
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 07 April 2025
Sec. Intensive Care Medicine and Anesthesiology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1571631
This article is part of the Research Topic Nutrition in Pancreatic Diseases: The Role of Nutritional Status and Nutrition Therapy in the Management of Pancreatitis and Pancreatic Cancer View all 3 articles
Objective: To identify independent risk factors and predictive markers for acute pancreatitis (AP) in patients with diabetic ketoacidosis (DKA).
Methods: Here, we reported a 11-year experience in a single tertiary medical center and conduct a systematic review of acute pancreatitis in diabetic ketoacidosis patients (DKA-AP). 1,941 cases were included. First retrospective cohort study analyzed clinical data from 45 patients with DKA-AP and 45 matched controls with DKA alone (DKA-nonAP) admitted to Yangzhou University Affiliated Hospital (2013–2024). Baseline characteristics included BMI, hyperlipidemia history, and serological profiles. Secondly we retrieved clinical studies of DKA-AP from PubMed database system and analyzed these clinical data in depth.
Results: Significant differences were observed in BMI: median [IQR] = 26.30 [22.15–28.40] vs. 23.20 [20.70–25.35] kg/m2, p = 0.031, hyperlipidemia history (26.7% vs. 4.4%, p = 0.004), abdominal pain duration (3 vs. 0 days, p < 0.001), and lipid profiles TC: median [IQR] = 7.71 [5.60–11.66] vs. 4.86 [4.22–6.52] mmol/L, p < 0.001; TG: median [IQR] = 11.90 [4.71–15.84] vs. 1.60 [0.89–3.64] mmol/L, p < 0.001. Logistic regression identified TC (OR = 1.455, 95% CI: 1.196–1.769), TG (OR = 2.046, 95% CI: 1.202–3.484), and abdominal pain duration (OR = 3.892, 95% CI: 2.173–6.972) as independent risk factors. Receiver operating characteristic curve (ROC) analysis demonstrated strong predictive performance (combined AUC = 0.933, sensitivity = 93.3%, specificity = 73.3%). Moreover, we found 11 clinical studies investigating DKA-AP. Cumulatively, 1,851 patients were studied, including 3 interventional studies, 1 genetic observational study, and 7 cohort studies exploring risk factors.
Conclusion: Elevated TC, TG, and prolonged abdominal pain duration are key predictors of DKA-AP. These factors enhance early diagnosis and clinical management in Yangzhou, China.
The current clinical evidence supporting the existence of a distinct set of risk factors for the co-occurrence of diabetic ketoacidosis (DKA) and acute pancreatitis (AP), called DKA-AP, is inconclusive. The interplay between DKA and AP disease makes the clinical diagnosis of the DKA-AP more challenging, which can result in death. Nair and Satheesh have indicated that the incidence of unrecognized DKA-AP accounts for at least 15% of the DKA population and have broadly called for clinicians to screen for DKA-AP by serum amylase, lipase, and triglyceride assessment (1). An analysis of a U.S. population-based survey revealed a higher mortality rate in patients with DKA-AP compared to those with AP (aOR 2.3, p < 0.001; CI 1.8–3.0). It is noteworthy that this study demonstrated a national incidence of DKA-AP of 1% in the United States between 2003 and 2013, with a cumulative total of 33,356 patients with DKA-AP (2). The presented data indicates that DKA-AP is not an exceedingly uncommon occurrence. The reason for the failure to diagnose DKA-AP, which has resulted in fatalities among patients with DKA or AP, may be attributed to the absence of validated clinical predictive risk factors upon which to base clinical diagnostic decisions. The objective of this study was to examine the risk factors associated with DKA-AP through a retrospective analysis and to evaluate the predictive efficacy of the identified risk factors. This will contribute to the prevention and management of patients with DKA-AP and enhance diagnostic accuracy.
This study employed a retrospective design. The clinical data of 45 patients with DKA-AP who were admitted to the emergency department, gastroenterology department, endocrinology department, and critical care medicine department of Yangzhou University Affiliated Hospital between September 2013 and December 2024 were analyzed. To serve as a control group, DKA patients without AP (DKA-nonAP) with an equivalent number of cases during the same period were selected. The control group was matched to the DKA-AP group in terms of age, gender, and duration of diabetes, except for the absence of AP, in order to reduce the influence of confounding factors. The matching process was based on the propensity score matching method, whereby propensity scores were calculated based on demographics, medical history, symptoms, and laboratory parameters. DKA patients with similar scores were selected as the control group. The diagnosis of AP is based on the Guidelines for the Diagnosis and Treatment of Acute Pancreatitis in China (2021). AP diagnosis followed the 2021 Chinese guidelines for AP, which require at least two of the following: (1) characteristic abdominal pain; (2) serum amylase/lipase more than 3 times the upper limit of normal; (3) imaging findings consistent with AP (contrast-enhanced computed tomography or magnetic resonance imaging). The study was approved by the Institutional Ethics Committee of Yangzhou University Affiliated Hospital (2022-YKL3-06-004).
This study employed a mixed research methodology of retrospective cohort study and systematic review. This mixed research method was similar to that envisaged by Zhu et al. and Li et al. (3–7).
First retrospective cohort study included 90 patients (45 DKA-AP, 45 DKA-nonAP) admitted between 2013 and 2024. AP diagnosis followed the 2021 Chinese guidelines for acute pancreatitis. The idea of using the Chinese pancreatitis guidelines as a diagnostic criterion for AP is similar to the design of a Chinese DKA-AP study, which reduces study bias in China (8). Data were extracted from electronic health records using a standardized checklist, including demographics, medical history, symptoms, and laboratory parameters.
Yan Bo’s view was that every study should have a necessary literature review to complement the methodology or results (9). The literature related to the study of DKA-AP was then reviewed according to the PRISMA guidelines (10). As of February 2025, the terms “diabetic ketoacidosis” and “pancreatitis” were searched using the database PubMed. The search terms “diabetic ketoacidosis” and “pancreatitis” were selected as they pertain to the two diseases under investigation in this study. These terms were used to perform a search of the existing literature to identify studies that directly investigated the relationship between DKA and AP. Also, after the initial search, it was found that these two terms could cover most of the relevant studies. In addition, other synonyms or variants were not selected for the search because it was considered that too many search terms might introduce a large number of irrelevant literature and reduce the efficiency of the search.
Further additions can be made if subsequent studies reveal a need to expand the search scope. Only studies examining DKA-AP were included, including clinical randomized controlled studies, cohort studies and observational studies. This was done because we wanted to understand the progress of previous international research on identifying risk factors for DKA-AP. Articles may contain unifactorial analyses, multifactorial analyses, however case reports or case series alone are not sufficient to explore risk factors. Thus case reports and review articles that lacked exploration of risk factors were excluded. We ran backtracking methods applied to the included articles to capture potential literature. The method of performing systematic search and reverse tracking was informed by the method of Zhao et al., Ma et al., and Liang et al. (11–13), who argued that the heterogeneity of randomized controlled studies is the smallest of all clinical study types and should be considered first, and that the use of reverse tracking method may compensate for the shortcomings in the search formula. The search was limited to search terms appearing in the title, abstract and keywords to accurately screen the literature studying DKA-AP. Meanwhile, only English literature was included to ensure the quality of literature and language consistency. In addition, the retrieved literature was preliminarily screened to exclude literature that was not directly related to the study of DKA-AP, such as literature that only studied a single disease, DKA or AP, and did not address the association between the two.
Normality of continuous variables was assessed using Shapiro–Wilk tests. Non-normally distributed variables were analyzed with non-parametric tests (Mann–Whitney U test), while normally distributed variables were analyzed using t-tests. Categorical variables used Pearson’s χ2 test. For categorical variables with expected cell counts <5 in >25% of cells, Fisher’s exact test was applied. Multivariable logistic regression identified independent risk factors.
ROC assessed predictive performance (14). Variables included in the ROC analysis were selected based on three criteria: (1) significant association with DKA-AP in univariate analysis (p < 0.05) (2) clinical relevance supported by literature review; and (3) predefined cutoffs from prior studies (TG and TC). Optimal cutoffs were determined by maximizing Youden’s Index. To compare the discriminatory performance of individual variables and the combined model, DeLong’s test was employed, a non-parametric method for comparing the area under the curve (AUC) of two or more correlated ROC curves. This test accounts for the paired nature of the data and was robust to non-normal distributions. Statistical comparisons were performed using the pROC package in R (version 4.3.1). This idea was similar to the idea of Yang et al. (15).
Optimal cutoffs for ROC analysis were determined by maximizing Youden’s Index (16). Positive predictive value (PPV) and negative predictive value (NPV) (17) were calculated using the following formulas (Equations 1, 2).
Analyses were performed using SPSS 25.0. Statistical significance was set at bilateral p < 0.05.
A total of 90 patients diagnosed with DKA were included in the study, comprising 45 cases with AP and 45 cases without AP. The DKA-AP group had higher BMI [26.30 (22.15–28.40) vs. 23.20 (20.70–25.35) kg/m2, p = 0.031], hyperlipidemia prevalence (26.7% vs. 4.4%, p = 0.004), and abdominal pain duration (3 vs. 0 days, p < 0.001). Significant differences were observed in hematocrit (HCT), red blood cell count (RBC), pH, bicarbonate (HCO₃−), glucose (Glu), total cholesterol (TC), triglycerides (TG), and amylase (AMY) (p < 0.05; Table 1). Some of laboratory examination data demonstrated no statistically significant differences in the analyzed parameters between the DKA-AP and DKA-nonAP groups. Specifically, there were no notable disparities observed in white blood cell count (WBC, 109/L), neutrophil count (NEUT, 109/L), creatinine (Cr, μmol/L), urea (mmol/L), lactate (Lac, mmol/L), glycated hemoglobin (HbA1c, %), fasting C-peptide (pmol/L), 2-h postprandial C-peptide (pmol/L), sodium (Na+, mmol/L), potassium (K+, mmol/L), calcium (Ca2+, mmol/L), high-density lipoprotein (HDL, mmol/L), low-density lipoprotein (LDL, mmol/L), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), or total bilirubin (TBIL, mmol/L) levels between the two groups. These findings suggested comparable laboratory profiles in both cohorts regarding the measured analytes.
A total of 90 patients diagnosed with DKA were included in the study, comprising 45 cases with AP and 45 cases without AP. The DKA-AP group had higher BMI (26.30 [22.15–28.40] vs. 23.20 [20.70–25.35] kg/m2, p = 0.031), hyperlipidemia prevalence (26.7% vs. 4.4%, p = 0.004), and abdominal pain duration (3 vs. 0 days, p < 0.001). Significant differences were observed in hematocrit (HCT), red blood cell count (RBC), pH value (pH), carbon dioxide combining power (CO₂CP), bicarbonate (HCO₃−), glucose (Glu), total cholesterol (TC), triglyceride (TG), and amylase (AMY) (p < 0.05; Table 1).
Multivariable logistic regression analysis was conducted on the indicators with significant differences in the univariate analysis, as illustrated in Table 2. The findings revealed that cholesterol, triglyceride levels, and the abdominal pain duration were independent predictors of DKA-AP. Nagelkerke R2 = 0.621 indicating that the model explained 62.1% of the variance.
ROC analysis demonstrated strong predictive performance for individual variables and their combination. Using a TG cut-off of ≥11.90 mmol/L, the sensitivity and specificity for DKA-AP diagnosis were 84.4% and 80.0%, respectively, with a PPV of 81.0% and NPV of 83.7%. Similarly, a TC cut-off of ≥7.71 mmol/L achieved 71.1% sensitivity and 75.6% specificity (PPV 74.3%, NPV 72.5%). Abdominal pain duration ≥3 days showed 77.8% sensitivity and 86.7% specificity (PPV 85.2%, NPV 80.0%). The combined model exhibited the highest discriminative power (AUC = 0.933, 95% CI: 0.881–0.986), with 93.3% sensitivity and 73.3% specificity (PPV 80.6%, NPV 90.0%). ROC analysis showed high discriminative power (p < 0.05; Table 3; Figure 1).
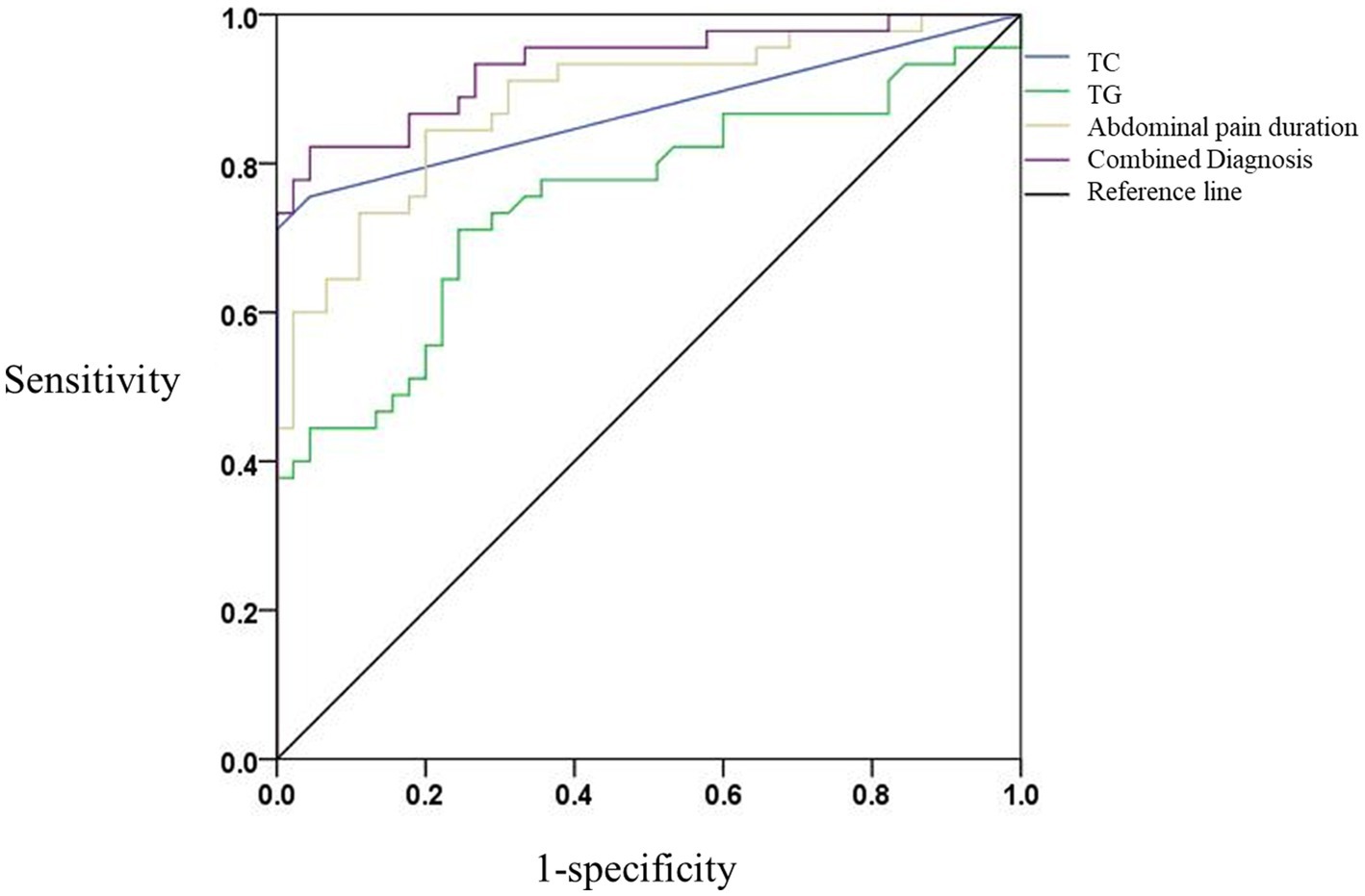
Figure 1. Comparison of ROC curves in subjects with different independent risk factors. Blue line: TC (AUC = 0.751, 95% CI: 0.648–0.855). Green line: TG (AUC = 0.884, 95% CI: 0.815–0.953). Yellow line: abdominal pain duration (AUC = 0.871, 95% CI: 0.791–0.951). Purple line: Combined model (AUC = 0.933, 95% CI: 0.881–0.986). The horizontal coordinate is the false positive rate (1-specificity) and the vertical coordinate is the true positive rate (sensitivity). The different curves have different meanings. The blue line is the TC predictive ROC curve for DKA-AP; the green line is the TG predictive ROC curve for DKA-AP; and the yellow line is the abdominal pain duration predictive ROC curve for DKA-AP; the purple line represents the ROC curve predicted by the combined diagnosis; the black line represents the reference line.
DeLong’s test showed that the combined model (TC + TG + abdominal pain duration) had a significantly higher AUC (0.933) than TC (0.751, p < 0.001), TG (0.884, p = 0.023) and abdominal pain duration (0.871, p = 0.015). Although the AUC of TG (0.884) was significantly higher than that of TC (0.751, p < 0.001), its specificity (80.0%) was lower than that of abdominal pain duration (86.7%, p = 0.035). The combined model, by integrating three significant variables, performed optimally in terms of sensitivity (93.3%) and negative predictive value (90.0%), suggesting its clinical predictive value in assessing the diagnosis of DKA-AP (Table 3; Figure 1).
After a systematic search in the PubMed database (Figure 2), we found 11 clinical studies investigating DKA-AP (1, 8, 18–26). Cumulatively, 1,851 patients were studied, including three interventional studies, 1 genetic observational study, and 7 cohort studies exploring risk factors (Table 4). Of the 7 cohort studies exploring risk factors, 2 studies analyzed risk factors using area under the ROC curve, 2 studies analyzed risk factors using a multifactorial approach, and the remaining 3 used a univariate or descriptive method of Analysis.
The results indicated the presence of numerous significant unifactorial differences in clinical symptoms, clinical assessment scores, laboratory tests and imaging in cases where DKA and AP developed concurrently. These differences are not universal and the main reason may be that the population investigated is so small that there is a large single-center bias. However, blood glucose values, total cholesterol levels, age, and pH derived from ROC curve analysis can alert the first clinician in the clinic. Khan et al. (24) showed that the younger the age the more likely to be comorbid with AP in patients with DKA.
The aim of this study was to investigate the risk factors for DKA-AP and to assess the predictive efficacy of the identified risk factors. The results of the study showed significant differences between the DKA-AP group and the DKA-nonAP group in a number of indices, with the differences in BMI, history of hyperlipidemia, duration of abdominal pain, and lipid profiles (TC, TG) being particularly prominent. TC, TG levels and duration of abdominal pain were identified as independent predictors of DKA-AP by multifactorial analysis, and ROC curve analysis showed that the combination of these factors had good predictive performance (AUC = 0.933, sensitivity = 93.3%, specificity = 73.3%). In addition, patients with DKA had elevated levels of TG (>11.97 mmol/L), reflecting disturbed lipid metabolism, which may trigger AP through free fatty acid-mediated pancreatic injury. Notably, abdominal pain duration over 3 days became a new predictor. This is in line with the aim of the study and provides an important basis for early clinical recognition of DKA-AP.
The identification of TC ≥ 7.71 mmol/L, TG ≥ 11.90 mmol/L, and abdominal pain duration ≥ 3 days as predictors enables clinicians to prioritize lipid-lowering therapies and expedite pancreatic imaging in high-risk DKA patients. This approach may reduce complications such as pancreatic necrosis or organ failure, aligning with guidelines for early intervention in hypertriglyceridemia-associated pancreatitis.
The high PPV and NPV of TG (81.0% and 83.7%) and abdominal pain duration (85.2% and 80.0%) suggest their utility as reliable screening tools in emergency settings. Clinicians should prioritize TG testing and abdominal pain assessment in DKA patients to expedite AP diagnosis.
There are some limitations to this study. Regarding the sample size, only 90 patients were included during the 11-year period, a small sample size, which limits the feasibility of subgroup analyses and may not accurately reflect the situation of patients in different subgroups. For example, it is difficult to carry out in-depth analyses of the differences in risk factors in different gender and age subgroups, which limits the precision of the study results.
On potential confounders, there were unmeasured confounders that could have affected the results, although some were considered in the study design. Patient dietary composition and physical activity were important potential confounders. Chronic high-triglyceride diets may increase the risk of AP development and are also associated with the development and poor control of DKA. Inadequate exercise may lead to metabolic disturbances that promote the development of DKA and AP. As this information was not collected in this study, it could not be adjusted in the analyses, which may have led to biased results. This bias may make the association between risk factors and DKA-AP overestimated or underestimated, interfering with the judgment of the true relationship.
In addition, this study was a single-center retrospective study with single-center bias. The single-center patient population has limitations in terms of geography, ethnicity and medical level, which may not be representative of the wider patient population and affect the generalizability of the study results.
In terms of the achievement of the study objectives, this study successfully targeted TC, TG and abdominal pain duration as the key predictors of DKA-AP. This result is important for achieving the goal of exploring risk factors and provides a key reference for early clinical identification of DKA-AP, which will help clinicians to take timely interventions to improve patients’ prognosis.
When compared with similar studies, the results of the present study present similarities and differences. In terms of hypertriglyceridemia as a risk factor for DKA-AP, the findings of several studies are consistent. Nair et al. conducted a prospective study on 100 patients with DKA and found that 11% of them had concomitant AP, and among them, 4 patients with AP had severe hypertriglyceridemia, and their triglyceride levels returned to near normal after remission of the DKA. Nair’s results strongly suggest a strong association between hypertriglyceridemia and DKA-AP (1). Nair’s results strongly demonstrated the strong association between hypertriglyceridemia and DKA-AP (1). Huang et al. (20) conducted a study on 6 patients with DKA and hypertriglyceridemia, and the findings of the present study showed similarities. Huang et al. (20) studied six patients with DKA, hypertriglyceridemia, and the AP triad and found that the patients’ triglyceride levels were extremely high, with a mean of 3282.17 ± 2975.43 mg/dL, further supporting this view. In addition, Yuan et al. showed a significantly higher incidence of hypertriglyceridemia in AP patients with combined DKA compared to AP patients without DKA. Fu et al. (23) also demonstrated that patients in the DKA group had significantly higher triglyceride levels than those in the non-DKA group. Together, these studies confirm the important role of hypertriglyceridemia in the pathogenesis of DKA-AP.
However, there were differences between the present study and other studies regarding the role of factors such as BMI and age. BMI was not identified as an independent risk factor in the present study, whereas Khan et al. (24) showed that BMI was significantly higher in patients with DKA-AP than in patients with DKA-nonAP. This may be due to the geographic and ethnic differences in the sample of this study, which was relatively small, resulting in an inadequate ability to detect the effect of BMI. Also, the different ways of measuring and categorizing BMI in the study methodology may have contributed to the difference in results.
Regarding age, the present study did not find any significant association between age and DKA-AP, whereas Khan et al. showed that age was a significant risk factor for DKA-AP and that patients with DKA-AP were older (24). However, a study by Fu et al. (23) found that among patients with type 2 diabetes combined with AP, those in the DKA group were younger than those in the non-DKA group. This difference may be related to the age range of patients included in each study, type of disease and study design. The age range of patients included in this study may have been narrower, making the effect of age difference on the results not fully apparent; and the different selection criteria for DKA and AP patients in different studies may have led to inconsistencies in the age-DKA-AP relationship. These could interfere with the results of studies of the association of the age factor with DKA-AP.
In addition, other studies have addressed some aspects that were not explored in depth in this study. For example, Wang et al. (18) investigated the effect of DKA on the clinical course and severity scores of hypertriglyceridemia-induced pancreatitis (HP), and found that DKA led to increased acute kidney injury and higher chance of ICU admission in patients with HP. Wang’s results are different from the risk factors that were focused on in the present study, but they also suggest that the different aspects of the disease need to be considered comprehensively in the assessment of DKA-AP. The results of Wang’s study are different from the present study but suggest that different aspects of the disease need to be considered together when assessing DKA-AP. Other studies have focused on the effect of treatment modalities on patients with DKA-AP. Wei et al. (21) found that insulin pumps combined with ustekin therapy reduced PCT, TG, and other markers in patients. Yin et al. (22) demonstrated that nutritional support combined with insulin therapy improved serum protein levels and reduced inflammatory response in patients. These studies provide a reference for the clinical management of DKA-AP from a therapeutic point of view, and also show that when interpreting the results of the risk factors in this study, it is necessary to make a comprehensive judgment in combination with the impact of different treatment modalities on the disease.
Multiple analyses were conducted in the study, and although methods such as multivariable logistic regression analyses were used to identify independent risk factors and ROC curves were used to assess the predictive performance, there may be some limitations and uncertainties in the different analytical methods. When combining the results of multiple analyses, there may also be contradictory or difficult-to-interpret situations.
Although this study has achieved some results in exploring the risk factors of DKA-AP, clinicians should be cautious in applying the results of this study due to the limitations of the study and the differences with similar studies. The results cannot be directly generalized to all DKA patients, but should be used in conjunction with the individual patient’s condition, the characteristics of the region, and other clinical evidence to formulate a more accurate and effective diagnosis and treatment plan. Future studies need to expand the sample size, conduct multicenter studies, and include more potential confounding factors to further validate and improve the findings of this study.
Elevated TC, TG, and prolonged abdominal pain duration are critical predictors of DKA-AP. These factors enhance diagnostic accuracy and guide targeted management, reducing morbidity in high-risk populations. Future multi-center studies with larger cohorts are warranted to validate these predictors and explore additional risk factors, such as genetic or lifestyle-related variables. At the same time, a prospective study design can be adopted to track the changes of patients’ conditions in depth and establish a better risk prediction model to provide a stronger basis for early clinical diagnosis and intervention.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The study was approved by the Institutional Ethics Committee of Yangzhou University Affiliated Hospital (2022-YKL3-06-004). Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
YC: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. YB: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. ZH: Writing – original draft, Writing – review & editing. MC: Conceptualization, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We thank the Emergency Department, Gastroenterology Department, Endocrinology Department, and Critical Care Medicine Department of Yangzhou University Affiliated Hospital for supporting our clinical research. We also thank Northwest Minzu University, Lanzhou, China, for providing evidence-based medicine assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nair, S, Yadav, D, and Pitchumoni, CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol. (2000) 95:2795–800. doi: 10.1111/j.1572-0241.2000.03188.x
2. Simons-Linares, CR, Jang, S, Sanaka, M, Bhatt, A, Lopez, R, Vargo, J, et al. The triad of diabetes ketoacidosis, hypertriglyceridemia and acute pancreatitis. How does it affect mortality and morbidity?: a 10-year analysis of the National Inpatient Sample. Medicine. (2019) 98:e14378. doi: 10.1097/MD.0000000000014378
3. Zhu, J, Wang, Z, Wang, W, Fan, J, Zhang, Y, Li, X, et al. Xanthomatous Hypophysitis: a case report and comprehensive literature review. Front Endocrinol. (2021) 12:735655. doi: 10.3389/fendo.2021.735655
4. Zhu, J, Wang, Z, Zhang, Y, Liu, J, Li, X, Deng, K, et al. Suprasellar pituitary adenomas: a 10-year experience in a single tertiary medical center and a literature review. Pituitary. (2020) 23:367–80. doi: 10.1007/s11102-020-01043-1
5. Li, X, Lynch, L, Xing, H, Wang, Z, Zhu, J, Deng, K, et al. Cosecreting TSH/GH pituitary adenomas-an 8-year experience in a single tertiary center. Pituitary. (2020) 23:573–81. doi: 10.1007/s11102-020-01064-w
6. Zhu, J, Wang, Z, Zhang, Y, Li, X, Liu, J, Deng, K, et al. Ectopic pituitary adenomas: clinical features, diagnostic challenges and management. Pituitary. (2020) 23:648–64. doi: 10.1007/s11102-020-01071-x
7. Li, X, Deng, K, Zhang, Y, Feng, M, Xing, B, Lian, W, et al. Pediatric pituitary neuroendocrine tumors-a 13-year experience in a tertiary center. Front Oncol. (2023) 13:1270958. doi: 10.3389/fonc.2023.1270958
8. Ma, LP, Liu, X, Cui, BC, Liu, Y, Wang, C, and Zhao, B. Diabetic ketoacidosis with acute pancreatitis in patients with type 2 diabetes in the emergency department: a retrospective study. Front Med. (2022) 9:813083. doi: 10.3389/fmed.2022.813083
9. Bo, Y, Lu, Q, Li, B, Sha, R, Yu, H, and Miao, C. The role of platelets in central hubs of inflammation: a literature review. Medicine. (2024) 103:e38115. doi: 10.1097/MD.0000000000038115
10. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Ma, Z-R, Ma, L-L, Zhao, F, and Bo, Y. Effects of oral calcium on reproduction and postpartum health in cattle: a meta-analysis and quality assessment. Front Vet Sci. (2024) 11:1357640. doi: 10.3389/fvets.2024.1357640
12. Zhao, F, Guo, Z, Bo, Y, Feng, L, and Zhao, J. Is cognitive behavioral therapy an efficacious treatment for psychological interventions in body dysmorphic disorders? A meta-analysis based on current evidence from randomized controlled trials. J Affect Disord. (2024) 352:237–49. doi: 10.1016/j.jad.2024.02.004
13. Liang, Q, Tao, Y, He, J, Bo, Y, Xu, L, and Zhao, F. Effects of home-based telemedicine and mHealth interventions on blood pressure in stroke patients: a systematic evaluation and meta-analysis of randomized controlled trials. J Stroke Cerebrovasc Dis. (2024) 33:107928. doi: 10.1016/j.jstrokecerebrovasdis.2024.107928
14. Fawcett, T. An introduction to ROC analysis. Pattern Recogn Lett. (2006) 27:861–74. doi: 10.1016/j.patrec.2005.10.010
15. Yang, J-M, Zhang, N, Luo, T, Yang, M, Shen, W-K, Tan, Z-L, et al. TCellSI: a novel method for T cell state assessment and its applications in immune environment prediction. iMeta. (2024) 3:e231. doi: 10.1002/imt2.231
16. Lai, C-Y, Tian, L, and Schisterman, EF. Exact confidence interval estimation for the Youden index and its corresponding optimal cut-point. Comput Stat Data Anal. (2012) 56:1103–14. doi: 10.1016/j.csda.2010.11.023
17. Altman, DG, and Bland, JM. Diagnostic tests 2: predictive values. BMJ. (1994) 309:102. doi: 10.1136/bmj.309.6947.102
18. Wang, Y, Attar, BM, Hinami, K, Jaiswal, P, Yap, JE, Jaiswal, R, et al. Concurrent diabetic ketoacidosis in hypertriglyceridemia-induced pancreatitis: how does it affect the clinical course and severity scores? Pancreas. (2017) 46:1336–40. doi: 10.1097/MPA.0000000000000937
19. Yuan, S, Liao, J, Cai, R, Xiong, Y, Zhan, H, and Zheng, Z. Acute pancreatitis concomitant with diabetic ketoacidosis: a cohort from South China. J Int Med Res. (2020) 48:300060520912128. doi: 10.1177/0300060520912128
20. Huang, Z, Xu, Z, Xu, R, Huang, L, Xu, X, and Lai, X. Whole exome sequencing identifies three novel gene mutations in patients with the triad of diabetic ketoacidosis, hypertriglyceridemia, and acute pancreatitis. J Diabetes. (2021) 13:200–10. doi: 10.1111/1753-0407.13100
21. Wei, D, Yin, C, Lu, S, Xiong, J, Zhu, L, Yan, S, et al. The effect of insulin pump combined with ulinastatin on the levels of PCT, TG, PTX-3, and CX3CL1 in patients with diabetic ketoacidosis and pancreatitis. Medicine. (2021) 100:e25141. doi: 10.1097/MD.0000000000025141
22. Yin, C, Lu, S, Wei, D, Xiong, J, Zhu, L, Yan, S, et al. Effects of nutritional support combined with insulin therapy on serum proteins, inflammatory factors, pentraxin-3, and serum amylase levels in patients with diabetic ketoacidosis complicated with acute pancreatitis. Medicine. (2021) 100:e27920. doi: 10.1097/MD.0000000000027920
23. Fu, Y, Liu, X, Cui, B, Wang, C, Liu, Z, and Zhao, B. Clinical characteristics of concomitant diabetic ketoacidosis in type 2 diabetes patients with acute pancreatitis. Diabetes Metab Syndr Obes. (2022) 15:111–9. doi: 10.2147/DMSO.S336619
24. Khan, AA, Ata, F, Yousaf, Z, Aljafar, MS, Seijari, MN, Matarneh, A, et al. A retrospective study on comparison of clinical characteristics and outcomes of diabetic ketoacidosis patients with and without acute pancreatitis. Sci Rep. (2023) 13:4347. doi: 10.1038/s41598-023-31465-3
25. Li, L, and Li, L. Risk factors for diabetic ketoacidosis in acute pancreatitis patients with type 2 diabetes. BMC Gastroenterol. (2023) 23:257. doi: 10.1186/s12876-023-02869-2
Keywords: diabetic ketoacidosis, acute pancreatitis, pancreatitis, risk factors, diagnosis
Citation: Chen Y, Bo Y, Han Z and Chen M (2025) Risk factors associated with acute pancreatitis in diabetic ketoacidosis patients: a 11-year experience in a single tertiary medical center and comprehensive literature review. Front. Med. 12:1571631. doi: 10.3389/fmed.2025.1571631
Received: 08 February 2025; Accepted: 25 March 2025;
Published: 07 April 2025.
Edited by:
Mustafa Agah Tekindal, Izmir Kâtip Çelebi University, TürkiyeReviewed by:
İbrahim Sarbay, Gaziosmanpaşa Training and Research Hospital, TürkiyeCopyright © 2025 Chen, Bo, Han and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifei Chen, MDkwNDg0QHl6dS5lZHUuY24=; Yan Bo, dGhvc2Vmb3JteWxvdmVkcGVvcGxlQG91dGxvb2suY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.