
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 03 April 2025
Sec. Dermatology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1585167
Introduction: Mucous membrane pemphigoid (MMP) is an autoimmune disease affecting the oral mucosa, conjunctivae and other mucous membranes. The mainstay treatment options are local and systemic corticosteroids and immunomodulatory therapies. Current research on cancer risk in MMP is scarce and has yielded conflicting results.
Methods: The aim of this study was to investigate the risk of developing skin cancer in patients with MMP by performing a large-scale, retrospective matched cohort study utilizing data from over 117 million US individuals. The risk of skin cancer in patients with MMP was observed within a 5-year follow-up period, along with three temporal difference analyses and stratification for disease severity.
Results: MMP was associated with an increased risk of several types of skin cancers within the first 5 years of follow-up, particularly squamous cell carcinoma, basal cell carcinoma, and non-melanoma skin cancer. Stratification by disease severity showed significantly elevated risks in severe cases.
Discussion: These findings underscore the importance of regular skin cancer screening and risk-based monitoring in MMP patients, particularly those with severe disease. Integrating dermatologic surveillance into routine care could facilitate early detection and timely intervention. Additionally, these results highlight the need for further research into cancer risks in other autoimmune blistering diseases, helping to refine long-term management strategies.
Mucous membrane pemphigoid (MMP) is a rare autoimmune blistering disease (AIBD) that causes erosions in the mouth, pharynx, larynx, esophagus, trachea, nose, genitalia and perianal area resulting in pain and strictures (1–5). In addition, conjunctival lesions lead to visual impairment and finally, blindness (6). In about a quarter of patients, skin lesions arise in addition to mucosal involvement. MMP is associated with autoantibodies that target components of the basement membrane zone attaching the epithelium/epidermis to the lamina propria/epidermis leading to its detachment and blister formation (4, 7, 8). The etiology of MMP is not yet fully understood and is likely rooted in a combination of genetic factors and various environmental triggers (7–9).
The mainstay treatment for mild MMP is local corticosteroids. More severe forms require treatment with systemic corticosteroids, anti-inflammatory agents, immunosuppressants, or intravenous immunoglobulins, among others (8, 10, 11). The most common autoantigen in MMP is BP180 (also known as type XVII collagen). In about 5% of MMP patients, autoantibodies react with type VII collagen, while anti-BP230 reactivity can be found in some patients with anti-BP180 antibodies. In individual MMP patients, IgG against α6β4 integrin can be detected (2, 7–10).
Current insights on the topic of MMP and cancer are limited in scope and often yield conflicting results (12–17). Limitations include low sample sizes, lack of adequate control groups, and imperfect methodological rigor. The association between anti-laminin 332 reactivity and malignancies, mostly solid cancers, has been clearly established in recent studies following the original descriptions by Leverkus et al. and Egan et al. (13, 18–23). This observation was facilitated by the development of standardized, sensitive, and specific detection systems for serum anti-laminin 332 IgG (20, 24). Consequently, national and international guidelines recommend testing for circulating anti-laminin 332 IgG in newly diagnosed MMP patients (2, 4, 10). The mechanisms responsible for the cancer risk in patients with MMP are not yet fully understood and large-scale studies investigating the possible correlation between cancer and MMP are lacking. Using the extensive TriNetX Analytics Network, this study set out to specifically examine the relationship between MMP and skin cancer.
Electronic health record (EHR) data from the US Collaborative Network of the federated TriNetX platform was used in this large-scale propensity-score matched cohort study, following previously used designs (25–27). TriNetX provides secure, computerized access to EHRs in real-time (28). Data from almost 118 million EHRs in the US Collaborative Network was sampled and retrieved in December of 2024 (Figure 1).
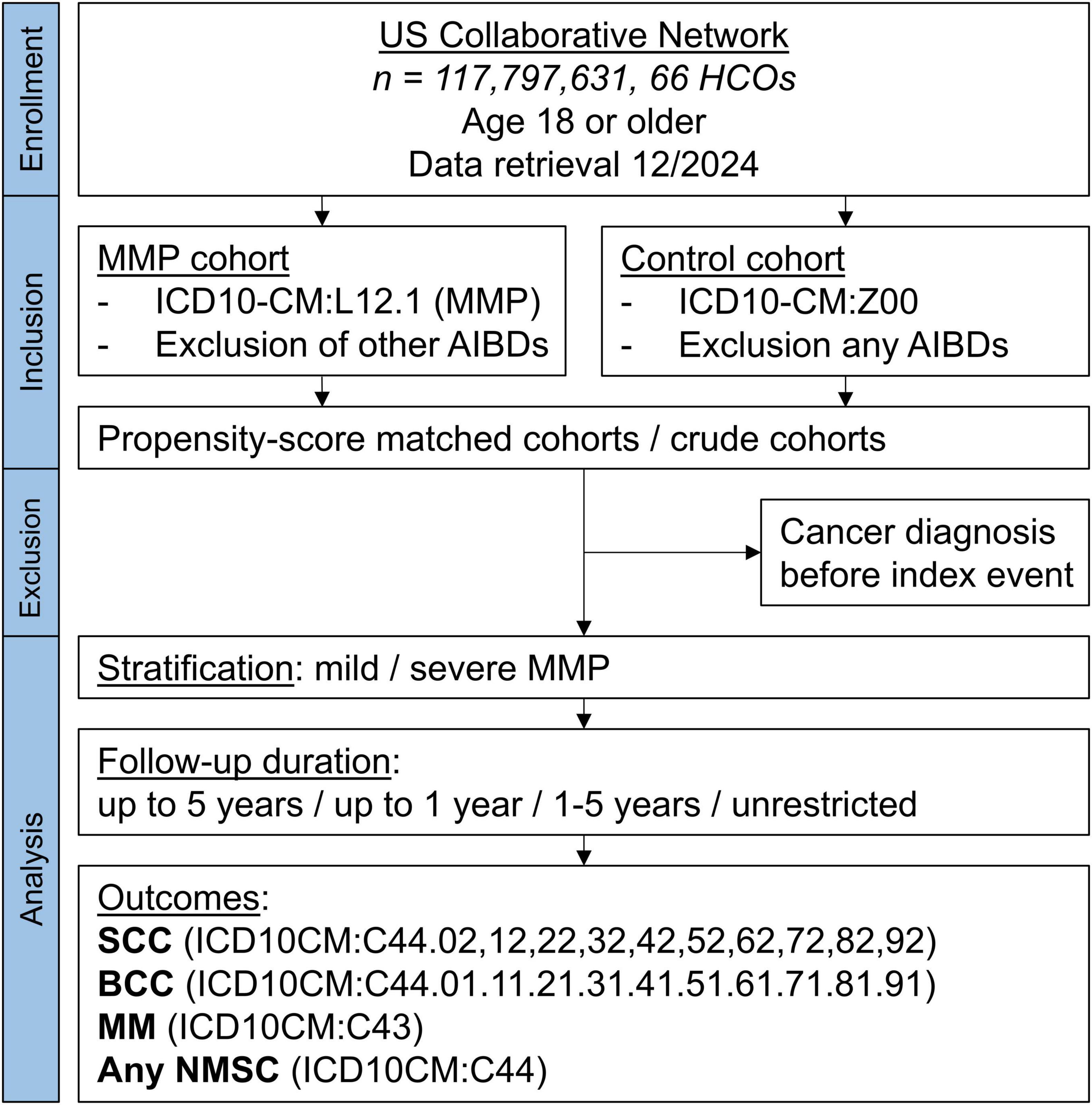
Figure 1. Study flow chart. AIBD, autoimmune blistering disease; BCC, basal cell carcinoma; HCO, health care organization; MM, malignant melanoma; MMP, mucous membrane pemphigoid; NMSC, non-melanoma skin cancer.
A cohort of individuals with a diagnosis of MMP was defined and compared with individuals without a diagnosis of MMP. The start of the study period, marking the index event and entry of each participant in the study, was defined as a diagnosis of MMP for cases and a diagnosis of ICD-10CM:Z00 “Encounter for general examination without complaint, suspected or reported diagnosis” for controls. Only individuals aged 18 years and older were included.
A total of 3,812 patients with MMP were enrolled in the study. The patient cohort was defined by the inclusion of an ICD-10CM code L12.1 and the exclusion of the following codes: L10, L11, L12.0, L13, and L14 (other AIBDs), to ensure a high probability of defining a cohort truly consisting of MMP patients. Controls (n = 12,777,220) were defined by ICD-10CM:Z00 and by the exclusion of any AIBD L10-L14. In addition, a subgroup analysis for mild and severe MMP was performed. Severe MMP was defined as the prescription of any systemic drug commonly known to be used in the treatment of MMP (Supplementary Table 1), while mild MMP was defined as the exclusion of any such medications except prednisone and tetracyclines. Only medications from the time of diagnosis were included. The index event, marking the start of the study and follow-up, was defined by the time of diagnosis.
The study outcomes were squamous cell carcinoma (SCC) of the skin, basal cell carcinoma (BCC), malignant melanoma (MM), and any non-melanoma skin cancer (NMSC) (Supplementary Table 2).
A crude analysis was performed, not accounting for any covariates, as well as rigorous propensity-score matching (PSM) to balance the cohorts and optimize comparability. A covariate matrix for PSM was established, including demographic information and potentially influential confounders, including the following covariates: age at index (continuous variable), white race (binary), female sex (binary), personal history of nicotine dependence (ICD-10CM:Z87.891, binary), nicotine dependence (ICD-10CM:F17, binary), and overweight and obesity (ICD-10CM:E66, binary), and family history of primary malignant neoplasm (ICD-10CM:Z80, binary). In addition, a large number of demographic variables and relevant comorbidities were extracted for the baseline characteristics in Table 1 but not matched for. The matrix row order was randomized after data retrieval. A propensity-score for each patient was generated by logistic regression analysis (with exposure as the dependent variable) using the Python package scikit-learn. Matching was performed for cases: controls on a 1:1 ratio using the greedy nearest neighbor approach with a cut-off distance of 0.1 pooled standard deviations of the logit of the propensity-score. Baseline characteristics were re-evaluated and reported after matching, differences were compared by t-test for continuous and z-test for binary or categorical variables.
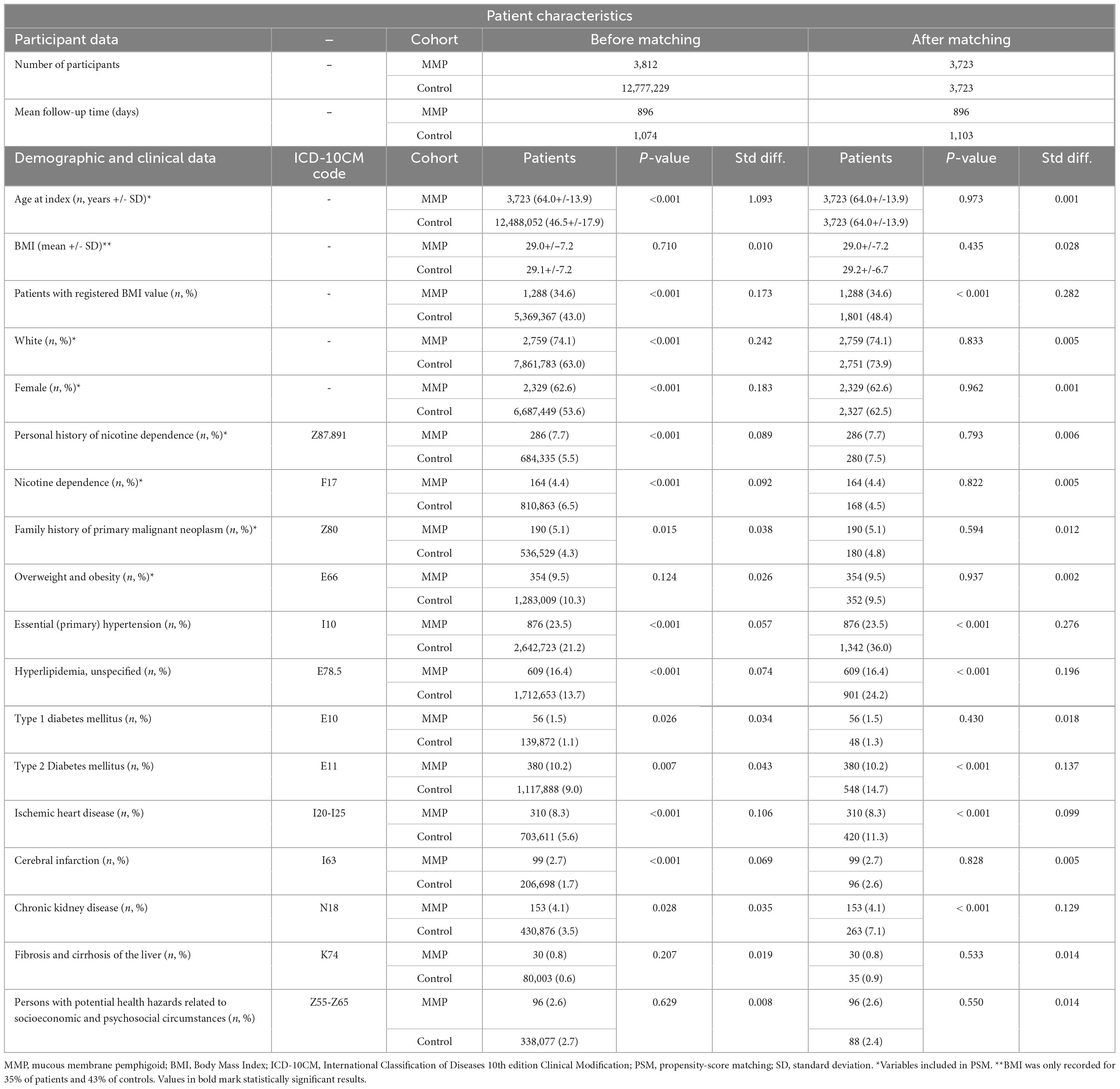
Table 1. Baseline characteristics of participants in the primary analysis before and after propensity-score matching.
The primary analysis investigated outcomes for crude and matched analyses 1 day to 5 years after index. To test the robustness of the results in the primary analysis, three matched analyses on temporal differences were performed on all MMP: (1) only outcomes 1 day to 1 year after index were considered to examine short-term effects, (2) outcomes 1-5 years after index were analyzed to reduce the potential influence of detection bias and reverse causality, and (3) outcomes 1 day to any time after index were examined for long-term associations. Any outcomes prior to the index event were excluded in all analyses. The subgroup analysis for mild and severe MMP was performed only for the primary follow-up time of 5 years.
Risk ratios, odds ratios, and risk differences were calculated. Survival analyses were performed using Kaplan–Meier (KM) analysis. The proportionality assumption was tested by the coxph function in R’s Survival package using Schoenfeld residuals and χ2 tests. KM-curves were compared using the Log-rank test. A univariate Cox proportional hazards regression was used to express hazard ratios (HR)s with 95%-confidence intervals (CI)s.
TriNetX data is presented solely in aggregated form and only contains anonymized data complying with the de-identification standard as defined by the US Health Insurance Portability and Accountability Act (HIPAA) in section §164,514(a). TriNetX is certified to the ISO 27001:2013 standard and maintains a so-called Information Security Management System to ensure rigorous protection of the healthcare data it has access to, following the HIPAA Security Rule. In addition, the Swedish Ethical Review Authority has granted ethical approval for this study (diary number 2024-06878-02).
Data from a total of 117,797,631 patients from 66 health care organizations were screened (Figure 1). The number of participants after successful PSM was 3,723 MMP patients and an equal number of controls. No significant differences were found in any of the covariates after PSM (Table 1).
MMP was associated with a significantly increased risk of several types of skin cancers within the first 5 years of follow-up (Figure 2) and more so in the crude analysis (Supplementary Table 3). The highest risk was observed for SCC which was more than tripled in the crude analysis and almost doubled in the matched analysis (HR 1.892, 95% CI 1.211–2.955, p = 0.004). An increased risk was also seen for BCC in the crude and matched analysis (HR 1.501, 95% CI 1.061–2.125, p = 0.021). Diagnosed MMs showed a significantly increased risk in the crude analysis, but not in the matched analysis. The risk of any NMSC was significantly increased in the crude and matched analysis (HR 1.807, 95% CI 1.373–2.378, p < 0.001) (Figure 2 and Supplementary Table 3). Since the risk of skin cancer, especially melanoma, is known to be increased in individuals with a history of previous skin cancers (29), an analysis with the addition of “Personal history of malignant neoplasm of skin” (ICD-10CM:Z85.82) as a covariate for PSM was performed, showing no significant change in risk relationships (results not shown).
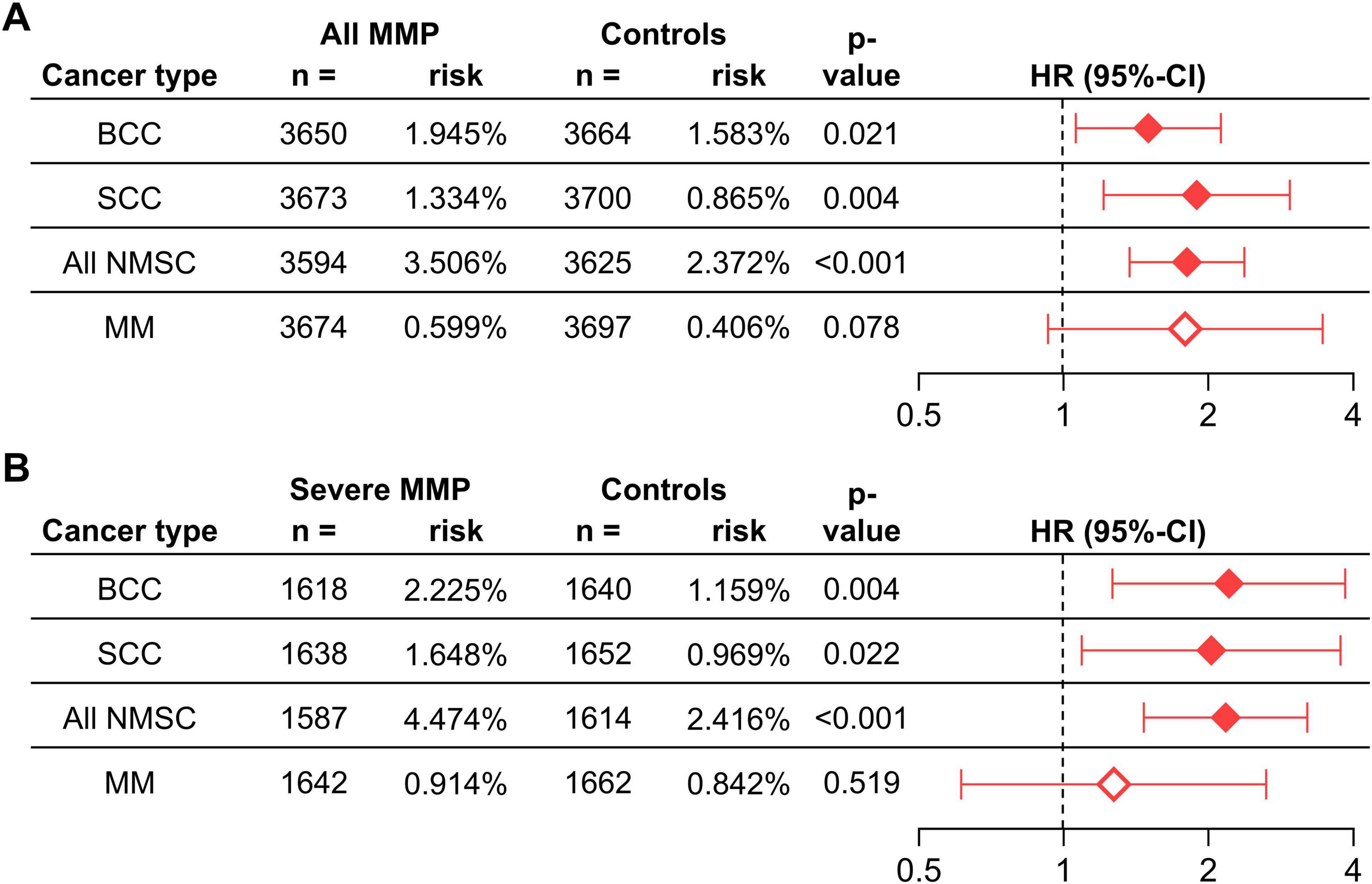
Figure 2. The risk of skin cancers in individuals with any MMP (A) and severe MMP (B) compared with controls without MMP. BCC, basal cell carcinoma; CI, confidence interval; HR, hazard ratio; MM, malignant melanoma; MMP, mucous membrane pemphigoid; NMSC, non-melanoma skin cancer.
When stratifying the analysis by disease severity for outcomes with a 5-year follow-up, significant risk increases for mild MMP were only found for SCC, BCC, and NMSC in the crude analysis but not for any outcome in the matched analysis (Supplementary Tables 3, 4). Interestingly, the observed risks were substantially augmented in patients with severe MMP. SCC showed an almost 4 times increased risk in the crude analysis, and the risk was doubled in the matched analysis (HR 2.026, 95% CI 1.091–3.763, p = 0.022). Risk increases for BCC were 2.5 in the crude and 2.2 times in the matched analysis (HR 2.206, 95% CI 1.264–3.848, p = 0.004). MM displayed a significantly increased risk in the crude analysis but was not significant in the matched analysis. Any NMSC, however, had an almost tripled risk in the crude analysis, and doubled risk in matched analysis (HR 2.171, 95% CI 1.468–3.211, p < 0.001) (Figure 2 and Supplementary Table 3).
All outcomes were analyzed for a 1-year follow-up with matched analyses. A significant risk was observed for SCC (HR 2.339, 95% CI 1.009–5.420, p = 0.041), while BCC and MM showed trends towards increased risks, albeit not significant. Significant risks remained for any NMSC (HR 1.710, 95% CI 1.137–2.578, p = 0.009). When analyzing for a 1–5-year follow-up, there were no significant increased risks for SCC, BCC, or MM. However, there was a significant increase in any NMSC in this group (HR 1.594, 95% CI 1.115–2.277, p = 0.010). When extending the follow-up to any time after index, significant risk increases for SCC (HR 1.594, 95% CI 1.115–2.277, p = 0.010), MM (HR 1.665, 95% CI 1.015–2.731, p = 0.041), and any NMSC (HR 1.492, 95% CI 1.184–1.880, p = 0.001) were observed, while BCC showed no significance (Table 2).
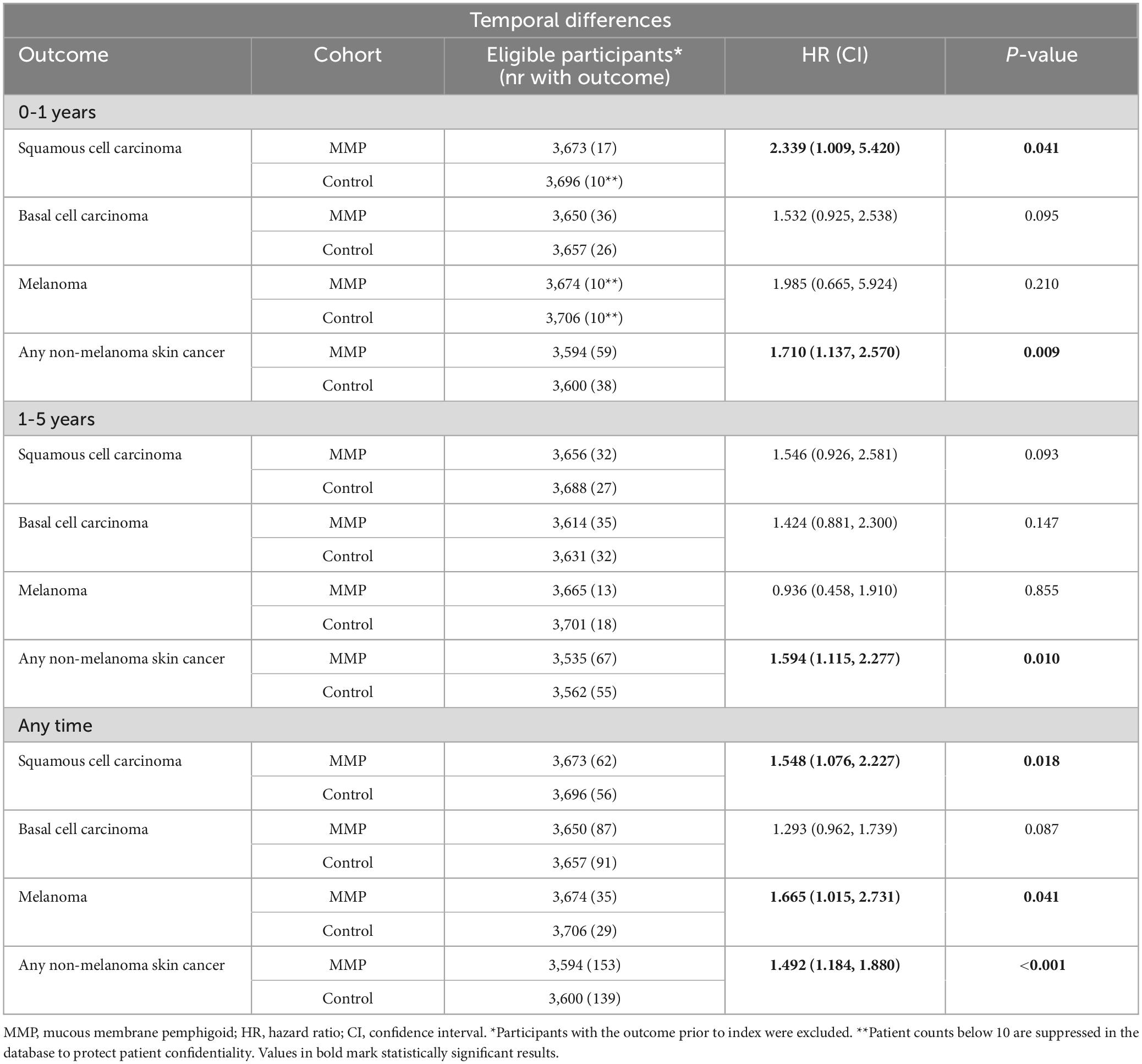
Table 2. Propensity-score matched analyses of temporal differences in patients with mucous membrane pemphigoid.
A significant association between MMP and specific skin cancers, particularly SCC, BCC, and total NMSC, was found in this large-scale propensity-score matched cohort study. The findings highlighted that the risks were most pronounced in patients with severe forms of MMP.
The mechanisms responsible for the risk of cancer in patients with MMP are not yet fully understood, with some research suggesting that the associated serotypes may confer anything from increased to reduced risk to having no influence. Most studies have focused on anti-laminin 332 MMP, despite it being a minority among MMP patients (12). Evidence primarily suggests an increased risk of internal malignancies, particularly adenocarcinomas (12–14, 16), with this risk potentially being higher within the first year of blister onset (13). Other forms of MMP include serotypes which produce antibodies against the different subunits of the α6β4-integrin heterodimer, which instead is thought to be linked to a decreased risk of cancer (15, 16), and a serotype with production of antibodies against BP180 and BP230 which seems to have no effect on cancer risk (12, 16).
The role of different antigens regarding tumor development, survival, proliferation, invasion, and prognosis is complex. Some research has shown that the expression of laminin 332 is increased in some tumor cell lines, while being decreased in others. However, it is generally believed that laminin 332 in most cases acts as a tumor suppressant by promoting tissue homeostasis, which would explain the increased risk of cancer in patients with anti-laminin 332 MMP (13). Some studies even suggest a direct correlation between tumor burden and disease severity (14), with some cases even reporting significant clinical improvement of the disease after successful treatment of the primary tumor (13, 18, 30, 31). In contrast, the α6β4 heterodimer is most often associated with promoting tumor growth and spread. This might explain why patients with anti-α6β4 integrin MMP show a decreased risk of cancer in some studies. Interestingly, the α6 and β4-integrin antibody titers seem to be correlated with disease activity and decrease as the symptoms improve and may even become negative with remission (16, 32–34). Of note, only individual MMP patients were shown to react with α6β4 integrin, and no reliable test system is available for these autoantibodies. While the main target antigen of MMP is BP180, the presence of these antibodies did not correlate with disease activity or severity or an increased risk of malignancy, which is why it can be believed to be a secondary phenomenon (12, 16).
Patients with MMP, especially individuals with more severe forms of the disease, are likely to have a higher level of autoimmunity compared to individuals of the general population, which could speculatively lead to an increased risk of cancer. This, coupled with a higher degree of inflammation causing long-term tissue damage and requiring constant cellular changes and repair, is likely to lead to the promotion of malignant transformation. Most medications used as treatment of severe MMP are immunosuppressives that reduce immune surveillance and weaken the immune system’s ability to detect and eliminate cancerous cells. However, this risk typically manifests after a timeline of more than 3-5 years (35, 36) and because of this, the elevated cancer prevalence observed in severe MMP cannot be explained solely by the effects of these treatments.
Overall different risk relationships were seen between NMSC and MM. Possible explanations might be that both MMP and NMSC involve dysfunction of the basement membrane zone and share autoimmune mechanisms affecting epithelial integrity (33, 37), while MM develops from melanocytes, being less affected by the autoimmune process in MMP compared to keratinocytes. Second, it could be speculated that the prevalence of MM in patients with more severe forms of MMP is higher due to broader immune system dysfunction that affects melanoma surveillance.
The present study described patients with MMP to be at an increased risk of developing skin cancer, particularly SCC, BCC, and NMSC, with the risks being most pronounced in severe cases. This underscores the importance of implementing tailored follow-up protocols and regular cancer screenings for MMP patients to facilitate early detection and timely intervention. Personalized care strategies that account for disease severity and cancer risk could significantly improve patient outcomes. Furthermore, these findings highlight the potential value of investigating similar cancer risks in other autoimmune blistering diseases and, more broadly, across autoimmune disorders. Such research could contribute to a deeper understanding of the complex interplay between chronic inflammation, autoimmunity, and cancer development.
The main strength of this study lies in the use of a large and diverse cohort, allowing for detailed propensity-score matching (PSM) to minimize confounding bias. The extensive sample size enhances the generalizability of our findings, and the inclusion of rigorous temporal differences across various follow-up periods strengthens the robustness of the results. The large sample size also allows for a comparison of mild and severe MMP, emphasizing the correlation to disease severity. This comprehensive approach provides valuable insights into cancer risks in patients with MMP, making the findings relevant for clinical practice.
A limitation of the study is the absence of specific autoantibody data, such as anti-laminin 332, which prevented examination into potential differences between MMP subtypes. However, since subtype testing is not routine in most clinical settings, the findings still offer a broad and clinically meaningful understanding of cancer risk in MMP. Additionally, although detection bias is a potential concern, given that MMP patients might undergo more frequent monitoring near diagnosis, analysis of temporal differences revealed this impact is likely small. While PSM controlled for many important variables, residual confounding from unmeasured factors such as UV radiation therapy and immunosuppressive post-MMP diagnosis, remains possible. The use of medications as a proxy for severity stratification could also constitute a limitation, as exposure to these may confound skin cancer risk. While statistically significant, the sometimes wide confidence intervals warrants caution when interpreting the strengths of the observed risk associations. Finally, the retrospective design and use of ICD-10CM codes introduces the risk of miscoding and misclassification, as well as limits causal inferences.
This study demonstrates a significant correlation between MMP and SCC, BCC, and NMSC in general, and in severe cases in particular. While no correlation was found between MMP and MM in the 5-year follow-up for mild cases, significant associations were observed in severe MMP and in longer-term analyses. Current research regarding the risk of cancer in different MMP subtypes provides conflicting results, and these relationships likely involve complex mechanisms including autoimmunity and inflammation. Highlighting these risks is crucial for improving awareness and early detection. Future research is warranted to explore the underlying mechanisms driving these associations, including the roles of chronic inflammation, immune dysregulation, and potential genetic predispositions.
The data analyzed in this study is subject to the following licenses/restrictions: Specific institutional accesses. Requests to access these datasets should be directed to PC, cGhpbGlwLmN1cm1hbkBraS5zZQ==.
The Swedish Ethical Review Authority has granted ethical approval for this study (diary number 2024-06878-02). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AN: Investigation, Visualization, Writing – original draft, Writing – review & editing. HO: Software, Visualization, Writing – original draft, Writing – review & editing. ES: Supervision, Writing – original draft, Writing – review & editing. RL: Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. PC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
RL and HO have received support for travelling from TriNetX.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that Generative AI was used in the creation of this manuscript. During the preparation of this manuscript the authors used GPT4o (OpenAI, San Francisco, CA) to improve readability in some sections. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1585167/full#supplementary-material
AIBD, Autoimmune Blistering Disease; BCC, basal cell carcinoma; CI, confidence interval; EHR, electronic health care record; HCO, health care organization; HIPAA, Health Insurance Portability and Accountability Act; HR, hazard ratio; KM, Kaplan-Meier; MM, malignant melanoma; MMP, mucous membrane pemphigoid; NMSC, non-melanoma skin cancer; SCC, squamous cell carcinoma.
1. Du G, Patzelt S, van Beek N, Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev. (2022) 21:103036. doi: 10.1016/j.autrev.2022.103036
2. Hofmann S, Günther C, Böckle B, Didona D, Ehrchen J, Gaskins M, et al. S2k Guideline for the diagnosis and treatment of mucous membrane pemphigoid. J Dtsch Dermatol Ges. (2022) 20:1530–50. doi: 10.1111/ddg.14905
3. Rashid H, Meijer J, Diercks G, Sieben N, Bolling M, Pas H, et al. Assessment of diagnostic strategy for mucous membrane pemphigoid. JAMA Dermatol. (2021) 157:780–7. doi: 10.1001/jamadermatol.2021.1036
4. Rashid H, Lamberts A, Borradori L, Alberti-Violetti S, Barry R, Caproni M, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part I. J Eur Acad Dermatol Venereol. (2021) 35:1750–64. doi: 10.1111/jdv.17397
5. Olbrich H, Patzelt S, Gaffal E, Kridin K, Curman P, Schmidt E, et al. Risk of oesophageal strictures in mucous membrane pemphigoid: Insights from a real-world cohort study. J Eur Acad Dermatol Venereol. (2025) [Online ahead of print]. doi: 10.1111/jdv.20603
6. Olbrich H, Patzelt S, Sadik C, Schmidt E, Ludwig R. Risk of blindness in a global cohort of patients with mucous membrane pemphigoid. J Eur Acad Dermatol Venereol. (2024) [Online ahead of print]. doi: 10.1111/jdv.20499
7. Kamaguchi M, Iwata H. The diagnosis and blistering mechanisms of mucous membrane pemphigoid. Front Immunol. (2019) 10:34. doi: 10.3389/fimmu.2019.00034
8. Chan L, Ahmed A, Anhalt G, Bernauer W, Cooper K, Elder M, et al. The first international consensus on mucous membrane pemphigoid: Definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. (2002) 138:370–9. doi: 10.1001/archderm.138.3.370
9. Buonavoglia A, Leone P, Dammacco R, Di Lernia G, Petruzzi M, Bonamonte D, et al. Pemphigus and mucous membrane pemphigoid: An update from diagnosis to therapy. Autoimmun Rev. (2019) 18:349–58. doi: 10.1016/j.autrev.2019.02.005
10. Schmidt E, Rashid H, Marzano A, Lamberts A, Di Zenzo G, Diercks G, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part II. J Eur Acad Dermatol Venereol. (2021) 35:1926–48. doi: 10.1111/jdv.17395
11. Patel P, Jones V, Murray T, Amber K. A review comparing international guidelines for the management of bullous pemphigoid, pemphigoid gestationis, mucous membrane pemphigoid, and epidermolysis bullosa acquisita. Am J Clin Dermatol. (2020) 21:557–65. doi: 10.1007/s40257-020-00513-3
12. Bernard P, Antonicelli F, Bedane C, Joly P, Le Roux-Villet C, Duvert-Lehembre S, et al. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. (2013) 149:533–40. doi: 10.1001/jamadermatol.2013.1434
13. Egan C, Lazarova Z, Darling T, Yee C, Yancey K. Anti-epiligrin cicatricial pemphigoid: clinical findings, immunopathogenesis, and significant associations. Medicine. (2003) 82:177–86. doi: 10.1097/01.md.0000076003.64510.00
14. Sadler E, Lazarova Z, Sarasombath P, Yancey KB. A widening perspective regarding the relationship between anti-epiligrin cicatricial pemphigoid and cancer. J Dermatol Sci. (2007) 47:1–7. doi: 10.1016/j.jdermsci.2007.02.012
15. Malik M, Gürcan H, Christen W, Ahmed A. Relationship between cancer and oral pemphigoid patients with antibodies to alpha6-integrin. J Oral Pathol Med. (2007) 36:1–5. doi: 10.1111/j.1600-0714.2006.00483.x
16. Letko E, Gürcan H, Papaliodis G, Christen W, Foster C, Ahmed A. Relative risk for cancer in mucous membrane pemphigoid associated with antibodies to the beta4 integrin subunit. Clin Exp Dermatol. (2007) 32:637–41. doi: 10.1111/j.1365-2230.2007.02463.x
17. La Placa M, Balestri R, Tartari F, Sechi A, Ferrara F, Loi C, et al. Mucous membrane pemphigoid-associated malignancies: Case series and a brief overview of the literature. Dermatol Pract Concept. (2019) 9:119–25. doi: 10.5826/dpc.0902a07
18. Leverkus M, Schmidt E, Lazarova Z, Bröcker E, Yancey K, Zillikens D. Antiepiligrin cicatricial pemphigoid: An underdiagnosed entity within the spectrum of scarring autoimmune subepidermal bullous diseases? Arch Dermatol. (1999) 135:1091–8. doi: 10.1001/archderm.135.9.1091
19. Matsushima S, Horiguchi Y, Honda T, Fujii S, Okano T, Tanabe M, et al. A case of anti-epiligrin cicatricial pemphigoid associated with lung carcinoma and severe laryngeal stenosis: Review of Japanese cases and evaluation of risk for internal malignancy. J Dermatol. (2004) 31:10–5. doi: 10.1111/j.1346-8138.2004.tb00497.x
20. Goletz S, Probst C, Komorowski L, Schlumberger W, Fechner K, van Beek N, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. (2019) 180:149–56. doi: 10.1111/bjd.17202
21. Qian H, Natsuaki Y, Koga H, Kawakami T, Tateishi C, Tsuruta D, et al. The second study of clinical and immunological findings in anti-laminin 332-type mucous membrane pemphigoid examined at Kurume University-diagnosis criteria suggested by summary of 133 cases. Front Immunol. (2021) 12:771766. doi: 10.3389/fimmu.2021.771766
22. van Beek N, Kridin K, Bühler E, Kochan A, Ständer S, Ludwig R, et al. Evaluation of site- and autoantigen-specific characteristics of mucous membrane pemphigoid. JAMA Dermatol. (2022) 158:84–9. doi: 10.1001/jamadermatol.2021.4773
23. Patzelt S, Schmidt E. Autoimmunity against laminin 332. Front Immunol. (2023) 14:1250115. doi: 10.3389/fimmu.2023.1250115
24. Giurdanella F, Nijenhuis A, Diercks G, Jonkman M, Pas H. Keratinocyte footprint assay discriminates antilaminin-332 pemphigoid from all other forms of pemphigoid diseases. Br J Dermatol. (2020) 182:373–81. doi: 10.1111/bjd.18129
25. Curman P, Kridin K, Zirpel H, Hernandez G, Akyuz M, Thaçi D, et al. COVID-19 infection is associated with an elevated risk for autoimmune blistering diseases while COVID-19 vaccination decreases the risk: A large-scale population-based cohort study of 112 million individuals. J Am Acad Dermatol. (2025) 92:452–63. doi: 10.1016/j.jaad.2024.10.063
26. Olbrich H, Kridin K, Zirpel H, Sadik C, Terheyden P, Thaçi D, et al. Cutaneous lupus erythematosus is associated with an increased risk of cardiac and vascular diseases: A large-scale, propensity-matched global retrospective cohort study. EBioMedicine. (2023) 93:104639. doi: 10.1016/j.ebiom.2023.104639
27. Ludwig R, Anson N, Zirpel H, Thaci D, Olbrich H, Bieber K, et al. A comprehensive review of methodologies and application to use the real-world data and analytics platform TriNetX. Front Immunol. (2025) 16:1516126. doi: 10.3389/fphar.2025.1516126
28. Palchuk M, London J, Perez-Rey D, Drebert Z, Winer-Jones J, Thompson C, et al. A global federated real-world data and analytics platform for research. JAMIA Open. (2023) 6:ooad035. doi: 10.1093/jamiaopen/ooad035
29. Pastorino L, Andreotti V, Dalmasso B, Vanni I, Ciccarese G, Mandalà M, et al. Insights into genetic susceptibility to melanoma by gene panel testing: Potential pathogenic variants in ACD, ATM, BAP1, and POT1. Cancers. (2020) 12:1007. doi: 10.3390/cancers12041007
30. Uchiyama K, Yamamoto Y, Taniuchi K, Matsui C, Fushida Y, Shirao Y. Remission of antiepiligrin (laminin-5) cicatricial pemphigoid after excision of gastric carcinoma. Cornea. (2000) 19:564–6. doi: 10.1097/00003226-200007000-00033
31. Ding D, Chu T, Hsu Y. Remission of anti-epiligrin cicatricial pemphigoid after excision of cervical adenocarcinoma. J Cutan Pathol. (2014) 41:692–3. doi: 10.1111/cup.12348
32. Letko E, Bhol K, Foster S, Ahmed R. Influence of intravenous immunoglobulin therapy on serum levels of anti-beta 4 antibodies in ocular cicatricial pemphigoid. A correlation with disease activity. A preliminary study. Curr Eye Res. (2000) 21:646–54.
33. Chan R, Bhol K, Tesavibul N, Letko E, Simmons R, Foster C, et al. The role of antibody to human beta4 integrin in conjunctival basement membrane separation: Possible in vitro model for ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci. (1999) 40:2283–90.
34. Rashid K, Foster C, Ahmed A. Identification of epitopes within integrin β4 for binding of auto-antibodies in ocular cicatricial and mucous membrane pemphigoid: Preliminary report. Invest Ophthalmol Vis Sci. (2013) 54:7707–16. doi: 10.1167/iovs.12-11404
35. Granata S, Tessari G, Stallone G, Zaza G. Skin cancer in solid organ transplant recipients: Still an open problem. Front Med. (2023) 10:1189680. doi: 10.3389/fmed.2023.1189680
36. Collins L, Quinn A, Stasko T. Skin cancer and immunosuppression. Dermatol Clin. (2019) 37:83–94. doi: 10.1016/j.det.2018.07.009
Keywords: autoimmunity, autoimmune, Pemphigus, pemphigoid, skin cancer, cancer, matched cohort study, mucous membrane pemphigoid
Citation: Nouri A, Olbrich H, Schmidt E, Ludwig RJ and Curman P (2025) Increased risk of skin cancers in mucous membrane pemphigoid: a large-scale matched cohort study of 117 million US individuals. Front. Med. 12:1585167. doi: 10.3389/fmed.2025.1585167
Received: 28 February 2025; Accepted: 20 March 2025;
Published: 03 April 2025.
Edited by:
Laura Atzori, University of Cagliari, ItalyReviewed by:
Giulia Ciccarese, San Martino Hospital (IRCCS), ItalyCopyright © 2025 Nouri, Olbrich, Schmidt, Ludwig and Curman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip Curman, cGhpbGlwLmN1cm1hbkBraS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.