
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 02 April 2025
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1548067
Background: The genus Gardnerella is commonly found in the vaginal ecosystem and is considered a covert pathogen of the urinary tract. However, Gardnerella vaginalis had been the only recognized species of the genus Gardnerella for decades. Cases regarding the clinical relevance of Gardnerella leopoldii have rarely been reported, which is crucial for fully understanding the various species within the genus Gardnerella.
Case presentation: A 72-year-old female patient was admitted to the hospital with gross hematuria and complaints of waist soreness. Physical examinations, including those of the head, chest, and abdomen, along with routine laboratory tests such as white blood cell (WBC) count and proportion, liver function, and renal function, yielded normal results. However, the patient also exhibited significantly elevated levels of serum C-reactive protein (CRP) and abnormal urinary test findings, which revealed positive results for occult blood and leukocyte esterase, and increased counts of erythrocyte and leukocyte. To further evaluate the urinary system, computerized tomography urography (CTU) was performed. The CTU results revealed multiple weakly enhanced foci in the right kidney and thickening of the right ureter, renal pelvis, calyces, and bladder walls. Based on the above findings, the initial diagnosis included hematuria, hydronephrosis, and urinary tract infection (UTI). To identify the causative pathogens, we employed a comprehensive approach that included microscopic morphology, Sanger sequencing, and metagenomic next-generation sequencing (mNGS). Finally, both Mycobacterium tuberculosis and G. leopoldii were identified as the co-infecting etiological agents responsible for the patient’s urinary tract infection.
Conclusion: This case represents the first documented isolation of clinically relevant G. leopoldii, guided by morphological and molecular evidence from a clinical urine sample. It highlights the potential of mNGS as a promising tool for identifying previously unrecognized species and offers valuable insights to enhance the understanding of clinically relevant microorganisms.
The genus Gardnerella has been extensively reported as a key component of polymicrobial biofilms (1) and is strongly associated with bacterial vaginosis in women (2, 3). Although the genus Gardnerella is commonly found in both the vagina and urine (4), emerging research suggests a relationship between the presence of Gardnerella in urine and recurrent urinary tract infections (UTIs). Accordingly, the clinical significance of Gardnerella spp. may be underestimated, and its role as a potential covert pathogen in urine warrants further investigation (5). For nearly four decades, Gardnerella vaginalis was the only recognized species within the genus Gardnerella (6). However, in 2019, Gardnerella leopoldii, Gardnerella swidsinskii, and Gardnerella piotii were delineated separately for the first time (6, 7) due to technical advancements in whole-genome sequencing analysis. Therefore, previous studies on UTIs and Gardnerella have mainly focused on G. vaginalis. There have been limited reports on the clinical relevance of G. leopoldii, which is essential for a comprehensive understanding of the relationship between different species of Gardnerella and their potential associations with clinical conditions.
Herein, we present a case of a female patient with UTI and gross hematuria. Through the use of gold-standard culture, microscopic morphology, and molecular detection, the causes of UTI were identified as a co-infection of Mycobacterium tuberculosis and G. leopoldii. To the best of our knowledge, this is the first clinically relevant isolation of G. leopoldii, which could be essential for enhancing the understanding of the species characteristics of the Gardnerella genus.
A 72-year-old female patient was admitted to the hospital for 1 day due to gross hematuria and a primary complaint of waist soreness. Initial evaluation at the outpatient clinic using B-mode ultrasound revealed signs of right hydronephrosis. Upon physical examination, no apparent abnormalities were detected in the chest or abdomen, but positive percussion pain was observed in the right renal area.
Laboratory tests were also performed, revealing the following results. Routine blood tests indicated a white blood cell (WBC) count of 5.92 × 109/L (within the normal range of 3.5–9.5 × 109/L), with a proportion of 64.7% categorized as neutrophils (normal range 40–75%). Serum C-reactive protein (CRP) levels were found to be elevated at 35.50 mg/L (normal range 0–6.00 mg/L). Liver function indicators were found to be normal. Urinalysis revealed 3+ for occult blood and leukocyte esterase, with an elevated urinary erythrocyte count of 84.5/μL (normal range 0–25.0/μL), a markedly high urinary leukocyte count of 20103.1/μL (normal range 0–30.0/μL), and an increased epithelial cell count of 63.1/μL (normal range 0–21.4/μL). The glomerular filtration rates (GFR, normal range >90.0 mL/min) were measured at 25.32 mL/min for the right kidney, indicating moderate function with reduced excretion, and 36.38 mL/min for the left kidney, suggesting normal function with slightly delayed excretion. Given the suggestive finding of localized inflammation on the outpatient ultrasound, a computerized tomography (CT) scan was performed. Chest CT revealed mixed ground-glass nodules in the upper lobe of the right lung, multiple small nodules in the left lung, and evidence of emphysema in both lungs. Abdominal CT revealed a gross bladder wall, thickening of the right ureter, renal pelvis, and calyces, right-sided hydronephrosis and ureter dilation, multiple enlarged retroperitoneal lymph nodes, and gallbladder stones (Figure 1A). CT urography (CTU) further confirmed hydronephrosis of the right kidney and ureter, the presence of a cyst in the right kidney, and inflammation of the bladder (Figure 1B). Based on previous clinical experience, the initial diagnosis was hematuria and hydronephrosis secondary to a urinary tract infection (UTI).
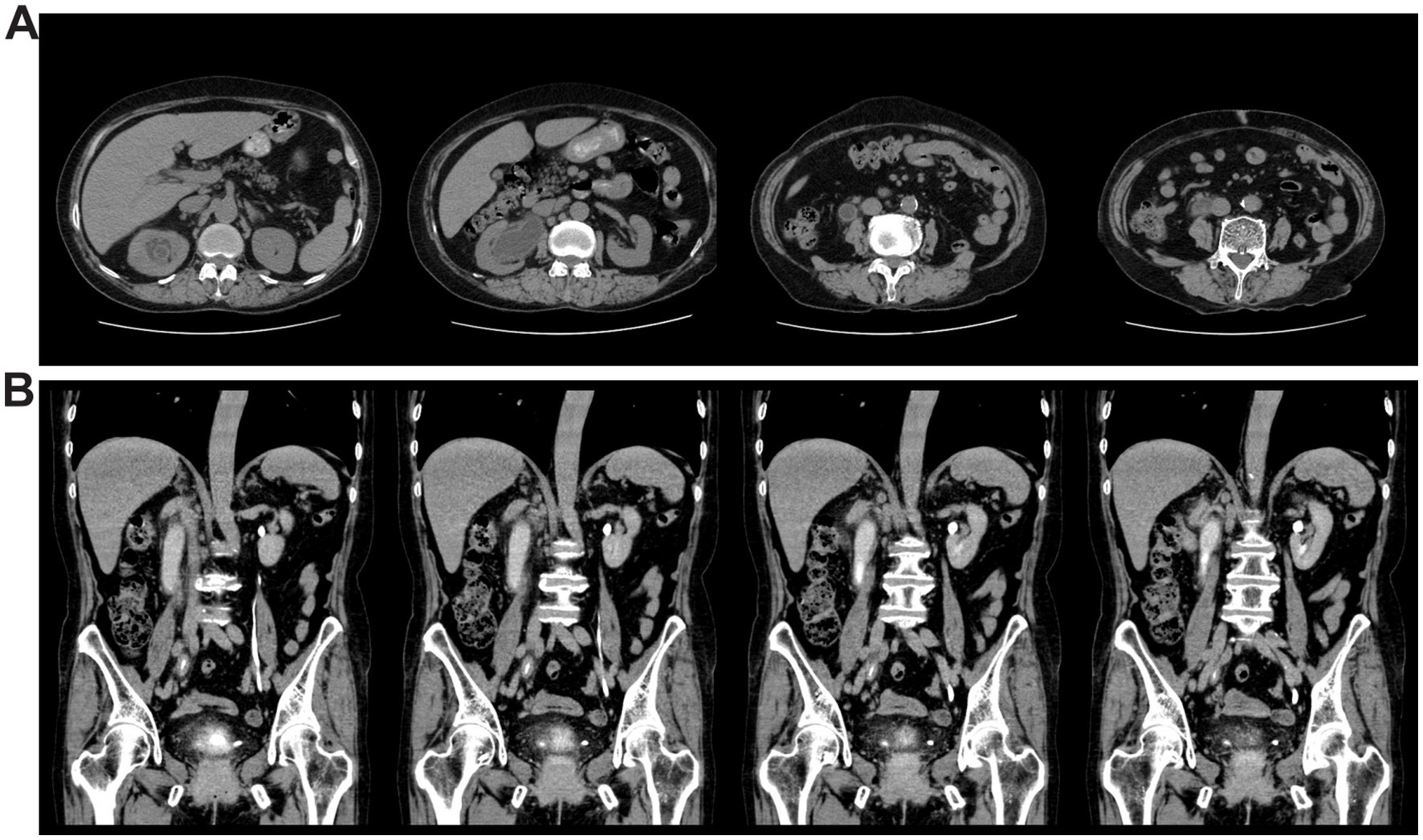
Figure 1. CT images showed thickening and hydronephrosis of the right kidney and right ureter, as well as inflammation of the bladder. (A) Whole abdominal CT of the patient. (B) CT urography of the patient.
Considering the complexity of microbial colonization in the urinary tract, a clean-catch midstream urine sample was collected for pathogen identification. DNA pellets from the urine sample were extracted for metagenomic next-generation sequencing (mNGS). Meanwhile, microbiological detection, including culture and smear examinations, was also conducted. The microscopic examination of the stained urine smear revealed coccobacilli being phagocytosed by immune cells (Figure 2A), confirming a bacterial infection in the urinary tract (8). White colonies were successfully cultured on a blood agar plate (Figure 2B) and were initially reported as G. vaginalis using the VITEK® Mass Spectrometry Identification System (Bio Mérieux, France).
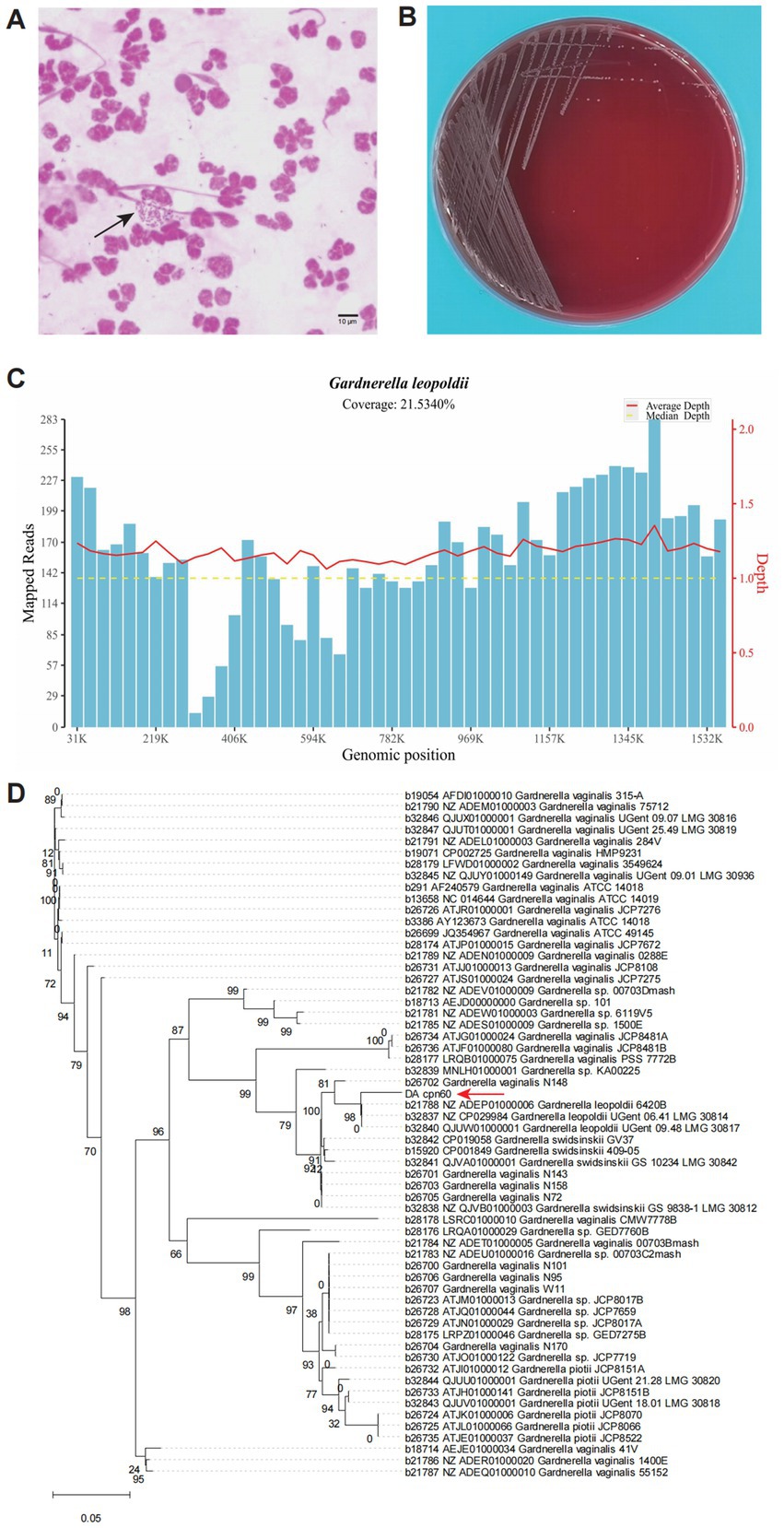
Figure 2. Morphological and molecular evidence revealed a urinary tract infection caused by G. leopoldii. (A) Microscopic field of the urinary smear exhibited Gram-negative coccobacilli internalized by neutrophils (black array marked). (B) Colonies growing on a blood agar plate, which were later molecularly confirmed as G. leopoldii. (C) Genome coverage distribution of the mapped reads sequenced using mNGS. (D) Phylogenetic tree derived from the cpn60 gene sequences of the isolate in this study (DA_cpn60, red array marked) and others of Gardnerella spp. from cpnDB (https://www.cpndb.ca/).
To confirm the identification, colony DNA was extracted using the TIANamp Bacteria DNA Kit (TIANGEN Biotech, China) for Sanger sequencing of the 16S rRNA and chaperonin60 (cpn60) genes. BLAST analysis of the 16S rRNA gene amplicon sequence revealed a high similarity to both G. leopoldii and G. vaginalis (Table 1), according to the National Center for Biotechnology Information (NCBI, https://blast.ncbi.nlm.nih.gov/Blast.cgi). For mNGS sequencing, we utilized the Universal Plus DNA Library Prep Kit for MGI (GeneDian, China) to prepare the libraries, which were then sequenced with MGISEQ200 (BGI, China). However, the mNGS results revealed Mycobacterium tuberculosis complex (2 reads) and G. leopoldii (1,832 reads) (Table 2). The genomic coverage of the mapped reads (Figure 2C) and the phylogenetic tree of the cpn60 gene (Figure 2D) confirmed the isolates as G. leopoldii, rather than G. vaginalis.
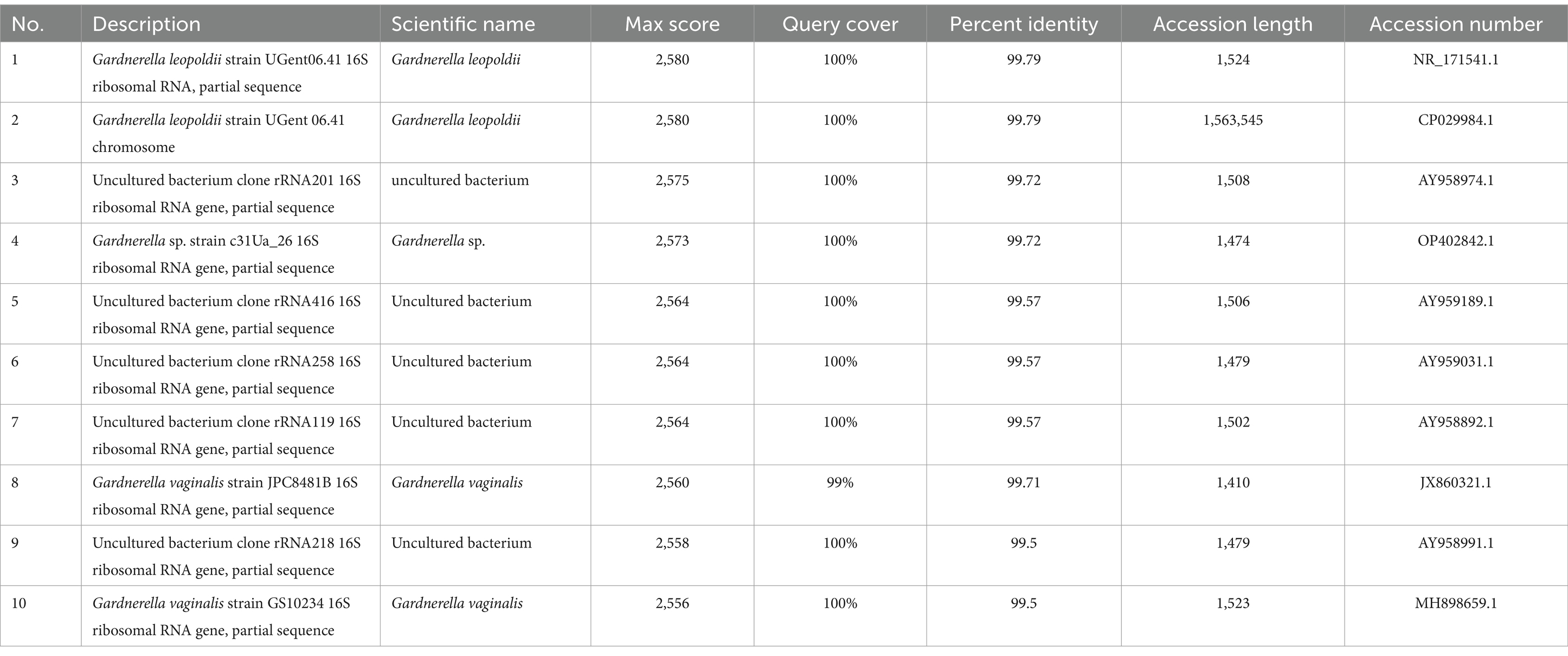
Table 1. BLAST results of the 16S rRNA gene sequences of the isolated single colony (top 10 BLAST in the NCBI).
In addition, the results of the GeneXpert MTB/RIF assay and interferon-gamma release assay (IGRA) confirmed the diagnosis of tuberculosis. Although urogenital tuberculosis (UGTB) could explain hematuria, the presence of phagocytosed bacteria suggested G. leopoldii was also clinically relevant. Therefore, the UTI was attributed to a co-infection of M. tuberculosis and G. leopoldii. The sequencing data from Sanger sequencing and mNGS were submitted to the China National GeneBank Database (CNGBdb) (9) under accession numbers CNP0004870 and CNP0005073.
Metronidazole (400 mg/8 h p.o.) was empirically administered for 7 days to treat urinary anaerobic bacterial infections. After confirming the co-infection of M. tuberculosis and G. leopoldii, the patient was transferred to a specialized tuberculosis hospital due to regulatory requirements for tuberculosis treatment. After a 4-month follow-up, the patient is currently receiving active triple anti-tuberculosis therapy with isoniazid, rifampicin, and ethambutol.
Herein, we present a case of urinary tract co-infection with M. tuberculosis and G. leopoldii. Notably, this could be the first documented clinically relevant isolation of a pathogenic strain of G. leopoldii, identified through microscopic morphology and molecular detection.
The genus Gardnerella was first reported in 1980 as a significant contributor to puerperal infections (10). Subsequent studies have found Gardnerella as a core component of the urinary microbiota in postmenopausal women (11) and ranked it as the second most commonly detected genus in both vaginal and urine samples (4). Interestingly, although Gardnerella was not a dominant urinary microbe in premenopausal women (11), women in a younger cohort exhibited a higher prevalence and abundance of Gardnerella in urine samples from the urethra compared to the older cohort (12). Gardnerella could be detected in the urine samples of women with low absolute but high relative abundance. Based on high-throughput sequencing of the 16S rRNA gene, pathogenic Gardnerella has been reported to be associated with bacterial vaginosis in women (13) and with severe complications following UTIs in both men (13) and women (14), especially involving G. vaginalis. The presence of Gardnerella in the urinary tract is considered a risk factor for UTIs and is increasingly recognized as a covert pathogen (5). In this case, the mNGS-detected reads of Gardnerella were over 10-fold higher compared to the reads of Prevotella and Enterococcus (Table 1). Furthermore, the microscopic examination of the urinary staining smear demonstrated immune phagocytosis of the microbes (Figure 2A), providing evidence of a local infection (8). However, the staining results of the microbes revealed them to be Gram-negative, which seemed inconsistent with the taxonomic status of G. leopoldii. This discrepancy can be attributed to the special cell wall structure of G. leopoldii, which can result in variable Gram staining, despite its taxonomic classification as a Gram-positive bacterium (7). Based on the above evidence, we believe that the genus Gardnerella was proliferating abnormally and acting as an opportunistic pathogen rather than as a commensal microorganism in this patient.
For nearly four decades, G. vaginalis was the only recognized species in the genus Gardnerella, possibly due to the high genetic similarity among species, as was initially suspected in this case. However, advancements in molecular techniques have led to the identification and classification of additional species, including G. leopoldii, G. swidsinskii, and G. piotii, which were delineated as distinct species in 2019 (7, 15). Studies on G. leopoldii have mainly focused on genetic heterogeneity and taxonomic diversity within the Gardnerella genus (6, 7) or the resolution and co-occurring patterns in the vaginal microbiome (16). Cases of clinically relevant G. leopoldii remain scarce. In this study, the Gram staining of the urine smear revealed Gram-negative coccobacilli internalized by neutrophils. Using molecular detection techniques, including Sanger sequencing and mNGS, the coccobacilli were definitively identified as G. leopoldii. This case suggests that the presence of urinary G. leopoldii could be pathogenic under specific circumstances, such as co-infection with urinary tuberculosis.
Urogenital tuberculosis (UGTB) is typically caused by M. tuberculosis or Mycobacterium bovis, which leads to infectious inflammation of the urogenital system. Clinical classifications of UGTB mainly include tuberculosis of the kidney, urinary tract, and genital region (17). In addition, disseminated tuberculosis could also affect the urogenital system (17, 18). Symptoms of UGTB include frequent voiding, dysuria, pyuria, back or abdominal pain, and microscopic or macroscopic hematuria (19). In cases of disseminated tuberculosis, systematic symptoms may also rise, such as malaise, fever, and anorexia (20). A history of M. tuberculosis infection (17), particularly pulmonary tuberculosis (21), is a significant risk factor for UGTB. Other risk factors include acquired immunodeficiency syndrome (AIDS) (19) and recurrent UTIs (17). UGTB can affect all ages, although it is rare in patients under the age of 20 years because of the long latent period (20). Due to the non-specific presentation and limited sensitivity of a single test to diagnose tuberculosis, a precise diagnosis requires the performance of multiple tests. Therefore, previous publications on urogenital tuberculosis primarily address the challenges of challenges, treatment options, and clinical features (22–24). To the best of our knowledge, only one article reported a co-infection of Mycoplasma genitalium and Chlamydia trachomatis in an infertile female patient with genital tuberculosis (25). In this case study, urinary tract infection caused by both M. tuberculosis and G. leopoldii was reported. The clinical team found no evidence of acquired immunodeficiency or a previous history of UTIs; however, the CT images did show a mixed ground-glass nodule in the right lung and a nodular area of increased density in the left lung, both with clear margins. The patient also reported a long history of pulmonary tuberculosis spanning several decades, which may contribute to renal symptoms and coinfections with G. leopoldii.
In conclusion, this case illustrates the first documented isolation of clinically relevant Gardnerella leopoldii, which was identified as a coinfection with M. tuberculosis. In addition, it highlights the utility of mNGS for the rapid and accurate identification of complicated UTIs. It is believed that mNGS technology is a promising tool for identifying and guiding the discovery of previously unrecognized species. When combined with conventional microbiological methods, mNGS can enhance the discovery and understanding of clinically relevant species, as exemplified by the identification and characterization of G. leopoldii in this case.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://db.cngb.org/cnsa/, CNP0004870, https://db.cngb.org/cnsa/, CNP0005073.
The studies involving humans were approved by Ethics Committee of Affiliated Jinhua Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LC: Conceptualization, Data curation, Funding acquisition, Writing – original draft. WW: Project administration, Resources, Supervision, Validation, Writing – original draft. DL: Formal analysis, Methodology, Software, Visualization, Writing – original draft. WX: Methodology, Supervision, Project administration, Validation, Writing – review & editing. LL: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing. SL: Conceptualization, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Jinhua City Science and Technology Bureau Social Development Class Key Project (No. 2022-3-076).
We would like to express our gratitude to the patient for the participation.
DL, WX, LL, and SL were employed by Dian Diagnostics Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jung, H, Ehlers, MM, Peters, RPH, Lombaard, H, Redelinghuys, MJ, Bezuidenhoudt, JE, et al. Growth forms of Gardnerella spp. and Lactobacillus spp. on vaginal cells. Front Cell Infect Microbiol. (2020) 10:71. doi: 10.3389/fcimb.2020.00071
2. Fredricks, DN, Fiedler, TL, Thomas, KK, Oakley, BB, and Marrazzo, JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. (2007) 45:3270–6. doi: 10.1128/jcm.01272-07
3. Onderdonk, AB, Delaney, ML, and Fichorova, RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. (2016) 29:223–38. doi: 10.1128/cmr.00075-15
4. Komesu, YM, Dinwiddie, DL, Richter, HE, Lukacz, ES, Sung, VW, Siddiqui, NY, et al. Defining the relationship between vaginal and urinary microbiomes. Am J Obstet Gynecol. (2020) 222:154.e1–154.e10. doi: 10.1016/j.ajog.2019.08.011
5. Yoo, JJ, Song, JS, Kim, WB, Yun, J, Shin, HB, Jang, MA, et al. Gardnerella vaginalis in recurrent urinary tract infection is associated with dysbiosis of the bladder microbiome. J Clin Med. (2022) 11:2295. doi: 10.3390/jcm11092295
6. Castro, J, Jefferson, KK, and Cerca, N. Genetic heterogeneity and taxonomic diversity among Gardnerella species. Trends Microbiol. (2020) 28:202–11. doi: 10.1016/j.tim.2019.10.002
7. Vaneechoutte, M, Guschin, A, Van Simaey, L, Gansemans, Y, Van Nieuwerburgh, F, and Cools, P. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol. (2019) 69:679–87. doi: 10.1099/ijsem.0.003200
8. Kasper, DL, Fauci, AS, and Harrison, TR. Harrison's infectious diseases. New York: McGraw-Hill (2010).
9. Chen, FZ, You, LJ, Yang, F, Wang, LN, Guo, XQ, Gao, F, et al. CNGBdb: China National GeneBank DataBase. Yi Chuan. (2020a) 42:799–809. doi: 10.16288/j.yczz.20-080
10. Eschenbach, DA, and Wager, GP. Puerperal infections. Clin Obstet Gynecol. (1980) 23:1003–38. doi: 10.1097/00003081-198012000-00006
11. Ammitzbøll, N, Bau, BPJ, Bundgaard-Nielsen, C, Villadsen, AB, Jensen, AM, Leutscher, PDC, et al. Pre- and postmenopausal women have different core urinary microbiota. Sci Rep. (2021) 11:2212. doi: 10.1038/s41598-021-81790-8
12. Chen, YB, Hochstedler, B, Pham, TT, Acevedo-Alvarez, M, Mueller, ER, and Wolfe, AJ. The urethral microbiota: a missing link in the female urinary microbiota. J Urol. (2020b) 204:303–9. doi: 10.1097/ju.0000000000000910
13. Boyanova, L, Marteva-Proevska, Y, Gergova, R, and Markovska, R. Gardnerella vaginalis in urinary tract infections, are men spared? Anaerobe. (2021) 72:102438. doi: 10.1016/j.anaerobe.2021.102438
14. Rosales-Castillo, A, Jiménez-Guerra, G, Ruiz-Gómez, L, Expósito-Ruíz, M, Navarro-Marí, JM, and Gutiérrez-Fernández, J. Emerging presence of culturable microorganisms in clinical samples of the genitourinary system: systematic review and experience in specialized care of a regional hospital. J Clin Med. (2022) 11:1348. doi: 10.3390/jcm11051348
15. Potter, RF, Burnham, CD, and Dantas, G. In silico analysis of Gardnerella genomospecies detected in the setting of bacterial vaginosis. Clin Chem. (2019) 65:1375–87. doi: 10.1373/clinchem.2019.305474
16. Hill, JE, and Albert, AYK. Resolution and cooccurrence patterns of Gardnerella leopoldii, G. swidsinskii, G. piotii, and G. vaginalis within the vaginal microbiome. Infect Immun. (2019) 87:e00532. doi: 10.1128/iai.00532-19
17. Kulchavenya, E. Urogenital tuberculosis: definition and classification. Ther Adv Infect Dis. (2014) 2:117–22. doi: 10.1177/2049936115572064
18. Abbara, A, and Davidson, RN. Etiology and management of genitourinary tuberculosis. Nat Rev Urol. (2011) 8:678–88. doi: 10.1038/nrurol.2011.172
19. Merchant, S, Bharati, A, and Merchant, N. Tuberculosis of the genitourinary system-urinary tract tuberculosis: renal tuberculosis-part I. Indian J Radiol Imaging. (2013) 23:46–63. doi: 10.4103/0971-3026.113615
20. Dias, N, Pina-Vaz, T, Abreu-Mendes, P, Azeredo-Costa, T, Rodrigues-Pereira, P, Silva, C, et al. Review of 175 cases of tuberculosis infections affecting the urogenital system. Turk J Urol. (2022) 48:440–5. doi: 10.5152/tud.2022.22148
21. Figueiredo, AA, and Lucon, AM. Urogenital tuberculosis: update and review of 8961 cases from the world literature. Rev Urol. (2008) 10:207–17.
22. Barreto, AA, Lopes, HE, Netto, JMB, and Figueiredo, AA. Urogenital tuberculosis and delayed diagnosis: a qualitative study. Urol Res Pract. (2024) 50:198–202. doi: 10.5152/tud.2024.24028
23. Figueiredo, AA, Truzzi, JC, Barreto, AA, Siqueira, EC, Lucon, M, Broglio, M, et al. Urogenital tuberculosis: a narrative review and recommendations for diagnosis and treatment. Int Braz J Urol. (2025) 51:e20240590. doi: 10.1590/s1677-5538.Ibju.2024.0590
24. Lahouar, R, Ben Khalifa, B, Baba, NE, Gazzah, W, Naouar, S, Braiek, S, et al. Unusual presentations of urogenital tuberculosis. Int J Surg Case Rep. (2020) 77:769–72. doi: 10.1016/j.ijscr.2020.11.080
Keywords: urinary tract infection (UTI), Gardnerella leopoldii , metagenomic next-generation sequencing (mNGS), microscopic morphology, case report
Citation: Chen L, Weng W, Li D, Xie W, Lu L and Li S (2025) Case Report: A clinically relevant isolation of Gardnerella leopoldii guided by morphological and molecular evidence from a urinary tract infection case. Front. Med. 12:1548067. doi: 10.3389/fmed.2025.1548067
Received: 19 December 2024; Accepted: 10 March 2025;
Published: 02 April 2025.
Edited by:
António Machado, University of the Azores, PortugalReviewed by:
Shabnam Momtahen, Dartmouth College, United StatesCopyright © 2025 Chen, Weng, Li, Xie, Lu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingling Lu, bHVsbDFAZGF6ZC5jbg==; Shuo Li, bGlzaHVvN0BkYXpkLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.