- Institute for Molecular and Cell Biology, University of Porto, Porto, Portugal
An ample understanding of the complex interactions between host and pathogen will improve our ability to develop new prophylactic and therapeutic measures against infection. Precise classification of infectious agents in regards to their infective lifestyles in the host and corresponding pathogenic implications are required because clear concepts are essential to plan fruitful research. Classically, pathogenic bacteria are classified as extracellular, facultative intracellular, and obligate intracellular. In my opinion, this classification is inadequate because, as concluded from data here discussed, it is based on inconsistencies and hyper-valorizes the capacity of the infectious agent replicate in vitro in cell-free media. For a microbial pathogen, what matters is whether intra- or extracellularity is in the context of the in vivo life and in association with pathogenicity. When living as a pathogen in association with its host, what is relevant in microbiological terms is not the ability to grow in artificial cell-free bacteriological media or in environmental niches but whether the intracellular infectious agent, besides the phase of intracellular growth which is behind its label, also is able to live extracellularly in the natural settings of the extracellular territories of their hosts. To eliminate the inconsistencies associated with the classical labeling of bacterial pathogens, I propose that bacterial pathogens be labeled exclusive extracellular, dual intracellular/extracellular and exclusive intracellular based on their infective lifestyle in the host, not in the ability to grow in artificial bacteriological media.
Introduction
Infectious diseases continue to be a major threat to human health. Factors that contribute to this include the changes in life style such as the increasing migration, the rising resistance of pathogens to antibiotherapy, the increasing number of immunosuppressed patients, and potential bioterrorism threats. Thus, the need for the development of improved antimicrobial chemotherapeutics and prophylaxis strategies is increasing. An ample understanding of the complex interactions between host and pathogen will substantially improve our ability to develop such new measures. In this sense, the classification of infectious agents in regards to their infective lifestyles in the host and corresponding pathogenic implications must be precisely defined because clear concepts are essential to direct fruitful research including in prophylactic and therapeutic areas.
In symbiotic associations, parasitism is characterized by the cause of host disease by the microbe. Thus, pathogenic microbes are infective when they are associated with their hosts and live from pathogenicity producing host damage, although they may have separate ways of life like survival or replication in the environment. Residence and multiplication of bacterial pathogens at environmental niches originates a route for transmission to human and animal hosts through water, food, air, and vectors, and promotes interaction with free-living protozoa (Yildiz, 2007), therefore being contributing factors for microbial survival that increase the changes of a pathogen being a disease producer but that do not operate as factors of pathogenicity during the in vivo infection. Moreover, the ability to replicate extracellularly in vitro requires metabolic pathways and corresponding genes different from those used in the extracellular growth in the host (Fuchs et al., 2012). In the present text the analysis is focused on the pathogenic life of bacterial parasites since the discussion is related to the traditional classification of bacteria while pathogens. Classically, infectious agents are labeled as extracellular, facultative intracellular, and obligate intracellular pathogens (Goodpasture, 1936; Moulder, 1962; Brubaker, 1985). Discussion of published data revealed inconsistencies with that classification of bacterial pathogens. These inconsistencies (Box 1) will be analyzed in this article and alternatives proposed.
Box 1. Inconsistencies in the classical labeling of bacterial pathogens.
1. The ability of the pathogen to grow in artificial cell-free media has been hyper-valorized.
2. Some extracellular bacterial pathogens have been shown to have an intracellular in vivo phase which has pathogenic implications.
3. In the labels “facultative intracellular pathogen” or “facultative intracellular parasite” the use of the term “facultative” is not adequate since the ability to grow intracellularly during the infectious process is considered facultative while it is obligate and indispensable for disease production as the term “facultative” is used in the context of the infectious process produced by the pathogen.
4. The term “obligate” in “obligate intracellular pathogens” is based on the incapability to grow in artificial conditions while what counts for an infectious agent living from pathogenicity is what happens in the infected host.
Extracellular Pathogens
Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli are typical examples of bacteria which have been labeled extracellular pathogens, and wound infections, osteomyelitis, scarlet fever, certain forms of pneumonia, urinary tract infections are examples of infections caused by these pathogens (Nahm et al., 1999; Weiser and Nahm, 2008). To produce disease, extracellular pathogens use any portal of entry provided a satisfactory fluid medium be established at the site of injury. Typically, these pathogens multiply in the host at extracellular sites such as mucosal surfaces, vascular, lymphatic, and body cavity fluids, and in interstitial spaces (Stuart and Ezekowitz, 2005), although they may be occasionally seen within phagocytes when these attempt to kill them as a host defense mechanism.
Extracellular pathogens use virulence mechanisms to evade the antimicrobial capabilities of humoral immunity and phagocytosis thus promoting extracellular multiplication (Weiser and Nahm, 2008), in contrast with intracellular pathogens that promote the entry into host cells including macrophages and non-professional phagocytes such as epithelial cells (Sansonetti, 2001).
Important in the present context is that examples have been accumulating of bacterial pathogens classically classified as extracellular which promote their entry into host cells in vivo resulting in a phase of intracellular residence which is relevant for the initiation of the typical extracellular infection where diverse virulence factors, specific to each pathogen, produce the pathology typical of the disease (Weiser and Nahm, 2008). Examples of extracellular pathogens that use an initial intracellular phase include S. aureus (Garzoni and Kelley, 2009), S. pyogenes (Medina et al., 2003; Cole et al., 2010), Streptococcus pneumoniae (Bergmann et al., 2009), Bacillus anthracis (Russell et al., 2008), E. coli pathovars [causing neonatal meningitis (NMEC) Croxen and Finlay, 2010, enteroinvasive (EIEC; same as Shigella (Lan and Reeves, 2002) Croxen and Finlay, 2010), adherent invasive (AIEC; Barnich et al., 2007), and uropathogenic (UPEC; Hunstad and Justice, 2010)], Bordetella pertussis (Saukkonen et al., 1991), and Helicobacter pylori (Allen, 1999). In agreement with those in vivo results, the above extracellular pathogens have been shown to replicate or survive within cells cultured in vitro and have the capacity to survive/replicate in amoeba (Winiecka-Krusnell et al., 2002; Alsam et al., 2006; Huws et al., 2008; Evstigneeva et al., 2009; Saeed et al., 2009).
Intracellular Pathogens
Classical examples of intracellular pathogens are Brucella abortus, Listeria monocytogenes, Chlamydia trachomatis, Coxiella burnetii, Mycobacterium tuberculosis, Salmonella enterica, and typical infectious diseases caused by them include brucellosis, listeriosis, tuberculosis, and salmonellosis (Pamer, 2008). Intracellular pathogenic bacteria have the ability to establish a relationship in the susceptible host which includes a stage of intracellular multiplication (Moulder, 1962, 1985). To establish an infection, these pathogens have to make contact with the appropriated type of host cell that affords suitable intracellular conditions for growth. Classically, intracellular pathogens have been classified as obligate or facultative (Moulder, 1985). This classification has been based on the presence (in facultative intracellular bacteria) or absence (in obligate intracellular bacteria) of the capacity to multiply in a cell-free environment (Suter, 1956; Moulder, 1985). This feature has been usually evaluated by the ability to multiply in artificial bacteriological media (Moulder, 1985; Renesto et al., 2003; Toft and Andersson, 2010), a perspective which introduces a serious caveat in that classification (see next section).
Intracellular bacterial pathogens use a wide range of host cells both professional (macrophages) and non-professional phagocytes like epithelial and endothelial cells and hepatocytes (Moulder, 1985; Pamer, 2008). After entering the target host cell, the intracellular pathogen may follow diverse vacuolar or cytosolic pathways (examples and references in Knodler and Celli, 2010).
As will be later discussed in detail, a central issue here is that the intracellular stage of infection by facultative intracellular pathogens is indispensable for the pathogenicity of these bacteria whenever this has been investigated (reviewed in Leung and Finlay, 1991; Kaufmann, 1999; Bogdan, 2008).
Facultative and obligate intracellular pathogens are located in the extracellular space before they enter cells and in progressing intracellular infections the pathogen must transit from one host cell to the next to spread the infection. Although a few intracellular pathogens are capable of intercellular transmission without an extracellular phase, most have extracellular passages when in transit (Hybiske and Stephens, 2008). However, several facultative intracellular pathogens have another type of extracellular life in the host independent from the cell–cell transit (Figure 1), which represents a particular disease stage and confers a dual intracellular/extracellular lifestyle that provides those infectious agents with survival and pathogenic advantages. The recognition of this implies that we have to consider that “in vivo bacteria” may include intracellular and extracellular lifestyles (Hazlett and Cirillo, 2009). In some cases, this extracellular stage involves systemic dissemination and multiplication and may be exuberant and responsible for the severity of the disease, as, for example, in plague (Sebbane et al., 2005), tularemia (Parmely et al., 2009), melioidosis (Wiersinga et al., 2006). During this second phase, diverse additional virulence factors enter into play to produce pathology specific for each disease. One frequent complication of the extracellular phase of infection by facultative intracellular bacterial pathogens when it becomes serious is severe sepsis, a clinical condition with a very high mortality rate, that represents a patient’s dysregulated response to a severe infection (Bone et al., 1992; Stearns-Kurosawa et al., 2010).
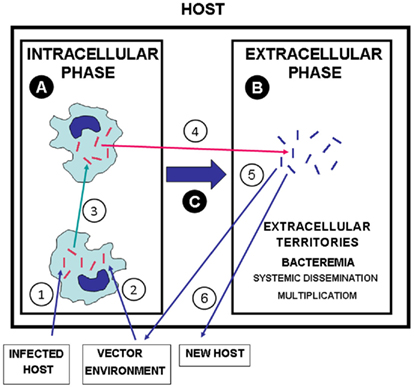
Figure 1. The dual lifestyle of intracellular/extracellular bacterial pathogens. (1) Transmission of bacterial pathogens to a new host from infected hosts, (2) from vectors and environment (water, soil, etc.). (3) Intercellular transit in the INTRACELLULAR PHASE (A). (4) Egress of intracellular pathogens to extracellular territories of the host leading to systemic dissemination and extracellular replication [EXTRACELLULAR PHASE (B)]. (C) Switch from the INTRACELLULAR PHASE to the EXTRACELLULAR PHASE, which may be essential for the pathogenesis. (5) Transmission of extracellular bacterial pathogens from infected hosts to vector and environment. (6) Transmission of extracellular pathogens from an infected host to a new host. Red bacteria: bacteria with phenotypes adapted to intracellular life and intercellular transit. Blue bacteria: bacteria with phenotypes adapted to extracellular multiplication in the host.
Diverse facultative bacterial intracellular pathogens have briefly been reported to exhibit the dual style of infectivity showing in the host a dual biphasic intracellular/extracellular microbiological behavior. This includes, among others, L. monocytogenes (Hof et al., 1997), Brucella sp. (Anderson et al., 1986), Erysipelothrix rhusiopathiae (Bender et al., 2009), Tropheryma whipplei (Renesto et al., 2003), and Shigella (or E. coli pathovar EIEC; Lan and Reeves, 2002; Croxen and Finlay, 2010). However, the extracellular behavior of most of these pathogens has not been fully analyzed in vivo and the relevance for pathogenicity of their extracellular phases in the host is not yet acceptably clarified.
Examples of infectious diseases where a dual intracellular/extracellular style of infectivity has been well studied include infections by Yersinia pestis (Perry and Fetherston, 1997; Sebbane et al., 2005), Francisella tularensis (Eisen et al., 2009; Parmely et al., 2009), Burkholderia pseudomallei (Reckseidler-Zenteno et al., 2005), Burkholderia cenocepacia (Wiersinga et al., 2006), S. enterica serovar Typhimurium (S. Typhimurium; MacLennan et al., 2008; Albaghdadi et al., 2009), Edwardsiella tarda (Wang et al., 2005; Mohanty and Sahoo, 2007), and M. tuberculosis (Dannenberg Jr., 1994; Bru and Cardona, 2010). These pathogens have the ability to produce extracellular infections as a second phase after the initial intracellular stage (Figure 1). These infections may include escape from its initial intracellular niche back into the gut or gall bladder lumen as part of its infectious cycle, as in the case of Salmonella infection, a step that is essential for dissemination and transmission to other hosts (Knodler et al., 2010), but in the above infections the extracellular phase may play a critical role in the pathogenesis of the infection and may lead to the terminal infectious episode preceding the death of the infected host.
Problems with the Classical Classification of Bacterial Pathogens
Extracellular in vitro and in vivo Growth and the Classification of Bacterial Pathogens
In the life of a microbe, the intracellularity and extracellularity are vague designations unless clearly related to the setting where it is living. The ability of the pathogen to grow in artificial cell-free media has been hyper-valorized as a parameter for the classification of pathogens. Isolation and identification of bacteria in vitro in pure culture in an artificial medium is invaluable and represents a major historical landmark of the pioneer studies by Koch, Pasteur and followers. In vitro cultivation of bacteria provides several important functions: (1) isolation and identification of bacteria in pure culture, (2) study of basic microbiology, (3) antibiotic sensitivity testing, (4) production of antigens and vaccines. However, it is not possible to duplicate in artificial cell-free bacteriological media the complex environment bacteria encounter in vivo when growing extracellularly, environment that involves much more than nutritional needs. It is interesting that Koch (1880) himself recognized that there are no better cultivation conditions for pathogenic bacteria than the animal body itself. The metabolic and other physiological parameters that bacteria require to grow extracellularly in vivo are not the same they use to grow in vitro as the two environments are quite diverse and impossible to adequately duplicate. In vitro pathogen proteomes are different from those present in host conditions.
Legionella are considered facultative intracellular pathogens because they are able to grow in artificial cell-free media (Pine et al., 1979; Toft and Andersson, 2010). However, when viewed as pathogens, Legionella enter and replicate in host cells (Newton et al., 2010), and there is no available data showing that they are able to multiply extracellularly in this setting.
Some intracellular bacterial pathogens are able to replicate or to survive in environmental niches. Residence and multiplication of bacterial pathogens at environmental niches originates a route for transmission to human and animal hosts through water, food, air, and vectors, and promotes interaction with free-living protozoa (Yildiz, 2007), therefore being contributing factors for microbial survival. However, a central issue here is that, for a microbial pathogen, what matters is whether intra- or extracellularity is in the context of the in vivo life and in association with pathogenicity. When analyzing a bacterial pathogen as an infectious agent that is producing disease, what is relevant is its behavior in the host. Thus, intra- or extracellularity should be considered in the host setting, and the ability to grow in artificial cell-free media is not relevant. When living as a pathogen in association with its host, important in microbiological terms is whether the intracellular infectious agent, besides the phase of intracellular growth which is behind its label, also is able to live extracellularly in the settings of the extracellular territories of their hosts.
The Inconsistency of the Label Facultative Intracellular Pathogen
In the term “facultative intracellular pathogens” the adjective “facultative” refers to “intracellular” to indicate that these infectious agents are able to replicate inside and outside cells (Moulder, 1985). But the problem is that the use of the term “facultative” is in the context of pathogenicity since that adjective is part of the label “facultative intracellular pathogens.” And, when living as pathogens, the facultative intracellular parasites must have a phase of intracellular life in the host it infects, phase which is obligate as it is required for pathogenicity and involves specific virulent genes (reviewed in References Leung and Finlay, 1991; Bogdan, 2008; Alkhuder et al., 2010; Fuchs et al., 2012). This has been specifically demonstrated for infectious diseases due to Y. pestis (Oyston et al., 2000; Bliska and Casadevall, 2009), F. tularensis (Bar-Haim et al., 2008; Qin et al., 2009), B. pseudomallei (Pilatz et al., 2006), E. tarda (Okuda et al., 2006), S. Typhimurium (Fields et al., 1986; Leung and Finlay, 1991), M. tuberculosis (Kaufmann, 1993). This stage of the life cycle of these pathogens (phase A in Figure 1) has been the preferred topic of most studies and the subject of many comprehensive reviews (Casadevall, 2008; Malik-Kale et al., 2011).
Therefore, the use of “facultative” in the label “facultative intracellular pathogens” results in a misleading title as it states that the pathogen may or may not be intracellular during the in vivo infection. I propose the label “dual intracellular/extracellular bacterial pathogens” to refer to infectious agents among those classically labeled extracellular, facultative intracellular, or obligate intracellular which exhibit the dual pathogenicity-associated capacity to replicate intracellularly and extracellularly in the host during progressive in vivo infections (Figure 2). The capacity to invade and replicate within a variety of host cells and to multiply in host environments exploited by extracellular parasites, makes several intracellular/extracellular pathogens capable of causing some of the more severe human diseases (Moulder, 1962; Brubaker, 1985). This is the case, for example, of septicemic plague (Perry and Fetherston, 1997), tularemia (Eisen et al., 2009; Parmely et al., 2009), and edwardsiellosis (Janda and Abbott, 1993).
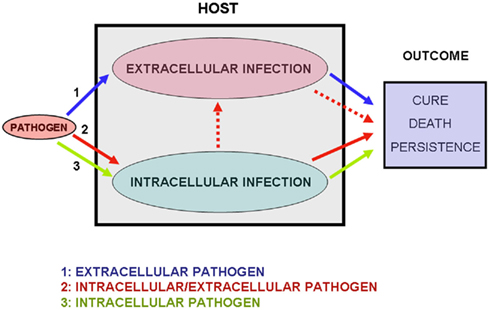
Figure 2. The three modes of microbial infection. In situation 1 (blue arrows) only the extracellular phase of infection occurs. I label the bacteria following this infectious process “exclusive extracellular pathogens.” In situation 2 (red arrows) extracellular replication occurs after a phase of intracellular multiplication. I label these infectious agents “dual intracellular/extracellular pathogens.” In some infections the extracellular phase may originate new phases of intracellular replication, as is the case of pulmonary tuberculosis (Dannenberg Jr., 1994; see main text). In situation 3 (green arrows) only the intracellular phase occurs. I label these infectious agents “exclusive intracellular pathogens.” The outcome of the infections will depend on the virulence of the pathogen and on the immune competence of the host.
The Label Obligate Intracellular Pathogen also is Inconsistent
The characterization of obligate intracellular parasitism based on the inability to grow in bacteriological media raises several problems. First, that parameter is flawed by its dependency on the state of the cultivation art (Moulder, 1985). Several bacterial pathogens considered paradigms of obligate intracellular parasitism have later been shown to be able to multiply in improved cell-free bacteriological media as in the classical example with Mycobacterium lepraemurium (Ishaque, 1981). This is also the case, among others, with Ehrlichia chaffeensis that was shown to undergo DNA replication and cell division in vitro when incubated in plasma (Li and Winslow, 2003), and with T. whipplei (Renesto et al., 2003) and C. burnetii (Omsland et al., 2009) that exhibits significant growth in new complex bacteriological media. Moreover, as discussed above, bacteriological media provide artificial conditions which do not adequately allow the assessment the pathogen’s ability to grow in a cell-free mode in natural settings like the host extracellular territories. The ability to grow in vitro outside host cells suggests the possibility of intracellular pathogens having a phase of extracellular life with pathogenic implications in the infected host, but the inability to grow extracellularly in vitro is not proof that extracellular multiplication is not occurring in the host as this has not been thoroughly investigated. Furthermore, in some intracellular pathogens like T. whipleii, the presence of intact cells promotes extracellular bacterial growth when co-cultured in vitro with cells (La Scola et al., 2001). The presence of large aggregates of bacteria including dividing cells in these culture supernatants suggested that T. whipplei may not be a strictly intracellular bacterium (La Scola et al., 2001) and, indeed, this pathogen was latter able to grow axenically (Renesto et al., 2003). This promotion of in vitro extracellular growth of T. whipplei by the presence of intact host cells indicates that extracellular multiplication in the host may occur even with pathogens that have not been cultivated in cell-free media. However, the likely pathogenetic implications of the capacity of obligate intracellular bacterial pathogens to replicate in bacteriological media have not been thoroughly evaluated (Box 2).
Box 2. Important questions for future research.
Further in vivo research is needed to clarify the following points:
1. What is the actual relevance of the occurrence of pathogenicity-associated intracellular life among the classically labeled extracellular pathogens?
2. What is the actual relevance of the occurrence of extracellular infective life of classically labeled facultative intracellular pathogens? How important for pathogenesis are the incomplete descriptions of extracellular residence/replication of some facultative intracellular pathogens, including L. monocytogenes, Brucella sp., E. rhusiopathiae, T. whipplei and Shigella (or E. coli pathovar EIEC)?
3. What is the actual relevance of the occurrence of extracellular infective life of classically labeled obligate intracellular pathogens? Is it possible to have in vivo extracellular replication in infections by obligate intracellular parasites that have so far been impossible to grow in vitro in cell-free media?
Conclusion
In the life of a microbe, intracellularity and extracellularity are vague designations unless clearly related to the setting where it is living. For a microbial pathogen when living as a pathogen, what matters is whether intra- or extracellularity is in the context of the in vivo life and in association with virulence not the ability to survive/replicate in the environment or artificial cell-free media.
In my opinion, a major point is that the characterization of bacteria as pathogenic agents should be based on the infective lifestyles they exhibit in infected hosts or in adequate experimental hosts as these are the relevant environments to evaluate pathogenicity. From this perspective, the classical classification of microbial pathogens in extracellular, facultative intracellular, and obligate intracellular is inadequate and I propose, according to their lifestyles in the host, to label these groups as exclusive extracellular, dual intracellular/extracellular, and exclusive intracellular pathogens, respectively (Figure 2). The distribution of bacterial pathogens following this classification is not a static process since it will be dependent on the likely discovery of new cases of dual intracellular/extracellular pathogens.
The ability for a dual life style with pathogenic implications of bacterial infectious agents in the host appears as an important trait which has been underappreciated and not thoroughly analyzed. Information in this topic is largely incomplete creating an important area of ignorance that urges for further research (see Box 2) which likely will be an expanding process revealing new cases of dual intra/extracellular pathogens among the three types of bacterial parasites (Figure 3).
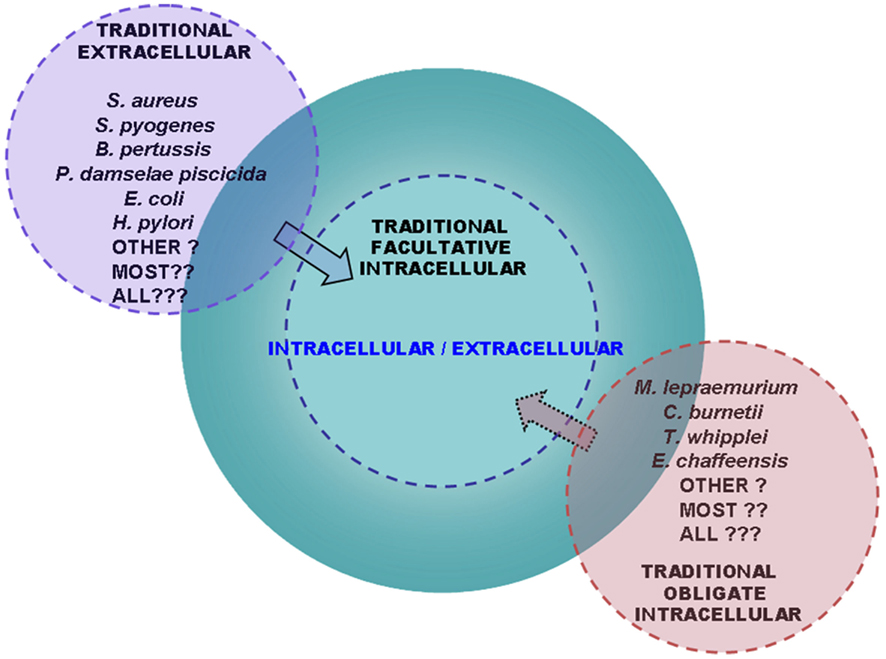
Figure 3. The group of bacterial pathogens with a dual intracellular/extracellular life style in the host is widespread and expanding. Scheme showing the advanced perspective that the group enclosing intracellular/extracellular bacterial pathogens is continuously enlarging. With advances in the research in the field, traditionally labeled extracellular bacterial pathogens (left) have been transferred to the group of dual intracellular/extracellular pathogens (center) through the demonstration that they use to their advantage the virulence-associated ability to grow intracellularly in the host. On the other hand, traditionally labeled obligate intracellular pathogens (right) have been found to be capable of cell-free replication in vitro although the implications for pathogenicity in vivo of this attribute have not been assessed. Perspectives similar to those in this scheme have previously been hypothesized in relation to the traditionally labeled obligate intracellular or extracellular parasites by Moulder (1985) and Casadevall (2008), respectively. Emerging evidence discussed in this review has strengthened the view of a trend toward the progressive enlargement of the group of intracellular/extracellular bacterial pathogens at the expenses of extracellular and obligate intracellular infectious agents and by discovering new cases of intracellular/extracellular bacteria among facultative intracellular pathogens.
Precise classification of infectious agents in regards to their infective lifestyles in the host and understanding the immunological and pathogenic implications of those lifestyles, are required because clear concepts are essential to direct fruitful research and to define prophylactic and therapeutic measures. Research on microbial and host factors that contribute to the success of the pathogens with a dual lifestyle may lead to novel strategies for prevention and treatment.
Obtaining relevant information about the lifestyles of pathogens in their hosts calls for studies exploiting in vivo investigations of infectious diseases in natural or representative experimental hosts using physiological conditions such as small inoculum, natural infection routes, and susceptible, untreated hosts. “Science is Nature, accurately seen” (Proper, 1989).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I’m grateful to Nazaré Pestana, David O’Callaghan, Annette Vergunst, A. Gil Castro, and Margarida Correia-Neves for helpful discussions and for reviewing the manuscript, and to José Pestana and Anabela Costa for editorial assistance.
References
Albaghdadi, H., Robinson, N., Finlay, B., Krishnan, L., and Sad, S. (2009). Selectively reduced intracellular proliferation of Salmonella enterica serovar typhimurium within APCs limits antigen presentation and development of a rapid CD8 T cell response. J. Immunol. 183, 3778–3787.
Alkhuder, K., Meibom, K. L., Dubail, I., Dupuis, M., and Charbit, A. (2010). Identification of trkH, encoding a potassium uptake protein required for Francisella tularensis systemic dissemination in mice. PLoS ONE 5, e8966. doi:10.1371/journal.pone.0008966
Allen, L. A. (1999). Intracellular niches for extracellular bacteria: lessons from Helicobacter pylori. J. Leukoc. Biol. 66, 753–756.
Alsam, S., Jeong, S. R., Sissons, J., Dudley, R., Kim, K. S., and Khan, N. A. (2006). Escherichia coli interactions with Acanthamoeba: a symbiosis with environmental and clinical implications. J. Med. Microbiol. 55(Pt 6), 689–694.
Anderson, T. D., Cheville, N. F., and Meador, V. P. (1986). Pathogenesis of placentitis in the goat inoculated with Brucella abortus. II. Ultrastructural studies. Vet. Pathol. 23, 227–239.
Bar-Haim, E., Gat, O., Markel, G., Cohen, H., Shafferman, A., and Velan, B. (2008). Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog. 4, e1000211. doi:10.1371/journal.ppat.1000211
Barnich, N., Carvalho, F. A., Glasser, A. L., Darcha, C., Jantscheff, P., Allez, M., Peeters, H., Bommelaer, G., Desreumaux, P., Colombel, J. F., and Darfeuille-Michaud, A. (2007). CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J. Clin. Invest. 117, 1566–1574.
Bender, J. S., Kinyon, J. M., Kariyawasam, S., Halbur, P. G., and Opriessnig, T. (2009). Comparison of conventional direct and enrichment culture methods for Erysipelothrix spp. from experimentally and naturally infected swine. J. Vet. Diagn. Invest. 21, 863–868.
Bergmann, S., Lang, A., Rohde, M., Agarwal, V., Rennemeier, C., Grashoff, C., Preissner, K. T., and Hammerschmidt, S. (2009). Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J. Cell. Sci. 122(Pt 2), 256–267.
Bliska, J. B., and Casadevall, A. (2009). Intracellular pathogenic bacteria and fungi – a case of convergent evolution? Nat. Rev. Microbiol. 7, 165–171.
Bogdan, C. (2008). Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell. Microbiol. 10, 1221–1234.
Bone, R. C., Balk, R. A., Cerra, F. B., Dellinger, R. P., Fein, A. M., Knaus, W. A., Schein, R. M., Sibbald, W. J., and ACCP/SCCM Consensus Conference Committee. (1992). Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101, 1644–1655.
Bru, A., and Cardona, P. J. (2010). Mathematical modeling of tuberculosis bacillary counts and cellular populations in the organs of infected mice. PLoS ONE 5, e12985. doi:10.1371/journal.pone.0012985
Cole, J. N., Pence, M. A., von Köckritz-Blickwede, M., Hollands, A., Gallo, R. L., Walker, M. J., and Nizet, V. (2010). M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. MBio 1, e00191-10.
Croxen, M. A., and Finlay, B. B. (2010). Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38.
Dannenberg, A. M. Jr. (1994). “Pathogenesis of pulmonary tuberculosis: an interplay of time-damaging and macrophage activating immune responses. Dual mechanisms that control bacillary multiplication,” in Tuberculosis: Protection, Pathogenesis and Control ed. B. R. Bloom (Washington, D. C.: American Society for Microbiology), 459–483.
Eisen, R. J., Yockey, B., Young, J., Reese, S. M., Piesman, J., Schriefer, M. E., Beard, C. B., and Petersen, J. M. (2009). Short report: time course of hematogenous dissemination of Francisella tularensis A1, A2, and Type B in laboratory mice. Am. J. Trop. Med. Hyg. 80, 259–262.
Evstigneeva, A., Raoult, D., Karpachevskiy, L., and La Scola, B. (2009). Amoeba co-culture of soil specimens recovered 33 different bacteria, including four new species and Streptococcus pneumoniae. Microbiology 155(Pt 2), 657–664.
Fields, P. I., Swanson, R. V., Haidaris, C. G., and Heffron, F. (1986). Mutants of Salmonella Typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. U.S.A. 83, 5189–5193.
Fuchs, T. M., Eisenreich, W., Heesemann, J., and Goebel, W. (2012). Metabolic adaptation of human pathogenic and related nonpathogenic bacteria to extra- and intracellular habitats. FEMS Microbiol. Rev. 36, 435–462.
Garzoni, C., and Kelley, W. L. (2009). Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 17, 59–65.
Goodpasture, E. W. (1936). Intracellular parasitism and the cytotropism of viruses. South. Med. J. 29, 297–303.
Hazlett, K. R., and Cirillo, K. A. (2009). Environmental adaptation of Francisella tularensis. Microbes Infect. 11, 828–834.
Hof, H., Nichterlein, T., and Kretschmar, M. (1997). Management of listeriosis. Clin. Microbiol. Rev. 10, 345–357.
Hunstad, D. A., and Justice, S. S. (2010). Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 64, 203–221.
Huws, S. A., Morley, R. J., Jones, M. V., Brown, M. R., and Smith, A. W. (2008). Interactions of some common pathogenic bacteria with Acanthamoeba polyphaga. FEMS Microbiol. Lett. 282, 258–265.
Hybiske, K., and Stephens, R. S. (2008). Exit strategies of intracellular pathogens. Nat. Rev. Microbiol. 6, 99–110.
Ishaque, M. (1981). In vitro cultivation of Mycobacterium lepraemurium and its identification by animal inoculation. Can. J. Microbiol. 27, 788–794.
Janda, J. M., and Abbott, S. L. (1993). Infections associated with the genus Edwardsiella: the role of Edwardsiella tarda in human disease. Clin. Infect. Dis. 17, 742–748.
Kaufmann, S. H. E. (1999). “Immunity to intracellular bacteria,” in Fundamental Immunology, ed. W. E. Paul (New York, NY: Lippincott-Raven), 1335–1371.
Knodler, L. A., and Celli, J. (2010). Eating the strangers within: host control of intracellular bacteria via xenophagy. Cell. Microbiol. 13, 1319–1327.
Knodler, L. A., Vallance, B. A., Celli, J., Winfree, S., Hansen, B., Montero, M., and Steele-Mortimer, O. (2010). Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. U.S.A. 107, 17733–17738.
Koch, R. (1880). Investigations into the Etiology of Traumatic Infective Diseases. London: The New Sydenham society.
La Scola, B., Fenollar, F., Fournier, P. E., Altwegg, M., Mallet, M. N., and Raoult, D. (2001). Description of Tropheryma whipplei gen. nov., sp. nov., the Whipple’s disease bacillus. Int. J. Syst. Evol. Microbiol. 51(Pt 4), 1471–1479.
Lan, R., and Reeves, P. R. (2002). Escherichia coli in disguise: molecular origins of Shigella. Microbes Infect. 4, 1125–1132.
Leung, K. Y., and Finlay, B. B. (1991). Intracellular replication is essential for the virulence of Salmonella Typhimurium. Proc. Natl. Acad. Sci. U.S.A. 88, 11470–11474.
Li, J. S., and Winslow, G. M. (2003). Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect. Immun. 71, 4229–4237.
MacLennan, C. A., Gondwe, E. N., Msefula, C. L., Kingsley, R. A., Thomson, N. R., White, S. A., Goodall, M., Pickard, D. J., Graham, S. M., Dougan, G., Hart, C. A., Molyneux, M. E., and Drayson, M. T. (2008). The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118, 1553–1562.
Malik-Kale, P., Jolly, C. E., Lathrop, S., Winfree, S., Luterbach, C., and Steele-Mortimer, O. (2011). Salmonella – at home in the host cell. Front. Microbiol. 2:125. doi:10.3389/fmicb.2011.00125
Medina, E., Goldmann, O., Toppel, A. W., and Chhatwal, G. S. (2003). Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 187, 597–603.
Mohanty, B. R., and Sahoo, P. K. (2007). Edwardsiellosis in fish: a brief review. J. Biosci. 32, 1331–1344.
Moulder, J. W. (1962). The Biochemistry of Intracellular Parasitism. Chicago: The University of Chicago Press.
Moulder, J. W. (1985). Comparative biology of intracellular parasitism. Microbiol. Rev. 49, 298–337.
Nahm, M. H., Apicella, M. A., and Briles, D. E. (1999). “Immunity to extracellular bacteria,” in Fundamental Immunology, ed. W. E. Paul (Philadelphia, PA, Lippincott-Raven), 1373–1386.
Newton, H. J., Ang, D. K., van Driel, I. R., and Hartland, E. L. (2010). Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 23, 274–298.
Okuda, J., Arikawa, Y., Takeuchi, Y., Mahmoud, M. M., Suzaki, E., Kataoka, K., Suzuki, T., Okinaka, Y., and Nakai, T. (2006). Intracellular replication of Edwardsiella tarda in murine macrophage is dependent on the type III secretion system and induces an up-regulation of anti-apoptotic NF-kappaB target genes protecting the macrophage from staurosporine-induced apoptosis. Microb. Pathog. 41, 226–240.
Omsland, A., Cockrell, D. C., Howe, D., Fischer, E. R., Virtaneva, K., Sturdevant, D. E., Porcella, S. F., and Heinzen, R. A. (2009). Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U.S.A. 106, 4430–4434.
Oyston, P. C., Dorrell, N., Williams, K., Li, S. R., Green, M., Titball, R. W., and Wren, B. W. (2000). The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68, 3419–3425.
Pamer, E. (2008). “Immune responses to intracellular bacteria,” in Fundamental Immunology, ed. W. E. Paul (Philadelphia: Lippincott Williams & Wilkins), 1165–1181.
Parmely, M. J., Fischer, J. L., and Pinson, D. M. (2009). Programmed cell death and the pathogenesis of tissue injury induced by type A Francisella tularensis. FEMS Microbiol. Lett. 301, 1–11.
Perry, R. D., and Fetherston, J. D. (1997). Yersinia pestis – etiologic agent of plague. Clin. Microbiol. Rev. 10, 35–66.
Pilatz, S., Breitbach, K., Hein, N., Fehlhaber, B., Schulze, J., Brenneke, B., Eberl, L., and Steinmetz, I. (2006). Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect. Immun. 74, 3576–3586.
Pine, L., George, J. R., Reeves, M. W., and Harrell, W. K. (1979). Development of a chemically defined liquid medium for growth of Legionella pneumophila. J. Clin. Microbiol. 9, 615–626.
Qin, A., Scott, D. W., Thompson, J. A., and Mann, B. J. (2009). Identification of an essential Francisella tularensis subsp. tularensis virulence factor. Infect. Immun. 77, 152–161.
Reckseidler-Zenteno, S. L., DeVinney, R., and Woods, D. E. (2005). The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect. Immun. 73, 1106–1115.
Renesto, P., Crapoulet, N., Ogata, H., La Scola, B., Vestris, G., Claverie, J. M., and Raoult, D. (2003). Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet 362, 447–449.
Russell, B. H., Liu, Q., Jenkins, S. A., Tuvim, M. J., Dickey, B. F., and Xu, Y. (2008). In vivo demonstration and quantification of intracellular Bacillus anthracis in lung epithelial cells. Infect. Immun. 76, 3975–3983.
Saeed, A., Abd, H., Edvinsson, B., and Sandström, G. (2009). Acanthamoeba castellanii an environmental host for Shigella dysenteriae and Shigella sonnei. Arch. Microbiol. 191, 83–88.
Sansonetti, P. (2001). Phagocytosis of bacterial pathogens: implications in the host response. Semin. Immunol. 13, 381–390.
Saukkonen, K., Cabellos, C., Burroughs, M., Prasad, S., and Tuomanen, E. (1991). Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J. Exp. Med. 173, 1143–1149.
Sebbane, F., Gardner, D., Long, D., Gowen, B. B., and Hinnebusch, B. J. (2005). Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 166, 1427–1439.
Stearns-Kurosawa, D. J., Osuchowski, M. F., Valentine, C., Kurosawa, S., and Remick, D. G. (2010). The pathogenesis of sepsis. Annu. Rev. Pathol. 6, 19–48.
Suter, E. (1956). Interaction between phagocytes and pathogenic microorganisms. Bacteriol. Rev. 20, 94–132.
Toft, C., and Andersson, S. G. (2010). Evolutionary microbial genomics: insights into bacterial host adaptation. Nat. Rev. Genet. 11, 465–475.
Wang, I. K., Kuo, H. L., Chen, Y. M., Lin, C. L., Chang, H. Y., Chuang, F. R., and Lee, M. H. (2005). Extraintestinal manifestations of Edwardsiella tarda infection. Int. J. Clin. Pract. 59, 917–921.
Weiser, J. N., and Nahm, M. H. (2008). “Immunity to extracellular bacteria,” in Fundamental Immunology, ed. W. E. Paul (Philadelphia: Lippincot Williams & Wilkins), 1182–1203.
Wiersinga, W. J., van der Poll, T., White, N. J., Day, N. P., and Peacock, S. J. (2006). Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4, 272–282.
Winiecka-Krusnell, J., Wreiber, K., von Euler, A., Engstrand, L., and Linder, E. (2002). Free-living amoebae promote growth and survival of Helicobacter pylori. Scand. J. Infect. Dis. 34, 253–256.
Keywords: extracellular pathogens, facultative intracellular pathogens, obligate intracellular pathogens, dual intra/extracellular pathogens
Citation: Silva MT (2012) Classical labeling of bacterial pathogens according to their lifestyle in the host: inconsistencies and alternatives. Front. Microbio. 3:71. doi: 10.3389/fmicb.2012.00071
Received: 08 January 2012;
Accepted: 11 February 2012;
Published online: 29 February 2012.
Edited by:
Hao Shen, University of Pennsylvania School of Medicine, USAReviewed by:
Sing Sing Way, University of Minnesota, USASunny Shin, University of Pennsylvania, USA
Copyright: © 2012 Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Manuel T. Silva, Instituto de Biologia Molecular e Celular, Rua do Campo Alegre 823, Porto, Portugal. e-mail:bXRzaWx2YUBpYm1jLnVwLnB0
