- 1Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Division of Hematology, Department of Medicine, Blood Research Center, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3Division of Hematology and Oncology, Department of Medicine, The University of Virginia, Charlottesville, VA, United States
- 4Division of Hematology, St. Paul’s Hospital, University of British Columbia, Vancouver, BC, Canada
- 5Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, United States
- 6Division of Hematology and Medical Oncology, Weill Cornell Medicine, New York, NY, United States
- 7Medical Oncology and Hematology, Marin Cancer Care, Greenbrae, CA, United States
- 8University of Washington Medical Center, Seattle, WA, United States
- 9Princess Margaret Cancer Centre, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 10PharmaEssentia Corporation, Taipei, Taiwan
- 11Our Lady of the Lake Medical Center, Baton Rouge, LA, United States
- 12Stony Brook University Medical Center, Stony Brook, NY, United States
- 13Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
- 14Department of Hematology and Oncology, Montefiore Einstein Comprehensive Cancer Center, Albert Einstein College of Medicine, Bronx, NY, United States
- 15Department of Medicine, Duke University School of Medicine, Durham, NC, United States
- 16Astera Cancer Care, East Brunswick, NJ, United States
- 17Dana Farber Cancer Institute/Massachusetts General Hospital, Boston, MA, United States
- 18Fort Wayne Medical Oncology & Hematology, Fort Wayne, IN, United States
- 19Greater Baltimore Medical Center, Baltimore, MD, United States
- 20Division of Hematology and Hematologic Malignancies, University of Calgary, Calgary, AB, Canada
- 21Division of Hematology, Department of Medicine, Washington University School of Medicine, St. Louis, MO, United States
- 22Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, United States
- 23Hematology Section, Department of Internal Medicine, Yale School of Medicine, New Haven, CT, United States
- 24O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham, Birmingham, AL, United States
- 25Hematologic Malignancies and Cellular Therapeutics, University of Kansas Medical Center, Kansas, MO, United States
- 26Division of Hematology-Oncology, Lombardi Comprehensive Cancer Center, Medstar Georgetown University Hospital, Washington, DC, United States
- 27Department of Oncology, McMaster University, Hamilton, ON, Canada
- 28Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, Duarte, CA, United States
- 29PharmaEssentia USA, Burlington, MA, United States
- 30Everest Clinical Research, Markham, ON, Canada
Clinical trial registration: No new drugs have been approved for essential thrombocythemia (ET) treatment since the anagrelide approval in 1997. Ropeginterferon alfa-2b-njft (ropeg) is approved for polycythemia vera, providing a rationale for its use in ET. Its current dosing schema requires dose up-titrations with 50 mcg every 2 weeks and takes approximately 20 weeks to reach a plateau. The goal of this study is to assess the efficacy and safety of ropeg in ET using a higher initial dose and accelerated titration (HDAC) regimen. This is a single-arm, multicenter study in the US and Canada. Patients with ET receive ropeg at 250 mcg on Day 0, 350 mcg at Week 2, and 500 mcg from Week 4 onward with flexibility of dose adjustment. The primary endpoint is: platelets ≤400×109/L, white blood cells <10×109/L, improvement or non-progression of spleen size or major symptoms, and absence of hemorrhagic or thrombotic events, at months 10 and 13. Secondary endpoints include molecular response, safety and tolerability. A total of 91 patients were enrolled with 77 (84.6%) patients in the US and 14 (15.4%) in Canada. The last patient was enrolled on March 28, 2024. JAK2V617F was found in 52 (57.1%) patients while CALR and MPL mutations in 34 (37.4%) and 5 (5.5%), respectively. As of November 12, 2024, the discontinuation rate was 8.8%. The study results will be available in mid-2025. This study will provide efficacy, tolerability and safety, molecular response and quality of life data that will be critical in assessing ropeg for ET treatment.
ClinicalTrials.gov, identifier NCT05482971.
Introduction
Essential thrombocythemia (ET) is a Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) associated with thrombocytosis, symptoms, and increased risk of developing thrombo-hemorrhagic events and transformation to myelofibrosis or acute myeloid leukemia (1, 2). Most cases of ET (~55%) carry the constitutively active point mutation in Janus kinase 2, JAK2V617F. Calreticulin (CALR) and myeloproliferative leukemia virus oncogene (MPL) mutations occur in approximately 15–24 and 4% of ET patients, respectively (1). Treatment decisions in ET are based upon thrombosis risk and symptoms. Patients at lower thrombosis risk are usually managed with aspirin alone while hydroxyurea (HU) is used as the first-line therapy in high-risk or symptomatic patients (3). HU treatment is associated with adverse events (AEs) including fever, rash, stomatitis, gastrointestinal upset, oral and leg ulcers, and increased risk of non-melanoma skin cancer. Patients receiving HU treatment can become intolerant or resistant to therapy, and HU resistance is associated with an increased risk of disease progression and reduced overall survival (4, 5). Anagrelide, an oral imidazoquinazoline derivative, was approved by the US Food and Drug Administration (FDA) to treat ET in 1997 (3). No new drugs have been approved for ET treatment since. Available data suggests that anagrelide therapy does not increase patient overall survival and is associated with toxicity and possibly increases risk of fibrosis progression on long-term follow up (3). Therefore, there is a strong rationale for developing disease-modifying therapies that can not only control hematologic parameters but also reduce mutational allelic burden and potentially provide a survival advantage in the treatment of ET.
Clinical studies suggest that interferon alfa (IFN-α)-based therapies are effective in treating thrombocytosis and leukocytosis in patients with ET or polycythemia vera (PV) (6–8). Recombinant IFN-α has also been shown to reduce JAK2V617F allele burden, inhibit disease progression, and prolong event-free survival and overall survival of patients with PV (9–11). It is recommended by the National Comprehensive Cancer Network (NCCN) for the management of ET, but does not have regulatory approval for this indication (12). The AEs and cumbersome dosing schedules of conventional IFNs have been significantly reduced with polyethylene glycol (PEG)-conjugation technologies (8, 13). Ropeginterferon alfa-2b-njft (ropeg) is a novel PEGylated IFN-based, anti-neoplastic agent which was approved in the US in November 2021 for the treatment of PV regardless of prior treatment, becoming the first approved IFN-based therapy for the treatment of a Philadelphia chromosome-negative MPN (14). Ropeg has a favorable in vivo pharmacokinetic (PK) profile that renders its dosing once biweekly or monthly, improves drug tolerability, and induces durable complete hematologic response (CHR) in the treatment of PV (15–17). In contrast to a high discontinuation rate of 34% due to AEs at 2 years with prior PEGylated IFN-based therapies (18), only 10% of the patients during the ropeg treatment discontinued due to drug-related events over 5 years (19, 20). The standard ropeg dosing schema favors a low starting dose and slow titration in an effort to maximize tolerability and with this strategy, approximately 20–28 weeks of treatment pass before the maximal dose plateau is reached. The dose-exposure-response profile of ropeg is consistent among different ethnic groups and higher PK exposure increases probability of achieving a CHR and reducing the JAK2V617F allele burden in patients with PV (21, 22). The reduction of JAK2V617F allele burden or variant allele frequency (VAF) is being recognized as an indicator of treatment effect in MPNs as it is associated with a lower risk of thrombotic events and prolongation of event-free survival or even overall survival (23–25). Hematologic control of thrombocytosis and leukocytosis is also relevant as their elevations are associated with thrombosis and disease progression (1, 26, 27). As compared to traditional low dose and slow titration regimens, a higher initial dose and accelerated dose titration (HIDAT) regimen of ropeg induces a more rapid and greater rate of CHR and molecular remission of JAK2V617F in patients with PV (28–30). Given its mechanism of action, ropeg could also potentially provide anti-clonal benefits in ET cases including the ~30% of them driven by mutations of CALR or MPL (31). Ropeg is currently being evaluated in a global randomized Phase 3 study, SURPASS-ET (NCT04285086), in patients with ET who are resistant or intolerant to HU under the HIDAT regimen (32).
There is a high unmet clinical need for effective, safe and tolerable treatment options for patients with ET, both HU treatment-naïve and pre-exposed. Ropeg may be a suitable therapy with the ability to provide clinical benefits as measured by the durable clinical/hematologic response with the potential to not only reduce thrombotic complications, but also to prevent progression to post-ET myelofibrosis and/or secondary acute myeloid leukemia.
Methods and analysis
Study design
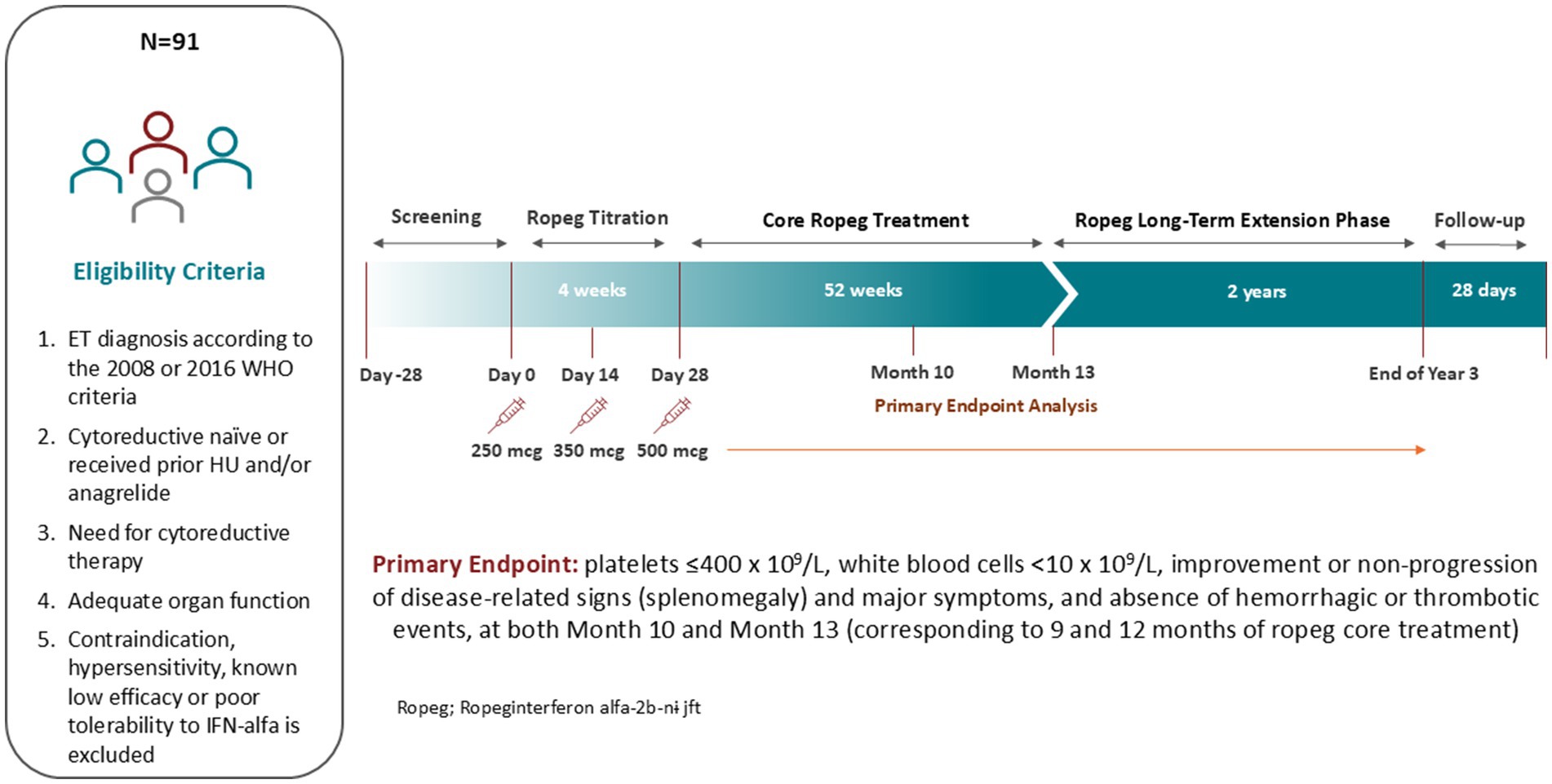
This is a single-arm, multicenter study to evaluate the efficacy, safety, and tolerability of ropeg for ET patients in the US and Canada in need of cytoreductive therapy. The indication for cytoreductive treatment for treatment-naïve patients is defined as progressive leukocytosis and/or thrombocytosis, disease-related symptoms (i.e., pruritus, night sweats, fatigue), vasomotor/microvascular disturbances including headache, chest pain or erythromelalgia that are not responsive to aspirin, history of thrombosis at any age, or age > 60 years with JAK2 mutation. Both treatment-naïve and HU- or anagrelide-pretreated patients are enrolled. The schematic study design is shown in Figure 1.
Selection of patients
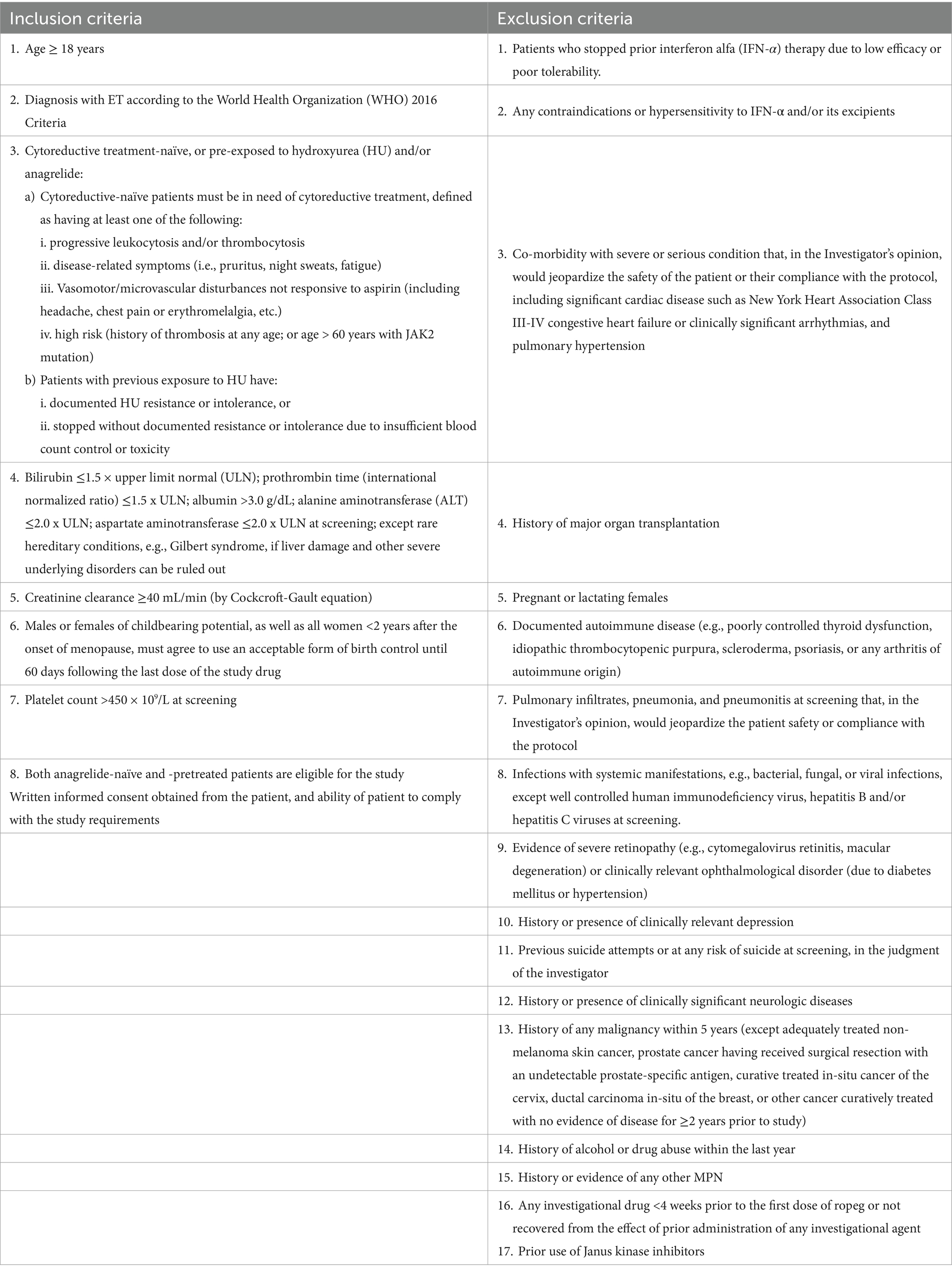
Eligibility criteria include ET diagnosis according to the 2008 or 2016 WHO criteria; adequate organ function defined as bilirubin ≤1.5 × upper limit normal (ULN), prothrombin time or international normalized ratio ≤1.5 x ULN, albumin >3.0 g/dL, alanine aminotransferase ≤2.0 x ULN, aspartate aminotransferase ≤2.0 x ULN, creatinine clearance ≥40 mL/min. Males and females of childbearing potential must agree to use an acceptable form of birth control. Exclusion criteria include any contraindication to IFN-α or prior hypersensitivity, known low efficacy or poor tolerability to IFN-α treatment; severe or serious conditions that may affect the participation, compliance and result assessment; history of major organ transplantation; pregnancy or lactation; history of any malignancy within 5 years not curatively treated; and use of any investigational drug <4 weeks prior to the first dose of study drug or on-going effects of prior administration of any investigational drug. The key inclusion and exclusion criteria are listed in Table 1.
Study treatment
Eligible patients receive ropeg subcutaneously every 2 weeks with the starting dose of 250 mcg on Day 0, 350 mcg at Week 2, and 500 mcg from Week 4 if tolerable. The dose can be adjusted according to safety or tolerability. If 250 mcg leads to toxicities, the following dose reductions are allowed upon discussion and approval by the Sponsor: dose levels −1 (200 mcg), −2 (150 mcg), and − 3 (100 mcg).
Patients entering the study receiving HU or anagrelide must discontinue their treatment prior to the initiation of ropeg therapy on Day 0. After stopping HU or anagrelide, neither drug is allowed to be concomitantly administered with ropeg.
Dose reduction and dose interruption
Dose reduction or interruption of ropeg is recommended in patients experiencing AEs. If major intolerance persists after dose reduction or interruption, therapy should be discontinued. These dose changes, date and time must be recorded on the Dosage Administration Record and AE electronic case report form (eCRF) page.
Dose reduction of ropeg is driven exclusively by safety and tolerability. If a certain dose is poorly tolerated and drug-related toxicities arise, the dose must be reduced to the prior dose, or interrupted as follows:
• If a patient has a severe (Grade 3 or 4) toxicity, or a drop in absolute neutrophil count (ANC) to below 0.5 × 109/L, temporary interruption must be implemented until recovery of the condition (i.e., Grade 0 or 1). Treatment re-initiation occurs from the prior lower dose. For example, if a Grade 3 AE occurs at 500 mcg, treatment re-initiation will take place with 350 mcg. If there is an absence of response (platelet count >400 × 109/L) at this decreased dose after 3 months, the dose can be increased to the prior level.
• If a patient has a Grade 2 toxicity, or a drop in ANC to below 0.75 × 109/L but higher than or equal to 0.5 × 109/L, dose reduction without treatment interruption should be considered.
• Grade 1 toxicity does not lead to dose reduction or interruption.
Assessments and data analysis
Patient visits are scheduled every 14 days (±3 days) during the titration period and the 12-month core study. In the core study, quarterly major assessment visits for the measurement of hematologic parameters and safety are held as in-office visits. Minor assessment visits can be substituted with phone visits under conditions of stable ropeg dose, ability to self-administer, and no need for intensive safety follow-up for AEs. Patients who are benefiting from treatment after the core study can receive ropeg up to a total of 3 years. A safety follow-up, or end of study (EoS) visit takes place 28 days after the end of treatment (EoT) visit.
The primary study endpoint is defined as: platelets ≤400 × 109/L, white blood cells (WBC) <10 × 109/L, improvement or non-progression in disease-related signs (splenomegaly) based on palpation and ultrasound, major symptom improvement or non-progression based on the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS), and absence of hemorrhagic or thrombotic events.
Secondary endpoints include hematologic response, durability of response, change of JAK2, CALR and MPL mutation allelic burden, symptomatic improvement, and occurrence of thromboembolic events and progressive disease.
Evaluation of efficacy includes clinical laboratory assessments, allelic burden measurements of JAK2, CALR, and MPL mutations; spleen size measurements, MPN-SAF TSS completion, and optional bone marrow sampling. Hematologic parameters are assessed by local labs and quantitative measurements of JAK2, CALR, and MPL VAF are performed by a central laboratory. They are assessed quarterly during the core study.
Safety endpoints included incidence, causality and intensity of AEs, serious AEs, and discontinuation of study treatment due to an AE, incidence of AEs of special interest (e.g., thrombotic and bleeding events); incidence of abnormalities of vital signs, clinical laboratory tests, results of physical examinations, electrocardiograms, Eastern Cooperative Oncology Group (ECOG) performance status and Hospital Anxiety and Depression Scale (HADS) score. PK parameters including, but not limited to, minimum concentration (Cmin), time to reach the maximum concentration (Tmax), maximum concentration (Cmax), and area under the drug concentration time curve (AUC) will be derived using population PK analysis and the relationship between exposure and efficacy and safety endpoints will be examined using efficacy and response analysis.
Based on the literature review and analysis of available data from a subset of the ropeg-treated patients with PV whose baseline platelets were ≥ 450 × 109/L, we expect that no treatment has minimal effect in inducing durable response and conservatively estimate a 30% durable response rate by Month 13 as measured at Month 10 and 13 according to the modified ELN response criteria with the ropeg treatment. Assuming a distance from this rate to the lower limit of the 95% one-side confidence interval is ≤10%, the study needs 56 evaluable patients. Further assuming a 12% dropout rate, a total of 64 patients are needed for the study. Final analysis of the primary endpoint will be based on the 13-month dataset. No formal hypothesis is planned to be tested for the extension of the study. Only patients who benefit from 13 months of active treatment and are willing to continue the ropeg treatment are enrolled into the extension. Secondary efficacy analyses will be performed for exploratory purposes using descriptive analysis and standard statistical tests. The secondary analyses are intended to be conducted for all patient subgroups.
Discussion
Recruitment was completed with 91 patients enrolled. 77 (84.6%) patients were enrolled in the US and 14 (15.4%) in Canada with the last patient enrolled on March 28, 2024. The study was initially planned to enroll 64 patients, but 91 eligible patients were enrolled due to rapid enrollment and a high interest level among the investigators and patient community. JAK2V617F was found in 52 (57.1%) patients while CALR and MPL mutations in 34 (37.4%) and 5 (5.5%), respectively. As of November 12, 2024, the discontinuation rate was 8.8%. Therefore, the study will be completed, and results are scheduled to be available in the middle of 2025.
There is a current need for new effective and tolerable therapeutics for ET treatment in the US and Canada, and in particular for therapies that can target neoplastic clones and induce durable hematologic response with the potential to prevent disease progression. HU is commonly used as a first line option, but it has limitations due to known toxicities and lack of disease-modifying effect. There is also a lack of viable second line option since anagrelide is not considered to be a suitable option by many physicians and patients because of the risk for AEs such as cardiovascular toxicity.3 IFN-based treatment has a great potential and has shown clinical efficacy in the treatment of ET.6, 7 Despite not being formally approved, it is a recommended treatment option for ET by the NCCN, European Leukemia Net [ELN], and other treatment guidelines. In almost all ET trials utilizing IFN therapy, patients experienced the normalization of platelet count and correction of leukocytosis as part of the hematologic responses.6 The toxicity associated with initial recombinant IFN preparations was significant, however, leading to a high treatment discontinuation rate. The issue of poor tolerability of IFN-based therapies is addressed by the improved PEGylation technology, as in case of ropeg. The EXCEED ET study will generate valuable data on the efficacy and safety of ropeg for the treatment of patients with ET.
Therefore, the development of a new ET treatment that can target neoplastic clones and induce durable hematologic response with the potential to prevent disease progression is highly needed. This study will contribute to valuable insights into optimizing the dosing strategy that other research efforts are also involved in (33), and together with the ongoing randomized controlled SURPASS-ET study (NCT04285086), help determine the disease-modifying potential of ropeg for the treatment of ET.
Ethics statement
The studies involving humans were approved by University of Texas MD Anderson Cancer Center; Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill; Division of Hematology and Oncology, Department of Medicine, The University of Virginia; Division of Hematology, St. Paul’s Hospital, University of British Columbia; Huntsman Cancer Institute, University of Utah; Division of Hematology and Medical Oncology, Weill Cornell Medicine; Medical Oncology and Hematology, Marin Cancer Care; University of Washington Medical Center; Princess Margaret Cancer Centre, Temerty Faculty of Medicine, University of Toronto; Our Lady of the Lake Medical Center, Baton Rouge; Stony Brook University Medical Center; Keck School of Medicine, University of Southern California; Montefiore Einstein Comprehensive cancer center, Albert Einstein College of Medicine; Duke University School of Medicine; Astera Cancer Care, East Brunswick; Dana Farber Cancer Institute/Massachusetts General Hospital; Fort Wayne Medical Oncology & Hematology; Greater Baltimore Medical Center; Division of Hematology and Hematologic Malignancies, University of Calgary; Washington University School of Medicine; Winship Cancer Institute of Emory University; Yale School of Medicine; O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham; Lombardi Comprehensive Cancer Center, Medstar Georgetown University Hospital; McMaster University; City of Hope National Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. BR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. FC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GA-Z: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AHa: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. DM: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AQ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HS: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. FL: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CO'C: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. SG: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. LR: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. BF: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JH: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. SB: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. ZL: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. SC: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. StO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AHu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. PV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AY: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JC: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CH: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. SaO: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. OZ: Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. HC: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. PB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. PharmaEssentia Corporation is the sponsor of the study and has provided support to the study and the development of this manuscript.
Conflict of interest
LM served on advisory boards for Cogent, GSK, MorphoSys, and PharmaEssentia. BR received consulting fees from PharmaEssentia, CTI BioPharma, and Incyte. FC serves as a consultant for SPD Oncology, Amgen, CTI BioPharma, AbbVie, MorphoSys, and the Association of Community Cancer Centers; received clinical trial grant support (PI) to the University of Virginia from Amgen, BMS, Celgene, SPD Oncology, Sanofi, Bristol Myers Squibb, FibroGen, PharmaEssentia, BioSight, MEI Pharma, Novartis, and Arog Pharmaceuticals; and received a travel grant from DAVA Oncology. LF received honoraria from AbbVie, BMS, GSK, Medison, and Novartis Research, and received funding from AbbVie, BMS, Constellation, CTI Biopharma, Incyte, PharmaEssentia, and Sumitomo Pharma. TT served as a consultant for Blueprint, PharmaEssentia, Cogent, and CITI Biopharma, and received funding from Blueprint, PharmaEssentia, Cogent, Italfarmaco, and Telios. GA-Z received research support from PharmaEssentia. DM received research support from Novartis, Celgene/BMS, PharmaEssentia, Takeda, and Kronos Bio, Inc.; received honoraria from Novartis, Celgene/BMS, and Jazz; served on advisory boards for Novartis, Celgene/BMS, and Jazz; and is a consultant for Pfizer and GSK. CO'C received research funding from Astex and Genentech and served on the scientific advisory board for GSK. AQ serves as chief medical officer of PharmaEssentia Corporation. LR received research funding from Kiadis Pharma. JH serves as a consultant for PharmaEssentia and Merck. SB received honoraria from Janssen Scientific Affairs, Pfizer, Novartis, and Cornerstone Healthcare Group; holds a consulting or advisory role with Cornerstone Specialty Network, E.R. Squibb Sons, LLC, Novartis, Kite (a Gilead company), Taiho Oncology, AstraZeneca, and Janssen Oncology; and received research funding: fromBristol-Myers Squibb, Novartis, Genentech/Roche, AstraZeneca/MedImmune, Janssen Oncology, Amgen, Abbvie, Lilly, Alexion Pharmaceuticals, Merck, Syndax, Nektar, Sanofi, Argenx, Gilead Sciences, BeiGene, Torl Biotherapeutics, 1200 Pharma, Takeda, Aptose Biosciences, and Aptose Biosciences. ZL holds a consulting or advisory role with AstraZeneca and Bristol Myers Squibb. SC served as a speaker and received honoraria from Novartis, Celgene, BMS, Incyte, Pfizer, Takeda, GSK, and Medison Pharma Canada Inc, and received consulting fees from Novartis, Celgene, BMS, Incyte, Pfizer, Abbvie, Medlior Health Outcomes Research, and Medison Pharma Canada Inc. StO served as a consultant for Kartos Therapeutics, CTIBioPharma, Celgene/Bristol Myers Squibb, Disc Medicine, Blueprint Medicines, PharmaEssentia, Constellation, Geron, Abbvie, Sierra Oncology, and Incyte. AHu received honoraria or consulting fees from GSK, Cogent Biosciences, PharmaEssentia, Blueprint Medicines, CTI Biopharma (now Sobi), and Incyte, and received research funding from Incyte, Cogent Biosciences, Ascentage Pharma, Blueprint Medicines, Syntrix Biosystems, Novartis, and PharmaEssentia. NP consulted for and received honoraria from Blueprint Medicines, Incyte, Novartis, PharmaEssentia, CTI BioPharma/Sobi, Constellation Pharmaceuticals/MorphoSys, AbbVie, Aptose Biosciences, and Karyopharm Therapeutics, and received honoraria as IDMC Chair from Cogent Biosciences. PV received consulting fees fromAbbVie, Amgen, Blueprint Medicines, Cogent Biosciences, Incyte, CTI BioPharma Corp, Daiichi Sankyo, GlaxoSmith Kline, Karyopharm, Novartis, Pfizer, Genentech, Servier, Stemline, MorphoSys, and LAVA Therapeutics, and honoraria for lectures/advisory boards from Incyte, Blueprint Medicines, and CTI Biopharma. AY provides consultancy for Incyte, CTI Pharma (SOBI), PharmaEssentia, Pfizer, Novartis, Servier, ABBVIE, Karyopharm Therapeutics, GSK, Blueprint Medicine, Apellis, Gilead, Notable Labs, and Protagonist, and received research funding from CTI Pharma (SOBI) and Stemline Therapeutics. JC received honoraria from Bioverativ (a Sanofi company), TrueNorth therapeutics, and CTI BioPharma. CH provided consultancy for Novartis, BMS, Abbvie, Beigene, Pfizer, and Eli Lilly. OZ works for PharmaEssentia USA. HC works for Everest, which is providing the CRO service for the EXCEED-ET study. PB consulted for or received honoraria from ABBVie, Blueprint, BMS, Cogent, CTI BioPharma Corp., (a Sobi company), Disc Medicine, Geron, GSK, Incyte, Ionis, Jubilant, Karyopharm, Keros, Morphic, MorphoSys, Novartis, Ono, PharmaEssentia, Raythera, and Sumitomo, and received research funding from Ajax, Blueprint, BMS, Cogent, CTI BioPharma Corp. (a Sobi company), DISC Medicine, Geron, Incyte, Ionis, Janssen, Kartos, Karyopharm, MorphoSys, Sumitomo, and Telios.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from PharmaEssentia. The funder had the following involvement in the study: study design, data collection and analysis. It was not involved in the decision to publish or preparation of the manuscript, outside of the work carried out by the authors.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tefferi, A, and Barbui, T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol. (2019) 94:133–43. doi: 10.1002/ajh.25303
2. Mesa, R, Palmer, J, Eckert, R, and Huberty, J. Quality of life in myeloproliferative neoplasms: symptoms and management implications. Hematol Oncol Clin North Am. (2021) 35:375–90. doi: 10.1016/j.hoc.2020.12.006
3. Tefferi, A, Szuber, N, Vallapureddy, RR, Begna, KH, Patnaik, MM, Elliott, MA, et al. Decreased survival and increased rate of fibrotic progression in essential thrombocythemia chronicled after the FDA approval date of anagrelide. Am J Hematol. (2019) 94:5–9. doi: 10.1002/ajh.25294
4. Hernández-Boluda, JC, Alvarez-Larrán, A, Gómez, M, Angona, A, Amat, P, Bellosillo, B, et al. Clinical evaluation of the European LeukaemiaNet criteria for clinicohaematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br J Haematol. (2011) 152:81–8. doi: 10.1111/j.1365-2141.2010.08430.x
5. Harrison, CN, Mead, AJ, Panchal, A, Fox, S, Yap, C, Gbandi, E, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. (2017) 130:1889–97. doi: 10.1182/blood-2017-05-785790
6. Bewersdorf, JP, Giri, S, Wang, R, Podoltsev, N, Williams, RT, Tallman, MS, et al. Interferon alpha therapy in essential thrombocythemia and polycythemia vera-a systematic review and meta-analysis. Leukemia. (2021) 35:1643–60. doi: 10.1038/s41375-020-01020-4
7. Masarova, L, Patel, KP, Newberry, KJ, Cortes, J, Borthakur, G, Konopleva, M, et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet Haematol. (2017) 4:e165–75. doi: 10.1016/S2352-3026(17)30030-3
8. Vachhani, P, Mascarenhas, J, Bose, P, Hobbs, G, Yacoub, A, Palmer, JM, et al. Interferons in the treatment of myeloproliferative neoplasms. Thera Adv Hematol. (2024) 15:20406207241229588. doi: 10.1177/20406207241229588
9. Kiladjian, JJ, Klade, C, Georgiev, P, Krochmalczyk, D, Gercheva-Kyuchukova, L, Egyed, M, et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon alfa-2b. Leukemia. (2022) 36:1408–11. doi: 10.1038/s41375-022-01528-x
10. Abu-Zeinah, G, Krichevsky, S, Cruz, T, Hoberman, G, Jaber, D, Savage, N, et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia. (2021) 35:2592–601. doi: 10.1038/s41375-021-01183-8
11. Gisslinger, H, Klade, C, Georgiev, P, Krochmalczyk, D, Gercheva-Kyuchukova, L, Egyed, M, et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia. (2023) 37:2129–32. doi: 10.1038/s41375-023-02008-6
12. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: myeloproliferative neoplasms. Version 3. (2022).
13. How, J, and Hobbs, G. Interferons as the first choice of cytoreduction in essential thrombocythemia and polycythemia vera. J Natl Compr Cancer Netw. (2022) 20:1063–8. doi: 10.6004/jnccn.2022.7026
14. United States Food & Drug Administration. FDA news release: FDA approves treatment for rare blood disease. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-rare-blood-disease (Accessed December 21, 2021).
15. Miyachi, N, Zagrijtschuk, O, Kang, L, Yonezu, K, and Qin, A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin Drug Investig. (2021) 41:391–404. doi: 10.1007/s40261-021-01026-5
16. Huang, YW, Qin, A, Fang, J, Wang, TF, Tsai, CW, Lin, KC, et al. Novel long-acting ropeginterferon alfa-2b: pharmacokinetics, pharmacodynamics and safety in a phase I clinical trial. Br J Clin Pharmacol. (2022) 88:2396–407. doi: 10.1111/bcp.15176
17. Gisslinger, H, Klade, C, Georgiev, P, Krochmalczyk, D, Gercheva-Kyuchukova, L, Egyed, M, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomized, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. (2020) 7:e196–208. doi: 10.1016/S2352-3026(19)30236-4
18. Knudsen, TA, Skov, V, Stevenson, K, Werner, L, Duke, W, Laurore, C, et al. Genomic profiling of a randomized trial of interferon-α vs hydroxyurea in MPN reveals mutation-specific responses. Blood Adv. (2022) 6:2107–19. doi: 10.1182/bloodadvances.2021004856
19. Gisslinger, H, Klade, C, Georgiev, P, Krochmalczyk, D, Gercheva-Kyuchukova, L, Egyed, M, et al. Polycythemia vera patients respond better to ropeginterferon alfa-2b than HU/BAT irrespective of pretreatment or mutational status; results from 5 years’ treatment in a randomized, controlled setting in the PROUD-PV/Continuation-PV trials. Blood. (2021) 138:3660. doi: 10.1182/blood-2021-152328
20. Gisslinger, H. Change in polycythemia Vera treatment: Ropeginterferon alfa-2b in light of current trials. Turk J Haematol. (2023) 40:266–8. doi: 10.4274/tjh.galenos.2023.2023.0419
21. Qin, A, Wu, D, Liao, J, Xie, S, Chen, H, Gao, Y, et al. Ethnic sensitivity analyses of pharmacokinetics, efficacy and safety in polycythemia vera treatment with ropeginterferon alfa-2b. Front Pharmacol. (2024) 15:1455979. doi: 10.3389/fphar.2024.1455979
22. Qin, A, Wu, D, Li, Y, Zhang, J, Wang, W, Shen, W, et al. Exposure-efficacy and exposure-safety analyses of ropeginterferon alfa-2b treatment in patients with polycythaemia vera. Br J Clin Pharmacol. (2024) 90:1493–502. doi: 10.1111/bcp.16043
23. Stein, BL, Oh, ST, Berenzon, D, Hobbs, GS, Kremyanskaya, M, Rampal, RK, et al. Polycythemia vera: an appraisal of the biology and management 10 years after the discovery of JAK2 V617F. J Clin Oncol. (2015) 33:3953–60. doi: 10.1200/jco.2015.61.6474
24. Moliterno, AR, Kaizer, H, and Reeves, BN. JAK2 V617F allele burden in polycythemia vera: burden of proof. Blood. (2023) 141:1934–42. doi: 10.1182/blood.2022017697
25. Harrison, CN, Nangalia, J, Boucher, R, Jackson, A, Yap, C, O’Sullivan, J, et al. Ruxolitinib versus best available therapy for polycythemia vera intolerant or resistant to hydroxycarbamide in a randomized trial. J Clin Oncol. (2023) 41:3534–44. doi: 10.1200/JCO.22.01935
26. De Stefano, V, Za, T, Rossi, E, Vannucchi, AM, Ruggeri, M, Elli, E, et al. Leukocytosis is a risk factor for recurrent arterial thrombosis in young patients with polycythemia vera and essential thrombocythemia. Am J Hematol. (2010) 85:97–100. doi: 10.1002/AJH.21593
27. Gerds, AT, Mesa, R, Burke, JM, Grunwald, MR, Scherber, R, Yu, J, et al. P1062: a real-world evaluation of the association between elevated blood counts and thrombotic events in polycythemia vera: an analysis of data from the REVEAL study. Hema. (2022) 6:952–3. doi: 10.1097/01.HS9.0000847116.74895.d8
28. Jin, J, Zhang, L, Qin, A, Wu, D, Shao, Z, Bai, J, et al. A new dosing regimen of ropeginterferon alfa-2b is highly effective and tolerable: findings from a phase 2 study in Chinese patients with polycythemia vera. Exp Hematol Oncol. (2023) 12:55. doi: 10.1186/s40164-023-00415-0
29. Suo, SS, Fu, RF, Qin, A, Shao, Z, Bai, J, Zhou, H, et al. Molecular remission uncoupled with complete haematologic response in polycythaemia vera treatment with ropeginterferon alfa-2b. Br J Haematol. (2024) 205:2510–4. doi: 10.1111/bjh.19846
30. Suo, SS, Fu, RF, Qin, A, Shao, ZH, Bai, J, Chen, SN, et al. Effective management of polycythemia vera with ropeginterferon alfa-2b treatment. J Hematol. (2024) 13:12–22. doi: 10.14740/jh1245
31. Qin, A. Mechanism of action of ropeginterferon alfa-2b in polycythemia vera treatment. Clin Ther. (2024) 46:439–40. doi: 10.1016/j.clinthera.2024.03.005
32. Verstovsek, S, Komatsu, N, Gill, H, Jin, J, Lee, SE, Hou, HA, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. (2022) 18:2999–3009. doi: 10.2217/fon-2022-0596
Keywords: ropeginterferon alfa-2b-njft (ropeg), essential thrombocythemia (ET), higher initial dose and accelerated titration (HDAC) regimen, complete hematologic response, molecular response, clinical trial
Citation: Masarova L, Reeves BN, El Chaer F, Foltz L, Tashi T, Abu-Zeinah G, Lucas J, Halpern AB, Maze D, Qin A, Safah H, Lan F, O'Connell CL, Goel S, Rein L, Fang B, How J, Babu S, Li Z, Cerquozzi S, Oh ST, Hunter AM, Podoltsev N, Vachhani P, Yacoub A, Cunningham JM, Hillis C, Otoukesh S, Zagrijtschuk O, Castro H and Bose P (2025) A multicenter study to assess efficacy, safety, and tolerability of ropeginterferon alfa-2b-njft in patients with essential thrombocythemia in the US and Canada: EXCEED-ET trial. Front. Med. 12:1548590. doi: 10.3389/fmed.2025.1548590
Edited by:
Shinobu Matsuura, Boston University, United StatesReviewed by:
Ken-Hong Lim, Mackay Memorial Hospital, TaiwanKazuya Shimoda, University of Miyazaki, Japan
Copyright © 2025 Masarova, Reeves, El Chaer, Foltz, Tashi, Abu-Zeinah, Lucas, Halpern, Maze, Qin, Safah, Lan, O’Connell, Goel, Rein, Fang, How, Babu, Li, Cerquozzi, Oh, Hunter, Podoltsev, Vachhani, Yacoub, Cunningham, Hillis, Otoukesh, Zagrijtschuk, Castro and Bose. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Masarova, lmasarova@mdanderson.org
 Lucia Masarova
Lucia Masarova