
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 04 March 2025
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1560635
Mycoplasma hominis is a rare cause of adult central nervous system infections, posing significant diagnostic challenges due to its fastidious growth requirements and high false-negative rate in conventional cultures. We report a case of Mycoplasma hominis meningoencephalitis in a postpartum female, diagnosed via metagenomic next-generation sequencing (mNGS) of cerebrospinal fluid (CSF). The patient presented with fever, headache, and progressive neurological deficits following a cesarean section. Neuroimaging revealed a subdural hematoma, and CSF analysis demonstrated an inflammatory response. mNGS identified Mycoplasma hominis, prompting targeted antimicrobial therapy with moxifloxacin and doxycycline, which led to significant clinical improvement. This case underscores the utility of mNGS in detecting rare intracranial infections and highlights the critical role of early pathogen identification in optimizing treatment outcomes.
Mycoplasma hominis is a member of the Mycoplasma family, commonly found as a commensal in the human oral cavity, respiratory tract, and urogenital tract, and is a frequent pathogen in neonatal infections (1). Mycoplasma hominis is associated with pelvic inflammatory disease (PID) and can contribute to pregnancy-related and postpartum complications, including preterm birth, chorioamnionitis, and postpartum endometritis. Its role in these conditions is linked to its ability to colonize the female genital tract and trigger inflammatory responses (2). Mycoplasma hominis is capable of symbiotically residing and replicating within Trichomonas vaginalis. Research has shown that M. hominis can persist in the cytoplasm of T. vaginalis, utilizing the host’s metabolites for growth. This symbiotic relationship may provide M. hominins with a protective niche against the host immune response while potentially modulating the pathogenicity of T. vaginalis (3).
While Mycoplasma hominis is often associated with infections of the external genitalia in adults, postoperative wound infections, and septic arthritis, its involvement in central nervous system infections is relatively rare (1, 4). Due to the absence of a cell wall, Mycoplasma hominis is challenging to grow in conventional in vitro cultures. It has long detection cycles and a high rate of false positives (5).
Metagenomic next-generation sequencing (mNGS) is an effective method for pathogen detection and can be used to identify Mycoplasma (6, 7). This study reports a case of Mycoplasma hominis meningoencephalitis diagnosed through cerebrospinal fluid (CSF) mNGS in a patient after cesarean section. This case contributes to a better understanding of the clinical characteristics of Mycoplasma meningoencephalitis, helping reduce misdiagnosis and missed diagnoses by clinicians.
A female patient in her 30s was admitted to our hospital due to persistent headaches and fever for 10 days, along with weakness in the right limbs for 3 days. The patient had a history of cesarean section 13 days prior and denied any history of trauma. Physical examination revealed a temperature of 38.2°C, a heart rate of 104 beats and a respiratory rate of 20 breaths per minute, and blood pressure of 135/85 mmHg. Muscle strength in the right limbs was 4+, with reduced pain and temperature sensation on the right side and positive neck stiffness. Blood tests showed a white blood cell (WBC) count of 12.19 × 109/L and a neutrophil count of 10.87 × 109/L, with normal coagulation and biochemical results. After the physical examination, the patient was preliminarily diagnosed with a high likelihood of intracranial infection. Given that she is a postpartum female presenting with headache and focal neurological deficits, further head Magnetic Resonance Imaging (MRI) examination was recommended to assist in differentiating intracranial venous sinus thrombosis. Additionally, to rule out the possibility of stroke, a head MRI with Diffusion Weighted Imaging (DWI) was performed. We also recommended additional diagnostic procedures, including lumbar puncture, head MRI, and pathogen testing. Head MRI and Magnetic Resonance Venography (MRV) indicated a left frontal-temporal–parietal subdural hematoma ruptured into the subarachnoid space, and MRV revealed abnormal development of the right transverse and sigmoid sinuses (Figure 1). On the second day of admission, the patient’s right limb weakness worsened, accompanied by persistent high fever. Examination revealed right-sided facial weakness with a shallow nasolabial fold and tongue deviation, grade 2 muscle strength in the right limbs, positive Babinski sign, reduced pain and temperature sensation, and neck stiffness. Head CT showed unclear gray-white matter boundaries and brain swelling in the left cerebral hemisphere (Figure 1). On the third day, a lumbar puncture revealed a pressure of 240 mmH2O. CSF analysis showed a total protein concentration of 706 mg/L and a WBC count of 197 × 106. The mNGS of CSF identified Mycoplasma hominis with 148 sequences detected (Figure 2). The patient was diagnosed with Mycoplasma hominis meningoencephalitis.
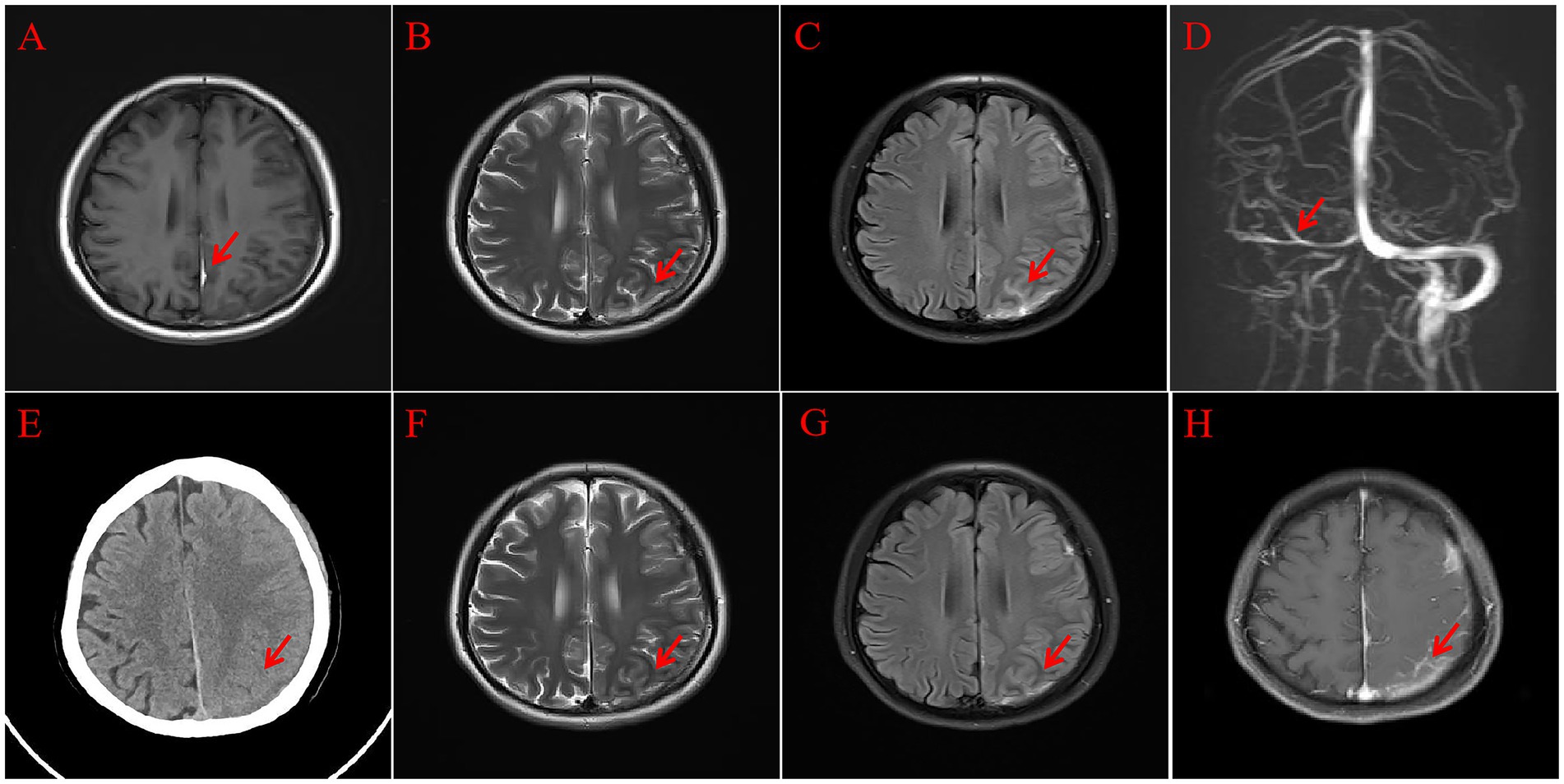
Figure 1. Head MRI. T1 (A) Shows a subdural hematoma (red arrow); T2 (B) shows cortical swelling in the left cerebral hemisphere (red arrow); Flair (C) shows the subdural hematoma rupturing into the subarachnoid space (red arrow); MRV (D) shows abnormal development of the right transverse and sigmoid sinuses (red arrow); follow-up head CT (E) shows improvement in the left cortical swelling (red arrow); follow-up head MRI, T2 (F), and Flair (G) show reduced left cortical swelling (red arrow); enhanced MRI (H) shows abnormal enhancement in the subdural area and some sulci in the left frontal-temporal–parietal region (red arrow).
The treatment strategy was adjusted to moxifloxacin 400 mg (intravenous injection) once daily and doxycycline 100 mg (oral administration) every 12 h. The patient’s headache resolved one week after admission, and body temperature returned to normal. MRI on the 11th day showed improved left frontal-temporal–parietal subdural and sulcal hyperintensity. Abnormal enhancement was observed in the left frontal-temporal–parietal subdural area and some sulci (Figure 1). A lumbar puncture on the 12th day showed a pressure of 120 mmH2O, with no significant abnormalities in CSF routine and biochemical tests. The patient was discharged on the 14th day after improvement. Upon discharge, a slightly shallow right nasolabial fold and slight right tongue deviation were noted; muscle strength in the right limbs was grade 4, and the right Babinski sign remained positive. One month post-discharge, follow-up showed the patient’s limb strength had fully recovered. The patient’s detailed diagnosis and treatment process is summarized in Figure 3.
Mycoplasma hominis is a pathogenic species of Mycoplasma in humans, commonly causing urogenital infections such as urethritis, cervicitis, pelvic inflammatory disease, pregnancy complications, and neonatal infections (8, 9). With advances in diagnostic technology, there have been reports of Mycoplasma causing central nervous system changes in children, including encephalitis, optic neuritis, acute psychiatric symptoms, stroke, cranial nerve palsies, aseptic meningitis, Acute Disseminated Encephalomyelitis (ADEM), Guillain-Barré Syndrome (GBS), and transverse myelitis (10). However, reports of intracranial infections caused by Mycoplasma hominis in adults remain relatively rare.
In this study, the patient presented with fever and headache accompanied by positive meningeal signs. Lumbar puncture CSF analysis indicated an inflammatory response, which is not significantly different from the clinical features of intracranial infections caused by other pathogens. Thus, diagnosing intracranial Mycoplasma infections based solely on clinical symptoms and laboratory tests is challenging. Additionally, detecting Mycoplasma is difficult, time-consuming, and has a high false-negative rate (5).
mNGS is a novel genetic testing method that performs high-throughput sequencing of nucleic acids directly from clinical samples, comparing them with databases to identify the types of pathogens present in the sample. It can quickly detect specific pathogens. Study have shown that mNGS plays an important auxiliary role in diagnosing unexplained encephalitis (11). mNGS enables the unbiased detection of nucleic acids from various pathogens in clinical specimens, including bacteria, fungi, viruses, and parasites. In 2014, the first reports emerged on the use of mNGS for diagnosing central nervous system (CNS) infections (12). Since 2015, CSF mNGS has been increasingly utilized in China for the diagnosis of CNS infectious diseases (13). Subsequent studies have reported that the detection rate of pathogenic organisms in CSF using mNGS ranges from 15.7 to 57.0% (11, 14). Among CNS infection cases, the concordance rate between mNGS and conventional pathogen detection methods ranges from 22.5 to 52.6%. The sensitivity and specificity of CSF mNGS in diagnosing encephalitis and meningitis have been reported as 73 and 99%, respectively (15). These findings underscore the practical value of CSF mNGS in the etiological diagnosis of CNS infectious diseases. Although mNGS technology is associated with high medical expenses, we strictly regulate its clinical indications, recommending CSF mNGS only for patients with unknown etiology, poor response to empirical treatment, severe disease, or immunodeficiency (or immunosuppression) presenting with encephalitis, meningitis, or brain abscess. Additionally, technological advancements have led to a gradual reduction in mNGS costs. Through continuous technological refinement, clinical application research, and accumulated experience, CSF mNGS has now become a crucial and practical tool for the differential diagnosis of CNS infections.
Currently, a consensus has been reached on the use of mNGS in patients with acute encephalitis and meningitis in clinical practice: (1) For community-acquired acute encephalitis, meningitis, and non-severe cases, conventional CSF microbiological testing is generally recommended as the initial approach. (2) For severe cases, mNGS is recommended for the initial CSF analysis (16). (3) For patients with a high suspicion of infection by emerging or rare pathogens, mNGS should be prioritized in the initial CSF analysis (17). (4) For patients with chronic CNS infections of unknown etiology, mNGS is the preferred method for pathogen detection (16). (5) For patients with primary immunodeficiency, neutropenia, AIDS, or those receiving immunosuppressive therapy, mNGS should be performed during the initial CSF evaluation (18). (6) In cases of strong clinical suspicion of RNA virus infection, mNGS should be the first-line diagnostic method (17). The patient’s condition worsened, and conventional empirical treatment proved ineffective. Traditional diagnostic methods for Mycoplasma infections face significant challenges in identifying the pathogen, as they are time-consuming and associated with a high false-negative rate, making a definitive diagnosis extremely difficult. This patient was ultimately diagnosed with Mycoplasma infection through CSF mNGS. This case demonstrated the diagnostic value of mNGS in intracranial infections caused by Mycoplasma hominis. Furthermore, the rapid adjustment of antibiotic therapy based on sequencing results led to a quick improvement in the patient’s clinical symptoms and a good prognosis, further confirming the advantage of mNGS in assisting the rapid adjustment of treatment strategies.
The mechanisms by which Mycoplasma invades the central nervous system mainly include the following hypotheses: (1) direct contamination during trauma or surgery; (2) dissemination and implantation in the brain following bacteremia secondary to urogenital surgical procedures; (3) direct spread of respiratory tract colonizing bacteria to open wounds through artificial airways (19).
Previous reports have shown that adult Mycoplasma encephalitis is often accompanied by intracranial hemorrhage or subarachnoid hemorrhage (SAH), with most patients having a history of head trauma followed by cranial surgery (20, 21). However, after consulting the patient’s family, no history of trauma was reported, and the patient had no vascular malformations or coagulation disorders. Currently, there is a lack of research directly linking subdural hematoma (SDH) and SAH to Mycoplasma infection. In this patient, the pathway for intracranial Mycoplasma infection might have involved Mycoplasma entering the bloodstream during the cesarean section, causing bacteremia. Coupled with the presence of SAH, this ultimately led to the dissemination and implantation of Mycoplasma hominis in the cerebral cortex. However, the etiology of this patient’s subdural hematoma and SAH remains unclear. Further research is needed to confirm the possibility of Mycoplasma entering the bloodstream.
Studies on successful treatment of central nervous system infections caused by Mycoplasma hominis often recommend using tetracyclines combined with quinolones, with doxycycline, minocycline, and moxifloxacin showing the best efficacy. The treatment dosage is the same as routine regimens, with the duration for meningitis treatment typically 2–3 weeks (1). In this study, we administered moxifloxacin combined with doxycycline for two weeks, rapidly improving symptoms. The CSF WBC count, protein, and glucose levels returned to normal. The patient’s improvement may be related to the good blood–brain barrier permeability and drug activity of quinolones, especially moxifloxacin. This also highlights the importance and urgency of early identification of the pathogen and timely medication adjustment in patients with intracranial Mycoplasma infections.
Due to the extreme rarity of intracranial infections caused by Mycoplasma, clinicians often find it difficult to diagnose without microbiological evidence. Additionally, the lack of specific clinical manifestations makes distinguishing Mycoplasma meningitis from bacterial or viral meningitis challenging. However, CSF mNGS technology can quickly provide etiological evidence of Mycoplasma hominis infection. Mycoplasma is resistant to most commonly used first-line antibiotics and requires specialized treatment, making early diagnosis crucial.
Although mNGS has shown great promise in clinical applications, it still has certain limitations. For instance, mNGS is highly prone to contamination from human host DNA and environmental microbes. Thus, strict adherence to established guidelines and protocols is essential at every stage, including sample collection and processing, library preparation, sequencing, bioinformatics analysis, and clinical interpretation. Optimizing workflows, strengthening quality control, and training specialized personnel are crucial strategies to minimize contamination risks. Moreover, the high cost of mNGS remains a significant obstacle to its widespread adoption. In China, this technology has not yet been incorporated into the medical insurance system, making cost reduction difficult in the short term. Furthermore, as a novel molecular diagnostic tool, mNGS still lacks broad patient acceptance. Its relatively long turnaround time also leads many patients to prefer PCR-based methods, which are faster and more affordable. Thus, expanding the clinical adoption of mNGS will require continuous technological advancements, cost-reduction strategies, and greater awareness among healthcare providers and patients.
Although mNGS currently faces challenges in terms of cost, pricing, and technology in clinical applications, its overall positive detection rate and sensitivity are markedly superior to traditional pathogen detection methods. CSF mNGS has become an essential and practical technique for the etiological identification of CNS infections. Therefore, for patients with encephalitis, meningitis, or brain abscesses of unknown etiology—especially those with poor responses to empirical treatment, severe disease, or immunodeficiency (or immunosuppression)—we recommend prioritizing CSF mNGS testing. In the future, adopting differentiated, standardized testing workflows tailored to the characteristics of various patients and specimens, combined with analysis strategies based on the specific microbial distribution of individual hospitals, may represent the direction for achieving precise etiological diagnosis of CNS infections.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Harrison International Peace Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent has been obtained from the participant(s) for the publication of this case report.
K-MW: Writing – original draft, Investigation. NM: Writing – review & editing, Software. H-BW: Conceptualization, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Natural Science Foundation of Hebei Province (H2020304171).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang, N-L, Cai, X, Que, Q, Zhao, H, Zhang, K-L, and Lv, S. Mycoplasma hominis meningitis after operative neurosurgery: a case report and review of literature. World J Clin Cases. (2022) 10:1131–9. doi: 10.12998/wjcc.v10.i3.1131
2. Murakami, S, Suemori, K, Uchikura, Y, Saito, M, Tamaki, M, Ochi, F, et al. Incidence and antimicrobial susceptibilities of Mycoplasma hominis in pregnant females, Ehime University Hospital. Rinsho Biseibutsu Jinsoku Shindan Kenkyukai shi. (2020) 29:53–8.
3. Margarita, V, Rappelli, P, Dessi, D, Pintus, G, Hirt, RP, and Fiori, PL. Symbiotic association with Mycoplasma hominis can influence growth rate, Atp production, cytolysis and inflammatory response of trichomonas vaginalis. Front Microbiol. (2016) 7:953. doi: 10.3389/fmicb.2016.00953
4. Potruch, A, Rosenthal, G, Michael-Gayego, A, Temper, V, Abdelrahman, M, Ayalon, O, et al. A case report of Mycoplasma hominis subdural empyema following decompressive craniotomy, and a review of central nervous system Mycoplasma hominis infections. Front Med. (2022) 9:792323. doi: 10.3389/fmed.2022.792323
5. Whitson, WJ, Ball, PA, Lollis, SS, Balkman, JD, and Bauer, DF. Postoperative Mycoplasma hominis infections after neurosurgical intervention: a review. J Neurosurg Pediatr. (2014) 14:212–8. doi: 10.3171/2014.4.PEDS13547
6. Cai, S, Pan, J, Wang, S, Bao, R, Zhou, C, Gao, X, et al. Accurate etiological diagnosis of Mycoplasma hominis Mediastinitis in immunocompetent patients using metagenomic next-generation sequencing: a case series and literature review. J Thorac Dis. (2024) 16:2499–509. doi: 10.21037/jtd-24-286
7. Dong, Y, He, Y, Zhou, X, Lv, X, Huang, J, Li, Y, et al. Diagnosis of Mycoplasma hominis meningitis with metagenomic next-generation sequencing: a case report. Infection Drug Resist. (2022) 15:4479–86. doi: 10.2147/IDR.S371771
8. Qiu, Y, Mao, S, Li, X, Chen, Y, Chen, W, Wen, Y, et al. Chinese advances in understanding and managing genitourinary tract infections caused by Mycoplasma Genitalium, Mycoplasma hominis, and Ureaplasma Urealyticum. Arch Microbiol. (2025) 207:5. doi: 10.1007/s00203-024-04204-z
9. Zhou, M, Wang, P, Chen, S, Du, B, Du, J, Wang, F, et al. Meningitis in a Chinese adult patient caused by Mycoplasma hominis: a rare infection and literature review. BMC Infect Dis. (2016) 16:1–7. doi: 10.1186/s12879-016-1885-4
10. Kammer, J, Ziesing, S, Davila, LA, Bültmann, E, Illsinger, S, Das, AM, et al. Neurological manifestations of Mycoplasma pneumoniae infection in hospitalized children and their long-term follow-up. Neuropediatrics. (2016) 47:308–17. doi: 10.1055/s-0036-1584325
11. Xing, X-W, Yu, S-F, Zhang, J-T, Tan, R-S, Ma, Y-B, Tian, X, et al. Metagenomic next-generation sequencing of cerebrospinal fluid for the diagnosis of cerebral Aspergillosis. Front Microbiol. (2021) 12:787863. doi: 10.3389/fmicb.2021.787863
12. Wilson, MR, Naccache, SN, Samayoa, E, Biagtan, M, Bashir, H, Yu, G, et al. Actionable diagnosis of Neuroleptospirosis by next-generation sequencing. N Engl J Med. (2014) 370:2408–17. doi: 10.1056/NEJMoa1401268
13. Guan, H, Shen, A, Lv, X, Yang, X, Ren, H, Zhao, Y, et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol. (2016) 22:240–5. doi: 10.1007/s13365-015-0390-7
14. Zhang, Y, Cui, P, Zhang, H-C, Wu, H-L, Ye, M-Z, Zhu, Y-M, et al. Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J Transl Med. (2020) 18:1–13. doi: 10.1186/s12967-020-02360-6
15. Miller, S, Naccache, SN, Samayoa, E, Messacar, K, Arevalo, S, Federman, S, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. (2019) 29:831–42. doi: 10.1101/gr.238170.118
16. Liu, Y, Zhu, W, Jiao, M, Guo, W, and Luo, Y. Clinical application value of metagenomic next-generation sequencing in the diagnosis of central nervous system infections. Front Bioeng Biotechnol. (2023) 11:885877. doi: 10.3389/fbioe.2023.885877
17. Yuan, L, Zhu, XY, Lai, LM, Chen, Q, Liu, Y, and Zhao, R. Clinical application and evaluation of metagenomic next-generation sequencing in pathogen detection for suspected central nervous system infections. Sci Rep. (2024) 14:16961. doi: 10.1038/s41598-024-68034-1
18. Benoit, P, Brazer, N, de Lorenzi-Tognon, M, Kelly, E, Servellita, V, Oseguera, M, et al. Seven-year performance of a clinical metagenomic next-generation sequencing test for diagnosis of central nervous system infections. Nat Med. (2024) 12:1–12. doi: 10.1093/ofid/ofae631.089
19. Pailhories, H, Rabier, V, Eveillard, M, Mahaza, C, Joly-Guillou, M-L, Chennebault, J-M, et al. A case report of Mycoplasma hominis brain abscess identified by Maldi-Tof mass spectrometry. Int J Infect Dis. (2014) 29:166–8. doi: 10.1016/j.ijid.2014.08.004
20. Chen, L, Zheng, L, Lin, J, and Huang, X. One case of Mycoplasma hominis-related intracranial infection after Craniocerebral injury. Chinese J Lab Med. (2022) 2:197–9.
Keywords: Mycoplasma hominis, adults, meningoencephalitis, metagenomic next-generation sequencing, diagnosis, case report
Citation: Wang K-M, Mu N and Wang H-B (2025) Intracranial infection in an adult caused by Mycoplasma hominis, diagnosed using mNGS technology: a case report. Front. Med. 12:1560635. doi: 10.3389/fmed.2025.1560635
Received: 17 January 2025; Accepted: 17 February 2025;
Published: 04 March 2025.
Edited by:
Muhammad Sohaib Asghar, AdventHealth, United StatesReviewed by:
Abdelrahman Abdalla, AdventHealth Sebring, United StatesCopyright © 2025 Wang, Mu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He-Bo Wang, d2FuZ2hiaG9wZUBoZWJtdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.