- Bacterial Genomics and Evolution Laboratory, Council of Scientific and Industrial Research – Institute of Microbial Technology, Chandigarh, India
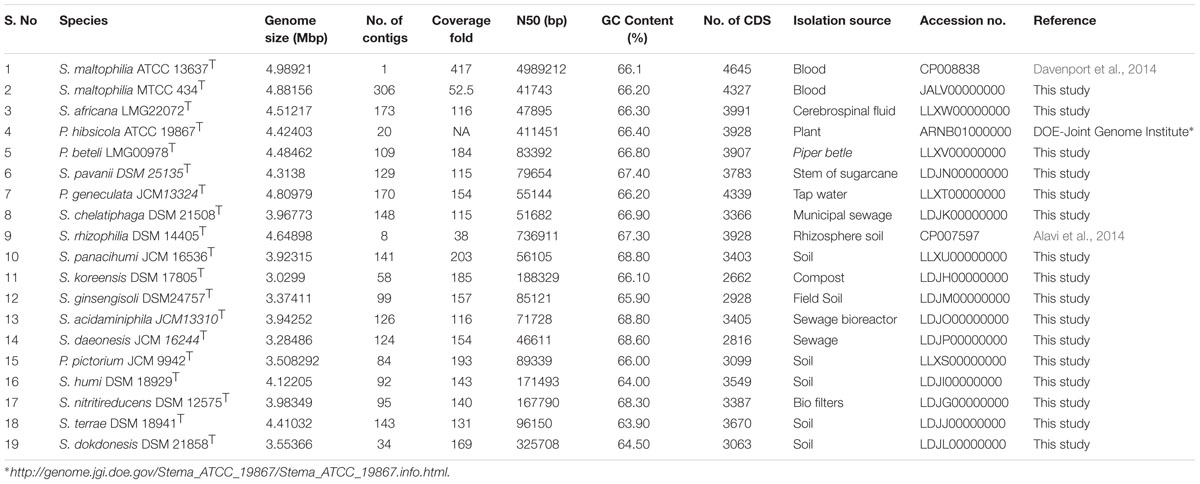
Genomic resource of type strains and historically important strains of genus Stenotrophomonas allowed us to reveal the existence of 18 distinct species by applying modern phylogenomic criterions. Apart from Stenotrophomonas maltophilia, S. africana represents another species of clinical importance. Interestingly, Pseudomonas hibsicola, P. beteli, and S. pavani that are of plant origin are closer to S. maltophilia than the majority of the environmental isolates. The genus has an open pan-genome. By providing the case study on genes encoding metallo-β-lactamase and Clustered Regularly Interspaced Short Palindrome Repeats (CRISPR) regions, we have tried to show the importance of this genomic dataset in understanding its ecology.
Background
The members of the genus Stenotrophomonas are widespread in the diverse habitats with biotechnological applications and clinical relevance (Ryan et al., 2009). According to a recent report by WHO, S. maltophilia is a leading drug-resistant pathogen in hospitals worldwide (Brooke, 2014). Currently, the genus Stenotrophomonas compromises 12 validated species in the List of Prokaryotic Names Standing in Nomenclature1 from diverse habitats. The type species of the genus S. maltophilia was initially isolated from the pleural fluid and named as Bacterium bookeri and was then reclassified as P. maltophilia (Hugh and Ryschenkow, 1961). Further, this species was transferred to genus Xanthomonas as X. maltophilia (Swings et al., 1983) and later it was designated as a distinct and new genus Stenotrophomonas (Palleroni and Bradbury, 1993). Remaining 11 species of this genus were isolated from distinct environmental sources, S. rhizophila (Wolf et al., 2002), S. pavanii (Ramos et al., 2011), S. humi, S. terrae (Heylen et al., 2007), S. ginsengisoli (Kim et al., 2010), and S. panacihumi (Yi et al., 2010), S. koreensis (Yang et al., 2006), S. nitritireducens (Finkmann et al., 2000), S. acidaminiphila (Assih et al., 2002), S. chelatiphaga (Kaparullina et al., 2009), and S. daejeonensis (Lee et al., 2011). Additionally, there are many taxonomical revisions in the genus Stenotrophomonas. S. africana was initially described as a novel species of this genus, isolated from human cerebrospinal fluid (Drancourt et al., 1997). But later based on the whole cell protein and DNA–DNA hybridization analysis S. africana was proposed as a synonym of S. maltophilia (Coenye et al., 2004). S. dokdonesis (Yoon et al., 2006) a former species of the genus Stenotrophomonas, was assigned to the new genus Pseudoxanthomonas (Lee et al., 2008). Apart from this, there are three species of genus Pseudomonas, i.e., P. beteli, P. hibiscicola, and P. geniculata which are transferred to the genus Stenotrophomonas considered as synonyms of the S. maltophilia (Van den Mooter and Swings, 1990; Anzai et al., 2000). P. pictorium, is also considered to be closer to Stenotrophomonas (Svensson-Stadler et al., 2012).
Herein we generated draft genomes of 16 type strains which include 11 currently validated species of the genus Stenotrophomonas and five genomes from the different genera which are historically associated or grouped with the Stenotrophomonas. Whole genome sequence of type strains of the S. rhizophila and P. hibisicola are available in the public database (Table 1). The complete genome of S. maltophilia type strain is available publically (Davenport et al., 2014), but we had also sequenced independently and hence included in this study. The genome sequence of the type strains will be valuable in taxonomic and evolutionary studies of genus Stenotrophomonas and its relatives.
Methods
Bacterial Strains and Culture Conditions
Type strains of genus Stenotrophomonas and related species (Table 1) were procured from different culture collection centers, Microbial Type Culture Collection (MTCC), Belgian Coordinated Collections of Microorganisms/LMG (BCCM/LMG) and The Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures GmbH (DSM). The high molecular weight genomic DNA of S. africana LMG 22072 was procured from BCCM/LMG for whole genome sequencing. All isolates were grown as per the media and conditions recommended by the respective culture collection centers.
Genome Sequencing, Assembly, and Annotation
Genomic DNA was extracted by using ZR Fungal/Bacterial DNA MiniPrep Kit (Zymo Research Corporation, Irvine, CA, USA) and quantified using Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Illumina sequencing library of genomic DNA was prepared using Nextera XT sample preparation kit (Illumina, Inc., San Diego, CA, USA) with dual indexing adapters. Illumina sequencing library was sequenced using in-house Illumina Miseq (Illumina, Inc., San Diego, CA, USA) platform using paired-end sequencing kits. The Illumina adapters were trimmed by the internal software during the base calling. In addition, to that adapter contamination identified by NCBI during the submission was removed by manual trimming. Raw reads were assembled using CLC Genomics Workbench v7.5 (CLC Bio-Qiagen, Aarhus, Denmark) and annotation was using NCBI Prokaryotic Genome Annotation Pipeline through NCBI2. CRISPR was identified using the CRISPR recognition tool (Bland et al., 2007).
Genome Similarity Assessment
For genome similarity assessment we used BLAST-based average nucleotide identity (ANIb) and Genome to Genome Distance calculator or digital DNA-DNA hybridization (dDDH) values. Pairwise ANI was calculated using JSpecies (Richter and Rosselló-Móra, 2009) and digital DDH (Auch et al., 2010) was calculated using web tool GGDC 2.03. Xanthomonas campestris pv. campestries ATCC 33913 and P. aeruginosa DSM 50071 were included as outgroups.
Pan-Genome Analysis
Pan-genome analysis of representatives of the genus Stenotrophomonas and related species under study was carried out by using the PGAP pipeline version 1.12 (Zhao et al., 2012). The MultiParanoid (MP) method was used for Pan-genome analysis with minimum score value 40 and e-value e1 × 10-10 used as cut off for BLAST. Pan-genome was visualized by using PanGP (Zhao et al., 2014). The flower pot diagram to represent the core and unique genes was drawn by using python script of Matplotlib (Hunter, 2007).
Data Deposition
The genome sequence data of the 16 type strains sequenced under this study has been deposited in NCBI GenBank and their accession numbers are mentioned in Table 1. Single point access to download genomes in FASTA format is also available at Figshare4.
Interpretation of Data Set
Genome Sequences and Phylogenic Inference
The general features of the newly sequenced genomes of type strains and their assembly statistics are summarized in Table 1. The ANI and dDDH values of representatives of the genus Stenotrophomonas are summarized in Supplementary Table S1. In microbial taxonomy for species delineation, 94 and 70% cut-off is used for ANI values and dDDH values, respectively, (Richter and Rosselló-Móra, 2009; Auch et al., 2010). No two strains have >94% ANI and >70% dDDH values suggesting that all the 18 members belong to distinct species.
Stenotrophomonas africana was initially described as a novel species but later reclassified as a synonym of S. maltophilia. Interestingly ANI and dDDH values of S. africana with the type strain of S. maltophilia are 90 and 49%, respectively, suggesting that the S. africana is a separate species.
The taxonomic status of the misclassified species P. genicualata, P. hibsicola, and P. betele is unclear and they are considered as synonyms of the S. maltophilia. But based on ANI and dDDH values with the type strain of the S. maltophilia, these species P. genicualata, P. hibsicola, and P. betele do not belong to S. maltophilia and represent separate species.
Pseudomonas pictorium is also considered as a misclassified Pseudomonas and is closely related to Stenotrophomonas sp. It shows <94% ANI and <70% dDDH with type strains of all Stenotrophomonas species and should be reclassified as a distinct species of the genus Stenotrophomonas.
Stenotrophomonas dokdonensis which was transferred to a new genus Pseudoxanthomonas exhibits ANI in the range of 73–75% and dDDH around 20% with the type strain of the species of Stenotrophomonas. Hence, there is need to re-examine its classification into a separate genus.
Pangenome Analysis of the Genus Stenotrophomonas
Genome sequences of the 18 Stenotrophomonas species under study were used to analyze the pan-genome and core genome. A total of 11052 genes were identified and among them, 1328 (12%) genes make the core genome of genus Stenotrophomonas. The pan and core genome sizes were plotted against the number of genomes under study. The pan-genome curve shows that the power trend line has not attended the plateau (Figure 1) suggesting that Stenotrophomonas displays an open pan-genome and that the number of genomes analyzed here is not sufficient to describe the complete gene repertoire and it requires more sequencing in order to describe all genes of the genus. The remaining 9724 gene clusters make the accessory genome which also includes the species-specific unique genes that range from 146 to 501 genes (Figure 1).
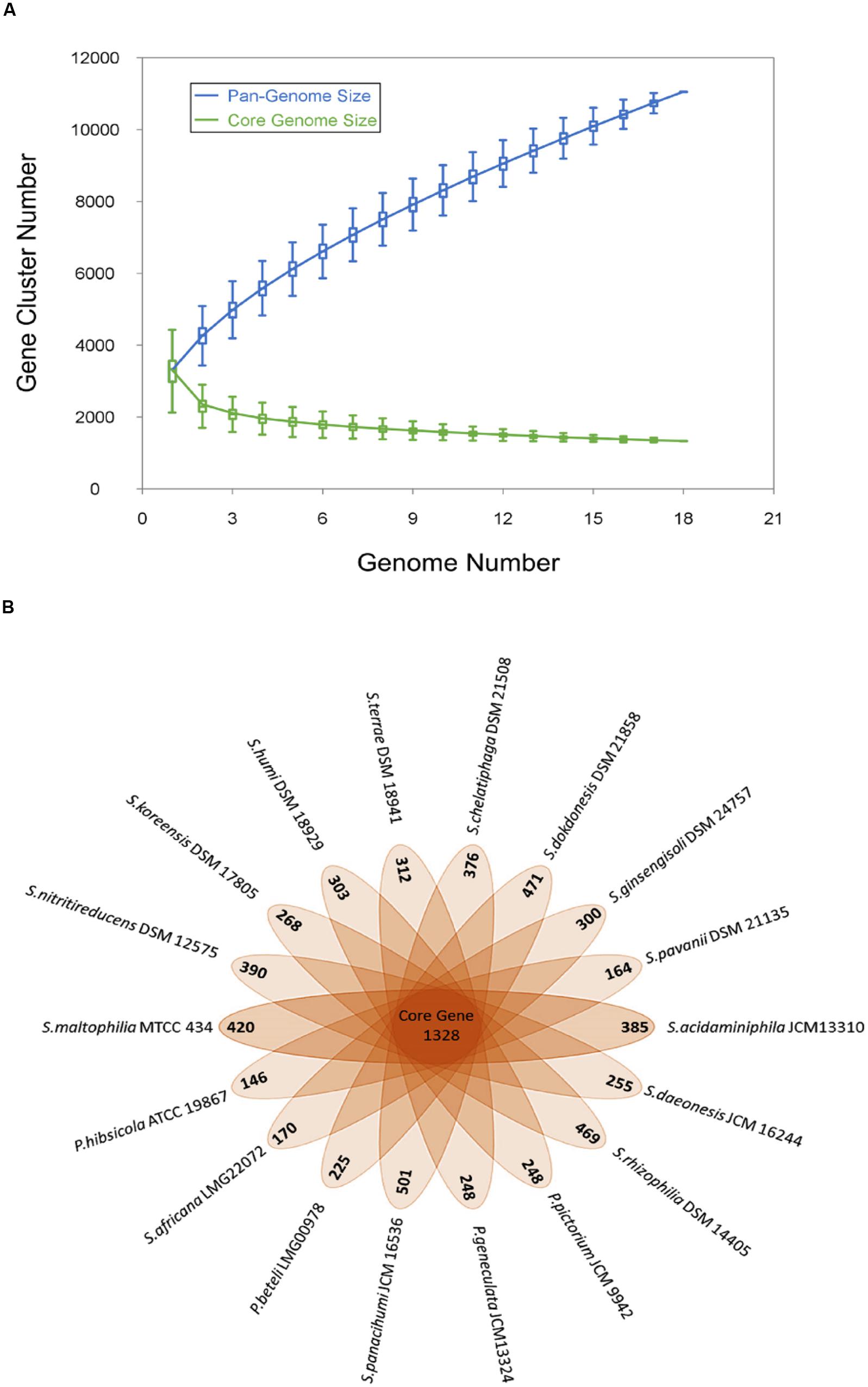
FIGURE 1. Pan and core genome of the genus Stenotrophomonas. (A) The number of gene clusters in pan-genome and core-genome are plotted against number of Stenotrophomonas genomes sequenced. (B) Flower plot diagram showing numbers of unique genes in each Stenotrophomonas species in the petals and Stenotrophomonas core orthologous gene number in the center.
Distribution of Chromosomal Metallo-β-Lactamase in Genus Stenotrophomonas
Type species of the genus Stenotrophomonas, i.e., S. maltophila has two chromosomally encoded β-lactamases, L1 and L2 which are characteristics of S. maltophilia and gives resistance to almost all β-lactam group of antibiotics (Denton and Kerr, 1998). L1 is a metallo-β-lactamase (Walsh et al., 1994; Ullah et al., 1998) and L2 is a clavulanic acid sensitive serine β-lactamases (Walsh et al., 1997). Here in we accessed the distribution of the L1 metallo-β-lactamase in the representative of the species of the genus. L1 metallo-β-lactamase is exclusively present only in the S. africana, S. pavani, P. genicualata, P. hibsicola, and P. betele along with the S. maltophilia and absent in the other species of the genus. The G+C content of the L1 metallo-β-lactamase is same as that of the genomic GC content, i.e., 67% suggesting that it is not acquired through the lateral gene transfer.
CRISPR-cas System in Stenotrophomonas
CRISPR-cas system is important to the bacteria for the adaptive immunity against the invasive elements in bacteria (Barrangou et al., 2007). Among 18 genomes analyzed representing 12 validated species and newly identified species of genus Stenotrophomonas, CRISPR loci are absent in the S. humi, S. koreensis, S. chelatiphaga, S. dokdonesis, S. daeonesis, P. pictorium, S. panacihumi, P. hibsicola and P. geniculata. Interestingly S. terrae, and S. acidaminiphila have multiple distinct CRISPR locus. The distribution of CRISPR locus across Stenotrophomonas genus along with repeat sequences and a number of repeats is mentioned in the Supplementary Table S2. It is important to note that the CRISPR loci are not widespread in this genus as 9 out of 18 genomes of the representative of the genus Stenotrophomonas are not having any CRISPR repeats.
Author Contributions
PP and SM carried out whole genome sequencing and prepared sequin files for submissions. PP and SK carried out the genome analysis. PP drafted the manuscript. PBP conceived the study and participated in its design and coordination. All authors have read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PP is supported by the fellowship from UGC and SM is supported by the fellowship from CSIR. We acknowledge the funding from CSIR Network projects (BSC-402H and BSC-119E/HUM) and OLP-0062.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00309
Footnotes
- ^ http://www.bacterio.net/stenotrophomonas.html
- ^ http://www.ncbi.nlm.nih.gov/genome/annotation_prok
- ^ http://ggdc.dsmz.de/distcalc2.php
- ^ https://figshare.com/s/7cbcaa37451dab19563d
References
Alavi, P., Starcher, M. R., Thallinger, G. G., Zachow, C., Müller, H., and Berg, G. (2014). Stenotrophomonas comparative genomics reveals genes and functions that differentiate beneficial and pathogenic bacteria. BMC Genomics 15:482. doi: 10.1186/1471-2164-15-482
Anzai, Y., Kim, H., Park, J.-Y., Wakabayashi, H., and Oyaizu, H. (2000). Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 50, 1563–1589. doi: 10.1099/00207713-50-4-1563
Assih, E. A., Ouattara, A. S., Thierry, S., Cayol, J.-L., Labat, M., and Macarie, H. (2002). Stenotrophomonas acidaminiphila sp. nov., a strictly aerobic bacterium isolated from an upflow anaerobic sludge blanket (UASB) reactor. Int. J. Syst. Evol. Microbiol. 52, 559–568. doi: 10.1099/00207713-52-2-559
Auch, A. F., Von Jan, M., Klenk, H.-P., and Göker, M. (2010). Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genomic Sci. 2:117. doi: 10.4056/sigs.531120
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712. doi: 10.1126/science.1138140
Bland, C., Ramsey, T. L., Sabree, F., Lowe, M., Brown, K., Kyrpides, N. C., et al. (2007). CRISPR recognition tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinform. 8:209. doi: 10.1186/1471-2105-8-209
Brooke, J. S. (2014). New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev. Anti-Infect. Ther. 12, 1–4. doi: 10.1586/14787210.2014.864553
Coenye, T., Vanlaere, E., Falsen, E., and Vandamme, P. (2004). Stenotrophomonas africana Drancourt et al. 1997 is a later synonym of Stenotrophomonas maltophilia (Hugh 1981) Palleroni and Bradbury 1993. Int. J. Syst. Evol. Microbiol. 54, 1235–1237. doi: 10.1099/ijs.0.63093-0
Davenport, K., Daligault, H., Minogue, T., Broomall, S., Bruce, D., Chain, P., et al. (2014). Complete genome sequence of Stenotrophomonas maltophilia type strain 810-2 (ATCC 13637). Genome Announc. 2:e974. doi: 10.1128/genomeA.00974-14
Denton, M., and Kerr, K. G. (1998). Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11, 57–80.
Drancourt, M., Bollet, C., and Raoult, D. (1997). Stenotrophomonas africana sp. nov., an opportunistic human pathogen in Africa. Int. J. Syst. Bacteriol. 47, 160–163. doi: 10.1099/00207713-47-2-606
Finkmann, W., Altendorf, K., Stackebrandt, E., and Lipski, A. (2000). Characterization of N2O-producing Xanthomonas-like isolates from biofilters as Stenotrophomonas nitritireducens sp. nov., Luteimonas mephitis gen. nov., sp. nov. and Pseudoxanthomonas broegbernensis gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 50, 273–282. doi: 10.1099/00207713-50-1-273
Heylen, K., Vanparys, B., Peirsegaele, F., Lebbe, L., and De Vos, P. (2007). Stenotrophomonas terrae sp. nov. and Stenotrophomonas humi sp. nov., two nitrate-reducing bacteria isolated from soil. Int. J. Syst. Evol. Microbiol. 57, 2056–2061. doi: 10.1099/ijs.0.65044-0
Hugh, R., and Ryschenkow, E. (1961). Pseudomonas maltophilia, an Alcaligenes-like species. J. Gen. Microbiol. 26, 123–132. doi: 10.1099/00221287-26-1-123
Hunter, J. D. (2007). Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95. doi: 10.1109/MCSE.2007.55
Kaparullina, E., Doronina, N., Chistyakova, T., and Trotsenko, Y. (2009). Stenotrophomonas chelatiphaga sp. nov., a new aerobic EDTA-degrading bacterium. Syst. Appl. Microbiol. 32, 157–162. doi: 10.1016/j.syapm.2008.12.003
Kim, H.-B., Srinivasan, S., Sathiyaraj, G., Quan, L.-H., Kim, S.-H., Bui, T. P. N., et al. (2010). Stenotrophomonas ginsengisoli sp. nov., isolated from a ginseng field. Int. J. Syst. Evol. Microbiol. 60, 1522–1526. doi: 10.1099/ijs.0.014662-0
Lee, D. S., Ryu, S. H., Hwang, H. W., Kim, Y.-J., Park, M., Lee, J. R., et al. (2008). Pseudoxanthomonas sacheonensis sp. nov., isolated from BTEX-contaminated soil in Korea, transfer of Stenotrophomonas dokdonensis Yoon et al. 2006 to the genus Pseudoxanthomonas as Pseudoxanthomonas dokdonensis comb. nov. and emended description of the genus Pseudoxanthomonas. Int. J. Syst. Evol. Microbiol. 58, 2235–2240. doi: 10.1099/ijs.0.65678-0
Lee, M., Woo, S.-G., Chae, M., Shin, M.-C., Jung, H.-M., and Ten, L. N. (2011). Stenotrophomonas daejeonensis sp. nov., isolated from sewage. Int. J. Syst. Evol. Microbiol. 61, 598–604. doi: 10.1099/ijs.0.017780-0
Palleroni, N. J., and Bradbury, J. F. (1993). Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int. J. Syst. Bacteriol. 43, 606–609. doi: 10.1099/00207713-43-3-606
Ramos, P. L., Van Trappen, S., Thompson, F. L., Rocha, R. C., Barbosa, H. R., De Vos, P., et al. (2011). Screening for endophytic nitrogen-fixing bacteria in Brazilian sugar cane varieties used in organic farming and description of Stenotrophomonas pavanii sp. nov. Int. J. Syst. Evol. Microbiol. 61, 926–931. doi: 10.1099/ijs.0.019372-0
Richter, M., and Rosselló-Móra, R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U.S.A. 106, 19126–19131. doi: 10.1073/pnas.0906412106
Ryan, R. P., Monchy, S., Cardinale, M., Taghavi, S., Crossman, L., Avison, M. B., et al. (2009). The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 7, 514–525. doi: 10.1038/nrmicro2163
Svensson-Stadler, L. A., Mihaylova, S. A., and Moore, E. R. (2012). Stenotrophomonas interspecies differentiation and identification by gyrB sequence analysis. FEMS Microbiol. Lett. 327, 15–24. doi: 10.1111/j.1574-6968.2011.02452.x
Swings, J., De Vos, P., Den Mooter, M. V., and De Ley, J. (1983). Transfer of Pseudomonas maltophilia Hugh 1981 to the Genus Xanthomonas as Xanthomonas maltophilia (Hugh 1981) comb. nov. Int. J. Syst. Bacteriol. 33, 409–413. doi: 10.1099/00207713-33-2-409
Ullah, J., Walsh, T., Taylor, I., Emery, D., Verma, C., Gamblin, S., et al. (1998). The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 Å resolution. J. Mol. Biol. 284, 125–136. doi: 10.1006/jmbi.1998.2148
Van den Mooter, M., and Swings, J. (1990). Numerical analysis of 295 phenotypic features of 266 Xanthomonas strains and related strains and an improved taxonomy of the genus. Int. J. Syst. Bacteriol. 40, 348–369. doi: 10.1099/00207713-40-4-348
Walsh, T., Macgowan, A., and Bennett, P. (1997). Sequence analysis and enzyme kinetics of the L2 serine beta-lactamase from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 41, 1460–1464.
Walsh, T. R., Hall, L., Assinder, S. J., Nichols, W. W., Cartwright, S. J., Macgowan, A. P., et al. (1994). Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta 1218, 199–201. doi: 10.1016/0167-4781(94)90011-6
Wolf, A., Fritze, A., Hagemann, M., and Berg, G. (2002). Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int. J. Syst. Evol. Microbiol. 52, 1937–1944. doi: 10.1099/00207713-52-6-1937
Yang, H.-C., Im, W.-T., Kang, M. S., Shin, D.-Y., and Lee, S.-T. (2006). Stenotrophomonas koreensis sp. nov., isolated from compost in South Korea. Int. J. Syst. Evol. Microbiol. 56, 81–84. doi: 10.1099/ijs.0.63826-0
Yi, H., Srinivasan, S., and Kim, M. K. (2010). Stenotrophomonas panacihumi sp. nov., isolated from soil of a ginseng field. J. Microbiol. 48, 30–35. doi: 10.1007/s12275-010-0006-0
Yoon, J.-H., Kang, S.-J., Oh, H. W., and Oh, T.-K. (2006). Stenotrophomonas dokdonensis sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 56, 1363–1367. doi: 10.1099/ijs.0.64070-0
Zhao, Y., Jia, X., Yang, J., Ling, Y., Zhang, Z., Yu, J., et al. (2014). PanGP: a tool for quickly analyzing bacterial pan-genome profile. Bioinformatics 30, 1297–1299. doi: 10.1093/bioinformatics/btu017
Keywords: Stenotrophomonas, Type Strains, phylogenomics, Average Nucleotide Identity (ANI), evolution
Citation: Patil PP, Midha S, Kumar S and Patil PB (2016) Genome Sequence of Type Strains of Genus Stenotrophomonas. Front. Microbiol. 7:309. doi: 10.3389/fmicb.2016.00309
Received: 10 December 2015; Accepted: 25 February 2016;
Published: 10 March 2016.
Edited by:
Jae-Ho Shin, Kyungpook National University, South KoreaReviewed by:
Shannon Lyn Johnson, Los Alamos National Laboratory, USADaniel Yero, Universitat Autónoma de Barcelona, Spain
Copyright © 2016 Patil, Midha, Kumar and Patil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prabhu B. Patil, cGJwYXRpbEBpbXRlY2gucmVzLmlu
 Prashant P. Patil
Prashant P. Patil Samriti Midha
Samriti Midha Sanjeet Kumar
Sanjeet Kumar Prabhu B. Patil
Prabhu B. Patil