
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 02 December 2013
Sec. Extreme Microbiology
Volume 4 - 2013 | https://doi.org/10.3389/fmicb.2013.00361
 Yoko Ohtomo1†
Yoko Ohtomo1† Akira Ijiri1,2†
Akira Ijiri1,2† Yojiro Ikegawa2,3
Yojiro Ikegawa2,3 Masazumi Tsutsumi1
Masazumi Tsutsumi1 Hiroyuki Imachi2,4
Hiroyuki Imachi2,4 Go-Ichiro Uramoto1,2
Go-Ichiro Uramoto1,2 Tatsuhiko Hoshino1,2
Tatsuhiko Hoshino1,2 Yuki Morono1,2
Yuki Morono1,2 Sanae Sakai4
Sanae Sakai4 Yumi Saito4
Yumi Saito4 Wataru Tanikawa1,2
Wataru Tanikawa1,2 Takehiro Hirose1,2
Takehiro Hirose1,2 Fumio Inagaki1,2*
Fumio Inagaki1,2*Geological CO2 sequestration in unmineable subsurface oil/gas fields and coal formations has been proposed as a means of reducing anthropogenic greenhouse gasses in the atmosphere. However, the feasibility of injecting CO2 into subsurface depends upon a variety of geological and economic conditions, and the ecological consequences are largely unpredictable. In this study, we developed a new flow-through-type reactor system to examine potential geophysical, geochemical and microbiological impacts associated with CO2 injection by simulating in-situ pressure (0–100 MPa) and temperature (0–70°C) conditions. Using the reactor system, anaerobic artificial fluid and CO2 (flow rate: 0.002 and 0.00001 ml/min, respectively) were continuously supplemented into a column comprised of bituminous coal and sand under a pore pressure of 40 MPa (confined pressure: 41 MPa) at 40°C for 56 days. 16S rRNA gene analysis of the bacterial components showed distinct spatial separation of the predominant taxa in the coal and sand over the course of the experiment. Cultivation experiments using sub-sampled fluids revealed that some microbes survived, or were metabolically active, under CO2-rich conditions. However, no methanogens were activated during the experiment, even though hydrogenotrophic and methylotrophic methanogens were obtained from conventional batch-type cultivation at 20°C. During the reactor experiment, the acetate and methanol concentration in the fluids increased while the δ13Cacetate, H2 and CO2 concentrations decreased, indicating the occurrence of homo-acetogenesis. 16S rRNA genes of homo-acetogenic spore-forming bacteria related to the genus Sporomusa were consistently detected from the sandstone after the reactor experiment. Our results suggest that the injection of CO2 into a natural coal-sand formation preferentially stimulates homo-acetogenesis rather than methanogenesis, and that this process is accompanied by biogenic CO2 conversion to acetate.
In addition to causing dramatic increases in the surface temperature of the Earth, the release of anthropogenic greenhouse gasses into the atmosphere is considered to have had a major effect on changes to the oceans and climate (e.g., sea-level changes, ocean acidification) (Crowley, 2000; Alley et al., 2003; Karl and Trenberth, 2003). A variety of potential methods have been proposed to reduce anthropogenic CO2 emissions. Of the methods proposed to date, CO2 capture and storage followed by geologic CO2 sequestration (GCS) have been proposed as effective means to prevent the potentially dangerous consequences of climate change (White et al., 2003; Anderson and Newell, 2004; IPCC Special Reports, 2005; Davison, 2007; Zakkour and Haines, 2007; Benson and Cole, 2008; Kirk, 2011). As a result, a number of potential CO2 storage sites have been identified in a wide variety of geological settings, and feasibility assessments have been undertaken by some corporations and national governments (e.g., European Union, United States and Australia), which leads to draft a variety of legal instruments to accommodate commercial interest in GCS projects (White et al., 2004; Zakkour and Haines, 2007). One such example is the Utsira sandstone formation, an aquifer 800 m beneath the North Sea, into which 1 Mt of CO2 has been injected per year by Statoil since 1996 (Eiken et al., 2000).
Preventing CO2 leaking from geologic formations is an essential component of minimizing maintenance costs and avoiding the extensive environmental damage that would result from a large-scale leak. Some studies have examined admissible quantities of CO2 leakage and operational costs, as well as the efficiencies of impermeable cap-rock layers and sealing mechanisms (Anderson and Newell, 2004; Davison, 2007; Van der Zwaan and Gerlagh, 2009; Song and Zhang, 2013). To prevent CO2 leakage and reduce costs, the offshore subsurface has been considered as potential realm of GCS. In the deep subseafloor environment, CO2 can exist as a liquid/supercritical phase or it can dissolve into ambient seawater under conditions of high pressure and low temperature, facilitating migration into subsurface geologic formations. Since CO2 dissolved in seawater and supercritical CO2 are less dense than ambient seawater, they are buoyant after sequestration, whereas liquid CO2 has a higher density than ambient seawater, causing it to sink (House et al., 2006; Benson and Cole, 2008). However, at water depths of <3000 m and a few hundred meters of sediment, the generation of CO2 hydrate disturbs the seepage of high-density liquid CO2 through deep-sea sediments (House et al., 2006), suggesting superiority of deep-sea subsurface environment as a GCS site.
At present, the major techniques employed for trapping CO2 are capillary trapping, solubility trapping and mineral trapping (Mitchell et al., 2010; Jun et al., 2013). All of these mechanisms require empty space inside geological formations for storing gaseous/liquid CO2 or precipitated carbonates. Sandstones are considered to be well suited for GCS because of their high porosity and their ubiquity (Bachu, 2003). Hydrocarbon reservoirs (e.g., unmineable subsurface oil/gas fields and coal beds) have also been considered for use as potential geologic CO2 repositories. Recovery of coal-bed CH4 (CBM) associated with hydrocarbon reservoirs can be facilitated by CO2 injection, which potentially contributes to decreasing the energy costs associated with such a venture (Gunter et al., 1997; White et al., 2005).
The geophysical, geochemical and ecological impacts of CO2 sequestration in the natural subsurface environment have largely remained unknown. The injection of CO2 can promote the precipitation of carbonates through a reaction between the CO2 and surrounding rocks (Oelkers et al., 2008; Rosenbauer et al., 2012), probably decreasing rock porosity/permeability and resulting in secondary cap-rock generation (Kharaka et al., 2006), however, compared to capillary trapping or solubility trapping, carbonate precipitation occurs too slowly for it to be considered as an effective means of capturing CO2 (Gilfillan et al., 2009).
On the other hand, the changes in the chemical environment associated with GCS by CO2 injection can have a marked impact on the microbial consortia within sediments. Supercritical CO2 or water containing large amounts of dissolved CO2 kills microorganisms by disrupting their cell membranes (Bertoloni et al., 2006; Wu et al., 2010), whereas the maximum limits of microbial CO2 tolerance are still poorly understood. Numerous studies have confirmed that methanogens are capable of growth in aqueous media containing dissolved CO2 concentrations that are considerably higher than those of natural conditions (Yakimov et al., 2002; Videmsek et al., 2009; Oppermann et al., 2010). The microbiological and geochemical characteristics of the deep-sea CO2 seep site at the Yonaguni Knoll IV hydrothermal system, which is characterized by CO2 seepage, suggest that habitat segregation of anaerobic bacteria and methanogens occurs in response to differences in CO2 concentrations and associated chemical conditions in marine sediments (Inagaki et al., 2006; Konno et al., 2006; Yanagawa et al., 2013). It is also expected that some microbes would be activated by CO2 injection under subsurface conditions, because CO2 is an important carbon source for autotrophic and mixotrophic microorganisms Previous studies on the activation of microbes associated with Fe3+, SO2−4 reduction and methanogenesis by CO2 injection suggest that microbial CO2 conversion to available carbon species lead to novel sustainable CO2 recycling system (Kirk, 2011; Mayumi et al., 2013).
Although laboratory-based GCS experiments could potentially clarify the impacts of CO2 injection on geologic formations, such studies have not yet been attempted. On the other hand, high-pressure incubators have been developed to culture microbes under in-situ conditions (Zobell and Oppenheimer, 1950; Yayanos et al., 1979; Orcutt et al., 2008; Sauer et al., 2012) and to simulate subsurface hydrothermal alteration of basaltic rocks (Seyfried and Janecky, 1985). In an attempt to simulate GCS conditions, a high-pressure flow-through reactor system was developed at the Kochi Institute for Core Sample Research, Japan Agency for Marine-Earth Science and Technology (JAMSTEC) in Kochi, Japan, by referring to previous studies on high-pressure instruments.
In this study, we investigated changes in the geophysical features, constituent minerals and microbial community structures in a column comprised of bituminous coal and sand before and after CO2 injection under simulating in-situ subsurface conditions and discussed various impacts of CO2 injection to geologic formations. CO2 was supplemented with anaerobic artificial fluids into a coal-sand column, which were sub-sampled after passing through the column. Concentrations and carbon isotope compositions of dissolved gases and volatile organic carbons in the sub-sampled fluids were determined to monitor CO2 impact during experiment. Sub-sampled fluids were also incubated to determine microbes survived through a CO2 injection experiment. Conventional batch-type cultivation was performed using same bituminous coal sample to investigate the potential for biological carbon conversion in sample pre-coal.
Fresh bituminous coal and associated sandstone were obtained from a subterranean coal mine and used as an analog of a subsurface coal-sand formation. As mentioned above, coal and sandstone are both considered to be well suited for use as CO2 repositories. In addition to the recovery of CBM by CO2 injection, immature hydrocarbon reservoirs (e.g., oil, bituminous coal and lignite) contain a variety of organic molecules and gases (e.g., H2 and CO2) generated during maturation of carbonaceous compounds in the hydrocarbon reservoirs accompanied by sedimentation. These compounds, in turn, are utilized by a variety of microorganisms in the nutrient-limited subsurface sediments. Consequently, development of microbial communities that consist of various bacteria, fungi and methanogenic archaea has been reported in these hydrocarbon reservoirs (Fakoussa, 1988, 1990; Edwards and Grbic-Galic, 1994; Nazina et al., 1995; Krüger et al., 2008; Strapoć et al., 2008), suggesting the possibility of biological CO2 conversion system (Bio-CCS) that responds to the GCS.
The geobio-reactor system consists of four flow-through, high-pressure vessels. The temperature and pressure of these vessels can be independently controlled up to 70°C and 100 MPa using controller (Teledyne Iso, D-series Pump Controller, Nebraska, USA) (Figures 1A, 2A,B). By connecting the four vessels either directly and/or in parallel, the movement of CO2 in the subsurface environment, and the associated mineralogical, geochemical and biological reactions that the CO2 may be involved in, can be experimentally simulated in the geobio-reactor system. The CO2 and/or fluid (e.g., artificial seawater) are supplemented through a mixer into the high-pressure reactor vessels. The pressure conditions and flow rate of the fluid in each vessel (i.e., confined pressure, pore pressure) are regulated by four cylinder pumps (Teledyne Isco, Nebraska, USA, 65D; 68 ml, Figure 1B). CO2 and fluid are first introduced into the upper cylinder pumps (pumps a and b, respectively, Figure 1B). The CO2 lines from the CO2 tank to the cylinder pump are cooled to 5°C by a condenser (F33, Julabo, Baden-Württemberg, Germany) to stabilize liquid CO2 phase in the lines (Figures 1B, 2C). The CO2 and fluid are then pressurized at the same level and injected into the vessel through a mixer by the cylinder pumps. After passing through the sediment column in the vessel, the CO2-injected fluid is stored in the lower pump (pump c; Figures 1B, 2D,E). During reactor operation, the CO2-injected fluid can be sub-sampled from a stainless-steel pressure-keeping cell connected to an outlet line from pump C. All of the parameters are displayed and monitored on computers in-situ (Figure 2A). All lines are made from Inconel (Special Metals, West Virginia, USA) and separated using several valves at appropriate positions. Waste solution is cleared from lines using a vacuum pump attached to a water trap (Figures 1A, 2C).
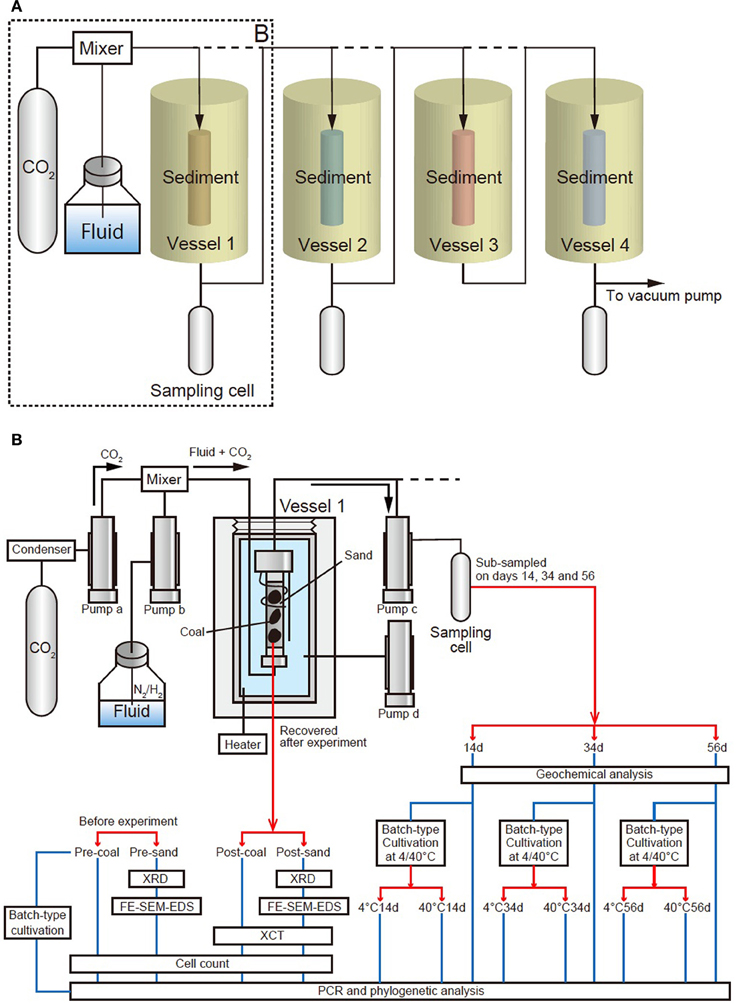
Figure 1. Schematic diagram of the geobio-reactor system. The system simulates in-situ subsurface pressure and temperature conditions ranging from 0 to 100 MPa and 0–70°C. (A) A schematic view of entire the reactor system, comprising four vessels and three sampling cells. CO2 and fluid are supplemented into a maximum of four vessels through a mixer. (B) Detail of the area dashed box (‘B’) in Figure 1A. Vessel 1 is connected to two upper cylinder pumps which supply CO2 and fluid (CO2: pump a, fluid: pump b). Another pump below the vessel (pump c) receives CO2-injected fluid that has passed through the sediment. Confined pressure is controlled by a cylinder pump d associated with the vessel filled with water (pressure medium), which is disconnected from the main line. CO2-injected fluids in pump c were sub-sampled by pressure-keeping sampling cell on days 14, 34, and 56 (Samples 14d, 34d, and 56d, respectively). These fluid samples were injected into tube filled with medium and incubated for 2 weeks at 4 and 40°C (4°C: 4°C14d, 4°C34d and 4°C56d; 40°C: 40°C14d, 40°C34d and 40°C56d). The coal-sand column was recovered and analyzed to determine geophysical/mineralogical characteristics and cell number. Coal and sand before experiment (Sample pre-coal and pre-sand) were also analyzed for comparison in the same way as sample post-coal and post-sand. Batch-type cultivation was performed using pre-coal. PCR and phylogenetic analysis were performed on all samples.
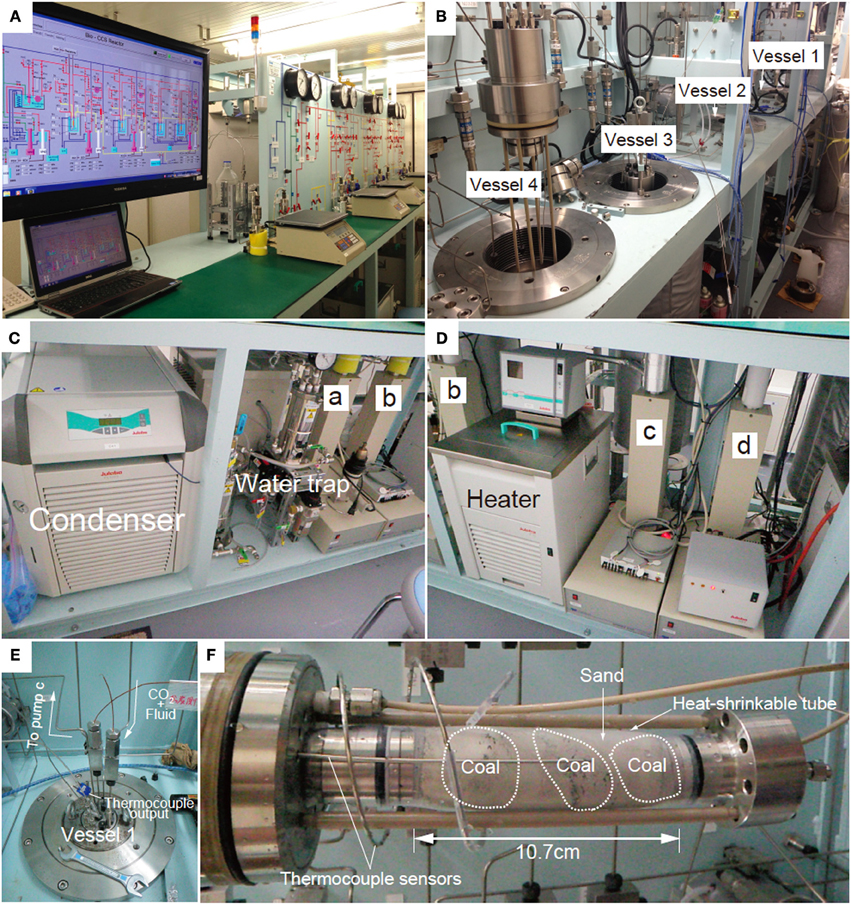
Figure 2. Photographs of the geobio-reactor system. (A) Photo of the entire system, (B) Photo of the four vessels, (C) Cylinder pumps for CO2 and fluid supply (pumps a and b). CO2 passes through a chiller which keeps the CO2 in the liquid phase until it reaches the pump a. (D) Cylinder pumps for sub-sampling and controlling confined pressure of the vessel (pumps c and d). (E) Photo of the top of the vessel. CO2 and fluid are supplemented through the upper line into the vessel. Fluid passing through the sediments is stored in pump c. (F) Coal-sand column unit used for CO2 injection experiment. Entire length of the column is 10.7 cm and the volume is ca. 81 cm3.
Each reaction vessel is constructed from stainless steel. The vessel is filled with a pressure medium (e.g., water) and sediment in the form of a column is inserted from the top. The vessel is then sealed tightly using a thick, threaded stainless-steel lid before conducting each experiment. When conducting an experiment, Mixture fluid of liquid CO2 and water is supplemented into the sediment column through polyetheretherketone (PEEK) lines from the bottom part of the column (Figure 1B). Confined pressure is controlled by the cylinder pump (pump d) (Figures 1B, 2D). The pressure medium is circulated through a heater (Julabo, Baden-Württemberg, Germany, HE; up to 70°C) and the vessel to stabilize the temperature in the vessel (Figure 2D). The in-situ temperature is monitored by a thermocouple sensor attached to stoppers of the column (Figures 1B, 2E,F).
CO2 injection experiment was performed using only vessel 1 of geobio-reactor system (Figure 1B). All lines of the geobio-reactor were vacuumed before experiment. CO2 and fluid were respectively introduced into the upper cylinder pumps (CO2: pump a, fluid: pump b). Pore and confined pressure were raised while maintaining the confined pressure higher than pore pressure to prevent fluid leakage from a column. Temperature, pore pressure and confined pressure were set to 40°C, 40 and 41 MPa, respectively. The flow rate of the fluid was 0.002 ml/min. CO2 was supplemented into lines from day 14 at 0.00001 ml/min (0.5 vol% vs. fluid) after sampling on day 14. The CO2 injection rate was sufficiently lower than the CO2 solubility [ca. 8.2 vol%; National Institute of Standards and Technology (NIST); (Duan and Sun, 2003)] to keep the microbes alive in the coal-sand column due to a marked decrease in the pH and destruction of cell structures associated with abundant CO2. The experiment was continued for 56 days and total amount of supplied CO2 into the coal-sandstone column was 0.81 ml.
The fluids passed through the column were sub-sampled on days 14, 34, and 56 (Sample 14d, 34d, and 56d; Figure 1B) by pressure-keeping sampling cell through pump c (Figure 1B). Dissolved gases and volatile organic carbons in the sub-sampled fluids were analyzed using Gas Chromatography-Isotope Ratio Mass spectrometry (GC-IRMS) and Isotope-Ratio-Monitoring Liquid Chromatography Mass Spectrometry (IRM-LCMS). The samples were also added into sealed test tubes filled by mediums under anaerobic conditions and incubated at 4 and 40°C, respectively. Samples inoculated from a sub-sampled fluid on day 14 and cultivated at 4 and 40°C were named as 4°C14d and 40°C14d. Samples inoculated from a sub-sampled fluid on day 34 and cultivated at 4 and 40°C were named as 4°C34d and 40°C34d. Samples inoculated from a sub-sampled fluid on day 56 and cultivated at 4 and 40°C were named as 4°C56d and 40°C56d (Figure 1B). PCR and phylogenetic analysis was also performed for these fluid samples.
After experiment, coal-sand column was recovered from vessel 1 (Sample post-coal and post-sand, Figure 1B) and analyzed to determine changes of geophysical and mineralogical characteristics using micro X-ray computed tomography (μ XCT scan), X-ray diffraction analysis (XRD), field emission scanning electron microscopy/energy-dispersive X-ray spectrometry (FE-SEM-EDS). Coal and sand were clearly separated based on grain size. Samples post-coal and post-sand were divided into three parts under anaerobic condition for geophysical/mineralogical analysis, cell counts and PCR/phylogenetic analysis. Samples for geophysical/mineralogical analysis were preserved at ambient temperature under aerobic condition. Samples for cell counts were suspended in paraformaldehyde for cell fixation, washed using phosphate buffered saline (PBS) and preserved at −20°C after addition of ethanol (99%). Samples for PCR/phylogenetic analysis were refrigerated at −80°C. Coal and sand before CO2 experiment (Sample pre-coal and pre-sand; Figure 1B) were also analyzed for comparison. Conventional batch-type cultivation was also performed using sample pre-coal. All sample names and performed analyses were summarized in Figure 1B. Construction of coal-sand column, composition of artificial fluid in CO2 injection experiment, detail of analyses and batch-type cultivations are as follows.
Eocene bituminous coal and sandstone were collected from exposed outcrops in pits 185 m below the ground surface in the Kushiro Coal Mine, Kushiro, Hokkaido on May 31, 2011. The coal layers belong to the Harudori Coal Formation, part of the Urahoro Group comprising a coal bearing formation, sandstone and black shale deposited in the Paleogene period (Hyakkoku, 1966). Collected coal was preserved in an anaerobic bag at 4°C and sandstone was preserved under aerobic conditions at 4°C.
Columned sediment was prepared from chips of Kushiro coal (1–3 cm in diameter) and the coal-bearing sandstone. These samples were enveloped by cylindrical, water-impermeable, heat-shrinkable tube under anaerobic condition as a column (7.5 × 10.7 cm3 in volume; Figure 2F). Both ends of the heat-shrinkable tube were sealed using a heat gun to prevent infiltration of the pressure medium from outside.
Since the pore water in the coal layer at the Kushiro Coal Mine was almost fresh (Electric conductivity: 1 mS/cm), we determined the composition of the anaerobic artificial fluid by referring to the nutrient medium used for methanogens in fresh anaerobic water (Sakai et al., 2012); specifically, we dissolved the following components into 1 L of Milli Q water (Merck Millipore, Massachusetts, USA): 0.14 g KH2PO4, 0.54 g NH4Cl, 0.24 g MgSO4· l,2O, 0.13 g CaCl2, 0.05 g NaCl, 2 g BD BBL™ yeast extract (BD, New Jersey, USA), 2.5 g NaHCO3, 10 ml methanol (50% v/v), 0.01 g Na-acetate, 0.2 g Na-formate, 0.001 g resazurin, 15 ml vitamin solution (4.9 mg biotin, 8.8 mg folic acid, 4.1 mg pyridoxine· HCl, 6.7 mg thiamine· HCl, 7.5 mg riboflavin, 2.4 mg nicotinic acid, 9.5 mg D-pantothenate (Calcium salt), 0.1 mg vitamin B12, 2.7 mg p-aminobenzoic acid and 4.1 mg lipoic acid per litre of Milli Q H2O) and 1 ml trace element solution (1.27 g FeCl2, 0.198 g MnCl2·4H2O, 0.136 g ZnCl2, 0.0062 g H3BO3, 0.025 g NiCl2·6H2O, 0.0133 g AlCl3, 0.001 g NaMoO4· 2H2O, 0.0017 g NaSeO3, 0.0003 g NaWO4·2H2O and 0.0013 g CuCl2 per litre of Milli Q H2O). Resazurin was added to the fluids as a redox indicator to monitor anaerobic conditions in the artificial fluid. The compositions of the vitamin and trace element solutions were a slight modification of Widdel's medium (Widdel, 1986). The pH of the fluid was adjusted to 7. Na2S (5 wt%, pH adjusted to 7) was added to the artificial fluid to maintain anaerobic conditions. After anaerobic treatment, the gas phase of the bottled fluid was replaced with N2 and the bottles were sealed tightly with butyl rubber stoppers and screw caps. The headspace of the bottle was filled with N2/H2 (80/20 [v/v]) before removed into geobio-reactor-system.
Samples post-coal and post-sand (ca. 1 cm in diameter) were roughly crushed and scanned by μ XCT (HMX225 Microfocus X-ray CT scanner, TESCO Corporation, Kanagawa, Japan) into ~1 mm3-size to estimate the porosity of the coal and sand after the experiment. The porosities of sample pre-sand and pre-coal could not be measured due to their inherently brittle properties. Samples pre-sand and post-sand were finely powdered and analyzed by XRD (XRD Phillips X'pert PRO, PANalycal, Almelo, Netherlands) to determine constituent minerals. Detailed chemical changes of constituent minerals were observed by FE-SEM-EDS (JEOL, JSM-6500F, Tokyo, Japan). Samples for FE-SEM-EDS observation were crushed into course powder and fixed on carbon tape of the sample stage.
Cells were separated from suspensions of samples pre-coal, pre-sand, post-coal and post-sand by heavy-solution separation (Nycodenz 30, 50, 80% [w/v] [1.159, 1.265 and 1.426 g/ml] and Na6H2W12O40· H2O 65% [w/w]) and concentrated to accumulate sufficient cells for counting, respectively (Morono et al., 2013). Concentrated cells were filtrated through Isopore™ membrane filters (0.2 μm GTBP, Merck Millipore, Massachusetts, USA) and stained with a fluorescent dye (SYBR Green, Takara Bio, Kyoto, Japan). Cells on membrane filters were then counted by observation under a fluorescence microscope (Olympus BX51Wl, Olympus, Tokyo, Japan) (Morono et al., 2009).
Sub-sampled fluids (Samples 14d, 34d, and 56d) were recovered and preserved in sealed tubes under anaerobic condition. The presence of microbes in these samples was confirmed by observation under an optical microscope (Olympus BX51Wl, Olympus, Tokyo, Japan). Then, 5 ml medium was prepared in 15 ml tubes and inoculated with 1 ml samples 14d, 34d, and 56d under anaerobic conditions, respectively. Composition of the medium was the same as artificial fluids used in CO2 injection experiment. After inoculation, the tubes were incubated at 4 and 40°C for 2 weeks under an atmosphere of N2/CO2 (80/20 [v/v]).
DNA extraction was performed using samples of pre-coal, pre-sand, post-coal and post-sand. DNA was manually extracted from approximately 10 g of the ground samples using a hot alkaline extraction method (Morono et al., in preparation). Briefly, the sample was pre-treated with alkaline lysis solution consisting 1M NaOH, 5 mM EDTA (pH 8.0), and 1% SDS at 70°C for 20 min, and then DNA was recovered by phonol:chloroform:isoamyl alcohol (25:24:1) and chloroform:isoamyl alcohol (24:1). The extracted DNA was then further purified and concentrated by NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Düren, Germany) followed by ethanol precipitation.
PCR amplification of bacterial and archaeal 16S rRNA genes was performed using SYBR premix ExTaq (Takara Bio, Kyoto, Japan) with a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA) after determining the optimum number of PCR cycles using multiple primer sets (Table 1). Of the primers tested, only 27F/338R, 341F/926R and 806F/958R could amplify the 16S rRNA gene with an annealing temperature of 56°C. The number of PCR cycles was 32 and 42 cycles for bacteria and archaea, respectively. The PCR products were amplified with the primer sets 341F/926R and 806F/958R and purified using a NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Düren, Germany), and the purified products were cloned into pCR2.1 TOPO vector (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instruction. The recombinant vector was transformed into Ecos competent Escherichia coli DH5α cells (Nippon Gene, Tokyo, Japan). The sequences of the inserts were determined using an ABI3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) and the obtained 16S rRNA gene sequences were classified using Mothur Utility package (Schloss et al., 2009). The 16S rRNA gene sequences reported in this study were deposited in the GenBank/EMBL/DDBJ database under accession numbers AB40305 to AB40587. In this study, samples pre14d, pre34d and pre56d in the GenBank/EMBL/DDBJ database were renamed as 14d, 34d and 56d, respectively.
Detection of the methyl co-enzyme M reductase (mcrA) gene was attempted using two primer sets (Table 1). The PCR consisted of 50 cycles and an annealing temperature of 54°C. In order to increase the detection sensitivity, multiple displacement amplification (MDA) was performed using Phi29 DNA polymerase (New England Biolabs, Beverly, MA, USA) according to the manufacturer's instructions. The amplification was performed at 30°C for 29 h. MDA products were purified using an Amicon Ultra-0.5 ml 30 K filter unit (Millipore, Bedford, MA, USA). Subsequently, the mcrA gene in the MDA product was amplified by PCR as described above.
To cultivate methanogens from sample pre-coal, batch-type cultivation was performed in 500 ml bottles containing 300 ml media under an atmosphere of N2/CO2 (80/20 [v/v]) without shaking (Figure 7). The basal medium was the same medium used for the geobio-reactor system without organic compounds and contained the following energy sources: (i) approximately 100 kPa H2 (in the head space) and 20 mM formate to obtain hydrogenotrophic methanogens; (ii) 10 mM acetate to enrich aceticlastic methanogens; and (iii) 5 mM methanol and 5 mM trimethylamine to cultivate methylotrophic methanogens. We also added coenzyme M (2-mercaptoethanesulfonic acid) to the medium at a final concentration of 0.5 mM, because the growth of some methanogens is stimulated/required by coenzyme M (e.g., Bräuer et al., 2011). For the hydrogen plus formate culture, acetate was also added as a carbon source at a final concentration of 1 mM. The coal samples were crushed using a sterilized hummer and then placed in a bottle containing the anaerobic medium. The bottles were then tightly closed with butyl rubber stoppers and screw caps and strictly anaerobic conditions were created by the addition of reducing agents. All of the cultures were incubated at 20°C. Separate culture bottles were used for each substrate condition. To monitor anaerobic conditions in the media, resazurin was added to the medium as a redox indicator. Methane concentration was determined by gas chromatography (GC3200G, GL Science) with a thermal conductivity detector. An Olympus microscope (Olympus BX51F, Olympus, Tokyo, Japan) with a colour CCD camera system (Olympus DP72) was used to examine cell morphology and epifluorescence.
Total DNA extraction, PCR amplification and sequencing were performed as described previously (Miyashita et al., 2009). For PCR amplification, primers 21F and r912R were used to obtain the archaeal 16S rRNA gene sequence of methanogens in the coal enrichment cultures. Phylogenetic analysis using 16S rRNA gene sequences was performed with the ARB program (Ludwig et al., 2004). A 16S rRNA gene-based tree was constructed using the neighbour-joining method for sequences >1000 nucleotides. Shorter sequences (i.e., sequences obtained in this study) were inserted into this tree without changing the tree topology by using the parsimony insertion tool of the ARB program (Ludwig et al., 2004). Bootstrap analysis was performed with the MEGA 5 program (Tamura et al., 2011) for 1000 resamplings to estimate the confidence of the 16S rRNA gene tree topology. The 16S rRNA gene sequences reported in this study were deposited in the GenBank/EMBL/DDBJ database under accession numbers AB828144 and AB828145.
To determine concentrations and isotopic compositions of dissolved gases in sub-sampled fluids (Sample 14d, 34d and 56d), we extracted the dissolved gases in the fluid under vacuum (Saegusa et al., 2006). The gas extraction procedure was as follows: 28.5–39 ml of the sub-sampled fluids was recovered in a gas-tight cylinder and transferred to a pre-evacuated glass extraction bottle (~370 ml), leaving 331–341.5 ml headspace in the extraction bottle. In order to facilitate the extraction of the dissolved gases from the fluid into the headspace, the extraction bottle was ultrasonicated at 25°C for 5 min. To determine the total gas volume in the fluid, the pressure was measured by a pressure gauge. The extracted headspace gas was then sub-sampled into vacuumed glass vials. After extraction of dissolved gases, the fluid was further processed for the analysis of dissolved components, dissolved inorganic carbon (DIC), formate, acetate, and methanol.
CBM in pre-coal was extracted to determine carbon isotopic composition of CH4 (δ13CCH4). For the extraction, ca. 3 cm3 of coal fragments of pre-coal were introduced into a 21.5 ml vial, which was filled with 5 ml Milli Q water sealed with a butyl stopper and crimp capped. The vial was shaken by vortexing and then heated in an oven at 70°C for 2 h. Headspace gas (~0.5 ml) was then extracted with a gas-tight syringe for the isotopic analysis.
H2, CH4, and CO2 concentrations in the extracted gas from the sub-sampled fluids were analyzed by gas chromatography with a He ionization detector (HID) using a SRI 8610C (SRI Instruments, California, USA). The standard deviation obtained for repeated analysis of the laboratory standard gas was <4%. Carbon isotopic composition of CH4 (δ13CCH4) was determined by gas chromatography isotope-ratio-mass spectrometry (GC-IRMS; Thermo-Finnigan Delta Plus XP isotope-ratio mass spectrometer connected to TRACE GC and GC-COMBUSTION III). DIC concentration and δ13CDIC in sub-sampled fluids were measured with a ThermoFinnigan Delta Plus XP IRMS instrument connected to Thermo Scientific TC/EA via a ConFlo III interface in a similar way to that described by Miyajima et al. (1995) and Toki et al. (2004). The standard deviation obtained for repeated carbon isotope analysis of the laboratory standard (NaHCO3 solution) was <0.2‰. We calculated the ΣCO2 concentrations by adding the DIC concentrations with CO2 concentrations.
The concentrations and δ13C of formate, acetate, and methanol were determined by Isotope-Ratio-Monitoring Liquid Chromatography Mass Spectrometry (IRM-LCMS); Thermo-Finnigan Delta Plus XP isotope-ratio mass spectrometer connected to LC IsoLink), as described by Heuer et al. (2006) and Ijiri et al. (2012).
To study theoretical constraints for the habitability of bacteria and methanogens, we calculated Gibbs free energies (ΔG) of a possible microbial reaction using in-vitro geochemical data obtained in the present study. Enthalpy (ΔH°), standard Gibbs free energy (ΔG°) (25°C, 1 atm) and Gibbs free energy at 40°C and 41 MPa at equilibrium (ΔG°40,41) were estimated using the SUPCRT92 database (Shock and Helgeson, 1990; Johnson et al., 1992). ΔG at 40°C and 41 MPa was calculated using as following reaction equation: ΔG40,41 = ΔG°40,41 – RTlnQ, where R is the universal gas constant 8.31 J· K−1· 1wh−1, T is the temperature in kelvin, and Q is the reaction quotient. Activity coefficients of all dissolved chemical species involved in the calculation of Q were estimated from geochemical data using Spec E8 of the Geochemist's Workbench 7.0 (summarized in Table 5).
The redox state of fluid was continuously monitored by the color of resazurin, showing that anaerobic condition was successfully maintained during the geobio-reactor operation and subsampling. The total and effective porosity of post-coal and post-sand are shown in Table 2. Post-sand showed a high total and effective porosity, which is much higher than that of post-coal. These results suggest that pore water could pass all the way through the column without considerable stress since the fine coal was entirely enveloped by sand in the heat-shrinkable tube (Figure 2F).
XRD analysis suggests that post-sand comprises quartz, albite and chlorite (clinochlore), and has a similar volume ratio of minerals to that of pre-sand (Figure 3). On the other hand, FE-SEM-EDS observations of post-sand revealed the existence of minor minerals containing high amounts of C, Ca, Mg, and Fe as the major elements, and Si and Al as the minor elements (measurement points 2 and 3 in Table 3 and Figures 4B–D), while chlorite aggregates are dominant in pre- and post-sand (measurement points 1 and 4 in Table 3, Figures 4A,D). Considering the absence of major anion species except CO2−3, the minor minerals are most likely carbonates. These results show that carbonation of the surface of chlorite aggregates proceeded slowly in post-sand and that no carbonate existed in pre-sand.
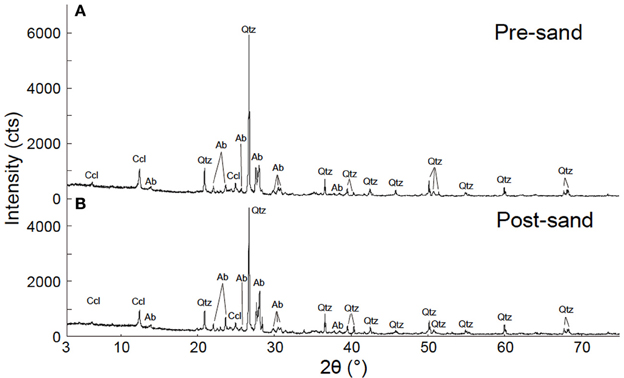
Figure 3. Result of XRD analysis. (A) XRD pattern of pre-sand. (B) XRD pattern of post-sand. Qtz, quartz; Ab, albite; Ccl, clinochlore.
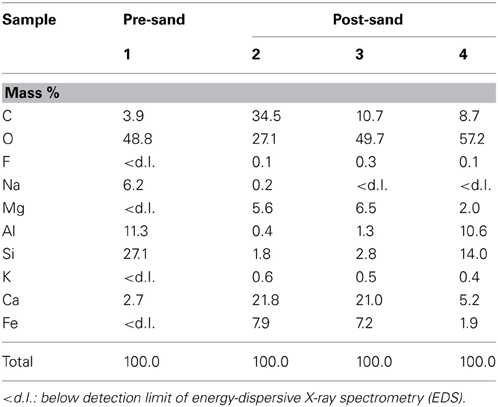
Table 3. Chemical compositions of representative carbonate and chlorite in samples pre-sand and post-sand.
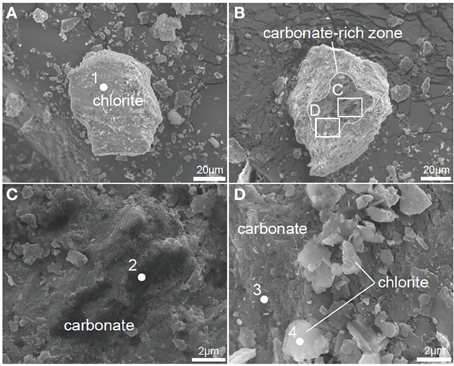
Figure 4. FE-SEM images of powdered pre-sand and post-sand. (A) Chlorite in pre-sand. (B) Carbonated chlorite aggregate in post-sand. (C) Magnified view of inset in (B) to show carbonate and chlorite aggregate in post-sand, respectively. (D) Magnified view of inset in (B) to show chlorite grains and the carbonated part of the chlorite aggregate.
The microbial community in pre-coal was dominated by Lysinibacillus and Bacillus, which was similar to the community structure in post-coal (Figure 5). Aerobic bacteria (e.g., Saccharopolyspora and Pseudonocardia) inhabited pre-sand because they were preserved in aerobic condition after sampling from Kushiro Coal Mine. The microbial community in post-sand was similar to that in pre- and post-coal, suggesting that bacteria in pre-coal migrated to surrounding sand together with the CO2-injected fluid during the experiment. Compared to pre-coal and pre-sand, cell numbers decreased slightly in post-coal and post-sand (Table 2). Given the high porosity of sand, cells in the coal-sand column were transported by the CO2-injected fluid.
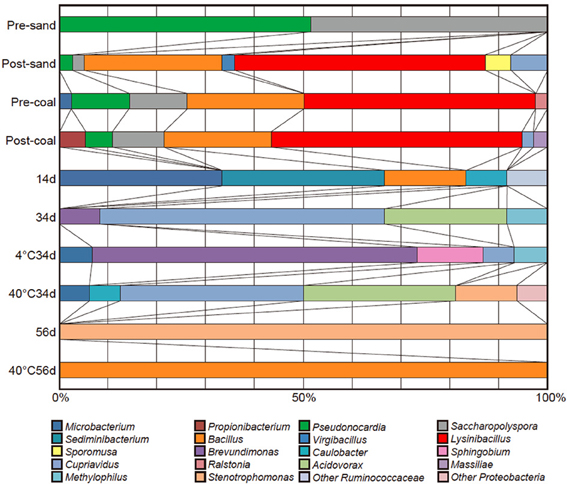
Figure 5. Cloning analyses of pre-sand, post-sand, pre-coal, post-coal, sup-sampled fluids (Samples 14d, 34d, and 56d), and the cultivated samples (Samples 4°C34d, 40°C34d and 40°C56d).
Microbial growth was confirmed in sub-sampled fluids (Samples 14d, 34d, and 56d) and the cultivated samples (Samples 4°C34d, 40°C34d, and 40°C56d). Cloning analysis was also performed on these samples to identify the microbial community in the coal-sand column (Figure 5; Table 4). Bacillus, Cupriavidus, and Microbacterium were observed with sequencing in the post-coal and post-sand, as well as in the sub-sampled fluids and the cultivated samples; however, the community structure of these samples differed markedly from each other (Figure 5). In sample 14d, Sediminibacterium, Microbacterium and Bacillus were dominant. Microbial communities in 34d, as well as samples 4°C34d and 40°C34d were relatively similar and were dominated by Cupriavidus and Brevundimonas. Bacterial diversity decreased dramatically in sample 56d and in 40°C56d. No bacterial growth was observed in 4°C14d and 40°C14d, or in 4°C56d. Archaeal clones belonging to South African Gold Mine Euryarchaeotic Group (SAGMEG), Gulf of Mexico arc I group (GOM arc I) and Misscelaneous Crenarchaeotal Group (MCG) were obtained by means of 42 cycles for PCR from the samples of pre-coal, post-coal, post-samd and 40°C34d (data is not shown, accession number AB840549-AB840587). The PCR amplification of archaeal 16S rRNA genes required higher number of the reaction cycle than those for bacteria (i.e., 32 cycles), indicating that bacteria dominate microbial communities we examined and the archaeal components are relatively minor. In archaeal clone libraries, no known methanogenic 16S rRNA sequence and mcrA gene were detected. Phylogenetic tree of the bacterial 16S rRNA gene sequences obtained from all analyzed samples were shown in Figure 6.
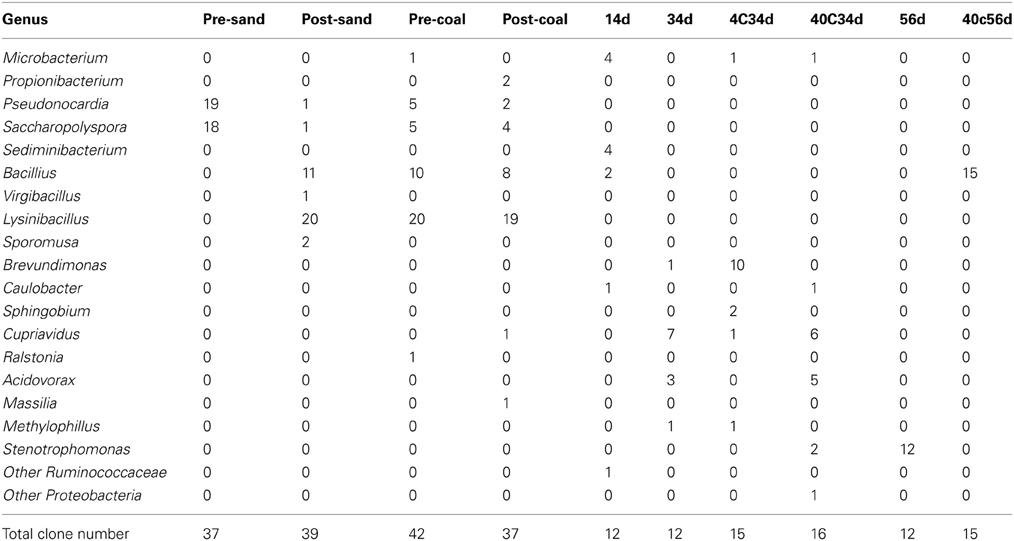
Table 4. Results of cloning analysis of bacteria in samples pre-sand, post-sand, pre-coal, post-coal, 14d, 34d, 4C34d, 40C34d, 56d, and 40C56d.
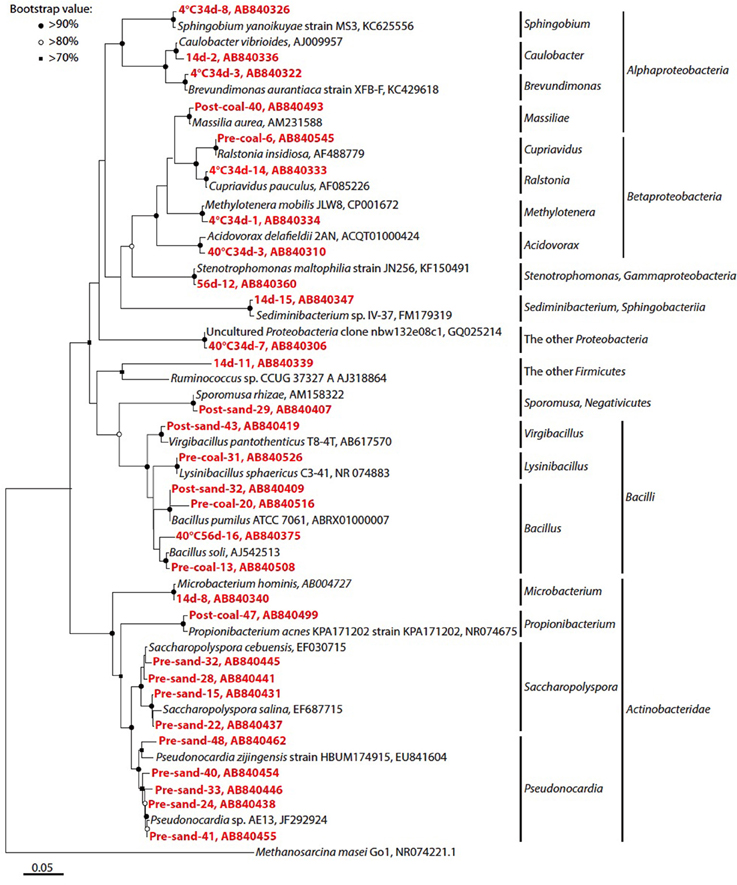
Figure 6. Phylogenetic analysis of bacterial 16S rRNA gene sequences. Only representative sequences from each operational taxonomic unit (OTU) at a 97% cut-off were presented. Accession numbers are also shown after each strain or clone name. Red colored number prefixed the each clone name indicate the clone number of each sample. Neighbor-joining tree was constructed using the MEGA 5.10 software (Tamura et al., 2011). Bootstrap values (>60%) obtained after 1000 iterations are indicated at nodes.
To investigate the potential for biological CO2 reduction to methane in Kushiro coal, we anaerobically incubated sample pre-coal using conventional batch-type cultivation technique in a medium for methanogens (Figure 7A). We prepared three cultures; i.e., H2 + formate-fed, acetate-fed, and methanol + trimethylamine-fed cultures, based on the three major groups of methanogens, i.e., hydrogenotrophic, acetoclastic, and methylotrophic methanogens (Liu and Whitman, 2008). After 1 year of incubation at 20°C, methane was detected in all of the cultures except the acetate-fed culture. Microscopic observations showed that almost all the cells in these methane-producing cultures consisted of F420-autofluorescence methanogen-like cells. Rod-shaped and coccoid-shaped cells having F420-like autofluorescence were dominant in the H2 + formate-fed and methanol-trimethylamine-fed cultures, respectively (Figures 7C,E). To identify the methanogens in the cultures, we sequenced archaeal 16S rRNA genes amplified by PCR using a universal archaeal primer set; 16S rRNA gene-based clone analysis was not used because the methanogen-like cells in each culture exhibited the same morphology. The sequence obtained from the H2 + formate-fed culture was affiliated with the hydrogenotrophic methanogen genus Methanobacterium. The archaeal 16S rRNA gene sequence obtained for the methanol-trimethylamine culture belonged to the methylotrophic methanogen genus Methanosarcina. These sequences had high sequence similarities (>99%) to known methanogens (Figure 8). For the acetate-fed culture, no cell proliferation or methane-production was observed after 2 years of incubation.
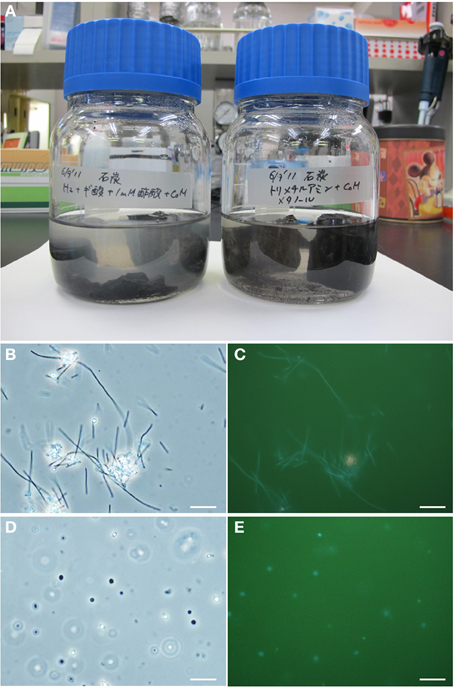
Figure 7. Micrographs of methanogenic enrichment cultures established from the coal sample. (A) Culture bottles. (B–E) Photomicrographs of the enrichment cultures. Phase-contrast micrographs (B,D) and fluorescence micrographs (C,E) of the same fields are shown. (B,C) Rod-shaped methanogen cells grown on H2/CO2-formate medium; and (D,E) coccoid-shaped methanogen cells grown on methanol-trimethylamine medium. Bars represent 10 μm.
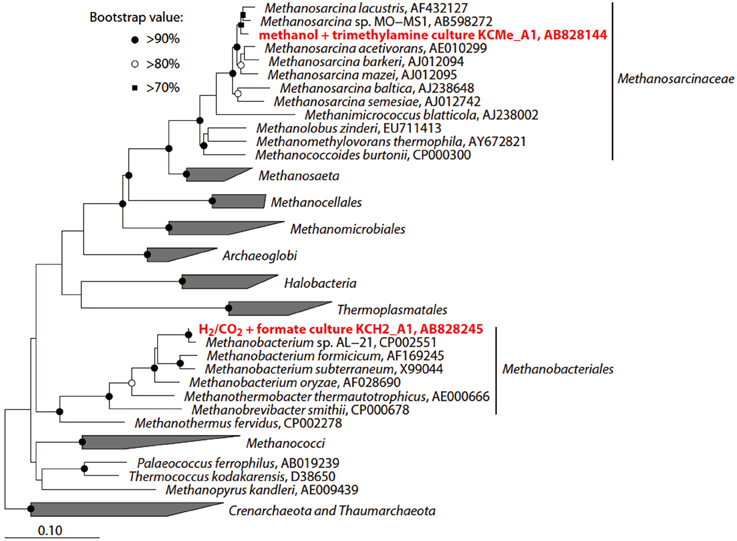
Figure 8. Phylogenetic tree showing the position of 16S rRNA gene sequences obtained from the methanogenic enrichment cultures. The initial tree was constructed with sequences greater than 1000 nucleotides using the neighbor-joining method. Subsequently, the sequences obtained in this study were inserted into the tree by the using the parsimony insertion tool of the ARB program. Accession numbers are also shown after each strain or sequence name. The scale bar indicates the estimated number of base changes per nucleotide position. The symbols at the branch nodes show the bootstrap values (>70%) obtained after 1000 resamplings.
The geochemical characteristics of the sub-sampled fluids are summarized in Table 5. Over the course of the experiment, the dissolved CH4 concentrations increased (63.6–186 μ M) while δ13CCH4 remained constant (−68.7 to −68.3‰, Figures 9A,C). The δ13CCBM value of sample pre-coal was −71.4‰ at day 0 (Figure 9C), which was similar to the dissolved δ13CCH4 value in all of the sub-sampled fluids. Dissolved CO2, DIC and ΣCO2 increased during the experiment, with ΣCO2 (22.1–125.6 mM) consistently lower than expected amount of ΣCO2 (initial concentration: 138.4 mM) in all samples (Figure 9B). δ13CDIC was depleted during experiment (−19.4 to −24.4‰). Dissolved H2 decreased from 217.2 to 0 μ M between days 14 and 56 (Figure 9A). An increase in the acetate concentration was observed (0.8–7.0 mM), whereas δ13Cacetate was depleted during experiment (−28.9 to −40.1‰) (Figures 9D,E). On the other hand, the formate concentration decreased and δ13Cformate was enriched in 13C. In addition, the methanol concentration also increased, while the δ13Cmethanol values remained constant for the duration of the experiment.
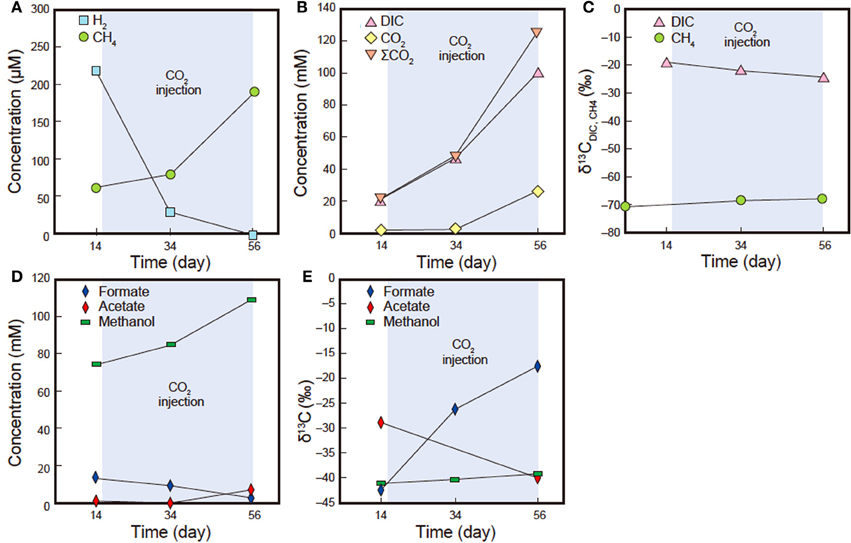
Figure 9. H2, CH4, CO2, DIC, ΣCO2, formate, acetate, methanol concentrations and carbon isotope compositions (δ13C) of DIC and CH4 insub-sampled fluids (samples Pre14d, Pre34d and Pre56d). (A) Dissolved H2 and CH4 concentrations. (B) Dissolved CO2, DIC and ΣCO2 concentrations. (C) δ13CDIC and δ13CCH4. (D) Dissolved formate, acetate and methanol concentrations. (E) δ13Cformate, δ13Cacetate and δ13Cmethanol. Standard deviation obtained after repeated analysis of the laboratory standard gas was <4%. Standard deviation obtained from repeated carbon isotope analysis of the laboratory standard (NaHCO3 solution) was <0.2‰.
Cloning analysis of samples pre-coal, pre-sand, post-coal and post-sand revealed that bacteria migrated from coal to surrounding sand. Furthermore, some of these bacteria were moved to the sub-sampling cell by CO2-containing fluid due to the high porosity of the sand. The mechanisms of microbial migration have been investigated in a variety of natural environments, either for purposes of bioremediation or to prevent microbial contamination (e.g., drinking water pumped from an aquifer) (Ferguson et al., 2003; Smith and Perdek, 2004). Numerous laboratory experiments and field observations of microbial migration have been examined using various mathematical models (Harvey et al., 1995; Johnson et al., 2001; Harvey and Harms, 2002; Tufenkji, 2007). However, the change of microbial communities associated with migration is still poorly understood. Cloning analyses of sub-sampled fluids and the cultivated samples showed that, with the exception of a few genera, the microbial communities differed markedly from those in the coal-sand column. These results can be explained by two sorting events of bacterial communities as follows: microbial effluence to CO2-injected fluid from the coal-sand column and decompression after sample recovery. The coal-sand column would retain some bacteria and prevent them from being transported in the flow of CO2-injected fluids. Cultivation experiments using sub-sampled fluids revealed that Bacillus, Cupriavidus, and Microbacterium were capable of surviving pressurization/decompression, suggesting that certain terrestrial bacteria in coal-sand formations have the ability to resist marked changes in pressure. Furthermore, it is conceivable that spore formation might be a possible function to survive drastic environmental changes associated with the GCS.
Although anaerobic conditions were maintained in all lines and vessels, aerobic Lysinibacillus bacteria were dominant in the microbial community of coal-sand column. It is possible that Lysinibacillus was able to survive the anaerobic conditions in coal-sand column by producing spores. Indeed, Lysinibacillus was not observed in any of the fluid samples collected, suggesting that Lysinibacillus does not produce spores under ambient pressure conditions or they could not migrate with fluid flow and remained in the column.
Microbial migration associated with fluid flow is expected to occur in natural subsurface formations if the formation has enough pore-throat connectivity. In addition, it is considered that artificial CO2 injected will positively enhance microbial migration. Thus, considering that the microbial communities in coal and sand became similar after experiment, it is possible that bacterial communities may be very homogeneous in porous subsurface coal-sand formations used as geological CO2 repositories.
The relationship between δ13CCH4 and δ13CDIC is good indicator of the biogenic production of CH4 during experiment (e.g., Whiticar, 1999). When the CH4 is produced by hydrogenotrophic methanogenesis or acetoclastic methanogenesis during experiment, the δ13CCH4 value should be affected by the δ13C values of precursors of CH4 such as δ13CDIC and δ13Cacetate. However, the δ13CCH4 remained constant through the experiment and was similar to δ13CCBM, even though the δ13CDIC and δ13Cacetate were depleted during the experiment. Those carbon isotopic compositions suggest that the CH4 dissolved in fluids not originated from biogenic but from CBM in pre-coal. Biogenic CH4 was not detected likely because of the slow metabolic rate or inactivation of methanogens in the experimental condition. It is most likely that CH4 discharge was enhanced by the CO2-injected fluid that was supplemented into the coal-sand column as previously reported (Gunter et al., 1997; White et al., 2005). Indeed, the results of the PCR and cloning analysis showed that known methanogen was not observed in any examined samples, which is consistent with the lack of any biogenic changes in δ13CCH4 values.
The CO2 concentration in sub-sampled fluids was consistently lower than the total amount of CO2 that was added to the system. This decrease in CO2 can be explained by adsorption on the coal, mineral trapping, or consumption by bacteria. Previous reports have suggested that several gases are adsorbed by immature coal (White et al., 2005; Bustin and Clarkson, 1998; Pan and Connell, 2007). The FE-SEM-EDS results of this study suggested that mineral trapping of CO2 by carbonate precipitation could have accounted for the slightly lower than expected CO2 concentrations. On the other hand, the increase of acetate concentration in the sub-sampled fluids was observed during the experiment, which, in conjunction with decreases in H2 and CO2 concentrations, implies that homo-acetogenesis occurred during the experiment. The lower δ13C value of increased acetate during the experiment than that in normal total organic carbon (−20 to −30‰) also suggests that acetate was produced by homo-acetogenesis. This is because, acetate synthesized via the acetyl-CoA pathway during homo-acetogenesis by acetogen is depleted in 13C compared with its precursor (House et al., 2003). Indeed, the presence of Sporomusa-related 16S rRNA genes, a homo-acetogenic bacterium, detected in the cloning analysis of post-sand also supports this interpretation. δ13CCO2 simply reflects the value of supplied CO2 and bicarbonate in pore water, which ranged from −24.4 to −19.2‰. A decrease of formate concentrations and enrichment of 13C in δ13Cformate also likely indicate bacterial consumption of formate in which 12C would be preferentially consumed.
Interestingly, we observed increase of methanol concentration during the CO2 injection experiment (Figure 9; Table 5). The carbon isotopic compositions of methanol were relatively constant at around −40‰ although the δ13C values were notably lower than those of DIC. Given the available data set, it is still difficult to identify the source and/or production mechanisms of methanol at this point; however, we infer that continuous injection of the CO2 and fluid might abiotically release absorbed methanol from the coal-formation sample. It might also be conceivable that microbial activity stimulated by the CO2 and fluid supply might contribute to methanol production (e.g., as a secondary product via degradation of organic matter).
Batch-type cultivation of methanogens at ambient temperature and pressure followed by cloning analysis revealed that Methanobacterium and Methanosarcina were indigenous to the Kushiro bituminous coal examined in this study. This finding is consistent with previous reports describing the presence of methanogens in coal (Krüger et al., 2008; Strapoć et al., 2008). However, no methanogens were activated during CO2 injection experiment under in-situ subsurface conditions. To constrain the habitability of bacteria and methanogens, we calculated ΔG of acetoclastic methanogenesis, hydrogenotrophic methanogenesis and homo-acetogenesis using in-situ geochemical data obtained in the present study (Tables 5, 6; Figure 10). We could not calculate ΔG of acetoclastic methanogenesis on day 34, hydrogenotrophic methanogenesis on day 56, and homo-acetogenesis on days 34 and 56 because the concentrations of acetate in sample 34d and the dissolved H2 in sample 56d were below detection limit. On days 14 and 34, the ΔG of hydrogenotrophic methanogenesis was the lowest of all reactions, indicating that hydrogenotrophic methanogenesis is most favorable under the conditions examined (Figure 10). It is thus enigmatic that hydrogenotrophic methanogenesis did not occur, even though methanogens are indigenous to the examined coal and sufficient H2 and CO2 were supplemented into the coal-sand column during experiment. Slow metabolic rates of methanogens might be responsible for the result. Under conditions of supplemented H2 and CO2, homo-acetogens and hydrogenotrophic methanogens would compete against each other because both utilize H2 and CO2. Sporomusa, the homo-acetogenic bacterium observed in post-sand, has been reported to be capable of demethylating aromatic compounds and of breaking the bonds of aromatic rings (Mechichi et al., 1999), many of the aromatic organic compounds supplied by pyrolysed/pressurized bituminous coal could be used by Sporomusa as nutrients, resulting in the predominance of homo-acetogenesis in the system. Alternatively, despite the sub-sampling and reactor experiment were carried out under the anaerobic condition, we cannot deny the possibility that small oxygen contamination during the initial sampling might negatively affect methanogenesis and if so, it would be outcompeted by homo-acetogenesis under trace oxygen conditions (Leadbetter and Breznak, 1996).
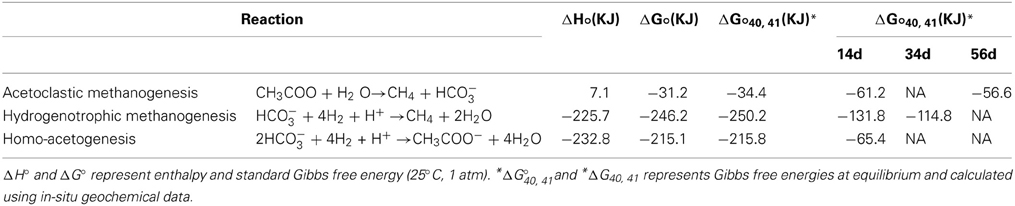
Table 6. Gibbs free energies and enthalpies of possible microbial reactions in CO2 injection experiment.
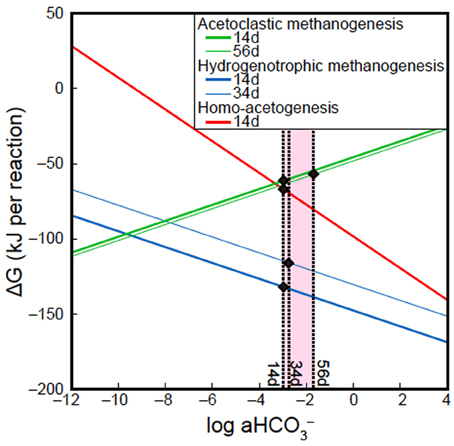
Figure 10. Effects of HCO−3 activity (a HCO−3) on ΔG of acetoclastic methanogenesis, hydrogenotrophic methanogenesis and homo-acetogenesis. All lines in figure were calculated using geochemical data of samples 14d, 34d, and 56d, except Dissolved inorganic carbon (DIC) concentrations. Solid circles indicate ΔG of acetoclastic methanogenesis, hydrogenotrophic methanogenesis and homo-acetogenesis at the DIC concentrations examined in this study.
A previous study suggested that an increase in the partial pressure of CO2 could promote acetoclastic methanogenesis in crude oil reservoirs (Mayumi et al., 2013). However, in this study, an increase in DIC concentration and a decrease in the acetate concentration associated with acetoclastic methanogenesis were not observed, indicating that acetoclastic methanogenesis did not occur even though the fluid supplemented into coal-sand column contained sufficient acetate for growth. Higher ΔG values of the acetoclastic methanogenesis on days 14and 56 than those obtained for hydrogenotrophic methanogenesis and homo-acetogenesis is also consistent with our results, which can be explained by differences H2 utilization in each system. The influence of dissolved H2 concentration on the ΔG of hydrogenotrophic methanogenesis was also observed in this study (blue lines in 14d and 34d; Figure 10). The steep gradient of the curves in Figure 10 suggest that an increase in HCO−3 activity is more effective for promoting homo-acetogenesis than hydrogenotrophic methanogenesis.
In summary, our results suggest that homo-acetogenesis is possible reaction in GCS settings involving unmineable subsurface coal-sand formations. Aromatic organic compounds supplied by bituminous coal can activate homo-acetogenic bacteria. These findings indicate that microbial conversion of CO2 to acetate under subsurface conditions is feasible. However, to gain our knowledge of the potential response of subsurface microbial ecosystem to CO2 sequestration, more detailed comparative geochemical and microbiological studies using tracer incubation experiments and high-throughput sequencing will be necessary for in-situ and ex-situ conditions. In addition, to accelerate the biological CO2 conversion to reduced compounds (i.e., Bio-CCS), supply of electron and/or molecular hydrogen would be essential. In this regard, we need to investigate the place where natural H2 concentration is remarkably high due to the thermal degradation of organic matter (Head et al., 2003) or other H2-producing geologic systems (e.g., serpentinization), or to consider the utilization of natural electric resources for electromethanogenesis (Cheng et al., 2009; Kuramochi et al., 2013). These are our on-going foci.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank A. Imajo and T. Terada for their technical assistance with analytical aspects of this study. The authors are grateful to the Kushiro Coal Mine Co. Ltd. for the submarine coal samples. This study was supported by the Japan Society for the Promotion of Science (JSPS) Strategic Fund for Strengthening Leading-Edge Research and Development (to JAMSTEC and Fumio Inagaki), the JSPS Funding Program for Next Generation World-Leading Researchers (NEXT Program, to Fumio Inagaki). Part of this work was conducted in cooperation with Research Institute of Innovative Technology for the Earth (RITE) in the Innovative Zero-emission Coal Gasification Power Generation Project funded by the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Alley, R. B., Marotzke, J., Nordhaus, W. D., Overpeck, J. T., Peteet, D. M., Pielke, R. A. Jr., et al. (2003). Abrupt climate change. Science 299, 2005–2010. doi: 10.1126/science.1081056
Anderson, S., and Newell, R. (2004). Prospects for carbon capture and storage technologies. Annu. Rev. Environ. Res. 29, 109–42. doi: 10.1146/annurev.energy.29.082703.145619
Bachu, S. (2003). Screening and ranking of sedimentary basins for sequestration of CO2 in geological media in response to climate change. Environ. Geol. 44, 277–289. doi: 10.1007/s00254-003-0762-9
Benson, S. M., and Cole, D. R. (2008). CO2 sequestration in deep sedimentary formations. Elements 4, 325–331. doi: 10.2113/gselements.4.5.325
Bertoloni, G., Bertucco, A., De Cian, V., and Parton, T. (2006). A study on the inactivation of micro-organisms and enzymes by high pressure CO2. Biotechnol. Bioeng. 95, 155–160. doi: 10.1002/bit.21006
Bräuer, S. L., Hinsby Cadillo-Quiroz, H., Ward, R. J., Yavitt, J. B., and Zinder, S. H. (2011). Methanoregula boonei gen. nov., sp. nov., an acidiphilic methanogen isolated from an acidic peat bog. Int. J. Syst. Evol. Microbiol. 61, 45–52. doi: 10.1099/ijs.0.021782-0
Bustin, R. M., and Clarkson, C. R. (1998). Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 38, 3–26. doi: 10.1016/S0166-5162(98)00030-5
Cheng, S., Xing, D., Call, D. F., and Logan, B. E. (2009). Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 43, 3953–3958. doi: 10.1021/es803531g
Crowley, T. J. (2000). Causes of climate change over the past 100 years. Science 289, 270–277. doi: 10.1126/science.289.5477.270
Davison, J. (2007). Performance and costs of power plants with capture and storage of CO2. Energy 32, 1163–1176. doi: 10.1016/j.energy.2006.07.039
DeLong, E. F. (1992). Archaea in coastal marine environments. Proc. Natl. Acad. Sci. U.S.A. 89, 5685–5689. doi: 10.1073/pnas.89.12.5685
Duan, Z., and Sun, S. (2003). An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 193, 257–271. doi: 10.1016/S0009-2541(02)00263-2
Edwards, E. A., and Grbic-Galic, D. (1994). Anaerobic degradation of toluene and oxylene by a methanogenic consortium. Appl. Environ. Microbiol. 60, 313–322.
Edwards, U., Rogall, T., Blöker, H., Emde, M., and Böttger, C. (1989). Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17, 7843–7853. doi: 10.1093/nar/17.19.7843
Eiken, O., Brevik, I., Arts, R., and Lindeberg, E. (2000). “Seismic monitoring of CO2 injected into a marine aquifer,” in Presented at the 5th International Conference on Greenhouse Gas Control Technologies, Cairns.
Fakoussa, R. M. (1988). Production of water-soluble coal substances by partial microbial liquefaction of untreated coal. Resour. Conserv. Recycling 1, 251–260. doi: 10.1016/0921-3449(88)90020-1
Fakoussa, R. M. (1990). “Microbiological treatment of German hard coal,” in Bioprocessing and Biotreatment of Coal, ed D. L. Wise (NewYork, NY: Marcel Dekker), 95–107.
Ferguson, C., de Roda Husman, A. M., Altavilla, N., Deere, D., and Ashbolt, N. (2003). Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33, 299–361. doi: 10.1080/10643380390814497
Gilfillan, S. M. V., Lollar, B. S., Holland, G., Blagburn, D., Stevens, S., Schoell, M., et al. (2009). Solubility trapping in formation water as dominant CO2 sink in natural gas fields. Nature 458, 614–618. doi: 10.1038/nature07852
Gunter, W. D., Gentzis, T., Rottenfusser, B. A., and Richardson, R. J. H. (1997). Deep coal-bed methane in Alberta, Canada: a fossil fuel resource with the potential of zero greenhouse gas emissions. Energy Conversion Manage. 38(suppl.), S217–S222. doi: 10.1016/S0196-8904(96)00272-5
Hales, B. A., Edwards, C., Ritchie, D. A., Hall, G., Pickup, R. W., and Saunders, J. R. (1996). Isolation and identification of methanogen-specific DNA from blanket bog peat by PCR amplification and sequence analysis. Appl. Environ. Microbiol. 62, 668–675.
Harvey, R. W., and Harms, H. (2002). “Transport of microorganisms in the terrestrial subsurface: in situ and laboratory methods,” in Manual of Environmental microbiology, ed C. J. Hurst (Washington, DC: ASM Press), 1138.
Harvey, R. W., Kinner, N. E., Bunn, A., MacDonald, D., and Metge, D. W. (1995). Transport behavior of groundwater protozoa and protozoan-sized microspheres in sandy aquifer sediments. Appl. Environ. Microbiol. 61, 209–217.
Head, I. M., Jones, D. M., and Larter, S. R. (2003). Biological activity in the deep subsurface and the origin of heavy oil. Nature 426, 344–352. doi: 10.1038/nature02134
Heuer, V., Elvert, M., Tille, S., Krummen, M., Prieto Mollar, X., Hmelo, L. R., et al. (2006). Online δ 13C analysis of volatile fatty acids in sediment/pore water systems by liquid chromatography-isotope ratio-mass spectrometry. Limnol. Oceanogr. Methods 4, 346–357. doi: 10.4319/lom.2006.4.346
House, C. H., Schopf, J. W., and Stetter, K. O. (2003). Carbon isotopic fractionation by Archaeans and other thermophilic prokaryotes. Org. Geochem. 34, 345–356. doi: 10.1016/S0146-6380(02)00237-1
House, K. Z., Schrag, D. P., Harvey, C. F., and Lackner, K. S. (2006). Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. U.S.A. 103, 12291–12295. doi: 10.1073/pnas.0605318103
Hyakkoku, H. (1966). Studies on the sedimentation of the Harutori formation in the eastern half of the Kushiro coal field. Mining Geol. 16, 172–182.
Ijiri, A., Harada, N., Hirota, A., Tsunogai, U., Ogawa, N. O., Itaki, T., et al. (2012). Biogeochemical processes involving acetate in sub-seafloor sediments from the Bering Sea shelf break. Org. Geochem. 48, 47–55. doi: 10.1016/j.orggeochem.2012.04.004
Inagaki, F., Kuypers, M. M. M., Tsunogai, U., Ishibashi, J., Nakamura, K., Treude, T., Ohkubo, S., et al. (2006). Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system. Proc. Natl. Acad. Sci. U.S.A. 103, 14164–14169. doi: 10.1073/pnas.0606083103
Johnson, J. W., Oelkers, E. H., and Helgeson, H. C. (1992). SUPCRT92: a software package for calculating the standard molal properties of minerals, gases, aqueous species and reactions among them from 1 to 5000 bars and 0 to 1000°C. Comp. Geosci. 18, 899–947. doi: 10.1016/0098-3004(92)90029-Q
Johnson, W. P., Zhang, P., Fuller, M. E., Scheibe, T. D., Mailloux, B. J., Onstott, T. C., et al. (2001). Ferrographic tracking of bacterial transport in the field at the Narrow Channel Focus Area, Oyster, VA. Environ. Sci. Technol. 35, 182–191. doi: 10.1021/es001170e
Jun, Y. S., Giammar, D. E., Werth, C. J., and Dzombak, D. A. (2013). Environmental and geochemical aspects of geologic carbon sequestration: a special issue. Environ. Sci. Technol. 47, 1–2. doi: 10.1021/es304681x
Karl, T. R., and Trenberth, K. E. (2003). Modern global climate change. Science 302, 1719–23. doi: 10.1126/science.1090228
Kharaka, Y. K., Cole, D. R., Hovorka, S. D., Gunter, W. D., Knauss, K. G., and Freifeld, B. M. (2006). Gas-water-rock interactions in Frio Formation following CO2 injection: implications for the storage of green–house gases in sedimentary basins. Geology 34, 577–580. doi: 10.1130/G22357.1
Kirk, M. F. (2011). Variation in energy available to populations of subsurface anaerobes in response to geological carbon storage. Environ. Sci. Technol. 45, 6676–6682. doi: 10.1021/es201279e
Konno, U., Tsunogai, U., Nakagawa, F., Nakaseama, M., Ishibashi, J., et al. (2006). Liquid CO2 venting on the seafloor: Yonaguni Knoll IV hydrothermal system, Okinawa Trough. Geophys. Res. Lett. 33, L16607. doi: 10.1029/2006GL026115
Krüger, M., Beckmann, S., Engelen, B., Thielemann, T., Cramer, B., Schippers, A., et al. (2008). Microbial methane formation from hard coal and timber in an abandoned coal mine. Geomicrobiol. J. 25, 315–321. doi: 10.1080/01490450802258402
Kuramochi, Y., Fu, Q., Kobayashi, H., Ikarashi, M., Wakayama, T., Kawaguchi, H., et al. (2013). Electromethanogenic CO2 conversion by subsurface-reservoir microorganisms. Energy Procedia 37, 7014–7020. doi: 10.1016/j.egypro.2013.06.636
Leadbetter, J. R., and Breznak, J. A. (1996). Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl. Environ. Microbiol. 62, 3620-3631.
Liu, Y., and Whitman, W. B. (2008). Metabolic, phylogenetic, and ecological diversity of the methanogenic Archaea. Ann. N. Y. Acad. Sci. 1125, 171–189. doi: 10.1196/annals.1419.019
Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar, et al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371. doi: 10.1093/nar/gkh293
Mayumi, D., Dolfing, J., Sakata, S., Maeda, H., Miyagawa, Y., Ikarashi, M., et al. (2013). Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 4, 1998. doi: 10.1038/ncomms2998
Mechichi, T., Labat, M., Patel, B. K. C., Woo, T. H. S., Thomas, P., and Garcia, J-L. (1999). Clostridium methoxybenzovorans sp. nov., a new aromatic odemethylating homoacetogen from an olive mill wastewater treatment digester. Int. J. Syst. Bacteriol. 49, 1201–1209. doi: 10.1099/00207713-49-3-1201
Metz, B., Davidson, O., de Coninck, H., Loos, M., and Meyer, L. (eds.) (2005). “Carbon dioxide capture and storage.” in IPCC Special Reports, New York, NY: IPCC
Mitchell, A. C., Dideriksen, K., Spangler, L. H., Cunningham, A. B., and Gerlach, R. (2010). Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 44, 5270–5276. doi: 10.1021/es903270w
Miyajima, T., Yamada, Y., Hanba, Y. T., Yoshii, K., Koitabashi, T., and Wada, E. (1995). Determining the stable-isotope ratio of total dissolved inorganic carbon in lake water by GC/C/IRMS. Limnol. Oceanogr. 40, 994–1000. doi: 10.4319/lo.1995.40.5.0994
Miyashita, A., Mochimaru, H., Kazama, H., Ohashi, A., Yamaguchi, T., Nunoura, T., et al. (2009). Development of 16S rRNA gene-targeted primers for detection of archaeal anaerobic methanotrophs (ANMEs). FEMS Microbiol. Lett. 297, 31–37. doi: 10.1111/j.1574-6968.2009.01648.x
Morono, Y., Terada, T., Kallmeyer, J., and Inagaki, F. (2013). An improved cell separation technique for marine subsurface sediments: applications for high-throughput analysis using flow cytometry and cell sorting. Envion. Microbiol. 15, 2841–2849. doi: 10.1111/1462-2920.12153
Morono, Y., Terada, T., Masui, N., and Inagaki, F. (2009). Discriminative detection and enumeration of microbial life in marine subsurface sediments. ISME J. 3, 503–511. doi: 10.1038/ismej.2009.1
Muyzer, G., Brinkhoff, T., Nübel, U., Santegoeds, C., Schäfer, H., and Wawer, C. (1998). “Denaturing gradient gel electrophoresis (DGGE) in microbial ecology,” in Molecular Microbial Ecology Manual, eds A. D. van Elsas and F. J. de Bruijn (Dordrecht: Kluwer Academic Publishers), 1–27.
Muyzer, G., Teske, A., Wirsen, C. O., and Jannasch, H. W. (1995). Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164, 165–172. doi: 10.1007/BF02529967
Nazina, T. N., Ivanova, A. E., Borzenkov, I. A., Belyaev, S. S., and Ivanov, M. V. (1995). Occurrence and geochemical activity of microorganisms in high-temperature, water-flooded oil fields of Kazakhstan and Western Siberia. Geomicrobiol. J. 13, 181–192. doi: 10.1080/01490459509378016
Oelkers, E. H., Gislason, S. R., and Matter, J. (2008). Mineral carbonation of CO2. Elements 4, 333–337. doi: 10.2113/gselements.4.5.333
Oppermann, B. I., Michaelis, W., Blumenberg, M., Frerichs, J., Schulz, H. M., Schippers, A., et al. (2010). Soil microbial community changes as a result of long-term exposure to a natural CO2 vent. Geochim. et Cosmochimica Acta 74, 2697–2716. doi: 10.1016/j.gca.2010.02.006
Orcutt, B., Samarkin, V., Boetius, A., and Joye, S. (2008). On the relationship between methane production and oxidation by anaerobic methanotrophic communities from cold seeps of the Gulf of Mexico. Environ. Microbiol. 10, 1108–1117. doi: 10.1111/j.1462-2920.2007.01526.x
Pan, Z., and Connell, L. D. (2007). A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 69, 243–252. doi: 10.1016/j.coal.2006.04.006
Rosenbauer, R. J., Thomas, B., Bischoff, J. L., and Palandri, J. (2012). Carbon sequestration via reaction with basaltic rocks: geochemical modeling and experimental results. Geochim. et Cosmochimica Acta 89, 116–133. doi: 10.1016/j.gca.2012.04.042
Saegusa, S., Tsunogai, U., Nakagawa, F., and Kaneko, S. (2006). Development of a multibottle gas-tight fluid sampler WHATS II for Japanese submersibles/ROVs. Geofluids 6, 234–240. doi: 10.1111/j.1468-8123.2006.00143.x
Sakai, S., Ehara, M., Tseng, I., Yamaguchi, T., Bräuer, S. L., Cadillo-Quiroz, H., et al. (2012). Methanolinea mesophila sp. nov., a hydrogenotrophic methanogen isolated from rice field soil, and proposal of the archaeal family Methanoregulaceae fam. nov. within the order Methanomicrobiales. Int. J. Syst. Evolut. Microbiol. 62, 1389–1395. doi: 10.1099/ijs.0.035048-0
Sauer, P., Glombitza, C., and Kallmeyer, J. (2012). A system for incubations at high gas partial pressure. Front. Microbiol. 3, 1–9. doi: 10.3389/fmicb.2012.00025
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Seyfried, W. E., and Janecky, D. R. (1985). Heavy metal and sulfur transport during subcritical and supercritical hydrothermal alteration of basalt: influence of fluid pressure and basalt composition and crystallinity. Geochim. et Cosmochimica Acta 49, 2545–2560. doi: 10.1016/0016-7037(85)90123-1
Shock, E. L., and Helgeson, H. C. (1990). Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: standard partial molal properties of organic species. Geochim. et Cosmochimica Acta 54, 915–945. doi: 10.1016/0016-7037(90)90429-O
Smith, J. E., and Perdek, J. M. (2004). Assessment and management of watershed microbial contaminants. Crit. Rev. Environ. Sci. Technol. 34, 109–39. doi: 10.1080/10643380490430663
Song, J., and Zhang, D. (2013). Comprehensive review of caprock-sealing mechanisms for geologic carbon sequestration. Environ. Sci. Technol. 47, 9-22. doi: 10.1021/es301610p
Strapoć, D., Picardal, F. W., Turich, C., Schaperdoth, I., Macalady, J. L., Lipp, J. S., et al. (2008). Methane-producing microbial community in a coal bed of the Illinois Basin. Appl. Environ. Microbiol. 74, 2424–2432. doi: 10.1128/AEM.02341-07
Suzuki, M. T., and Giovannoni, S. J. (1996). Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62, 625–630.
Takai, K., and Horikoshi, K. (2000). Rapid detection and quantification of members of the Archaeal community by quantitative PCR using fluorogenic probes. Appl. Environ. Microbiol. 66, 5066–5072. doi: 10.1128/AEM.66.11.5066-5072.2000
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evolut. 28, 2731–2739. doi: 10.1093/molbev/msr121
Toki, T., Tsunogai, U., Gamo, T., Kuramoto, S., and Ashi, J. (2004). Detection of low-chloride waters beneath a cold seep field on the Nankai accretionary wedge off Kumano, south of Japan. Earth Planet. Sci. Lett. 228, 37–47. doi: 10.1016/j.epsl.2004.09.007
Tufenkji, N. (2007). Modeling microbial transport in porous media: traditional approaches and recent developments. Adv. Water Resour. 30, 1455–1469. doi: 10.1016/j.advwatres.2006.05.014
Van der Zwaan, B., and Gerlagh, R. (2009). Economics of geological CO2 storage and leakage. Climatic Change 93, 285–309. doi: 10.1007/s10584-009-9558-6
Videmsek, U., Hagn, A., Suhadolc, M., Radl, V., Knicker, H., Schloter, M., and Vodnik, D. (2009). Abundance and diversity of CO2-fixing bacteria in grassland soils close to natural carbon dioxide springs. Microb. Ecol. 58, 1–9. doi: 10.1007/s00248-008-9442-3
Weisburg, W., Barns, S. M., Pelletier, D. A., and Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703.
White, C. M., Smith, D. H., Jones, K. L., Goodman, A. L., Jikich, S. A., LaCount, R. B., et al. (2005). Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery–A review. Energy Fuels 19, 599–724. doi: 10.1021/ef040047w
White, C. M., Strazisar, B. R., Granite, E. J., Hoffman, J. S., and Pennline, H. W. (2003). Separation and capture of CO2 from large stationary sources and sequestration in geological formations Coalbeds and deep saline aquifers. J. Air Waste Manage. Assoc. 53, 645–715. doi: 10.1080/10473289.2003.10466206
White, D. J., Burrowes, G., Davis, T., Hajnal, Z., Hirsche, K., Hutcheon, I., et al. (2004). Greenhouse gas sequestration in abandoned oil reservoirs: the International energy agency Weyburn pilot project. GSA Today 14, 4–10. doi: 10.1130/1052-5173(2004)014<004:GGSIAO>2.0.CO;2
Whiticar, M. J. (1999). Carbon and hydrogen isotopesystemetics of bacterial formation and oxidation of methane. Chem. Geol. 161, 291–314. doi: 10.1016/S0009-2541(99)00092-3
Widdel, F. (1986). Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl. Environ. Microbiol. 51, 1056–1062.
Wu, B., Shao, H. B., Wang, Z. P., Hu, Y. D., Tang, Y. J. J., and Jun, Y. S. (2010). Viability and metal reduction of Shewanella oneidensis MR-1 under CO2 stress: implications for ecological effects of CO2 leakage from geologic CO2 sequestration. Environ. Sci. Technol. 44, 9213–9218. doi: 10.1021/es102299j
Yakimov, M. M., Giuliano, L., Crisafi, E., Chernikova, T. N., Timmis, K. N., and Golyshin, P. N. (2002). Microbial community of a saline mud volcano at San Biagio-Belpasso, Mt. Etna (Italy). Environ. Microbiol. 4, 249–256. doi: 10.1046/j.1462-2920.2002.00293.x
Yanagawa, K., Morono, Y., De Beer, D., Haeckel, M., Sunamura, M., Urabe, T., et al. (2013). Metabolically active microbial communities in marine sediment under high-CO2 and low-pH extremes. ISME J. 7, 555–567. doi: 10.1038/ismej.2012.124
Yanagawa, K., Sunamura, M., Lever, M. A., Morono, Y., Hiruta, A., Ishizaki, O., et al. (2011). Niche separation of methanotrophic archaea (ANME-1 and -2) in methane-seep sediments of the eastern Japan Sea offshore Joetsu. Geomicrobiol. J. 28, 118–129. doi: 10.1080/01490451003709334
Yayanos, A. A., Dietz, A. S., and Van Boxtel, R. (1979). Isolation of a deep-sea barophilic bacterium and some of its growth characteristics, Science 205, 808–810. doi: 10.1126/science.205.4408.808
Zakkour, P., and Haines, M. (2007). Permitting issues for CO2 capture, transport and geological storage: a review of Europe, USA, Canada and Australia. Int. J. Greenhouse Gas Control 1, 94–100. doi: 10.1016/S1750-5836(06)00008-9
Keywords: geological CO2 sequestration, bituminous coal, geobio-reactor system, coal-bed methane, methanogen, homo-acetogenesis
Citation: Ohtomo Y, Ijiri A, Ikegawa Y, Tsutsumi M, Imachi H, Uramoto G-I, Hoshino T, Morono Y, Sakai S, Saito Y, Tanikawa W, Hirose T and Inagaki F (2013) Biological CO2 conversion to acetate in subsurface coal-sand formation using a high-pressure reactor system. Front. Microbiol. 4:361. doi: 10.3389/fmicb.2013.00361
Received: 11 October 2013; Paper pending published: 31 October 2013;
Accepted: 13 November 2013; Published online: 02 December 2013.
Edited by:
Mark A. Lever, Aarhus University, DenmarkReviewed by:
William D. Orsi, Woods Hole Oceanographic Institution, USACopyright © 2013 Ohtomo, Ijiri, Ikegawa, Tsutsumi, Imachi, Uramoto, Hoshino, Morono, Sakai, Saito, Tanikawa, Hirose and Inagaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fumio Inagaki, Kochi Institute for Core Sample Research, Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Monobe B200, Nankoku, Kochi 783-8502, Japan e-mail:aW5hZ2FraUBqYW1zdGVjLmdvLmpw
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.