- National Leading Research Laboratory of Drug Resistance Proteomics, Department of Biological Sciences, Myongji University, Yongin, South Korea
The increase of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) poses a worldwide and serious health threat. Although new antibiotics, such as daptomycin and linezolid, have been developed for the treatment of infections of Gram-positive pathogens, the emergence of daptomycin-resistant and linezolid-resistant strains during therapy has now increased clinical treatment failures. In the past few years, studies using quantitative proteomic methods have provided a considerable progress in understanding antibiotic resistance mechanisms. In this review, to understand the resistance mechanisms to four clinically important antibiotics (methicillin, vancomycin, linezolid, and daptomycin) used in the treatment of Gram-positive pathogens, we summarize recent advances in studies on resistance mechanisms using quantitative proteomic methods, and also examine proteins playing an important role in the bacterial mechanisms of resistance to the four antibiotics. Proteomic researches can identify proteins whose expression levels are changed in the resistance mechanism to only one antibiotic, such as LiaH in daptomycin resistance and PrsA in vancomycin resistance, and many proteins simultaneously involved in resistance mechanisms to various antibiotics. Most of resistance-related proteins, which are simultaneously associated with resistance mechanisms to several antibiotics, play important roles in regulating bacterial envelope biogenesis, or compensating for the fitness cost of antibiotic resistance. Therefore, proteomic data confirm that antibiotic resistance requires the fitness cost and the bacterial envelope is an important factor in antibiotic resistance.
Introduction
Antibiotic resistance has posed a serious threat to the worldwide public health in the past two decades. The gradual increase in resistance rates of several important pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), multidrug-resistant (MDR) Pseudomonas aeruginosa, imipenem-resistant Acinetobacter baumannii, and third-generation cephalosporin-resistant Escherichia coli and Klebsiella pneumonia, has become an increasingly severe problem in many hospitals worldwide (Lee et al., 2013). However, the decline in novel antibiotics that are introduced in the market weakens the hope of overcoming this threat by the development of new antibiotics. Most of the antibiotic classes used in hospitals today were discovered during the period 1930–1960. Only two new systemic classes of antibiotics that were developed during the past 30 years were linezolid and daptomycin, which are used only in the treatment of Gram-positive pathogens (Lee et al., 2013). Because many Gram-positive pathogens increasingly develop resistance against currently available antibiotics such as methicillin and vancomycin, these new antibiotics have become valuable for the treatment of various infections of methicillin- or vancomycin-resistant S. aureus and Streptococcus pneumonia (Ament et al., 2002; Mendes et al., 2014). However, the emergence of daptomycin-resistant or linezolid-resistant strains has recently been described in some Gram-positive pathogens (Fischer et al., 2011; Mendes et al., 2014). In this review, we summarize resistance mechanisms to four clinically important antibiotics (methicillin, vancomycin, linezolid, and daptomycin) used in the treatment of Gram-positive pathogens, and highlights recent important studies using comparative proteomic tools to understand resistance mechanisms of these antibiotics in more detail.
Action and Resistance Mechanisms of Methicillin, Vancomycin, Linezolid, and Daptomycin Resistance
Methicillin
Methicillin is a narrow-spectrum β-lactam antibiotic of the penicillin class. Like other β-lactam antibiotics, methicillin prevents the synthesis of bacterial cell walls by inhibiting peptidic cross-linkage between the linear peptidoglycan polymer chains, which provides rigidity to the cell wall of Gram-positive bacteria (Chambers, 1997) (Table 1). Methicillin and other β-lactam antibiotics are structural analogs of D-Ala-D-Ala, which is the terminus of a short amino acid chain attached in N-acetylmuramic acids; so, they interact with and irreversibly inhibit the transpeptidase enzyme [also called penicillin-binding protein (PBP)] that crosslinks the linear peptidoglycan polymer chains (Lee et al., 2012). This process leads to loss of osmotic integrity and makes the bacterial cells susceptible to lysis. Although most β-lactam antibiotics are inhibited by bacterial enzymes that hydrolyze the β-lactam ring (named β-lactamases), due to a modification of the original penicillin structure methicillin is resistant to β-lactamases (Lee et al., 2012). Therefore, since the late 1950s when methicillin was first introduced in markets, this antibiotic has been used to treat infections caused by Staphylococcus pathogens such as Staphylococcus aureus, most of which produces β-lactamase (Newsom, 2004).
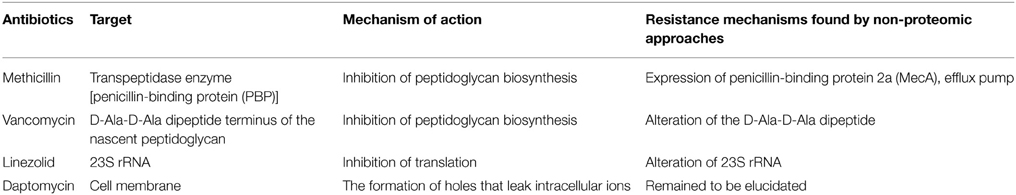
Table 1. Modes of action of four clinically important antibiotics (methicillin, vancomycin, linezolid, and daptomycin) and resistance mechanisms to these antibiotics.
Today, methicillin is not as effective against these organisms due to resistance (Cordwell et al., 2002; Newsom, 2004). Although the resistance phenotype of methicillin is influenced by numerous factors, including mecA, glmM, fmtAB, murE, llm, β-lactamase (bla) regulatory elements, and fem factors (Chambers, 1997; Cordwell et al., 2002; Hao et al., 2012), one major reason for methicillin resistance is the expression of the mecA gene, encoding penicillin-binding protein 2a (PBP 2a) that is not inhibited by classical β-lactam antibiotics including methicillin (Katayama et al., 2004) (Table 1). PBP 2a works in a similar manner to other PBPs, but it is bound by β-lactams with very low affinity (Katayama et al., 2004). Expression of PBP 2a confers resistance to all β-lactams. A variety of factors such as MecI and MecR1 controlled the mecA expression (Chambers, 1997). Resistance to methicillin exhibited by strains lacking the mecA gene is associated with modifications in native PBPs, β-lactamase hyperproduction, or possibly a methicillinase (Chambers, 1997). In pathogenesis, it has been reported that some virulence factors (Panton-Valentine leukocidin, phenol-soluble modulin, arginine catabolic mobile element, and other toxin elements) and two-component regulation systems (agr, saeRS, and vraRS) involved in pathogenesis can enhance the fitness of methicillin-resistant pathogens (Hao et al., 2012).
Vancomycin
Vancomycin made by the soil bacterium Amycolatopsis orientalis is a member of the glycopeptide antibiotic class and has an important role in the treatment of serious infections caused by Gram-positive bacteria such as Staphylococcus and Streptococcus (Woodford, 1998). It is a complex compound consisting of a branched tricyclic glycosylated peptide and is a rare example of a halo-organic natural compound containing two covalently bonded chlorine atoms (Levine, 2006). Vancomycin inhibits the peptidoglycan synthesis by binding at the D-Ala-D-Ala dipeptide terminus of the nascent peptidoglycan in Gram-positive bacteria (Healy et al., 2000; Levine, 2006). This binding of vancomycin to the D-Ala-D-Ala prevents the peptidic cross-linking between the linear peptidoglycan polymer chains by inhibiting the proper interaction with the transpeptidase enzyme (Healy et al., 2000) (Table 1).
Most Gram-negative bacteria are intrinsically resistant to vancomycin because it cannot penetrate the outer membrane of Gram-negative bacteria. In Gram-positive bacteria, one mechanism of resistance to vancomycin is the alteration of the terminal amino acid residues (D-Ala-D-Ala), to which vancomycin binds (Table 1). The D-Ala-D-Ala dipeptide terminus of the nascent peptidoglycan is replaced by D-Ala-D-Lac or D-Ala-D-Ser. The D-Ala-D-Lac variation results in a 1000-fold decrease in the affinity between vancomycin and the peptide, and the D-Ala-D-Ser variation causes a 6-fold loss of affinity, most likely due to steric hindrance (Courvalin, 2005). These alterations of the D-Ala-D-Ala dipeptide terminus require the coordinate action of several enzymes encoded by the van genes. Alternative ligases catalyze the formation of the D-Ala-D-Lac peptide (VanA, B, and D type enzymes) or D-Ala-D-Ser peptide (VanC, E, and G type enzymes) in peptidoglycan synthesis. VanH protein (α-keto acid reductase) reduces pyruvate to D-Lac, and the D,D-dipeptidase VanX selectively removes the D-Ala-D-Ala produced by the native ligase to enhance the incorporation of the D-Ala-D-Lac or D-Ala-D-Ser into the peptidoglycan precursor. VanR and VanS constitute a two-component regulatory system that activates the transcription of the van gene cluster (Marcone et al., 2010).
Linezolid
Linezolid is a first synthetic oxazolidinone antibiotic used to treat infections caused by VRE and MRSA. Although the mechanism of action of linezolid is not fully understood, it seems to bind to the 50S subunit of the bacterial ribosome through interaction with the central loop of the 23S rRNA and block the formation of protein synthesis initiation complexes (Swaney et al., 1998; Ament et al., 2002) (Table 1). Because linezolid binds to the 23S portion of the 50S subunit different from the binding sites of other ribosome-binding antibiotics such as chloramphenicol, cross-resistance between linezolid and other protein synthesis inhibitors is highly rare (Herrmann et al., 2008). The crystal structures of linezolid bound to the 50S subunit in 2008 showed that linezolid binds to the A site of the 50S ribosomal subunit and induces a conformational change perturbing the correct positioning of tRNAs on the ribosome (Ippolito et al., 2008; Wilson et al., 2008).
Most Gram-negative bacteria have an intrinsic resistance to linezolid due to the high activity of efflux pumps, which actively pump linezolid out of the cell (Schumacher et al., 2007). In Gram-positive bacteria, the acquired resistance to linezolid was first reported in 1999 in multidrug-resistant Enterococcus faecium (Mendes et al., 2014). High-resolution structures of linezolid with the 50S ribosomal subunit showed that it binds to a deep cleft that is surrounded by the central loop of domain V of 23S rRNA (Long and Vester, 2012). Therefore, the most common resistance mechanism of Gram-positive bacteria to linezolid was a point mutation known as G2576T, in which the G2576 position of 23S ribosomal RNA is converted to thymine (Mendes et al., 2014). In addition to mutations in 23S rRNA, other mechanisms have been identified in Gram-positive bacteria, including a six base pair deletion in the ribosomal protein L4, mutations in the ribosomal protein L3, mutations in an RNA methyltransferase (encoded by the cfr gene) that methylates G2445 of the 23S rRNA, and mutations causing increased expression of ABC transporter genes (patA and patB).
Daptomycin
Daptomycin is a lipopeptide antibiotic consisting of a lipid molecule conjugated with anionic peptide and is a natural compound found in the soil bacterium Streptomyces roseosporus (Miao et al., 2005). Daptomycin absolutely requires Ca2+ for activity, making this agent a cationic antimicrobial peptide functionally (Baltz, 2009). The poorly calcium-decorated form of daptomycin is 10 times less active microbiologically than the heavily calcium-decorated form (Baltz, 2009). The calcium-bound daptomycin interacts with phosphatidylglycerol in the bacterial membrane and inserts into the cell membrane, leading to the formation of holes that leak intracellular ions (Pogliano et al., 2012). A loss of membrane potential causes inhibition of protein, DNA, and RNA synthesis, which results in bacterial cell death (Pogliano et al., 2012). Because of a distinct mechanism of action of daptomycin, it is used in the treatment of life-threatening infections caused by multiple drug-resistant Gram-positive bacteria (Baltz, 2009). Because vancomycin and daptomycin have molecular weight (MWs) of more than 1000 Da (vancomycin of 1449 Da and daptomycin of 1620 Da), they cannot penetrate the outer membrane of Gram-negative bacteria (Lee et al., 2013). Therefore, two antibiotics are used to control infections caused by Gram-positive bacteria.
Although daptomycin was clinically introduced in 2003, clinical treatment failures by the emergence of daptomycin-resistant strains during therapy have now been described (Hobbs et al., 2008; Fischer et al., 2011). Up to now, specific genetic determinant of the daptomycin-resistant strain remained to be elucidated, despite the finding of several phenotypic and genetic determinants (altered phospholipid synthesis, thickened cell walls, alteration of cell membrane fluidity, and the acquisition of mutations within the mprF or yycG gene) (Mishra et al., 2009; Fischer et al., 2011). The mprF gene encodes a dual functional enzyme that catalyzes the coupling of lysine to phosphatidylglycerol (PG) and transfers the lysyl-PG (LPG) to the outer leaflet of the membrane. The LPG is less acidic than PG, and membranes lacking LPG are more acidic than those containing PG and LPG (Baltz, 2009). Daptomycin-resistant strains with mprF mutations have membranes with increased levels of LPG (Jones et al., 2008). Therefore, the increased positive charge caused by increased LPG in the mprF mutant (gain-of-function) reduces the binding of Ca2+-bound daptomycin to bacterial membranes by a less favorable electrostatic interaction. YycG is a membrane spanning sensor histidine kinase of a two-component signal transduction system that partners with the YycF response regulator. YycFG functions as a master regulatory system for cell wall metabolism and biofilm formation and is the only two-component system required for viability in many Gram-positive bacteria (Winkler and Hoch, 2008; Baltz, 2009).
Comparative Proteomic Analyses of Methicillin, Vancomycin, Linezolid, and Daptomycin Resistance
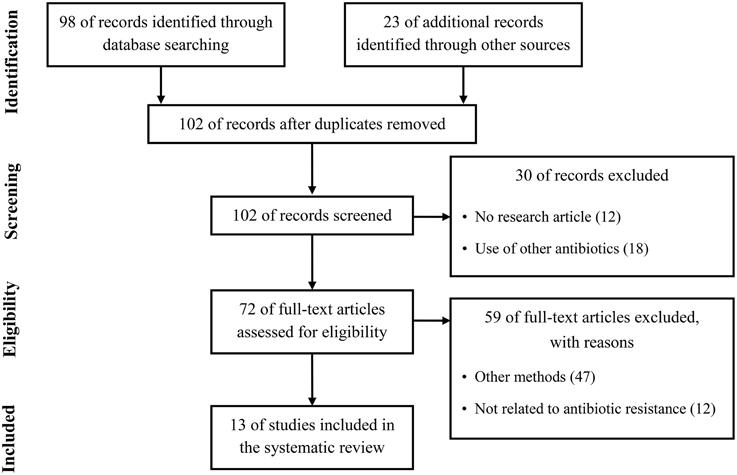
Quantitative proteomics have been considerably improved during the past decade and have been employed for investigation of the differences in whole protein expression dynamics of cells grown under a variety of growth conditions or stress conditions such as antibiotics (Radhouani et al., 2012). Therefore, by studies using quantitative proteomic approaches in the past few years, a considerable progress has recently been made in the study of antibiotic resistance mechanism. To summarize recent updates to understand the resistance mechanism to four clinically important antibiotics used in the treatment of Gram-positive pathogens, we used the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) in our review (Figure 1) (Moher et al., 2009). We conducted a systematic literature search in the following databases: Medline via PubMed and Embase. We used keywords as search terms. We combined terms for selected indications (methicillin, vancomycin, linezolid, daptomycin, and proteomics). The literature search included all studies published in English between 2000 and 2015. We identified 13 proteomics studies comparing proteomic profiles in antibiotic-resistant and antibiotic-sensitive strains or exploring proteomic profiles in cells treated with or without antibiotics.
Methicillin
Two studies exploring proteomic profiles of methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) in the absence of methicillin were reported (Cordwell et al., 2002; Enany et al., 2014). Cordwell et al. compared the protein profiles between S. aureus strains COL (methicillin-resistant) and 8325 (methicillin-susceptible) in the absence of methicillin (Cordwell et al., 2002). Interestingly, among proteins previously known as resistance-related factors (e.g., mecA, glmM, fmtAB, murE, llm, bla, and fem factors), only FemA protein, which is known as a host-mediated factor essential for methicillin resistance in S. aureus (Berger-Bächi et al., 1989), was more highly expressed in methicillin-resistant cells (Cordwell et al., 2002). However, upon growth of both strains in the presence of Triton X-100 (TX-100), a detergent that has been shown to reduce methicillin resistance, no difference on the production of the essential methicillin-resistance factor FemA was detected (Cordwell et al., 2002). Instead, expression levels of stress-related proteins including cold-shock proteins (CspABC) and alkaline-shock protein 23 (Asp23) increased in the methicillin-resistant S. aureus strain COL (Cordwell et al., 2002). Notably, the amount of CspB, CspC, and Asp23 proteins was affected in cases of vancomycin and daptomycin antibiotics, despite being down-regulated in the vancomycin-resistant strain and up-regulated in the daptomycin-resistant strain (Table 7). This study also showed that three proteins linked to the alternative sigma factor σB, Asp23, anti-anti- σB factor RsbV, and conserved hypothetical protein SA0772, were also present at significantly higher levels in methicillin-resistant cells (Cordwell et al., 2002). In the presence of TX-100 weakening the methicillin resistance, the comparative proteomic analysis showed that proteins of the σB and SarA (a regulator of virulence genes) regulons are involved in methicillin resistance of S. aureus (Cordwell et al., 2002). The level of SarA protein also increased in vancomycin-resistant and daptomycin-resistant cells (Table 7). This study also showed that the stage V sporulation protein G (SpoVG), originally identified in Bacillus subtilis as being involved in the formation of the spore cortex (Matsuno and Sonenshein, 1999), was up-regulated in the methicillin-resistant S. aureus strain COL. In the non-sporulating S. aureus, SpoVG contributes to stimulate capsule synthesis, and was recently shown to regulate a small σB-subregulon comprising mainly excreted virulence factors including the highly up-regulated virulence factor EsxA (Schulthess et al., 2012). Recently, it has been reported that SpoVG was involved in resistance mechanisms to methicillin and glycopeptide (Schulthess et al., 2009). Together with this report, a comparative proteome analysis showed that the expression level of SpoVG increased in strains resistant to methicillin, vancomycin, and daptomycin (Table 5), indicating that SpoVG may be involved in resistance mechanisms to other antibiotics as well as methicillin and glycopeptide.
Another report explored proteome profiles of extracellular proteins in methicillin-sensitive and methicillin-resistant S. aureus (Enany et al., 2014). They identified some proteins increased in MRSA; Asp23 (10-fold more in MRSA than MSSA), alkyl hydroperoxide reductase subunit C (AhpC) (2-fold), D-lactate dehydrogenase (LdhD) (2-fold), general stress protein 20U (3-fold), L-lactate dehydrogenase (LdhA) (2-fold), pyruvate dehydrogenase E1 component beta subunit (PdhB) (2-fold), superoxide dismutase (SodA) (2-fold), triacylglycerol lipase precursor (LipA) (2-fold), triosephosphate isomerase (TpiA) (2-fold), and universal stress protein family protein (7-fold) (Enany et al., 2014). Notably, among them, most proteins (AhpC, SodA, LdhA, LipA, and TipA) also have altered expression levels in other antibiotic-resistant strains (Table 7). In addition, elongation factor G (encoded by the fusA gene) was also increased in MRSA. Our analysis showed that PusA is one of the three proteins affected in all four antibiotic-resistant strains (Table 5). Although elongation factor G is a major target of fusidic acid which has been used as a topical agent for skin infection and for some systemic infections caused by S. aureus (Howden and Grayson, 2006), and had a contribution to fusidic acid resistance mechanisms evolved in MRSA (Koripella et al., 2012), the relationship between elongation factor G and resistance mechanisms of other antibiotics has not yet been identified.
Vancomycin
There were two studies exploring proteomic profiles in vancomycin-susceptible S. aureus (VSSA) and vancomycin-intermediate S. aureus (VISA) with a minimal inhibitory concentration (MIC) of 4–8 μg/ml, one study exploring proteomic profiles in VSSA and heterogeneous vancomycin-intermediate S. aureus (hVISA) with a vancomycin MIC of ≤2 μg/ml, one study exploring proteomic profiles in VISA and vancomycin-resistant S. aureus (VRSA) with MIC of ≥8 μg/ml, one study analyzing global proteomes of vancomycin stress in S. aureus, and two studies examining vancomycin-induced proteomes of Enterococcus faecalis under vancomycin treatment (Pieper et al., 2006; Scherl et al., 2006; Drummelsmith et al., 2007; Wang et al., 2010; Chen et al., 2013; Hessling et al., 2013; Ramos et al., 2015). Many proteins previously known as resistance-related factors, including VanA, VanB, VanX, and VanR, were also identified in comparative proteomic analyses (Table 2). Scherl et al. (2006) showed that a total of 155 proteins are differentially expressed between two vancomycin-susceptible S. aureus strains (MRGR3 and 14-4Rev) and the vancomycin-intermediate S. aureus strain 14-4, and most proteins play a role in energy metabolism, cell envelope biosynthesis, protein turnover, amino acids transport, and metabolism, and inorganic ion transport. Genes or gene products known to be involved in resistance mechanisms to different antibiotics, such as PBP 2a (MecA), O-nucleotidyltransferase(9) [Ant(9)], UDP-N-acetylmuramyl tripeptide synthetase (MurE), and penicillin-binding methicillin resistant-related protein (FmtA), were up-regulated in the VISA strain (Scherl et al., 2006). All of them are involved in peptidoglycan biosynthesis. Levels of many other proteins involved in peptidoglycan metabolism also increased in the VISA strain, such as glycosyltransferase (SgtB) and CHAP (Cysteine, Histidine-dependent Amidohydrolases/Peptidases)-domain amidase (SsaA). SsaA belongs to the CHAP amidase family, members of which such as LysK and LytA have been shown to have D-alanyl-glycyl endopeptidase activity, cleaving between the crossbridge and the stem peptide (Delaune et al., 2011), and protein levels of SsaA were also changed in cases of methicillin and linezolid (Table 6), indicating the importance of this protein on peptidoglycan metabolism and antibiotic resistance.
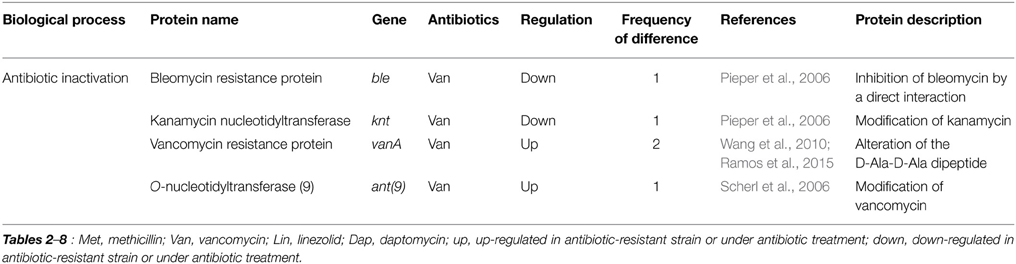
Table 2. Differentially expressed proteins identified by the quantitative proteomic approach: proteins involved in resistance mechanisms.
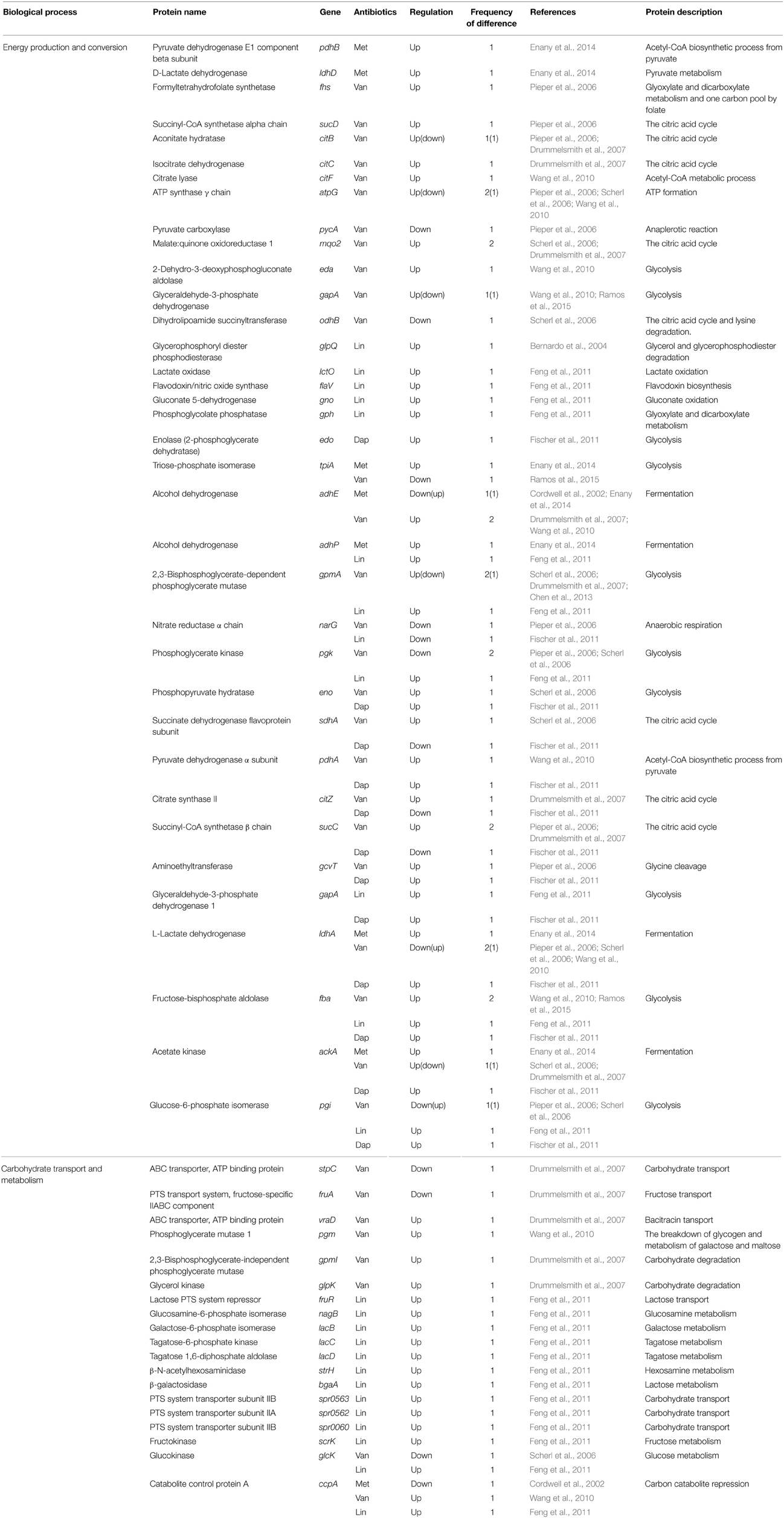
Table 3. Differentially expressed proteins identified by the quantitative proteomic approach: proteins involved in energy metabolism.
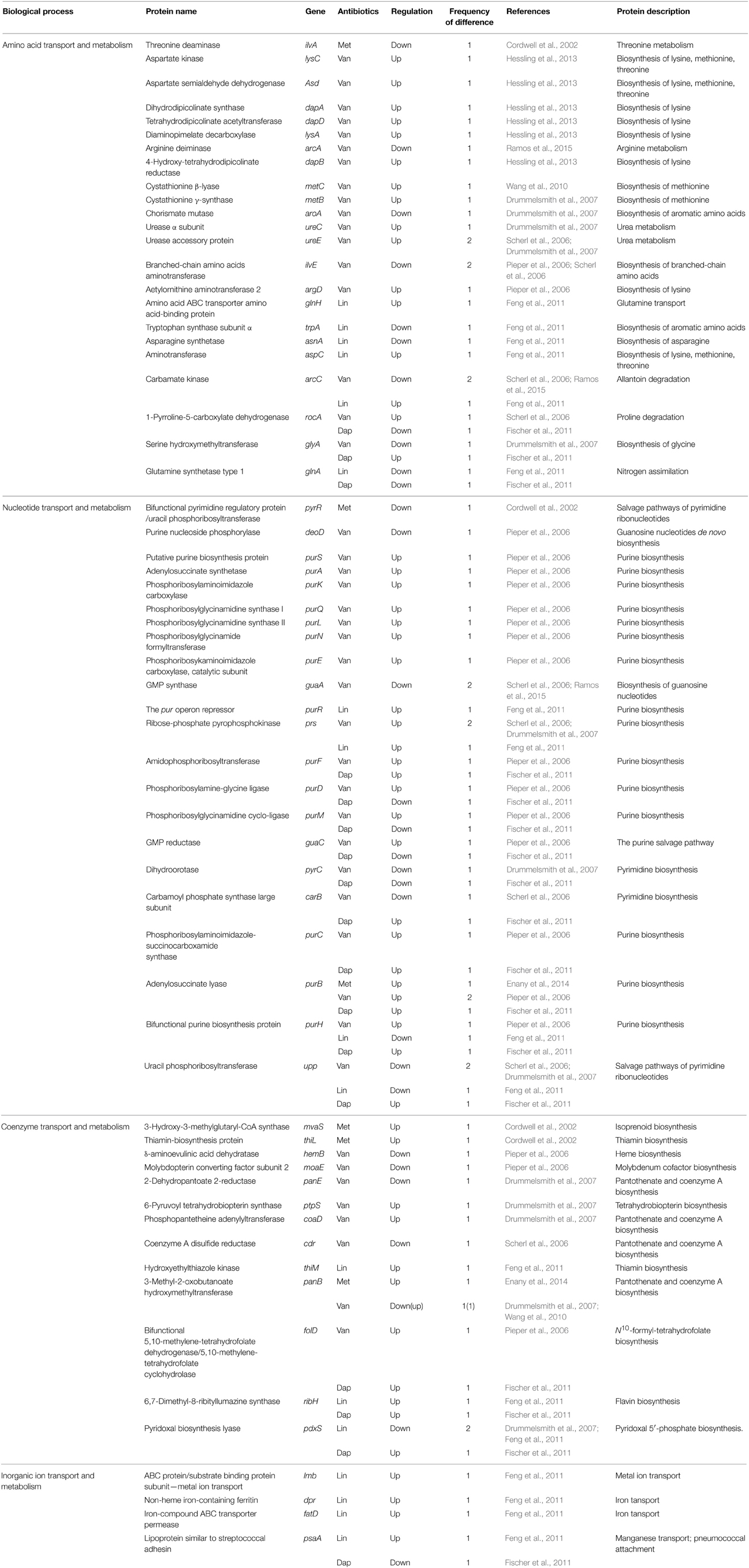
Table 4. Differentially expressed proteins identified by the quantitative proteomic approach: proteins involved in amino acid, nucleotide, coenzyme, and inorganic ion metabolisms.
They also identified several regulatory systems contributing to the VISA phenotype, such as the two-component system (VraSR) regulating expression of a set of genes involved in the cell wall biosynthesis or degradation (Boyle-Vavra et al., 2013), the signal transduction protein TRAP acting on quorum sensing (Gov et al., 2004), the DivIVA protein known to regulate cell division in B. subtilis (Perry and Edwards, 2004), and putative transcription factors SA2296 and SarH1. VraSR (vancomycin resistance associated regulator) was up-regulated under vancomycin treatment (Kuroda et al., 2003) and in the VISA strain when compared with an isogenic vancomycin-susceptible strain (Kuroda et al., 2000). In addition, inactivation of the vraSR gene increased vancomycin susceptibility (Kuroda et al., 2003). Interestingly, VraSR was also induced by other antibiotic classes that target the cell wall, including β-lactam (Gardete et al., 2006; Yin et al., 2006), mersacidin (Sass et al., 2008), certain cationic peptides (Pietiäinen et al., 2009), and daptomycin (Muthaiyan et al., 2008). Inactivation of the vraSR gene attenuates resistance to various antibiotics, such as vancomycin (Kuroda et al., 2003; Gardete et al., 2006), daptomycin (Mehta et al., 2012), and β-lactams (Kuroda et al., 2003; Boyle-Vavra et al., 2006; Gardete et al., 2006). The expression of many genes, such as ctpA, drp35, fmtA, opuD, pbp2, prsA, sgtB, and vraX, is regulated by VraSR (Utaida et al., 2003; McAleese et al., 2006; Dengler et al., 2011). Among them, FmtA is typically known as a factor involved in methicillin-resistant phenotype of S. aureus (Fan et al., 2007), and PrsA (foldase precursor) was recently reported to be involved in both glycopeptide and oxacillin resistance in S. aureus (Jousselin et al., 2012). Similarly, at three independent studies of comparative proteomic analysis, it has been proven that the expression level of PrsA is up-regulated in VISA when compared with VSSA (Table 5), indicating that proteomic studies can support the identification of targets involved in antibiotic resistance. They also identified another important protein VraX (a hypothetical protein which encodes a 55-amino acids protein) differentially expressed between vancomycin-susceptible S. aureus strains and the vancomycin-intermediate S. aureus strain 14-4 (Scherl et al., 2006). This gene was up-regulated by multiple cell wall and/or membrane active compounds (bacitracin, d-cycloserine, oxacillin, tunicamycin, flavomycin, fosfomycin, teicoplanin, vancomycin, daptomycin, lysostaphin, epicatechin gallate, ranalexin, and antimicrobial peptides) (Utaida et al., 2003; Pietiäinen et al., 2009; Dengler et al., 2011; Cuaron et al., 2013). The vraX gene belongs to the vra operon together with the vraA gene encoding for a long chain fatty acid-CoA ligase, which was up-regulated in the VISA. Additionally, this gene seems to be involved in resistance mechanism to vancomycin (Hanaki et al., 1998; Buntaran et al., 2013). Finally, stress-related proteins such as proteinases (CtpA), methionine sulfoxide reductase A (MsrA2), and the methionine sulfoxide reductase regulator MsrR, were over-expressed in the vancomycin-intermediate S. aureus strain 14-4 (Scherl et al., 2006). In other studies, MsrA2 was also up-regulated in hVISA (Chen et al., 2013).
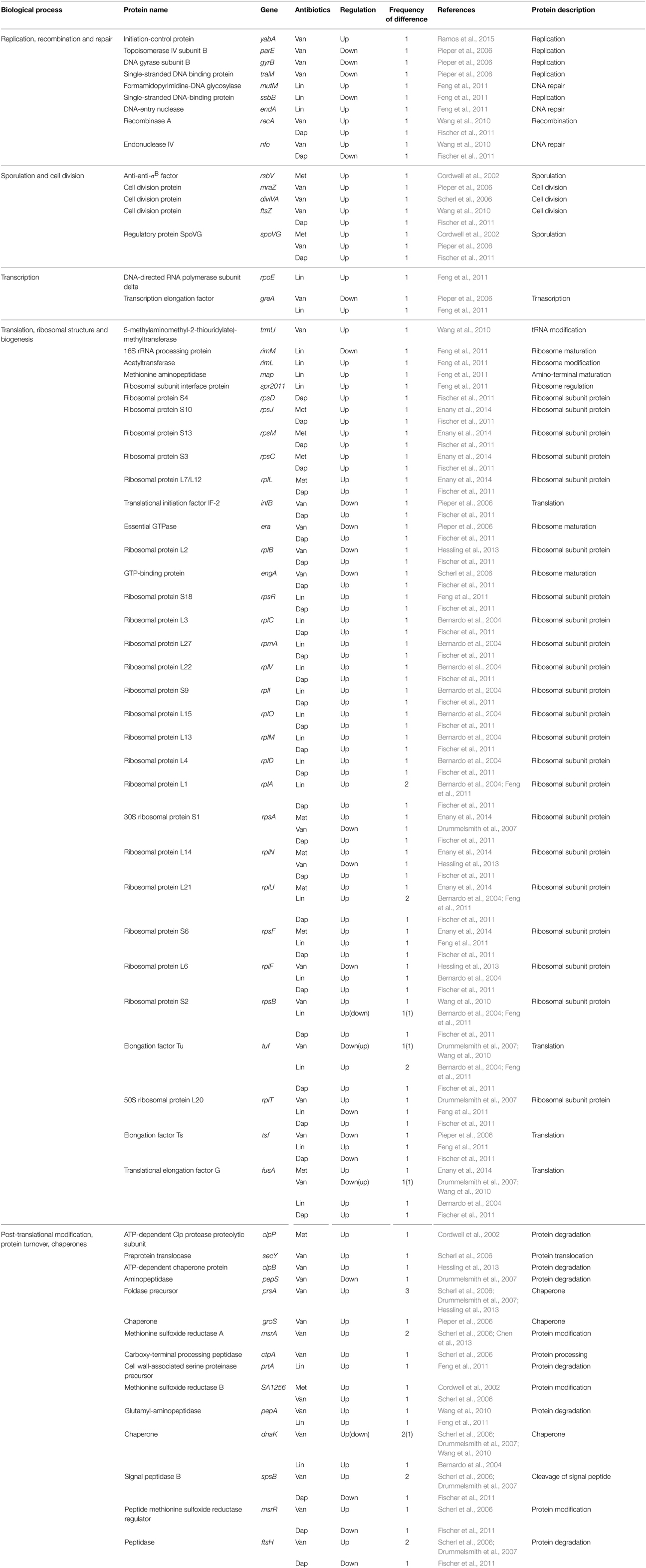
Table 5. Differentially expressed proteins identified by the quantitative proteomic approach: proteins involved in replication, cell division, transcription, translation, and protein turnover.
Pieper et al. showed that purine ribonucleotide biosynthesis (PRNBS) pathway enzymes, which are under the control of the PurR regulator, strongly increased in protein abundance in the vancomycin-resistant S. aureus strain VP32 having a vancomycin MIC of 32 μg/ml when compared with the vancomycin-intermediate S. aureus strain HIP5827 (MIC = 8 μg/ml) (Pieper et al., 2006). Notably, among them, several proteins such as amidophosphoribosyltransferase (PurF), phosphoribosylamine-glycine ligase (PurD), phosphoribosylglycinamidine cyclo-ligase (PurM), phosphoribosylaminoimidazole-succinocarboxamide synthase (PurC), adenylosuccinate lyase (PurB), and bifunctional purine biosynthesis protein (PurH), were also changed in protein abundance in cases of other antibiotics such as daptomycin and linezolid (Table 4). Microarray transcription analysis of clinical VISA isolates already showed that among the 35 genes with increased transcription in vancomycin-resistant S. aureus strain VP32 when compared with those of their VISA parent strains HIP5827 and P100, 15 were involved in purine biosynthesis or transport (Mongodin et al., 2003). They hypothesized that increased energy (ATP) is required to generate the thicker cell walls that characterize resistant mutants (Mongodin et al., 2003). However, contrary to these results, other comparative proteomic analyses between vancomycin-susceptible strains and vancomycin-intermediate S. aureus strains did not show similar results (Scherl et al., 2006; Drummelsmith et al., 2007; Chen et al., 2013). Therefore, these results imply that VRSA may more efficiently compensate for a fitness cost of antibiotic resistance such as ATP requirement than VISA.
Abundance changes were also found in proteins such as the single-stranded DNA binding protein (TraM), DNA gyrase subunit B (GyrB), and topoisomerase IV subunit B (ParE), which catalyze or influence the fidelity of DNA replication and repair (Table 5). This result is consistent with the increasing evidence that exposure to antibiotics in bacteria leads to increased mutation rates in the genome, to favor their survivals under antibiotic pressure (Napolitano et al., 2000; Friedberg et al., 2002; Pieper et al., 2006). Expression levels of many enzymes involved in energy metabolisms, including L-lactate dehydrogenase (LdhA), glucose-6-phosphate isomerase (Pgi), succinyl-CoA synthetase (SucCD), phosphoglycerate kinase (Pgk), nitrate reductase alpha chain (NarG), and aconitate hydratase (CitB), were also changed. In fact, comparative proteomic analyses show that proteins involved in energy metabolism, protein synthesis, and envelope biogenesis, most frequently exhibit abundance change in antibiotic-resistant strains (Table 3). In many cases, proteins playing a role in energy metabolism were up-regulated in antibiotic-resistant strains (Table 3). This phenomenon may be explained by a prior hypothesis that increased energy (ATP) is required to generate the thicker cell walls or to pump antibiotics out of the cell using efflux pumps. This study also showed the changes of proteins involved in cell envelope biogenesis, such as D-Ala-D-Ala ligase (Ddl), D-Ala-D-Lac ligase (VanA), peptidoglycan hydrolase (LytM), cell division and cell wall biosynthesis protein (MraZ), putative cell wall transglycosylase (SceD), and glucosamine-fructose-6-phosphate aminotransferase (GlmS) (Pieper et al., 2006).
Similar to prior reports, Drummelsmith et al. showed the high level inductions of cell wall metabolism-related proteins such as MecA, LytM, GlmS, and SceD in the VISA type strain Mu50 when compared with the vancomycin-sensitive strain CMRSA-2 (Drummelsmith et al., 2007). In particular, they selected SceD for further study based on its high level of induction (approximately 16-fold) in VISA, and relative sceD mRNA expression levels were compared between 25 VSSA and VISA clinical isolates by real-time RT-PCR (Drummelsmith et al., 2007). The sceD mRNA was significantly induced in all VISA isolates relative to all VSSA strains, and they suggest that SceD expression level could serve as a molecular diagnostic marker for the rapid detection of VISA (Drummelsmith et al., 2007). Interestingly, SceD was also up-regulated in both daptomycin-resistant (Song et al., 2013) and linezolid-resistant strains (Bernardo et al., 2004), suggesting the importance of this protein in antibiotic resistance. They also identified other proteins involved in cell envelope metabolism as a highly up-regulated protein in VISA; UDP-GlcNAc 1-carboxyvinyltransferase 1 (MurA), bifunctional autolysin (Atl), immunodominant antigen A (IsaA), UDP-glucose/GDP-mannose dehydrogenase (CapO), and UDP-N-acetyltalosamine 2-epimerase (CapG) (Table 6). Among them, IsaA was also up-regulated in VISA at other two studies (Scherl et al., 2006; Chen et al., 2013). In addition, its expression level increased in both methicillin-resistant and daptomycin-resistant strains (Cordwell et al., 2002; Fischer et al., 2011), and decreased in linezolid-resistant strains (Bernardo et al., 2004), suggesting the importance of this protein. The housekeeping protein IsaA is a highly immunogenic, non-covalently cell wall-bound lytic transglycosylase that is co-regulated with a glycylglycine endopeptidase LytM (Stapleton et al., 2007; Lorenz et al., 2011). S. aureus has two putative peptidoglycan hydrolases, IsaA and SceD, and SceD can compensate for the loss of IsaA (Stapleton et al., 2007). The fact that both peptidoglycan hydrolases (IsaA and SceD) are involved in antibiotic resistance strongly indicates the importance of cell wall dynamics in antibiotic resistance mechanism.
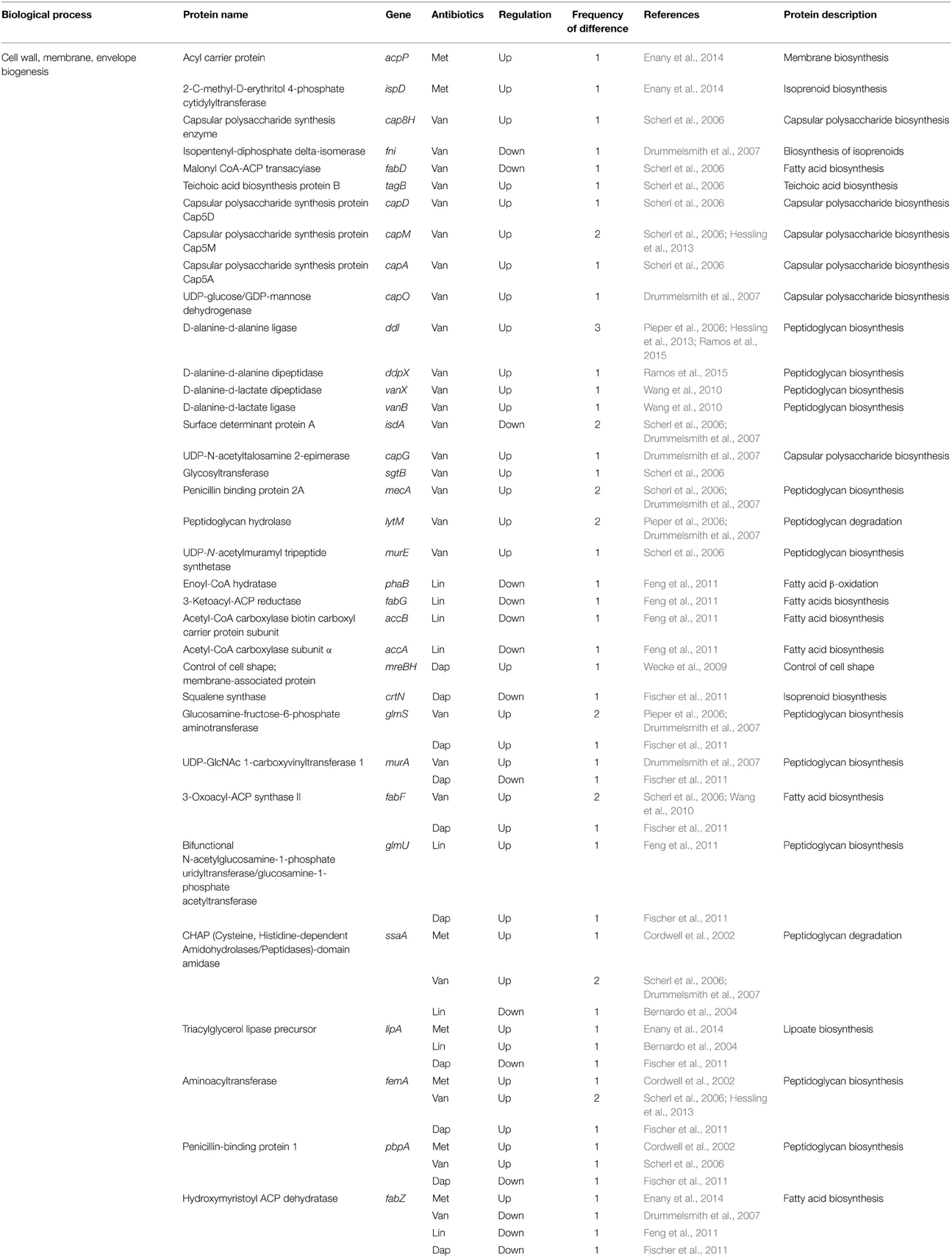
Table 6. Differentially expressed proteins identified by the quantitative proteomic approach: proteins involved in envelope biogenesis.
To identify the resistance mechanisms of hVISA with a vancomycin MIC of ≤2 μg/ml, Chen et al. compared proteomic profiles of six pairs of isogenic hVISA and VSSA strains and unrelated hVISA (n = 24) and VSSA stains (n = 30) (Chen et al., 2013). They identified five proteins up-regulated in the hVISA strains; IsaA, MsrA, Asp32, 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase (GpmA), and AhpC. Consistent with this result, MsrA was up-regulated in a prior study using comparative proteomics (Scherl et al., 2006) and in the DNA microarray study, and the msrA gene was also over-expressed in VISA strains (Cui et al., 2005). MsrA, catalyzing the reversible oxidation-reduction of methionine sulfoxide to methionine, has a key function as a repair enzyme for proteins inactivated by oxidation (Chen et al., 2013). The msrA gene is highly induced by cell wall-active antibiotics, such as oxacillin and vancomycin (Chen et al., 2013). The increased level of MsrA can enhance peptidoglycan biosynthesis which results in cell wall thickening, and gene knockout of the msrA gene weakened vancomycin and β-lactam resistance of S. aureus strains (Cui et al., 2005). In addition, MsrA is involved in virulence in several bacteria (Sasindran et al., 2007). Taken together, these observations suggest the important role of methionine sulfoxide in antibiotic resistance. Although in other studies, the abundance of GpmA, which plays a physiological role in glycolysis, has been reported to be changed in VISA (Table 3), its exact role in antibiotic resistance has not been determined. AhpC, an alkyl hydroperoxide reductase subunit C, plays an important role in oxidative-stress resistance of S. aureus (Cosgrove et al., 2007). Interestingly, it was reported that AhpC is up-regulated in strains resistant to methicillin, vancomycin, and daptomycin antibiotics (Table 7). However, up to now, there is no report investigating the direct role of AhpC in antibiotic resistance. It is noteworthy that several proteins involved in oxidative-stress resistance, such as AhpC, SodA, catalase (KatA), and superoxide dismutase (SodM), show the abundance change of proteins in antibiotic-resistant strains (Table 7), and in most cases, their expression is up-regulated. In spite of these interesting results, the relationship between these proteins and antibiotic resistance was not determined.
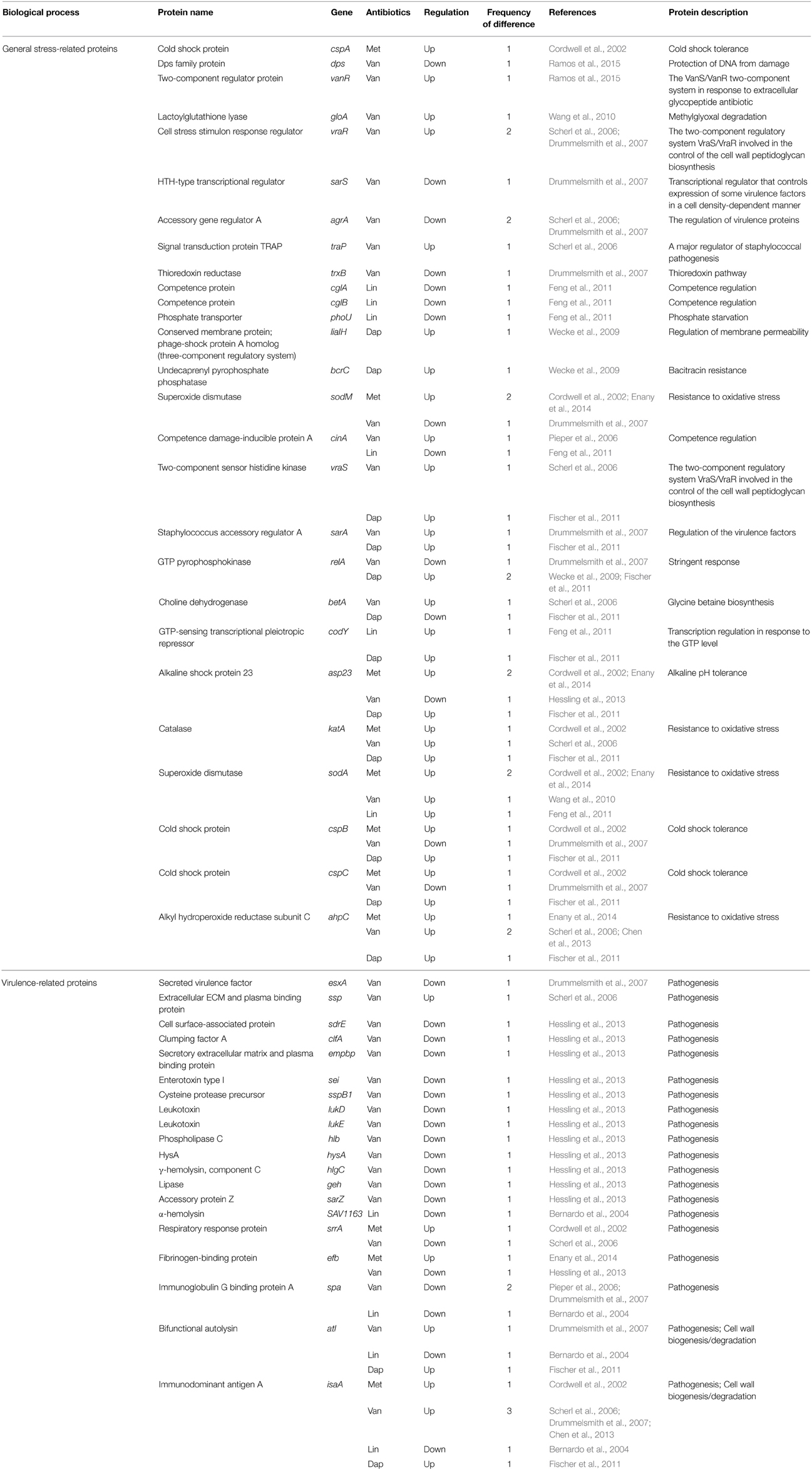
Table 7. Differentially expressed proteins identified by the quantitative proteomic approach: proteins involved in stress response.
Hassling et al. analyzed proteomic profiles of vancomycin-susceptible S. aureus strain COL under the sublethal vancomycin exposure (4.5 μg/ml) (Hessling et al., 2013). They found the specific increase of proteins involved in the synthesis of lysine which are essential for the synthesis of the peptidoglycan precursor pentapeptide; aspartate kinase (LysC), aspartate semialdehyde dehydrogenase (Asd), dihydrodipicolinate synthase (DapA), 4-hydroxy-tetrahydrodipicolinate reductase (DapB), diaminopimelate decarboxylase (LysA), and tetrahydrodipicolinate acetyltransferase (DapD). An increase of lysine synthesis proteins can lead to an overall increase of peptidoglycan synthesis. Induction of genes involved in lysine synthesis under cell wall stress conditions have been documented before by two transcriptome studies (Kuroda et al., 2003; Sobral et al., 2007). Consistent with the previous report (Scherl et al., 2006), this report also showed that several proteins regulated by the two-component system VraSR increased in amount after vancomycin addition (Hessling et al., 2013). Additionally, they identified two important regulators (the alternative sigma factor σB and the two-component system SaeRS regulating numerous virulence genes) that play a role in vancomycin stress response. The cluster of proteins under positive σB control mainly increased, whereas negatively regulated proteins primarily decreased in amount after vancomycin addition (Hessling et al., 2013). The induction of σB regulon by vancomycin has been found in another report (Chen et al., 2013). Increase of the σB activity has also been observed in strains resistant to teicoplanin (Bischoff and Berger-Bächi, 2001) or methicillin (Cordwell et al., 2002). Hassling et al. also found decreased expression levels of most proteins with a virulence related function (Hessling et al., 2013). However, because the great majority of virulence genes in previous transcriptome studies under cell wall stress in S. aureus have been shown to be up-regulated (Kuroda et al., 2003; Utaida et al., 2003; Sobral et al., 2007), the role of virulence genes in antibiotic resistance needs to be determined.
Lastly, Wang et al. and Ramos et al. performed proteomic analysis of vancomycin-resistant E. faecalis strains (V583, V306, and SU18) under 64 μg/ml vancomycin treatment (Wang et al., 2010; Ramos et al., 2015). Vancomycin induced expression of vancomycin resistance-related proteins such as VanA, VanX, D-Ala-D-Ala dipeptidase (DdpX), VanR, and VanB (Wang et al., 2010; Ramos et al., 2015). Distinctively, Wang et al. found that six proteins (Pgm, Ldh, Gap-2, RpsB, EF2076, and sex pheromone cAD1 precursor lipoprotein) exhibited clear post-translational modifications and vancomycin induced phosphorylation of Ser/Thr in Ldh, Gap-2, and sex pheromone cAD1 precursor lipoprotein (EF3256) (Wang et al., 2010). Ramos et al. showed that metabolism-related proteins, such as TipA, GMP synthase (GuaA), and glyceraldehyde-3-phosphate dehydrogenase (GapB), were down-regulated under vancomycin treatment (Ramos et al., 2015).
Linezolid
There was one study exploring comparative proteomic profiles in linezolid-susceptible S. pneumonia strains and linezolid-resistant S. pneumonia strains, and one study analyzing global proteomes of a linezolid- susceptible S. aureus under linezolid stresses (Bernardo et al., 2004; Feng et al., 2011). Through the comparison between linezolid-susceptible S. pneumonia strains (1974 and R6) with linezolid MICs of 0.5–0.75 μg/ml and linezolid-resistant S. pneumonia strains (1974M2-LZD and R6M2-LZD) with MIC of 32 μg/ml, Feng et al. showed that the proteomic and transcriptomic approaches were poorly correlated with previously known resistance factors (23S rRNA, ribosomal proteins L3 and L4, RNA methyltransferase Cfr, and ABC transporter PatA and PatB), as modulated proteins rarely had significant concomitant changes at the expression level (Feng et al., 2011). They found increased expression of proteins involved in the metabolism and transport of carbohydrates in linezolid-resistant S. pneumoniae strains (Feng et al., 2011). Through inactivation of target genes in the linezolid-resistant strains (1974M2-LZD and R6M2-LZD), they identified two ABC transporter substrate-binding proteins (Spr0083 and Spr1527) and the catabolite control protein A (CcpA) as factors associated with resistance to linezolid (Feng et al., 2011). CcpA is known to function as the global regulator controlling the efficient utilization of sugars through carbon catabolite repression (CCR) in Gram-positive bacteria (Stülke and Hillen, 2000). Inactivation of the ccpA gene in S. aureus affected growth, glucose metabolism, and expression of virulence genes (Seidl et al., 2006). CcpA inactivation was also linked to the down-regulation of glycolytic genes in Bacillus cereus (van der Voort et al., 2008; Feng et al., 2011). Therefore, the increased level of CcpA may cause the increased expression of glycolytic enzymes in linezolid-resistant S. pneumonia strains. In S. aureus, the correlation between antibiotic resistance and CcpA has already been reported, as CcpA inactivation significantly reduced the oxacillin resistance levels in MRSA and the teicoplanin resistance level in a glycopeptide-intermediate-resistant S. aureus strain (Seidl et al., 2006). Table 3 shows the possibility that CcpA may also be involved in methicillin and vancomycin resistance. Together with CcpA, inactivation of two ABC transporters putatively involved in the sugar transport (Spr0083 and Spr1527) also reduced resistance to linezolid of S. pneumonia (Feng et al., 2011). Notably, S. pneumoniae is predicted to be highly dependent on external sugars to fulfill its energy requirements by substrate-level phosphorylation as it lacks functional electron transport chain and tricarboxylic acid cycle (Tettelin et al., 2001; Feng et al., 2011). This process eventually leads to the formation of lactate and acetate by the lactate dehydrogenase and lactate oxidase enzymes and these proteins were also found to be overexpressed in linezolid-resistant S. pneumonia strains (Tettelin et al., 2001; Feng et al., 2011). Therefore, these results imply increased energy requirements associated with resistance mechanism to linezolid in S. pneumonia (Feng et al., 2011). To sustain a fitness cost associated with resistance mechanisms such as the 23S rRNA mutations (Besier et al., 2008), S. pneumonia seems to select an increased metabolism of sugars as a secondary adaptation.
This study also showed that several genes involved in the biosynthesis of fatty acids, including enoyl-CoA hydratase (PhaB), 3-ketoacyl-ACP reductase (FabG), acetyl-CoA carboxylase biotin carboxyl carrier protein subunit (AccB), acetyl-CoA carboxylase subunit alpha (AccA), and hydroxymyristoyl-ACP dehydratase (FabZ), were down-regulated in linezolid-resistant strains (Feng et al., 2011). Whether this is directly related to linezolid resistance remains to be established, but it is intriguing that the cell wall inhibitor penicillin also causes a down-regulation of several genes of this pathway in S. pneumoniae (Rogers et al., 2007; Feng et al., 2011). Interestingly, expression levels of FabZ are changed in all cases of the four antibiotics (Table 6), even though its expression increased in methicillin-resistant strains and decreased in strains resistant to other antibiotics. Many numbers of ribosomal proteins were found to be overexpressed or down-regulated in linezolid-resistant strains, but whether this pattern is due to the mechanism of action of linezolid (which targets the ribosome) remains to be established. Although recent several lines of evidence indicate the presence of functional selective ribosomal subpopulations that exhibit variations in the RNA or the protein components and modulate the translational program in response to environmental changes (Byrgazov et al., 2013), it is difficult to obtain any information from variation patterns of ribosomal proteins in this study.
Bernardo et al. compared the change of proteomic profiles of a linezolid- susceptible S. aureus strain ATCC 29213 (MIC = 2.5 μg/ml) under linezolid stresses (12.5, 25, 50, and 90% of MIC) (Bernardo et al., 2004). They found that linezolid reduced in a dose-dependent manner the secretion of specific virulence factors, including bifunctional autolysin (Atl), immunoglobulin G binding protein A (Spa), and α-hemolysin (SAV1163), CHAP-domain amidase (SsaA), and immunodominant antigen A (IsaA). This result is similar to the proteomic result that analyzes protein profiles of S. aureus under the sublethal vancomycin exposure (Hessling et al., 2013).
Daptomycin
There were one study examining comparative proteomic profiles in daptomycin-susceptible and daptomycin-resistant S. aureus strains, and one study analyzing global proteomes of daptomycin-susceptible B. subtilis under daptomycin stress (Wecke et al., 2009; Fischer et al., 2011). Unlike other three antibiotics (methicillin, vancomycin, and linezolid), specific genetic determinant of the daptomycin-resistant strain was not determined. Probable daptomycin resistance-related proteins (MprF, YycG, RpoB, and RpoC) identified in previous reports (Jones et al., 2008; Baltz, 2009) were not identified in comparative proteomic analyses (Tables 2–8). In 2011, Fisher et al. compared proteomic profiles in the daptomycin-susceptible S. aureus strain 616 with a daptomycin MIC of 0.5 μg/ml and the daptomycin-resistant S. aureus strain 701 with MIC of 2 μg/ml (Fischer et al., 2011). Comparative proteomics and transcriptomic approach revealed a differential abundance of proteins in various functional categories, including cell wall-associated targets and biofilm formation proteins (Fischer et al., 2011). Phenotypically, daptomycin-susceptible strains, and daptomycin-resistant strains showed major differences in their ability to develop bacterial biofilms in the presence of the antibacterial lipid, oleic acid (Fischer et al., 2011). Transcriptomic approach showed different expressions of some important genes, such as the key genes (yycFGHI) affecting cell membrane lipid homeostasis, cell wall metabolism and biofilm formation, and two-component regulation system genes (agr, saeRS, and vraRS) involved in pathogenesis of methicillin-resistant strains (Fischer et al., 2011). However, through proteomic research, only several proteins, including Asp23, 3-oxoacyl-ACP synthase II (FabF), GTP-sensing transcriptional pleiotropic repressor (CodY), and PurH, was identified as proteins involved in daptomycin resistance.
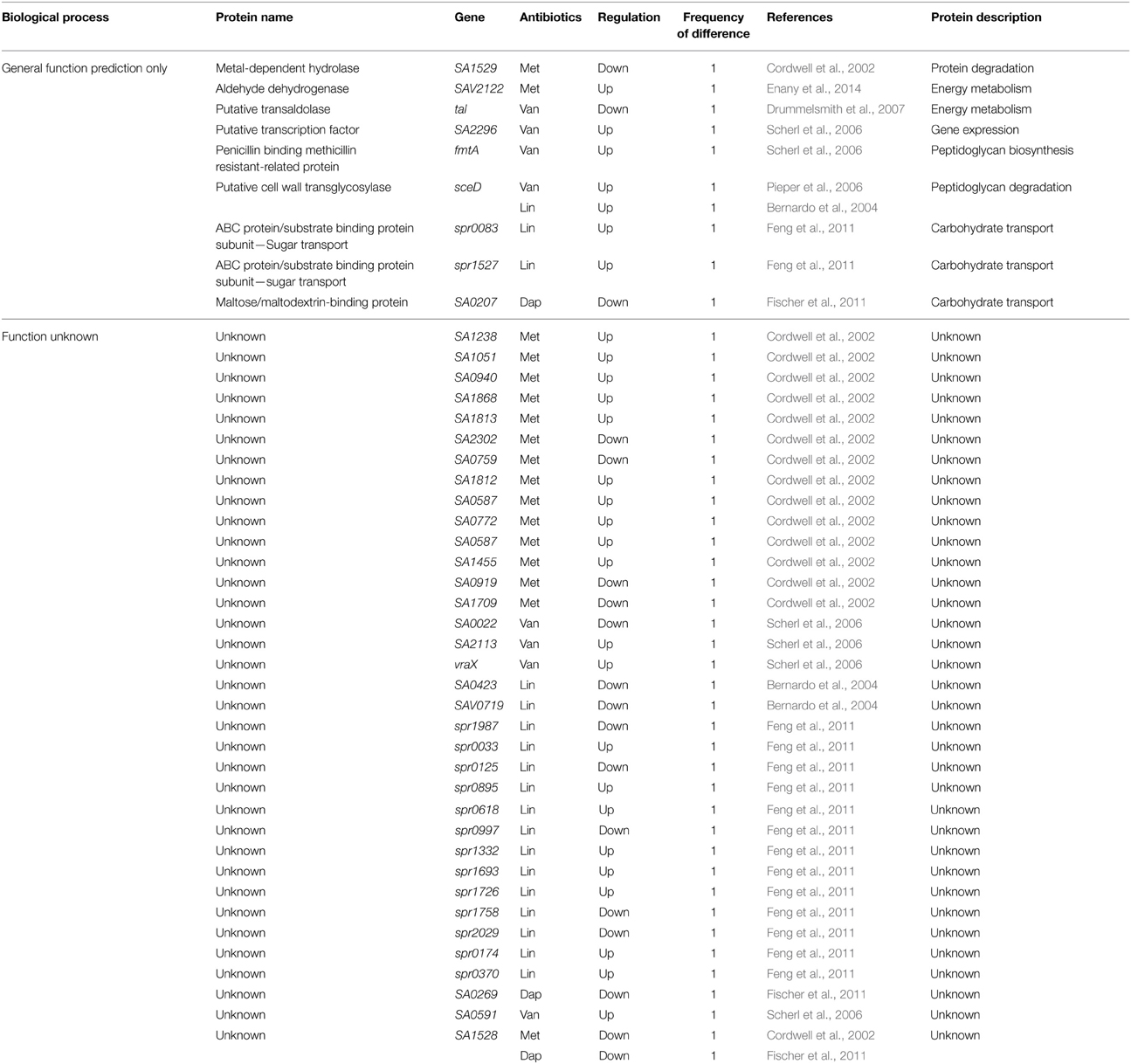
Table 8. Differentially expressed proteins identified by the quantitative proteomic approach: proteins of unknown function.
Wecke et al. searched proteins induced by daptomycin, through the proteomic approach of a daptomycin-susceptible B. subtilis strain W168 under daptomycin treatment of sublethal amount (1 μg/ml) (Wecke et al., 2009). They identified LiaI and LiaH proteins exclusively and strongly induced (429-fold) by daptomycin. This result is in good agreement with data analyzing genes induced by daptomycin through transcriptome profiling (Muthaiyan et al., 2008). LiaH is a conserved membrane protein similar to a phage shock protein A (PspA) of E. coli, and its expression is regulated by the cell envelope stress-sensing two-component system LiaRS (Jordan et al., 2006; Hachmann et al., 2009; Wecke et al., 2009). Inactivation of liaH leads to 3-fold increased susceptibility to daptomycin and this susceptibility was further exacerbated in cells additionally lacking the paralogous gene pspA (Hachmann et al., 2009). In E. coli, the pspA gene is induced upon phage infection, osmotic shock, exposure to ethanol, or temperature increase, and functions to help cells manage the impacts of agents impairing cell membrane function (Joly et al., 2010). A recent report showed that deletion of the response regulator LiaR regulating expression of liaIH in daptomycin-resistant E. faecalis reversed resistance to daptomycin, and resulted in hypersusceptibility to daptomycin (Reyes et al., 2015). Therefore, these results indicate that LiaR is a master regulator protecting cell membrane to diverse antimicrobial agents, through regulating expression of various genes such as the liaH gene (Reyes et al., 2015).
Conclusion
Although specific genetic determinants of resistance mechanisms to methicillin, vancomycin, and linezolid were identified through non-proteomic approaches (e.g., van genes in vancomycin resistance) (Table 1), recent comparative proteomic methods provide new opportunities to understand the antibiotic resistance mechanism. In particular, in the case of recently used antibiotics such as daptomycin, specific genetic determinant(s) of antibiotic resistance was not fully determined through non-proteomic approaches. Therefore, quantitative proteomic methods can be a good tool to find an important protein involved in daptomycin resistance. Actually, a proteomic research identified LiaH as a highly induced protein by daptomycin treatment (Muthaiyan et al., 2008) and a subsequent report showed that the expression level of this protein is important to daptomycin-resistant phenotype (Reyes et al., 2015). These results show that quantitative proteomic analysis can be used as an effective tool to find novel resistance mechanisms.
Interestingly, comparative proteomic approaches in methicillin, linezolid, and daptomycin, except for vancomycin, were poorly correlated with known resistance-related factors found by non-proteomic approaches (Table 2). This result may be caused by a lack of comparative proteomic studies in three antibiotics, or imply the existence of novel resistance mechanisms different from previously known resistance mechanisms found by non-proteomic approaches. Through summarizing recent data of comparative proteomic researches of four clinically important antibiotics, we can find proteins of which expression levels are changed only in the resistance mechanism to specific antibiotic, such as LiaH in daptomycin resistance and PrsA in vancomycin resistance. It is necessary to determine whether these proteins affect antibiotic resistance through regulating previously known resistance-related determinants or by a novel mechanism. Another interesting result is that many proteins identified by comparative proteomic analyses seem to be simultaneously involved in resistance mechanism to two or more antibiotics (Tables 2–8). These proteins include cold shock proteins (CspABC), sporulation protein G (SpoVG), alkyl hydroperoxide reductase subunit C (AhpC), L-lactate dehydrogenase (LdhA), triacylglycerol lipase precursor (LipA), superoxide dismutase (SodA), catalase (KatA), elongation factor G (FusA), CHAP-domain amidase (SsaA), two component system (VraSR), penicillin binding methicillin resistant-related protein (FmtA), adenylosuccinate lyase (PurB), glucose-6-phosphate isomerase (Pgi), catabolite control protein A (CcpA), putative cell wall transglycosylase (SceD), immunodominant antigen A (IsaA), bifunctional autolysin (Atl), the σB regulon, and hydroxymyristoyl-ACP dehydratase (FabZ). These proteins can be divided into two groups, proteins involved in bacterial envelope regulation and proteins compensating for a fitness cost of antibiotic resistance. Proteins such as LipA, VraSR, FmtA, SsaA, SceD, IsaA, Atl, and FabZ, are directly or indirectly involved in envelope regulation. In order to modify or thicken the bacterial cell wall for antibiotic resistance, cells require abundant energy, and proteins involved in stress adaptation are necessary to neutralize various damages by antibiotic. To sustain these fitness costs associated with resistance mechanisms, proteins involved in energy metabolism (LdhA, FusA, Pgi, PurB, and CcpA) and stress-related proteins (CspABC, SpoVG, AhpC, SodA, KatA, and the σB regulon) seem to be identified in resistance mechanisms to several antibiotics. Therefore, these proteomic results confirm that antibiotic resistance requires a fitness cost.
Detailed studies on the mechanism by which these proteins affect antibiotic resistance are required. In particular, because these proteins can act as the global factor affecting resistance mechanisms to most antibiotics, it is necessary to examine whether they affect resistance mechanism of other antibiotics having different action modes. These studies will provide important clues for understanding and managing antibiotic resistance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Research Laboratory Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. 2011-0027928); the Cooperative Research Program for Agriculture Science and Technology Development (No. PJ01103103) of Rural Development Administration in Republic of Korea; and the National Research Foundation of the Ministry of Education, Republic of Korea (2012R1A1A2044184).
References
Ament, P. W., Jamshed, N., and Horne, J. P. (2002). Linezolid: its role in the treatment of gram-positive, drug-resistant bacterial infections. Am. Fam. Physician. 65, 663–671. Available online at: http://www.aafp.org/afp/2002/0215/p663.html
Baltz, R. H. (2009). Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13, 144–151. doi: 10.1016/j.cbpa.2009.02.031
Berger-Bächi, B., Barberis-Maino, L., Strässle, A., and Kayser, F. H. (1989). FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol. Gen. Genet. 219, 263–269.
Bernardo, K., Pakulat, N., Fleer, S., Schnaith, A., Utermöhlen, O., Krut, O., et al. (2004). Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob. Agents Chemother. 48, 546–555. doi: 10.1128/AAC.48.2.546-555.2004
Besier, S., Ludwig, A., Zander, J., Brade, V., and Wichelhaus, T. A. (2008). Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob. Agents Chemother. 52, 1570–1572. doi: 10.1128/AAC.01098-07
Bischoff, M., and Berger-Bächi, B. (2001). Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 45, 1714–1720. doi: 10.1128/AAC.45.6.1714-1720.2001
Boyle-Vavra, S., Yin, S., and Daum, R. S. (2006). The VraS/VraR two-component regulatory system required for oxacillin resistance in community-acquired methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 262, 163–171. doi: 10.1111/j.1574-6968.2006.00384.x
Boyle-Vavra, S., Yin, S., Jo, D. S., Montgomery, C. P., and Daum, R. S. (2013). VraT/YvqF is required for methicillin resistance and activation of the VraSR regulon in Staphylococcus aureus. Antimicrob. Agents Chemother. 57, 83–95. doi: 10.1128/AAC.01651-12
Buntaran, L., Hatta, M., Sultan, A. R., Dwiyanti, R., and Sabir, M. (2013). Sccmec type II gene is common among clinical isolates of methicillin-resistant Staphylococcus aureus in Jakarta, Indonesia. BMC Res. Notes 6:110. doi: 10.1186/1756-0500-6-110
Byrgazov, K., Vesper, O., and Moll, I. (2013). Ribosome heterogeneity: another level of complexity in bacterial translation regulation. Curr. Opin. Microbiol. 16, 133–139. doi: 10.1016/j.mib.2013.01.009
Chambers, H. F. (1997). Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10, 781–791.
Chen, H., Liu, Y., Zhao, C., Xiao, D., Zhang, J., Zhang, F., et al. (2013). Comparative proteomics-based identification of genes associated with glycopeptide resistance in clinically derived heterogeneous vancomycin-intermediate Staphylococcus aureus strains. PLoS ONE 8:e66880. doi: 10.1371/journal.pone.0066880
Cordwell, S. J., Larsen, M. R., Cole, R. T., and Walsh, B. J. (2002). Comparative proteomics of Staphylococcus aureus and the response of methicillin-resistant and methicillin-sensitive strains to Triton X-100. Microbiology. 148, 2765–2781. doi: 10.1099/00221287-148-9-2765
Cosgrove, K., Coutts, G., Jonsson, I. M., Tarkowski, A., Kokai-Kun, J. F., Mond, J. J., et al. (2007). Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 189, 1025–1035. doi: 10.1128/JB.01524-06
Courvalin, P. (2005). Genetics of glycopeptide resistance in gram-positive pathogens. Int. J. Med. Microbiol. 294, 479–486. doi: 10.1016/j.ijmm.2004.10.002
Cuaron, J. A., Dulal, S., Song, Y., Singh, A. K., Montelongo, C. E., Yu, W., et al. (2013). Tea tree oil-induced transcriptional alterations in Staphylococcus aureus. Phytother. Res. 27, 390–396. doi: 10.1002/ptr.4738
Cui, L., Lian, J. Q., Neoh, H. M., Reyes, E., and Hiramatsu, K. (2005). DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 49, 3404–3413. doi: 10.1128/AAC.49.8.3404-3413.2005
Delaune, A., Poupel, O., Mallet, A., Coic, Y. M., Msadek, T., and Dubrac, S. (2011). Peptidoglycan crosslinking relaxation plays an important role in Staphylococcus aureus WalKR-dependent cell viability. PLoS ONE 6:e17054. doi: 10.1371/journal.pone.0017054
Dengler, V., Meier, P. S., Heusser, R., Berger-Bächi, B., and McCallum, N. (2011). Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics. BMC Microbiol. 11:16. doi: 10.1186/1471-2180-11-16
Drummelsmith, J., Winstall, E., Bergeron, M. G., Poirier, G. G., and Ouellette, M. (2007). Comparative proteomics analyses reveal a potential biomarker for the detection of vancomycin-intermediate Staphylococcus aureus strains. J. Proteome Res. 6, 4690–4702. doi: 10.1021/pr070521m
Enany, S., Yoshida, Y., and Yamamoto, T. (2014). Exploring extra-cellular proteins in methicillin susceptible and methicillin resistant Staphylococcus aureus by liquid chromatography-tandem mass spectrometry. World J. Microbiol. Biotechnol. 30, 1269–1283. doi: 10.1007/s11274-013-1550-7
Fan, X., Liu, Y., Smith, D., Konermann, L., Siu, K. W., and Golemi-Kotra, D. (2007). Diversity of penicillin-binding proteins. Resistance factor FmtA of Staphylococcus aureus. J. Biol. Chem. 282, 35143–35152. doi: 10.1074/jbc.M706296200
Feng, J., Billal, D. S., Lupien, A., Racine, G., Winstall, E., Légaré, D., et al. (2011). Proteomic and transcriptomic analysis of linezolid resistance in Streptococcus pneumoniae. J. Proteome Res. 10, 4439–4452. doi: 10.1021/pr200221s
Fischer, A., Yang, S. J., Bayer, A. S., Vaezzadeh, A. R., Herzig, S., Stenz, L., et al. (2011). Daptomycin resistance mechanisms in clinically derived Staphylococcus aureus strains assessed by a combined transcriptomics and proteomics approach. J. Antimicrob. Chemother. 66, 1696–1711. doi: 10.1093/jac/dkr195
Friedberg, E. C., Wagner, R., and Radman, M. (2002). Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 296, 1627–1630. doi: 10.1126/science.1070236
Gardete, S., Wu, S. W., Gill, S., and Tomasz, A. (2006). Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob. Agents Chemother. 50, 3424–3434. doi: 10.1128/AAC.00356-06
Gov, Y., Borovok, I., Korem, M., Singh, V. K., Jayaswal, R. K., Wilkinson, B. J., et al. (2004). Quorum sensing in Staphylococci is regulated via phosphorylation of three conserved histidine residues. J. Biol. Chem. 279, 14665–14672. doi: 10.1074/jbc.M311106200
Hachmann, A. B., Angert, E. R., and Helmann, J. D. (2009). Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 53, 1598–1609. doi: 10.1128/AAC.01329-08
Hanaki, H., Inaba, Y., Sasaki, K., and Hiramatsu, K. (1998). A novel method of detecting Staphylococcus aureus heterogeneously resistant to vancomycin (hetero-VRSA). Jpn. J. Antibiot. 51, 521–530.
Hao, H., Dai, M., Wang, Y., Huang, L., and Yuan, Z. (2012). Key genetic elements and regulation systems in methicillin-resistant Staphylococcus aureus. Future Microbiol. 7, 1315–1329. doi: 10.2217/fmb.12.107
Healy, V. L., Lessard, I. A., Roper, D. I., Knox, J. R., and Walsh, C. T. (2000). Vancomycin resistance in enterococci: reprogramming of the D-Ala-D-Ala ligases in bacterial peptidoglycan biosynthesis. Chem. Biol. 7, R109–R119. doi: 10.1016/s1074-5521(00)00116-2
Herrmann, D. J., Peppard, W. J., Ledeboer, N. A., Theesfeld, M. L., Weigelt, J. A., and Buechel, B. J. (2008). Linezolid for the treatment of drug-resistant infections. Expert Rev. Anti. Infect. Ther. 6, 825–848. doi: 10.1586/14787210.6.6.825
Hessling, B., Bonn, F., Otto, A., Herbst, F. A., Rappen, G. M., Bernhardt, J., et al. (2013). Global proteome analysis of vancomycin stress in Staphylococcus aureus. Int. J. Med. Microbiol. 303, 624–634. doi: 10.1016/j.ijmm.2013.08.014
Hobbs, J. K., Miller, K., O'Neill, A. J., and Chopra, I. (2008). Consequences of daptomycin-mediated membrane damage in Staphylococcus aureus. J. Antimicrob. Chemother. 62, 1003–1008. doi: 10.1093/jac/dkn321
Howden, B. P., and Grayson, M. L. (2006). Dumb and dumber-the potential waste of a useful antistaphylococcal agent: emerging fusidic acid resistance in Staphylococcus aureus. Clin. Infect. Dis. 42, 394–400. doi: 10.1086/499365
Ippolito, J. A., Kanyo, Z. F., Wang, D., Franceschi, F. J., Moore, P. B., Steitz, T. A., et al. (2008). Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 51, 3353–3356. doi: 10.1021/jm800379d
Joly, N., Engl, C., Jovanovic, G., Huvet, M., Toni, T., Sheng, X., et al. (2010). Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiol. Rev. 34, 797–827. doi: 10.1111/j.1574-6976.2010.00240.x
Jones, T., Yeaman, M. R., Sakoulas, G., Yang, S. J., Proctor, R. A., Sahl, H. G., et al. (2008). Failures in clinical treatment of Staphylococcus aureus Infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52, 269–278. doi: 10.1128/AAC.00719-07
Jordan, S., Junker, A., Helmann, J. D., and Mascher, T. (2006). Regulation of LiaRS-dependent gene expression in bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system. J. Bacteriol. 188, 5153–5166. doi: 10.1128/JB.00310-06
Jousselin, A., Renzoni, A., Andrey, D. O., Monod, A., Lew, D. P., and Kelley, W. L. (2012). The posttranslocational chaperone lipoprotein PrsA is involved in both glycopeptide and oxacillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 56, 3629–3640. doi: 10.1128/AAC.06264-11
Katayama, Y., Zhang, H. Z., and Chambers, H. F. (2004). PBP 2a mutations producing very-high-level resistance to β-lactams. Antimicrob. Agents Chemother. 48, 453–459. doi: 10.1128/AAC.48.2.453-459.2004
Koripella, R. K., Chen, Y., Peisker, K., Koh, C. S., Selmer, M., and Sanyal, S. (2012). Mechanism of elongation factor-G-mediated fusidic acid resistance and fitness compensation in Staphylococcus aureus. J. Biol. Chem. 287, 30257–30267. doi: 10.1074/jbc.M112.378521
Kuroda, M., Kuroda, H., Oshima, T., Takeuchi, F., Mori, H., and Hiramatsu, K. (2003). Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49, 807–821. doi: 10.1046/j.1365-2958.2003.03599.x
Kuroda, M., Kuwahara-Arai, K., and Hiramatsu, K. (2000). Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem. Biophys. Res. Commun. 269, 485–490. doi: 10.1006/bbrc.2000.2277
Lee, C. R., Cho, I. H., Jeong, B. C., and Lee, S. H. (2013). Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health. 10, 4274–4305. doi: 10.3390/ijerph10094274
Lee, J. H., Bae, I. K., and Lee, S. H. (2012). New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med. Res. Rev. 32, 216–232. doi: 10.1002/med.20210
Levine, D. P. (2006). Vancomycin: a history. Clin. Infect. Dis. 42(Suppl. 1), S5–S12. doi: 10.1086/491709
Long, K. S., and Vester, B. (2012). Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob. Agents Chemother. 56, 603–612. doi: 10.1128/AAC.05702-11
Lorenz, U., Lorenz, B., Schmitter, T., Streker, K., Erck, C., Wehland, J., et al. (2011). Functional antibodies targeting IsaA of Staphylococcus aureus augment host immune response and open new perspectives for antibacterial therapy. Antimicrob. Agents Chemother. 55, 165–173. doi: 10.1128/AAC.01144-10
Marcone, G. L., Beltrametti, F., Binda, E., Carrano, L., Foulston, L., Hesketh, A., et al. (2010). Novel mechanism of glycopeptide resistance in the A40926 producer Nonomuraea sp. ATCC 39727. Antimicrob. Agents Chemother. 54, 2465–2472. doi: 10.1128/AAC.00106-10
Matsuno, K., and Sonenshein, A. L. (1999). Role of SpoVG in asymmetric septation in Bacillus subtilis. J. Bacteriol. 181, 3392–3401.
McAleese, F., Wu, S. W., Sieradzki, K., Dunman, P., Murphy, E., Projan, S., et al. (2006). Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J. Bacteriol. 188, 1120–1133. doi: 10.1128/JB.188.3.1120-1133.2006
Mehta, S., Cuirolo, A. X., Plata, K. B., Riosa, S., Silverman, J. A., Rubio, A., et al. (2012). VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56, 92–102. doi: 10.1128/AAC.00432-10
Mendes, R. E., Deshpande, L. M., and Jones, R. N. (2014). Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist. Updat. 17, 1–12. doi: 10.1016/j.drup.2014.04.002
Miao, V., Coëffet-Legal, M. F., Brian, P., Brost, R., Penn, J., Whiting, A., et al. (2005). Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 151, 1507–1523. doi: 10.1099/mic.0.27757-0
Mishra, N. N., Yang, S. J., Sawa, A., Rubio, A., Nast, C. C., Yeaman, M. R., et al. (2009). Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53, 2312–2318. doi: 10.1128/AAC.01682-08
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Mongodin, E., Finan, J., Climo, M. W., Rosato, A., Gill, S., and Archer, G. L. (2003). Microarray transcription analysis of clinical Staphylococcus aureus isolates resistant to vancomycin. J. Bacteriol. 185, 4638–4643. doi: 10.1128/JB.185.15.4638-4643.2003
Muthaiyan, A., Silverman, J. A., Jayaswal, R. K., and Wilkinson, B. J. (2008). Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob. Agents Chemother. 52, 980–990. doi: 10.1128/AAC.01121-07
Napolitano, R., Janel-Bintz, R., Wagner, J., and Fuchs, R. P. (2000). All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J. 19, 6259–6265. doi: 10.1093/emboj/19.22.6259
Newsom, S. W. (2004). MRSA—past, present, future. J. R. Soc. Med. 97, 509–510. doi: 10.1258/jrsm.97.11.509
Perry, S. E., and Edwards, D. H. (2004). Identification of a polar targeting determinant for Bacillus subtilis DivIVA. Mol. Microbiol. 54, 1237–1249. doi: 10.1111/j.1365-2958.2004.04363.x
Pieper, R., Gatlin-Bunai, C. L., Mongodin, E. F., Parmar, P. P., Huang, S. T., Clark, D. J., et al. (2006). Comparative proteomic analysis of Staphylococcus aureus strains with differences in resistance to the cell wall-targeting antibiotic vancomycin. Proteomics. 6, 4246–4258. doi: 10.1002/pmic.200500764
Pietiäinen, M., François, P., Hyyryläinen, H. L., Tangomo, M., Sass, V., Sahl, H. G., et al. (2009). Transcriptome analysis of the responses of Staphylococcus aureus to antimicrobial peptides and characterization of the roles of vraDE and vraSR in antimicrobial resistance. BMC Genomics. 10:429. doi: 10.1186/1471-2164-10-429
Pogliano, J., Pogliano, N., and Silverman, J. A. (2012). Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J. Bacteriol. 194, 4494–4504. doi: 10.1128/JB.00011-12
Radhouani, H., Pinto, L., Poeta, P., and Igrejas, G. (2012). After genomics, what proteomics tools could help us understand the antimicrobial resistance of Escherichia coli? J. Proteomics. 75, 2773–2789. doi: 10.1016/j.jprot.2011.12.035
Ramos, S., Chafsey, I., Silva, N., Hébraud, M., Santos, H., Capelo-Martinez, J. L., et al. (2015). Effect of vancomycin on the proteome of the multiresistant Enterococcus faecium SU18 strain. J. Proteomics. 113, 378–387. doi: 10.1016/j.jprot.2014.10.012
Reyes, J., Panesso, D., Tran, T. T., Mishra, N. N., Cruz, M. R., Munita, J. M., et al. (2015). A liaR deletion restores susceptibility to daptomycin and antimicrobial peptides in multidrug-resistant Enterococcus faecalis. J. Infect. Dis. 211, 1317–1325. doi: 10.1093/infdis/jiu602
Rogers, P. D., Liu, T. T., Barker, K. S., Hilliard, G. M., English, B. K., Thornton, J., et al. (2007). Gene expression profiling of the response of Streptococcus pneumoniae to penicillin. J. Antimicrob. Chemother. 59, 616–626. doi: 10.1093/jac/dkl560
Sasindran, S. J., Saikolappan, S., and Dhandayuthapani, S. (2007). Methionine sulfoxide reductases and virulence of bacterial pathogens. Future Microbiol. 2, 619–630. doi: 10.2217/17460913.2.6.619
Sass, P., Jansen, A., Szekat, C., Sass, V., Sahl, H. G., and Bierbaum, G. (2008). The lantibiotic mersacidin is a strong inducer of the cell wall stress response of Staphylococcus aureus. BMC Microbiol. 8:186. doi: 10.1186/1471-2180-8-186
Scherl, A., François, P., Charbonnier, Y., Deshusses, J. M., Koessler, T., Huyghe, A., et al. (2006). Exploring glycopeptide-resistance in Staphylococcus aureus: a combined proteomics and transcriptomics approach for the identification of resistance-related markers. BMC Genomics. 7:296. doi: 10.1186/1471-2164-7-296
Schulthess, B., Bloes, D. A., and Berger-Bachi, B. (2012). Opposing roles of σB and σB-controlled SpoVG in the global regulation of esxA in Staphylococcus aureus. BMC Microbiol. 12:17. doi: 10.1186/1471-2180-12-17
Schulthess, B., Meier, S., Homerova, D., Goerke, C., Wolz, C., Kormanec, J., et al. (2009). Functional characterization of the σB-dependent yabJ-spoVG operon in Staphylococcus aureus: role in methicillin and glycopeptide resistance. Antimicrob. Agents Chemother. 53, 1832–1839. doi: 10.1128/AAC.01255-08
Schumacher, A., Trittler, R., Bohnert, J. A., Kümmerer, K., Pages, J. M., and Kern, W. V. (2007). Intracellular accumulation of linezolid in Escherichia coli, Citrobacter freundii and Enterobacter aerogenes: role of enhanced efflux pump activity and inactivation. J. Antimicrob. Chemother. 59, 1261–1264. doi: 10.1093/jac/dkl380
Seidl, K., Stucki, M., Ruegg, M., Goerke, C., Wolz, C., Harris, L., et al. (2006). Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50, 1183–1194. doi: 10.1128/AAC.50.4.1183-1194.2006
Sobral, R. G., Jones, A. E., Des Etages, S. G., Dougherty, T. J., Peitzsch, R. M., Gaasterland, T., et al. (2007). Extensive and genome-wide changes in the transcription profile of Staphylococcus aureus induced by modulating the transcription of the cell wall synthesis gene murF. J. Bacteriol. 189, 2376–2391. doi: 10.1128/JB.01439-06
Song, Y., Rubio, A., Jayaswal, R. K., Silverman, J. A., and Wilkinson, B. J. (2013). Additional routes to Staphylococcus aureus daptomycin resistance as revealed by comparative genome sequencing, transcriptional profiling, and phenotypic studies. PLoS ONE 8:e58469. doi: 10.1371/journal.pone.0058469
Stapleton, M. R., Horsburgh, M. J., Hayhurst, E. J., Wright, L., Jonsson, I. M., Tarkowski, A., et al. (2007). Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J. Bacteriol. 189, 7316–7325. doi: 10.1128/JB.00734-07
Stülke, J., and Hillen, W. (2000). Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 54, 849–880. doi: 10.1146/annurev.micro.54.1.849
Swaney, S. M., Aoki, H., Ganoza, M. C., and Shinabarger, D. L. (1998). The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42, 3251–3255.
Tettelin, H., Nelson, K. E., Paulsen, I. T., Eisen, J. A., Read, T. D., Peterson, S., et al. (2001). Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293, 498–506. doi: 10.1126/science.1061217
Utaida, S., Dunman, P. M., Macapagal, D., Murphy, E., Projan, S. J., Singh, V. K., et al. (2003). Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149, 2719–2732. doi: 10.1099/mic.0.26426-0
van der Voort, M., Kuipers, O. P., Buist, G., de Vos, W. M., and Abee, T. (2008). Assessment of CcpA-mediated catabolite control of gene expression in Bacillus cereus ATCC 14579. BMC Microbiol. 8:62. doi: 10.1186/1471-2180-8-62
Wang, X., He, X., Jiang, Z., Wang, J., Chen, X., Liu, D., et al. (2010). Proteomic analysis of the Enterococcus faecalis V583 strain and clinical isolate V309 under vancomycin treatment. J. Proteome Res. 9, 1772–1785. doi: 10.1021/pr901216e
Wecke, T., Zuhlke, D., Mäder, U., Jordan, S., Voigt, B., Pelzer, S., et al. (2009). Daptomycin versus Friulimicin B: in-depth profiling of Bacillus subtilis cell envelope stress responses. Antimicrob. Agents Chemother. 53, 1619–1623. doi: 10.1128/AAC.01046-08
Wilson, D. N., Schluenzen, F., Harms, J. M., Starosta, A. L., Connell, S. R., and Fucini, P. (2008). The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. U.S.A. 105, 13339–13344. doi: 10.1073/pnas.0804276105
Winkler, M. E., and Hoch, J. A. (2008). Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J. Bacteriol. 190, 2645–2648. doi: 10.1128/JB.01682-07
Woodford, N. (1998). Glycopeptide-resistant enterococci: a decade of experience. J. Med. Microbiol. 47, 849–862.
Keywords: quantitative proteomics, methicillin resistance, vancomycin resistance, linezolid resistance, daptomycin resistance
Citation: Lee C-R, Lee JH, Park KS, Jeong BC and Lee SH (2015) Quantitative proteomic view associated with resistance to clinically important antibiotics in Gram-positive bacteria: a systematic review. Front. Microbiol. 6:828. doi: 10.3389/fmicb.2015.00828
Received: 04 June 2015; Accepted: 27 July 2015;
Published: 11 August 2015.
Edited by:
Antonio C. M. Correia, Universidade de Aveiro, PortugalReviewed by:
Dmitri Debabov, NovaBay Pharmaceuticals, USAAnnalisa Pantosti, Istituto Superiore di Sanità, Italy
Copyright © 2015 Lee, Lee, Park, Jeong and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang Hee Lee, National Leading Research Laboratory of Drug Resistance Proteomics, Department of Biological Sciences, Myongji University, 116 Myongjiro, Yongin, Gyeonggido 17058, South Korea,c2FuZ2hlZWxlZUBtanUuYWMua3I=
†These authors have contributed equally to this work.
 Chang-Ro Lee
Chang-Ro Lee Jung Hun Lee
Jung Hun Lee Kwang Seung Park
Kwang Seung Park Byeong Chul Jeong
Byeong Chul Jeong Sang Hee Lee
Sang Hee Lee