- 1 Department of Molecular and Applied Microbiology, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Jena, Germany
- 2 Department of Microbiology and Molecular Biology, Friedrich Schiller University, Jena, Germany
- 3 Department of Infection Biology, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Jena, Germany
- 4 Electron Microscopic Centre, Clinics of the Friedrich Schiller University, Jena, Germany
Aspergillus fumigatus is the most important air-borne fungal pathogen of humans. The interaction of the pathogen with the host’s immune system represents a key process to understand pathogenicity. For elimination of invading microorganisms, they need to be efficiently phagocytosed and located in acidified phagolysosomes. However, as shown previously, A. fumigatus is able to manipulate the formation of functional phagolysosomes. Here, we demonstrate that in contrast to pigmentless pksP mutant conidia of A. fumigatus, the gray-green wild-type conidia inhibit the acidification of phagolysosomes of alveolar macrophages, monocyte-derived macrophages, and human neutrophil granulocytes. Therefore, this inhibition is independent of the cell type and applies to the major immune effector cells required for defense against A. fumigatus. Studies with melanin ghosts indicate that the inhibitory effect of wild-type conidia is due to their dihydroxynaphthalene (DHN)-melanin covering the conidia, whereas the hydrophobin RodA rodlet layer plays no role in this process. This is also supported by the observation that pksP conidia still exhibit the RodA hydrophobin layer, as shown by scanning electron microscopy. Mutants defective in different steps of the DHN-melanin biosynthesis showed stronger inhibition than pksP mutant conidia but lower inhibition than wild-type conidia. Moreover, A. fumigatus and A. flavus led to a stronger inhibition of phagolysosomal acidification than A. nidulans and A. terreus. These data indicate that a certain type of DHN-melanin that is different in the various Aspergillus species, is required for maximal inhibition of phagolysosomal acidification. Finally, we identified the vacuolar ATPase (vATPase) as potential target for A. fumigatus based on the finding that addition of bafilomycin which inhibits vATPase, led to complete inhibition of the acidification whereas the fusion of phagosomes containing wild-type conidia and lysosomes was not affected.
Introduction
Filamentous fungi of the genus Aspergillus are ubiquitously found in nature and generally play important roles in the degradation of organic matter (Latge, 2001). Among the more than 240 Aspergillus species described until now, ca. 10–20% are regarded as pathogenic or to have other adverse effects.
The most prominent species of the pathogenic aspergilli is Aspergillus fumigatus. The main route of infections caused by A. fumigatus is via inhalation of conidia. It is assumed that we inhale several hundreds of A. fumigatus conidia during routine daily activities (Chazalet et al., 1998; Hospenthal et al., 1998; Latge, 2001). In immunocompetent hosts, conidia that reach the lung alveoli, are effectively cleared by the host immune system (Latge, 1999; Brakhage et al., 2010). However, as a consequence of continuous medical improvement, especially with regard to bone-marrow or solid organ transplantation, chemotherapy, and long-term corticosteroid therapy, the number of patients with a severely suppressed immune system significantly increased in the last decades. These patients have a high risk of an often fatal infection with A. fumigatus, designated as invasive aspergillosis. As a consequence of a compromised immune system, inhaled spores are not eliminated completely and can germinate and grow invasively within the lung tissue. After invasion of blood vessels, hyphae can disseminate throughout the host and infect all organs (Ruchel and Reichard, 1999), resulting in a lethality of up to 90% in case of invasive aspergillosis. Therefore, a much more detailed understanding of the infection process with special focus on the interaction of immune effector cells with conidia of A. fumigatus is of great interest.
Alveolar macrophages (AMs) are involved in the defense against an A. fumigatus infection. The phagocytosis and intracellular degradation of conidia by AMs contributes to the fungal clearance as well as the concerted secretion of proinflammatory cytokines and chemokines to recruit further phagocytes such as neutrophil granulocytes, that are essential for the defense, at the site of infection (Ibrahim-Granet et al., 2003; Philippe et al., 2003; Steele et al., 2005; Behnsen et al., 2007; Brakhage et al., 2010). A number of studies have been performed to identify mechanisms by which A. fumigatus interacts with the innate immune system. Pattern recognition receptors (PRRs) like dectin-1, TLR-2, and TLR-4 have been proposed to play a crucial role in sensing A. fumigatus. They appear to allow the immune defense to discriminate between different spore states like resting and swollen conidia, different morphological forms and certain cell wall components (Meier et al., 2003; Netea et al., 2003; Steele et al., 2005; Chai et al., 2011).
Recent studies indicate that A. fumigatus possesses strategies to evade these recognition processes, e.g., by hiding immunogenic structures of the conidial surface with an immunologically inert proteinaceous layer consisting of the hydrophobin RodA (Aimanianda et al., 2009). Swelling and germination of conidia, however, unmasks this protective layer and exposes β-1,3-glucan on their surface. This cell wall polysaccharide is recognized by the dectin-1 receptor on the surface of macrophages (Luther et al., 2007). The finding that dectin-1−/− and TLR2−/− mice that exhibit impaired production of cytokines and insufficient recruitment of neutrophil granulocytes, are more susceptible toward an A. fumigatus infection, further highlights the importance of macrophages in coordinating initial inflammatory reactions in response to pathogen recognition (Balloy et al., 2005; Werner et al., 2009; Ibrahim-Granet et al., 2010).
Nevertheless, beside the proper recognition of conidia by macrophages or phagocytes in general, a functional intracellular degradation of conidia when processed by the endocytic pathway, plays a decisive role in fungal clearance. A critical step in killing phagocytosed conidia is the fusion of lysosomes and the conidia-containing phagosome. The resulting phagolysosome contains degrading enzymes and generates an acidic pH after fusion (Forlenza et al., 2008). Until now, only a few studies addressed the intracellular fate of conidia, whereas exact mechanisms still remain to be elucidated (Jahn et al., 2002; Ibrahim-Granet et al., 2003; Kasperkovitz et al., 2010). Conidia of the A. fumigatus pksP mutant lack 1,8-dihydroxynaphthalene (DHN)-melanin, resulting in white conidia with a smooth surface (Jahn et al., 1997; Langfelder et al., 1998). Interestingly, such conidia were shown to be found in a much higher percentage in phagolysosomes than gray-green wild-type conidia. This finding well correlates with a more effective killing of pksP conidia by macrophages and their significant attenuation in virulence in a mouse infection model (Jahn et al., 1997, 2002). However, it remained unclear which mechanism of the phagolysosome maturation was inhibited by wild-type conidia and which components of the conidia were responsible for these effects.
Here, we comprehensively investigated the interaction of A. fumigatus conidia with macrophages and neutrophil granulocytes. We were able to show that conidia of the wild type but not the pksP mutant inhibited acidification of the phagolysosome. In the course of swelling and germination of conidia, this ability was decreased. The effect was shown to be independent of the presence of the conidial hydrophobin RodA. Data obtained with melanin ghosts, A. fumigatus mutants defective in different steps of the DHN-melanin biosynthesis as well as the investigation of Aspergillus species containing different melanin types indicate, that melanin is decisive for this effect. Our finding might also explain differences in virulence of various Aspergillus species.
Materials and Methods
Fungal Strains and Culture Conditions
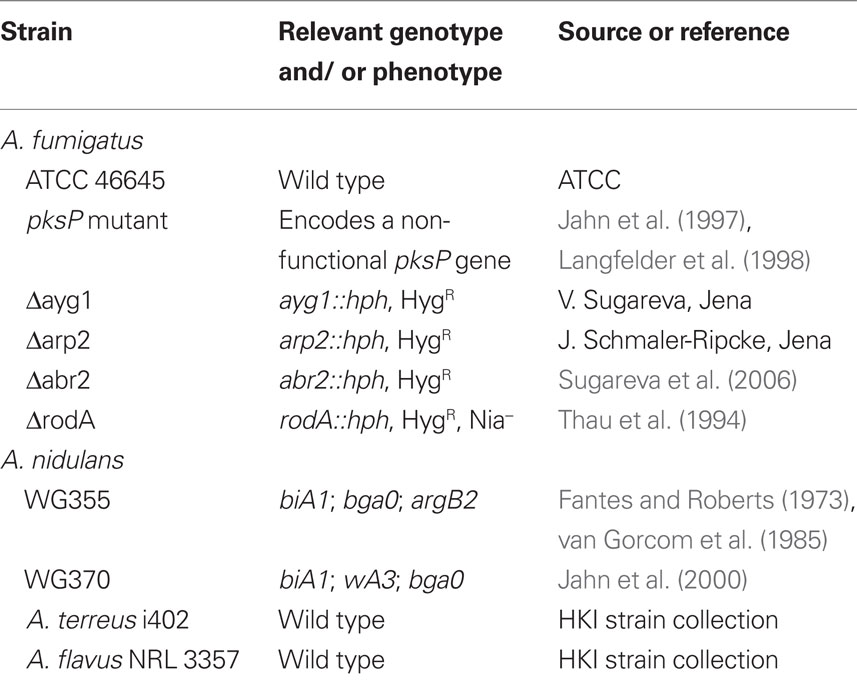
Aspergillus strains used in this study are listed in Table 1. All strains were cultivated for 5 days at 37°C on Aspergillus minimal medium (AMM) agar plates as described previously (Weidner et al., 1998). For propagation of A. nidulans, AMM was supplemented with 871 μg/ml arginine and 60 μg/ml biotin. Conidia were harvested in sterile 0.9% (wt/vol) NaCl, 0.1% (vol/vol) Tween 20, and filtered through 40-μm cell strainers (BD Biosciences, Heidelberg, Germany). Swollen conidia were prepared by incubating conidia in RPMI 1640 medium, supplemented with 10% (vol/vol) heat inactivated FCS (Lonza, Verviers, Belgium). Resting spores were fixed with PBS, 2.5% (vol/vol) p-formaldehyde at 4°C overnight, neutralized with 0.1 M NH4Cl, and resuspended in PBS.
FITC-/Calcofluor White-Labeling of Conidia
Prior to infection, resting, swollen or formaldehyde-fixed spores were stained with 250 μg/ml calcofluor white (CFW; Sigma-Aldrich, Germany) and washed three times with PBS, 0.1% (vol/vol) Tween 20. For intraphagosomal pH measurements, conidia were labeled with FITC as previously described (Sturtevant and Latge, 1992). Briefly, resting conidia were harvested and labeled with 0.1 mg/ml FITC (Sigma-Aldrich, Germany) in 0.1 M Na2CO3 for 30 min at 37°C and then washed three times with PBS, 0.1% (v/v) Tween 20. Spore concentration was determined using a Thoma counting chamber.
Preparation of Melanin Ghosts
Melanin ghosts from A. fumigatus wild-type conidia were prepared according to a method previously described (Youngchim et al., 2004). 2 × 109 conidia were washed three times with PBS. Cell walls were digested overnight with 10 mg/ml Glucanex® (Sigma-Aldrich, Germany) in 0.1 M sodium citrate and 1 M sorbitol, pH 5.5 at 30°C. The protoplasts generated were washed three times with PBS and incubated with 4 M guanidine thiocyanate solution (Sigma-Aldrich, Germany) overnight at room temperature. After three washing steps with PBS, dark particles were treated with 1 mg/ml proteinase K from Tritirachium album (Boehringer Mannheim, Germany) in 10 mM Tris, 1 mM CaCl2 and 0.1% (wt/vol) SDS overnight at 37°C. Particles were washed three times with PBS and boiled in 6 M hydrochloric acid for 1.5 h. The obtained particles designated melanin ghosts, were separated from the solution by centrifugation with Ultrafree-MC Centrifugal Filter Units with Microporous Membrane (Millipore, Germany) and washed extensively with PBS. After lyophilization, melanin ghosts were resuspended in PBS.
Cy5.5-Labeling of Melanin Ghosts
Melanin ghosts were washed three times in panning buffer (PB; 1 mM CaCl2, 50 mM Tris, 150 mM NaCl) and blocked for 1 h with PB, 2% (wt/vol) BSA to prevent non-specific binding. After two washing steps with PB, melanin ghosts were labeled by incubation with biotinylated anti-melanin camelid antibody (Schmaler-Ripcke et al., unpublished data) in high salt PB (HS-PB) (1 mM CaCl2, 50 mM Tris, 0.5 M NaCl), 2% (wt/vol) BSA, followed by three washes with HS-PB. Bound primary antibodies were labeled by incubation with Cy5.5-conjugated streptavidin in HS-PB, 2% (wt/vol) BSA. Labeled melanin ghosts were washed three times in HS-PB, 0.05% (vol/vol) Tween 20 and suspended in PB. Melanin ghost concentration was determined using a Thoma counting chamber.
Cell Lines
Murine alveolar MH-S macrophages (ATCC: CRL-2019) and human monocytic THP-1 cells (DSMZ: ACC16) were cultivated in RPMI 1640 supplemented with 10% (vol/vol) heat inactivated fetal calf serum (PAA), 1% (wt/vol) ultraglutamine 1 (Lonza, Verviers, Belgium), and 27.5 μg/ml gentamicin sulfate (Lonza, Verviers, Belgium) at 37°C in 5% (vol/vol) CO2. THP-1 macrophages were prepared by stimulating the monocytic cells with 0.4 μg/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, Germany) for 16 h. For infection experiments, macrophages were seeded in 24-well plates with or without glass cover slips at a density of 4 × 105 and 2 × 105 cells per well for MH-S and THP-1 cells, respectively, to grow adherently overnight.
Isolation of Human Neutrophils
Human neutrophils were isolated from peripheral blood of healthy donors according to the protocol of Wozniok et al. (2008). Briefly, neutrophils were collected after a gradient centrifugation of the peripheral blood in Polymorphprep® (Axis Shield, UK) at 550 × g. Subsequent erythrocyte lysis was carried out in ACK buffer following washing in HBSS buffer. Neutrophils were finally resuspended in RPMI media with 5% (vol/vol) heat inactivated FCS. For coincubation with A. fumigatus conidia, neutrophil granulocytes were plated on glass cover slips in 24-well plates at a density of 4 × 105 cells per well. After brief centrifugation, cells were immediately subjected to coincubation.
Fluorescence Image Analysis by Confocal Laser Scanning Microscopy
Quantitation of phagolysosomal acidification
Prior to infection, macrophages, monocyte-derived macrophages or neutrophils were preloaded with 50 nM LysoTracker Red DND-99 (Invitrogen, Darmstadt, Germany) in RPMI medium for 2 or 1 h for macrophages or neutrophil granulocytes, respectively. The cells were washed with prewarmed RPMI medium without LysoTracker and infected with CFW-labeled conidia at a multiplicity of infection (MOI) of 2. Synchronization of phagocytosis was performed by incubating the cells at 4°C for 30 min. The infection was started by washing the coincubation with prewarmed medium containing LysoTracker to additionally remove unbound conidia and shifting the cells to 37°C in a humidified CO2 incubator. The cells were allowed to take up conidia for 4 h. Phagocytosis was stopped by incubating the cells with ice-cold PBS and several washing steps with PBS. Samples were immediately subjected to microscopy and were visualized using a Zeiss LSM 5 Live (Carl Zeiss, Jena, Germany). Conidia surrounded by a ring-like LysoTracker-derived fluorescence signal were considered as conidia residing in an acidified compartment, designated as acidified conidia. At least 400 conidia were counted and scored for acidification or no acidification. The values represent mean ± SD of three experiments.
Quantitation of phagolysosomal fusion
To evaluate phagolysosome fusion, confluent monolayers of macrophages at a density of 4 × 105 cells per well were incubated with 10% (vol/vol) FCS/RPMI 1640 in the presence of 100 μg/ml Oregon Green® 488- or TMR-Dextran, 70,000 MW (Invitrogen, Darmstadt, Germany) for 18 h, then washed and loaded with dextran for 2 h allowing accumulation of labeled dextran in lysosomes. Macrophages were infected with CFW-labeled live conidia at an MOI of 2 and immediately incubated at 4°C for 30 min to synchronize phagocytosis. Unbound conidia were removed by washing with prewarmed RPMI medium and phagocytosis was initiated by shifting the cells to 37°C in a humidified CO2 incubator. Conidial uptake was stopped after 4 h by washing the cells with ice-cold PBS, followed by washing with PBS. For vacuolar ATPase (vATPase)-inhibition studies, 100 nM bafilomycin A1 (Sigma-Aldrich, Germany) was added during coincubation. Samples were visualized using a Zeiss LSM 5 Live. The presence of a periconidial ring-like structure surrounding the ingested conidium was considered as fusion of the conidia-containing phagosome with the dextran-labeled lysosome. At least 400 intracellular conidia were counted and scored for phagolysosomal fusion or lack of fusion. The values represent mean ± SD of three independent experiments.
Determination of Intraphagosomal pH
Confluent monolayers of macrophages were grown in 24 Lumox® well plate (Sarstedt, Nümbrecht, Germany) in RPMI medium without phenol red. They were infected with live FITC-labeled conidia at an MOI of 5 and phagocytosis was allowed to synchronize. The coincubation was shifted to 37°C and internalization was stopped after 4 h. After removal of non-adherent conidia by gentle washing with prewarmed medium without phenol red, the cells were rinsed with PBS. The intracellular pH was calculated by linear regression analysis from the fluorescence intensity ratio at Ex495 nm/Ex450 nm (Ohkuma and Poole, 1978) in comparison to a standard curve using a Tecan Infinite® M200 plate reader (Tecan, Crailsheim, Germany). This standard curve was generated by determination of fluorescence intensity ratios of macrophages infected by FITC-labeled conidia, permeabilized by incubation with PBS, 10 μM nigericin (Sigma-Aldrich, Germany) at pH values ranging from 4 to 7. The values represent mean ± SD of three independent experiments.
Scanning Electron Microscopy
For preparation of scanning electron microscopy (SEM) samples, resting conidia grown on AMM agar plates were collected on a electrically conductive and adhesive tag (Leit-Tab, Plano GmbH, Wetzlar, Germany). Because of the low water content of spores a critical point drying was not performed. To avoid surface charging the samples were coated with platinum (thickness approximately 2 nm) by high vacuum evaporation using a BAF 400 D (BALTEC, Liechtenstein). This technique provides a much better surface quality at high magnification than the common sputter coating. Finally the specimen were investigated with a field emission (FE) SEM LEO-1530 Gemini (Carl Zeiss NTS GmbH, Oberkochen, Germany) at electron energy of 8 keV using the in-lens secondary electron detector and magnifications of 50,000× to 200,000×.
Blast Analyses
BLAST analyses (www.ncbi.nlm.nih.gov/BLAST/) using the blastP protein–protein BLAST algorithm, were performed to identify homologs of A. fumigatus PksP in other aspergilli. Identities were calculated in % referring to the A. fumigatus PksP sequence as 100%.
Results
The Inhibition of Acidification of Phagolysosomes by A. fumigatus Conidia Occurs in Alveolar Macrophages, Monocyte-Derived Macrophages, and Human Neutrophils
In a previous study, we showed that conidia of A. fumigatus are capable to influence the phagolysosomes of human monocyte-derived macrophages thereby leading to higher survival of conidia (Jahn et al., 2002). Here, to analyze whether this effect is of general importance also for other cell types, we (i) improved the detection method to measure phagolysosome fusion and acidification and, (ii) extended the investigations to other cell types. As shown in Figure 1, compared to pigmentless pksP mutant conidia, the pigmented wild-type conidia led to a strong reduction of acidified compartments in which the conidia reside in all three cell types analyzed, i.e., the AM cell line MH-S, human monocyte-derived macrophage cell line THP-1, and also of human neutrophil granulocytes. These data indicate that A. fumigatus conidia are able to inhibit the phagolysosomal acidification of immune effector cells, irrespective whether they derived from mouse or humans, or from primary cells or cell lines. Interestingly, the inhibitory effect was also detected in human neutrophils which, besides AMs, are essential for the defense against A. fumigatus.
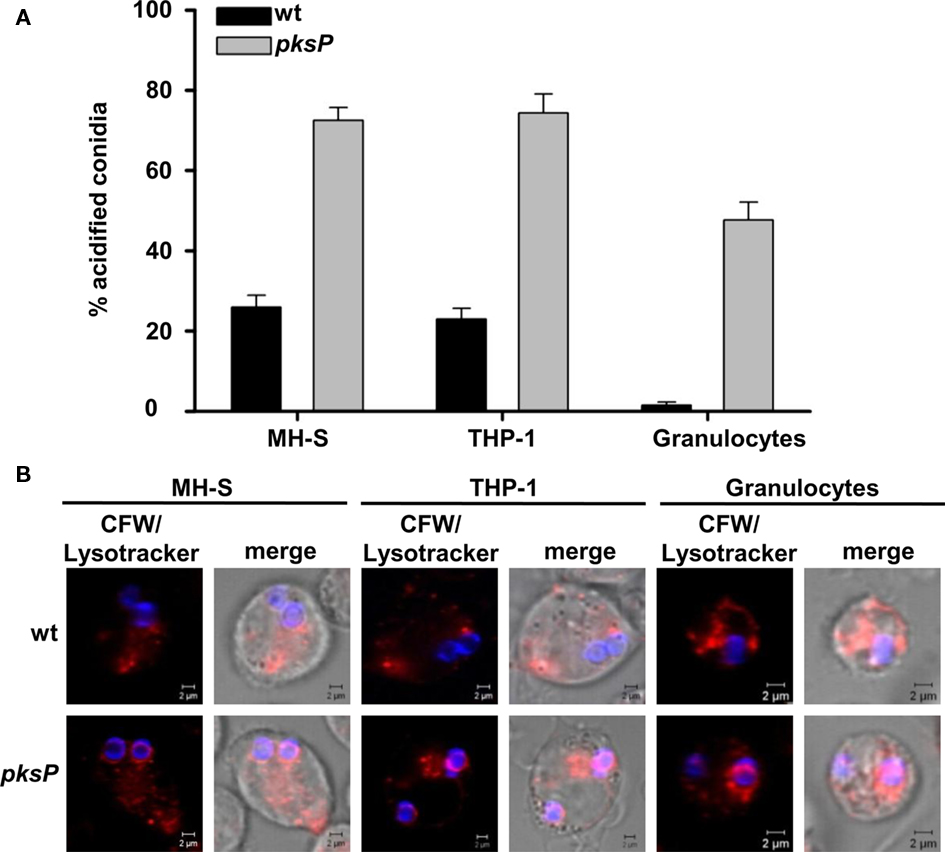
Figure 1. Detection of conidia in acidified compartments after phagocytosis by MH-S and THP-1 cells, and neutrophil granulocytes. (A) Percentages of conidia present in acidic phagolysosomes. Calcofluor white (CFW)-labeled conidia were intracellularly colocalized with LysoTracker Red-DND99. Data represent the mean values and SD of three experiments. (B) Representative micrographs showing colocalization of conidia stained using CFW (blue) with acidified compartments visualized by LysoTracker labeling (red). Size bar, 2 μm.
A. fumigatus Conidia Inhibit Acidification of Phagolysosomes but not Fusion of Phagosomes and Lysosomes
Previously, we demonstrated that a high percentage of wild-type conidia ended up in non-acidified phagosomes. We hypothesized that conidia inhibited the phagolysosome fusion. Now, to distinguish the possibilities that A. fumigatus conidia either inhibit phagolysosome fusion or the acidification of phagolysosomes, we measured both processes independently of each other. Since the maturation status of a phagosome correlates with its capacity to fuse with secondary lysosomes (Pitt et al., 1992; Desjardins et al., 1994; Desjardins, 1995), it is a common procedure to study phagolysosomal fusion events by tracing lysosomes labeled with dextran. Therefore, phagolysosome fusion was followed by loading lysosomes with Oregon Green 488-dextran. Then, we monitored whether phagosomes containing conidia fused with green-labeled lysosomes (Figure 2). Acidification of phagolysosomes was measured using LysoTracker, a dye giving red fluorescence when present in an acidic compartment. Thus, we analyzed whether any of the conidia were surrounded by red fluorescence (Figure 2). Our data clearly show that the percentage of wild-type conidia and pksP mutant conidia ending up in phagolysosomes of both THP-1 and MH-S cells was similar (Figures 2A,B). However, when we measured the intraphagosomal pH, a different picture emerged (Figure 2C). pksP mutant conidia were located in phagolysosomes with an intracellular phagolysosomal pH of 4.9, whereas wild-type conidia displayed a pH of 6 in their surrounding. This data indicates that both types of conidia are targeted to phagolysosomes but mainly pksP mutant conidia were located in an acidic environment which coincides with a more effective killing of these conidia (Jahn et al., 2002). This finding suggests that wild-type conidia are able to inhibit acidification of the phagolysosome.
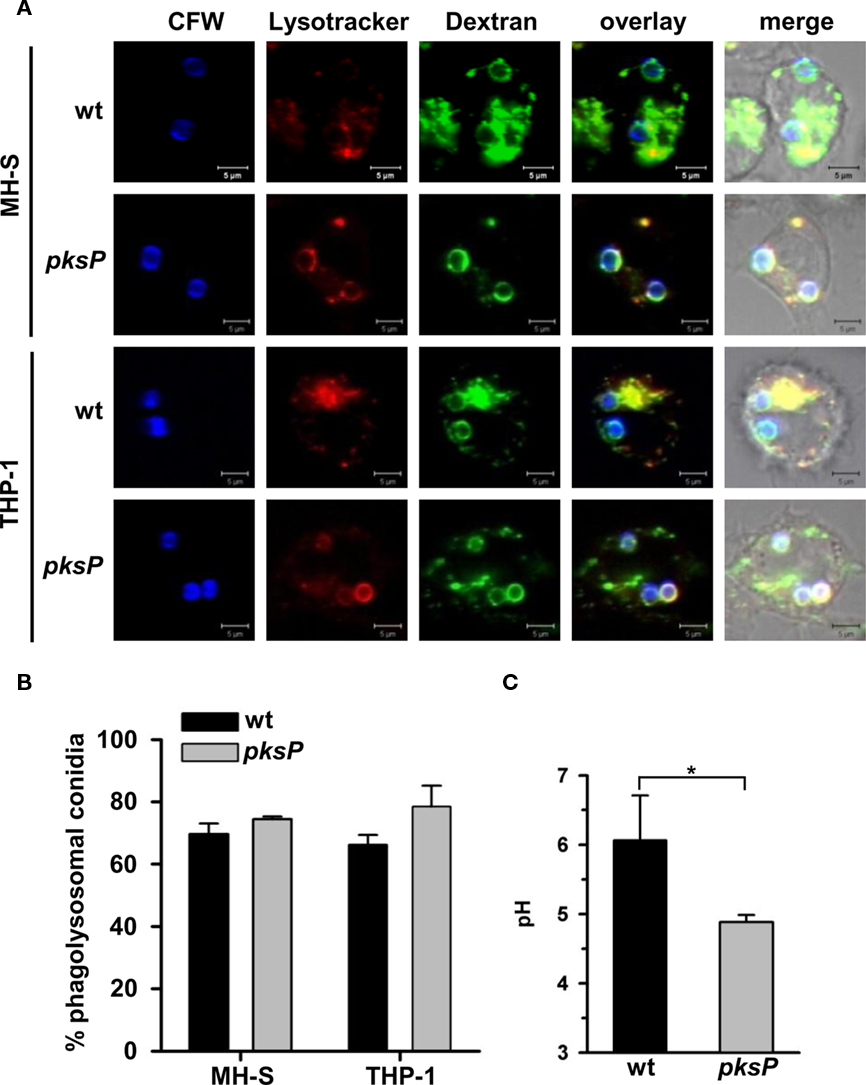
Figure 2. Monitoring of phagolysosomal fusion, acidification of conidia-containing phagosomes, and of intraphagosomal pH. (A) Lysosomes of MH-S and THP-1 cells were chased with Oregon Green (OG) 488-Dextran. Colocalization of calcofluor white (CFW)-stained conidia (blue) with OG488 (green) showing fusion of phagosomes with lysosomes. Acidification of phagolysosomes was studied by LysoTracker (red). Images show representative microscopic pictures. Size bar, 5 μm. (B) Quantification of intracellular conidia residing in mature phagolysosomes of MH-S cells. Data represent the mean + SD from three experiments. (C) Determination of the intraphagosomal pH of conidia-containing phagosomes of MH-S cells. MH-S cells were allowed to phagocytose FITC-labeled conidia. Intracellular pH was calculated by linear regression analysis based on the fluorescence intensity ratio at Ex495 nm/Ex450 nm in comparison to a pH standard curve. Values represent the mean + SD of three experiments. *P < 0.05.
DHN-Melanin is Responsible for Inhibition of Acidification of Phagolysosomes
The difference observed between pksP and wild-type conidia strongly suggests that DHN-melanin plays a decisive role in this process. To address this question, we generated melanin ghosts, i.e., the melanin envelope of conidia was isolated. As shown in Figure 3, in contrast to pksP conidia, melanin ghosts exhibited the same effect as wild-type conidia, i.e., they inhibited the phagolysosome acidification to the same extent as wild-type conidia. Both wild-type conidia and melanin ghosts showed the same percentage of particles in acidified phagolysosomes. Furthermore, exogenous melanin particles consisting of synthetic DOPA-melanin resided in acidified compartments (data not shown), indicating that only DHN-melanin is responsible for this effect. As expected, latex beads used as a control were efficiently phagocytosed and resided to almost 100% in acidified phagolysosomes.
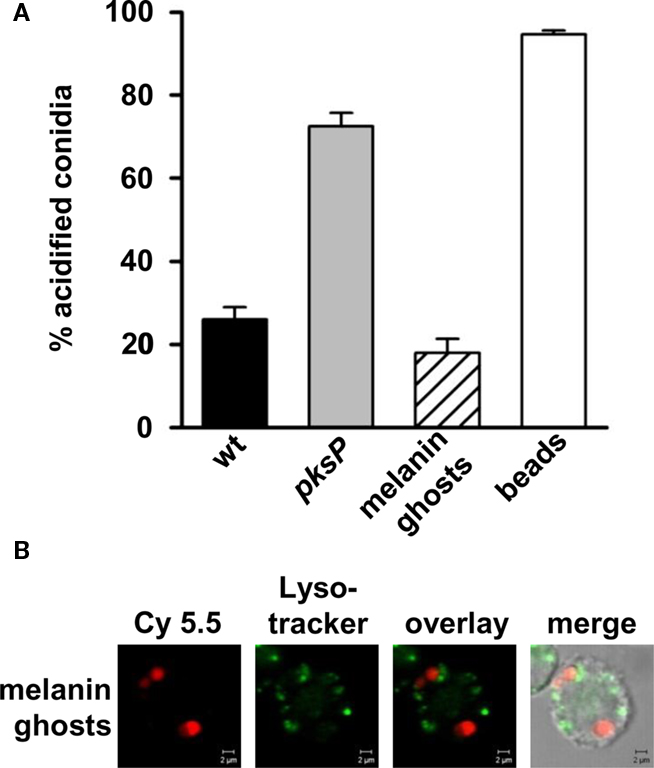
Figure 3. Influence of A. fumigatus wild-type-derived melanin ghosts on phagolysosomal acidification. (A) Percentage of intracellular conidia residing in acidified phagolysosomes after ingestion by MH-S cells. Latex beads were used as a positive control. Values represent the mean + SD of three experiments. (B) Representative micrographs showing colocalization of melanin ghosts, stained prior to phagocytosis with biotinylated anti-melanin camelid antibody and Cy5.5 conjugated streptavidin (red), with acidified compartments visualized by LysoTracker labeling (green). Size bar, 2 μm.
Absence of Conidial Surface Hydrophobin Does not Alter Phagolysosomal Acidification
The surface of conidia is not only covered by melanin, but also by other proteins such as the very prominent protein hydrophobin RodA that was shown to render conidia immunologically inert (Aimanianda et al., 2009). It was thus conceivable that also RodA plays a role in the inhibition of the phagolysosome acidification. Therefore, we analyzed a rodA deletion mutant. As shown in Figure 4A, there was no difference in the acidification of phagolysosomes of MH-S cells irrespective whether wild-type or ΔrodA conidia were phagocytosed. Furthermore, SEM revealed that the pksP mutant still contains the conidial surface hydrophobin RodA despite higher acidification of phagolysosomes in case of phagocytosis of pksP conidia (Figure 4B). This excludes an involvement of RodA in the inhibition of phagolysosome acidification.
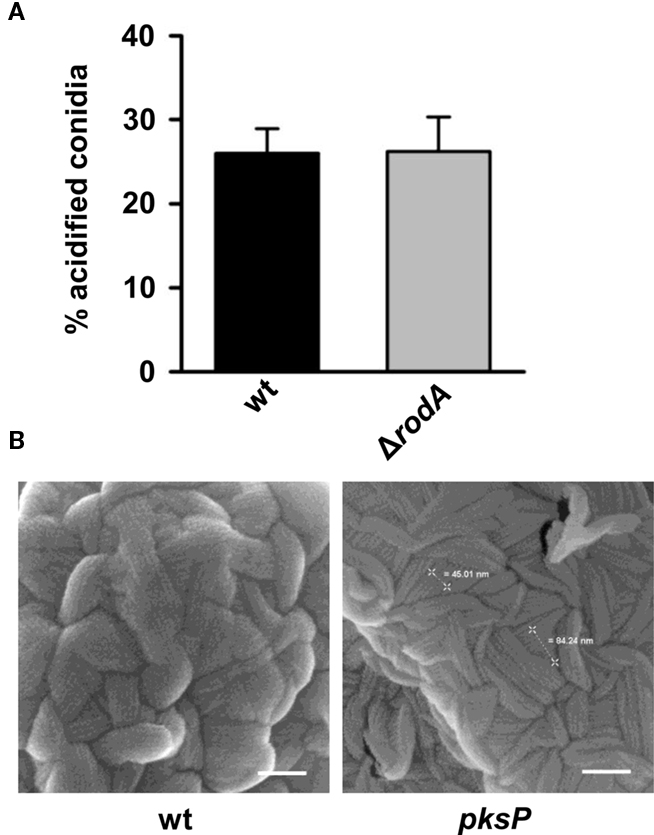
Figure 4. Absence of conidial surface hydrophobin RodA does not alter phagolysosomal acidification. (A) Percentage of intracellular conidia residing in acidified phagolysosomes after phagocytosis by MH-S cells. Values represent the mean + SD of three experiments. (B) Scanning electron microscopy (SEM) micrographs of resting conidia. Resting conidia exhibit conidial surface hydrophobin layer (rodlet structure). Scale bars = 100 nm.
Resting Conidia Led to Stronger Inhibition of Phagolysosomal Acidification than Swollen Conidia of A. fumigatus
When A. fumigatus conidia are inhaled, they are regarded as resting conidia. Upon contact with an appropriate environment like the lung, conidia swell and subsequently germinate. In liquid medium in vitro, swelling takes about 2–3 h and germination occurs after 5–6 h. The proteome of the different morphotypes differs considerably (Teutschbein et al., 2010). It is thus interesting to study whether the various morphotypes display differences in their ability to inhibit phagolysosome fusion. As shown in Figure 5, resting conidia and conidia fixed by p-formaldehyde showed the strongest inhibition of phagolysosome acidification. By contrast, swollen conidia showed a reduced capability to inhibit phagolysosome acidification. This further supports our conclusion that the DHN-melanin that is loosened in swollen conidia and subsequently degraded, plays an important role for this process. Furthermore, the fact that also fixed conidia have the same ability as live wild-type conidia to inhibit the acidification suggests, that an active metabolism, de novo protein synthesis or active secretion of proteins or small molecules are unlikely to influence the process.
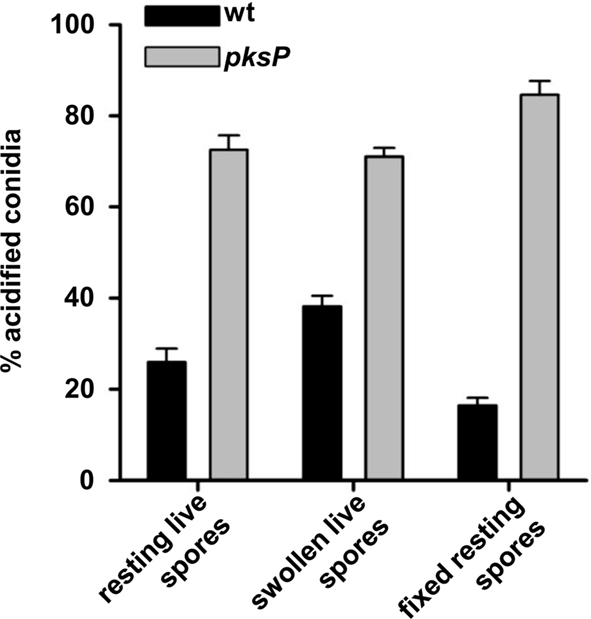
Figure 5. Swelling of conidia affects phagolysosomal acidification. Resting conidia, swollen conidia or formaldehyde-fixed conidia of the wild type or the pksP mutant were subjected to MH-S coincubation. The percentage of intracellular conidia residing in acidified phagolysosomes after ingestion by MH-S cells was estimated. Values represent the mean + SD of three experiments.
Aspergillus Species Vary in Their Capability to Inhibit Phagolysosomal Acidification
Although homologs of pksP and other genes involved in DHN-melanin biosynthesis are found in several aspergilli, the presence of DHN-melanin was unequivocally shown only for A. fumigatus. To get further hints on the meaning of DHN-melanin for this process, we tested conidia of different Aspergillus species with potentially different melanin types (Langfelder et al., 2003) for their ability to inhibit the acidification.
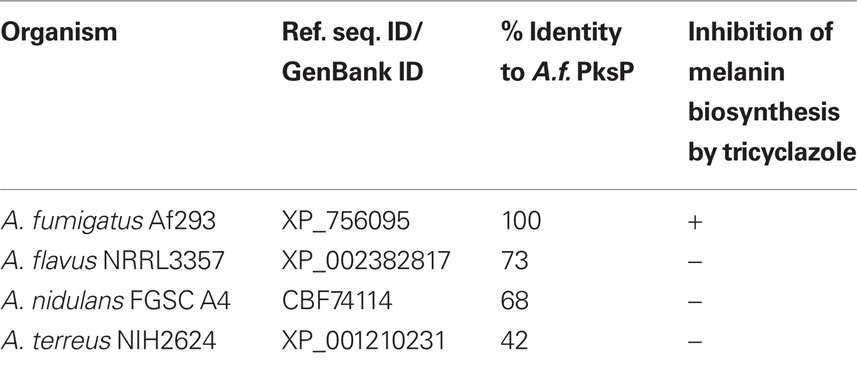
In A. fumigatus, all genes necessary for the synthesis of DHN-melanin are organized in a cluster. This cluster is not present in the other aspergilli that were tested here, i.e., A. flavus, A. nidulans, and A. terreus. Nevertheless, homologs of the polyketide synthase PksP that is essential for the first step of DHN-melanin biosynthesis, the synthesis of naphthopyrone, can be found in several, but not all aspergilli. A. nidulans and A. flavus harbor a PksP homolog with 68 and 73%, respectively, of identical amino acids to PksP of A. fumigatus (Table 2). By contrast, A. terreus is lacking a PksP homolog. BlastP analysis revealed that the closest relative polyketide synthase of A. terreus only has 42% identity to A.f. PksP.
Data on the phagolysosome acidification are shown in Figure 6A. Conidia of A. fumigatus and even more, of A. flavus were most effective in inhibiting the acidification of phagolysosomes. Only 26 and 12% of conidia of both species, respectively, ended up in acidified phagolysosomes. By contrast, about 48 and 66% of conidia of A. nidulans and A. terreus, respectively, were located in acidified phagolysosomes. Hence, there is a major difference between conidia of Aspergillus species in their ability to inhibit acidification of phagolysosomes. Although the conidial pigment of A. nidulans has a much lower capacity to inhibit acidification of phagolysosomes, the analysis of the white wA mutant whose conidia almost completely ended up in acidified phagolysosomes, indicate that the A. nidulans pigment has still an influence on the acidification of the phagolysosome. It is tempting to speculate that the virulence of A. fumigatus and also A. flavus is, at least in part, associated with the ability of their conidia to inhibit the acidification of phagolysosomes and thus enable them a better survival in immune effector cells.
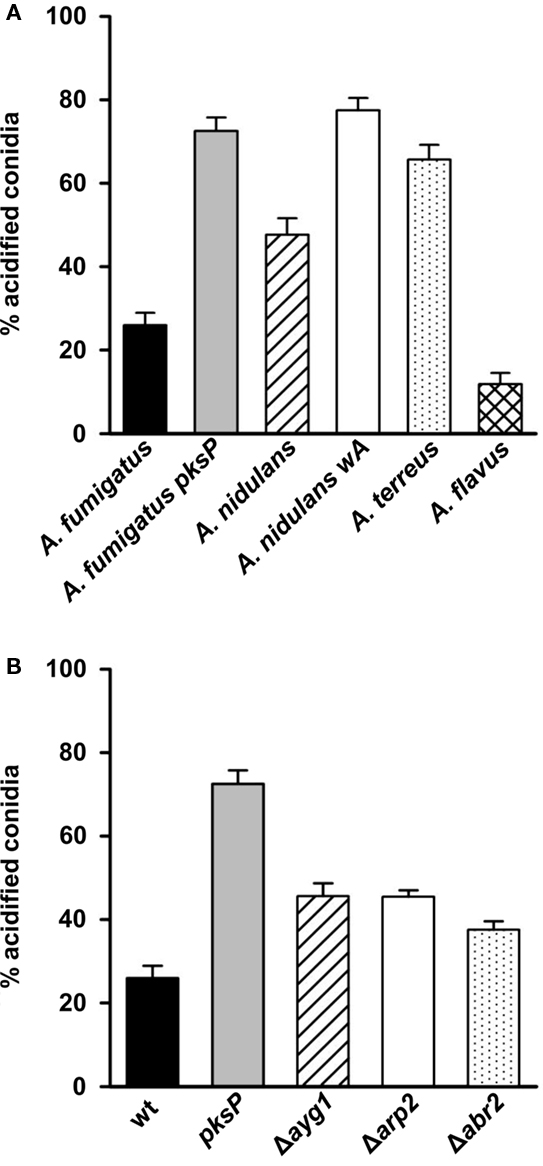
Figure 6. Impact on phagolysosome acidification of (A) different Aspergillus species and (B) A. fumigatus mutant strains accumulating different DHN-melanin precursor molecules. The percentage of intracellular conidia residing in acidified phagolysosomes after phagocytosis by MH-S cells was calculated. Values represent the mean + SD of three experiments.
The Complete DHN-melanin Biosynthesis of A. fumigatus is Required for Maximal Inhibition of Phagolysosomal Acidification
The polyketide synthase PksP represents the key enzyme of DHN-melanin biosynthesis. The enzyme is responsible for catalyzing the synthesis of the heptaketide YWA1 (naphthopyrone) from malonyl CoA and acetyl CoA. In subsequent steps, shortening of the heptaketide occurs catalyzed by the Ayg1 protein followed by reduction and dehydratation of the generated pentaketide catalyzed by Arp2 and Arp1, respectively. The generated product is reduced by Abr1, the resulting product vermelone dehydrated to 1,8-DHN which is polymerized to melanin by the laccase Abr2 (Langfelder et al., 2003). To obtain further hints that DHN-melanin and DHN-melanin precursors contribute to inhibition of phagolysosomal acidification, mutants of A. fumigatus with defects in different steps of the DHN-melanin biosynthesis, i.e., ayg1, arp2, and abr2 (Tsai et al., 1999; Sugareva et al., 2006) that produce yellow, reddish-pink and brown conidia, respectively, were analyzed. Compared with the pigmentless pksP conidia, all these mutants showed a lower percentage of conidia in acidified phagolysosomes (Figure 6B). However, this percentage was higher than that observed for the fully pigmented wild-type conidia. Thus, the mutants showed an intermediate phenotype further suggesting that the complete DHN-melanin biosynthesis is required for inhibition of phagolysosome acidification and that intermediates of DHN-melanin already affect the acidification.
Analysis of Bafilomycin on Fusion of Phagosome-Containing Conidia with Lysosomes
Since our data indicated that both pksP and wild-type conidia localize in phagolysosomes but pksP conidia lost the ability to prevent acidification of this compartment, we investigated whether acidification via the vATPase is required for the acidification of pksP mutant conidia-containing phagolysosomes. Therefore, we studied acidification as well as fusion of phagosomes with lysosomes in the presence of bafilomycin A1, a potent and selective vATPase inhibitor (Bowman et al., 1988). As shown in Figure 7, acidification of phagolysosomal compartments was completely abolished by the addition of bafilomycin in the nanomolar range, independently of ingested wild-type or pksP conidia. Nevertheless, inhibition of the vATPase by bafilomycin had no effect on the phagolysosomal fusion, since treated cells showed the same colocalization pattern of conidia-containing phagosomes with dextran-labeled lysosomes as untreated cells (Figure 7) and, furthermore, did not affect fusion rates (data not shown). This findings suggests that the wild type is able to prevent acidification of the phagolysosome by influencing the activation of the vATPase.
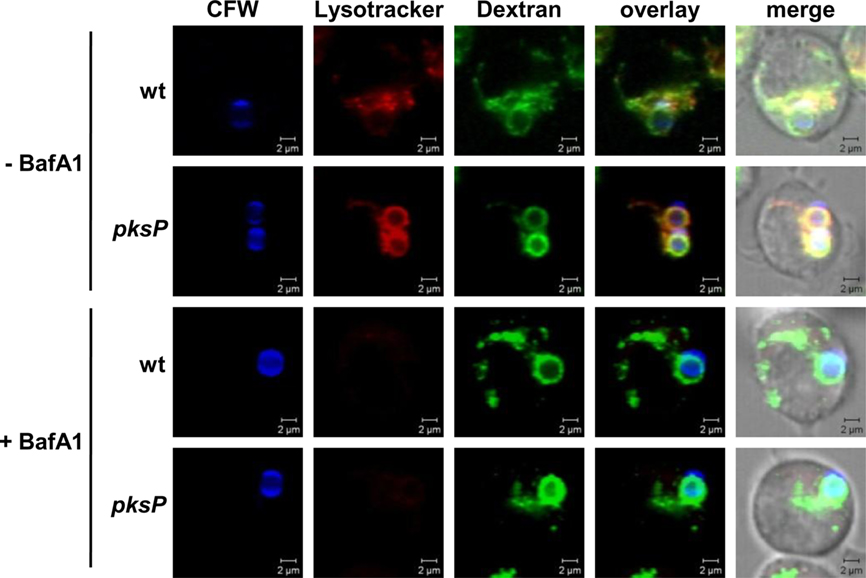
Figure 7. Effect of bafilomycin A1. Lysosomes were loaded with Oregon Green (OG) 488-Dextran. Colocalization of calcofluor white (CFW)-stained conidia (blue) with OG488 (green) showing fusion of phagosomes with lysosomes. Acidification of phagolysosomes was monitored by LysoTracker (red). Images show representative microscopic pictures. Size bar, 2 μm.
Discussion
The interaction of invading microorganisms with phagocytes is of central importance for the innate immune defense against microorganisms. This complex process consists of many different stages and can be separated into two main parts: First, the recognition of conidia by phagocytes as non-self leading to phagocytosis, and second, the intracellular processing of the conidia including the formation of acidified phagolysosomes that ultimately lead to killing of phagocytosed microorganisms. In recent years, the process by which conidia are recognized and ingested by professional phagocytes (Hohl et al., 2005; Luther et al., 2008; Bhatia et al., 2011) as well as non-professional phagocytes (Paris et al., 1997; Filler and Sheppard, 2006) has been studied extensively. Unraveling that PRRs like dectin-1, TLR-2, and TLR-4 play an important role in recognizing fungal structures, i.e., mainly conidial cell wall components, e.g., β-1,3-glucan (Meier et al., 2003; Luther et al., 2007; Chai et al., 2009). Recent studies revealed that beside mediating fungal recognition, TLR-2 and TLR-4 are also involved in evasion mechanisms of the fungus by which cell wall components diminish the proinflammatory response (Chai et al., 2011). Until now, only few studies concentrated on the intracellular fate of conidia following phagocytosis mainly focusing on the host (Ibrahim-Granet et al., 2003; Kasperkovitz et al., 2010). Whereas the mechanisms and the underlying fungal structures that mediate or interfere with these processes remained hardly understood. Previously, we could demonstrate that the processing of A. fumigatus by human monocyte-derived macrophages was inhibited when wild-type conidia were analyzed in comparison to pigmentless pksP mutant conidia (Jahn et al., 2002). Here, we further specified the mechanism by which A. fumigatus alters the endocytotic pathway, preventing full phagolysosomal maturation by inhibiting acidification. It was previously shown that A. fumigatus wild-type conidia are taken up quickly by macrophages and furthermore reside immediately in matured phagolysosomes (Ibrahim-Granet et al., 2003). In contrast to the fast processing, this study revealed that approximately only 20% of wild-type conidia are found in acidified compartments, which was confirmed by our results. This data implied, that the full maturation of wild-type conidia-containing phagosomes is disturbed.
Beside the protective effect of conidial melanin-pigmentation against reactive oxygen species (ROS; Jahn et al., 1997), melanin is responsible for the interference of wild-type conidia with the endocytosis pathway, by blocking phagolysosomal acidification. In contrast to the wild type, the non-pigmented pksP mutant showed a drastical increase in the number of conidia residing in acidified compartments. Moreover, this phenotype was not restricted to a certain cell type or organism, since we could observe wild-type dependent inhibition of phagolysosomal acidification in murine macrophages, human monocyte-derived macrophages and even in human neutrophil granulocytes. This observation indicated that the mechanism underlying this effect seems to represent a general evasion mechanism of A. fumigatus, contributing to its virulence. Surprisingly, this inhibition of acidification by the wild type is not due to an arrest or modulation of the phagosomal maturation as observed for several other intracellular pathogens such as Mycobacterium tuberculosis or Listeria monocytogenes. Phagosomes containing conidia either of the wild type or the pksP mutant fused to the same extent with lysosomal vesicles, revealing that the maturation of A. fumigatus wild-type conidia-containing phagosomes allowed the formation of the phagolysosome but not their final acidification. These data demonstrate that A. fumigatus as an opportunistic pathogen evolved a different evasion mechanism to support its intracellular survival compared with facultative intracellular bacterial pathogens. For example, M. tuberculosis inhibits phagolysosomal maturation by hydrolyzing PI(3)P to PI (Fratti et al., 2001; van der Wel et al., 2007) and furthermore, promotes fusion of endosomes with M. tuberculosis-containing phagosomes by a yet unknown mechanism. By contrast, L. monocytogenes applies a different mechanism to survive intracellularly by modifying and subsequently escaping from phagosomes. Listeriolysin O generates pores in the phagosomal membrane leading to perturbation of the phagosomal ion gradients, which promotes the escape of the bacteria from the phagosome (Singh et al., 2008). By the additional secretion of two specific membrane-active phospholipases, L. monocytogenes enhances the breakdown of the surrounding phagosomal membrane and enables the bacteria to escape and to propagate in the cytosol (Smith et al., 1995; Birmingham et al., 2008). Comparison with more related organisms reveals that Histoplasma capsulatum as a pathogenic intracellular fungus exhibits a similar evasion strategy as A. fumigatus. After phagocytosis of H. capsulatum yeast forms by macrophages, a selective inhibition of acidification of the yeast-containing phagolysosome was described, that resulted in an intracellular pH of above 6 (Eissenberg et al., 1993; Newman et al., 2006), similar to that observed here for A. fumigatus. However, until now fungal effectors of H. capsulatum causing this inhibition have not been identified. Here, by using DHN-melanin particles, swollen conidia, fixed conidia, and mutants with defects in various steps of the DHN-melanin biosynthesis, we showed that the DHN-melanin pigment of A. fumigatus is exclusively responsible for this inhibition. This conclusion is supported by the finding that exogenous DOPA-melanin particles did not decrease the phagolysosomal acidification. DOPA-melanin is produced via a PksP-independent biosynthesis. Thus, we identified a major effector for the intracellular survival of this fungus within phagocytes.
Since the melanin-layer of conidia is thought to be connected to the hydrophobic rodlet layer, which renders the wild-type spores immunologically inert (Aimanianda et al., 2009), we reasoned that the loss of melanin affected the structures of the rodlet layer, leading to loss of this protective layer. The fact that pksP conidia still contain a rodlet layer similar to that of the wild type and, in addition, that we could show that the ΔrodA mutant exhibits the same phenotype as the wild type in blocking acidification, this finding clearly proves that the rodlet layer is dispensable for inhibition of acidification. Interestingly, we observe a rather normal rodlet structure on the pksP mutant conidia, whereas Pihet et al. (2009) suggested, based on a study employing atomic force microscopy (AFM), that the rodlet structure was missing on pigmentless mutant conidia. Here, the SEM technique was applied, since it is a direct imaging method without mechanical interaction with the sample. The influence of the preparation process was minimized by applying exclusively a high vacuum platinum coating after collecting of the dry conidia. Thus, potential effects of the wet handling steps of the AFM preparation were avoided.
Blocking of the dectin-1 receptor and masking of β-1,3-glucan layer of A. fumigatus conidia using laminarin and antibodies, respectively, did not influence the acidification of phagolysosomes, making an involvement of the dectin-1 receptor unlikely (unpublished data).
To further investigate the impact of DHN-melanin of A. fumigatus on the phagolysosomal maturation, we tested whether mutants defective in DHN-melanin biosynthesis were able to affect the acidification of the phagolysosome. Molecular analyses showed that A. fumigatus conidia contain a DHN-melanin pigment (Jahn et al., 1997; Langfelder et al., 1998; Tsai et al., 1998). The nature of the conidial pigment produced in the other aspergilli tested here, has not been elucidated yet. Studies employing the antifungal compound tricyclazole implied that neither A. terreus, nor A. nidulans and A. flavus produce DHN-melanin but rather a similar but not identical melanin. Tricyclazole is an inhibitor of tetrahydroxynaphthalene (THN)-reductases, e.g., Arp2, involved in DHN-melanin biosynthesis. Consistently, tricyclazole effectively inhibited DHN-melanin production in A. fumigatus, that resulted in formation of yellow-brownish spores. However, the pigment color was not affected by tricyclazole in A. terreus, A. nidulans, and also A. flavus, indicating that the melanin types of these fungi might differ (Wheeler and Klich, 1995; Heinekamp, unpublished).
In general, the DHN-melanin biosynthesis gene cluster, as it can be found in A. fumigatus, is not conserved among aspergilli. From the Aspergillus species that were included in this study, a clear homolog of the central polyketide synthase PksP was only identified in the genomes of A. fumigatus, A. flavus, and A. nidulans but not A. terreus. Furthermore, the genome of A. terreus does not contain any putative homologous gene to the DHN-gene cluster, whereas A. nidulans and A. flavus both encode a homolog for Ayg1, that catalyzes the second step in DHN-melanin biosynthesis, i.e., the conversion of naphthopyrone to 1,3,6,8-THN. Further analysis of other genes involved in DHN-melanin biosynthesis revealed that homologs of the THN-reductase Arp2 cannot be identified in A. flavus or A. nidulans. This finding might explain that the spore color in these fungi is not affected by tricyclazole. The reductase found in A. flavus with the highest identity (52%) to the A. fumigatus Arp2 protein was AflM (XP_002379941). However, this reductase is part of the aflatoxin biosynthesis gene cluster and was previously found to be involved in aflatoxin biosynthesis rather than melanin biosynthesis (Yu et al., 2004).
All these data suggest that A. flavus and A. nidulans conidia contain a melanin derived from naphthopyrone and 1,3,6,8-THN and that this melanin is more similar to DHN-melanin of A. fumigatus than the melanin produced by A. terreus, that completely lacks naphthopyrone and 1,3,6,8-THN biosynthesis genes. We propose that these variations in melanin contribute to the differences observed for phagolysosomal acidification induced by conidia of the aspergilli tested here.
Inhibition studies with bafilomycin A1 indicated that the wild type is able to prevent acidification of the phagolysosome by influencing the activation of the vATPase. Therefore, it is conceivable that the DHN-melanin of wild-type conidia leads to deregulation or even inhibition of the vATPase which needs to be in the assembled form to be active. Similarly, it was shown that the vATPase in its active assembled form was localized to a lesser extent in H. capsulatum-containing phagolysosomes than in phagolysosomes containing cells of the yeast Saccharomyces cerevisiae (Strasser et al., 1999). In the same line, Legionella pneumoniae possesses a protein which inhibits vATPase activity (Xu et al., 2010).
In summary, we demonstrate that A. fumigatus conidia have the capability to inhibit the acidification of phagolysosomes of professional phagocytes that are of major importance for defense against an infection by A. fumigatus. All our data point to the conclusion that the DHN-melanin type present in A. fumigatus specifically inhibits the activity of the vATPase. It is tempting to speculate about the precise underlying mechanism. Our data will also contribute to understand the pathogenic potential of A. fumigatus compared to other aspergilli.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Venelina Sugareva for providing the ayg1 deletion strain. This work was supported by the International Leibniz Research School (ILRS) for Microbial and Biomolecular Interactions as part of the excellence graduate school Jena School for Microbial Communication (JSMC).
References
Aimanianda, V., Bayry, J., Bozza, S., Kniemeyer, O., Perruccio, K., Elluru, S. R., Clavaud, C., Paris, S., Brakhage, A. A., Kaveri, S. V., Romani, L., and Latge, J. P. (2009). Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460, 1117–1121.
Balloy, V., Si-Tahar, M., Takeuchi, O., Philippe, B., Nahori, M. A., Tanguy, M., Huerre, M., Akira, S., Latge, J. P., and Chignard, M. (2005). Involvement of toll-like receptor 2 in experimental invasive pulmonary aspergillosis. Infect. Immun. 73, 5420–5425.
Behnsen, J., Narang, P., Hasenberg, M., Gunzer, F., Bilitewski, U., Klippel, N., Rohde, M., Brock, M., Brakhage, A. A., and Gunzer, M. (2007). Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans. PLoS Pathog. 3, e13. doi: 10.1371/journal.ppat.0030013
Bhatia, S., Fei, M., Yarlagadda, M., Qi, Z., Akira, S., Saijo, S., Iwakura, Y., Van Rooijen, N., Gibson, G. A., St Croix, C. M., Ray, A., and Ray, P. (2011). Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS ONE 6, e15943. doi: 10.1371/journal.pone.0015943
Birmingham, C. L., Canadien, V., Kaniuk, N. A., Steinberg, B. E., Higgins, D. E., and Brumell, J. H. (2008). Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451, 350–354.
Bowman, E. J., Siebers, A., and Altendorf, K. (1988). Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U.S.A. 85, 7972–7976.
Brakhage, A. A., Bruns, S., Thywissen, A., Zipfel, P. F., and Behnsen, J. (2010). Interaction of phagocytes with filamentous fungi. Curr. Opin. Microbiol. 13, 409–415.
Chai, L. Y., Kullberg, B. J., Vonk, A. G., Warris, A., Cambi, A., Latge, J. P., Joosten, L. A., Van Der Meer, J. W., and Netea, M. G. (2009). Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect. Immun. 77, 2184–2192.
Chai, L. Y., Vonk, A. G., Kullberg, B. J., Verweij, P. E., Verschueren, I., Van Der Meer, J. W., Joosten, L. A., Latge, J. P., and Netea, M. G. (2011). Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 13, 151–159.
Chazalet, V., Debeaupuis, J. P., Sarfati, J., Lortholary, J., Ribaud, P., Shah, P., Cornet, M., Vu Thien, H., Gluckman, E., Brucker, G., and Latge, J. P. (1998). Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J. Clin. Microbiol. 36, 1494–1500.
Desjardins, M. (1995). Biogenesis of phagolysosomes: the ‘kiss and run’ hypothesis. Trends Cell Biol. 5, 183–186.
Desjardins, M., Huber, L. A., Parton, R. G., and Griffiths, G. (1994). Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124, 677–688.
Eissenberg, L. G., Goldman, W. E., and Schlesinger, P. H. (1993). Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 177, 1605–1611.
Fantes, P. A., and Roberts, C. F. (1973). Beta-galactosidase activity and lactose utilization in Aspergillus nidulans. J. Gen. Microbiol. 77, 417–486.
Filler, S. G., and Sheppard, D. C. (2006). Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2, e129. doi: 10.1371/journal.ppat.0020129
Forlenza, M., Scharsack, J. P., Kachamakova, N. M., Taverne-Thiele, A. J., Rombout, J. H., and Wiegertjes, G. F. (2008). Differential contribution of neutrophilic granulocytes and macrophages to nitrosative stress in a host–parasite animal model. Mol. Immunol. 45, 3178–3189.
Fratti, R. A., Backer, J. M., Gruenberg, J., Corvera, S., and Deretic, V. (2001). Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154, 631–644.
Hohl, T. M., Van Epps, H. L., Rivera, A., Morgan, L. A., Chen, P. L., Feldmesser, M., and Pamer, E. G. (2005). Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 1, e30. doi: 10.1371/journal.ppat.0010030
Hospenthal, D. R., Kwon-Chung, K. J., and Bennett, J. E. (1998). Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med. Mycol. 36, 165–168.
Ibrahim-Granet, O., Jouvion, G., Hohl, T. M., Droin-Bergere, S., Philippart, F., Kim, O. Y., Adib-Conquy, M., Schwendener, R., Cavaillon, J. M., and Brock, M. (2010). In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol. 10, 105. doi: 10.1186/1471-2180-10-105
Ibrahim-Granet, O., Philippe, B., Boleti, H., Boisvieux-Ulrich, E., Grenet, D., Stern, M., and Latge, J. P. (2003). Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71, 891–903.
Jahn, B., Boukhallouk, F., Lotz, J., Langfelder, K., Wanner, G., and Brakhage, A. A. (2000). Interaction of human phagocytes with pigmentless Aspergillus conidia. Infect. Immun. 68, 3736–3739.
Jahn, B., Koch, A., Schmidt, A., Wanner, G., Gehringer, H., Bhakdi, S., and Brakhage, A. A. (1997). Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect. Immun. 65, 5110–5117.
Jahn, B., Langfelder, K., Schneider, U., Schindel, C., and Brakhage, A. A. (2002). PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell. Microbiol. 4, 793–803.
Kasperkovitz, P. V., Cardenas, M. L., and Vyas, J. M. (2010). TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J. Immunol. 185, 7614–7622.
Langfelder, K., Jahn, B., Gehringer, H., Schmidt, A., Wanner, G., and Brakhage, A. A. (1998). Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 187, 79–89.
Langfelder, K., Streibel, M., Jahn, B., Haase, G., and Brakhage, A. A. (2003). Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38, 143–158.
Luther, K., Rohde, M., Sturm, K., Kotz, A., Heesemann, J., and Ebel, F. (2008). Characterisation of the phagocytic uptake of Aspergillus fumigatus conidia by macrophages. Microbes Infect. 10, 175–184.
Luther, K., Torosantucci, A., Brakhage, A. A., Heesemann, J., and Ebel, F. (2007). Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell. Microbiol. 9, 368–381.
Meier, A., Kirschning, C. J., Nikolaus, T., Wagner, H., Heesemann, J., and Ebel, F. (2003). Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 5, 561–570.
Netea, M. G., Warris, A., Van Der Meer, J. W., Fenton, M. J., Verver-Janssen, T. J., Jacobs, L. E., Andresen, T., Verweij, P. E., and Kullberg, B. J. (2003). Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 188, 320–326.
Newman, S. L., Gootee, L., Hilty, J., and Morris, R. E. (2006). Human macrophages do not require phagosome acidification to mediate fungistatic/fungicidal activity against Histoplasma capsulatum. J. Immunol. 176, 1806–1813.
Ohkuma, S., and Poole, B. (1978). Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. U.S.A. 75, 3327–3331.
Paris, S., Boisvieux-Ulrich, E., Crestani, B., Houcine, O., Taramelli, D., Lombardi, L., and Latge, J. P. (1997). Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 65, 1510–1514.
Philippe, B., Ibrahim-Granet, O., Prevost, M. C., Gougerot-Pocidalo, M. A., Sanchez Perez, M., Van Der Meeren, A., and Latge, J. P. (2003). Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71, 3034–3042.
Pihet, M., Vandeputte, P., Tronchin, G., Renier, G., Saulnier, P., Georgeault, S., Mallet, R., Chabasse, D., Symoens, F., and Bouchara, J. P. (2009). Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 9, 177. doi: 10.1186/1471-2180-9-177
Pitt, A., Mayorga, L. S., Schwartz, A. L., and Stahl, P. D. (1992). Transport of phagosomal components to an endosomal compartment. J. Biol. Chem. 267, 126–132.
Ruchel, R., and Reichard, U. (1999). Pathogenesis and clinical presentation of aspergillosis. Contrib. Microbiol. 2, 21–43.
Singh, R., Jamieson, A., and Cresswell, P. (2008). GILT is a critical host factor for Listeria monocytogenes infection. Nature 455, 1244–1247.
Smith, G. A., Marquis, H., Jones, S., Johnston, N. C., Portnoy, D. A., and Goldfine, H. (1995). The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63, 4231–4237.
Steele, C., Rapaka, R. R., Metz, A., Pop, S. M., Williams, D. L., Gordon, S., Kolls, J. K., and Brown, G. D. (2005). The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1, e42. doi: 10.1371/journal.ppat.0010042
Strasser, J. E., Newman, S. L., Ciraolo, G. M., Morris, R. E., Howell, M. L., and Dean, G. E. (1999). Regulation of the macrophage vacuolar ATPase and phagosome–lysosome fusion by Histoplasma capsulatum. J. Immunol. 162, 6148–6154.
Sturtevant, J. E., and Latge, J. P. (1992). Interactions between conidia of Aspergillus fumigatus and human complement component C3. Infect. Immun. 60, 1913–1918.
Sugareva, V., Hartl, A., Brock, M., Hubner, K., Rohde, M., Heinekamp, T., and Brakhage, A. A. (2006). Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch. Microbiol. 186, 345–355.
Teutschbein, J., Albrecht, D., Potsch, M., Guthke, R., Aimanianda, V., Clavaud, C., Latge, J. P., Brakhage, A. A., and Kniemeyer, O. (2010). Proteome profiling and functional classification of intracellular proteins from conidia of the human-pathogenic mold Aspergillus fumigatus. J. Proteome Res. 9, 3427–3442.
Thau, N., Monod, M., Crestani, B., Rolland, C., Tronchin, G., Latge, J. P., and Paris, S. (1994). Rodletless mutants of Aspergillus fumigatus. Infect. Immun. 62, 4380–4388.
Tsai, H. F., Chang, Y. C., Washburn, R. G., Wheeler, M. H., and Kwon-Chung, K. J. (1998). The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180, 3031–3038.
Tsai, H. F., Wheeler, M. H., Chang, Y. C., and Kwon-Chung, K. J. (1999). A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181, 6469–6477.
van der Wel, N., Hava, D., Houben, D., Fluitsma, D., Van Zon, M., Pierson, J., Brenner, M., and Peters, P. J. (2007). M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129, 1287–1298.
van Gorcom, R. F., Pouwels, P. H., Goosen, T., Visser, J., Van Den Broek, H. W., Hamer, J. E., Timberlake, W. E., and Van Den Hondel, C. A. (1985). Expression of an Escherichia coli beta-galactosidase fusion gene in Aspergillus nidulans. Gene 40, 99–106.
Weidner, G., D’enfert, C., Koch, A., Mol, P. C., and Brakhage, A. A. (1998). Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33, 378–385.
Werner, J. L., Metz, A. E., Horn, D., Schoeb, T. R., Hewitt, M. M., Schwiebert, L. M., Faro-Trindade, I., Brown, G. D., and Steele, C. (2009). Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182, 4938–4946.
Wheeler, M. H., and Klich, M. A. (1995). The effects of tricyclazole, pyroquilon, phtalide, and related fungicides on the production of conidial wall pigments by Penicillium and Aspergillus species. Pestic. Biochem. Physiol. 52, 125–136.
Wozniok, I., Hornbach, A., Schmitt, C., Frosch, M., Einsele, H., Hube, B., Loffler, J., and Kurzai, O. (2008). Induction of ERK-kinase signalling triggers morphotype-specific killing of Candida albicans filaments by human neutrophils. Cell. Microbiol. 10, 807–820.
Xu, L., Shen, X., Bryan, A., Banga, S., Swanson, M. S., and Luo, Z. Q. (2010). Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 6, e1000822. doi: 10.1371/journal.ppat.1000822
Youngchim, S., Morris-Jones, R., Hay, R. J., and Hamilton, A. J. (2004). Production of melanin by Aspergillus fumigatus. J. Med. Microbiol. 53, 175–181.
Keywords: Aspergillus fumigatus, endocytosis, melanin, neutrophils, macrophages, phagolysosome, virulence
Citation: Thywißen A, Heinekamp T, Dahse H-M, Schmaler-Ripcke J, Nietzsche S, Zipfel PF and Brakhage AA (2011) Conidial dihydroxynaphthalene melanin of the human pathogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway. Front. Microbio. 2:96. doi: 10.3389/fmicb.2011.00096
Received: 11 March 2011; Paper pending published: 29 March 2011;
Accepted: 19 April 2011; Published online: 03 May 2011.
Edited by:
Vishnu Chaturvedi, New York State Department of Health, USAReviewed by:
Gustavo Henrique Goldman, Universidade de Sao Paulo, BrazilWilliam J. Steinbach, Duke University Medical Center, USA
Copyright: © 2011 Thywißen, Heinekamp, Dahse, Schmaler-Ripcke, Nietzsche, Zipfel and Brakhage. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Axel A. Brakhage, Department of Molecular and Applied Microbiology, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Beutenbergstrasse 11a, 07745 Jena, Germany. e-mail:YXhlbC5icmFraGFnZUBoa2ktamVuYS5kZQ==


 Hans-Martin Dahse3
Hans-Martin Dahse3