- 1Drug Development Diagnostic and Biotechnology Division, Council of Scientific and Industrial Research-Indian Institute of Chemical Biology, Kolkata, India
- 2Structural Biology and Bioinformatics Division, Council of Scientific and Industrial Research-Indian Institute of Chemical Biology, Kolkata, India
This study catalogs production of industrially important enzymes and changes in transcript expression caused by 2-deoxy D-glucose (2-DG) treatment in Arthrinium malaysianum cultures. Carbon Catabolite Repression (CCR) induced by 2-DG in this species is cAMP independent unlike many other organisms. Higher levels of secreted endoglucanase (EG), β-glucosidase (BGL), β-xylosidase (BXL), and filter paper activity assay (FPase) enzymes under 2-DG treatment can be exploited for commercial purposes. An integrated RNA sequencing and quantitative proteomic analysis was performed to investigate the cellular response to 2-DG in A. malaysianum. Analysis of RNASeq data under 2-DG treated and control condition reveals that 56% of the unigenes do not have any known similarity to proteins in non-redundant database. Gene Ontology IDs were assigned to 36% of the transcripts (13260) and about 5207 (14%) were mapped to Kyoto Encyclopedia of Genes and Genomes pathway (KEGG). About 1711 genes encoding 2691 transcripts were differentially expressed in treated vs. control samples. Out of the 2691 differentially expressed transcripts, only 582 have any known function. The most up regulated genes belonged to Pentose Phosphate Pathways and carbohydrate degradation class as expected. In addition, genes involved in protein folding, binding, catalytic activity, DNA repair, and secondary metabolites were up-regulated under 2-DG treatment. Whereas genes encoding glycosylation pathways, growth, nutrient reservoir activity was repressed. Gene ontology analysis of the differentially expressed genes indicates metabolic process (35%) is the pre-dominant class followed by carbohydrate degradation (11%), protein folding, and trafficking (6.2%) and transport (5.3%) classes. Unlike other organisms, conventional unfolded protein response (UPR) was not activated in either control or treated conditions. Major enzymes secreted by A. malaysianum are those degrading plant polysaccharides, the most dominant ones being β-glucosidase, as demonstrated by the 2D gel analysis. A set of 7 differentially expressed mRNAs were validated by qPCR. Transmission electron microscopy analyses demonstrated that the 2-DG treated cell walls of hyphae showed significant differences in the cell-wall thickness. Overall 2-DG treatment in A. malaysianum induced secretion of large amount of commercially viable enzymes compared to other known species.
Introduction
Endophytes represent a promising group of organisms, as they are mostly untapped reservoirs of metabolic diversity. They are often able to degrade cellulose and produce an extraordinary diversity of metabolites (Gianoulis et al., 2012). Arthrinium sp. are endophytes grown in diverse ecological niches, also known to produce bioactive compounds that have pharmacological and medicinal applications (Crous and Groenewald, 2013). The first Arthrinium sp., A. saccharicola was isolated from Saccharum officinarum and was later reported from various habitats such as plants, air and seawater (Miao et al., 2006). A. malaysianum is a known contaminant on Termitomyces cultures (Sawhasan et al., 2012) and in the present study, this organism is isolated from Termitomyces mycellial mats. Reports of several other endophytes belonging to Arthrinium sp. (A. arundinis, A. saccharicola) isolated from marine brown algae Sargassum sp. or from a mixed culture of Termitomyces isolated from termite comb, displayed considerable cellulolytic activities along with antifungal and antioxidant activity (Hong et al., 2015). Arthrinium saccharicola, Arthrinium affsacchari, Arthrinium phaeospermum isolated from Miscanthus sp. are known to produce industrially important enzymes (Shrestha et al., 2015). However, protein secretory pathways and the spectrum of proteins natively secreted by this fungus to the extracellular medium still remain virtually unexplored. The production and storage of several secondary metabolites from Arthrinium sp. have been reported earlier. Bostrycin, ergosterol, succinic acid, various phenolic compounds and antibiotics such as 1-threo-β-hydroxiaspartic acid are some of the known pharmaceutically important products from these genera. Papulacandins, with inhibitory and antifungal activity against bacteria, molds, yeasts, and fungi are also some of the most commonly found secreted products from this group (Traxler et al., 1980; Larrondo et al., 1996; Aissaoui et al., 1998; Adelantado et al., 2005; Calvo et al., 2005).
Carbohydrates are the primary and preferred source of metabolic carbon for most organisms and are used for generating energy and producing biomolecules (de Sousa Lima et al., 2014). The gene Mig1/CreA/CRE1, a zinc-finger transcription factor conserved in most fungal species mediates carbon catabolite repression (CCR) in presence of easily metabolized carbohydrates such as glucose (Ruijter and Visser, 1997). Relief of CCR by glucose depletion or deletion of creA was found to activate expression of genes involved in cell wall-degrading enzymes (CWDEs). Besides being required for CWDE gene expression, alleviation of CCR is also needed for the activation of alternative metabolic pathways like ethanol assimilation and virulence. These proteins that are responsive to carbon catabolite (de) repression, are generally soluble. CAZymes are released under CC (de) Repression. Low molecular weight carbohydrates can act as inducers for the expression of other genes encoding CAZymes, either working individually or together (Delmas et al., 2012). In Neurospora crassa, a number of genes that encode cellulolytic and pectinolytic enzymes show increased transcription levels upon carbon starvation as well as during early exposure to cellulose or pectin (Coradetti et al., 2012; Benz et al., 2014).
In recent years, high throughput next generation sequencing technologies like Roche, Illumina, and Applied Biosystems SOLiD, Ion Torrent are increasingly being used for non-model organisms or where reference genome is not available. Several investigations have been successfully carried out using these techniques lately (Zhou et al., 2014). Recently several de novo transcriptome sequencing using Illumina platform has been reported in species such as Safflower (Lulin et al., 2012), Anopheles (Crawford et al., 2010); Eucalyptus (Mizrachi et al., 2010); making it an attractive option for researchers.
In this study, 2-deoxy D-glucose was used in the growth medium as metabolite for down regulating carbon catabolic repression in A. malaysianum followed by induction of CWDE. Glucose is a major structural precursor in filamentous fungi as the dominant monosaccharide component for cell wall β-glucans. When glycolysis is inhibited by 2-DG in aerobic condition, cells continue to produce ATP using alternative energy sources, i.e., amino and fatty acids metabolism. Pentose Phosohate Pathway plays an important role in the production of proteins and therefore this pathway is expected to be relevant especially in an organism with an efficient protein production system (Wick et al., 1957; Chen and Gueron, 1992; Limón et al., 2011).
This is a first of its kind study in endophytic fungus A. malaysianum cataloging the metabolic and transcriptional changes taking place under 2-DG regulation. This study further substantiates if 2-DG can be used as a precursor for production of commercially applicable enzymes.
Materials and Methods
2, 2-Diphenyl-1-pycrylhydrazyl, glucose 6-P dehydrogenase (EC 1.1.1.49), p-nitrophenyl-β-D-glucopyranoside (pNPG), CM-Chitin-RBV substrates, D-(+)-glucose, laminarin, 2-deoxy-Dglucose, fructose-1,6-bisphosphate, NADP, ATP, NADH, D-L-isocitrate, phenyl hydrazine-HCl, cystein-HCl, EDTA, cAMP Enzyme Immunoassay Kit (Cat no 100 CA201) were purchased from Sigma Chemical Company, USA. Other chemicals of analytical grade were purchased locally.
Growing Cultures in Control and Treated Conditions
A. malaysianum was isolated as a contaminant from the mat of Termitomyces clypeatus MTCC 5091 and cultured in potato dextrose agar. The mycelia of A. malaysianum was later transferred to 100 ml of growth media in 500 ml shake-flasks containing (%, w/v), Glucose-5, Malt extract-5, Potato extract-20%. Apart from this, other components and micronutrients such as ammonium dihydrogen phosphate 2.5, (%, w/v) of CaCl2.2H2O, 0.037; KH2PO4, 0.087; MgSO4.7H2O, 0.05; Boric acid,0.057; FeSO4.7H2O, 0.025; MnCl2.4H2O, 0.0036; NaMoO4.4H2O, 0.0032; ZnSO4.7H2O 0.03 was added to the culture at pH 5.0, and incubated at 30°C under shaking condition (250 rpm) in orbital shaker. The mycelia mats were harvested by filtration through a double-layered muslin cloth and washed several times with sterile distilled water. The mycelia mats were then stored in −80°C (with excess liquid squeezed out), for 18s RNA sequence extraction. For determination of dry weight, samples collected at various time intervals were centrifuged at 9000 × g for 30 min, and the resulting supernatant was filtered through a 0.45 μ (Millipore). The dry weight of mycelia were measured after repeated washing of the mycelial pellets with distilled water and drying overnight at 70°C. The biomass concentration was measured as grams of dry weight.
For studying 2-DG response on the cultures, mycelium of A. malaysianum was added to the media containing a single carbon source (1% of glucose or cellobiose). pH of the medium was adjusted to 5.0. The cultures were grown under shaking conditions at 30 ± 2°C for requisite number of days. A separate set containing the above media ingredients along with addition of 2-DG (0.05%) was also done simultaneously.
Free-Radical Scavenging Activity Assay
Each culture was incubated for 7 days and filtered to separate the mycelium from the media. 100 mg wet mycelial mats were extracted and thoroughly washed with distilled water and homogenized on ice, for 5 mins. The mycelial suspension was centrifuged at 15,000 rpm for 20 min at 4°C. The pellet was resuspended in distilled water and sonicated on ice 4 times for 1 min using ultrasonic sonicator at full amplitude. The suspension was centrifuged at 8000 rpm for 10 min at 4°C to precipitate the coarse particle. The precipitate was then washed, air dried and stored at 4°C. The antioxidant activity of aqueous fungal extracts and the standards were assessed on the basis of the radical scavenging effect of the stable DPPH (2, 2-Diphenyl-1-pycrylhydrazyl; Leung et al., 2006). The DPPH, a stable free radical with the characteristic absorption at 517 nm, was used to study the radical scavenging effects on the fungus. The image of hyphae was captured by using light microscopy.
β-Glucosidase (E.C.3.2.1.21) assay was carried out in the reaction mixture containing 2 mM pNPG as substrate in 0.1 M sodium acetate buffer at pH 5.0 and an appropriate amount of enzyme. One unit of β-Glucosidase activity was defined as the amount of enzyme needed to liberate 1 μM of p-nitrophenol (pNP) per minute under the standard assay conditions. β-Xylosidase (E.C.3.2.1.37) was assayed using a similar method with p-nitrophenyl-β-D-xylopyranoside (pNPX) as substrate. Xylanase was assayed using birch wood xylan as substrate. The solution of 0.9 ml xylan (1%, w/v) and the 0.1 ml of enzyme at appropriate dilution were incubated at 50°C for 10 mins. The reducing sugar was determined by the DNS method at 540 nm with xylose as standard. Filter paper degrading activity (FPA) was assayed according to the IUPAC method using 1 × 6 cm (50 mg) Whatman No.1 filter paper strips as a substrate.
Determination of Enzyme Activities
The extracellular β-1, 3-glucanase, β-glucosidase, and chitinase activities were measured using laminarin, p-nitrophenyl-β-D-glucose and CM-Chitin-RBV substrates, respectively. cAMP assay was done according to the technical bulletin provided by the cAMP Enzyme Immunoassay Kit. Hexokinase activity assay was based on the reduction of NADP+ through a coupled reaction with glucose-6-phosphate dehydrogenase (10U ml−1) in 1 ml reaction mixture containing Tris–HCl buffer (500 mM, pH 7.5), NADP (6.5 mM), ATP (10 mM), MgCl2 (70 mM), and 100 mM glucose. Increase in absorbance at 340 nm was determined spectrophotometrically at 37°C and activity of the enzyme (U ml−1) was measured using the value of molar extinction coefficient of the liberated product (€ = 6.22 mM−1 cm−1). The phosphoglucose isomerase reaction mixture contained 0.4 mM NADP and 0.9 U ml−1 glucose-6-phosphate dehydrogenase with 20 mM fructose-6-phosphate as the substrate. Fructose-1,6-bisphosphatase activity was determined in 1 ml assay mixture containing fructose-1,6-bisphosphate (0.8 mM), Tris–HCl buffer (100 mM, pH 7.5), EDTA (2.4 mM), MgCl2 (74 mM), glucose-6-phosphate dehydrogenase (0.7 U ml−1) and phosphoglucose isomerase (3.5 U ml−1). Isocitrate Lyase activity was measured by adding 200 μl-D-L-isocitrate (10 μmol) in 1 ml assay mixture containing KH2PO4 (200 μmole, pH 6.8), phenyl hydrazine-HCl (10 μmol), cystein- HCl (6 μmol) and MgCl2 (15 μmol). The glucose-6-phosphate dehydrogenase reaction mixture contained 0.4 mM NADP with 5 mM glucose-6-phosphate (Kuby et al., 1974).
Total RNA Isolation from Control and Experimental Fungus Samples
Total RNA was isolated from 100 mg of tissue using Xcelgen Plant RNA Miniprep kit. The quality of RNA was analyzed on 1% denaturing agarose gel. The paired-end cDNA sequencing libraries were prepared for both samples, separately using Illumina TruSeq RNA Library Preparation Kit. Library preparation was started with mRNA fragmentation followed by reverse transcription, second-strand synthesis, pair-end adapter ligation, and finally ended by index PCR amplification of adaptor-ligated library. Library quantification and qualification was performed on Caliper LabChip GX using HT DNA High Sensitivity Assay Kit.
High Throughput Transcript Sequencing
Sequencing was done using Illumina Hiseq platform (Paired end, 101 bases long) generating 50.6 million paired-end reads (12.4 Gbp) from mycelium (control) and 77.14 million reads (18.4 GB) from 2-DG treated samples. Sequences were assembled separately for control and treated samples as well as together generating merged assembly (~128 million reads after merging 50.6 million control reads and 77.14 million 2-DG treated reads). Assembly was done using several assemblers such as Trinity (Grabherr et al., 2011), Ray (Boisvert et al., 2010), Mira (Chevreux et al., 2004), and ABYSS (Birol et al., 2009). Annotation of the assembled transcripts was carried out using Blast (Altschul et al., 1990), KEGG (Kanehisa and Goto, 2000), Interproscan (Jones et al., 2014), Gene Ontology (Conesa et al., 2005) etc. The expression levels of assembled unigenes/transcripts were measured using fragments per kilobases per million reads (FPKM) method. We mapped treated and control assemblies back into merged assembly for annotation consistency using BLAT (Kent, 2002).
Generating CDS From Unigenes Using Codon Preference
The identification of coding sequences (CDS) from expressed transcripts still remains a challenge. Although many methods exist, a modified log likelihood approach based on codon preference (Staden and McLachlan, 1982) works best. Initially, we generated a codon usage table using CUSP from EMBOSS (Rice et al., 2000), where the entire transcript set was used for generating training dataset. Then we iterated this process several times using the modified outputs from the previous dataset and extracted codon preference data from that and used them for training in the subsequent prediction. Fourth iteration produced the best CDS that were subsequently used for analysis.
qPCR Protocols
Based on their functional importance, seven genes were selected for validating the results of the RNA-Seq analysis. The primers for these genes were designed with Primer Express 3.0 software (Applied Biosystems). Total RNA was extracted from both sets of independent cultures grown in absence (control) and presence (treated) of 2-DG in cellobiose media as described above, and then converted to cDNA by random priming. PCR reactions were run in four replicates. The transcription level of genes was determined according to the 2−ΔΔCT method, using actin as a reference gene for the normalization of gene expression levels.
Second Dimension Gel Electrophoresis (2DGE) and Protein Visualization, MALDI-TOF MS/MS
Tri Caboxylic Acid precipitated protein (120 μg,) were loaded onto pH 3-10, 7 cm IPG strips with active rehydration for 12 h in rehydration buffer [7 mol/l urea, 2 mol/l theorem, 4% (w/v) CHAPS, 65 mmol/l DTT, 0.2% (v/v) IPG buffer (pH 3–10), trace bromophenol blue]. Isoelectric focusing (IEF) procedure was performed at 25°C using the following setting: S1 linear 250 V 30 min, S2 rapid 500 V 30 min, S3 rapid 1000 V 1 h, S4 linear 10,000 V 4 h, S5 rapid 10,000 V 60 kVh, S6 rapid 500 V 24 h. The second-dimensional SDS-PAGE was performed with 10% polyacrylamide gel in Protein cell IEF (BIO-RAD, USA). The parameter of electrophoresis: 100 V 30 min, 180 V 6 h. The gels were stained in the base of colloidal Coomassie Brilliant Blue G-250. The gel images were imported into the PDQuest 8.0.1 (Bio-Rad, Hercules CA, USA) image software for analysis. The generated peak-lists were searched against fungal database using the MASCOT software package (Version 2.1, Matrix Sciences, U.K.; www.matrixscience.com).
Study of A. malaysianum Ultra-Structure by Transmission Electron Microscopy (TEM)
Ultra structural damage of 2-DG treated mycelia was ascertained through TEM studies carried out with a TECNAI SPIRIT (FEI, The Netherlands) instrument having an accelerating voltage of 60 kV. Culture aliquots were centrifuged at 10,000 rpm for 10 min; the mycelial pellet was fixed with 2.5% glutaraldehyde in 0.25 M phosphate buffer (pH 7.2) for 24 h, washed thrice with the same buffer and post fixed with 1% w/v osmium tetroxide (2 h). This was followed by 2 h incubation with 0.5% w/v aqueous uranyl acetate solution followed by dehydration using a graded alcohol series (from 50 to 100% v/v) and embedding in spurr. Mycelial thin sections (60 nm) were cut with a diamond knife (DUPONT, Germany) in a UC6 Leica Ultra microtome (Germany) and mounted on copper grids before examination by TEM.
Results
Significant Increase in the Production of Cellulolyitc enzymes Under 2-Deoxy D-Glucose Treatment
A. malaysianum was grown in cellobiose and glucose medium (1%) with (treated) and without 2-Deoxy D-Glucose it (control) (Figure 1). To investigate the potential cellulolytic enzyme activities of the fungus secreted in cellobiose medium, the filter paper activity assay (FPase), endoglucanase (EG), β-glucosidase (BGL), Xylanase (X) and β-xylosidase (BXL) activities were measured. This data was compared with another Arthrinium sp. e.g., A. saccharicola and other cellulolytic fungi such as Penicillium sp., Aspergillus sp., Trichoderma sp. Significantly improved activity of FPase (0.67U/ml), BGL (4.06U/ml), EG (1.38U/ml), Xylanase (3.02U/ml), and BXL(0.53U/ml) was detected in A. malaysianum compared to 0.39 U/ml FPase, 1.04 U/ml BGL, 0.38 U/ml EG and negligible BXL with no Xylanase activity in A. saccharicola (Table 1A). In vitro addition of 2-DG in cellobiose medium resulted in further increase of β-glucosidase (14.02U/ml), endoglucanase (4.02 U/ml), β 1, 3 glucanase (1.78 U/ml), chitinase (1.75 U/ml) activity by several folds (Table 1B).
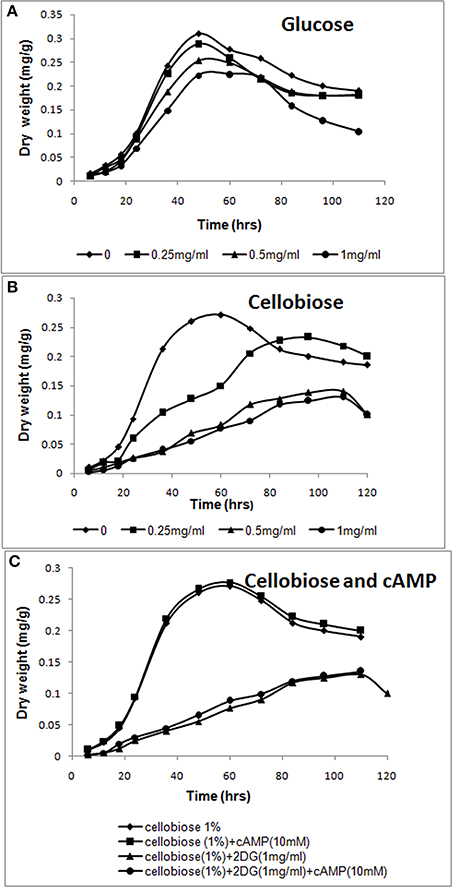
Figure 1. cAMP-independent CCR by 2-deoxyglucose. (A,B) Increasing amounts of 2-deoxyglucose (0–1 mg/ml) was added to A. malaysianum cultures supplemented with glucose (1%) or cellobiose (1%). (C) cAMP and 2-deoxyglucose were added to A. malaysianum. cultures supplemented with cellobiose (1%), cellobiose + cAMP (10 Mm), and cellobiose+2DG (1 mg/ml) and + cellobiose+ 2DG (1 mg/ml) +cAMP (10 mM).
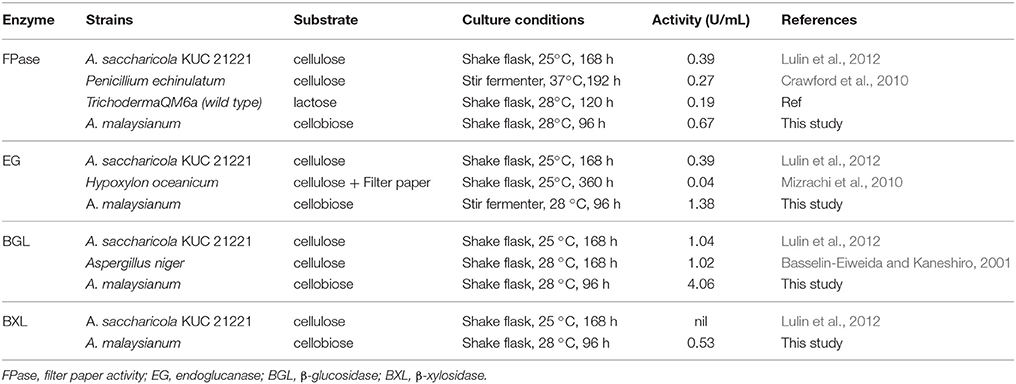
Table 1A. Quantitative comparison of enzyme activities of A. saccharicola KUC21221 with A. malaysianum and other terrestrial fungi.
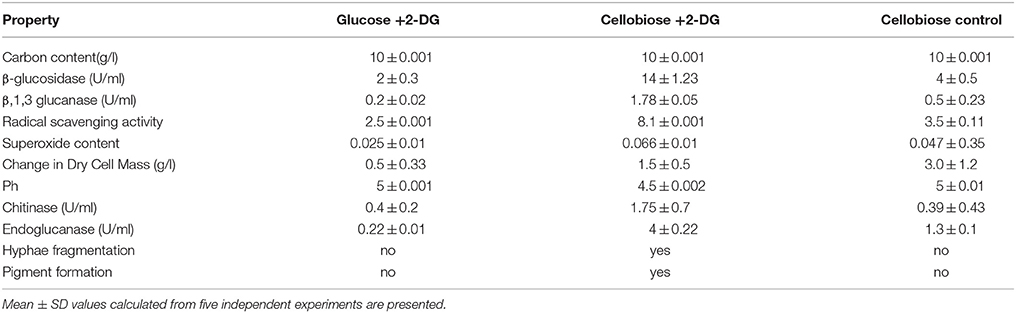
Table 1B. Characteristic properties of A. malaysianum cultures: Mycelia from exponential phase of A. malaysianum (approximately after 24 h) were washed and transferred into either glucose containing 2-deoxy D-glucose or cellobiose containing 2-deoxyglucose or in minimal medium containing just cellobiose.
Increased Secretion of β-Glucosidase in 2-DG Treated Condition
Cellobiose consumption and β-glucosidase activity were monitored during cultivation of the A. malaysianum strains in cellobiose (1%) + 2-DG (0.5 mg/ml) media.
In presence of 2-DG, intracellular β-glucosidase activity appeared within 48 h of cultivation (0.067 U/ml), when 40% of the cellobiose still remained in the culture medium. Maximum activity of 1.0 U/ml was achieved at 72 h after which no intracellular β-glucosidase was detected. Extracellular β-glucosidase activity appeared after 48 h and increased gradually until 96 h and attained a maximum value 3 U/ml. Rise in extracellular β-glucosidase was observed till 96 h (Figure 2A). In the attempt to identify enzymatic reactions associated with the cell walls which correlate with high release of extracellular β-glucosidase into the medium, the cell wall bound activities of the hydrolytic enzymes like β,1-3 glucanase, chitinase, endoglucanase were measured during growth on glucose +2-DG, cellobiose +2-DG and cellobiose media (Table 1B; Figure 3). The β, 1-3 glucanase activity was highest in cellobiose+2-DG media (1.78 U/ml) and lowest in glucose +2-DG media (0.2 U/ml). Similarly chitinase and endoglucanase activity was highest in cellobiose+2-DG medium (1.7 and 4U/ml, respectively).
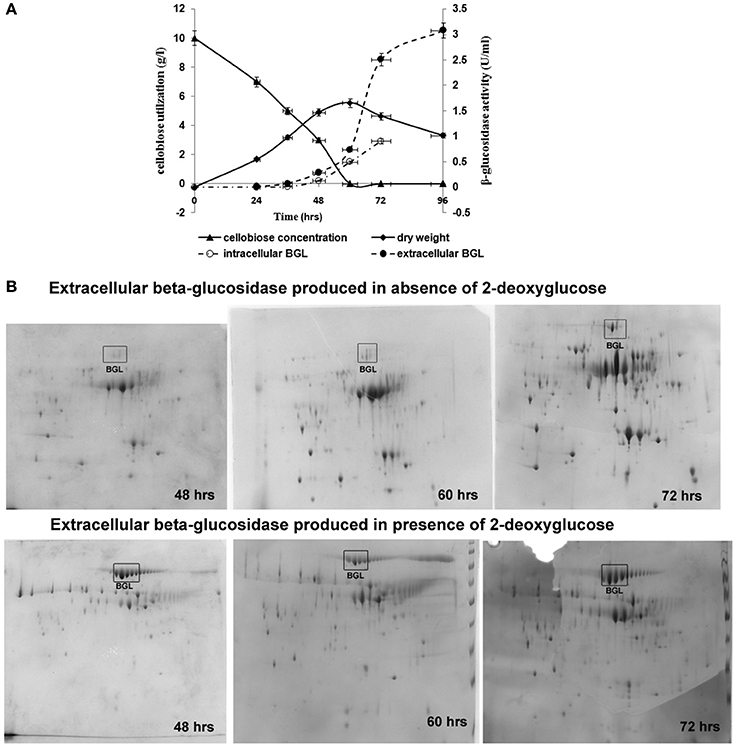
Figure 2. Growth, carbon consumption, and synthesis of β-glucosidase in A. malaysianum in presence of 0.5 mg/ml of 2DG. (A) Time dependent profile of fungal growth, cellobiose utilization, and kinetics of intracellular and extracellular β-glucosidase production, (B) 2D Gel electrophoresis analysis of the secreted proteins of A. malaysianum at three different hours. Extracellular β-glucosidase secreted into the culture medium in absence (A,B) in presence of 2DG (0.5 mg/ml) at 48, 60, and 72 h is highlighted as (Muller et al., 2015).
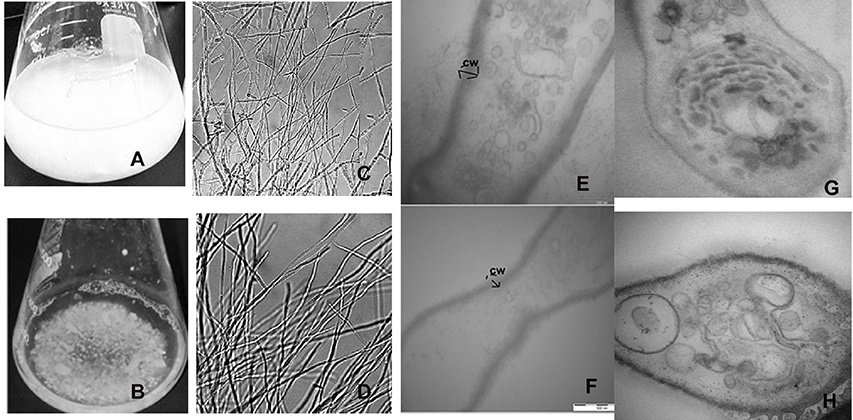
Figure 3. Formation of aberrant hyphae structure in treated mycelia cultured. (A) Cellobiose cultures in absence of 2DG and (B) in presence of 2DG after 48 h. (B) Morphological changes in the hyphae of A. malaysianum grown in (C) absence and (D) presence of 2-deoxyglucose as observed in simple microscope, Longitudinal (E,F) and transverse section (G,H) of the hyphae in absence (E,G) and presence (F,H) of 2DG.
Water extracts of mycelia of both treated (cellobiose +2-DG) and control (cellobiose) of A. malaysianum were subjected to possible antioxidant activity by the DPPH free radical scavenging method. The model of scavenging the stable DPPH radical is widely used to evaluate antioxidant activities over a relatively short time compared to other methods. Scavenging effect of mycelia extracts on DPPH radicals was found to be concentration dependent. Free radical scavenging effect of 8.1% at a lower concentration of 2 mg/ml of mycelia extracts was achieved in 2-DG treated mycelium as shown in Table 1B.
Proteome Analysis of β-Glucosidase Secretion
Total proteins secreted to extracellular milieu during cultivation of A. malaysianum in treated (cellobiose in presence of 2-DG) and control (cellobiose in absence of 2-DG) medium was extracted at different time interval (48, 60, 72 h) and was analyzed by 2-DGE analysis, (Figure 2B). Most of the protein spots were concentrated in pI range 4–7 and between molecular mass of 30 and 120 kDa. It was clear from the gel images that extracellular β-glucosidase in absence of 2-DG was produced after 60 h of cultivation whereas extracellular β-glucosidase in presence of 2-DG was produced within 48 h and secreted into the medium (Figure 2B).
Transcriptome Assembly and Annotation
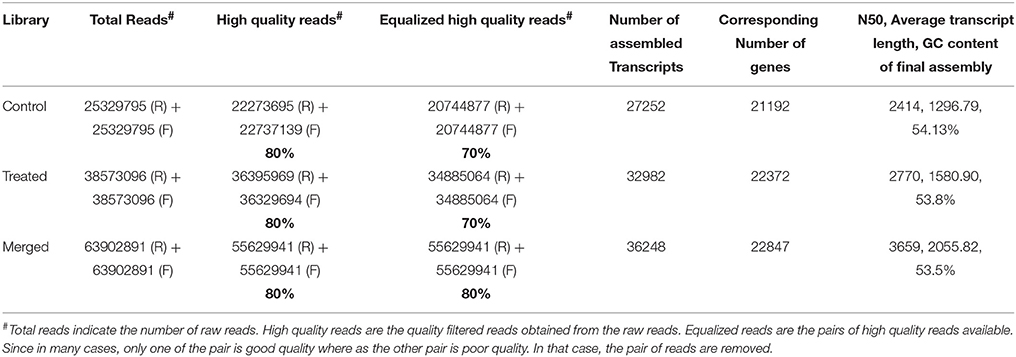
We generated deep transcriptome sequences with an approximate coverage of 250X from control and 2-DG treated samples using Illumina platform. Transcript reads from control and treated samples were assembled separately and together (merged assembly) for analyzing differential expression. Out of the several assemblers used, Trinity assembler (Grabherr et al., 2011) generated the best results. Further, downstream data analysis was carried out using merged assembly. We used dinucleotide relative abundance (Gentles and Karlin, 2001) to calculate possible contaminants and removed about 84 sequences from the merged assembly. Final assembly resulted in 21192 genes (termed as unigenes) coding for 27252 transcripts from control samples, 22372 unigenes coding for 32982 transcripts from 2-DG treated samples and 22847 unigenes encoding 36248 transcripts from merged samples (Table 2). The length distribution of the assembled transcripts are represented in Figure 4A.
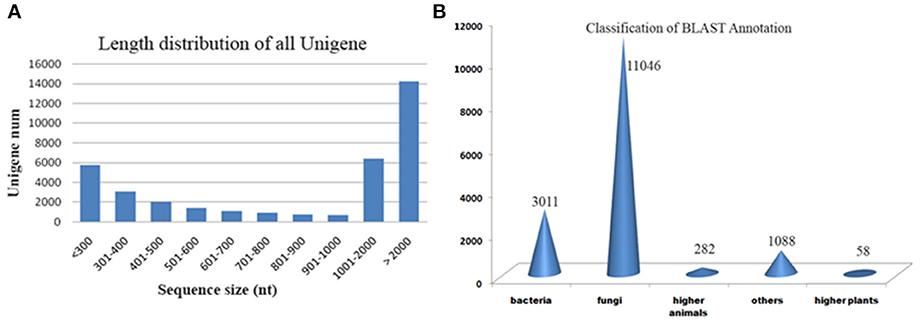
Figure 4. (A) Length distribution of transcripts. (B) Blast results of A. malaysianum against non-redundant database.
Putative functions were assigned to 44% (16,096) transcripts based on their similarities to non redundant database (Supplementary Table 1). Out of which, 152 (0.4%) transcripts matched with ribosomal genes. A significantly large number of orthologs were detected with Ascomycetes fungus Eutypa followed by Bacteria (Figure 4B).
Taxonomy analysis A. malaysianum unigenes indicated that it had 55% sequence similarity with Eutypa lata UCREL1 (3588) species, followed by Colletotrichum higginsianum (1499), Gaeumannomyces sp. (304), Glomerella sp. (546), Thielavia sp (312), Togninia sp. (984), respectively. The secretome analysis was also done to identify the diverse array of secretory proteins of A. malysianum (Supplementary Table 2). A large part of the secretome was classified as hypothetical proteins followed by phosphorylation related proteins, Glycosyl hydrolases and transferase. The major gene ontology processes in A. malaysianum were consistent with those of Eutypa lata, Gaeumannomyces graminis, Thielavia sp., Togninia, and Colletotrichum (O'Connell et al., 2012; Jaramillo et al., 2015).
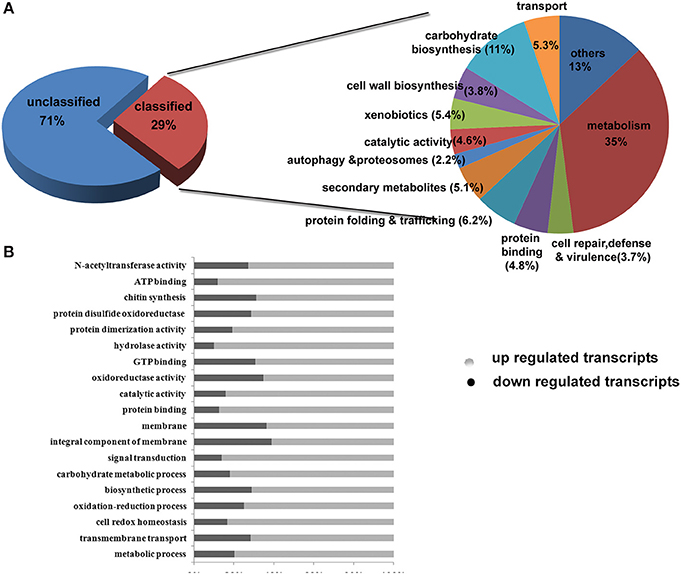
Among the GO classifications, 7351 transcripts were assigned to molecular process; 230 under cellular component and 838 under biological process. Under each of the three main categories (biological process, cellular component and molecular function), “metabolic process,”, “cell,” “cellular process,” “binding,” and “catalytic activity” terms occupied the largest proportion in both the control and experimental samples. We also noticed a high percentage of experimental transcripts from categories of “transporter activity,” “organelle” and “protein binding.” “Nutrient reserve activity,” “viral reproduction” and “protease activity” was more prominent in 2-DG treated transcripts.
About 7.3% Genes are Differentially Expressed Under 2-DG Treated Conditions
We used a fold change value of >=2 for estimating differentially expressed genes. A total of 2691 transcripts encoded by 1711 genes were differentially expressed under control and treated conditions. Out of this, 1150 transcripts were down regulated and 1540 transcripts were up-regulated. The list of genes and their individual functions with their KO id's are provided in Supplementary Table 3. Out of the differentially expressed genes, the number of carbohydrate metabolism (11%) genes was the highest in number followed by secondary metabolites (5.1%), transporters (5.3%), binding proteins (4.8%), catalytic activity (4.6%), and cell rescue and virulence genes (3.7%; Figure 5). Differentially expressed transcripts were analyzed further on several key functions such as (i) carbohydrate-active enzymes(CAZymes) and cell wall biogenesis (ii) Secondary metabolism, unfolded protein response and virulence genes iii) metabolic pathways and enzymes, and (iv) Other functions mainly on cellular transporters and cell repair.
Enhanced Expression of Cazymes and Cell Wall Homeostasis During 2-DG Treatment
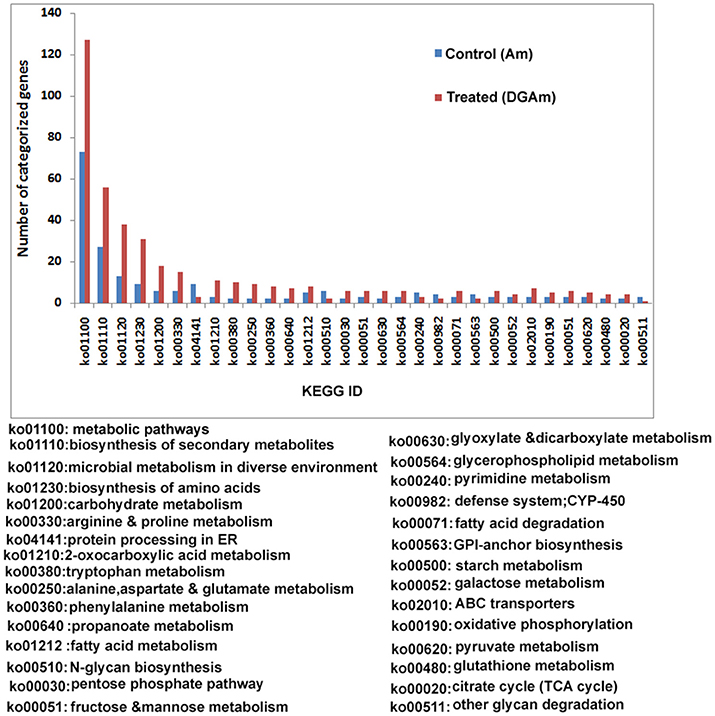
Enzymes that break down plant cell wall can be categorized into the following classes: glycoside hydrolases (GH), glycosyl transferases (GT) polysaccharide lyases (PL), carbohydrate esterases (CE), and auxiliary activities (AA). All CAZymes and auxiliary activity enzymes can be attached to carbohydrate-binding modules (CBMs). Our transcriptomic analysis revealed a vast majority of carbohydrate-active enzymes encoded by A. malaysianum. About 3447 CAZymes were detected from the merged transcriptome (Supplementary Table 4).
We predicted carbohydrate degrading enzymes and their annotations using CAZymes Analysis Toolkit (CAT) that uses CAZY database (Cantarel et al., 2009) (Table 3; Supplementary Table 4). A core set of 302 genes encoding CAZymes was up-regulated in response to 2-DG. The results show that 16 β-glucosidase (bglX) belonging to GH 1 and 6 glucan 1,3-betaglucosidase (elgA) belonging to GH 5 family was significantly upregulated. Both theses enzymes are involved in cell wall biogenesis/degradation. Other glycosidases upregulated were α-glucosidases (2), β-galactosidases (2), 1 polygalacturonases and 5 chitinases. Beside this, a large number of cytochrome superfamily (CYP5A CYP1A1) and esterase were also upregulated (Table 4). In addition, 30 polyketide related genes, 6 non-Ribosomal peptide along with many bioactive compound producing genes are up-regulated making this fungus an industrially important strain to both biofuel and pharmaceutical industry (Supplementary Table 1).
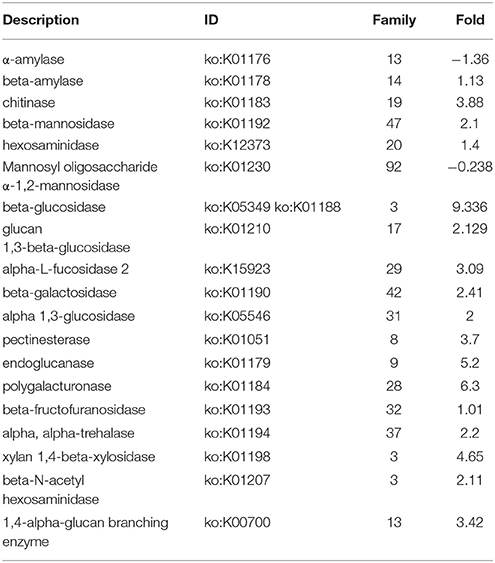
Table 3. Up- and down-Fold increased in presence of 2DG of differentially expressed genes encoding CAZymes of A. malaysianum.
Majority of CAZymes belonged to GH (117) and GT (62). Few others were categorized under PL (2), CE (27), CBM(35), and AA (52). A increased set of GH, GT and CBM (22) domains can be regarded as a hallmark of convergent evolution of A. malaysianum caused due to mixed culture with Termitomyces clypeatus.
2-DG Triggers Expression of Virulence Associated Genes, Unfolded Protein Response And Biosynthesis of Secondary Metabolites
Virulence factors are difficult to define by classical terminology. Intrinsically associated with pathogenicity, such as endotoxin and molecules referred to as modulins (e.g., cell wall polysaccharides and lipids), can contribute to the pathogenic process. Transcriptome analysis A. malaysianum in 2-DG treated condition revealed the presence of potential virulence associated genes (Table 4). Some requisite virulence factors, such as the toxins or polysaccharide capsules proteins, beta-lactamase like protein were expressed suggesting the pathogenic nature of the organism in stressed environmental condition. Constitutively expressed components of microorganisms, such as endotoxin and molecules referred to as modulins (e.g., cell wall polysaccharides and lipids), that contribute to the pathogenic process were also expressed.
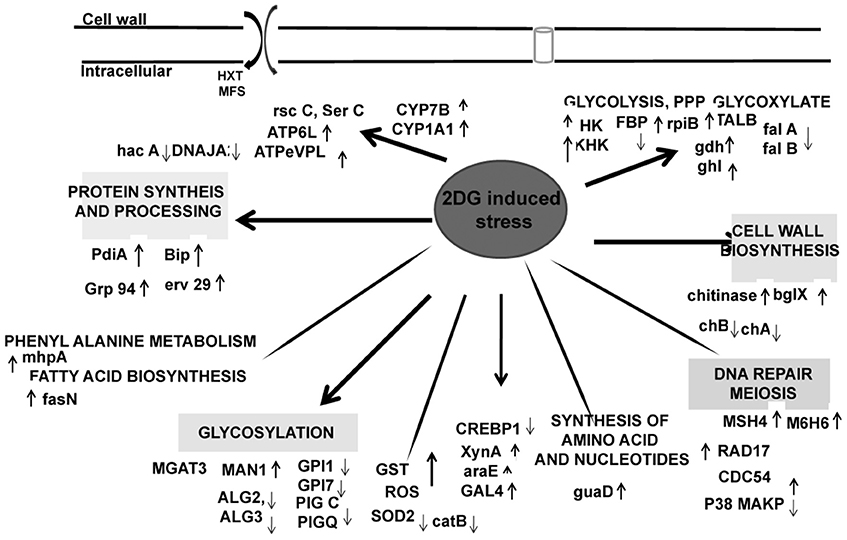
Surprisingly, several genes with important functions in protein synthesis and trafficking were also upregulated (Table 3). Many of them were proteins involved in folding and maturation like pdi-1, ER resident chaperones, bip-1, grp 78, and several heat shock proteins. These genes were induced in response to increased up regulation of CWDE influenced by 2-DG. These data suggested that the secretory pathway in this fungus regulates increased lignocellulase synthesis and secretion by up regulating the ER stress responsive proteins to reduce ER load (Figures 6, 7). Protein disulphide isomerase (PDI) is one of the major ER foldases. The enzyme catalyses the formation and rearrangement of disulphide bridges in proteins during folding. The pdi gene was upregulated in treated condition.and its expression level was qPCR (Figure 8B). The differential expression levels of several chaperons and binding protein were also upregulated (Figure 6), although the expression of bZIP transcription factor and HAC 1 showed minor increase (Figures 6, 8B).
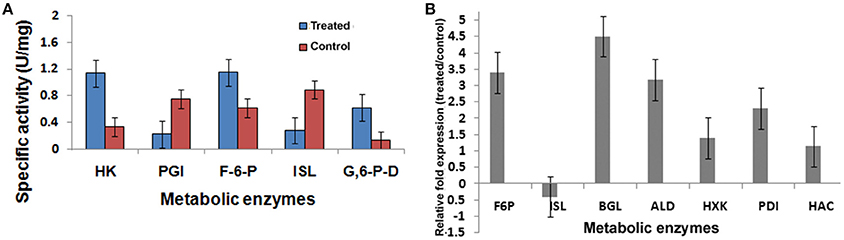
Figure 8. Metabolic enzyme profile induced by 2DG. (A) Specific activity of Hexokinase (HK), Phosphoglucoisomerase (PGI), Fructose bis phosphate (F6P), Isocitrate lyase (ISL), and Glucose 6 phosphate dehydrogenase (G6PD) and (B) Relative fold change of seven genes using qPCR methods. Data represents four replicates with standard error bar.
Secondary metabolites are involved in intracellular, intercellular and interspecific interactions. A. is known to produces a wide variety of secondary metabolites, and this motivated us to find the molecular basis of this production by de novo transcriptomics. We identified 30 core genes related to secondary metabolism including 12 polyketide synthase (PKSs), four putative PKS-like enzymes, seven fatty-acid synthases, (Monooxygenase FAD-binding protein etc. Supplementary Table 1; Octave et al., 2006).
Other Artifacts of 2-DG Treatments in the Culture Media
2-DG Reduced Cell Viability Followed by Cell Death
A large number cell repair, senescence, p38, mapk, glutathione-s-transferase, pho81 was induced by 2-DG (Supplementary Table 4). This is in concurrence to the condition induced by carbon starvation in fungi (Emri et al., 2004; Pócsi et al., 2004; Katz et al., 2006; Szilágyi et al., 2013). This data suggest that macrophagy is a part of late response to 2-DG in A. malaysianum as observed in old cultures by gradual pigmentation and mycelia fragmentation. However, a more detailed study is required to further establish this effect. The production of cell wall hydrolyzing enzymes may also trigger cell death process in old carbon starved cultures (Emri et al., 2013). In our study, there was up regulation of cell wall integrity pathway genes such as WSC, SNF-1, and other map kinases that are usually present in carbon starvation or depletion like condition (Table 3). The degradation of glutathione is significant, because glutathione is utilized as an energy source, supports intracellular amino acid transport and protects cells against ROS. Interestingly, 2DG resulted in up regulation of the expression of glutathione-s-transferase but down regulation of superoxide (Figure 6).
Expanded Transporter Gene Families in Treated Condition
In plant pathogens, cellular transporters are responsible not only for export of compounds involved in pathogenesis and virulence, but they also may play an essential role in protection against plant defense compounds (e.g., secondary metabolites) during pathogenesis, possibly by exporting host-derived antimicrobial compounds out of the cell (Pao et al., 1998; Schoonbeek et al., 2002). The transportation system is involved in uptake of essential nutrients and ions, excretion of metabolic end products, and deleterious substances, and communication between cells and the environment. Transporters comprised of 5.3% of our classified transcripts (the second largest cluster after carbohydrate biosynthesis). Specific transporters elevated such as those of ABC, hexoses, solute, sugar:H+ symporter, MFS transporters. A total of 46 genes encoding transporters were identified in the merged transcriptome highest order of MFS transporters. MFS transporters are involved in the transport of monosaccharides, oligosaccharides, inositols, drugs, amino acids, nucleosides, organophosphate esters, Krebs cycle metabolites, and a large variety of organic and inorganic anions and cations.Compared to the control, a significant increase in MFS transporters was observed in the transcriptome of treated. The gene number of sugar transporter (SP) family of MFS subfamily was higher indicating the uptake of more carbohydrate-produced nutrients. Comparative analysis with other fungi revealed that the MFS alpha-glucoside transporter, MFS multidrug transporter is overrepresented, suggesting export of more metabolism products in the extracellular. Presence of a high affinity nicotinate permease that catalyzes nicotinic acid (vitamin B3) uptake, vitamin H transporter, putative vitamin b6 transporter, bsu1 protein, vitamin H transporter 1/biotin symporter vht1, reflecting that might be dependent from the host plant for vitamin supply (Table 3).
Validating RNAseq Results with qPCR Techniques
Seven differentially expressed transcripts, of which most of them were related to metabolism (Hexokinase, Fructose bisphosphate, Isocitrate lyase, Transaldolase), protein folding (Protein disulfide isomerase, HAC) and cell wall degrading enzyme (β-glucosidase) were selected for validating the results obtained RNAseq analyses using qPCR (Table 5). In this analysis, a comparison has been made for the expression of the chosen genes between the Control (absence of 2-DG) and treated (presence of 2-DG) as illustrated in Figure 8. This study showed that most of the gene related to metabolism and protein folding were found to be upregulated in 2-DG treatment compared to the control, which showed no such up-regulation.
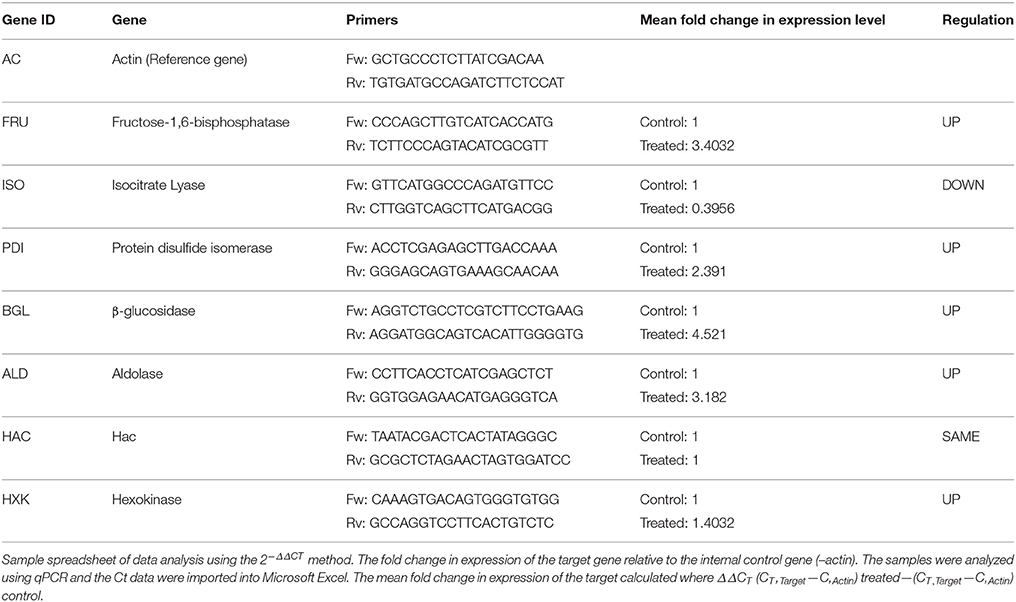
Table 5. List of genes selected for differential expression analysis and the oligonucleotides used in this study.
We examined transcript levels of β-glucosidase differentially expressed gene through qPCR, which were also found to be differentially expressed at 2-D proteomic analysis (Figure 2B). The expression levels of two well-known UPR maker genes such as PDI and HACA was determined using qPCR and was found that though there was increased expression of Protein disulfide isomerase the expression of HAC remained unaltered. The results obtained from qPCR data are concordant with our transcriptomics data, confirming the reliability of the results with minimum discrepancies.
Discussion
Cellulose Degrading Enzyme Production in Response to 2-Deoxyglucose are Results of Shunting of Catabolite Repression
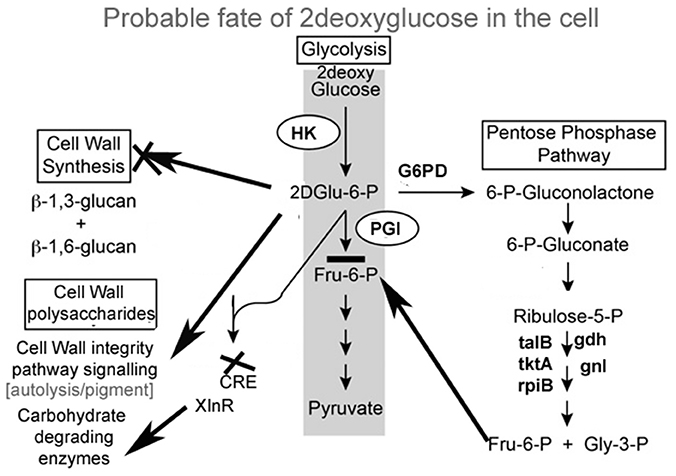
A. malaysianum has an innate ability to secrete lignocellulose-degrading enzymes in the extracellular medium (Table 1A). The higher level of production of these enzymes makes A. malaysianum an industrially important strain for biofuel production that can be rapidly exploited. In vitro addition of 2-DG in cellobiose medium resulted in further increase of β-glucosidase and endoglucanase activity by several folds (Table 1B). It may be proposed that such rise in β-glucosidase results from the combined action of two mechanisms acting simultaneously; (i) Shunting down of the catabolite repression of the cell by glucose anti-metabolite 2-DG in the medium; (ii) cellobiose induces lignocellulolytic enzymes. The non- metabolizable glucose analog 2-DG has been used extensively to induce catabolite repression in bacteria and fungi (Kornberg and Lambourne, 1994). Moreover, the use of 2-deoxyglucose (2-DG), as an antimetabolite has been routinely practiced for selection of the carbohydrate degrading enzyme hyper-producing mutants. The addition of 2-DG to cells causes a glucose starvation-like response, inhibiting growth and reducing viability even in the presence of abundant glucose. 2-DG is taken up and converted to 2-deoxyglucose-6-phosphate (2-DG-6P). However, the absence of a hydroxyl group on C-2 prevents the further catabolism of 2DG-6P by phosphogluco-isomerase. Accumulation of 2DG-6P in the cytoplasm result in inhibition of growth by repressing early glycolytic enzymes, thereby inhibiting glycolysis. Whether these are the only means by which 2-DG short-circuits normal glucose utilization needs to be explored further.
Exogeneous addition of 2-DG in a growth media containing a sole carbon source extended the lag phase, and inhibited growth in fungi (Zhu et al., 2015). In presence of preferred carbon source (glucose), sugar uptake and utilization was unaffected and maximum growth was observed in 48 h in absence as well as in presence of 2-DG. However, in cellobiose medium 2-DG caused delayed growth, indicating that 2-deoxyglucose led to CCR of cellobiose utilization (Figures 1A,B). Similar result was observed in Thermoanaerobacterium aotearoense where 0.5 mg/ml of 2-DG strongly inhibited growth (Zhu et al., 2015). The CCR is generally controlled by the second messenger cyclic adenosine 3',5'-monophosphate (cAMP), which has a fundamental role in global gene regulation (Lin et al., 2013). Catabolite repression by 2-DG was not reversed by adding exogenous cAMP (10 mM; Figure 1C). Similar studies on another Ascomycetous Aspergillus nidulans also corroborates our observation of no CCR relief in presence of cAMP (Borgia and Sypherd, 1977; Lee et al., 1996).
Since 2-DG hampers microorganism's growth, it is widely used as an inducer of CCR (Zhu et al., 2015). We added different concentration of 2-DG (0.25–1 mg/ml) to two different carbon source (1% cellobiose and glucose media) to detect the CCR by monitoring the culture growth. 2-DG delayed growth in cellobiose but not in glucose cultures. In glucose medium maximum growth (measured as mycelia dry weight) was observed in 48 h both in absence and presence of 2DG (Figure 1A). The growth rate measured in absence of 2-DG was 0.31 mg/g and 0.22 mg/g at 1 mg/ml concentration. In cellobiose medium maximum growth in absence of 2DG was observed at 60 h (0.271 mg/g) and was lagged upto 36 h with increasing concentration of 2DG (0.25–1 mg/ml). The growth density was also visibly reduced with increasing 2DG concentration (Figures 3A,B). Addition of 10 mM cAMP showed no effect on the growth delay caused by 2-DG in cellobiose medium (Figure 1C), unlike in E. coli where exogenous addition of cAMP in the medium provided CCR relief (Ullmann and Monod, 1968; Isaacs et al., 1994). This proves that in A. malaysianum, 2-DG-induced CCR is cAMP-independent.
Release of Extracellular β-Glucoside in Response to 2-DG is Involved in Cellular Signaling
In filamentous fungus, signal transduction pathways have been found to be largely associated with cell wall weakening (Wang et al., 2014). In N. crassa addition of 1 mM cellobiose and 2-deoxy-glucose inhibited the secretion of β-glucoside. Although the actual mechanism behind this was not discussed it was hypothesized that 2-DG was nearly as effective as glucose in blocking induction. Segregated and pellet like hyphae is probably the consequence of hyphae fragmentation due to production of cell wall hydrolytic enzymes like endochitinase and endo β-1,3 glucanases (Szilágyi et al., 2012, 2013) (Figures 3A–D).
Since 2-DG is a non-metabolizable glucose analog and also a glycosylation inhibitor, it markedly reduced the amount of cell wall components resulting in declining dry cell mass and progressive fragmentation of the hyphae in A. malaysianum (Figures 3E–H, 10). Cell wall of S. cerevisae grown in presence of 0.2%, 2-DG contained less glucose and mannose compared to the normal cell (McCartney et al., 2014). In the present study, we gained a more complex view of the changes taking place in the cell wall homeostasis. We detected the induction of several genes encoding hydrolytic enzymes such as chitinase, glucan β-1,3-glucosidase (Table 1B) and down regulation of chitin synthase in 2-DG treated condition (Figure 6; Table 4). Similar induction of several genes encoding cell wall hydrolyzing enzymes (chitinase, α and β-glucanases and mannanases) has been detected in carbon starved cultures of Aspergillus niger (Nitsche et al., 2012). Up regulation of putative β-glucosidase encoding genes might also contribute to the degradation of the fungal cell wall, as observed by enhanced extracellular β-glucosidase activity in presence of 2-DG (Figure 2; Table 3; Szilágyi et al., 2010).
Interestingly the degree in depression in growth rate and cell wall composition was dose dependent. In the culture with 0.025% 2-DG, the decrease in cell viability was much lesser compared to 0.05 and 0.1% 2-DG concentration (data not shown). In both 0.05 and 0.1% 2-DG cell lysis took places from late exponential phase to early stationary phase. 2-DG (0.1% w/v) added at inoculation time to cultures of Aspergillus prevents α-l, 3-glucan, α-l, 3-glucanase, and cleistothecium formation. 2-DG given after α-1,3-glucan synthesis, inhibits a-l, 3-glucanase and glucan breakdown partially in Aspergillus (Lee et al., 1996). By RNA sequencing and secretome analysis, we identified seven fungal hydrophobin, five chitinase, two endochitinase, one chitinase 18-18, proteases, one chitinasechi18-5, one Chitinase precursor, one Chitin-binding type1 and 2 chitin synthase genes in the transcriptome of A. malaysianum (Supplementary Tables 1, 2).
Distinctive Expansion of Gene Families Associated with Plant Cell Wall Degradation, Secondary Metabolism, and Nutrient Uptake in the Transcriptome of A. malaysianum
The expanded CAZyme arsenals of A. malaysianum were similar to those of Eutypa lata, Thielavia terrestris, and Colletotrichum sp. (Table 6). Interestingly, these fungi are known to be pathogen on host plants and have been isolated as endophytes from other sources also. Glycoside hydrolases (GHs) emerges as a large family under differentially expressed gene category. Unlike pathogens that thrive on pectin-rich tissue (Zhao et al., 2013), which possesses high numbers of pectolytic enzymes, A. malaysianum showed a wider array of enzymes that target cellulose and hemicellulose. This includes endo-β-1,4-cellulases (GH5), β-glucosidases (GH3), xyloglucan transglucosylase/hydrolases (GH16), and β-xylosidases (GH43). We found significant expansion of genes coding for CAZymes containing the CBM1 domain, a carbohydrate-binding module. CBM1 strongly binds to crystalline cellulose and that is required for full activity of fungal cellulases similar to the findings from E. lata that has a similar CBM1gene family expansion pattern (Klosterman et al., 2011).
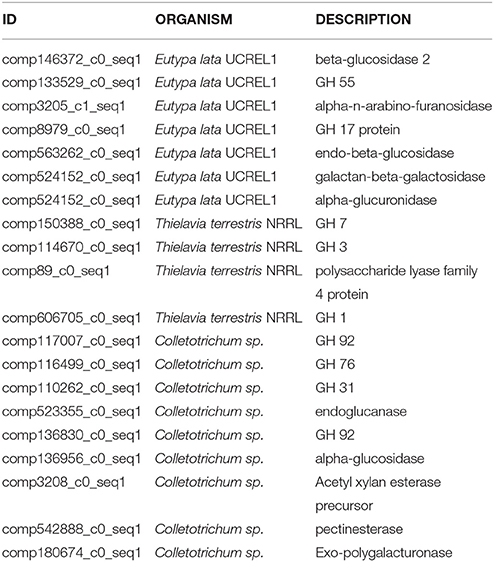
Table 6. CAZymes showing maximum similarity with Eutypa lata UCREL1, Thielavia terrestris NRRL, and Colletotrichum sp.
P450s have a broad spectrum of functions in fungi, from housekeeping activities, such as synthesis of essential membrane lipids, to synthesis of secondary metabolites, and detoxification of xenobiotic compounds (Chen et al., 2014). Differences in the number of genes belonging to the different P450 classes were observed among species, particularly between the Ascomycetes and Basidiomycete (Brakhage, 2013). A. malaysianum harbors high copies of antifungal, antioxidant, monooxygenases and cytochomeP450 superfamily genes including the genes responsible for the production of rare alkaloid—Loleine (Wang et al., 2008; Table 3). Three different types of CYP families were identified in A. malaysianum: cytochrome c, b560, and the P450. The high diversity of secondary metabolites is related to the diversity of the CYP genes. For example, the 219 CYP genes in the Ganoderma lucidum genome resulted in a large number of different secondary metabolites (Yu et al., 2012).
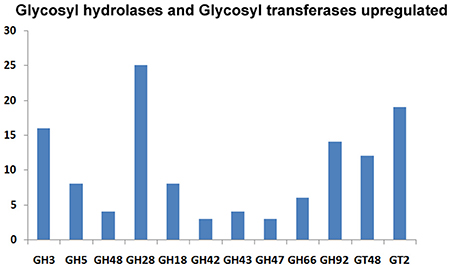
The CAZymes-encoding genes that are significantly up-regulated in treated as compared to the control condition include many glycosyl hydrolases (GH classes; Cantarel et al., 2009) and glycosyl transferases: GH5 (8), GH3 (16), GH28 (25), GH18 (8) and GH66 (6), GT48, GT2 (19), GT17 (5), GT22 (5), and GT55 (5) (Figure 9). Polygalacturonases belonging to Glycosyl hydrolases family 28 was highly upregulated. Eight genes encoding different GH28 family pectinolytic enzymes (pectinesterase, polygalacturonase, pectate lyase proteins) were expressed.
Metabolic Pathways Involved in Carbon Flow Cycle are Perturbed in Response to 2-DG
To gain an insight into the altered metabolic status imposed by 2-DG, four different enzymes involved in the interlinked pathways of glycolysis (hexokinase), gluconeogenesis (fructose-bisphosphatase), Krebs' cycle (isocitrate lyase, malate dehydrogenase), and glyoxalate cycle (isocitrate lyase) were ascertained and compared in qPCR (Figure 8B; Table 5).
Pathways associated with Hexokinase, Transaldolase, F-6-phosphate, and glucose-6 phosphate dehydrogenase and alcohol dehydrogenase production were induced whereas Isocitrate lyase and Phosphogluco-isomerase were suppressed (Figures 8A,B). The activity and expression fold of these marker metabolic enzymes was quantified by enzymatic assay and also determined by qPCR data reinforcing our suggested carbon flow cycle (Livak and Schmittgen, 2001; Figure 10). The accumulation of non metabolizable 2DG-6-P in the cytoplasm forced the cell to recover from the glucose sink by entering into Pentose phosphate Pathway and gluconeogenesis pathway. This leads to up regulation of several metabolic enzymes like transaldolase (talB), transketolase (tktA), fructose bis phosphate (fbp), glucose 1 dehydrogenase (gdh), gluconolactonase (gnl), ribose 5 phosphate isomerase (rpiB; Figure 6).
CreA/Cre1- knock out mutants display severe phenotypic changes such as reduced growth, abnormal hyphal morphology and sporulation. Up regulation of different genes linked to carbohydrate metabolism (e.g., glycoside hydrolases and glycoside transferases), cellular transport, signaling molecules like MAPK and SNF2 as well as certain chaperons and heat shock protein that is induced by several stresses, including glucose limitation (Portnoy et al., 2011). It may be presumed that 2-DG works in a manner homologous to these Cre mutants creating a similar environment and displays similar traits (Figure 10).
Conclusion
Tricking A. malaysianum into producing industrially important enzymes with 2-Deoxy D-Glucose treatment is a novel way to increase commercial usability of this fungi. The ability of A. malaysianum to produce hemicellulolytic enzymes renders it an attractive organism for the production of fuels and renewable energy. We investigated 2-DG induced carbon metabolism and carbohydrate degrading enzymes secretion in this organism in order to better understand the regulatory mechanism of this non metabolized sugar and how to manipulate it. Transcriptomics study and our experimental data indicate that A. malaysianum is regulated in a CCR independent mechanism in presence of 2DG. The induced synthesis of secondary metabolites, fatty acid and lipids, and proteins associated with cell wall degrading enzymes demonstrated the benefits of addition of 2-DG in the culture medium. 2-DG induces a carbon starving like environment causing increased production of proteins and extracellular enzymes. As a result, endoplasmic reticulum activity increases considerably leading in further up regulation of transporters, disulfide isomerases, and binding proteins. Plethora of secretory enzymes linked to carbohydrate degradation and several metabolites having economic values can further be explored for commercialization.
Data Availability
Sequence data is publicly available in Genbank with SRA id SRR746797. The Bioproject id is PRJNA186407 and Biosample id is SAMN01923006.
Author Contribution
SK and ST conceived the project. SM and SK designed the project. SM conducted the experiment. AP, MC, and ST analyzed the data. SM, ST, and MC. wrote the manuscript.
Funding
ST would like to acknowledge DBT - Ramalingaswamy fellowship for supporting this work. SM would like to acknowledge CSIR, Govt of India for funding (Award no. 31/2 (901)/2012-EMR-I). MMC. would like to acknowledge DST for fellowship.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Support from Prof. Siddhartha Roy, former director, CSIR-IICB is gratefully acknowledged.
Abbreviations
2-DG, 2-deoxy D-glucose; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene ontology; CAZY:Carbohydrate Active enzymes; UPR, Unfolded protein response; qPCR, Quantitative Real time PCR; GPI-anchored, Glycophosphatidylinositol anchored protein; cAMP, Cyclic adenosine monophosphate; ORF:Open reading frame.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00596
Supplementary Table 1. Parsed output of blast results of merged transcripts (control + treated) against Non Redundant database.
Supplementary Table 2. Catalog of secretory proteins under 2-DG treated and control conditions.
Supplementary Table 3. Differential expression analysis of 2-DG treated samples with control. Positive values indicate up-regulation under 2-DG and negative values indicate down-regulation. CAZY annotation, interproscan, and blast annotations are provided alongside.
Supplementary Table 4. Pathway analysis and KO IDs of the entire merged (Control + treated) transcripts.
References
Adelantado, C., Naranjo, C., and Calvo, M. A. (2005). Evaluation of culture conditions that Minimize 3-nitropropionic acid production by Arthrinium Strains. Pak. J. Biol. Sci. 8, 550–552. doi: 10.3923/pjbs.2005.550.552
Aissaoui, H., Agut, M., and Calvo, T. M. (1998). Effect of the raw extract of Arthrinium strains (Hyphomycetes, Dematiaceae) on the growth of pathogenic bacteria in poultry feed. Microbios 100, 109–116.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Basselin-Eiweida, M., and Kaneshiro, E. S. (2001). Detection of two distinct transporter systems for 2-deoxyglucose uptake by the opportunistic pathogen Pneumocystis carinii. Biochim. Biophys. Acta 1515, 177–188. doi: 10.1016/S0005-2736(01)00412-6
Benz, J. P., Chau, B. H., Zheng, D., Bauer, S., Glass, N. L., and Somerville, C. R. (2014). A comparative systems analysis of polysaccharide-elicited responses in Neurospora crassa reveals carbon source-specific cellular adaptations. Mol. Microbiol. 91, 275–299. doi: 10.1111/mmi.12459
Birol, I., Jackman, S. D., Nielsen, C. B., Qian, J. Q., Varhol, R., Stazyk, G., et al. (2009). De novo transcriptome assembly with ABySS. Bioinformatics 25, 2872–2877. doi: 10.1093/bioinformatics/btp367
Boisvert, S., Laviolette, F., and Corbeil, J. (2010). Ray: simultaneous assembly of reads from a mix of high-throughput sequencing technologies. J. Comput. Biol. 17, 1519–1533. doi: 10.1089/cmb.2009.0238
Borgia, P., and Sypherd, P. S. (1977). Control of beta-glucosidase synthesis in Mucor racemosus. J. Bacteriol. 130, 812–817.
Brakhage, A. A. (2013). Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 11, 21–32. doi: 10.1038/nrmicro2916
Calvo, M., Arosemena, L., Agut, M., Adelantado, C., and Aissaoui, H. (2005). Biological control of bayoud in the palm trees by strains of Arthrinium. J. Biol. Sci. 5, 236–238. doi: 10.3923/jbs.2005.236.238
Cantarel, B. L., Coutinho, P. M., Rancurel, C., Bernard, T., Lombard, V., and Henrissat, B. (2009). The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37(Suppl. 1), D233–D238. doi: 10.1093/nar/gkn663
Chen, W., and Gueron, M. (1992). The inhibition of bovine heart hexokinase by 2-deoxy-d-glucose-6-phosphate: characterization by 31 P NMR and metabolic implications. Biochimie 74, 867–873. doi: 10.1016/0300-9084(92)90070-U
Chen, W., Lee, M.-K., Jefcoate, C., Kim, S.-C., Chen, F., and Yu, J.-H. (2014). Fungal cytochrome p450 monooxygenases: their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 6, 1620–1634. doi: 10.1093/gbe/evu132
Chevreux, B., Pfisterer, T., Drescher, B., Driesel, A. J., Müller, W. E., Wetter, T., et al. (2004). Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res. 14, 1147–1159. doi: 10.1101/gr.1917404
Conesa, A., Gotz, S., Garcia-Gomez, J. M., Terol, J., Talon, M., and Robles, M. (2005). Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676. doi: 10.1093/bioinformatics/bti610
Coradetti, S. T., Craig, J. P., Xiong, Y., Shock, T., Tian, C., and Glass, N. L. (2012). Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc. Natl. Acad. Sci. 109, 7397–7402. doi: 10.1073/pnas.1200785109
Crawford, J. E., Guelbeogo, W. M., Sanou, A., Traoré, A., Vernick, K. D., Sagnon, N., et al. (2010). De novo transcriptome sequencing in Anopheles funestus using Illumina RNA-seq technology. PLoS ONE 5:e14202. doi: 10.1371/journal.pone.0014202
Crous, P. W., and Groenewald, J. Z. (2013). A phylogenetic re-evaluation of Arthrinium. IMA Fungus 4, 133. doi: 10.5598/imafungus.2013.04.01.13
Delmas, S., Pullan, S. T., Gaddipati, S., Kokolski, M., Malla, S., Blythe, M. J., et al. (2012). Uncovering the genome-wide transcriptional responses of the filamentous fungus Aspergillus niger to lignocellulose using RNA sequencing. PLoS Genet. 8:e1002875. doi: 10.1371/journal.pgen.1002875
de Sousa Lima, P., Casaletti, L., Bailão, A. M., de Vasconcelos, A. T. R., da Rocha Fernandes, G., and de Almeida Soares, C. M. (2014). Transcriptional and proteomic responses to carbon starvation in Paracoccidioides. PLoS Negl. Trop. Dis. 8:e2855. doi: 10.1371/journal.pntd.0002855
Emri, T., Majoros, L., Tóth, V., and Pócsi, I. (2013). Echinocandins: production and applications. Appl. Microbiol. Biotechnol. 97, 3267–3284. doi: 10.1007/s00253-013-4761-9
Emri, T., Molnár, Z., Pusztahelyi, T., and Pócsi, I. (2004). Physiological and morphological changes in autolyzing Aspergillus nidulans cultures. Folia Microbiol. 49, 277–284. doi: 10.1007/BF02931043
Gentles, A. J., and Karlin, S. (2001). Genome-scale compositional comparisons in eukaryotes. Genome Res. 11, 540–546. doi: 10.1101/gr.163101
Gianoulis, T. A., Griffin, M. A., Spakowicz, D. J., Dunican, B. F., Sboner, A., Sismour, A. M., et al. (2012). Genomic analysis of the hydrocarbon-producing, cellulolytic, endophytic fungus Ascocoryne sarcoides. PLoS Genet. 8:e1002558. doi: 10.1371/journal.pgen.1002558
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 29, 644. doi: 10.1038/nbt.1883
Hong, J.-H., Jang, S., Heo, Y. M., Min, M., Lee, H., Lee, Y. M., et al. (2015). Investigation of marine-derived fungal diversity and their exploitable biological activities. Mar. Drugs 13, 4137–4155. doi: 10.3390/md13074137
Isaacs, H., Chao, D., Yanofsky, C., and Saier, M. H. (1994). Mechanism of catabolite repression of tryptophanase synthesis in Escherichia coli. Microbiology 140, 2125–2134. doi: 10.1099/13500872-140-8-2125
Jaramillo, V. D. A., Sukno, S. A., and Thon, M. R. (2015). Identification of horizontally transferred genes in the genus Colletotrichum reveals a steady tempo of bacterial to fungal gene transfer. BMC Genomics 16:2. doi: 10.1186/1471-2164-16-2
Jones, P., Binns, D., Chang, H. Y., Fraser, M., Li, W., McAnulla, C., et al. (2014). InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240. doi: 10.1093/bioinformatics/btu031
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Katz, M. E., Gray, K.-A., and Cheetham, B. F. (2006). The Aspergillus nidulans xprG (phoG) gene encodes a putative transcriptional activator involved in the response to nutrient limitation. Fungal Genet. Biol. 43, 190–199. doi: 10.1016/j.fgb.2005.12.001
Kent, W. J. (2002). BLAT–the BLAST-like alignment tool. Genome Res. 12, 656–664. doi: 10.1101/gr.229202
Klosterman, S. J., Subbarao, K. V., Kang, S., Veronese, P., Gold, S. E., Thomma, B., et al. (2011). Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 7:e1002137. doi: 10.1371/journal.ppat.1002137
Kornberg, H., and Lambourne, L. T. (1994). The role of phosphoenolpyruvate in the simultaneous uptake of fructose and 2-deoxyglucose by Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 91, 11080–11083. doi: 10.1073/pnas.91.23.11080
Kuby, S. A., Wu, J. T., and Roy, R. N. (1974). Glucose 6-phosphate dehydrogenase from brewers' yeast (Zwischenferment). Further observations on the ligand-induced macromolecular association phenomenon: kinetic properties of the two-chain protein species; and studies on the enzyme-substrate interactions. Arch. Biochem. Biophys. 165, 153–178. doi: 10.1016/0003-9861(74)90153-2
Larrondo, J., Agut, M., and Calvo, M. (1996). Influence of the culture medium on the inhibitory activity of Arthrinium strains. Microbios 87, 39–42.
Lee, J., Kwon, K.-S., and Hah, Y. C. (1996). Regulation of β-glucosidase biosynthesis in Aspergillus nidulans. FEMS Microbiol. Lett. 135, 79–84. doi: 10.1016/0378-1097(95)00433-5
Leung, P. H., Zhang, Q. X., and Wu, J. Y. (2006). Mycelium cultivation, chemical composition and antitumour activity of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. J. Appl. Microbiol. 101, 275–283. doi: 10.1111/j.1365-2672.2006.02930.x
Limón, M. C., Pakula, T., Saloheimo, M., and Penttilä, M. (2011). The effects of disruption of phosphoglucose isomerase gene on carbon utilisation and cellulase production in Trichoderma reesei Rut-C30. Microb. Cell Fact. 10:40. doi: 10.1186/1475-2859-10-40
Lin, C. T., Chen, Y. C., Jinn, T. R., Wu, C. C., Hong, Y. M., and Wu, W. H. (2013). Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS ONE. 8:e54430. doi: 10.1371/journal.pone.0054430
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lulin, H., Xiao, Y., Pei, S., Wen, T., and Shangqin, H. (2012). The first Illumina-based de novo transcriptome sequencing and analysis of safflower flowers. PLoS ONE. 7:e38653. doi: 10.1371/journal.pone.0038653
McCartney, R. R., Chandrashekarappa, D. G., Zhang, B. B., and Schmidt, M. C. (2014). Genetic analysis of resistance and sensitivity to 2-deoxyglucose in Saccharomyces cerevisiae. Genetics 198, 635–646. doi: 10.1534/genetics.114.169060
Miao, L., Kwong, T. F., and Qian, P.-Y. (2006). Effect of culture conditions on mycelial growth, antibacterial activity, and metabolite profiles of the marine-derived fungus Arthrinium cf saccharicola. Appl. Microbiol. Biotechnol. 72, 1063–1073. doi: 10.1007/s00253-006-0376-8
Mizrachi, E., Hefer, C. A., Ranik, M., Joubert, F., and Myburg, A. A. (2010). De novo assembled expressed gene catalog of a fast-growing Eucalyptus tree produced by Illumina mRNA-Seq. BMC Genomics 11:681. doi: 10.1186/1471-2164-11-681
Muller, M., Wasson, C. W., Bhatia, R., Boxall, S., Millan, D., Goh, G. Y., et al. (2015). YIP1 family member 4 (YIPF4) is a novel cellular binding partner of the papillomavirus E5 proteins. Sci. Rep. 5:12523. doi: 10.1038/srep12523
Nitsche, B. M., Jørgensen, T. R., Akeroyd, M., Meyer, V., and Ram, A. F. (2012). The carbon starvation response of Aspergillus niger during submerged cultivation: insights from the transcriptome and secretome. BMC Genomics. 13:380. doi: 10.1186/1471-2164-13-380
O'Connell, R. J., Thon, M. R., Hacquard, S., Amyotte, S. G., Kleemann, J., Torres, M. F., et al. (2012). Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 44, 1060–1065. doi: 10.1038/ng.2372
Octave, S., Roblin, G., Vachaud, M., and Fleurat-Lessard, P. (2006). Polypeptide metabolites secreted by the fungal pathogen Eutypa lata participate in Vitis vinifera cell structure damage observed in Eutypa dieback. Funct. Plant Biol. 33, 297–307. doi: 10.1071/FP05230
Pao, S. S., Paulsen, I. T., and Saier, M. H. (1998). Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62, 1–34.
Pócsi, I., Prade, R. A., and Penninckx, M. J. (2004). Glutathione, altruistic metabolite in fungi. Adv. Microb. Physiol. 49, 1–76. doi: 10.1016/S0065-2911(04)49001-8
Portnoy, T., Margeot, A., Linke, R., Atanasova, L., Fekete, E., Sándor, E., et al. (2011). The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: a master regulator of carbon assimilation. BMC Genomics 12:269. doi: 10.1186/1471-2164-12-269
Rice, P., Longden, I., and Bleasby, A. (2000). EMBOSS: the European molecular biology open software suite. Trends Genet. 16, 276–277. doi: 10.1016/S0168-9525(00)02024-2
Ruijter, G. J., and Visser, J. (1997). Carbon repression in Aspergilli. FEMS Microbiol. Lett. 151, 103–114. doi: 10.1111/j.1574-6968.1997.tb12557.x
Sawhasan, P., Worapong, J., Flegel, T., and Vinijsanun, T. (2012). Fungal partnerships stimulate growth of Termitomyces clypeatus stalk mycelium in vitro. World J. Microbiol. Biotechnol. 28, 2311–2318. doi: 10.1007/s11274-012-1038-x
Schoonbeek, H.-J., Raaijmakers, J. M., and De Waard, M. A. (2002). Fungal ABC transporters and microbial interactions in natural environments. Mol. Plant Microbe Interact. 15, 1165–1172. doi: 10.1094/MPMI.2002.15.11.1165
Shrestha, P., Ibanez, A. B., Bauer, S., Glassman, S. I., Szaro, T. M., Bruns, T. D., et al. (2015). Fungi isolated from Miscanthus and sugarcane: biomass conversion, fungal enzymes, and hydrolysis of plant cell wall polymers. Biotechnol. Biofuels. 8, 38. doi: 10.1186/s13068-015-0221-3
Staden, R., and McLachlan, A. D. (1982). Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 10, 141–156. doi: 10.1093/nar/10.1.141
Szilágyi, M., Anton, F., Forgács, K., Yu, J.-H., Pócsi, I., and Emri, T. (2012). Antifungal activity of extracellular hydrolases produced by autolysing Aspergillus nidulans cultures. J. Microbiol. 50, 849–854. doi: 10.1007/s12275-012-2001-0
Szilágyi, M., Kwon, N. J., Dorogi, C., Pócsi, I., Yu, J. H., and Emri, T. (2010). The extracellular β-1, 3-endoglucanase EngA is involved in autolysis of Aspergillus nidulans. J. Appl. Microbiol. 109, 1498–1508. doi: 10.1111/j.1365-2672.2010.04782.x
Szilágyi, M., Miskei, M., Karányi, Z., Lenkey, B., Pócsi, I., and Emri, T. (2013). Transcriptome changes initiated by carbon starvation in Aspergillus nidulans. Microbiology 159(Pt 1), 176–190. doi: 10.1099/mic.0.062935-0
Traxler, P., Fritz, H., Fuhrer, H., and Richter, W. J. (1980). Papulacandins, a new family of antibiotics with antifungal activity. Structures of papulacandins A, B, C and D. J. Antibiot.(Tokyo) 33, 967–978.
Ullmann, A., and Monod, J. (1968). Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 2, 57–60. doi: 10.1016/0014-5793(68)80100-0
Wang, F., Hou, Z., Wang, C., Li, P., and Shi, D. (2008). Bioactive metabolites from Penicillium sp., an endophytic fungus residing in Hopea hainanensis. World J. Microbiol. Biotechnol. 24, 2143–2147. doi: 10.1007/s11274-008-9720-8
Wang, M., Dong, Y., Zhao, Q., Wang, F., Liu, K., Jiang, B., et al. (2014). Identification of the role of a MAP kinase Tmk2 in Hypocrea jecorina (Trichoderma reesei). Sci. Rep. 4:6732. doi: 10.1038/srep06732
Wick, A. N., Drury, D. R., Nakada, H. I., and Wolfe, J. B. (1957). Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 224, 963–969.
Yu, G.-J., Wang, M., Huang, J., Yin, Y.-L., Chen, Y.-J., Liang, Y., et al. (2012). Deep insight into the Ganoderma lucidum by comprehensive analysis of its transcriptome. PLoS ONE 7:e44031. doi: 10.1371/journal.pone.0044031
Zhao, Z., Liu, H., Wang, C., and Xu, J.-R. (2013). Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics 14:274. doi: 10.1186/1471-2164-14-274
Zhou, Y., Chen, L., Fan, X., and Bian, Y. (2014). De novo assembly of Auricularia polytricha transcriptome using Illumina sequencing for gene discovery and SSR marker identification. PLoS ONE 9:e91740. doi: 10.1371/journal.pone.0091740
Keywords: 2-deoxy d-glucose, anti-metabolite, Carbon catabolic repressor, Carbohydrate active enzymes, Cell wall degrading enzymes, unfolded protein response
Citation: Mukherjee S, Chandrababunaidu MM, Panda A, Khowala S and Tripathy S (2016) Tricking Arthrinium malaysianum into Producing Industrially Important Enzymes Under 2-Deoxy D-Glucose Treatment. Front. Microbiol. 7:596. doi: 10.3389/fmicb.2016.00596
Received: 30 October 2015; Accepted: 11 April 2016;
Published: 13 May 2016.
Edited by:
Weiwen Zhang, Tianjin University, ChinaCopyright © 2016 Mukherjee, Chandrababunaidu, Panda, Khowala and Tripathy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sucheta Tripathy, dHN1Y2hldGFAaWljYi5yZXMuaW47
Suman Khowala, c3VtYW5raG93YWxhQGlpY2IucmVzLmlu
 Soumya Mukherjee1
Soumya Mukherjee1 Sucheta Tripathy
Sucheta Tripathy