
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 12 January 2016
Sec. Virology
Volume 6 - 2015 | https://doi.org/10.3389/fmicb.2015.01444
This article is part of the Research Topic HIV and illicit drugs of abuse View all 28 articles
As the threat of Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS) persists to rise, effective drug treatments are required to treat the infected people. Even though combination antiretroviral therapy (cART) provides stable viral suppression, it is not devoid of undesirable side effects, especially in persons undergoing long-term treatment. The present therapy finds its limitations in the emergence of multidrug resistance and accordingly finding new drugs and novel targets is the need of the hour to treat the infected persons and further to attack HIV reservoirs in the body like brain, lymph nodes to achieve the ultimate goal of complete eradication of HIV and AIDS. Natural products such as plant-originated compounds and plant extracts have enormous potential to become drug leads with anti-HIV and neuroprotective activity. Accordingly, many research groups are exploring the biodiversity of the plant kingdom to find new and better anti-HIV drugs with novel mechanisms of action and for HIV-associated neurocognitive disorders (HAND). The basic challenge that still persists is to develop viral replication-targeted therapy using novel anti-HIV compounds with new mode of action, accepted toxicity and less resistance profile. Against this backdrop, the World Health Organization (WHO) suggested the need to evaluate ethno-medicines for the management of HIV/AIDS. Consequently, there is need to evaluate traditional medicine, particularly medicinal plants and other natural products that may yield effective and affordable therapeutic agents. Although there are a good number of reports on traditional uses of plants to treat various diseases, knowledge of herbal remedies used to manage HIV/AIDS and HAND are scanty, vague and not well documented. In this review, plant substances showing a promising action that is anti-HIV and HAND will be explored along with what they interact. Since some plant substances are also known to modulate several cellular factors which are also involved in the replication of HIV and hence their role as potential candidates will be discussed. HIV/AIDS being an exceptional epidemic, demands an exceptional approach and that forms very much focus for the current review.
Since its first discovery in 1981, Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS) has killed more than 25 million people worldwide and is today the major threat to human health. However, due to the introduction of highly active antiretroviral therapy (HAART) as well as the impact of preventive measures, the prevalence and incidence of HIV have declined globally over the last decade except for parts of Eastern Europe and Central Asia where a slight increase has been observed (UNAIDS, 2010; Cohen, 2012; Futterman, 2012; Kumari and Singh, 2012; Saxena et al., 2012). Even though HAART provides stable viral suppression, it is not devoid of undesirable side effects, especially in persons undergoing long-term treatment. The present therapy finds its limitations in the emergence of multidrug resistance and accordingly finding new drugs and novel targets is the need of the hour to treat the infected persons and further to attack HIV reservoirs in the body like brain, lymph nodes to achieve the ultimate goal of complete eradication of HIV and AIDS (Vermani and Garg, 2002; Chinsembu and Hedimbi, 2010). Nature has always been considered a basis for providing resources that can be used to fight off infections and treat diseases. Among these resources there have been findings of medicinal plants that have anti-HIV properties with low levels of toxicity. For the management of HIV/AIDS, in 1989 the World Health Organization (WHO) had declared the necessity to assess ethnomedicines and other natural products. To quote: “In this context, there is need to evaluate those elements of traditional medicine, particularly medicinal plants and other natural products that might yield effective and affordable therapeutic agents. This will require a systematic approach” (World Health Organization [WHO], 1989a,b). Moreover, because they are fairly not as expensive and have less side effects, phytomedicines are retrieving patient approval (Vermani and Garg, 2002; Short, 2006). Medicinal effects of plants incline to stabilize physiological function and fix the fundamental cause of the condition (Murray and Pizzorno, 1999). Recently, there has been a sustained bio prospective effort to isolate the active leads from plants and other natural products for treatment of HIV and management of AIDS (Asres et al., 2001; Vermani and Garg, 2002) and also screening of plants based on ethnopharmacological data enhances the ability of discovering novel anti-HIV compounds (Fabricant and Farnsworth, 2001; Farnsworth, 2007). Indigenous knowledge of medicinal plants further provides leads toward therapeutic concept, thereby accelerating drug discovery. Thus, it is essential to explore for new antiretroviral agents which can be combined with or substitute the present resource of drugs against HIV (Klos et al., 2009).
Also, due to the widespread infection of HIV there has been a determination for the discovery and use of medicinal agents that can inhibit the virus. HIV type-1 (HIV-1) displays extraordinary genetic variation and can be phylogenetically classified into three distinct groups and several subgroups (A–K) across the globe. While HIV-1 clade C dominates the HIV-1 epidemic worldwide with more than 50% of the total viral infections, predominantly in Southern Africa, India, and other parts of Asia, HIV-1 subtype B is mainly found in (Western) Europe and in the Americas, accounting for about 10% of the total infections (Rambaut et al., 2001; Hemelaar et al., 2011; UNAIDS, 2011; Hemelaar, 2012; Reddy et al., 2012). In places like Brazil, clade C viruses have been spreading rapidly to replace the previously dominant clade B epidemic (Buonaguro et al., 2007; Gandhi et al., 2009; de Oliveira et al., 2010). The virus basically utilizes the host’s cellular machinery for its own replication leading to extensive viral and host interactions during its life cycle (Kitchen et al., 2001; Lawn et al., 2001; Valenti, 2001; An et al., 2004; Resh, 2005; Broder, 2009; Gandhi et al., 2010). Some of the cellular factors aid replication while others are inhibitory (Balzarini, 1994; Mohri et al., 2001; Benito et al., 2004; Petrovas et al., 2006; Appay et al., 2007; Samikkannu et al., 2010). All these mechanisms lead to chronic immune activation, the key factor in HIV-1 immunopathogenesis. The disease progression (Clerici et al., 1996; Rodriguez et al., 2006; Schader and Wainberg, 2011; Wayengera, 2011) is associated with the gradual T lymphocyte decline, first taking toll of helper CD4+ T cells and then the cytotoxic CD8+ T cells. The pronounced decline of CD4+ T cells occurs due to the destruction of both infected and uninfected cells mainly because of apoptosis of activated cells, diminished hematopoiesis and thymic maturation. Apart from inducing immune dysfunction, HIV-1 infection affects neuronal function by significantly increasing several cellular proteins in the brain, including amyloid precursor protein (APP), especially in the axons present in the subcortical white matter tracts (Esiri et al., 1998; Green et al., 2005; Soontornniyomkij et al., 2012). During HAART therapy HIV persists in the brain and local inflammatory responses to the virus can lead to higher APP production and β-amyloid deposition which may lead to the progression of Alzheimer’s disease and HAND (Giunta et al., 2011). Accordingly, finding new drugs and novel targets is the need of the hour to treat the infected persons and further to attack HIV reservoirs in the brain to achieve the ultimate goal of complete eradication of HIV and AIDS.
Extensive attention has been given to the pathogenesis of HIV-1 infection and associated immunologic mechanisms, however, the basic challenge that still persists is to develop viral replication-targeted therapy using novel anti-HIV compounds with new mode of action, accepted toxicity, and less resistance profile. Accordingly, in this review, plant substances showing a promising anti-HIV activity and HAND will be summarized along with the targets they interact including the specific objectives for the pursuit of plants and other natural products with identified active substances and mechanisms as well as those with unidentified active mechanisms against HIV, AIDS, and other neurocognitive disorders.
Individuals infected with HIV have conditions of fluctuating degrees of impairment of cognition and associated functioning and can develop HIV-associated neurocognitive disorders (HAND; Antinori et al., 2007). Invasion and replication of HIV in the brain parenchyma is accomplished by brain perivascular macrophages, endogenous microglia, and some astrocytes that are infected and can initiate the neuropathogenesis of HAND (Kaul, 2009; McArthur et al., 2010). Immune activation of resident glia and neuroinflammation are associated with this infection and neuronal injury. The general occurrence of HAND and related diseases continue to be at high levels even with the extensive use of antiretroviral therapy (ART) that has radically reduced the occurrence of the severest form of HAND, HIV-associated dementia (HAD; Sacktor et al., 2002; McArthur et al., 2004; Nath et al., 2008; Heaton et al., 2010). The persistence of this high risk for HAND in individuals experiencing effective control of systemic HIV viral load is incompletely explained, and suggested factors include effects of aging on brain vulnerability, persistence of HIV replication in brain macrophages, evolution of highly neurovirulent CNS HIV strains, and even long-term CNS toxicity of ART (Heaton et al., 2010, 2011). The features linked with increasing the inflammatory setting within the CNS and HIV replication are what drive the pathogenesis of HAND. Neuronal damage associated with HAND can be augmented by subordinate effects of aging, movement of activated monocytes, systemic immune activation and drug abuse and can continue in spite of the effective systemic control of HIV replication by ART (Gannon et al., 2013). Active complementary neuroprotectants are substances that can overturn systemic immune activation and associated inflammation both systemically and within the CNS (Kaul and Lipton, 2006; Kraft-Terry et al., 2009; Grovit-Ferbas and Harris-White, 2010; McArthur et al., 2010). Drugs that aim toward these cellular pathways could quickly ease testing and application of possible complementary neuroprotective approaches against HAND. Prevention and treatment of HAND requires strategies aimed at suppressing CNS HIV replication and effects of systemic and CNS inflammation in aging and substance-abusing HIV populations. Use of improved CNS-penetrating ART must be accompanied by evaluation of potential ART neurotoxicity.
After initial infection and seroconversion, HIV usually reaches the brain. Infected monocytes migrating across the blood–brain barrier (BBB), which can be seen as a “Trojan horse” mechanism, is one concept suggested for potential viral entrance. After these infected monocytes migrate across the endothelium, they settle as infected perivascular macrophages. It has been proposed that the virus is spread by cell-to-cell interaction between microglia cells and macrophages (Kaul et al., 2001). The main cell types that upkeep viral replication in the brain are multinucleated giant cells (fused microglia cells and macrophages) and macrophages in the perivascular spaces (Gonzalez-Scarano and Martin-Garcia, 2005). A multifaceted cascade is prompted within the brain with recurrent exposures and heightened viral replication within microglia. Inside the brain tissue is where viral proteins are released which stimulate astrocytes and microglia to produce cytokines, chemokines, and other inflammatory agents (Nottet and Gendelman, 1995; Achim and Wiley, 1996). Release of viral proteins is further stimulated by these cell-encoded indicators that support the increase of HIV replication in microglia cells. This can produce a toxic effect on neurons, causing neuronal dysfunction and neuronal loss. Nevertheless, when a collection of these factors are existing along with the HIV infection, a distinctive neuropathological signature is seen. Pathological alterations are usually minor with irregularities mostly taking place in basal ganglia, brain stem, central white matter, frontal cortices, thalamus, and eventually leading to HAND (Sharer, 1992). There are studies exploring the associated risk factors for the neurocognitive disorders in HIV patients and found that in some but not in all studies, CD4 nadir count (Munoz-Moreno et al., 2008; Ellis et al., 2011; Heaton et al., 2011) and detectable HIV plasma viral load (Tozzi et al., 1999; Chang et al., 2002; Devlin et al., 2012), have been associated with neurocognitive impairment (Gonzalez et al., 2003; Paul et al., 2005; Vitiello et al., 2007; Ettenhofer et al., 2009; Simioni et al., 2010). Shortening in telomere length (TL), which has been used as a biomarker of aging, has also been reported in individuals with an HIV+ status (Zanet et al., 2012; Malan-Muller et al., 2013; Pathai et al., 2013). Also, shortened TL reported to increase the neurocognitive impairment and risk of dementia in non-HIV populations (von Zglinicki et al., 2000; Panossian et al., 2003; Honig et al., 2006) which can be a marker for atypical age-related cognitive decline (Martin-Ruiz et al., 2006; Valdes et al., 2008; Yaffe et al., 2009).
The following natural products namely Calanolides (Coumarins), Betulinic acid (a Triterpene), Baicalin (a Flavonoid), Polycitone A (an Alkaloid), Lithospermic acid (a Polyphenolic) can be mentioned as promising for anti-HIV agents (Figure 1) whereas Withanolides (Steroidal lactones; Figure 2) for HIV-associated neurocognitive disorders.
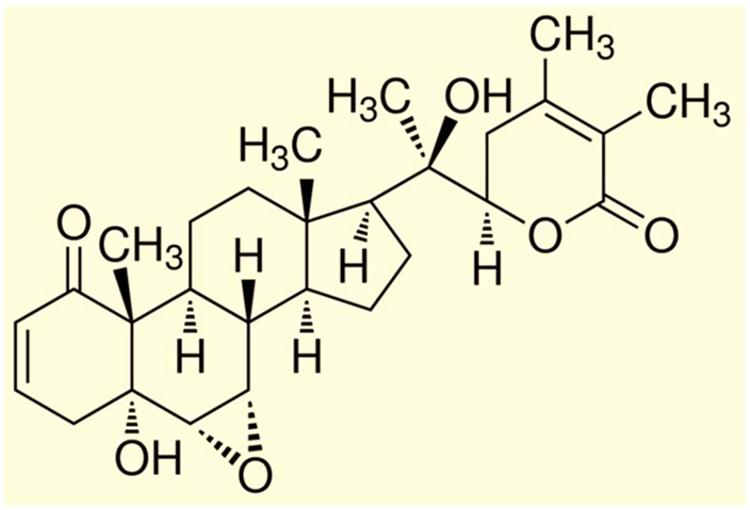
FIGURE 2. Chemical structure of Withanolide A. [Reproduced with permission from Kurapati et al. (2014).]
An example of non-nucleoside specific reverse transcriptase inhibitors (NNRTI) of the virus are calanolides which are a type of coumarin derived from different tropical tree species of the Calophyllum (Clusiaceae family; Dharmaratne et al., 2002). Calanolide A (Figure 1), (Vlietinck et al., 1998), Calanolide B and its derivative 7,8-dihydrocalanolide B can prevent cytopathogenic effects of HIV-1 on host cells and are attained from the Calophyllum lanigerum. Cordatolide A and B are similar in structure to Calanolides and can inhibit replication of HIV-1. These compounds are extracted from the Calophyllum cordato-oblongum (Lee et al., 1994). Suksdorfin (Yu et al., 2003) is another compound that also has inhibitory effects on replication of the virus in the T cell line and is a pyrocoumarin derivative that can be extracted from the Angelica morii and Lomatium suksdorfii fruits from the Apiaceae family (Zhou et al., 2000).
Antiretroviral activity with diverse mechanisms of action have been observed with some triterpenoids. Betulinic acid (Figure 1), platanic and oleanolic acid tested in H9 lymphocyte cells have shown inhibition of HIV and can be obtained from the leaves of Syzigium claviflorum (Min et al., 1999). Inhibition of HIV-1 replication in these cells were observed with oleanolic acid which can be attained from methanolic extract of the Xanthoceras sorbifolia, wood from the Sapindaceae family (Kashiwada et al., 1998). Potent inhibitory activity against HIV-1 protease has been seen with maslinic acid derived from the Geum japonicum (Xu et al., 1996). Anti-HIV replication activity in H9 cells is observed with Celasdin B that is derived from ethanolic extract of the Celastrus hindsii which is from the Celastraceae family (Yao-Haur and Li-Ming Yang, 1997). The protostanes, garcisaterpenes A and C can suppress HIV-1 RTase activity and are obtained from the ethyl acetate extract of the stems and bark of Garcinia speciosa (Rukachaisirikul et al., 2003). Lanostane-type triterpene has also shown inhibition of HIV replication in H9 cells and is a suberosol obtained from ethanolic extract of the leaves and stems of Polyalthia suberosa from the Annonaceae family (Li et al., 1993). Triterpene lactone, lancilactone C extracted from the roots and stems of Kadsura lancilimba is another compound that restrains HIV replication in these cells (Chen et al., 1999). The 12-O-tetradecanoylphorbol-13-acetate (TPA), a phorbol diester, can inhibit cytopathogenic effects of HIV-1 and is attained from methanolic extract of Croton tiglium from the Euphorbiaceae family. Prostratin, a phorbol ester also has anti-HIV properties and is extracted from Homalanthus nutans from the Euphorbiaceaen family (El-Mekkawy et al., 2000).
Favorable anti-HIV activity have been exhibited by flavonoids and associated polyphenols. They are known for having antioxidant properties and have been found to show antiviral activity in different cell cultures (Orhan et al., 2010). Baicalin (Figure 1) inhibits HIV replication in PBMC in a dose dependent fashion and is an anti-HIV flavonoid extracted from Scutellaria baicalensis (Ohtake et al., 2004). The 6,8-diprenylaromadendrin and 6,8-diprenylkaempferol, prenylated flavonoids, also show anti-HIV activity in the XTT-based, whole-cell screen and are derived from the extract of Monotes africanus (Meragelman et al., 2001). Flavonoid gallate ester and quercetin 3-O-(2-galloyl) a-L-arbinopyranose can inhibit integrase activity of HIV-1 and are obtained from ethanolic extract of Acer okamotoanum from the Aceraceae family (Kim et al., 1998). Hinokiflavone, robustaflavone, and biflavonoids have demonstrated inhibition of the polymerase of HIV-1 reverse transcriptase (RT) and are attained from methanolic extracts of leaves and twigs of Rhus succedanea from the Anacardiaceae family (Lin et al., 1997). Wikstrol B, a biflavonoid, also shows anti-HIV activity and can be isolated from extracts of roots of Wikstroemia indica from the Thymelaeaceae family (Hu et al., 2000). Xanthohumol, a prenylchalcone that has demonstrated inhibition of HIV-1 and is extracted from hops Humulus lupulus (Wang et al., 2004).
Different types of alkaloids have shown anti-HIV activity. One of the natural products with interesting activity on RT is polycitone A (Figure 1), an aromatic alkaloid isolated from the marine ascidian Polycitor sp. Polycitone A exhibits potent inhibitory activity on both RNA- and DNA-directed DNA polymerases (Loya et al., 1999). Papaverine, an alkaloid can inhibit HIV replication and is extracted from Papaver sominiferum from the Papaveraceae family. Buchapine is a type of quinolone that has shown inhibition of cytopathogenic effects of HIV-1 and is isolated from Eodia roxburghiana (McCormick et al., 1996). Nitidine also shows anti-HIV activity and is extracted from the roots of Toddalia asiatica of the Rutaceae family (Tan et al., 1991). A piperidine flavone related alkaloid O-demethylbuchenavianine shows a hindrance to the activity of HIV and is attained from Buchenavia capitata of the Combretaceae family (Beutler et al., 1992). Harmine has shown inhibition of HIV replication in H9 cells and is derived from Symplocos setchuensis (Ishida et al., 2001). 1-Methoxy canthionone has anti-HIV properties and is obtained from Leitneria floridana (Xu et al., 2000). Hypoglaumine B, Troponine B, and Troponine A are sesquiterpene pyridine alkaloids that have shown to also possess anti-HIV properties and are isolated from Tripterygium wilfordii and Tripterygium hypoglaucum (Duan et al., 2000).
Because of heightened phytohaemagglutinin-induced lymphocytes proliferation, prolonged administration of polyphenol-rich fruit juices is believed to be promising to HIV-positive individuals. There are several tannins and related phenolic substances which show virucidal effects in several viral systems. Lithospermic acid (Figure 1) isolated from Salvia miltiorrhiza has strong anti-HIV activity in H9 cells (Abd-Elazem et al., 2002). Punicalagin, chebulagic acid, and punicalin are hydrolysable tannins that demonstrate anti-HIV activity and come from Terminalia chebula (Lim et al., 1997). Repandusinic acid has shown inhibition of HIV-1 RTase and is extracted from Phyllanthus niruri of the Euphorbiaceae family (Ogata et al., 1992). Monopotassium and monosodium salts of isomeric caffeic acid tetramer have shown inhibition of HIV replication and are obtained from the aqueous acetone extract of Arnebia euchroma of the Boraginaceae family (Kashiwada et al., 1995). Camellia-tannin H shows inhibition of the HIV-1 protease and is extracted from the pericarp of Camellia japonica (Fuller et al., 1999). Galloyl glucoses and gallic acid exhibited a hindrance of HIV integrase and are extracted from Terminalia chebula of the Combretaceae family (Ahn et al., 2002; Park et al., 2002). Mallotojaponin is a dimeric phloroglucinol derivative that inhibits activity of HIV-1 RTase and is extracted from the pericarps of Mallotus japonicus. The curcuminoids have shown inhibition of HIV-1 and HIV-2 protease and are extracted from rhizomes of Curcuma longa (Roth et al., 1998). Peltatol A is a prenylated catechol dimer that shows anti-HIV activity and is extracted from Pothomorphe peltata of the Piperaceae family (Gustafson et al., 1992).
Several lignans have antiviral properties (Charlton, 1998). Phyllamyricin B and its lactone retrojusticidin B display inhibition of HIV-RTase activity and are obtained from chloroform extract of Phyllanthus myrtifolius/P. urinaria of the Euphorbiaceae famly (Liu and Li, 1995). Anolignan B, anolignan A, and dibenzylbutadiene lignans have shown inhibition of HIV-1 RTase and are extracted from Anogeissus acuminata (Rimando et al., 1994). Gomisin has been found to be one of the strongest inhibitors of HIV replication and is obtained from Kadsura interior (Chen et al., 1997).
Plumbagin, 1,4-naphthoquinone, juglone, and vitamin K3 are naphthoquinones that all demonstrate HIV inhibitory activity (Min et al., 2002). Conocurvone is a trimeric naphthoquinone that shows effective anti-HIV activity and is extracted from Conospermum incurvum of the Proteaceae family (Decosterd et al., 1993).
Actein is a tetracyclic triterpenoid saponin that exhibits strong anti-HIV activity and derives from the rhizome of Cimicifuga racemosa (black cohosh), (Sakurai et al., 2004).
Swertifrancheside is a flavonone–xanthone glucoside that has shown inhibition of HIV-1 RTase and is extracted from Swertia franchetiana (Wang et al., 1994). Macluraxanthone B is a prenylated xanthone that also shows anti-HIV activity and is extracted from Maclura tinctoria of the Moraceae family (Groweiss et al., 2000).
Table 1 list the ongoing or completed clinical trials of anti-HIV effect of natural substances.
It is well known that use of alcohol and drugs of abuse are associated with increased risk of attaining HIV-1 infection as well as augmented disease progression (Nair et al., 2004, 2009; Dhillon et al., 2007; Atluri et al., 2014). Further, studies have shown that use of alcohol and drugs of abuse are also risk factors for dementia and other cognitive disorders (Brandt et al., 1983; Darke et al., 2000; Atluri et al., 2015; Hidalgo et al., 2015; Roy et al., 2015; Sagar et al., 2015; Samikkannu et al., 2015a,b). Along this line, impacting more than 15 million people worldwide, Alzheimer’s disease (AD) is the most frequent form of senile dementia (Kim et al., 2011). This number will surely increase quickly in the future with augmented life expectancy. The pathological hallmarks of AD are complex and include neuronal degeneration, neuro-inflammation, toxic β-amyloid (AB) plaques, abnormal neurofibrillary tangles, and a decline of neurochemicals which are essential for neuronal transmission (McArthur et al., 2003, 2010; Robertson et al., 2007; Heaton et al., 2010, 2011). The precise mechanisms of β-amyloid that lead to neurotoxicity continue to be a question but its cytotoxicity to neuronal cells has been acknowledged as one of the main features in AD pathology (Sacktor et al., 2001). In AB-induced cytotoxicity it seems that some of the possible mechanisms involved are oxidative stress and free radical formation (Xu and Ikezu, 2009). In HIV infection, neuronal degeneration is also a main feature. In AIDS patients, a noteworthy increase in brain APP has been observed especially in the axons found in the subcortical white matter tracts (Esiri et al., 1998; Green et al., 2005; Anthony et al., 2006; Lindl et al., 2010). HIV can persist in the brain during HAART therapy and local inflammatory responses can cause an increase of APP production and exposure to amyloid deposition (Anthony and Bell, 2008). During HIV induced neuronal degeneration and development of Alzheimer’s disease, β- amyloid buildup may be used as an indicator of early neuronal degeneration.
A medicinal plant that has many therapeutic properties such as antioxidant and immunomodulatory properties, memory enhancer, nerve tonic and antistress is Withania somnifera (L.) Dunal, also known as ‘ashwagandha’ (ASH) or ‘Indian ginseng’ and has had a significant upsurge in pharmacological studies in the recent years (Mishra et al., 2000; Kulkarni and Dhir, 2008). In cultured neurons and in rodents injected with Aβ 25–35, neurite outgrowth has been induced by withanoside IV and withanolide A extracted from the roots (Kuboyama et al., 2005). Root extracts from this species have also been shown to significantly reduce the number of hippocampal degenerating cells in the brains of stressed rodents (Jain et al., 2001) and were neuro-protective in animal models of Parkinson’s disease (Sankar et al., 2007). Accumulation of oligomers and β-amyloid peptides (Aβ), behavioral deficits and plaque pathology have been upturned in the brains of old and middle-aged APP/PS1 Alzheimer’s disease transgenic mice with the oral dispensation of a semi-purified extract of the root of W. somnifera mainly involving withanosides and withanolides (Sehgal et al., 2012). However, there is a scarcity of data on the mechanisms related to the possible defensive effects of W. somnifera root against HIV-1Ba-L (clade B) infection and β-amyloid (1–42)-induced cytotoxicity. In a confocal microscopic analysis, β-amyloid treated SK-N-MC cells (neuronal cell line) showed a reduction in spine area, dendrite diameter, spine density, total dendrite, and a loss of spines when compared to untreated cells (Kurapati et al., 2013). Nonetheless, the toxic effects were counteracted when ASH root extract was added to β-amyloid treated cells (Kurapati et al., 2013). Further, as demonstrated by increased trypan blue stained cells, β-amyloid prompted cytotoxic effects in SK-N-MC cells. Nevertheless, the toxic effects were neutralized when ASH root extract was added to β-amyloid treated cells (Figure 3), (Kurapati et al., 2014). This observation was supported by the levels of acetylcholinesterase activity, cellular localization of β-amyloid, and MTT formazan exocytosis, confirming the defensive effects of ASH root extract against β-amyloid prompted toxicity (Kurapati et al., 2013, 2014). Also, Withanolide A (WA; Figure 2) demonstrated the same pattern using MTT assay as a parameter and is a refined component of ASH (Figure 4) (Kurapati et al., 2014). These observations suggests that neuroprotective properties of ASH root extract may offer some clarification for the ethnopharmacological usages of ASH in traditional medicine for cognitive and other HIV associated neurodegenerative disorders and additionally ASH could be a possible new drug to reduce the brain amyloid load and/or improve the HIV-1 associated neurocognitive damages.
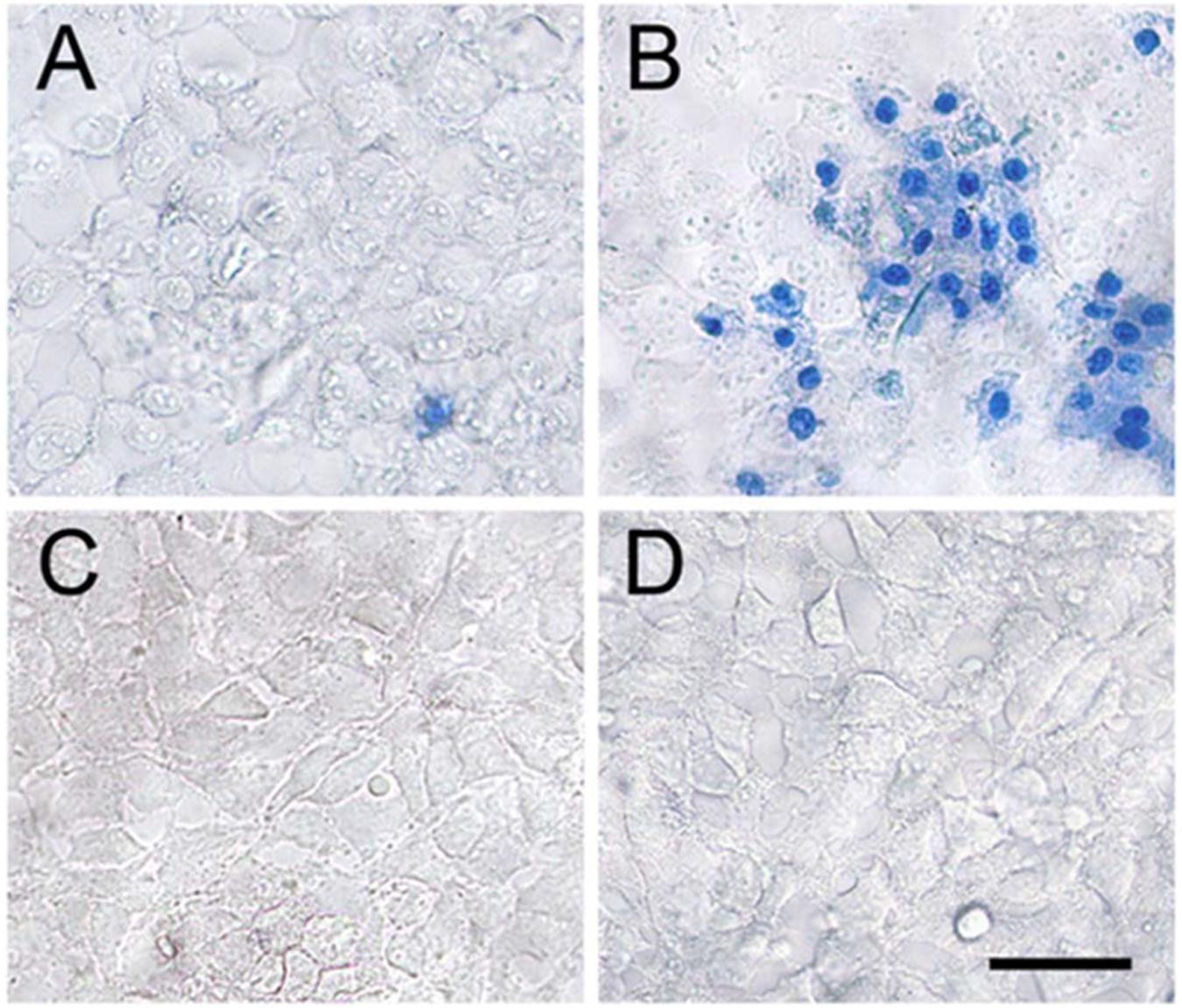
FIGURE 3. Effect of ASH in reversal of β-Amyloid induced cell death. Treating the SK-N-MC cells with β-Amyloid (B) resulted in significant cell death when compared to the untreated control cells (A). Treating the cells with ASH (C) and ASH plus β-Amyloid (D) exhibited good viability as evidenced by exclusion of trypan blue dye. [Reproduced with permission from (Kurapati et al., 2014).]
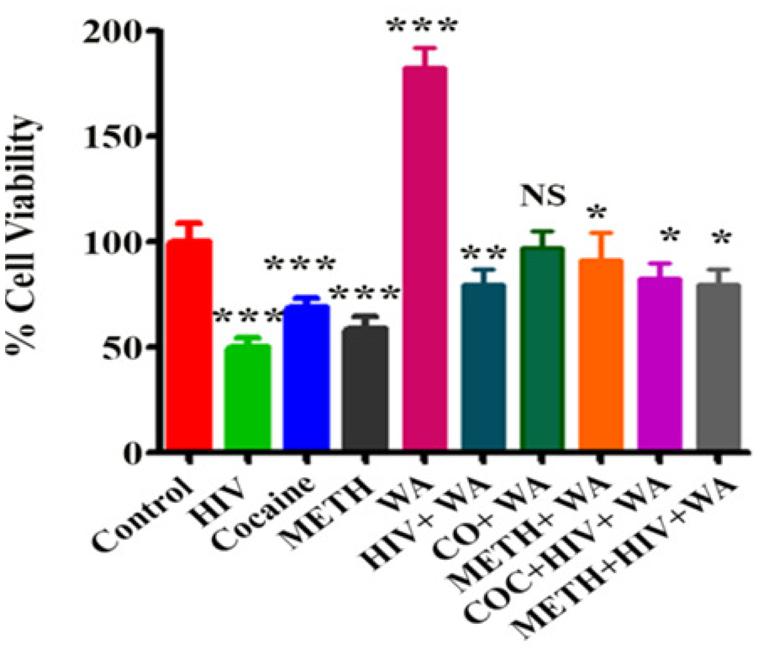
FIGURE 4. Effect of Withanolide A on viability of SK-N-MC cells treated with HIV-1Ba-L (clade B), Cocaine and Methamphetamine (METH): SK-N-MC cells were infected with HIV in the presence of Cocaine/METH. The MTT assay is showing that Withanolide A reverse the neurotoxic effects on cell viability induced by HIV, Cocaine, METH and combined treatment. Withanolide A alone showed significant increase in cell viability. The values were expressed as percentage of cell viability compared to control cells and are the mean ± SD of four experiments. ∗∗∗, ∗∗, ∗ indicates a statistically significant difference (p,0.0001), (p,0.0006), and (p,0.0185), respectively, compared to controls. WA, Withanolide A; CO, COC, Cocaine; METH, Methamphetamine. [Reproduced with permission from Kurapati et al. (2014).]
Apart from our studies on ASH, reports are scanty on the effect of natural compounds for the treatment of HIV associated neurocognitive disorders. Ginkgo biloba has been known as a traditional medicine for people with dementia. Zou et al. (2007) reported that administration of EGb 761, a standardized formulation of Ginkgo biloba extract, reported to protect Tat transgenic mice from Tat-induced neuro toxic effects like developmental retardation, inflammation, death, astrocytosis, and neuron loss by down-regulating the glial fibrillary acidic protein (GFAP) expression. In GFAP null Tat-transgenic mice model, reduced Tat-induced neuropathological phenotypes including macrophage/microglia activation, central nervous system infiltration of T lymphocytes, and oxidative has been reported.
Hypericum perforatum (St John’s Wort) extracts are generally used for the treatment of mild to moderately severe depression. Role of P27SJ, a protein present in a laboratory callus culture of Hypericum perforatum was explored to control HIV transcription and replication in microglia and astrocytes (Darbinian-Sarkissian et al., 2006). This study reported that P27SJ suppress transcription of the HIV-1 Genome/viral replication (50%) by interacting, inhibiting the DNA-binding activity and altering subcellular localization of CCAAT/enhancer-binding protein beta (C/EBPb), a transcription factor that regulates expression of the HIV-1 genome. Also, P27SJ reported to interact with HIV-1 Tat and inhibits its activity on transcription of HIV-1 LTR and relocalizing it to the cytoplasm. Therefore P27SJ is a potential therapeutic agent for inhibiting the HIV-1 induced neurotoxicity in the brain by suppressing its replication and inhibiting inflammatory response induced by proinflammatory Tat-inducible chemokines such as MCP-1. Few studies reported the potential adverse St. John’s Wort extracts drug reactions and interactions especially with other antidepressants, with coumarin-type anticoagulants, the immunosuppressants cyclosporine and tacrolimus, protease and RT inhibitors used in anti-HIV treatment (Schulz, 2006).
For inhibiting the HIV replication in the brain or nullifying the neurotoxicity induced by HIV replication and its proteins, further studies are necessary to explore the neuroprotective effect of natural compounds like Sage (Salvia officinalis, Salvia lavandulaefolia, Salvia lavandulifolia), Bacopa (Bacopa monnieri), Gotu kola (Centella asiatica), which are being used as a therapeutic alternative medicines for memory and cognitive improvement.
In the study of plant extracted anti-HIV compounds there have been significant improvements that are centered on their activity in vitro and the mechanisms that are used. Some of these compounds will be discussed in this review along with the viral targets they interact with. Covering all the natural substances that possess these anti-HIV properties is still a work in progress. Most of these compounds interfere with early steps in the HIV replication, such as the virus entry steps and on the viral enzymes RT and integrase.
Many protein interactions are involved in HIV entry and have the potential to become targets for the development of new entry blockers. The HIV fusion inhibitor, Enfuvirtide (Jiang et al., 2002) does not have oral bioavailability but requires two injections per day and can cause significant local side effects. Compounds that are recognized as strong inhibitors of HIV-1 and HIV-2 replication in vitro are sulfated polysaccharides (Witvrouw and De Clercq, 1997; Lüscher-Mattli, 2000). These compounds exert their anti-HIV properties by shielding off the positively charged amino acids in the V3 loop of the glycoprotein gp120 from the viral envelope of HIV (Witvrouw and De Clercq, 1997). The V3 loop is necessary for virus attachment to cell surface heparan sulfate, a primary binding site, before more specific binding occurs to the CD4 receptor of CD4+ cells. Ursolic acid, oleanolic acid, betulinic acid and their derivatives have been the focus of recent research on triterpenoids. Inhibition of HIV-1 protease has been observed in vitro with these triterpenes. One of the many betulinic acid derivatives that were synthesized in a structure-activity relationship study, RPR103611, was chosen for additional mechanistic research. Numerous important steps of the HIV life cycle have been inhibited by flavonoids. Viral protease has been inhibited by taxifolin which is a flavanone with an OH group at position C-3′. Aromadendrin does not inhibit viral protease or RT but can inhibit interactions between CD4 and gp120, is more definite in its antiviral activity and is a flavanone that lacks an OH group at position C-3′ (Mahmood et al., 1997). The (-)-epigallocatechin 3-O-gallate has demonstrated activity against HIV-1 (De Clercq, 1995) such as a damaging effect of virus particles and post-adsorption entry and inhibition of RT and viral protease (Yamaguchi et al., 2002). Flavonoids are recognized as inhibitors of several enzymes including RT which is crucial for HIV replication (Kitamura et al., 1998) viral protease (Xu et al., 2000) and integrase (Kim et al., 1998).
The viral reproductive cycle begins when the enzymes within the nucleoprotein complex are activated after the virus enters the cell. The viral RT transcribes the RNA genome of HIV into a double-stranded DNA form after the nucleoprotein core of the virus is disrupted. RNA reverse transcription to DNA, degradation of RNA template by RN-ase H and duplication of the remaining DNA strand are the three consecutive functions that are controlled by the HIV-1- RT. The 4-propyldipyranocoumarins or calanolides are natural products that have been considered to be potential anti-HIV agents. (+)-Calanolide A (Chan and Kim, 1998) is significantly more active on inhibition of HIV-1 RT when compared to cordatolides A (Jiang et al., 2002) and B (Lüscher-Mattli, 2000). Currently, to evaluate its pharmacokinetics and safety, (+)-calanolide A is being tested in clinical trials for both HIV-infected and healthy volunteers (Creagh et al., 2001).
Besides protease and RT, integrase is the only enzyme that is encoded by HIV-1. Strand transfer and 3′-processing are the two steps for integration of viral DNA into host DNA and are catalyzed by integrase. This enzyme first cleaves the last two nucleotides from each 3′-end of the linear viral DNA. Then the nucleophilic attack of these 3′-ends on host chromosomal DNA are involved in the subsequent DNA strand transfer reaction. The l-chicoric acid was the most active inhibitor of HIV integrase out of all the bis-catechols tested. Recently, due to its compelling anti-HIV potential, MAP30 received a lot of attention and is a plant protein of 30 kDa extracted from Momordica charantia L., (Lee-Huang et al., 1995; Sun et al., 2001). Moreover, this plant protein toxicity is definite to HIV-infected cells, does not exhibit opposing effects on normal cells and is an inhibitor of HIV-1 integrase by rendering the HIV LTR an unsuitable substrate for HIV integrase as well as DNA gyrase.
Until levels of its trans-activator protein Tat rise, HIV-1 DNA transcription is low after integration of viral DNA into the host genome. By binding to the transactivation response element (TAR) at the 5′-end of the viral mRNA, Tat, an 86-amino acid polypeptide, can increase the capacity of host RNA polymerase (Ptak, 2002). The transcriptional activity of HIV-1 long terminal repeat (LTR) can be stimulated by the Tat protein after being released from HIV-1-infected cells and has entered new cells in an active form (Mann and Frankel, 1991). Between positions 133 and 104 of the HIV promoter, tannic acid has been observed to have found a putative tannic acid-responsive element and suppress 12-O-tetradecanoylphorbol 13-acetate (TPA) – inducing HIV promoter activity (Uchiumi et al., 1996). Furthermore, a number of natural substances related to tannins were assessed for their HIV promoter repression effects (Uchiumi et al., 2003). Chalcones, 3-phenylcoumarins and isoflavones have all successfully repressed TPA-induced HIV promoter activity in a structure-activity relationship study.
Although, antiretroviral drugs can bring about the repression of the serum load of the virus to undetectable levels, economical, commercial, and political barriers have limited their accessibility to a good part of the population suffering from the diseases. Natural products, particularly those in traditional medicine have supplied a basis of new drug candidates for many diseases including HIV and other neurocognitive disorders. The number of compounds exhibiting anti-HIV activity isolated from plants is increasing gradually and due to their explicit activity, there are several natural products have been used as primary compounds. Since the resistance of HIV-1 to the antiretroviral drugs has been increasing along with the necessity of agents that are not as costly and toxic as the ones being used now, new treatments with these natural products are needed. The basic challenge that still persists is to develop viral replication-targeted therapy using novel anti-HIV compounds with new mode of action, accepted toxicity, and less resistance profile. Against this backdrop, the World Health Organization (WHO) suggested the need to evaluate ethno-medicines for the management of HIV/AIDS. Accordingly, there is need to evaluate traditional medicine, particularly medicinal plants and other natural products that may yield effective and affordable therapeutic agents (World Health Organization [WHO], 2002; Jaco et al., 2004; Hedimbi and Chinsembu, 2012). In this context, the above mentioned results demonstrate that several plants, the majority of which are usually utilized for the treatment of various illnesses, are active against HIV. Since they are the indicators for the decision making at various levels and the finding of new lead substances, these reports are invaluable. The preparation, cytotoxicity, or selectivity profile of an antiviral substance should also be carefully assessed before any further attention is given to it. However, in repressing HIV progression and replication it should be stressed that several natural products primarily extracted from plants have demonstrated to be effective. HIV/AIDS being an exceptional epidemic, demands an exceptional approach and that forms very much our current and future research.
This study was supported by National Institutes Health grants R01MH085259, R037DA025576, R01DA021537, and R01DA027049 to MN.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abd-Elazem, I. S., Chen, H. S., Bates, R. B., and Huang, R. C. (2002). Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antiviral Res. 55, 91–106. doi: 10.1016/S0166-3542(02)00011-6
Achim, C. L., and Wiley, C. A. (1996). Inflammation in AIDS and the role of the macrophage in brain pathology. Curr. Opin. Neurol. 9, 221–225. doi: 10.1097/00019052-199606000-00013
Ahn, M.-J., Kim, C. Y., Lee, J. S., Kim, T. G., Kim, S. H., Lee, C.-K., et al. (2002). Inhibition of HIV-1 integrase by galloyl glucoses from terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 68, 457–459. doi: 10.1055/s-2002-32070
An, P., Bleiber, G., Duggal, P., Nelson, G., May, M., Mangeat, B., et al. (2004). APOBEC3G genetic variants and their influence on the progression to AIDS. J. Virol. 78, 11070–11076. doi: 10.1128/jvi.78.20.11070-11076.2004
Anthony, I., Ramage, S., Carnie, F., Simmonds, P., and Bell, J. (2006). Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 111, 529–538. doi: 10.1007/s00401-006-0037-0
Anthony, I. C., and Bell, J. E. (2008). The neuropathology of HIV/AIDS. Int. Rev. Psychiatry 20, 15–24. doi: 10.1080/09540260701862037
Antinori, A., Arendt, G., Becker, J. T., Brew, B. J., Byrd, D. A., Cherner, M., et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b
Appay, V., Almeida, J. R., Sauce, D., Autran, B., and Papagno, L. (2007). Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 42, 432–437. doi: 10.1016/j.exger.2006.12.003
Asres, K., Bucar, F., Kartnig, T., Witvrouw, M., Pannecouque, C., and De Clercq, E. (2001). Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother. Res. 15, 62–69. doi: 10.1002/1099-1573(200102)15:1<62::AID-PTR956>3.0.CO;2-X
Atluri, V. S., Hidalgo, M., Samikkannu, T., Kurapati, K. R., Jayant, R. D., Sagar, V., et al. (2015). Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front. Cell Neurosci. 9:212. doi: 10.3389/fncel.2015.00212
Atluri, V. S., Pilakka-Kanthikeel, S., Samikkannu, T., Sagar, V., Kurapati, K. R., Saxena, S. K., et al. (2014). Vorinostat positively regulates synaptic plasticity genes expression and spine density in HIV infected neurons: role of nicotine in progression of HIV-associated neurocognitive disorder. Mol. Brain 7:37. doi: 10.1186/1756-6606-7-37
Balzarini, J. (1994). Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16, 113–126. doi: 10.1007/BF01880662
Benito, J. M., Lopez, M., and Soriano, V. (2004). The role of CD8+ T-cell response in HIV infection. AIDS Rev. 6, 79–88.
Beutler, J. A., Cardellina, J. H., Mcmahon, J. B., Boyd, M. R., and Cragg, G. M. (1992). Anti-HIV and cytotoxic alkaloids from Buchenavia capitata. J. Nat. Prod. 55, 207–213. doi: 10.1021/np50080a008
Brandt, J., Butters, N., Ryan, C., and Bayog, R. (1983). Cognitive loss and recovery in long-term alcohol abusers. Arch. Gen. Psychiatry 40, 435–442. doi: 10.1001/archpsyc.1983.01790040089012
Broder, S. (2009). The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 85, 1–18. doi: 10.1016/j.antiviral.2009.10.002
Buonaguro, L., Tornesello, M. L., and Buonaguro, F. M. (2007). Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: pathogenetic and therapeutic implications. J. Virol. 81, 10209–10219. doi: 10.1128/JVI.00872-07
Chan, D. C., and Kim, P. S. (1998). HIV entry and its inhibition. Cell 93, 681–684. doi: 10.1016/S0092-8674(00)81430-0
Chang, L., Ernst, T., Witt, M. D., Ames, N., Gaiefsky, M., and Miller, E. (2002). Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-Naïve HIV patients. NeuroImage 17, 1638–1648. doi: 10.1006/nimg.2002.1254
Charlton, J. L. (1998). Antiviral activity of lignans. J. Nat. Prod. 61, 1447–1451. doi: 10.1021/np980136znp980136z
Chen, D.-F., Zhang, S.-X., Wang, H.-K., Zhang, S.-Y., Sun, Q.-Z., Cosentino, L. M., et al. (1999). Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 62, 94–97. doi: 10.1021/np980291d
Chen, D. F., Zhang, S. X., Xie, L., Xie, J. X., Chen, K., Kashiwada, Y., et al. (1997). Anti-AIDS agents–XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg. Med. Chem. 5, 1715–1723. doi: 10.1016/S0968-0896(97)00118-1
Chinsembu, K., and Hedimbi, M. (2010). Ethnomedicinal plants and other natural products with anti-HIV active compounds and their putative modes of action. Int. J. Biotechnol. Mol. Biol. Res. 1, 74–91.
Clerici, M., Balotta, C., Salvaggio, A., Riva, C., Trabattoni, D., Papagno, L., et al. (1996). Human immunodeficiency virus (HIV) phenotype and interleukin-2/ interleukin-10 ratio are associated markers of protection and progression in HIV infection. Blood 88, 574–579.
Cohen, J. (2012). The many states of HIV in America. Science 337, 168–171. doi: 10.1126/science.337.6091.168
Creagh, T., Ruckle, J. L., Tolbert, D. T., Giltner, J., Eiznhamer, D. A., Dutta, B., et al. (2001). Safety and pharmacokinetics of single doses of (+)-calanolide a, a novel, naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy, human immunodeficiency virus-negative human subjects. Antimicrob. Agents Chemother. 45, 1379–1386. doi: 10.1128/AAC.45.5.1379-1386.2001
Darbinian-Sarkissian, N., Darbinyan, A., Otte, J., Radhakrishnan, S., Sawaya, B. E., Arzumanyan, A., et al. (2006). p27(SJ), a novel protein in St John’s Wort, that suppresses expression of HIV-1 genome. Gene Ther. 13, 288–295. doi: 10.1038/sj.gt.3302649
Darke, S., Sims, J., Mcdonald, S., and Wickes, W. (2000). Cognitive impairment among methadone maintenance patients. Addiction 95, 687–695. doi: 10.1046/j.1360-0443.2000.9556874.x
De Clercq, E. (1995). Antiviral therapy for human immunodeficiency virus infections. Clin. Microbiol. Rev. 8, 200–239.
Decosterd, L. A., Parsons, I. C., Gustafson, K. R., Cardellina, J. H., Mcmahon, J. B., Cragg, G. M., et al. (1993). HIV inhibitory natural products. 11. Structure, absolute stereochemistry, and synthesis of conocurvone, a potent, novel HIV-inhibitory naphthoquinone trimer from a Conospermum sp. J. Am. Chem. Soc. 115, 6673–6679. doi: 10.1021/ja00068a026
de Oliveira, T., Pillay, D., Gifford, R. J., and UK Collaborative Group on Hiv Drug Resistance (2010). The HIV-1 subtype c epidemic in South America is linked to the United Kingdom. PLoS ONE 5:e9311. doi: 10.1371/journal.pone.0009311
Devlin, K. N., Gongvatana, A., Clark, U. S., Chasman, J. D., Westbrook, M. L., Tashima, K. T., et al. (2012). Neurocognitive effects of HIV, hepatitis C, and substance use history. J. Int. Neuropsychol. Soc. 18, 68–78. doi: 10.1017/s1355617711001408
Dharmaratne, H. R. W., Tan, G. T., Marasinghe, G. P. K., and Pezzuto, J. M. (2002). Inhibition of HIV-1 reverse transcriptase and HIV-1 replication by Calophyllum coumarins and Xanthones. Planta Med. 68, 86–87. doi: 10.1055/s-2002-20058
Dhillon, N., Williams, R., Peng, F., Tsai, Y.-J., Dhillon, S., Nicolay, B., et al. (2007). Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J. Neurovirol. 13, 483–495. doi: 10.1080/13550280701528684
Duan, H., Takaishi, Y., Imakura, Y., Jia, Y., Li, D., Cosentino, L. M., et al. (2000). Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: a new class of potent Anti-HIV agents. J. Nat. Prod. 63, 357–361. doi: 10.1021/np990281s
Ellis, R. J., Badiee, J., Vaida, F., Letendre, S., Heaton, R. K., Clifford, D., et al. (2011). CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 25, 1747–1751.
El-Mekkawy, S., Meselhy, M. R., Nakamura, N., Hattori, M., Kawahata, T., and Otake, T. (2000). Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry 53, 457–464. doi: 10.1016/S0031-9422(99)00556-7
Esiri, M. M., Biddolph, S. C., and Morris, C. S. (1998). Prevalence of alzheimer plaques in AIDS. J. Neurol. Neurosurg. Psychiatry 65, 29–33. doi: 10.1136/jnnp.65.1.29
Ettenhofer, M. L., Hinkin, C. H., Castellon, S. A., Durvasula, R., Ullman, J., Lam, M., et al. (2009). Aging, neurocognition, and medication adherence in HIV infection. Am. J. Geriatr. Psychiatry 17, 281–290. doi: 10.1097/JGP.0b013e31819431bd00019442-200904000-00004
Fabricant, D. S., and Farnsworth, N. R. (2001). The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 109, 69–75. doi: 10.2307/3434847
Farnsworth, N. R. (2007). “Ethnopharmacology and drug development,” in Ciba Foundation Symposium 185 – Ethnobotany and the Search for New Drugs, eds D. J. Chadwick and J. Marsh (Hoboken, NJ: John Wiley & Sons, Ltd), 42–59.
Fuller, R. W., Westergaard, C. K., Collins, J. W., Cardellina, J. H., and Boyd, M. R. (1999). Vismiaphenones D–G, new prenylated benzophenones from Vismia cayennensis. J. Nat. Prod. 62, 67–69. doi: 10.1021/np980152w
Fundacio Lluita Contra la SIDA (2011). Clinicaltrials.gov, ‘Drug Interactions Between Echinacea Purpurea and Etravirine.’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Futterman, D. (2012). HIV/AIDS: the next generation. Science 337:799. doi: 10.1126/science.337.6096.799-a
Gandhi, N., Saiyed, Z., Napuri, J., Samikkannu, T., Reddy, P. B., Agudelo, M., et al. (2010). Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J. Neurovirol. 16, 294–305. doi: 10.3109/13550284.2010.499891
Gandhi, N., Saiyed, Z., Thangavel, S., Rodriguez, J., Rao, K. V. K., and Nair, M. P. N. (2009). Differential effects of HIV type 1 clade B and clade C tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res. Hum. Retrovir. 25, 691–699. doi: 10.1089/aid.2008.0299
Gannon, P., Khan, M. Z., and Kolson, D. L. (2013). Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr. Opin. Neurol. 24, 275–283. doi: 10.1097/WCO.0b013e32834695fb
Giunta, B., Ehrhart, J., Obregon, D. F., Lam, L., Le, L., Jin, J., et al. (2011). Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol. Brain 4, 23–23. doi: 10.1186/1756-6606-4-23
Gonzalez, R., Heaton, R. K., Moore, D. J., Letendre, S., Ellis, R. J., Wolfson, T., et al. (2003). Computerized reaction time battery versus a traditional neuropsychological battery: detecting HIV-related impairments. J. Int. Neuropsychol. Soc. 9, 64–71. doi: 10.1017/S1355617703910071
Gonzalez-Scarano, F., and Martin-Garcia, J. (2005). The neuropathogenesis of AIDS. Nat. Rev. Immunol. 5, 69–81. doi: 10.1038/nri1527
Green, D. A., Masliah, E., Vinters, H. V., Beizai, P., Moore, D. J., and Achim, C. L. (2005). Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19, 407–411. doi: 10.1097/01.aids.0000161770.06158.5c
Grovit-Ferbas, K., and Harris-White, M. E. (2010). Thinking about HIV: the intersection of virus, neuroinflammation and cognitive dysfunction. Immunol. Res. 48, 40–58. doi: 10.1007/s12026-010-8166-x
Groweiss, A., Cardellina, J. H., and Boyd, M. R. (2000). HIV-Inhibitory prenylated Xanthones and flavones from Maclura tinctoria. J. Nat. Prod. 63, 1537–1539. doi: 10.1021/np000175m
Gustafson, K. R., Cardellina, J. H., Mcmahon, J. B., Pannell, L. K., Cragg, G. M., and Boyd, M. R. (1992). HIV inhibitory natural products. 6. The peltatols, novel HIV-inhibitory catechol derivatives from Pothomorphe peltata. J. Organ. Chem. 57, 2809–2811. doi: 10.1021/jo00036a010
Heaton, R. K., Clifford, D. B., Franklin, D.R. Jr, Woods, S. P., Ake, C., Vaida, F., et al. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096. doi: 10.1212/WNL.0b013e318200d727
Heaton, R. K., Franklin, D. R., Ellis, R. J., Mccutchan, J. A., Letendre, S. L., Leblanc, S., et al. (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17, 3–16. doi: 10.1007/s13365-010-0006-1
Hedimbi, M., and Chinsembu, K. C. (2012). Ethnomedicinal study of plants used to manage HIV/AIDS-related disease conditions in the Ohangwena region, Namibia. Int. J. Med. Plants Res. 1, 4–11.
Hemelaar, J. (2012). The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 18, 182–192. doi: 10.1016/j.molmed.2011.12.001
Hemelaar, J., Gouws, E., Ghys, P. D., and Osmanov, S. (2011). Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS 25, 679–689. doi: 10.1097/QAD.0b013e328342ff93
Hidalgo, M., Atluri, V. S. R., and Nair, M. (2015). Drugs of abuse in HIV infection and neurotoxicity. Front. Microbiol. 6:217. doi: 10.3389/fmicb.2015.00217
Honig, L. S., Schupf, N., Lee, J. H., Tang, M. X., and Mayeux, R. (2006). Shorter telomeres are associated with mortality in those with APOE 𝜖4 and dementia. Ann. Neurol. 60, 181–187. doi: 10.1002/ana.20894
Hu, K., Kobayashi, H., Dong, A., Iwasaki, S., and Yao, X. (2000). Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 66, 564–567. doi: 10.1055/s-2000-8601
Ishida, J., Wang, H.-K., Oyama, M., Cosentino, M. L., Hu, C.-Q., and Lee, K.-H. (2001). Anti-AIDS agents. 46.1 anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its Derivatives. J. Nat. Prod. 64, 958–960. doi: 10.1021/np0101189
Jaco, H., King, R. L., Tenywa, T., Kyeyune, P., and Opio, A. (2004). Defining minimum standards of practice for incorporating african traditional medicine into HIV/AIDS prevention, care, and support: a regional initiative in eastern and Southern Africa. J. Altern. Complement. Med. 10, 905–910. doi: 10.1089/acm.2004.10.905
Jiang, S., Zhao, Q., and Debnath, A. K. (2002). Peptide and non-peptide HIV fusion inhibitors. Curr. Pharm. Des. 8, 563–580. doi: 10.2174/1381612024607180
Jain, S., Shukla, S. D., Sharma, K., and Bhatnagar, M. (2001). Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother. Res. 15, 544–548. doi: 10.1002/ptr.802
Kashiwada, Y., Nishizawa, M., Yamagishi, T., Tanaka, T., Nonaka, G.-I., Cosentino, L. M., et al. (1995). Anti-AIDS Agents, 18. sodium and potassium salts of caffeic acid tetramers from Arnebia euchroma as anti-HIV agents. J. Nat. Prod. 58, 392–400. doi: 10.1021/np50117a007
Kashiwada, Y., Wang, H.-K., Nagao, T., Kitanaka, S., Yasuda, I., Fujioka, T., et al. (1998). Anti-AIDS agents. 30. anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 61, 1090–1095. doi: 10.1021/np9800710
Kaul, M. (2009). HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr. Opin. Neurol. 22, 315–320. doi: 10.1097/WCO.0b013e328329cf3c
Kaul, M., Garden, G. A., and Lipton, S. A. (2001). Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994. doi: 10.1038/3507366735073667
Kaul, M., and Lipton, S. A. (2006). Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J. Neuroimmune Pharmacol. 1, 138–151. doi: 10.1007/s11481-006-9011-9
Kim, H. J., Woo, E.-R., Shin, C.-G., and Park, H. (1998). A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J. Nat. Prod. 61, 145–148. doi: 10.1021/np970171q
Kim, S. R., Jeong, H. Y., Yang, S., Choi, S. P., Seo, M. Y., Yun, Y. K., et al. (2011). Effects of chronic alcohol consumption on expression levels of APP and abeta-producing enzymes. BMB Rep. 44, 135–139. doi: 10.5483/BMBRep.2011.44.2.135
Kitamura, K., Honda, M., Yoshizaki, H., Yamamoto, S., Nakane, H., Fukushima, M., et al. (1998). Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res. 37, 131–140. doi: 10.1016/S0166-3542(97)00069-7
Kitchen, C. M., Kitchen, S. G., Dubin, J. A., and Gottlieb, M. S. (2001). Initial virological and immunologic response to highly active antiretroviral therapy predicts long-term clinical outcome. Clin. Infect. Dis. 33, 466–472. doi: 10.1086/321900
Klos, M., Van De Venter, M., Milne, P. J., Traore, H. N., Meyer, D., and Oosthuizen, V. (2009). In vitro anti-HIV activity of five selected South African medicinal plant extracts. J. Ethnopharmacol. 124, 182–188. doi: 10.1016/j.jep.2009.04.043
Kraft-Terry, S. D., Buch, S. J., Fox, H. S., and Gendelman, H. E. (2009). A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64, 133–145. doi: 10.1016/j.neuron.2009.09.042
Kuboyama, T., Tohda, C., and Komatsu, K. (2005). Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br. J. Pharmacol. 144, 961–971. doi: 10.1038/sj.bjp.0706122
Kulkarni, S. K., and Dhir, A. (2008). Withania somnifera: an Indian ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1093–1105. doi: 10.1016/j.pnpbp.2007.09.011
Kumari, G., and Singh, R. K. (2012). Highly active antiretroviral therapy for treatment of HIV/AIDS patients: current status and future prospects and the Indian scenario. HIV AIDS Rev. 11, 5–14. doi: 10.1016/j.hivar.2012.02.003
Kurapati, K. R., Atluri, V. S., Samikkannu, T., and Nair, M. P. (2013). Ashwagandha (Withania somnifera) reverses beta-amyloid1-42 induced toxicity in human neuronal cells: implications in HIV-associated neurocognitive disorders (HAND). PLoS ONE 8:e77624. doi: 10.1371/journal.pone.0077624PONE-D-13-22227
Kurapati, K. R., Samikkannu, T., Atluri, V. S., Kaftanovskaya, E., Yndart, A., and Nair, M. P. (2014). beta-Amyloid1-42, HIV-1Ba-L (clade B) infection and drugs of abuse induced degeneration in human neuronal cells and protective effects of ashwagandha (Withania somnifera) and its constituent Withanolide A. PLoS ONE 9:e112818. doi: 10.1371/journal.pone.0112818PONE-D-14-30964
Lawn, S. D., Butera, S. T., and Folks, T. M. (2001). Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14, 753–777. doi: 10.1128/CMR.14.4.753-777.2001
Lee, T. T.-Y., Kashiwada, Y., Huang, L., Snider, J., Cosentino, M., and Lee, K.-H. (1994). Suksdorfin: an anti-HIV principle from Lomatium suksdorfii, its structure-activity correlation with related coumarins, and synergistic effects with anti-AIDS nucleosides. Bioorg. Med. Chem. 2, 1051–1056. doi: 10.1016/S0968-0896(00)82054-4
Lee-Huang, S., Huang, P. L., Huang, P. L., Bourinbaiar, A. S., Chen, H. C., and Kung, H. F. (1995). Inhibition of the integrase of human immunodeficiency virus (HIV) type 1 by anti-HIV plant proteins MAP30 and GAP31. Proc. Natl. Acad. Sci. U.S.A. 92, 8818–8822.
Li, H.-Y., Sun, N.-J., Kashiwada, Y., Sun, L., Snider, J. V., Cosentino, L. M., et al. (1993). Anti-AIDS agents, 9. Suberosol, a new C31 lanostane-type triterpene and Anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 56, 1130–1133. doi: 10.1021/np50097a017
Li, T. (2014). Clinicaltrials.gov, ‘Study on the Impact of Triptolide Woldifiion on HIV-1 Reservoir In Acute HIV-1 Infection.’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Lim, Y. A., Mei, M. C., Kusumoto, I. T., Miyashiro, H., Hattori, M., Gupta, M. P., et al. (1997). HIV-1 reverse transcriptase inhibitory principles from Chamaesyce hyssopifolia. Phytother. Res. 11, 22–27. doi: 10.1002/(SICI)1099-1573(199702)11:1<22::AID-PTR951>3.0.CO;2-3
Lin, Y.-M., Anderson, H., Flavin, M. T., Pai, Y.-H. S., Mata-Greenwood, E., Pengsuparp, T., et al. (1997). In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 60, 884–888. doi: 10.1021/np9700275
Lindl, K. A., Marks, D. R., Kolson, D. L., and Jordan-Sciutto, K. L. (2010). HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J. Neuroimmune Pharmacol. 5, 294–309. doi: 10.1007/s11481-010-9205-z
Liu, J.-S., and Li, L. (1995). Kadsulignans L-N, three dibenzocyclooctadiene lignans from Kadsura coccinea. Phytochemistry 38, 241–245. doi: 10.1016/0031-9422(94)00557-A
Loya, S., Rudi, A., Kashman, Y., and Hizi, A. (1999). Polycitone A, a novel and potent general inhibitor of retroviral reverse transcriptases and cellular DNA polymerases. Biochem. J. 344, 85–92. doi: 10.1042/bj3440085
Lu, W. (2013). Clinicaltrials.gov, ‘Impact on T Cell Immune Activation and Inflammation of Triptolide Woldifii in HIV-infected Immunological Non-Responders (CACTrip12).’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Lüscher-Mattli, M. (2000). Polyanions–a lost chance in the fight against HIV and other virus diseases? Antivir. Chem. Chemother. 11, 249–259. doi: 10.1177/095632020001100401
Mahmood, N., Piacente, S., Burke, A., Khan, A. I., and Pizza, C. (1997). Constituents of Cuscuto reflexa are anti-HIV Agents. Antivir. Chem. Chemother. 8, 70–74. doi: 10.1177/095632029700800108
Malan-Muller, S., Hemmings, S. M., Spies, G., Kidd, M., Fennema-Notestine, C., and Seedat, S. (2013). Shorter telomere length - a potential susceptibility factor for HIV-associated neurocognitive impairments in South African women [corrected]. PLoS ONE 8:e58351. doi: 10.1371/journal.pone.0058351PONE-D-12-29635
Mann, D. A., and Frankel, A. D. (1991). Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 10, 1733–1739.
Martin-Ruiz, C., Dickinson, H. O., Keys, B., Rowan, E., Kenny, R. A., and Von Zglinicki, T. (2006). Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 60, 174–180. doi: 10.1002/ana.20869
McArthur, J. C., Haughey, N., Gartner, S., Conant, K., Pardo, C., Nath, A., et al. (2003). Human immunodeficiency virus-associated dementia: an evolving disease. J. Neurovirol. 9, 205–221. doi: 10.1080/135502803901941094J811MK1R26FL3NR
McArthur, J. C., Mcdermott, M. P., Mcclernon, D., St Hillaire, C., Conant, K., Marder, K., et al. (2004). Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch. Neurol. 61, 1687–1696. doi: 10.1001/archneur.61.11.1687
McArthur, J. C., Steiner, J., Sacktor, N., and Nath, A. (2010). Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann. Neurol. 67, 699–714. doi: 10.1002/ana.22053
McCormick, J. L., Mckee, T. C., Cardellina, J. H., and Boyd, M. R. (1996). HIV inhibitory natural products. 26. Quinoline alkaloids from Euodia roxburghiana. J. Nat. Prod. 59, 469–471. doi: 10.1021/np960250m
Meragelman, K. M., Mckee, T. C., and Boyd, M. R. (2001). Anti-HIV prenylated flavonoids from Monotes africanus1. J. Nat. Prod. 64, 546–548. doi: 10.1021/np0005457
Min, B. S., Jung, H. J., Lee, J. S., Kim, Y. H., Bok, S. H., Ma, C. M., et al. (1999). Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med. 65, 374–375. doi: 10.1055/s-2006-960792
Min, B. S., Miyashiro, H., and Hattori, M. (2002). Inhibitory effects of quinones on RNase H activity associated with HIV-1 reverse transcriptase. Phytother. Res. 16(Suppl. 1), S57–S62. doi: 10.1002/ptr.808
Mishra, L. C., Singh, B. B., and Dagenais, S. (2000). Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern. Med. Rev. 5, 334–346.
Mohri, H., Perelson, A. S., Tung, K., Ribeiro, R. M., Ramratnam, B., Markowitz, M., et al. (2001). Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 194, 1277–1288. doi: 10.1084/jem.194.9.1277
Molto, J. (2010). Clinicaltrials.gov, ‘Drug Interactions Between Echinacea Purpurea and Darunavir/Ritonavir.’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Munoz-Moreno, J. A., Fumaz, C. R., Ferrer, M. J., Prats, A., Negredo, E., Garolera, M., et al. (2008). Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res. Hum. Retrovir. 24, 1301–1307. doi: 10.1089/aid.2007.0310
Murray, M. T., and Pizzorno, J. E. (1999). Textbook of Natural Medicine. London: Churchill Livingstone.
Murray, S. M., Down, C. M., Boulware, D. R., Stauffer, W. M., Cavert, W. P., Schacker, T. W., et al. (2010). Reduction of immune activation with chloroquine therapy during chronic HIV infection. J. Virol. 84, 12082–12086. doi: 10.1128/JVI.01466-10
Nair, M. P. N., Saiyed, Z. M., Nair, N., Gandhi, N. H., Rodriguez, J. W., Boukli, N., et al. (2009). Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J. Neuroimmune Pharmacol. 4, 129–139. doi: 10.1007/s11481-008-9128-0
Nair, M. P. N., Schwartz, S. A., Mahajan, S. D., Tsiao, C., Chawda, R. P., Whitney, R., et al. (2004). Drug abuse and neuropathogenesis of HIV infection: role of DC-SIGN and IDO. J. Neuroimmunol. 157, 56–60. doi: 10.1016/j.jneuroim.2004.08.040
Nath, A., Schiess, N., Venkatesan, A., Rumbaugh, J., Sacktor, N., and Mcarthur, J. (2008). Evolution of HIV dementia with HIV infection. Int. Rev. Psychiatry 20, 25–31. doi: 10.1080/09540260701861930
Nottet, H. S., and Gendelman, H. E. (1995). Unraveling the neuroimmune mechanisms for the HIV-1-associated cognitive/motor complex. Immunol. Today 16, 441–448. doi: 10.1016/0167-5699(95)80022-0
Ogata, T., Higuchi, H., Mochida, S., Matsumoto, H., Kato, A., Endo, T., et al. (1992). HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Res. Hum. Retrovir. 8, 1937–1944. doi: 10.1089/aid.1992.8.1937
Ohtake, N., Nakai, Y., Yamamoto, M., Sakakibara, I., Takeda, S., Amagaya, S., et al. (2004). Separation and isolation methods for analysis of the active principles of Sho-saiko-to (SST) oriental medicine. J. Chromatogr. B 812, 135–148. doi: 10.1016/j.jchromb.2004.06.051
Orhan, D. D., Ozcelik, B., Ozgen, S., and Ergun, F. (2010). Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 165, 496–504. doi: 10.1016/j.micres.2009.09.002
Panossian, L. A., Porter, V. R., Valenzuela, H. F., Zhu, X., Reback, E., Masterman, D., et al. (2003). Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol. Aging 24, 77–84. doi: 10.1016/S0197-4580(02)00043-X
Park, J. C., Hur, J. M., Park, J. G., Hatano, T., Yoshida, T., Miyashiro, H., et al. (2002). Inhibitory effects of Korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 16, 422–426. doi: 10.1002/ptr.919
Pathai, S., Lawn, S. D., Gilbert, C. E., Mcguinness, D., Mcglynn, L., Weiss, H. A., et al. (2013). Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS 27, 2375–2384. doi: 10.1097/QAD.0b013e328363bf7f
Paul, R., Flanigan, T. P., Tashima, K., Cohen, R., Lawrence, J., Alt, E., et al. (2005). Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. J. Neuropsychiatry Clin. Neurosci. 17, 114–118. doi: 10.1176/jnp.17.1.114
Petrovas, C., Casazza, J. P., Brenchley, J. M., Price, D. A., Gostick, E., Adams, W. C., et al. (2006). PD-1 is a regulator of virus-specific CD8(+) T cell survival in HIV infection. J. Exp. Med. 203, 2281–2292. doi: 10.1084/jem.20061496
Piconi, S., Parisotto, S., Rizzardini, G., Passerini, S., Terzi, R., Argenteri, B., et al. (2011). Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 118, 3263–3272. doi: 10.1182/blood-2011-01-329060
Ptak, R. G. (2002). HIV-1 regulatory proteins: targets for novel drug development. Expert Opin. Investig. Drugs 11, 1099–1115. doi: 10.1517/13543784.11.8.1099
Rambaut, A., Robertson, D. L., Pybus, O. G., Peeters, M., and Holmes, E. C. (2001). Human immunodeficiency virus: phylogeny and the origin of HIV-1. Nature 410, 1047–1048. doi: 10.1038/35074179
Reddy, P. V., Pilakka-Kanthikeel, S., Saxena, S. K., Saiyed, Z., and Nair, M. P. (2012). Interactive effects of morphine on HIV infection: role in HIV-associated neurocognitive disorder. AIDS Res. Treat. 2012:953678. doi: 10.1155/2012/953678
Resh, M. D. (2005). Intracellular trafficking of HIV-1 Gag: how Gag interacts with cell membranes and makes viral particles. AIDS Rev. 7, 84–91.
Rimando, A. M., Pezzuto, J. M., Farnsworth, N. R., Santisuk, T., Reutrakul, V., and Kawanishi, K. (1994). New lignans from Anogeissus acuminata with HIV-1 reverse transcriptase inhibitory activity. J. Nat. Prod. 57, 896–904. doi: 10.1021/np50109a004
Robertson, K. R., Smurzynski, M., Parsons, T. D., Wu, K., Bosch, R. J., Wu, J., et al. (2007). The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21, 1915–1921. doi: 10.1097/QAD.0b013e32828e4e2700002030-200709120-00011
Rodriguez, B., Sethi, A. K., Cheruvu, V. K., Mackay, W., Bosch, R. J., Kitahata, M., et al. (2006). Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 296, 1498–1506. doi: 10.1001/jama.296.12.1498
Roth, G. N., Chandra, A., and Nair, M. G. (1998). Novel bioactivities of Curcuma longa constituents. J. Nat. Prod. 61, 542–545. doi: 10.1021/np970459f
Roy, U., Atluri, V. S., Agudelo, M., Yndart, A., Huang, Z., and Nair, M. (2015). DJ1 expression downregulates in neuroblastoma cells (SK-N-MC) chronically exposed to HIV-1 and cocaine. Front. Microbiol. 6:749. doi: 10.3389/fmicb.2015.00749
Rukachaisirikul, V., Pailee, P., Hiranrat, A., Tuchinda, P., Yoosook, C., Kasisit, J., et al. (2003). Anti-HIV-1 protostane triterpenes and digeranylbenzophenone from trunk bark and stems of Garcinia speciosa. Planta Med. 69, 1141–1146. doi: 10.1055/s-2003-818006
Sacktor, N., Lyles, R. H., Skolasky, R., Kleeberger, C., Selnes, O. A., Miller, E. N., et al. (2001). HIV-associated neurologic disease incidence changes: multicenter AIDS cohort study, 1990-1998. Neurology 56, 257–260. doi: 10.1212/WNL.56.2.257
Sacktor, N., Mcdermott, M., Marder, K., Schifitto, G., Selnes, O., Mcarthur, J., et al. (2002). HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 8, 136–142. doi: 10.1080/13550280290049615
Sagar, V., Pilakka-Kanthikeel, S., Atluri, V. S. R., Ding, H., Arias, A. Y., Jayant, R. D., et al. (2015). Therapeutical neurotargeting via magnetic nanocarrier: implications to opiate-induced neuropathogenesis and NeuroAIDS. J. Biomed. Nanotechnol. 11, 1722–1733. doi: 10.1166/jbn.2015.2108
Sakurai, N., Wu, J. H., Sashida, Y., Mimaki, Y., Nikaido, T., Koike, K., et al. (2004). Anti-AIDS agents. Part 57: actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorg Med. Chem. Lett. 14, 1329–1332. doi: 10.1016/j.bmcl.2003.12.035S0960894X03013222
Samikkannu, T., Ranjith, D., Rao, K. V., Atluri, V. S., Pimentel, E., El-Hage, N., et al. (2015a). HIV-1 gp120 and morphine induced oxidative stress: role in cell cycle regulation. Front. Microbiol. 6:614. doi: 10.3389/fmicb.2015.00614
Samikkannu, T., Rao, K. V., Salam, A. A., Atluri, V. S., Kaftanovskaya, E. M., Agudelo, M., et al. (2015b). HIV subtypes B and C gp120 and methamphetamine interaction: dopaminergic system implicates differential neuronal toxicity. Sci. Rep. 5:11130. doi: 10.1038/srep11130
Samikkannu, T., Rao, K. K., Gandhi, N., Saxena, S., and Nair, M. N. (2010). Human immunodeficiency virus type 1 clade B and C Tat differentially induce indoleamine 2,3-dioxygenase and serotonin in immature dendritic cells: implications for neuroAIDS. J. Neurovirol. 16, 255–263. doi: 10.3109/13550284.2010.497809
Sankar, S. R., Manivasagam, T., Krishnamurti, A., and Ramanathan, M. (2007). The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: an analysis of behavioral and biochemical variables. Cell Mol. Biol. Lett. 12, 473–481. doi: 10.2478/s11658-007-0015-0
Saxena, S. K., Tiwari, S., and Nair, M. P. (2012). A global perspective on HIV/AIDS. Science 337:798. doi: 10.1126/science.337.6096.798
Schader, S. M., and Wainberg, M. A. (2011). Insights into HIV-1 pathogenesis through drug discovery: 30 years of basic research and concerns for the future. HIV AIDS Rev. 10, 91–98. doi: 10.1016/j.hivar.2011.09.003
Schulz, V. (2006). Safety of St. John’s Wort extract compared to synthetic antidepressants. Phytomedicine 13, 199–204. doi: 10.1016/j.phymed.2005.07.005
Sebit, M. B., Chandiwana, S. K., Latif, A. S., Gomo, E., Acuda, S. W., Makoni, F., et al. (2000). Quality of life evaluation in patients with HIV-I infection: the impact of traditional medicine in Zimbabwe. Cent. Afr. J. Med. 46, 208–213.
Sehgal, N., Gupta, A., Valli, R. K., Joshi, S. D., Mills, J. T., Hamel, E., et al. (2012). Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. U.S.A. 109, 3510–3515.
Sharer, L. R. (1992). Pathology of HIV-1 infection of the central nervous system. A review. J. Neuropathol. Exp. Neurol. 51, 3–11. doi: 10.1097/00005072-199201000-00002
Short, R. V. (2006). New ways of preventing HIV infection: thinking simply, simply thinking. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 811–820. doi: 10.1098/rstb.2005.1781
Simioni, S., Cavassini, M., Annoni, J. M., Rimbault Abraham, A., Bourquin, I., Schiffer, V., et al. (2010). Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24, 1243–1250. doi: 10.1097/QAD.0b013e3283354a7b
Soontornniyomkij, V., Moore, D. J., Gouaux, B., Soontornniyomkij, B., Tatro, E. T., Umlauf, A., et al. (2012). Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS 26, 2327–2335. doi: 10.1097/QAD.0b013e32835a117c
Sun, Y., Huang, P. L., Li, J. J., Huang, Y. Q., Zhang, L., Huang, P. L., et al. (2001). Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis in AIDS-related lymphoma cells infected with Kaposi’s sarcoma-associated virus. Biochem. Biophys. Res. Commun. 287, 983–994. doi: 10.1006/bbrc.2001.5689
Tan, G. T., Pezzuto, J. M., Kinghorn, A. D., and Hughes, S. H. (1991). Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 54, 143–154. doi: 10.1021/np50073a012
Tozzi, V., Balestra, P., Galgani, S., Narciso, P., Ferri, F., Sebastiani, G., et al. (1999). Positive and sustained effects of highly active antiretroviral therapy on HIV-1-associated neurocognitive impairment. AIDS 13, 1889–1897. doi: 10.1097/00002030-199910010-00011
Uchiumi, F., Hatano, T., Ito, H., Yoshida, T., and Tanuma, S. (2003). Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res. 58, 89–98. doi: 10.1016/S0166-3542(02)00186-9
Uchiumi, F., Maruta, H., Inoue, J., Yamamoto, T., and Tanuma, S. (1996). Inhibitory effect of tannic acid on human immunodeficiency virus promoter activity induced by 12-O-tetra decanoylphorbol-13-acetate in Jurkat T-cells. Biochem. Biophys. Res. Commun. 220, 411–417. doi: 10.1006/bbrc.1996.0419
Valdes, A. M., Deary, I. J., Gardner, J., Kimura, M., Lu, X., Spector, T. D., et al. (2008). Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol. Aging 31, 986–992. doi: 10.1016/j.neurobiolaging.2008.07.012
Vermani, K., and Garg, S. (2002). Herbal medicines for sexually transmitted diseases and AIDS. J. Ethnopharmacol. 80, 49–66. doi: 10.1016/S0378-8741(02)00009-0
Vitiello, B., Goodkin, K., Ashtana, D., Shapshak, P., Atkinson, J. H., Heseltine, P. N., et al. (2007). HIV-1 RNA concentration and cognitive performance in a cohort of HIV-positive people. AIDS 21, 1415–1422. doi: 10.1097/QAD.0b013e328220e71a00002030-200707110-00004
Vlietinck, A. J., De Bruyne, T., Apers, S., and Pieters, L. A. (1998). Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 64, 97–109. doi: 10.1055/s-2006-957384
von Zglinicki, T., Serra, V., Lorenz, M., Saretzki, G., Lenzen-Grossimlighaus, R., Gessner, R., et al. (2000). Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab. Invest. 80, 1739–1747. doi: 10.1038/labinvest.3780184
Wang, J. (2009a). Clinicaltrials.gov, ‘Clinical Trials on the Effect of Immunity 1 (Fuzheng 1) On Immune Reconstitution of HIV Patients.’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Wang, J. (2009b). Clinicaltrials.gov, ‘Research on Effect of Traditional Chinese Medicine (TCM) on Immune Reconstitution of HIV/AIDS Patients After Highly Active Antiretroviral Therapy (HAART).’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Wang, J. (2009c). Clinicaltrials.gov, ‘The Effect of Combination of Traditional Chinese Medicine (TCM) and Highly Active Antiretroviral Therapy (HAART) on Immune Reconstitution of HIV/AIDS Patients.’ Available at: Clinicaltrials.gov [accessed December 1, 2015].
Wang, J. N., Hou, C. Y., Liu, Y. L., Lin, L. Z., Gil, R. R., and Cordell, G. A. (1994). Swertifrancheside, an HIV-reverse transcriptase inhibitor and the first flavone-xanthone dimer, from Swertia franchetiana. J. Nat. Prod. 57, 211–217. doi: 10.1021/np50104a003
Wang, Q., Ding, Z.-H., Liu, J.-K., and Zheng, Y.-T. (2004). Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antiviral Res. 64, 189–194. doi: 10.1016/j.antiviral.2004.08.005
Wayengera, M. (2011). Targeting persistent HIV infection: where and how, if possible? HIV AIDS Rev. 10, 1–8. doi: 10.1016/j.hivar.2011.01.002
Witvrouw, M., and De Clercq, E. (1997). Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 29, 497–511. doi: 10.1016/S0306-3623(96)00563-0
World Health Organization [WHO] (1989a). In vitro screening of traditional medicines for anti-HIV activity: memorandum from a WHO meeting. Bull. World Health Organ. 67, 613–618.
World Health Organization [WHO] (1989b). Report of a WHO Informal Consultation on Traditional Medicine and AIDS: In Vitro Screening for Anti-HIV Activity, Geneva, 6-8 February 1989. Geneva: World Health Organization.
World Health Organization [WHO] (2002). WHO Traditional Medicine Strategy 2002-2005. Geneva: World Health Organization, 1–61.
Xu, H. X., Wan, M., Dong, H., But, P. P., and Foo, L. Y. (2000). Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol. Pharm. Bull. 23, 1072–1076. doi: 10.1248/bpb.23.1072
Xu, H.-X., Zeng, F.-Q., Wan, M., and Sim, K.-Y. (1996). Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 59, 643–645. doi: 10.1021/np960165e
Xu, J., and Ikezu, T. (2009). The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J. Neuroimmune Pharmacol. 4, 200–212. doi: 10.1007/s11481-008-9136-0
Yaffe, K., Lindquist, K., Kluse, M., Cawthon, R., Harris, T., Hsueh, W.-C., et al. (2009). Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol. Aging 32, 2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006
Yamaguchi, K., Honda, M., Ikigai, H., Hara, Y., and Shimamura, T. (2002). Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antiviral Res. 53, 19–34. doi: 10.1016/S0166-3542(01)00189-9
Yao-Haur, K., and Li-Ming Yang, K. (1997). Antitumour and anti-AIDS triterpenes from Celastrus hindsii. Phytochemistry 44, 1275–1281. doi: 10.1016/S0031-9422(96)00719-4
Yu, D., Suzuki, M., Xie, L., Morris-Natschke, S. L., and Lee, K.-H. (2003). Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 23, 322–345. doi: 10.1002/med.10034
Zanet, D. L., Thorne, A., Singer, J., Maan, E. J., Sattha, B., Le Campion, A., et al. (2012). Association between short leukocyte telomere length and HIV infection in a cohort study: no evidence of a relationship with antiretroviral therapy. Clin. Infect. Dis. 58, 1322–1332. doi: 10.1093/cid/ciu051
Zhou, P., Takaishi, Y., Duan, H., Chen, B., Honda, G., Itoh, M., et al. (2000). Coumarins and bicoumarin from Ferula sumbul: anti-HIV activity and inhibition of cytokine release. Phytochemistry 53, 689–697. doi: 10.1016/S0031-9422(99)00554-3
Keywords: HIV, neurocognitive disorders, natural products, anti-HIV agents
Citation: Kurapati KV, Atluri VS, Samikkannu T, Garcia G and Nair MPN (2016) Natural Products as Anti-HIV Agents and Role in HIV-Associated Neurocognitive Disorders (HAND): A Brief Overview. Front. Microbiol. 6:1444. doi: 10.3389/fmicb.2015.01444
Received: 12 April 2015; Accepted: 03 December 2015;
Published: 12 January 2016.
Edited by:
Francois Villinger, Emory University School of Medicine, USAReviewed by:
Sara Louise Cosby, Queen’s University Belfast, UKCopyright © 2016 Kurapati, Atluri, Samikkannu, Garcia and Nair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhavan P. N. Nair, bmFpcm1AZml1LmVkdQ==; Venkata S. Atluri, ZHJhdGx1cmlAYW9sLmNvbQ==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.