- 1Division of Molecular Biology and Human Genetics, Faculty Medicine and Health Sciences, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, SAMRC Centre for Tuberculosis Research, Stellenbosch University, Cape Town, South Africa
- 2The Gade Research Group for Infection and Immunity, Department of Clinical Science, University of Bergen, Bergen, Norway
- 3Max Planck Institute for Biochemistry, Munich, Germany
- 4Faculty of Health Sciences, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa
- 5Norway Proteomics Core Facility, Department of Immunology, Oslo University, Oslo, Norway
Reversible protein phosphorylation, regulated by protein kinases and phosphatases, mediates a switch between protein activity and cellular pathways that contribute to a large number of cellular processes. The Mycobacterium tuberculosis genome encodes 11 Serine/Threonine kinases (STPKs) which show close homology to eukaryotic kinases. This study aimed to elucidate the phosphoproteomic landscape of a clinical isolate of M. tuberculosis. We performed a high throughput mass spectrometric analysis of proteins extracted from an early-logarithmic phase culture. Whole cell lysate proteins were processed using the filter-aided sample preparation method, followed by phosphopeptide enrichment of tryptic peptides by strong cation exchange (SCX) and Titanium dioxide (TiO2) chromatography. The MaxQuant quantitative proteomics software package was used for protein identification. Our analysis identified 414 serine/threonine/tyrosine phosphorylated sites, with a distribution of S/T/Y sites; 38% on serine, 59% on threonine and 3% on tyrosine; present on 303 unique peptides mapping to 214 M. tuberculosis proteins. Only 45 of the S/T/Y phosphorylated proteins identified in our study had been previously described in the laboratory strain H37Rv, confirming previous reports. The remaining 169 phosphorylated proteins were newly identified in this clinical M. tuberculosis Beijing strain. We identified 5 novel tyrosine phosphorylated proteins. These findings not only expand upon our current understanding of the protein phosphorylation network in clinical M. tuberculosis but the data set also further extends and complements previous knowledge regarding phosphorylated peptides and phosphorylation sites in M. tuberculosis.
Introduction
According to the World Health Organization (WHO), tuberculosis (TB) ranks as the second leading cause of death from an infectious disease worldwide, after HIV (WHO|Global tuberculosis report 2014, 2014). It is estimated that one third of the world's population is infected with Mycobacterium tuberculosis, the causative agent of TB and 8.6 million new TB cases were reported in 2012 alone (WHO|Global tuberculosis report 2013, 2013). In order to control this epidemic there is a critical need for the development of effective and affordable anti-TB therapy and diagnostic tools.
Harnessing the power of the field of proteomics provides a unique opportunity to identify novel protein candidates for diagnosis and drug targets of pathogenic bacteria. Of particular interest is the identification of proteins with post-translational modifications (PTMs) as these modifications are often critical to protein functions, such as regulating protein-protein interactions, subcellular localization or modification of catalytic sites (Seo and Lee, 2004; Gupta et al., 2007). Protein phosphorylation is an important reversible PTM that directly or indirectly regulates signal transduction cascades linking the intracellular and extracellular environments. In bacteria, protein phosphorylation plays a fundamental role in the regulation of key processes ranging from metabolism and cellular homeostasis to cellular signaling which can be mediated by two classes of phosphorylation events (Cozzone, 1998). The underlying molecular mechanisms regulating protein phosphorylation and dephosphorylation is of great physiological importance due to its ability to ultimately affect protein activity, function, half-life or subcellular localization (McConnell and Wadzinski, 2009). Until recently it was thought that histidine/aspartate phosphorylation was the main mediator of signal transduction in bacteria (Frasch and Dworkin, 1996). However, with the advancement of mass spectrometry-based analyses serine/threonine and tyrosine kinases have been identified in a number of different bacteria (Macek et al., 2007; Macek and Mijakovic, 2011; Mijakovic and Macek, 2012).
The M. tuberculosis genome encodes 11 Serine/Threonine kinases (STPK's) (PknA, PknB, PknD, PknE, PknF, PknG, PknH, PknI, PknJ, PknK, PknL), two tyrosine phosphatases (PtpA, PtpB) and 11 two-component systems, highlighting the complexity of signaling network mediated by protein phosphorylation and thereby their potential as drug targets (Chopra et al., 2003; Koul et al., 2004; Sharma et al., 2004; Sala and Hartkoorn, 2011). Prisic et al. described the Serine/Threonine (S/T) phosphorylation profiles of the laboratory strain M. tuberculosis H37Rv under 6 different culture conditions (Prisic et al., 2010). This study identified 301 phosphorylated proteins after combining data from six different culture conditions (Prisic et al., 2010) and identified four phosphorylated STPKs, ribosomal and ribosome-associated proteins as well as phosphorylated substrates which suggest that protein phosphorylation provides a mechanism for regulating key physiological process during infection. A more recent study of H37Rv further expanded the knowledge of the phosphoproteome by identifying novel tyrosine (Y) phosphorylated proteins in M. tuberculosis further supporting the broad regulation of its physiology by phosphorylation (Kusebauch et al., 2014).
In this study we report the phosphoproteome of a previously described clinical Beijing genotype M. tuberculosis isolate at early-logarithmic growth phase in liquid culture to provide further insight the influence of S/T/Y phosphorylation events on bacterial growth and virulence (de Souza et al., 2010). We used a combination of strong cation exchange (SCX) with Titanium dioxide (TiO2) enrichment in a mass spectrometry-based phosphoproteomic analysis of a hyper-virulent clinical M. tuberculosis isolate (de Souza et al., 2010). We confirmed the presence of previously described M. tuberculosis phosphorylated proteins and also identified novel phosphorylated proteins and sites. In addition, this dataset identified novel tyrosine phosphorylation events, and thereby confirmed that there are multiple tyrosine kinase targets in this clinically relevant M. tuberculosis strain.
Materials and Methods
Cell Culture and Lysate Preparation
A previously described hyper-virulent clinical Beijing genotype Mycobacterium tuberculosis isolate, SAW5527, isolated from a TB patient attending a primary health care clinic in the Western Cape province, South Africa was used for this phosphoproteomics analysis (de Souza et al., 2010). Secondary cultures were inoculated into 50 ml 7H9 Middlebrooks medium supplemented with Dextrose and Catalase and incubated at 37°C until early-logarithmic phase (OD600 between 0.6 and 0.7). Mycobacterial cells were collected by centrifugation (2000 × g for 10 min at 4°C) and washed two times with cold lysis buffer containing 10 mM Tris-HCl (pH 7.4), 0.1% Tween-80, Complete Protease inhibitor cocktail (Roche, Mannheim Germany) and Phosphatase inhibitor cocktail (Roche, Mannheim Germany). An equal amount of 0.1 mm glass beads (Biospec Products Inc., Bartlesville, OK) was added to the cell pellet after centrifugation together with cold 300 μl lysis buffer and 10 μl DNaseI (2U/ml) (NEB, New England Laboratories). Lysis was achieved by mechanical bead-beating in a Rybolyser (Bio101 SAVANT, Vista, CA) for 6 cycles of 20 s at a speed of 4.0 m.s−1, with 1 min cooling periods on ice. The whole cell lysates were filter-sterilized with a sterile 0.22 μm pore acrodisc 25 mm PF syringe filter (Pall Life Sciences, Pall Corporation, Ann Arbour, MI) and stored at −80°C. The protein concentration of the whole cell lysate was determined using the RC DC Protein assay according to manufacturer's instructions (BioRad). A single biological replicate was analyzed in triplicate for downstream phosphoproteomic analysis.
Filter Aided Sample Preparation and Trypsin Digestion
Four milligrams of concentrated whole cell lysate proteins was heated in 4% SDS buffer and 0.1 M dithiothreitol (DTT) in 100 mM Tris/HCl pH 7.5. The samples were processed using Filter Aided Sample Preparation (FASP) (Wiśniewski et al., 2009). In brief, 4 mg dried whole cell lysate protein was resuspended in 250 μl of urea (UA) and loaded onto a 15 ml Amicon filtration device (30 kDa MWCO) and centrifuged at 2000 × g for 40 min at 25°C. After centrifugation, the flow-through was collected in a clean falcon tube and discarded. The concentrated whole cell lysate proteins in the filter unit were diluted in 2 ml 8 M Urea in 0.1 M Tris/HCl (pH 8.5) and re-centrifuged to remove the SDS. The flow-through was discarded and the remaining proteins in the filter unit were alkylated by mixing with 1.5 ml 50 mM iodacetamide (IAA) and incubated in the dark for 20 min to irreversibly modify cysteine. The alkylated proteins were equilibrated with 2 ml 50 mM ammonium bicarbonate (ABC) and digested with trypsin (Promega) in a protein to enzyme ratio of 100:1 at 37°C overnight. After trypsin digestion the filter unit is transferred to a clean collection tube and the peptides were eluted by centrifuged at 14 000 × g for 10 min. The eluted peptides were diluted in 50 μl water to avoid desalting for further processing of the peptide and acidified with trifluoroacetic acid (TFA).
Fractionation of Peptides by Strong Cation Exchange (SCX)
Extracted trypsin digested peptides were diluted to a volume of 7 ml in Solvent A (5 mM monopotassium phosphate (KH2PO4) 30% acetonitrile (ACN), pH 2.7). The pH of the diluted peptide samples was adjusted to 2.7 and made up to a volume of 10 ml with 100% ACN. The respective peptide samples were then separated at pH 2.7 by strong cation exchange (SCX ) by loading each peptide mixture onto a cation exchanger column (3.0 mm × 20 cm) (Poly LC, Columbia, MD) containing 5 μm polysulfoethyl aspartamine beads with a 200 Å pore size and a flow rate of 350 μl/min−1 equilibrated with SCX solvent A. The flow-through was collected and the bound peptides were eluted from the columns using an increasing salt gradient (0–30%) containing 5 mM KH2PO4 pH and 150 mM KCl. A total of 9 fractions were collected; 5 fractions generated by SCX based on UV absorbance (220 and 280), 3 from the flow-through and 1 salvage fraction (containing washes from the cation exchanger column) from the SCX column as an additional fraction.
Enrichment of Phosphopeptides with TiO2 Beads
All nine fractions (5 SCX, 3 SCX flow-through, 1 salvage fraction) were subjected to Titanium dioxide (TiO2) phosphopeptide enrichment. The TiO2-beads,10 μm in size, (GL Sciences, Inc., Japan) was resuspended 30 mg/ml dihydrobenzoic acid (DHB) (Sigma) to prevent non-specific binding. Each of the 9 fractions was incubated 4 times with TiO2 at a peptide to bead ratio of 1:2–1:8. Each fraction was rotated for 30 min, and then briefly centrifuged (14,000 g × 30 s). The supernatants were aspirated and transferred to a new labeled tube and the phosphopeptides bound to the TiO2beads were washed twice with 30% ACN and 3% TFA followed by washing twice with 75% ACN and 0.3% TFA. The enriched phosphorylated peptides were eluted with elution buffer containing 25% ammonia and ACN pH10. The eluted phosphopeptides were desalted using in house prepared C18 Stage tips.
LC-MS/MS Analysis
The peptides were separated on a column packed in-house with C18 beads (reprosil-AQ Pur, Rd Maisch) on an Proxeon Easy-nLC system (Proxeon Biosystems, Odense, Denmark) using a binary gradient with buffer A (0.5% acetic acid in water) and buffer B (0.5% acetic acid and 80% ACN). The 4 μl of the enriched phosphopeptides from each of the 9 fractions were injected 3 times and were loaded directly without any trapping column with buffer A at a flow rate of 500 nl/min. Elution was carried out at a flow rate of 250 nl/min, with a linear gradient from 10 to 35% buffer B over a period of 95 min followed by 50% buffer B for 15 min. At the end of the gradient the column was washed with 90% buffer B and equilibrated with 5% buffer B for 10 min. The LC system was directly coupled in-line with the LTQ-Orbitrap Velos instrument (Thermo Fisher Scientific) via the Proxeon Biosystems nanoelectrospray source. The mass spectrometer was programmed to acquire in a data-dependant mode with a resolution of 30,000 at 400 m/z with lock mass option enabled for the 445.120025. However, the target lock mass abundance was set to 0% in order to save the injection time for lock mass. For full scans 1E6 ions were accumulated within a maximum injection time of 250 ms in the C trap and detected in the Orbitrap mass analyser. The 10 most intense ions with charge states ≥2 were sequentially isolated and fragmented by high-energy collision dissociation (HCD) mode in the collision cell with normalized collision energy of 40% and detected in the Orbitrap analyser at 75,000 resolution. For HCD based method, the activation time option in the Xcalibur file was set to 0.1 ms. For the high-low strategy, full scans were acquired in the Orbitrap analyser at 60,000 resolution as parallel acquisition is enabled in the high-low mode. Up to the 10 most intense peaks with charge states ≥2 were selected for sequencing to a target value of 5000 with a maximum injection time of 25 ms and fragmented in the ion trap by HCD with normalized collision energy of 35%, activation of 0.25 and activation time of 10 ms.
Database Search
The raw data acquired were processed using MaxQuant software version (1.4.1.2) and processed as per default workflow. Since HCD spectra were acquired in profile mode, deisotoping was performed similar to survey MS scans to obtain singly charged peak lists and searched against the M. tuberculosis H37Rv protein database (version R11 tuberculist.epfl.ch). The search included cysteine carbamidomethylation as a fixed modification while N-acetylation, oxidation of methionine and phosphorylation at serine, threonine and tyrosine were set as variable modifications. Up to two missed cleavages were allowed for protease digestion and a peptide had to be fully tryptic. Identifications were filtered at 1% FDR at three levels namely; site, peptide and protein using reversed sequences. As such there is no fixed cut-off score threshold but instead spectra were accepted until the 1% false discovery rate (FDR) is reached. Only peptides with a minimum length of 7 amino acids were considered for identification and detected in at least one or more of the replicates. All phosphopeptide spectra were manually validated by applying stringent acceptance criteria: only phosphorylation events on S/T/Y with a localization probability of ≥0.75 and PEP ≤ 0.01 were used for further analysis and reported as high confidence localized phosphosites.
Gene Ontology Analysis
The categorization of identified phosphorylated proteins in terms of functional categorization, molecular function, biological processes and cellular components was carried out using TubercuList-Mycobacterium tuberculosis Database.
Results
In this study we set out to analyse the phosphoproteome of a hyper-virulent Beijing genotype M. tuberculosis isolate by extracting whole cell lysate proteins at early-logarithmic growth (OD600 of 0.8) (Figure 1) which resulted in the identification of 619 MS/MS spectra. The 274 LC-MS/MS spectra that fulfilled the criteria for high confidence phosphosites identified a total of 414 (38:59:3%) S/T/Y phosphorylation sites present in 214 M. tuberculosis H37Rv proteins (Supplementary Table S2; Supplementary Figure S1).
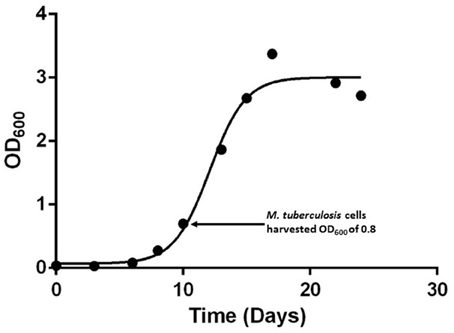
Figure 1. Growth curves of hyper-virulent M. tuberculosis Beijing strain. Growth monitored over a 24 day period using OD600 measurements in 7H9 Middlebrooks liquid media supplemented with dextrose and catalase.
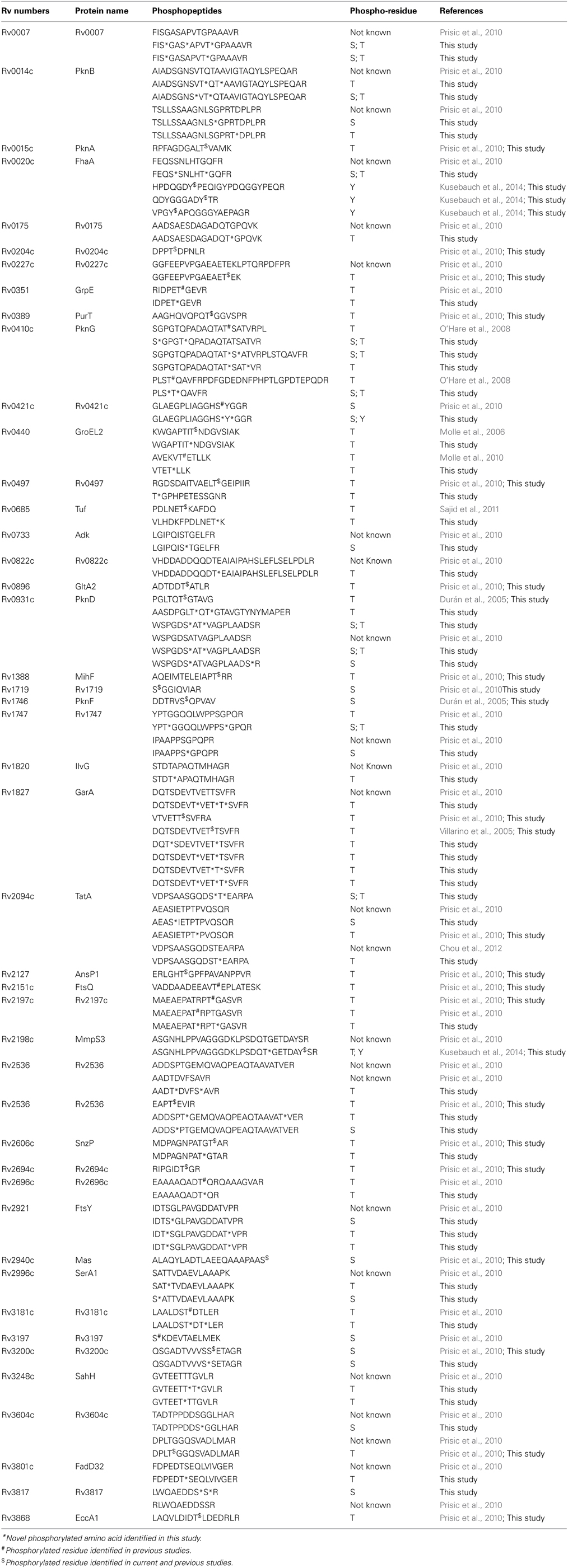
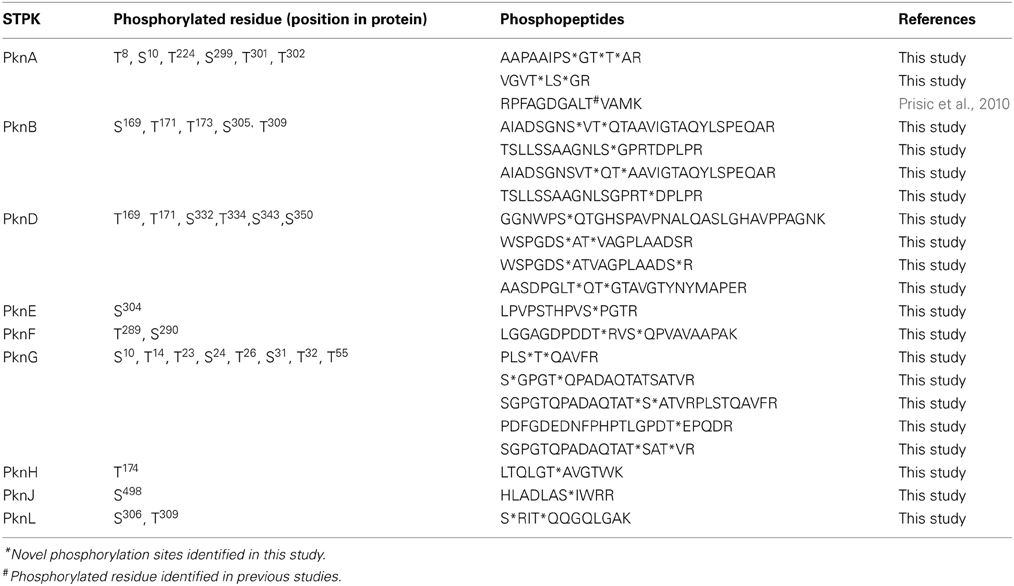
Of the 401 serine/threonine phosphorylation sites (pS/T), only 156 had been previously described for M. tuberculosis (Table 1). Only 6 of 13 tyrosine phosphorylation sites (pY) has been previously described for M. tuberculosis (Kusebauch et al., 2014). The remaining 245 pS/T and 7 pY were uniquely identified in this study (Supplemental Table S2). To determine whether phosphorylated proteins were differentially represented in any particular cellular process, we grouped the phosphorylated proteins based on their functional category according to Tuberculist (Lew et al., 2011) (Figure 2). One hundred and seventy (79.4%) of the phosphorylated proteins had an annotated function, while the remaining 59 phosphorylated proteins (20.5%) were assigned as hypothetical. The biological function of the annotated proteins varied from transcription, translation, protein biosynthesis, fatty acid metabolism to phosphorylation. Our analysis identified phosphorylated forms of the 9 of the 11 M. tuberculosis STPK's: PknA, PknB, PknD, PknF, PknG PknE, PknH, PknJ, and PknL (Table 2). Of these, phosphorylated forms of PknE, PknH, PknJ, and PknL had not been previously described in M. tuberculosis.
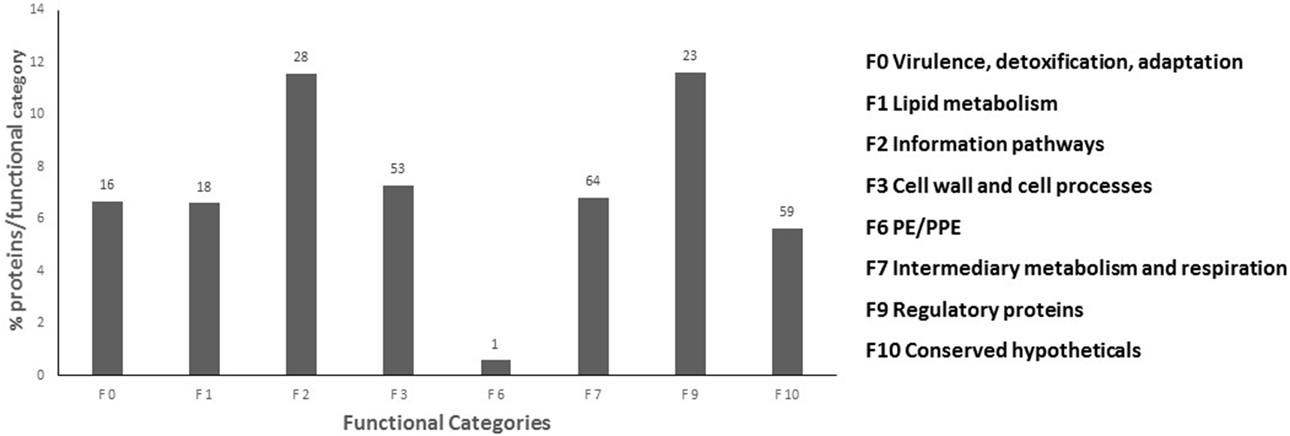
Figure 2. Functional classification of phosphorylated proteins. Percentage phosphorylated proteins per functional category were classified according to Tuberculist. Number of phosphorylated proteins identified in each functional category depicted above the black bars.
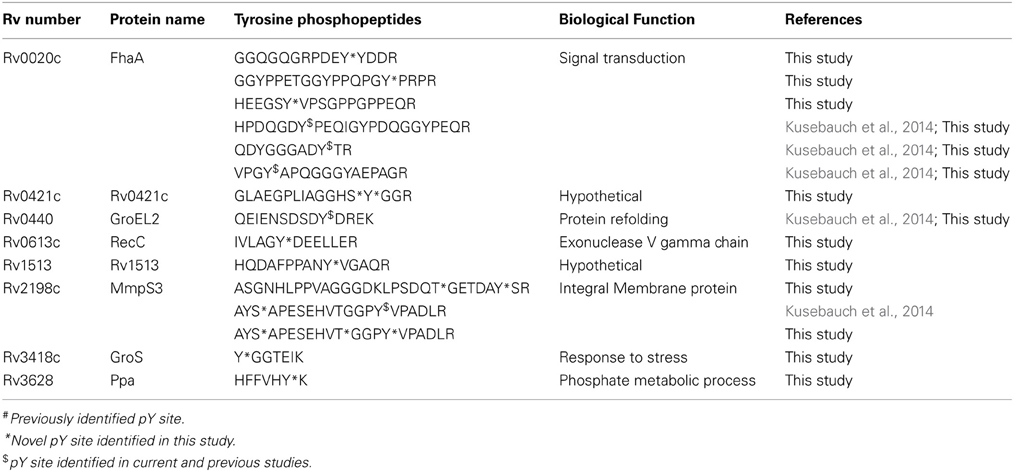
We detected 13 Y phosphorylation sites located on 10 proteins in M. tuberculosis during early-logarithmic growth. Six of the 13 Y phosphorylation sites (Table 3) were located on two proteins, FhaA and MmpS3 (Kusebauch et al., 2014) and have recently been described in a M. tuberculosis H37Rv at stationary growth phase. The remaining 7 Y phosphorylated sites were uniquely identified in this study. Amongst these with known annotations were 2 virulence factors (GroS and GroEL2) and Ppa involved in macromolecule biosynthesis.
A large number of proteins involved in intermediary metabolism and respiration processes such as lipid and fatty acid metabolism (KasB, FadD32, AccD4, and MmaA3) were found to be phosphorylated in this study (Supplemental Table S2). In addition, several proteins from the ESX-1 type seven secretion system (T7SS) in M. tuberculosis including EspR, EccA, CFP10, EspI, EspL, EspB were amongst the identified phosphorylated proteins (Supplemental Table S1). We also identified virulence factors such Pks15, AceA5, FadD5, EsxB, KatG, GlpX, Rv2032, and PtbB that were phosphorylated in this hyper-virulent M. tuberculosis strain (Supplemental Table S2).
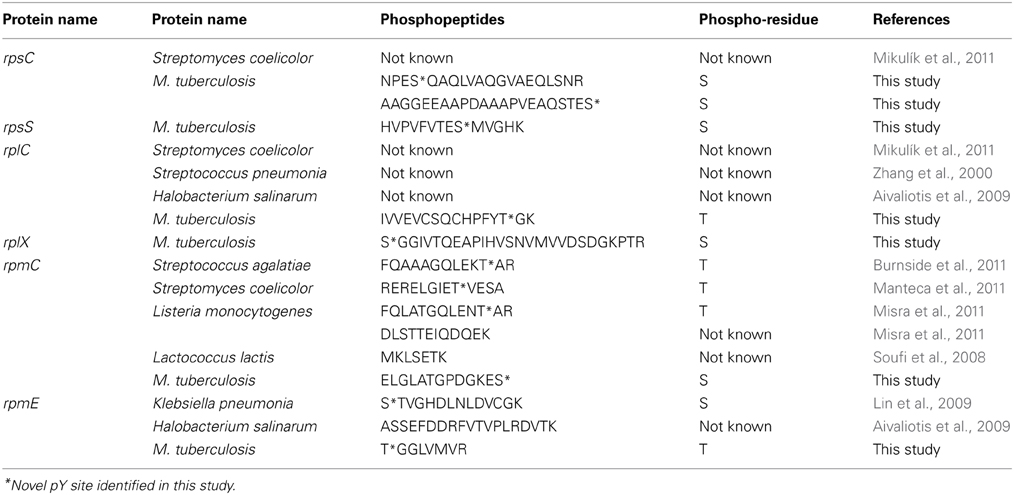
Of the 21 phosphorylated proteins involved in information pathways, we identified 6 phosphorylated ribosomal proteins; two phosphorylated small subunit (30S) ribosomal proteins (S3, S19), and four large subunit (50S) ribosomal proteins (L3, L24, L29, L31) with a total of 8 S/T phosphorylation sites. In addition, phosphorylated sites on the ribosomal proteins RpsS and RplX were also identified (Table 4).
Discussion
Here we report 214 phosphorylated proteins extracted during early-logarithmic growth phase from a hyper-virulent clinical Beijing genotype Mycobacterium tuberculosis isolate. These proteins can be categorized into different biological functions according to Tuberculist (Lew et al., 2011). The impact of phosphorylation on these Hank's type Ser/Thr kinases (STPKs), phosphatases and their substrates, and the functional role of phosphorylated residues still remains to be elucidated. However, as in previous studies, the identification of phosphorylated residues clearly suggests functional importance.
Regulatory Proteins
In recent years, bacterial S/T/Y kinases and phosphatases have been extensively investigated, with indications that they might play a crucial role in pathogenicity. These Hank's type kinases have the ability to short-circuit the host defense mechanism since they are mostly involved in key biological processes. We identified phosphopeptides from 9 of the 11 STPKs encoded by the M. tuberculosis genome. This included both previously described S/T phosphorylation sites (Boitel et al., 2003; Young et al., 2003; Durán et al., 2005; Villarino et al., 2005; Molle et al., 2006; O'Hare et al., 2008; Prisic et al., 2010; Sajid et al., 2011; Chou et al., 2012; Kusebauch et al., 2014) and novel sites on these STPKs thereby highlighting the complexity of the signal transduction mechanism of this pathogen. Phosphorylated peptides were not detected for PknI and PknK. However MS/MS spectra for these peptides for these proteins were detected with mass spectrometry thereby confirming the presence of these proteins (data not shown).
Of the phosphorylated STPKs, PknA, PknB, and PknG have been shown to be essential for in vitro growth (Sassetti et al., 2003) and to regulate cell growth and cell division and interfere with host signaling pathways (Fernandez et al., 2006). PknE, PknH, PknJ, and PknL have been implicated in the adaptation to the extracellular environment or intracellular survival of M. tuberculosis (Sharma et al., 2006; Lakshminarayan, 2009; Arora et al., 2010; Parandhaman et al., 2014) which is in agreement with reports that during early growth the bacilli undergo a period of adaptation to their external environment (Stock et al., 1989; Soares et al., 2013). PknE is involved in the suppression of apoptosis during nitrate stress (Kumar and Narayanan, 2012) and intracellular survival and adaptation to hostile environments (Parandhaman et al., 2014). In M. tuberculosis, PknH controls the expression of a variety of cell wall related enzymes and regulates in vivo growth in mice (Zheng et al., 2007). PknJ undergoes autophosphorylation and phosphorylates the Thr168, Thr171, and Thr173 residues of Embr (a transcriptional regulator), MmaA4/Hma (a methyltransferase involved in mycolic acid biosynthesis) and PepE (a peptidase located adjacent to the pknJ gene in the M. tuberculosis genome), respectively (Jang et al., 2010). Lastly, PknL is involved in an adaptive response to nutrient starvation. This kinase regulates transcription which allows the bacilli to maintain metabolic activity without sourcing energy from elsewhere (Lakshminarayan, 2009). Furthermore, we identified a number of STPK substrates that were phosphorylated in clinical hyper-virulent M. tuberculosis strain (list not shown) thereby highlighting the complexity of the phosphorylation regulatory network in M. tuberculosis. Even though the role of STPKs in bacterial physiology is not yet fully understood the data presented here could underpin a targeted approach to improving our understanding of STPK-mediated signal transduction mechanisms in M. tuberculosis.
Tyrosine Phosphorylation
The M. tuberculosis genome encodes for two putative tyrosine phosphatases (PtpA and PtpB) but is not predicted to encode tyrosine kinases (Cole et al., 1998; Bach et al., 2009). Most bacterial phosphorylation sites are on serine and threonine; a survey of 11 bacterial phosphoproteomes revealed that S/T phosphorylation accounted for an average of 48 and 40% of phosphorylated sites, respectively, while tyrosine phosphorylation events account for less than 10% of the overall phosphoproteome (Ge and Shan, 2011). Tyrosine phosphorylated proteins have been previously shown to play important regulatory roles through their involvement in biological functions such as exopolysaccharide production, DNA metabolism, stress responses (Ge et al., 2011; Whitmore and Lamont, 2012). Recently, Kusebauch et al. identified tyrosine phosphorylated proteins in M. tuberculosis and demonstrated that a number of STPKs can phosphorylate tyrosine in either cis or trans (Kusebauch et al., 2014). This suggests that STPKs have the ability to phosphorylate S/T/Y. In this study we identified 13 tyrosine phosphorylation sites in 8 proteins (Table 3). An overlap of three proteins (FhaA, MmpS3, and GroES) and 6 tyrosine phosphorylated sites we similar between this and the previous study (Kusebauch et al., 2014). Five of the tyrosine phosphorylated proteins (FhaA, GroEL2, MmpS3, GroES, and Ppa) identified in this study are essential for in vitro growth (Sassetti et al., 2003; Griffin et al., 2011) and involved in a variety of functions.
This study confirmed and expanded work by Kusebauch et al., where multiple tyrosine phosphopeptides were identified for FhaA. FhaA is a regulatory protein which has been implicated in cell wall biosynthesis (Fernandez et al., 2006) and has a strong association with PknA and PknB (Pallen et al., 2002; Roumestand et al., 2011). We found that the highly S/T/Y phosphorylated FHA-domain contained 6 tyrosine phosphopeptides of which 4 were previously been identified in H37Rv (Kusebauch et al., 2014). FhaA is a major substrate of PknB and has been implicated in the formation of a regulatory complex with MviN required for peptidoglycan biosynthesis (Warner and Mizrahi, 2012). In our dataset, we found that all three of the proteins (PknB, FhaA, and MviN) in the regulator complex were phosphorylated.
We also confirm the presence of a previously reported Y phosphopeptide of MmpS3 and identified a second phosphopeptide. MmpS3 forms part of the mycobacterial membrane protein small family and is an essential protein for mycobacterial growth and cholesterol metabolism (Griffin et al., 2012). The role of phosphorylation of this protein has yet to be determined.
The two proteins GroEL2 and GroES have been identified as potential candidates for antituberculosis treatment (Al-Attiyah et al., 2006). We confirmed the presence of the Y phosphopeptide in GroEL2 identified in H37Rv (Kusebauch et al., 2014). Recently it has been shown that the antigen GroES is sufficient to protect BALB/c mice against challenge infection (Lima et al., 2003) and up-regulated in kanamycin and amikacin resistant isolates (Kumar et al., 2013). Ppa is an inorganic pyrophosphate and is involved in macromolecule biosynthesis. The M. tuberculosis Ppa is highly similar to a well conserved homolog of Legionella. pneumophila PPase which is induced in macrophages, although the M. tuberculosis PPa promotor is not responsive to any specific intracellular triggers (Triccas and Gicquel, 2001).
Virulence Factors
The identification of virulence factors is crucial in order to improve our understanding of the mechanisms involved in pathogenesis of M. tuberculosis. Several of the phosphorylated virulence factors identified in this study were found to be involved in basic metabolic pathways such a lipid and fatty acid metabolism, secretion systems and response and adaptation to environmental changes. The virulence factor KasB and key enzymes (FadD32, AccD4, and MmaA3) in the mycolic acids biosynthesis pathway were phosphorylated in this hyper-virulent M. tuberculosis strain. The kasB gene is not essential for growth, however, the deletion mutant, ΔkasB, resulted in an alteration in growth morphology and loss of acid-fast staining (Bhatt et al., 2007). This suggests that modification of this protein could influence the synthesis of mycolic acids and thereby the pathogenicity of the bacilli.
The specialized ESX-1 Type VII secretion system (T7SS), unique to pathogenic mycobacteria is responsible for the secretion of two culture filtrate proteins EsxA and EsxB (ESAT-6 and CFP-10). These secretion systems have been shown to be involved in virulence and are critical for intracellular survival (Bitter and Kuijl, 2014) due to their ability to secrete proteins that lack classical signal peptides across the complex cell envelope to host cells during infection (Houben et al., 2014, p. 5). M. tuberculosis have several different ESX regions (ESX-1 to ESX-5) (Daleke et al., 2012) with varying gene numbers and size for each of these secretion machinery. In this study we found 6 T7SS proteins to be phosphorylated in the hyper-virulent strain. In a previous study, proteomics of whole cell extracts of this hyper-virulent M. tuberculosis strain revealed an under-representation of virulence factors such as ESAT-6 and Esx-like proteins (de Souza et al., 2010). The authors showed the abundance of ESAT-6 gene expression was reduced in the hyper-virulent M. tuberculosis suggesting that the low levels of this protein might be as a result of its ability to export these proteins more efficiently into the extracellular environment (de Souza et al., 2010).
Protein Synthesis and Interactions
The impact of phosphorylation on the functionality of ribosomal proteins is not fully understood. Mikulik et al. hypothesized that phosphorylation of ribosomal proteins induces or stabilizes conformational changes during proteins synthesis which could allow modification of subunit association or changes in interactions with proteins and RNAs (Mikulík et al., 2011). According to the protein phosphorylation database, phosphopeptides of RpmC have been identified in four different bacteria (Soufi et al., 2008; Burnside et al., 2011; Manteca et al., 2011; Misra et al., 2011). The implication of phosphorylation on RpmC has not been investigated. However, in E.coli, RpmC, RplW and Trigger factor are located at the exit tunnel in the ribosome, suggesting that phosphorylation may impact on multiple stages of transcription (Kramer et al., 2002). In our study we identified phosphopeptides for 7 ribosomal proteins. We also identified unique phosphopeptides on ribosomal proteins RpsS and RplX (Table 3).
Overlap of Phosphorylated Proteins with other Bacteria
Twenty-five of phosphorylated proteins identified in our study were also identified in phosphoproteomics studies of other bacteria such as Klebsiella pneumonia (Lin et al., 2009), Helicobacter pylori (Ge et al., 2011), Steptococcus pneumonia (Sun et al., 2010), Bacillus subtillis (Macek et al., 2007), Halobacterium salinarum (Aivaliotis et al., 2009), etc. (Table 4). In our dataset the distribution of S/T/Y seem to be bias toward pT and is in accordance with previously described phosphoproteomes of M. tuberculosis (Prisic et al., 2010; Kusebauch et al., 2014). Manual evaluation of the genome found an over-representation of Threonine relative to Serine (52:48%). This compared to other bacteria such as Acinetobacter baumannii (Soares et al., 2014), Bacillus subtilis (Macek et al., 2007), Escherichia coli (Macek et al., 2008; Soares et al., 2013) and Halobacterium salinarum (Aivaliotis et al., 2009), Pseudomonas aeruginosa (Ravichandran et al., 2009), and Streptomyces coelicolor (Parker et al., 2010) which demonstrate a bias toward pS.
Forty-five of the phosphorylated proteins identified in our study were previously described for M. tuberculosis H37Rv (Boitel et al., 2003; Young et al., 2003; Molle et al., 2004, 2006; Durán et al., 2005; Kang et al., 2005; Villarino et al., 2005; O'Hare et al., 2008; Thakur et al., 2008; Prisic et al., 2010; Sajid et al., 2011; Gee et al., 2012). The reason for not identifying all of the previously identified phosphorylated proteins in the protein phosphorylation database could be ascribed to different genetic backgrounds of the analyzed M. tuberculosis strains, culture conditions, sample preparation and different MS-based proteomics approaches used in each of the studies. Our analysis was performed on a hyper-virulent clinical isolate of M. tuberculosis and a member of the Beijing genotype which is genetically distinct from the laboratory strain M. tuberculosis H37Rv analyzed by Prisic et al. and Kusebauch et al. In addition, the Prisic et al. study reported on the combined phosphoproteome from 6 different conditions (5 different culture conditions and 2 different growth phases) (Prisic et al., 2010) while Kusebauch et al. reported on the phosphoproteome of late-logarithmic phase cultures (Kusebauch et al., 2014), whereas our study analyzed early-logarithmic phase cultures. Even though the overlap between our study of clinical M. tuberculosis and that of the previously described laboratory M. tuberculosis H37Rv is low this work substantially extends our knowledge of the M. tuberculosis phosphoproteome. During logarithmic growth phase of bacterial growth the cells are adapting to the environment of the growth media and biological process such as RNA synthesis, DNA replication and synthesis of micro- and macromolecules are up-regulated. It is important to note that in this study the whole cell lysate proteins were enriched for phosphopeptides and we detected a number of phosphorylated proteins involved in these biological processes such as fatty acid- and lipid biosynthetic metabolism; RNA modification and translation; DNA repair, replication and modification. It is believed that environmental conditions, cell density and growth phase influence the expression of virulence factors by a pathogen (McIver et al., 1995). This is consistent other bacterial phosphoproteomes, thereby emphasizing that S/T/Y phosphorylation is an important process required for the regulation of numerous cellular processes.
Conclusion
Recent developments in the methodology and mass spectrometry technology for phosphoproteomics have highlighted the need to explore the involvement of phosphorylation in disease development and progression. However, the impact of the protein phosphorylation cascade on the physiology of pathogenic bacteria such as M. tuberculosis has yet to be fully elucidated. Improved preparative techniques and more sensitive instrumentation are required to fully appreciate the complexity of protein modification. This can only be achieved if concomitant methods are developed to elucidate the impact of phosphorylation on protein function. Although this qualitative study was done in clinical hyper-virulent M. tuberculosis, without any follow-up validation studies it still provides a valuable resource for further investigating and understanding the impact of protein phosphorylation regulation in M. tuberculosis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was sponsored by the National Research Foundation Norway/RSA research cooperation programme, the Medical research Council of South Africa, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research Stellenbosch University (Professor Paul van Helden). We further want to acknowledge Prof. M. Mann who permitted us to use the proteomics and mass spectrometry facilities at the Max Planck Institute, Munich, Germany.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmicb.2015.00006/abstract
References
Aivaliotis, M., Macek, B., Gnad, F., Reichelt, P., Mann, M., and Oesterhelt, D. (2009). Ser/Thr/Tyr protein phosphorylation in the archaeon Halobacterium salinarum—a representative of the third domain of life. PLoS ONE 4:e4777. doi: 10.1371/journal.pone.0004777
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Al-Attiyah, R., Madi, N. M., El-Shamy, A. M., Wiker, H. G., Andersen, P., and Mustafa, A. S. (2006). Cytokine profiles in tuberculosis patients and healthy subjects in response to complex and single antigens of Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol. 47, 254–261. doi: 10.1111/j.1574-695X.2006.00110.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Arora, G., Sajid, A., Gupta, M., Bhaduri, A., Kumar, P., Basu-Modak, S., et al. (2010). Understanding the role of PknJ in Mycobacterium tuberculosis: biochemical characterization and identification of novel substrate pyruvate kinase A. PLoS ONE 5:10772. doi: 10.1371/journal.pone.0010772
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bach, H., Wong, D., and Av-Gay, Y. (2009). Mycobacterium tuberculosis PtkA is a novel protein tyrosine kinase whose substrate is PtpA. Biochem. J. 420, 155–160. doi: 10.1042/BJ20090478
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bhatt, A., Fujiwara, N., Bhatt, K., Gurcha, S. S., Kremer, L., Chen, B., et al. (2007). Deletion of kasB in Mycobacterium tuberculosis causes loss of acid-fastness and subclinical latent tuberculosis in immunocompetent mice. Proc. Natl. Acad. Sci. U.S.A. 104, 5157–5162. doi: 10.1073/pnas.0608654104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bitter, W., and Kuijl, C. (2014). Targeting bacterial virulence: the coming out of type VII secretion inhibitors. Cell Host Microbe 16, 430–432. doi: 10.1016/j.chom.2014.09.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Boitel, B., Ortiz-Lombardía, M., Durán, R., Pompeo, F., Cole, S. T., Cerveñansky, C., et al. (2003). PknB kinase activity is regulated by phosphorylation in two Thr residues and dephosphorylation by PstP, the cognate phospho-Ser/Thr phosphatase, in Mycobacterium tuberculosis. Mol. Microbiol. 49, 1493–1508. doi: 10.1046/j.1365-2958.2003.03657.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Burnside, K., Lembo, A., Harrell, M. I., Gurney, M., Xue, L., BinhTran, N.-T., et al. (2011). Serine/threonine phosphatase Stp1 mediates post-transcriptional regulation of hemolysin, autolysis, and virulence of group B Streptococcus. J. Biol. Chem. 286, 44197–44210. doi: 10.1074/jbc.M111.313486
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chopra, P., Meena, L. S., and Singh, Y. (2003). New drug targets for Mycobacterium tuberculosis. Indian J. Med. Res. 117, 1–9.
Chou, M. F., Prisic, S., Lubner, J. M., Church, G. M., Husson, R. N., and Schwartz, D. (2012). Using bacteria to determine protein kinase specificity and predict target substrates. PLoS ONE 7:e52747. doi: 10.1371/journal.pone.0052747
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544. doi: 10.1038/31159
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cozzone, A. J. (1998). Post-translational modification of proteins by reversible phosphorylation in prokaryotes. Biochimie 80, 43–48.
Daleke, M. H., Ummels, R., Bawono, P., Heringa, J., Vandenbroucke-Grauls, C. M. J. E., Luirink, J., et al. (2012). General secretion signal for the mycobacterial type VII secretion pathway. Proc. Natl. Acad. Sci. U.S.A. 109, 11342–11347. doi: 10.1073/pnas.1119453109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
de Souza, G. A., Fortuin, S., Aguilar, D., Pando, R. H., McEvoy, C. R. E., van Helden, P. D., et al. (2010). Using a label-free proteomics method to identify differentially abundant proteins in closely related hypo- and hypervirulent clinical Mycobacterium tuberculosis Beijing isolates. Mol. Cell. Proteomics 9, 2414–2423. doi: 10.1074/mcp.M900422-MCP200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Durán, R., Villarino, A., Bellinzoni, M., Wehenkel, A., Fernandez, P., Boitel, B., et al. (2005). Conserved autophosphorylation pattern in activation loops and juxtamembrane regions of Mycobacterium tuberculosis Ser/Thr protein kinases. Biochem. Biophys. Res. Commun. 333, 858–867. doi: 10.1016/j.bbrc.2005.05.173
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fernandez, P., Saint-Joanis, B., Barilone, N., Jackson, M., Gicquel, B., Cole, S. T., et al. (2006). The Ser/Thr protein kinase PknB is essential for sustaining mycobacterial growth. J. Bacteriol. 188, 7778–7784. doi: 10.1128/JB.00963-06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frasch, S. C., and Dworkin, M. (1996). Tyrosine phosphorylation in Myxococcus xanthus, a multicellular prokaryote. J. Bacteriol. 178, 4084–4088.
Ge, R., and Shan, W. (2011). Bacterial phosphoproteomic analysis reveals the correlation between protein phosphorylation and bacterial pathogenicity. Genomics Proteomics Bioinformatics 9, 119–127. doi: 10.1016/S1672-0229(11)60015-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ge, R., Sun, X., Xiao, C., Yin, X., Shan, W., Chen, Z., et al. (2011). Phosphoproteome analysis of the pathogenic bacterium Helicobacter pylori reveals over-representation of tyrosine phosphorylation and multiply phosphorylated proteins. Proteomics 11, 1449–1461. doi: 10.1002/pmic.201000649
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gee, C. L., Papavinasasundaram, K. G., Blair, S. R., Baer, C. E., Falick, A. M., King, D. S., et al. (2012). A phosphorylated pseudokinase complex controls cell wall synthesis in Mycobacteria. Sci. Signal. 5, ra7. doi: 10.1126/scisignal.2002525
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Griffin, J. E., Gawronski, J. D., Dejesus, M. A., Ioerger, T. R., Akerley, B. J., and Sassetti, C. M. (2011). High-resolution phenotypic profiling defines genes essential for Mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7:e1002251. doi: 10.1371/journal.ppat.1002251
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Griffin, J. E., Pandey, A. K., Gilmore, S. A., Mizrahi, V., McKinney, J. D., Bertozzi, C. R., et al. (2012). Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 19, 218–227. doi: 10.1016/j.chembiol.2011.12.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gupta, N., Tanner, S., Jaitly, N., Adkins, J. N., Lipton, M., Edwards, R., et al. (2007). Whole proteome analysis of post-translational modifications: applications of mass-spectrometry for proteogenomic annotation. Genome Res. 17, 1362–1377. doi: 10.1101/gr.6427907
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Houben, E. N. G., Korotkov, K. V., and Bitter, W. (2014). Take five—Type VII secretion systems of Mycobacteria. Biochim. Biophys. Acta 1843, 1707–1716. doi: 10.1016/j.bbamcr.2013.11.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jang, J., Stella, A., Boudou, F., Levillain, F., Darthuy, E., Vaubourgeix, J., et al. (2010). Functional characterization of the Mycobacterium tuberculosis serine/threonine kinase PknJ. Microbiol. Read. Engl. 156, 1619–1631. doi: 10.1099/mic.0.038133-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kang, C.-M., Abbott, D. W., Park, S. T., Dascher, C. C., Cantley, L. C., and Husson, R. N. (2005). The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 19, 1692–1704. doi: 10.1101/gad.1311105
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koul, A., Herget, T., Klebl, B., and Ullrich, A. (2004). Interplay between mycobacteria and host signalling pathways. Nat. Rev. Microbiol. 2, 189–202. doi: 10.1038/nrmicro840
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kramer, G., Rauch, T., Rist, W., Vorderwülbecke, S., Patzelt, H., Schulze-Specking, A., et al. (2002). L23 protein functions as a chaperone docking site on the ribosome. Nature 419, 171–174. doi: 10.1038/nature01047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kumar, B., Sharma, D., Sharma, P., Katoch, V. M., Venkatesan, K., and Bisht, D. (2013). Proteomic analysis of Mycobacterium tuberculosis isolates resistant to kanamycin and amikacin. J. Proteomics 94, 68–77. doi: 10.1016/j.jprot.2013.08.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kumar, D., and Narayanan, S. (2012). pknE, a serine/threonine kinase of Mycobacterium tuberculosis modulates multiple apoptotic paradigms. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 12, 737–747. doi: 10.1016/j.meegid.2011.09.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kusebauch, U., Ortega, C., Ollodart, A., Rogers, R. S., Sherman, D. R., Moritz, R. L., et al. (2014). Mycobacterium tuberculosis supports protein tyrosine phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 111, 9265–9270. doi: 10.1073/pnas.1323894111
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lakshminarayan, H. (2009). Involvement of serine threonine protein kinase PknL, from Mycobacterium tuberculosis H37Rv in starvation response of Mycobacteria. J. Microb. Biochem. Technol. 1, 30–36.
Lew, J. M., Kapopoulou, A., Jones, L. M., and Cole, S. T. (2011). TubercuList—10 years after. Tuberc. Edinb. Scotl. 91, 1–7. doi: 10.1016/j.tube.2010.09.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lima, K. M., Santos, S. A., Lima, V. M. F., Coelho-Castelo, A. A. M., Rodrigues, J. M., and Silva, C. L. (2003). Single dose of a vaccine based on DNA encoding mycobacterial hsp65 protein plus TDM-loaded PLGA microspheres protects mice against a virulent strain of Mycobacterium tuberculosis. Gene Ther. 10, 678–685. doi: 10.1038/sj.gt.3301908
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lin, M.-H., Hsu, T.-L., Lin, S.-Y., Pan, Y.-J., Jan, J.-T., Wang, J.-T., et al. (2009). Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol. Cell. Proteomics 8, 2613–2623. doi: 10.1074/mcp.M900276-MCP200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Macek, B., Gnad, F., Soufi, B., Kumar, C., Olsen, J. V., Mijakovic, I., et al. (2008). Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol. Cell. Proteomics 7, 299–307. doi: 10.1074/mcp.M700311-MCP200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Macek, B., and Mijakovic, I. (2011). Site-specific analysis of bacterial phosphoproteomes. Proteomics 11, 3002–3011. doi: 10.1002/pmic.201100012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Macek, B., Mijakovic, I., Olsen, J. V., Gnad, F., Kumar, C., Jensen, P. R., et al. (2007). The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6, 697–707. doi: 10.1074/mcp.M600464-MCP200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Manteca, A., Ye, J., Sánchez, J., and Jensen, O. N. (2011). Phosphoproteome analysis of Streptomyces development reveals extensive protein phosphorylation accompanying bacterial differentiation. J. Proteome Res. 10, 5481–5492. doi: 10.1021/pr200762y
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McConnell, J. L., and Wadzinski, B. E. (2009). Targeting protein serine/threonine phosphatases for drug development. Mol. Pharmacol. 75, 1249–1261. doi: 10.1124/mol.108.053140
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McIver, K. S., Heath, A. S., and Scott, J. R. (1995). Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect. Immun. 63, 4540–4542.
Mijakovic, I., and Macek, B. (2012). Impact of phosphoproteomics on studies of bacterial physiology. FEMS Microbiol. Rev. 36, 877–892. doi: 10.1111/j.1574-6976.2011.00314.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mikulík, K., Bobek, J., Ziková, A., Smětáková, M., and Bezoušková, S. (2011). Phosphorylation of ribosomal proteins influences subunit association and translation of poly (U) in Streptomyces coelicolor. Mol. Biosyst. 7, 817–823. doi: 10.1039/c0mb00174k
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Misra, S. K., Milohanic, E., Aké, F., Mijakovic, I., Deutscher, J., Monnet, V., et al. (2011). Analysis of the serine/threonine/tyrosine phosphoproteome of the pathogenic bacterium Listeria monocytogenes reveals phosphorylated proteins related to virulence. Proteomics 11, 4155–4165. doi: 10.1002/pmic.201100259
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Molle, V., Brown, A. K., Besra, G. S., Cozzone, A. J., and Kremer, L. (2006). The condensing activities of the Mycobacterium tuberculosis type II fatty acid synthase are differentially regulated by phosphorylation. J. Biol. Chem. 281, 30094–30103. doi: 10.1074/jbc.M601691200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Molle, V., Leiba, J., Zanella-Cléon, I., Becchi, M., and Kremer, L. (2010). An improved method to unravel phosphoacceptors in Ser/Thr protein kinase-phosphorylated substrates. Proteomics 10, 3910–3915. doi: 10.1002/pmic.201000316
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Molle, V., Soulat, D., Jault, J.-M., Grangeasse, C., Cozzone, A. J., and Prost, J.-F. (2004). Two FHA domains on an ABC transporter, Rv1747, mediate its phosphorylation by PknF, a Ser/Thr protein kinase from Mycobacterium tuberculosis. FEMS Microbiol. Lett. 234, 215–223. doi: 10.1016/j.femsle.2004.03.033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
O'Hare, H. M., Durán, R., Cerveñansky, C., Bellinzoni, M., Wehenkel, A. M., Pritsch, O., et al. (2008). Regulation of glutamate metabolism by protein kinases in mycobacteria. Mol. Microbiol. 70, 1408–1423. doi: 10.1111/j.1365-2958.2008.06489.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pallen, M., Chaudhuri, R., and Khan, A. (2002). Bacterial FHA domains: neglected players in the phospho-threonine signalling game? Trends Microbiol. 10, 556–563. doi: 10.1016/S0966-842X(02)02476-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Parandhaman, D. K., Hanna, L. E., and Narayanan, S. (2014). PknE, a serine/threonine protein kinase of Mycobacterium tuberculosis initiates survival crosstalk that also impacts HIV coinfection. PLoS ONE 9:e83541. doi: 10.1371/journal.pone.0083541
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Parker, J. L., Jones, A. M. E., Serazetdinova, L., Saalbach, G., Bibb, M. J., and Naldrett, M. J. (2010). Analysis of the phosphoproteome of the multicellular bacterium Streptomyces coelicolor A3(2) by protein/peptide fractionation, phosphopeptide enrichment and high-accuracy mass spectrometry. Proteomics 10, 2486–2497. doi: 10.1002/pmic.201000090
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Prisic, S., Dankwa, S., Schwartz, D., Chou, M. F., Locasale, J. W., Kang, C.-M., et al. (2010). Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc. Natl. Acad. Sci. U.S.A. 107, 7521–7526. doi: 10.1073/pnas.0913482107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ravichandran, A., Sugiyama, N., Tomita, M., Swarup, S., and Ishihama, Y. (2009). Ser/Thr/Tyr phosphoproteome analysis of pathogenic and non-pathogenic Pseudomonas species. Proteomics 9, 2764–2775. doi: 10.1002/pmic.200800655
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roumestand, C., Leiba, J., Galophe, N., Margeat, E., Padilla, A., Bessin, Y., et al. (2011). Structural insight into the Mycobacterium tuberculosis Rv0020c protein and its interaction with the PknB kinase. Structure 19, 1525–1534. doi: 10.1016/j.str.2011.07.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sajid, A., Arora, G., Gupta, M., Singhal, A., Chakraborty, K., Nandicoori, V. K., et al. (2011). Interaction of Mycobacterium tuberculosis elongation factor Tu with GTP is regulated by phosphorylation. J. Bacteriol. 193, 5347–5358. doi: 10.1128/JB.05469-11
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sala, C., and Hartkoorn, R. C. (2011). Tuberculosis drugs: new candidates and how to find more. Future Microbiol. 6, 617–633. doi: 10.2217/fmb.11.46
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sassetti, C. M., Boyd, D. H., and Rubin, E. J. (2003). Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84. doi: 10.1046/j.1365-2958.2003.03425.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seo, J., and Lee, K.-J. (2004). Post-translational modifications and their biological functions: proteomic analysis and systematic approaches. J. Biochem. Mol. Biol. 37, 35–44. doi: 10.5483/BMBRep.2004.37.1.035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sharma, K., Chopra, P., and Singh, Y. (2004). Recent advances towards identification of new drug targets for Mycobacterium tuberculosis. Expert Opin. Ther. Targets 8, 79–93. doi: 10.1517/14728222.8.2.79
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sharma, K., Gupta, M., Pathak, M., Gupta, N., Koul, A., Sarangi, S., et al. (2006). Transcriptional control of the mycobacterial embCAB operon by PknH through a regulatory protein, EmbR, in vivo. J. Bacteriol. 188, 2936–2944. doi: 10.1128/JB.188.8.2936-2944.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Soares, N. C., Spät, P., Krug, K., and Macek, B. (2013). Global dynamics of the Escherichia coli proteome and phosphoproteome during growth in minimal medium. J. Proteome Res. 12, 2611–2621. doi: 10.1021/pr3011843
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Soares, N. C., Spät, P., Méndez, J. A., Nakedi, K., Aranda, J., and Bou, G. (2014). Ser/Thr/Tyr phosphoproteome characterization of Acinetobacter baumannii: comparison between a reference strain and a highly invasive multidrug-resistant clinical isolate. J. Proteomics 102, 113–124. doi: 10.1016/j.jprot.2014.03.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Soufi, B., Gnad, F., Jensen, P. R., Petranovic, D., Mann, M., Mijakovic, I., et al. (2008). The Ser/Thr/Tyr phosphoproteome of Lactococcus lactis IL1403 reveals multiply phosphorylated proteins. Proteomics 8, 3486–3493. doi: 10.1002/pmic.200800069
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stock, J. B., Ninfa, A. J., and Stock, A. M. (1989). Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53, 450–490.
Sun, X., Ge, F., Xiao, C.-L., Yin, X.-F., Ge, R., Zhang, L.-H., et al. (2010). Phosphoproteomic analysis reveals the multiple roles of phosphorylation in pathogenic bacterium Streptococcus pneumoniae. J. Proteome Res. 9, 275–282. doi: 10.1021/pr900612v
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thakur, M., Chaba, R., Mondal, A. K., and Chakraborti, P. K. (2008). Interdomain interaction reconstitutes the functionality of PknA, a eukaryotic type Ser/Thr kinase from Mycobacterium tuberculosis. J. Biol. Chem. 283, 8023–8033. doi: 10.1074/jbc.M707535200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Triccas, J. A., and Gicquel, B. (2001). Analysis of stress- and host cell-induced expression of the Mycobacterium tuberculosis inorganic pyrophosphatase. BMC Microbiol. 1:3. doi: 10.1186/1471-2180-1-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Villarino, A., Duran, R., Wehenkel, A., Fernandez, P., England, P., Brodin, P., et al. (2005). Proteomic identification of M. tuberculosis protein kinase substrates: PknB recruits GarA, a FHA domain-containing protein, through activation loop-mediated interactions. J. Mol. Biol. 350, 953–963. doi: 10.1016/j.jmb.2005.05.049
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Warner, D. F., and Mizrahi, V. (2012). A pseudokinase debut at the mycobacterial cell wall. Sci. Signal. 5, pe3–pe3. doi: 10.1126/scisignal.2002785
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitmore, S. E., and Lamont, R. J. (2012). Tyrosine phosphorylation and bacterial virulence. Int. J. Oral Sci. 4, 1–6. doi: 10.1038/ijos.2012.6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
WHO|Global tuberculosis report 2013. (2013). WHO. Available online at: http://www.who.int/tb/publications/global_report/en/ [Accessed October 1, 2014].
Wiśniewski, J. R., Zougman, A., Nagaraj, N., and Mann, M. (2009). Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362. doi: 10.1038/nmeth.1322
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Young, T. A., Delagoutte, B., Endrizzi, J. A., Falick, A. M., and Alber, T. (2003). Structure of Mycobacterium tuberculosis PknB supports a universal activation mechanism for Ser/Thr protein kinases. Nat. Struct. Mol. Biol. 10, 168–174. doi: 10.1038/nsb897
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, W., Li, L., Jiang, W., Zhao, G., Yang, Y., and Chiao, J. (2000). A novel transmembrane serine/threonine protein kinase gene from a rifamycin SV-producing amycolatopsis mediterranei U32. Eur. J. Biochem. FEBS 267, 3744–3752. doi: 10.1046/j.1432-1327.2000.01410.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zheng, X., Papavinasasundaram, K. G., and Av-Gay, Y. (2007). Novel substrates of Mycobacterium tuberculosis PknH Ser/Thr kinase. Biochem. Biophys. Res. Commun. 355, 162–168. doi: 10.1016/j.bbrc.2007.01.122
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: M. tuberculosis, phosphoproteomics, tyrosine phosphorylation, serine phosphorylation, threonine phosphorylation
Citation: Fortuin S, Tomazella GG, Nagaraj N, Sampson SL, Gey van Pittius NC, Soares NC, Wiker HG, de Souza GA and Warren RM (2015) Phosphoproteomics analysis of a clinical Mycobacterium tuberculosis Beijing isolate: expanding the mycobacterial phosphoproteome catalog. Front. Microbiol. 6:6. doi: 10.3389/fmicb.2015.00006
Received: 31 October 2014; Accepted: 04 January 2015;
Published online: 10 February 2015.
Edited by:
Ivan Mijakovic, Chalmers University of Technology, SwedenReviewed by:
Lei Shi, Chalmers University of Technology, SwedenBoumediene Soufi, Proteome Center Tuebingen, Germany
Copyright © 2015 Fortuin, Tomazella, Nagaraj, Sampson, Gey van Pittius, Soares, Wiker, de Souza and Warren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin M. Warren, Division of Molecular Biology and Human Genetics, Faculty Medicine and Health Sciences, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, SAMRC Centre for Tuberculosis Research, Stellenbosch University, Francie van Zijl drive, Tygerberg, Cape Town 7505, South Africa e-mail:cncxQHN1bi5hYy56YQ==
†These authors have contributed equally to this work.
 Suereta Fortuin
Suereta Fortuin Gisele G. Tomazella2
Gisele G. Tomazella2 Samantha L. Sampson
Samantha L. Sampson Nelson C. Soares
Nelson C. Soares Gustavo A. de Souza
Gustavo A. de Souza Robin M. Warren
Robin M. Warren