
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Microbiol. , 31 March 2016
Sec. Systems Microbiology
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.00455
Most of the Human diseases affecting westernized countries are associated with dysbiosis and loss of microbial diversity in the gut microbiota. The Western way of life, with a wide use of antibiotics and other environmental triggers, may reduce the number of bacterial predators leading to a decrease in microbial diversity of the Human gut. We argue that this phenomenon is similar to the process of ecosystem impoverishment in macro ecology where human activity decreases ecological niches, the size of predator populations, and finally the biodiversity. Such pauperization is fundamental since it reverses the evolution processes, drives life backward into diminished complexity, stability, and adaptability. A simple therapeutic approach could thus be to reintroduce bacterial predators and restore a bacterial diversity of the host microbiota.
The gut is the largest interface (200 m2) between the host and its external milieu. It plays an important part in the metabolism of nutriments and water absorption. Furthermore, immune cells of the Gut Associated Lymphoid Tissue (GALT) are more numerous than those from other secondary lymphoid tissues contained in the whole body. They interact with trillions of bacteria, archaea and eukaryotes forming a complex ecosystem: the gut microbiota. Almost 80% of microbes observed by microscopic examination of fecal specimens are not recoverable by culture (Suau et al., 1999). Thus, until the recent development of culture-independent methods, which combine isolation and sequencing of nucleic acids and powerful bioinformatics analyses, the gut microbiota was seen as an ignored organ or a black box.
Our knowledge on the Human gut microbiota is quickly increasing. In a given individual, the microbiota is relatively stable after the first months of life (Koenig et al., 2011). At the population level, the microbiota is also supposed to be stable and selected by evolution (Ochman et al., 2010; Jalanka-Tuovinen et al., 2011). However, the commensal microbiota can be qualitatively and quantitatively modulated by the environment (Huttenhower et al., 2012). Recent changes in the environment may thus have altered the mutually beneficial interaction toward another stable but harmful balance (Lozupone et al., 2012; Shade et al., 2012).
Sometimes and unexpectedly, links have been established between diseases of industrialized countries and altered patterns of the gut microbial ecosystem collectively known as intestinal dysbiosis. The concept of dysbiosis refers to an unbalanced microbiota, which is most of the time supposed to be harmful. The effectiveness of fecal microbiota transplantation in the cases of Clostridium difficile associated colitis or T2D supports this opinion (Vrieze et al., 2012; Sha et al., 2014). As a result, “understanding the structure and function of the human symbiont communities might become the first great breakthrough of twenty-first century medicine” (Guarner, 2014).
Loss of microbiota diversity (LOMD) appears as the most constant finding of intestinal dysbiosis. By analogy with macro ecosystems, we propose here that it is related to the loss of bacterial predators associated with our modern Western lifestyle. Interestingly, this hypothesis suggests that the reintroduction of bacterial predators in our digestive ecosystem may be an option for improving/restoring the gut microbiota diversity and for treating at-risk people (Jakobsson et al., 2014).
In the past decades, the prevalence of numerous diseases has sharply increased, a phenomenon initially observed in Western countries and more recently in developing countries (Figure 1, Table 1). These complex genetic disorders include “immune related” diseases like allergy, inflammatory bowel diseases (IBD), type 1 diabetes mellitus (T1D), multiple sclerosis (MS) but also colorectal cancer or metabolic disorders like obesity and type 2 diabetes mellitus (T2D; Mayr et al., 2003; Eder et al., 2006; Harjutsalo et al., 2008; Andersson et al., 2014). Unrecognized environmental changes necessarily explain these outbreaks. The higher occurrence of some of these diseases (i.e., asthma, T1D, MS, obesity, T2D, colorectal cancer) among migrants (Bodansky et al., 1992; Hammond et al., 2000; Grüber et al., 2002; Pinheiro et al., 2009; Creatore et al., 2010), especially when emigrating before the age of 5 years (Kuehni et al., 2007) confirms a role of early environmental risk factors.
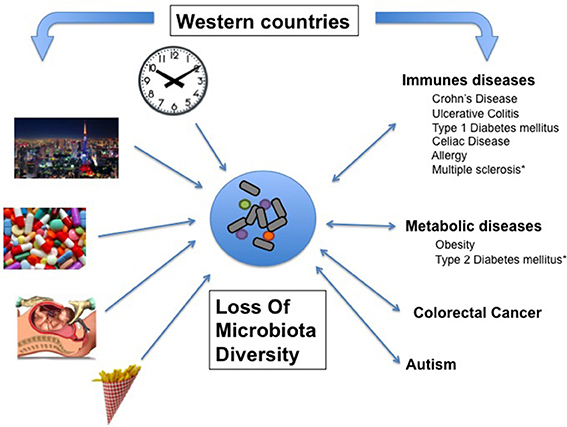
Figure 1. Associative links between Western lifestyle, Human conditions, and loss of microbial diversity (LOMD). On one hand, most of the Human diseases affecting westernized countries are associated with LOMD and on the other hand, some western lifestyle patterns cause LOMD. Then, LOMD appears to play a central role linking western lifestyle and western chronic human conditions (see also Table 1). *LOMD not assessed.
Among the huge body of literature describing disease-associated microbiota, LOMD appears as a common feature of most dysbioses. In digestive diseases such as Crohn's disease (CD) (an IBD condition; Sha et al., 2013; Matsuoka and Kanai, 2015), irritable bowel syndrome (IBS), with (Carroll et al., 2012) or without diarrhea (Durbán et al., 2012) and colorectal cancer (Ahn et al., 2013), LOMD is constantly observed. The duodenum-associated microbiota of celiac disease patients is also less diversified (Schippa et al., 2010). LOMD is a risk factor of relapse in Clostridium difficile colitis (Chang et al., 2008). Obesity is associated with altered representations of specific bacterial species, which often differ between studies, but LOMD is constantly retrieved with up to 20% loss of phylogenetic diversity and one third difference in terms of gene number (Turnbaugh et al., 2009; Cotillard et al., 2013; Tagliabue and Elli, 2013). Finally and more surprisingly, LOMD is also reported in non-digestive diseases such as autism (Kang et al., 2013). It thus appears that LOMD is a general feature associated with several Human conditions.
However, these associations might be the consequence rather than the cause of the disease. Several authors have questioned this chicken-egg problem and found arguments for a causal effect of LOMD on Human diseases. LOMD was found in CD (54 ribotypes in CD patients vs. 88 in healthy controls) not only in case of flare but also in case of remission (Manichanh et al., 2006) suggesting that it is not a consequence of gut inflammation. In healthy people investigated for T1D markers, the microbial diversity was lower in fecal samples of children with at least two disease-associated autoantibodies when compared to autoantibody-negative children matched for age, sex, early feeding history, and HLA-genotyping (De Goffau et al., 2013). In two studies following children at risk for T1D longitudinally from birth, a decrease of microbial diversity occurred just before the occurrence of anti-islet cell antibodies and subsequently T1D (Giongo et al., 2010; Kostic et al., 2015). Among obese individuals, a decrease of richness assessed by a low microbiome gene count was predictive of response to diet (Cotillard et al., 2013).
Consistently, in two prospective studies, LOMD measured during the first week of life was predictive of allergic manifestations at ages of 12 and 18 months (Wang et al., 2008; Ismail et al., 2012) and asthma at the age of 7 years (Abrahamsson et al., 2014). Decrease of microbial diversity also predicts the response to treatments. For example, in Ulcerative Colitis (UC), children who responded to corticosteroids had a more diverse microbiota than non-responders (Michail et al., 2012). LOMD is also a risk factor of relapse after intestinal resection in CD (Dey et al., 2013). Finally, it seems that CD can be triggered by a dysbiotic gut microbiota in a mouse model (Schaubeck et al., 2015).
Altogether, these observations strongly argue for a causal effect of LOMD in several Human conditions.
LOMD is a feature of industrialized countries. Among the many candidate risk factors causing the LOMD, some of them can be highlighted, such as lifestyle, eating behaviors, disruption of biological clock and antibiotic consumption (Figure 1).
The microbiota of Malawian and Venezuelan people are more diversified than their US children and adult counterparts (Yatsunenko et al., 2012). Similar differences were found when comparing Bangladeshi to American children (Lin et al., 2013). More recently, the analysis of gut microbiota patterns of rural Papua New Guineans compared with those of people from USA showed that westernization may decrease bacterial dispersal rates and alter the microbiota structure (Martínez et al., 2015). The Human hunter-gatherers Hadza of Tanzania—where people still live outside without access to antibiotics and treated water—had higher levels of microbial richness and biodiversity than Italian urban controls (Schnorr et al., 2014).
Different eating behaviors that are known to rapidly affect the intestinal microbiome (David et al., 2013) are obvious possible explanations. For example, fiber consumption may explain the differences observed between children from Burkina Faso and Italy (De Filippo et al., 2010). Recently, the impact of diet on the microbiota has been reviewed, and it turns out that fiber rich diet enhances gut microbiota diversity (Simpson and Campbell, 2015; for a more complete critical review on gut microbiota and diet, see Graf et al., 2015). However, these differences might not only be due to diet (Wu et al., 2016) and alternative hypotheses may be raised. For example, the practice of cesarean section has grown steadily since the second half of the twentieth century in Western countries, reaching an average of 26.9% of birth in OECD countries (OECD, 2013) but only 5% in developing countries (Betrán et al., 2007). Children born by cesarean section have a LOMD when compared to those born vaginally (Jakobsson et al., 2014).
Modern lifestyle might trigger a disruption of the biological clock with consequences for the host and the microbiota. Several characteristics of modern life style such as working shifts, stress, jet lag, unusual feeding patterns have been shown to disrupt the biological clock. The link between microbiota, its host and diurnal oscillation was well-described for the squid-Vibrio fischerii symbiosis (Wier et al., 2010). More recently, the intestinal microbiota in both mice and humans has been shown to exhibit diurnal oscillations, that when disrupted, led to a LOMD and dysbiosis (Thaiss et al., 2014).
Antibiotics (ATB) are other candidate risk factors. Naturally produced by many microorganisms, ATB have been developed by humans after the 30's. Beyond human medicine, antibiotics are now widely used especially for animal rearing, to the point where they are now markers of human economic activity. Humans cause a massive spread of ATB in the environment with significant impacts on the bacterial ecosystems of water, soil, and plants (Sarmah et al., 2006; Dolliver and Gupta, 2008; Martinez, 2009). ATB profoundly alter the structure of the intestinal microbiota (Buffie et al., 2012) and reduce the bacterial diversity (Dethlefsen et al., 2008; Pérez-Cobas et al., 2013; Zhao et al., 2014). Of note, ATB use during infancy and childhood is associated with increased incidences of asthma, atopic dermatitis, MS, IBD, juvenile idiopathic arthritis and obesity (Zeissig and Blumberg, 2014; Horton et al., 2015; Schwartz et al., 2015).
Whatever the relevant environmental risk factor(s) playing a role in industrialized countries, the way it impacts the composition of the gut microbiota remains to be explained.
Microbial communities harbor a wide range of ecological interactions which may be of five different types including (i) mutualism, where both the participants are benefited; (ii) amensalism, where one organism is inhibited or destroyed and the other is unaffected; (iii) commensalism, where one partner gets the advantage without any help or harm to the other; (iv) competition, where both the participants harm each other; and (v) predation and parasitism, where one gets benefited out of the other (Faust and Raes, 2012). All of these interactions are supposed to shape the community assembly. For instance, cooperation through metabolic exchange between species has been shown to be predictive for some co-occurrences (Zelezniak et al., 2015). However, the way diversity is shaped and persists is still poorly understood for the complex network of interactions within the gut.
Thank to phylogenetic approaches, knowledge on mechanisms governing microbial communities has improved over the last few years, filling the gap between knowledge on macrobial and microbial ecosystems. According to Vellend's conceptual synthesis of community ecology (Vellend, 2010), diversity at local scales (alpha diversity) is shaped by a combination of four processes: dispersal, local diversification, environmental selection and ecological drift (or demographic stochasticity; Costello et al., 2012). Inside the gut ecosystem, such processes could drive the microbiota community and specifically the microbiota diversity toward short-term and long-term co-evolution. Then, we thought to be inspired by macro ecological theories to encompass to what extend predation could play a role in driving gut microbiota diversity.
In macro ecology, it is now recognized that human activity causes a decrease in biodiversity of unprecedented proportions (Butchart et al., 2010). Among the many parameters that are under the pressure from Human activities, a major one is the loss of large predators, i.e., those who are at the top of the food chain in a given ecosystem. In 1966, Paine was the first to observe a decrease of mussel species in a marine ecosystem when removing the starfishes (Paine, 1966). He hypothesized that “the local biodiversity was directly related to the efficiency with which predators prevent the monopolization of the major environmental requisites by one species.” Similarly, sea otters protect kelp forests from damage by sea urchins (Szpak et al., 2013). Australian natives introduced dingo (Canis lupus Dingo) at least 3500 years ago. As it preyed upon livestock, the dingo has been the target of extermination programs for the last two centuries. Hereby, it was seen that the loss of dingoes has resulted in the depletion of plant biomass through a dysregulation of herbivore population (Letnic et al., 2012). Indeed, the impact on ecosystems of the decline of the seven largest carnivores in the world has been recorded in recent decades and the disappearance of a large carnivore always alters deeply the ecosystem and more particularly, it reduces its biodiversity (Ripple et al., 2014).
It thus appears that experimentally, species diversity can be maintained by predation. In the absence of predators, a few dominant species can grow rapidly and then supplant many of the other species, limiting the amount of their available resources. Conversely, the presence of predators limits the population of dominant species. The current working paradigm for community dynamics -understudied in the human gut- is the Lotka Voltera model. Colloquially known as Kill-the-Winner, the Lotka Voltera model predicts that predators will rapidly and drastically reduce the population of the most abundant species, preventing the best competitors from building up a high biomass. The role of a predator population in maintaining species diversity has been assessed by computer simulations, which displayed the robustness of the “Lotka-Volterra-like” cycle and implies that frequent predation might be a mechanism to maintain species diversity in nature. Moreover, combining the classical Lotka–Volterra model of population dynamics with regression techniques provides a mechanistic scheme that can be used to construct predictive models of ecosystem dynamics. Such an approach has been successfully applied to the gut microbiota ecosystem to show which network elements are disrupted during ATB course and predict to what extend Clostridium difficile infection (CDI) can arise (Stein et al., 2013). In the same way, it should be possible to design a mathematical models including predation within the gut microbial community (Figure 2). Such model would be validated by new in vitro data issued from studies on microbial predation in animal's gut.
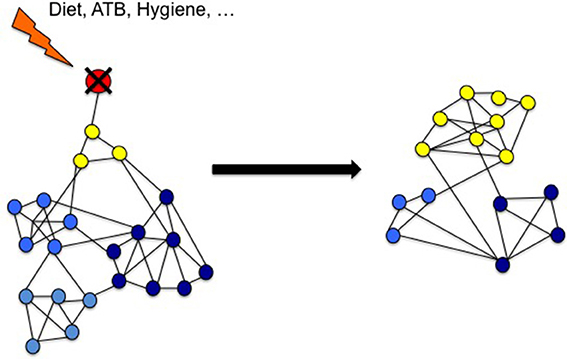
Figure 2. Environmental factors reduce microbial diversity because of the loss of predatory species. Gut microbiota may be seen as a complex network of many interacting species (nodes) with several kinds of interactions (links). Predators (in red) are key species that maintain the diversity of the microbiota by direct impact on preys (yellow) and indirect effect on other related species (blue). According to Voltera equations, loss of predators causes an increase number of preys but a loss of diversity. We propose that in industrialized countries environmental risk factors reduces the predators in the network causing LOMD.
The stability of microbial ecosystem can be analyzed through predictive or mechanistic models. On one hand, microbial communities tend to be more phylogenetically clustered than expected by chance. For instance, in aquatic ecosystems, the response to disturbance has been linked to the community network (Hunt and Ward, 2015). Then, a predictive model can be drawn to predict reactions to disturbance in a particular community. Within the gut microbiota community, where microbes compete and cooperate, such network has also been highlighted. Indeed, co-occurrence of microbial species are commonly measured in the gut microbiota (Faust et al., 2012).
On the other hand, in large ecosystems, a decrease of resilience is often associated with the lack of biodiversity. Resilience is defined as the amount of disturbance a system can absorb while remaining in the same functional state. It also refers to the ability of an altered ecosystem to reorganize and renew in case of failure. Through the diversity/versatility of each species response to external or internal changes, a high biodiversity helps to maintain a stable ecosystem function called “robustness” or stability (Elmqvist et al., 2003). Coral reefs around Jamaica are an example of such a phenomenon (Hughes, 1994). Overfishing led to a loss of diversity in the functional group of “grazers,” but that loss was made up by an increase in sea urchins, which offsets the loss of other herbivores and maintained corals as in “healthy state.” However, when a pathogen reduced the population of sea urchins (Lessios et al., 1984; Nyström et al., 2000) there were no grazing species left to prevent the invasion of algae that leads to the “undesirable state.” In other words, the “diversity of response increases the tolerance for management of mistakes” (Elmqvist et al., 2003; Figures 3A–C).
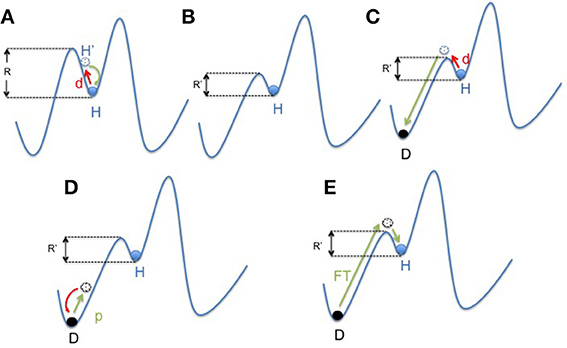
Figure 3. Mapping concept of microbiota dysbiosis and resilience. (A) In a healthy state, the gut microbiota ecosystem (represented by a ball) is in a steady state (H). The depth of the well in which the ball is located represents its resilience (R). In case of disturbance (d; i.e., ATB course or digestive infection), the gut microbiota changes (H′) and then get back to its anterior state (H). (B) Environmental factors (i.e., western lifestyle) negatively impact the diversity of the microbiota resulting in a decrease of resilience (R′ < R). However, the microbiota still remains in an apparent healthy state, due to the functional redundancy of the ecosystem (H). (C) But in this new situation, the same disturbance (d) results in a shift of the gut microbiota balance to an other state called dysbiosis (D). This new state is also a steady state, but it impacts negatively the individual condition. It represents an “unhealthy” state. (D,E) “Rebiosis” with respective effect of probiotics administration and fecal transplantation on the the gut microbiota ecosystem. A single strain (probiotic) fails to restore a “healthy” ecosystem (D), but a radical change of the ecosystem through fecal transplantation could be able to achieve this goal (E). p, probiotics; FT, Fecal Transplant.
Similarly to macro-ecosystems where the influence of human activity reduces biodiversity by the reduction of large predators, we hypothesize that the Western lifestyle reduces the diversity of the intestinal microbiota by loss of bacterial predation. Several microorganisms such as protists, bacteriophages and predatory bacteria are known to be predators of components of the gut microbiota.
Amoebas have developed complex interactions with bacteria during evolution (Cosson and Soldati, 2008). Basically, their interaction is a predator-prey model for which several parameters have been described. Differences in susceptibility to predation differentiate bacterial species (e.g., medium-sized bacteria are most vulnerable to predation while bacteria with small and large filamentous forms are partially resistant to predation). Direct and indirect influences of predation on the conditions of bacterial growth (substrate supply) or bacterial competition (elimination of competitors) are also important. In soils, this relationship seems even more complex, since under favorable environmental conditions, the interaction between amoebae and non-virulent bacteria causes lysis of the bacteria, while the interaction with low-virulent bacteria causes a symbiotic relationship or amoebic lysis (Ronn et al., 2002). Interestingly, the hypothesis that protist predation could shape the gut microbiota diversity has been recently supported by the discovery of an association between the absence of commensal amoebas in the intestine of West African subjects and LOMD (Morton et al., 2015).
Bacteriophages, or phages, are the most abundant biological group on Earth. Phages essentially belong to two categories, virulent and temperate. The virulent phage lifecycle consists in replicating in, and then lysing its bacterial host. Temperate phages can alternate between two lifestyles: they either lyse their host, like virulent phages, or establish a symbiosis with it, the so-called “lysogeny state.” In most ecosystems, where phages outnumber bacteria by a 10-fold factor, predation by phages allows maintaining bacterial diversity (Rodriguez-Valera et al., 2009). However, in humans, only 108 phages vs. 1012 bacteria /g of feces were reported with a preferentially lysogenic phenotype in the colon of healthy people (Mills et al., 2013). Moreover, phages cannot move to go and meet their prey and are therefore subject to a “random collision.” Consequently, viral-bacterial predator dynamic, evident in a number of other characterized ecosystems as aquatic environment (Rodriguez-Valera et al., 2009), could be absent in the distal intestine of healthy individuals (Reyes et al., 2010, 2013). Thus, they may not be considered “key predatory.”
To date, the most studied predatory bacteria belong to the Bdellovibrio-and-like organisms group called BALOs. The first report of B. bacteriovorus, gen. et sp. n. was made by Stolp and Starr in 1963 (Stolp and Starr, 1963) as a predatory and bacteriolytic microorganism. Bdellovibrio bacteriovorus (Bdello) is a microaerophilic species from the δ Proteobacteria group naturally present in soils (rhizosphere) and freshwater but also in the human intestine (Iebba et al., 2013). It is a highly motile bacterium that seeks out a prey bacterium and invades it. The ensuing intracellular growth and replication lead to the lysis of the prey bacterium and then motile predators are released (Strauch et al., 2007). It is a non-specific predator of Gram-negative bacteria but it is not pathogenic for higher organisms (Dashiff et al., 2011). Beyond this direct predation against gram-negative bacteria, it even impacts the growth of Gram positive bacteria such as Staphylococcus aureus (Iebba et al., 2014; Monnappa et al., 2014).
Despite its broad spectrum of activity on many gram negative bacteria (Dashiff et al., 2011; Kadouri et al., 2013), its impact on the intestinal microbiota has not been studied. So far, it has mainly been studied as a potential alternative to antibiotics (Sockett and Lambert, 2004; Dwidar et al., 2012) for ocular (Shanks et al., 2013), periodontal (Van Essche et al., 2011), or respiratory infections (Iebba et al., 2014). The only report of oral administration of Bdello showed a 10-fold decrease of Salmonella enteritidis and a reduction of morphological abnormalities associated with the cecum infection in infected chicken (Atterbury et al., 2011). The effects of Bdello on oral microbiota were recently analyzed using high throughput-sequencing methods (Loozen et al., 2015). Ex vivo experiments performed on sub-gingival plaque and saliva samples from periodontitis patients demonstrated changes in the overall ecology. Unfortunately, the effect on bacterial diversity was not specifically assessed. However, the evidence of coevolution of Bdello and its preys according to the Red Queen theory has been made by a long-term experiment involving Bdello and its prey Pseudomonas fluorescens (Gallet et al., 2009), indicating that Bdello could drive the biodiversity in the ecosystem when present.
Predatory-preys interactions were operating among bacteria long before the appearance of animals on earth. Then, animals provided new surroundings for bacteria, resulting in a more complex interaction between host cells and microorganisms. For instance, gut microbiota in vertebrates' gut adapts to the host diet over daily time scales, as we already mentioned (Graf et al., 2015), but even over evolutionary scales. This allowed animals to face environmental changes as they were able to digest a broad variety of biomolecules (Ley et al., 2008). Moreover, it has been proposed that this microbial functional diversity would have been beneficial for the host in an evolutionary perspective since it might have shaped the adaptive immune system (McFall-Ngai, 2007; McFall-Ngai et al., 2013).
Although many studies assessed the impact of single bacterial species on inflammatory markers, few of them looked at the relationship between microbiota diversity and its consequences on the immune system. One of the major components of the adaptive immune system are the CD4+ regulatory T (Treg) cells, which express the transcription factor Foxp3 and play an essential role in maintaining the immune homeostasis. Gnotobiotic mice colonized with 3 strains of Clostridium were shown to display a profile of Treg induction intermediate between axenic animals and mice inoculated with 46 strains. This observation suggests that an optimal induction of Tregs requires a set of metabolites that are more efficiently produced by a panel of Clostridium strains (Atarashi et al., 2011). On the other hand, axenic mice and those with low microbiota diversity develop high levels of serum IgE early in life (Cahenzli et al., 2013). Finally, an antibiotic treatment with vancomycin reduced the microbial diversity and increased the severity of asthmatic disease in mice (Russell et al., 2012).
CD typically represents a chronic inflammatory disease whose incidence increased sharply in Western countries (Molodecky et al., 2012) with large variations between Westernized and developing countries (Ng et al., 2013). It is also a disease for which epidemiological studies have linked low exposure to infectious agents and disease occurrence. For example, the farm life in childhood would protect against the disease (Amre et al., 2006). ATB taken in childhood increase the risk of CD (Shaw et al., 2010; Hviid et al., 2011; Ungaro et al., 2014). CD is also associated with a dysbiosis with a significant reduction in bacterial diversity (Manichanh et al., 2006; Dey et al., 2013; Sha et al., 2013). Finally, the presence of Bdello in stools and intestinal biopsies of children with CD has recently been studied. Only one out of nine patients carried Bdello while healthy eight controls were all positive (Iebba et al., 2013).
What would be the consequences of Bdello decrease in CD? Firstly, Bdello having a predation predominant on Gram negative bacteria, its absence could result in a proliferation of “pathobiont” bacteria, like Adherent and Invasive Escherichia coli (AIEC) or Yersinia species which are suspected to play a role in CD lesions (Carrière, 2014). Secondly, it can explain the observed increased proportion of Gram-negative symbionts (for example Bacteroides from the Bacteroidetes phylum) with a subsequent decrease of Gram positive bacteria such as Faecalibacterium prausnitzii from the Clostridium leptum group within the Firmicutes observed in CD patients. As a whole, CD could thus represent a prototypic example of the proposed theory.
Since the discovery of disease-associated dysbiosis, it is now accepted that a therapeutic goal is to restore a “healthy” or balanced microbiota, notably by improving the gut microbiota diversity. Several therapeutic attempts have been considered. Elie Metchnikoff first wrote about the health benefits of probiotics. He observed that Bulgarian peasants who consumed large amounts of fermented milk, the early form of yogurt, lived longer and healthier. For over a century, probiotics have been used in a large number of diseases, but with disappointing results (Quigley, 2010), even in CD (Orel, 2014). The rationale could be that providing one or a handful of strains may not be sufficient to restore a functionally compromised ecosystem (Figure 3D). As a matter of fact, it was recently shown that some probiotic strains delivered to the mothers failed to increase the microbiota diversity in their offspring's (Dotterud et al., 2015).
To restore the diversity, transplantation of fecal microbiota has been proposed. This drastic therapy, described in China as early as in the fourth century, was occasionally used as a treatment for severe and ATB resistant Clostridium difficile colitis during the twentieth century (Vrieze et al., 2013). Recently, the European Society of Clinical Microbiology and Infectious Diseases has included this approach for the treatment of CDI (Debast et al., 2014) (Figure 3E). In the same line, the “Repopulate” study proposed a cocktail of 33 species cultured from stools of a single donor. Two patients were effectively treated for CDI and the bacterial populations persisted in the recipient colon for over 6 months after the transplantation.
Alternatively, If our theory is true, an option could be to reintroduce predators in order to restore the microbial diversity and health.
Over the last few years, data linking LOMD and Human diseases associated with the westernized lifestyle has been accumulating. We hypothesize that the modern western lifestyle may be associated with a loss of predators within the microbiota ecosystem as demonstrated in macro-ecological models. According to this hypothesis, the lack of predation and LOMD could lead to an unstable microbial ecosystem. The consequent instability could favor the emergence of dysbiotic microbiota giving rise to immunological or metabolic diseases. If demonstrated, this point of view might bring a significant impact on understanding and treating these diseases.
AM, Hypothesis conception and drafting manuscript; ML, Critically revising the manuscript; JH, Critically revising the manuscript.
We acknowledge the financial support of the “Investissements d'Avenir programme ANR-11-IDEX-0005-02, Sorbonne Paris Cite,” Laboratoire d'excellence INFLAMEX, Inserm and Assistance Publique des Hôpitaux de Paris.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abrahamsson, T. R., Jakobsson, H. E., Andersson, A. F., Björkstén, B., Engstrand, L., and Jenmalm, M. C. (2014). Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 44, 842–850. doi: 10.1111/cea.12253
Ahn, J., Sinha, R., Pei, Z., Dominianni, C., Wu, J., Shi, J., et al. (2013). Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 105, 1907–1911. doi: 10.1093/jnci/djt300
Allison, S. D., and Martiny, J. B. H. (2008). Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. U.S.A. 105, 11512–11519. doi: 10.1073/pnas.0801925105
Amre, D. K., Lambrette, P., Law, L., Krupoves, A., Chotard, V., Costea, F., et al. (2006). Investigating the hygiene hypothesis as a risk factor in pediatric onset crohn's disease: a case-control study. Am. J. Gastroenterol. 101, 1005–1011. doi: 10.1111/j.1572-0241.2006.00526.x
Andersson, T., Ahlbom, A., Magnusson, C., and Carlsson, S. (2014). Prevalence and incidence of diabetes in stockholm county 1990-2010. PLoS ONE 9:e104033. doi: 10.1371/journal.pone.0104033
Atarashi, K., Tanoue, T., Shima, T., Imaoka, A., Kuwahara, T., Momose, Y., et al. (2011). Induction of colonic regulatory t cells by indigenous clostridium species. Science 331, 337–341. doi: 10.1126/science.1198469
Atladottir, H. O., Gyllenberg, D., Langridge, A., Sandin, S., Hansen, S. N., Leonard, H., et al. (2015). The increasing prevalence of reported diagnoses of childhood psychiatric disorders: a descriptive multinational comparison. Eur. Child Adolesc. Psychiatry 24, 173–183. doi: 10.1007/s00787-014-0553-8
Atterbury, R. J., Hobley, L., Till, R., Lambert, C., Capeness, M. J., Lerner, T. R., et al. (2011). Effects of orally administered Bdellovibrio bacteriovorus on the well-being and salmonella colonization of young chicks. Appl. Environ. Microbiol. 77, 5794–5803. doi: 10.1128/AEM.00426-11
Betrán, A. P., Merialdi, M., Lauer, J. A., Bing-Shun, W., Thomas, J., Van Look, P., et al. (2007). Rates of caesarean section: analysis of global, regional and national estimates. Paediatr. Perinat. Epidemiol. 21, 98–113. doi: 10.1111/j.1365-3016.2007.00786.x
Bhargava, P., and Mowry, E. M. (2014). Gut microbiome and multiple sclerosis. Curr. Neurol. Neurosci. Rep. 14:492. doi: 10.1007/s11910-014-0492-2
Bodansky, H. J., Staines, A., Stephenson, C., Haigh, D., and Cartwright, R. (1992). Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ 304, 1020–1022. doi: 10.1136/bmj.304.6833.1020
Buffie, C. G., Jarchum, I., Equinda, M., Lipuma, L., Gobourne, A., Viale, A., et al. (2012). Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to clostridium difficile-induced colitis. Infect. Immun. 80, 62–73. doi: 10.1128/IAI.05496-11
Butchart, S. H. M., Walpole, M., Collen, B., van Strien, A., Scharlemann, J. P. W., Almond, R. E. A., et al. (2010). Global biodiversity: indicators of recent declines. Science 328, 1164–1168. doi: 10.1126/science.1187512
Cahenzli, J., Köller, Y., Wyss, M., Geuking, M. B., and McCoy, K. D. (2013). Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570. doi: 10.1016/j.chom.2013.10.004
Carrière, J. (2014). Infectious etiopathogenesis of Crohn's disease. World J. Gastroenterol. 20:12102. doi: 10.3748/wjg.v20.i34.12102
Carroll, I. M., Ringel-Kulka, T., Siddle, J. P., and Ringel, Y. (2012). Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 24, 521–530. doi: 10.1111/j.1365-2982.2012.01891.x
Chang, J. Y., Antonopoulos, D. A., Kalra, A., Tonelli, A., Khalife, W. T., Schmidt, T. M., et al. (2008). Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 197, 435–438. doi: 10.1086/525047
Cosson, P., and Soldati, T. (2008). Eat, kill or die: when amoeba meets bacteria. Curr. Opin. Microbiol. 11, 271–276. doi: 10.1016/j.mib.2008.05.005
Costello, E. K., Stagaman, K., Dethlefsen, L., Bohannan, B. J. M., and Relman, D. A. (2012). The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262. doi: 10.1126/science.1224203
Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., et al. (2013). Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588. doi: 10.1038/nature12480
Creatore, M. I., Moineddin, R., Booth, G., Manuel, D. H., DesMeules, M., McDermott, S., et al. (2010). Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. Can. Med. Assoc. J. 182, 781–789. doi: 10.1503/cmaj.091551
Dashiff, A., Junka, R. A., Libera, M., and Kadouri, D. E. (2011). Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus: predation by M. aeruginosavorus and B. bacteriovorus. J. Appl. Microbiol. 110, 431–444. doi: 10.1111/j.1365-2672.2010.04900.x
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2013). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
Debast, S. B., Bauer, M. P., Kuijper, E. J., and on behalf of the Committee (2014). European society of clinical microbiology and infectious diseases: update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 20, 1–26. doi: 10.1111/1469-0691.12418
De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., et al. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U.S.A. 107, 14691–14696. doi: 10.1073/pnas.1005963107
De Goffau, M. C., Luopajarvi, K., Knip, M., Ilonen, J., Ruohtula, T., Harkonen, T., et al. (2013). Fecal microbiota composition differs between children with -cell autoimmunity and those without. Diabetes 62, 1238–1244. doi: 10.2337/db12-0526
Dethlefsen, L., Huse, S., Sogin, M. L., and Relman, D. A. (2008). The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6:e280. doi: 10.1371/journal.pbio.0060280
Dey, N., Soergel, D. A., Repo, S., and Brenner, S. E. (2013). Association of gut microbiota with post-operative clinical course in Crohn's disease. BMC Gastroenterol. 13:131. doi: 10.1186/1471-230X-13-131
Dolliver, H., and Gupta, S. (2008). Antibiotic losses in leaching and surface runoff from manure-amended agricultural land. J. Environ. Qual. 37, 1227–1237. doi: 10.2134/jeq2007.0392
Dotterud, C. K., Avershina, E., Sekelja, M., Simpson, M. R., Rudi, K., Storrø, O., et al. (2015). Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J. Pediatr. Gastroenterol. Nutr. 61, 200–207. doi: 10.1097/MPG.0000000000000781
Durbán, A., Abellán, J. J., Jiménez-Hernández, N., Salgado, P., Ponce, M., Ponce, J., et al. (2012). Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome: microbial diversity in irritable bowel syndrome. Environ. Microbiol. Rep. 4, 242–247. doi: 10.1111/j.1758-2229.2012.00327.x
Dwidar, M., Monnappa, A. K., and Mitchell, R. J. (2012). The dual probiotic and antibiotic nature of Bdellovibrio bacteriovorus. BMB Rep. 45, 71–78. doi: 10.5483/BMBRep.2012.45.2.71
Eder, W., Ege, M. J., and von Mutius, E. (2006). The asthma epidemic. N. Engl. J. Med. 355, 2226–2235. doi: 10.1056/NEJMra054308
Elmqvist, T., Folke, C., Nyström, M., Peterson, G., Bengtsson, J., Walker, B., et al. (2003). Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 1:488–494. doi: 10.1890/1540-9295(2003)001[0488:RDECAR]2.0.CO;2
Faust, K., and Raes, J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550. doi: 10.1038/nrmicro2832
Faust, K., Sathirapongsasuti, J. F., Izard, J., Segata, N., Gevers, D., Raes, J., et al. (2012). Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 8:e1002606. doi: 10.1371/journal.pcbi.1002606
Gallet, R., Tully, T., and Evans, M. E. K. (2009). Ecological conditions affect evolutionary trajectory in a predator-prey system. Evolution 63, 641–651. doi: 10.1111/j.1558-5646.2008.00559.x
Giongo, A., Gano, K. A., Crabb, D. B., Mukherjee, N., Novelo, L. L., Casella, G., et al. (2010). Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91. doi: 10.1038/ismej.2010.92
Graf, D., Di Cagno, R., Fåk, F., Flint, H. J., Nyman, M., Saarela, M., et al. (2015). Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26:26164. doi: 10.3402/mehd.v26.26164
Grüber, C., Illi, S., Plieth, A., Sommerfeld, C., and Wahn, U. (2002). Cultural adaptation is associated with atopy and wheezing among children of Turkish origin living in Germany. Clin. Exp. Allergy 32, 526–531. doi: 10.1046/j.0954-7894.2002.01331.x
Guarner, F. (2014). Decade in review—gut microbiota: the gut microbiota era marches on. Nat. Rev. Gastroenterol. Hepatol. 11, 647–649. doi: 10.1038/nrgastro.2014.156
Hammond, S. R., English, D. R., and McLeod, J. G. (2000). The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain J. Neurol. 123(Pt. 5), 968–974. doi: 10.1093/brain/123.5.968
Harjutsalo, V., Sjöberg, L., and Tuomilehto, J. (2008). Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 371, 1777–1782. doi: 10.1016/S0140-6736(08)60765-5
Holling, C. S. (1973). Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 4, 1–23. doi: 10.1146/annurev.es.04.110173.000245
Horton, D. B., Scott, F. I., Haynes, K., Putt, M. E., Rose, C. D., Lewis, J. D., et al. (2015). Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics 136, e333–e343. doi: 10.1542/peds.2015-0036
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551. doi: 10.1126/science.265.5178.1547
Hunt, D. E., and Ward, C. S. (2015). A network-based approach to disturbance transmission through microbial interactions. Front. Microbiol. 6:1182. doi: 10.3389/fmicb.2015.01182
Huttenhower, C., Gevers, D., Knight, R., Abubucker, S., Badger, J. H., Chinwalla, A. T., et al. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Hviid, A., Svanstrom, H., and Frisch, M. (2011). Antibiotic use and inflammatory bowel diseases in childhood. Gut 60, 49–54. doi: 10.1136/gut.2010.219683
Iebba, V., Santangelo, F., Totino, V., Nicoletti, M., Gagliardi, A., De Biase, R. V., et al. (2013). Higher prevalence and abundance of Bdellovibrio bacteriovorus in the human gut of healthy subjects. PLoS ONE 8:e61608. doi: 10.1371/annotation/b08ddcc9-dfdb-4fc1-b2ac-5a4af3051a91
Iebba, V., Totino, V., Santangelo, F., Gagliardi, A., Ciotoli, L., Virga, A., et al. (2014). Bdellovibrio bacteriovorus directly attacks Pseudomonas aeruginosa and Staphylococcus aureus Cystic fibrosis isolates. Front. Microbiol. 5:280. doi: 10.3389/fmicb.2014.00280
Ismail, I. H., Oppedisano, F., Joseph, S. J., Boyle, R. J., Licciardi, P. V., Robins-Browne, R. M., et al. (2012). Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high-risk infants: microbial diversity and infant eczema. Pediatr. Allergy Immunol. 23, 674–681. doi: 10.1111/j.1399-3038.2012.01328.x
Jakobsson, H. E., Abrahamsson, T. R., Jenmalm, M. C., Harris, K., Quince, C., Jernberg, C., et al. (2014). Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63, 559–566. doi: 10.1136/gutjnl-2012-303249
Jalanka-Tuovinen, J., Salonen, A., Nikkilä, J., Immonen, O., Kekkonen, R., Lahti, L., et al. (2011). Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE 6:e23035. doi: 10.1371/journal.pone.0023035
Jenq, R. R., Ubeda, C., Taur, Y., Menezes, C. C., Khanin, R., Dudakov, J. A., et al. (2012). Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 209, 903–911. doi: 10.1084/jem.20112408
Kadouri, D. E., To, K., Shanks, R. M. Q., and Doi, Y. (2013). Predatory bacteria: a potential ally against multidrug-resistant gram-negative pathogens. PLoS ONE 8:e63397. doi: 10.1371/journal.pone.0063397
Kang, D.-W., Park, J. G., Ilhan, Z. E., Wallstrom, G., LaBaer, J., Adams, J. B., et al. (2013). Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 8:e68322. doi: 10.1371/journal.pone.0068322
Koenig, J. E., Spor, A., Scalfone, N., Fricker, A. D., Stombaugh, J., Knight, R., et al. (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4578–4585. doi: 10.1073/pnas.1000081107
Kostic, A. D., Gevers, D., Siljander, H., Vatanen, T., Hyötyläinen, T., Hämäläinen, A.-M., et al. (2015). The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17, 260–273. doi: 10.1016/j.chom.2015.01.001
Kuehni, C. E., Strippoli, M.-P., Low, N., and Silverman, M. (2007). Asthma in young south Asian women living in the United Kingdom: the importance of early life. Clin. Exp. Allergy 37, 47–53. doi: 10.1111/j.1365-2222.2006.02627.x
Latvala, J., von Hertzen, L., Lindholm, H., and Haahtela, T. (2005). Trends in prevalence of asthma and allergy in Finnish young men: nationwide study, 1966-2003. BMJ 330, 1186–1187. doi: 10.1136/bmj.38448.603924.AE
Lehtinen, P., Ashorn, M., Iltanen, S., Jauhola, R., Jauhonen, P., Kolho, K.-L., et al. (2011). Incidence trends of pediatric inflammatory bowel disease in Finland, 1987-2003, a nationwide study. Inflamm. Bowel Dis. 17, 1778–1783. doi: 10.1002/ibd.21550
Lessios, H. A., Robertson, D. R., and Cubit, J. D. (1984). Spread of diadema mass mortality through the Caribbean. Science 226, 335–337. doi: 10.1126/science.226.4672.335
Letnic, M., Ritchie, E. G., and Dickman, C. R. (2012). Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol. Rev. 87, 390–413. doi: 10.1111/j.1469-185X.2011.00203.x
Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R., and Gordon, J. I. (2008). Worlds within worlds: evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 6, 776–788. doi: 10.1038/nrmicro1978
Lin, A., Bik, E. M., Costello, E. K., Dethlefsen, L., Haque, R., Relman, D. A., et al. (2013). Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 8:e53838. doi: 10.1371/journal.pone.0053838
Lohi, S., Mustalahti, K., Kaukinen, K., Laurila, K., Collin, P., Rissanen, H., et al. (2007). Increasing prevalence of coeliac disease over time: increasing Prevalence Of Coeliac Disease. Aliment. Pharmacol. Ther. 26, 1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x
Loozen, G., Boon, N., Pauwels, M., Slomka, V., Rodrigues Herrero, E., Quirynen, M., et al. (2015). Effect of Bdellovibrio bacteriovorus HD100 on multispecies oral communities. Anaerobe 35, 45–53. doi: 10.1016/j.anaerobe.2014.09.011
Lovell, R. M., and Ford, A. C. (2012). Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4. doi: 10.1016/j.cgh.2012.02.029
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., and Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. doi: 10.1038/nature11550
Manichanh, C., Rigottier-Gois, L., Bonnaud, E., Gloux, K., Pelletier, E., Frangeul, L., et al. (2006). Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55, 205–211. doi: 10.1136/gut.2005.073817
Martínez, I., Stegen, J. C., Maldonado-Gómez, M. X., Eren, A. M., Siba, P. M., Greenhill, A. R., et al. (2015). The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep. 11, 527–538. doi: 10.1016/j.celrep.2015.03.049
Martinez, J. L. (2009). Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 157, 2893–2902. doi: 10.1016/j.envpol.2009.05.051
Matsuoka, K., and Kanai, T. (2015). The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 37, 47–55. doi: 10.1007/s00281-014-0454-4
Mayr, W. T., Pittock, S. J., McClelland, R. L., Jorgensen, N. W., Noseworthy, J. H., and Rodriguez, M. (2003). Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985-2000. Neurology 61, 1373–1377. doi: 10.1212/01.WNL.0000094316.90240.EB
McFall-Ngai, M. (2007). Adaptive immunity: care for the community. Nature 445, 153–153. doi: 10.1038/445153a
McFall-Ngai, M., Hadfield, M. G., Bosch, T. C., Carey, H. V., Domazet-Lošo, T., Douglas, A. E., et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. U.S.A. 110, 3229–3236. doi: 10.1073/pnas.1218525110
Michail, S., Durbin, M., Turner, D., Griffiths, A. M., Mack, D. R., Hyams, J., et al. (2012). Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 18, 1799–1808. doi: 10.1002/ibd.22860
Mills, S., Shanahan, F., Stanton, C., Hill, C., Coffey, A., and Ross, R. P. (2013). Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes 4, 4–16. doi: 10.4161/gmic.22371
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42. doi: 10.1053/j.gastro.2011.10.001
Monnappa, A. K., Dwidar, M., Seo, J. K., Hur, J.-H., and Mitchell, R. J. (2014). Bdellovibrio bacteriovorus inhibits staphylococcus aureus biofilm formation and invasion into human epithelial cells. Sci. Rep. 4:3811. doi: 10.1038/srep03811
Morton, E. R., Lynch, J., Froment, A., Lafosse, S., Heyer, E., Przeworski, M., et al. (2015). Variation in rural african gut microbiota is strongly correlated with colonization by entamoeba and subsistence. PLoS Genet. 11:e1005658. doi: 10.1371/journal.pgen.1005658
Ng, S. C., Bernstein, C. N., Vatn, M. H., Lakatos, P. L., Loftus, E. V., Tysk, C., et al. (2013). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 62, 630–649. doi: 10.1136/gutjnl-2012-303661
Nyström, M., Folke, C., and Moberg, F. (2000). Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 15, 413–417. doi: 10.1016/S0169-5347(00)01948-0
Ochman, H., Worobey, M., Kuo, C.-H., Ndjango, J.-B. N., Peeters, M., Hahn, B. H., et al. (2010). Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 8:e1000546. doi: 10.1371/journal.pbio.1000546
OECD. (2013). “Caesarean sections,” in Health at a Glance (Organisation for Economic Co-operation and Development), 98–99. Available online at: http://www.oecd-ilibrary.org/content/chapter/health_glance-2013-39-en (Accessed September 9, 2014).
Orel, R. (2014). Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 20:11505. doi: 10.3748/wjg.v20.i33.11505
Paine, R. (1966). Food web complexity and species diversity. Am. Nat. 100, 65–75. doi: 10.1086/282400
Pérez-Cobas, A. E., Artacho, A., Knecht, H., Ferrús, M. L., Friedrichs, A., Ott, S. J., et al. (2013). Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS ONE 8:e80201. doi: 10.1371/journal.pone.0080201
Petersen, C., and Round, J. L. (2014). Defining dysbiosis and its influence on host immunity and disease: how changes in microbiota structure influence health. Cell. Microbiol. 16, 1024–1033. doi: 10.1111/cmi.12308
Pinheiro, P. S., Sherman, R. L., Trapido, E. J., Fleming, L. E., Huang, Y., Gomez-Marin, O., et al. (2009). Cancer Incidence in First Generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and New Latinos. Cancer Epidemiol. Biomarkers Prev. 18, 2162–2169. doi: 10.1158/1055-9965.EPI-09-0329
Quigley, E. M. M. (2010). Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol. Res. 61, 213–218. doi: 10.1016/j.phrs.2010.01.004
Reyes, A., Haynes, M., Hanson, N., Angly, F. E., Heath, A. C., Rohwer, F., et al. (2010). Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 466, 334–338. doi: 10.1038/nature09199
Reyes, A., Wu, M., McNulty, N. P., Rohwer, F. L., and Gordon, J. I. (2013). Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc. Natl. Acad. Sci.U.S.A. 110, 20236–20241. doi: 10.1073/pnas.1319470110
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world's largest carnivores. Science 343, 1241484. doi: 10.1126/science.1241484
Rodriguez-Valera, F., Martin-Cuadrado, A.-B., Rodriguez-Brito, B., Pašić, L., Thingstad, T. F., Rohwer, F., et al. (2009). Explaining microbial population genomics through phage predation. Nat. Rev. Microbiol. 7, 828–836. doi: 10.1038/nrmicro2235
Ronn, R., McCaig, A. E., Griffiths, B. S., and Prosser, J. I. (2002). Impact of protozoan grazing on bacterial community structure in soil microcosms. Appl. Environ. Microbiol. 68, 6094–6105. doi: 10.1128/AEM.68.12.6094-6105.2002
Russell, S. L., Gold, M. J., Hartmann, M., Willing, B. P., Thorson, L., Wlodarska, M., et al. (2012). Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447. doi: 10.1038/embor.2012.32
Sarmah, A. K., Meyer, M. T., and Boxall, A. B. A. (2006). A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65, 725–759. doi: 10.1016/j.chemosphere.2006.03.026
Schaubeck, M., Clavel, T., Calasan, J., Lagkouvardos, I., Haange, S. B., Jehmlich, N., et al. (2015). Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence. Gut 65, 225–237. doi: 10.1136/gutjnl-2015-309333
Schippa, S., Iebba, V., Barbato, M., Di Nardo, G., Totino, V., Checchi, M. P., et al. (2010). A distinctive “microbial signature” in celiac pediatric patients. BMC Microbiol. 10:175. doi: 10.1186/1471-2180-10-175
Schnorr, S. L., Candela, M., Rampelli, S., Centanni, M., Consolandi, C., Basaglia, G., et al. (2014). Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 5:3654. doi: 10.1038/ncomms4654
Schwartz, B. S., Pollak, J., Bailey-Davis, L., Hirsch, A. G., Cosgrove, S. E., Nau, C., et al. (2015). Antibiotic use and childhood body mass index trajectory. Int. J. Obes. doi: 10.1038/ijo.2015.218. [Epub ahead of print].
Sha, S., Liang, J., Chen, M., Xu, B., Liang, C., Wei, N., et al. (2014). Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment. Pharmacol. Ther. 39, 1003–1032. doi: 10.1111/apt.12699
Sha, S., Xu, B., Wang, X., Zhang, Y., Wang, H., Kong, X., et al. (2013). The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn. Microbiol. Infect. Dis. 75, 245–251. doi: 10.1016/j.diagmicrobio.2012.11.022
Shade, A., Peter, H., Allison, S. D., Baho, D. L., Berga, M., Bürgmann, H., et al. (2012). Fundamentals of microbial community resistance and resilience. Front. Microbiol. 3:417. doi: 10.3389/fmicb.2012.00417
Shanks, R. M. Q., Davra, V. R., Romanowski, E. G., Brothers, K. M., Stella, N. A., Godboley, D., et al. (2013). An eye to a kill: using predatory bacteria to control gram-negative pathogens associated with ocular infections. PLoS ONE 8:e66723. doi: 10.1371/journal.pone.0066723
Shaw, S. Y., Blanchard, J. F., and Bernstein, C. N. (2010). Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 105, 2687–2692. doi: 10.1038/ajg.2010.398
Simpson, H. L., and Campbell, B. J. (2015). Review article: dietary fibre–microbiota interactions. Aliment. Pharmacol. Ther. 42, 158–179. doi: 10.1111/apt.13248
Sockett, R. E., and Lambert, C. (2004). Bdellovibrio as therapeutic agents: a predatory renaissance? Nat. Rev. Microbiol. 2, 669–675. doi: 10.1038/nrmicro959
Stein, R. R., Bucci, V., Toussaint, N. C., Buffie, C. G., Rätsch, G., Pamer, E. G., et al. (2013). Ecological modeling from time-series inference: insight into dynamics and stability of intestinal microbiota. PLoS Comput. Biol. 9:e1003388. doi: 10.1371/journal.pcbi.1003388
Stewart, C. J., Marrs, E. C. L., Nelson, A., Lanyon, C., Perry, J. D., Embleton, N. D., et al. (2013). Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS ONE 8:e73465. doi: 10.1371/journal.pone.0073465
Stolp, H., and Starr, M. P. (1963). Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek 29, 217–248. doi: 10.1007/BF02046064
Strauch, E., Schwudke, D., and Linscheid, M. (2007). Predatory mechanisms of Bdellovibrio and like organisms. Future Microbiol. 2, 63–73. doi: 10.2217/17460913.2.1.63
Suau, A., Bonnet, R., Sutren, M., Godon, J.-J., Gibson, G. R., Collins, M. D., et al. (1999). Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65, 4799–4807.
Szpak, P., Orchard, T. J., Salomon, A. K., and Gröcke, D. R. (2013). Regional ecological variability and impact of the maritime fur trade on nearshore ecosystems in southern Haida Gwaii (British Columbia, Canada): evidence from stable isotope analysis of rockfish (Sebastes spp.) bone collagen. Archaeol. Anthropol. Sci. 5, 159–182. doi: 10.1007/s12520-013-0122-y
Tagliabue, A., and Elli, M. (2013). The role of gut microbiota in human obesity: recent findings and future perspectives. Nutr. Metab. Cardiovasc. Dis. 23, 160–168. doi: 10.1016/j.numecd.2012.09.002
Thaiss, C. A., Zeevi, D., Levy, M., Zilberman-Schapira, G., Suez, J., Tengeler, A. C., et al. (2014). Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529. doi: 10.1016/j.cell.2014.09.048
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540
Ungaro, R., Bernstein, C. N., Gearry, R., Hviid, A., Kolho, K.-L., Kronman, M. P., et al. (2014). Antibiotics associated with increased risk of new-onset crohn's disease but not ulcerative colitis: a meta-analysis. Am. J. Gastroenterol. 109, 1728–1738. doi: 10.1038/ajg.2014.246 (Accessed November 6, 2014).
Van Essche, M., Quirynen, M., Sliepen, I., Loozen, G., Boon, N., Van Eldere, J., et al. (2011). Killing of anaerobic pathogens by predatory bacteria: bacterial predation on anaerobic pathogens. Mol. Oral Microbiol. 26, 52–61. doi: 10.1111/j.2041-1014.2010.00595.x
Vellend, M. (2010). Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206. doi: 10.1086/652373
Vrieze, A., de Groot, P. F., Kootte, R. S., Knaapen, M., van Nood, E., and Nieuwdorp, M. (2013). Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract. Res. Clin. Gastroenterol. 27, 127–137. doi: 10.1016/j.bpg.2013.03.003
Vrieze, A., Van Nood, E., Holleman, F., Salojärvi, J., Kootte, R. S., Bartelsman, J. F. W. M., et al. (2012). Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7. doi: 10.1053/j.gastro.2012.06.031
Wang, M., Karlsson, C., Olsson, C., Adlerberth, I., Wold, A. E., Strachan, D. P., et al. (2008). Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 121, 129–134. doi: 10.1016/j.jaci.2007.09.011
Wier, A. M., Nyholm, S. V., Mandel, M. J., Massengo-Tiassé, R. P., Schaefer, A. L., Koroleva, I., et al. (2010). Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc. Natl. Acad. Sci. U.S.A. 107, 2259–2264. doi: 10.1073/pnas.0909712107
Wu, G. D., Compher, C., Chen, E. Z., Smith, S. A., Shah, R. D., Bittinger, K., et al. (2016). Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65, 63–72. doi: 10.1136/gutjnl-2014-308209
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486, 222–227. doi: 10.1038/nature11053
Zeissig, S., and Blumberg, R. S. (2014). Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat. Immunol. 15, 307–310. doi: 10.1038/ni.2847
Zelezniak, A., Andrejev, S., Ponomarova, O., Mende, D. R., Bork, P., and Patil, K. R. (2015). Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. U.S.A. 112, 6449–6454. doi: 10.1073/pnas.1421834112
Zhao, J., Murray, S., and LiPuma, J. J. (2014). Modeling the impact of antibiotic exposure on human microbiota. Sci. Rep. 4:4345. doi: 10.1038/srep04345
- Intestinal dysbiosis: Distinct definitions of dysbiosis can be found from the literature. In practice, dysbiosis refers to microbiota that differs from the one of healthy people. We chose the definition of Petersen and Round (Petersen and Round, 2014) because it seems relevant here since it is linked to its impact on host immunity. According to, dysbiosis can be defined by three main features, (i) a loss of beneficial microbial organisms, (ii) the expansion of pathobionts, and (iii) a loss of microbial diversity.
- Diversity metrics:
- Richness: Number of taxonomic units (e.g., species) in a community, standardized to the number of individuals sampled.
- Alpha diversity: An inventory metric that expresses the amount of local diversity or diversity at the smallest spatial scale of analysis (e.g., Intra individual gut microbiota diversity). Assessed by Shannon or Simplon index for example. LOMD refers to alpha diversity.
- Beta diversity: A differentiation diversity metric that refers to turnover at the landscape scale, i.e., turnover between local populations (e.g., inter-individual gut microbiota diversity within the same population): not used in this paper.
- Gamma diversity: is a similar metric used to refer to diversity at the regional scale (e.g., between different population: westernized vs. non westernized): not used in this paper).
- Community: A group of potentially interacting species that co-occur in space and time (e.g., gut microbiota).
-Resilience and stability: The stability of an ecosystem is defined as a community's response to disturbance. It depends on the resistance (insensitivity to disturbance) and the resilience of the ecosystem (Shade et al., 2012). The resilience is the rate at which a microbial community returns to its original composition after being disturbed (Allison and Martiny, 2008). Then, it represents a measure of the ability of this system to absorb changes and still persist. In this definition, resilience is the property of the system and persistence or probability of extinction is the result (Holling, 1973).
Keywords: dysbiosis, ecosystem, predator, western lifestyle, chronic human conditions, Bdellovibrio bacteriovorus
Citation: Mosca A, Leclerc M and Hugot JP (2016) Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 7:455. doi: 10.3389/fmicb.2016.00455
Received: 16 November 2015; Accepted: 21 March 2016;
Published: 31 March 2016.
Edited by:
Lois Maignien, University of Western Brittany, FranceReviewed by:
Damien Eveillard, Université de Nantes, FranceCopyright © 2016 Mosca, Leclerc and Hugot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexis Mosca, YWxleGlzLm1vc2NhQGFwaHAuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.