
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 02 April 2025
Sec. Ophthalmology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1572461
This article is part of the Research TopicNew Concepts, Advances, and Future Trends in Clinical Research on Eye DiseasesView all 30 articles
Amiodarone, a highly effective yet lipophilic antiarrhythmic drug with prolonged half-life, is associated with systemic and ocular complications. While keratopathy being the most prevalent, affecting 70–100% of long-term users, amiodarone-associated optic neuropathy (AAON), though rare (incidence: 0.36–2%), can induce diverse visual impairments, ranging from mild deficits to profound vision loss. Given that patients on amiodarone frequently possess the risk factors of vascular diseases, it is essential to differentiate the diagnosis of AAON from non-arteritic anterior ischemic optic neuropathy (AION). This study reports a 61-year-old man who developed both corneal deposition and optic neuropathy during systemic amiodarone therapy. We further analyze the clinical features of keratopathy and optic neuropathy caused by amiodarone through a comprehensive literature review, aiming to enhance diagnostic recognition and management strategies.
Amiodarone is a class III antiarrhythmic drug with properties of class I, II, and IV, commonly used to treat ventricular and supraventricular arrhythmias (1). The compound exhibits oral bioavailability between 30 and 80%, with a half-life spanning 20 to 100 days (2). Amiodarone’s lipophilicity and strong tissue affinity contribute to its widespread toxicity, impacting various organs such as the lungs, thyroid, liver, skin, and nervous system, particularly with prolonged or high-dose usage (3, 4). Moreover, ocular side effects have been noted including keratopathy, lens opacities, retinopathy and optic neuropathy, among which the most common is vortex keratopathy, occurring in 70–100% of the patients (5). This form of keratopathy is typically benign, potentially reversible, and generally does not result in visual impairment. Amiodarone-associated optic neuropathy (AAON) is a relatively rare condition, with an incidence rate ranging from 0.36 to 2%. It has the potential to cause visual impairment, which can vary from mild to severe permanent vision loss (6). Several case reports and case series have linked the use of amiodarone to optic neuropathy or keratopathy (7, 8). However, there are relatively few reports documenting the co-occurrence of optic neuropathy and keratopathy in previous studies (9, 10). We present a case of a 61-year-old male with a history of ventricular tachycardia treated with amiodarone, who developed concurrent corneal and optic neuropathy in both eyes.
In November 2023, a 61-year-old male patient was admitted to our hospital presenting with a complaint of bilateral blurred vision persisting for over 20 days. The patient had a four-year history of hypertension treated with metoprolol and a two-year history of hypertrophic non-obstructive cardiomyopathy without specific treatment. Four months ago, the patient received a dual-chamber implantable cardioverter-defibrillator (ICD) for persistent ventricular tachycardia, after which he began taking 200 mg of amiodarone daily to reduce the occurrence of ventricular tachycardia. The patient reported no history of systemic or ocular diseases and no history of ocular trauma.
A comprehensive ophthalmologic examination was conducted, encompassing best-corrected visual acuity (BCVA), slit-lamp examination, applanation tonometry, perimetry, and fundoscopy. Utilizing the international standard visual acuity chart, the BCVA was determined to be 0.08 for the right eye and 0.5 for the left eye. Slit-lamp examination revealed bilateral, symmetric, whorl-like brown deposits in the inferocentral cornea, with no fluorescein staining observed (Figures 1A,B). Intraocular pressures were normal, and mild lens opacities were noted in both eyes.
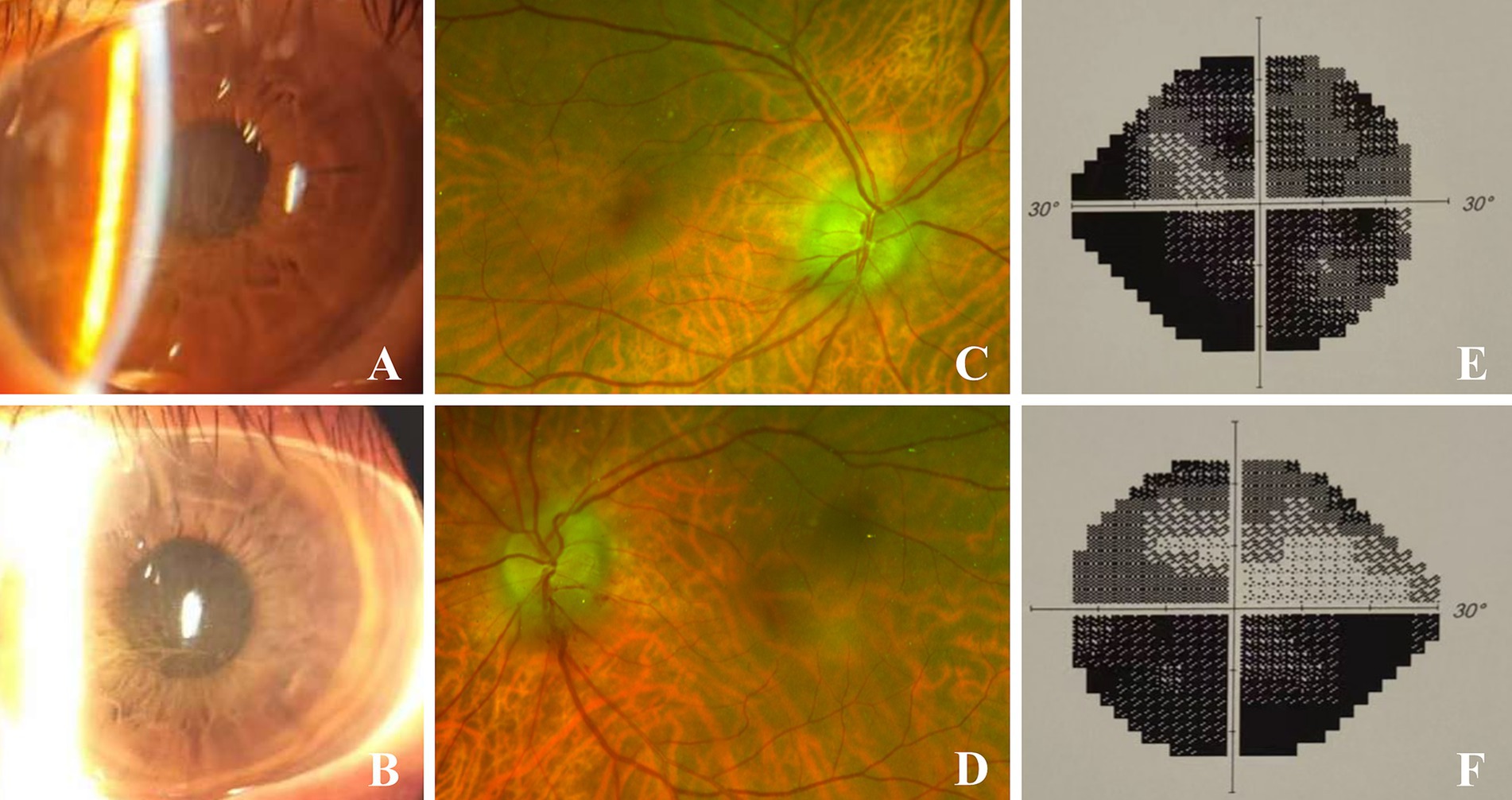
Figure 1. Binocular images on initial presentation. (A,B) were slit-lamp images of the right and left eyes respectively, showing greyish brown corneal epithelial opacities. (C,D) were fundus images of the right and left eyes respectively, showing disc edema and peridisk bleeding. (E,F) showed binocular perimetry indicating diffuse total defect in the right eye, inferior altitudinal defect and upper arcuate blind spots in the left eye.
The pupil measures approximately 3.5 mm in diameter and exhibits a slow response to both direct and indirect light, with no relative afferent pupillary defect (RAPD) observed. The fundus examination revealed bilateral optic disc edema accompanied by peripapillary hemorrhages (Figures 1C,D), while no significant abnormalities were detected in the macular region. Perimetry revealed a diffuse defect in the right eye and inferior altitudinal as well as superior arcuate defects in the left eye (Figures 1E,F).
Upon admission, systemic examinations were conducted. Laboratory findings indicated an erythrocyte sedimentation rate of 2.0 mm/h (reference range: 0–21.00 mm/h), C-reactive protein level of 4.68 mg/L (0–5.00 mg/L), international normalized ratio of 1.03 (0.75–1.10), antistreptolysin O titer of 33.3 IU/mL (0–116.0 IU/mL), and rheumatoid factor of less than 20 IU/mL (0–20 IU/mL). Tests for tuberculin, hepatitis markers, toxoplasma, cytomegalovirus, herpes simplex virus (HSV), varicella zoster virus (VZV), human immunodeficiency virus (HIV), syphilis, aquaporin-4 antibodies (NMO), and myelin oligodendrocyte glycoprotein antibodies (MOG) were all negative. Both cervical artery ultrasonography and a non-contrast brain CT scan yielded normal results. Despite empirical treatment with 500 mg/day intravenous methylprednisolone for three days, the patient’s visual acuity did not show significant improvement. The patient had taking amiodarone therapy for four months. Cardiology was consulted. A diagnosis of amiodarone induced optic neuropathy and keratopathy was made. Amiodarone was discontinued with the consent of the patient’s cardiologists, and corticosteroid treatment was gradually tapered. After two months, the corneal deposits resolved (Figures 2A,B), and BCVA was 0.1 in the right eye and 0.4 in the left eye. However, disc swelling and peridisk hemorrhage persisted in both eyes. A follow-up examination at 5 months demonstrated complete resolution of optic disc edema (Figures 2C,D) and slight progression of visual field defect in both eyes (Figures 2E,F). After one year of follow-up, BCVA and visual field remained stable, and the papilledema had fully regressed without recurrence. The patient’s diagnosis with the relevant data about the care and follow-up was summarized in Table 1.
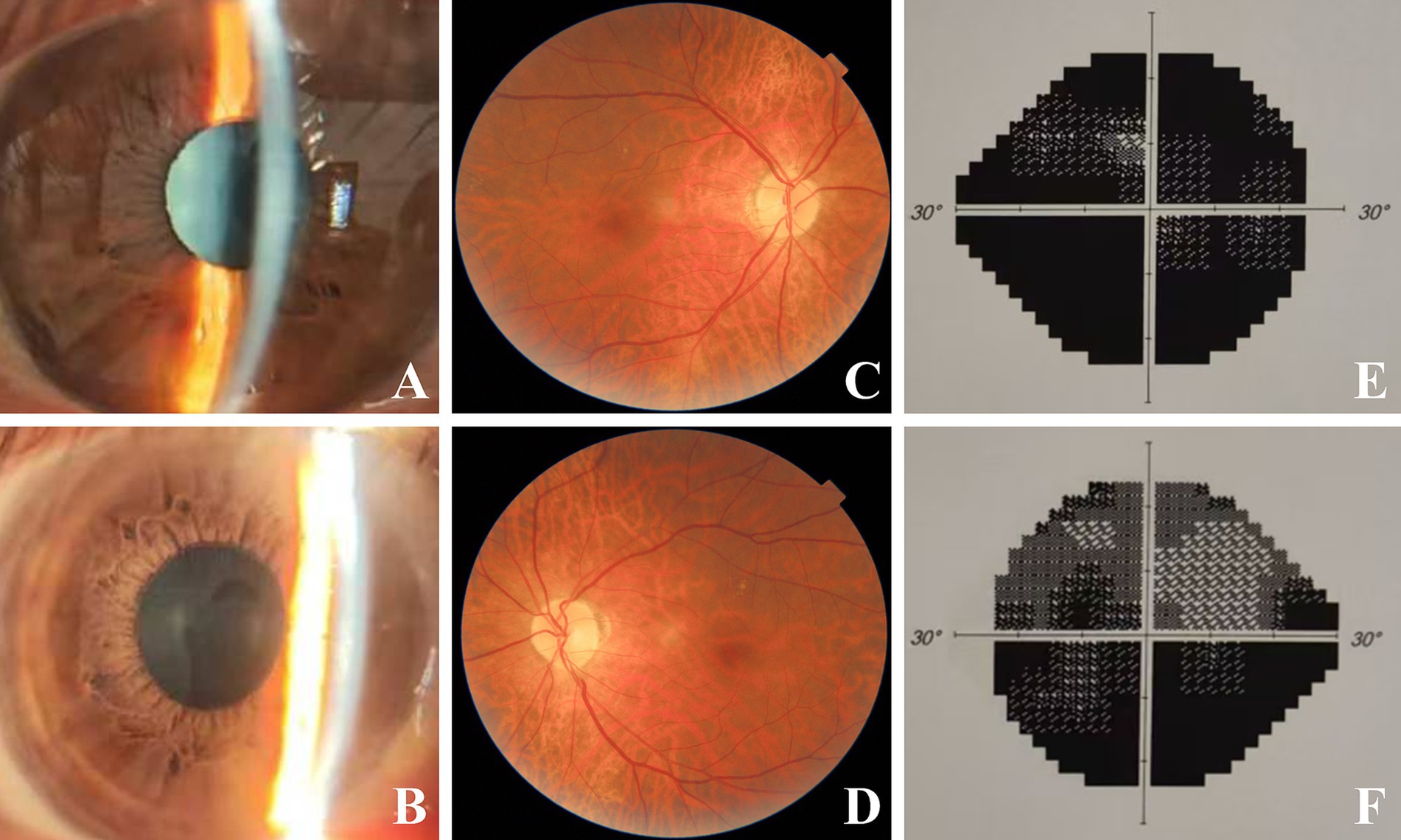
Figure 2. Binocular images during follow-up. Two months later after discontinuing of amiodarone, vortex keratopathy of right (A) and left (B) eye regressed absolutely. Five months after discontinuing of amiodarone, complete resolution of papilledema and hemorrhages were observed in right (C) and left eye (D). Slight progression of visual field defect was observed in right (E) and left (F) eye.
Amiodarone is increasingly utilized as an effective antiarrhythmic medication. However, due to its high lipophilicity, low bioavailability and prolonged half-life, various systemic and ocular complications have emerged. Desethyl-amiodarone, the primary metabolite of amiodarone, exhibits identical pharmacological effects to the parent compound. Amiodarone and its active metabolite form a complex with lipid substrates of lysozyme, leading to deposition in lysosomal inclusion bodies (11). These lamellar bodies had been observed in nearly all ocular tissues, including the cornea, crystalline epithelium, retina, and optic nerve, causing various amiodarone-induced ocular tissue complications (12). Among these complications, amiodarone-induced keratopathy and optic neuropathy represent two clinically significant ocular manifestations. While keratopathy is the most prevalent (affecting 70–100% of long-term users), optic neuropathy, though rare (0.36–2% incidence), may lead to irreversible vision loss, underscoring the need for vigilant monitoring.
Amiodarone-induced ocular lesions were firstly described in the 1960s, with vortex keratopathy being the most prevalent manifestation. The formation of corneal deposits may be due to the secretion of amiodarone and its active metabolites in tears into the tear film. With the increase of drug concentration in tear film and prolonged exposure to ultraviolet light, the drug-lipid complex is deposited in the corneal tissue (13, 14).
Vortex keratopathy is characterized by a distinctive, linear, branching pattern of grayish or golden-brown deposits located in the lower two-thirds of the corneal epithelium. One hypothesis posits that the predominant horizontal line in this region of the cornea aligns with the lid closure line, with deposits formation due to drug concentration in the tears (15–17). Alternatively, it is suggested that these lines arise from the centripetal migration of epithelial cells (15, 18). Keratopathy is categorized into four stages based on the extent of lesion involvement through slitlamp biomicroscopy (15). Grade I is characterized by a ‘dusting’ of the cornea at the inferior pupillary margin in the midperiphery; Grade II presents with deposits arranged in a linear pattern extending from the inferior pupillary margin toward the limbus; Grade III is marked by an increased number of branching patterns in the inferior pupillary area extending into the visual axis; Grade IV is distinguished by the presence of additional clumps of deposits compared to grade III. These four grades represent a sequential progression and are associated with the dosage and duration of amiodarone therapy. The examination of all corneal layers by confocal microscopy has revealed that amiodarone keratopathy is not confined to the epithelium, but also affects the entire corneal structure, including the Bowman’s layer, stroma, and endothelium (19, 20).
Keratopathy can develop as early as 6 days following the initiation medication, although it typically manifests within 1 to 3 months (18). The severity of keratopathy correlates with both the daily dosage and the duration of amiodarone use. A higher total intake is associated with more advanced keratopathy (21, 22). Dovie et al. (23) reported a case who took 100 mg of amiodarone daily for 6 years without developing keratopathy. However, keratopathy appeared after 6 weeks when the dosage was increased from 100 mg/d to 300 mg/d. Another report indicated that grade I keratopathy was observed after 5 months of taking 400 mg of amiodarone daily, grade II keratopathy was noted at a 12-month follow up, and grade IV keratopathy emerged at a 40-month follow-up (24).
Amiodarone-induced corneal deposits are generally bilateral and symmetrical (3, 25), although some exceptional cases have been documented. Chilov et al. (13) reported a unilateral case of amiodarone keratopathy alongside corneal dysplasia in the contralateral eye, suggesting that the rapid epithelial cell turnover associated with corneal dysplasia prevents the drug-lipid complex from adhering to the corneal tissue, thereby inhibiting the development of keratopathy. Bhatt and Ramaesh (26) documented another case of unilateral lesions, where a contact lens was present on the contralateral eye. The authors suggested that the contact lens inhibited the interaction between ultraviolet light and concentrated amiodarone, thereby preventing keratopathy in the opposite eye. Another research indicated that keratopathy in donor corneas resolves entirely within two weeks following transplantation. This rapid resolution may be attributed to either the redistribution of the drug from the donor tissue into a drug-free reservoir or the swift repopulation of the donor cornea by recipient-derived epithelium (27). Another study reported that the recipient was administered amiodarone after a corneal transplant, keratopathy manifested in one eye, while no significant changes were observed in the donor cornea. A possible reason was that disruption at the graft-host interface may impede the centripetal migration of deposit-laden epithelial cells forming at the limbus (28).
Corneal deposits rarely lead to visual loss, yet, some patients experience photosensitivity, colored halos, and glare (25, 29). In case that more severe adverse reactions such as vision loss occur, discontinuation of the drug may be considered, with the keratopathy potentially resolving completely within 3 to 20 months thereafter (5, 30). Our patient did not experience any obvious foreign body sensation, photophobia and other symptoms in either eye. Consequently, the keratopathy was not subjected to any specific treatment; instead, the medication was discontinued for observation. Expectedly, the corneal pigmentation completely resolved following a two-month period of drug withdrawal.
AAON is an uncommon condition with an incidence of 0.36–2%. The exact mechanism of optic neuropathy remains to be determined, yet one potential hypothesis suggests that lipid inclusion body deposition in optic nerve axons may impede axonal blood flow, leading to optic disc edema (31). Vision loss in AAON patients ranges from mild and reversible to severe and permanent (32, 33).
Patients on amiodarone usually have risk factors for vascular disease, which is the primary cause of non-arteritic anterior ischemic optic neuropathy (NAION), leading to frequent misdiagnosis of AAON as NAION due to their similar clinical features. However, AAON and NAION differ in treatment and prognosis, necessitating careful differentiation.
Previous studies have documented the clinical manifestations of AAON. Macaluso et al. (34) analyzed 73 AAON patients, identifying their clinical characteristics as insidious onset, gradual progression, bilateral visual impairment, and optic disc swelling. A study by Passman et al. (35) involving 296 AAON patients showed the average duration of amiodarone use prior to vision loss was 9 months. They reported that 44% of patients experienced hidden onset, one-third exhibited no obvious symptoms, and 85% presented with optic disc edema. Nevertheless, NAION is marked by sudden, one-sided disc swelling accompanied by hemorrhage, typically resolving within several weeks (34, 36).
Purvin et al. (36) reported that 14 out of 19 patients with AAON had bilateral disc edema, while Johnson et al. (37) showed that 43 out of 55 patients experienced binocular disc edema, indicating that binocular disease was relatively common in AAON, accounting for about 80% of cases. NAION typically manifests as a unilateral condition. In a prospective study including 388 NAION patients, 18% of the cases experienced the second eye involvement within one year, increasing to 23% within two years (38).
AAON is more frequently observed in males. Johnson et al. (37) reported that 87% of the 55 patients diagnosed with AAON were male, and Cheng’s study (39) indicated that males treated with amiodarone had nearly a threefold increased risk of developing optic neuropathy compared to females. In contrast, NAION does not exhibit a sex preference.
Optic disc edema can persist for 1–8 months in AAON cases, with a median duration of approximately 3 months (37). Conversely, optic disc swelling in NAION typically resolves within 2 to 6 weeks. The prolonged half-life of amiodarone, which can be up to 100 days, may account for the extended duration of optic nerve swelling in AAON. Furthermore, NAION occurs in patients with crowded small optic disc most commonly, AAON is not associated with any specific cup-disc ratio (40–43). Based on these distinguishing features, the diagnostic criteria for AAON are delineated in the literature as follows: (1) a documented history of amiodarone use; (2) gradual onset; (3) slow progression; (4) bilateral vision defect; and (5) optic disc edema. Clinicians should be alert to the possibility of AAON in patients who do not present with the typically small, crowded optic disc associated with NAION (4, 44). The characteristics of our case aligned with these criteria, thereby corroborating the diagnosis of AAON.
When evaluating patients undergoing amiodarone therapy who develop optic disc swelling or optic neuropathy, it is crucial to consider that these patients often have significant cardiovascular conditions. Therefore, it is prudent to consult the cardiologist regarding the potential discontinuation or dosage reduction of amiodarone. Prognostic outcomes following the cessation of amiodarone vary according to several case studies. Passman et al. (37) reported that 58% of the patients experienced visual improvement, while 21% showed no change, and another 21% suffered further visual deterioration after discontinuing amiodarone. The study by Purvin et al. (39) showed nearly half of the 14 patients exhibited decreased vision during follow-up.
In the present case, although the BCVA in the right eye improved slightly after discontinuation of amiodarone, the damage to the visual field in both eyes was further aggravated. It is considered that the progressive visual field deficiency may be attributed to the underlying risk factors predisposing to secondary optic disc ischemia or the direct toxic effects of amiodarone due to given its prolonged half-life of up to 100 days. Although the pathogenesis of amiodarone-related cytotoxicity has not been fully elucidated, several studies have proposed potential mechanisms. For example, Golli-Bennour et al. (45) reported that amiodarone cytotoxicity was caused by reactive oxygen species (ROS)-related oxidative stress, another report from Bayrakçeken et al. (46) showed that ATP can antagonize amiodarone-caused oxidative damage, it is known that ATP is involved in the synthesis of antioxidants scavenging and clearing ROS. Therefore, we believe that antioxidative treatment in the future may be beneficial in preventing amiodarone-induced optic neuropathy.
In conclusion, amiodarone-related ocular conditions are frequently encountered in clinical practice, with keratopathy being the most prevalent due to its typical clinical manifestations, which facilitate straightforward diagnosis. However, the incidence of AAON is relatively low, and its onset is insidious. The clinical diagnosis of AAON is challenging due to its similarities with NAION and its unclear pathogenesis. Consequently, further prospective studies are warranted to elucidate the potential pathophysiological and pathogenic mechanisms of optic neuropathy associated with amiodarone, thereby enhancing our understanding of AAON.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by the Ethics Committee of Affiliated People’s Hospital, Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual (s) for the publication of any potentially identifiable images or data included in this article.
NM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft. LX: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft. YG: Data curation, Investigation, Resources, Validation, Writing – review & editing. PL: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Zhenjiang City Social Development Project (FZ2023062, SH2023041, JC2024033), the Bethune Medical Scientific Research Foundation (2023-YJ-119-J-031).
The authors thank the studies which were included in this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AAON, Amiodarone-associated optic neuropathy; BCVA, Best-corrected visual acuity; RAPD, Relative afferent pupillary defect; NAION, Non-arteritic anterior ischemic optic neuropathy.
1. Mitchell, R, and Chacko, J. Clinical and mechanistic review of amiodarone-associated optic neuropathy. Biomol Ther. (2022) 12:1298. doi: 10.3390/biom12091298
2. Latini, R, Tognoni, G, and Kates, RE. Clinical pharmacokinetics of amiodarone. Clin Pharmacokinet. (1984) 9:136–56. doi: 10.2165/00003088-198409020-00002
3. Turk, U, Turk, BG, Yılmaz, SG, Tuncer, E, Alioğlu, E, and Dereli, T. Amiodarone-induced multiorgan toxicity with ocular findings on confocal microscopy. Middle East Afr J Ophthalmol. (2015) 22:258–60. doi: 10.4103/0974-9233.154411
4. Nagra, PK, Foroozan, R, Savino, PJ, Castillo, I, and Sergott, RC. Amiodarone induced optic neuropathy. Br J Ophthalmol. (2003) 87:420–2. doi: 10.1136/bjo.87.4.420
5. Ingram, DV, Jaggarao, NS, and Chamberlain, DA. Ocular changes resulting from therapy with amiodarone. Br J Ophthalmol. (1982) 66:676–9. doi: 10.1136/bjo.66.10.676
6. Gittinger, JW Jr, and Asdourian, GK. Papillopathy caused by amiodarone. Archives Ophthalmol. (1987) 105:349–51. doi: 10.1001/archopht.1987.01060030069028
7. Inoue, H, Toriyama, K, Joko, T, and Shiraishi, A. In vivo confocal microscopic images of atypical amiodarone-induced keratopathy in patient with epithelial basement membrane dystrophy. Am J. Ophthalmol Case Reports. (2021) 22:101105. doi: 10.1016/j.ajoc.2021.101105
8. Alshehri, M, and Joury, A. Ocular adverse effects of amiodarone: a systematic review of case reports. Optometry Vision Sci. (2020) 97:536–42. doi: 10.1097/opx.0000000000001534
9. Tan, Y, Sia, P, and Simon, S. Optic neuropathy secondary to Perhexiline and amiodarone. BMJ Case Rep. (2021) 14:e237727. doi: 10.1136/bcr-2020-237727
10. Chassang, B, Bonnin, N, Moisset, X, Citron, B, Clavelou, P, and Chiambaretta, F. Two cases of bilateral amiodarone-associated optic neuropathy. Journal francais d'ophtalmologie. (2014) 37:231–6. doi: 10.1016/j.jfo.2013.12.004
11. Lüllmann, H, Lüllmann-Rauch, R, and Wassermann, O. Drug-induced Phospholipidoses. CRC Crit Rev Toxicol. (1975) 4:185–218. doi: 10.1080/10408447509164014
12. Palmeri, S, Battisti, C, Malandrini, A, and Federico, A. Amiodarone induced Lipidosis similar to Niemann-pick C disease. Biochemical and morphological study. Life Sci. (1995) 57:1963–71. doi: 10.1016/0024-3205(95)02129-7
13. Chilov, MN, Moshegov, CN, and Booth, F. Unilateral amiodarone keratopathy. Clin Experiment Ophthalmol. (2005) 33:666–8. doi: 10.1111/j.1442-9071.2005.01121.x
14. Pollak, PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. (1999) 84:37R–45R. doi: 10.1016/s0002-9149(99)00700-6
16. Gass, JD. The Iron lines of the superficial. Cornea Archives Ophthalmol. (1964) 71:348–58. doi: 10.1001/archopht.1964.00970010364010
17. Feiler-Ofry, V, Lazar, M, Solomon, A, and Godel, V. Amiodarone keratopathy. Ophthalmologica. (1980) 180:257–61. doi: 10.1159/000308983
18. Orlando, RG, Dangel, ME, and Schaal, SF. Clinical experience and grading of amiodarone keratopathy. Ophthalmology. (1984) 91:1184–7. doi: 10.1016/s0161-6420(84)34165-3
19. Ciancaglini, M, Carpineto, P, Zuppardi, E, Nubile, M, Doronzo, E, and Mastropasqua, L. In vivo confocal microscopy of patients with amiodarone-induced keratopathy. Cornea. (2001) 20:368–73. doi: 10.1097/00003226-200105000-00007
20. Erdurmus, M, Selcoki, Y, Yagci, R, and Hepsen, IF. Amiodarone-induced keratopathy: full-thickness corneal involvement. Eye Contact Lens. (2008) 34:131–2. doi: 10.1097/ICL.0b013e31814934c0
21. Kaplan, LJ, and Cappaert, WE. Amiodarone Keratopathy: correlation to dosage and duration. Archives Ophthalmol. (1982) 100:601–2. doi: 10.1001/archopht.1982.01030030603011
22. Nielsen, CE, Andreasen, F, and Bjerregaard, P. Amiodarone induced cornea Verticillata. Acta Ophthalmol. (1983) 61:474–80. doi: 10.1111/j.1755-3768.1983.tb01447.x
23. Dovie, JM, and Gurwood, AS. Acute onset of halos and glare: bilateral corneal epithelial edema with cystic eruptions--atypical presentation of amiodarone keratopathy. Optometry. (2006) 77:76–81. doi: 10.1016/j.optm.2005.12.002
24. Rivera, RP, Younge, BR, and Dyer, JA. Atypical amiodarone-induced keratopathy in a patient wearing soft contact lenses. CLAO J. (1989) 15:219–21.
25. Frings, A, and Schargus, M. Recovery from amiodarone-induced cornea Verticillata by application of topical heparin. Cornea. (2017) 36:1419–22. doi: 10.1097/ico.0000000000001306
26. Bhatt, PR, and Ramaesh, K. Unilateral amiodarone keratopathy: occlusive contact Lens spares development in the contralateral eye. Eye. (2007) 21:296–8. doi: 10.1038/sj.eye.6702553
27. Garrett, SN, Waterhouse, WJ, and Parmley, VC. Amiodarone keratopathy in the donor cornea. Am J Ophthalmol. (1988) 105:425–7. doi: 10.1016/0002-9394(88)90315-7
28. Davitt, SP, and Mannis, MJ. Delayed development of amiodarone keratopathy in a corneal graft. Cornea. (1997) 16:695–7. doi: 10.1097/00003226-199711000-00017
29. Chan, TC, and Jhanji, V. Images in clinical medicine. Amiodarone-induced Vortex keratopathy. N Engl J Med. (2015) 372:1656. doi: 10.1056/NEJMicm1406501
30. Dolan, BJ, Flach, AJ, and Peterson, JS. Amiodarone keratopathy and Lens opacities. J Am Optom Assoc. (1985) 56:468–70.
31. Chen, D, and Hedges, TR. Amiodarone optic neuropathy--review. Semin Ophthalmol. (2003) 18:169–73. doi: 10.1080/08820530390895163
32. Patel, S, and Mahmood, R. Amiodarone-associated optic neuropathy in a patient with associated arrhythmia. Cureus. (2024) 16:e55819. doi: 10.7759/cureus.55819
33. Mason, RH, Handzic, A, Nichani, PAH, and Margolin, EA. Amiodarone producing insidious optic neuropathy. Can J Neurol Sci. (2024) 51:564–5. doi: 10.1017/cjn.2023.269
34. Macaluso, DC, Shults, WT, and Fraunfelder, FT. Features of amiodarone-induced optic neuropathy. Am J Ophthalmol. (1999) 127:610–2. doi: 10.1016/s0002-9394(99)00016-1
35. Passman, RS, Bennett, CL, Purpura, JM, Kapur, R, Johnson, LN, Raisch, DW, et al. Amiodarone-associated optic neuropathy: a critical review. Am J Med. (2012) 125:447–53. doi: 10.1016/j.amjmed.2011.09.020
36. Purvin, V, Kawasaki, A, and Borruat, FX. Optic neuropathy in patients using amiodarone. Archives Ophthalmol. (2006) 124:696–701. doi: 10.1001/archopht.124.5.696
37. Johnson, LN, Krohel, GB, and Thomas, ER. The clinical Spectrum of amiodarone-associated optic neuropathy. J Natl Med Assoc. (2004) 96:1477–91.
38. Beri, M, Klugman, MR, Kohler, JA, and Hayreh, SS. Anterior Ischemic Optic Neuropathy: Vii. Incidence of Bilaterality and various influencing factors. Ophthalmology. (1987) 94:1020–8. doi: 10.1016/s0161-6420(87)33350-0
39. Cheng, HC, Yeh, HJ, Huang, N, Chou, YJ, Yen, MY, and Wang, AG. Amiodarone-associated optic neuropathy: a Nationwide study. Ophthalmology. (2015) 122:2553–9. doi: 10.1016/j.ophtha.2015.08.022
40. Wang, AG, and Cheng, HC. Amiodarone-associated optic neuropathy: clinical review. Neuro Ophthalmol. (2017) 41:55–8. doi: 10.1080/01658107.2016.1247461
41. Rucker, JC, Biousse, V, and Newman, NJ. Ischemic optic neuropathies. Curr Opin Neurol. (2004) 17:27–35. doi: 10.1097/00019052-200402000-00006
42. Beck, RW, Servais, GE, and Hayreh, SS. Anterior Ischemic Optic Neuropathy: Ix. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. (1987) 94:1503–8. doi: 10.1016/S0161-6420(87)33263-4
43. Burde, RM. Optic disk risk factors for Nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. (1993) 116:759–64. doi: 10.1016/s0002-9394(14)73478-6
44. Luneau, K, Newman, NJ, and Biousse, V. Ischemic optic neuropathies. Neurologist. (2008) 14:341–54. doi: 10.1097/NRL.0b013e318177394b
45. Golli-Bennour, EE, Bouslimi, A, Zouaoui, O, Nouira, S, Achour, A, and Bacha, H. Cytotoxicity effects of amiodarone on cultured cells. Exp Toxicol Pathol. (2012) 64:425–30. doi: 10.1016/j.etp.2010.10.008
Keywords: amiodarone, optic neuropathy, keratopathy, amiodarone-associated optic neuropathy, anterior ischemic optic neuropathy
Citation: Meng N, Xia L, Gong Y and Lu P (2025) Amiodarone-related keratopathy and optic neuropathy: case report and literature review. Front. Med. 12:1572461. doi: 10.3389/fmed.2025.1572461
Received: 07 February 2025; Accepted: 21 March 2025;
Published: 02 April 2025.
Edited by:
Qi Dai, Wenzhou Medical University, ChinaReviewed by:
Amany El-Shazly, Ain Shams University, EgyptCopyright © 2025 Meng, Xia, Gong and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peirong Lu, bHVwZWlyb25nQHN1ZGEuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.