- 1Department of General Medicine, The Yuelushan Area of Hunan Provincial People’s Hospital, Changsha, China
- 2Department of Radiology, The Third Xiangya Hospital of Central South University, Changsha, China
- 3Department of Cardiology, The Third Xiangya Hospital of Central South University, Changsha, China
- 4Department of Respiratory and Critical Care Medicine, The Third Xiangya Hospital of Central South University, Changsha, China
- 5Department of Respiratory and Critical Care Medicine, The Second Xiangya Hospital of Central South University, Changsha, China
Background: With this study, we aimed to explore the clinical features, laboratory examinations, imaging features, and severe predictors of Chlamydia psittaci pneumonia to identify the disease early, shorten the course of illness, and improve prognosis.
Methods: We retrospectively reviewed the clinical data of 39 patients diagnosed with Chlamydia psittaci pneumonia and 39 patients with non-psittacosis community-acquired pneumonia at the Third Xiangya Hospital of Central South University from December 2018 to April 2021. We collected the remaining medical serum to analyze cytokines that are associated with disease-related inflammation. We used the R software to perform statistical analysis.
Results: Compared to the non-psittacosis community-acquired pneumonia group, the common Chlamydia psittaci pneumonia group exhibited more severe symptoms, including a longer duration of hyperthermia. Most patients experienced dyspnea, as well as extrapulmonary symptoms such as fatigue, muscle soreness, and diarrhea. There were also significant increases in levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), C-reactive protein, and procalcitonin, while hemoglobin (Hb) and albumin (ALB) levels decreased significantly. Primary lung imaging features included consolidation and exudation, with nodules and cavities being rare. These changes were even more severe in the severe Chlamydia psittaci pneumonia group, with further increased levels of myoglobin and a larger spread of lesions in the lungs. Additionally, the Th1 inflammatory factor INF-γ was elevated in the Chlamydia psittaci pneumonia group.
Conclusion: Fatigue, myalgia, low Hb, low ALB, high ALT, and high AST are predictors of Chlamydia psittaci pneumonia. Fast respiratory rates, low Hb, high LDH, significant involvement of multiple lobes, high Sequential Organ Failure scores, and high Acute Physiological and Chronic Health scores are predictors of severe Chlamydia psittaci pneumonia. The increase of INF-γ may be related to the condition.
Background
Chlamydial pneumonia is a zoonotic infection caused by Chlamydia psittaci, which has been reported in sporadic cases worldwide (1–5). This prokaryotic intracellular bacterium was first identified in parrots, its most common host, and can infect birds, poultry, and humans through contact with or inhalation of infected birds’ excretions (6). The clinical symptoms of this infection are non-specific and can vary (7–11). Psittacosis pneumonia, a type of community-acquired pneumonia (CAP), is often difficult to distinguish from other types of pneumonia. Currently, a definitive diagnosis of this disease requires identifying its underlying cause. However, traditional methods such as culture and serologic antibody testing can be challenging and may delay early diagnosis. This can lead to rapid progression to critical illness or even death (12). Fortunately, new detection technology, such as metagenomic next-generation sequencing (mNGS), allows for the identification of pathogens in bronchoalveolar lavage fluid (BALF) (13, 14). In fact, in our study, mNGS successfully diagnosed all confirmed cases of Chlamydia psittaci pneumonia (15). However, mNGS is expensive and requires a high level of skill, making it difficult to implement in most primary hospitals (16). Therefore, it is crucial to understand the clinical characteristics of Chlamydia psittaci pneumonia. Currently, there are inadequate researches on potential peripheral blood biomarkers for this disease (17). Previous studies have shown that Chlamydia psittaci infection can activate Th1 cells, which are responsible for immune responses against intracellular microorganisms. Further research is needed to determine if Th1 cell-related cytokines, such as interferon-γ (INF-γ), interleukin-2 (IL-2), and interleukin-12 (IL-12), could serve as biomarkers for this disease. Thus, the objective of this study was to describe the clinical characteristics of psittacosis pneumonia and identify potential predictors for critically ill patients. Additionally, we aimed to identify possible peripheral blood biomarkers. Improving our understanding of this disease and identifying it early can greatly improve prognoses.
Methods
Study design
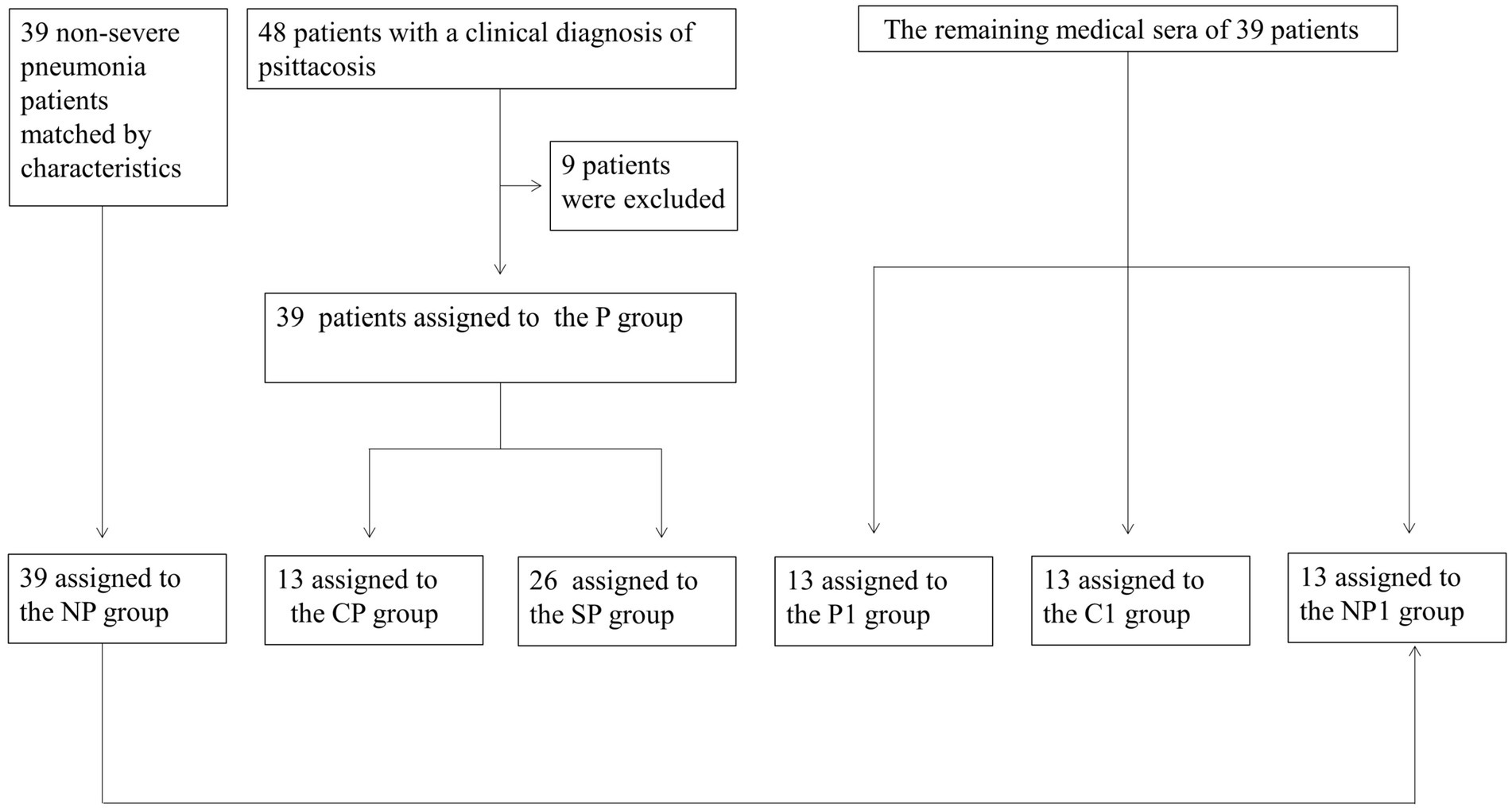
We collected data from December 2018 to April 2021 on 48 patients diagnosed with Chlamydia psittaci pneumonia at the Third Xiangya Hospital of Central South University. Nine patients were excluded, leaving a total of 39 cases in the Chlamydia psittaci pneumonia group (P group). This group was further divided into 13 patients with common Chlamydia psittaci pneumonia (CP group) and 26 patients with severe Chlamydia psittaci pneumonia (SP group). Additionally, 39 non-severe pneumonia patients were matched based on characteristics and assigned to the non-psittacosis community-acquired pneumonia group (NP group). The remaining medical sera from some patients were collected prospectively and divided into three groups: 13 cases in the Chlamydia psittaci pneumonia sera group (P1 group), 13 cases in the healthy control sera group (C1 group), and 13 cases in the non-psittacosis community-acquired pneumonia sera group (NP1 group; Figure 1). The study was approved by the ethics committee of the Third Xiangya Hospital of Central South University (Fast I 21036), registered with the Chinese Clinical Trials Registry (ChiCTR2100045952), and conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Patient inclusion and exclusion criteria
Inclusion criteria
(1) All cases met the diagnostic and treatment guidelines for CAP in adults set by the Chinese Medical Association (18). (2) Patients with Chlamydia psittaci pneumonia were diagnosed by metagenomic next-generation sequencing (mNGS). (3) Severe cases of Chlamydia psittaci pneumonia met the above diagnostic criteria for severe pneumonia.
Exclusion criteria
(1) Cases with incomplete medical records. (2) Cases where mNGS indicates the presence of other major pathogenic bacteria.
The mNGS detection of bronchoalveolar lavage fluid
The bronchoalveolar lavage fluid (BALF), from each participant, were collected from the lung segments indicated by the CT scan. Instill 20 mL of sterile saline into the affected lung segment and recover at least 10 mL of bronchoalveolar lavage fluid as a test sample.
DNA from each BALF sample (~1 mL) was extracted using the QIAamp DNA Micro Kit (Qiagen, Germany). Total RNA from 15 BALF samples was isolated with the QIAamp Viral RNA Kit (Qiagen, Germany), and rRNA was removed using the Ribo-Zero rRNA Removal Kit (Illumina, United States). cDNA was synthesized with reverse transcriptase and dNTPs (Thermo Fisher, United States). DNA and cDNA libraries were prepared using the QIAseq Ultralow Input Library Kit (Qiagen, Germany) and assessed for quality with a Qubit fluorometer and Agilent 2100 Bioanalyzer. The final libraries were sequenced on a NextSeq 550 platform (Illumina, United States) with 75-bp single-end reads, yielding approximately 20 million reads per library. The following bioinformatics analysis were conducted and reported by Vision Medicals (Guangzhou, China).
Data collection
We utilized the electronic medical record system to gather comprehensive data, including general information, symptoms, signs, laboratory tests, pulmonary images, complications, treatments, scores, course of illness, and prognoses. The laboratory tests consisted of blood routine, inflammatory markers, blood coagulation, liver and kidney function, electrolyte levels, and enzyme levels. The scores used were the Sequential Organ Failure (SOFA) score, Acute Physiological and Chronic Health (APACHE II) score, and Glasgow Coma Scale (GCS) score. To measure the concentrations of Th1 cell-associated factors INF-γ, IL-2, and IL-12 in the remaining medical sera of different groups, we employed enzyme-linked immunosorbent assay (ELISA) kits from Thermo Fisher Scientific.
Statistical analysis
We used R software (The R Foundation, http://www.r-project.org, version 3.6.1). We expressed the continuous variables as medians (interquartile range) and compared them using a t-test or a Mann–Whitney test, depending on whether they conformed to the normal distribution. We represented the classification variables with n (%) and compared them with the Fisher exact chi-square test. We analyzed potential predictors with multiple logistic regression analyses. We drew a scatter plot to explore the concentration of Th1 cell-related factors (INF-r, IL2, and IL12) in each group. p < 0.05 was considered statistically significant.
Results
Patient baseline characteristics
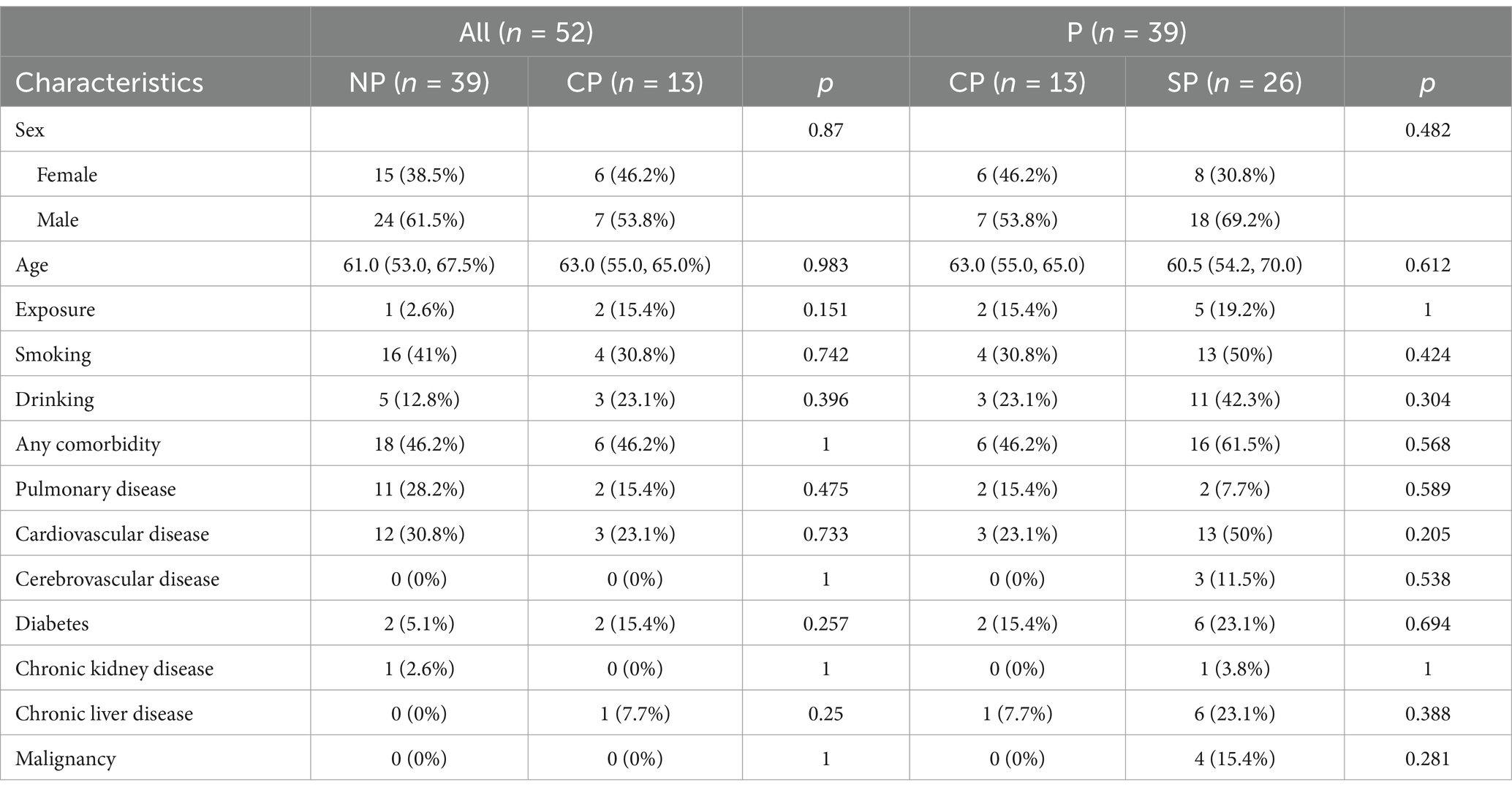
The CP and NP groups had similar sociodemographic characteristics and history of comorbidities. Among the patients in the CP group, 15.4% had a history of exposure to birds and poultry, and 23.1% had a history of alcohol consumption. The SP group had a higher number of comorbidities compared to the CP group (Table 1).
Clinical symptoms and physical signs
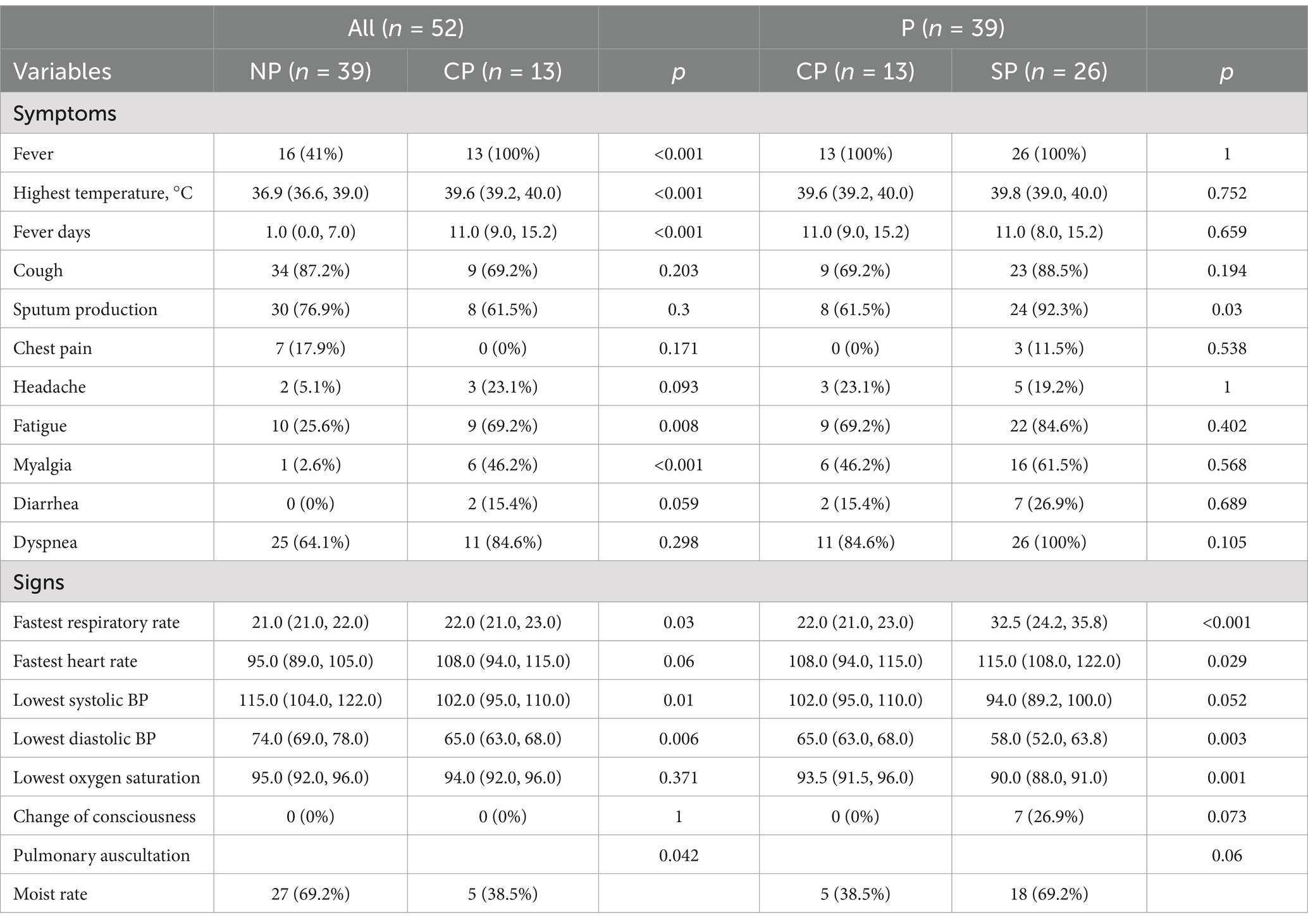
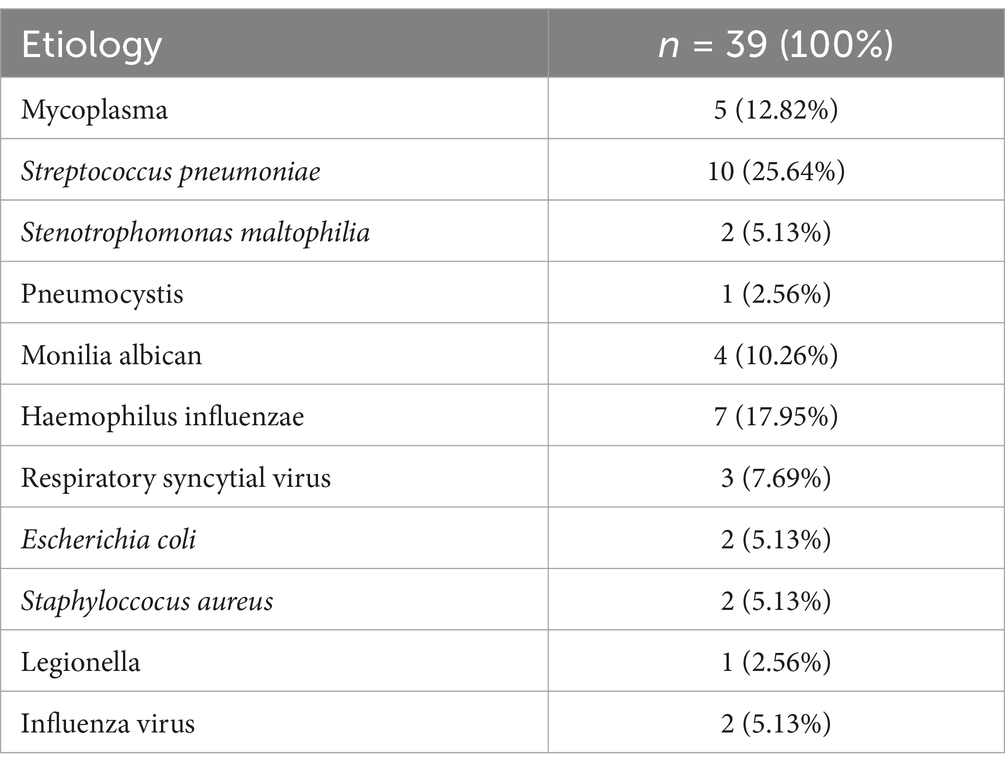
All patients in the CP group had a fever, with a mean temperature of 39.6°C. The duration of fever in the CP group was longer than in the NP group, while cough and sputum were similar between the two groups. Patients in the CP and SP groups were more likely to experience dyspnea and extrapulmonary symptoms such as fatigue, muscle soreness, and diarrhea compared to the NP group. Additionally, the CP group had a faster respiratory rate, a relatively slow pulse, and lower blood pressure compared to the NP group. The SP group had a more rapid respiratory rate, lower blood pressure, and lower oxygen saturation than the CP group (Table 2). The etiology data of NP group participants are listed in Table 3.
Laboratory test results
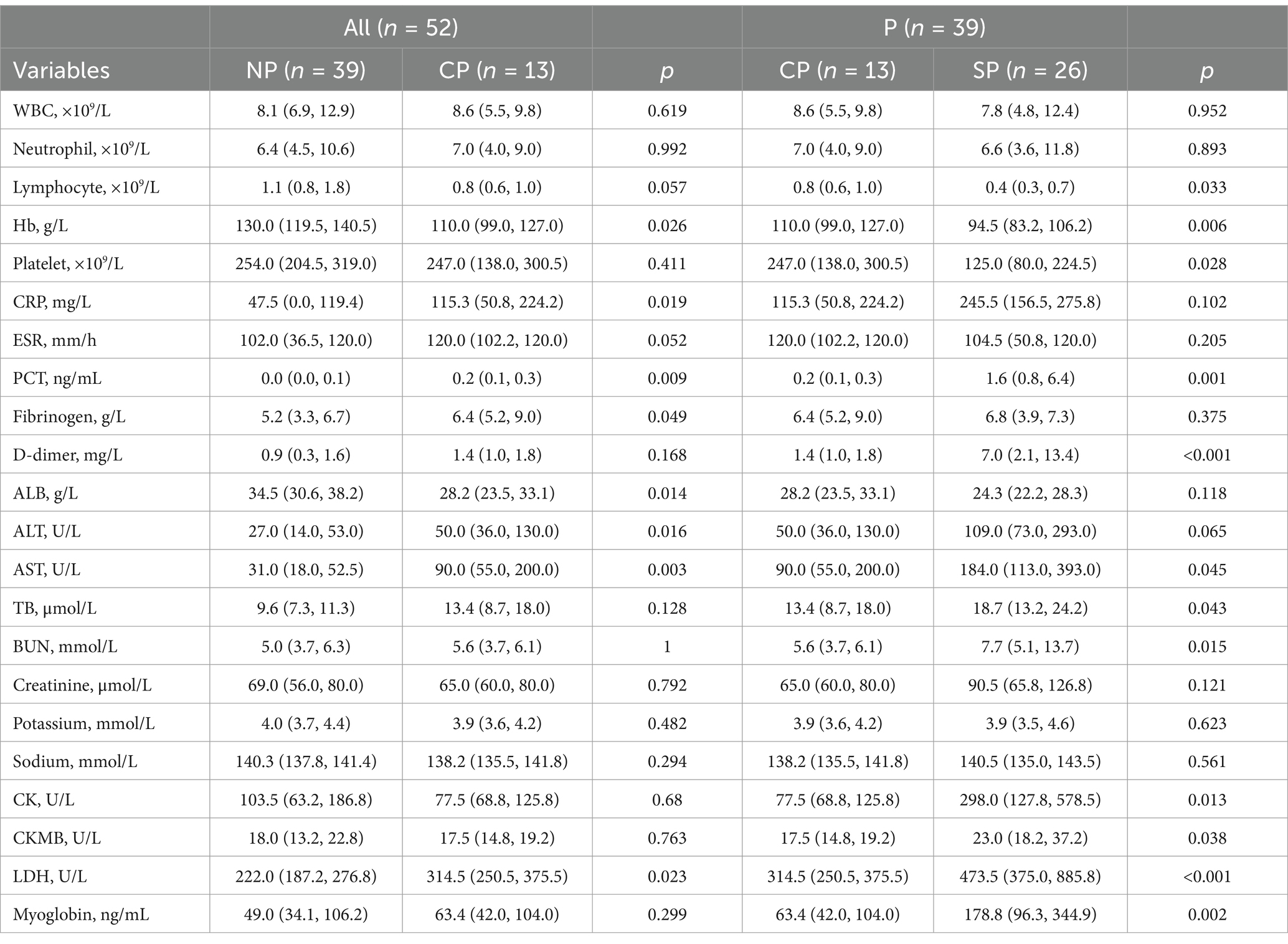
Compared to the NP group, the CP group showed a decrease in average blood lymphocytes and Hb levels. However, there was no significant difference in platelet, leukocyte, and neutrophil levels. The SP group had lower lymphocyte and platelet levels, and their Hb levels fell to the diagnostic criteria for anemia. Additionally, the inflammatory indicator C-reactive protein (CRP) increased significantly in both the CP and SP groups. The CP group also showed a decrease in liver function, with a decrease in albumin (ALB) levels and an increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. There was also an increase in the myocardial enzyme lactate dehydrogenase (LDH). In comparison, the SP group showed a significant increase in ALT, AST, and LDH levels, as well as an increase in myoglobin levels (Table 4).
Pulmonary CT images changes
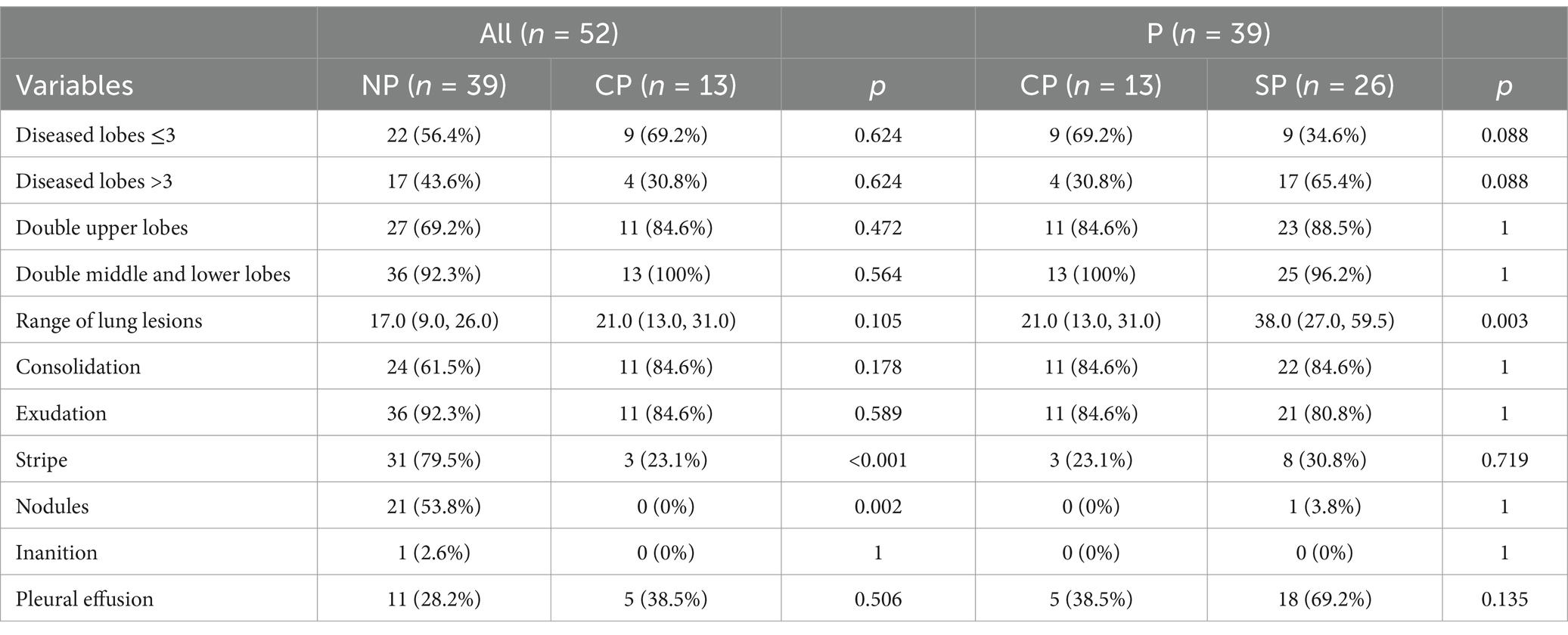
The most common pulmonary CT features in the P group were consolidation and exudation, which are similar to other cases of community-acquired pneumonia (CAP). However, nodules and cavities were not present in any of the cases. Furthermore, the rate of Chlamydia psittaci pneumonia complicated with pleural effusion increased as the condition worsened. In the SP group, there was a higher likelihood of involvement in multiple lobes compared to the CP group, with 65.4% of patients having 4–5 lobes affected (Table 5 and Figure 2).
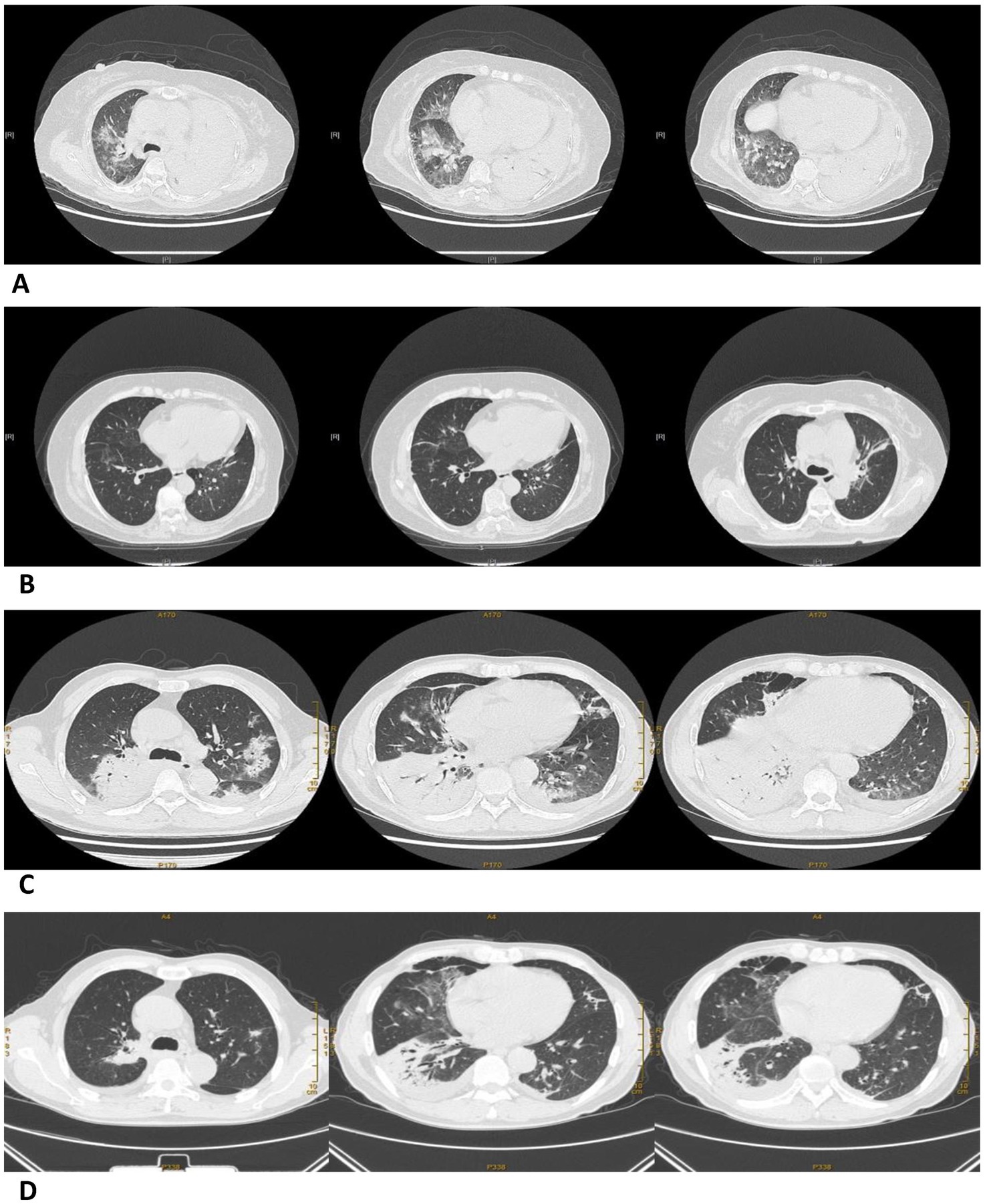
Figure 2. Pulmonary CT images before and after treatment in a severe and a common Chlamydia psittaci pneumonia patient. (A) Large consolidation of the left lung and patchy exudation shadow of the right lung before treatment (07/02/2021). (B) The area of consolidation and patchy exudation shadow significantly reduced after treatment (01/03/2021). Pulmonary CT images before and after treatment in a common Chlamydia psittaci pneumonia patient. (C) Large areas of exudation and consolidation in the right upper lobe and right lower lobe, bronchial inflation signs, and minor pleural effusion before treatment (18/09/2020). (D) Lesion clearly absorbed after treatment (29/09/2020).
Complications and treatments
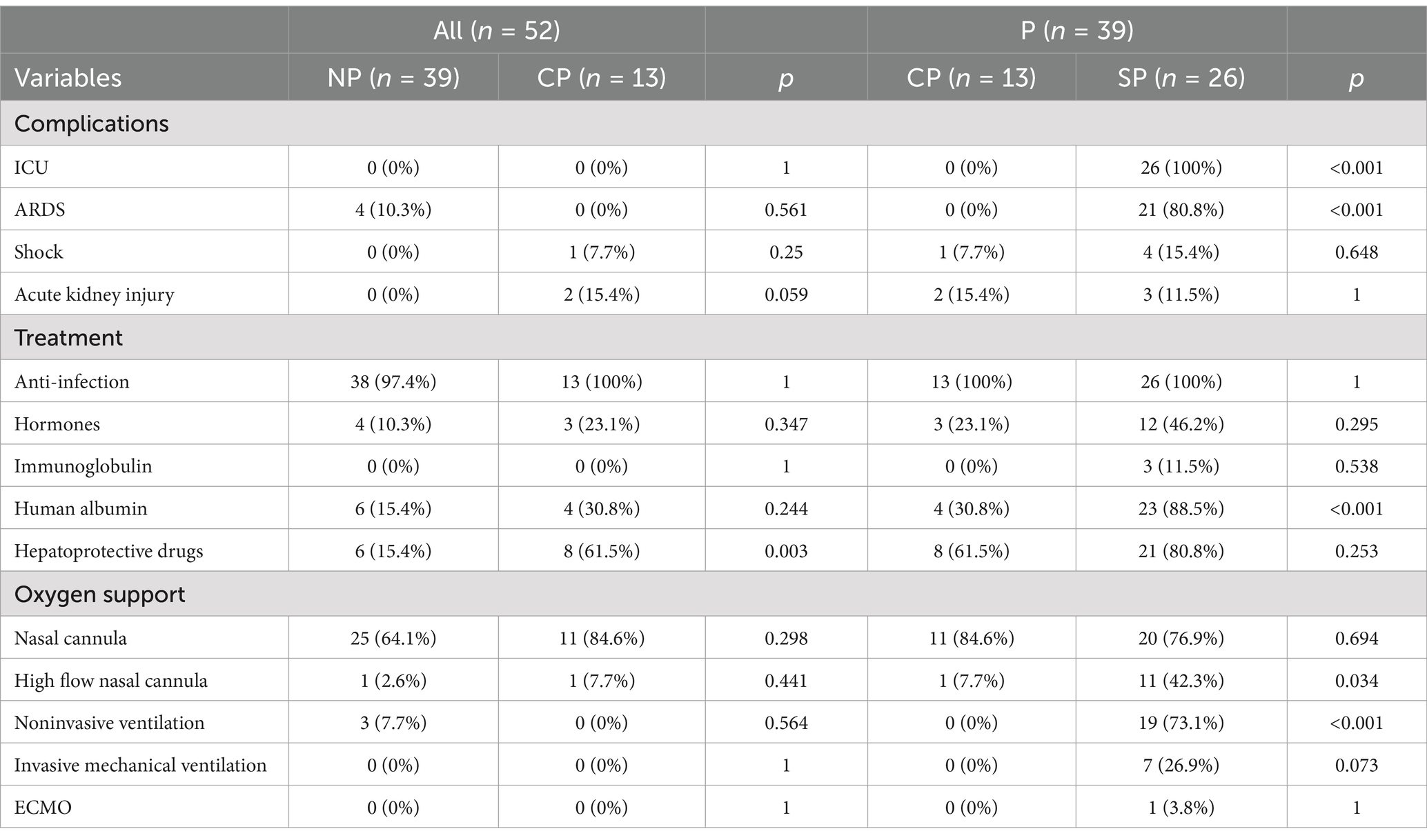
Compared to the CP group, all patients in the SP group were admitted to the intensive care unit (ICU). Additionally, 80.8% of these patients were diagnosed with acute respiratory distress syndrome (ARDS). The use of respiratory support therapies, including high-flow oxygen, noninvasive assisted ventilation, invasive assisted ventilation, and extracorporeal membrane oxygenation (ECMO), significantly increased in the SP group. Furthermore, there was a higher frequency of treatment with glucocorticoids, albumin, and liver protection therapy in the SP group (Table 6).
Score, duration, and prognosis
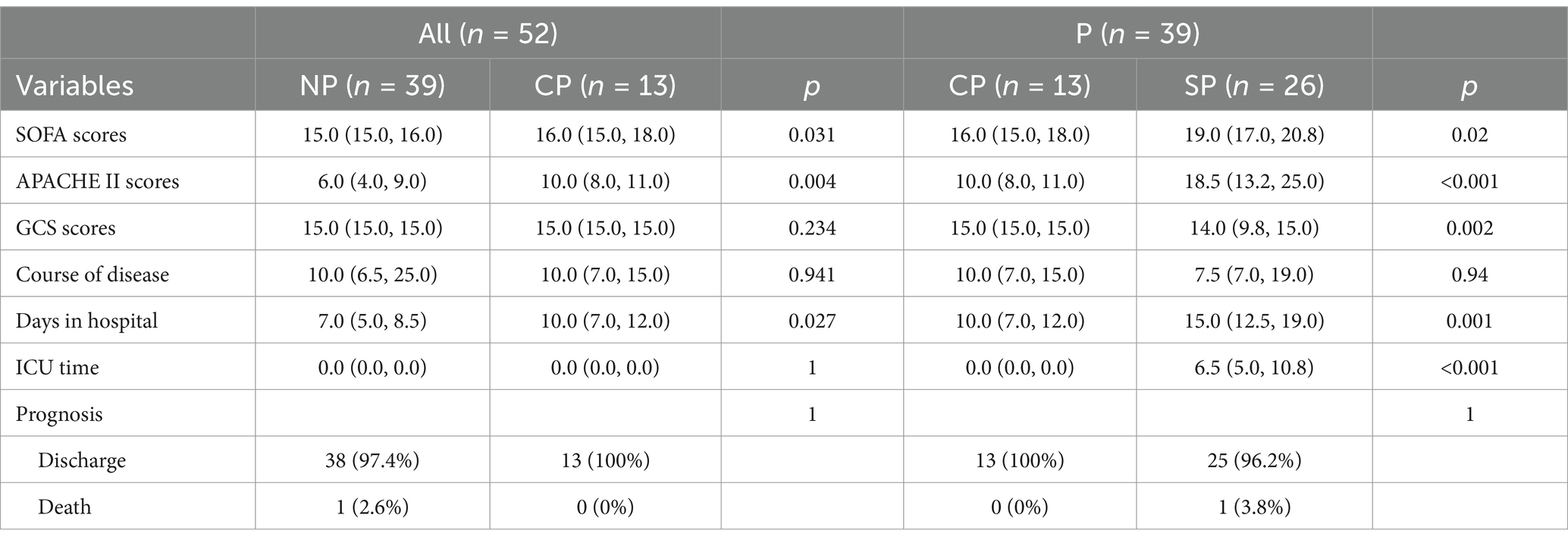
The SP group had significantly higher SOFA and APACHE II scores compared to the CP group, and their hospitalization and ICU admission lengths were longer. However, the course of disease was shorter in the SP group compared to the CP group (Table 7).
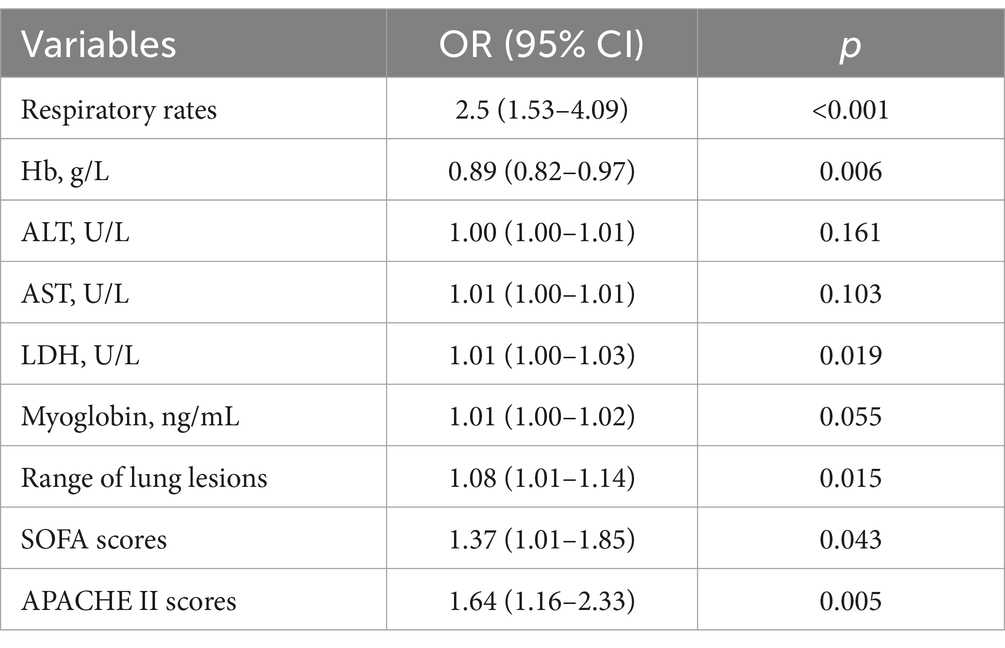
Predictors of Chlamydia psittaci pneumonia
Multiple logistic regression analysis and receiver operating characteristic (ROC) curve found that fatigue, myalgia, low hemoglobin levels, low albumin levels, elevated ALT levels, and elevated AST levels are all predictors for Chlamydia psittaci pneumonia (Table 8), with excellent predictive performance (Figure 3D). The area under the curve (AUC) of each predictor.
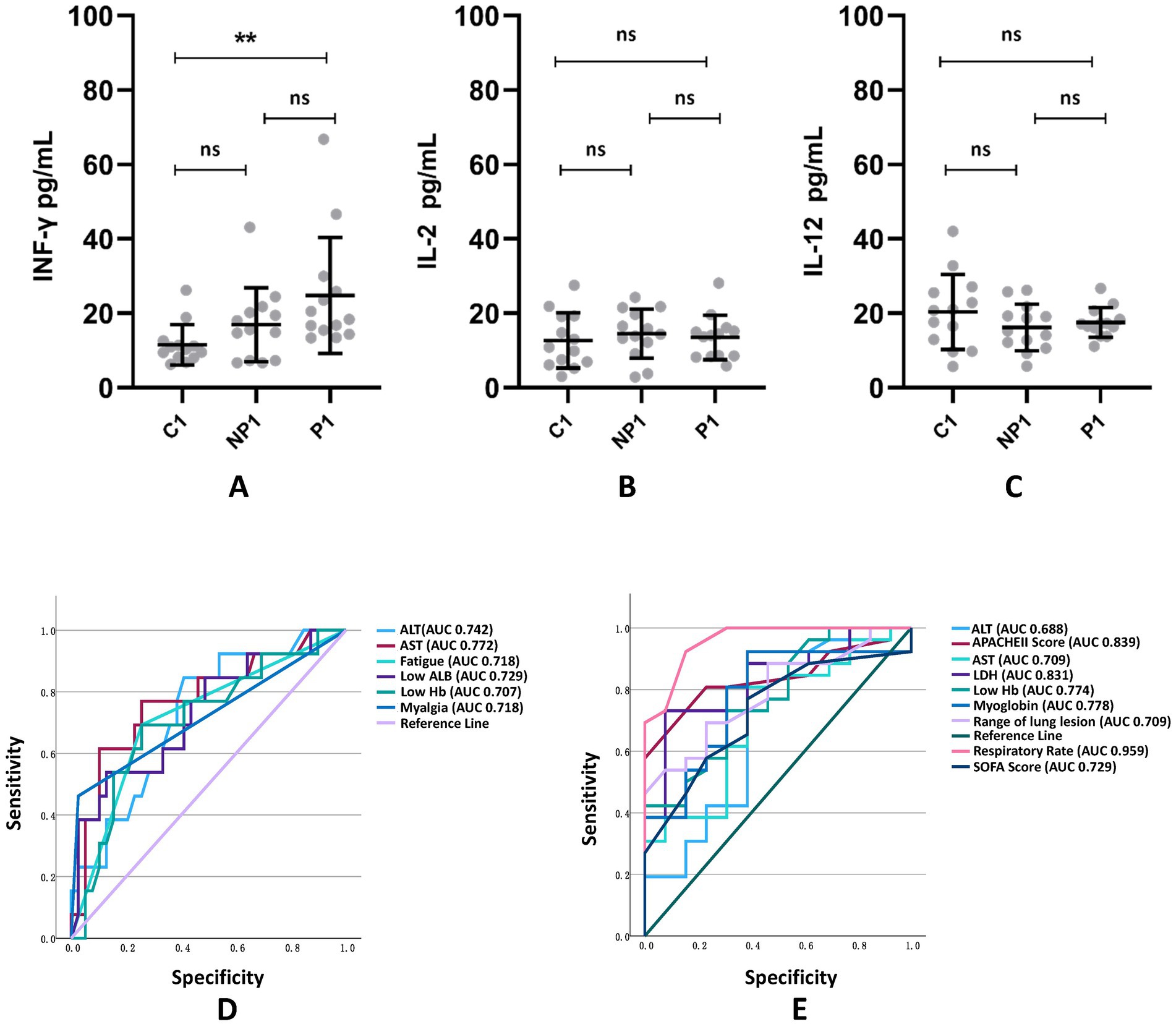
Figure 3. Comparison of Th1-related cytokines INF-γ (A), IL-2 (B), and IL-12 (C) among C1 healthy population group, NP1 non-psittacosis community-acquired pneumonia group, and P1 Chlamydia psittaci pneumonia group. The ROC curve and related AUC of CP pneumonia (D) and severe CP pneumonia (E) predictors are exhibited. **p < 0.01; ns, the difference was not statistically significant.
Predictors of severe Chlamydia psittaci pneumonia
Multiple logistic regression analysis (Table 9) and ROC curve (Figure 3E) revealed that fast respiratory rates, low Hb, high LDH, significant involvement of multiple lobes, high Sequential Organ Failure scores, and high Acute Physiological and Chronic Health scores were predictors for severe Chlamydia psittaci pneumonia.
Th1-related cytokines
Compared to the C1 and NP1 groups, the P1 group showed a significant increase in INF-γ levels. However, there was no significant difference in IL-2 and IL-12 levels among the groups (see Figure 3).
Discussion
Concerning Chlamydia psittaci pneumonia, previous studies have suggested that its clinical manifestations are similar to other cases of community-acquired pneumonia (CAP) (19). However, due to the difficulty in isolating and identifying Chlamydia psittaci using traditional cultural methods, diagnosing the pathogen can be challenging (20, 21). Treatment with common drugs used for CAP, such as cephalosporins, penicillins, and other β-lactam antibiotics, is often ineffective. In some cases, patients may even develop critical conditions or die without timely and effective treatment (12). Therefore, early recognition of the infection is crucial for improving prognosis. In this study, current cases were diagnosed using mNGS, a massively parallel sequencing technique for determining nucleotide sequences (13, 14). The analysis of 39 Chlamydia psittaci patients using mNGS confirms the validity of this test for diagnosing the infection. However, mNGS is an expensive and unpopular method. As a result, it is important to distinguish this disease early on from other cases of CAP, promptly identify severe patients, and search for possible peripheral blood markers as urgent clinical tasks. Besides, real-time polymerase chain reaction (RT-PCR) is also one of the effective tests for CP pneumonia and this method which is more time-, money- and labor-saving, especially in the duration of CP pneumonia outbreak (22). However, when dealing with sporadic cases, mNGS has greater diagnostic value, as it enables the timely detection of nearly any lower respiratory tract pathogen.
Previous case reports and a few studies have summarized some features of Chlamydia psittaci pneumonia (23–25). However, this study is the first to compare the clinical features, laboratory tests, and imaging characteristics of Chlamydia psittaci pneumonia with those of non-psittacosis community-acquired pneumonia (CAP). Our findings indicate that the majority of cases with avian exposure were caused by Chlamydia psittaci. This bacterium primarily infects birds and poultry (12), and humans typically contract it through inhalation or contact with the secretions of sick birds and poultry (6, 26–29). Human-to-human transmission is rare (30). Previous studies have identified bird and poultry exposure as the main risk factor (3), highlighting the importance of collecting thorough medical histories. Additionally, 23.1% of the CP group had a history of chronic alcohol consumption, and nearly 7.7% had chronic liver disease. Further these individuals. In our study, 61.5% of the SP group had comorbidities, as Chlamydia psittaci often infects immunocompromised hosts (31). Therefore, we must remain vigilant for severe infections in patients with underlying diseases who are infected with Chlamydia psittaci.
According to previous case reports, Chlamydia psittaci pneumonia can affect multiple systems, including the respiratory, digestive, cardiovascular, blood, urinary, nervous, and skin barrier systems (11, 32–40). The clinical symptoms of Chlamydia psittaci pneumonia lack specificity and can vary in severity, with the potential to rapidly progress from asymptomatic infection to severe pneumonia with multiple organ failure (7–10, 12, 39). In our study, we observed that hyperthermia, dyspnea, and extrapulmonary manifestations such as fatigue, myalgia, and diarrhea were more common in the CP group compared to the NP group. We also found that CP pneumonia patients are more likely to display fatigue and myalgia. Therefore, patients with community-acquired pneumonia (CAP) who present with extrapulmonary symptoms of fatigue and myalgia should be monitored for possible Chlamydia psittaci infection. Interestingly, we did not observe any abnormal electrolyte levels in patients with psittacosis, which differs from Legionella pneumophilia pneumonia, which often presents with similar extrapulmonary symptoms (19, 41). In contrast, patients with Legionella pneumophilia pneumonia often have polyhyponatremia and low blood phosphorus levels (42). In terms of physical signs, we noted that significantly higher respiratory rate (tachypnea) in the CP group, with more pronounced abnormalities in the SP group. Our statistical analysis revealed that tachypnea is very likely to be associated with severe Chlamydia psittaci pneumonia. Additionally, we found that a high proportion of severe patients (66.7%) required admission to the intensive care unit (ICU), highlighting the importance of early recognition and effective treatment in improving outcomes for patients with Chlamydia psittaci pneumonia.
This study not only analyzed laboratory examinations such as blood routine, inflammatory indexes, liver function, enzymology, coagulation function, renal function, and electrolytes, but also explored the concentrations of Th1 cell-associated factors INF-γ, IL-2, and IL-12 in the remaining medical sera. We found that compared with the NP group, the lymphocytes and hemoglobin (Hb) levels in the blood routine of the CP group were decreased. In the SP group, these decreases were more significant, with most patients experiencing anemia and a significant decrease in platelet levels within the normal range. However, leukocyte and neutrophil levels did not increase, which is not consistent with the symptoms of hyperthermia. These abnormal changes in the blood routine may be related to the spread of Chlamydia psittaci in the blood and its involvement in the blood system (43). Statistical analysis found that low Hb is a predictor and risk factor for Chlamydia psittaci pneumonia and severe cases. When Hb levels decrease in patients with CAP, excluding obvious acute blood loss and chronic anemia, Chlamydia psittaci pneumonia should be considered, as the decline may be related to the severity of the infection. Inflammatory parameters such as CRP were found to be significantly increased in the CP group and even more so in the SP group, indicating a severe infection. The liver enzymes ALT and AST were also significantly elevated in the CP group and even more so in the SP group, which may be related to liver injury (7, 9). Clinical reports have shown elevated liver enzymes in patients with psittacosis (20, 44), and veterinary pathology studies have shown liver involvement in infected parrots (45). This suggests that the liver may be a relatively susceptible site for Chlamydia psittaci pneumonia. We also found reduced levels of albumin (ALB) in patients with Chlamydia psittaci pneumonia. In addition to liver damage, Chlamydia psittaci is a specialized intracellular pathogen that lacks many essential nutrients and requires amino acids from eukaryotic host cells (46), leading to hypoalbuminemia. Statistical analysis found that low Hb, high ALT, and high AST were predictors for Chlamydia psittaci pneumonia, and high ALT and AST were also predictors of severe cases. Therefore, when patients with CAP have impaired liver function, decreased Hb, and increased liver enzymes ALT and AST, they should be alert to the possibility of infection with Chlamydia psittaci. High ALT and high AST levels suggest a severe infection. Unlike previous studies that found abnormal levels of CK and BNP to be predictors for severe Chlamydia psittaci pneumonia (31), we found that LDH levels were increased in the CP group, especially in the SP group, and myoglobin levels were also increased. These could be associated with rhabdomyolysis and cardiac involvement (12, 47, 48). Statistical analysis found that high LDH levels were predictors and predictors for severe Chlamydia psittaci pneumonia, and high myoglobin levels were also predictors of severe cases. The increase in LDH and myoglobin levels may suggest a serious condition in patients with Chlamydia psittaci pneumonia. In contrast, there were no obvious abnormalities in coagulation function, renal function, and electrolytes in patients with Chlamydia psittaci pneumonia, which may serve as exclusionary diagnostic references and differentiation points. In this study, the Th1 cell-associated factor INF-γ was found to be elevated in the P1 group. Previous studies have shown that INF-γ can induce the iron deficiency pathway by upregulating the expression of divalent metal ion transporter 1 in monocyte macrophages and downregulating the transferrin receptor of infected cells and ferrous transporter in monocyte macrophages (49). This limits the uptake of iron, an essential element for chlamydia growth, and has an anti-chlamydia infection effect (50, 51). Therefore, the increase in INF-γ levels may be related to the condition and should be investigated in the future as a possible peripheral blood marker. Besides, another study also explored the immune reactions in Chlamydia psittaci pneumonia and they found psittacosis cases with pneumonia had higher levels of serum cytokines (G-CSF, IL-2, IL-6, IL-10, IL-18, IP-10, MCP-3, and TNF-α) than bronchitis cases (17). Among them, IL-6 contributes to both pneumonia pathogenesis and iron restriction during infection by increasing hepcidin that causes iron restriction in blood and traps the iron intracellularly (52). This effect may lead to the lower availability of iron for Hb synthesis thus leading to lower Hb levels but paradoxically promote the multiplication of intracellular pathogens like Chlamydia psittaci (53).
The CT findings in this study were generally consistent with those of previous studies (44, 54). The predominant imaging pattern of psittacosis was consolidation and exudative (55). Nodules and cavities were rare, which could be used as exclusionary diagnostic criteria and a differential point for Chlamydia psittaci pneumonia (55). Furthermore, we observed that psittacosis often involved multiple lobes and resulted in more pleural effusion, indicating a more severe disease. Statistical analysis revealed that extensive involvement of multiple lobes is a predictor and risk factor for severe Chlamydia psittaci pneumonia, which can aid in determining the severity of the disease. In the SP group, most patients also had acute respiratory distress syndrome (ARDS), which may be attributed to the fact that Chlamydia psittaci primarily affects the lungs. Additionally, some patients had multiple organ dysfunction and sepsis in the past (10), and severe cases can be life-threatening.
Anti-infective treatment for Chlamydia psittaci pneumonia is most effective with tetracyclines, followed by macrolides, and quinolones (56, 57). However, conventional CAP anti-infective drugs such as cephalosporins, penicillins, and other β-lactam antibiotics have poor treatment effects due to the intracellular nature of the pathogenic bacteria. In our study, patients with Chlamydia psittaci pneumonia were treated with the aforementioned anti-infective therapy and showed significant improvement, with most being discharged. Out of 39 patients, only one severe case resulted in death, accounting for 2.6%. In addition to anti-infective treatment, a comprehensive respiratory support regimen, glucocorticoids, albumin, and hepatoprotective treatment were also utilized for patients with Chlamydia psittaci pneumonia. The intensity and abundance of treatment increased with the severity of the condition. Previous studies have reported that fulminant Chlamydia psittaci pneumonia can lead to multiple organ failures, requiring additional treatments such as cardiopulmonary bypass and hemodialysis (12). Timely and effective drug treatment has shown to have a positive response in Chlamydia psittaci pneumonia cases (25, 37). Early diagnosis is crucial for prompt and effective treatment.
Patients in the SP group had higher SOFA and APACHE II scores compared to the CP group. According to our analysis, high SOFA and APACHE II scores were found to be predictors for severe Chlamydia psittaci pneumonia. These scores can serve as reference indexes to aid in determining the severity of Chlamydia psittaci pneumonia. The SP group had longer hospital stays and were more likely to be admitted to the ICU, indicating a heavier disease burden. However, the SP group had a shorter disease course compared to the CP group, suggesting a faster progression of severe psittacosis pneumonia.
In summary, this study identified predictors for Chlamydia psittaci pneumonia and severe cases, as well as explored potential peripheral blood biomarkers. These findings can aid in early detection of Chlamydia psittaci pneumonia, prompt identification of severe cases, and potentially lead to shorter disease courses and improved outcomes.
However, this study has some limitations. Firstly, it was a single-center study conducted in a tertiary-care teaching hospital, which may introduce bias. Secondly, it was a retrospective study and some patients were difficult to track. Lastly, the sample size was small and may not fully represent the clinical features of Chlamydia psittaci pneumonia. Future prospective studies with larger, multicenter samples should be conducted.
Conclusion
Fatigue, myalgia, low Hb, low ALB, high ALT, and high AST are predictors of Chlamydia psittaci pneumonia. Fast respiratory rates, low Hb, high LDH, significant involvement of multiple lobes, high Sequential Organ Failure scores, and high Acute Physiological and Chronic Health scores are predictors of severe Chlamydia psittaci pneumonia. The increase of INF-γ may be related to the condition.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Third Xiangya Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. LC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. LP: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CL: Conceptualization, Resources, Writing – review & editing. SH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. LX: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82070048), National Natural Science Foundation of China (the Youth Foundation, Grant No. 82400051), Natural Science Foundation of Hunan Province of China (Grant No. 2023JJ30849), Natural Science Foundation of Hunan Province of China (the Youth Foundation, Grant No. 2023JJ40820) and the Scientific Research Launch Project for New Employees of the Second Xiangya Hospital of Central South University (to SH). The National Key Clinical Specialty Construction Projects of China.
Acknowledgments
The authors thank all study participants for their involvement in the current study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reisinger, AC, Schilcher, G, Prattes, J, Zollner-Schwetz, I, and Eller, P. Acute respiratory distress syndrome during a pandemic-an obvious diagnosis? Lancet Infect Dis. (2020) 20:873. doi: 10.1016/S1473-3099(20)30468-0
2. Shaw, KA, Szablewski, CM, Kellner, S, Kornegay, L, Bair, P, Brennan, S, et al. Psittacosis outbreak among workers at chicken slaughter plants, Virginia and Georgia, USA, 2018. Emerg Infect Dis. (2019) 25:2143–5. doi: 10.3201/eid2511.190703
3. Kozuki, E, Arima, Y, Matsui, T, Sanada, Y, Ando, S, Sunagawa, T, et al. Human psittacosis in Japan: notification trends and differences in infection source and age distribution by gender, 2007 to 2016. Ann Epidemiol. (2020) 44:60–3. doi: 10.1016/j.annepidem.2020.03.001
4. Mattmann, P, Marti, H, Borel, N, Jelocnik, M, Albini, S, and Vogler, BR. Chlamydiaceae in wild, feral and domestic pigeons in Switzerland and insight into population dynamics by Chlamydia psittaci multilocus sequence typing. PLoS One. (2019) 14:e0226088. doi: 10.1371/journal.pone.0226088
5. Stokes, HS, Martens, JM, Jelocnik, M, Walder, K, Segal, Y, Berg, ML, et al. Chlamydial diversity and predictors of infection in a wild Australian parrot, the Crimson Rosella (Platycercus elegans). Transbound Emerg Dis. (2021) 68:487–98. doi: 10.1111/tbed.13703
6. de Gier, B, Hogerwerf, L, Dijkstra, F, and van der Hoek, W. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. (2018) 146:303–5. doi: 10.1017/S0950268817003065
7. Petrovay, F, and Balla, E. Two fatal cases of psittacosis caused by Chlamydophila psittaci. J Med Microbiol. (2008) 57:1296–8. doi: 10.1099/jmm.0.2008/001578-0
8. Pandeli, V, and Ernest, D. A case of fulminant psittacosis. Crit Care Resusc. (2006) 8:40–2. doi: 10.1016/S1441-2772(23)02203-2
9. Kong, CY, Zhu, J, Lu, JJ, and Xu, ZH. Clinical characteristics of Chlamydia psittaci pneumonia. Chin Med J. (2021) 134:353–5. doi: 10.1097/CM9.0000000000001313
10. Radomski, N, Einenkel, R, Muller, A, and Knittler, MR. Chlamydia-host cell interaction not only from a bird's eye view: some lessons from Chlamydia psittaci. FEBS Lett. (2016) 590:3920–40. doi: 10.1002/1873-3468.12295
11. Meijer, R, van Biezen, P, Prins, G, and Boiten, H-J. Multi-organ failure with necrotic skin lesions due to infection with Chlamydia psittaci. Int J Infect Dis. (2021) 106:262–4. doi: 10.1016/j.ijid.2021.03.091
12. Zhang, H, Zhan, D, Chen, D, Huang, W, Yu, M, Li, Q, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med. (2020) 8:401. doi: 10.21037/atm.2020.03.17
13. Xie, G, Zhao, B, Wang, X, Bao, L, Xu, Y, Ren, X, et al. Exploring the clinical utility of metagenomic next-generation sequencing in the diagnosis of pulmonary infection. Infect Dis Ther. (2021) 10:1419–35. doi: 10.1007/s40121-021-00476-w
14. Wu, X, Li, Y, Zhang, M, Li, M, Zhang, R, Lu, X, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect Dis Ther. (2020) 9:1003–15. doi: 10.1007/s40121-020-00353-y
15. Miao, Q, Ma, Y, Wang, Q, Pan, J, Zhang, Y, Jin, W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. (2018) 67:S231–40. doi: 10.1093/cid/ciy693
16. Chen, X, Cao, K, Wei, Y, Qian, Y, Liang, J, Dong, D, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. (2020) 48:535–42. doi: 10.1007/s15010-020-01429-0
17. Zhang, Z, Wang, P, Ma, C, Wang, J, Li, W, Quan, C, et al. Host inflammatory response is the major factor in the progression of Chlamydia psittaci pneumonia. Front Immunol. (2022) 13:929213. doi: 10.3389/fimmu.2022.929213
18. Cao, B, Huang, Y, She, D-Y, Cheng, Q-J, Fan, H, Tian, X-L, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. (2018) 12:1320–60. doi: 10.1111/crj.12674
19. Gacouin, A, Revest, M, Letheulle, J, Fillatre, P, Jouneau, S, Piau, C, et al. Distinctive features between community-acquired pneumonia (CAP) due to Chlamydophila psittaci and CAP due to Legionella pneumophila admitted to the intensive care unit (ICU). Eur J Clin Microbiol Infect Dis. (2012) 31:2713–8. doi: 10.1007/s10096-012-1618-6
20. Li, N, Li, S, Tan, W, Wang, H, Xu, H, and Wang, D. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerg Microbes Infect. (2021) 10:1418–28. doi: 10.1080/22221751.2021.1948358
21. Fraeyman, A, Boel, A, Van Vaerenbergh, K, and De Beenhouwer, H. Atypical pneumonia due to Chlamydophila psittaci: 3 case reports and review of literature. Acta Clin Belg. (2010) 65:192–6. doi: 10.1179/acb.2010.040
22. McGovern, OL, Kobayashi, M, Shaw, KA, Szablewski, C, Gabel, J, Holsinger, C, et al. Use of real-time PCR for Chlamydia psittaci detection in human specimens during an outbreak of psittacosis—Georgia and Virginia, 2018. MMWR Morb Mortal Wkly Rep. (2021) 70:505–9. doi: 10.15585/mmwr.mm7014a1
23. Shen, L, Tian, XJ, Liang, RZ, Cheng, Y, Kong, XL, He, F, et al. Clinical and imaging features of Chlamydia psittaci pneumonia: an analysis of 48 cases in China. Zhonghua Jie He He Hu Xi Za Zhi. (2021) 44:886–91. doi: 10.3760/cma.j.cn112147-20210127-00082
24. Xiao, Q, Shen, W, Zou, Y, Dong, S, Tan, Y, Zhang, X, et al. Sixteen cases of severe pneumonia caused by Chlamydia psittaci in South China investigated via metagenomic next-generation sequencing. J Med Microbiol. (2021) 70. doi: 10.1099/jmm.0.001456
25. Yang, F, Li, J, Qi, B, Zou, L, Shi, Z, Lei, Y, et al. Clinical symptoms and outcomes of severe pneumonia caused by Chlamydia psittaci in Southwest China. Front Cell Infect Microbiol. (2021) 11:727594. doi: 10.3389/fcimb.2021.727594
26. Rybarczyk, J, Versteele, C, Lernout, T, and Vanrompay, D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. (2020) 75:42–8. doi: 10.1080/17843286.2019.1590889
27. Dickx, V, Beeckman, DSA, Dossche, L, Tavernier, P, and Vanrompay, D. Chlamydophila psittaci in homing and feral pigeons and zoonotic transmission. J Med Microbiol. (2010) 59:1348–53. doi: 10.1099/jmm.0.023499-0
28. Beeckman, DS, and Vanrompay, DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. (2009) 15:11–7. doi: 10.1111/j.1469-0691.2008.02669.x
29. Li, X, Zuo, Z, Wang, Y, Hegemann, JH, and He, C. Polymorphic membrane protein 17G of Chlamydia psittaci mediated the binding and invasion of bacteria to host cells by interacting and activating EGFR of the host. Front Immunol. (2021) 12:818487. doi: 10.3389/fimmu.2021.818487
30. Zhao, W, He, L, Xie, X-Z, Liao, X, Tong, D-J, Wu, S-J, et al. Clustering cases of Chlamydia psittaci pneumonia mimicking COVID-19 pneumonia. World J Clin Cases. (2021) 9:11237–47. doi: 10.12998/wjcc.v9.i36.11237
31. Su, S, Su, X, Zhou, L, Lin, P, Chen, J, Chen, C, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. (2021) 10:8051–60. doi: 10.21037/apm-21-1502
32. Schinkel, AF, Bax, JJ, van der Wall, EE, and Jonkers, GJ. Echocardiographic follow-up of Chlamydia psittaci myocarditis. Chest. (2000) 117:1203–5. doi: 10.1378/chest.117.4.1203
33. Devlin, RK, Andrews, M-M, and von Reyn, CF. Recent trends in infective endocarditis: influence of case definitions. Curr Opin Cardiol. (2004) 19:134–9. doi: 10.1097/00001573-200403000-00012
34. Chau, S, Tso, EYK, Leung, WS, and Fung, KSC. Three cases of atypical pneumonia caused by Chlamydophila psittaci. Hong Kong Med J. (2015) 21:272–5. doi: 10.12809/hkmj144321
35. De Boeck, C, Dehollogne, C, Dumont, A, Spierenburg, M, Heijne, M, Gyssens, I, et al. Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in the Netherlands. Epidemiol Infect. (2016) 144:1710–6. doi: 10.1017/S0950268815003106
36. Yin, X-W, Mao, Z-D, Zhang, Q, Ou, Q-X, Liu, J, Shao, Y, et al. Clinical metagenomic sequencing for rapid diagnosis of pneumonia and meningitis caused by Chlamydia psittaci. World J Clin Cases. (2021) 9:7693–703. doi: 10.12998/wjcc.v9.i26.7693
37. Yang, Z, Wang, S, Xing, D, and Zhang, H. Pregnancy combined with severe pneumonia caused by Chlamydia psittaci infection—a case report. Ginekol Pol. (2021) 92:743–4. doi: 10.5603/GP.a2021.0184
38. Davar, K, Wilson, MR, Miller, S, Chiu, CY, and Vijayan, T. A rare bird: diagnosis of psittacosis meningitis by clinical metagenomic next-generation sequencing. Open Forum Infect Dis. (2021) 8:ofab555. doi: 10.1093/ofid/ofab555
39. Jia, Q, Sun, J, Wang, D, Xu, J, Li, X, Zhang, S, et al. Clinical features and prognostic predictors of C. psittaci pneumonia: a systematic review and individual patient meta-analysis. BMC Pulm Med. (2025) 25:55. doi: 10.1186/s12890-025-03511-5
40. Shao, S, Liu, J, Wu, Z, and Wu, S. Chlamydophila psittaci pneumonia followed by lower gastrointestinal ischemic necrosis: a case report. Front Med. (2024) 11:1394897. doi: 10.3389/fmed.2024.1394897
41. Teng, XQ, Gong, WC, Qi, TT, Li, GH, Qu, Q, Lu, Q, et al. Clinical analysis of metagenomic next-generation sequencing confirmed Chlamydia psittaci pneumonia: a case series and literature review. Infect Drug Resist. (2021) 14:1481–92. doi: 10.2147/IDR.S305790
42. Cunha, BA, Burillo, A, and Bouza, E. Legionnaires’ disease. Lancet. (2016) 387:376–85. doi: 10.1016/S0140-6736(15)60078-2
43. Wichert, A, Lukasewitz, P, Häuser, MH, Bittersohl, J, and Lennartz, H. ARDS in fulminant ornithosis and treatment with extracorporeal lung assist. Int J Artif Organs. (2000) 23:371–4. doi: 10.1177/039139880002300605
44. Shi, Y, Chen, J, Shi, X, Hu, J, Li, H, Li, X, et al. A case of Chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis. (2021) 21:621. doi: 10.1186/s12879-021-06205-5
45. Chaber, AL, Jelocnik, M, and Woolford, L. Undiagnosed cases of human pneumonia following exposure to Chlamydia psittaci from an infected rosella parrot. Pathogens. (2021) 10:968. doi: 10.3390/pathogens10080968
46. Jordan, SJ, Bakshi, RK, Brown, LT, Chi, X, and Geisler, WM. Stimulated peripheral blood mononuclear cells from chlamydia-infected women release predominantly Th1-polarizing cytokines. Cytokine. (2019) 113:458–61. doi: 10.1016/j.cyto.2018.06.017
47. Matsushima, H, Takayanagi, N, Ubukata, M, Tokunaga, D, Mori, S, Sato, N, et al. A case of fulminant psittacosis with rhabdomyolysis. Nihon Kokyuki Gakkai Zasshi. (2002) 40:612–6.
48. Zhang, A, Xia, X, Yuan, X, Liu, Y, Niu, H, Zhang, Y, et al. Severe Chlamydia psittaci pneumonia complicated by rhabdomyolysis: a case series. Infect Drug Resist. (2022) 15:873–81. doi: 10.2147/IDR.S355024
49. Nairz, M, Haschka, D, Demetz, E, and Weiss, G. Iron at the interface of immunity and infection. Front Pharmacol. (2014) 5:152. doi: 10.3389/fphar.2014.00152
50. Brinkworth, AJ, Wildung, MR, and Carabeo, RA. Genomewide transcriptional responses of iron-starved Chlamydia trachomatis reveal prioritization of metabolic precursor synthesis over protein translation. mSystems. (2018) 3:e00184. doi: 10.1128/msystems.00184-17
51. Prasad, P, Singh, N, Das, B, Raisuddin, S, Dudeja, M, and Rastogi, S. Cytokine-induced expression of nitric oxide synthases in Chlamydia trachomatis-infected spontaneous aborters. J Matern Fetal Neonatal Med. (2019) 32:3511–9. doi: 10.1080/14767058.2018.1465914
52. Michels, K, Nemeth, E, Ganz, T, and Mehrad, B. Hepcidin and host defense against infectious diseases. PLoS Pathog. (2015) 11:e1004998. doi: 10.1371/journal.ppat.1004998
53. Paradkar, PN, De Domenico, I, Durchfort, N, Zohn, I, Kaplan, J, and Ward, DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. (2008) 112:866–74. doi: 10.1182/blood-2007-12-126854
54. Yung, AP, and Grayson, ML. Psittacosis—a review of 135 cases. Med J Aust. (1988) 148:228–33. doi: 10.5694/j.1326-5377.1988.tb99430.x
55. Yang, N, Ou, Z, Sun, Q, Pan, J, Wu, J, and Xue, C. Chlamydia psittaci pneumonia—evolutionary aspects on chest CT. BMC Infect Dis. (2025) 25:11. doi: 10.1186/s12879-024-10374-4
56. Stewardson, AJ, and Grayson, ML. Psittacosis. Infect Dis Clin N Am. (2010) 24:7–25. doi: 10.1016/j.idc.2009.10.003
Keywords: Chlamydia psittaci pneumonia, community-acquired pneumonia, clinical characteristics, predictors, severe pneumonia
Citation: Wu L, Chen L, Peng L, Liu C, He S and Xie L (2025) Clinical characteristics of Chlamydia psittaci pneumonia and predictors analysis of severe patients: a retrospective observational study. Front. Med. 12:1565254. doi: 10.3389/fmed.2025.1565254
Edited by:
Zheng Jin Tu, Cleveland Clinic, United StatesReviewed by:
Barbara Camilloni, University of Perugia, ItalyRoshan Pais, Rajiv Gandhi University of Health Sciences, India
Copyright © 2025 Wu, Chen, Peng, Liu, He and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengyang He, aGVybW1vb25AY3N1LmVkdS5jbg==; Lihua Xie, eGllbGlodWFAY3N1LmVkdS5jbg==
 Ling Wu1
Ling Wu1 Chun Liu
Chun Liu Shengyang He
Shengyang He