- 1Horn Point Laboratory, Center for Environmental Science, University of Maryland, Cambridge, MD, USA
- 2Cooperative Oxford Laboratory, National Centers for Coastal Ocean Science, National Ocean Service, Oxford, MD, USA
- 3College of Earth, Ocean, and Atmospheric Science, Oregon State University, Corvallis, OR, USA
To determine if a storm event (i.e., high winds, large volumes of precipitation) could alter concentrations of Vibrio vulnificus and V. parahaemolyticus in aquacultured oysters (Crassostrea virginica) and associated surface water and sediment, this study followed a sampling timeline before and after Hurricane Irene impacted the Chesapeake Bay estuary in late August 2011. Aquacultured oysters were sampled from two levels in the water column: surface (0.3 m) and near-bottom (just above the sediment). Concentrations of each Vibrio spp. and associated virulence genes were measured in oysters with a combination of real-time PCR and most probable number (MPN) enrichment methods, and in sediment and surface water with real-time PCR. While concentration shifts of each Vibrio species were apparent post-storm, statistical tests indicated no significant change in concentration for either Vibrio species by location (surface or near bottom oysters) or date sampled (oyster tissue, surface water, and sediment concentrations). V. vulnificus in oyster tissue was correlated with total suspended solids (r = 0.41, P = 0.04), and V. vulnificus in sediment was correlated with secchi depth (r = -0.93, P <0.01), salinity (r = -0.46, P = 0.02), tidal height (r = -0.45, P = 0.03), and surface water V. vulnificus (r = 0.98, P <0.01). V. parahaemolyticus in oyster tissue did not correlate with environmental measurements, but V. parahaemolyticus in sediment and surface water correlated with several measurements including secchi depth [r = -0.48, P = 0.02 (sediment); r = -0.97, P <0.01 (surface water)] and tidal height [r = -0.96, P <0.01 (sediment), r = -0.59, P <0.01 (surface water)]. The concentrations of Vibrio spp. were higher in oysters relative to other studies (average V. vulnificus 4 × 105 MPN g-1, V. parahaemolyticus 1 × 105 MPN g-1), and virulence-associated genes were detected in most oyster samples. This study provides a first estimate of storm-related Vibrio density changes in oyster tissues, sediment, and surface water at an aquaculture facility in the Chesapeake Bay.
Introduction
Storm events are thought to be important mechanisms for the distribution of benthic Vibrio populations into the water column via resuspension of sediments associated with high winds, and flushing due to large volumes of precipitation (Randa et al., 2004; Fries et al., 2008; Wetz et al., 2008; Johnson et al., 2010). Frequent storm events in the Chesapeake Bay are associated with the summer season, a time when Vibrio vulnificus and V. parahaemolyticus, autochthonous bacteria known to cause human illness, are at their highest densities in surface waters (Wright et al., 1996; Parveen et al., 2008; Jacobs et al., 2010; Johnson et al., 2012). The frequency and intensity of storm events are predicted to escalate in response to global climate change (Goldenberg et al., 2001), with increases in peak wind intensities and near-storm precipitation (Meehl et al., 2007) likely impacting mid-Atlantic areas such as the Chesapeake Bay. In the Chesapeake Bay, a shallow, partially mixed estuary prone to tidal circulation (average depth 6.5 m), storm events may be expected to increase the overall Vibrio density in surface waters with relatively moderate wind speed and associated wave action. Increases in post-hurricane Vibrio infection has been documented (e.g., Hurricane Katrina), with a resultant need for heightened clinical awareness, particularly of wound infections, following exposure to flood waters (Centers for Disease Control and Prevention [CDC], 2005). Based on the reported increases in storm-related Vibriosis in other areas of the United States, it is conceivable that storm-induced increases in Chesapeake Bay Vibrio density may be linked to future Vibriosis outbreaks.
According to the U.S. Environmental Protection Agency, the Chesapeake Bay is home to 25% of the total approved shellfish harvesting waters in the United States (Environmental Protection Agency [EPA], 2011). Recently, the Chesapeake Bay has become a site of interest for oyster (Crassostrea virginica) aquaculture production to supplement the dwindling wild harvest, both through on-bottom (submerged land) and off-bottom (water column) leases (Maryland Department of Natural Resources, Shellfish Aquaculture Program). As of January 2013, 169 aquaculture operation permit applications (~4000 acres) were submitted to Maryland Department of Natural Resources for water-column and submerged-land leases (Webster, University of Maryland Extension, personal communication), and a total of 300 submerged-land leases (~3500 acres) and 23 water-column leases (~94 acres) permitted. A small number of new aquaculture operations are in year-round production of retail oysters, with the supposition that many new operations will soon be joining their ranks.
Summer is generally considered to be a viable oyster harvest season in Maryland, but summer is also when Vibrio populations reach their peak in the Bay (Wright et al., 1996; Parveen et al., 2008; Jacobs et al., 2010; Johnson et al., 2012). Studies are currently being conducted to determine ways to reduce Vibrio concentrations in oysters (e.g., high salinity relay), but factors influencing the accumulation of high numbers or virulent strains of Vibrio in oysters are not completely understood (Warner and Oliver, 2008; Johnson et al., 2010; Froelich and Oliver, 2013). Thus, the harvest of oysters during seasons when surface water Vibrio populations are at high densities could become a pressing issue for seafood safety. If Vibrio density in oysters increases after storm events, shellfish managers may need to institute shellfish harvest closure periods to allow for oyster depuration or wait for suitable environmental conditions that favor a reduction in Vibrio concentrations, such as cooler water temperatures.
This study was conducted to test the hypothesis that a storm event, using Hurricane Irene as a proxy, generates enough wave energy to cause resuspension of sediment that would cause an increase in oyster-tissue density of V. vulnificus and V. parahaemolyticus. Oysters were tested in Taylor-style surface-water floats (Luckenbach et al., 1999) and in on-bottom cages, to determine if there was an accumulation difference based on water column position. Results from this study provide a first estimate of storm-related Vibrio density changes in oyster tissues, sediment and surface water at an aquaculture facility in the Chesapeake Bay.
Materials and Methods
Sampling Site
The study was conducted at an oyster aquaculture facility in a mesohaline tributary of the Chesapeake Bay. The oyster farm was approximately 250,000 m2(6 acres) with a water depth of approximately 1.2 m (4 ft) at low tide and 2.1 m (7 ft) at high tide. Sediment types at the farm ranged from predominantly sand to predominantly silt. The sampling location within the oyster farm was chosen for the predominance of silty sediment (20.4% sand: 66.6% silt: 13.0% clay; Owens, Cornwell, University of Maryland Center for Environmental Science, personal communication), which is representative of the biodeposition typically produced by oysters (Haven and Morales-Alamo, 1972). Three sampling sub-locations were selected along the outermost matrix of oyster floats, which covered approximately 1 acre, both for sediment composition and the likelihood of the area being unprotected from wind events and resultant resuspension activity. Estimates of wind speeds and resultant wave height were made using equations from Young and Verhagen (1996). Calculations of maximum bottom-sheer stress were made according to (Sanford, 1994) incorporating an approximate bottom depth of 1 m and sand grain roughness of 0.0005 m. Sand grain roughness is a measurement of characteristic bottom roughness height for use in hydrodynamic calculations. Erosion rate was calculated using the equation E (g m-2 h-1) = Mo (kg m-2 s-1 Pa-1) × 3600 s h-1 × 1000 g kg-1 × (τb–τc) (Pa), with site-specific estimates of τc = 0.025 Pa and Mo = 0.000315 kg m-2 s-1 Pa-1 (τb: bottom-related sheer stress; τc: current-related shear stress; Pascal (Pa); Mo is erosion rate constant; Sanford, Kwon, University of Maryland Center for Environmental Science, personal communication). These calculations do not acknowledge the potential for a wave-dampening effect by the large array of oyster floats tied together at the aquaculture site, although a physical oceanographer conducting experiments at the same site shares that long period waves at the bottom of the water column are damped out by perhaps as much as 50% by the floats, but not so much that resuspension would be negated (Sanford, University of Maryland, personal communication).
Environmental Sample Collection
Baseline surface water, oyster, and sediment samples were collected from the field location on August 26, 2011, the day before Hurricane Irene and associated storm impacts were forecast to be present along the Maryland coastline. Subsequent samples were taken at time points 1, 4, and 8 days after Hurricane Irene. All samples were collected at approximately 10:00 A.M. to approximate a uniform water and air temperature at the time of sampling due to solar irradiation.
Surface-water samples were collected at each sampling location in sterile wide mouth polypropylene 1 L bottles (Nalgene Thermo Scientific 2105-0032) following the methods described by Jacobs et al. (2009). Surface water (200 mL) was filtered through a 0.22 μm Sterivex-GP polyethersulfone filter (Millipore, Billerica, MA, USA) using a 60 mL BD luer lock syringe (BD, Franklin Lakes, NJ, USA), wrapped in Parafilm M laboratory wrapping film (Bemis Flexible Packaging, Oshkosh, WI, USA), and sealed in a labeled 7 oz Whirlpak bag (Nasco, Fort Atkinson, WI, USA). Filters were stored on ice until return to the laboratory (~1 h), where they were stored at -20°C until DNA extraction.
Physical/Chemical Measurements
Temperature, salinity, conductivity, and dissolved oxygen were measured using a YSI Model 85 (YSI, Yellow Springs, OH, USA) at 0.3 m depth and near-bottom (~0.3 m off bottom). Secchi depth was recorded to the nearest 0.05 m. Total suspended solids (TSS) measurements were completed using 250–400 mL of surface water, filtered onto pre-weighed 47 mm glass fiber filters (Whatman GF/F, GE Healthcare Life Sciences, Piscataway, NJ, USA).
Sample Size
Based on standard deviations reported in Johnson et al. (2010), sample size needed was calculated for a statistical power of 0.8, significance criterion of 0.05, and preferred detection difference of 500 CFU g-1. Based on this calculation, three samples were required for each depth (top and bottom), per sampling period.
Oyster Sample Collection
Oyster samples (C. virginica) were collected from the top (n = 3) and bottom (n = 3) of the water column on each of the four sampling dates. Collected oysters [six per sample (Kaufman et al., 2003)] had shell heights (oyster hinge to opposite edge periphery) of ~ 8 cm (3.1 in). Surface water oyster samples were collected from Taylor-style floats, which remained submerged in water continuously, and bottom-water oyster samples were enclosed in 1.3 cm mesh bags deployed inside of crab pots to keep the oysters at the bottom of the water column, but out of the sediment layer. Bottom oysters, collected from identical resident oyster stock as surface oyster samples, were deployed 1 month before the commencement of this study for acclimation purposes. Collected oysters were immediately placed on ice and processed within an hour.
Crab pots consistently had a coating of top layer sediment (~1 cm) on the bottom of the pot from being deployed in the sediment. That sediment was collected at each of the three sites by filling a 50 mL Falcon sterile polypropylene conical centrifuge tube (BD Vacutainer Labware Medical 352070). Sediment samples were placed on ice, and stored frozen at -20°C.
Oyster Processing
On each sampling date, a total of 36 oysters were examined, divided into six samples, for a total of 144 analyzed oysters over the four sampling periods. One sample of n = 6 oysters were collected from both the top and bottom layers at each of three sampling strata (Kaufman et al., 2003) and were homogenized following the three-tube MPN method described in the U.S. Food and Drug Administration Bacteriological Analytical Manual (BAM; DePaola and Kaysner, 2004) with slight modifications. Briefly, oysters were scrubbed, shucked with a sterile knife into a sterile blender, diluted with an equal weight of sterile phosphate-buffered-saline (Food and Drug Administration [FDA], 1998) and blended for 90 s to create a 1:1 (wt:wt) shellfish:diluent homogenate. A 1:20 dilution of oyster homogenate was made in triplicate by adding 1 mL of the 1:1 diluted homogenate to 9 mL alkaline peptone water (APW; 1% peptone, 1% NaCl, pH 8.5 ± 0.2). Additional triplicate 10-fold dilutions to 5 × 10-7 were prepared volumetrically by transferring 1 mL portions into 9 mL APW. Following overnight incubation at 35 ± 2°C, the top 1 mL of tubes showing growth was collected and frozen at -20°C.
DNA Extraction, Detection, and Quantification
DNA from surface water was extracted following a modified MO BIO Powersoil extraction protocol (Jacobs et al., 2009), and DNA from sediments was extracted using the standard MO BIO Powersoil extraction protocol. Extracted DNA was stored at -80°C. Quantitative PCR was used to quantify CFU mL-1 in water and CFU g-1 in sediment. The reported extraction efficiency of surface water and sediment samples using their respective methods were comparable (Jacobs et al., 2010; Lloyd et al., 2010).
DNA template was obtained from MPN cultures by producing crude cell lysates by boiling 1 mL aliquots of APW cultures in 2 mL micro-centrifuge tubes for 10 min. Following boiling, tubes were plunged into ice until cool and then centrifuged at 14,000 × g for 2 min. Supernatant template was added to real-time PCR reactions (3–5 uL; see PCR methods) to determine presence or absence of V. vulnificus and V. parahaemolyticus in cultured samples. Bio-rad CFX96 TouchTM Real-Time PCR Detection System (Bio-rad, Hercules, CA, USA) was used to confirm the species with primers designed to detect V. vulnificus (Panicker and Bej, 2005) or V. parahaemolyticus (Nordstrom et al., 2007). Following initial detection, samples testing positive for either species were subjected to further PCR testing for virulence genes (V. vulnificus: virulence correlated gene, clinical variant (vcgC; Baker-Austin et al., 2010); V. parahaemolyticus: thermostable direct hemolysin (tdh), thermostable related hemolysin (trh) genes (Nordstrom et al., 2007).
Quantitative PCR was performed on surface water and sediment sample extracts by using 2.50 uL of 10X PCR Buffer (Qiagen, Valencia, CA, USA), 1.25 uL of 25 mM MgCl2 (Qiagen), 0.50 uL of 10 mM dNTP’s solution (Qiagen), 5 uL Q solution (Qiagen), 0.45 uL of 5 U/uL TopTaq DNA polymerase (Qiagen), 0.188 uL of 10 uM internal control primers (each), 0.375 uL of 10 uM internal control probe, 2 uL internal control DNA, 0.50 uL of 10 uM primer (each), 0.188 uL of 10 uM probe, and 3 uL DNA template per reaction, with the exception of the V. vulnificus vcgC assay, in which 5 uL of DNA template was used. DNase–RNase free water was added in a quantity sufficient for a 25 uL total reaction volume. Two-stage qPCR cycling parameters were optimized to the conditions as described in Shaw et al. (2014). A unique internal control, including a primer set, probe and internal control DNA, was incorporated simultaneously into each assay, excluding V. vulnificus vcgC, to test for the presence and influence of inhibitors (Nordstrom et al., 2007). Positive controls used for each qPCR were V. parahaemolyticus USFDA TX2103 and V. vulnificus ATCC 27562. Standard curves were constructed as reported in Jacobs et al. (2010) from spiked environmental water and used during each qPCR analysis with appropriate parameters. Cycle threshold (Ct) value was plotted against the slope of the standard curve to determine PCR unit quantity.
Most Probable Number Calculation Using QPCR Results
Corresponding qPCR-MPN values were derived using the U.S. Food and Drug Administration MPN calculator, downloaded from the online publication “Bacteriological Analytical Manual, Appendix 2: Most Probable Number from Serial Dilutions.”1
Statistical Analysis
Statistical analysis was completed using Intercooled Stata 9.1 for Macintosh statistical software (StataCorp LP, College Station, TX, USA). Oyster MPN g-1, sediment and surface water data (CFU mL-1) were log transformed (log10) to equalize variances. Each data set was analyzed for normality. Normally distributed oyster MPN g-1data were analyzed with multivariate analysis of variance (MANOVA) to test for differences in sampling location (top vs. bottom oyster concentrations) and sampling date for each species of Vibrio. Surface water and sediment samples were tested with one-way analysis of variance (ANOVA). Data sets not meeting normality criteria were analyzed with Kruskal–Wallis non-parametric rank test for differences in sampling location and sampling date. Pearson pairwise correlation analysis was conducted for the experimental variables of oyster MPN g-1, surface water CFU mL-1, sediment CFU g-1, MPN g-1, salinity, temperature, TSS, dissolved oxygen, tidal height, and secchi depth. Spearman’s rank correlation analysis was used for non-normally distributed data. Due to low sample numbers, virulence associated gene (tdh and vcgC) concentrations were not included in correlation analysis.
Results
Hurricane Details
During the early morning hours of August 28, 2011, Hurricane Irene was just off the Delmarva coastline and the associated winds and rain impacted the Chesapeake Bay region. At the study site, there were ~18.4 cm (7.23 in) of rainfall (NOAA, 2011). Wind gusts were recorded in excess of 26 m s-1(58 MPH). Highest sustained winds were measured at 19.5 ms-1 (44 MPH) at 23:30 h on August 27, 2011 (Avila and Cangialosi, 2011; Figure 1A). Barometric pressure over the area reached a minimum of 976.2 mb at ~18:40 h on August 28, 2011 (Figure 1B). Tidal height did not deviate from the predicted normal height on the first day of sampling, so there was no hurricane-related tidal forcing at the first sampling time point.
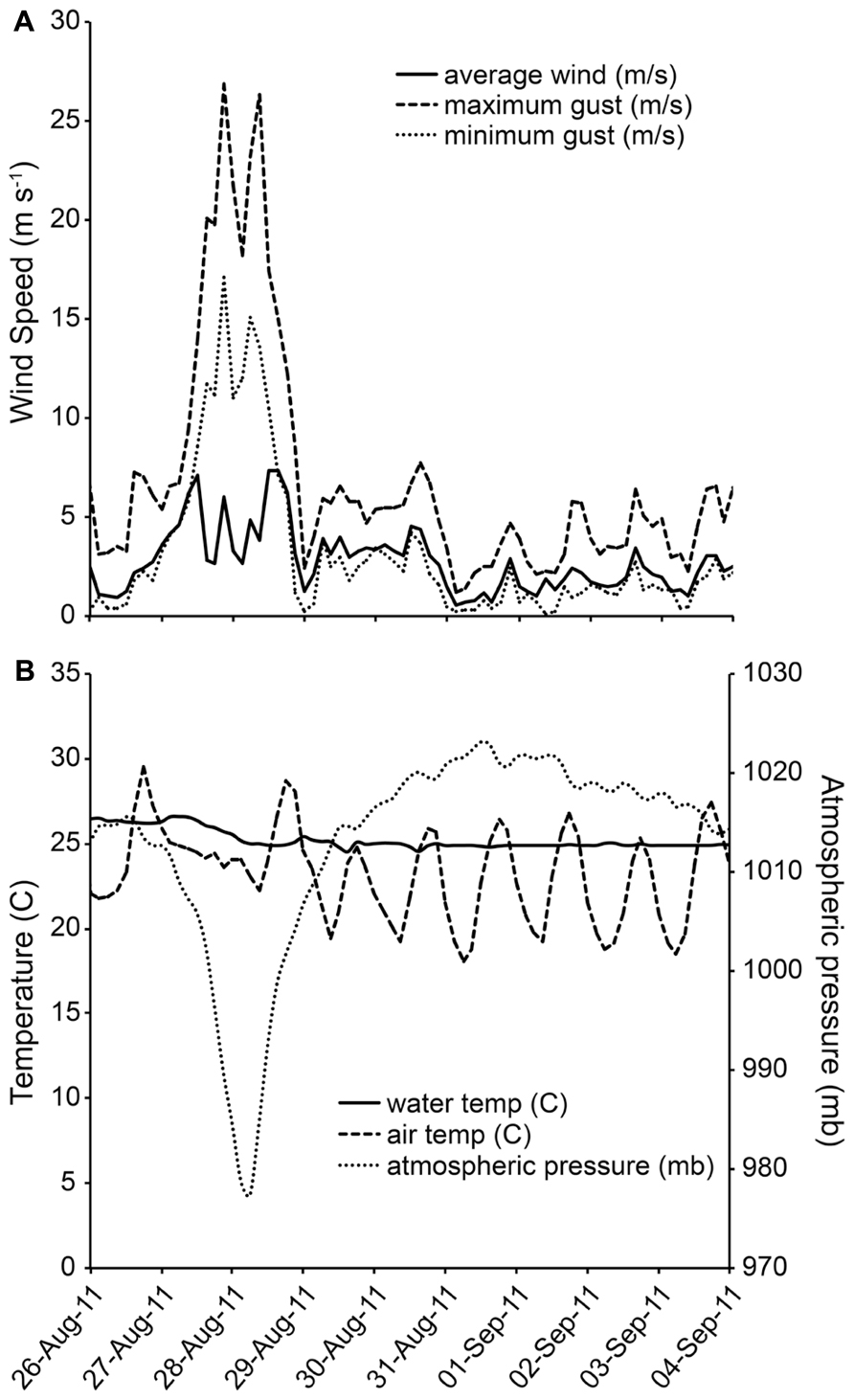
FIGURE 1. (A,B) Wind speed and direction at study site during Hurricane Irene Data from NOAA station CAMM2.
Physical and Chemical Conditions
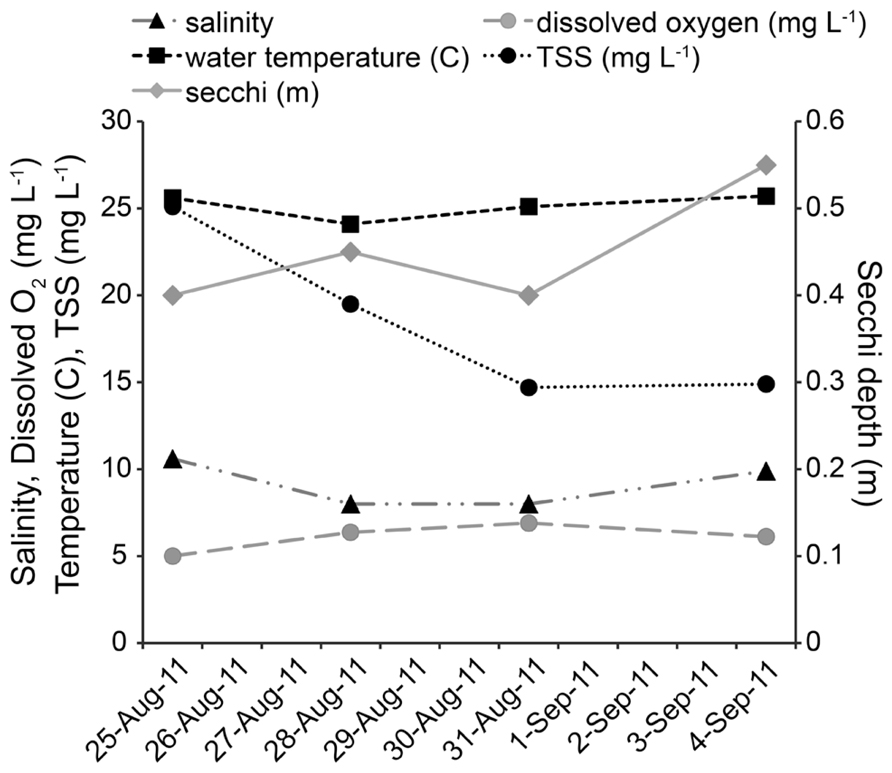
All physical and chemical measurements, whether taken at ~0.3 m below the surface or ~0.3 m from bottom, were found to be the same on each sampling date. As no water column stratification was detected, only one value per parameter is reported for each sampling date. Twenty-four hours after Hurricane Irene, salinity at the study site decreased from 10.6 to 8.0, and by day 8 returned to 9.9. Dissolved oxygen increased from 5.01 mg L-1 to 6.37 mg L-1 after the storm, and remained above 6 mg L-1. Water temperature decreased from 25.6°C to 24.1°C after the storm and by day 8 increased to 25.7°C. Secchi depth increased from 0.4 to 0.45 m on the day after the storm, returned to 0.4 m on day 4, and increased to 0.55 m on day 8 (Figure 2). TSS started at 25.1 mg L-1 and decreased over the course of the study to 19.5 mg L-1 (day 1), 14.7 mg L-1 (day 4), and 14.9 mg L-1 (day 8). Tidal height ranged from low tide during initial sampling efforts [pre-storm: 0.20 m above mean lower low water (MLLW), Day 1: 0.15 m above MLLW] to high tide (day 4: 0.38 m above MLLW; day 8: 0.55 m above MLLW). While changes in temperature, salinity, dissolved oxygen, secchi depth, and TSS were small, tidal height was significantly correlated with temperature (P = 0.001, r = 0.6251), TSS (P <0.001, r = -0.7512), and secchi depth (P <0.001, r = 0.6621).
Resuspension Calculations
Rates of erosion were calculated based on highest wind gusts (26.9 and 22.6 m s-1) and highest sustained wind speeds (9-9.8 m s-1). Most winds during the storm were moving in a north-northeast or northeast direction. Erosion rates were predicted to range from 2,343 to 3,616 g m-2 h-1 during periods of wind gusts and 487 to 730 g m-2 h-1 during highest sustained winds. Given the lowest wind speed (m s-1) during the height of the storm, the oyster farm would have expected an erosion rate of ~3 × 105 g sediment h-1.
Vibrio vulnificus
Oyster MPN
Vibrio vulnificus oyster MPN g-1 data were not normally distributed and Kruskal–Wallis non-parametric rank test determined no statistical difference in oyster V. vulnificus (MPN g-1) by location (top vs. bottom) or by date sampled. Spearman’s rank correlation analysis of oyster V. vulnificus MPN g-1 showed significant associations with TSS (P = 0.0455, r = 0.4119; Table 1).
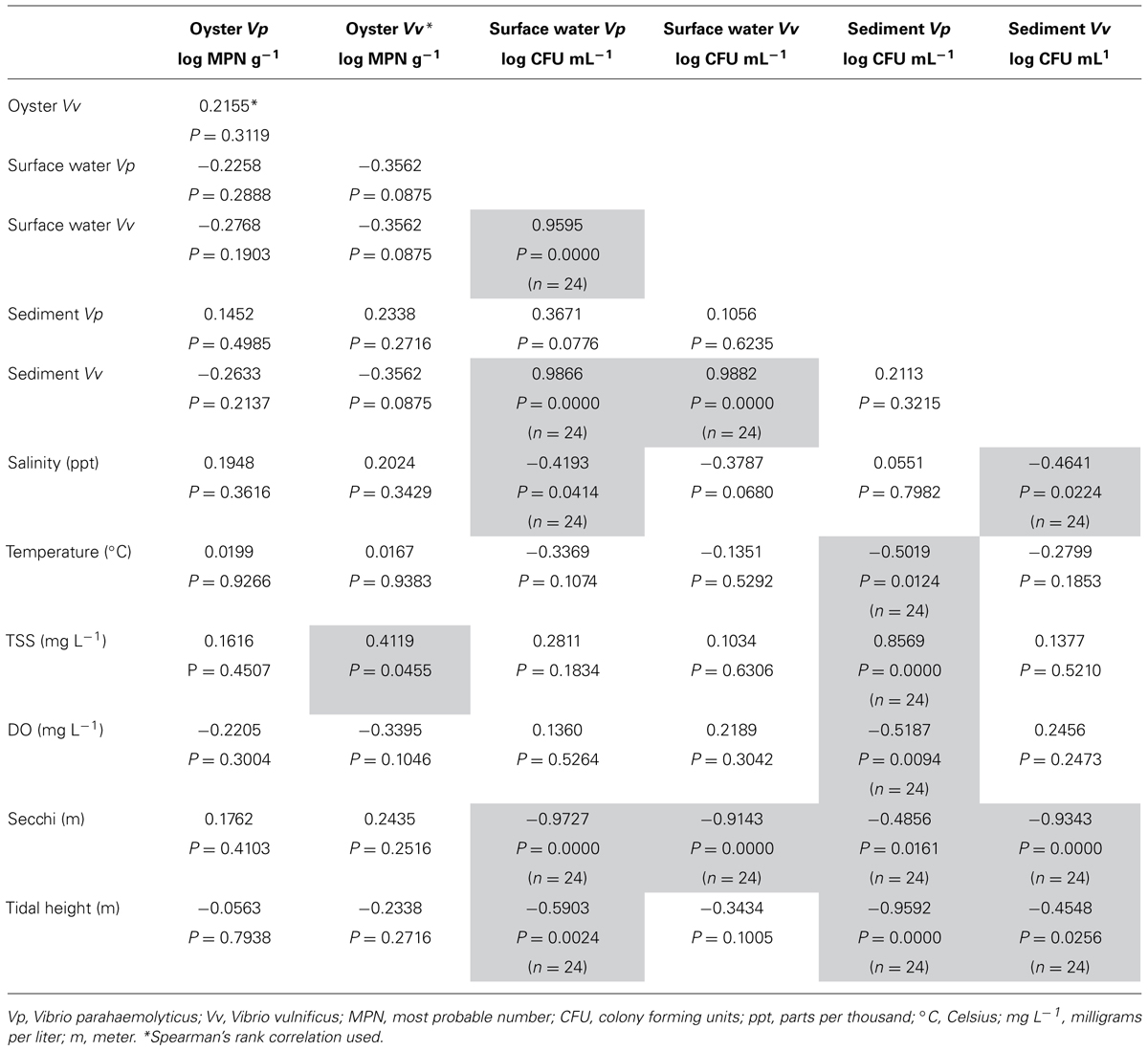
TABLE 1. Correlation table of environmental parameters and Vibrio concentrations in oysters, sediment and surface water.
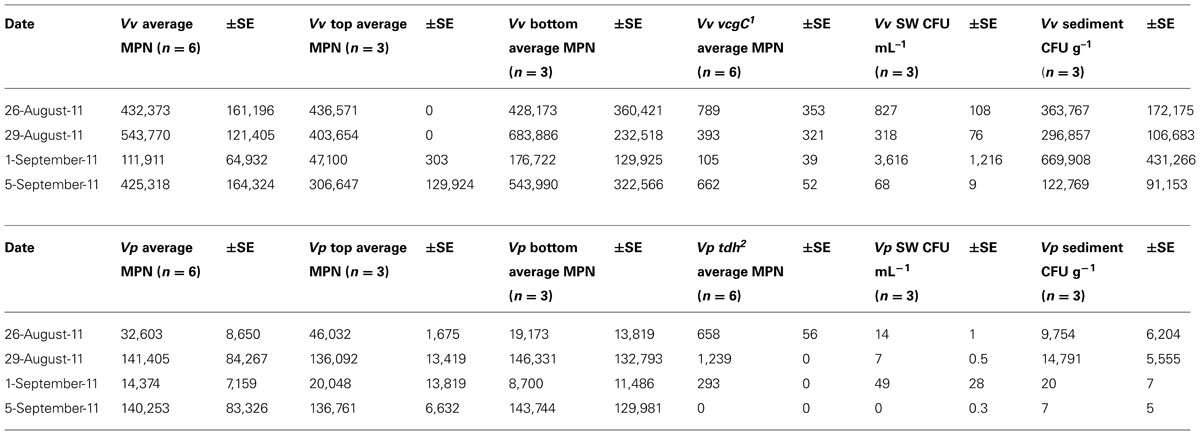
Although non-significant statistically, a small concentration increase in average V. vulnificus in oysters (MPN g-1) was detected between the first sampling pre-storm (August 26, 2011) and 1 day after the storm (August 29, 2011; Table 2). Average V. vulnificus decreased approximately between day 1 and day 4 post-storm, and then increased between day 4 and day 8. Despite these shifts, a very small change (1.6%) was measured in total V. vulnificus in oysters the entire study period.
Surface water and sediment
One-way ANOVA analysis of sediment and surface water CFU mL-1 determined no statistically significant difference between dates for either sediment or surface water. Pearson’s correlation analysis of sediment V. vulnificus revealed significant negative relationships with the environmental variables of salinity (P = 0.0224, r = -0.4641), secchi depth (P <0.0001, r = -0.9343) and tidal height (P = 0.0256, r = -0.4548). Correlation analysis of surface water V. vulnificus found significant associations with sediment V. vulnificus concentrations (P <0.0001, r = 0.9882) and secchi depth (P <0.0001, r = -0.8917; Table 1).
While concentration changes detected were non-significant, average V. vulnificus decreased in surface waters and sediment on day 1 post-storm, increased on day 4, and decreased again to the lowest of this study’s detected V. vulnificus concentrations for either substrate on day 8 (Table 2).
Vibrio vulnificus virulence correlated gene
The V. vulnificus vcgC was detected in oysters during each of the sampling dates, but concentrations were reduced during the day 1 and 4 sampling time points (393 and 105 MPN g-1, respectively) relative to concentrations pre-storm (789 MPN g-1) and on day 8 (622 MPN g-1; Table 2). The percentage V. vulnificus vcgC MPN g-1 of overall V. vulnificus MPN g-1 was appreciably the same on all sampled dates (0.2%). V. vulnificus vcgC was detected in both surface and bottom sampled oysters, but not in sediment or surface waters during this study.
Vibrio parahaemolyticus
Oyster MPN
Multivariate analysis of variance found no statistical difference between the sampling locations or sampling dates for V. parahaemolyticus MPN g-1values of oysters. Oyster V. parahaemolyticus MPN g-1 did not correlate significantly (Pearson’s correlation) with any of the environmental variables tested (Table 1).
While not significant statistically, concentration changes of average overall V. parahaemolyticus MPN g-1 increased 1 day post-storm from pre-storm concentrations and decreased 4 days post-storm, with a final increase on day 8 post-storm.
Surface water and sediment
One-way ANOVA analysis of difference among sampling dates for sediment and surface water CFU mL-1 showed no statistically significant difference between dates for either sediment or surface water. Correlation analysis of sediment V. parahaemolyticus CFU g-1 revealed significant associations with the environmental variables of temperature (P = 0.0124, r = -0.5019), TSS (P <0.0001, r = 0.8569), dissolved oxygen (P = 0.0094, r = -0.5187), secchi depth (P = 0.0161, r = -0.4856), and tidal height (P <0.0001, r = -0.9592). Correlation analysis of surface water V. parahaemolyticus CFU mL-1 found a significant negative relationship with salinity (P = 0.0414, r = -0.4193), secchi depth (P <0.0001, r = -0.9727), and tidal height (P = 0.0024, r = -0.5903). Conversely, a strong and statistically positive association was found between surface water V. parahaemolyticus and V. vulnificus CFU mL-1 (P <0.0001, r = 0.9595) and between surface water V. parahaemolyticus CFU mL-1 and sediment V. vulnificus CFU g-1 (P <0.0001, r = 0.9866; Table 1).
While not statistically significant, concentration changes of average V. parahaemolyticus were detected, with decreases in surface waters, but increases in sediment, 1 day after the storm. Surface water V. parahaemolyticus then increased on day 4 post-storm and decreased on day 8 post-storm. Conversely, sediment V. parahaemolyticus decreased on day 4 and decreased further on day 8 (Table 2).
Vibrio parahaemolyticus tdh/trh
The trh gene was not detected in any of the oyster MPN cultures, nor the sediment or surface water samples. The tdh gene was detected in oyster MPN cultures at all time points except on day 8. Two samples were positive for tdh during pre-storm sampling (average 658 MPN g-1), and three samples were positive post-storm (day 1, 1239 MPN g-1; day 8, 294 MPN g-1). Concentrations of tdh decreased over the sampling period, although overall percent V. parahaemolyticus tdh MPN g-1, when compared to total V. parahaemolyticus MPN g-1, was greatest at day 4 (2.9%). The percent of sampled oysters positive for tdh was lowest on day 8 [(2/6) = 33%].
Discussion
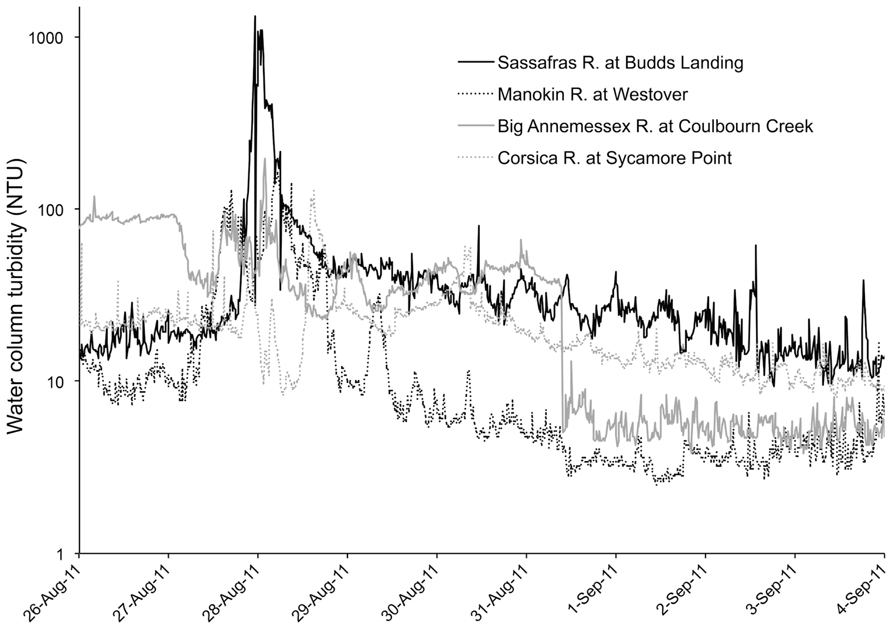
Hurricane Irene produced a significant wind event for the Chesapeake Bay region and wave action was sufficient to cause sediment resuspension at the studied aquaculture facility, according to estimates of erosion based on wind speed and direction. Additionally, there was a large amount of precipitation (18 cm) during the storm event. Although our data lacks a sampling time point during the storm, in situ continuous monitoring data archives of turbidity (accessed at Maryland Department of Natural Resources “Eyes on the Bay;”2 depict sharp spikes in nephelometric turbidity units (NTU) during the peak of the storm winds and a rapid subsequent decrease of NTU, most likely due to the large amount of rainfall experienced during the storm and a resultant flushing effect (Figure 3). This flushing effect may be the cause of reduced turbidity and lowered surface water CFU mL-1 for both Vibrio species 1 day after the storm.
In general, many concentrations of V. vulnificus and V. parahaemolyticus detected during this study were greater than those found in similar studies documenting the detection of these species in the same sampled matrices in the Chesapeake Bay. Maximum concentrations of Vibrio detected in previous studies of oyster tissue were considerably lower [V. parahaemolyticus: 6.0 × 102 CFU g-1 (Parveen et al., 2008), 1.0 × 104CFU g-1(Johnson et al., 2012); V. vulnificus: 1.2 × 104CFU g-1 (Johnson et al., 2012)] than the findings of this study (V. parahaemolyticus: 4.1 × 105 MPN g-1; V. vulnificus: 1.14 × 106MPN g-1). In addition, Johnson et al. (2012) detected lower surface water and sediment
V. vulnificus concentrations [surface water: 150 CFU mL-1 vs. 1.2 × 103 CFU mL-1(this study); sediment: 3.5 × 104 CFU g-1 vs. 3.6 × 105 MPN g-1(this study)], although V. parahaemolyticus concentrations found in Johnson et al. (2012) were approximately double the concentrations detected in this study [surface water: 60 CFU mL-1 vs. 17.5 CFU mL-1 (this study); sediment: 1.5 × 104 CFU g-1vs. 6.0 × 103 MPN g-1 (this study)]. The lower oyster MPN g-1 and surface water/sediment V. vulnificus values from previous studies may be due to a difference in sampling depth for oysters (i.e., natural oyster bar depth and open water versus near shore shallows) or a difference in recovery efficiencies of methodologies used in either study, such as under-detection (culture-based methods, previous studies) or detection of non-viable cells by qPCR (direct detection, this study) in sampled surface water and sediment matrixes.
While there was large variation in the average V. vulnificus and V. parahaemolyticus cell densities in oysters, surface water, and sediment, the values quantified in each of these substrates was not significantly different over the course of the study. There was a species difference in oyster tissue concentration immediately after the storm, with V. parahaemolyticus increasing substantially, but V. vulnificus increasing only slightly. A recent, similar study (i.e., sampling frequency, salinity, and temperature range) comparing oyster, sediment, and water concentrations of V. vulnificus and V. parahaemolyticus in the Gulf of Mexico reported comparable changes in oyster tissue Vibrio concentrations for both species over the course of the study (Givens et al., 2014). These findings contrast with the post-storm Vibrio concentration changes seen in this study, suggesting a species-specific dynamic post-storm during this study. Additionally, it has been shown that V. vulnificus outnumbers V. parahaemolyticus in sediment, oyster tissue and the water column (Johnson et al., 2010). During this study, V. parahaemolyticus cell g-1 was approximately 5% of the total V. vulnificus cell g-1 in sediment, which is consistent with the findings of Johnson et al. (2010). However, despite the relative dominance of V. vulnificus in sediments, post-storm increases in Vibrio were dominated by V. parahaemolyticus, suggesting species-specific variation during this study in the degree to which these bacteria were resuspended from sediments or were retained in oyster tissues, perhaps differing from V. vulnificus in properties of adhesion to marine aggregates, which may have been subsequently filtered by oysters.
Interestingly, on day 4 post-storm, oyster tissue Vibrio MPN g-1decreased precipitously from pre-storm concentrations (-74%, V. vulnificus; -56% V. parahaemolyticus), while surface water CFU mL-1 and sediment CFU g-1increased substantially (+337 and +84%, respectively; Table 2). On day 8, oyster tissue V. vulnificus concentrations returned to pre-storm concentrations (-1.6%), while V. parahaemolyticus MPN g-1 concentrations approximately quadrupled. Conversely, surface water and sediment concentrations decreased to a fraction of their original concentrations at day 8 post-storm (-92, -66% V. vulnificus, respectively; -100% for both sediment and surface water, V. parahaemolyticus). One possible explanation for these changes is a bacterial response to the flushing effect from the wind and rain at the study site, but more likely is storm-induced changes in oyster filtration rates over the course of this study. In Givens et al. (2014), changes in Vibrio concentration were seen to be approximately replicated in surface waters and oyster tissues, suggesting that the opposing patterns of oyster and water Vibrio concentration detected in the days following Hurricane Irene were atypical.
Oysters have been shown to reduce or halt filtration during periods of high suspended solids, recommencing filtration at a normal or increased rate when water clarity returns to ambient conditions (Loosanoff and Tommers, 1948). If filtration stalled during the height of the storm and then resumed after sediment resuspension ceased, it may have explained the concomitant decrease in oyster Vibrio concentrations by 5–10 times (Table 2), while surface water Vibrio concentrations increased by 7–11 times on the fourth day post-Hurricane Irene (Table 2; Figure 2). However, filtration rates were not directly measured in this study and other factors, such as population turnover and physical transport, cannot be excluded as potentially important mechanisms for changes in Vibrio concentrations. Similar to Fries et al. (2008), who noted an increase in sediment concentrations of total Vibrio when Hurricane Ophelia impacted the Neuse River Estuary, NC, USA; there was also an increase in the sediment concentrations of both Vibrio species during the first four days post-storm (Table 2). However, this pattern then reversed with an overall decrease in sediment CFU g-1(-100%, V. parahaemolyticus; -66%, V. vulnificus). Whether this was due to a change in oyster filtration or a difference in how each Vibrio species was introduced into the water column as a function of resuspension, and associated particle adhesion, remains to be understood. In contrast to other studies (Fries et al., 2008; Hsieh et al., 2008; Wetz et al., 2008; Johnson et al., 2010), surface water CFU mL-1 decreased following the storm (Table 2).
Notably, virulence-associated genes of V. vulnificus and V. parahaemolyticus were not detected in surface waters or sediment during the course of this study, possibly due to limitations of the direct extraction method (sediment, water) in relation to the MPN enrichment method (oyster samples). This is counter to other study findings, such as Johnson et al. (2010), which reported virulence-associated V. parahaemolyticus genes at similar frequencies in sediment, surface water and oysters. The V. vulnificus vcgC gene was found routinely in oyster tissues, but the percentage of V. vulnificus carrying vcgC was elevated at the beginning and end of the study (0.2%), and reduced one day after the storm and on day 4 (0.09%). Similarly, the percentage of V. parahaemolyticus carrying the tdh virulence-associated gene was elevated before the storm and on day 4 (2%) and reduced one day after the storm (0.7%). Incidence and concentration of virulent V. parahaemolyticus was at its lowest point at day 8 (0%). These findings are in contrast to previous, laboratory-based studies, examining the relationship between V. vulnificus’ virulence associated genes in oysters. These previous studies found no change in V. vulnificus virulence associated genes during the passage through the oyster (Groubert and Oliver, 1994; Staley et al., 2011). It is possible that the changes in virulence-associated genes percentages in this study are associated with population turnover within the oyster during the storm period.
Movement towards increased aquaculture production of oysters in the Chesapeake Bay, in combination with forecasted environmental responses to global climate change (e.g., warmer surface waters, increased frequency and/or intensity of storm events), may create a situation of higher Vibrio density in oysters, especially during the summer harvest season. An inventory of the last decade of tropical storms (2001–2011)3 in the Chesapeake Bay elucidates that at least one tropical storm or depression is routinely seen in the region each year, and at least one hurricane within each decade, with an anticipated increase in tropical weather influenced by climate change conditions. Further research is needed to determine if patterns of adherence to oyster tissues is different between V. parahaemolyticus and V. vulnificus, as well as among virulent subsets of each species. As the storm event in this study consisted of both high winds and large amounts of precipitation, it would be useful to examine storm events with a range of wind speeds and precipitation to account for the individual response variables of resuspension and surface water flushing. Additionally, the role of nutrient introduction from terrestrial sources and the impact of plankton dynamics on Vibrio populations should be investigated in future studies to elucidate the impact of either variable on Vibrio concentration in the measured substrates. Such information would help managers of shellfish harvest decide if there should be a cessation or modification (e.g., post-harvest treatment) of harvest post-storm, what winds or rainfall would be significant for a given aquaculture site, and how long that suspension or modification of harvest should be recommended.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Financial support for this research was graciously provided by National Oceanic and Atmospheric Administration award EA133C07CN0163.
We would like to thank Dr. Lawrence Harding and Mr. Steve Suttles (University of Maryland Center for Environmental Science, Horn Point Laboratory) for helpful conversations regarding the sampling site physical dynamics and how to appropriately calculate erosion rates for the site. We would also like to thank Dr. Jeffrey Cornwell and Mr. Michael Owens for information related to study site sediment properties and Dr. Jessica Jones (Food and Drug Administration, Gulf Coast Seafood Laboratory, Dauphin Island, AL, USA), Dr. Salina Parveen, Ms. Chanelle White (University of Maryland, Eastern Shore), and Mr. Matt Rhodes (NOAA Cooperative Oxford Laboratory) for assistance in determining appropriate microbiological, molecular, and quantitative techniques for Vibrio enumeration. We are appreciative of thoughtful edits provided by Ms. Carol McCollough (Maryland Department of Natural Resources). We are indebted to Dr. John Bowers (Food and Drug Administration, Center for Food Safety and Nutrition, College Park, MD, USA) for providing helpful assistance during statistical analyses of data. We thank Dr. Jessica Jones (Food and Drug Administration) and Dr. Carrie Givens (University of Georgia) for sharing data for comparison purposes. Lastly, we are extremely grateful to the aquaculture facility and its staff for all of their assistance and support.
Footnotes
- ^ http://www.fda.gov/Food/scienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm109656.htm
- ^ http://mddnr.chesapeakebay.net/eyesonthebay/index.cfm
- ^ www.weather.gov/lwx/tropical
References
Avila, L. A., and Cangialosi, J. (2011). “Tropical Cyclone Report: Hurricane Irene (AL092011), August 2011.” National Hurricane Center Summaries and Reports. Oxford, MD: NOAA.
Baker-Austin, C., Gore, A., Oliver, J. D., Rangdale, R., Mcarthur, J. V., and Lees, D. N. (2010). Rapid in situ detection of virulent Vibrio vulnificus strains in raw oyster matrices using real-time PCR. Environ. Microbiol. Rep. 2, 76–80. doi: 10.1111/j.1758-2229.2009.00092.x
Centers for Disease Control and Prevention [CDC]. (2005). Vibrio illnesses after Hurricane Katrina – multiple states, August–September 2005. MMWR Morb. Mortal. Wkly. Rep. 54, 928–931.
DePaola, A., and Kaysner, C. A. (2004). “Vibrio,” in Bacteriological Analytical Manual, Chap. 9 (Washington, DC: U.S. Food and Drug Administration).
Environmental Protection Agency [EPA]. (2011). The Great Waters Program: Introduction to the Issues and Ecosystesms, Chesapeake Bay. Available at: http://www.epa.gov/oaqps001/gr8water/xbrochure/chesapea.html [accessed February 10, 2013].
Food and Drug Administration [FDA]. (1998). R59: Phosphate-Buffered Saline (PBS), pH 7.4. Available at: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm062268.htm.
Fries, J. S., Characklis, G. W., and Noble, R. T. (2008). Sediment-water exchange of Vibrio sp. and fecal indicator bacteria: implications for persistence and transport in the Neuse River Estuary, North Carolina, USA. Water Res. 42, 941–950. doi: 10.1016/j.watres.2007.09.006
Froelich, B., and Oliver, J. D. (2013). The interactions of Vibrio vulnificus and the oyster Crassostrea virginica. Microb. Ecol. 65, 807–816. doi: 10.1007/s00248-012-0162-3
Givens, C. E., Bowers, J. C., DePaola, A., Hollibaugh, J. T., and Jones, J. L. (2014). Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus – potential roles for fish, oyster, sediment and water. Lett. Appl. Microbiol. doi: 10.1111/lam.12226 [Epub ahead of print].
Goldenberg, S. B., Landsea, C. W., Mestas-Nunez, A. M., and Gray, W. M. (2001). The recent increase in Atlantic hurricane activity: causes and implications. Science 293, 474–479. doi: 10.1126/science.1060040
Groubert, T. N., and Oliver, J. D. (1994). Interaction of Vibrio vulnificus and the eastern oyster, Crassostrea virginica. J. Food Prot. 57, 224–228.
Haven, D. S., and Morales-Alamo, R. (1972). “Biodeposition as a factor in sedimentation of fine suspended solids in estuaries,” in Geological Society of America Memoirs, Vol. 133, Environmental Framework of Coastal Plain Estuaries, ed. B. W. Nelson (Boulder, CO: Geological Society of America), 121–130.
Hsieh, J. L., Fries, S., and Noble, R. T. (2008). Dynamics and predictive modelling of Vibrio spp. in the Neuse River Estuary, North Carolina, USA. Environ. Microbiol. 10, 57–64. doi: 10.1111/j.1462-2920.2007.01429.x
Jacobs, J. M., Rhodes, M. R., Brown, C. W., Hood, R. R., Leight, A. K., Long, W., et al. (2010). Predicting the distribution of Vibrio vulnificus in Chesapeake Bay. NOAA Technical Memorandum NOS NCOOS 112. Oxford, MD: NOAA National Centers for Coastal Ocean Science, Center for Coastal Environmental Health and Biomolecular Research, Cooperative Oxford Laboratory.
Jacobs, J., Rhodes, M., Sturgis, B., and Wood, B. (2009). Influence of environmental gradients on the abundance and distribution of Mycobacterium spp. in a coastal lagoon estuary. Appl. Environ. Microbiol. 75, 7378–7384. doi: 10.1128/AEM.01900-09
Johnson, C. N., Bowers, J. C., Griffitt, K. J., Molina, V., Clostio, R. W., Pei, S., et al. (2012). Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington, United States. Appl. Environ. Microbiol. 78, 7249–7257. doi: 10.1128/AEM.01296-12
Johnson, C. N., Flowers, A. R., Noriea, N. F. III, Zimmerman, A. M., Bowers, J. C., DePaola, A., et al. (2010). Relationships between environmental factors and pathogenic Vibrios in the Northern Gulf of Mexico. Appl. Environ. Microbiol. 76, 7076–7084. doi: 10.1128/AEM.00697-10
Kaufman, G. E., Bej, A. K., Bowers, J., and DePaola, A. (2003). Oyster-to-oyster variability in levels of Vibrio parahaemolyticus. J. Food Prot. 66, 125–129.
Lloyd, K. G., Macgregor, B. J., and Teske, A. (2010). Quantitative PCR methods for RNA and DNA in marine sediments: maximizing yield while overcoming inhibition. FEMS Microbiol. Ecol. 72, 143–151. doi: 10.1111/j.1574-6941.2009.00827.x
Loosanoff, V. L., and Tommers, F. D. (1948). Effect of suspended silt and other substances on rate of feeding of oysters. Science 107, 69–70. doi: 10.1126/science.107.2768.69
Luckenbach, M. W., O’Beirn, F. X., and Taylor, J. (1999). An Introduction to Culturing Oysters in Virginia. Gloucester Point, VA: School of Marine Science, Virginia Institute of Marine Science, College of William & Mary.
Meehl, G. A., Stocker, T. F., Collins, W. D., Friedlingstein, P., Gaye, A. T., Gregory, J. M., et al. (2007). “Global climate projections,” in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, et al. (Cambridge: Cambridge University Press).
NOAA, N. W. S. (2011). Post Tropical Storm Report: Hurricane Irene [Online]. Available: http://www.erh.noaa.gov/akq/wx_events/hur/Irene/index.html [accessed February 2, 2013].
Nordstrom, J. L., Vickery, M. C., Blackstone, G. M., Murray, S. L., and DePaola, A. (2007). Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 73, 5840–5847. doi: 10.1128/AEM.00460-07
Panicker, G., and Bej, A. K. (2005). Real-time PCR detection of Vibrio vulnificus in oysters: comparison of oligonucleotide primers and probes targeting vvhA. Appl. Environ. Microbiol. 71, 5702–5709. doi: 10.1128/AEM.71.10.5702-5709.2005
Parveen, S., Hettiarachchi, K. A., Bowers, J. C., Jones, J. L., Tamplin, M. L., Mckay, R., et al. (2008). Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. Int. J. Food Microbiol. 128, 354–361. doi: 10.1016/j.ijfoodmicro.2008.09.019
Randa, M. A., Polz, M. F., and Lim, E. (2004). Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl. Environ. Microbiol. 70, 5469–5476. doi: 10.1128/AEM.70.9.5469-5476.2004
Sanford, L. P. (1994). Wave-forced resuspension of upper Chesapeake Bay muds. Estuaries 17, 148–165. doi: 10.2307/1352564
Shaw, K. S., Rosenberg Goldstein, R. E., He, X., Jacobs, J. M., Crump, B. C., and Sapkota, A. R. (2014). Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Chesapeake Bay and Maryland Coastal Bays. PLoS ONE 9:e89616. doi: 10.1371/journal.pone.0089616
Staley, C., Jones, M. K., Wright, A. C., and Harwood, V. J. (2011). Genetic and quantitative assessment of Vibrio vulnificus populations in oyster (Crassostrea virginica) tissues. Environ. Microbiol. Rep. 3, 543–549. doi: 10.1111/j.1758-2229.2011.00256.x
Warner, E., and Oliver J. D. (2008). Population structures of two genotypes of Vibrio vulnificus in oysters (Crassostrea virginica) and seawater. Appl. Environ. Microbiol. 74, 80–85. doi: 10.1128/AEM.01434-07
Wetz, J., Blackwood, A., Fries, J., Williams, Z., and Noble, R. (2008). Trends in total Vibrio spp. and Vibrio vulnificus concentrations in the eutrophic Neuse River Estuary, North Carolina, during storm events. Aquat. Microb. Ecol. 53, 141–149. doi: 10.3354/ame01223
Wright, A. C., Hill, R. T., Johnson, J. A., Roghman, M. C., Colwell, R. R., and Morris, J. G. (1996). Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62, 717–724.
Keywords: aquacultured oyster, Vibrio vulnificus, Vibrio parahaemolyticus, sediment resuspension, wind event, Chesapeake Bay, estuary, storm event
Citation: Shaw KS, Jacobs JM and Crump BC (2014) Impact of Hurricane Irene on Vibrio vulnificus and Vibrio parahaemolyticus concentrations in surface water, sediment and cultured oysters in the Chesapeake Bay, MD, USA. Front. Microbiol. 5:204. doi: 10.3389/fmicb.2014.00204
Received: 15 November 2013; Paper pending published: 16 December 2013;
Accepted: 17 April 2014; Published online: 07 May 2014.
Edited by:
Daniela Ceccarelli, University of Maryland, USAReviewed by:
Bradd Haley, Environmental Microbial and Food Safety Laboratory – United States Department of Agriculture, USACrystal N. Johnson, Louisiana State University, USA
Copyright © 2014 Shaw, Jacobs and Crump. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristi S. Shaw, Horn Point Laboratory, Center for Environmental Science, University of Maryland, P.O. Box 775, Cambridge, MD 21613, USA e-mail:a3Jpc3Rpc3RldmVuc3NoYXdAZ21haWwuY29t
†Present address: Byron C. Crump, College of Earth, Ocean, and Atmospheric Science, Oregon State University, Corvallis, OR, USA
 Kristi S. Shaw
Kristi S. Shaw John M. Jacobs
John M. Jacobs Byron C. Crump
Byron C. Crump