- Department of Dermatology, Affiliated Hospital of Jiangsu University, Zhenjiang, China
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a high prevalence worldwide and multifaceted pathogenesis. In general, patients with moderate to severe AD often experience relapse after discontinuing treatment. Therefore, to understand the possible factors of chronic relapse of AD and to look for biological markers that predict the relapse or poor prognosis of AD will be helpful for clinical treatment. Mutations in genes such as FLG, SPINK5, STAT, KIF3A, claudin-1, Ovol1, and HLA-DRB1 offer new insights into the genetic basis of AD. Routine factors may help improve patient lifestyle, highlight the importance of environmental influences (including psychological stress), and support clinicians in optimizing anti-infective treatment strategies. The inflammatory axis (CD30–CD30L axis, IL-9-IL-18 axis) provides new insights into the inflammatory pathways of AD and may be a target for future therapies. Low NKG2D expression may have adverse effects on prognosis. Prognostic biomarkers can play an important role in treatment monitoring, disease progression and recurrence, and provide the possibility for more personalized treatment.
1 Introduction
Atopic dermatitis (AD) is a chronic, recurrent inflammatory skin disease that affects the quality of life of patients, characterized by recurrent eczematous skin lesions, severe pruritus and sensitive, dry skin (1). AD is a heterogenous disease with primary T helper 2 cells (Th2)/Th22 skewing, and variable Th1/Th17 contribution (2). AD is diagnosed according to the criteria developed by Hanifin& Rajka. The pathogenesis of AD is complex, and it is evident that a strong genetic predisposition (filaggrin mutations), epidermal dysfunction, skin microbiome abnormalities (Staphylococcus aureus colonization), immune dysregulation, and the neuroimmune system are critical in AD development (3). AD management is multifaceted, involving emollient moisturizers, topical corticosteroid, topical calcineurin inhibitor, phosphodiesterase type 4 inhibitors, antimicrobial treatment, antihistamines, phototherapy, systemic corticosteroid, systemic immunomodulatory treatments and targeted therapies (4). Nonetheless, there is no cure for AD currently, the goals of AD treatment are to reduce pruritus and skin inflammation, restore barrier function, prevent infection and establish long-term disease control. After treatment discontinuation, AD recurs in up to 70% of cases, with a 7-year recurrence rate reaching 75.9% (5). Patients with recurrence (eczema area and severity index, EASI ≥ 16) within 3 months after cessation of treatment should be considered as a recurrent AD (6). Therefore, understanding the possible factors of AD exacerbation, chronic recurrence, and looking for biomarkers to predict AD recurrence or poor treatment response will help clinical decision-making, decrease the risk of developing comorbidities related to disease progression, and reduce the costs of AD for the health system.
2 Gene mutations
Research has elucidated the significant role of the filaggrin gene (FLG) as a prominent genetic risk factor in atopic dermatitis (7). Loss-of-function mutations in the gene encoding the epidermal protein filaggrin can lead to the loss or reduction of filaggrin synthesis (8). The deficiency of filaggrin in AD patients impacts on the function of the epidermis and increases the risk for microbial infection or development of other atopic diseases (9). FLG mutations has been associated with more persistent disease (10). Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects (11).
Mutations in SPINK5 have already been shown to be responsible for the development of Netherton syndrome, which can severely affect the skin in the form of refractory severe AD. It was clearly demonstrated that rs2303067 in SPINK5 is predisposing to early onset AD, and similar to FLG mutations, rs2303067 is also predisposing to prolonged AD duration, the need for hospitalization and more affected body parts (12, 13).
Signal transducer and activator of transcription (STAT) proteins are critical mediators of cytokine signals that regulate gene expression programs underlying key processes involved in the immune system. Case analyses indicate that gain-of-function mutations in STAT5B and STAT6 may contribute to treatment-resistant severe AD (14, 15).
Genetic variation in the cilia structural gene kinesin family number 3A (KIF3A) has been associated with AD, asthma, and the atopic march by numerous studies. A study in mice found KIF3A deficiency causes skin barrier dysfunction and contributes to AD susceptibility. KIF3A is required for skin barrier homeostasis whereby decreased KIF3A skin expression causes disrupted skin barrier function and promotes development of AD (16).
Tight junctions (TJs) are cell–cell connections located under the stratum corneum (SC), which contribute to epidermal barrier integrity. Epithelial TJs are composed of proteins including claudin, occludin and zonula occludens. Claudin 1 is integral to TJs functionality in the skin barrier. Claudin 1 mutations are associated with skin xerosis, pruritus and AD development (17, 18).
Ovol1 has also been identified as a susceptibility gene for AD by genome-wide association studies, in addition to FLG (19). Ovol1 impairment downregulates FLG, contributing to AD pathogenesis (20). Ovol1 deficient in keratinocytes impairs the barrier-promoting function of aryl hydrocarbon receptor (AhR), exacerbating AD-like inflammation (21). Ovol1 deficient affect the AhR-Ovol1-Id1 regulatory axis which promotes both epidermal and immune homeostasis in the context of skin inflammation.
Human leukocyte antigen (HLA)-DRB1 was regarded as a susceptibility factor associated with AD (22). Amino acid variations at peptide-binding pockets of HLA-DRB1 were associated with the persistence of AD in African-American children (23).
3 General factors
AD comprises multiple endotypes with distinct inflammatory pathways influenced by disease stage, age, and ethnicity. Th2-dominant immunity is central to AD, while Th1, Th17, and Th22 pathways also contribute (24). With regard to disease stage, Th2 activity predominates during the acute phase, whereas Th1, Th17, and Th22 responses become more prominent in chronic disease (25). With aging, Th2 activity significantly declines and the expression of Th1 and Th17 related markers increases. Elderly AD patients show reduced Th2/Th22 and increased Th1/Th17 inflammation in both the skin and blood, still presents as a type 2 inflammatory dominant disease (26). A single-center prospective cohort finding that age > 60 predicted poor response of dupilumab (27). It is speculated that the endotype shift in the elderly may be one of the reasons for the poor outcome. In the meantime, this cohort is a cross-sectional study, although there are data suggesting that type 2 inflammation decreases with age, there are no data in clinical practice suggesting that the efficacy of these drugs decreases over time. Ethnic differences further shape AD endotypes. Asian AD patients exhibit mixed Th2/Th22/Th17 inflammation, whereas white-skinned individuals predominantly display Th2/Th22-driven inflammation (26, 28). Current inclusion criteria for AD clinical trials are mostly based on disease severity rather than AD phenotyping. An attempt to define the patient’s endotype before treatment should be made to optimize therapeutic responses moving toward precision medicine based on the different clinical and molecular disease subsets.
Obesity is considered a risk factor for AD, contributing to skin’s epidermal barrier, increased transepidermal water loss and dry skin, immune dysregulation, and alterations in the skin microbiome and gut flora, exacerbating inflammation (29). Leptin, a cytokine cascade activator secreted by white adipose tissue (30), can enhance Th2 responses, promoting interleukin (IL)-4, IL-5, and IL-13 release, immunoglobin E (IgE) production, and skin inflammation in AD (31) (Figure 1). Obesity also affects dupilumab efficacy. Higher body mass index reduces both short-term and long-term treatment effectiveness (32, 33). Real-world studies indicated that higher body mass index was associated with a lower probability of reaching an improvement of Investigator’s Global Assessment ≥2 points at Week 52 (34) and body mass index <24 was identified as potential predictive factors of good response (27). Given these associations, nutritional interventions may not only mitigate obesity-related metabolic comorbidities but also improve AD outcomes, making timely weight management essential for obese AD patients.
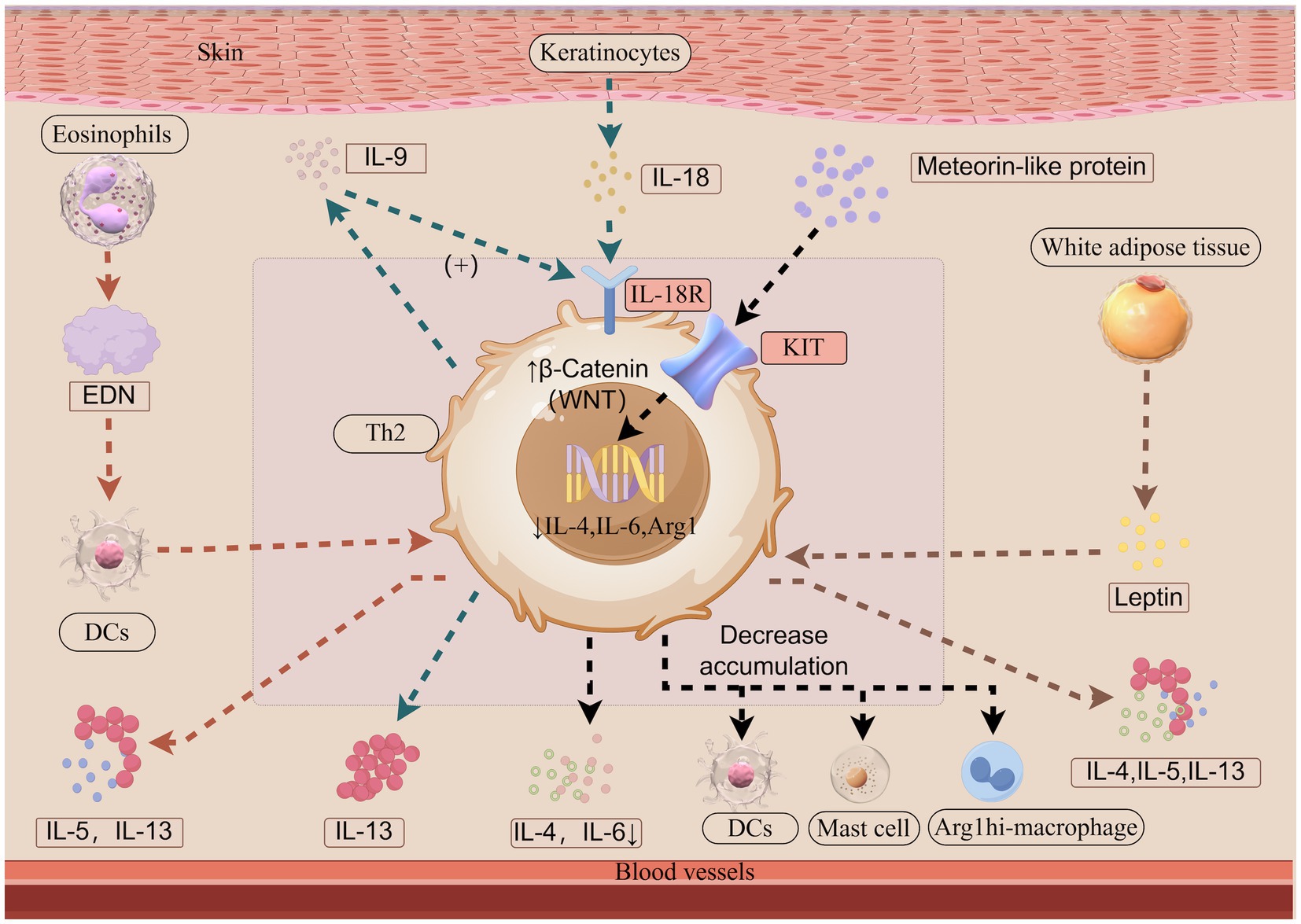
Figure 1. The roles of leptin, IL-9-IL-18 axis, EDN and Meteorin-like protein in atopic dermatitis (Illustration created with Figdraw). Leptin, primarily secreted by white adipose tissue, enhances Th2 responses by stimulating the release of IL-4, IL-5, and IL-13 from Th2 cells. IL-18 is predominantly produced by keratinocytes, while IL-18R is primarily expressed on T cells. The interaction between IL-18 and IL-18R activates downstream signaling pathways. IL-9 is mainly secreted by recently activated Th2 cells during the early phases of allergic skin inflammation. IL-9 promotes IL-18R expression in Th2 cells, while IL-18 functions as an upstream regulator of Th2 cells by inducing IL-13 secretion. EDN, released from eosinophil granules, activates dendritic cells, thereby amplifying antigen-specific Th2 immune responses through the enhanced secretion of IL-5 and IL-13. METRNL binds to the KIT receptor and enhances the levels of the active WNT pathway molecule β-Catenin, thereby downregulating the expression of inflammatory genes, including IL-4, IL-6, and Arginase-1 mRNA expression. Consequently, the accumulation of Arg1hi macrophages, DCs, and activated mast cells and the level of IL-4 and IL-6 are reduced. Arg1, Arginase-1; DCs, dendritic cells; EDN, eosinophil-derived neurotoxin; IL, interleukin; IL-18R, interleukin-18 receptor; Th, T helper cell type.
Head and neck dermatitis (HND) is a refractory phenotype of AD (35), that is often resistant to first-line topical therapies. And biologic treatments, such as Dupilumab, may cause the development or exacerbation of HND, defined as dupilumab-associated HND (36, 37). Malassezia overgrowth, driven by ceramide deficiency, is prevalent in HND, contributing to disease severity and treatment resistance (35, 38). In addition, the constant exposure of the head-and-neck area to several types of allergens might be a contributing factor to AD persistence (39). Persistent HND has been associated with lower dupilumab response rates (39, 40), and baseline Malassezia-specific IgE is proposed as a potential biomarker for predicting the development or exacerbation of HND (37, 41).
A positive family history of AD, particularly parental AD or other allergic diseases (e.g., rhinitis), increases the probability of the onset, recurrence and persistence of AD after puberty (42). High socioeconomic status (≥12 years of parental education), a positive skin prick test at baseline, and employment in high-risk occupations (nursing, healthcare, bakery, cleaning) are significant predictors of AD recurrence and persistence (42). Pregnancy may exacerbate AD (43). Increasing levels of progesterone and estrogen have been associated with an enhanced Th2 immune response, which supports pregnancy maintenance but may also promote atopic disease development (43, 44).
Pruritus is a common and distressing symptom of AD, with nocturnal pruritus significantly impairing sleep, leading to anxiety and daytime somnolence (45). AD skin lesions release various substances (pruritogens), including cytokines and chemokines (e.g., IL-31), and chemical mediators, triggering itching and subsequent scratching behavior. Scratching further aggravates AD, perpetuating the itch–scratch cycle. Irritation from scratching is extremely important as an exacerbation factor of AD. In addition to the therapeutic ladder for pruritus in atopic dermatitis to break the itch–scratch cycle, cutting nails short, and wearing gloves, long sleeves and long pants while sleeping, if necessary, so that scratching does not cause skin damage, may be helpful in some cases (45).
Exposure to environmental factors including allergens and stimuli in the work place and daily environment, is associated with maintenance and exacerbation of AD. Non-specific irritation present in daily-life such as contact with saliva, sweat, hair, and friction against clothes may exacerbate AD. Exposure to chlorine in water, formaldehyde, benzene, particulate matter 10 (PM10), nitrogen oxide compounds, and carbo monoxide in air was associated with AD worsening. Certain foods such as milk, eggs and wheat can trigger AD. Soap usage can thin the SC in both normal and non-lesional atopic skin, while alterations in skin pH further compromise barrier function. Additionally, disturbances in perspiration as well as excess sweat remaining on the skin surface exposed to high-temperatures and humidity may worsen symptoms of AD (46, 47).
Patients with AD have an increased risk of bacterial, viral and fungal infections of the skin, mainly due to impaired barrier function, altered immune response, certain systemic treatments and frequent scratching, which can have negative consequences on the disease course (48). Bacterial infections, particularly those caused by Staphylococcus aureus, are the most common and can be challenging to diagnose due to symptom overlap with AD (48). Recurrent viral infections, though less frequent, include herpes simplex virus infection, which can lead to eczema herpeticum (48). Fungal infections are also common in patients with AD. Findings suggest that Malassezia decreases the effectiveness of treatment with ruxolitinib and dupilumab (49), and C. albicans colonization of the gastrointestinal tract results in antigen sensitization which may potentially lead to chronic AD (48). These can also lead to infections of other organ systems including the lungs and the urinary tract. Recurrent bacterial infections can favor disease chronicisation and cause disease flares with significant morbidity, and possible mortality in patients with AD. For the management of infections in patients with AD, it is important to identify and treat the underlying infection, optimize AD treatment to improve skin barrier function and immune system regulation and implement infection prevention strategies. The risk of infection should also be considered when selecting topical or systemic immunosuppressive therapies, as some of these agents can increase the risk of viral infections.
Psychological stress contributes to skin barrier dysfunction in AD and is a well-established trigger for disease exacerbation (47). Poorly controlled AD can lead to psychological burden and secondary cognitive disturbances, reflecting the complex psycho-neuro-immunological interactions involved in eczema. Clinicians should adopt a comprehensive treatment approach that considers psychosomatic interactions. Stress-reduction interventions improve both skin manifestations and overall patient well-being (50).
Poor treatment adherence is a significant barrier to optimal outcomes in AD. Key factors contributing to nonadherence include frustration with treatment efficacy, inconvenience, and concerns about side effects. Additional barriers include forgetfulness, financial constraints, distrust in physicians, dislike of prescribed medications, and insufficient understanding of the disease or treatment (51).
Topical corticosteroids (TCS) are a common treatment for AD; however, prolonged use may increase recurrence risk. Studies have suggested that barrier defects are not repaired by steroid use and treated skin become up to 70% thinner. Additionally, TCS may increase expression of desquamatory protease KLK7, which is linked to increased AD lesions (47). TCS therapy is also associated with steroid phobia, leading to nonadherence and undertreatment. Misinformation, fear of side effects, and inadequate education contribute to this phenomenon (52). Topical steroid withdrawal syndrome (TSW), characterized by TCS dependence and skin disease worsening after TCS discontinuation, can further exacerbate steroid phobia. However, overdiagnosis of TSW may result in unnecessary treatment avoidance, leading to prolonged and intensified AD flares (53).
Patient education plays a crucial role in the long-term management of AD. Educational strategies vary, including printed materials, structured courses, and digital interventions, each influencing patient outcomes differently. Despite the prevalence of misinformation on social media, web-based interventions, smartphone applications, and teledermatology have been shown to enhance patient awareness. Additionally, social media can support online interventions, which, in some cases, may be as effective as face-to-face consultations (54).
4 Axis
OX40 is a co-stimulatory immune checkpoint molecule that promotes the activation and the effector function of T lymphocytes through interaction with its ligand (OX40L) on antigen-presenting cells (55). OX40–OX40L axis plays a crucial role in Th1 and Th2 cell expansion, particularly during the late phases or long-lasting response (55). Activation of the OX40–OX40L axis can promote Th2 differentiation in the absence of Th1 cytokines. A close relationship exists between this co-stimulatory pathway and the principal proinflammatory cytokines that initiate the AD inflammatory cascade, such as Thymic stromal lymphopoietin and IL-33. OX40–OX40L signaling contributes to AD chronicity by enhancing T cell expansion and survival with subsequent memory T cell development, responsible for chronic relapsing inflammation (55). OX40-OX40L pathway inhibitors, such as rocatinlimab, amlitelimab and telazorlimab, present an innovative approach by modulating T cell activity.
IL-18, produced by keratinocytes, binds interleukin-18 receptor (IL-18R) on skin immune cells, activating signaling pathways, modulating Th1/Th2 responses (56). Studies in murine models indicate that IL-18R is specifically expressed in the skin, where it triggers IL-13 secretion upon IL-18 stimulation (57). IL-9 belongs to the family of common γ chain cytokines and is linked to type 2 immunity and immunopathology (58). In human skin, IL-9 is mainly secreted by recently activated Th2 cells during early phases of allergic skin inflammation (59). IL-9 induces IL-18R expression in Th2 cells through the activation of the JAK1/JAK3-STAT1 signaling cascade. In this setting, IL-13, a well-established pathogenic cytokine in AD, production appears to be enhanced (60) (Figure 1). In conclusion, the novel IL-9-IL-18 axis provides new insights into the inflammatory pathways of AD and may represent a potential therapeutic target for its treatment. A study in mice found IL-18 blocking agent substantially controlled skin inflammation in a model of atopic dermatitis (61). However anti-IL-9 monoclonal antibody was not associated with any improvement in asthma (62).
CD30 was originally described as a Th2 cell surface-expressed molecule that also aided Th2 cell development (63). The interaction between CD30 and CD30L has been shown to regulate the generation of CD4 effector and memory T cells, in conjunction with OX40/OX40L (64, 65). CD30, similar to OX40, can activate signaling pathways, including NF-κB and PI3K/Akt, which influence T cell proliferation, survival, and cytokine production. Study confirmed upregulated expression of CD30 transcripts in CD4 T cells in the skin lesions from AD patients and found that these partially coincided with expression of CD30L, as well as with OX40 and OX40L transcripts (63). CD30–CD30L signaling may contribute to AD chronicity as well as OX40–OX40L axis.
5 Prognostic biomarkers
Prognostic biomarkers are used to determine the possibility or risk of progression or recurrence of a disease and the response to a given therapy. The expression of accurate and reliable biomarkers is essential for effective predictive, preventive, and personalized AD management.
AD is characterized by elevated production of the type 2 cytokines IL-4, IL-5, and IL-13, which promote AD pathogenesis. Despite initial interest in IL-5 blockade for AD treatment, clinical trials with mepolizumab (anti-IL-5 monoclonal antibody) have failed to demonstrate efficacy (66), suggesting a limited role of IL-5 in AD. In contrast, IL-31 has emerged as a key mediator of pruritus (67), while IL-4 and IL-13 remain central therapeutic targets. Dupilumab, the first biologic agent targeting IL-4 receptor α, and tralokinumab, an IL-13 inhibitor, have demonstrated significant clinical efficacy, highlighting the critical roles of IL-4 and IL-13 in AD pathogenesis and treatment. Various biological agents targeting Interleukin-4, IL-13 receptors are also being studied (68).
Total serum IgE, extensively studied as a biomarker in AD, may represent allergic diathesis rather than short-term disease activity in AD (46). High baseline IgE (≥10,000 IU/mL) was the most important patient-related factor for a poor long-term outcome, negatively impacting treatment response (69). High serum IgE levels in adult patients with AD have been associated with ongoing eczema after 10 years (70). IgE autoantibodies may serve as potential predictive biomarkers for the course of AD (71). One early study found IgE receptor antagonists (omalizumab) have not demonstrated efficacy in atopic dermatitis (72), indicating that the development of AD-like skin inflammation was largely dependent on IL-4/13 signaling but not on IgE (73). However, a small phase 2 study with 12 patients about IgE receptor antagonists showed promising results in decreasing IgE levels and percent change in Scoring Atopic Dermatitis (68). More adequate clinical studies may be needed to demonstrate a correlation between IgE receptor antagonists and AD.
Thymus and activation-regulated chemokine/chemokine C-C motif ligand 17 (TARC/CCL17) is a reliable clinical biomarker for AD severity used to monitor the efficacy of treatment (24). Elevated serum CCL17/TARC levels are associated with frequent AD relapses, even after clinical resolution (74). Persistently high TARC levels indicate inadequate treatment (75), and their increase often precedes relapse following ciclosporin tapering (76). A decrease in TARC levels to ≤837 pg/mL within 1–3 months is predictive of long-term symptom control (77). Additionally, elevated TARC levels in 2-month-old infants have been linked to a higher risk of developing AD within the initial 2 years of life (78).
Pulmonary and activation-regulated chemokine (PARC/CCL18) have been described correlating with AD severity and monitoring of disease activity during treatment (79). The rapid decline in CCL18/PARC levels was associated with good responses to abroxitinib treatment, including head and neck dermatitis (80).
Thymic stromal lymphopoietin protein is a crucial mediator of atopic inflammation, as its overexpression in murine skin models induces eczematous lesions resembling AD (81). It can induce the proliferation of Th2 memory cells that are crucial in maintaining chronic-relapse inflammation (82, 83).
Recent clinical data indicate that AD patients with low baseline lactate dehydrogenase (LDH) levels (<400 U/L) achieve higher EASI-75 response rates to dupilumab than those with elevated LDH (≥400 U/L) (84). Higher baseline LDH is also associated with poorer long-term treatment efficacy, suggesting its potential as a predictive marker for dupilumab response (33).
CXCL9 (Th1/interferon-related cytokine) and CXCL2 (Th17-related cytokine) have been suggested as treatment-specific predictive biomarkers for cyclosporine and dupilumab, respectively (85).
Macrophage-derived chemokine (MDC/CCL22) serves as the best biomarker of disease response across studies using different therapeutics (85).
The recent study found that high levels of vascular endothelial growth factor (VEGF) were associated with a higher rate of disease remission, while low VEGF has a significant impact on prediction of disease persistence (86).
Fatty-acid-binding protein 5, elevated serum eosinophil percentages and IL-13, and increased transepidermal water loss levels were identified as a potential biomarker in atopic march (87–90).
Eosinophilic granulocytes and eosinophil-derived neurotoxin (EDN) which is released from eosinophil (91) are actively involved in the regulation of allergic inflammation (92, 93). Research on dupilumab therapy revealed a significant inverse correlation between the percentage decrease in EASI scores and baseline blood eosinophil count. Furthermore, patients with low (<500/μL) baseline blood eosinophil counts showed a higher EASI-75 response rate (84). EDN can activate dendritic cells (DCs) to enhance antigen-specific Th2 immune responses, such as the release of IL-5 and IL-13 (93) (Figure 1). Research findings indicate that EDN positively correlate with the scoring AD and are notably elevated among individuals experiencing relapse. A cutoff value of 64.5 ng/mL for EDN has been identified as predictive of relapse (94).
IL-31 has been shown to induce late-onset itch in human AD patients and is thought to be involved in promoting the pathophysiology of AD and pruritus via the “scratch-itch cycle” (68). Low levels of IL-31 might predict poor efficacy of dupilumab treatment (24).
In a study based on Chinese patients, low levels of IL-36β might predict poor efficacy of dupilumab treatment (24). But, a study based on European populations has found that IL-36β levels in skin lesions of patients with AD are not increased, which may be related to cytokine profiles in the population. Even studies based on Asian populations have had conflicting results on baseline serum levels of IL-36β in patients with AD (24, 95). So the generalizability of IL-36β is still open to question.
Indoleamine 2,3-dioxygenase expression and activity in langerhans cells seems involved in the pathophysiology of eczema herpeticum in AD and could represent a predictive biomarker for patients with risk to develop eczema herpeticum and other viral complications (96).
High serum periostin and dipeptidyl peptidase-4 levels in AD patients have been reported as significant biomarkers to predict a good response to anti-IL-13 (tralokinumab) treatment (97).
Meteorin-like protein (METRNL) which is a cytokine known for its anti-inflammatory properties is significantly upregulated in the lesional skin and serum of both AD mice and patients (98). Murine studies suggest that the activation of WNT/β-catenin signaling can initiate the differentiation and expansion of CD103 + DCs (99), hamper DCs recruitment (100), decrease mast cells and pro-inflammatory factor levels (101), and drive keratinocyte proliferation for tissue repair (102). Additionally, study in murine models indicates that METRNL can bind to KIT receptor, a receptor tyrosine kinase type III (103), to mitigate allergic inflammation in AD. This effect is achieved by inhibiting immune cell expansion, enhancing β-catenin activation, and downregulating Th2-related gene expression, including IL-4, IL-6, and Arginase-1 mRNA expression. Consequently, the accumulation of Arg1hi macrophages, DCs, and activated mast cells, as well as the levels of IL-4 and IL-6, are reduced (98) (Figure 1). METRNL appears to positively affect patient outcomes, its precise mechanisms require further investigation.
Ceramides in the SC play critical roles in cutaneous barrier function. A recent study found that the carbon chain lengths of ceramides containing nonhydroxy fatty acids and dihydrosphingosines (NDS), ceramides containing nonhydroxy fatty acids and sphingosines (NS), and ceramides containing nonhydroxy fatty acids and 6-hydroxysphingosines (NH) were shorter in the exacerbated AD compared to those in the maintained AD (104). Thus, SC ceramide profiling, particularly NDS, NS, and NH chain lengths, may serve as potential biomarkers for AD remission and predicting future exacerbations (104). Despite its promise, the clinical application of SC ceramide profiling remains under investigation. While liquid chromatography-mass spectrometry provides precise ceramide analysis, its use in routine practice is limited by cost, equipment requirements, and technical expertise. Future advancements in non-invasive, cost-effective analytical methods will be crucial for its integration into dermatological assessments. Combining SC ceramide profiling with other biomarkers, such as TARC, may further enhance its clinical utility, allowing for timely, objective treatment adjustments.
Stratum corneum hydration (SCH) is one of the commonly used parameters to evaluate skin barrier function (105). SCH levels in lesional skin have shown moderate predictive value for sensitization to food allergens in children with AD (106). We observed that lower increases in SCH on noninvolved skin and on eczematous lesions are factors that predict failure to dupilumab, independently of eczema area and severity index at baseline and treatment duration (107). As a non-invasive and simple method, SCH measurement of skin barrier function is safer and has shown that epidermal changes could be happening before clinical changes (108), making it a valuable predictor of treatment outcomes.
6 NK and NKG2D
Reduced frequency and impaired cytolytic function of circulating natural killer (NK) cells contribute to AD pathogenesis (109). Murine studies suggest that NK cells mitigate eczema and allergic inflammation by counteracting type 2 inflammation via interferon-γ (110), while NK cell deficiency exacerbates skin inflammation (109). Notably, IL-15 superagonist therapy has been shown to improve AD-like disease in mice (109), underscoring NK cell modulation as a potential immunotherapeutic strategy. AD patients exhibit NK cell deficiencies with diagnostic value that improve with treatment. A longitudinal study of children with AD found that more severe disease and increased allergen sensitivity were associated with a selective loss of NKG2D expression on NK cells, while other immune cells remained unaffected. Additionally, decreased NKG2D expression on NK cells was associated with impaired cytolytic function but an increased release of the proinflammatory cytokine tumor necrosis factor-α. Furthermore, lower NKG2D expression correlated with compromised skin barrier integrity, as indicated by increased transepidermal water loss. Reduced NKG2D expression also impaired the ability of NK cells to combat infections. Moreover, NKG2D expression on NK cells was inversely associated with AD severity and the presence of allergic comorbidities. NK cell dysregulation in AD may play a role in the progression of the atopic march (111). Low NKG2D expression provides novel cytological insights into AD initiation and progression, which may have adverse effects on prognosis.
An in-depth analysis of biomarkers in relation to the established factors contributing to poor prognosis in AD is essential. Biomarkers such as TARC, CCL18, and LDH levels are closely linked to disease severity, relapse rates, and treatment efficacy. Elevated TARC and CCL18 levels have been associated with persistent inflammation and inadequate treatment response, while high LDH and IgE levels predict poorer long-term outcomes. These biomarkers reflect the influence of environmental triggers, infections, and impaired skin barrier, which exacerbate AD. Understanding the interplay between these factors and biomarkers can guide personalized treatment strategies for AD, improving patient prognosis.
7 Discussion
In summary, mutations in FLG, SPINK5, STAT, KIF3A, claudin-1, Ovol1, and HLA-DRB1 genes contribute to AD susceptibility, persistence, and severity. These genetic factors affect skin barrier function, immune responses, and disease progression, highlighting the complexity of AD’s genetic basis. AD involves various endotypes with distinct inflammatory pathways, influenced by disease stage, age, and ethnicity. Obesity, environmental factors, infections, and psychological stress exacerbate the condition. Adherence enhancement, patient education, and the management of comorbidities like infections are key to improving AD outcomes. Biomarkers not only assist in assessing the severity of AD but also predict the long-term prognosis of patients, offering more precise individualized treatment strategies. A comprehensive assessment combining multiple biomarkers can help clinicians manage AD more effectively, improve treatment outcomes, and reduce the risk of relapse.
Author contributions
ZL: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. MG: Writing – original draft. YL: Supervision, Writing – review & editing. HX: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Glickman, JW, Dubin, C, Han, J, Dahabreh, D, Garcet, S, Krueger, JG, et al. Comparing cutaneous molecular improvement with different treatments in atopic dermatitis patients. J Allergy Clin Immunol. (2020) 145:1285–8. doi: 10.1016/j.jaci.2020.01.005
3. Li, H, Zhang, Z, Zhang, H, Guo, Y, and Yao, Z. Update on the pathogenesis and therapy of atopic dermatitis. Clin Rev Allergy Immunol. (2021) 61:324–38. doi: 10.1007/s12016-021-08880-3
4. Goh, MS, Yun, JS, and Su, JC. Management of atopic dermatitis: a narrative review. Med J Aust. (2022) 216:587–93. doi: 10.5694/mja2.51560
5. Zhang, L, Chen, N, Ye, J, and Lin, H. Evaluation of the efficacy of intervention in the management of AD chronic disease based on the theory of “prevention of the recrudescence of disease”. Technol Health Care. (2024) 32:107–14. doi: 10.3233/THC-248009
6. Kim, JE, Shin, MK, Park, G-H, Lee, UH, Lee, JH, Han, T-Y, et al. 2019 consensus Korean diagnostic guidelines to define severity classification and treatment refractoriness for atopic dermatitis: objective and subjective assessment of severity. Ann Dermatol. (2019) 31:654–61. doi: 10.5021/ad.2019.31.6.654
7. Chiesa Fuxench, ZC. Atopic dermatitis: disease background and risk factors. Adv Exp Med Biol. (2017) 1027:11–9. doi: 10.1007/978-3-319-64804-0_2
8. Tsakok, T, Woolf, R, Smith, CH, Weidinger, S, and Flohr, C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol. (2019) 180:464–74. doi: 10.1111/bjd.16934
9. Cabanillas, B, and Novak, N. Atopic dermatitis and filaggrin. Curr Opin Immunol. (2016) 42:1–8. doi: 10.1016/j.coi.2016.05.002
10. Margolis, DJ, Apter, AJ, Gupta, J, Hoffstad, O, Papadopoulos, M, Campbell, LE, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. (2012) 130:912–7. doi: 10.1016/j.jaci.2012.07.008
11. Margolis, DJ, Gupta, J, Apter, AJ, Ganguly, T, Hoffstad, O, Papadopoulos, M, et al. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. (2014) 133:784–9. doi: 10.1016/j.jaci.2013.09.015
12. Dežman, K, Korošec, P, Rupnik, H, and Rijavec, M. SPINK5 is associated with early-onset and CHI3L1 with late-onset atopic dermatitis. Int J Immunogenet. (2017) 44:212–8. doi: 10.1111/iji.12327
13. Wilson, BE, Grebe, TA, Prakash, S, and Bauer, CS. Systemic JAK inhibitors for treatment of cutaneous manifestations in a patient with SPINK5 variants: a case report and review of the literature. Pediatr Allergy Immunol. (2025) 36:e70039. doi: 10.1111/pai.70039
14. Kasap, N, Aslan, K, Karakurt, LT, Bozkurt, H, Canatan, H, Cavkaytar, O, et al. A novel gain-of-function mutation in STAT5B is associated with treatment-resistant severe atopic dermatitis. Clin Exp Allergy. (2022) 52:907–10. doi: 10.1111/cea.14148
15. Takeuchi, I, Yanagi, K, Takada, S, Uchiyama, T, Igarashi, A, Motomura, K, et al. STAT6 gain-of-function variant exacerbates multiple allergic symptoms. J Allergy Clin Immunol. (2023) 151:1402–1409.e6. doi: 10.1016/j.jaci.2022.12.802
16. Stevens, ML, Zhang, Z, Johansson, E, Ray, S, Jagpal, A, Ruff, BP, et al. Disease-associated KIF3A variants alter gene methylation and expression impacting skin barrier and atopic dermatitis risk. Nat Commun. (2020) 11:4092. doi: 10.1038/s41467-020-17895-x
17. De Benedetto, A, Rafaels, NM, McGirt, LY, Ivanov, AI, Georas, SN, Cheadle, C, et al. Tight junction defects in atopic dermatitis. J Allergy Clin Immunol. (2011) 127:773–786.e7. doi: 10.1016/j.jaci.2010.10.018
18. Jin, S-P, Han, SB, Kim, YK, Park, EE, Doh, EJ, Kim, KH, et al. Changes in tight junction protein expression in intrinsic aging and photoaging in human skin in vivo. J Dermatol Sci. (2016) 84:99–101. doi: 10.1016/j.jdermsci.2016.07.002
19. Tsuji, G, Ito, T, Chiba, T, Mitoma, C, Nakahara, T, Uchi, H, et al. The role of the OVOL1-OVOL2 axis in normal and diseased human skin. J Dermatol Sci. (2018) 90:227–31. doi: 10.1016/j.jdermsci.2018.02.005
20. Tsuji, G, Hashimoto-Hachiya, A, Kiyomatsu-Oda, M, Takemura, M, Ohno, F, Ito, T, et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. (2017) 8:e2931. doi: 10.1038/cddis.2017.322
21. Chen, Z, Dragan, M, Sun, P, Haensel, D, Vu, R, Cui, L, et al. The AhR-Ovol1-Id1 regulatory axis in keratinocytes promotes epidermal and immune homeostasis in atopic dermatitis-like skin inflammation. Cell Mol Immunol. (2025) 22:300–15. doi: 10.1038/s41423-025-01264-z
22. Park, H, Ahn, K, Park, MH, and Lee, SI. The HLA-DRB1 polymorphism is associated with atopic dermatitis, but not egg allergy in Korean children. Allergy Asthma Immunol Res. (2012) 4:143–9. doi: 10.4168/aair.2012.4.3.143
23. Margolis, DJ, Mitra, N, Kim, B, Gupta, J, Hoffstad, OJ, Papadopoulos, M, et al. Association of HLA-DRB1 genetic variants with the persistence of atopic dermatitis. Hum Immunol. (2015) 76:571–7. doi: 10.1016/j.humimm.2015.08.003
24. Wu, Y, Gu, C, Wang, S, Yin, H, Qiu, Z, Luo, Y, et al. Serum biomarker-based endotypes of atopic dermatitis in China and prediction for efficacy of dupilumab. Br J Dermatol. (2023) 188:649–60. doi: 10.1093/bjd/ljad032
25. Czarnowicki, T, He, H, Krueger, JG, and Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. (2019) 143:1–11. doi: 10.1016/j.jaci.2018.10.032
26. Wang, S, Zhu, R, Gu, C, Zou, Y, Yin, H, Xu, J, et al. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J Eur Acad Dermatol Venereol. (2020) 34:2346–52. doi: 10.1111/jdv.16346
27. Gu, C, Wu, Y, Luo, Y, Wang, S, Yin, H, Gao, Y, et al. Real-world efficacy and safety of dupilumab in Chinese patients with atopic dermatitis: a single-Centre, prospective, open-label study. J Eur Acad Dermatol Venereol. (2022) 36:1064–73. doi: 10.1111/jdv.18109
28. Wen, H-C, Czarnowicki, T, Noda, S, Malik, K, Pavel, AB, Nakajima, S, et al. Serum from Asian patients with atopic dermatitis is characterized by TH2/TH22 activation, which is highly correlated with nonlesional skin measures. J Allergy Clin Immunol. (2018) 142:324–328.e11. doi: 10.1016/j.jaci.2018.02.047
29. Seremet, T. New bricks in the wall regarding the link between obesity and atopic dermatitis. J Eur Acad Dermatol Venereol. (2025) 39:27–8. doi: 10.1111/jdv.20403
30. Dopytalska, K, Baranowska-Bik, A, Roszkiewicz, M, Bik, W, and Walecka, I. The role of leptin in selected skin diseases. Lipids Health Dis. (2020) 19:215. doi: 10.1186/s12944-020-01391-8
31. Jiménez-Cortegana, C, Ortiz-García, G, Serrano, A, Moreno-Ramírez, D, and Sánchez-Margalet, V. Possible role of leptin in atopic dermatitis: a literature review. Biomol Ther. (2021) 11:1642. doi: 10.3390/biom11111642
32. Patruno, C, Potestio, L, Cecere, D, Cosenza, A, Brescia, C, and Napolitano, M. The impact of body mass index on dupilumab treatment outcomes in adult atopic dermatitis patients. J Eur Acad Dermatol Venereol. (2024) 39:e71–4. doi: 10.1111/jdv.20111
33. Kato, A, Kamata, M, Ito, M, Uchida, H, Nagata, M, Fukaya, S, et al. Higher baseline serum lactate dehydrogenase level is associated with poor effectiveness of dupilumab in the long term in patients with atopic dermatitis. J Dermatol. (2020) 47:1013–9. doi: 10.1111/1346-8138.15464
34. Melé-Ninot, G, Curto-Barredo, L, Bonfill-Ortí, M, Expósito-Serrano, V, Munera-Campos, M, Figueras Nart, I, et al. Assessment of potential predictive factors of dupilumab response in patients with moderate-to-severe atopic dermatitis. Australas J Dermatol. (2024) 65:153–62. doi: 10.1111/ajd.14196
35. Chu, H, Kim, SM, Zhang, K, Wu, Z, Lee, H, Kim, JH, et al. Head and neck dermatitis is exacerbated by Malassezia furfur colonization, skin barrier disruption, and immune dysregulation. Front Immunol. (2023) 14:1114321. doi: 10.3389/fimmu.2023.1114321
36. Chong, AC, Navarro-Triviño, FJ, Su, M, and Park, CO. Fungal head and neck dermatitis: current understanding and management. Clin Rev Allergy Immunol. (2024) 66:363–75. doi: 10.1007/s12016-024-09000-7
37. Kozera, E, Stewart, T, Gill, K, De La Vega, MA, and Frew, JW. Dupilumab-associated head and neck dermatitis is associated with elevated pretreatment serum Malassezia-specific IgE: a multicentre, prospective cohort study. Br J Dermatol. (2022) 186:1050–2. doi: 10.1111/bjd.21019
38. Darabi, K, Hostetler, SG, Bechtel, MA, and Zirwas, M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. (2009) 60:125–36. doi: 10.1016/j.jaad.2008.07.058
39. Gori, N, Ippoliti, E, Peris, K, and Chiricozzi, A. Head and neck atopic dermatitis: still a challenging manifestation in the biologic era. Expert Opin Biol Ther. (2023) 23:575–7. doi: 10.1080/14712598.2023.2224499
40. Uchida, H, Kamata, M, Kato, A, Mizukawa, I, Watanabe, A, Agematsu, A, et al. One-year real-world clinical effectiveness, safety, and laboratory safety of dupilumab in Japanese adult patients with atopic dermatitis: a single-center retrospective study. J Am Acad Dermatol. (2021) 84:547–50. doi: 10.1016/j.jaad.2020.05.102
41. See Tow, HX, and Yew, YW. Malassezia specific IgE in head and neck dermatitis of eczema: a systematic review & meta-analysis. Exp Dermatol. (2024) 33:e15108. doi: 10.1111/exd.15108
42. Peters, AS, Kellberger, J, Vogelberg, C, Dressel, H, Windstetter, D, Weinmayr, G, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. (2010) 126:590–3. doi: 10.1016/j.jaci.2010.06.020
43. Balakirski, G, and Novak, N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. (2022) 149:1185–94. doi: 10.1016/j.jaci.2022.01.010
44. Bláfoss, J, Hansen, AV, Malchau Lauesgaard, SS, Ali, Z, and Ulrik, CS. Female asthma and atopy - impact on fertility: a systematic review. J Asthma Allergy. (2019) 12:205–11. doi: 10.2147/JAA.S203576
45. Farmer, WS, and Marathe, KS. Atopic dermatitis: managing the itch. Adv Exp Med Biol. (2024) 1447:191–207. doi: 10.1007/978-3-031-54513-9_16
46. Katoh, N, Ohya, Y, Ikeda, M, Ebihara, T, Katayama, I, Saeki, H, et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int. (2020) 69:356–69. doi: 10.1016/j.alit.2020.02.006
47. Boothe, WD, Tarbox, JA, and Tarbox, MB. Atopic dermatitis: pathophysiology In: SR Feldman, LC Strowd, and KK Lovell, editors. Management of Atopic Dermatitis. Advances in experimental medicine and biology. Cham: Springer International Publishing (2024). 21–35.
48. Napolitano, M, Esposito, M, Fargnoli, MC, Girolomoni, G, Romita, P, Nicoli, E, et al. Infections in patients with atopic dermatitis and the influence of treatment. Am J Clin Dermatol. (2025) 26:183–97. doi: 10.1007/s40257-025-00917-z
49. Lee, Y-J, Yassa, C, Park, S-H, Song, SW, Jung, WH, Lee, YW, et al. Interactions between Malassezia and new therapeutic agents in atopic dermatitis affecting skin barrier and inflammation in recombinant human epidermis model. Int J Mol Sci. (2023) 24:6171. doi: 10.3390/ijms24076171
50. Katsarou, A, and Armenaka, M. Atopic dermatitis in older patients: particular points. J Eur Acad Dermatol Venereol. (2011) 25:12–8. doi: 10.1111/j.1468-3083.2010.03737.x
51. Patel, N, and Feldman, S. Adherence in atopic dermatitis. Adv Exp Med Biol. (2024) 1447:169–90. doi: 10.1007/978-3-031-54513-9_15
52. Fitzmaurice, W, and Silverberg, NB. Systematic review of steroid phobia in atopic dermatitis®. Dermatitis. (2024) 35:664–8. doi: 10.1089/derm.2023.0213
53. Larney, C, Courtney, A, Yazdabadi, A, and Su, JC. Topical steroid withdrawal: an emerging challenge in the treatment of atopic dermatitis. J Paediatr Child Health. (2025). doi: 10.1111/jpc.70018 [Epub ahead of print].
54. Sy, W, Bhayana, M, and Lamb, AJ. Atopic dermatitis disease education. Adv Exp Med Biol. (2024) 1447:209–15. doi: 10.1007/978-3-031-54513-9_17
55. Schettini, N, Pacetti, L, Corazza, M, and Borghi, A. The role of OX40-OX40L Axis in the pathogenesis of atopic dermatitis. Dermatitis. (2024) 36:28–36. doi: 10.1089/derm.2024.0058
56. Nakanishi, K, Yoshimoto, T, Tsutsui, H, and Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. (2001) 19:423–74. doi: 10.1146/annurev.immunol.19.1.423
57. Ricardo-Gonzalez, RR, Van Dyken, SJ, Schneider, C, Lee, J, Nussbaum, JC, Liang, H-E, et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol. (2018) 19:1093–9. doi: 10.1038/s41590-018-0201-4
58. Angkasekwinai, P, and Dong, C. IL-9-producing T cells: potential players in allergy and cancer. Nat Rev Immunol. (2021) 21:37–48. doi: 10.1038/s41577-020-0396-0
59. Micossé, C, von Meyenn, L, Steck, O, Kipfer, E, Adam, C, Simillion, C, et al. Human “TH9” cells are a subpopulation of PPAR-γ+ TH2 cells. Sci Immunol. (2019) 4:eaat5943. doi: 10.1126/sciimmunol.aat5943
60. Schärli, S, Luther, F, Di Domizio, J, Hillig, C, Radonjic-Hoesli, S, Thormann, K, et al. IL-9 sensitizes human TH2 cells to proinflammatory IL-18 signals in atopic dermatitis. J Allergy Clin Immunol. (2024) 155:491–504.e9. doi: 10.1016/j.jaci.2024.10.027
61. Jang, Y-S, Lee, K, Park, M, Joo Park, J, Choi, GM, Kim, C, et al. Albumin-binding recombinant human IL-18BP ameliorates macrophage activation syndrome and atopic dermatitis via direct IL-18 inactivation. Cytokine. (2023) 172:156413. doi: 10.1016/j.cyto.2023.156413
62. Oh, CK, Leigh, R, McLaurin, KK, Kim, K, Hultquist, M, and Molfino, NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res. (2013) 14:93. doi: 10.1186/1465-9921-14-93
63. Gupta, RK, Figueroa, DS, Ay, F, Causton, B, Abdollahi, S, and Croft, M. Comparison of CD30L and OX40L reveals CD30L as a promising therapeutic target in atopic dermatitis. Allergy. (2024) 80:500–12. doi: 10.1111/all.16412
64. Gaspal, F, Bekiaris, V, Kim, M-Y, Withers, DR, Bobat, S, MacLennan, ICM, et al. Critical synergy of CD30 and OX40 signals in CD4 T cell homeostasis and Th1 immunity to Salmonella. J Immunol. (2008) 180:2824–9. doi: 10.4049/jimmunol.180.5.2824
65. Gaspal, FMC, Kim, M-Y, McConnell, FM, Raykundalia, C, Bekiaris, V, and Lane, PJL. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. (2005) 174:3891–6. doi: 10.4049/jimmunol.174.7.3891
66. Antosz, K, Batko, J, Błażejewska, M, Gawor, A, Sleziak, J, and Gomułka, K. Insight into IL-5 as a potential target for the treatment of allergic diseases. Biomedicines. (2024) 12:1531. doi: 10.3390/biomedicines12071531
67. Hawro, T, Saluja, R, Weller, K, Altrichter, S, Metz, M, and Maurer, M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. (2014) 69:113–7. doi: 10.1111/all.12316
68. Lovell, K, Patel, N, Rao, S, and Strowd, LC. The future of atopic dermatitis treatment. Adv Exp Med Biol. (2024) 1447:227–44. doi: 10.1007/978-3-031-54513-9_19
69. Kiiski, V, Karlsson, O, Remitz, A, and Reitamo, S. High serum total IgE predicts poor long-term outcome in atopic dermatitis. Acta Derm Venereol. (2015) 95:943–7. doi: 10.2340/00015555-2126
70. Bakker, D, de Bruin-Weller, M, Drylewicz, J, van Wijk, F, and Thijs, J. Biomarkers in atopic dermatitis. J Allergy Clin Immunol. (2023) 151:1163–8. doi: 10.1016/j.jaci.2023.01.019
71. Krohn, K I, FMS, B, Herrmann, N, Maintz, L, De Vriese, S, Ring, J, et al. Immunoglobulin E autoantibodies in atopic dermatitis associate with Type-2 comorbidities and the atopic march. Allergy. (2023) 78:3178–92. doi: 10.1111/all.15822
72. Heil, PM, Maurer, D, Klein, B, Hultsch, T, and Stingl, G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. (2010) 8:990–8. doi: 10.1111/j.1610-0387.2010.07497.x
73. Tai, Y, Sakaida, Y, Kawasaki, R, Kanemaru, K, Akimoto, K, Brombacher, F, et al. Foxp3 and Bcl6 deficiency synergistically induces spontaneous development of atopic dermatitis-like skin disease. Int Immunol. (2023) 35:423–35. doi: 10.1093/intimm/dxad018
74. Renert-Yuval, Y, Thyssen, JP, Bissonnette, R, Bieber, T, Kabashima, K, Hijnen, D, et al. Biomarkers in atopic dermatitis-a review on behalf of the international eczema Council. J Allergy Clin Immunol. (2021) 147:1174–1190.e1. doi: 10.1016/j.jaci.2021.01.013
75. Kataoka, Y. Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J Dermatol. (2014) 41:221–9. doi: 10.1111/1346-8138.12440
76. Radonjic-Hoesli, S, Pavlov, N, Simon, H-U, and Simon, D. Are blood cytokines reliable biomarkers of allergic disease diagnosis and treatment responses? J Allergy Clin Immunol. (2022) 150:251–8. doi: 10.1016/j.jaci.2022.06.008
77. Saito, R, Tanaka, A, Hiragun, T, Iwamoto, K, Takahagi, S, Takahashi, M, et al. Reduction of serum thymus and activation-regulated chemokine after short-term intensive therapy may predict better prognosis of moderate to severe atopic dermatitis. J Dermatol. (2019) 46:e486–7. doi: 10.1111/1346-8138.15036
78. Halling, A-S, Rinnov, MR, Ruge, IF, Gerner, T, Ravn, NH, Knudgaard, MH, et al. Skin TARC/CCL17 increase precedes the development of childhood atopic dermatitis. J Allergy Clin Immunol. (2023) 151:1550–1557.e6. doi: 10.1016/j.jaci.2022.11.023
79. Mastraftsi, S, Vrioni, G, Bakakis, M, Nicolaidou, E, Rigopoulos, D, Stratigos, AJ, et al. Atopic dermatitis: striving for reliable biomarkers. J Clin Med. (2022) 11:4639. doi: 10.3390/jcm11164639
80. Li, Z, Wang, Y, Wu, Y, Yin, H, Wang, S, Wu, H, et al. Real-world efficacy and safety of Abrocitinib in Chinese atopic dermatitis patients: a single-center prospective study. Allergy. (2025). doi: 10.1111/all.16495 [Epub ahead of print].
81. Yoo, J, Omori, M, Gyarmati, D, Zhou, B, Aye, T, Brewer, A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. (2005) 202:541–9. doi: 10.1084/jem.20041503
82. Wang, Q, Du, J, Zhu, J, Yang, X, and Zhou, B. Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol. (2015) 135:781–791.e3. doi: 10.1016/j.jaci.2014.09.015
83. Wang, Y-H, Ito, T, Wang, Y-H, Homey, B, Watanabe, N, Martin, R, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. (2006) 24:827–38. doi: 10.1016/j.immuni.2006.03.019
84. Lee, Y, Kim, M-E, and Nahm, D-H. Real clinical practice data of monthly Dupilumab therapy in adult patients with moderate-to-severe atopic dermatitis: clinical efficacy and predictive markers for a favorable clinical response. Allergy Asthma Immunol Res. (2021) 13:733–45. doi: 10.4168/aair.2021.13.5.733
85. Glickman, JW, Han, J, Garcet, S, Krueger, JG, Pavel, AB, and Guttman-Yassky, E. Improving evaluation of drugs in atopic dermatitis by combining clinical and molecular measures. J Allergy Clin Immunol Pract. (2020) 8:3622–3625.e19. doi: 10.1016/j.jaip.2020.07.015
86. Lauffer, F, Baghin, V, Standl, M, Stark, SP, Jargosch, M, Wehrle, J, et al. Predicting persistence of atopic dermatitis in children using clinical attributes and serum proteins. Allergy. (2021) 76:1158–72. doi: 10.1111/all.14557
87. Lee, J, Kim, B, Chu, H, Zhang, K, Kim, H, Kim, JH, et al. FABP5 as a possible biomarker in atopic march: FABP5-induced Th17 polarization, both in mouse model and human samples. EBioMedicine. (2020) 58:102879. doi: 10.1016/j.ebiom.2020.102879
88. Lee, E, Lee, SH, Kwon, JW, Kim, YH, Cho, HJ, Yang, SI, et al. Atopic dermatitis phenotype with early onset and high serum IL-13 is linked to the new development of bronchial hyperresponsiveness in school children. Allergy. (2016) 71:692–700. doi: 10.1111/all.12844
89. Noh, G, Jin, H, Lee, J, Noh, J, Lee, WM, and Lee, S. Eosinophilia as a predictor of food allergy in atopic dermatitis. Allergy Asthma Proc. (2010) 31:18–24. doi: 10.2500/aap.2010.31.3312
90. Simpson, EL, Irvine, AD, Eichenfield, LF, and Friedlander, SF. Update on epidemiology, diagnosis, and disease course of atopic dermatitis. Semin Cutan Med Surg. (2016) 35:S84–8. doi: 10.12788/j.sder.2016.041
91. Rosenberg, HF, Dyer, KD, and Foster, PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. (2013) 13:9–22. doi: 10.1038/nri3341
92. Kiehl, P, Falkenberg, K, Vogelbruch, M, and Kapp, A. Tissue eosinophilia in acute and chronic atopic dermatitis: a morphometric approach using quantitative image analysis of immunostaining. Br J Dermatol. (2001) 145:720–9. doi: 10.1046/j.1365-2133.2001.04456.x
93. Yang, D, Chen, Q, Su, SB, Zhang, P, Kurosaka, K, Caspi, RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. (2008) 205:79–90. doi: 10.1084/jem.20062027
94. Kim, HS, Kim, JH, Seo, YM, Chun, YH, Yoon, J-S, Kim, HH, et al. Eosinophil-derived neurotoxin as a biomarker for disease severity and relapse in recalcitrant atopic dermatitis. Ann Allergy Asthma Immunol. (2017) 119:441–5. doi: 10.1016/j.anai.2017.06.022
95. Komaki, R, Miyagaki, T, Tanaka, M, Nakajima, K, Okano, T, Takeuchi, S, et al. Increased interleukin-36β expression promotes angiogenesis in Japanese atopic dermatitis. Int J Mol Sci. (2023) 24:11104. doi: 10.3390/ijms241311104
96. Staudacher, A, Hinz, T, Novak, N, von Bubnoff, D, and Bieber, T. Exaggerated IDO1 expression and activity in Langerhans cells from patients with atopic dermatitis upon viral stimulation: a potential predictive biomarker for high risk of eczema herpeticum. Allergy. (2015) 70:1432–9. doi: 10.1111/all.12699
97. Libon, F, Caron, J, and Nikkels, AF. Biomarkers in atopic dermatitis. J Allergy Clin Immunol. (2024) 14:1729–38. doi: 10.1007/s13555-024-01193-1
98. Huang, D, Liu, X, Gao, X, Choi, CK, Giglio, G, Farah, L, et al. Meteorin-like protein/METRNL/Interleukin-41 ameliorates atopic dermatitis-like inflammation. Allergy. (2024) 80:474–88. doi: 10.1111/all.16150
99. Cohen, SB, Smith, NL, McDougal, C, Pepper, M, Shah, S, Yap, GS, et al. β-Catenin signaling drives differentiation and Proinflammatory function of IRF8-dependent dendritic cells. J Immunol. (2015) 194:210–22. doi: 10.4049/jimmunol.1402453
100. Mortezaee, K. WNT/β-catenin regulatory roles on PD-(L)1 and immunotherapy responses. Clin Exp Med. (2024) 24:15. doi: 10.1007/s10238-023-01274-z
101. Fang, W, Song, Q, Lv, T, Lv, J, Cai, Z, Wang, Z, et al. Serpina3n/serpina3 alleviates cyclophosphamide-induced interstitial cystitis by activating the Wnt/β-catenin signal. Int Urol Nephrol. (2023) 55:3065–75. doi: 10.1007/s11255-023-03726-7
102. Wang, Y, Ding, H, Bai, R, Li, Q, Ren, B, Lin, P, et al. Exosomes from adipose-derived stem cells accelerate wound healing by increasing the release of IL-33 from macrophages. Stem Cell Res Ther. (2025) 16:80. doi: 10.1186/s13287-025-04203-x
103. Reboll, MR, Klede, S, Taft, MH, Cai, C-L, Field, LJ, Lavine, KJ, et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science. (2022) 376:1343–7. doi: 10.1126/science.abn3027
104. Sho, Y, Sakai, T, Sato, T, Sonezaki, M, Taima, H, Taguchi, H, et al. Stratum Corneum ceramide profiles provide reliable indicators of remission and potential flares in atopic dermatitis. J Invest Dermatol. (2022) 142:3184–3191.e7. doi: 10.1016/j.jid.2022.06.012
105. Pretel-Lara, C, Sanabria-de la Torre, R, Arias-Santiago, S, and Montero-Vilchez, T. Skin barrier function and microtopography in patients with atopic dermatitis. J Clin Med. (2024) 13:5861. doi: 10.3390/jcm13195861
106. Tran, NLH, Ly, NTM, Trinh, HKT, Le, MK, Vo, NVT, and Pham, DL. Prediction of food sensitization in children with atopic dermatitis based on disease severity and epidermal layer impairment. Int Arch Allergy Immunol. (2024) 185:43–55. doi: 10.1159/000533492
107. Montero-Vilchez, T, Rodriguez-Pozo, J-A, Cuenca-Barrales, C, Sanabria-de-la-Torre, R, Torres-de-Pinedo, J-M, and Arias-Santiago, S. Stratum Corneum hydration as a potential marker of response to Dupilumab in atopic dermatitis®: a prospective observational study. Dermatitis. (2024) 35:250–7. doi: 10.1089/derm.2023.0176
108. Ferrucci, S, Romagnuolo, M, Maronese, CA, Germiniasi, F, Tavecchio, S, Angileri, L, et al. Skin barrier status during dupilumab treatment in patients with severe atopic dermatitis. Ther Adv Chronic Dis. (2021) 12:20406223211058332. doi: 10.1177/20406223211058332
109. Mack, MR, Brestoff, JR, Berrien-Elliott, MM, Trier, AM, Yang, T-LB, McCullen, M, et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci Transl Med. (2020) 12:eaay1005. doi: 10.1126/scitranslmed.aay1005
110. Bi, J, Cui, L, Yu, G, Yang, X, Chen, Y, and Wan, X. NK cells alleviate lung inflammation by negatively regulating group 2 innate lymphoid cells. J Immunol. (2017) 198:3336–44. doi: 10.4049/jimmunol.1601830
111. Ochayon, DE, DeVore, SB, Chang, W-C, Krishnamurthy, D, Seelamneni, H, Grashel, B, et al. Progressive accumulation of hyperinflammatory NKG2Dlow NK cells in early childhood severe atopic dermatitis. Sci Immunol. (2024) 9:eadd3085. doi: 10.1126/sciimmunol.add3085
Glossary
AD - Atopic dermatitis
EDN - Eosinophil-derived neurotoxin
FLG - Filaggrin gene
HLA - Human leukocyte antigen
HND - Head and neck dermatitis
IgE - Immunoglobin E
IL - Interleukin
IL-18R - Interleukin-18 receptor
KIF3A - Kinesin family number 3A
METRNL - Meteorin-like protein
NDS - Ceramides containing nonhydroxy fatty acids and dihydrosphingosines
NH - Ceramides containing nonhydroxy fatty acids and 6-hydroxysphingosines
NK - Natural killer cells
NS - Ceramides containing nonhydroxy fatty acids and sphingosines
SC - Stratum corneum
SCH - Stratum corneum hydration
STAT - Signal transducer and activator of transcription
TARC - Thymus and activation-regulated chemokine
TCS - Topical corticosteroids
Th - T helper cell type
TJs - Tight junctions
TSW - Topical steroid withdrawal syndrome
AhR - Aryl hydrocarbon receptor
CCL - Chemokine C-C motif ligand
DCs - Dendritic cells
EASI - Eczema area and severity index
LDH - Lactate dehydrogenase
MDC - Macrophage-derived chemokine
PARC - Pulmonary and activation-regulated chemokine
VEGF - Vascular endothelial growth factor
Keywords: atopic dermatitis, signal transducer and activator of transcription, general factors, IL-9-IL-18 axis, prognostic biomarkers, ceramide profiles, NKG2D
Citation: Liu Z, Guo M, Li Y and Xu H (2025) A multidimensional analysis of prognostic factors in atopic dermatitis. Front. Med. 12:1554669. doi: 10.3389/fmed.2025.1554669
Edited by:
Luis F. Santamaria-Babí, University of Barcelona, SpainReviewed by:
Xuming Mao, University of Pennsylvania, United StatesOzge Uluckan, Allmiral R&D, Spain
Copyright © 2025 Liu, Guo, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Xu, eHVodWlyYWlhbkB1anMuZWR1LmNu
 Zhenggang Liu
Zhenggang Liu Mengnan Guo
Mengnan Guo Yumei Li
Yumei Li