
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 23 March 2016
Sec. Infectious Agents and Disease
Volume 7 - 2016 | https://doi.org/10.3389/fmicb.2016.00329
This article is part of the Research Topic Mycoplasma pneumoniae clinical manifestations, microbiology and immunology View all 18 articles
 Patrick M. Meyer Sauteur1,2,3*
Patrick M. Meyer Sauteur1,2,3* Wendy W. J. Unger2
Wendy W. J. Unger2 David Nadal3
David Nadal3 Christoph Berger3
Christoph Berger3 Cornelis Vink4
Cornelis Vink4 Annemarie M. C. van Rossum1
Annemarie M. C. van Rossum1“Atypical” pneumonia was described as a distinct and mild form of community-acquired pneumonia (CAP) already before Mycoplasma pneumoniae had been discovered and recognized as its cause. M. pneumoniae is detected in CAP patients most frequently among school-aged children from 5 to 15 years of age, with a decline after adolescence and tapering off in adulthood. Detection rates by polymerase chain reaction (PCR) or serology in children with CAP admitted to the hospital amount 4–39%. Although the infection is generally mild and self-limiting, patients of every age can develop severe or extrapulmonary disease. Recent studies indicate that high rates of healthy children carry M. pneumoniae in the upper respiratory tract and that current diagnostic PCR or serology cannot discriminate between M. pneumoniae infection and carriage. Further, symptoms and radiologic features are not specific for M. pneumoniae infection. Thus, patients may be unnecessarily treated with antimicrobials against M. pneumoniae. Macrolides are the first-line antibiotics for this entity in children younger than 8 years of age. Overall macrolides are extensively used worldwide, and this has led to the emergence of macrolide-resistant M. pneumoniae, which may be associated with severe clinical features and more extrapulmonary complications. This review focuses on the characteristics of M. pneumoniae infections in children, and exemplifies that simple clinical decision rules may help identifying children at high risk for CAP due to M. pneumoniae. This may aid physicians in prescribing appropriate first-line antibiotics, since current diagnostic tests for M. pneumoniae infection are not reliably predictive.
The clinical entity of “atypical” pneumonia was recognized in the 1930s many years before the etiological agent was established (McCoy, 1946). The term separated this entity of pneumonia from classical pneumococcal pneumonia due to its lack of response to available antibiotics and the distinct clinical presentation without typical lobar pneumonia and a less severe disease course. That is why the term “walking pneumonia” has been introduced to denote this mild form of pneumonia.
It was in a patient with “atypical” pneumonia in 1944, where Mycoplasma pneumoniae was first isolated from sputum in tissue culture by Eaton et al. (1944). At that time, it was believed to be a virus because it was resistant to penicillin and sulfonamides and passed through bacteria-retaining filters. Experiments with Marine recruits and adult prisoners demonstrated that the so-called Eaton agent caused lower respiratory tract infections in humans (Chanock et al., 1961a,b). In 1963, it was first cultured on cell-free medium and classified as M. pneumoniae (Chanock et al., 1962; Chanock, 1963). Today we know that mycoplasmas are prokaryotes that lack a cell wall and represent the smallest self-replicating organisms (Figure 1). With a size of 816,394 base pairs, the genome of M. pneumoniae is at least five times smaller than that of Escherichia coli (Himmelreich et al., 1996). The absence of a cell wall and the specialized attachment organelle facilitate close contact with the host respiratory epithelium, which supplies the bacterium with the necessary nutrients for its growth and proliferation.
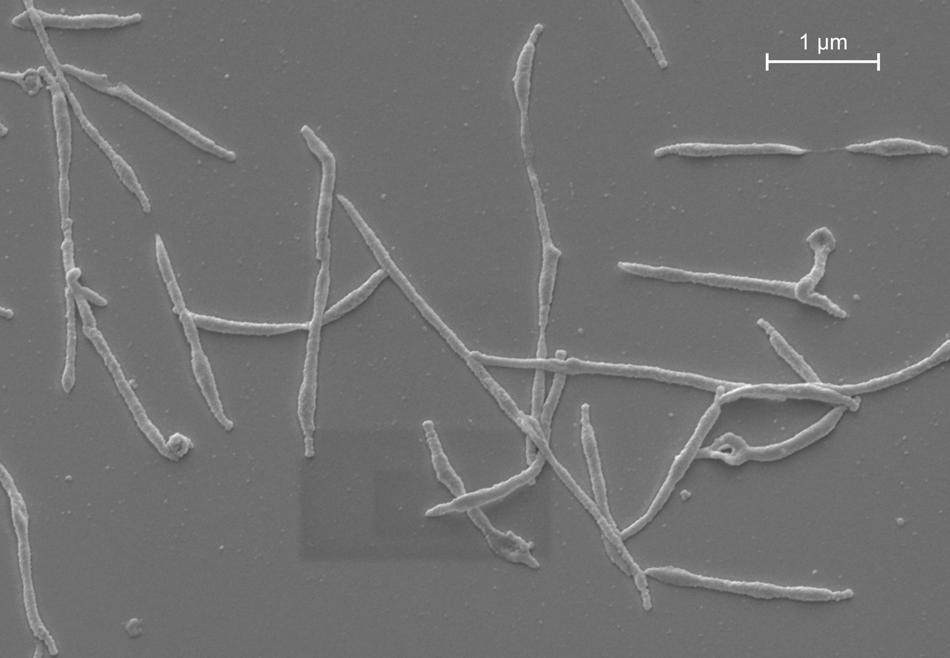
FIGURE 1. M. pneumoniae morphology in vitro. Scanning electron micrograph of M. pneumoniae strain Mac (subtype 2).
Mycoplasma pneumoniae causes both upper and lower respiratory tract infections, with community-acquired pneumonia (CAP) as the major burden of disease. Although M. pneumoniae infections are generally mild and self-limiting, patients of every age can develop severe and fulminant disease (Kannan et al., 2012). M. pneumoniae can also cause extrapulmonary manifestations that affect almost every organ (Narita, 2010).
In children, M. pneumoniae infections were first reported in 1960 when 16% of 110 children with lower respiratory tract disease were tested positive by a fourfold rise in antibody titers against the Eaton agent (Chanock et al., 1960). To date, it is known that the incidence of M. pneumoniae infections is generally higher in children than in adults (Foy et al., 1979). This review focuses on the characteristics of M. pneumoniae infections in children, and discusses simple clinical decision rules that may further aid clinicians in identifying patients at high risk for M. pneumoniae CAP.
Mycoplasma pneumoniae is transmitted by respiratory droplets through close contact. The incubation period can be long from 1 up to 3 weeks. Outbreaks have been reported within families, schools, universities, institutions, camps, and military bases. Family members of index patients with acute respiratory infection and detection of M. pneumoniae in the upper respiratory tract were found positive in 15% by polymerase chain reaction (PCR) (Dorigo-Zetsma et al., 2001). Thereof, 75% were <16 years of age and 44% did not develop any respiratory symptoms. At universities, the largest outbreak within 35 years in the U.S. was observed during September 1–December 4, 2012, where a total of 83 CAP cases were identified among students, and 12 out of 19 tested cases (63%) were positive for M. pneumoniae by quantitative real-time PCR (Centers for Disease Control and Prevention [CDC], 2013).
Outbreaks appear mainly during M. pneumoniae epidemics that occur in 3–7 years cycles, in addition to a background endemic pattern (Jacobs, 2012). The most recent epidemic in Europe occurred in 2010–2012 with a peak incidence in Finland of 145/100,000 cases in 2011 (Polkowska et al., 2012; Jacobs et al., 2015). The cyclic occurrence of epidemics may be facilitated by a decreasing herd immunity and different M. pneumoniae genotypes circulating in the human population (Jacobs, 2012). The two major circulating genotypes, or subtypes, of M. pneumoniae are indicated as subtype 1 and 2. Differences between these subtypes in the amino acid sequence of the major adhesion protein P1 are believed to play a role in the epidemiology of infections with M. pneumoniae (Su et al., 1990; Vink et al., 2012). The differences between the 169-kDa P1 proteins of subtype 1 and 2 isolates were found to be concentrated in two specific amino acid stretches within the protein. These regions are encoded by two DNA elements within the P1 gene, i.e., repetitive elements RepMP2/3 and RepMP4. The RepMP2/3 and RepMP4 are not unique to the P1 gene, but are also found at other sites within the bacterial genome (Spuesens et al., 2009). Homologous recombination events between these repetitive elements, which are similar to each other, but not identical, may form the basis of antigenic variation of the P1 protein of M. pneumoniae (Vink et al., 2012). While such recombination events may induce antigenic variation within subtype 1 or subtype 2 strains, M. pneumoniae strains cannot switch from one subtype to the other, as the entire set of RepMP elements found in one subtype differs significantly from those found in the other subtype. Moreover, changes in the proportion of the two subtypes of M. pneumoniae were not observed between 2003 and 2012 in Europe (Jacobs et al., 2015).
Although CAP is the major burden of disease, milder clinical presentations of M. pneumoniae respiratory infections may be much more common than CAP. These include acute bronchitis and upper respiratory tract infections (Esposito et al., 2000, 2002). M. pneumoniae could be detected by PCR and/or serology in 24% of non-streptococcal pharyngitis cases (Esposito et al., 2002).
It is estimated that 3–10% of children with M. pneumoniae respiratory infection develop CAP and that <5% of CAP cases are severe enough to require hospitalization (Waites and Talkington, 2004). Between 1963 and 1975, M. pneumoniae was detected by culture of respiratory specimens and/or a fourfold titer rise in complement fixation test (CFT) in 15–20% of radiologically confirmed CAP cases in Seattle, U.S. (Foy et al., 1979). In subsequent etiological studies, M. pneumoniae accounted for 4–39% of the isolates identified by PCR and/or serology in children with CAP admitted to the hospital (Juven et al., 2000; Principi et al., 2001; Baer et al., 2003; Michelow et al., 2004). M. pneumoniae was first reported as the most common bacterial cause of CAP in children requiring hospitalization in a U.S. multicenter study from 2011 to 2012 in Nashville and Salt Lake City (Jain et al., 2015). In this study, M. pneumoniae could be detected by PCR in 178 (8%) out of 2179 cases with CAP, whereas Streptococcus pneumoniae was found in 79 cases (4%).
Manifest upper and/or lower respiratory tract infections with M. pneumoniae occur at all ages (Foy et al., 1979). Recent observations have indicated that M. pneumoniae has also a relatively high prevalence in the respiratory tract of children <5 years (Principi et al., 2001; Gadsby et al., 2012). M. pneumoniae CAP, however, was reported to be most frequent among school-aged children from 5 to 15 years of age, with a decline after adolescence and tapering off in adulthood (Figure 2) (Foy et al., 1979). This notion was corroborated in the recent CAP study in the U.S., where M. pneumoniae was detected significantly more frequent in children ≥5 years of age than in younger children (19% vs. 3%) (Jain et al., 2015).
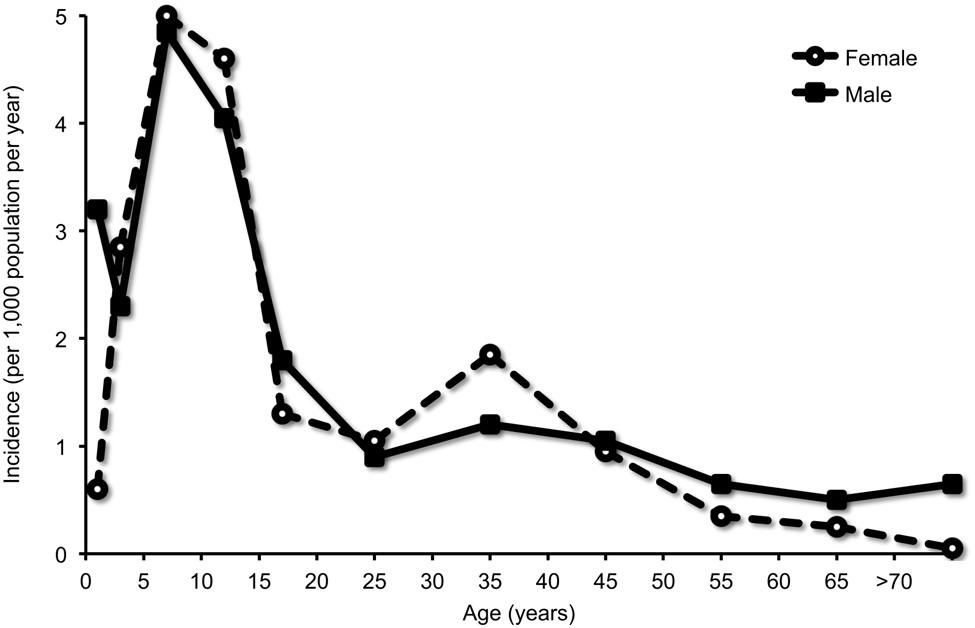
FIGURE 2. Detection of M. pneumoniae in community-acquired pneumonia (CAP) according to age group. Infection was diagnosed by culture of respiratory specimens and/or a fourfold titer rise in complement fixation test (CFT). Adapted with permission from Foy et al. (1979).
In addition to the presentation at school-age, children with CAP due to M. pneumoniae have been found to present with a significantly longer duration of fever compared with other children with CAP (Fischer et al., 2002). Other symptoms that may be associated with M. pneumoniae CAP are the absence of wheeze and the presence of chest pain (Wang et al., 2012). However, there is still a paucity of high quality data regarding clinical signs and symptoms associated with M. pneumoniae infections. A recent Cochrane review therefore concluded that the absence or presence of individual clinical symptoms or signs cannot be used to help clinicians accurately diagnose M. pneumoniae in children and adolescents with CAP (Wang et al., 2012).
Pathogenic effects in the respiratory tract may be caused by M. pneumoniae either directly (by active infection), indirectly (by infection-induced immune mechanisms), or both (Narita, 2010). M. pneumoniae causes direct injury through the generation of activated oxygen. A potential candidate protein of M. pneumoniae that may be involved in causing direct damage to the respiratory tract is a pertussis toxin-like protein termed Community-Acquired Respiratory Distress Syndrome (CARDS) toxin (Kannan and Baseman, 2006; Becker et al., 2015). A recombinant version of the CARDS toxin has been shown to bind with high affinity to surfactant protein A and to exhibit mono-ADP ribosyltransferase and vacuolating activities, which causes disruption of the respiratory epithelium in animal models (Kannan and Baseman, 2006).
In addition to the direct damage resulting from infection by M. pneumoniae, the immunological response following infection generates inflammatory reactions that may cause pulmonary and extrapulmonary symptoms. More severe symptoms of CAP have been observed in older children and adolescents (Waites and Talkington, 2004). This suggests that the age-dependent magnitude and nature of inflammatory responses in childhood may be a major factor contributing to the development of M. pneumoniae-associated disease, similar to what is observed, e.g., in infectious mononucleosis or rheumatic fever. In fact, the severity of M. pneumoniae CAP in children was closely associated with increased concentrations of interleukin (IL)-8 and IL-18 in acute phase serum and pleural fluid samples (Narita and Tanaka, 2007). In addition, it has been demonstrated that cell-mediated immunity contributes to the pathogenesis of M. pneumoniae CAP, as it was shown that the severity of CAP correlated positively with the size of a cutaneous induration following intradermal injection of M. pneumoniae antigens (Mizutani et al., 1971). This study described 20 patients with CAP, of which 19 were children 4–15 years of age, diagnosed by a significant rise in antibody titers against M. pneumoniae with CFT. The strongest skin reactions were seen in patients with severe CAP.
Mycoplasma pneumoniae and other “atypical bacteria” have long been implicated in the pathogenesis of asthma (Atkinson, 2013). There are many studies that have addressed this issue in the recent past. In an observational study on children and adults with asthma, M. pneumoniae infection was diagnosed in 9% of children with asthma (24/256) and was found more frequent in patients with chronic asthma (14%) than in those with asthma exacerbations (7%; p = 0.10) (Bebear et al., 2014). The diagnosis of M. pneumoniae infection in this study was performed by PCR and/or serology. Another recent study diagnosed M. pneumoniae in children with acute asthma (64%, 34/53) and refractory asthma (65%, 17/26), as well as in healthy controls (56%, 36/64), but did not find significant differences between these three groups (Wood et al., 2013). The high detection rates reported in this study were obtained using novel diagnostic methods [CARDS toxin enzyme immunoassay (EIA) and CARDS gene-specific PCR] (Wood et al., 2013). In a recent Taiwanese study (Yeh et al., 2015), 1591 children and adults with M. pneumoniae infection, diagnosed by positive immunoglobulin (Ig) M or fourfold IgG titer increase, but without prior asthma history were included from 2000 to 2008 and followed until the diagnosis of asthma or the end of 2011. Compared to matched 6364 patients without M. pneumoniae infection, the cumulative incidence of asthma was significantly higher in the M. pneumoniae cohort than in the control cohort (p < 0.0001). Patients with M. pneumoniae infection were at higher risk of having early-onset asthma (age at asthma diagnosis <12 years) and late-onset asthma (age at asthma diagnosis ≥12 years). These most recent findings suggested that M. pneumoniae can induce airway inflammation and contribute to incident asthma. Interestingly, exposure to recombinant CARDS toxin resulted in an allergic-type inflammatory response and airway hyperreactivity in mice and baboons (Hardy et al., 2009; Medina et al., 2012). It will be interesting to investigate whether CARDS toxins induce a similar allergic response during M. pneumoniae infections.
Apart from the respiratory tract infection, M. pneumoniae can cause extrapulmonary manifestations in almost every organ, including the skin and the hematologic, cardiovascular, musculoskeletal, and nervous systems (Narita, 2010). These manifestations may be caused either by direct local effects of M. pneumoniae, after dissemination of the bacteria throughout the body, or indirect effects, such as autoimmune reactions. The most frequent manifestations are diseases of the dermatologic and nervous system.
Skin manifestations occur in up to 25% of all M. pneumoniae infections, including mostly non-specific exanthems, erythema nodosum, urticaria, Stevens–Johnson syndrome, and a rare but distinct disorder with prominent mucous membrane involvement denominated as M. pneumoniae-associated mucositis (MPAM) (Schalock and Dinulos, 2009; Meyer Sauteur et al., 2012). This condition was first described by Fuchs (1876), and therefore also referred to as Fuchs syndrome (Meyer Sauteur et al., 2011). A recent review identified 32 patients with MPAM at a median age of 13.5 years at presentation (range 3–38 years, 23 children or young adolescents ≤18 years) (Meyer Sauteur et al., 2012). All patients presented with prodromal respiratory symptoms with a median duration of 7 days, and pneumonia was found in chest radiography in 79%. Oral lesions were present in all cases (Figure 3), ocular lesions in 97%, and urogenital lesions in 78%. There were no skin lesions in 69%. Although 12% of the patients were admitted to the intensive care unit, no one suffered from long-term sequelae.
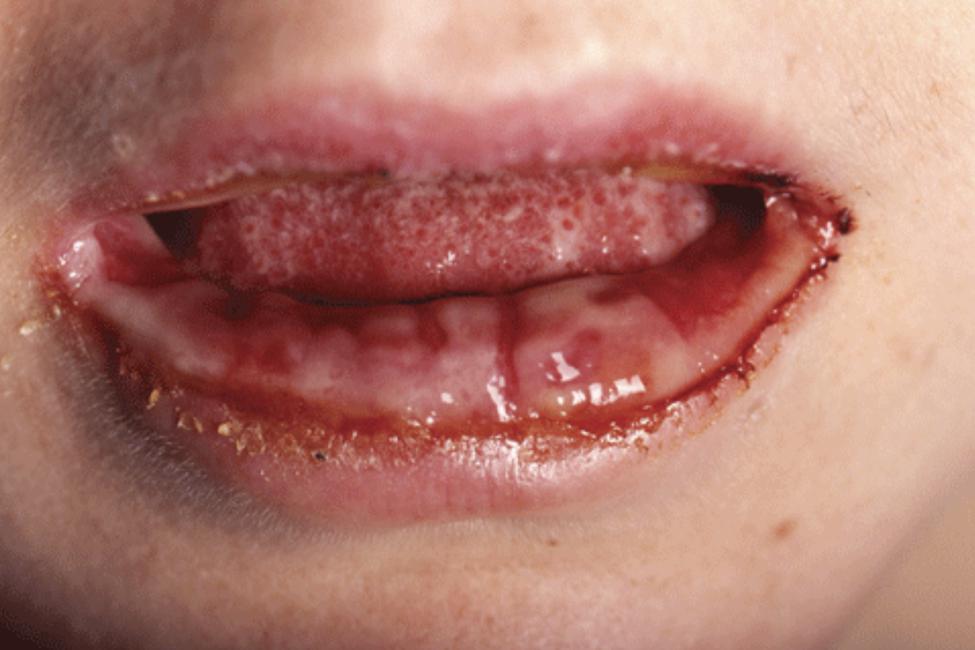
FIGURE 3. M. pneumoniae-associated mucositis (MPAM). Erosive oral lesions limited to the mucosa in this form of MPAM in a 24-year-old woman. Reprinted with permission from Meyer Sauteur et al. (2012).
Encephalitis and Guillain–Barré syndrome (GBS) constitute the most common and severe neurologic manifestations, where M. pneumoniae infection is established in up to 10 and 15% of patients, respectively (Bitnun et al., 2001; Sinha et al., 2007). In M. pneumoniae-associated encephalitis, both a direct infection of the central nervous system (CNS) and an immune-mediated process have been implied to be involved (Narita, 2009). Because the detection rate of M. pneumoniae by PCR in cerebrospinal fluid (CSF) of M. pneumoniae encephalitis patients is relatively low (0–14%) (Bitnun et al., 2001; Daxboeck et al., 2004; Christie et al., 2007a; Domenech et al., 2009), a significant proportion of the cases is believed to be immune-mediated. This is supported by the finding that various cases with M. pneumoniae encephalitis in which bacterial DNA could not be detected in CSF had a more prolonged duration of respiratory symptoms before the onset of encephalitis (>5–7 days) (Bitnun et al., 2001; Narita and Yamada, 2001; Daxboeck et al., 2004). These cases indicate that M. pneumoniae encephalitis represents a postinfectious disorder, which manifests after clearance of the bacteria from the CNS or respiratory tract by the immune system (Meyer Sauteur et al., 2014b). A recent study presented 365 children with M. pneumoniae detected in the respiratory tract or CSF by PCR, 22 (6%) of whom had encephalitis (1996–2013, Toronto, ON, Canada) (Al-Zaidy et al., 2015). Interestingly, patients in which M. pneumoniae was detectable in the respiratory tract but not in CSF showed pulmonary infiltrates on chest radiograph more frequently than patients with positive PCR in CSF (77% vs. 33%). This suggests that pneumonia may be an indicator for a remote inflammatory process in M. pneumoniae encephalitis patients, which was also shown in 83% (5/6) of children observed during a national surveillance, all with negative PCR in CSF (2010–2015, Switzerland) (Meyer Sauteur et al., 2016) (Figure 4).
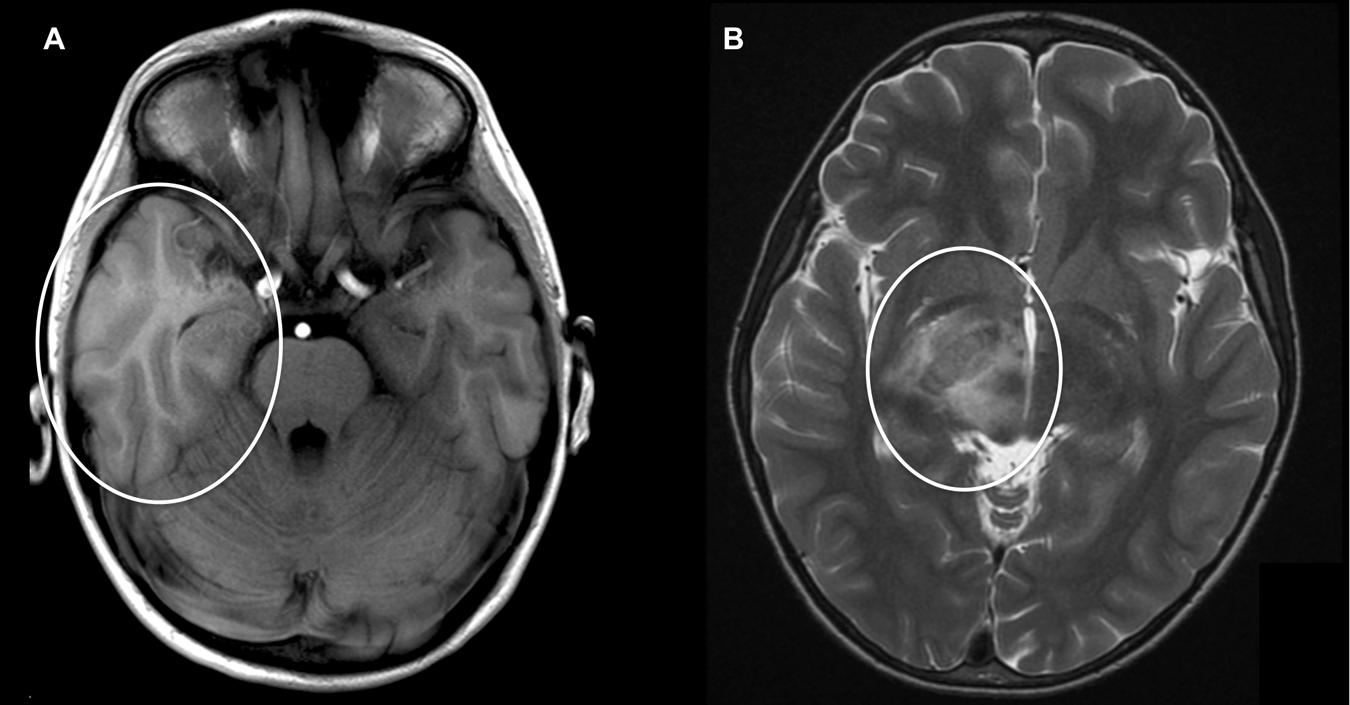
FIGURE 4. M. pneumoniae-associated encephalitis. Axial cranial magnetic resonance imaging (MRI) in two children with encephalitis during M. pneumoniae infection: (A) 5-year-old boy with hyperintensity and generalized edema of the right temporal lobe [T1 weight MRI; patient 1 published in Meyer Sauteur et al. (2016)]. (B) 9-year-old boy with generalized edema of crus posterior of capsula interna [T2 weight MRI; reprinted with permission from Meyer Sauteur et al. (2014c)].
Mycoplasma pneumoniae expresses adhesion proteins and glycolipids that share structural homology with a variety of host cells (molecular mimicry) and may induce cross-reactive antibodies (Meyer Sauteur et al., 2014b). In children with M. pneumoniae encephalitis, intrathecal antibodies directed against galactocerebroside (GalC) were found (Christie et al., 2007b; Meyer Sauteur et al., 2015a). Of note, all these patients had a negative PCR in CSF. GalC is a major glycolipid antigen in the myelin sheath of both the peripheral and CNS neurons (Menge et al., 2005). In fact, antibodies against M. pneumoniae infection have been found to cross-react with GalC in GBS patients (Kusunoki et al., 2001; Ang et al., 2002). Moreover, anti-GalC antibodies caused demyelinating neuropathy in rabbits (Saida et al., 1979) and have been associated with demyelination in GBS (Ang et al., 2002), but also in encephalitis (Christie et al., 2007b) and encephalomyelitis (Samukawa et al., 2012). The detection of intrathecal antibodies against M. pneumoniae and GalC may also be regarded as a promising new diagnostic tool for M. pneumoniae-associated CNS disease (Meyer Sauteur et al., 2014b, 2015b).
Like many other respiratory pathogens, M. pneumoniae can be carried asymptomatically in the respiratory tract (Foy, 1993). Recent studies have demonstrated that asymptomatic carriage of M. pneumoniae is highly prevalent. Detection rates of M. pneumoniae DNA in the respiratory tract of healthy children without respiratory symptoms were 21% in a Dutch study (2008–2011, Rotterdam, The Netherlands) (Spuesens et al., 2013) and 56% in a U.S. study (2009–2011, San Antonio, TX, U.S.) (Wood et al., 2013). Longitudinal sampling of M. pneumoniae–positive asymptomatic children demonstrated that M. pneumoniae can be present in the upper respiratory tract without causing disease, for up to 4 months (Spuesens et al., 2013). The prevalence of M. pneumoniae in the upper respiratory tract of asymptomatic children varied considerably between years and seasons. For example, asymptomatic carriage rates of 3% and 58% were reported in the spring of 2009 and the summer of 2010, respectively (Spuesens et al., 2013). These data suggest that carriage follows an epidemic pattern. It is tempting to speculate that this fluctuation in prevalence is related to the cyclic epidemics of M. pneumoniae infections. Apart from M. pneumoniae, children were found to simultaneously carry many pathogens in their nose and throat (Spuesens et al., 2013). These pathogens include the bacteria S. pneumoniae, Staphylococcus aureus, Moraxella catarrhalis, and Haemophilus influenzae, and the viruses influenza A/B, human metapneumovirus, respiratory syncytial virus, parainfluenzavirus, rhinovirus, coronavirus, bocavirus, and adenovirus. The simultaneous presence of two or more of these pathogens was detected in 56% of asymptomatic children (Spuesens et al., 2013).
In children with M. pneumoniae CAP, co-existence of M. pneumoniae with other pathogens has also been described (Juven et al., 2000; Michelow et al., 2004), and was recently reported in 28% of the patients (Jain et al., 2015). The impact of co-infections in M. pneumoniae CAP on disease severity is not yet determined.
Because the mere presence of M. pneumoniae in the upper respiratory tract is neither indicative nor predictive for respiratory disease, the routine diagnostic procedures to detect acute respiratory infections with M. pneumoniae need to be reconsidered. An overview of diagnostic tests with their advantages and drawbacks is shown in Table 1.
Current guidelines (Bradley et al., 2011; Harris et al., 2011) recommend PCR and single-sample serological tests to diagnose M. pneumoniae infections. The sensitivity of serological tests depends on the time point of the first serum and on the availability of paired sera for seroconversion to IgG and/or rise in antibody titer. Specific serum IgM emerges within 1 week after initial infection and about 2 weeks before IgG (Meyer Sauteur et al., 2014b). Although specific serum IgA arises even earlier than IgM, it could be detected only in 2% of PCR-positive children with symptomatic respiratory tract infection (Spuesens et al., 2013). Cross-reactions with other pathogens and non-infectious disease has been described for CFT and particle agglutination assay, but also some EIAs lack the required sensitivity and specificity (Beersma et al., 2005). Further, it is intriguing that the detection of IgM, as well as IgG and IgA by EIA could not discriminate between the asymptomatic and symptomatic groups of children (Spuesens et al., 2013). The demonstrated positive serological results in asymptomatic M. pneumoniae PCR-positive children (n = 66; IgM in 17%, IgG in 24%, and IgA in 6%) may simply reflect one or more previous encounters with M. pneumoniae and are not necessarily related to the presence of M. pneumoniae in the respiratory tract. Thus, it is questionable whether or not a positive result in these tests actually indicates the etiological role of M. pneumoniae in all cases. In that sense, the positive predictive value of these tests may be overestimated, whereas the negative predictive value may be acceptable (Bradley et al., 2011).
The “gold standard” for diagnosis of M. pneumoniae infections is still considered to be a fourfold increase in antibody titer as measured in paired sera (Gardiner et al., 2015). However, the use of convalescent sera is not useful in clinical practice because it is too time-consuming and does not allow clinicians to initiate treatment protocols in a timely fashion. Clinicians therefore need to be aware of the implications and clinical significance of a positive PCR or serology test result.
While diagnostic tests may not be reliably predictive for a symptomatic M. pneumoniae infection, the clinical assessment of this entity is being revisited. The British Thoracic Society guidelines recommend that bacterial pneumonia should be considered in children when there is persistent or repetitive fever >38.5°C together with chest recession and a raised respiratory rate (Harris et al., 2011). A chest radiograph should not be considered a routine investigation in children thought to have CAP. In fact, although bilateral, diffuse infiltrates are common, none of the radiographic findings associated with M. pneumoniae CAP is specific (John et al., 2001).
A fast-and-frugal clinical decision tree provided a rapid probability estimate of the cause of CAP in 253 children (1 months–16 years; 1997–1999, Zurich, Switzerland) (Fischer et al., 2002). M. pneumoniae infection was diagnosed in 13% (n = 32) of these children by PCR in respiratory specimens and serology (seroconversion and/or fourfold rise in antibody titer). Compared with other children with CAP, patients with M. pneumoniae were older and had a longer duration of fever (p < 0.001). Asking the simple question regarding the age of the child and the duration of fever allowed identification of the following group at high risk for CAP due to M. pneumoniae: children with CAP who have had fever >2 days and who were >3 years of age. The score model placed 75% of all patients with M. pneumoniae infection into the high-risk group (Figure 5). These simple rules may further aid physicians in prescribing appropriate first-line antibiotics.
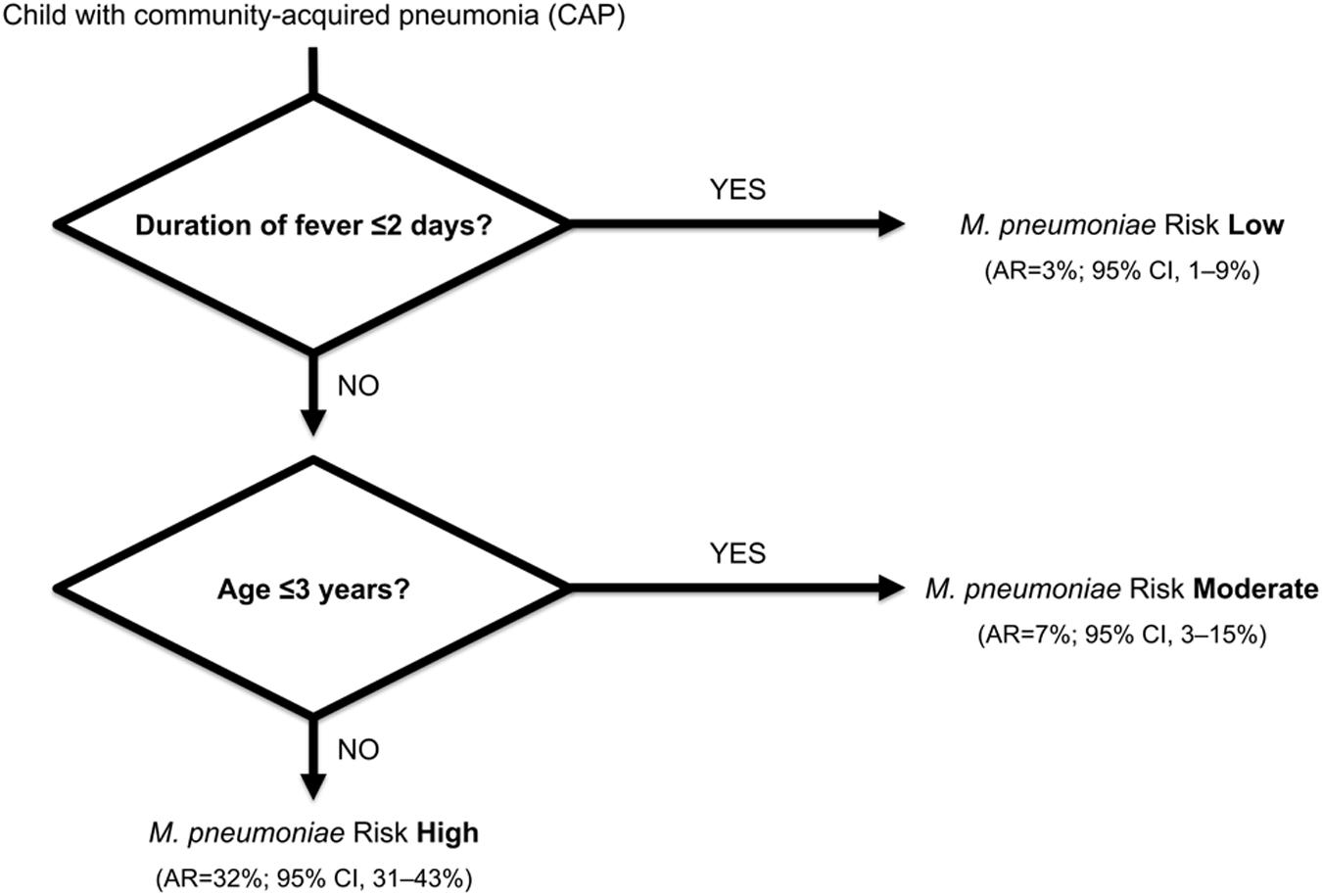
FIGURE 5. A fast-and-frugal clinical decision tree for ruling out M. pneumoniae infection in children with community-acquired pneumonia (CAP). Clinical features are considered sequentially, with a possible stop decision after each question. Abbreviations: AR, absolute risk; CI, confidence interval. Adapted from Fischer et al. (2002).
In consequence of the diagnostic uncertainty for M. pneumoniae infections, the British Thoracic Society guidelines suggest empiric macrolide treatment at any age if there is no response to first-line β-lactam antibiotics or in the case of very severe disease (Harris et al., 2011). The lack of a cell wall makes M. pneumoniae resistant to cell wall synthesis inhibitors such as β-lactam antibiotics. The antibiotics with the best minimum inhibitory concentration values against M. pneumoniae include macrolides, tetracyclines, and fluoroquinolones (Waites and Talkington, 2004). Although the latter two have a good in vitro inhibitory effect against M. pneumoniae, tetracyclines may cause teeth discoloration (Waites and Talkington, 2004) and fluoroquinolones may affect the developing cartilage in young children (Adefurin et al., 2011). Thus, they are not recommended by current guidelines in young children; the age limit for tetracyclines is ≥8 years, while that of fluoroquinolones is adolescence with skeletal maturity (Bradley et al., 2011). The occurrence of arthropathy due to fluoroquinolones, however, is uncertain, and all musculoskeletal adverse effects reported in the literature had been reversible following withdrawal of treatment (Adefurin et al., 2011). The protein synthesis inhibitors of the macrolide class have a more favorable side effect profile and are therefore the first-line antibiotics for M. pneumoniae infections in children (Bradley et al., 2011).
Although antibiotics are effective against M. pneumoniae in vitro (Bebear et al., 2011), there is lack of evidence on their in vivo efficacy. Observational data indicated that children with CAP due to M. pneumoniae have a shorter duration of symptoms and fewer relapses when treated with an antimicrobial agent active against M pneumoniae (McCracken, 1986; Waites and Talkington, 2004). A recent Cochrane review evaluated seven studies on the effectiveness of antibiotic treatment for M. pneumoniae lower respiratory tract infections in children (Gardiner et al., 2015). However, the diagnostic criteria, the type and duration of treatment, inclusion criteria, and outcome measures differed significantly, making it difficult to draw any specific conclusions, although one trial suggested that macrolides may be efficacious in some cases (Esposito et al., 2005). It is clear that studies on the efficacy of antibiotics rely on a correct diagnosis of M. pneumoniae infections. Given the aforementioned shortcomings of current diagnostic tests, conclusions on the efficacy of antibiotic treatment will have to be re-examined.
Since 2000, the extensive macrolide use led to an alarming worldwide increase in the prevalence of macrolide-resistant M. pneumoniae (MRMP) strains (Bebear et al., 2011). Resistance is based on specific point mutations in domain V of the 23S rRNA (at positions 2063, 2064, and 2617), which reduce the affinity of macrolides to the large subunit (50S) of the bacterial ribosome (Bebear et al., 2011). MRMP has been observed during macrolide treatment as a result of antibiotic selective pressure (Cardinale et al., 2011; Chironna et al., 2011; Saegeman et al., 2012). To date, macrolide resistance has been detected on a worldwide scale. MRMP had developed in Asia (Hong et al., 2013), where MRMP rates have risen as high as 97% in China (Zhao et al., 2013). MRMP has now also been reported from North America and Europe (Figure 6).
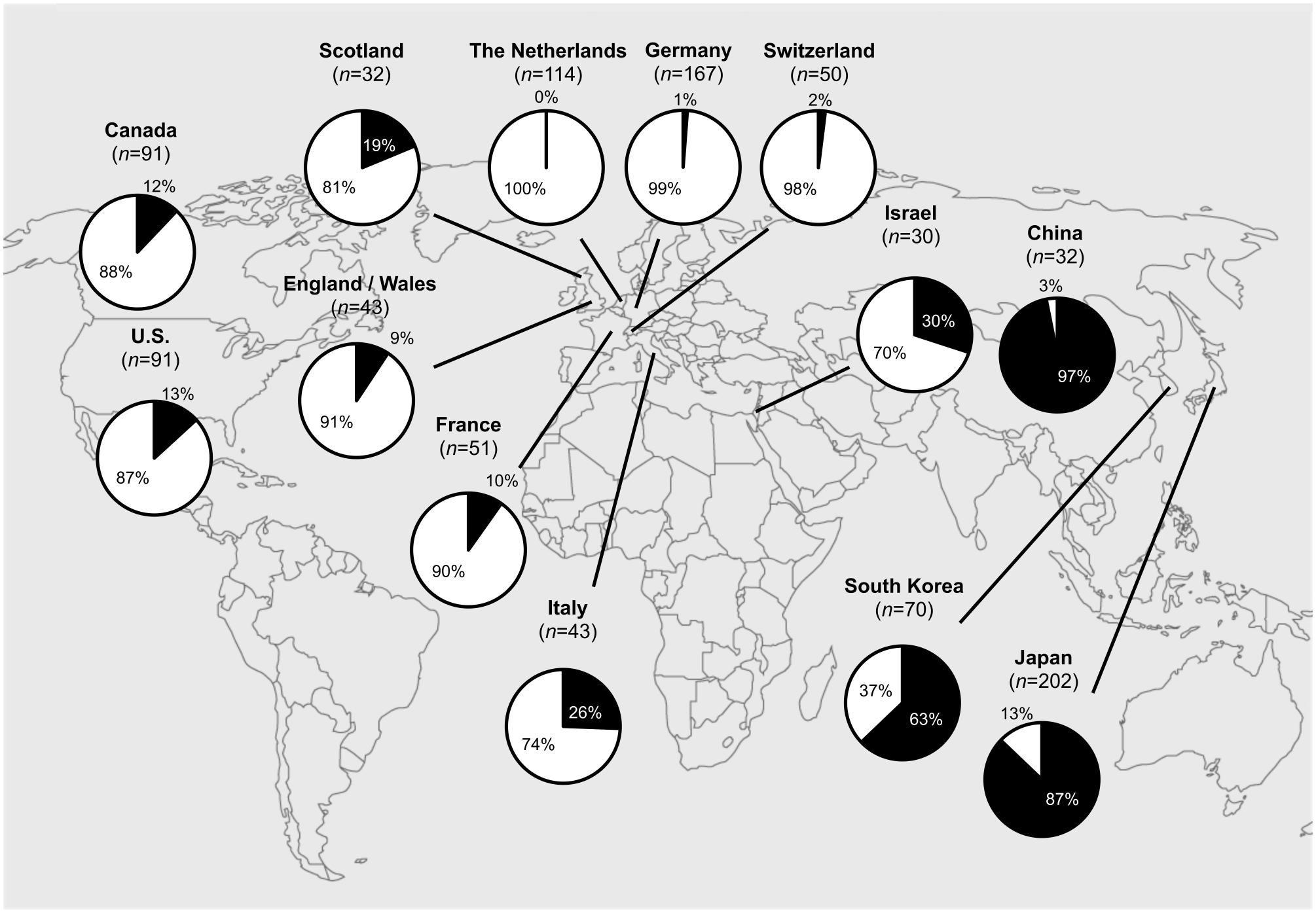
FIGURE 6. Worldwide macrolide-resistant M. pneumoniae (MRMP) rates. Actual MRMP rates are punctually depicted in pie charts (in black) over the world map. Asia: Japan (2011): 87% (176/202) (Okada et al., 2012), South Korea (2011): 63% (44/70) (Hong et al., 2013), China (2012): 97% (31/32) (Zhao et al., 2013), Israel (2010): 30% (9/30) (Averbuch et al., 2011); North America: U.S. (2012–2014): 13% (12/91) (Zheng et al., 2015), Canada (2010–2012): 12% (11/91) (Eshaghi et al., 2013); Europe: The Netherlands (1997–2008): 0% (0/114) (Spuesens et al., 2012), Germany (2003–2008): 1% (2/167) (Dumke et al., 2010), France (2005–2007): 10% (5/51) (Peuchant et al., 2009), Italy (2010): 26% (11/43) (Chironna et al., 2011), Scotland (2010–2011): 19% (6/32) (Ferguson et al., 2013), Switzerland (2011–2013): 2% (1/50) (Meyer Sauteur et al., 2014a), England and Wales (2014–2015): 9% (4/43) (Brown et al., 2015).
The clinical relevance of macrolide resistance in hospitalized children with CAP may lie in prolonging the symptoms of the disease (Okada et al., 2012; Cardinale et al., 2013; Zhou et al., 2014). Zhou et al. (2014) found that an increase in MRMP may also have serious clinical consequences in children, leading to more severe radiological findings of CAP and even an increase in extrapulmonary manifestations. In this study, hospitalized children with CAP due to MRMP developed more often extrapulmonary disease than children with CAP caused by macrolide-sensitive strains (30% vs. 10%; p = 0.03) (Zhou et al., 2014). These manifestations included skin diseases and nervous system complications in 18% and 7%, respectively, of the MRMP-infected children. Serum inflammatory cytokine levels (INF-γ, IL-6, and IP-10) were higher in patients infected with MRMP than in patients infected with macrolide-sensitive strains (Matsuda et al., 2013). This suggests that the higher and more persistent inflammatory stimulation by MRMP may increase the possibility of severe lung lesions and extrapulmonary complications.
While previous attempts to produce vaccines on the basis of inactivated bacteria resulted in limited efficacy against CAP and various adverse effects (Linchevski et al., 2009), the recent use of recombinant proteins as potential vaccines was found to be promising: The immunization of mice with a immunogenic recombinant protein encompassing the C-terminal part of the P1 protein (RP14) induced strong mucosal and systemic antibody responses against M. pneumoniae, and reduced lung inflammation in infected mice (Zhu et al., 2012). Another study showed that immunization of guinea pigs with a chimeric protein consisting of RP14 and the P30 adhesion protein of M. pneumoniae resulted in a robust antibody response that led to a reduction in bacterial loads in the respiratory tract (Hausner et al., 2013).
The increasing prevalence of MRMP has become a significant issue, since MRMP can potentially cause more severe and even extrapulmonary disease. Because symptoms and radiologic features of M. pneumoniae CAP seem to be unspecific and current diagnostic procedures cannot discern between carriage and infection in a clinically relevant time frame, simple clinical rules may further aid physicians in prescribing appropriate first-line antibiotics. Thus, empiric macrolide treatment may be restricted to children at high risk for M. pneumoniae CAP, i.e., children with CAP who have fever >2 days and who are >3 years of age, or in the case of very severe disease. Future research should focus on novel aspects of M. pneumoniae-related pathogenesis resulting in more precise diagnostic tools and tailored treatment that prevents the emergence of antimicrobial resistance.
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
PMMS received grants of the Swiss National Science Foundation (PBZHP3_147290), the Sophia Scientific Research Foundation (SSWO 2014–150/WO), the Promedica Foundation (Chur, Switzerland), and a Fellowship Award of the European Society for Paediatric Infectious Diseases (ESPID), outside the submitted work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Steve Gschmeissner (Bedford, UK) for performing scanning electron microscopy of the M. pneumoniae strain.
Adefurin, A., Sammons, H., Jacqz-Aigrain, E., and Choonara, I. (2011). Ciprofloxacin safety in paediatrics: a systematic review. Arch. Dis. Child. 96, 874–880. doi: 10.1136/adc.2010.208843
Al-Zaidy, S. A., MacGregor, D., Mahant, S., Richardson, S. E., and Bitnun, A. (2015). Neurological complications of PCR-proven M. pneumoniae infections in children: prodromal illness duration may reflect pathogenetic mechanism. Clin. Infect. Dis. 61, 1092–1098. doi: 10.1093/cid/civ473
Ang, C. W., Tio-Gillen, A. P., Groen, J., Herbrink, P., Jacobs, B. C., Van Koningsveld, R., et al. (2002). Cross-reactive anti-galactocerebroside antibodies and Mycoplasma pneumoniae infections in Guillain-Barré syndrome. J. Neuroimmunol. 130, 179–183. doi: 10.1016/S0165-5728(02)00209-6
Atkinson, T. P. (2013). Is asthma an infectious disease? New evidence. Curr. Allergy Asthma Rep. 13, 702–709. doi: 10.1007/s11882-013-0390-8
Averbuch, D., Hidalgo-Grass, C., Moses, A. E., Engelhard, D., and Nir-Paz, R. (2011). Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg. Infect. Dis. 17, 1079–1082. doi: 10.3201/eid/1706.101558
Baer, G., Engelcke, G., Abele-Horn, M., Schaad, U. B., and Heininger, U. (2003). Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community-acquired pneumonia in hospitalised children and adolescents. Eur. J. Clin. Microbiol. Infect. Dis. 22, 742–745. doi: 10.1007/s10096-003-1037-9
Bebear, C., Pereyre, S., and Peuchant, O. (2011). Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol. 6, 423–431. doi: 10.2217/fmb.11.18
Bebear, C., Raherison, C., Nacka, F., de Barbeyrac, B., Pereyre, S., Renaudin, H., et al. (2014). Comparison of Mycoplasma pneumoniae infections in asthmatic children versus asthmatic adults. Pediatr. Infect. Dis. J. 33, e71–e75. doi: 10.1097/INF.0000000000000063
Becker, A., Kannan, T. R., Taylor, A. B., Pakhomova, O. N., Zhang, Y., Somarajan, S. R., et al. (2015). Structure of CARDS toxin, a unique ADP-ribosylating and vacuolating cytotoxin from Mycoplasma pneumoniae. Proc. Natl. Acad. Sci. U.S.A. 112, 5165–5170. doi: 10.1073/pnas.1420308112
Beersma, M. F., Dirven, K., van Dam, A. P., Templeton, K. E., Claas, E. C., and Goossens, H. (2005). Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard”. J. Clin. Microbiol. 43, 2277–2285. doi: 10.1128/JCM.43.5.2277-2285.2005
Bitnun, A., Ford-Jones, E. L., Petric, M., MacGregor, D., Heurter, H., Nelson, S., et al. (2001). Acute childhood encephalitis and Mycoplasma pneumoniae. Clin. Infect. Dis. 32, 1674–1684. doi: 10.1086/320748
Bradley, J. S., Byington, C. L., Shah, S. S., Alverson, B., Carter, E. R., Harrison, C., et al. (2011). The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76. doi: 10.1093/cid/cir531
Brown, R. J., Macfarlane-Smith, L., Phillips, S., and Chalker, V. J. (2015). Detection of macrolide resistant Mycoplasma pneumoniae in England, September 2014 to September 2015. Euro Surveill. 20, 30078. doi: 10.2807/1560-7917.ES.2015.20.48.30078
Cardinale, F., Chironna, M., Chinellato, I., Principi, N., and Esposito, S. (2013). Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J. Clin. Microbiol. 51, 723–724. doi: 10.1128/JCM.02840-12
Cardinale, F., Chironna, M., Dumke, R., Binetti, A., Daleno, C., Sallustio, A., et al. (2011). Macrolide-resistant Mycoplasma pneumoniae in paediatric pneumonia. Eur. Respir. J. 37, 1522–1524. doi: 10.1183/09031936.00172510
Centers for Disease Control and Prevention [CDC] (2013). Mycoplasma pneumoniae outbreak at a university – Georgia, 2012. Morb. Mortal. Wkly. Rep. 62, 603–606.
Chalker, V. J., Pereyre, S., Dumke, R., Winchell, J., Khosla, P., Sun, H., et al. (2015). International Mycoplasma pneumoniae typing study: interpretation of M. pneumoniae multilocus variable-number tandem-repeat analysis. New Microbes New Infect. 7, 37–40. doi: 10.1016/j.nmni.2015.05.005
Chanock, R. M. (1963). Mycoplasma pneumoniae: proposed nomenclature for atypical pneumonia organism (Eaton agent). Science 140, 662. doi: 10.1126/science.140.3567.662
Chanock, R. M., Cook, M. K., Fox, H. H., Parrott, R. H., and Huebner, R. J. (1960). Serologic evidence of infection with Eaton agent in lower respiratory illness in childhood. N. Engl. J. Med. 262, 648–654. doi: 10.1056/NEJM196003312621303
Chanock, R. M., Hayflick, L., and Barile, M. F. (1962). Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc. Natl. Acad. Sci. U.S.A. 48, 41–49. doi: 10.1073/pnas.48.1.41
Chanock, R. M., Mufson, M. A., Bloom, H. H., James, W. D., Fox, H. H., and Kingston, J. R. (1961a). Eaton agent pneumonia. JAMA 175, 213–220. doi: 10.1001/jama.1961.03040030037007
Chanock, R. M., Rifkind, D., Kravetz, H. M., Kinght, V., and Johnson, K. M. (1961b). Respiratory disease in volunteers infected with Eaton agent: a preliminary report. Proc. Natl. Acad. Sci. U.S.A. 47, 887–890. doi: 10.1073/pnas.47.6.887
Chironna, M., Sallustio, A., Esposito, S., Perulli, M., Chinellato, I., Di Bari, C., et al. (2011). Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J. Antimicrob. Chemother. 66, 734–737. doi: 10.1093/jac/dkr003
Christie, L. J., Honarmand, S., Talkington, D. F., Gavali, S. S., Preas, C., Pan, C. Y., et al. (2007a). Pediatric encephalitis: what is the role of Mycoplasma pneumoniae? Pediatrics 120, 305–313. doi: 10.1542/peds.2007-0240
Christie, L. J., Honarmand, S., Yagi, S., Ruiz, S., and Glaser, C. A. (2007b). Anti-galactocerebroside testing in Mycoplasma pneumoniae-associated encephalitis. J. Neuroimmunol. 189, 129–131. doi: 10.1016/j.jneuroim.2007.06.017
Daxboeck, F., Blacky, A., Seidl, R., Krause, R., and Assadian, O. (2004). Diagnosis, treatment, and prognosis of Mycoplasma pneumoniae childhood encephalitis: systematic review of 58 cases. J. Child Neurol. 19, 865–871.
Domenech, C., Leveque, N., Lina, B., Najioullah, F., and Floret, D. (2009). Role of Mycoplasma pneumoniae in pediatric encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 28, 91–94. doi: 10.1007/s10096-008-0591-6
Dorigo-Zetsma, J. W., Wilbrink, B., van der Nat, H., Bartelds, A. I., Heijnen, M. L., and Dankert, J. (2001). Results of molecular detection of Mycoplasma pneumoniae among patients with acute respiratory infection and in their household contacts reveals children as human reservoirs. J. Infect. Dis. 183, 675–678. doi: 10.1086/318529
Dumke, R., Strubel, A., Cyncynatus, C., Nuyttens, H., Herrmann, R., Luck, C., et al. (2012). Optimized serodiagnosis of Mycoplasma pneumoniae infections. Diagn. Microbiol. Infect. Dis. 73, 200–203. doi: 10.1016/j.diagmicrobio.2012.02.014
Dumke, R., von Baum, H., Luck, P. C., and Jacobs, E. (2010). Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin. Microbiol. Infect. 16, 613–616. doi: 10.1111/j.1469-0691.2009.02968.x
Eaton, M. D., Meiklejohn, G., and van Herick, W. (1944). Studies on the etiology of primary atypical pneumonia: a filterable agent transmissible to cotton rats, hamsters, and chick embryos. J. Exp. Med. 79, 649–668. doi: 10.1084/jem.79.6.649
Eshaghi, A., Memari, N., Tang, P., Olsha, R., Farrell, D. J., Low, D. E., et al. (2013). Macrolide-resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010-2011. Emerg. Infect. Dis. 19, 1525–1527.
Esposito, S., Blasi, F., Arosio, C., Fioravanti, L., Fagetti, L., Droghetti, R., et al. (2000). Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur. Respir. J. 16, 1142–1146. doi: 10.1034/j.1399-3003.2000.16f21.x
Esposito, S., Bosis, S., Faelli, N., Begliatti, E., Droghetti, R., Tremolati, E., et al. (2005). Role of atypical bacteria and azithromycin therapy for children with recurrent respiratory tract infections. Pediatr. Infect. Dis. J. 24, 438–444. doi: 10.1097/01.inf.0000160949.99560.8d
Esposito, S., Cavagna, R., Bosis, S., Droghetti, R., Faelli, N., and Principi, N. (2002). Emerging role of Mycoplasma pneumoniae in children with acute pharyngitis. Eur. J. Clin. Microbiol. Infect. Dis. 21, 607–610. doi: 10.1007/s10096-002-0780-7
Ferguson, G. D., Gadsby, N. J., Henderson, S. S., Hardie, A., Kalima, P., Morris, A. C., et al. (2013). Clinical outcomes and macrolide resistance in Mycoplasma pneumoniae infection in Scotland, UK. J. Med. Microbiol. 62, 1876–1882. doi: 10.1099/jmm.0.066191-0
Fischer, J. E., Steiner, F., Zucol, F., Berger, C., Martignon, L., Bossart, W., et al. (2002). Use of simple heuristics to target macrolide prescription in children with community-acquired pneumonia. Arch. Pediatr. Adolesc. Med. 156, 1005–1008. doi: 10.1001/archpedi.156.10.1005
Foy, H. M. (1993). Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin. Infect. Dis. 17(Suppl. 1), S37–S46. doi: 10.1093/clinids/17.Supplement_1.S37
Foy, H. M., Kenny, G. E., Cooney, M. K., and Allan, I. D. (1979). Long-term epidemiology of infections with Mycoplasma pneumoniae. J. Infect. Dis. 139, 681–687. doi: 10.1093/infdis/139.6.681
Gadsby, N. J., Reynolds, A. J., McMenamin, J., Gunson, R. N., McDonagh, S., Molyneaux, P. J., et al. (2012). Increased reports of Mycoplasma pneumoniae from laboratories in Scotland in 2010 and 2011 – impact of the epidemic in infants. Euro Surveill. 17, 20110.
Gardiner, S. J., Gavranich, J. B., and Chang, A. B. (2015). Antibiotics for community-acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database Syst. Rev. 1, CD004875. doi: 10.1002/14651858.CD004875.pub5
Granerod, J., Cunningham, R., Zuckerman, M., Mutton, K., Davies, N. W., Walsh, A. L., et al. (2010). Causality in acute encephalitis: defining aetiologies. Epidemiol. Infect. 138, 783–800. doi: 10.1017/S0950268810000725
Hardy, R. D., Coalson, J. J., Peters, J., Chaparro, A., Techasaensiri, C., Cantwell, A. M., et al. (2009). Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS ONE 4:e7562. doi: 10.1371/journal.pone.0007562
Harris, M., Clark, J., Coote, N., Fletcher, P., Harnden, A., McKean, M., et al. (2011). British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66(Suppl. 2), ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598
Hausner, M., Schamberger, A., Naumann, W., Jacobs, E., and Dumke, R. (2013). Development of protective anti-Mycoplasma pneumoniae antibodies after immunization of guinea pigs with the combination of a P1-P30 chimeric recombinant protein and chitosan. Microb. Pathog. 64, 23–32. doi: 10.1016/j.micpath.2013.07.004
Himmelreich, R., Hilbert, H., Plagens, H., Pirkl, E., Li, B. C., and Herrmann, R. (1996). Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24, 4420–4449. doi: 10.1093/nar/24.22.4420
Hong, K. B., Choi, E. H., Lee, H. J., Lee, S. Y., Cho, E. Y., Choi, J. H., et al. (2013). Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg. Infect. Dis. 19, 1281–1284. doi: 10.3201/eid1908.121455
Jacobs, E. (2012). Mycoplasma pneumoniae: now in the focus of clinicians and epidemiologists. Euro Surveill. 17, 1–3.
Jacobs, E., Ehrhardt, I., and Dumke, R. (2015). New insights in the outbreak pattern of Mycoplasma pneumoniae. Int. J. Med. Microbiol. 305, 705–708. doi: 10.1016/j.ijmm.2015.08.021
Jain, S., Williams, D. J., Arnold, S. R., Ampofo, K., Bramley, A. M., Reed, C., et al. (2015). Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845. doi: 10.1056/NEJMoa1405870
John, S. D., Ramanathan, J., and Swischuk, L. E. (2001). Spectrum of clinical and radiographic findings in pediatric mycoplasma pneumonia. Radiographics 21, 121–131. doi: 10.1148/radiographics.21.1.g01ja10121
Juven, T., Mertsola, J., Waris, M., Leinonen, M., Meurman, O., Roivainen, M., et al. (2000). Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 19, 293–298. doi: 10.1097/00006454-200004000-00006
Kannan, T. R., and Baseman, J. B. (2006). ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc. Natl. Acad. Sci. U.S.A. 103, 6724–6729. doi: 10.1073/pnas.0510644103
Kannan, T. R., Hardy, R. D., Coalson, J. J., Cavuoti, D. C., Siegel, J. D., Cagle, M., et al. (2012). Fatal outcomes in family transmission of Mycoplasma pneumoniae. Clin. Infect. Dis. 54, 225–231. doi: 10.1093/cid/cir769
Kusunoki, S., Shiina, M., and Kanazawa, I. (2001). Anti-Gal-C antibodies in GBS subsequent to mycoplasma infection: evidence of molecular mimicry. Neurology 57, 736–738. doi: 10.1212/WNL.57.4.736
Linchevski, I., Klement, E., and Nir-Paz, R. (2009). Mycoplasma pneumoniae vaccine protective efficacy and adverse reactions–Systematic review and meta-analysis. Vaccine 27, 2437–2446. doi: 10.1016/j.vaccine.2009.01.135
Loens, K., Goossens, H., and Ieven, M. (2010). Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur. J. Clin. Microbiol. Infect. Dis. 29, 1055–1069. doi: 10.1007/s10096-010-0975-2
Matsuda, K., Narita, M., Sera, N., Maeda, E., Yoshitomi, H., Ohya, H., et al. (2013). Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniae infection in Fukuoka, Japan. BMC Infect. Dis. 13:591. doi: 10.1186/1471-2334-13-591
McCoy, W. C. (1946). Primary atypical pneumonia. South Med. J. 39, 696–706. doi: 10.1097/00007611-194609000-00005
McCracken, G. H. Jr. (1986). Current status of antibiotic treatment for Mycoplasma pneumoniae infections. Pediatr. Infect. Dis. 5, 167–171. doi: 10.1097/00006454-198601000-00054
Medina, J. L., Coalson, J. J., Brooks, E. G., Winter, V. T., Chaparro, A., Principe, M. F., et al. (2012). Mycoplasma pneumoniae CARDS toxin induces pulmonary eosinophilic and lymphocytic inflammation. Am. J. Respir. Cell Mol. Biol. 46, 815–822. doi: 10.1165/rcmb.2011-0135OC
Menge, T., Lalive, P. H., von Budingen, H. C., Cree, B., Hauser, S. L., and Genain, C. P. (2005). Antibody responses against galactocerebroside are potential stage-specific biomarkers in multiple sclerosis. J. Allergy Clin. Immunol. 116, 453–459. doi: 10.1016/j.jaci.2005.03.023
Meyer Sauteur, P. M., Bleisch, B., Voit, A., Maurer, F. P., Relly, C., Berger, C., et al. (2014a). Survey of macrolide-resistant Mycoplasma pneumoniae in children with community-acquired pneumonia in Switzerland. Swiss Med. Wkly. 144, w14041.
Meyer Sauteur, P. M., Gansser-Kalin, U., Lautenschlager, S., and Goetschel, P. (2011). Fuchs syndrome associated with Mycoplasma pneumoniae (Stevens-Johnson syndrome without skin lesions). Pediatr. Dermatol. 28, 474–476. doi: 10.1111/j.1525-1470.2010.01200.x
Meyer Sauteur, P. M., Goetschel, P., and Lautenschlager, S. (2012). Mycoplasma pneumoniae and mucositis–part of the Stevens-Johnson syndrome spectrum. J. Dtsch. Dermatol. Ges. 10, 740–746. doi: 10.1111/j.1610-0387.2012.07951.x
Meyer Sauteur, P. M., Hackenberg, A., Tio-Gillen, A. P., van Rossum, A. M., Berger, C., and Jacobs, B. C. (2015a). Intrathecal anti-GalC antibodies in Bickerstaff brain stem encephalitis. Neuropediatrics 46, 428–430. doi: 10.1055/s-0035-1566730
Meyer Sauteur, P. M., Jacobs, B. C., Spuesens, E. B., Jacobs, E., Nadal, D., Vink, C., et al. (2014b). Antibody responses to Mycoplasma pneumoniae: role in pathogenesis and diagnosis of encephalitis? PLoS Pathog. 10:e1003983. doi: 10.1371/journal.ppat.1003983
Meyer Sauteur, P. M., Moeller, A., Relly, C., Berger, C., Plecko, B., Nadal, D., et al. (2016). Swiss national prospective surveillance of paediatric Mycoplasma pneumoniae-associated encephalitis. Swiss Med. Wkly. 145, w14222. doi: 10.4414/smw.2016.14222
Meyer Sauteur, P. M., Relly, C., Hackenberg, A., Stahr, N., Berger, C., Bloemberg, G. V., et al. (2014c). Mycoplasma pneumoniae intrathecal antibody responses in Bickerstaff brain stem encephalitis. Neuropediatrics 45, 61–63. doi: 10.1055/s-0033-1348150
Meyer Sauteur, P. M., Roodbol, J., Hackenberg, A., de Wit, M. C., Vink, C., Berger, C., et al. (2015b). Severe childhood Guillain-Barré syndrome associated with Mycoplasma pneumoniae infection: a case series. J. Peripher. Nerv. Syst. 20, 72–78. doi: 10.1111/jns.12121
Michelow, I. C., Olsen, K., Lozano, J., Rollins, N. K., Duffy, L. B., Ziegler, T., et al. (2004). Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113, 701–707. doi: 10.1542/peds.113.4.701
Mizutani, H., Kitayama, T., Hayakawa, A., and Nagayama, E. (1971). Delayed hypersensitivity in Mycoplasma pneumoniae infections. Lancet 1, 186–187. doi: 10.1016/S0140-6736(71)91956-8
Monteyne, P., Albert, F., Weissbrich, B., Zardini, E., Ciardi, M., Cleator, G. M., et al. (1997). The detection of intrathecal synthesis of anti-herpes simplex IgG antibodies: comparison between an antigen-mediated immunoblotting technique and antibody index calculations. European Union Concerted Action on Virus Meningitis and Encephalitis. J. Med. Virol. 53, 324–331. doi: 10.1002/(SICI)1096-9071(199712)53:4<324::AID-JMV3>3.0.CO;2-9
Narita, M. (2009). Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection. Pediatr. Neurol. 41, 159–166. doi: 10.1016/j.pediatrneurol.2009.04.012
Narita, M. (2010). Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J. Infect. Chemother. 16, 162–169. doi: 10.1007/s10156-010-0044-x
Narita, M., and Tanaka, H. (2007). Cytokines involved in the severe manifestations of pulmonary diseases caused by Mycoplasma pneumoniae. Pediatr. Pulmonol. 42, 397. doi: 10.1002/ppul.20445
Narita, M., and Yamada, S. (2001). Two distinct patterns of central nervous system complications due to Mycoplasma pneumoniae infection. Clin. Infect. Dis. 33, 916–917. doi: 10.1086/322709
Okada, T., Morozumi, M., Tajima, T., Hasegawa, M., Sakata, H., Ohnari, S., et al. (2012). Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin. Infect. Dis. 55, 1642–1649. doi: 10.1093/cid/cis784
Peuchant, O., Menard, A., Renaudin, H., Morozumi, M., Ubukata, K., Bebear, C. M., et al. (2009). Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J. Antimicrob. Chemother. 64, 52–58. doi: 10.1093/jac/dkp160
Polkowska, A., Harjunpaa, A., Toikkanen, S., Lappalainen, M., Vuento, R., Vuorinen, T., et al. (2012). Increased incidence of Mycoplasma pneumoniae infection in Finland, 2010-2011. Euro Surveill. 17, 20072.
Principi, N., Esposito, S., Blasi, F., Allegra, L., and Mowgli study group (2001). Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin. Infect. Dis. 32, 1281–1289. doi: 10.1086/319981
Reiber, H. (1994). Flow rate of cerebrospinal fluid (CSF)–a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122, 189–203. doi: 10.1016/0022-510X(94)90298-4
Saegeman, V., Proesmans, M., and Dumke, R. (2012). Management of macrolide-resistant Mycoplasma pneumoniae infection. Pediatr. Infect. Dis. J. 31, 1210–1211. doi: 10.1097/INF.0b013e3182611cee
Saida, T., Saida, K., Dorfman, S. H., Silberberg, D. H., Sumner, A. J., Manning, M. C., et al. (1979). Experimental allergic neuritis induced by sensitization with galactocerebroside. Science 204, 1103–1106. doi: 10.1126/science.451555
Samukawa, M., Hirano, M., Tsugawa, J., Sakamoto, H., Tabata, E., Takada, K., et al. (2012). Refractory acute disseminated encephalomyelitis with anti-galactocerebroside antibody. Neurosci. Res. 74, 284–289. doi: 10.1016/j.neures.2012.09.006
Schalock, P. C., and Dinulos, J. G. (2009). Mycoplasma pneumoniae-induced cutaneous disease. Int. J. Dermatol. 48, 673–680; quiz 680–671. doi: 10.1111/j.1365-4632.2009.04154.x
Sinha, S., Prasad, K. N., Jain, D., Pandey, C. M., Jha, S., and Pradhan, S. (2007). Preceding infections and anti-ganglioside antibodies in patients with Guillain-Barré syndrome: a single centre prospective case-control study. Clin. Microbiol. Infect. 13, 334–337. doi: 10.1111/j.1469-0691.2006.01636.x
Spuesens, E. B., Fraaij, P. L., Visser, E. G., Hoogenboezem, T., Hop, W. C., van Adrichem, L. N., et al. (2013). Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med. 10:e1001444. doi: 10.1371/journal.pmed.1001444
Spuesens, E. B., Meijer, A., Bierschenk, D., Hoogenboezem, T., Donker, G. A., Hartwig, N. G., et al. (2012). Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae in respiratory specimens collected between 1997 and 2008 in The Netherlands. J. Clin. Microbiol. 50, 1999–2004. doi: 10.1128/JCM.00400-12
Spuesens, E. B., Oduber, M., Hoogenboezem, T., Sluijter, M., Hartwig, N. G., van Rossum, A. M., et al. (2009). Sequence variations in RepMP2/3 and RepMP4 elements reveal intragenomic homologous DNA recombination events in Mycoplasma pneumoniae. Microbiology 155, 2182–2196. doi: 10.1099/mic.0.028506-0
Su, C. J., Chavoya, A., Dallo, S. F., and Baseman, J. B. (1990). Sequence divergency of the cytadhesin gene of Mycoplasma pneumoniae. Infect. Immun. 58, 2669–2674.
Vink, C., Rudenko, G., and Seifert, H. S. (2012). Microbial antigenic variation mediated by homologous DNA recombination. FEMS Microbiol. Rev. 36, 917–948. doi: 10.1111/j.1574-6976.2011.00321.x
Waites, K. B., and Talkington, D. F. (2004). Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 17, 697–728. doi: 10.1128/CMR.17.4.697-728.2004
Wang, K., Gill, P., Perera, R., Thomson, A., Mant, D., and Harnden, A. (2012). Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst. Rev. 10, CD009175. doi: 10.1002/14651858.CD009175.pub2
Wood, P. R., Hill, V. L., Burks, M. L., Peters, J. I., Singh, H., Kannan, T. R., et al. (2013). Mycoplasma pneumoniae in children with acute and refractory asthma. Ann. Allergy Asthma Immunol. 110, 321.e1–334.e1. doi: 10.1016/j.anai.2013.01.022
Yeh, J. J., Wang, Y. C., Hsu, W. H., and Kao, C. H. (2015). Incident asthma and Mycoplasma pneumoniae: a nationwide cohort study. J. Allergy Clin. Immunol. doi: 10.1016/j.jaci.2015.09.032 [Epub ahead of print].
Zhao, F., Liu, G., Wu, J., Cao, B., Tao, X., He, L., et al. (2013). Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob. Agents Chemother. 57, 1521–1523. doi: 10.1128/AAC.02060-12
Zheng, X., Lee, S., Selvarangan, R., Qin, X., Tang, Y. W., Stiles, J., et al. (2015). Macrolide-Resistant Mycoplasma pneumoniae, United States. Emerg. Infect. Dis. 21, 1470–1472. doi: 10.3201/eid2108.150273
Zhou, Y., Zhang, Y., Sheng, Y., Zhang, L., Shen, Z., and Chen, Z. (2014). More complications occurred in macrolide-resistant Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 58, 1034–1038. doi: 10.1128/AAC.01806-13
Keywords: Mycoplasma pneumoniae, pneumonia, carriage, children, diagnosis
Citation: Meyer Sauteur PM, Unger WWJ, Nadal D, Berger C, Vink C and van Rossum AMC (2016) Infection with and Carriage of Mycoplasma pneumoniae in Children. Front. Microbiol. 7:329. doi: 10.3389/fmicb.2016.00329
Received: 15 January 2016; Accepted: 02 March 2016;
Published: 23 March 2016.
Edited by:
Frederic Lamoth, Lausanne University Hospital, SwitzerlandReviewed by:
Marta Palusinska-Szysz, Maria Curie-Sklodowska University, PolandCopyright © 2016 Meyer Sauteur, Unger, Nadal, Berger, Vink and van Rossum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick M. Meyer Sauteur, cGF0cmljay5tZXllckBraXNwaS51emguY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.