- 1Department of Microbiology, School of Life Sciences, Sikkim University, Tadong, India
- 2Department of Animal Science and Technology, National Taiwan University, Taipei, Taiwan
- 3Advance Green Energy and Environment Institute, Handong Global University, Pohang-si, South Korea
Culturalable and non-culturable microorganisms naturally ferment majority of global fermented foods and beverages. Traditional food fermentation represents an extremely valuable cultural heritage in most regions, and harbors a huge genetic potential of valuable but hitherto undiscovered strains. Holistic approaches for identification and complete profiling of both culturalable and non-culturable microorganisms in global fermented foods are of interest to food microbiologists. The application of culture-independent technique has thrown new light on the diversity of a number of hitherto unknown and non-cultural microorganisms in naturally fermented foods. Functional bacterial groups (“phylotypes”) may be reflected by their mRNA expression in a particular substrate and not by mere DNA-level detection. An attempt has been made to review the microbiology of some fermented foods and alcoholic beverages of the world.
Introduction
Traditionally, boiled rice is a staple diet with fermented and non-fermented legume (mostly soybeans) products, vegetables, pickles, fish, and meat in Far-East Asia, South Asia, North Asia, and the Indian subcontinent excluding Western and Northern India; while wheat/barley-based breads/loaves comprise a staple diet followed by milk and fermented milk products, meat, and fermented meats (sausages) in the Western and Northern part of India, West Asian continent, Europe, North America, and even in Australia and New Zealand (Tamang and Samuel, 2010). Sorghum/maize porridges, on the other hand, are the main courses of diet with many fermented and non-fermented sorghum/maize/millets, cassava, wild legume seeds, meat, and milk products in Africa and South America. Fermented foods are the hub of consortia of microorganisms, since they are either present as natural indigenous microbiota in uncooked plant or animal substrates, utensils, containers, earthen pots, and the environment (Hesseltine, 1979; Franz et al., 2014), or add starter culture(s) containing functional microorganisms (Holzapfel, 1997; Stevens and Nabors, 2009) which modify the substrates biochemically, and organoleptically into edible products that are culturally and socially acceptable to the consumers (Campbell-Platt, 1994; Steinkraus, 1997; Tamang, 2010b). Microorganisms convert the chemical composition of raw materials during fermentation, which enrich the nutritional value in some fermented foods, and impart health-benefits to the consumers (Steinkraus, 2002; Farhad et al., 2010; Tamang, 2015a).
Several researchers have reviewed the microbiology, biochemistry, and nutrition of fermented foods and beverages from different countries of Asia (Hesseltine, 1983; Steinkraus, 1994, 1996; Nout and Aidoo, 2002; Tamang et al., 2015); Africa (Odunfa and Oyewole, 1997; Olasupo et al., 2010; Franz et al., 2014); Europe (Pederson, 1979; Campbell-Platt, 1987; Wood, 1998); South America (Chaves-López et al., 2014), and North America (Doyle and Beuchat, 2013). Many genera/species of microorganisms have been reported in relation to various fermented foods and beverages across the world; the usage of molecular tools in recent years have helped to clarify, at least in part, the nomenclatural confusion and generalization caused by conventional (phenotypic) taxonomic methods. The present paper is an attempt to collate and review the updated information on microbiology of some globally fermented foods and beverages.
Microorganisms in Fermented Foods
Lactic acid bacteria (LAB) are widely present in many fermented foods and beverages (Stiles and Holzapfel, 1997; Tamang, 2010b). Major genera of the LAB such as Alkalibacterium, Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus, and Weissella (Salminen et al., 2004; Axelsson et al., 2012; Holzapfel and Wood, 2014) have been isolated from various globally fermented foods and beverages.
Bacillus is present in alkaline-fermented foods of Asia and Africa (Parkouda et al., 2009; Tamang, 2015b). Species of Bacillus that are present, mostly in legume-based fermented foods, are Bacillus amyloliquefaciens, Bacillus circulans, Bacillus coagulans, Bacillus firmus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus, Bacillus subtilis, Bacillus subtilis variety natto, and Bacillus thuringiensis (Kiers et al., 2000; Kubo et al., 2011), while strains of Bacillus cereus have been isolated from the fermentation of Prosopis africana seeds for the production of okpehe in Nigeria (Oguntoyinbo et al., 2007). Some strains of B. subtilis produce λ-polyglutamic acid (PGA) which is an amino acid polymer commonly present in Asian fermented soybean foods, giving the characteristic of a sticky texture to the product (Urushibata et al., 2002; Nishito et al., 2010).
The association of several species of Kocuria, Micrococcus (members of the Actinobacteria), and Staphylococcus (belonging to the Firmicutes) has been reported for fermented milk products, fermented sausages, meat, and fish products (Martín et al., 2006; Coton et al., 2010). Species of Bifidobacterium, Brachybacterium, Brevibacterium, and Propionibacterium are isolated from cheese, and species of Arthrobacter and Hafnia from fermented meat products (Bourdichon et al., 2012). Enterobacter cloacae, Klebsiella pneumoniae, K. pneumoniae subsp. ozaenae, Haloanaerobium, Halobacterium, Halococcus, Propionibacterium, Pseudomonas, etc. are also present in many global fermented foods (Tamang, 2010b).
Genera of yeasts reported for fermented foods, alcoholic beverages and non-food mixed amylolytic starters are mostly Brettanomyces, Candida, Cryptococcus, Debaryomyces, Dekkera, Galactomyces, Geotrichum, Hansenula, Hanseniaspora, Hyphopichia, Issatchenkia, Kazachstania, Kluyveromyces, Metschnikowia, Pichia, Rhodotorula, Rhodosporidium, Saccharomyces, Saccharomycodes, Saccharomycopsis, Schizosaccharomyces, Sporobolomyces, Torulaspora, Torulopsis, Trichosporon, Yarrowia, and Zygosaccharomyces (Watanabe et al., 2008; Tamang and Fleet, 2009; Lv et al., 2013).
Major role of filamentous molds in fermented foods and alcoholic beverages is the production of enzymes and the degradation of anti-nutritive factors (Aidoo and Nout, 2010). Species of Actinomucor, Amylomyces, Aspergillus, Monascus, Mucor, Neurospora, Parcilomyces, Penicillium, Rhizopus, and Ustilago are reported for many fermented foods, Asian non-food amylolytic starters and alcoholic beverages (Nout and Aidoo, 2002; Chen et al., 2014).
Taxonomic Tools for Identification of Microorganisms from Fermented Foods
Use of culture media may ignore several unknown non-culturable microorganisms that may play major or minor functional roles in production of fermented foods. Direct DNA extraction from samples of fermented foods, commonly known as culture-independent methods, is nowadays frequently used in food microbiology to profile both culturable and non-culturable microbial populations from fermented foods (Cocolin and Ercolini, 2008; Alegría et al., 2011; Cocolin et al., 2013; Dolci et al., 2015), provided that the amplification efficiency is high enough. PCR-DGGE analysis is the most popular culture-independent technique used for detecting microorganisms in fermented foods and thereby profiling both bacterial populations (Cocolin et al., 2011; Tamang, 2014) and yeast populations in fermented foods (Cocolin et al., 2002; Jianzhonga et al., 2009). Both culturable and non-culturable microorganisms from any fermented food and beverage may be identified using culture-dependent and -independent methods to document a complete profile of microorganisms, and also to study both inter- and intra-species diversity within a particular genus or among genera (Ramos et al., 2010; Greppi et al., 2013a,b; Yan et al., 2013). A combination of Propidium MonoAzide (PMA) treatment on samples before DNA extraction and molecular quantifying method can be used to accurately enumerate the viable microorganisms in fermented foods (Desfossés-Foucault et al., 2012; Fujimoto and Watanabe, 2013).
Molecular identification is emerging as an accurate and reliable identification tool, and is widely used in identification of both culture-dependent and culture-independent microorganisms from fermented foods (Giraffa and Carminati, 2008; Dolci et al., 2015). Species-specific PCR primers are used for species level identification (Tamang et al., 2005); this technique is widely applied in the identification of LAB isolated from fermented foods (Robert et al., 2009). The application of real-time quantitative PCR (qPCR) with specific primers enables the specific detection and quantification of LAB species in fermented foods (Park et al., 2009).
Random amplification of polymorphic DNA (RAPD) is a typing method based on the genomic DNA fragment profiles amplified by PCR, and is commonly used for disintegration of LAB strains from fermented foods (Coppola et al., 2006; Chao et al., 2008). The repetitive extragenic palindromic sequence-based PCR (rep-PCR) technique permits typing at subspecies level and reveals significant genotypic differences among strains of the same bacterial species from fermented food samples (Tamang et al., 2008). Amplified fragment length polymorphism (AFLP) is a technique based on the selective amplification and separation of genomic restriction fragments, and its applicability in identification and to discriminate has been demonstrated for various LAB strains (Tanigawa and Watanabe, 2011).
Techniques of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) have been developed to profile microbial communities directly from fermented foods, and are based on sequence-specific distinctions of 16S rDNA and 26S rDNA amplicons produced by PCR (Ercolini, 2004; Flórez and Mayo, 2006; Alegría et al., 2011). However, DGGE has some disadvantages as well like it is time consuming, unable to determine the relative abundance of dominant species and distinguish between viable and nonviable cells, as well as it has difficulties in interpretation of multi-bands (Dolci et al., 2015). DGGE is also limited to detect specific species as it may only reveal some of the major bacterial species such as B. licheniformis and Bacillus thermoamylovorans in chungkokjang (sticky fermented soybean food of Korea) and not detect a large number of predominant or diverse rare bacterial species identified in pyrosequencing analysis (Nam et al., 2011).
The amplified ribosomal DNA restriction analysis (ARDRA) technique using restriction enzymes is also useful in identification of microorganisms from fermented foods (Jeyaram et al., 2010).
Multilocus sequence analysis (MLSA), using housekeeping genes as molecular markers alternative to the 16S rRNA genes, is used for LAB species identification: rpoA and pheS genes for Enterococcus and Lactobacillus, atpA and pepN for Lactococcus species, and dnaA, gyrB, and rpoC for species of Leuconostoc, Oenococcus, and Weissella (de Bruyne et al., 2007, 2008b, 2010; Diancourt et al., 2007; Picozzi et al., 2010; Tanigawa and Watanabe, 2011).
Effective tools of next generation sequencing (NGS) such as metagenomics, phylobiomics, and metatranscriptomics are nowadays applied for documentation of cultures in traditionally fermented products (Mozzi et al., 2013; van Hijum et al., 2013). However, NGS as a sophisticated tool needs well-trained hands and a well-equipped molecular laboratory, which may not always be available. Application of metagenomic approaches, by using parallel pyrosequencing of tagged 16S rRNA gene amplicons, provide information on microbial communities as profiled in kimchi, a naturally fermented vegetable product of Korea (Jung et al., 2011; Park et al., 2012), nukadoko, a fermented rice bran of Japan (Sakamoto et al., 2011), narezushi, a fermented salted fish and cooked rice of Japan (Kiyohara et al., 2012), and ben-saalga, a traditional gruel of pearl millet of Burkina Faso (Humblot and Guyot, 2009). Pyrosequencing has revealed the presence of numerous and even minor bacterial groups in fermented foods, but DNA-level detection does not distinguish between metabolically “active” and “passive” organisms. “Functionally relevant phylotypes” in an ecosystem may be specifically detected by, e.g., weighted UniFrac principal coordinate analysis based on 454 pyrosequencing of 16S rRNA genes, as applied in studies on gut microbiota (Wang et al., 2015). The 16S rRNA gene sequence based pyrosequencing method enables a comprehensive and high-throughput analysis of microbial ecology (Sakamoto et al., 2011), and this method has been applied to various traditionally fermented foods (Oki et al., 2014).
A proteomics identification method based on protein profiling using matrix-assisted laser desorption ionizing-time of flight mass spectrometry (MALDI-TOF MS) has been used to identify species of Bacillus in fermented foods of Africa (Savadogo et al., 2011), and species of LAB isolated from global fermented foods (Tanigawa et al., 2010; Dušková et al., 2012; Sato et al., 2012; Nguyen et al., 2013a; Kuda et al., 2014).
Global Fermented Foods
Campbell-Platt (1987) reported around 3500 global fermented foods and beverages, and had divided them into about 250 groups. There might be more than 5000 varieties of common and uncommon fermented foods and alcoholic beverages being consumed in the world today by billions of people, as staple and other food components (Tamang, 2010b). Global fermented foods are classified into nine major groups on the basis of substrates (raw materials) used from plant/animal sources: (1) fermented cereals, (2) fermented vegetables and bamboo shoots, (3) fermented legumes, (4) fermented roots/tubers, (5) fermented milk products, (6) fermented and preserved meat products, (7) fermented, dried and smoked fish products, (8) miscellaneous fermented products, and (9) alcoholic beverages (Steinkraus, 1997; Tamang, 2010b,c).
Fermented Milk Products
Fermented milk products (Table 1) are classified into two major groups on the basis of microorganisms: (A) lactic fermentation, dominated by species of LAB, comprising the “thermophilic” type (e.g., yogurt, Bulgarian buttermilk), probiotic type (e.g., acidophilus milk, bifidus milk), and the mesophilic type (e.g., natural fermented milk, cultured milk, cultured cream, cultured buttermilk); and (B) fungal-lactic fermentations, where LAB and yeasts cooperate to generate the final product, which include alcoholic milks (e.g., acidophilus-yeast milk, kefir, koumiss), and moldy milks (e.g., viili; Mayo et al., 2010). Natural fermentation is one of the oldest methods of milk processing using raw and boiled milk to ferment spontaneously, or of using the back-slopping method where a part of the previous batch of a fermented product is used to inoculate the new batch (Holzapfel, 2002; Josephsen and Jespersen, 2004). Cheese and cheese products derived from the fermentation of milk are of major nutritional and commercial importance throughout the world (de Ramesh et al., 2006). Starter cultures in milk fermentation are of two types: primary cultures that are mostly Lactococcus lactis subsp. cremoris, Lc. lactis subsp. lactis, Lactobacillus delbrueckii subsp. delbrueckii, Lb. delbrueckii subsp. lactis, Lb. helveticus, Leuconostoc spp., and Streptococcus thermophilus to participate in the acidification (Parente and Cogan, 2004); and secondary cultures that are used in cheese-making are Brevibacterium linens, Propionibacterium freudenreichii, Debaryomyces hansenii, Geotrichum candidum, Penicillium camemberti, and P. roqueforti for development of flavor and texture during ripening of cheese (Coppola et al., 2006; Quigley et al., 2011). Some non-starter lactic acid bacteria (NSLAB) microbiota are usually present in high numbers in fermented milk, which include Enterococcus durans, Ent. faecium, Lb. casei, Lb. plantarum, Lb. salivarius, and Staphylococcus spp. (Briggiler-Marcó et al., 2007).
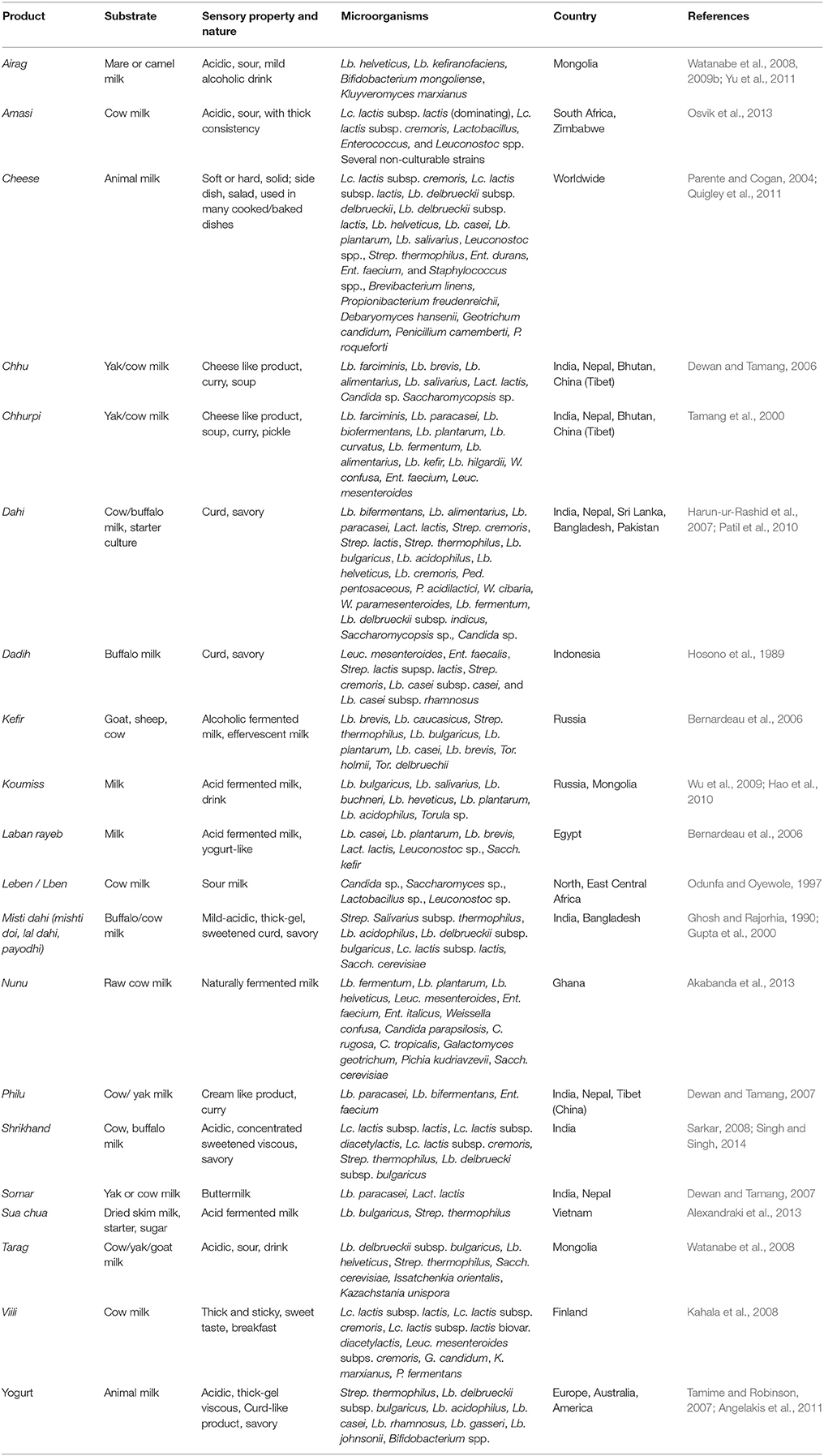
Table 1. Microorganisms isolated from some common and uncommon fermented milk products of the world.
Fermented Cereal Foods
In most of the Asian countries, rice is fermented either by using mixed-culture(s) into alcoholic beverages or by using food beverages (Tamang, 2010c), whereas in Europe, America, and Australia, most cereals like wheat, rye, barley and maize are fermented by natural fermentation or by adding commercial baker's yeast into the batter for dough breads/loaves (Guyot, 2010). In Africa, fermented cereal foods are traditionally used as staples as well as complementary and weaning foods for infants and young children (Tou et al., 2007). In Europe, people still practice the old traditional method of preparation of breads or loaves without using any commercial strains of baker's yeast (Hammes and Ganzle, 1998). Yeasts and LAB conduct dough fermentation, mostly San Francisco sourdough, and the resultant products are generally called sourdough breads because they have higher contents of lactic acid and acetic acid due to the bacterial growth (Brandt, 2007; de Vuyst et al., 2009).
Cereal fermentation is mainly represented by species of LAB and yeasts (Corsetti and Settanni, 2007). Enterococcus, Lactococcus, Lactobacillus, Leuconostoc, Pediococcus, Streptococcus, and Weissella are common bacteria associated with cereal fermentations (Table 2; de Vuyst et al., 2009; Guyot, 2010; Moroni et al., 2011). Native strains of Saccharomyces cerevisiae are the principal yeast of most bread fermentations (Hammes et al., 2005), but other non-Saccharomyces yeasts are also significant in many cereal fermentations including Candida, Debaryomyces, Hansenula, Kazachstania, Pichia, Trichosporon, and Yarrowia (Iacumin et al., 2009; Weckx et al., 2010; Johnson and Echavarri-Erasun, 2011).
Fermented Vegetable Foods
Perishable and seasonal leafy vegetables, radish, cucumbers including young edible bamboo tender shoots are traditionally fermented into edible products (Table 3). Fermentation of vegetables is mostly dominated by species of Lactobacillus and Pediococcus, followed by Leuconostoc, Weissella, Tetragenococcus, and Lactococcus (Chang et al., 2008; Watanabe et al., 2009a). A complete microbial profile of LAB in kimchi has been characterized using different molecular identification tools (Shin et al., 2008; Nam et al., 2009; Park et al., 2010; Jung et al., 2011, 2013a). Natural fermentations during production of sauerkraut, a fermented cabbage product of Germany, had been studied and a species of LAB were reported. (Johanningsmeier et al., 2007; Plengvidhya et al., 2007). Species of LAB constitute the native population in the Himalayan fermented vegetable products such as gundruk, sinki, goyang, khalpi, and inziangsang (Karki et al., 1983; Tamang et al., 2005, 2009; Tamang and Tamang, 2007, 2010) and in several naturally fermented bamboo products of India and Nepal (Tamang and Sarkar, 1996; Tamang et al., 2008; Tamang and Tamang, 2009; Jeyaram et al., 2010; Sonar and Halami, 2014).
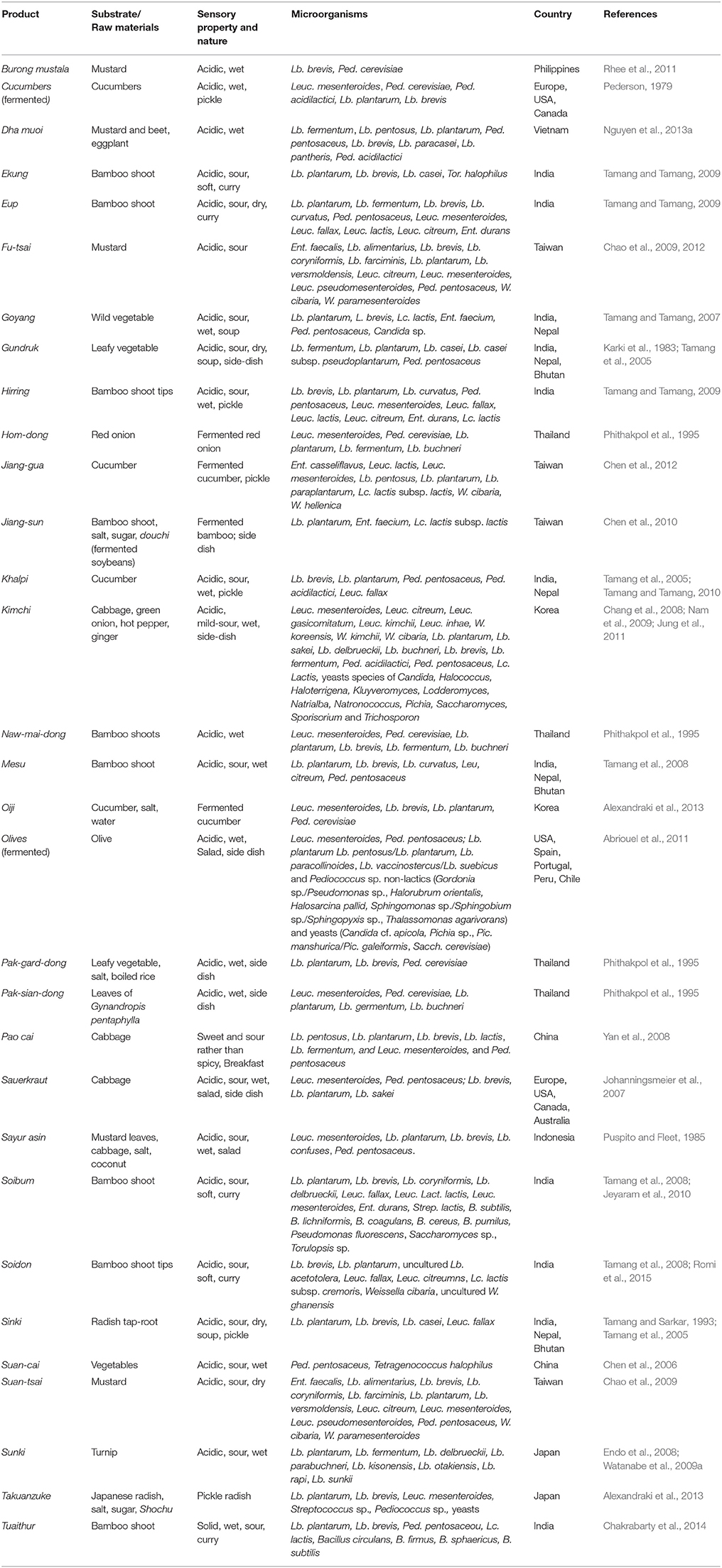
Table 3. Microorganisms isolated from some common and uncommon fermented vegetable products of the world.
Fermented Soybeans and Other Legumes
Two types of fermented soybean foods are produced: soybean foods fermented by Bacillus spp. (mostly B. subtilis) with the stickiness characteristic, and soybean foods fermented by filamentous molds, mostly Aspergillus, Mucor, Rhizopus (Tamang, 2010b). Bacillus-fermented, non-salty and sticky soybean foods are concentrated in an imaginary triangle with three vertices lying each on Japan (natto), east Nepal and north-east India (kinema and its similar products), and northern Thailand (thua-nao), named as “natto triangle” (Nakao, 1972) and renamed as “kinema-natto-thuanao (KNT)-triangle” (Tamang, 2015b). Within the KNT-triangle-bound countries, Bacillus-fermented sticky non-salty soybean foods are consumed such as natto of Japan, chungkokjang of Korea, kinema of India, Nepal and Bhutan, aakhune, bekang, hawaijar, peruyaan, and tungrymbai of India, thua nao of Thailand, pepok of Myanmar, and sieng of Cambodia and Laos (Nagai and Tamang, 2010; Tamang, 2015b; Table 4). Although, the method of production and culinary practices vary from product to product, plasmids, and phylogenetic analysis of B. subtilis showed the similarity among the strains of B. subtilis isolated from common sticky fermented soybean foods of Asia (Hara et al., 1986, 1995; Tamang et al., 2002; Meerak et al., 2007) suggesting the common stock of Bacillus. Mould-fermented soybean products are miso and shoyu of Japan, tempe of Indonesia, douchi and sufu of China, and doenjang of Korea (Sugawara, 2010). Some common non-soybean fermented legumes (e.g., locust beans) are bikalga, dawadawa, iru, okpehe, soumbala, and dugba of Africa (Ouoba et al., 2004, 2008, 2010; Amoa-Awua et al., 2006; Azokpota et al., 2006; Oguntoyinbo et al., 2007, 2010; Meerak et al., 2008; Parkouda et al., 2009; Ahaotu et al., 2013), fermented black-grams products such as dhokla, papad, and wari of India (Nagai and Tamang, 2010), and maseura of India and Nepal (Chettri and Tamang, 2008).
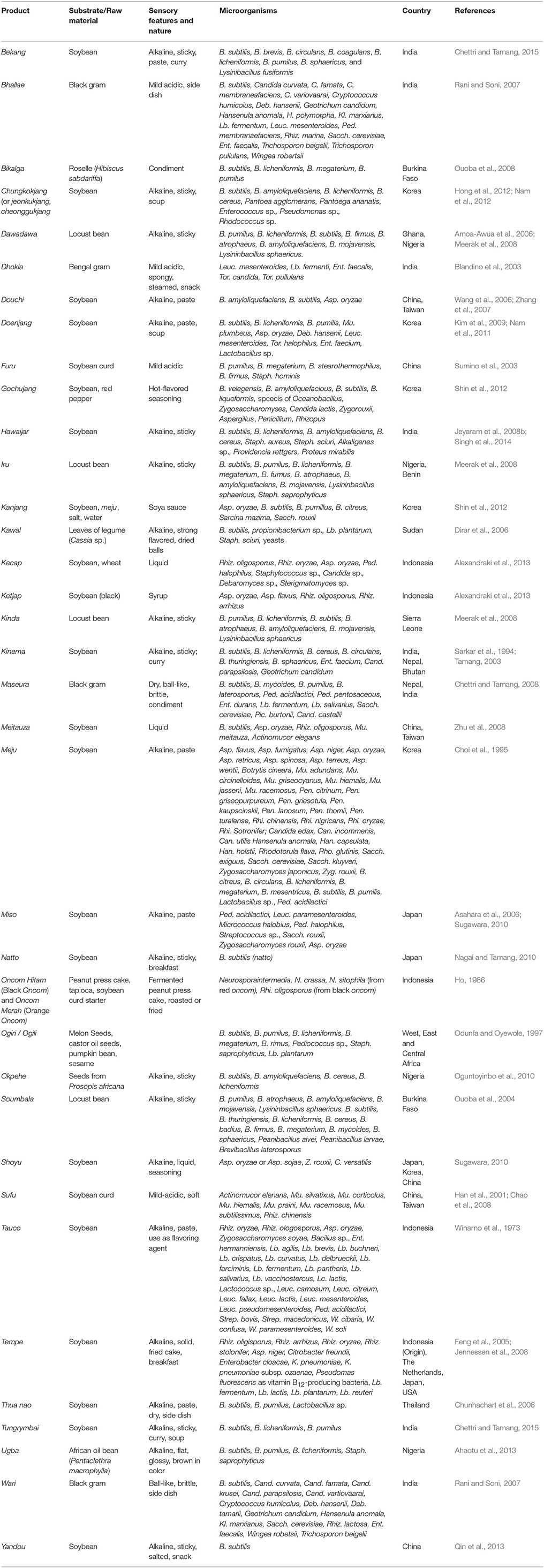
Table 4. Microorganisms isolated from some common and uncommon fermented legume (soybeans and non-soybean) products of the world.
Species of Bacillus have been reported for several Asian fermented soybean foods (Sarkar et al., 2002; Tamang et al., 2002; Tamang, 2003; Park et al., 2005; Inatsu et al., 2006; Choi et al., 2007; Kimura and Itoh, 2007; Shon et al., 2007; Jeyaram et al., 2008b; Dajanta et al., 2009; Kwon et al., 2009; Kubo et al., 2011; Singh et al., 2014; Wongputtisin et al., 2014; Chettri and Tamang, 2015). However, B. subtilis is the dominant functional bacterium in Asian fermented soybean foods (Sarkar and Tamang, 1994; Tamang and Nikkuni, 1996; Dajanta et al., 2011). Japanese natto is the only Bacillus-fermented soybean food now produced by commercial monoculture starter B. natto, earlier isolated from naturally fermented natto by Sawamura (Sawamura, 1906). Ent. Faecium, as a minor population group, is also present in kinema (Sarkar et al., 1994), in okpehe (Oguntoyinbo et al., 2007), and in chungkukjang (Yoon et al., 2008).
Fermented Root and Tuber Products
Cassava (Manihot esculenta) root is traditionally fermented into staple foods such as gari in Nigeria; fufu in Togo, Burkina Faso, Benin and Nigeria; agbelima in Ghana; chikawgue in Zaire; kivunde in Tanzania; kocho in Ethiopia; and foo foo in Nigeria, Benin, Togo, and Ghana, respectively (Franz et al., 2014; Table 5). The initial stage of cassava fermentation is dominated by Corynebacterium manihot (Oyewole et al., 2004) with LAB succession (Lb. acidophilus, Lb. casei, Lb. fermentum, Lb. pentosus, Lb. plantarum, Oguntoyinbo and Dodd, 2010). Cassava root is also traditionally fermented into sweet dessert known as tapé in Indonesia (Tamang, 2010b).
Fermented Meat Products
Fermented meat products are divided into two categories: those made from whole meat pieces or slices such as dried meat and jerky; and those made by chopping or comminuting the meat, usually called sausages (Adams, 2010). Traditionally fermented meat products of many countries have been well-documented (Table 6), such as fermented sausages (Lücke, 2015) and salami (Toldra, 2007) of Europe, jerky of America and Africa (Baruzzi et al., 2006), nham of Thailand (Chokesajjawatee et al., 2009), and nem chua of Vietnam (Nguyen et al., 2013b). The main microbial groups involved in meat fermentation are LAB (Albano et al., 2009; Cocolin et al., 2011; Khanh et al., 2011; Nguyen et al., 2013b), followed by coagulase-negative staphylococci, micrococci and Enterobacteriaceae (Cocolin et al., 2011; Marty et al., 2011), and depending on the product, some species of yeasts (Encinas et al., 2000; Tamang and Fleet, 2009), and molds, which may play a role in meat ripening (Lücke, 2015).
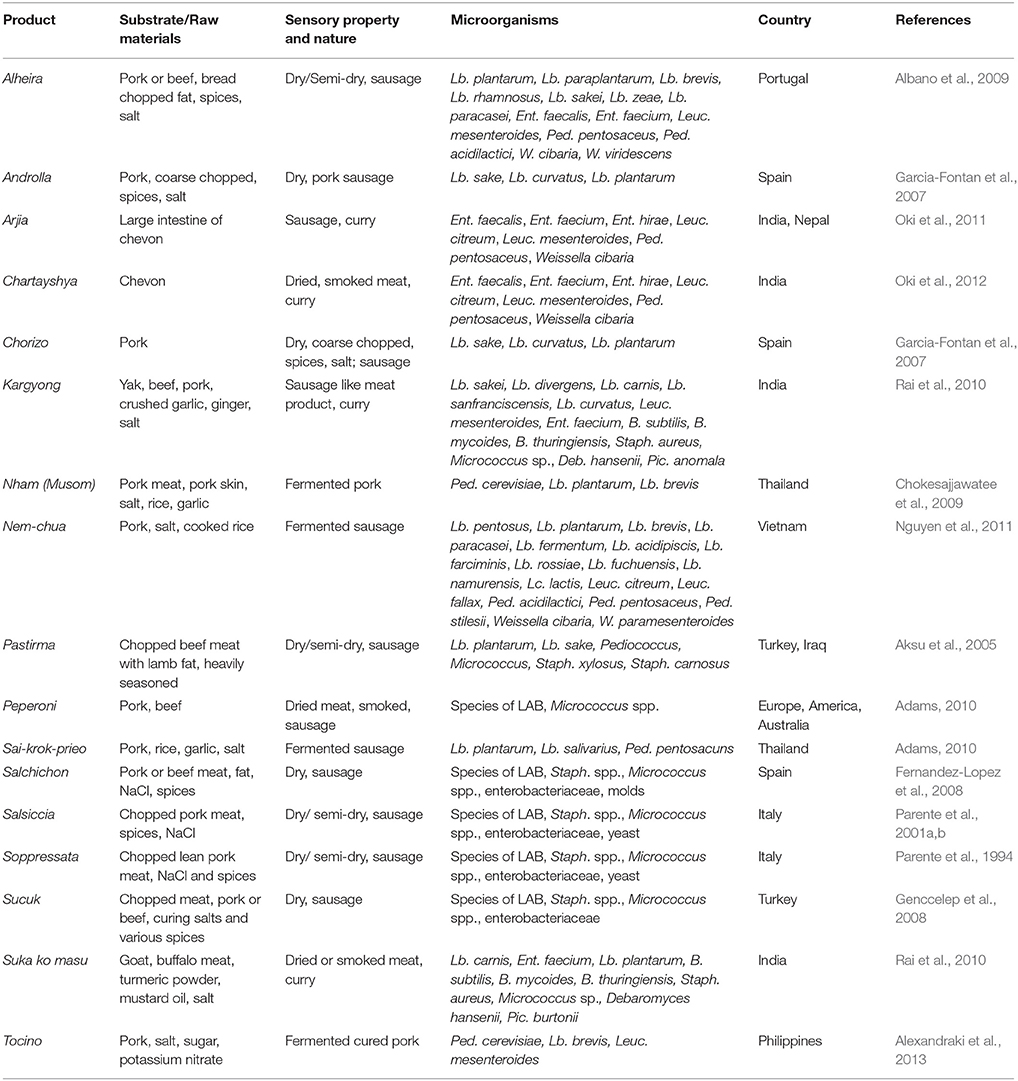
Table 6. Microorganisms isolated from some common and uncommon fermented meat products of the world.
Fermented Fish Products
Preservation of fish through fermentation, sun/smoke drying and salting (Table 7) is traditionally practiced by people living nearby coastal regions, lakes, and rivers and is consumed as seasoning, condiments, and side dishes (Salampessy et al., 2010). Several species of bacteria and yeasts have been reported from fermented and traditionally preserved fish products of the world (Kobayashi et al., 2000a,b,c; Wu et al., 2000; Thapa et al., 2004, 2006, 2007; Saithong et al., 2010; Hwanhlem et al., 2011; Romi et al., 2015).
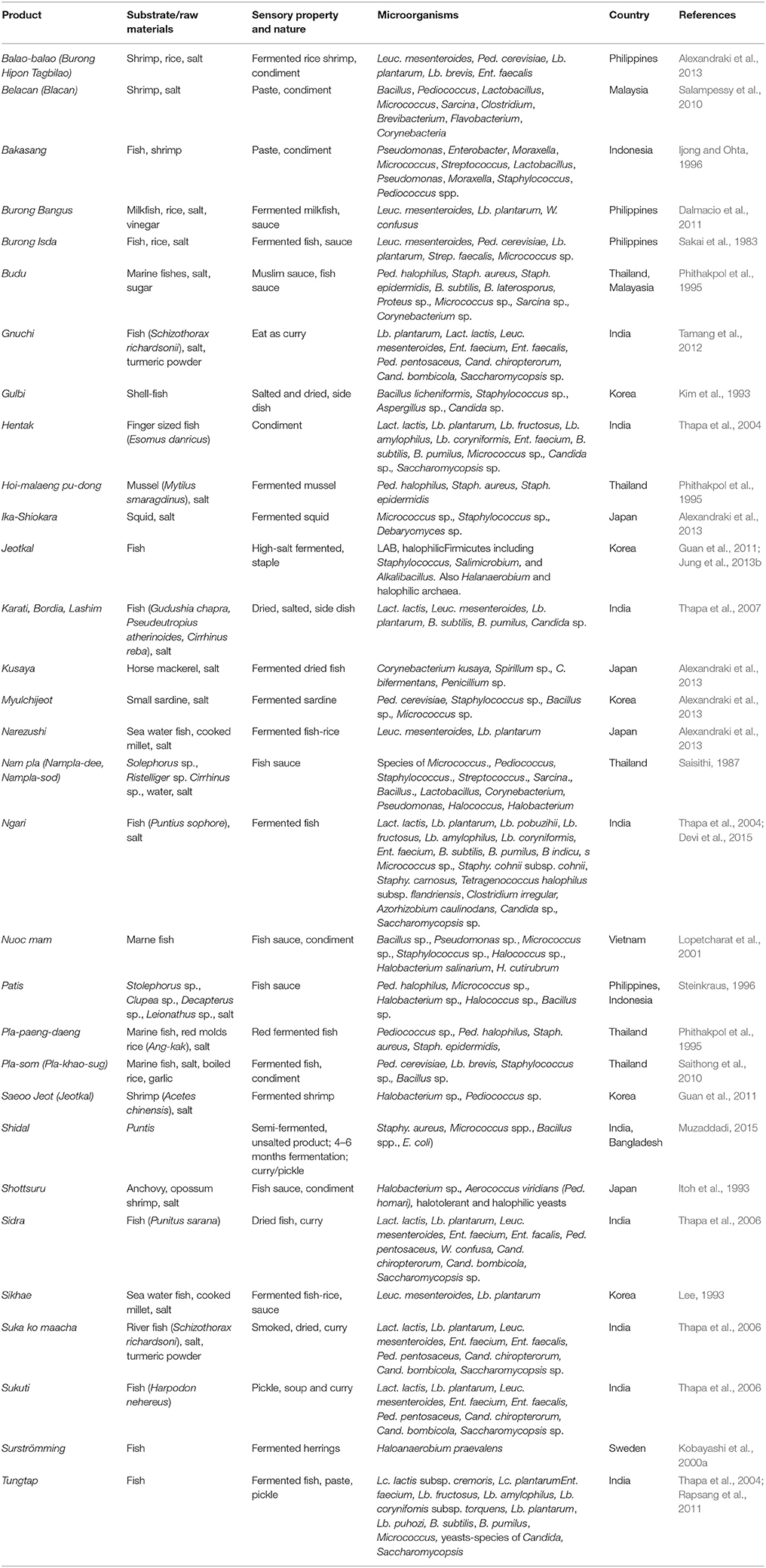
Table 7. Microorganisms isolated from some common and uncommon fermented fish products of the world.
Miscellaneous Fermented Products
Vinegar is one of the most popular condiments in the world and is prepared from sugar or ethanol containing substrates and hydrolyzed starchy materials by aerobic conversion to acetic acid (Solieri and Giudici, 2008). Acetobacter aceti subsp. aceti, Acetobacter pasteurianus, Acetobacter polyxygenes, Acetobacter xylinum, Acetobacter malorum, Acetobacter pomorum dominate during vinegar production (Haruta et al., 2006), while yeast species such as Candida lactis-condensi, Candida stellata, Hanseniaspora valbyensis, Hanseniaspora osmophila, Saccharomycodes ludwigii, Sacch. cerevisiae, Zygosaccharomyces bailii, Zygosaccharomyces bisporus, Zygosaccharomyces lentus, Zygosaccharomyces mellis, Zygosaccharomyces Pseudorouxii, and Zygosaccharomyces Rouxii have also been reported (Sengun and Karabiyikli, 2011).
Though normal black tea is consumed everywhere, some ethnic Asian communities enjoy special fermented teas such as miang of Thailand (Tanasupawat et al., 2007) and puer tea, fuzhuan brick, and kombucha of China (Mo et al., 2008). Aspergillus niger is the predominant fungus in puer tea while Blastobotrys adeninivorans, Asp. glaucus, species of Penicillium, Rhizopus, and Saccharomyces and the bacterial species Actinoplanes and Streptomyces are isolated (Jeng et al., 2007; Abe et al., 2008). Brettanomyces bruxellensis, Candida stellata, Rhodotorula mucilaginosa, Saccharomyces spp., Schizosaccharomyces pombe, Torulaspora delbrueckii, Zygosaccharomyces bailii, Zygosaccharomyces bisporus, Zygosaccharomyces kombuchaensis, and Zygosaccharomyces microellipsoides are also isolated from kombucha (Kurtzman et al., 2001; Teoh et al., 2004). Major bacterial genera present in kombucha are Gluconacetobacter. However, Marsh et al. (2014) reported the predominance of Lactobacillus, Acetobacter, and Zygosaccharomyces. Lb. thailandensis, Lb. camelliae, Lb. plantarum, Lb. pentosus, Lb. vaccinostercus, Lb. pantheris, Lb. fermentum, Lb. suebicus, Ped. siamensis, Ent. casseliflavus and Ent. camelliae in the fermentation of miang production (Sukontasing et al., 2007; Tanasupawat et al., 2007). Species of Aspergillus, Penicillium, and Eurotium are major fungi for fermentation of fuzhuan brick tea (Mo et al., 2008).
Nata or bacterial cellulose produced by Acetobacter xylinum is a delicacy of the Philippines, eaten as candy (Chinte-Sanchez, 2008; Jagannath et al., 2010; Adams, 2014). Two types of nata are well-known: nata de piña, produced on the juice from pineapple trimmings, and nata de coco, produced on coconut water or coconut skim milk (Adams, 2014). Bacterial cellulose has significant potential as a food ingredient in view of its high purity, in situ change of flavor and color, and having the ability to form various shapes and textures (Shi et al., 2014).
Chocolate is a product of cocoa bean fermentation where Lb. fermentum and Acetobacter pasteurianus are reported as the predominating bacterial species (Lefeber et al., 2010; Papalexandratou et al., 2011). Diverse LAB species appear to be typically associated with the fermentation of cocoa beans in Ghana, which include Lb. ghanensis (Nielsen et al., 2007), Weissella ghanensis (de Bruyne et al., 2008a), Lb. cacaonum, and Lb. fabifermentans (de Bruyne et al., 2009), and Weissella fabaria (de Bruyne et al., 2010). Fructobacillus pseudoficulneus, Lb. plantarum, Acetobacter senegalensis, and the enterobacteria Tatumella ptyseos and Tatumella citrea are among the prevailing species during the initial phase of cocoa fermentations (Papalexandratou et al., 2011). Yeasts involved during spontaneous cocoa fermentation are Hanseniaspora uvarum, Hanseniaspora quilliermundii, Issatchenkia orientalis (Candida krusei), Pichia membranifaciens, Sacch. Cerevisiae, and Kluyveromyces species for flavor development (Schillinger et al., 2010).
Pidan is a preserved egg prepared from alkali-treated fresh duck eggs and is consumed by the Chinese, and has a strong hydrogen sulfide and ammonia smell (Ganasen and Benjakul, 2010). The main alkaline chemical reagent used for making pidan is sodium hydroxide, which is produced by the reaction of sodium carbonate, water, and calcium oxide of pickle or coating mud. B. cereus, B. macerans, Staph. cohnii, Staph. epidermidis, Staph. Haemolyticus, and Staph. warneri are predominant in pidan (Wang and Fung, 1996).
Amylolytic Starters
Traditional way of culturing the essential microorganisms (consortia of filamentous molds, amylolytic, and alcohol-producing yeasts and LAB) with rice or wheat as the base in the form of dry, flattened or round balls, for production of alcoholic beverages is a remarkable discovery in the food history of Asian people, which is exclusively practiced in South-East Asia including the Himalayan regions of India, Nepal, Bhutan, and China (Tibet; Hesseltine, 1983; Tamang, 2010a). Around 1–2% of previously prepared amylolytic starters are inoculated into the dough, and mixed cultures are allowed to develop for a short time, then dried, and used to make either alcohol or fermented foods from starchy materials (Tamang et al., 1996). Asian amylolytic starters have different vernacular names such as marcha in India and Nepal; hamei, humao, phab in India; mana and manapu of Nepal; men in Vietnam; ragi in Indonesia; bubod in Philippines; chiu/chu in China and Taiwan; loogpang in Thailand; mae/dombae/buh/puh in Cambodia; and nuruk in Korea (Hesseltine and Kurtzman, 1990; Nikkuni et al., 1996; Sujaya et al., 2004; Thanh et al., 2008; Yamamoto and Matsumoto, 2011; Tamang et al., 2012).
Microbial profiles of amylolytic starters of India, Nepal, and Bhutan are filamentous molds like, Mucor circinelloides forma circinelloides, Mucor hiemalis, Rhi. chinensis, and Rhi. stolonifer variety lyococcus (Tamang et al., 1988); yeasts like Sacch. cerevisiae, Sacch. bayanus, Saccharomycopsis (Sm.) fibuligera, Sm. capsularis, Pichia anomala, Pic. burtonii, and Candida glabrata; (Tamang and Sarkar, 1995; Shrestha et al., 2002; Tsuyoshi et al., 2005; Tamang et al., 2007; Jeyaram et al., 2008a, 2011; Chakrabarty et al., 2014); and species of LAB namely Ped. pentosaceus, Lb. bifermentans, and Lb. brevis (Hesseltine and Ray, 1988; Tamang and Sarkar, 1995; Tamang et al., 2007; Chakrabarty et al., 2014). A diversity of yeasts (Candida tropicalis, Clavispora lusitaniae, Pichia anomala, Pichia ranongensis, Saccharomycopsis fibuligera, Sacch. cerevisiae, Issatchenkia sp.); filamentous molds (Absidia corymbifera, Amylomyces rouxii, Botryobasidium subcoronatum, Rhizopus oryzae, Rhi. microsporus, Xeromyces bisporus); LAB (Ped. pentosaceus, Lb. plantarum, Lb. brevis, Weissella confusa, Weissella paramesenteroides); amylase-producing bacilli (Bacillus subtilis, B. circulans, B. amyloliquefaciens, B. sporothermodurans); and acetic acid bacteria (Acetobacter orientalis, A. pasteurianus) is present in men, a starter culture of Vietnam (Dung et al., 2006, 2007; Thanh et al., 2008).
A combination of Asp. oryzae and Asp. sojae is used in koji in Japan to produce alcoholic beverages including saké (Zhu and Trampe, 2013). Koji (Chinese chu, shi, or qu) also produces amylases that convert starch to fermentable sugars, which are then used for the second stage yeast fermentation to make non-alcoholic fermented soybean miso and shoyu (Sugawara, 2010). Asp. awamori, Asp. kawachii, Asp. oryzae, Asp. shirousamii, and Asp. sojae have been widely used as the starter in preparation of koji for production of miso, saké, shoyu, shochu (Suganuma et al., 2007).
Alcoholic Beverages
Tamang (2010c) classified alcoholic beverages of the world into 10 types:
(1) Non-distilled and unfiltered alcoholic beverages produced by amylolytic starters e.g., kodo ko jaanr (fermented finger millets; Thapa and Tamang, 2004) and bhaati jaanr (fermented rice) of India and Nepal (Tamang and Thapa, 2006), makgeolli (fermented rice) of Korea (Jung et al., 2012).
(2) Non-distilled and filtered alcoholic beverages produced by amylolytic starters e.g., saké of Japan (Kotaka et al., 2008).
(3) Distilled alcoholic beverages produced by amylolytic starter e.g., shochu of Japan, and soju of Korea (Steinkraus, 1996).
(4) Alcoholic beverages produced by involvement of amylase in human saliva e.g., chicha of Peru (Vallejo et al., 2013).
(5) Alcoholic beverages produced by mono- (single-strain) fermentation e.g., beer (Kurtzman and Robnett, 2003).
(6) Alcoholic beverages produced from honey e.g., tej of Ethiopia (Bahiru et al., 2006).
(7) Alcoholic beverages produced from plant parts e.g., pulque of Mexico (Lappe-Oliveras et al., 2008), toddy of India (Shamala and Sreekantiah, 1988), and kanji of India (Kingston et al., 2010).
(8) Alcoholic beverages produced by malting (germination) e.g., sorghum (“Bantu”) beer of South Africa (Kutyauripo et al., 2009), pito of Nigeria, and Ghana (Kolawole et al., 2013), and tchoukoutou of Benin (Greppi et al., 2013a).
(9) Alcoholic beverages prepared from fruits without distillation e.g., wine, cider.
(10) Distilled alcoholic beverages prepared from fruits and cereals e.g., whisky and brandy.
Non-Distilled Mild-Alcoholic Food Beverages Produced by Amylolytic Starters
The biological process of liquefaction and saccharification of cereal starch by filamentous molds and yeasts, supplemented by amylolytic starters, under solid-state fermentation is one of the two major stages of production of alcoholic beverages in Asia (Tamang, 2010c). These alcoholic beverages are mostly considered as food beverage and eaten as staple food with high calorie in many parts of Asia, e.g., kodo ko jaanr of the Himalayan regions in India, Nepal, Bhutan, and China (Tibet) with 5% alcohol content (Thapa and Tamang, 2004). Saccharifying activities are mostly shown by Rhizopus spp. and Sm. fibuligera whereas, liquefying activities are shown by Sm. fibuligera and Sacch. cerevisiae (Thapa and Tamang, 2006). Rhizopus, Amylomyces, Torulopsis, and Hansenula are present in lao-chao, a popular ethnic fermented rice beverage of China (Wei and Jong, 1983). During fermentation of Korean makgeolli (prepared from rice by amylolytic starter nuruk), the proportion of the Saccharomycetaceae family increases significantly and the major bacterial phylum of the samples shifts from γ-Proteobacteria to Firmicutes (Jung et al., 2012).
Non-Distilled and Filtered Alcoholic Beverages Produced by Amylolytic Starters
Alcoholic beverages produced by amylolytic starter (koji) are not distilled but the extract of fermented cereals is filtered into clarified high alcohol-content liquor, like in sake, which is a national drink of Japan containing 15–20% alcohol (Tamang, 2010c). Improved strains of Asp. oryzae are used for saké production in industrial scale (Kotaka et al., 2008; Hirasawa et al., 2009).
Distilled Alcoholic Beverages Produced by Amylolytic Starters
This category of alcoholic drinks is the clear distillate of high alcohol content prepared as drink from fermented cereal beverages by using amylolytic starters. Raksi is an ethnic alcoholic (22–27% v/v) drink of the Himalayas with aromatic characteristic, and distilled from the traditionally fermented cereal beverages (Kozaki et al., 2000).
Alcoholic Beverages Produced by Human Saliva
Chicha is a unique ethnic fermented alcoholic (2–12% v/v) beverage of Andes Indian race of South America mostly in Peru, prepared from maize by human salivation process (Hayashida, 2008). Sacch. cerevisiae, Sacch. apiculata, Sacch. pastorianus, species of Lactobacillus and Acetobacter are present in chicha (Escobar et al., 1996). Sacch. cerevisiae was isolated from chicha and identified using MALDI-TOF (Vallejo et al., 2013). Species of Lactobacillus, Bacillus, Leuconostoc, Enterococcus, Streptomyces, Enterobacter, Acinetobacter, Escherichia, Cronobacter, Klebsiella, Bifidobacterium, and Propioniobacterium have been reported from chicha of Brazil (Puerari et al., 2015).
Alcoholic Beverages Produced from Honey
Some alcoholic beverages are produced from honey e.g., tej of Ethiopia. It is a yellow, sweet, effervescent and cloudy alcoholic (7–14% v/v) beverage (Steinkraus, 1996). Sacch. cerevisiae, Kluyvermyces bulgaricus, Debaromyces phaffi, and Kl. veronae, and LAB species of Lactobacillus, Streptococcus, Leuconostoc, and Pediococcus are responsible for tej fermentation (Bahiru et al., 2006).
Alcoholic Beverages Produced from Plant Parts
Pulque is one of the oldest alcoholic beverages prepared from juices of the cactus (Agave) plant of Mexico (Steinkraus, 2002). Bacteria present during the fermentation of pulque were LAB (Lc. lactis subsp. lactis, Lb. acetotolerans, Lb. acidophilus, Lb. hilgardii, Lb. kefir, Lb. plantarum, Leuc. citreum, Leuc. kimchi, Leuc. mesenteroides, Leuc. pseudomesenteroides), the γ-Proteobacteria (Erwinia rhapontici, Enterobacter spp., and Acinetobacter radioresistens, several α-Proteobacteria), Zymomonas mobilis, Acetobacter malorum, A. pomorium, Microbacterium arborescens, Flavobacterium johnsoniae, Gluconobacter oxydans, and Hafnia alvei (Escalante et al., 2004, 2008). Yeasts isolated from pulque are Saccharomyces (Sacch. bayanus, Sacch. cerevisiae, Sacch. paradoxus) and non-Saccharomyces (Candida spp., C. parapsilosis, Clavispora lusitaniae, Hanseniaspora uvarum, Kl. lactis, Kl. marxianus, Pichia membranifaciens, Pichia spp., Torulaspora delbrueckii; Lappe-Oliveras et al., 2008).
Depending on the region, traditional alcoholic drinks prepared from palm juice called “palm wine” are known by various names, e.g., toddy or tari in India, mu, bandji, ogogoro, nsafufuo, nsamba, mnazi, yongo, taberna, tua, or tubak in West Africa and South America (Ouoba et al., 2012). Microorganisms that are responsible for toddy fermentation are Sacch. cerevisiae, Schizosaccharomyces pombe, Acetobacter aceti, A. rancens, A. suboxydans, Leuc. dextranicum (mesenteroides), Micrococcus sp., Pediococcus sp., Bacillus sp., and Sarcina sp. (Shamala and Sreekantiah, 1988).
Kanji is an ethnic Indian strong-flavored mild alcoholic beverage prepared from beet-root and carrot by natural fermentation (Batra and Millner, 1974). Hansenlu anomala, Candida guilliermondii, C. tropicalis, Geotrichium candidum, Leuc. mesenteroides, Pediococcus sp., Lb. paraplantarum, and Lb. pentosus are present in kanji (Batra and Millner, 1976; Kingston et al., 2010).
Alcoholic Beverages Produced by Malting or Germination
Bantu beer or sorghum beer of Bantu tribes of South Africa is an alcoholic beverage produced by malting or germination process (Taylor, 2003). Malted beer is common in Africa with different names e.g., as bushera or muramba in Uganda, chibuku in Zimbabwe, dolo, burkutu, and pito in West Africa and ikigage in Rwanda (Myuanja et al., 2003; Sawadogo-Lingani et al., 2007; Lyumugabe et al., 2012). Sorghum (Sorghum caffrorum or S. vulgare) is malted (Kutyauripo et al., 2009), characterized by a two-stage (lactic followed by alcoholic) fermentation, with Lb. fermentum as the dominating LAB species (Sawadogo-Lingani et al., 2007).
Alcoholic Beverages Produced from Fruits without Distillation
The most common example of alcoholic beverages produced from fruits without distillation is wine, which is initiated by the growth of various species of Saccharomyces and non-Saccharomyces (so-called “wild”) yeasts (e.g., Candida colliculosa, C. stellata, Hanseniaspora uvarum, Kloeckera apiculata, Kl. thermotolerans, Torulaspora delbrueckii, Metschnikowia pulcherrima; Pretorius, 2000; Moreira et al., 2005; Sun et al., 2014; Walker, 2014). Candida sp. and Cladosporium sp. were isolated from fermenting white wine using mCOLD-PCR-DGGE, but had not been detected by conventional PCR (Takahashi et al., 2014). Sacch. cerevisiae strains developed during wine fermentations play an active role in developing the characteristics of a wine (Capece et al., 2013). Saccharomyces Genome Database (SGD; www.yeastgenome.org) provides free of charge access or links to comprehensive datasets comprising genomic, transcriptomic, proteomic and metabolomic information (Pretorius et al., 2015).
Conclusions
Every community in the world has distinct food culture including fermented foods and alcoholic beverages, symbolizing the heritage and socio-cultural aspects of the ethnicity. The word “culture” denotes food habits of ethnicity; another meaning for the same word “culture” is a cluster of microbial cells or inoculum, an essential biota for fermentation, often used in the microbiology. The diversity of functional microorganisms ranges from filamentous molds to enzyme-producing and alcohol-producing yeasts, and from Gram-positive to a few Gram-negative bacteria, while even Archaea has been ascribed roles in some fermented foods and alcoholic beverages. However, consumption of lesser known and uncommon ethnic fermented foods is declining due to the change in lifestyles that is shifting from cultural food habits to commercial foodstuffs and fast foods, drastically affecting traditional culinary practices, and also due to the climate change in some environments such as the Sahel region in Africa and the vast areas adjacent to the Gobi desert in Asia.
Author Contributions
JT: contributed 50% of review works. WH, contributed 25% of review. KW contributed 25% of review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abe, M., Takaoka, N., Idemoto, Y., Takagi, C., Imai, T., and Nakasaki, K. (2008). Characteristic fungi observed in the fermentation process for Puer tea. Int. J. Food Microbiol. 124, 199–203. doi: 10.1016/j.ijfoodmicro.2008.03.008
Abriouel, H., Benomar, N., Lucas, R., and Gálvez, A. (2011). Culture-independent study of the diversity of microbial populations in brines during fermentation of naturally-fermented Aloreña green table olives. Int. J. Food Microbiol. 144, 487–496. doi: 10.1016/j.ijfoodmicro.2010.11.006
Abriouel, H., Omar, N. B., López, R. L., Martínez-Cañamero, M., Keleke, S., and Gálvez, A. (2006). Culture-independent analysis of the microbial composition of the African traditional fermented foods poto poto and dégué by using three different DNA extraction methods. Int. J. Food Microbiol. 111, 228–233. doi: 10.1016/j.ijfoodmicro.2006.06.006
Adams, M. R. (2010). “Fermented meat products,” in Fermented Foods and Beverages of the World, eds J. P. Tamang, and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 309–322.
Adams, M. R. (2014). “Vinegar,” in Encyclopaedia of Food Microbiology, 2nd Edn., eds C. Batt and M. A. Tortorello (Oxford: Elsevier Ltd.), 717–721.
Ahaotu, I., Anyogu, A., Njoku, O. H., Odu, N. N., Sutherland, J. P., and Ouoba, L. I. I. (2013). Molecular identification and safety of Bacillus species involved in the fermentation of African oil beans (Pentaclethra macrophylla Benth) for production of Ugba. Int. J. Food Microbiol. 162, 95–104.
Aidoo, K. E., and Nout, M. J. R. (2010). “Functional yeasts and molds in fermented foods and beverages,” in Fermented Foods and Beverages of the World, eds J. P. Tamang and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 127–148. doi: 10.1201/ebk1420094954-c4
Akabanda, F., Owusu-Kwarteng, J., Tano-Debrah, K., Glover, R. L. K., Nielsen, and, D. S., and Jespersen, L. (2013). Taxonomic and molecular characterization of lactic acid bacteria and yeasts in nunu, a Ghanaian fermented milk product. Food Microbiol. 34, 277–283. doi: 10.1016/j.fm.2012.09.025
Aksu, M. I., Kaya, M., and Ockerman, H. W. (2005). Effect of modified atmosphere packaging and temperature on the shelf life of sliced Pastirma produced from frozen/thawed meat. J. Muscle Foods 16, 192–206. doi: 10.1111/j.1745-4573.2005.08404.x
Albano, H., van-Reenen, C. A., Todorov, S. D., Cruz, D., Fraga, L., Hogg, T., et al. (2009). Phenotypic and genetic heterogeneity of lactic acid bacteria isolated from “Alheira”, a traditional fermented sausage produced in Portugal. Meat Sci. 82, 389–398. doi: 10.1016/j.meatsci.2009.02.009
Alegría, A., González, R., Díaz, M., and Mayo, B. (2011). Assessment of microbial populations dynamics in a blue cheese by culturing and denaturing gradient gel electrophoresis. Curr. Microbiol. 62, 888–893. doi: 10.1007/s00284-010-9799-7
Alexandraki, V., Tsakalidou, E., Papadimitriou, K., and Holzapfel, W. H. (2013). Status and Trends of the Conservation and Sustainable Use of Microorganisms in Food Processes. Commission on Genetic Resources for Food and Agriculture. FAO Background Study Paper No. 65.
Amoa-Awua, W. K., Terlabie, N. N., and Sakyi-Dawson, E. (2006). Screening of 42 Bacillus isolates for ability to ferment soybeans into dawadawa. Int. J. Food Microbiol. 106, 343–347. doi: 10.1016/j.ijfoodmicro.2005.08.016
Angelakis, E., Million, M., Henry, M., and Raoult, D. (2011). Rapid and accurate bacterial identification in probiotics and yoghurts by MALDI-TOF mass spectrometry. J. Food Sci. 76, M568–M572. doi: 10.1111/j.1750-3841.2011.02369.x
Asahara, N., Zhang, X. B., and Ohta, Y. (2006). Antimutagenicity and mutagen-binding activation of mutagenic pyrolyzates by microorganisms isolated from Japanese miso. J. Sci. Food Agric. 58, 395–401. doi: 10.1002/jsfa.2740580314
Axelsson, L., Rud, I., Naterstad, K., Blom, H., Renckens, B., Boekhorst, J., et al. (2012). Genome sequence of the naturally plasmid-free Lactobacillus plantarum strain NC8 (CCUG 61730). J. Bacteriol. 194, 2391–2392. doi: 10.1128/JB.00141-12
Azokpota, P., Hounhouigan, D. J., and Nago, M. C. (2006). Microbiological and chemical changes during the fermentation of African locust bean (Parkia biglobosa) to produce afitin, iru, and sonru, three traditional condiments produced in Benin. Int. J. Food Microbiol. 107, 304–309. doi: 10.1016/j.ijfoodmicro.2005.10.026
Bahiru, B., Mehari, T., and Ashenafi, M. (2006). Yeast and lactic acid flora of tej, an indigenous Ethiopian honey wine: variations within and between production units. Food Microbiol. 23, 277–282. doi: 10.1016/j.fm.2005.05.007
Baruzzi, F., Matarante, A., Caputo, L., and Marea, M. (2006). Molecular and physiological characterization of natural microbial communities isolated from a traditional Southern Italian processed sausage. Meat Sci. 72, 261–269. doi: 10.1016/j.meatsci.2005.07.013
Batra, L. R., and Millner, P. D. (1974). Some Asian fermented foods and beverages and associated fungi. Mycologia 66, 942–950. doi: 10.2307/3758313
Batra, L. R., and Millner, P. D. (1976). Asian fermented foods and beverages. Developments in >Indus. Microbiol. 17, 117–128.
Bernardeau, M., Guguen, M., and Vernoux, J. P. (2006). Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol. Rev. 30, 487–513. doi: 10.1111/j.1574-6976.2006.00020.x
Blandino, A., Al-Aseeri, M. E., Pandiella, S. S., Cantero, D., and Webb, C. (2003). Cereal-based fermented foods and beverages. Food Res. Int. 36, 527–543. doi: 10.1016/S0963-9969(03)00009-7
Bourdichon, F., Casaregola, S., Farrokh, C., Frisvad, J. C., Gerds, M. L., Hammes, W. P., et al. (2012). Food fermentations: microorganisms with technological beneficial use. Int. J. Food Microbiol. 154, 87–97. doi: 10.1016/j.ijfoodmicro.2011.12.030
Brandt, M. J. (2007). Sourdough products for convenient use in baking. Food Microbiol. 24, 161–164. doi: 10.1016/j.fm.2006.07.010
Briggiler-Marcó, M., Capr, M. L., Quiberoni, A., Vinderola, G., Reinheimer, J. A., and Hynes, E. (2007). Nonstarter Lactobacillus strains as adjunct cultures for cheese making: in vitro characterization and performance in two model cheese. J. Dairy Sci. 90, 4532–4542. doi: 10.3168/jds.2007-0180
Campbell-Platt, G. (1987). Fermented Foods of the World: A Dictionary and Guide. London: Butterworths.
Campbell-Platt, G. (1994). Fermented foods - a world perspective. Food Res. Int. 27, 253–257. doi: 10.1016/0963-9969(94)90093-0
Capece, A., Siesto, G., Poeta, C., Pietrafesa, R., and Romano, P. (2013). Indigenous yeast population from Georgian aged wines produced by traditional “Kakhetian” method. Food Microbiol. 36, 447–455. doi: 10.1016/j.fm.2013.07.008
Chakrabarty, J., Sharma, G. D., and Tamang, J. P. (2014). Traditional technology and product characterization of some lesser-known ethnic fermented foods and beverages of North Cachar Hills District of Assam. Indian J. Tradit. Knowl. 13, 706–715.
Chang, H. W., Kim, K. H., Nam, Y. D., Roh, S. W., Kim, M. S., Jeon, C. O., et al. (2008). Analysis of yeast and archaeal population dynamics in kimchi using denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 126, 159–166. doi: 10.1016/j.ijfoodmicro.2008.05.013
Chao, S. H., Kudo, Y., Tsai, Y. C., and Watanabe, K. (2012). Lactobacillus futsaii sp. nov., isolated from traditional fermented mustard products of Taiwan, fu-tsai and suan-tsai. Int. J. Syst. Evol. Microbiol. 62, 489–494. doi: 10.1099/ijs.0.030619-0
Chao, S. H., Tomii, Y., Watanabe, K., and Tsai, Y. C. (2008). Diversity of lactic acid bacteria in fermented brines used to make stinky tofu. Int. J. Food Microbiol. 123, 134–141. doi: 10.1016/j.ijfoodmicro.2007.12.010
Chao, S. H., Wu, R. J., Watanabe, K., and Tsai, Y. C. (2009). Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 135, 203–210. doi: 10.1016/j.ijfoodmicro.2009.07.032
Chaves-López, C., Serio, A., Grande-Tovar, C. D., Cuervo-Mulet, R., Delgado-Ospina, J., and Paparella, A. (2014). Traditional fermented foods and beverages from a microbiological and nutritional perspective: the Colombian heritage. Compr. Rev. Food Sci. Food Saf. 13, 1031–1048. doi: 10.1111/1541-4337.12098
Chen, B., Wu, Q., and Xu, Y. (2014). Filamentous fungal diversity and community structure associated with the solid state fermentation of Chinese Maotai-flavor liquor. Int. J. Food Microbiol. 179, 80–84. doi: 10.1016/j.ijfoodmicro.2014.03.011
Chen, Y. S., Wu, H. C., Liu, C. H., Chen, H. C., and Yanagida, F. (2010). Isolation and characterization of lactic acid bacteria from jiang-sun (fermented bamboo shoots), a traditional fermented food in Taiwan. J. Sci. Food Agric. 90, 1977–1982. doi: 10.1002/jsfa.4034
Chen, Y. S., Wu, H. C., Lo, H. Y., Lin, W. C., Hsu, W. H., Lin, C. W., et al. (2012). Isolation and characterisation of lactic acid bacteria from jiang-gua (fermented cucumbers), a traditional fermented food in Taiwan. J. Sci. Food Agric. 92, 2069–2075. doi: 10.1002/jsfa.5583
Chen, Y. S., Yanagida, F., and Hsu, J. S. (2006). Isolation and characterization of lactic acid bacteria from suan-tsai (fermented mustard), a traditional fermented food in Taiwan. J. Appl. Microbiol. 101, 125–130. doi: 10.1111/j.1365-2672.2006.02900.x
Chettri, R., and Tamang, J. P. (2008). Microbiological evaluation of maseura, an ethnic fermented legume-based condiment of Sikkim. J. Hill Res. 21, 1–7.
Chettri, R., and Tamang, J. P. (2015). Bacillus species isolated from Tungrymbai and Bekang, naturally fermented soybean foods of India. Int. J. Food Microbiol. 197, 72–76. doi: 10.1016/j.ijfoodmicro.2014.12.021
Chinte-Sanchez, P. (2008). Philippine Fermented Foods: Principles and Technology. Quezon: The University of the Philippines Press.
Choi, S. H., Lee, M. H., Lee, S. K., and Oh, M. J. (1995). Microflora and enzyme activity of conventional meju and isolation of useful mould. J. Agric. Sci. Chungnam Natl. Univ. Korea 22, 188–197.
Choi, U. K., Kim, M. H., and Lee, N. H. (2007). The characteristics of cheonggukjang, a fermented soybean product, by the degree of germination of raw soybeans. Food Sci. Biotechnol. 16, 734–739.
Chokesajjawatee, N., Pornaem, S., Zo, Y. G., Kamdee, S., Luxananil, P., Wanasen, S., et al. (2009). Incidence of Staphylococcus aureus and associated risk factors in Nham, a Thai fermented pork product. Food Microbiol. 26, 547–551. doi: 10.1016/j.fm.2009.02.009
Chunhachart, O., Itoh, T., Sukchotiratana, M., Tanimoto, H., and Tahara, Y. (2006). Characterization of ©-glutamyl hydrolase produced by Bacillus sp. isolated from Thai thua-nao. Biosci. Biotechnol. Biochem. 70, 2779–2782. doi: 10.1271/bbb.60280
Cocolin, L., Aggio, D., Manzano, M., Cantoni, C., and Comi, G. (2002). An application of PCR-DGGE analysis to profile the yeast populations in raw milk. Int. Dairy J. 12, 407–411. doi: 10.1016/S0958-6946(02)00023-7
Cocolin, L., Alessandria, V., Dolci, P., Gorra, R., and Rantsiou, R. (2013). Culture independent methods to assess the diversity and dynamics of microbiota during food fermentation. Int. J. Food Microbiol. 167, 29–43. doi: 10.1016/j.ijfoodmicro.2013.05.008
Cocolin, L., Dolci, P., and Rantsiou, K. (2011). Biodiversity and dynamics of meat fermentations: the contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 89, 296–302. doi: 10.1016/j.meatsci.2011.04.011
Cocolin, L., and Ercolini, D. (eds.). (2008). Molecular Techniques in the Microbial Ecology of Fermented Foods. New York, NY: Springer. doi: 10.1007/978-0-387-74520-6
Coppola, S., Fusco, V., Andolfi, R., Aponte, M., Aponte, M., Blaiotta, G., et al. (2006). Evaluation of microbial diversity during the manufacture of Fior di Latte di Agerola, a traditional raw milk pasta-filata cheese of the Naples area. J. Dairy Res. 73, 264–272. doi: 10.1017/S0022029906001804
Corsetti, A., and Settanni, L. (2007). Lactobacilli in sourdough fermentation. Food Res. Int. 40, 539–558. doi: 10.1016/j.foodres.2006.11.001
Coton, E., Desmonts, M. H., Leroy, S., Coton, M., Jamet, E., Christieans, S., et al. (2010). Biodiversity of coagulase-negative staphylococci in French cheeses, dry fermented sausages, processing environments and clinical samples. Int. J. Food Microbiol. 137, 221–229. doi: 10.1016/j.ijfoodmicro.2009.11.023
Dajanta, K., Apichartsrangkoon, A., Chukeatirote, E., Richard, A., and Frazier, R. A. (2011). Free-amino acid profiles of thua nao, a Thai fermented soybean. Food Chem. 125, 342–347. doi: 10.1016/j.foodchem.2010.09.002
Dajanta, K., Chukeatirote, E., Apichartsrangkoon, A., and Frazier, R. A. (2009). Enhanced aglycone production of fermented soybean products by Bacillus species. Acta Biol. Szegediensis 53, 93–98.
Dalmacio, L. M. M., Angeles, A. K. J., Larcia, L. L. H., Balolong, M., and Estacio, R. (2011). Assessment of bacterial diversity in selected Philippine fermented food products through PCR-DGGE. Benef. Microbes 2, 273–281. doi: 10.3920/BM2011.0017
de Bruyne, K., Camu, N., De Vuyst, L., and Vandamme, P. (2009). Lactobacillus fabifermentans sp. nov. and Lactobacillus cacaonum sp. nov., isolated from Ghanaian cocoa fermentations. Int. J. Syst. Evol. Microbiol. 59, 7–12. doi: 10.1099/ijs.0.001172-0
de Bruyne, K., Camu, N., de Vuyst, L., and Vandamme, P. (2010). Weissella fabaria sp. nov., from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 60, 1999–2005. doi: 10.1099/ijs.0.019323-0
de Bruyne, K., Camu, N., Lefebvre, K., De Vuyst, L., and Vandamme, P. (2008a). Weissella ghanensis sp. nov., isolated from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 58, 2721–2725. doi: 10.1099/ijs.0.65853-0
de Bruyne, K., Franz, C. M., Vancanneyt, M., Schillinger, U., Mozzi, F., de Valdez, G. F., et al. (2008b). Pediococcus argentinicus sp. nov. from Argentinean fermented wheat flour and identification of Pediococcus species by pheS, rpoA and atpA sequence analysis. Int. J. Sys. Evo. Microbiol. 58, 2909–2916. doi: 10.1099/ijs.0.65833-0
de Bruyne, K., Schillinger, U., Caroline, L., Boehringer, B., Cleenwerck, I., Vancanneyt, M., et al. (2007). Leuconostoc holzapfelii sp. nov., isolated from Ethiopian coffee fermentation and assessment of sequence analysis of housekeeping genes for delineation of Leuconostoc species. Int. J. Sys. Evo. Microbiol. 57, 2952–2959. doi: 10.1099/ijs.0.65292-0
de Ramesh, C. C., White, C. H., Kilara, A., and Hui, Y. H. (2006). Manufacturing Yogurt and Fermented Milks. Oxford, Blackwell Publishing.
Desfossés-Foucault, E., Dussault-Lepage, V., Le Boucher, C., Savard, P., LaPointe, G., and Roy, D. (2012). Assessment of probiotic viability during Cheddar cheese manufacture and ripening using propidium monoazide-PCR quantification. Front. Microbiol. 3:350. doi: 10.3389/fmicb.2012.00350
Devi, K. R., Deka, M., and Jeyaram, K. (2015). Bacterial dynamics during yearlong spontaneous fermentation for production of ngari, a dry fermented fish product of Northeast India. Int. J. Food Microbiol. 199, 62–71. doi: 10.1016/j.ijfoodmicro.2015.01.004
de Vuyst, L., Vrancken, G., Ravyts, F., Rimaux, T., and Weckx, S. (2009). Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 26, 666–675. doi: 10.1016/j.fm.2009.07.012
Dewan, S., and Tamang, J. P. (2006). Microbial and analytical characterization of Chhu, a traditional fermented milk product of the Sikkim Himalayas. J. Sci. Indus. Res. 65, 747–752.
Dewan, S., and Tamang, J. P. (2007). Dominant lactic acid bacteria and their technological properties isolated from the Himalayan ethnic fermented milk products. Antonie van Leeuwenhoek 92, 343–352. doi: 10.1007/s10482-007-9163-5
Diancourt, L., Passet, V., Chervaux, C., Garault, P., Smokvina, T., and Brisse, S. (2007). Multilocus sequence typing of Lactobacillus casei reveals a clonal population structure with low levels of homologous recombination. Appl. Environ. Microbiol. 73, 6601–6611. doi: 10.1128/AEM.01095-07
Díaz-Ruiz, G., Guyot, J. P., Ruiz-Teran, F., Morlon-Guyot, J., and Wacher, C. (2003). Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: a functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 69, 4367–4374. doi: 10.1128/AEM.69.8.4367-4374.2003
Dirar, H. A., Harper, D. B., and Collins, M. A. (2006). Biochemical and microbiological studies on kawal, a meat substitute derived by fermentation of Cassia obtusifolia leaves. J. Sci. Food Agric. 36, 881–892. doi: 10.1002/jsfa.2740360919
Dolci, P., Alessandria, V., Rantsiou, K., and Cocolin, L. (2015). “Advanced methods for the identification, enumeration, and characterization of microorganisms in fermented foods,” in Advances in Fermented Foods and Beverages, ed W. H. Holzapfel (London: Elsevier), 157–176. doi: 10.1016/b978-1-78242-015-6.00007-4
Doyle, M. P., and Beuchat, L. R. (2013). Food Microbiology: Fundamentals and Frontiers, 4th Edn. Washington, DC: ASM Press. doi: 10.1128/9781555818463
Dung, N. T. P., Rombouts, F. M., and Nout, M. J. R. (2006). Functionality of selected strains of moulds and yeasts from Vietnamese rice wine starters. Food Microbiol. 23, 331–340. doi: 10.1016/j.fm.2005.05.002
Dung, N. T. P., Rombouts, F. M., and Nout, M. J. R. (2007). Characteristics of some traditional Vietnamese starch-based rice wine starters (Men). LWT Food Sci. Technol. 40, 130–135. doi: 10.1016/j.lwt.2005.08.004
Dušková, M., Šedo, O., Kšicová, K., Zdráhal, Z., and Karpíšková, R. (2012). Identification of lactobacilli isolated from food by genotypic methods and MALDI-TOF MS. Int. J. Food Microbiol. 159, 107–114. doi: 10.1016/j.ijfoodmicro.2012.07.029
Encinas, J. P., Lopez-Diaz, T. M., Garcia-Lopez, M. L., Otero, A., and Moreno, B. (2000). Yeast populations on Spanish fermented sausages. Meat Sci. 54, 203–208.
Endo, A., Mizuno, H., and Okada, S. (2008). Monitoring the bacterial community during fermentation of sunki, an unsalted, fermented vegetable traditional to the Kiso area of Japan. Letters Appl. Microbiol. 47, 221–226. doi: 10.1111/j.1472-765X.2008.02404.x
Ercolini, D. (2004). PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J. Microbiol. Methods 56, 297–314. doi: 10.1016/j.mimet.2003.11.006
Escalante, A., Giles-Gómez, M., Hernández, G., Córdova-Aguilar, M. S., López-Munguía, A., Gosset, G., et al. (2008). Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food Microbiol. 124, 126–134. doi: 10.1016/j.ijfoodmicro.2008.03.003
Escalante, A., Rodríguez, M. E., Martínez, A., López-Munguía, A., Bolívar, F., and Gosset, G. (2004). Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 2, 273–279. doi: 10.1111/j.1574-6968.2004.tb09599.x
Escobar, A., Gardner, A., and Steinkraus, K. H. (1996). “Studies of South American chichi” in Handbook of Indigenous Fermented Food, 2nd Edn., ed K. H. Steinkraus (New York, NY: Marcel Dekker, Inc.), 402–406.
Farhad, M., Kailasapathy, K., and Tamang, J. P. (2010). “Health aspects of fermented foods,” in Fermented Foods and Beverages of the World, eds J. P. Tamang and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 391–414.
Feng, X. M., Eriksson, A. R. B., and Schnürer, J. (2005). Growth of lactic acid bacteria and Rhizopus oligosporus during barley tempeh fermentation. Int. J. Food Microbiol. 104, 249–256. doi: 10.1016/j.ijfoodmicro.2005.03.005
Fernandez-Lopez, J., Sendra, E., Sayas-Barbera, E., Navarro, C., and Perez-Alvarez, J. A. (2008). Physico-chemical and microbiological profiles of “Salchichon” (Spanish dry fermented sausage) enriched with orange fiber. Meat Sci. 80, 410–417. doi: 10.1016/j.meatsci.2008.01.010
Flórez, A. B., and Mayo, B. (2006). Microbial diversity and succession during the manufacture and ripening of traditional, Spanish, blue-veined Cabrales cheese, as determined by PCR261 DGGE. Int. J. Food Microbiol. 110, 165–171. doi: 10.1016/j.ijfoodmicro.2006.04.016
Franz, C. M. A. P., Huch, M., Mathara, J. M., Abriouel, H., Benomar, N., Reid, G., et al. (2014). African fermented foods and probiotics. Int. J. Food Microbiol. 190, 84–96. doi: 10.1016/j.ijfoodmicro.2014.08.033
Fujimoto, J., and Watanabe, K. (2013). Quantitative detection of viable Bifidobacterium bifidum BF-1 in human feces by using propidium monoazide and strain-specific primers. Appl. Environ. Microbiol. 79, 2182–2188. doi: 10.1128/AEM.03294-12
Ganasen, P., and Benjakul, S. (2010). Physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cations. LWT Food Sci. Technol. 43, 77–85. doi: 10.1016/j.lwt.2009.06.007
Gänzle, M. G., Ehmann, M., and Hammes, W. P. (1998). Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl. Environ. Microbiol. 64, 2616–2623.
Garcia-Fontan, M. C., Lorenzo, J. M., Parada, A., Franco, I., and Carballo, J. (2007). Microbiological characteristics of “Androlla”, a Spanish traditional pork sausage. Food Microbiol. 24, 52–58. doi: 10.1016/j.fm.2006.03.007
Genccelep, H., Kaban, G., Aksu, M. I., Oz, F., and Kaya, M. (2008). Determination of biogenic amines in sucuk. Food Control 19, 868–872. doi: 10.1016/j.foodcont.2007.08.013
Ghosh, J., and Rajorhia, G. S. (1990). Selection of starter culture for production of indigenous fermented milk product (Misti dahi). Lait 70, 147–154. doi: 10.1051/lait:1990213
Giraffa, G., and Carminati, D. (2008). “Molecular techniques in food fermentation: principles and applications, Chap. 1” in Molecular Techniques in the Microbial Ecology of Fermented Foods, eds L. Cocolin, and D. Ercolini (New York, NY: Springer Science+Business Media, LCC), 1–30. doi: 10.1007/978-0-387-74520-6_1
Greppi, A., Rantsiou, K., Padonou, W., Hounhouigan, J., Jespersen, L., Jakobsen, M., et al. (2013a). Determination of yeast diversity in ogi, mawè, gowé and tchoukoutou by using culture-dependent and -independent methods. Int. J. Food Microbiol. 165, 84–88. doi: 10.1016/j.ijfoodmicro.2013.05.005
Greppi, A., Rantsiou, K., Padonou, W., Hounhouigan, J., Jespersen, L., Jakobsen, M., et al. (2013b). Yeast dynamics during spontaneous fermentation of mawè and tchoukoutou, two traditional products from Benin. Int. J. Food Microbiol. 165, 200–207. doi: 10.1016/j.ijfoodmicro.2013.05.004
Guan, L., Cho, K. H., and Lee, J. H. (2011). Analysis of the cultivable bacterial community in jeotgal, a Korean salted and fermented seafood, and identification of its dominant bacteria. Food Microbiol. 28, 101–113. doi: 10.1016/j.fm.2010.09.001
Gupta, M., Khetarpaul, N., and Chauhan, B. M. (1992). Rabadi fermentation of wheat: changes in phytic acid content and in vitro digestibility. Plant Foods Human Nutr. 42, 109–116. doi: 10.1007/BF02196463
Gupta, R. C., Mann, B., Joshi, V. K., and Prasad, D. N. (2000). Microbiological, chemical and ultrastructural characteristics of misti doi (sweetened dahi). J. Food Sci. Technol. 37, 54–57.
Guyot, J. P. (2010). “Fermented cereal products,” in Fermented Foods and Beverages of the World, eds J. P. Tamang and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 247–261. doi: 10.1201/ebk1420094954-c8
Hamad, S. H., Dieng, M. M. C., Ehrmann, M. A., and Vogel, R. F. (1997). Characterisation of the bacterial flora of Sudanese sorghum flour and sorghum sourdough. J. Appl. Microbiol. 83, 764–770. doi: 10.1046/j.1365-2672.1997.00310.x
Hammes, W. P., Brandt, M. J., Francis, K. L., Rosenheim, J., Seitter, M. F. H., and Vogelmann, S. A. (2005). Microbial ecology of cereal fermentations. Trends Food Sci. Technol. 16, 4–11. doi: 10.1016/j.tifs.2004.02.010
Hammes, W. P., and Ganzle, M. G. (1998). “Sourdough breads and related products,” in Microbiology of fermented foods, 2nd Edn., ed B. J. B. Wood (Glasgow: Blackie Academic and Professional), 199–216.
Han, B. Z., Beumer, R. R., Rombouts, F. M., and Nout, M. J. R. (2001). Microbiological safety and quality of commercial sufu- a Chinese fermented soybean food. Food Control 12, 541–547. doi: 10.1016/S0956-7135(01)00064-0
Hao, Y., Zhao, L., Zhang, H., and Zhai, Z. (2010). Identification of the bacterial biodiversity in koumiss by denaturing gradient gel electrophoresis and species-specific polymerase chain reaction. J. Dairy Sci. 93, 1926–1933. doi: 10.3168/jds.2009-2822
Hara, T., Chetanachit, C., Fujio, Y., and Ueda, S. (1986). Distribution of plasmids in polyglutamate-producing Bacillus strains isolated from “natto”–like fermented soybeans, “thua nao,” in Thailand. J. Gen. Appl. Microbiol. 32, 241–249. doi: 10.2323/jgam.32.241
Hara, T., Hiroyuki, S., Nobuhide, I., and Shinji, K. (1995). Plasmid analysis in polyglutamate-producing Bacillus strain isolated from non-salty fermented soybean food, “kinema”, in Nepal. J. Gen. Appl. Microbiol. 41, 3–9. doi: 10.2323/jgam.41.3
Harun-ur-Rashid, M., Togo, K., Useda, M., and Miyamoto, T. (2007). Probiotic characteristics of lactic acid bacteria isolated from traditional fermented milk “Dahi” in Bangladesh. Pakistan J. Nutr. 6, 647–652. doi: 10.3923/pjn.2007.647.652
Haruta, S., Ueno, S., Egawa, I., Hashiguchi, K., Fujii, A., Nagano, M., et al. (2006). Succession of bacterial and fungal communities during a traditional pot fermentation of rice vinegar assessed by PCR-mediated denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 109, 79–87. doi: 10.1016/j.ijfoodmicro.2006.01.015
Hayashida, F. M. (2008). Ancient beer and modern brewers: ethnoarchaeological observations of chicha production in two regions of the North Coast of Peru. J. Anthropol. Archaeol. 27, 161–174 doi: 10.1016/j.jaa.2008.03.003
Hesseltine, C. W. (1979). Some important fermented foods of Mid-Asia, the Middle East, and Africa. J. Am. Oil Chem. Soc. 56, 367–374. doi: 10.1007/BF02671501
Hesseltine, C. W. (1983). Microbiology of oriental fermented foods. Ann. Rev. Microbiol. 37, 575–601. doi: 10.1146/annurev.mi.37.100183.003043
Hesseltine, C. W., and Kurtzman, C. P. (1990). Yeasts in amylolytic food starters. Anales del instituto de biologia de la universidad nacional autonoma de Mexico. Serie Botanica 60, 1–7.
Hesseltine, C. W., and Ray, M. L. (1988). Lactic acid bacteria in murcha and ragi. J. Appl. Bacteriol. 64, 395–401. doi: 10.1111/j.1365-2672.1988.tb05096.x
Hirasawa, T., Yamada, K., Nagahisa, K., Dinh, T. N., Furusawa, C., Katakura, Y., et al. (2009). Proteomic analysis of responses to osmotic stress in laboratory and sake-brewing strains of Saccharomyces cerevisiae. Process Biochem. 44, 647–653. doi: 10.1016/j.procbio.2009.02.004
Ho, C. C. (1986). Identity and characteristics of Neurospora intermedia responsible for oncom fermentation in Indonesia. Food Microbiol. 3, 115–132. doi: 10.1016/S0740-0020(86)80035-1
Holzapfel, W. (2002). Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 75, 197–212. doi: 10.1016/S0168-1605(01)00707-3
Holzapfel, W. H. (1997). Use of starter cultures in fermentation on a household scale. Food Control 8, 241–258. doi: 10.1016/S0956-7135(97)00017-0
Holzapfel, W. H., and Wood, B. J. B. (2014). Lactic Acid Bacteria: Biodiversity And Taxonomy. New York, NY: Wiley-Blackwell, 632. doi: 10.1002/9781118655252
Hong, S. W., Choi, J. Y., and Chung, K. S. (2012). Culture-based and denaturing gradient gel electrophoresis analysis of the bacterial community from chungkookjang, a traditional Korean fermented soybean food. J. Food Sci. 77, M572–578. doi: 10.1111/j.1750-3841.2012.02901.x
Hosono, A., Wardoyo, R., and Otani, H. (1989). Microbial flora in “dadih”, a traditional fermented milk in Indonesia. Lebensm Wiss Technol. 22, 20–24.
Humblot, C., and Guyot, J. P. (2009). Pyrosequencing of tagged 16S rRNA gene amplicons for rapid deciphering of the microbiomes of fermented foods such as pearl millet slurries. Appl. Environ. Microbiol. 75, 4354–4361. doi: 10.1128/AEM.00451-09
Hwanhlem, N., Buradaleng, S., Wattanachant, S., Benjakul, S., Tani, A., and Maneerat, S. (2011). Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control 22, 401–407. doi: 10.1016/j.foodcont.2010.09.010
Iacumin, L., Cecchini, F., Manzano, M., Osualdini, M., Boscolo, D., Orlic, S., et al. (2009). Description of the microflora of sourdoughs by culture-dependent and culture-independent methods. Food Microbiol. 26, 128–135. doi: 10.1016/j.fm.2008.10.010
Ijong, F. G., and Ohta, Y. (1996). Physicochemical and microbiological changes associated with bakasang processing - a traditional Indonesian fermented fish sauce. J. Sci. Food Agric. 71, 69–74.
Inatsu, Y., Nakamura, N., Yuriko, Y., Fushimi, T., Watanasritum, L., and Kawanmoto, S. (2006). Characterization of Bacillus subtilis strains in Thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 43, 237–242. doi: 10.1111/j.1472-765X.2006.01966.x
Itoh, H., Tachi, H., and Kikuchi, S. (1993). “Fish fermentation technology in Japan,” in Fish Fermentation Technology, eds C. H. Lee, K. H. Steinkraus, and P. J. Alan Reilly (Tokyo: United Nations University Press), 177–186.
Jagannath, A., Raju, P. S., and Bawa, A. S. (2010). Comparative evaluation of bacterial cellulose (natta) as a cryoprotectant and carrier support during the freeze drying process of probiotic lactic acid bacteria. LWT Food Sci. Technol. 43, 1197–1203. doi: 10.1016/j.lwt.2010.03.009
Jeng, K. C., Chen, C. S., Fang, Y. P., Hou, R. C. W., and Chen, Y. S. (2007). Effect of microbial fermentation on content of statin, GABA, and polyphenols in Puerh tea. J. Agric. Food Chem. 55, 8787–8792. doi: 10.1021/jf071629p
Jennessen, J., Schnürer, J., Olsson, J., Samson, R. A., and Dijiksterhuis, J. (2008). Morphological characteristics of sporangiospores of the tempe fungus Rhizopus oligosporus differentiate it from other taxa of the R. microsporus group. Mycol. Res. 112, 547–563. doi: 10.1016/j.mycres.2007.11.006
Jeyaram, K., Mohendro Singh, W., Capece, A., and Romano, P. (2008a). Molecular identification of yeast species associated with ‘Hamei”- a traditional starter used for rice wine production in Manipur, India. Int. J. Food Microbiol. 124, 115–125. doi: 10.1016/j.ijfoodmicro.2008.02.029
Jeyaram, K., Mohendro Singh, W., Premarani, T., Ranjita Devi, A., Selina Chanu, K., Talukdar, N. C., et al. (2008b). Molecular identification of dominant microflora associated with ‘Hawaijar’ – a traditional fermented soybean (Glycine max L.) food of Manipur, India. Int. J. Food Microbiol. 122, 259–268. doi: 10.1016/j.ijfoodmicro.2007.12.026
Jeyaram, K., Romi, W., Ah Singh, T., Devi, A. R., and Devi, S. S. (2010). Bacterial species associated with traditional starter cultures used for fermented bamboo shoot production in Manipur state of India. Int. J. Food Microbiol. 143, 1–8. doi: 10.1016/j.ijfoodmicro.2010.07.008
Jeyaram, K., Tamang, J. P., Capece, A., and Romano, P. P. (2011). Geographical markers for Saccharomyces cerevisiae strains with similar technological origins domesticated for rice-based ethnic fermented beverages production in North East India. Antonie van Leeuwenhoek 100, 569–578. doi: 10.1007/s10482-011-9612-z
Jianzhonga, Z., Xiaolia, L., Hanhub, J., and Mingshengb, D. (2009). Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 26, 770–775. doi: 10.1016/j.fm.2009.04.009
Johanningsmeier, S., McFeeters, R. F., Fleming, H. P., and Thompson, R. L. (2007). Effects of Leuconostoc mesenteroides starter culture on fermentation of cabbage with reduced salt concentrations. J. Food Sci. 72, M166–M172. doi: 10.1111/j.1750-3841.2007.00372.x
Johnson, E. A., and Echavarri-Erasun, C. (2011). “Yeast Biotechnology,” in The Yeasts: A Taxonomic Study 5th Edn., Vol. 1, eds C. Kurtzman, J. W. Fell, and T. Boekhout (Amsterdam: Elsevier), 23. doi: 10.1016/b978-0-444-52149-1.00003-3
Josephsen, J., and Jespersen, L. (2004). “Handbook of Food and Beverage Fermentation Technology,” in Starter Cultures and Fermented Products, eds Y. H. Hui, L. Meunier-Goddik, Å. S. Hansen, J. Josephsen, W. K. Nip, P. S. Stanfield, F. Toldrá (New York, NY: Marcel Dekker, Inc.), 23–49.
Jung, J. Y., Lee, S. H., Jin, H. M., Hahn, Y., Madsen, E. L., and Jeon, C. O. (2013a). Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int. J. Food Microbiol. 163, 171–179. doi: 10.1016/j.ijfoodmicro.2013.02.022
Jung, J. Y., Lee, S. H., Kim, J. M., Park, M. S., Bae, J. W., Hahn, Y., et al. (2011). Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 77, 2264–2274. doi: 10.1128/AEM.02157-10
Jung, J. Y., Lee, S. H., Lee, H. J., and Jeon, C. O. (2013b). Microbial succession and metabolite changes during fermentation of saeu-jeot: traditional Korean salted seafood. Food Microbiol. 34, 360–368. doi: 10.1016/j.fm.2013.01.009
Jung, M. J., Nam, Y. D., Roh, S. W., and Bae, J. W. (2012). Unexpected convergence of fungal and bacterial communities during fermentation of traditional Korean alcoholic beverages inoculated with various natural starters. Food Microbiol. 30, 112–123. doi: 10.1016/j.fm.2011.09.008
Kahala, M., Mäki, M., Lehtovaara, A., Tapanainen, J. M., Katiska, R., Juuruskorpi, M., et al. (2008). Characterization of starter lactic acid bacteria from the Finnish fermented milk product viili. J. Appl. Microbiol. 105, 1929–1938. doi: 10.1111/j.1365-2672.2008.03952.x
Karki, T., Okada, S., Baba, T., Itoh, H., and Kozaki, M. (1983). Studies on the microflora of Nepalese pickles gundruk. Nippon Shokuhin Kogyo Gakkaishi 30, 357–367. doi: 10.3136/nskkk1962.30.357
Khanh, T. M., May, B. K., Smooker, P. M., Van, T. T. H., and Coloe, P. J. (2011). Distribution and genetic diversity of lactic acid bacteria from traditional fermented sausage. Food Res. Int. 44, 338–344. doi: 10.1016/j.foodres.2010.10.010
Kiers, J. L., Van laeken, A. E. A., Rombouts, F. M., and Nout, M. J. R. (2000). In vitro digestibility of Bacillus fermented soya bean. Int. J. Food Microbiol. 60, 163–169. doi: 10.1016/S0168-1605(00)00308-1
Kim, T. W., Lee, J. W., Kim, S. E., Park, M. H., Chang, H. C., and Kim, H. Y. (2009). Analysis of microbial communities in doenjang, a Korean fermented soybean paste, using nested PCR-denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 131, 265–271. doi: 10.1016/j.ijfoodmicro.2009.03.001
Kim, Y. B., Seo, Y. G., and Lee, C. H. (1993). “Growth of microorganisms in dorsal muscle of gulbi during processing and their effect on its quality” in Fish Fermentation Technology, eds C. H. Lee, K. H. Steinkraus, and P. J. Alan Reilly (Tokyo: United Nations University Press), 281–289
Kimura, K., and Itoh, Y. (2007). Determination and characterization of IS4Bsu1-insertion loci and identification of a new insertion sequence element of the IS256 family in a natto starter. Biosci. Biotechnol. Biochem. 71, 2458–2464. doi: 10.1271/bbb.70223
Kingston, J. J., Radhika, M., Roshini, P. T., Raksha, M. A., Murali, H. S., and Batra, H. V. (2010). Molecular characterization of lactic acid bacteria recovered from natural fermentation of beet root and carrot Kanji. Indian J. Microbiol. 50, 292–298. doi: 10.1007/s12088-010-0022-0
Kiyohara, M., Koyanagi, T., Matsui, H., Yamamoto, K., Take, H., Katsuyama, Y., et al. (2012). Changes in microbiota population during fermentation of Narezushi as revealed by pyrosequencing analysis. Biosci. Biotechnol. Biochem. 76, 48–52. doi: 10.1271/bbb.110424
Kobayashi, T., Kimura, B., and Fujii, T. (2000a). Strictly anaerobic halophiles isolated from canned Swedish fermented herrings (Suströmming). Int. J. Food Microbiol. 54, 81–89. doi: 10.1016/S0168-1605(99)00172-5
Kobayashi, T., Kimura, B., and Fujii, T. (2000b). Haloanaerobium fermentans sp. nov., a strictly anaerobic, fermentative halophile isolated from fermented puffer fish ovaries. Int. J. Syst. Evol. Microbiol. 50, 1621–1627. doi: 10.1099/00207713-50-4-1621
Kobayashi, T., Kimura, B., and Fujii, T. (2000c). Differentiation of Tetragenococcus populations occurring in products and manufacturing processes of puffer fish ovaries fermented with rice-bran. Int. J. Food Microbiol. 56, 211–218. doi: 10.1016/S0168-1605(00)00214-2
Kolawole, O. M., Kayode, R. M. O., and Akinduyo, B. (2013). Proximate and microbial analyses of burukutu and pito produced in Ilorin, Nigeria. Afr. J. Microbiol. 1, 15–17.
Kotaka, A., Bando, H., Kaya, M., Kato-Murai, M., Kuroda, K., Sahara, H., et al. (2008). Direct ethanol production from barley β-glucan by sake yeast displaying Aspergillus oryzae β-glucosidase and endoglucanase. J. Biosci. Bioeng. 105, 622–627. doi: 10.1263/jbb.105.622
Kozaki, M., Tamang, J. P., Kataoka, J., Yamanaka, S., and Yoshida, S. (2000). Cereal wine (jaanr) and distilled wine (raksi) in Sikkim. J. Brew. Soc. Japan 95, 115–122. doi: 10.6013/jbrewsocjapan1988.95.115
Kubo, Y., Rooney, A. P., Tsukakoshi, Y., Nakagawa, R., Hasegawa, H., and Kimura, K. (2011). Phylogenetic analysis of Bacillus subtilis Strains applicable to natto (fermented soybean) production. Appl. Environ. Microbiol. 77, 6463–6469. doi: 10.1128/AEM.00448-11
Kuda, T., Izawa, Y., Yoshida, S., Koyanagi, T., Takahashi, H., and Kimura, B. (2014). Rapid identification of Tetragenococcus halophilus and Tetragenococcus muriaticus, important species in the production of salted and fermented foods, by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Food Control 35, 419–425. doi: 10.1016/j.foodcont.2013.07.039
Kurtzman, C. P., and Robnett, C. J. (2003). Phylogenetic relationship among yeasts of the “Saccharomyces complex” determined from multigene sequence analyses. FEMS Yeast Res. 3, 417–432. doi: 10.1016/S1567-1356(03)00012-6
Kurtzman, C. P., Robnett, C. J., and Basehoar-Powers, E. (2001). Zygosaccharomyces kombuchaensis, a new ascosporogenous yeast from ‘Kombucha tea’. FEMS Yeast Res. 1, 133–138. doi: 10.1111/j.1567-1364.2001.tb00024.x
Kutyauripo, J., Parawira, W., Tinofa, S., Kudita, I., and Ndengu, C. (2009). Investigation of shelf-life extension of sorghum beer (Chibuku) by removing the second conversion of malt. Int. J. Food Microbiol. 129, 271–276. doi: 10.1016/j.ijfoodmicro.2008.12.008
Kwon, G. H., Lee, H. A., Park, J. Y., Kim, J. S., Lim, J., Park, C. S., et al. (2009). Development of a RAPD-PCR method for identification of Bacillus species isolated from Cheonggukjang. Int. J. Food Microbiol. 129, 282–287. doi: 10.1016/j.ijfoodmicro.2008.12.013
Lappe-Oliveras, P., Moreno-Terrazas, R., Arrizón-Gaviño, J., Herrera-Suárez, T., Garcia-Mendoza, A., and Gschaedler-Mathis, A. (2008). Yeasts associated with the production of Mexican alcoholic non distilled and distilled Agave beverages. FEMS Yeast Res. 8, 1037–1052. doi: 10.1111/j.1567-1364.2008.00430.x
Lee, C. H. (1993). “Fish fermentation technology in Korea,” in Fish Fermentation Technology, eds C. H., Lee, K. H. Steinkraus, and P. J. Alan Reilly (Tokyo: United Nations University Press), 187–201.
Lefeber, T., Janssens, M., Camu, N., and De Vuyst, L. (2010). Kinetic analysis of strains of lactic acid bacteria and acetic acid bacteria in cocoa pulp simulation media toward development of a starter culture for cocoa bean fermentation. Appl. Environ. Microbiol. 76, 7708–7716. doi: 10.1128/AEM.01206-10
Lopetcharat, K., Choi, Y. J., Park, J. W., and Daeschel, M. A. (2001). Fish sauce products and manufacturing: a review. Food Rev. Int. 17, 65–88. doi: 10.1081/FRI-100000515
Lücke, F. K. (2015). “Quality improvement and fermentation control in meat products,” in Advances in Fermented Foods and Beverages. Improving Quality, Technologies and Health Benefits. Woodhead Publishing Series in Food Science, Technology and Nutrition No. 265. ed W. H. Holzapfel (Cambridge: Woodhead Publishing Ltd.), 357–376. doi: 10.1016/b978-1-78242-015-6.00015-3
Lv, X.-C., Huang, X.-L., Zhang, W., Rao, P.-F., and Ni, L. (2013). Yeast diversity of traditional alcohol fermentation starters for Hong Qu glutinous rice wine brewing, revealed by culture-dependent and culture-independent methods. Food Control 34, 183–190. doi: 10.1016/j.foodcont.2013.04.020
Lyumugabe, F., Gros, J., Nzungize, J., Bajyana, E., and Thonart, P. (2012). Characteristics of African traditional beers brewed with sorghum malt: a review. Biotechnol. Agron. Soc. Environ. 16, 509–530.
Marsh, A. J., O'sullivan, O., Hill, C. R., Ross, R. P., and Cotter, D. (2014). Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 38, 171–178. doi: 10.1016/j.fm.2013.09.003
Martín, B., Garriga, M., Hugas, M., Bover-Cid, S., Veciana-Noqués, M. T., and Aymerich, T. (2006). Molecular, technological and safety characterization of Gram-positive catalase-positive cocci from slightly fermented sausages. Int. J. Food Microbiol. 107, 148–158. doi: 10.1016/j.ijfoodmicro.2005.08.024
Marty, E., Buchs, J., Eugster-Meier, E., Lacroix, C., and Meile, L. (2011). Identification of staphylococci and dominant lactic acid bacteria in spontaneously fermented Swiss meat products using PCR–RFLP. Food Microbiol. 29, 157–166. doi: 10.1016/j.fm.2011.09.011
Mayo, B., Ammor, M. S., Delgado, S., and Alegría, A. (2010). “Fermented milk products,” in Fermented Foods and Beverages of the World, eds J. P. Tamang, and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 263–288. doi: 10.1201/ebk1420094954-c9
Meerak, J., Iida, H., Watanabe, Y., Miyashita, M., Sato, H., Nakagawa, Y., et al. (2007). Phylogeny of ©-polyglutamic acid-producing Bacillus strains isolated from fermented soybean foods manufactured in Asian countries. J. Gen. Appl. Microbiol. 53, 315–323. doi: 10.2323/jgam.53.315
Meerak, J., Yukphan, P., Miyashita, M., Sato, H., Nakagawa, Y., and Tahara, Y. (2008). Phylogeny of ©-polyglutamic acid-producing Bacillus strains isolated from a fermented locust bean product manufactured in West Africa. J. Gen. Appl. Microbiol. 54, 159–166. doi: 10.2323/jgam.54.159
Merican, Z., and Yeoh, Q. L. (1989). “Tapai proceeding in Malaysia: a technology in transition,” in Industrialization Of Indigenous Fermented Foods, ed K. H. Steinkraus (New York, NY: Marcel Dekker, Inc.), 169–189.
Mo, H., Zhu, Y., and Chen, Z. (2008). Microbial fermented tea – a potential source of natural food preservatives. Trends Food Sci. Technol. 19, 124–130. doi: 10.1016/j.tifs.2007.10.001
Moreira, N., Mendes, F., Hogg, T., and Vasconcelos, I. (2005). Alcohols, esters and heavy sulphur compounds produced by pure and mixed cultures of apiculture wine yeasts. Int. J. Food Microbiol. 103, 285–294. doi: 10.1016/j.ijfoodmicro.2004.12.029
Moroni, A. V., Arendt, E. K., and Bello, F. D. (2011). Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiol. 28, 497–502. doi: 10.1016/j.fm.2010.10.016
Mozzi, F., Eugenia Ortiz, M., Bleckwedel, J., De Vuyst, L., and Micaela, P. (2013). Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 54, 1152–1161. doi: 10.1016/j.foodres.2012.11.010
Mugula, J. K., Ninko, S. A. M., Narvhus, J. A., and Sorhaug, T. (2003). Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 80, 187–199. doi: 10.1016/S0168-1605(02)00141-1
Muzaddadi, A. U. (2015). Minimisation of fermentation period of shidal from barbs (Puntius spp.). Fishery Technol. 52, 34–41.
Myuanja, C. M. B. K., Narvhus, J. A., Treimo, J., and Langsrud, T. (2003). Isolation, characterisation and identification of lactic acid bacteria from bushera: a Ugandan tradition al fermented beverage. Int. J. Food Microbiol. 80, 201–210. doi: 10.1016/S0168-1605(02)00148-4
Nagai, T., and Tamang, J. P. (2010). “Fermented soybeans and non-soybeans legume foods,” in Fermented Foods and Beverages of the World, eds J. P. Tamang, and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 191–224.
Nakao, S. (1972). “Mame no ryori,” in Ryori no kigen, ed S. Nakao (Tokyo: Japan Broadcast Publishing), 115–126.
Nam, Y. D., Chang, H. W., Kim, K. H., Roh, S. W., and Bae, J. W. (2009). Metatranscriptome analysis of lactic acid bacteria during kimchi fermentation with genome-probing microarrays. Int. J. Food Microbiol. 130, 140–146. doi: 10.1016/j.ijfoodmicro.2009.01.007
Nam, Y. D., Lee, S. Y., and Lim, S. I. (2011). Microbial community analysis of Korean soybean pastes bynext-generation sequencing. Int. J. Food Microbiol. 155, 36–42. doi: 10.1016/j.ijfoodmicro.2012.01.013
Nam, Y. D., Yi, S. H., and Lim, S. I. (2012). Bacterial diversity of cheonggukjang, a traditional Koreanfermented food, analyzed by barcoded pyrosequencing. Food Control 28, 135–142. doi: 10.1016/j.foodcont.2012.04.028
Nguyen, D. T. L., Van Hoorde, K., Cnockaert, M., de Brandt, E., Aerts, M., Thanh, and, L. B., et al. (2013a). A description of the lactic acid bacteria microbiota associated with the production of traditional fermented vegetables in Vietnam. Int. J. Food Microbiol. 163, 19–27. doi: 10.1016/j.ijfoodmicro.2013.01.024
Nguyen, D. T. L., Van Hoorde, K., Cnockaert, M., de Brandt, E., de Bruyne, K., Le, B. T., et al. (2013b). A culture-dependent and -independent approach for the identification of lactic acid bacteria associated with the production of nem chua, a Vietnamese fermented meat product. Food Res. Int. 50, 232–240. doi: 10.1016/j.foodres.2012.09.029
Nguyen, H. T., Elegado, F. B., Librojo-Basilio, N. T., Mabesa, R. C., and Dozon, E. I. (2011). Isolation and characterisation of selected lactic acid bacteria for improved processing of Nem chua, a traditional fermented meat from Vietnam. Benef. Microbes 1, 67–74. doi: 10.3920/BM2009.0001
Nielsen, D. S., Schillinger, U., Franz, C. M. A. P., Bresciani, J., Amoa-Awua, W., Holzapfel, W. H., et al. (2007). Lactobacillus ghanensis sp. nov., a motile lactic acid bacterium isolated from Ghanaian cocoa fermentations. Int. J. Syst. Evol. Microbiol. 57, 1468–1472. doi: 10.1099/ijs.0.64811-0
Nikkuni, S., Karki, T. B., Terao, T., and Suzuki, C. (1996). Microflora of mana, a Nepalese rice koji. J. Fermen Bioeng. 81, 168–170. doi: 10.1016/0922-338X(96)87597-0
Nishito, Y., Osana, Y., Hachiya, T., Popendorf, K., Toyoda, A., Fujiyama, A., et al. (2010). Whole genome assembly of a natto production strain Bacillus subtilis natto from very short read data. BMC Genomics 11:243. doi: 10.1186/1471-2164-11-243
Nout, M. J. R., and Aidoo, K. E. (2002). “Asian fungal fermented food,” in The Mycota, ed H. D. Osiewacz (New York, NY: Springer-Verlag), 23–47. doi: 10.1007/978-3-662-10378-4_2
Odunfa, S. A., and Oyewole, O. B. (1997). African fermented Foods. London: Blackie Academic and Professional.
Oguntoyinbo, F. A., and Dodd, C. E. R. (2010). Bacterial dynamics during the spontaneous fermentation of cassava dough in gari production. Food Control 21, 306–312. doi: 10.1016/j.foodcont.2009.06.010
Oguntoyinbo, F. A., Huch, M., Cho, G. S., Schillinger, U., Holzapfel, W. H., Sanni, A. I., et al. (2010). Diversity of Bacillus species isolated from okpehe, a traditional fermented soup condiment from Nigeria. J. Food Protect. 73, 870–878.
Oguntoyinbo, F. A., Tourlomousis, P., Gasson, M. J., and Narbad, A. (2011). Analysis of bacterial communities of traditional fermented West African cereal foods using culture independent methods. Int. J. Food Microbiol. 145, 205–210. doi: 10.1016/j.ijfoodmicro.2010.12.025
Oguntoyinbo, F. A., Sanni Abiodun, I. S., Franz, C. M. A. P., and Holzapfel, W. H. (2007). In vitro fermentation studies for selection and evaluation of Bacillus strains as starter cultures for the production of okpehe, a traditional African fermented condiment. Int. J. Food Microbiol. 113, 208–218. doi: 10.1016/j.ijfoodmicro.2006.07.006
Oki, K., Dugersuren, J., Demberel, S., and Watanabe, K. (2014). Pyrosequencing analysis on the microbial diversity in Airag, Khoormog and Tarag, traditional fermented dairy products of Mongolia. Biosci. Microbiota Food Health 33, 53–64. doi: 10.12938/bmfh.33.53
Oki, K., Kudo, Y., and Watanabe, K. (2012). Lactobacillus saniviri sp. nov. and Lactobacillus senioris sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 62, 601–607. doi: 10.1099/ijs.0.031658-0
Oki, K., Rai, A. K., Sato, S., Watanabe, K., and Tamang, J. P. (2011). Lactic acid bacteria isolated from ethnic preserved meat products of the Western Himalayas. Food Microbiol. 28, 1308–1315. doi: 10.1016/j.fm.2011.06.001
Olasupo, N. A., Odunfa, S. A., and Obayori, O. S. (2010). “Ethnic African fermented foods,” in Fermented Foods and Beverages of the World, eds J. P. Tamang and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 323–352. doi: 10.1201/ebk1420094954-c12
Osvik, R. D., Sperstad, S., Breines, E., Hareide, E., Godfroid, J., Zhou, Z., et al. (2013). Bacterial diversity of a Masi, a South African fermented milk product, determined by clone library and denaturing gradient gel electrophoresis analysis. African J. Microbiol. Res. 7, 4146–4158.
Ouoba, L. I., Diawara, B., Wk, A. A., Traore, A., and Moller, P. (2004). Genotyping of starter cultures of Bacillus subtilis and Bacillus pumilus for fermentation of African locust bean (Parkia biglobosa) to produce Soumbala. Int. J. Food Microbiol. 90, 197–205. doi: 10.1016/S0168-1605(03)00302-7
Ouoba, L. I., Kando, C., Parkouda, C., Sawadogo-Lingani, H., Diawara, B., and Sutherland, J. P. (2012). The microbiology of Bandji, palm wine of Borassus akeassii from Burkina Faso: identification and genotypic diversity of yeasts, lactic acid and acetic acid bacteria. J. Appl. Microbiol. 113, 1428–1441. doi: 10.1111/jam.12014
Ouoba, L. I., Nyanga-Koumou, C. A., Parkouda, C., Sawadogo, H., Kobawila, S. C., Keleke, S., et al. (2010). Genotypic diversity of lactic acid bacteria isolated from African traditional alkaline-fermented foods. J. Appl. Microbiol. 108, 2019–2029. doi: 10.1111/j.1365-2672.2009.04603.x
Ouoba, L. I., Parkouda, C., Diawara, B., Scotti, C., and Varnam, A. (2008). Identification of Bacillus spp. from Bikalga, fermented seeds of Hibiscus sabdariffa: phenotypic and genotypic characterization. J. Appl. Microbiol. 104, 122–131. doi: 10.1111/j.1365-2672.2007.03550.x
Oyewole, O. B., Olatunji, O. O., and Odunfa, S. A. (2004). A process technology for conversion of dried cassava chips into ‘gari’. Nigerian Food J. 22, 65–76.
Papalexandratou, Z., Vrancken, G., De Bruyne, K., Vandamme, P., and de Vuyst, L. (2011). Spontaneous organic cocoa bean box fermentations in Brazil are characterized by a restricted species diversity of lactic acid bacteria and acetic acid bacteria. Food Microbiol. 28, 1326–1338. doi: 10.1016/j.fm.2011.06.003
Parente, E., and Cogan, T. M. (2004). “Starter cultures: general aspects,” in Cheese: Chemistry, Physics and Microbiology, 3rd Edn, ed P. O. Fox (Oxford: Elsevier), 123–147. doi: 10.1016/S1874-558X(04)80065-4
Parente, E., Martuscelli, M., Gardini, F., Grieco, S., Crudele, M. A., and, G., and Suzzi, G. (2001b). Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J. Appl. Microbiol. 90, 882–891. doi: 10.1046/j.1365-2672.2001.01322.x
Parente, E. S., Di Matteo, M., Spagna Musso, S., and Crudele, M. A. (1994). Use of commercial starter cultures in the production of soppressa lucana, a fermented sausage from Basilicata. Italian J. Sci. 6, 59–69.
Parente, E. S., Grieco, S., and Crudele, M. A. (2001a). Phenotypic diversity of lactic acid bacteria isolated from fermented sausages produced in Basilicata (Southern Italy). J. Appl. Microbiol. 90, 943–952. doi: 10.1046/j.1365-2672.2001.01328.x
Park, C., Choi, J. C., Choi, Y. H., Nakamura, H., Shimanouchi, K., Horiuchi, T., et al. (2005). Synthesis of super-high-molecular-weight poly-©-glutamic acid by Bacillus subtilis subsp. chungkookjang. J. Mol. Catal. B. Enzym. 35, 128–133. doi: 10.1016/j.molcatb.2005.06.007
Park, E. J., Chang, H. W., Kim, K. H., Nam, Y. D., Roh, S. W., and Bae, J. W. (2009). Application of quantitative real-time PCR for enumeration of total bacterial, archaeal, and yeast populations in kimchi. J. Microbiol. 47, 682–685. doi: 10.1007/s12275-009-0297-1
Park, E. J., Chun, J., Cha, C. J., Park, W. S., Jeon, C. O., and Bae, J. W. (2012). Bacterial community analysis during fermentation of ten representative kinds of kimchi with barcoded pyrosequencing. Food Microbiol. 30, 197–204. doi: 10.1016/j.fm.2011.10.011
Park, J. M., Shin, J. H., Lee, D. W., Song, J. C., Suh, H. J., Chang, U. J., et al. (2010). Identification of the lactic acid bacteria in kimchi according to initial and over-ripened fermentation using PCR and 16S rRNA gene sequence analysis. Food Sci. Biotechnol. 19, 541–546. doi: 10.1007/s10068-010-0075-1
Parkouda, C., Nielsen, D. S., Azokpota, P., Ouoba, L. I. I., Amoa-Awua, W. K., Thorsen, L., et al. (2009). The microbiology of alkaline-fermentation of indigenous seeds used as food condiments in Africa and Asia. Critical Rev. Microbiol. 35, 139–156. doi: 10.1080/10408410902793056
Patil, M. M., Pal, A., Anand, T., and Ramana, K. V. (2010). Isolation and characterization of lactic acid bacteria from curd and cucumber. Indian J. Biotechnol. 9, 166–172.
Pederson, C. S. (1979). Microbiology of Food Fermentations, 2nd edition. Westport, AVI Publishing Company.
Phithakpol, B., Varanyanond, W., Reungmaneepaitoon, S., and Wood, H. (1995). The Traditional Fermented Foods of Thailand. Kuala Lumpur: ASEAN Food Handling Bureau.
Picozzi, C., Bonacina, G., Vigentini, I., and Foschino, R. (2010). Genetic diversity in Italian Lactobacillus sanfranciscensis strains assessed by multilocus sequence typing and pulsed field gel electrophoresis analyses. Microbiol. 156, 2035–2045. doi: 10.1099/mic.0.037341-0
Plengvidhya, V., Breidt, F., and Fleming, H. P. (2007). Use of RAPD-PCR as a method to follow the progress of starter cultures in sauerkraut fermentation. Int. J. Food Microbiol. 93, 287–296. doi: 10.1016/j.ijfoodmicro.2003.11.010
Pretorius, I. S. (2000). Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16, 675–729. doi: 10.1002/1097-0061(20000615)16:8&<675::AID-YEA585&>3.0.CO;2-B
Pretorius, I. S., Curtin, C. D., and Chambers, P. J. (2015). “Designing wine yeast for the future, Chap. 9,” in Advances in fermented foods and beverages. Improving quality, technologies and health benefits. Woodhead Publishing Series in Food Science, Technology and Nutrition No. 265. ed W. H. Holzapfel (Cambridge: Woodhead Publishing Ltd.), 197–226.
Puerari, C., Magalhâes-Guedes, T. M., and Schwan, R. F. (2015). Physicochemical and microbiological characterization of chicha, a rice-based fermented beverage produced by Umutina Brazilian Amerindians. Food Microbiol. 46, 210–217. doi: 10.1016/j.fm.2014.08.009
Puspito, H., and Fleet, G. H. (1985). Microbiology of sayur asin fermentation. Appl. Microbiol. Biotechnol. 22, 442–445. doi: 10.1007/BF00252788
Qin, H., Yang, H., Qiao, Z., Gao, S., and Liu, Z. (2013). Identification and characterization of a Bacillus subtilis strain HB-1 isolated from Yandou, a fermented soybean food in China. Food Control 31, 22–27. doi: 10.1016/j.foodcont.2012.10.004
Quigley, L., O'sullivan, O., Beresford, T. P., Ross, R. P., Fitzgerald, G. F., and Cotter, P. D. (2011). Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 150, 81–94. doi: 10.1016/j.ijfoodmicro.2011.08.001
Rai, A. K., Palni, U., and Tamang, J. P. (2010). Microbiological studies of ethnic meat products of the Eastern Himalayas. Meat Sci. 85, 560–567. doi: 10.1016/j.meatsci.2010.03.006
Ramos, C. L., de Almeida, E. G., de Melo Pereira, G. V., Cardoso, P. G., Dias, E. S., and Schwan, R. F. (2010). Determination of dynamic characteristics of microbiota in a fermented beverage produced by Brazilian Amerindians using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 140, 225–231. doi: 10.1016/j.ijfoodmicro.2010.03.029
Rani, D. K., and Soni, S. K. (2007). “Applications and commercial uses of microorganisms,” in Microbes: a source of energy for 21st century. ed S. K. Soni (Delhi: Jai Bharat Printing Press), 71–126.
Rapsang, G. F., Kumar, R., and Joshi, S. R. (2011). Identification of Lactobacillus puhozihii from tungtap: A traditionally fermented fish food, and analysis of its bacteriocinogenic potential. African J. Biotechnol. 10, 12237–12243.
Rhee, S. J., Lee, J. E., and Lee, C. H. (2011). Importance of lactic acid bacteria in Asian fermented foods. Microbial Cell Factories 10, 1–13. doi: 10.1186/1475-2859-10-S1-S5
Robert, H., Gabriel, V., and Fontagné-Faucher, C. (2009). Biodiversity of lactic acid bacteria in French wheat sourdough as determined by molecular characterization using species-specific PCR. Int. J. Food Microbiol. 135, 53–59. doi: 10.1016/j.ijfoodmicro.2009.07.006
Romi, W., Ahmed, G., and Jeyaram, K. (2015). Three-phase succession of autochthonous lactic acid bacteria to reach a stable ecosystem within 7 days of natural bamboo shoot fermentation as revealed by different molecular approaches. Mol. Ecol. 13, 3372–3389. doi: 10.1111/mec.13237
Saisithi, P. (1987). Traditional fermented fish products with special reference to Thai products. ASEAN Food J. 3, 3–10
Saithong, P., Panthavee, W., Boonyaratanakornkit, M., and Sikkhamondhol, C. (2010). Use of a starter culture of lactic acid bacteria in plaa-som, a Thai fermented fish. J. Biosci. Bioeng. 110, 553–557. doi: 10.1016/j.jbiosc.2010.06.004
Sakai, H., Caldo, G. A., and Kozaki, M. (1983).Yeast-flora in red burong-isda a fermented fish food from the Philippines. J. Agric. Sci. (Tokyo) 28, 181–185.
Sakamoto, N., Tanaka, S., Sonomoto, K., and Nakayama, J. (2011). 16S rRNA pyrosequencing-based investigation of the bacterial community in nukadoko, a pickling bad of fermented rice bran. Int. J. Food Microbiol. 144, 352–359. doi: 10.1016/j.ijfoodmicro.2010.10.017
Salampessy, J., Kailasapathy, K., and Thapa, N. (2010). Fermented fish products. in Fermented Foods and Beverages of the World, eds J. P. Tamang and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 289–307.
Salminen, S., Wright, A. V., and Ouwehand, A. (2004). Lactic Acid Bacteria Microbiology and Functional Aspects, 3rd Edn., New York, NY: Marcel Dekker.
Sarkar, P. K., Hasenack, B., and Nout, M. J. R. (2002). Diversity and functionality of Bacillus and related genera isolated from spontaneously fermented soybeans (Indian Kinema) and locust beans (African Soumbala). Int. J. Food Microbiol. 77, 175–186. doi: 10.1016/S0168-1605(02)00124-1
Sarkar, P. K., and Tamang, J. P. (1994). The influence of process variables and inoculum composition on the sensory quality of kinema. Food Microbiol. 11, 317–325. doi: 10.1006/fmic.1994.1036
Sarkar, P. K., Tamang, J. P., Cook, P. E., and Owens, J. D. (1994). Kinema-a traditional soybean fermented food: proximate composition and microflora. Food Microbiol. 11, 47–55. doi: 10.1006/fmic.1994.1007
Sarkar, S. (2008). Innovations in Indian fermented milk products-a review. Food Biotechnol. 22, 78–97. doi: 10.1080/08905430701864025
Sato, H., Torimura, M., Kitahara, M., Ohkuma, M., Hotta, Y., and Tamura, H. (2012). Characterization of the Lactobacillus casei group based on the profiling of ribosomal proteins coded in S10-spc-alpha operons as observed by MALDI-TOF MS. Sys. Appl. Microbiol. 35, 447–454. doi: 10.1016/j.syapm.2012.08.008
Savadogo, A., Tapi, A., Chollet, M., Wathelet, B., Traoré, A. S., and Jacques, P. (2011). Identification of surfactin producing strains in Soumbala and Bikalga fermented condiments using Polymerase chain reaction and matrix assisted laser desorption/ionization-mass spectrometry methods. Int. J. Food Microbiol. 151, 299–306. doi: 10.1016/j.ijfoodmicro.2011.09.022
Sawadogo-Lingani, H., Lei, V., Diawara, B., Nielsen, D. S., Møller, P. L., Traoré, A. S., et al. (2007). The biodivesity of predominant lactic acid bacteria in dolo and pito wort for the production of sorghum beer. J. Appl. Microbiol. 103, 765–777. doi: 10.1111/j.1365-2672.2007.03306.x
Sawamura, S. (1906). On the microorganisms of natto. Bull. Coll. Agri. Tokyo Imperial Univ. 7, 107–110.
Schillinger, U., Ban-Koffi, L., and Franz, C. M. A. P. (2010). “Tea, coffee and cacao,” in Fermented Foods and Beverages of the World, eds J. P. Tamang, and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 353–375. doi: 10.1201/ebk1420094954-c13
Sengun, I. Y., and Karabiyikli, S. (2011). Importance of acetic acid bacteria in food industry. Food Control 22, 647–665 doi: 10.1016/j.foodcont.2010.11.008
Sengun, I. Y., Nielsen, D. S., Karapinar, M., and Jakobsen, M. (2009). Identification of lactic acid bacteria isolated from Tarhana, a traditional Turkish fermented food. Int. J. Food Microbiol. 135, 105–111. doi: 10.1016/j.ijfoodmicro.2009.07.033
Shamala, T. R., and Sreekantiah, K. R. (1988). Microbiological and biochemical studies on traditional Indian palm wine fermentation. Food Microbiol. 5, 157–162. doi: 10.1016/0740-0020(88)90014-7
Shrestha, H., Nand, K., and Rati, E. R. (2002). Microbiological profile of murcha starters and physico-chemical characteristics of poko, a rice based traditional food products of Nepal. Food Biotechnol. 16, 1–15. doi: 10.1081/FBT-120004198
Shi, Z., Zhang, Y., Phillips, G. O., and Yang, G. (2014). Utilization of bacterial cellulose in food. Food Hydrocolloids 35, 539–545. doi: 10.1016/j.foodhyd.2013.07.012
Shin, D. H., Kwon, D. Y., Kim, Y. S., and Jeong, D. Y. (2012). Science and Technology of Korean Gochujang. Seoul: Public Health Edu.
Shin, M. S., Han, S. K., Ryu, J. S., Kim, K. S., and Lee, W. K. (2008). Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2 isolated from kimchi. J. Appl. Microbiol. 105, 331–339. doi: 10.1111/j.1365-2672.2008.03770.x
Shon, M. Y., Lee, J., Choi, J. H., Choi, S. Y., Nam, S. H., Seo, K. I., et al. (2007). Antioxidant and free radical scavenging activity of methanol extract of chungkukjang. J. Food Comp. Anal. 20, 113–118. doi: 10.1016/j.jfca.2006.08.003
Singh, D., and Singh, J. (2014). Shrikhand: a delicious and healthful traditional Indian fermented dairy dessert. Trends Biosci. 7, 153–155.
Singh, T. A., Devi, K. R., Ahmed, G., and Jeyaram, K. (2014). Microbial and endogenous origin of fibrinolytic activity in traditional fermented foods of Northeast India. Food Res. Int. 55, 356–362. doi: 10.1016/j.foodres.2013.11.028
Solieri, L., and Giudici, P. (2008). Yeasts associated to traditional balsamic vinegar: ecological and technological features. Int. J. Food Microbiol. 125, 36–45. doi: 10.1016/j.ijfoodmicro.2007.06.022
Sonar, R. N., and Halami, P. M. (2014). Phenotypic identification and technological attributes of native lactic acid bacteria present in fermented bamboo shoot products from North-East India. J. Food Sci. Technol. doi: 10.1007/s13197-014-1456-x
Soni, S. K., Sandhu, D. K., Vilkhu, K. S., and Kamra, N. (1986). Microbiological studies on Dosa fermentation. Food Microbiol. 3, 45–53. doi: 10.1016/S0740-0020(86)80025-9
Sridevi, J., Halami, P. M., and Vijayendra, S. V. N. (2010). Selection of starter cultures for idli batter fermentation and their effect on quality of idli. J. Food Sci. Technol. 47, 557–563. doi: 10.1007/s13197-010-0101-6
Steinkraus, K. H. (1994). Nutritional significance of fermented foods. Food Res. Int. 27, 259–267. doi: 10.1016/0963-9969(94)90094-9
Steinkraus, K. H. (1996). Handbook of Indigenous Fermented Food, 2nd Edn. New York, NY: Marcel Dekker, Inc.
Steinkraus, K. H. (1997). Classification of fermented foods: worldwide review of household fermentation techniques. Food Control 8, 331–317. doi: 10.1016/S0956-7135(97)00050-9
Steinkraus, K. H. (2002). Fermentations in world food processing. Comprehensive Rev. Food Sci. Food Safety 1, 23–32. doi: 10.1111/j.1541-4337.2002.tb00004.x
Steinkraus, K. H. (2004). Industrialization of Indigenous Fermented Foods. New York, NY: Marcel Dekker, Inc.
Steinkraus, K. H., van Veer, A. G., and Thiebeau, D. B. (1967). Studies on idli-an Indian fermented black gram-rice food. Food Technol. 21, 110–113.
Stevens, H. C., and Nabors, L. (2009). Microbial food cultures: a regulatory update. Food Technol. (Chicago) 63, 36–41.
Stiles, M. E., and Holzapfel, W. H. (1997). Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 36, 1–29. doi: 10.1016/S0168-1605(96)01233-0
Suganuma, T., Fujita, K., and Kitahara, K. (2007). Some distinguishable properties between acid-stable and neutral types of α-amylases from acid-producing koji. J. Biosci. Bioeng. 104, 353–362. doi: 10.1263/jbb.104.353
Sugawara, E. (2010). “Fermented soybean pastes miso and shoyu with reference to aroma,” in Fermented Foods and Beverages of the World, eds J. P. Tamang and K. Kailasapathy, (New York, NY: CRC Press, Taylor and Francis Group), 225–245. doi: 10.1201/ebk1420094954-c7
Sujaya, I., Antara, N., Sone, T., Tamura, Y., Aryanta, W., Yokota, A., et al. (2004). Identification and characterization of yeasts in brem, a traditional Balinese rice wine. World J. Microbiol. Biotechnol. 20, 143–150. doi: 10.1023/B:WIBI.0000021727.69508.19
Sukontasing, S., Tanasupawat, S., Moonmangmee, S., Lee, J. S., and Suzuki, K. (2007). Enterococcus camelliae sp. nov., isolated from fermented tea leaves in Thailand. Int. J. Sys. Evo. Microbiol. 57, 2151–2154. doi: 10.1099/ijs.0.65109-0
Sumino, T., Endo, E., Kageyama, A. S., Chihihara, R., and Yamada, K. (2003). Various Components and Bacteria of Furu (Soybean Cheese). J. Cookery Sci. Japan 36, 157–163. doi: 10.11402/cookeryscience1995.36.2_157
Sun, S. Y., Gong, H. S., Jiang, X. M., and Zhao, Y. P. (2014). Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 44, 15–23. doi: 10.1016/j.fm.2014.05.007
Suprianto Ohba, R., Koga, T., and Ueda, S. (1989). Liquefaction of glutinous rice and aroma formation in tapé preparation by ragi. J. Ferment Bioeng. 64, 249–252. doi: 10.1016/0922-338X(89)90227-4
Takahashi, M., Masaki, K., Mizuno, A., and Goto-Yamamoto, N. (2014). Modified COLD-PCR for detection of minor microorganisms in wine samples during the fermentation. Food Microbiol. 39, 74–80. doi: 10.1016/j.fm.2013.11.009
Tamang, B., and Tamang, J. P. (2007). Role of lactic acid bacteria and their functional properties in Goyang, a fermented leafy vegetable product of the Sherpas. J. Hill Res. 20, 53–61.
Tamang, B., and Tamang, J. P. (2009). Lactic acid bacteria isolated from indigenous fermented bamboo products of Arunachal Pradesh in India and their functionality. Food Biotechnol. 23, 133–147. doi: 10.1080/08905430902875945
Tamang, B., and Tamang, J. P. (2010). In situ fermentation dynamics during production of gundruk and khalpi, ethnic fermented vegetables products of the Himalayas. Indian J. Microbiol. 50, 93–98. doi: 10.1007/s12088-010-0058-1
Tamang, B., Tamang, J. P., Schillinger, U., Franz, C. M. A. P., Gores, M., and Holzapfel, W. H. (2008). Phenotypic and genotypic identification of lactic acid bacteria isolated from ethnic fermented tender bamboo shoots of North East India. Int. J. Food Microbiol. 121, 35–40. doi: 10.1016/j.ijfoodmicro.2007.10.009
Tamang, J. P. (2003). Native microorganisms in fermentation of kinema. Indian J. Microbiol. 43, 127–130.
Tamang, J. P. (2010a). Himalayan Fermented Foods: Microbiology, Nutrition, and Ethnic Values. New York, NY: CRC Press, Taylor and Francis Group.
Tamang, J. P. (2010b). Diversity of fermented foods, In: Tamang JP, Kailasapathy, K. (Eds.) Fermented Foods and Beverages of the World, CRC Press, Taylor and Francis Group, New York, 41–84. doi: 10.1201/ebk1420094954-c2
Tamang, J. P. (2010c). “Diversity of fermented beverages,” in Fermented Foods and Beverages of the World, eds J. P. Tamang, and K. Kailasapathy (New York, NY: CRC Press, Taylor and Francis Group), 85–125.
Tamang, J. P. (2014). “Biochemical and modern identification techniques - microfloras of fermented foods,” in: Encyclopaedia of Food Microbiology, 2nd Edn., eds C. Batt, and M. A. Tortorello (Oxford: Elsevier Ltd.), 250–258.
Tamang, J. P. (2015a). Health Benefits of Fermented Foods and Beverages. New York, NY: CRC Press, Taylor and Francis Group
Tamang, J. P. (2015b). Naturally fermented ethnic soybean foods of India. J. Ethnic Foods 2, 8–17. doi: 10.1016/j.jef.2015.02.003
Tamang, J. P., Dewan, S., Tamang, B., Rai, A., Schillinger, U., and Holzapfel, W. H. (2007). Lactic acid bacteria in Hamei and Marcha of North East India. Indian J. Microbiol. 47, 119–125. doi: 10.1007/s12088-007-0024-8
Tamang, J. P., Dewan, S., Thapa, S., Olasupo, N. A., Schillinger, U., Wijaya, A., et al. (2000). Identification and enzymatic profiles of predominant lactic acid bacteria isolated from soft-variety chhurpi, a traditional cheese typical of the Sikkim Himalayas. Food Biotechnol. 14, 99–112. doi: 10.1080/08905430009549982
Tamang, J. P., and Fleet, G. H. (2009). “Yeasts diversity in fermented foods and beverages,” in Yeasts Biotechnology: Diversity and Applications, eds T. Satyanarayana, and G. Kunze, (New York, NY: Springer), 169–198. doi: 10.1007/978-1-4020-8292-4_9
Tamang, J. P., and Nikkuni, S. (1996). Selection of starter culture for production of kinema, fermented soybean food of the Himalaya. World J. Microbiol. Biotechnol. 12, 629–635. doi: 10.1007/BF00327727
Tamang, J. P., and Samuel, D. (2010). “Dietary cultures and antiquity of fermented foods and beverages,” in Fermented Foods and Beverages of the World eds J. P. Tamang, and K. Kailasapathy (London: CRC press), 1–40. doi: 10.1201/ebk1420094954-c1
Tamang, J. P., and Sarkar, P. K. (1993). Sinki - a traditional lactic acid fermented radish tap root product. J. Gen. Appl. Microbiol. 39, 395–408. doi: 10.2323/jgam.39.395
Tamang, J. P., and Sarkar, P. K. (1995). Microflora of murcha: an amylolytic fermentation starter. Microbios 81, 115–122.
Tamang, J. P., and Sarkar, P. K. (1996). Microbiology of mesu, a traditional fermented bamboo shoot product. Int. J. Food Microbiol. 29, 49–58. doi: 10.1016/0168-1605(95)00021-6
Tamang, J. P., Sarkar, P. K., and Hesseltine, C. W. (1988). Traditional fermented foods and beverages of Darjeeling and Sikkim - a review. J. Sci. Food Agric. 44, 375–385. doi: 10.1002/jsfa.2740440410
Tamang, J. P., Tamang, B., Schillinger, U., Franz, C. M. A. P., Gores, M., and Holzapfel, W. H. (2005). Identification of predominant lactic acid bacteria isolated from traditional fermented vegetable products of the Eastern Himalayas. Int. J. Food Microbiol. 105, 347–356. doi: 10.1016/j.ijfoodmicro.2005.04.024
Tamang, J. P., Tamang, B., Schillinger, U., Guigas, C., and Holzapfel, W. H. (2009). Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Int. J. Food Microbiol. 135, 28–33. doi: 10.1016/j.ijfoodmicro.2009.07.016
Tamang, J. P., Tamang, N., Thapa, S., Dewan, S., Tamang, B. M., Yonzan, H., et al. (2012). Microorganisms and nutritional value of ethnic fermented foods and alcoholic beverages of North East India. Indian J. Traditional Know. 11, 7–25.
Tamang, J. P., Thapa, N., Tamang, B., Rai, A., and Chettri, R. (2015). “Microorganisms in fermented foods and beverages, Chap. 1,” in Health Benefits of Fermented Foods ed J. P. Tamang, (New York, NY: CRC Press, Taylor and Francis Group), 1–110.
Tamang, J. P., and Thapa, S. (2006). Fermentation dynamics during production of bhaati jaanr, a traditional fermented rice beverage of the Eastern Himalayas. Food Biotechnol. 20, 251–261. doi: 10.1080/08905430600904476
Tamang, J. P., Thapa, S., Dewan, S., Jojima, Y., Fudou, R., and Yamanaka, S. (2002). Phylogenetic analysis of Bacillus strains isolated from fermented soybean foods of Asia: kinema, chungkokjang and natto. J. Hill Res. 15, 56–62.
Tamang, J. P., Thapa, S., Tamang, N., and Rai, B. (1996). Indigenous fermented food beverages of Darjeeling hills and Sikkim: process and product characterization. J. Hill Res. 9, 401–411.
Tamime, A. Y., and Robinson, R. K. (2007). Yoghurt Science and Technology. Cambridge: Woodhead Publishing Ltd.
Tanasupawat, S., Pakdeeto, A., Thawai, C., Yukphan, P., and Okada, S. (2007). Identification of lactic acid bacteria from fermented tea leaves (miang) in Thailand and proposals of Lactobacillus thailandensis sp. nov., Lactobacillus camelliae sp. nov., and Pediococcus siamensis sp. nov. J. Gen. Appl. Microbiol. 53, 7–15. doi: 10.2323/jgam.53.7
Tanigawa, K., Kawabata, H., and Watanabe, K. (2010). Identification and typing of Lactococcus lactis by matrix-assisted laser desorption ionization – time-of-flight mass spectrometry. Appl. Environ. Microbiol. 76, 4055–4062. doi: 10.1128/AEM.02698-09
Tanigawa, K., and Watanabe, K. (2011). Multilocus sequence typing reveals a novel subspeciation of Lactobacillus delbrueckii. Microbiol. 157, 727–738. doi: 10.1099/mic.0.043240-0
Taylor, J. R. N. (2003). “Beverages from sorghum and millet,” in Encyclopedia of Food Sciences and Nutrition, 2nd Edn., eds B. Caballero, L. C. Trugo, P. M. Finglas (London: Academic Press), 2352–2359. doi: 10.1016/B0-12-227055-X/00454-5
Teoh, A. L., Heard, G., and Cox, J. (2004). Yeasts ecology of Kombucha fermentation. Int. J. Food Microbiol. 95, 119–126. doi: 10.1016/j.ijfoodmicro.2003.12.020
Thanh, V. N., Mai, L. T., and Tuan, D. A. (2008). Microbial diversity of traditional Vietnamese alcohol fermentation starters (banh men) as determined by PCR-mediated DGGE. Int. J. Food Microbiol. 128, 268–273. doi: 10.1016/j.ijfoodmicro.2008.08.020
Thapa, N., Pal, J., and Tamang, J. P. (2004). Microbial diversity in ngari, hentak and tungtap, fermented fish products of Northeast India. World J. Microbiol. Biotechnol. 20, 599–607. doi: 10.1023/B:WIBI.0000043171.91027.7e
Thapa, N., Pal, J., and Tamang, J. P. (2006). Phenotypic identification and technological properties of lactic acid bacteria isolated from traditionally processed fish products of the Eastern Himalayas. Int. J. Food Microbiol. 107, 33–38. doi: 10.1016/j.ijfoodmicro.2005.08.009
Thapa, N., Pal, J., and Tamang, J. P. (2007). Microbiological profile of dried fish products of Assam. Indian J. Fisheries 54, 121–125.
Thapa, S., and Tamang, J. P. (2004). Product characterization of kodo ko jaanr: fermented finger millet beverage of the Himalayas. Food Microbiol. 21, 617–622. doi: 10.1016/j.fm.2004.01.004
Thapa, S., and Tamang, J. P. (2006). Microbiological and physico-chemical changes during fermentation of kodo ko jaanr, a traditional alcoholic beverage of the Darjeeling hills and Sikkim. Indian J. Microbiol. 46, 333–341.
Toldra, F. (2007). Handbook of Fermented Meat and Poultry. Oxford: Blackwell Publishing. doi: 10.1002/9780470376430
Tou, E. H., Mouquet-River, C., Rochette, I., Traoré, A. S., Treche, S., and Guyot, J. P. (2007). Effect of different process combinations on the fermentation kinetics, microflora and energy density of ben-saalga, a fermented gruel from Burkina Faso. Food Chem. 100, 935–943. doi: 10.1016/j.foodchem.2005.11.007
Tsuyoshi, N., Fudou, R., Yamanaka, S., Kozaki, M., Tamang, N., Thapa, S., et al. (2005). Identification of yeast strains isolated from marcha in Sikkim, a microbial starter for amylolytic fermentation. Int. J. Food Microbiol. 99, 135–146. doi: 10.1016/j.ijfoodmicro.2004.08.011
Urushibata, Y., Tokuyama, S., and Tahara, Y. (2002). Characterization of the Bacillus subtilisyws C gene, involved in ⌊–polyglutamic acid production. J. Bacteriol. 184, 337–343. doi: 10.1128/JB.184.2.337-343.2002
Vallejo, J. A., Miranda, P., Flores-Félix, J. D., Sánchez-Juanes, F., Ageitos, J. M., González-Buitrago, J. M., et al. (2013). Atypical yeasts identified as Saccharomyces cerevisiae by MALDI-TOF MS and gene sequencing are the main responsible of fermentation of chicha, a traditional beverage from Peru. Syst. Appl. Microbiol. 36, 560–564. doi: 10.1016/j.syapm.2013.09.002
van Hijum, S. A. F. T., Vaughan, E. E., and Vogel, R. F. (2013). Application of state-of-art sequencing technologies to indigenous food fermentations. Curr. Opin. Biotechnol. 24, 178–186. doi: 10.1016/j.copbio.2012.08.004
Vieira-Dalodé, G., Jespersen, L., Hounhouigan, J., Moller, P. L., Nago, C. M., and Jakobsen, M. (2007). Lated with gowé production from sorghum in Bénin. J. Appl. Microbiol. 103, 342–349. doi: 10.1111/j.1365-2672.2006.03252.x
Walker, G. M. (2014). “Microbiology of Winemaking,” in Encyclopaedia of Food Microbiology, 2nd Edn., eds C. Batt and M. A Tortorello (Oxford: Elsevier Ltd.), 787–792. doi: 10.1016/B978-0-12-384730-0.00356-6
Wang, C. T., Ji, B. P., Li, B., Nout, R., Li, P. L., Ji, H., et al. (2006). Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from Chinese traditional Douchi. Indus. Microbiol. Biotechnol. 33, 750–758. doi: 10.1007/s10295-006-0111-6
Wang, J., and Fung, D. Y. C. (1996). Alkaline-fermented foods: a review with emphasis on pidan fermentation. Crit. Rev. Microbiol. 22, 101–138. doi: 10.3109/10408419609106457
Wang, J., Tang, H., Zhang, C., Zhao, Y., Derrien, M., Rocher, E., et al. (2015). Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 9, 1–15. doi: 10.1038/ismej.2014.99
Watanabe, K., Fujimoto, J., Sasamoto, M., Dugersuren, J., Tumursuh, T., and Demberel, S. (2008). Diversity of lactic acid bacteria and yeasts in airag and tarag, traditional fermented milk products from Mongolia. World J. Microbiol. Biotechnol. 24, 1313–1325. doi: 10.1007/s11274-007-9604-3
Watanabe, K., Fujimoto, J., Tomii, Y., Sasamoto, M., Makino, H., Kudo, Y., et al. (2009a). Lactobacillus kisonensis sp. nov., Lactobacillus otakiensis sp. nov., Lactobacillus rapi sp. nov. and Lactobacillus sunkii sp. nov., heterofermentative species isolated from sunki, a traditional Japanese pickle. Int. J. Syst. Evol. Microbiol. 59, 754–760. doi: 10.1099/ijs.0.004689-0
Watanabe, K., Makino, H., Sasamoto, M., Kudo, Y., Fujimoto, J., and Demberel, S. (2009b). Bifidobacterium mongoliense sp. nov., from airag, a traditional fermented mare's milk product from Mongolia. Int. J. Syst. Evol. Microbiol. 59, 1535–1540. doi: 10.1099/ijs.0.006247-0
Weckx, S., Meulen, van der., Maes, R., Scheirlinck, D., Huys, I., Vandamme, G. P., and De Vuyst, L. (2010). Lactic acid bacteria community dynamics and metabolite production of rye sourdough fermentations share characteristics of wheat and spelt sourdough fermentations. Food Microbiol. 27, 1000–1008. doi: 10.1016/j.fm.2010.06.005
Wei, D., and Jong, S. (1983). Chinese rice pudding fermentation: fungal flora of starter cultures and biochemical changes during fermentation. J. Ferment. Technol. 61, 573–579.
Winarno, F.G., Fardiaz, S., and Daulay, D. (1973). Indonesian Fermented Foods. Indonesia: Department of Agricultural Product Technology, Bogor Agricultural University.
Wongputtisin, P., Khanongnuch, C., Kongbuntad, W., Niamsup, P., Lumyong, S., and Sarkar, P. K. (2014). Use of Bacillus subtilis isolates from Tua-nao towards nutritional improvement of soya bean hull for monogastric feed application. Lett. Appl. Microbiol. 59, 328–333. doi: 10.1111/lam.12279
Wu, R., Wang, L., Wang, J., Li, H., Menghe, B., Wu, J., et al. (2009). Isolation and preliminary probiotic selection of lactobacilli from Koumiss in Inner Mongolia. J. Basic Microbiol. 49, 318–326. doi: 10.1002/jobm.200800047
Wu, Y. C., Kimura, B., and Fujii, T. (2000). Comparison of three culture methods for the identification of Micrococcus and Staphylococcus in fermented squid shiokara. Fish. Sci. 66, 142–146. doi: 10.1046/j.1444-2906.2000.00021.x
Yamamoto, S., and Matsumoto, T. (2011). Rice fermentation starters in Cambodia: cultural importance and traditional methods of production. Southeast Asian Stud. 49, 192–213.
Yan, P. M., Xue, W. T., Tan, S. S., Zhang, H., and Chang, X. H. (2008). Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control 19, 50–55. doi: 10.1016/j.foodcont.2007.02.008
Yan, Y., Qian, Y., Ji, F., Chen, J., and Han, B. (2013). Microbial composition during Chinese soy sauce koji-making based on culture dependent and independent methods. Food Microbiol. 34, 189–195. doi: 10.1016/j.fm.2012.12.009
Yonzan, H., and Tamang, J. P. (2010). Microbiology and nutritional value of selroti, an ethnic fermented cereal food of the Himalayas. Food Biotechnol. 2, 227–247. doi: 10.1080/08905436.2010.507133
Yonzan, H., and Tamang, J. P. (2013). Optimization of traditional processing of Selroti, a popular cereal-based fermented food. J. Sci. Indu. Res. 72, 43–47.
Yoon, M. Y., Kim, Y. J., and Hwang, H. J. (2008). Properties and safety aspects of Enterococcus faecium strains isolated from Chungkukjang, a fermented soy product. LWT Food Sci. Technol. 41, 925–933. doi: 10.1016/j.lwt.2007.05.024
Yousif, N. M. K., Huch, M., Schuster, T., Cho, G. S., Dirar, H. A., Holzapfel, W. H., et al. (2010). Diversity of lactic acid bacteria from Hussuwa, a traditional African fermented sorghum food. Food Microbiol. 27, 757–768. doi: 10.1016/j.fm.2010.03.012
Yu, J., Wang, W. H., Menghe, B. L., Jiri, M. T., Wang, H. M., Liu, W. J., et al. (2011). Diversity of lactic acid bacteria associated with traditional fermented dairy products in Mongolia. J. Dairy Sci. 94, 3229–3241. doi: 10.3168/jds.2010-3727
Zhang, J. H., Tatsumi, E., Fan, J. F., and Li, L. T. (2007). Chemical components of Aspergillus-type Douchi, a Chinese traditional fermented soybean product, change during the fermentation process. Int. J. Food Sci. Technol. 42, 263–268. doi: 10.1111/j.1365-2621.2005.01150.x
Zhu, Y. P., Cheng, Y. Q., Wang, L. J., Fan, J. F., and Li, L. T. (2008). Enhanced antioxidative activity of Chinese traditionally fermented Okara (Meitauza) prepared with various microorganism. Int. J. Food Prop. 11, 519–529. doi: 10.1080/10942910701472813
Keywords: global fermented foods, LAB, Bacillus, yeasts, filamentous molds
Citation: Tamang JP, Watanabe K and Holzapfel WH (2016) Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 7:377. doi: 10.3389/fmicb.2016.00377
Received: 14 December 2015; Accepted: 08 March 2016;
Published: 24 March 2016.
Edited by:
Abd El-Latif Hesham, Assiut University, EgyptReviewed by:
Catarina Prista, Instituto Superior de Agronomia, PortugalChiara Montanari, Centro Interdipartimentale di Ricerca Industriale Agroalimentare, Italy
Copyright © 2016 Tamang, Watanabe and Holzapfel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jyoti P. Tamang, anlvdGlfdGFtYW5nQGhvdG1haWwuY29t
 Jyoti P. Tamang
Jyoti P. Tamang Koichi Watanabe
Koichi Watanabe Wilhelm H. Holzapfel
Wilhelm H. Holzapfel