- 1BRD School of Biosciences, Sardar Patel University, Anand, India
- 2Laboratory of Cyanobacterial Biotechnology, Department of Biochemistry, Faculty of Science, Chulalongkorn University, Bangkok, Thailand
Cyanobacteria are ecologically one of the most prolific groups of phototrophic prokaryotes in both marine and freshwater habitats. Both the beneficial and detrimental aspects of cyanobacteria are of considerable significance. They are important primary producers as well as an immense source of several secondary products, including an array of toxic compounds known as cyanotoxins. Abundant growth of cyanobacteria in freshwater, estuarine, and coastal ecosystems due to increased anthropogenic eutrophication and global climate change has created serious concern toward harmful bloom formation and surface water contamination all over the world. Cyanobacterial blooms and the accumulation of several cyanotoxins in water bodies pose severe ecological consequences with high risk to aquatic organisms and global public health. The proper management for mitigating the worldwide incidence of toxic cyanobacterial blooms is crucial for maintenance and sustainable development of functional ecosystems. Here, we emphasize the emerging information on the cyanobacterial bloom dynamics, toxicology of major groups of cyanotoxins, as well as a perspective and integrative approach to their management.
Introduction
Cyanobacteria are considered the most primitive groups of photosynthetic prokaryotes (Bullerjahn and Post, 2014) and possibly appeared on the Earth about 3.5 billion years ago (Tomitani et al., 2006). They are ubiquitous in nature and thrive in a variety of ecological niches ranging from desert to hot springs and ice-cold water. Most cyanobacteria are an immense source of several secondary natural products with applications in the food, pharmaceuticals, cosmetics, agriculture, and energy sectors (Rastogi and Sinha, 2009). Moreover, some species of cyanobacteria grow vigorously and form a dominant microflora in terms of their biomass and productivity in specific ecosystems. Bloom formations (Figure 1) due to excessive growth of certain cyanobacteria followed by the production of toxic compounds have been reported in many eutrophic to hypertrophic lakes, ponds, and rivers throughout the world (Rastogi et al., 2014). A range of toxic secondary compounds, called cyanotoxins, have been reported from cyanobacteria inhabiting freshwater and marine ecosystems. These toxic compounds are highly detrimental for survival of several aquatic organisms, wild and/or domestic animals, and humans. Aquatic organisms, including plants and animals, and phyto/zoo-planktons inhabiting under toxic bloom rich ecosystems, are directly exposed to the harmful effects of different cyanotoxins. The intoxication occurring in wild and/or domestic animals and humans is either due to direct ingestion of cells of toxin producing cyanobacteria or the consumption of drinking water contaminated with cyanotoxins (Rastogi et al., 2014). The toxicity of different cyanotoxins is directly proportional to the growth of cyanobacteria and the extent of their toxin production. It has been shown that the growth of different cyanobacteria and their toxin biosynthesis is greatly influenced by different abiotic factors such as light intensity, temperature, short wavelength radiations, pH, and nutrients (Neilan et al., 2013; Häder et al., 2014; Rastogi et al., 2014). Global warming and temperature gradients can significantly change species composition and favor blooms of toxic phytoplanktons (El-Shehawy et al., 2012; Häder and Gao, 2015).

FIGURE 1. Examples of excessive nutrient enrichment and bloom dynamics in freshwater ponds. (A) Harmful algal blooms in a pond at Chulalongkorn University, Bangkok, Thailand, showing the life of turtles (in red circle) under the toxic blooms condition (B). (C) Harmful algal blooms in a large pond in Varanasi, India (Photograph by R.P. Rastogi).
It has been assumed that cyanotoxins play an important role in chemical defense mechanisms giving survival advantages to the cyanobacteria over other microbes or deterring predation by higher trophic levels (Vepritskii et al., 1991; Jang et al., 2007; Berry et al., 2008). Cyanotoxins may also take part in chemical signaling. Overall, information regarding the specific role(s) of cyanotoxins in the life of individual cyanobacteria or their ecological and biotechnological operations is still very limited and needs extensive research. In the present review, we summarize the recent advances on bloom dynamics, cyanotoxin production, and mitigation strategies as well as their consequences on environmental health perspectives.
Eutrophication, Global Climate Change, and Cyanobacterial Bloom Dynamics
Occurrence of toxic cyanobacterial blooms (cyanoblooms) is a serious global problem which affects the water quality due to the production and accumulation of different cyanotoxins and other malodorous compounds. These blooms may cause an increase of biological oxygen demand (BOD) and anoxia in the water bodies, and death of aquatic life (Havens, 2008; Brookes and Carey, 2011; Rastogi et al., 2014). The factors contributing to the worldwide occurrence of cyanobacterial blooms are still debatable. Nevertheless, cultural eutrophication from domestic, industrial, and agricultural wastes as well as global climate change can play a major role in the global expansion of harmful algal blooms and toxin production (Kaebernick and Neilan, 2001; Conley et al., 2009; Smith and Schindler, 2009; Paerl and Scott, 2010; Kleinteich et al., 2012; O’Neil et al., 2012; Paerl and Paul, 2012; Neilan et al., 2013; Gehringer and Wannicke, 2014; Figure 2). Excessive loads of certain inorganic and/or organic nutrient concentrations are considered as strong risk factors for bloom promotion both in fresh and marine water habitats (Smith, 2003; Heisler et al., 2008; Conley et al., 2009; Ruttenberg and Dyhrman, 2012; Michalak et al., 2013; Davidson et al., 2014; Beversdorf et al., 2015). The anthropogenically mediated change in the N/P ratio has frequently been interrelated to the appearance of cyanobacterial blooms (Glibert et al., 2004). The phosphorus concentration was found as a primary regulating factor for increased cyanobacterial growth and changes of genotypes, both of which were found to be closely related to the water temperature, signifying the role of eutrophication in the occurrence of toxic blooms (Joung et al., 2011). Recently, Molot et al. (2014) presented a novel conceptual model linking anoxia, phosphorus (P), nitrogen (N), iron (Fe), and sulfate to the formation of harmful cyanobacterial blooms across three gradients, i.e., nutrients, salinity, and acidity. Continued transfer of sediments to a water body may block the natural flow of water and enrich the dissolved organic carbon and other compounds leading to potential risk of bloom formation.
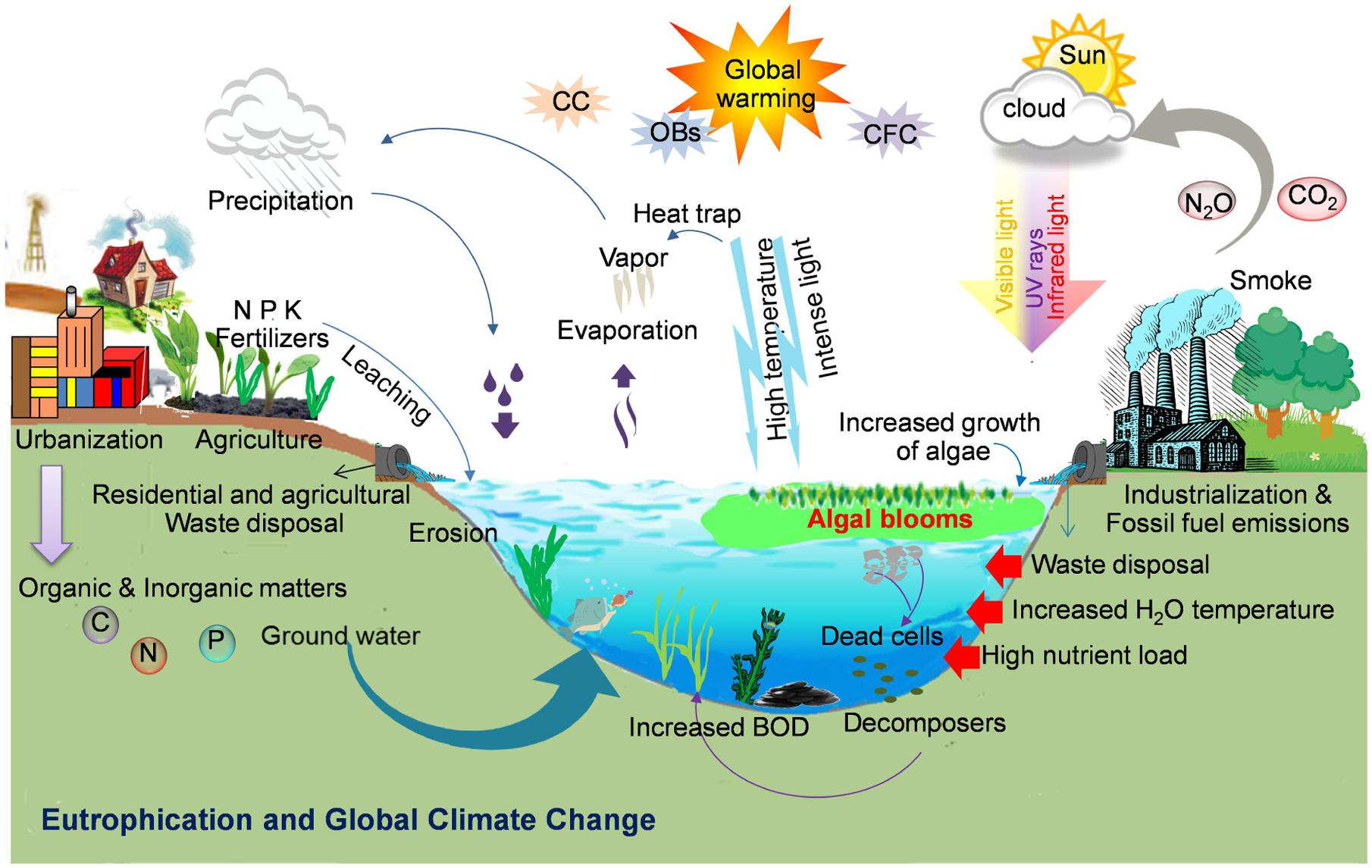
FIGURE 2. Formation of cyanobacterial blooms: Schematic illustration showing the key factors such as anthropogenic eutrophication, global climate change such as increased temperature and light or global warming due to an increase in ozone depleting substances (e.g., CO2, N2O, etc.), and other biotic and abiotic factors responsible for the worldwide bloom incidence (Illustration by R. P. Rastogi).
Global climate change followed by changes in air/water temperature gradients, as well as increased nutrient precipitation can affect the cyanobacterial bloom formation and production of different cyanotoxins (Kanoshina et al., 2003; Paerl and Huisman, 2009; El-Shehawy et al., 2012; Paerl and Paul, 2012). Several environmental factors related to the dynamics of the abundance of toxic cyanobacterial bloom formation have been verified (Joung et al., 2011; Neilan et al., 2013). Warm and calm weather and low turbulence can enhance the formation of cyanobacterial blooms (Paerl and Huisman, 2008). Increased emission of ozone depleting substances (ODSs), due to huge burning of fossil-fuels and concomitant changes in air temperature, may promote the water cyanobacterial growth. As a result of climate change, the frequent droughts in summer as well as flash-flooding may lead to abandoned nutrient discharges from urban areas to unloading water bodies such as ponds, lakes, ditches, and estuaries with the consequence of the augmentation of toxic blooms and the increase of the BOD of a water reservoir (Whitehead et al., 2009). Nitrogen limitation under drought condition may cause a shift from non-N2-fixing to N2-fixing cyanobacteria leading to an increase in biologically available nitrogen and a subsequent production of cyanotoxins (Posch et al., 2012). The increased salination due to summer droughts, rising sea levels, wind flow, and common practices of the use of freshwater for agricultural irrigation, all have led to the origin and existence of several salt tolerant freshwater toxic cyanobacteria as evidenced by an increased number of blooms in brackish waters (Kanoshina et al., 2003; Paerl and Huisman, 2009). Under increased temperature and low wind mixing, the water column becomes stagnant and a large number of buoyant cyanobacteria move upward at the water surface causing dense surface blooms to fulfill their photosynthetic needs (Huisman et al., 2004; Paerl and Huisman, 2008, 2009; Figure 2). It has been established that dense cyanobacterial blooms require excessive CO2 to support their photosynthetic growth (Paerl and Huisman, 2009). Furthermore, global climate change due to anthropogenically released ODS and increased atmospheric CO2 levels can minimize carbon limitation of photosynthetic growth leading to increased algal biomass productions in the water reservoirs (Paerl and Huisman, 2009). Moreover, increased CO2 levels may increase the problems associated with the harmful cyanobacteria in eutrophic lakes (Sandrini et al., 2015). Recently, Verspagen et al. (2014) reported that rising CO2 levels may result in a marked intensification of phytoplankton blooms in eutrophic and hypertrophic waters. Climate change, which is predicted to lead the changes in rainfall patterns along with an increase in temperature may also influence the occurrence and severity of toxic cyanobacterial blooms due to a significant impact on inland water resources (Reichwaldt and Ghadouani, 2012). It has been suggested that UV-B radiation may significantly influence strain composition of cyanobacterial blooms in favor of microcystin (MC) producers (Ding et al., 2013). Several species/strains of bloom forming cyanobacteria produce different toxic peptides and alkaloids (Table 1), which are a major threat to the safe drinking water and pose a serious threat to the global environmental and human health (Kaplan et al., 2012; Rastogi et al., 2014). Until now, a number of views have been given for world-wide occurrence of cyanobacterial blooms (Paerl and Huisman, 2009; Paerl and Paul, 2012; Neilan et al., 2013; Rastogi et al., 2014); however, the exact mechanisms and the role of different environmental factors regulating the bloom dynamics are disputable and yet to be understood.
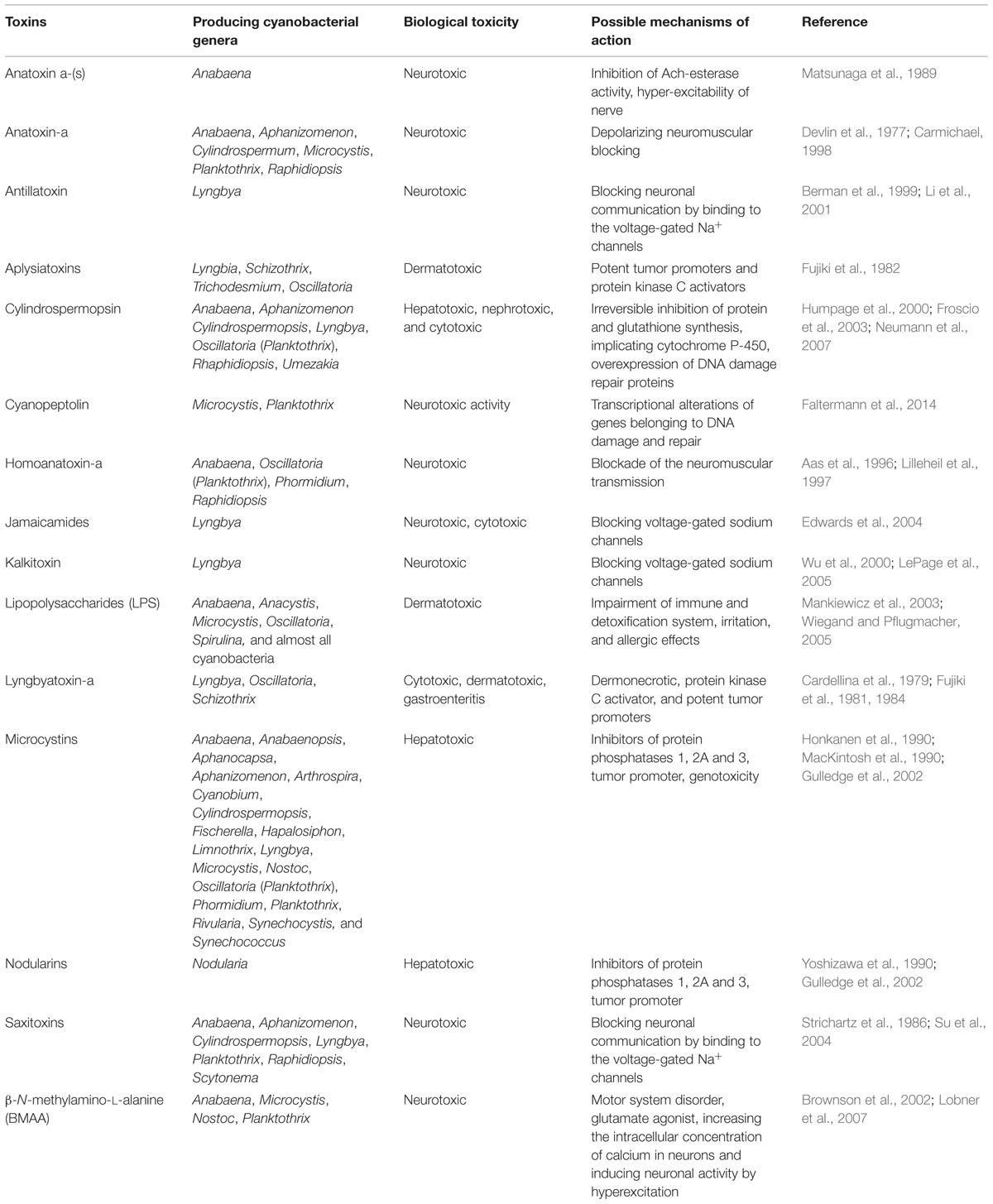
TABLE 1. Some common cyanotoxins found in different cyanobacteria and their possible toxicity and mode of actions.
Our understanding of the responses of various environmental factors associated with climate change and their impact on marine/freshwater ecosystems is based on several experimental and/or inferential data. From the above discussions, it is clear that the appearance of a cyanobacterial bloom is the consequence of several coherent signals. It is utmost important to unravel the specific effects of nutrient enrichment and other global climate change on our aquatic ecosystem, and to establish the facts on how the structure and function of an ecosystem can be maintained. Moreover, if the existing level of anthropogenically induced nutrient loading in the water bodies and environmental warming continues, multiple-fold increase in algal bloom followed by contamination of our aquatic ecosystem by several toxic substances is expected in future. Henceforth, most conceptual and empirical research on the triggers of cyanobacterial blooms is needed to understand the multifarious set of situations that influence the worldwide incidence of toxic cyanoblooms.
Toxins From Cyanobacteria
Cyanobacteria produce a wide range of toxic secondary compounds causing human and domestic/wildlife intoxication. A number of bloom forming cyanobacteria from diverse habitats have been reported to produce different toxins (Rastogi et al., 2014). Chemically, the cyanotoxins are divided into three main groups, i.e., cyclic peptides (MCs and nodularins), alkaloids (anatoxin-a, anatoxin-a(s), saxitoxins, cylindrospermopsin, aplysiatoxin, lyngbiatoxin-a), and lipopolysaccharides (LPSs; Kaebernick and Neilan, 2001). However, based on biological effects, the cyanobacterial toxins can be classified into five functional groups such as hepatotoxins, neurotoxins, cytotoxins, dermatotoxins, and irritant toxins (Sivonen and Jones, 1999; Codd et al., 2005).
Cyclic Peptides
Among the different cyanobacterial toxins, MCs are the most frequently occurring cyanotoxins in surface as well as drinking water and widely investigated hepatotoxins. MCs are cyclic heptapeptides (Figure 3) produced by several strains of cyanobacteria (Sivonen and Jones, 1999; Krienitz et al., 2002; Izaguirre et al., 2007; Aboal and Puig, 2009; Rastogi et al., 2014; Table 1). Currently, more than 90 variants of MCs are known, all with the general structure cyclo-(D-Ala-X-D-MeAsp-Z-Adda- D-Glu- Mdha), X and Z being variable L-amino acids. On the basis of acute toxicity, microcystin-LR (MC-LR) is considered the most potent hepatotoxin (Funari and Testai, 2008).
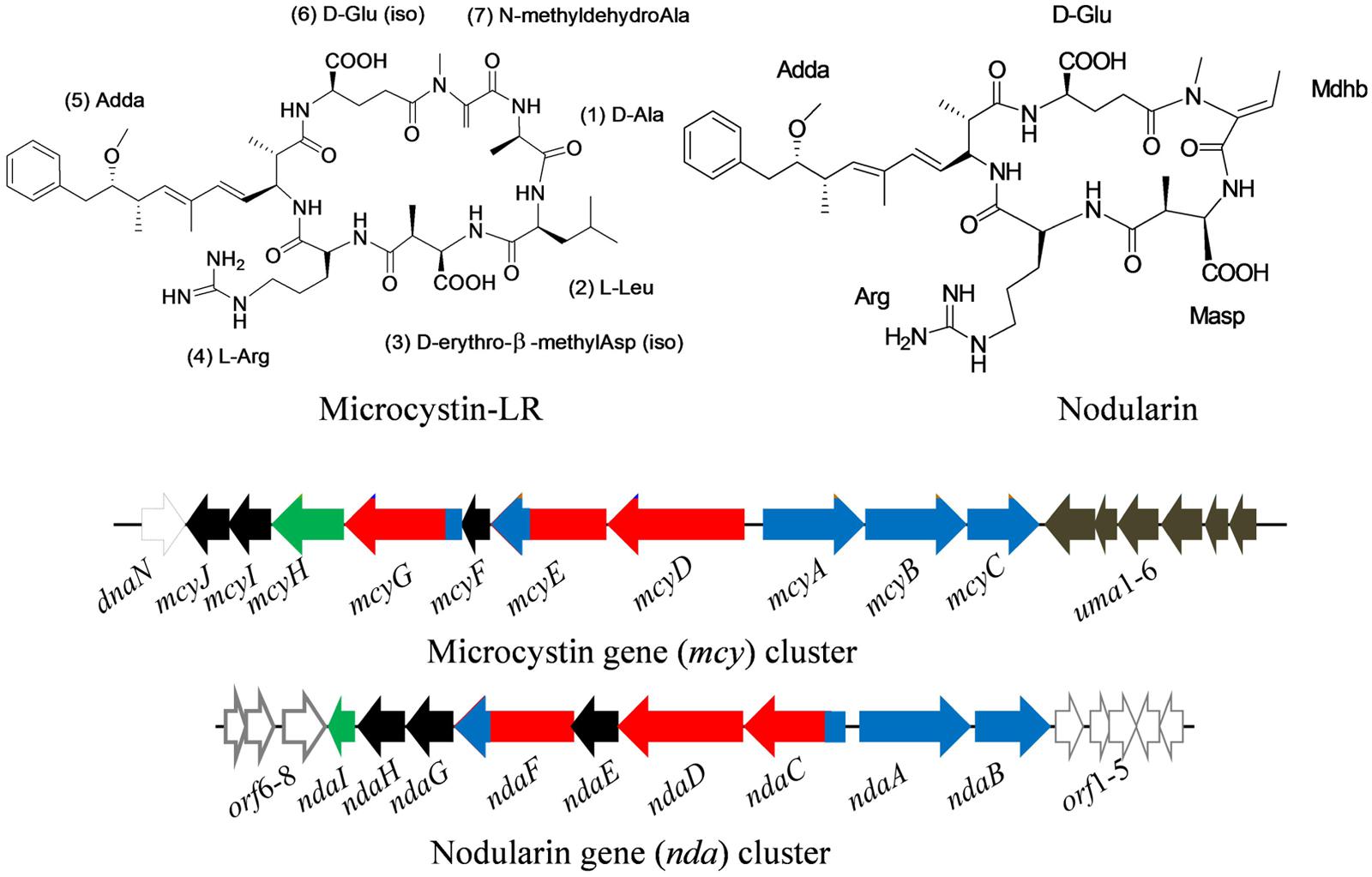
FIGURE 3. Chemical structure of microcystin (MC-LR) and nodularin (NOD), and their biosynthetic gene clusters, mcy and nda in the cyanobacteria Microcystis aeruginosa PCC7806 and Nodularia spumigena NSOR10, respectively. Black – tailoring enzymes, red – polyketide synthases, blue – non-ribosomal peptide synthetases, light black – non-microcystin synthetase, green – ABC transporter (adapted from Tillett et al., 2000; Moffitt and Neilan, 2004; Gehringer et al., 2012; Gehringer and Wannicke, 2014; Gene cluster not drawn to scale).
Microcystin is synthesized non-ribosomally by large multi-enzyme complexes comprising different modules including non-ribosomal peptide synthetases (NRPSs) as well as polyketide synthases (PKSs), and several tailoring enzymes. The gene cluster responsible for MC biosynthesis has been identified in different cyanobacteria (Tillett et al., 2000; Rouhiainen et al., 2004; Christiansen et al., 2008; Gehringer et al., 2012). In the cyanobacterium Microcystis aeruginosa PCC7806, the MC gene clusters spans 55 kb of DNA and is composed of 10 (mcyABCDEFGHIJ) bidirectionally transcribed open reading frames (ORFs) arranged in two divergently transcribed operons, mcyA-C and mcyD-J (Tillett et al., 2000; Figure 3). The assembly of MC begins with the activation of a phenylalanine-derived phenyl propionate starter unit at the NRPS/PKS hybrid enzyme McyG (Hicks et al., 2006). The gene clusters encoding MC biosynthesis sequence from the Microcystis (Tillett et al., 2000), Planktothrix (Christiansen et al., 2008), and Anabaena (Rouhiainen et al., 2004) species revealed that the arrangements of ORFs in the mcy cluster vary among different genera. However, a high sequence similarity between the mcy gene clusters of different genera suggests a common ancestor for MC synthesis (Rantala et al., 2004).
Similar to MCs, cyclic pentapeptide toxic compounds, nodularins (NODs; Figure 3) represent the second group of hepatotoxins produced by the cyanobacteria Nodularia and Nostoc. At present, more than seven variants of NOD have been reported. Both hepatotoxins (MCs and NODs) contain a unique hydrophobic amino acid, Adda (2S,3S,8S,9S-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-deca-4,6-dienoic acid). Chemically, NODs differ from MCs in terms of the absence of two core amino acids and have N-methyldehydrobutyrine (Mdhb) instead of N-methyldehydroalanine (Mdha; Rinehart et al., 1998). Similar to MCs, NODs are also produced non-ribosomally from nda gene clusters by means of NRPS-PKS enzyme systems (Moffitt and Neilan, 2004; Figure 3). In the cyanobacterium Nodularia spumigena NSOR10, the locus of nda gene clusters (48 kb) consists of nine ORFs (ndaA–I) transcribed from a bidirectional regulatory promoter region (Moffitt and Neilan, 2004). Moreover, MCs and NODs show similar biological activity in spite of their different chemical structures. These cyclic peptides inhibit the specific protein serine/threonine phosphatases-1 (PP1) and -2A (PP2A) which are important regulatory enzymes in eukaryotic cells (MacKintosh et al., 1990).
Alkaloids
A number of toxic alkaloids have been found in different cyanobacteria. The alkaloids anatoxin-a (MW = 165 Da) and its homolog homoanatoxin-a (MW = 179 Da) are fast-acting neurotoxins, also known as fast death factors (FDFs). Anatoxin-a (Figure 4) was first isolated from Anabaena flos-aquae and so far has been found in several cyanobacteria such as A. circinalis, A. planctonica, A. spiroides, Aphanizomenon, Cylindrospermum, Planktothrix, and M. aeruginosa (Edwards et al., 1992; Park et al., 1993; Table 1). The alkaloid homoanatoxin-a has a methylene group at C-2 instead of the acetyl group (Figure 4) and structurally resembles anatoxin-a. Homoanatoxin-a has been isolated from the cyanobacteria Oscillatoria (Planktothrix) formosa, Phormidium formosum, Anabaena, and Raphidiopsis mediterranea (Furey et al., 2003; Namikoshi et al., 2003; Watanabe et al., 2003). Another homolog of anatoxin, anatoxin-a(s) (MW 252 Da; Figure 4), isolated from A. flos-aquae and A. lemmermannii, is a potent acetylcholinesterase (AChE) inhibitor (Matsunaga et al., 1989) but more lethal than anatoxin-a (Carmichael et al., 1990; Méjean et al., 2014). It is synthesized in the cell from ornithine via putrescine catalyzed by the enzyme ornithine decarboxylase. Moreover, the partial genome sequencing demonstrated the presence of putative gene cluster (Méjean et al., 2014) encoding the biosynthetic pathway of anatoxin-a and homoanatoxin-a in cyanobacteria such as Oscillatoria PCC 6506 (Méjean et al., 2009) and Anabaena strain 37 (Rantala-Ylinen et al., 2011; Figure 4).
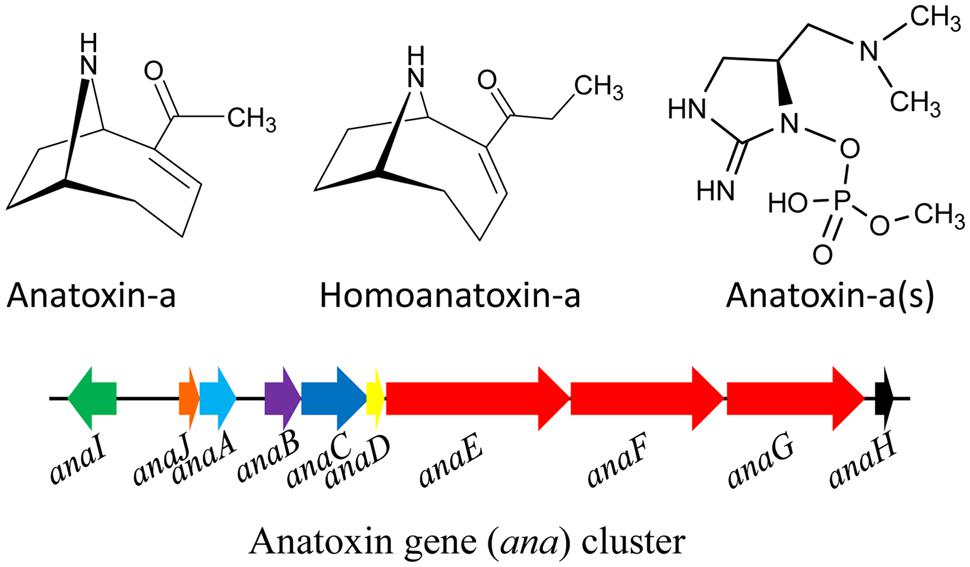
FIGURE 4. Chemical structure of anatoxins and its biosynthetic gene (ana) cluster in the cyanobacterium Oscillatoria sp. PCC6506. Green – transporter, orange – cyclase, light blue – thioesterase, purple – oxidase, blue- adenylation protein, yellow – acyl carrier protein, red – polyketide synthase, black – transposase (adapted from Rantala-Ylinen et al., 2011; Méjean et al., 2014; Gene cluster not drawn to scale).
Saxitoxin and its analogs (e.g., neosaxitoxin; Figure 5) are a group of carbamate alkaloid toxins which all are highly potent neurotoxins. These are tricyclic compounds, consisting of a tetrahydropurine group and two guanidine subunits, commonly called paralytic shellfish poisons (PSPs). Currently, about 27 variants of saxitoxins have been found in different cyanobacteria such as Aphanizomenon, Anabaena flos-aquae, Anabaena circinalis, Lyngbya wollei, and Cylindrospermopsis raciborskii (Table 1). Regulation of saxitoxin biosynthetic pathway and characterization of some enzymes involved are not well-studied (Soto-Liebe et al., 2010). However, it has been postulated that biosynthesis of saxitoxin depends on the multifunctional PKS enzyme, SxtA (Kellmann et al., 2008). The saxitoxin biosynthetic gene cluster (25.7–36 kb) includes 33 genes, reported in cyanobacteria such as Cylindrospermopsis raciborskii (strain T3), Anabaena circinalis (strain AWQC131C), Aphanizomenon strain NH-5, Lyngbya wollei, and Raphidiopsis brookii (strain D9; Kellmann et al., 2008; Mihali et al., 2009, 2011; Soto-Liebe et al., 2010; Stucken et al., 2010; Neilan et al., 2013; Figure 5). The positions of genes encoding biosynthetic enzymes, transporters, and regulatory proteins within the cluster differ among the different cyanobacterial strains dicsussed above. Moreover, the toxic profile expressed in different strains is determined by the position and presence, or absence, of specific genes in the respective clusters.
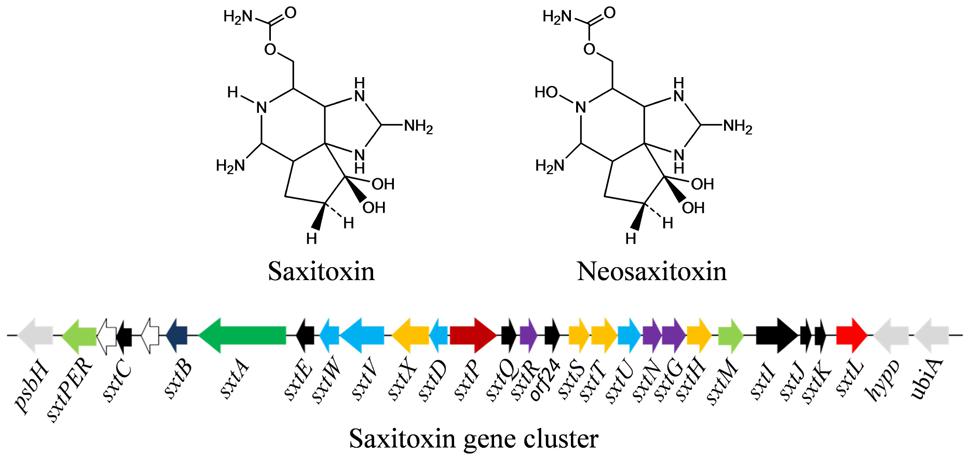
FIGURE 5. Chemical structure of saxitoxin and its biosynthetic gene cluster in the cyanobacterium Aphanizomenon sp. NH-5. Light green – transporter, white – transposase, black – unknown, dark blue – cyclase, green – polyketide synthase, light blue – oxido-reductase, orange – hydroxylase, dark red – putative regulator, orange – transferase, red – hydrolase (for details, see Kellmann et al., 2008; Mihali et al., 2009; Gene cluster not drawn to scale).
The cyanotoxin cylindrospermopsin (CYN: MW 415 Da) is a polyketide-alkaloid having a tricyclic guanidine moiety and sulfate groups (Figure 6). Presently, some analogs of CYN such as deoxy-cylindrospermopsin, demethoxy-cylindrospermopsin and 7-epicylindrospermopsin have been identified in the cyanobacteria C. raciborskii (Norris et al., 1999) and Aphanizomenon ovalisporum (Banker et al., 2000). The CYN variant 7-epicylindrospermopsin differs due to the orientation of the hydroxyl group close to the uracil moiety (Banker et al., 2000), and the other variant deoxy-cylindrospermopsin is characterized by a missing oxygen atom related to the initial hydroxyl group close to uracil moiety. Moreover, a number of cyanobacteria such as Cylindrospermopsis raciborskii, Aphanizomenon ovalisporum, Aphanizomenon flos-aquae, Anabaena lapponica, Anabaena bergii, Lyngbya wollei, Umezakia natans, Raphidiopsis curvata, and Oscillatoria (Planktothrix) have been reported to produce CYN and its analogs (Ohtani et al., 1992; Harada et al., 1994; Banker et al., 1997; Preussel et al., 2006; Spoof et al., 2006; Seifert et al., 2007; Mazmouz et al., 2010). McGregor et al. (2011) reported the presence of the cyanotoxins CYN and deoxy-CYN from the cyanobacterium Raphidiopsis mediterranea FSS1-150/1 of a eutrophic reservoir in Queensland, Australia. CYN shows hepatotoxic, nephrotoxic, and cytotoxic effects and is a potential carcinogen owing to the inhibition of glutathione, cytochrome P450 and protein synthesis (Humpage et al., 2000; Froscio et al., 2003; Neumann et al., 2007). The gene cluster (cyr) encoding the enzymes of the CYN biosynthesis (Figure 6) has been reported to be present in several cyanobacteria such as C. raciborskii (Mihali et al., 2008; Stucken et al., 2010; Jiang et al., 2012), Aphanizomenon strain 10E6 (Stuken and Jakobsen, 2010), and Oscillatoria PCC 6506 (Mazmouz et al., 2010). The arrangements of genes and flanking regions differ across genera; however, all the gene clusters are highly conserved with respect to the nucleotide sequence of orthologous genes (Neilan et al., 2013). In case of the cyanobacterium C. raciborskii AWT205, the cyr gene cluster (42 kb) encodes 15 ORFs (cyrA-O). The biosynthesis of CYN is initiated by an amidinotransferase and completed by NRPS-PKS-type enzymes in combination with tailoring enzymes (Muenchhoff et al., 2010). As stated above, the gene cluster for CYN biosynthesis has been sequenced from several cyanobacteria; however, few studies have been conducted on its transcriptional organization and promoter structure (Stuken and Jakobsen, 2010).
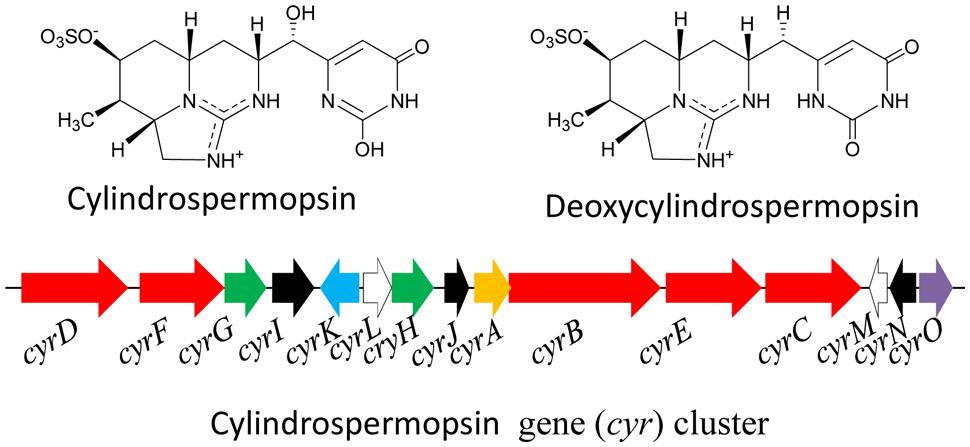
FIGURE 6. Chemical structure of cylindrospermopsin and its biosynthetic gene (cyr) cluster in the cyanobacterium Cylindrospermopsis raciborskii AWT205. Red – PKS/NRPS, green – uracil ring, black – tailoring, blue – transport, white – transposase, orange – amidinotransferase, purple – regulator (Adapted from Mihali et al., 2008).
Lipopolysaccharides
The endotoxins LPSs consist of an internal acylated glycolipid (lipid-A), core domain (an oligosaccharide) and an outer polysaccharide (O-antigen) chain (Raetz and Whitfield, 2002). In general, the fatty acid component (lipid-A) of LPS is responsible for the toxic actions such as irritant and allergenic responses in human and animal tissues (Mankiewicz et al., 2003). The LPSs present in cyanobacteria differ from those in enteric bacteria by having a larger variety of long chain unsaturated fatty acids and hydroxy fatty acids and the lack of phosphate. Moreover, there is substantial diversity of LPSs composition among the cyanobacteria, although variations are basically related to phylogeny. Different genera of cyanobacteria have distinct LPSs compositions conserved within the particular genus (Sivonen and Jones, 1999). Several cyanobacteria such as Anacystis nidulans, Microcystis, Anabaena, Spirulina, and Oscillatoria all have been reported to produce LPS toxin (Smith et al., 2008; Bláhová et al., 2013). The structure of the lipid-A subunit in the cyanobacterial LPS molecule has not been clearly identified, and furthermore, the exact mechanism of LPS toxicity produced by cyanobacteria is still unknown.
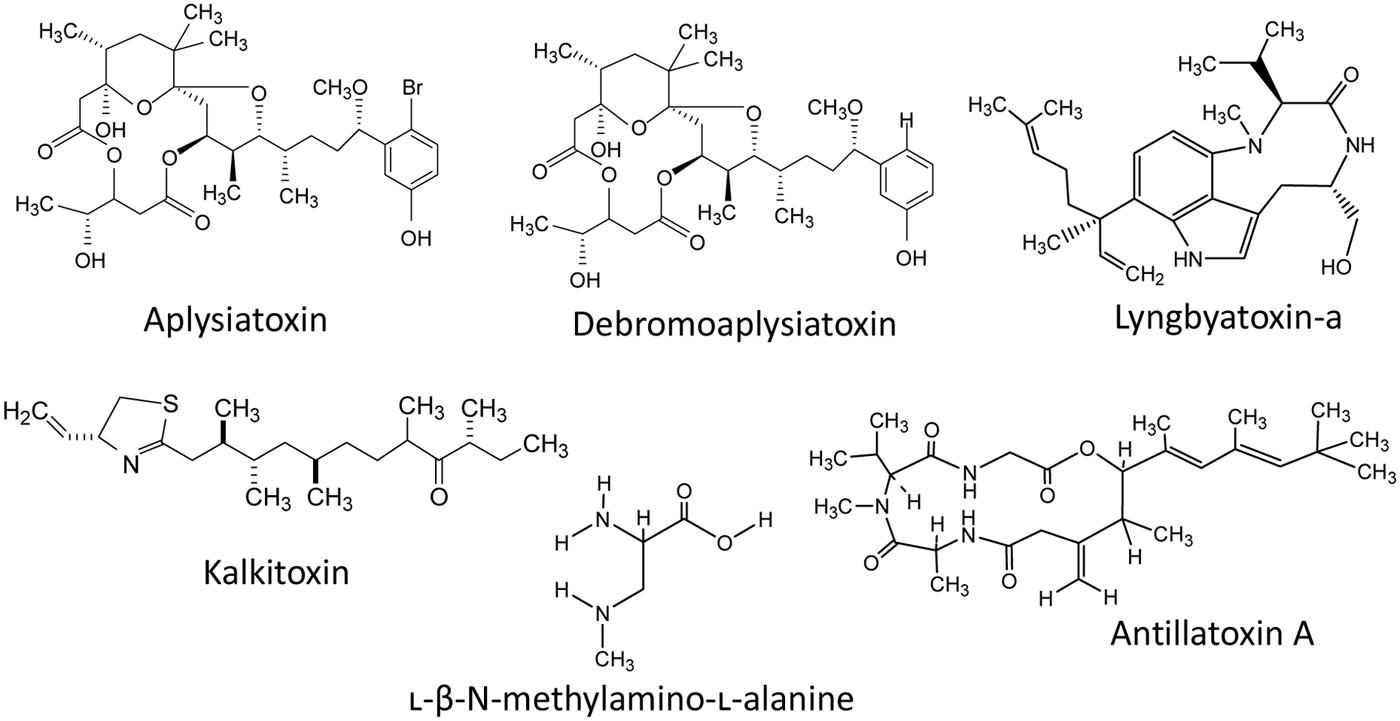
Besides the above mentioned cyanotoxins, a number of toxins such as aplysiatoxin, kalkitoxin, antillatoxin, lyngbyatoxins, cyanopeptolin, aurilides, and jamaicamides have been reported to be present in different cyanobacteria in fresh and/or marine water habitats (Figure 7). A phenolic bislactone alkaloid aplysiatoxin has been reported from several cyanobacteria such as Lyngbia majuscula, Schizothrix calcicola, Trichodesmium erythraeum, and Oscillatoria nigroviridis (Mynderse et al., 1977; Gupta et al., 2014). Aplysiatoxin and debromoaplysiatoxin (Figure 7) are potent tumor promoters and protein kinase C activators and show signs of several lethal effects. Moreover, an analog of the tumor-promoting aplysiatoxin has been reported as an antineoplastic agent rather than a tumor-promoting substance (Nakagawa et al., 2009). Recently, the analogs of aplysiatoxin debromoaplysiatoxin and anhydrodebromoaplysiatoxin, as well as two new analogs, 3-methoxyaplysiatoxin and 3-methoxydebromoaplysiatoxin have been reported from the marine cyanobacterium Trichodesmium erythraeum (Gupta et al., 2014). The alkaloid lyngbyatoxin, a prenylated cyclic dipeptide compound, was isolated from Lyngbya majuscula (Taylor et al., 2014) and has several similarities with aplysiatoxin in its mechanism of toxicity and both are potent tumor promoters. Kalkitoxin (Figure 7) is a lipopeptide neurotoxin produced by some species of cyanobacteria such as L. majuscula (Berman et al., 1999). The antillatoxin is an ichthyotoxic cyclic depsipeptide isolated from L. majuscula (Orjala et al., 1995). A number of bioactive peptides such as microviridins, microginins, cyanopeptolides, and β-N-methylamino-L-alanine (BMAA; Figure 7) have also been reported from diverse cyanobacteria, but their toxicological profiles and impacts on the environment as well as human health are not known (Downing et al., 2014). Moreover, the cyanobacterial neurotoxin, BMAA has been suggested to function as a causative agent for certain neurodegenerative diseases (Lobner et al., 2007). The compound curacin-A, isolated from L. majuscula (Gerwick et al., 1994), exhibited potent anti-proliferative and cytotoxic activity against colon, renal, and breast cancer derived cell lines (Verdier-Pinard et al., 1998). A cyanobacterial toxin cyanopeptolin (CP1020) produced by Microcystis and Planktothrix strains was found to cause transcriptional alterations of genes involved in DNA damage and repair (Faltermann et al., 2014). Recently, two new cyanobacterial peptides named micropeptins 1106 and 1120 were reported from cyanobacterial blooms in North Carolina’s Cape Fear River. However, their biological activities have not yet been determined (Isaacs et al., 2014). Moreover, several studies indicate the presence of several additional, still unidentified and not characterized biotoxins in cyanobacterial blooms.
Ecological Health Impacts of Cyanotoxins
The increased incidence of toxic cyanobacterial blooms is posing potential risks to aquatic ecosystem as well as human and animal health. Cyanotoxins may cause several harmful effects on humans or animals either through direct contact or by means of intake of untreated contaminated water and food (Miller et al., 2010; Papadimitriou et al., 2012; Rastogi et al., 2014; Sukenik et al., 2015). Aquatic organisms may be affected either through direct ingestion of toxic cyanobacterial cells or through contact with cyanotoxins. It has been established that intake of contaminated water or food is a key route for cyanotoxin intoxication (Zhang et al., 2009; Miller et al., 2010). Several secondary compounds have been reported to have their toxic effects on different organisms ranging from plant to animals. In the subsequent section we have focused on the adverse toxic effects of some common cyanotoxins on aquatic/wild animals and humans.
The cyanotoxin MCs are well-known for their toxic effects. MCs can affect the cellular system through disorganization of cytoskeleton, cell proliferation, genome damage, inhibition of enzyme activity, imprecise mitotic cell division, loss of membrane integrity, oxidative stress, and lipid peroxidation (Rastogi et al., 2014). To know the detailed mechanisms or mode of action of MCs, readers are referred to the recent review by Rastogi et al. (2014). MCs act by blocking protein PP1 and -2A, causing toxicity at the hepatic level. It has been demonstrated that MC-LR can induce reproductive (Chen et al., 2011; Zhou et al., 2012) as well as cardio-toxicity in animals (Qiu et al., 2009). MC-LR was found to cause normocyte anemia and the bone marrow injury, and also affected the immune system of rabbits (Zhang et al., 2011; Yuan et al., 2012). Moreover, a number of fatal poisonings of MCs regarding the health risk of domestic and wild animals, birds, fish, and several other aquatic as well as terrestrial organisms have been reported worldwide (Stewart et al., 2008; Rastogi et al., 2014). The mass mortalities of Lesser Flamingos were reported at Lake Bogoria, Kenya due to MC intoxication (Krienitz et al., 2003). A new episode of cyanotoxin (MC-LR, -YR, and -RR) intoxication and mass mortalities of Lesser Flamingos (Phoeniconaias minor Geoffroy) have also been reported at Lake Manyara in Tanzania (Nonga et al., 2011). At least 6,000 birds belonging to 47 species, including endangered species such as the marbled teal (Marmaronetta angustirostris) and white-headed duck (Oxyura leucocephala), died due to MC-LR intoxication at the Doñana National Park, Spain (Lopez-Rodas et al., 2008).
Despite numerous reports of cyanotoxins impact on the aquatic organisms and wild or domestic animals, the epidemiological facts for cyanotoxins intoxication in humans are very limited (Rastogi et al., 2014). Recent studies have established the cytotoxic and genotoxic potentials of various cyanotoxins including MCs (Žegura et al., 2011). The use of untreated water contaminated with cyanobacterial blooms and MCs resulted in normocytic anemia (Pouria et al., 1998), liver failure and several other symptoms such as nausea, vomiting, and acute liver damage leading to human death in a hemodialysis center in Caruaru, Brazil (Pouria et al., 1998; Hilborn et al., 2007). The use of MC-contaminated water can be a potential risk factor for liver and colorectal cancer among humans (Lun et al., 2002; Hernández et al., 2009). Moreover, MCs may cause hepatotoxicity and neurotoxicity, kidney impairment, allergies and eye, ear and skin irritation, and certain gastrointestinal disorders such as nausea/vomiting and diarrhea in humans (Torokne et al., 2001; Pilotto et al., 2004; Codd et al., 2005).
As stated above, the cyanotoxin NODs have chemical structure as well as mechanisms of action similar to those of MCs (Yoshizawa et al., 1990); however, NODs have not been studied as extensively as MCs (Funari and Testai, 2008). NODs are a potent inhibitor of protein phosphatase 1 and 2A (Ohta et al., 1994) and show accumulative toxicity and tumor formation (Ohta et al., 1994; Sueoka et al., 1997; Song et al., 1999). The toxic effects of NODs have also been investigated in fish (Sotton et al., 2015). In the flatfish Platichthys flesus, NODs induced oxidative stress as indicated by a decrease of GST and CAT activity resulting in increased vulnerability of the cells to reactive oxygen species (ROS; Persson et al., 2009). NOD can also induce apoptosis and hyperphosphorylation of signaling proteins in cultured rat hepatocytes (Ufelmann and Schrenk, 2015). Nevertheless, not much toxicological data are available for NODs carcinogenicity in humans.
A cytotoxic alkaloid CYN can irreversibly inhibit the biosynthesis of protein and glutathione leading to cell death (Ohtani et al., 1992; Terao et al., 1994; Froscio et al., 2003). A Cylindrospermopsis bloom episode was found to cause cattle mortalities and human poisonings in north Queensland (Saker et al., 1999; Griffiths and Saker, 2003). Moreover, a number of disorders such as damage to liver, kidney, thymus, and heart, as well as hepatic and renal toxicity were observed in mice (Terao et al., 1994; Falconer et al., 1999; Bernard et al., 2003; Froscio et al., 2003). CYN may induce DNA strand breaks and possibly disrupt the kinetochore spindle, leading to chromosome loss, specifying its clastogenic and aneugenic action (Humpage et al., 2000). In primary rat hepatocytes, CYN has been shown to inhibit protein and glutathione synthesis and induce apoptosis (López-Alonso et al., 2013). Recently, Huguet et al. (2014) studied the effects of CYN on human intestinal Caco-2 cells and reported that CYN can modulate different biological functions by overexpressing the genes encoding proteins involved in DNA damage repair and transcription including modifications of nucleosomal histones. It has been shown that CYN may cause a decrease in glutathione synthesis (Runnegar et al., 1994) and induce oxidative stress in fish (Guzmán-Guillén et al., 2013a,b). Indeed, CYN can accumulate in various organs of fish, leading to deleterious effects on their normal physiology and biochemistry (Sotton et al., 2015). CYN may interfere with the basic functions of fish phagocytic cells and as a consequence, influence the fish immunity (Sieroslawska and Rymuszka, 2015).
A number of neurotoxic alkaloids from cyanobacteria have been reported, exerting their action on the neuromuscular system by blocking skeletal and respiratory muscles leading to respiratory failure. The cyanotoxins such as anatoxins, saxitoxins, antillatoxin, kalkitoxin, and jamaicamide are major groups of neurotoxic compounds (Aráoz et al., 2010). It has been established that anatoxin-a is a potent depolarizing neuromuscular blocking agent which acts by binding to nicotinic receptors for acetylcholine in the central nervous system (CNS), peripheral nervous system (PNS) and in neuromuscular junctions (Carmichael, 1998). Several studies regarding the mechanisms of anatoxins toxicity were performed in mice using the sub-lethal or lethal doses of anatoxin-a. Anatoxin-a, well-known as a “Very Fast Death Factor,” can cause contraction, muscular paralysis, and respiratory arrest leading to death of mice in a very short time after intraperitoneal injection (i.p. mouse LD50: 250 to 375 μg/kg; Devlin et al., 1977). Anatoxin-a can impair blood pressure, heart rate and gas exchange triggering hypoxia, respiratory arrest and severe acidosis leading to death of the animals (Adeymo and Sirén, 1992). The toxicological properties of homoanatoxin-a are more or less identical to those of anatoxin-a (Namikoshi et al., 2003). The neurotoxic alkaloid saxitoxins are considered the most toxic compounds. The mode of action of all analogs of saxitoxins is more or less similar; however, they differ in toxicity (Funari and Testai, 2008). Saxitoxins may block voltage-gated sodium channels in nerve cells and discontinue the entry of sodium flow, preventing the generation of a proper action potential or electrical transmission in nerves and muscle fibers leading to paralysis of muscles and death by respiratory arrest in mammals (Strichartz et al., 1986; Su et al., 2004; Bricelj et al., 2005). Another neurotoxic cyanotoxin antillatoxin is a novel ichthyotoxic (LC50 = 0.1 μM) cyclic lipopeptide isolated from the marine cyanobacterium Lyngbya majuscula (Orjala et al., 1995). Antillatoxin-A prompted a rapid neuronal death in cerebellar granule cell cultures (LC50 = 0.18 μM; Berman et al., 1999). Voltage-gated sodium channels were shown as the main molecular target of antillatoxin (Li et al., 2001). The neurotoxic compound kalkitoxin isolated from L. majuscula is a thiazoline-containing lipopeptide compound (Wu et al., 2000). Lyngbyatoxin-A, a cyclic dipeptide found in L. majuscula, appears to have been responsible for a severe oral and gastrointestinal inflammation suffered by a person who accidentally ingested this cyanobacterium (Sims and Zandee Van Rillaud, 1981). Kalkitoxin was shown ichthyotoxic to the goldfish Carassius auratus and toxic to the aquatic crustacean brine shrimp (Artemia salina) with an LC50 700 and 170 nM, respectively (Wu et al., 2000). Kalkitoxin may also block voltage-gated sodium channels (LePage et al., 2005). The neurotoxic amino acid BMAA acts in mammals as a glutamate agonist (Corbel et al., 2014). BMAA increases the intracellular concentration of calcium in neurons and induces neuronal activity by hyperexcitation (Brownson et al., 2002).
The endotoxic LPSs are known to cause fever in mammals and are involved in septic shock syndrome and liver injury (Choi and Kim, 1998). LPS can impair the immune system and also affect the detoxification system of diverse organisms (Wiegand and Pflugmacher, 2005). Until now, very little is known about the LPS intoxication and its toxicity is assumed to be associated with the host-mediated factors (Stewart et al., 2006a,b). More extensive research is needed to clarify a definite toxicity mechanism of LPS. Overall, it is no doubt that the acute effects of several cyanotoxins represent the major concern for ecological health impacts.
Cyanoblooms and Cyanotoxins: Mitigation Strategies
The increased incidence of toxic cyanobacterial blooms (cyanoblooms) worldwide and their potential health risks have generated tremendous concern for dynamic management of toxic cyanoblooms. The economic cost of freshwater blooms in the United States was estimated to be about 2.2–4.6 billion dollars/annum (Dodds et al., 2009). Henceforth, advanced approaches or development of a new technology is needed to terminate or prevent/suppress the harmful cyanobacterial blooms for environmental sustainability and economic vitality (Hudnell, 2008, 2010; Srivastava et al., 2013; Harris et al., 2014; Koreivienë et al., 2014). Several factors boosting the incidence of harmful cyanobacterial blooms, such as nutrient input, wind velocity, sediment deposition, reduced water flow, increased salinity and temperature gradients, global warming and drought can be regulated to a certain extent to eliminate or minimize the bloom incidence. The approaches implemented for bloom suppression should be environmentally sustainable without adversely influencing the aquatic ecosystems. A number of strategies or approaches such as chemical, physical, biological, and other cognizance approaches came into consideration for mitigating the harmful cyanobacterial bloom incidences.
Chemical Approaches
Cyanoblooms can be controlled to a certain extent using some chemicals such as algicides, inhibitors or flocculants; however, use of these chemicals can inevitably recontaminate water bodies (Murray-Gulde et al., 2002; Van Hullebusch et al., 2002; Jančula and Maršálek, 2011). The use of certain pigments (aquashade) can reduce the amount of light availability, and inhibit the growth of harmful algae; however, this approach may not be effective due to growth inhibition of other beneficial microalgae, thereby undesirably influencing the aquatic ecosystems (Spencer, 1984). The use of some algicides has been reported to decline the bloom formation. The natural product cyanobacterin has been shown to be toxic to most cyanobacteria at a concentration of approximately 5 μM (Gleason and Baxa, 1986). Many biologically derived (but non-antibiotic) bioactive substances are known to inhibit the growth of aquatic bloom-forming cyanobacteria (Shao et al., 2013). Recently, Dai et al. (2012) have shown the fast removal (up to 98.99%) of MC-LR by a low-cytotoxic microgel- Fe(III) complex. Preoxidation with chlorine dioxide followed by flocculation and settling was found effective in removing cyanobacterial blooms and MCs (Bogialli et al., 2013). The use of aluminum salts can be used as algicides for nuisance algae and cyanobacteria control (Lelkova et al., 2008). The use of slaked lime [Ca(OH)2] or calcite (CaCO3) has also been reported to remove the algal communities, including cyanobacteria (Prepas et al., 2001; Zhang et al., 2001). Aluminum compounds can be used to remove the nutrients from industrial and domestic wastewaters (Auvray et al., 2006; Rodriguez et al., 2008; De Julio et al., 2010). Besides aluminum, several other metals such as iron and copper are used to remove the algal blooms. The salt of copper (CuSO4.5H2O) is widely used as an algicide (Murray-Gulde et al., 2002). The herbicide diuron together with copper sulfate as well as other copper-based compounds have been approved by the United States Environmental Protection Agency (USEPA) for use as algicides in fish production ponds (Schrader et al., 2004). Moreover, the use of synthetic compounds for bloom control has their own limitations, and therefore, a range of natural chemicals (e.g., anthraquinone, nostocarboline, and stilbenes) from diverse organisms have been derived as potent substituents of synthetic algicides (Schrader et al., 2003, 2004; Becher et al., 2005; Mizuno et al., 2008; Table 2). Recently, Jančula and Maršálek (2011) reviewed the availability of different chemical compounds for prevention and management of cyanobacterial blooms.
Physical Approaches
Bloom control by physical methods generally involves mechanical removal techniques or short wavelength radiation treatment to control the incidence of cyanobacteria. The use of new and improved technologies can eliminate industrial/agricultural/ household pollutants to a certain extent to minimize the environmental pollution, including the water pollution by the incidence of harmful algal blooms. Global climate change and rising fresh water demand for multipurpose usage caused a remarkable increase in drought frequency and decreased freshwater flow rates (Paerl and Huisman, 2008; Paul, 2008). However, increasing flow rates and decreasing water residence time can remove fresh water algal blooms of a reservoir even in nutrient-rich conditions (Paerl, 2008). The artificial circulation for increased water flow is reported to suppress the blooms, but it may also cause habitat disturbance (Visser and Ibelings, 1996; Jungo et al., 2001; Huisman et al., 2004; Hudnell et al., 2010). Moreover, a solar powered circulation (SPC) has been designed to create long-distances circulation of the epilimnion (>200 m) to suppress freshwater harmful algal blooms (Hudnell et al., 2010). Data obtained from a case study of nutrient-enriched, source-water reservoirs, revealed the role of SPC in reduction of cyanobacterial peak density by about 82 and 95% during the first and second year of SPC deployment, respectively (Hudnell et al., 2010). Intensity of light and temperature play a significant role in bloom incidence as mentioned above. However, the increase in incidence of light and temperature can hardly be controlled in a large water reservoir, where as it is energy intensive in smaller water bodies. Short wavelength ultraviolet radiation can bring about a rapid degradation of the cyanotoxins MCs (Tsuji et al., 1995; Kaya and Sano, 1998). Moreover, it has been concluded that photosensitized processes may play an important role in the photochemical transformation of cyanotoxins (e.g., MC-LR; cylindrospermopsin) in the natural water (Lawton et al., 1999; Song et al., 2007; Wörmer et al., 2010; He et al., 2012). Simulated waterfalls or fountains may also be effective to control the cyanobacterial blooms in smaller water bodies; however, it requires electric-grid power constantly (Clevely and Wooster, 2007). The use of hydraulic jet cavitation may be a good approach to cyanobacterial water-bloom management (Jančula et al., 2014). Moreover, cavitation treatment can disintegrate gas vesicles of cyanobacterial cells, and can remove up to 99% cyanobacteria growing in a lake, ponds or reservoirs (Jančula et al., 2014).
Biological Approaches
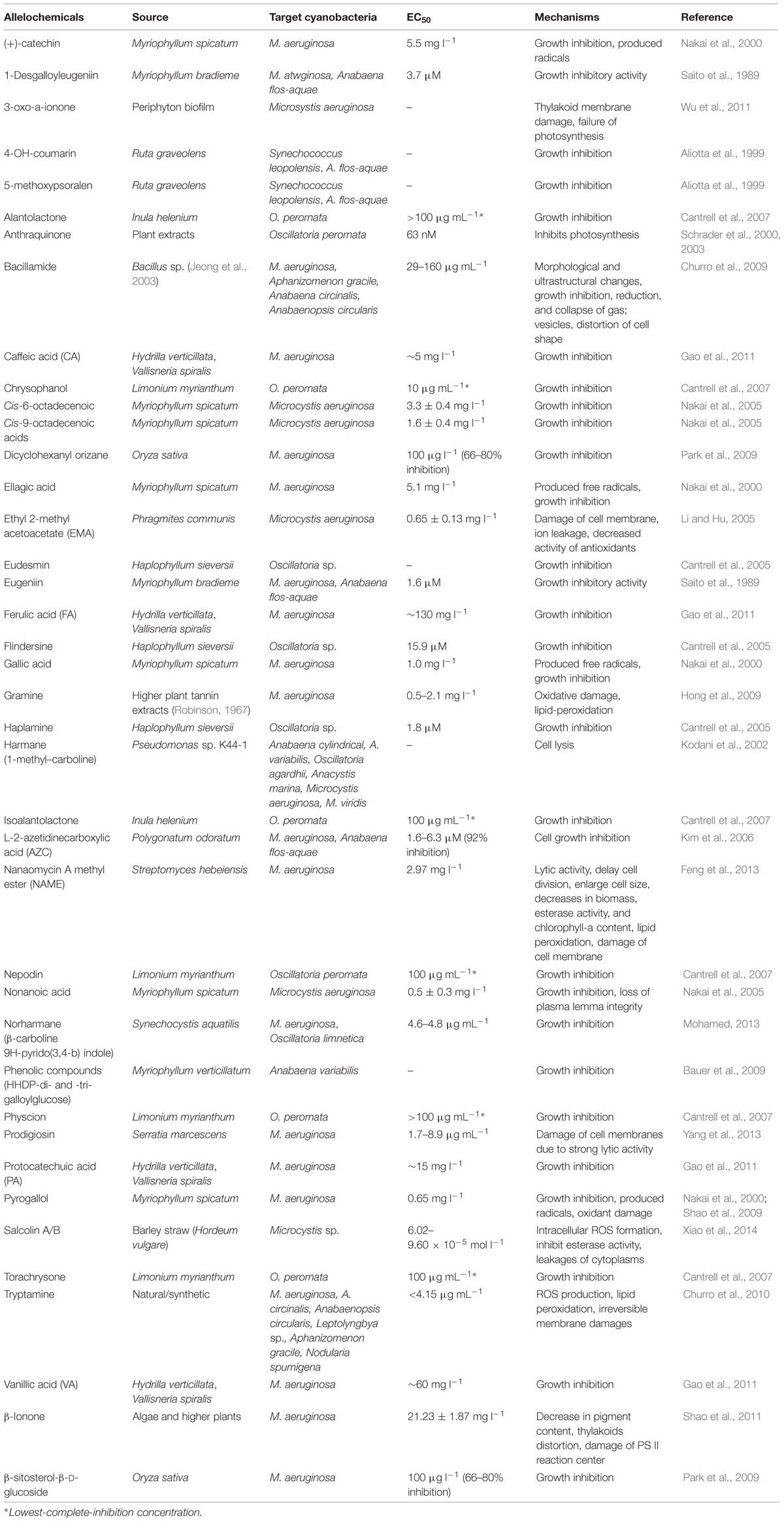
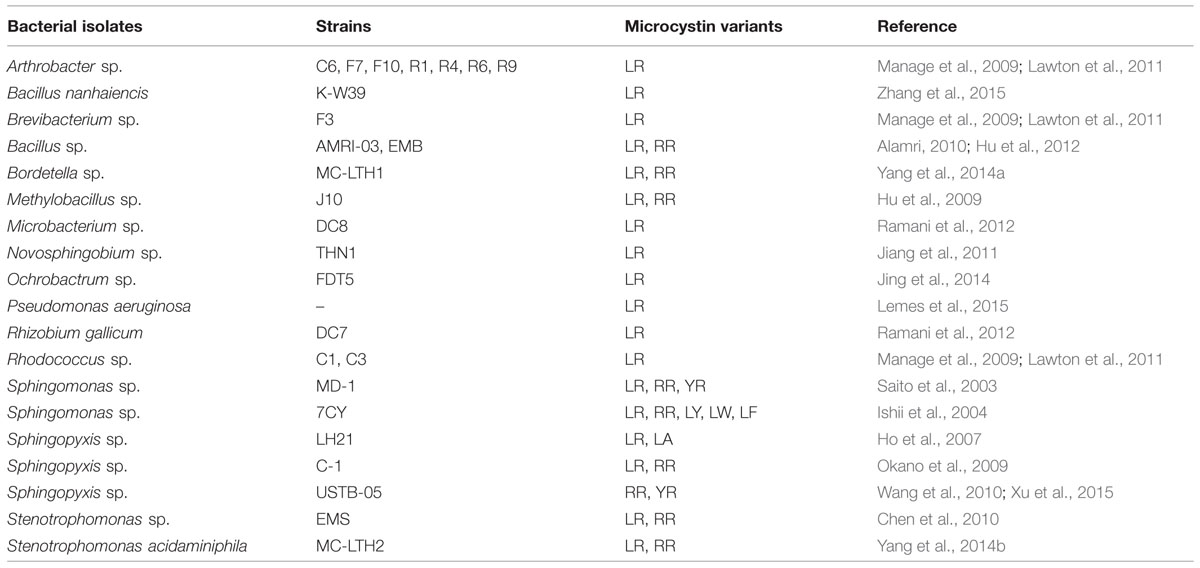
Control of cyanoblooms through biological mechanisms such as regulation of nutrient uptake or availability, alteration of normal physiology (such as a decrease in photosynthetic pigment), and/or direct feeding of cyanobacterial biomass by some aquatic organisms may be promising ways of ecological restoration (Bond and Lake, 2003; Qin et al., 2006; Zhang et al., 2008; Zhang et al., 2012, 2014). The gastropod Radix swinhoei can ingest cyanobacteria and survive well without loss in fecundity in the water reservoirs with cyanobacterial blooms (Zhang et al., 2012). The combined use of snails (R. swinhoei) and a submerged plant (Potamogeton lucens) in eutrophic waters can eliminate cyanobacterial bloom by minimizing the eutrophication; however, this method is under the preliminary stage due to the lack of the field study (Zhang et al., 2014). Occurrence and growth of aquatic plants are considered good candidates for limiting algal growth as the aquatic plants directly compete with algae for nutrients, light and space (Qiu et al., 2001; Wang et al., 2009). Some aquatic plants release different allelochemicals that can inhibit the growth of cyanobacteria and other phytoplanktons (Nakai et al., 2000; Körner and Nicklisch, 2002). Biodegradation using different species/strains of bacteria (Table 3) and other organisms may be the most efficient process to control the fate of some cyanotoxins in natural waters (Zhang et al., 2008; Manage et al., 2009; Lawton et al., 2011; Rastogi et al., 2014).
Research and Management
Development of wastewater research and management program is highly amenable to prevent or control the worldwide incidence of algal blooms and maintaining the ecological integrity and sustainability. Documentation of different environmental factors responsible for increased incidence of harmful cyanoblooms is crucial toward the development of demarcated management strategies. Moreover, interactive management of anthropogenic over nutrient-enrichment and global climate change is a major task for ensuring the protection and sustainability of aquatic ecosystems (Paerl et al., 2011a,b). The availability of phosphorus plays an important role in the growth of cyanobacteria and other microalgae or phytoplanktons (Schindler et al., 2008); henceforth, controlled input of phosphorus to the water reservoir may be an effective management strategy for restoration of aquatic ecosystems. In order to minimize the bloom boosting organic or inorganic nutrients coming from common practices such as excessive use of fertilizers (e.g., NPK) and detergents, prior wastewater treatment may be needed to reduce the incidence of cyanobacterial blooms (Conley et al., 2009; Paerl et al., 2011a; Jacquet et al., 2014). The modeling of different water bodies at risk of toxic blooms may be a good approach to develop a proactive algal bloom monitoring and management strategies (Tyler et al., 2009; Coad et al., 2014). Moreover, the fundamental research and quantitative ecological awareness toward the bloom incidence can be a supportive tool guiding large-scale water management against harmful bloom incidence.
Public Awareness Approaches
Public environmental awareness (PEA) is a fundamental approach for the attainment of sustainable environment (Xu et al., 2013; Kirkpatrick et al., 2014). PEA about the incidence and harmful effects of toxic cyanoblooms may be a dynamic approach to eradicate and avoid the blooms and their toxic effects. The edifying approaches will allow people to think about their practices in their day-to-day life, such as unregulated disposal of organic/inorganic domestic wastes in the water reservoir, thereby enhancing the risks of bloom formation. As discussed elsewhere, global climate change may potentially impact the success of cyanobloom incidence. An emphasis on social practice to minimize the bloom formation and intoxication can allow intellectuals actualizing the significant development to control the environmental pollution. A change in the PEA levels in response to the increased incidence of environmental pollution is indispensable for ensuring the effective environmental protection and restoration (Xu et al., 2013). An increase in public awareness regarding the environmental sustainability and ecosystem health can inform the policy or decision makers to develop the strategies or to set-up the environmental protection laws against anthropogenic environmental pollution (such as direct disposal of domestic or industrial waste in open water reservoir such as rivers, ponds, lakes, and catchments). Moreover, various means of environmental protection program should be launched worldwide by the concerned government or non-government organization (NGO) to spread the knowledge about different environmental issues such as harmful cyanobloom incidence (Mikami et al., 1995; Palmer et al., 1998; Li et al., 2008; Várkuti et al., 2008; Xu et al., 2013).
Overall, little is known concerning the formation of cyanoblooms and production of different variants of cyanotoxin in diverse water bodies. Furthermore, each of these strategies mentioned above has their own advantages and limitations, and more extensive collaborative work is needed to control or manage the occurrence of algal blooms worldwide. Since eutrophication is considered as the most immediate environmental consequence of cyanoblooms, the uncontrolled disposal of organic/inorganic nutrients in the water reservoir through agricultural runoff or through industrial and household sewage water must be diminished or even forbidden. The establishment of several dyes or chemical based industries are the source of several blooms forming substances and therefore the government law should strictly be implemented to sanitize unwanted industrial effluents before reaching the water bodies. Furthermore, the eutrophication of water reservoirs must be regularly checked for an increased prevalence of toxin producers mainly in the bloom sensitive areas of subtropical and temperate climates. Severity on global warming is also an important trigger that is likely to create toxic cyanoblooms, therefore a proper environmental management toward increasing global climate change is necessary for sustainability of the pollution-free aquatic ecosystems.
Conclusion and Perspective
Cyanobacterial blooms are an increasing issue in both the wastewater-treatment and drinking water systems. Eutrophication and global climate change is the key factors for the occurrence of cyanoblooms all over the world. Cyanoblooms and production of several cyanotoxins in water bodies may reduce the surface/drinking water quality leading to high health risk to the organisms in aquatic ecosystems as well as wild/domestic animals and humans. A number of cyanotoxins such as MCs, nodularins, cylindrospermopsins, anatoxins, saxitoxins, and LPSs have been recognized as the major environmental contaminants in the immediate aquatic ecosystems. Control of cyanobloom using the chemical approaches can be effective; however, some algicidal/herbicides chemicals can cause secondary pollution of aquatic ecosystems. Several mitigation strategies have been tested and employed at laboratory levels; however, their efficacy to remove the blooms has not been confirmed under field environments. Establishment of effective mitigation strategies such as chemical, biological as well as public cognizance approach toward environmental awareness may be the most realistic measure to overcome the worldwide incidence of algal blooms and the attainment of a sustainable environment. Some natural algicidal compounds are really very effective to control the cyanoblooms; however, their production and availability is still very limited. The cost-effective synthesis of these biochemicals would be highly valuable to control the cyanoblooms. Furthermore, several cyanobacteria may become resistant toward certain chemicals. The use of biocides or several different biological processes against cyanoblooms may also affect other non-target aquatic organisms. Hence, common ecotoxicological impacts should be sensibly evaluated in the milieu of the lack of ecological health risk assessment. Moreover, a combined policy should strictly be regulated to diminish the bloom-boosting cause such as massive eutrophication of aquatic ecosystems by anthropogenic sources.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
RR is thankful to the University Grant Commission (UGC), New Delhi, India, for Dr. D. S. Kothari Postdoctoral Research Grant. AI thanks Chulalongkorn University and Thailand Research Fund for financial support through Ratchadaphiseksomphote Endowment Fund (Food and Water cluster) and for research grant (IRG 5780008), respectively. We also thank Professor Peter Lindblad for critical reading and English language editing.
References
Aas, P., Eriksen, S., Kolderup, J., Lundy, P., Haugen, J. E., Skulberg, O. M., et al. (1996). Enhancement of acetylcholine release by homoanatoxin-a from Oscillatoria Formosa. Environ. Toxicol. Pharmacol. 2, 223–232. doi: 10.1016/S1382-6689(96)00059-2
Aboal, M., and Puig, M. A. (2009). Microcystin production in Rivularia colonies of calcareous streams from Mediterranean Spanish basins. Algol. Stud. 130, 39–52. doi: 10.1127/1864-1318/2009/0130-0039
Adeymo, O. M., and Sirén, A. L. (1992). Cardio-respiratory changes and mortality in the conscious rat induced by (+)- and (±)-anatoxin-a. Toxicon 30, 899–905. doi: 10.1016/0041-0101(92)90388-L
Alamri, S. A. (2010). Biodegradation of microcystin by a new Bacillus sp. isolated from a Saudi freshwater lake. Afr. J. Biotechnol. 9, 6552–6559.
Aliotta, G., Defeo, V., Pinto, G., and Pollio, A. (1999). In vitro inhibition of algal growth by Rut a graveolens L. extracts: biological and chemical aspects. Plant Biosys. 133, 185–191. doi: 10.1080/11263509909381547
Aráoz, R., Molgó, J., and Tandeau de Marsac, N. (2010). Neurotoxic cyanobacterial toxins. Toxicon 56, 813–828. doi: 10.1016/j.toxicon.2009.07.036
Auvray, F., van Hullebusch, E. D., Deluchat, V., and Baudu, M. (2006). Laboratory investigation of the phosphorus removal (SRP and TP) from eutrophic lake water treated with aluminium. Water Res. 40, 2713–2719. doi: 10.1016/j.watres.2006.04.042
Banker, R., Carmeli, S., Hadas, O., Teltsch, B., Porat, R., and Sukenik, A. (1997). Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from Lake Kinneret, Israel. J. Phycol. 33, 613–616. doi: 10.1111/j.0022-3646.1997.00613.x
Banker, R., Teltsch, B., Sukenik, A., and Carmeli, S. (2000). 7-Epicylindrospermopsin, a toxic minor metabolite of the cyanobacterium Aphanizomenon ovalisporum from Lake Kinneret, Israel. J. Nat. Prod. 63, 387–389. doi: 10.1021/np990498m
Bauer, N., Blaschke, U., Beutler, E., Gross, E. M., Jenett-Siems, K., Siems, K., et al. (2009). Seasonal and interannual dynamics of polyphenols in Myriophyllum verticillatum and their allelopathic activity on Anabaena variabilis. Aquat. Bot. 91, 110–116. doi: 10.1016/j.aquabot.2009.03.005
Becher, P. G., Beuchat, J., Gademann, K., and Juttner, F. (2005). Nostocarboline: isolation and synthesis of a new cholinesterase inhibitor from Nostoc 78–12A. J. Nat. Prod. 68, 1793–1795. doi: 10.1021/np050312l
Berman, F. W., Gerwick, W. H., and Murray, T. F. (1999). Antillatoxin and kalkitoxin, ichthyotoxins from the tropical cyanobacterium Lyngbya majuscula, induce distinct temporal patterns of NMDA receptor mediated neurotoxicity. Toxicon 37, 1645–1648. doi: 10.1016/S0041-0101(99)00108-7
Bernard, C., Harvey, M., Briand, J. F., Bire, R., Krys, S., and Fontaine, J. J. (2003). Toxicological comparison of diverse Cylindrospermopsis raciborskii strains: evidence of liver damage caused by a French C. raciborskii strain. Environ. Toxicol. 18, 176–186. doi: 10.1002/tox.10112
Berry, J. P., Gantar, M., Perez, M. H., Berry, G., and Noriega, F. G. (2008). Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 6, 117–146. doi: 10.3390/md20080007
Beversdorf, L. J., Miller, T. R., and McMahon, K. D. (2015). Long-term monitoring reveals carbon-nitrogen metabolism key to microcystin production in eutrophic lakes. Front. Microbiol 6:456. doi: 10.3389/fmicb.2015.00456
Bláhová, L., Adamovský, O., Kubala, L., Švihálková Šindlerová, L., Zounková, R., and Bláha, L. (2013). The isolation and characterization of lipopolysaccharides from Microcystis aeruginosa, a prominent toxic water bloom forming cyanobacteria. Toxicon 76, 187–196. doi: 10.1016/j.toxicon.2013.10.011
Bogialli, S., di Gregorio, F. N., Lucentini, L., Ferretti, E., Ottaviani, M., Ungaro, N., et al. (2013). Management of a toxic cyanobacterium bloom (Planktothrix rubescens) affecting an Italian drinking water basin: a case study. Environ. Sci. Technol. 47, 574–583. doi: 10.1021/es302260p
Bond, N. R., and Lake, P. S. (2003). Characterizing fish-habitat associations in streams as the first step in ecological restoration. Aust. J. Ecol. 28, 611–621. doi: 10.1046/j.1442-9993.2003.t01-1-01317.x
Bricelj, V. M., Connell, L., Konoki, K., MacQuarrie, S. P., Scheuer, T., Catterall, W. A., et al. (2005). Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 434, 763–767. doi: 10.1038/nature03415
Brookes, J. D., and Carey, C. C. (2011). Resilience to Blooms. Science 334, 46–47. doi: 10.1126/science.1207349
Brownson, D. M., Mabry, T. J., and Leslie, S. W. (2002). The cycad neurotoxic amino acid, beta-N-methylamino-L-alanine (BMAA), elevates intracellular calcium levels in dissociated rat brain cells. J. Ethnopharmacol. 82, 159–167. doi: 10.1016/S0378-8741(02)00170-8
Bullerjahn, G. S., and Post, A. F. (2014). Physiology and molecular biology of aquatic cyanobacteria. Front. Microbiol. 5:359. doi: 10.3389/fmicb.2014.00359
Cantrell, C. L., Mamonov, L. K., Ryabushkina, N., Kustova, T. S., Fischer, N. H., and Schrader, K. K. (2007). Bioassay-guided isolation of anti-algal constituents from Inula helenium and Limonium myrianthum. ARKIVOC 7, 65–75.
Cantrell, C. L., Schrader, K. K., Mamonov, L. K., Sitpaeva, G. T., Kustova, T. S., Dunbar, C., et al. (2005). Isolation and identification of antifungal and antialgal alkaloids from Haplophyllum sieversii. J. Agric. Food Chem. 53, 7741–7748. doi: 10.1021/jf051478v
Cardellina, J. H., Marner, F.-J., and Moore, R. E. (1979). Seaweed dermatitis: structure of lyngbyatoxin A. Science 204, 193–195. doi: 10.1126/science.107586
Carmichael, W. W. (1998). “Toxins of freshwater algae,” in Handbook of Natural Toxins, Marine Toxins and Venoms, Vol. 3, ed. A. T. Tu (New York, NY: Marcel Dekker), 121–147.
Carmichael, W. W., Mahmood, N. A., and Hyde, E. G. (1990). “Natural toxins from cyanobacteria (blue–green algae),” in Marine Toxins: Origin, Structure, and Molecular Pharmacology, eds S. Hall and G. Strichartz (Washington DC: American Chemical Society), 87–106.
Chen, J., Hu, L. B., Zhou, W., Yan, S. H., Yang, J. D., Xue, Y. F., et al. (2010). Degradation of microcystin-LR and RR by a Stenotrophomonas sp. strain EMS isolated from Lake Taihu. China. Int. J. Mol. Sci. 11, 896–911. doi: 10.3390/ijms11030896
Chen, Y., Xu, J., Li, Y., and Han, X. (2011). Decline of sperm quality and testicular function in male mice during chronic low-dose exposure to microcystin-LR. Reprod. Toxicol. 31, 551–557. doi: 10.1016/j.reprotox.2011.02.006
Choi, S. H., and Kim, S. G. (1998). Lipopolysaccharide inhibition of rat hepatic microsomal epoxide hydrolase and glutathione S-transferase gene expression irrespective of nuclear factor-kB activation. Biochem. Pharmacol. 56, 1427–1436. doi: 10.1016/S0006-2952(98)00204-4
Christiansen, G., Yoshida, W. Y., Blom, J. F., Portmann, C., Gademann, K., Hemscheidt, T., et al. (2008). Isolation and structure determination of two microcystins and sequence comparison of the McyABC adenylation domains in Planktothrix species. J. Nat. Prod. 71, 1881–1886. doi: 10.1021/np800397u
Churro, C., Alverca, E., Sam-Bento, F., Paulino, S., Figueira, V. C., Bento, A. J., et al. (2009). Effects of bacillamide and newly synthesized derivatives on the growth of cyanobacteria and microalgae cultures. J. Appl. Phycol. 21, 429–442. doi: 10.1007/s10811-008-9388-3
Churro, C., Fernandes, A. S., Alverca, E., Sam-Bento, F., Paulino, S., Figueira, V. C., et al. (2010). Effects of tryptamine on growth, ultrastructure, and oxidative stress of cyanobacteria and microalgae cultures. Hydrobiologia 649, 195–206. doi: 10.1007/s10750-010-0245-4
Coad, P., Cathers, B., Ball, J. E., and Kadluczka, R. (2014). Proactive management of estuarine algal blooms using an automated monitoring buoy coupled with an artificial neural network. Environ. Model. Softw. 61, 393–409. doi: 10.1016/j.envsoft.2014.07.011
Codd, G. A., Morrison, L. F., and Metcalf, J. S. (2005). Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharmacol. 203, 264–272. doi: 10.1016/j.taap.2004.02.016
Conley, D. J., Paerl, H. W., Howarth, R. W., Boesch, D. F., and Seitzinger, S. P. (2009). Controlling eutrophication: nitrogen and phosphorus. Science 323, 1014–1015. doi: 10.1126/science.1167755
Corbel, S., Mougin, C., and Bouaïcha, N. (2014). Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 96, 1–15. doi: 10.1016/j.chemosphere.2013.07.056
Dai, G., Quan, C., Zhang, X., Liu, J., Song, L., and Gan, N. (2012). Fast removal of cyanobacterial toxin microcystin-LR by a low-cytotoxic microgel-Fe(III) complex. Water Res. 46, 1482–1489. doi: 10.1016/j.watres.2011.11.010
Davidson, K., Gowen, R. J., Harrison, P. J., Fleming, L. E., Hoagland, P., and Moschonas, G. (2014). Anthropogenic nutrients and harmful algae in coastal waters. J. Environ. Manage. 146, 206–216. doi: 10.1016/j.jenvman.2014.07.002
De Julio, M., Fioravante, D. A., De Julio, T. S., Oroski, F. I., and Graham, N. J. D. (2010). A methodology for optimising the removal of cyanobacteria cells from a Brazilian eutrophic water. Braz. J. Chem. Eng. 27, 113–126. doi: 10.1590/S0104-66322010000100010
Devlin, J. P., Edwards, O. E., Gorham, P. R., Hunter, N. R., Pike, R. K., and Starvic, B. (1977). Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 55, 1367–1371. doi: 10.1139/v77-189
Ding, Y., Song, L., and Sedmak, B. (2013). UVB radiation as a potential selective factor favoring microcystin producing bloom forming cyanobacteria. PLoS ONE 8:e73919. doi: 10.1371/journal.pone.0073919
Dodds, W. K., Bouska, W. W., Eitzmann, J. L., Pilger, T. J., Pitts, K. L., Riley, A. J., et al. (2009). Eutrophication of U.S, freshwaters: analysis of potential economic damages. Environ. Sci. Technol. 43, 12–19. doi: 10.1021/es801217q
Downing, S., Contardo-Jara, V., Pflugmacher, S., and Downing, T. G. (2014). The fate of the cyanobacterial toxin β-N-methylamino-L-alanine in freshwater mussels. Ecotoxicol. Environ. Saf. 101, 51–58. doi: 10.1016/j.ecoenv.2013.11.028
Edwards, C., Beattie, K. A., Scrimgeour, C. M., and Codd, G. A. (1992). Identification of anatoxin-a in benthic cyanobacteria (blue–green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 30, 1165–1175. doi: 10.1016/0041-0101(92)90432-5
Edwards, D. J., Marquez, B. L., Nogle, L. M., McPhail, K., Goeger, D. E., Roberts, M. A., et al. (2004). Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 11, 817–833. doi: 10.1016/j.chembiol.2004.03.030
El-Shehawy, R., Gorokhova, E., Fernández-Piñas, F., and del Campo, F. F. (2012). Global warming and hepatotoxin production by cyanobacteria: what can we learn from experiments? Water Res. 46, 1420–1429. doi: 10.1016/j.watres.2011.11.021
Falconer, I. R., Hardy, S. J., Humpage, A. R., Froscio, S. M., Tozer, G. J., and Hawkins, P. R. (1999). Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male Swiss albino mice. Environ. Toxicol. 14, 143–150. doi: 10.1002/(SICI)1522-7278(199902)14:1<143::AID-TOX18>3.3.CO;2-8
Faltermann, S., Zucchi, S., Kohler, E., Blom, J. F., Pernthaler, J., and Fent, K. (2014). Molecular effects of the cyanobacterial toxin cyanopeptolin (CP1020) occurring in algal blooms: global transcriptome analysis in zebrafish embryos. Aqua. Toxicol. 149, 33–39. doi: 10.1016/j.aquatox.2014.01.018
Feng, Y., Chang, X., Zhao, L., Li, X., Li, W., and Jiang, Y. (2013). Nanaomycin A methyl ester, an actinomycete metabolite: algicidal activity and the physiological response of Microcystis aeruginosa. Ecol. Eng. 53, 306–312. doi: 10.1016/j.ecoleng.2012.12.066
Froscio, S. M., Humpage, A. R., Burcham, P. C., and Falconer, I. R. (2003). Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 18, 243–251. doi: 10.1002/tox.10121
Fujiki, H., Mori, M., Nakayasu, M., Terada, M., Sugimura, T., and Moore, R. E. (1981). Indole alkaloids: dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc. Natl. Acad. Sci. U.S.A. 78, 3872–3876. doi: 10.1073/pnas.78.6.3872
Fujiki, H., Suganuma, M., Hakii, H., Bartolini, G., Moore, R. E., Takayama, S., et al. (1984). A two-stage mouse skin carcinogenesis study of lyngbyatoxin A. J. Cancer Res. Clin. Oncol. 108, 174–176. doi: 10.1007/BF00390993
Fujiki, H., Suganuma, M., Nakayasu, M., Hoshino, H., Moore, R. E., and Sugimura, T. (1982). The third class of new tumor promoters, polyacetates (debromoaplysiatoxin and aplysiatoxin), can differentiate biological actions relevant to tumor promoters. Gan 73, 497–499.
Funari, E., and Testai, E. (2008). Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 38, 97–125. doi: 10.1080/10408440701749454
Furey, A., Crowley, J., Shuilleabhain, A. N., Skulberg, O. M., and James, K. J. (2003). The first identification of the rare cyanobacterial toxin, homoanatoxin-a, in Ireland. Toxicon 41, 297–303. doi: 10.1016/S0041-0101(02)00291-X
Gao, Y. N., Liu, B. Y., Zhou, Q. H., Hu, C. Y., Ge, F. J., Zhang, L. P., et al. (2011). Phenolic compounds extracted from two submerged freshwater macrophytes and their allelopathic effects on Microcystis aeruginosa. Pol. J. Environ. Stud. 20, 1153–1159.
Gehringer, M. M., Adler, L., Roberts, A. A., Moffitt, M. C., Mihali, T. K., Mills, T. J. T., et al. (2012). Nodularin, a cyanobacterial toxin, is synthesized in planta by symbiotic Nostoc sp. ISME J. 6, 1834–1847. doi: 10.1038/ismej.2012.25
Gehringer, M. M., and Wannicke, N. (2014). Climate change and regulation of hepatotoxin production in cyanobacteria. FEMS Microbiol. Ecol. 88, 1–25. doi: 10.1111/1574-6941.12291
Gerwick, W. H., Proteau, P. J., Nagle, D. G., Hamel, E., Blokhin, A., and Slate, D. (1994). Structure of curacin A, a novel antimitotic, antiproliferative, and brine shrimp toxic natural product from the marine cyanobacterium Lyngbya majuscula. J. Org. Chem. 59, 1243–1245. doi: 10.1021/jo00085a006
Gleason, F. K., and Baxa, C. A. (1986). Activity of the natural algicide, cyanobacterin, on eukaryotic microorganisms. FEMS Microbiol. Lett. 33, 85–88. doi: 10.1111/j.1574-6968.1986.tb01217.x
Glibert, P. M., Heil, C. A., Hollander, D., Revilla, M., Hoare, A., Alexander, J., et al. (2004). Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Prog. Ser. 280, 73–83. doi: 10.3354/meps280073
Griffiths, D. J., and Saker, M. L. (2003). The palm island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 18, 78–93. doi: 10.1002/tox.10103
Gulledge, B. M., Aggen, J. B., Huang, H.-B., Nairn, A. C., and Chamberlin, A. R. (2002). The microcystins and nodularins: cyclic polypeptide inhibitors of PP1 and PP2A. Curr. Med. Chem. 9, 1991–2003. doi: 10.2174/0929867023368845
Gupta, D. K., Kaur, P., Leong, S. T., Tan, L. T., Prinsep, M. R., and Hann Chu, J. J. (2014). Anti-chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs 12, 115–127. doi: 10.3390/md12010115
Guzmán-Guillén, R., Prieto, A. I., Vasconcelos, V. M., and Cameán, A. M. (2013a). Cyanobacterium producing cylindrospermopsin cause oxidative stress at environmentally relevant concentrations in sub-chronically exposed tilapia (Oreochromis niloticus). Chemosphere 90, 1184–1194. doi: 10.1016/j.chemosphere.2012.09.027
Guzmán-Guillén, R., Prieto, A. I., Vázquez, C. M., Vasconcelos, V., and Cameán, A. M. (2013b). The protective role of L-carnitine against cylindrospermopsin-induced oxidative stress in tilapia (Oreochromis niloticus). Aqua. Toxicol. 13, 141–150. doi: 10.1016/j.aquatox.2013.02.011
Häder, D.-P., and Gao, K. (2015). Interactions of anthropogenic stress factors on marine phytoplankton. Front. Environ. Sci. 3:14. doi: 10.3389/fenvs.2015.00014
Häder, D.-P., Villafañe, V. E., and Helbling, E. W. (2014). Productivity of aquatic primary producers under global climate change. Photochem. Photobiol. Sci. 13, 1370–1392. doi: 10.1039/c3pp50418b
Harada, K. I., Ohtani, I., Iwamoto, K., Suzuki, M., Watanabe, M. F., Watanabe, M., et al. (1994). Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon 32, 73–84. doi: 10.1016/0041-0101(94)90023-X
Harris, T. D., Wilhelm, F. M., Graham, J. L., and Loftin, K. A. (2014). Experimental manipulation of TN: TP ratios suppress cyanobacterial biovolume and microcystin concentration in large-scale in situ mesocosms. Lake Reser. Manage. 30, 72–83. doi: 10.1080/10402381.2013.876131
Havens, K. E. (2008). Cyanobacteria blooms: effects on aquatic ecosystems. Adv. Exp. Med. Biol. 619, 733–747. doi: 10.1007/978-0-387-75865-7_33
He, X., Pelaez, M., Westrick, J. A., O’Shea, K. E., Hiskia, A., Triantis, T., et al. (2012). Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water Res. 46, 1501–1510. doi: 10.1016/j.watres.2011.11.009
Heisler, J., Gilbert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., et al. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. doi: 10.1016/j.hal.2008.08.006
Hernández, J. M., López-Rodas, V., and Costas, E. (2009). Microcystins from tap water could be a risk factor for liver and colorectal cancer: a risk intensified by global change. Med. Hypotheses 72, 539–540. doi: 10.1016/j.mehy.2008.11.041
Hicks, L. M., Moffitt, M. C., Beer, L. L., Moore, B. S., and Kelleher, N. L. (2006). Structural characterization of in vitro and in vivo intermediates on the loading module of microcystin synthetase. ACS Chem. Biol. 1, 93–102. doi: 10.1021/cb500007v
Hilborn, E. D., Carmichael, W. W., Soares, R. M., Yuan, M., Servaites, J. C., Barton, H. A., et al. (2007). Serologic evaluation of human microcystin exposure. Environ. Toxicol. 22, 459–463. doi: 10.1002/tox.20281
Ho, L., Hoefel, D., Saint, C. P., and Newcombe, G. (2007). Isolation and identification of a novel microcystin degrading bacterium from a biological sand filter. Water Res. 41, 4685–4695. doi: 10.1016/j.watres.2007.06.057
Hong, Y., Hu, H.-Y., Xie, X., Sakoda, A., Sagehashi, M., and Li, F.-M. (2009). Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 91, 262–269. doi: 10.1016/j.aquatox.2008.11.014
Honkanen, R. E., Zwiller, J., Moore, R. E., Daily, S. L., Khatra, B. S., Dukelow, M., et al. (1990). Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatises. J. Biol. Chem. 265, 19401–19404.
Hu, L. B., Yang, J. D., Zhou, W., Yin, Y. F., Chen, J., and Shi, Z. Q. (2009). Isolation of a Methylobacillus sp. that degrades microcystin toxins associated with cyanobacteria. New Biotechnol. 26, 205–211. doi: 10.1016/j.nbt.2009.09.001
Hu, L. B., Zhang, F., Liu, C., and Wang, M. (2012). Biodegradation of microcystins by Bacillus sp. strain EMB. Energy Procedia 16, 2054–2059. doi: 10.1016/j.egypro.2012.01.312
Hudnell, H. K. (2008). Cyanobacterial harmful algal blooms: state of the science and research needs Adv. Exp. Med. Biol. 619, 1–16. doi: 10.1007/978-0-387-75865-7_1
Hudnell, H. K. (2010). The state of U.S. freshwater harmful algal blooms assessments, policy and legislation. Toxicon 55, 1024–1034. doi: 10.1016/j.toxicon.2009.07.021
Hudnell, H. K., Jones, C., Labisi, B., Lucero, V., Hill, D. R., and Eilers, J. (2010). Freshwater harmful algal bloom (FHAB) suppression with solar powered circulation (SPC). Harmful Algae 9, 208–217. doi: 10.1016/j.hal.2009.10.003
Huguet, A., Hatton, A., Villot, R., Quenault, H., Blanchard, Y., and Fessard, V. (2014). Modulation of chromatin remodelling induced by the freshwater cyanotoxin cylindrospermopsin in human intestinal Caco-2 Cells. PLoS ONE 9:e99121. doi: 10.1371/journal.pone.0099121
Huisman, J., Sharples, J., Stroom, J., Visser, P. M., Kardinaal, W. E. A., Verspagen, J. M. H., et al. (2004). Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85, 2960–2970. doi: 10.1890/03-0763
Humpage, A. R., Fenech, M., Thomas, P., and Falconer, I. R. (2000). Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 472, 155–161. doi: 10.1016/S1383-5718(00)00144-3
Isaacs, J. D., Strangman, W. K., Barbera, A. E., Mallin, M. A., McIver, M. R., and Wright, J. L. C. (2014). Microcystins and two new micropeptin cyanopeptides produced by unprecedented Microcystis aeruginosa blooms in North Carolina’s Cape Fear River. Harmful Algae 31, 82–86. doi: 10.1016/j.hal.2013.09.010
Ishii, H., Nishijima, M., and Abe, T. (2004). Characterization of degradation process of cyanobacterial hepatotoxins by a Gram negative aerobic bacterium. Water Res. 38, 2667–2676. doi: 10.1016/j.watres.2004.03.014
Izaguirre, G., Jungblut, A. D., and Neilan, B. A. (2007). Benthic cyanobacteria (Oscillatoriaceae) that produce microcystin-LR, isolated from four reservoirs in southern California. Water Res. 41, 492–498. doi: 10.1016/j.watres.2006.10.012
Jacquet, S. T. E., Kerimoglu, O., Rimet, F., Paolini, G. E., and Anneville, O. (2014). Cyanobacterial bloom termination: the disappearance of Planktothrix rubescens from Lake Bourget (France) after restoration. Freshwater Biol. 59, 2472–2487. doi: 10.1111/fwb.12444
Jančula, D., and Maršálek, B. (2011). Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85, 1415–1422. doi: 10.1016/j.chemosphere.2011.08.036
Jančula, D., Mikula, P., Maršálek, B., Rudolf, P., and Pochyly, F. (2014). Selective method for cyanobacterial bloom removal: hydraulic jet cavitation experience. Aquacult. Int. 22, 509–521. doi: 10.1007/s10499-013-9660-7
Jang, M.-H., Kyong, H., and Takamura, N. (2007). Reciprocal allelopathic responses between toxic cyanobacteria (Microcystis aeruginosa) and duckweed (Lemna japonica). Toxicon 49, 727–733. doi: 10.1016/j.toxicon.2006.11.017
Jeong, S., Ishida, K., Ito, Y., Okada, S., and Murakami, M. (2003). Bacillamide-a novel algicide from the marine bacterium, Bacillus sp. SY-1, against the harmful dinoflagellate, Cochlodinium polykrikoides. Tetrahedron Lett. 44, 8005–8007. doi: 10.1016/j.tetlet.2003.08.115
Jiang, Y., Shao, J., Wu, X., Xu, Y., and Li, R. (2011). Active and silent members in the mlr gene cluster of a microcystin degrading bacterium isolated from Lake Taihu. China. FEMS Microbiol. Lett. 322, 108–114. doi: 10.1111/j.1574-6968.2011.02337.x
Jiang, Y., Xiao, P., Yu, G., Sano, T., Pan, Q., and Li, R. (2012). Molecular basis and phylogenetic implications of deoxycylindrospermopsin biosynthesis in the cyanobacterium Raphidiopsis curvata. Appl. Environ. Microbiol. 78, 2256–2263. doi: 10.1128/AEM.07321-11
Jing, W., Sui, G., and Liu, S. (2014). Characteristics of a microcystin-LR biodegrading bacterial isolate: Ochrobactrum sp. FDT5. Bull. Environ. Contam. Toxicol. 92, 119–122. doi: 10.1007/s00128-013-1170-9
Joung, S. H., Oh, H. M., Ko, S. R., and Ahn, C. Y. (2011). Correlations between environmental factors and toxic and non-toxic Microcystis dynamics during bloom in Daechung Reservoir, Korea. Harmful Algae 10, 188–193. doi: 10.1016/j.hal.2010.09.005
Jungo, E., Visser, P. M., Stroom, J., and Mur, L. R. (2001). Artificial mixing to reduce growth of the blue-green alga Microcystis in Lake Nieuwe Meer, Amsterdam: an evaluation of 7 years of experience. Water Sci. Technol. 1, 17–23.
Kaebernick, M., and Neilan, B. A. (2001). Ecological and molecular investigations of cyano-toxin production. FEMS Microbiol. Ecol. 35, 1–9. doi: 10.1111/j.1574-6941.2001.tb00782.x
Kanoshina, I., Lips, U., and Leppänen, J. M. (2003). The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2, 29–41. doi: 10.1016/S1568-9883(02)00085-9
Kaplan, A., Harel, M., Kaplan-Levy, R. N., Hadas, O., Sukenik, A., and Dittmann, E. (2012). The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Microbiol. 3:138. doi: 10.3389/fmicb.2012.00138
Kaya, K., and Sano, T. (1998). A photodetoxification mechanism of the cyanobacterial hepatotoxin microcystin-LR by ultraviolet irradiation. Chem. Res. Toxicol. 11, 159–163. doi: 10.1021/tx970132e
Kellmann, R., Mihali, T. K., Jeon, Y. J., Pickford, R., Pomati, F., and Neilan, B. A. (2008). Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 74, 4044–4053. doi: 10.1128/AEM.00353-08
Kim, J.-S., Kim, J.-C., Lee, S., Lee, B.-H., and Cho, K. Y. (2006). Biological activity of L-2-azetidinecarboxylic acid, isolated from Polygonatum odoratum var. pluriflorum, against several algae. Aquat. Bot. 85, 1–6. doi: 10.1016/j.aquabot.2006.01.003
Kirkpatrick, B., Kohler, K., Byrne, M., Fleming, L. E., Scheller, K., Reich, A., et al. (2014). Human responses to Florida red tides: policy awareness and adherence to local fertilizer ordinances. Sci. Total Environ. 493, 898–909. doi: 10.1016/j.scitotenv.2014.06.083
Kleinteich, J., Wood, S. A., Kupper, F. C., Camacho, A., Quesada, A., Frickey, T., et al. (2012). Temperature-related changes in polar cyanobacterial mat diversity and toxin production. Nat. Clim. Chang. 2, 356–360. doi: 10.1038/nclimate1418
Kodani, S., Imoto, A., Mitsutani, A., and Murakami, M. (2002). Isolation and identification of the antialgal compound, harmane (1-methyl-β-carboline), produced by the algicidal bacterium, Pseudomonas sp. K44-1. J. Appl. Phycol. 14, 109–114. doi: 10.1023/A:1019533414018
Koreivienë, J., Anne, O., Kasperovičienë, J., and Burškytë, V. (2014). Cyanotoxin management and human health risk mitigation in recreational waters. Environ. Monit. Assess. 186, 4443–4459. doi: 10.1007/s10661-014-3710-0
Körner, S., and Nicklisch, A. (2002). Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. J. Phycol. 38, 862–871. doi: 10.1046/j.1529-8817.2002.t01-1-02001.x
Krienitz, L., Ballot, A., Kotut, K., Wiegand, C., Pütz, S., Metcalf, J. S., et al. (2003). Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 43, 141–148. doi: 10.1111/j.1574-6941.2003.tb01053.x
Krienitz, L., Ballot, A., Wiegand, C., Kotut, K., Codd, G. A., and Pflugmacher, S. (2002). Cyanotoxin-producing bloom of Anabaena flos-aquae, Anabaena discoidea and Microcystis aeruginosa (cyanobacteria) in Nyanza Gulf of Lake Victoria, Kenya. J. Appl. Bot. 76, 179–183. doi: 10.1128/AEM.05587-11
Lawton, L. A., Robertson, P. K. J., Cornish, B. J. P. A., and Jaspars, M. (1999). Detoxification of microcystins (cyanobacterial hepatotoxins) using TiO2 photocatalytic oxidation. Environ. Sci. Technol. 33, 771–775. doi: 10.1021/es9806682
Lawton, L. A., Welgamage, A., Manage, P. M., and Edwards, C. (2011). Novel bacterial strains for the removal of microcystins from drinking water. Water Sci. Technol. 63, 1137–1142. doi: 10.2166/wst.2011.352
Lelkova, E., Rulik, M., Hekera, P., Dobias, P., Dolejs, P., Borovickova, M., et al. (2008). The influence of the coagulant PAX-18 on Planktothrix agardhii bloom in a shallow eutrophic fishpond. Fottea 8, 147–154. doi: 10.5507/fot.2008.013
Lemes, G. A. F., Kist, L. W., Bogo, M. R., and Yunes, J. S. (2015). Biodegradation of [D-Leu1] microcystin-LR by a bacterium isolated from sediment of Patos Lagoon estuary, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 21:4. doi: 10.1186/s40409-015-0001-3
LePage, K. T., Goeger, D., Yokokawa, F., Asano, T., Shioiri, T., Gerwick, W. H., et al. (2005). The neurotoxic lipopeptide kalkitoxin interacts with voltage-sensitive sodium channels in cerebellar granule neurons. Toxicol. Lett. 158, 133–139. doi: 10.1016/j.toxlet.2005.03.007
Li, F. M., and Hu, H. Y. (2005). Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl. Environ. Microbiol. 71, 6545–6553. doi: 10.1128/AEM.71.11.6545-6553.2005
Li, F., Xiong, B., and Xu, B. (2008). Improving public access to environmental information in China. J. Environ. Manage. 88, 1649–1656. doi: 10.1016/j.jenvman.2007.08.002
Li, W. I., Berman, F. W., Okino, T., Yokokawa, F., Shioiri, T., Gerwick, W. H., et al. (2001). Antillatoxin is a marine cyanobacterial toxin that potently activates voltage-gated sodium channels. Proc. Natl. Acad. Sci. U.S.A. 98, 7599–7604. doi: 10.1073/pnas.121085898
Lilleheil, G., Andersen, R. A., Skulberg, O. M., and Alexander, J. (1997). Effects of a homoanatoxin-a-containing extract from oscillatoria formosa (cyanophyceae/cyanobacteria) on neuromuscular transmission. Toxicon 35, 1275–1289. doi: 10.1016/S0041-0101(97)00013-5
Lobner, D., Piana, P. M. T., Salous, A. K., and Peoples, R. W. (2007). β-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 25, 360–366. doi: 10.1016/j.nbd.2006.10.002
López-Alonso, H., Rubiolo, J. A., Vega, F., Vieytes, M. R., and Botana, L. M. (2013). Protein synthesis inhibition and oxidative stress induced by cylindrospermopsin elicit apoptosis in primary rat hepatocytes. Chem. Res. Toxicol. 26, 203–212. doi: 10.1021/tx3003438
Lopez-Rodas, V., Maneiro, E., Lanzarot, M. P., Perdigones, N., and Costas, E. (2008). Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Vet. Rec. 162, 317–318. doi: 10.1136/vr.162.10.317
Lun, Z., Hai, Y., and Kun, C. (2002). Relationship between microcystin in drinking water and colorectal cancer. Biomed. Environ. Sci. 15, 166–171.
MacKintosh, C., Beattie, K. A., Klumpp, S., Cohen, P., and Codd, G. A. (1990). Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 264, 187–192. doi: 10.1016/0014-5793(90)80245-E
Manage, P. M., Pathmalal, M., Edwards, C., Singh, B. K., and Lawton, L. A. (2009). Isolation and identification of novel microcystin degrading bacteria. Appl. Environ. Microbiol. 75, 6924–6928. doi: 10.1128/AEM.01928-09
Mankiewicz, J., Malgorzata, T. M., Walter, Z., and Maciej, Z. M. (2003). Natural toxins from cyanobacteria. Acta Biol. Cracovien. Ser. Bot. 45, 9–20.
Matsunaga, S., Moore, R. E., Niemczura, W. P., and Carmichael, W. W. (1989). Anatoxin-a(s), a potent anticholinesterase from Anabaena flos-aquae. J. Am. Chem. Soc. 111, 8021–8023. doi: 10.1021/ja00202a057
Mazmouz, R., Chapuis-Hugon, F., Mann, S., Pichon, V., Mejean, A., and Ploux, O. (2010). Biosynthesis of cylindrospermopsin and 7-epicylindrospermopsin in Oscillatoria sp. strain PCC 6506: identification of the cyr gene cluster and toxin analysis. Appl. Environ. Microbiol. 76, 4943–4949. doi: 10.1128/AEM.00717-10
McGregor, G. B., Sendall, B. C., Hunt, L. T., and Eaglesham, G. K. (2011). Report of the cyanotoxins cylindrospermopsin and deoxy-cylindrospermopsin from Raphidiopsis mediterranea Skuja (Cyanobacteria/Nostocales). Harmful Algae 10, 402–410. doi: 10.1016/j.hal.2011.02.002
Méjean, A., Mann, S., Maldiney, T., Vassiliadis, G., Lequin, O., and Ploux, O. (2009). Evidence that biosynthesis of the neurotoxic alkaloids anatoxin-a and homoanatoxin-a in the cyanobacterium Oscillatoria PCC 6506 occurs on a modular polyketide synthase initiated by L-proline. J. Am. Chem. Soc. 131, 7512–7513. doi: 10.1021/ja9024353
Méjean, A., Paci, G., Gautier, V., and Ploux, O. (2014). Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 91, 15–22. doi: 10.1016/j.toxicon.2014.07.016
Michalak, A. M., Anderson, E. J., Beletsky, D., Boland, S., and Bosch, N. S. (2013). Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. U.S.A. 110, 6448–6452. doi: 10.1073/pnas.1216006110
Mihali, T. K., Carmichael, W. W., and Neilan, B. A. (2011). A putative gene cluster from a Lyngbya wollei bloom that encodes paralytic shellfish toxin biosynthesis. PLoS ONE 6:e14657. doi: 10.1371/journal.pone.0014657
Mihali, T. K., Kellmann, R., Muenchhoff, J., Barrow, K. D., and Neilan, B. A. (2008). Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl. Environ. Microbiol. 74, 716–722. doi: 10.1128/AEM.01988-07
Mihali, T. K., Kellmann, R., and Neilan, B. A. (2009). Characterization of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5. BMC Biochem. 10:8. doi: 10.1186/1471-2091-10-8
Mikami, S., Takeshita, T., Nakada, M., and Kawabata, M. (1995). The media coverage and public awareness of environmental issues in Japan. Int. Commun. Gaz. 54, 209–226. doi: 10.1177/001654929505400302
Miller, M. A., Kudela, R. M., Mekebri, A., Crane, D., Oates, S. C., Tinker, M. T., et al. (2010). Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from land to Sea Otters. PLoS ONE 5:e12576. doi: 10.1371/journal.pone.0012576
Mizuno, C. S., Schrader, K. K., and Rimando, A. A. (2008). Algicidal activity of stilbene analogues. J. Agric. Food Chem. 56, 9140–9145. doi: 10.1021/jf801988p
Moffitt, M. C., and Neilan, B. A. (2004). Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 70, 6353–6362. doi: 10.1128/AEM.70.11.6353-6362.2004
Mohamed, Z. A. (2013). Allelopathic activity of the norharmane-producing cyanobacterium Synechocystis aquatilis against cyanobacteria and microalgae. Int. J. Oceanogr. Hydrobiol. 42, 1–7. doi: 10.2478/s13545-013-0053-3
Molot, L. A., Watson, S. B., Creed, I. F., Trick, C. G., Mccabe, S. K., Verschoor, M. J., et al. (2014). A novel model for cyanobacteria bloom formation: the critical role of anoxia and ferrous iron. Freshwater Biol. 59, 1323–1340. doi: 10.1111/fwb.12334
Muenchhoff, J., Siddiqui, K. S., Poljak, A., Raftery, M. J., Barrow, K. D., and Neilan, B. A. (2010). A novel prokaryotic L-arginine:glycine amidinotransferase is involved in cylindrospermopsin biosynthesis. FEBS J. 277, 3844–3860. doi: 10.1111/j.1742-4658.2010.07788.x
Murray-Gulde, C. L., Heatley, J. E., Schwartzman, A. L., and Rodgers, J. H. (2002). Algicidal effectiveness of clearigate, cutrine-plus, and copper sulfate and margins of safety associated with their use. Arch. Environ. Contam. Toxicol. 43, 19–27. doi: 10.1007/s00244-002-1135-1
Mynderse, J. S., Moore, R. E., Kashiwagi, M., and Norton, T. R. (1977). Antileukemia activity in the Osillatoriaceae: isolation of debromoaplysiatoxin from Lyngbya. Science 196, 538–540. doi: 10.1126/science.403608
Nakagawa, Y., Yanagita, R. C., Hamada, N., Murakami, A., Takahashi, H., Saito, N., et al. (2009). A simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J. Am. Chem. Soc. 131, 7573–7579. doi: 10.1021/ja808447r
Nakai, S., Inoue, Y., Hosomi, M., and Murakami, A. (2000). Myriophyllum spicatum- Released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 34, 3026–3032. doi: 10.1016/S0043-1354(00)00039-7
Nakai, S., Yamada, S., and Hosomi, M. (2005). Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia 543, 71–78. doi: 10.2166/wst.2012.272
Namikoshi, M., Murakami, T., Watanabe, M. F., Oda, T., Yamada, J., Tsujimura, S., et al. (2003). Simultaneous production of homoanatoxin-a, anatoxin-a, and a new nontoxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 42, 533–538. doi: 10.1016/S0041-0101(03)00233-2
Neilan, B. A., Pearson, L. A., Muenchhoff, J., Moffitt, M. C., and Dittmann, E. (2013). Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 15, 1239–1253. doi: 10.1111/j.1462-2920.2012.02729.x
Neumann, C., Bain, P., and Shaw, G. (2007). Studies of the comparative in vitro toxicology of the cyanobacterial metabolite deoxycylindrospermopsin. J. Toxicol. Environ. Health 70, 1679–1686. doi: 10.1080/15287390701434869
Nonga, H. E., Sandvik, M., Miles, C. O., Lie, E., Mdegela, R. H., Mwamengele, G. L., et al. (2011). Possible involvement of microcystins in the unexplained mass mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 678, 167–178. doi: 10.1007/s10750-011-0844-8
Norris, R. L. G., Eaglesham, G. K., Pierens, G., Shaw, G. R., Smith, M. J., Chiswell, R. K., et al. (1999). Deoxycylindrospermopsin, an analog of cylindrospermopsin from Cylindrospermopsis raciborskii. Environ. Toxicol. 14, 163–165. doi: 10.1002/(SICI)1522-7278(199902)14:1<163::AID-TOX21>3.3.CO;2-M
Ohta, T., Sueoka, E., Iida, N., Komori, A., Suganuma, M., Nishiwaki, R., et al. (1994). Nodularin, a potent inhibitor of protein phosphatase 1 and 2A, is a new environmental carcinogen in male F344 rat liver. Cancer Res. 54, 6402–6406.
Ohtani, I., Moore, R. E., and Runnegar, M. T. C. (1992). Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 114, 7941–7942. doi: 10.1021/ja00046a067
Okano, K., Shimizu, K., Kawauchi, Y., Maseda, H., Utsumi, M., Zhang, Z., et al. (2009). Characteristics of a microcystin-degrading bacterium under alkaline environmental conditions. J. Toxicol. 2009:954291. doi: 10.1155/2009/954291
O’Neil, J. M., Davis, T. W., Burford, M. A., and Gobler, C. J. (2012). The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14, 313–334. doi: 10.1016/j.hal.2011.10.027
Orjala, J., Nagle, D. G., Hsu, V., and Gerwick, W. H. (1995). Antillatoxin: an exceptionally ichthyotoxic cyclic lipopeptide from the tropical cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 117, 8281–8282. doi: 10.1021/ja00136a031
Paerl, H. W. (2008). “Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater-marine continuum,” in Cyanobacterial Harmful Algal Blooms, State of the Science and Research Needs, Vol. 619, ed. H. K. Hudnell (New York, NY: Springer Press), 218–237.
Paerl, H. W., Hall, N. S., and Calandrino, E. S. (2011a). Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 409, 1739–1745. doi: 10.1016/j.scitotenv.2011.02.001
Paerl, H. W., Xu, H., McCarthy, M. J., Zhu, G., Qin, B., Li, Y., et al. (2011b). Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res. 45, 1973–1983. doi: 10.1016/j.watres.2010.09.018
Paerl, H. W., and Huisman, J. (2008). Blooms like it hot. Science 320, 57–58. doi: 10.1126/science.1155398
Paerl, H. W., and Huisman, J. (2009). Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 1, 27–37. doi: 10.1111/j.1758-2229.2008.00004.x
Paerl, H. W., and Paul, V. J. (2012). Climate change: links to global expansion of harmful cyanobacteria. Water Res. 46, 1349–1363. doi: 10.1016/j.watres.2011.08.002
Paerl, H. W., and Scott, J. T. (2010). Throwing fuel on the fire: synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. Technol. 44, 7756–7758. doi: 10.1021/es102665e
Palmer, J. A., Suggate, J., Bajd, B., and Tsaliki, E. (1998). Significant influences on the development of adults’ environmental awareness in the UK. Slovenia and Greece. Environ. Edu. Res. 4, 429–444. doi: 10.1159/000353459
Papadimitriou, T., Kagalou, I., Stalikas, C., Pilidis, G., and Leonardos, I. D. (2012). Assessment of microcystin distribution and biomagnifications in tissues of aquatic food web compartments from a shallow lake. Ecotoxicology 21, 1155–1166. doi: 10.1007/s10646-012-0870-y
Park, H. D., Watanabe, M. F., Harada, K. I., Nagai, H., Suzuki, M., Watanabe, M., et al. (1993). Hepatotoxin (microcystin) and neurotoxin (anatoxin-a) contained in natural blooms and strains of cyanobacteria from Japanese waters. Nat. Toxins 1, 353–360. doi: 10.1002/nt.2620010606
Park, M.-H., Chung, I.-M., Ahmad, A., Kim, B.-H., and Hwang, S.-J. (2009). Growth inhibition of unicellular and colonial Microcystis strains (Cyanophyceae) by compounds isolated from rice (Oryza sativa) hulls. Aquat. Bot. 90, 309–314. doi: 10.1016/j.aquabot.2008.11.007
Paul, V. J. (2008). “Global warming and cyanobacterial harmful algal blooms,” in Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs, Advances in Experimental Medicine and Biology, Vol. 619, ed. H. K. Hudnell (New York, NY: Springer Press), 239–258.
Persson, K.-J., Legrand, C., and Olsson, T. (2009). Detection of nodularin in European flounder (Platichthys flesus) in the west coast of Sweden: evidence of nodularin mediated oxidative stress. Harmful Algae 8, 832–838. doi: 10.1016/j.hal.2009.03.003
Pilotto, L. S., Hobson, P., Burch, M. D., Ranmuthugala, G., Attewell, R., and Weightman, W. (2004). Acute skin irritant effects of cyanobacteria (blue-green algae) in healthy volunteers. Aust. N. Z. J. Public Health 28, 220–224. doi: 10.1111/j.1467-842X.2004.tb00479.x
Posch, T., Köster, O., Salcher, M. M., and Pernthaler, J. (2012). Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat. Clim. Change 2, 809–813. doi: 10.1038/nclimate1581
Pouria, S., de Andrade, A., Barbosa, J., Cavalcanti, R. L., Barreto, V. T. S., Ward, C. J., et al. (1998). Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 352, 21–26. doi: 10.1016/S0140-6736(97)12285-1
Prepas, E. E., Pinel-Alloul, B., Chambers, P. A., Murphy, T. P., Reedyk, S., Sandland, G., et al. (2001). Lime treatment and its effects on the chemistry and biota of hardwater eutrophic lakes. Freshwater Biol. 46, 1049–1060. doi: 10.1046/j.1365-2427.2001.00788.x
Preussel, K., Stüken, A., Wiedner, C., Chorus, I., and Fastner, J. (2006). First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon 47, 156–162. doi: 10.1016/j.toxicon.2005.10.013
Qin, B., Yang, L., Chen, F., Zhu, G., Zhang, L., and Chen, Y. (2006). Mechanism and control of lake eutrophication. Chinese Sci. Bull. 51, 2401–2412. doi: 10.1007/s11434-006-2096-y
Qiu, D., Wu, Z., Liu, B., Deng, J., Fu, G., and He, F. (2001). The restoration of aquatic macrophytes for improving water quality in a hypertrophic shallow lake in Hubei Province. China. Ecol. Eng. 1, 147–156. doi: 10.1016/S0925-8574(01)00074-X
Qiu, T., Xie, P., Liu, Y., Li, G., Xiong, Q., Hao, L., et al. (2009). The profound effects of microcystin on cardiac antioxidant enzymes, mitochondrial function and cardiac toxicity in rat. Toxicology 257, 86–94. doi: 10.1016/j.tox.2008.12.012
Raetz, C. R. H., and Whitfield, C. (2002). Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700. doi: 10.1146/annurev.biochem.71.110601.135414
Ramani, A., Rein, K., Shetty, K. G., and Jayachandran, K. (2012). Microbial degradation of microcystin in Florida’s freshwaters. Biodegradation 23, 35–45. doi: 10.1007/s10532-011-9484-y
Rantala, A., Fewer, D. P., Hisbergues, M., Rouhiainen, L., Vaitomaa, J., Börner, T., et al. (2004). Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. U.S.A. 101, 568–573. doi: 10.1073/pnas.0304489101
Rantala-Ylinen, A., Kana, S., Wang, H., Rouhiainen, L., Wahlsten, M., Rizzi, E., et al. (2011). Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 77, 7271–7278. doi: 10.1128/AEM.06022-11
Rastogi, R. P., and Sinha, R. P. (2009). Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 27, 521–539. doi: 10.1016/j.biotechadv.2009.04.009
Rastogi, R. P., Sinha, R. P., and Incharoensakdi, A. (2014). The cyanotoxin-microcystins: current overview. Rev. Environ. Sci. Bio/Technol. 13, 215–249. doi: 10.1007/s11157-014-9334-6
Reichwaldt, E. S., and Ghadouani, A. (2012). Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: between simplistic scenarios and complex dynamics. Water Res. 46, 1372–1393. doi: 10.1016/j.watres.2011.11.052
Rinehart, K. L., Harada, K., Namikoshi, M., Chen, C., and Harvis, C. A. (1998). Nodularin, microcystin, and the configuration of Adda. J. Am. Chem. Soc. 110, 8557–8558. doi: 10.1021/ja00233a049
Robinson, T. (1967). The Organic Constituents of Higher Plants. Minneapolis, MN: Burgess publishing Co.
Rodriguez, I. R., Amrhein, C., and Anderson, M. A. (2008). Reducing dissolved phosphorus loading to the Salton Sea with aluminum sulfate. Hydrobiologia 604, 37–44. doi: 10.1007/s10750-008-9313-4
Rouhiainen, L., Vakkilainen, T., Siemer, B. L., Buikema, W., Haselkorn, R., and Sivonen, K. (2004). Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Appl. Environ. Microbiol. 79, 686–692. doi: 10.1128/AEM.70.2.686-692.2004
Runnegar, M. T., Kong, S. M., Zhong, Y. Z., and Liu, S. C. (1994). The role of glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Biophys. Res. Commun. 201, 235–241. doi: 10.1006/bbrc.1994.1694
Ruttenberg, K. C., and Dyhrman, S. T. (2012). Dissolved organic phosphorus production during simulated phytoplankton blooms in a coastal upwelling system. Front. Microbiol. 3:274. doi: 10.3389/fmicb.2012.00274
Saito, K., Matsumoto, M., Sekine, T., and Murakoshi, I. (1989). Inhibitory substances from Myriophyllum brasiliense on growth of blue-green algae. J. Nat. Prod. 52, 1221–1226. doi: 10.1021/np50066a004
Saito, T., Okana, K., Park, H. D., Itayama, T., Inamori, Y., Neilan, B. A., et al. (2003). Detection and sequencing of the microcystin LR-degrading gene, mlrA, from new bacteria isolated from Japanese lakes. FEMS Microbiol. Lett. 229, 271–276. doi: 10.1016/S0378-1097(03)00847-4
Saker, M. L., Thomas, A. D., and Norton, J. H. (1999). Cattle mortality attributed to the toxic cyanobacterium Cylindrospermopsis raciborskii in an outback region of north Queensland. Environ. Toxicol. 14, 179–182. doi: 10.1002/(SICI)1522-7278(199902)14:1<179::AID-TOX23>3.0.CO;2-G
Sandrini, G., Cunsolo, S., Schuurmans, J. M., Matthijs, H. C. P., and Huisman, J. (2015). Changes in gene expression, cell physiology and toxicity of the harmful cyanobacterium Microcystis aeruginosa at elevated CO2. Front. Microbiol. 6:401. doi: 10.3389/fmicb.2015.00401
Schindler, D. W., Hecky, R. E., Findlay, D. L., Stainton, M. P., Parker, B. R., Paterson, M. J., et al. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37 year whole ecosystem experiment. Proc. Natl. Acad. Sci. U.S.A. 105, 11254–11258. doi: 10.1073/pnas.0805108105
Schrader, K. K., Dayan, F. E., Allen, S. N., de Regt, M. Q., Tucker, C. S., and Paul, R. N. Jr. (2000). 9,10-Anthraquinone reduces the photosynthetic efficiency of Oscillatoria perornata and modifies cellular inclusions. Int. J. Plant Sci. 161, 265–270. doi: 10.1086/314255
Schrader, K. K., Nanayakkara, N. P. D., Tucker, C. S., Rimando, A. M., Ganzera, M., and Schaneberg, B. T. (2003). Novel derivatives of 9,10-anthraquinone are selective algicides against the musty-odor cyanobacterium Oscillatoria perornata. Appl. Environ. Microbiol. 69, 5319–5327. doi: 10.1128/AEM.69.9.5319-5327.2003
Schrader, K. K., Rimando, A. M., Tucker, C. S., Glinski, J., Cutler, S. J., and Cutler, H. G. (2004). Evaluation of the natural product SeaKleen for controlling the musty-odor producing cyanobacterium Oscillatoria perornata in catfish ponds. N. Am. J. Aquacult. 66, 20–28. doi: 10.1577/A03-026
Seifert, M., McGregor, G., Eaglesham, G., Wickramasinghe, W., and Shaw, G. (2007). First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gomont) Speziale and Dyck. Harmful Algae 6, 73–80. doi: 10.1016/j.hal.2006.07.001
Shao, J., Li, R., Lepo, J. E., and Gu, J.-D. (2013). Potential for control of harmful cyanobacterial blooms using biologically derived substances: problems and prospects. J. Environ. Manage. 125, 149–155. doi: 10.1016/j.jenvman.2013.04.001
Shao, J., Wu, Z., Yu, G., Peng, X., and Li, R. (2009). Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): from views of gene expression and antioxidant system. Chemosphere 75, 924–928. doi: 10.1016/j.chemosphere.2009.01.021
Shao, J., Xu, Y., Wang, Z., Jiang, Y., Yu, G., Peng, X., et al. (2011). Elucidating the toxicity targets of b-ionone on photosynthetic system of Microcystis aeruginosa NIES- 843 (Cyanobacteria). Aquat. Toxicol. 104, 48–55. doi: 10.1016/j.aquatox.2011.03.014
Sieroslawska, A., and Rymuszka, A. (2015). Effects of cylindrospermopsin on a common carp leucocyte cell line. J. Appl. Toxicol. 35, 83–89. doi: 10.1002/jat.2990
Sims, J. K., and Zandee Van Rillaud, R. D. (1981). Escharotic stomatitis caused by the ‘stinging seaweed’ Microcoleus lyngbyaceus (formerly Lyngbya majuscula): a case report and review of literature. Hawaii Med. J. 40, 243–248.
Sivonen, K., and Jones, G. (1999). “Cyanobacterial toxins,” in Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, eds I. Chorus and J. Bartram (London: E and FN Spon), 41–111.
Smith, J. L., Boyer, G. L., and Zimba, P. V. (2008). A review of cyanobacterial odorous and bioactive metabolites: impacts and management alternatives in aquaculture. Aquaculture 280, 5–20. doi: 10.1016/j.aquaculture.2008.05.007
Smith, V. H. (2003). Eutrophication of freshwater and coastal marine ecosystems- a global problem. Environ. Sci. Pollut. Res. 10, 126–139. doi: 10.1065/espr2002.12.142
Smith, V. H., and Schindler, D. W. (2009). Eutrophication science: where do we go from here? Trends Ecol. Evol. 24, 201–207. doi: 10.1016/j.tree.2008.11.009
Song, K. Y., Lim, I. K., Park, S. C., Lee, S. O., Park, H. S., Choi, Y. K., et al. (1999). Effect of nodularin on the expression of glutathione S-transferase placental form and proliferating cell nuclear antigen in N-nitrosodiethylamine initiated hepato-carcinogenesis in the male Fischer 344 rat. Carcinogenesis 20, 1541–1548. doi: 10.1093/carcin/20.8.1541
Song, W., Bardowell, S., and O’shea, K. E. (2007). Mechanistic study and the influence of oxygen on the photosensitized transformations of microcystins (cyanotoxins). Environ. Sci. Technol. 41, 5336–5341. doi: 10.1021/es063066o
Soto-Liebe, K., Murillo, A. A., Krock, B., Stucken, K., Fuentes-Valdes, J. J., Trefault, N., et al. (2010). Reassessment of the toxin profile of Cylindrospermopsis raciborskii T3 and function of putative sulfotransferases in synthesis of sulfated and sulfonated PSP toxins. Toxicon 56, 1350–1361. doi: 10.1016/j.toxicon.2010.07.022
Sotton, B., Domaizon, I., Anneville, O., Cattanéo, F., and Guillard, J. (2015). Nodularin and cylindrospermopsin: a review of their effects on fish. Rev. Fish Biol. Fisheries 25, 1–19. doi: 10.1007/s11160-014-9366-6
Spencer, D. F. (1984). Influence of aquashade on growth, photosynthesis, and phosphorus uptake of microalgae. J. Aquat. Plant Manage. 22, 80–84.
Spoof, L., Berg, K. A., Rapala, J., Lahti, K., Lepistö, L., Metcalf, J. S., et al. (2006). First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environ. Toxicol. 21, 552–560. doi: 10.1002/tox.20216
Srivastava, A., Singh, S., Ahn, C.-Y., Oh, H.-M., and Asthana, R. K. (2013). Monitoring approaches for a toxic cyanobacterial bloom. Environ. Sci. Technol. 47, 8999–9013. doi: 10.1021/es401245k
Stewart, I., Seawright, A. A., and Shaw, G. R. (2008). “Cyanobacterial poisoning in livestock, wild mammals and birds–an overview,” in Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs, ed. H. H. Kenneth (Heidelberg: Springer), 613–638.
Stewart, I., Webb, P. M., Schluter, P. J., and Shaw, G. R. (2006a). Recreational and occupational field exposure to freshwater cyanobacteria - A review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment. Environ. Health 24:6. doi: 10.1186/1476-069X-5-6
Stewart, I., Webb, P. M., Schluter, P. J., and Shaw, G. R. (2006b). Cyanobacterial lipopolysaccharides and human health-A review. Environ. Health 24:7. doi: 10.1186/1476-069X-5-7
Strichartz, G., Rando, T., Hall, S., Gitschier, J., Hall, L., Magnani, B., et al. (1986). On the mechanism by which saxitoxin binds to and blocks sodium channels. Ann. N. Y. Acad. Sci. 479, 96–112. doi: 10.1111/j.1749-6632.1986.tb15564.x
Stucken, K., John, U., Cembella, A., Murillo, A. A., Soto-Liebe, K., Fuentes-Valdés, J. J., et al. (2010). The smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications. PLoS ONE 5:e9235. doi: 10.1371/journal.pone.0009235
Stuken, A., and Jakobsen, K. S. (2010). The cylindrospermopsin gene cluster Aphanizomenon sp. strain 10E6: organisation and recombination. Microbiology 156, 2438–2451. doi: 10.1099/mic.0.036988-0
Su, Z., Sheets, M., Ishida, H., Li, F., and Barry, W. H. (2004). Saxitoxin blocks L-Type/Ca. J. Pharmacol. Exp. Therapeut. 308, 324–329. doi: 10.1124/jpet.103.056564
Sueoka, E., Sueoka, N., Okabe, S., Kozu, T., Ohta, T., Suganuma, M., et al. (1997). Expression of the tumor necrosis factor alpha gene and early response genes by nodularin, a liver tumor promoter in primary cultured rat hepatocytes. J. Cancer Res. Clin. Oncol. 123, 413–419. doi: 10.1007/BF01372544
Sukenik, A., Quesada, A., and Salmaso, N. (2015). Global expansion of toxic and non-toxic cyanobacteria: effect on ecosystem functioning. Biodivers. Conserv. 24, 889–908. doi: 10.1007/s10531-015-0905-9
Taylor, M. S., Stahl-Timmins, W., Redshaw, C. H., and Osborne, N. J. (2014). Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae 31, 1–8. doi: 10.1016/j.hal.2013.09.003
Terao, K., Ohmori, S., Igarashi, K., Ohtani, I., Watanabe, M. F., Harada, K. I., et al. (1994). Electron-microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green-alga Umezakia natans. Toxicon 32, 833–843. doi: 10.1016/0041-0101(94)90008-6
Tillett, D., Dittmann, E., Erhard, M., von Döhren, H., Börner, T., and Neilan, B. A. (2000). Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 7, 753–764. doi: 10.1016/S1074-5521(00)00021-1
Tomitani, A., Knoll, A. H., Cavanaugh, C. M., and Ohno, T. (2006). The evolutionary diversification of cyanobacteria: molecular phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. U.S.A. 103, 5442–5447. doi: 10.1073/pnas.0600999103
Torokne, A., Palovics, A., and Bankine, M. (2001). Allergenic (sensitization, skin and eye irritation) effects of freshwater cyanobacteria-experimental evidence. Environ. Toxicol. 16, 512–516. doi: 10.1002/tox.10011.abs
Tsuji, K., Watanuki, T., Kondo, F., Watanabe, M. F., Suzuki, S., Nakazawa, H., et al. (1995). Stability of microcystins from cyanobacteria-II. Effect of UV light on decomposition and isomerization. Toxicon 33, 1619–1631. doi: 10.1016/0041-0101(95)00101-8
Tyler, A. N., Hunter, P. D., Carvalho, L., Codd, G. A., Elliott, J. A., Ferguson, C. A., et al. (2009). Strategies for monitoring and managing mass populations of toxic cyanobacteria in recreational waters: a multi-interdisciplinary approach. Environ. Health 8:S11. doi: 10.1186/1476-069X-8-S1-S11
Ufelmann, H., and Schrenk, D. (2015). Nodularin-triggered apoptosis and hyperphosphorylation of signaling proteins in cultured rat hepatocytes. Toxicol. In Vitro 29, 16–26. doi: 10.1016/j.tiv.2014.08.008
Van Hullebusch, E., Deluchat, V., Chazal, P. M., and Baudu, M. (2002). Environmental impact of two successive chemical treatments in a small shallow eutrophied lake, Part II: case of copper sulfate. Environ. Pollut. 120, 627–634. doi: 10.1016/S0269-7491(02)00191-4
Várkuti, A., Kovács, K., Stenger-Kovács, C., and Padisák, J. (2008). Environmental awareness of the permanent inhabitants of towns and villages on the shores of Lake Balaton with special reference to issues related to global climate change. Hydrobiologia 599, 249–257. doi: 10.1007/s10750-007-9190-2
Vepritskii, A. A., Gromov, B. V., Titota, N. N., and Mamkaeva, K. A. (1991). Production of the antibiotic algicide cyanobacterin LU-2 by a filamentous cyanobacterium Nostoc sp. Mikrobiologia 60, 21–25.
Verdier-Pinard, P., Lai, J.-Y., Yoo, H.-D., Yu, J., Marquez, B., Nagle, D. G., et al. (1998). Structure-activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol. Pharmacol. 53, 62–76.
Verspagen, J. M. H., Van de Waal, D. B., Finke, J. F., Visser, P. M., Donk, E. V., and Huisman, J. (2014). Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PLoS ONE 9:e104325. doi: 10.1371/journal.pone.0104325
Visser, P. M., and Ibelings, B. W. (1996). Artificial mixing prevents nuisance blooms of the cyanobacterium Microcystis in Lake Niewe Meer. The Netherlands. Freshwater Biol. 36, 435–450. doi: 10.1046/j.1365-2427.1996.00093.x
Wang, G., Zhang, L., Chua, H., Li, X., Xia, M., and Pu, P. (2009). A mosaic community of macrophytes for the ecological remediation of eutrophic shallow lakes. Ecol. Eng. 35, 582–590. doi: 10.1016/j.ecoleng.2008.06.006
Wang, J. F., Wu, P. F., Chen, J., and Yan, H. (2010). Biodegradation of microcystin-RR by a new isolated Sphingopyxis sp USTB- 05. Chin. J. Chem. Eng. 18, 108–112. doi: 10.1016/S1004-9541(08)60330-4
Watanabe, M. F., Tsujimura, S., Oishi, S., Niki, T., and Namikoshi, M. (2003). Isolation and identification of homoanatoxin-a from a toxic strain of the cyanobacterium Raphidiopsis meditarranea Skuja isolated from Lake Biwa. Japan. Phycol. 42, 364–369. doi: 10.2216/i0031-8884-42-4-364.1
Whitehead, P. G., Wilby, R. L., Battarbee, R. W., Kernan, M., and Wade, A. J. (2009). A review of the potential impacts of climate change on surface water quality. Hydrol. Sci. J. 54, 101–123. doi: 10.1623/hysj.54.1.101
Wiegand, C., and Pflugmacher, S. (2005). Ecotoxicological effects of selected cyanobacterial secondary metabolites: a short review. Toxicol. Appl. Pharmacol. 203, 201–218. doi: 10.1016/j.taap.2004.11.002
Wörmer, L., Huerta-Fontela, M., Cire’s, S., Carrasco, D., and Quesada, A. (2010). Natural photodegradation of the cyanobacterial toxins microcystin and cylindrospermopsin. Environ. Sci. Technol. 44, 3002–3007. doi: 10.1021/es9036012
Wu, M., Okino, T., Nogle, L. M., Marquez, B. L., Williamson, R. T., Sitachitta, N., et al. (2000). Structure, synthesis, and biological properties of kalkitoxin, a novel neurotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 122, 12041–12042. doi: 10.1021/ja005526y
Wu, Y., Liu, J., Yang, L., Chen, H., Zhang, S., Zhao, H., et al. (2011). Allelopathic control of cyanobacterial blooms by periphyton biofilms. Environ. Microbiol. 13, 604–615. doi: 10.1111/j.1462-2920.2010.02363.x
Xiao, X., Huang, H., Ge, Z., Rounge, T. B., Shi, J., Xu, X., et al. (2014). A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison of their anti-cyanobacterial activities. Environ. Microbiol. 16, 1238–1251. doi: 10.1111/1462-2920.12226
Xu, H., Wang, H., Xu, Q., Lv, L., Yin, C., Liu, X., et al. (2015). Pathway for biodegrading microcystin-YR by Sphingopyxis sp. USTB-05. PLoS ONE 10:e0124425. doi: 10.1371/journal.pone.0124425
Xu, L., Shen, J., Marinova, D., Guo, X., Sun, F., and Zhu, F. (2013). Changes of public environmental awareness in response to the Taihu blue-green algae bloom incident in China. Environ. Dev. Sustain. 15, 1281–1302. doi: 10.1007/s10668-013-9440-6
Yang, F., Wei, H. Y., Li, X. Q., Li, Y. H., Li, X. B., Yin, L. H., et al. (2013). Isolation and characterization of an algicidal bacterium indigenous to Lake Taihu with a red pigment able to lyse Microcystis aeruginosa. Biomed. Environ. Sci. 26, 148–154. doi: 10.3967/0895-3988.2013.02.009
Yang, F., Zhou, Y., Sun, R., Wei, H., Li, Y., Yin, L., et al. (2014a). Biodegradation of microcystin-LR and-RR by a novel microcystin-degrading bacterium isolated from Lake Taihu. Biodegradation 25, 447–457. doi: 10.1007/s10532-013-9673-y
Yang, F., Zhou, Y., Yin, L., Zhu, G., Liang, G., and Pu, Y. (2014b). Microcystin-degrading activity of an indigenous bacterial strain Stenotrophomonas acidaminiphila MCLTH2 isolated from Lake Taihu. PLoS ONE 9:e86216. doi: 10.1371/journal.pone.0086216
Yoshizawa, S., Matsushima, R., Watanabe, M. F., Harada, K.-I., Ichihara, A., Carmichael, W. W., et al. (1990). Inhibition of protein phosphatases by microcystis and nodularin associated with hepatotoxicity. J. Cancer Res. Clin. Oncol. 116, 609–614. doi: 10.1007/BF01637082
Yuan, G., Xie, P., Zhang, X., Tang, R., Gao, Y., Li, D., et al. (2012). In vivo studies on the immunotoxic effects of microcystins on rabbit. Environ. Toxicol. 27, 83–89. doi: 10.1002/tox.20615
Žegura, B., Štraser, A., and Filipič, M. (2011). Genotoxicity and potential carcinogenicity of cyanobacterial toxins-a review. Mutat. Res. 727, 16–41. doi: 10.1016/j.mrrev.2011.01.002
Zhang, D., Xie, P., Liu, Y., and Qiu, T. (2009). Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health. Sci. Total Environ. 407, 2191–2199. doi: 10.1016/j.scitotenv.2008.12.039
Zhang, J., Shi, H., Liu, A., Cao, Z., Hao, J., and Gong, R. (2015). Identification of a new microcystin-degrading bacterium isolated from lake Chaohu. China. Bull. Environ. Contam. Toxicol. 94, 661–666. doi: 10.1007/s00128-015-1531-7
Zhang, J., Wang, Z., Song, Z. Y., Xie, Z. C., Li, L., and Song, L. R. (2012). Bioaccumulation of microcystins in two freshwater gastropods from a cyanobacteria-bloom Plateau Lake, Lake Dianchi. Environ. Pollut. 164, 227–234. doi: 10.1016/j.envpol.2012.01.021
Zhang, J., Xie, Z., Jiang, X., and Wang, Z. (2014). Control of cyanobacterial blooms via synergistic effects of pulmonates and submerged plants. Clean Soil Air Water 42, 1–6.
Zhang, X., Hu, H. Y., Hong, Y., and Yang, J. (2008). Isolation of a Poterioochromonas capable of feeding on Microcystis aeruginosa and degrading microcystin-LR. FEMS Microbiol. Lett. 288, 241–246. doi: 10.1111/j.1574-6968.2008.01355.x
Zhang, X., Xie, P., Li, D., Shi, Z., Wang, J., Yuan, G., et al. (2011). Anemia induced by repeated exposure to cyanobacterial extracts with explorations of underlying mechanisms. Environ. Toxicol. 26, 472–479. doi: 10.1002/tox.20583
Zhang, Y., Ghadouani, A., Prepas, E. E., Pinel-Alloul, B., Reedyk, S., Chambers, P. A., et al. (2001). Response of plankton communities to whole-lake Ca(OH)(2) and CaCO3 additions in eutrophic hardwater lakes. Freshwater Biol. 46, 1105–1119. doi: 10.1046/j.1365-2427.2001.00793.x
Keywords: cyanobacteria, eutrophication, cyanobacterial blooms, cyanotoxins, ecotoxicology, mitigation strategies
Citation: Rastogi RP, Madamwar D and Incharoensakdi A (2015) Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 6:1254. doi: 10.3389/fmicb.2015.01254
Received: 18 June 2015; Accepted: 28 October 2015;
Published: 17 November 2015.
Edited by:
Federico Lauro, University of New South Wales, AustraliaReviewed by:
Xavier Mayali, Lawrence Livermore National Laboratory, USAGurjeet Singh Kohli, University of Technology Sydney, Australia
Copyright © 2015 Rastogi, Madamwar and Incharoensakdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aran Incharoensakdi, YXJhbi5pQGNodWxhLmFjLnRo
 Rajesh P. Rastogi
Rajesh P. Rastogi Datta Madamwar
Datta Madamwar Aran Incharoensakdi
Aran Incharoensakdi