- 1Medicinal Chemistry Laboratory, Department of Chemistry, Jamia Millia Islamia, New Delhi, India
- 2Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, New Delhi, India
Diarrhea is the manifestation of gastrointestinal infection and is one of the major causes of mortality and morbidity specifically among the children of less than 5 years age worldwide. Moreover, in recent years there has been a rise in the number of reports of intestinal infections continuously in the industrialized world. These are largely related to waterborne and food borne outbreaks. These occur by the pathogenesis of both prokaryotic and eukaryotic organisms like bacteria and parasites. The parasitic intestinal infection has remained mostly unexplored and under assessed in terms of therapeutic development. The lack of new drugs and the risk of resistance have led us to carry out this review on drug development for parasitic diarrheal diseases. The major focus has been depicted on commercially available drugs, currently synthesized active heterocyclic compounds and unique drug targets, that are vital for the existence and growth of the parasites and can be further exploited for the search of therapeutically active anti-parasitic agents.
Introduction
Diarrhea is a symptom of an infection in the intestinal tract, which can be caused by variety of bacterial, viral, and parasitic organisms. Infection spreads through contaminated food or drinking water or from person to person as a result of poor hygiene. Diarrheal diseases is the second leading cause of death in children under 5 years and is responsible for killing around 760,000 children every year (World Health Organization, 2013). The common symptoms include the frequent bowel movements, loose watery stool, incontinence, lower abdominal pain, or cramping and in severe cases it causes blood or flecks of mucus in the stool. The dreadful infection leads to the loss of appetite and patient is more likely to suffer from loss of weight. There are a number of non-infectious medical conditions that may cause diarrhea too. The inability in digesting dairy products which includes lactose intolerance, coeliac disease which is an intolerance of gluten in wheat and some other grains and pancreatic problems (cystic fibrosis) which interfere with production of important digestive substances (Haque et al., 2003). Several agents that cause diarrhea include viruses (rotavirus or Norwalk virus, enterovirus, or a hepatitis virus), bacteria (Shigella species, Escherichia coli, and Campylobacter jejuni) and parasites (Giardia lamblia, Entamoeba histolytica, and Cryptosporidium species; Shah et al., 2009). Diarrhea caused by parasites is unlike that of either bacterial or viral infections. For instance, Giardia has a slow onset of diarrhea and can be present for months, while most bacterial and viral infections are limited to 1–2 weeks (Petri, 2003). In addition, parasites are eukaryotic, which makes them larger and more complex than either viruses or bacteria and also more difficult to eradicate due to their similarity to the host. Though the infection is caused by a number of parasites which mainly include G. lamblia, E. histolytica, Cryptosporidium parvum, Cyclospora cayetanensis, Isospora belli, Blastocystis hominis but the enzyme immunoassays are only available for the testing of three parasites G. lamblia, E. histolytica, and C. parvum (Slack, 2012).
Many of the new drug targets are being discovered with much more ease as the genomic sequences of many parasitic organisms are becoming available. This advancement has enabled researchers not only to identify new biochemical pathways and gene families that can be short-listed as potential drug targets but also has significantly increased their basic understanding of parasites that cause some of the most severe diseases. Moreover, biochemical analysis and genome sequencing both have helped in identifying potential targets, enzymes, transporters, and metabolites that are distinct in parasites and their mammalian host (Loftus et al., 2005). As a consequence one can design even better and safer effective drugs that can be used in future to combat the disease caused by the organism. The heterocyclic compounds with different modifications have been synthesized and their biological evaluation have identified them to fight the diarrhea causing parasites. In the following discussion some of the most important parasites causing diarrhea, their drugs, important traced targets and the active heterocyclic compounds synthesized against them have been summarized.
Microsporidia
Enterocytozoon bieneusi, Encephalitozoon cuniculi, and Encephalitozoon intestinalis are the reported species of microsporidia which are the causative agents of chronic diarrhea predominantly in immunocompromised individuals such as AIDS patients (Blanshard et al., 1992; Chokephaibulkit et al., 2001; Goodgame, 2003). The diarrheal disease caused by microsporodia spp. is treated by drugs fumagilin (1) and albendazole (2) (Goodgame, 2003; Agholi et al., 2013). The polyamine analogs, fumagillin-related compounds and analogs, nikkomycins (3), fluoroquinolones (4–9) and benzimidazole-related compounds are the active sources of new compounds against microsporidiosis. Fumagillin, a natural product (1) is an antibiotic and anti-angiogenic compound produced by Aspergillus fumigatus and has been found potent against Encephalitozoon spp. and E. bieneusi in vitro (Bacchi and Weiss, 2004). The antimicrosporidial activity of various fluoroquinolones derivatives such as norfloxacin (4) and ofloxacin (5) is more than gatifloxacin (6), lomefloxacin (7), moxifloxacin (8), and nalidixic acid (9) and can be the effective option of chemotherapy (Didier et al., 2005). It was observed that TNP-470 (10), ovalicin (11) and its derivatives inhibited E. intestinalis replication by more than 70% in vitro which indicates that these compounds can be used as medication for this infection (Didier et al., 2006). It has been also reported that the use of chlorine and ozone as disinfectants kills the E. intestinalis in water (John et al., 2005).
The albendazole, a benzimidazole derivative inhibits the microtubule assembly in E. intestinalis but not in E. bieneusi infections because benzimidazoles bind to the colchicine-binding site of β-tubulin monomer prior to dimerization with α-tubulin which blocks subsequent microtubule formation (Macdonald et al., 2004). The β-tubulin subunit is the primary target of benzimidazole, where its predictive sensitive residues are Cys 165, Phe 167, Glu 198, Phe 200, Arg 242, and Val 268 (Macdonald et al., 2004; Tremoulet et al., 2004). It has been also reported that microtubule assembly gets inhibited due to the presence of hydrogen atom at the 1-position and a methoxycarbonylamino group at the 2-position of the benzimidazole ring (Friedman and Platzer, 1980). Fumagillin along with its analogs act on the methionine aminopeptidase type 2(MetAP2) by non-competitive inhibition, i.e., by irreversibly blocking the active site in E. cuniculi and it seems to be the potent inhibitor (Molina et al., 2002). Analysis of EcMetAP2 demonstrated conservation of the key residues associated with the active site of this class of enzymes including Asp251, Asp262, His331, Glu364, and Glu459 (involved in coordination of metal binding); His231 (to which fumagillin covalently binds); and Phe219, Leu328, Ile338, His339, Asp378, Tyr444, Leu447 (involved in binding substrates into the active site; Upadhya et al., 2006). The aspartic proteases are a family of protease enzymes that use two highly conserved aspartic acid residues in the active site for catalytic cleavage of their peptide substrates (Pozio and Morales, 2005). Ritonavir (12) and indinavir (13) drugs are inhibitors of aspartyl protease, used in the highly-active antiretroviral therapy cocktail, inhibited the growth of E. intestinalis in tissue culture due to intermolecular hydrogen bonding between -NH group of these drugs with aspartic acid residue (Menotti et al., 2005; Pozio and Morales, 2005). The polyamine analogs are important as they act by causing depletion of polyamines, due to excessive acetylation and excretion of intracellular amines, followed by cytostasis, apoptosis and cell death (Bacchi et al., 2001). In sporoblasts of E. cuniculi, the chitin deacetylase activity (EcCDA) is present in the inner part of the cell wall tightly associated with the plasma membrane. This location was analyzed by biochemical and sequence data, and suggests a role in cell-wall formation. Late sporogony was characterized by the thickening of the chitin-containing endospore, but the mechanism of chitin deposition is unknown. Since the EcCDA enzyme is regularly distributed at the plasma membrane throughout sporogony, chitin may be produced all around the cell (Brosson et al., 2005). The chitin is potent target of nikkomycins due to their deacetylation activity (Bacchi et al., 2001). The fluoroquinolones interact with E. cuniculi targets significantly with DNA topoisomerases, which are the enzymes that have evolved to solve the topological problems associated with DNA metabolisms such as transcription, replication, packing and unpacking of DNA in the cell (Hooper, 2000). So, present research studies are concentrating on compounds that target microsporidian polyamines (e.g., polyamine analogs), Chitin (e.g., nikkomycins), and DNA topoisomerases (e.g., fluoroquinolones; Bacchi et al., 2001; Zhang et al., 2005; Didier and Weiss, 2006).
Entamoeba histolytica
E. histolytica is the causative agent of amoebiasis, a contagious disease of the human gastrointestinal tract (Tengku and Norhayati, 2011; Watanabe et al., 2011). It is an organism implicated in both diarrheal disease and invasive disease such as liver abscesses. Metronidazole (14) is the first line medication used against the infection but long-term uses produce several side effects in patients (Ordaz-Pichardo et al., 2005). It is potentially carcinogenic to humans because it is genotoxic to human cells (Bendesky et al., 2002). Furthermore, resistance of E. histolytica to standard drug metronidazole and relapses of intestinal and hepatic amoebiasis have been reported (Becker et al., 2011; Hwang et al., 2011). The authors have earlier reported diverse functionalized organic compounds synthesized and screened against E. histolytica (Azam and Agarwal, 2007, 2015). Nitroimidazole compounds have been used to treat a number of anaerobic bacteria and pathogenic protozoan infections, including Trichomonas vaginalis, E. histolytica, and G. lamblia since decades (Müller, 1983; Upcroft et al., 1999). Presently, metronidazole, tinidazole (15) and ornidazole (16) are highly recommended drugs for the treatment of protozoal infections (Azam and Agarwal, 2007). The interesting fact about the metronidazole analogs as an alternative to the treatment of parasitic infections is facilitated by the fact that not only the metronidazole is an effective drug but it also has a side chain which provides an opportunity to carry out various modifications whose significant activity has been reported (Kucik et al., 2004).
Chalcones are the important class of antamoebic drugs and their analogs have been synthesized having better activity. A series of chalcones were synthesized bearing N-substituted ethanamine by aldol condensation reaction and the compound (17) was found to be very potent amoebicidal indicating that such compounds can be the effective therapeutic candidates (Zaidi et al., 2015). Metronidazole hydrazone conjugates were synthesized and screened in vitro for antiamoebic activity, the compound (18) was found more active (Ansari et al., 2015). A series of chloroquinoline-acetamide hybrids were synthesized and the compound (19) inhibit the growth of E. histolytica (Inam et al., 2015). The N-acylhydrazones derived from 7-chloro-4-piperazin-1-yl-quinoline were evaluated against E. histolytica and the compound (20) was more potent than metronidazole (Inam et al., 2014). Metronidazole-triazole hybrids (21) showed amoebicidal activity (Negi et al., 2014). Coordination complexes do exhibit the anti-amoebic activity and the efficacy depends on the selection of both central metal atom and ligands. The terpyridine ligand complexed with divalent metals viz Cu, Co, Mn, Ni, and Zn yield the coordination complexes of general formula [Metal(Fctpy)2][PF6]2. The complex Ni(Fctpy)2][PF6]2 (22) has been found to be having most promising activity against E. histolytica and therefore, can be used as significant drug candidates for amoebiasis (Juneja et al., 2014). Hydrazone and oxadiazoline derivatives of 2-methyl-5-nitro-1H-imidazole were being synthesized and evaluated in vitro against HM1:IMSS strain of E. histolytica and it was observed that compounds (23, 24) were more potent against amoebiasis (Wani et al., 2013). A series of pyrazoline derivatives were synthesized and their in vitro screening against HM1: IMSS strain of E. histolytica, results showed the compounds (2E)-1,3-bis(4-methylphenyl)prop-2-en-1-one (25) and 1-(4,5-dihydro-5-(4-methoxyphenyl)-3-phenylpyrazol-1-yl)- 2-(5-(4-methoxyphenyl)-1H-tetrazol-1-yl)ethanone (26) exhibited the excellent antiamoebic activity and were having the IC50-values of 4.19 and 1.16 μM, respectively (Wani et al., 2012b). Compounds bearing a tetrazole and triazine ring motifs in conjugation with a sulphonamide group were synthesized and N, N∕-6-(1,3-benzodioxol-5-yl)-(1,3,5-triazine-2,4-diyl)-bis-4-nitrobenzene sulphonamide (27) was found to be more potent antiamoebic in nature and was having IC50-value of 1.02 μM (Wani et al., 2012a). The in vitro antiamoebic activity of 4,6 aminopyrimidines and their sulphonamide derivatives was investigated against E. histolytica and it was found that the compound N-[4-(2-chlorophenyl)-6-phenylpyrimidin-2-yl]-benzenesulphonamide (28) having IC50-value 0.44 μM was most active (Siddiqui and Azam, 2014). Various derivatives of dioxazoles were synthesized and in vitro analysis showed that the compound 5-(4-methoxy-phenyl)-3-(5-nitro-2-thienyl)-1,4,2-dioxazole (29) was having IC50-value 1.60 μM and inhibited the growth of E. histolytica significantly (Irfan et al., 2010). Thiosemicarbazides are the focused moieties for antiamoebic drug synthesis and the attempt was made to synthesize a novel series of 4-substituted 1-{[4-(10,15,20-triphenylporphyrin-5-yl)phenyl]methylidene}thiosemicarbazide and their antiamoebic activity was investigated. The 4-(3-methylphenyl)-1-{[4-(10,15,20-triphenylporphyrin-5-yl)phenyl] methylidene}thiosemicarbazide (30) with an electron-withdrawing group attached to N(4) exhibited the best antiamoebic activity and was having IC50-value 0.538 μM (Bhat et al., 2008). A new series of thiosemicarbazones of 7-hydroxy-8-acetylcoumarin with different thiosemicarbazides were synthesized and were tested against E. histolytica. The 7-hydroxy-8-acetylcoumarin-N(4,4)methylbenzyl thiosemicarbazone (31) was having IC50-value 1.06 μM and found to be antiamoebic (Iqbal et al., 2009). Thiocarbamoyl bis-pyrazoline derivatives were synthesized by cyclization of chalcones with N-4 substituted thiosemicarbazides under basic conditions and the evaluation showed that the compound(4-nitrophenylamino)[5-(4-{1-[(4-nitrophenylamino)thioxomethyl]-3-phenyl(2-pyrazoline-5-yl-phenyl)}-3-phenyl(2-pyrazolinyl)]methane-1-thione (32) was having IC50-value 0.42 μM and predominantly active as an amoebicidal candidate (Bhat et al., 2009a). An attempt was made to synthesize the amino-5-substituted-(3-phenyl(2-pyrazolinyl))methane-1-thione derivatives, 2-(5-substituted-3-phenyl-2-pyrazolinyl)-1,3-thiazolino[5,4-b] quinoxaline derivatives, and various chalcones derivatives for evaluation against HM1:IMSS strain of E. histolytica. The quinoxaline2-{5-[2-(methylethyl)phenyl]-3-phenyl- 2-pyrazolinyl}-1,3-thiazolino[5,4-b]quinoxaline (33) showed most promising antiamoebic activity with IC50-value of 0.17 μM. The results suggested that the further modification of such compounds can enhance their efficacy (Budakoti et al., 2009). The synthesis of bis-ferrocenyl-substituted core-modified porphyrins derivatives were synthesized under acidic conditions and their in vitro screening was performed against HM1:IMSS strain of E. histolytica. The promising results showed the1,1∕-(10,15-diphenyl-21,23-dithiaporphine-5,20-diyl)bis[2-{[methyl(phenylmethyl)amino]methyl}-ferrocene] (34) having IC50-value of 0.59 μM was extremely potent (Bhat et al., 2009b). A library of isothiourea derivatives was synthesized and the maximum activity was shown by compound (35) with IC50-value 2.48 μM. These are the amphiphilic compounds and exist in cationic form which makes them active in biological systems (Kazimierczuk et al., 2010). Benzimidazoles proved to be having antiparasitic activity and 5(6)-chloro-1H-benzimidazole-2-(3H)-thione (36) was having IC50-value of 0.005 μM for E. histolytica which indicates that these derivatives are having higher activity than metronidazole (Valdez et al., 2002). A series of ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives were synthesized by Beirut reaction and evaluated against HM1:IMSS strain of E. histolytica. The methyl 2-acetyl-3-methyl-quinoxaline-7-carboxylate 1,4-di-N-oxide (37) and ethyl 2-amide-3-methylquinoxaline-7-carboxylate 1,4-di-N-oxide (38) were having IC50-value 1.41 and 1.47 μM, respectively and showed higher potency toward amoebicidal activity (Duque-Montaño et al., 2013). The benzologue derivative of nitazoxanide 2-{[(6-Nitro-1, 3-benzothiazol-2-yl)amino]carbonyl}phenyl acetate(39) having IC50-value 0.297 μM showed better antiamoebic activity as compared to metronidazole and indicated that such derivatives can be explored for the search of effective chemotherapy (Navarrete-Vazquez et al., 2011). The amide derivatives of trifluoromethionine (TFM) have been observed to have excellent activity against E. histolytica. Using the TFM as lead 3,4-dimethoxyanilide trifluoromethionine (40) with methoxy group at meta and para positions was having IC50-value 1.19 μM and was observed most active in vitro evaluation (Sato et al., 2010). The compound 1,5-bis[4-(5-methoxy-1H-benzimidazole-2-yl) phenoxy]pentane (41) was evaluated in vitro against E. histolytica showed remarkable IC50-value 0.109 μM for amoebicidal potency due to methoxy group attached to benzimidazole ring (Torres-Gomez et al., 2008b). Acetamide and sulfonamide derivatives of imidazole viz N-benzyl-2-(2-methyl-4-nitro-1H-imidazol-1-yl)acetamide (42) and 2-methyl-1-[(4-methylphenyl)sulfonyl]-4-nitro-1H-imidazole (43) are important class of antiparasitic agents and were having IC50-value 3.96 and 3.55 μM, respectively (Hernández-Nunez et al., 2009). Phenyl hydrazine derivatives were synthesized and it was observed that (E)-1-(3-nitrobenzylidene)-2-phenylhydrazine (44) was most potent against HM1:IMSS strain of E. histolytica in vitro having IC50-value 0.84 μM. This indicated that hydrazine derivatives can be explored for the search of effective chemotherapeutic agent for amoebiasis and their mechanism of action needs to be elucidated (Toledano-Magana et al., 2015).
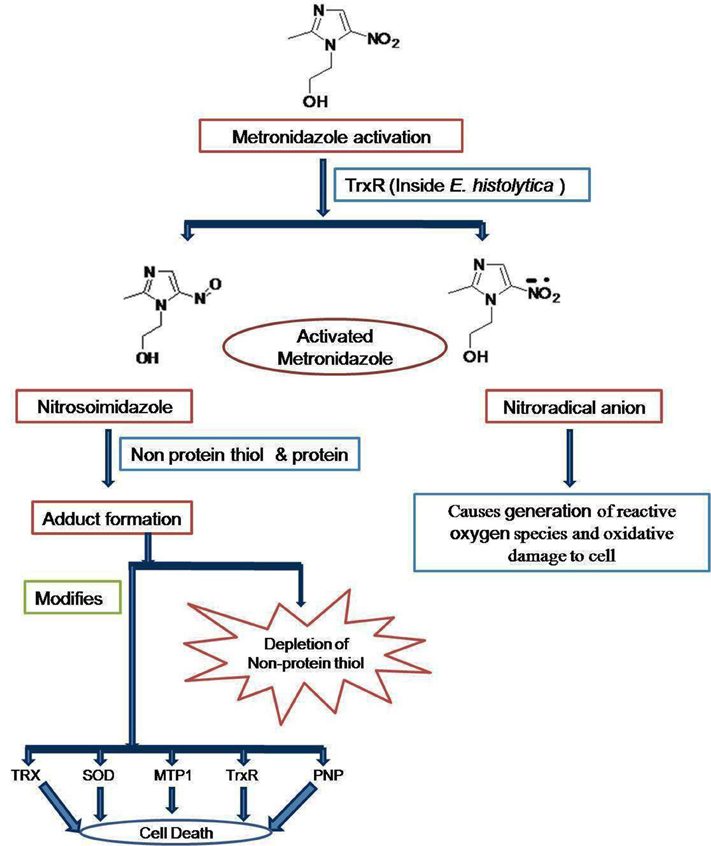
Emphasizing the elucidated drug targets and mechanism, the mode of action of metronidazole lies in that it gets reduced to nitroradical anion or nitrosoimidazole by thioredoxin reductase (TrxR) inside E. histolytica cell (Leitsch et al., 2007). The nitroradical anion formed reduces O2 and thereby generates reactive oxygen species, which are highly noxious to the cells of microaerophilic E. histolytica. However, the nitroso imidazole reacts with non-protein thiols or proteins to forms adduct. The adduct causes depletion of non-protein thiols and the modification of thioredoxin reductase (TrxR), thioredoxin (Trx), superoxide dismutase (SOD), metronidazole target protein 1 (Mtp1), and purine nucleoside phosphorylase (PNP). The combination of proteins with activated metronidazole makes the cells more vulnerable to oxidative stress and thereby kills E. histolytica (Figure 1; Samarawickrema et al., 1997; Leitsch et al., 2007). 5-Nitroimidazoles have been the mainstay of treatment, but resistance is a concern and new drug targets are needed. One potential target is the biosynthetic pathway for cysteine which is crucial for growth and various cellular activities (Diamond et al., 1978; Gilin and Diamond, 1980). Besides being precursor for protein biosynthesis, cysteine may compensate for the lack of glutathione, a major component of oxidative stress resistance in many organisms (Fahey et al., 1984; Loftus et al., 2005). It is also needed in the attachment to matrix, elongation and mobility of E. histolytica cells (Gilin and Diamond, 1980). In this organism, the condensation of O- acetylserine with sulfide is the major route of cysteine biosynthesis, which involves two key enzymes: O-acetyl-L-serine sulfhydrylase (OASS) and serine acetyltransferase (SATase; Nozaki et al., 1998). In general, in plants and most known organisms, the cysteine biosynthesis pathway is regulated both by the interaction of SAT and OASS to form the cysteine synthase complex (CSC) under sulfur sufficient condition but this type of regulation is absent in E. histolytica. These enzymes have been demonstrated to play a central role in controlling the intracellular cysteine concentration in E. Histolytica (Nozaki et al., 1999) and block of cysteine biosynthesis is therefore a possible strategy for inhibiting growth of E. histolytica (Ali and Nozaki, 2007). E. histolytica along with a number of other parasitic protists utilizes an unusual form of phosphofructo-1-kinase (PFK; EC 2.7.1.90) in a central step in carbohydrate metabolism. A homology model of E. histolytica PPi-PFK was constructed and screened with various bisphosphonates, which are analogs of pyrophosphate with a carbon instead of oxygen atom and synthetic pyrophosphate. It was found that the Compounds BA49280E (45) and CGP42446A (46) were good inhibitors of PFK in E. histolytica. Based on this study it has been suggested that PFK is attractive target for antiamoebic drugs (Reeves et al., 1974, 1976). Triosephosphate isomerase is another relatively well-studied glycolytic enzyme with both its sequence and structure known in a large number of organisms including E. histolytica (Rodriguez-Romero et al., 2002). It has been proposed to be a target for drug design due to presence of a cysteine residue (Cys 14) at the dimer interface in E. histolytica (Gómez-Puyou et al., 1995). Alcohol dehydrogenase 2 (EhADH2) is a bifunctionl 97 kDa polypeptide having the alcohol and aldehyde (ALDH) dehydrogenase activities utilize NAD and Fe2+ as cofactor (Bruchhaus and Tannich, 1994). This enzyme does not have a homolog in man. However, E. histolytica possesses other NADP dependent ADH and ALDH enzymes that could serve a similar function i.e., EhADH1, EhADH3 and EhALDH1. EhADH1 enzyme does not utilize acetyl CoA as substrate thereby suggesting that EhADH2 is solely responsible for the conversion of acetyl CoA to acetaldehyde (Zhang et al., 1994). Based on this data it has been suggested that EhADH2 could also serve as a target for antiamoebic drugs. Calcium is an important secondary messenger in many signal transduction pathways, is thought to be involved in the pathogenesis of amoebiasis and the prevention of the influx of Ca2+ can be the potent target (Meza, 2000). In E. histolytica, a number of calcium binding proteins have been identified. More importantly two distinct calcium binding proteins EhCaBP1 and EhCaBP2, have been characterized and their role has been simultaneously approached (Sahoo et al., 2003). It has been characterized that cyclosporine A inhibits calcineurin and P-glycoprotein activity and thereby inhibits proliferation of E. histolytica (Carrero et al., 2004). Recently it has been shown that when expression of EhCaBP1(a calmodulin containing 134 amino acid long proteins demonstrated to be essential for Entamoeba) is blocked, the cellular proliferation in E. histolytica gets inhibited (Sahoo et al., 2004).
Giardia lamblia
G. lamblia, an amitochondriate parasite is the major causative agent of human diarrheal disease, infecting an estimated 10% of the world's population both endemically and epidemically (Huang and White, 2006). For the treatment of the intestinal infections caused by G. lamblia at least six different classes of drugs are used, but the drugs containing 5-nitroimidazole moiety are most often prescribed due to their significant efficacy. However, the other classes are used when the former fails in treatment. Metronidazole (14), tinidazole (15), ornidazole (16) and secnidazole (47) are the 5-nitroimidazole moiety containing drugs. Furazolidone (48) a nitrofuran derivative, albendazole (2) and mebendazole (49) the benzimidazole derivatives, quinacrine (50) an acridine derivative, paromomycin (51) an aminoglycoside derivative and nitazoxanide (52) a 5-nitrothiazolyl derivative are also used for the treatment of G. lamblia diarrheal disease depending on the age of patient. But these drugs have various side effects (Reynoldson et al., 1992; Escobedo and Cimerman, 2007). The in vivo effects of benzyl and cyclohexyl derivatives like, 2-(1H-1-imidazolyl)-1-phenyl-1-ethanol, 2-(2–methyl–1 H-1-imidazolyl)–1-phenyl-1-ethanol, 2-(2-methyl-4-nitro-1H-1-imidazolyl)-1-phenyl-1-ethanol, 2-(1H-1-imidazolyl)-1-cyclohexanol and 1[bis-4-methoxyphenyl-phenylmethyl]-2-methyl-4-nitroimidazole (53–57) were studied on the G. lamblia trophozoite in the white Syrian mice model and were found to be active (Motazedian et al., 2014). The thieno[2,3-b]pyridine derivatives were synthesized and the 4-(4-methoxyphenylamino)thieno[2,3-b]pyridine-5-carbonitrile having the –OCH3 group at para position (58) showed the excellent giardicidal effect possibly due to the higher electron density concentrated over phenyl ring (Bernardino et al., 2006). A series of novel hybrids of benzimidazole-pentamidine were prepared and 1,5-bis[2-methoxy-4-(5-methyl-1H-benzimidazole-2-yl)phenoxy] pentane (59) was found 3- and 9-folds more potent against G. lamblia infection than metronidazole and pentamidine, respectively and was having IC50-value of 0.372 μM (Torres-Gomez et al., 2008a). The 2-(trifluoromethyl)benzimidazole derivatives like 5-chloro-1-methyl-2-(trifluoromethyl)benzimidazole (60), differently substituted at the 1-, 5-, and 6-positions proved to be active against G. lamblia in their in vitro analysis and IC50-value of 0.042 μM was observed for compound (60) (Gabriel Navarrete-Vaa Zquez et al., 2001). A library of chalcones was prepared by condensing substituted acetophenones with benzaldehydes using the Claisen–Schmidt base-catalyzed aldol condensation reaction. Among these chalcones, substituted with –OCH3, -H, -Cl, -F at different positions (E)-3-(6-flourophenyl)-1-(4-methoxyphenyl)prop-2-en 1-one (61) with IC50-value of 12.72 μM showed the excellent results toward the giardial infection (Montes-Avila et al., 2009). The benzimidazole derivative 5(6)-chloro-1H-benzimidazole-2-(3H)-thione (62) was reported active in vitro against G. lamblia with IC50-value of 0.005 μM and inhibits tubulin polymerization (Valdez et al., 2002). The albendazole analogs 1-methyl-6-(propylthio)-2-(trifluoromethyl)-1H-benzimidazole (63) and 5(6)-(propylthio) -2-(trifluoromethyl)-1H-benzimidazole (64) were having IC50-values 1.403 and 1.515 μM, respectively are active toward giardiasis. The mebendazole analogs 5-benzoyl-1-methyl-2-(trifluoromethyl)-1H-benzimidazole (65) and 6-benzoyl-1-methyl-2-(trifluoromethyl)-1H-benzimidazole (66) have also been found to be active against G. lamblia and these were having IC50-values 1.098 μM 1.285 μM, respectively (Navarrete-Vazquez et al., 2003). Among the series of 3-tetrazolylmethyl-4H-chromen-4-ones the compound 3-((1-cyclohexyl-1H-tetrazol-5-yl)((3,4,5-trimethoxyphenyl)amino)methyl)-4H-chromen-4-one (67) having IC50-value of 171.4 μM can represent the suitable alternatives against resistant G. lamblia parasites(Cano et al., 2014). Thiazole derivatives were synthesized and screened against G. intestinalis, two novel methyl 5-nitro-1,3-thiazol-2-ylcarbamate (68) and ethyl [(5-nitro-1,3-thiazol-2-yl)amino](oxo)acetate (69) proved to be potent giardicidal and were having IC50-value of 0.010 and 6.410 μM, respectively(Nava-Zuazo et al., 2014). By using the Ugi-azide multicomponent reaction, novel 3-tetrazolylmethyl-4H-chromen-4-ones were synthesized and evaluated against G. lamblia. The in vivo investigation demonstrated that these compounds like (70) could be specifically considered drug candidates for giardial infection (Cano et al., 2014). A series of novel hybrids from benzimidazole and pentamidine were prepared and tested in vitro against the protozoa and it was observed that the compound1,5-bis[2-methoxy-4-(5-methyl-1H-benzimidazole-2- yl)phenoxy] pentane (71) with IC50-value 0.372 μM showed 3- and 9-fold more activity against G. lamblia than metronidazole and pentamidine, respectively (Torres-Gomez et al., 2008b). Benzologues of nitazoxanide and tizoxanide were synthesized and tested in vitro against G. intestinalis. The 2-{[(6-nitro-1,3-benzothiazol-2-yl)amino]carbonyl}phenyl acetate (39) was having IC50-value 3.515 μM and was18-times more potent than metronidazole (Navarrete-Vazquez et al., 2011). Acetamide derivatives of imidazole like N-(4-cyanophenyl)-2-(2-methyl-4-nitro-1H-imidazol-1-yl) acetamide (72) and 2-methyl-4-nitro-1-[(4-nitrophenyl)sulfonyl]-1H-imidazole (73) were screened in vitro and were found active against G. lamblia with IC50-value 11.25 and 7.50 μM, respectively. These can be further modified to enhance their activity (Hernández-Nunez et al., 2009).
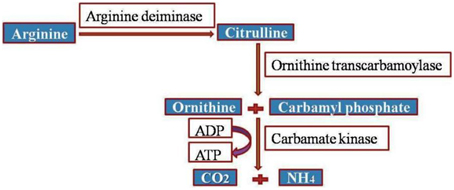
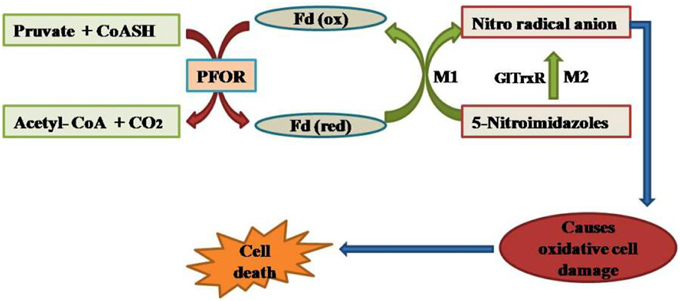
Accentuating the targets, G. lamblia utilizes the arginine dihydrolase pathway to produce ATP from ADP and L-arginine (Schofield et al., 1990), a pathway which is lacking in higher eukaryotes including humans. The arginine dihydrolase pathway employs three enzymes, arginine deiminase, ornithine transcarbamoylase, and carbamate kinase (CK; EC 2.7.2.2). CK catalyzes the last step of the pathway, converting carbamoyl phosphate and ADP into carbamate and ATP (Figure 2; Lim et al., 2013). Carbamate kinase is essential enzyme of Giardia lamblia, and the antialcoholism drug disulfiram (74) kills the trophozoites and inhibits this enzyme. Disulfiram and their analogs act by modifying Cys242 adjacent to the active site and cure of giardiasis in mice has also been reported (Galkin et al., 2014). Nitazoxanide, nitroimidazoles, metronidazole and tinidazole use nitroreductases such as pyruvate ferredoxin oxidoreductase (PFOR) as their target sites in Giardial infection (Hoffman et al., 2007; Müller et al., 2007). The 5-nitroimidazoles like metronidazole act in G. lamblia by two distinct mechanisms. In one mechanism metronidazole gets reduced to toxic nitro-radical anion via pyruvate ferredoxin oxidoreductase/ferredoxin (PFOR/Fd) couple in presence of pyruvate and the cofactor CoASH during the electron transport pathway in G. lamblia. In the second mechanism metronidazole reduction occurs due to the activity of G. lamblia thioredoxin reductase (GlTrxR). The radicals formed cause oxidative damage to G. lamblia cell and thereby kills parasite (Figure 3).
Protein disulfide isomerases, specifically PDI2 and PDI4 have recently been reported as promising targets for new drugs effective against giardiasis (Müller et al., 2007). Furazolidone gets reduced by NADH oxidase when the reduced products obtained disrupt the action of DNA in Giardial infection (Brown et al., 1996; Upcroft and Upcroft, 1998). Recently, Hsp90 inhibitors as potential leads for antiparasitic chemotherapy have been reported. Hsp90 inhibitor prodrug (SNX-5422) was evaluated in animal models to proof the principle that an oral drug could be effective (Debnath et al., 2014). Metronidazole analogs were more active than metronidazole in changing significantly the morphology and ultrastructure of the parasite G. lamblia. The analogs affected parasite cell vesicle trafficking, autophagy, and triggered differentiation into cysts of G. lamblia (Busatti et al., 2013). The novel nitroreductase target sites viz GlNR1 and GlNR2 have been identified to be the potent targets for nitroimidazole, metronidazole, nitrothiazolide and nitazoxanide in G. lamblia (Muller et al., 2015).
Cryptosporidium parvum
The protozoan C. parvum causes cryptosporidiosis which is associated with watery diarrhea that may sometimes be profuse and prolonged in children (Current and Garcia, 1991; Bouzid et al., 2013). It is the second most common protozoan pathogen responsible for severe diarrhea and was also associated with death in young children under 12–23 months of age (Kotloff et al., 2013). Currently nitazoxanide (52), paromomycin (51), and azithromycin are the most commonly used for the treatment of cryptosporidiosis. However, nitazoxanide (52) is effective in the immunocompetent and ineffective in the immunocompromised patients (Mead, 2002; Gargala, 2008). The available medication is not effective in all the patients besides having side effects of headache, nausea, stomach pain, severe or persistent dizziness, shortness of breath and unusual tiredness. Progress in the development of anti-cryptosporidial drugs has been very slow due to the difficulty in the in vitro culture of Cryptosporidium (Abrahamsen et al., 2004; Andrews et al., 2014). Many drug targets are not present in this parasite because it has completely lost the plastid derived apicoplast. Its mitochondrion lacks the citrate cycle and cytochrome based respiratory chain which directs the novel target identification for drug development. Recently a research group synthesized 11 derivatives of benzimidazole, out of which three compounds (75–77) showed anti-cryptosporidic effect equivalent to paromomycin (Graczyk et al., 2011). The dicationic carbazole compounds are active against C. parvum and compound (78) was elucidated to be potent against cryptosporidiosis (Blagburn et al., 1998). The macrolide antibiotics like spiramycin (79), roxithromycin (80), and clarithromycin (81) were thought to be promising drugs against cryptosporidiosis but the final clinical trials have not yet been completed. There is the intense need for the development of effective chemotherapy for cryptosporidiosis in immunodeficient patients (Saez-Llorens, 1989; Uip et al., 1998). The nitrogen-containing bisphosphonates (N-BPs) exhibit anticryptosporiudium activities in low micromolar concentrations. It has been observed that NBPs act on the novel non-specific polyprenyl pyrophosphate synthase that can synthesize isoprenoids from C20->C45 in Cryptosporidium spp (Artz et al., 2008).
In C. parvum, phosphofructokinase enzyme is pyrophosphate specific (PPi-PFK; Denton et al., 1996). The accepted dogma is that PPi-PFK is advantageous to anaerobic organisms because its economic effect on the cell's consumption of ATP means that the net production from glycolysis is three ATP molecules per glucose molecule rather than the more usual two ATP molecules (Coombs and Müller, 1995). The critical role played by PPi-PFK in energy metabolism together with difference from the human host PFK, make it an attractive target for drug. A mannitol cycle is an important part of energy metabolism for C. parvum. A key feature of the cycle is that the enzyme initiates mannitol biosynthesis, mannitol-1-phosphate dehydrogenase, is regulated by the binding of a specific inhibitor, a protein that has been found to belong to the 14–3–3 group of proteins. Inhibition of mannitol synthesis would be likely to have a severe impact upon the oocyst stage of the parasite and so reduces transmission (Schmatz, 1997). The inosine-5′-monophosphate dehydrogenase (IMPDH) and proteases are also proposed to be the target sites in cryptosporidial infection (Mandapati et al., 2014). It has a bacterial-type inosine-5′-monophosphate dehydrogenase (IMPDH) as the only means to convert AMP to GMP (Umejiego et al., 2008). The target site of the paromomycin is the A site of ribosome where upon action it disrupts the protein biosynthesis in C. parvum (Müller et al., 2007). Acyl-coenzyme-A synthetases have been observed to be inhibited by triacsin C in cryptosporidial infection in mice (Guo et al., 2014). The dicationic-substituted aromatic molecules act by binding to the minor groove of DNA in the Cryptosporidium and thereby affects. DNA-associated enzymes such as topoisomerases, endo and exonucleases or other processes, such as DNA replication, recombination, and repair (Hildebrandt et al., 1998). Mebendazole or albendazole acts by interacting with the colchicine site of tubuline in the microtubules of Cryptosporidium spp (Brown et al., 1996).
Blastocystis hominis
Blastocystis hominis is a unicellular anaerobic protozoon and infects the human gastrointestinal tract. It gets transmitted via animal contact, water and food contaminated with excreted cysts of the organism (Eroglu and Koltas, 2010). The pathogenesis of B. hominis leads to symptoms such as diarrhea, flatulence, anorexia, abdominal pain, vomiting, perianal pruritics, and bloating (Sheehan et al., 1986; Chen et al., 2014). The currently used drugs against this infection are metronidazole (14) or tinidazole (15) (Sohail and Fischer, 2005; Tan, 2008). Metronidazole commonly used for the treatment of blastocystosis induces programmed cell death in the B. hominis(Nasirudeen et al., 2004). The reduction of the nitro group cytotoxic radicals in mitochondria causes the death of concerned pathogen (Kulda, 1999). Trimethoprim-sulfamethoxazole (TMP-SMX) (82, 83) is used as the second line medication when the first line treatment by metronidazole (14) does not work (Ok et al., 1999). Other compounds being occasionally used include pentamidine (84), iodochlorhydroxyquin (85), furazolidone (48), iodoquinol (86), tinidazole (15), nitazoxanide (52), and emetine (87) (Moghaddam et al., 2005; Sohail and Fischer, 2005). Paramomycin (51), a broad spectrum aminoglycoside antibiotic has exhibited superior performance in comparison to metronidazole (14). Therefore, it can act as the therapeutic agent for B. hominis infections (Van Hellemond et al., 2013).
The mechanism of action of several drugs, included metronidazole, have been studied and found linked to the induction of programmed cell death in the parasite, when treated with a surface reactive cytotoxic monoclonal antibody (MAb 1D5). The central vacuole of B. hominis grown in normal physiological conditions may contain lipid granules or electron-dense particles. This central vacuole is used for programmed cell death (PCD) process and it acts as a repository where apoptotic bodies are stored before being released into the extracellular space. Central vacuole is potent target for apoptosis (Nasirudeen et al., 2001, 2004).
Isospora belli
Isosporiasis is human intestinal disease caused by the parasite I. belli. It is most commonly observed in Africa, Asia and South America. The currently used drugs against this infection is combination of trimethoprim (82) and sulfamethoxazole (83) (Goodgame, 2003; Hunter et al., 2004). The pyrimethamine (88) and sulfadiazine (89) also cure the diarrheal disease caused by I. Belli (Ferguson et al., 1980; Ebrahimzadeh and Bottone, 1996). Macrolide antibiotics like sirimamycin proved to be effective when pyrimethamine-sulfadoxine (88, 90) fails to cure the intestinal infection by I. belli specifically in AIDS patients (Ferguson et al., 1980). Similarly, roxithromycin (91) is also observed to be effective for chronic I. belli induced diarrhea when TMP-SMX (82, 83) or pyrimethamine (88) treatments become inadequate (Musey et al., 1988). It has been reported that metronidazole (14), tinidazole (15), quinacrine (50), and furazolidone (48) also cure the I. belli induced diarrhea but to lesser extent. However, the treatment with metronidazole is more efficient than tinidazole (15), quinacrine (50), and furazolidone (48) (Trier et al., 1974; Syrkis et al., 1975; Butler and De Boer, 1981; Weiss et al., 1988). The antimalarial compounds, primaquine phosphate (92) and chloroquine phosphate (93) may be helpful in immunocompetent patients with persistent I. belli infection (Trier et al., 1974). The synthesis of more potent drugs and tracing the valuable targets is the important area of research for I. belli intestinal infection.
Cyclospora cayetanensis
Cyclosporiasis is the gastrointestinal illness caused by the microscopic parasite C. cayetanensis. The extent of illness varies based on age, condition of the host and size of the infectious dose (Goodgame, 2003). Trimethoprim–sulfamethoxazole (82, 83) was at first used to treat cyclosporiasis in immunocompetent and immunocompromised patients (Madico et al., 1993; Guerrant et al., 2001). However, the patients who are allergic to sulpha drugs, the ciprofloxacin (94) or trimethoprim (82) is prescribed (Verdier et al., 2000). Nitazoxanide (52) had no severe side effects and was tolerated effectively and can be better option for intestinal infection caused by C. cayetanensis (Diaz et al., 2003). The development of effective drugs and the quest of essential targets are of prime importance to treat cyclosporiasis.
Conclusion
Drug development for parasite-induced diarrheal disease is in progress. There is a pressing need for the identification of compounds which are efficacious in in vivo animal studies and can be subjected to clinical trials. Drug development for C. parvum is particularly challenging because of the difficulty of in vitro screening. Maximum effort should be directed toward new compounds to treat cryptosporidiosis because of the limited availability of effective drugs. Resistance to the current drugs, metronidazole, paromomycin, and nitazoxanide is a major concern. A major research focus on key biochemical pathways, identification of essential targets, better assay methods, and in vivo testing are urgently needed to identify new chemotherapeutic agents for parasite-induced diarrheal disease. As per our contemplated study, there is a need for the determination of mechanism of action of the synthesized compounds against E. histolytica and G. lamblia. The synthesized heterocyclic scaffolds bearing different substituents that have been found to be active in vivo studies against the parasites causing diarrhea, can be further explored as future drug candidates with higher efficacy, resistance effectiveness, and lesser side effects.
The bold numbers in braces refer to the chemical structures of the respective compounds (see Supplementary Material).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was carried out due to the mutual effort of the authors and no funding agency has assisted in this direction.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.01183
References
Abrahamsen, M. S., Templeton, T. J., Enomoto, S., Abrahante, J. E., Zhu, G., Lancto, C. A., et al. (2004). Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304, 441–445. doi: 10.1126/science.1094786
Agholi, M., Hatam, G. R., and Motazedian, M. H. (2013). Microsporidia and coccidia as causes of persistence diarrhea among liver transplant children: incidence rate and species/genotypes. Pediatry Infect. Dis. J. 32, 185–187. doi: 10.1097/INF.0b013e318273d95f
Ali, V., and Nozaki, T. (2007). Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by amitochondriate protozoan parasites. Clin. Microbiol. Rev. 20, 164–187. doi: 10.1128/CMR.00019-06
Andrews, K. T., Fisher, G., and Skinner-Adams, T. S. (2014). Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 4, 95–111. doi: 10.1016/j.ijpddr.2014.02.002
Ansari, M. F., Siddiqui, S. M., Agarwal, S. M., Vikramdeo, K. S., Mondal, N., and Azam, A. (2015). Metronidazole hydrazone conjugates: design, synthesis, antiamoebic and molecular docking studies. Bioorg. Med. Chem. Lett. 25, 3545–3549. doi: 10.1016/j.bmcl.2015.06.091
Artz, J. D., Dunford, J. E., Arrowood, M. J., Dong, A., Chruszcz, M., Kavanagh, K. L., et al. (2008). Targeting a uniquely nonspecific prenyl synthase with bisphosphonates to combat cryptosporidiosis. Chem. Biol. 15, 1296–1306. doi: 10.1016/j.chembiol.2008.10.017
Azam, A., and Agarwal, S. (2007). Targeting Amoebiasis: status and developments. Curr. Bioact. Compd. 3, 121–133. doi: 10.2174/157340707780809590
Azam, A., and Agarwal, S. (2015). Amebiasis: Biology and Pathogenesis of Entamoeba. New Delhi: Springer.
Bacchi, C., and Weiss, L. (2004). “Chemotherapy of microsporidiosis: Benzimidazoles, fumagillin and polyamine analogues,” in Opportunistic Infections: Toxoplasma, Sarcocystis, and Microsporidia, ed R. Decampo (New York, NY: Springer), 159–188.
Bacchi, C. J., Lane, S., Weiss, L. M., Yarlett, N., Takvorian, P., and Wittner, M. (2001). Polyamine synthesis and interconversion by the microsporidian Encephalitozoon cuniculi. J. Eukaryot. Microbiol. 48, 374–381. doi: 10.1111/j.1550-7408.2001.tb00327.x
Becker, S., Hoffman, P., and Houpt, E. R. (2011). Efficacy of antiamebic drugs in a mouse model. Am. J. Trop. Med. Hyg. 84, 581–586. doi: 10.4269/ajtmh.2011.10-0580
Bendesky, A. S., Menécndez, D., and Ostrosky-Wegman, P. (2002). Is metronidazole carcinogenic? Mut. Res. 511, 133–144. doi: 10.1016/S1383-5742(02)00007-8
Bernardino, A. M. R., Da Silva Pinheiro, L. C., Rodrigues, C. R., Loureiro, N. I., Castro, H. C., Lanfredi-Rangel, A., et al. (2006). Design, synthesis, SAR, and biological evaluation of new 4-(phenylamino) thieno [2, 3-b] pyridine derivatives. Bioorg. Med. Chem. 14, 5765–5770. doi: 10.1016/j.bmc.2006.03.013
Bhat, A. R., Athar, F., and Azam, A. (2009a). Bis-pyrazolines: synthesis, characterization and antiamoebic activity as inhibitors of growth of Entamoeba histolytica. Eur. J. Med. Chem. 44, 426–431. doi: 10.1016/j.ejmech.2007.11.005
Bhat, A. R., Athar, F., Van Zyl, R. L., Chen, C. T., and Azam, A. (2008). Synthesis and biological evaluation of novel 4-substituted 1-{[4-(10,15,20-triphenylporphyrin-5-yl)phenyl]methylidene}thiosemicarbazides as new class of potential antiprotozoal agents. Chem. Biodivers. 5, 764–776. doi: 10.1002/cbdv.200890073
Bhat, A. R., Bhat, A. I., Athar, F., and Azam, A. (2009b). Synthesis, characterization, and anti-amoebic screening of core-modified 5, 20-Bis {2-{[(alkyl)(alkyl′) amino] methyl} ferrocen-1-yl}-10, 15-diphenyl-21, 23-dithiaporphyrin (= 1, 1″-(10, 15-Diphenyl-21, 23-dithiaporphine-5, 20-diyl) bis [2-{[(alkyl)(alkyl′) amino] methyl} ferrocene]) Derivatives. Helv. Chim. Acta 92, 1644–1656. doi: 10.1002/hlca.200800461
Blagburn, B. L., Drain, K. L., Land, T. M., Kinard, R. G., Moore, P. H., Lindsay, D. S., et al. (1998). Comparative efficacy evaluation of dicationic carbazole compounds, nitazoxanide, and paromomycin against Cryptosporidium parvum infections in a neonatal mouse model. Antimicrob. Agents Chemother. 42, 2877–2882.
Blanshard, C., Ellis, D. S., Tovey, D. G., Dowell, S., and Gazzard, B. G. (1992). Treatment of intestinal microsporidiosis with albendazole in patients with AIDS. Aids 6, 311–314. doi: 10.1097/00002030-199203000-00009
Bouzid, M., Hunter, P. R., Chalmers, R. M., and Tyler, K. M. (2013). Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 26, 115–134. doi: 10.1128/CMR.00076-12
Brosson, D., Kuhn, L., Prensier, G. R., Vivaré, S. C. P, and Texier, C. (2005). The putative chitin deacetylase of Encephalitozoon cuniculi: a surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol. Lett. 247, 81–90. doi: 10.1016/j.femsle.2005.04.031
Brown, D. M., Upcroft, J. A., and Upcroft, P. (1996). A H2O-Producing NADH oxidase from the protozoan parasite Giardia Duodenalis. Eur. J. Biochem. 241, 155–161. doi: 10.1111/j.1432-1033.1996.0155t.x
Bruchhaus, I., and Tannich, E. (1994). Purification and molecular characterization of the NAD(+)-dependent acetaldehyde/alcohol dehydrogenase from Entamoeba histolytica. Biochem. J. 303(Pt 3), 743–748. doi: 10.1042/bj3030743
Budakoti, A., Bhat, A. R., and Azam, A. (2009). Synthesis of new 2-(5-substituted-3-phenyl-2-pyrazolinyl)-1,3-thiazolino[5,4-b]quinoxaline derivatives and evaluation of their antiamoebic activity. Eur. J. Med. Chem. 44, 1317–1325. doi: 10.1016/j.ejmech.2008.02.002
Busatti, H. G., Alves, R. J., Santana-Anjos, K. G., Gil, F. F., Cury, M. C., Vannier-Santos, M. A., et al. (2013). Effects of metronidazole analogues on Giardia lamblia: experimental infection and cell organization. Diagn. Microbiol. Infect. Dis. 75, 160–164. doi: 10.1016/j.diagmicrobio.2012.11.001
Butler, T., and De Boer, W. G. (1981). Isospora belli infection in Australia. Pathology 13, 593–595. doi: 10.3109/00313028109059077
Cano, P. A., Islas-JáCome, A., Gonzalez-Marrero, J., Yepez-Mulia, L., Calzada, F., and Gamez-Montano, R. (2014). Synthesis of 3-tetrazolylmethyl-4H-chromen-4-ones via Ugi-azide and biological evaluation against Entamoeba histolytica, Giardia lamblia and Trichomona vaginalis. Bioorg. Med. Chem. 22, 1370–1376. doi: 10.1016/j.bmc.2013.12.069
Carrero, J. C., Lugo, H., Perez, D. G., Ortiz-Martinez, C., and Laclette, J. P. (2004). Cyclosporin A inhibits calcineurin (phosphatase 2B) and P-glycoprotein activity in Entamoeba histolytica. Int. J. Parasitol. 34, 1091–1097. doi: 10.1016/j.ijpara.2004.05.004
Chen, C.-H., Sun, H.-Y., Chien, H.-F., Lai, H.-S., and Chou, N.-K. (2014). Blastocystis hominis infection in a post-cardiotomy patient on extracorporeal membrane oxygenation support: a case report and literature review. Inter. J. Surg. Case Rep. 5, 637–639. doi: 10.1016/j.ijscr.2014.07.010
Chokephaibulkit, K., Wanachiwanawin, D., Tosasuk, K., Vanprapa, N., and Chearskul, S. (2001). A report case of Cyclospora and Cryptosporidium mixed infection in a HIV-negative child in Thailand. J. Med. Assoc. Thai. 84, 36–37. 589–592.
Coombs, G. H., and Müller, M. (1995). 3 – Energy metabolism in Anaerobic protozoa. Biochem. Mol. Biol. Parasites, 33–47. doi: 10.1016/B978-012473345-9/50004-0
Debnath, A., Shahinas, D., Bryant, C., Hirata, K., Miyamoto, Y., Hwang, G., et al. (2014). Hsp90 inhibitors as new leads to target parasitic diarrheal diseases. Antimicrob. Agents Chem. 58, 4138–4144. doi: 10.1128/AAC.02576-14
Denton, H., Brown, S. M., Roberts, C. W., Alexander, J., Mcdonald, V., Thong, K. W., et al. (1996). Comparison of the phosphofructokinase and pyruvate kinase activities of Cryptosporidium parvum, Eimeria tenella and Toxoplasma gondii. Mol. Biochem. Parasitol. 76, 23–29. doi: 10.1016/0166-6851(95)02527-8
Diamond, L. S., Harlow, D. R., and Cunnick, C. C. (1978). A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72, 431–432. doi: 10.1016/0035-9203(78)90144-X
Diaz, E., Mondragon, J., Ramirez, E., and Bernal, R. (2003). Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am. J. Trop. Med. Hyg. 68, 384–385.
Didier, E. S., and Weiss, L. M. (2006). Microsporidiosis: current status. Curr. Opin. Iinfect. Dis. 19, 485. doi: 10.1097/01.qco.0000244055.46382.23
Didier, E. S., Bowers, L., Stovall, M. E., Kuebler, D., Mittleider, D., Brindley, P. J., et al. (2005). Antimicrosporidial activity of (fluoro)quinolones in vitro and in vivo. Folia Parasitol. 52, 173–181. doi: 10.14411/fp.2005.022
Didier, P. J., Phillips, J. N., Kuebler, D. J., Nasr, M., Brindley, P. J., Stovall, M. E., et al. (2006). Antimicrosporidial activities of fumagillin, TNP-470, ovalicin, and ovalicin derivatives in vitro and in vivo. Antimicrob. Agents Chemother. 50, 2146–2155. doi: 10.1128/AAC.00020-06
Duque-Montaño, B. E., Gómez-Caro, L. C., Sanchez-Sanchez, M., Monge, A., Hernandez-Baltazar, E., Rivera, G., et al. (2013). Synthesis and in vitro evaluation of new ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide against Entamoeba histolytica. Bioorg. Med. Chem. 21, 4550–4558. doi: 10.1016/j.bmc.2013.05.036
Ebrahimzadeh, A., and Bottone, E. J. (1996). Presistent diarrhea caused by Isospora belli: therapeutic response to pyrimethamine and sulfadiazine. Diagn. Microb. Infetc. Dis. 26, 87–89. doi: 10.1016/S0732-8893(96)00175-7
Eroglu, F., and Koltas, I. S. (2010). Evaluation of the transmission mode of B. hominis by using PCR method. Parasitol. Res. 107, 841–845. doi: 10.1007/s00436-010-1937-4
Escobedo, A. A., and Cimerman, S. (2007). Giardiasis: a pharmacotherapy review. Expert Opin. Pharmacol. 8, 1885–1902. doi: 10.1517/14656566.8.12.1885
Fahey, R. C., Newton, G. L., Arrick, B., Overdank-Bogart, T., and Aley, S. B. (1984). Entamoeba histolytica: a eukaryote without glutathione metabolism. Science 224, 70–72. doi: 10.1126/science.6322306
Ferguson, D. J., Birch-Andersen, A., Hutchison, W. M., and Siim, J. C. (1980). Ultrastructural observations on microgametogenesis and the structure of the microgamete of Isospora felis. Acta Pathol. Microbiol. Scand. B 88, 151–159. doi: 10.1111/j.1699-0463.1980.tb02621.x
Friedman, P. A., and Platzer, E. G. (1980). Interaction of anthelmintic benzimidazoles with Ascaris suum embryonic tubulin. Biochim. Biophys. Acta 630, 271–278. doi: 10.1016/0304-4165(80)90431-6
Gabriel Navarrete-Vaa Zquez, A. R. C., Alicia Hernaandez-Campos, B., Liliaan, Y. B., Francisco Herna Ndez-Luis, A., Juan Valdez, A., Rau, L., et al. (2001). Synthesis and antiparasitic activity of 2-(Trichloromethyl)-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 11, 187–190. doi: 10.1016/S0960-894X(00)00619-3
Galkin, A., Kulakova, L., Lim, K., Chen, C. Z., Zheng, W., Turko, I. V., et al. (2014). Structural basis for inactivation of Giardia lamblia carbamate kinase by disulfiram. J. Biolog. Chem. 289, 10502–10509. doi: 10.1074/jbc.M114.553123
Gargala, G. (2008). Drug treatment and novel drug target against Cryptosporidium. Parasite 15, 275–281. doi: 10.1051/parasite/2008153275
Gilin, F. D., and Diamond, L. S. (1980). Attachment and short-term maintenance of motility and viability of Entamoeba histolytica in a defined medium. J. Protozool. 27, 220–225. doi: 10.1111/j.1550-7408.1980.tb04685.x
Gómez-Puyou, A., Saavedra-Lira, E., Becker, I., Zubillaga, R. A., Rojo-Dominguez, A., and Perez-Montfort, R. (1995). Using evolutionary changes to achieve species-specific inhibition of enzyme action–studies with triosephosphate isomerase. Chem. Biol. 2, 847–855. doi: 10.1016/1074-5521(95)90091-8
Goodgame, R. (2003). Emerging causes of travelers diarrhea: cryptosporidium, cyclospora, isospora, and microsporidia. Curr. Infect. Dis. Rep. 5, 66–73. doi: 10.1007/s11908-003-0067-x
Graczyk, Z., Chomicz, L., Kozlowska, M., Kazimierczuk, Z., and Graczyk, T. K. (2011). Novel and promising compounds to treat Cryptosporidium parvum infections. Parasitol. Res. 109, 591–594. doi: 10.1007/s00436-011-2290-y
Guerrant, R. L., Van Gilder, T., Steiner, T. S., Thielman, N. M., Slutsker, L., Tauxe, R. V., et al. (2001). Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32, 331–351. doi: 10.1086/318514
Guo, F., Zhang, H., Fritzler, J. M., Rider, S. D., Xiang, L., Mcnair, N. N., et al. (2014). Amelioration of Cryptosporidium parvum Infection in vitro and in vivo by targeting parasite fatty acyl-coenzyme a synthetases. J. Infect. Dis. 209, 1279–1287. doi: 10.1093/infdis/jit645
Haque, R., Huston, C. D., Hughes, M., Houpt, E., and Petri, W. A. Jr. (2003). Amebiasis. N. Engl. J. Med. 348, 1565–1573. doi: 10.1056/NEJMra022710
Hernández-Nunez, E., Tlahuext, H., Moo-Puc, R., Torres-Gomez, H., Reyes-Martinez, R., Cedillo-Rivera, R., et al. (2009). Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur. J. Med. Chem. 44, 2975–2984. doi: 10.1016/j.ejmech.2009.01.005
Hildebrandt, E., Boykin, D. W., Kumar, A., Tidwell, R. R., and Dykstra, C. C. (1998). Identification and characterization of an endo/exonuclease in Pneumocystis carinii that is inhibited by dicationic diarylfurans with efficacy against Pneumocystis pneumonia. J. Eukaryot. Microbiol. 45, 112–121. doi: 10.1111/j.1550-7408.1998.tb05078.x
Hoffman, P. S., Sisson, G., Croxen, M. A., Welch, K., Harman, W. D., Cremades, N., et al. (2007). Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chem. 51, 868–876. doi: 10.1128/AAC.01159-06
Hooper, D. C. (2000). Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 31, S24–S28. doi: 10.1086/314056
Huang, D. B., and White, A. C. (2006). An updated review on cryptosporidium and giardia. Gastroenterol. Clin. North Am. 35, 291–314. doi: 10.1016/j.gtc.2006.03.006
Hunter, P. R., Hughes, S., Woodhouse, S., Nicholas, R., Syed, Q., Chalmers, R. M., et al. (2004). Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin. Infect. Dis. 39, 504–510. doi: 10.1086/422649
Hwang, E. W., Cheung, L., Mojtahed, A., and Cartwright, C. A. (2011). Relapse of intestinal and hepatic amebiasis after treatment. Dig. Dis. Sci. 56, 677–680. doi: 10.1007/s10620-010-1492-y
Inam, A., Siddiqui, S. M., Macedo, T. S., Moreira, D. R., Leite, A. C., Soares, M. B., et al. (2014). Design, synthesis and biological evaluation of 3-[4-(7-chloro-quinolin-4-yl)-piperazin-1-yl]-propionic acid hydrazones as antiprotozoal agents. Eur. J. Med. Chem. 75, 67–76. doi: 10.1016/j.ejmech.2014.01.023
Inam, A., Van Zyl, R. L., Van Vuuren, N. J., Chen, C.-T., Avecilla, F., Agarwal, S. M., et al. (2015). Chloroquinoline–acetamide hybrids: a promising series of potential antiprotozoal agents. RSC Adv. 5, 48368–48381. doi: 10.1039/C5RA05472A
Iqbal, P. F., Bhat, A. R., and Azam, A. (2009). Antiamoebic coumarins from the root bark of Adina cordifolia and their new thiosemicarbazone derivatives. Eur. J. Med. Chem. 44, 2252–2259. doi: 10.1016/j.ejmech.2008.06.003
Irfan, I., Sawangjaroen, N., Bhat, A. R., and Azam, A. (2010). New dioxazole derivatives: synthesis and effects on the growth of Entamoeba histolytica and Giardia intestinalis. Eur. J. Med. Chem. 45, 1648–1653. doi: 10.1016/j.ejmech.2009.12.051
John, D. E., Haas, C. N., Nwachuku, N., and Gerba, C. P. (2005). Chlorine and ozone disinfection of Encephalitozoon intestinalis spores. Water Res. 39, 2369–2375. doi: 10.1016/j.watres.2005.04.013
Juneja, A., Macedo, T. S., Magalhaes Moreira, D. R., Pereira Soares, M. B., Lima Leite, A. C., Kelle De Andrade Lemoine Neves, J., et al. (2014). Synthesis of 4′-(2-ferrocenyl)-2,2′:6′2″-terpyridine: characterization and antiprotozoal activity of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes. Eur. J. Med. Chem. 75, 203–210. doi: 10.1016/j.ejmech.2014.01.051
Kazimierczuk, Z., Chalimoniuk, M., Laudy, A. E., Moo-Puc, R., Cedillo-Rivera, R., Starosciak, B. J., et al. (2010). Synthesis and antimicrobial and nitric oxide synthase inhibitory activities of novel isothiourea derivatives. Arch. Pharm. Res. 33, 821–830. doi: 10.1007/s12272-010-0604-8
Kotloff, K. L., Nataro, J. P., Blackwelder, W. C., Nasrin, D., Farag, T. H., Panchalingam, S., et al. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382, 209–222. doi: 10.1016/S0140-6736(13)60844-2
Kucik, C. J., Martin, G. L., and Sortor, B. V. (2004). Common intestinal parasites. Am. Fam. Phys. 69, 1161–1168.
Kulda, J. (1999). Trichomonads, hydrogenosomes and drug resistance. Int. J. Parasitol. 29, 199–212. doi: 10.1016/S0020-7519(98)00155-6
Leitsch, D., Kolarich, D., Wilson, I., Altmann, F., and Duchêne, M. (2007). Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 5:e211. doi: 10.1371/journal.pbio.0050211
Lim, K., Kulakova, L., Galkin, A., and Herzberg, O. (2013). Crystal structures of carbamate kinase from Giardia lamblia bound with citric acid and AMP-PNP. PLoS ONE 8:e64004. doi: 10.1371/journal.pone.0064004
Loftus, B., Anderson, I., Davies, R., Alsmark, U. C. M., Samuelson, J., Amedeo, P., et al. (2005). The genome of the protist parasite Entamoeba histolytica. Nature 433, 865–868. doi: 10.1038/nature03291
Macdonald, L. M., Armson, A., Thompson, A. R., and Reynoldson, J. A. (2004). Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol. Biochem. Parasitol. 138, 89–96. doi: 10.1016/j.molbiopara.2004.08.001
Madico, G., Gilman, R., Miranda, E., Cabrera, L., and Sterling, C. (1993). Treatment of Cyclospora infections with co-trimoxazole. Lancet 342, 122–123. doi: 10.1016/0140-6736(93)91330-O
Mandapati, K., Gorla, S. K., House, A. L., Mckenney, E. S., Zhang, M., Rao, S. N., et al. (2014). Repurposing Cryptosporidium inosine 5-monophosphate dehydrogenase inhibitors as potential antibacterial agents. ACS Med. Chem. Lett. 5, 846–850. doi: 10.1021/ml500203p
Mead, J. R. (2002). Cryptosporidiosis and the challenges of chemotherapy. Drug Resist. Updat. 5, 47–57. doi: 10.1016/S1368-7646(02)00011-0
Menotti, J., Santillana-Hayat, M., Cassinat, B., Sarfati, C., Derouin, F., and Molina, J.-M. (2005). Inhibitory activity of human immunodeficiency virus aspartyl protease inhibitors against Encephalitozoon intestinalis evaluated by cell culture-quantitative PCR assay. Antimicrob. Agents Chem. 49, 2362–2366. doi: 10.1128/AAC.49.6.2362-2366.2005
Meza, I. (2000). Extracellular matrix-induced signaling in Entamoeba histolytica: its role in invasiveness. Parasitol. Today 16, 23–28. doi: 10.1016/S0169-4758(99)01586-0
Moghaddam, D. D., Ghadirian, E., and Azami, M. (2005). Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol. Res. 96, 273–275. doi: 10.1007/s00436-005-1363-1
Molina, J.-M., Tourneur, M., Sarfati, C., Chevret, S., De Gouvello, A., Gobert, J.-G., et al. (2002). Fumagillin treatment of intestinal microsporidiosis. N Engl. J. Med. 346, 1963–1969. doi: 10.1056/NEJMoa012924
Montes-Avila, J., Diaz-Camacho, S. P., Sicairos-Felix, J., Delgado-Vargas, F., and Rivero, I. (2009). Solution-phase parallel synthesis of substituted chalcones and their antiparasitary activity against Giardia lamblia. Bioorg. Med. Chem. 17, 6780–6785. doi: 10.1016/j.bmc.2009.02.052
Motazedian, M. H., Mohammadpour, N., and Khabnadideh, S. (2014). “In vivo study of efficacy of some metronidazole derivatives on Giardia lamblia,” in 2nd International Conference on Innovation Challenges in Multidisciplinary Research and Practice (Shiraz: University of Medical Sciences).
Muller, J., Rout, S., Leitsch, D., Vaithilingam, J., Hehl, A., and Muller, N. (2015). Comparative characterisation of two nitroreductases from Giardia lamblia as potential activators of nitro compounds. Int. J. Parasitol. Drug. Drug Resist. 5, 37–43. doi: 10.1016/j.ijpddr.2015.03.001
Müller, J., Wastling, J., Sanderson, S., Müller, N., and Hemphill, A. (2007). A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chem. 51, 1979–1986. doi: 10.1128/AAC.01548-06
Müller, M. (1983). Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery 93, 165–171.
Musey, K. L., Chidiac, C., Beaucaire, G., Houriez, S., and Fourrier, A. (1988). Effectiveness of roxithromycin for treating Isospora belli infection. J. Infect. Dis. 158, 646. doi: 10.1093/infdis/158.3.646
Nasirudeen, A., Hian, Y. E., Singh, M., and Tan, K. S. (2004). Metronidazole induces programmed cell death in the protozoan parasite Blastocystis hominis. Microbiol. 150, 33–43. doi: 10.1099/mic.0.26496-0
Nasirudeen, A., Singh, M., Yap, E., and Tan, K. (2001). Blastocystis hominis: evidence for caspase-3-like activity in cells undergoing programmed cell death. Parasitol. Res. 87, 559–565. doi: 10.1007/s004360100427
Navarrete-Vazquez, G., Chávez-Silva, F., Argotte-Ramos, R., Rodríguez-Gutiérrez Mdel, C., Chan-Bacab, M. J., Cedillo-Rivera, R., et al. (2011). Synthesis of benzologues of Nitazoxanide and Tizoxanide: a comparative study of their in vitro broad-spectrum antiprotozoal activity. Bioorg. Med. Chem. Lett. 21, 3168–3171. doi: 10.1016/j.bmcl.2011.02.100
Navarrete-Vazquez, G., Yepez, L., Hernandez-Campos, A., Tapia, A., Hernandez-Luis, F., Cedillo, R., et al. (2003). Synthesis and antiparasitic activity of albendazole and mebendazole analogues. Bioorg. Med. Chem. 11, 4615–4622. doi: 10.1016/S0968-0896(03)00497-8
Nava-Zuazo, C., Chavez-Silva, F., Moo-Puc, R., Chan-Bacab, M. J., Ortega-Morales, B. O., Moreno-Diaz, H., et al. (2014). 2-acylamino-5-nitro-1,3-thiazoles: preparation and in vitro bioevaluation against four neglected protozoan parasites. Bioorg. Med. Chem. 22, 1626–1633. doi: 10.1016/j.bmc.2014.01.029
Negi, B., Raj, K. K., Siddiqui, S. M., Ramachandran, D., Azam, A., and Rawat, D. S. (2014). In vitro antiamoebic activity evaluation and docking studies of metronidazole-triazole hybrids. Chem. Med. Chem. 9, 2439–2444. doi: 10.1002/cmdc.201402240
Nozaki, T., Asai, T., Kobayashi, S., Ikegami, F., Noji, M., Saito, K., et al. (1998). Molecular cloning and characterization of the genes encoding two isoforms of cysteine synthase in the enteric protozoan parasite Entamoeba histolytica. Mol. Biochem. P arasitol. 97, 33–44. doi: 10.1016/S0166-6851(98)00129-7
Nozaki, T., Asai, T., Sanchez, L. B., Kobayashi, S., Nakazawa, M., and Takeuchi, T. (1999). Characterization of the gene encoding serine acetyltransferase, a regulated enzyme of cysteine biosynthesis from the protist parasites Entamoeba histolytica and Entamoeba dispar regulation and possible function of the cysteine biosynthetic pathway in entamoeba. J. Biol Chem. 274, 32445–32452. doi: 10.1074/jbc.274.45.32445
Ok, U. Z., Girginkardeşler, N., Balcioğlu, C., Ertan, P., Pirildar, T., and Kilimcioğlu, A. A. (1999). Effect of trimethoprim-sulfamethaxazole in Blastocystis hominis infection. Am. J. Gastroenterol. 94, 3245–3247. doi: 10.1111/j.1572-0241.1999.01529.x
Ordaz-Pichardo, C., Shibayama, M., Villa-Trevino, S., Arriaga-Alba, M., Angeles, E., and De La Garza, M. (2005). Antiamoebic and toxicity studies of a carbamic acid derivative and its therapeutic effect in a hamster model of hepatic amoebiasis. Antimicrob. Agents Chemother. 49, 1160–1168. doi: 10.1128/AAC.49.3.1160-1168.2005
Petri, W. A. Jr. (2003). Therapy of intestinal protozoa. Ternds Parasitol. 19, 523–526. doi: 10.1016/j.pt.2003.09.003
Pozio, E., and Morales, M. A. G. (2005). The impact of HIV-protease inhibitors on opportunistic parasites. Trends Parasitol. 21, 58–63. doi: 10.1016/j.pt.2004.11.003
Reeves, R. E., Serrano, R., and South, D. J. (1976). 6-phosphofructokinase (pyrophosphate). Properties of the enzyme from Entamoeba histolytica and its reaction mechanism. J. Biol. Chem. 251, 2958–2962.
Reeves, R. E., South, D. J., Blytt, H. J., and Warren, L. G. (1974). Pyrophosphate: d-fructose 6-phosphate 1-phosphotransferase a new enzyme with the glycolytic function of 6-phosphofructokinase. J. Biol Chem. 249, 7737–7741.
Reynoldson, J., Thompson, R., and Horton, R. (1992). Albendazole as a future antigiardial agent. Parasitol. Today 8, 412–414. doi: 10.1016/0169-4758(92)90193-6
Rodriguez-Romero, A., Hernandez-Santoyo, A., Del Pozo Yauner, L., Kornhauser, A., and Fernandez-Velasco, D. A. (2002). Structure and inactivation of triosephosphate isomerase from Entamoeba histolytica. J. Mol. Biol. 322, 669–675. doi: 10.1016/S0022-2836(02)00809-4
Saez-Llorens, X. (1989). Spiramycin for treatment of Cryptosporidium enteritis. J. Infect. Dis. 160, 342. doi: 10.1093/infdis/160.2.342
Sahoo, N., Bhattacharya, S., and Bhattacharya, A. (2003). Blocking the expression of a calcium binding protein of the protozoan parasite Entamoeba histolytica by tetracycline regulatable antisense-RNA. Mol. Biochem. Parasitol. 126, 281–284. doi: 10.1016/S0166-6851(02)00284-0
Sahoo, N., Labruyere, E., Bhattacharya, S., Sen, P., Guillen, N., and Bhattacharya, A. (2004). Calcium binding protein 1 of the protozoan parasite Entamoeba histolytica interacts with actin and is involved in cytoskeleton dynamics. J. Cell Sci. 117, 3625–3634. doi: 10.1242/jcs.01198
Samarawickrema, N., Brown, D., Upcroft, J., Thammapalerd, N., and Upcroft, P. (1997). Involvement of superoxide dismutase and pyruvate: ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J. Antimicrob. Chemother. 40, 833–840. doi: 10.1093/jac/40.6.833
Sato, D., Kobayashi, S., Yasui, H., Shibata, N., Toru, T., Yamamoto, M., et al. (2010). Cytotoxic effect of amide derivatives of trifluoromethionine against the enteric protozoan parasite Entamoeba histolytica. Int. J. Antimicrob. Agents 35, 56–61. doi: 10.1016/j.ijantimicag.2009.08.016
Schofield, P., Costello, M., Edwards, M., and O”Sullivan, W. (1990). The arginine dihydrolase pathway is present in Giardia intestinalis. Int. J. Parasitol. 20, 697–699. doi: 10.1016/0020-7519(90)90133-8
Shah, N., Dupont, H. L., and Ramsey, D. J. (2009). Global etiology of travelers diarrhea: systematic review from 1973 to the present. Am. J. Med. Hyg. 80, 609–614.
Sheehan, D. J., Raucher, B., and Mckitrick, J. C. (1986). Association of Blastocystis hominis with signs and symptoms of human disease. J. Clin. Microbiol. 24, 548–550.
Siddiqui, S. M., and Azam, A. (2014). Synthesis, characterization of 4,6-disubstituted aminopyrimidines and their sulphonamide derivatives as anti-amoebic agents. Med. Chem. Res. 23, 2976–2984. doi: 10.1007/s00044-013-0877-9
Slack, A. (2012). Parasitic causes of prolonged diarrhoea in travelers: diagnosis and management. Aust. Fam. Physician. 41, 782.
Sohail, M. R., and Fischer, P. R. (2005). Blastocystis hominis and travelers. Travel. Med. Infect. Dis. 3, 33–38. doi: 10.1016/j.tmaid.2004.06.001
Syrkis, I., Fried, M., Elian, I., Pietrushka, D., and Lengy, J. (1975). A case of severe human coccidiosis in Israel. Isr. J. Med. Sci. 11, 373–377.
Tan, K. S. (2008). New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 21, 639–665. doi: 10.1128/CMR.00022-08
Tengku, S., and Norhayati, M. (2011). Review Paper Public health and clinical importance of amoebiasis in Malaysia: a review. Trop. Biomed. 28, 194–222.
Toledano-Magana, Y., Garcia-Ramos, J. C., Navarro-Olivarria, M., Flores-Alamo, M., Manzanera-Estrada, M., Ortiz-Frade, L., et al. (2015). Potential amoebicidal activity of hydrazone derivatives: synthesis, characterization, electrochemical behavior, theoretical study and evaluation of the biological activity. Molecules 20, 9929–9948. doi: 10.3390/molecules20069929
Torres-Gomez, H., Hernandez-Nunez, E., Leon-Rivera, I., Guerrero-Alvarez, J., Cedillo-Rivera, R., Moo-Puc, R., et al. (2008a). Design, synthesis and in vitro antiprotozoal activity of benzimidazole-pentamidine hybrids. Bioorg. Med. Chem. Lett. 18, 3147–3151. doi: 10.1016/j.bmcl.2008.05.009
Torres-Gomez, H., Hernandez-Nunez, E., Leon-Rivera, I., Guerrero-Alvarez, J., Cedillo-Rivera, R., Moo-Puc, R., et al. (2008b). Design, synthesis and in vitro antiprotozoal activity of benzimidazole-pentamidine hybrids. Bioorg. Med. Chem. Lett. 18, 3147–3151. doi: 10.1016/j.bmcl.2008.05.009
Tremoulet, A. H., Avila-Aguero, M. L., Paris, M. M., Canas-Coto, A., Ulloa-Gutierrez, R., and Faingezicht, I. (2004). Albendazole therapy for Microsporidium diarrhea in immunocompetent Costa Rican children. Pediatry Infect. Dis. J. 23, 915–918. doi: 10.1097/01.inf.0000141724.06556.f9
Trier, J. S., Moxey, P. C., Schimmel, E. M., and Robles, E. (1974). Chronic intestinal coccidiosis in man: intestinal morphology and response to treatment. Gastroenterology 66, 923–935.
Uip, D. E., Lima, A. L., Amato, V. S., Boulos, M., Neto, V. A., and Bem David, D. (1998). Roxithromycin treatment for diarrhoea caused by Cryptosporidium spp. in patients with AIDS. J. Antimicrob. Chemother. 41(Suppl. B), 93–97. doi: 10.1093/jac/41.suppl_2.93
Umejiego, N. N., Gollapalli, D., Sharling, L., Volftsun, A., Lu, J., Benjamin, N. N., et al. (2008). Targeting a prokaryotic protein in a eukaryotic pathogen: identification of lead compounds against cryptosporidiosis. Chem. Biol. 15, 70–77. doi: 10.1016/j.chembiol.2007.12.010
Upadhya, R., Zhang, H. S., and Weiss, L. M. (2006). System for expression of microsporidian methionine amino peptidase type 2 (MetAP2) in the yeast Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 50, 3389–3395. doi: 10.1128/AAC.00726-06
Upcroft, J. A., Campbell, R. W., Benakli, K., Upcroft, P., and Vanelle, P. (1999). Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and-resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob. Agents Chemother. 43, 73–76.
Valdez, J., Cedillo, R., Hernandez-Campos, A., Yepez, L., Hernandez-Luis, F., Navarrete-Vazquez, G., et al. (2002). Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 12, 2221–2224. doi: 10.1016/S0960-894X(02)00346-3
Van Hellemond, J. J., Molhoek, N., Koelewijn, R., Wismans, P. J., and Van Genderen, P. J. (2013). Is paromomycin the drug of choice for eradication of Blastocystis in adults? J. Infect. Chemother. 19, 545–548. doi: 10.1007/s10156-012-0496-2
Verdier, R.-I., Fitzgerald, D. W., Johnson, W. D., and Pape, J. W. (2000). Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients: a randomized, controlled trial. Ann. Intern. Med. 132, 885–888. doi: 10.7326/0003-4819-132-11-200006060-00006
Wani, M. Y., Bhat, A. R., Azam, A., and Athar, F. (2013). Nitroimidazolyl hydrazones are better amoebicides than their cyclized 1,3,4-oxadiazoline analogues: in vitro studies and Lipophilic efficiency analysis. Eur. J. Med. Chem. 64, 190–199. doi: 10.1016/j.ejmech.2013.03.034
Wani, M. Y., Bhat, A. R., Azam, A., Choi, I., and Athar, F. (2012a). Probing the antiamoebic and cytotoxicity potency of novel tetrazole and triazine derivatives. Eur. J. Med. Chem. 48, 313–320. doi: 10.1016/j.ejmech.2011.12.033
Wani, M. Y., Bhat, A. R., Azam, A., Lee, D. H., Choi, I., and Athar, F. (2012b). Synthesis and in vitro evaluation of novel tetrazole embedded 1,3,5-trisubstituted pyrazoline derivatives as Entamoeba histolytica growth inhibitors. Eur. J. Med. Chem. 54, 845–854. doi: 10.1016/j.ejmech.2012.03.049
Watanabe, K., Gatanaga, H., Escueta-De Cadiz, A., Tanuma, J., Nozaki, T., and Oka, S. (2011). Amebiasis in HIV-1-infected Japanese men: clinical features and response to therapy. PLoS. Negl. Trop. Dis. 5:e1318. doi: 10.1371/journal.pntd.0001318
Weiss, L. M., Perlman, D. C., Sherman, J., Tanowitz, H., and Wittner, M. (1988). Isospora belli infection: treatment with pyrimethamine. Ann. Intern. Med. 109, 474–475. doi: 10.7326/0003-4819-109-6-474
Zaidi, S. L., Mittal, S., Rajala, M. S., Avecilla, F., Husain, M., and Azam, A. (2015). Synthesis, characterization and antiamoebic activity of chalcones bearing N-substituted ethanamine tail. Eur. J. Med. Chem. 98, 179–189. doi: 10.1016/j.ejmech.2015.05.013
Zhang, H., Huang, H., Cali, A., Takvorian, P. M., Feng, X., Zhou, G., et al. (2005). Investigations into microsporidian methionine aminopeptidase type 2: a therapeutic target for microsporidiosis. Folia Parasitol. 52, 182. doi: 10.14411/fp.2005.023
Keywords: diarrhea, causative parasitic agents, chemotherapy, drug targets, therapeutic developments
Citation: Azam A, Peerzada MN and Ahmad K (2015) Parasitic diarrheal disease: drug development and targets. Front. Microbiol. 6:1183. doi: 10.3389/fmicb.2015.01183
Received: 10 June 2015; Accepted: 12 October 2015;
Published: 27 October 2015.
Edited by:
Anjan Debnath, University of California, San Diego, USAReviewed by:
Ximin Zeng, University of Tennessee, USASharon Lee Reed, UC San Diego Health Sciences, USA
Copyright © 2015 Azam, Peerzada and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Azam, YW1pcl9zdW1idWxAeWFob28uY28uaW4=
 Amir Azam
Amir Azam Mudasir N. Peerzada
Mudasir N. Peerzada Kamal Ahmad
Kamal Ahmad