandsberg, J. W., Jones, R., and Hammerman, K. J. (1973). Pulmonary aspergillosis caused by Aspergillus fischeri var. spinosus: report of a case and value of serologic studies. Am. J. Clin. Pathol. 60, 861–866. doi: 10.1093/ajcp/60.6.861
Ghebremedhin, B., Bluemel, A., Neumann, K. H., Koenig, B., and Koenig, W. (2009). Peritonitis due to Neosartorya pseudofischeri in an elderly patient undergoing peritoneal dialysis successfully treated with voriconazole. J. Med. Microbiol. 58, 678–682. doi: 10.1099/jmm.0.005785-0
Gori, S., Pellegrini, G., Filipponi, F., Capanna, S. D., Biancofiore, G., Mosca, F., et al. (1998). Pulmonary aspergillosis caused by Neosartorya fischeri (Aspergillus fischeriani) in a liver transplant recipient. J. Mycol. Med. 8, 105–107.
Guarro, J., Kallas, E. G., Godoy, P., Karenina, A., Gene, J., Stchigel, A., et al. (2002). Cerebral aspergillosis caused by Neosartorya hiratsukae, Brazil. Emerg. Infect. Dis. 8, 989–991. doi: 10.3201/eid0809.020073
Gurcan, S., Tikvesli, M., Ustundag, S., and Ener, B. (2013). A Case Report On Aspergillus lentulus pneumonia. Balkan Med. J. 30, 429–431. doi: 10.5152/balkanmedj.2013.8572
Gyotoku, H., Izumikawa, K., Ikeda, H., Takazono, T., Morinaga, Y., Nakamura, S., et al. (2012). A case of bronchial aspergillosis caused by Aspergillus udagawae and its mycological features. Med. Mycol. 50, 631–636. doi: 10.3109/13693786.2011.639036
Hong, S. B., Go, S. J., Shin, H. D., Frisvad, J. C., and Samson, R. A. (2005). Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97, 1316–1329. doi: 10.3852/mycologia.97.6.1316
Horie, Y., Miyaji, M., Nishimura, K., Franco, M. F., and Coelho, K. I. (1995). Two new species of Neosartorya from Brazilian soil. Mycoscience 36, 159–165. doi: 10.1007/BF02268552
Jarv, H., Lehtmaa, J., Summerbell, R. C., Hoekstra, E. S., Samson, R. A., and Naaber, P. (2004). Isolation of Neosartorya pseudofischeri from blood: first hint of pulmonary Aspergillosis. J. Clin. Microbiol. 42, 925–928. doi: 10.1128/JCM.42.2.925-928.2004
Katz, M. E., Dougall, A. M., Weeks, K., and Cheetham, B. F. (2005). Multiple genetically distinct groups revealed among clinical isolates identified as atypical Aspergillus fumigatus. J. Clin. Microbiol. 43, 551–555. doi: 10.1128/JCM.43.2.551-555.2005
Khare, R., Gupta, S., Arif, S., Jentoft, M. E., Deziel, P. J., Roden, A. C., et al. (2014). Misidentification of Neosartorya pseudofischeri as Aspergillus fumigatus in a lung transplant patient. J. Clin. Microbiol. 52, 2722–2725. doi: 10.1128/JCM.00216-14
Kidd, S. E., Goeman, E., Meis, J. F., Slavin, M. A., and Verweij, P. E. (2015). Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58, 350–355. doi: 10.1111/myc.12324
Koutroutsos, K., Arabatzis, M., Bougatsos, G., Xanthaki, A., Toutouza, M., and Velegraki, A. (2010). Neosartorya hiratsukae peritonitis through continuous ambulatory peritoneal dialysis. J. Med. Microbiol. 59, 862–865. doi: 10.1099/jmm.0.019133-0
Lonial, S., Williams, L., Carrum, G., Ostrowski, M., and McCarthy, P. Jr. (1997). Neosartorya fischeri: an invasive fungal pathogen in an allogeneic bone marrow transplant patient. Bone Marrow Transplant. 19, 753–755. doi: 10.1038/sj.bmt.1700715
Matsumoto, N., Shiraga, H., Takahashi, K., Kikuchi, K., and Ito, K. (2002). Successful treatment of Aspergillus peritonitis in a peritoneal dialysis patient. Pediatr. Nephrol. 17, 243–245. doi: 10.1007/s00467-002-0821-6
Mellado, E., Alcazar-Fuoli, L., Cuenca-Estrella, M., and Rodriguez-Tudela, J. L. (2011). Role of Aspergillus lentulus 14-alpha sterol demethylase (Cyp51A) in azole drug susceptibility. Antimicrob. Agents Chemother. 55, 5459–5468. doi: 10.1128/AAC.05178-11
Miceli, M. H., and Kauffman, C. A. (2015). Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 61, 1558–1565. doi: 10.1093/cid/civ571
Montenegro, G., Sanchez Puch, S., Jewtuchowicz, V. M., Pinoni, M. V., Relloso, S., Temporitti, E., et al. (2009). Phenotypic and genotypic characterization of Aspergillus lentulus and Aspergillus fumigatus isolates in a patient with probable invasive aspergillosis. J. Med. Microbiol. 58, 391–395. doi: 10.1099/jmm.0.005942-0
Mortensen, K. L., Mellado, E., Lass-Florl, C., Rodriguez-Tudela, J. L., Johansen, H. K., and Arendrup, M. C. (2010). Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 54, 4545–4549. doi: 10.1128/AAC.00692-10
O’Gorman, C. M., Fuller, H., and Dyer, P. S. (2009). Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457, 471–474. doi: 10.1038/nature07528
Padhye, A. A., Godfrey, J. H., Chandler, F. W., and Peterson, S. W. (1994). Osteomyelitis caused by Neosartorya pseudofischeri. J. Clin. Microbiol. 32, 2832–2836.
Pelaez, T., Alvarez-Perez, S., Mellado, E., Serrano, D., Valerio, M., Blanco, J. L., et al. (2013). Invasive aspergillosis caused by cryptic Aspergillus species: a report of two consecutive episodes in a patient with leukaemia. J. Med. Microbiol. 62, 474–478. doi: 10.1099/jmm.0.044867-0
Peterson, S. W. (1992). Neosartorya pseudofischeri sp. nov. and its relationship to other species in Aspergilius section Fumigati. Mycol. Res. 96, 547–554. doi: 10.1016/S0953-7562(09)80979-9
Peterson, S. W. (2008). Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100, 205–226. doi: 10.3852/mycologia.100.2.205
Pinel, C., Arlotto, M., Issartel, J. P., Berger, F., Pelloux, H., Grillot, R., et al. (2011). Comparative proteomic profiles of Aspergillus fumigatus and Aspergillus lentulus strains by surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI-TOF-MS). BMC Microbiol. 11:172. doi: 10.1186/1471-2180-11-172
Posteraro, B., Mattei, R., Trivella, F., Maffei, A., Torre, A., De Carolis, E., et al. (2011). Uncommon Neosartorya udagawae fungus as a causative agent of severe corneal infection. J. Clin. Microbiol. 49, 2357–2360. doi: 10.1128/JCM.00134-11
Predari, S. C., de Paulis, A. N., Veron, D., Zucchini, A., and Santoianni, J. E. (2007). Fungal peritonitis in patients on peritoneal dialysis: twenty five years of experience in a teaching hospital in Argentina. Rev. Argent. Microbiol. 39, 213–217.
Sabino, R., Verissimo, C., Parada, H., Brandao, J., Viegas, C., Carolino, E., et al. (2014). Molecular screening of 246 Portuguese Aspergillus isolates among different clinical and environmental sources. Med. Mycol. 52, 519–529. doi: 10.1093/mmy/myu006
Samson, R. A., Hong, S., Peterson, S. W., Frisvad, J. C., and Varga, J. (2007). Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 59, 147–203. doi: 10.3114/sim.2007.59.14
Samson, R. A., Visagie, C. M., Houbraken, J., Hong, S. B., Hubka, V., Klaassen, C. H., et al. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 78, 141–173. doi: 10.1016/j.simyco.2014.07.004
Sanguinetti, M., and Posteraro, B. (2014). MALDI-TOF mass spectrometry: any use for Aspergilli? Mycopathologia 178, 417–426. doi: 10.1007/s11046-014-9757-1
Serrano, R., Gusmao, L., Amorim, A., and Araujo, R. (2011). Rapid identification of Aspergillus fumigatus within the section Fumigati. BMC Microbiol. 11:82. doi: 10.1186/1471-2180-11-82
Shigeyasu, C., Yamada, M., Nakamura, N., Mizuno, Y., Sato, T., and Yaguchi, T. (2012). Keratomycosis caused by Aspergillus viridinutans: an Aspergillus fumigatus-resembling mold presenting distinct clinical and antifungal susceptibility patterns. Med. Mycol. 50, 525–528. doi: 10.3109/13693786.2012.658875
Shivaprakash, M. R., Jain, N., Gupta, S., Baghela, A., Gupta, A., and Chakrabarti, A. (2009). Allergic fungal rhinosinusitis caused by Neosartorya hiratsukae from India. Med. Mycol. 47, 317–320. doi: 10.1080/13693780802562977
Staab, J. F., Balajee, S. A., and Marr, K. A. (2009). Aspergillus section Fumigati typing by PCR-restriction fragment polymorphism. J. Clin. Microbiol. 47, k2079–2083. doi: 10.1128/JCM.00551-09
Staab, J. F., Kahn, J. N., and Marr, K. A. (2010). Differential Aspergillus lentulus echinocandin susceptibilities are Fksp independent. Antimicrob. Agents Chemother. 54, 4992–4998. doi: 10.1128/AAC.00774-10
Steinbach, W. J., Marr, K. A., Anaissie, E. J., Azie, N., Quan, S. P., Meier-Kriesche, H. U., et al. (2012). Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 65, 453–464. doi: 10.1016/j.jinf.2012.08.003
Sugui, J. A., Peterson, S. W., Figat, A., Hansen, B., Samson, R. A., Mellado, E., et al. (2014). Genetic relatedness versus biological compatibility between Aspergillus fumigatus and related species. J. Clin. Microbiol. 52, 3707–3721. doi: 10.1128/JCM.01704-14
Sugui, J. A., Vinh, D. C., Nardone, G., Shea, Y. R., Chang, Y. C., Zelazny, A. M., et al. (2010). Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48, 220–228. doi: 10.1128/JCM.01556-09
Summerbell, R. C., de Repentigny, L., Chartrand, C., and St Germain, G. (1992). Graft-related endocarditis caused by Neosartorya fischeri var. spinosa. J. Clin. Microbiol. 30, 1580–1582.
Swilaiman, S. S., O’Gorman, C. M., Balajee, S. A., and Dyer, P. S. (2013). Discovery of a sexual cycle in Aspergillus lentulus, a close relative of A. fumigatus. Eukaryot. Cell 12, 962–969. doi: 10.1128/EC.00040-13
Symoens, F., Haase, G., Pihet, M., Carrere, J., Beguin, H., Degand, N., et al. (2010). Unusual Aspergillus species in patients with cystic fibrosis. Med. Mycol. 48(Suppl. 1), S10–S16. doi: 10.3109/13693786.2010.501345
Tamiya, H., Ochiai, E., Kikuchi, K., Yahiro, M., Toyotome, T., Watanabe, A., et al. (2015). Secondary metabolite profiles and antifungal drug susceptibility of Aspergillus fumigatus and closely related species, Aspergillus lentulus, Aspergillus udagawae, and Aspergillus viridinutans. J. Infect. Chemother. 21, 385–391. doi: 10.1016/j.jiac.2015.01.005
Toskova, M., Palousova, D., Kocmanova, I., Pavlovsky, Z., Timilsina, S., Lengerova, M., et al. (2013). Invasive mould disease involving the gastrointestinal tract caused by Neosartorya pseudofischeri in a haematological patient. Mycoses 56, 385–388. doi: 10.1111/myc.12038
van der Linden, J. W., Arendrup, M. C., Warris, A., Lagrou, K., Pelloux, H., Hauser, P. M., et al. (2015). Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis 21, 1041–1044. doi: 10.3201/eid2106.140717
Verwer, P. E., van Leeuwen, W. B., Girard, V., Monnin, V., van Belkum, A., Staab, J. F., et al. (2014). Discrimination of Aspergillus lentulus from Aspergillus fumigatus by Raman spectroscopy and MALDI-TOF MS. Eur. J. Clin. Microbiol. Infect. Dis. 33, 245–251. doi: 10.1007/s10096-013-1951-4
Vinh, D. C., Shea, Y. R., Jones, P. A., Freeman, A. F., Zelazny, A., and Holland, S. M. (2009a). Chronic invasive aspergillosis caused by Aspergillus viridinutans. Emerg. Infect. Dis. 15, 1292–1294. doi: 10.3201/eid1508.090251
Vinh, D. C., Shea, Y. R., Sugui, J. A., Parrilla-Castellar, E. R., Freeman, A. F., Campbell, J. W., et al. (2009b). Invasive aspergillosis due to Neosartorya udagawae. Clin. Infect. Dis. 49, 102–111. doi: 10.1086/599345
Yaguchi, T., Horie, Y., Tanaka, R., Matsuzawa, T., Ito, J., and Nishimura, K. (2007). Molecular phylogenetics of multiple genes on Aspergillus section Fumigati isolated from clinical specimens in Japan. Nippon Ishinkin Gakkai Zasshi 48, 37–46. doi: 10.3314/jjmm.48.37
Yoshida, H., Seki, M., Umeyama, T., Urai, M., Kinjo, Y., Nishi, I., et al. (2015). Invasive pulmonary aspergillosis due to Aspergillus lentulus: successful treatment of a liver transplant patient. J. Infect. Chemother. 21, 479–481. doi: 10.1016/j.jiac.2015.02.010
Keywords: Aspergillus section Fumigati, Aspergillus lentulus, Aspergillus udagawae, Aspergillus viridinutans, Aspergillus felis, Neosartorya fischeri, Neosartorya pseudofischeri, Neosartorya hiratsukae
Citation: Lamoth F (2016) Aspergillus fumigatus-Related Species in Clinical Practice. Front. Microbiol. 7:683. doi: 10.3389/fmicb.2016.00683
Received: 09 February 2016; Accepted: 26 April 2016;
Published: 17 May 2016.
Edited by:
Jörg Linde, Leibniz-Institute for Natural Product Research and Infection Biology – Hans-Knoell-Institute, GermanyReviewed by:
Amariliz Rivera, University of Medicine and Dentistry of New Jersey, USACopyright © 2016 Lamoth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frédéric Lamoth, ZnJlZGVyaWMubGFtb3RoQGNodXYuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.