
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 14 March 2025
Sec. Hepatobiliary Diseases
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1543903
Introduction: Transcatheter arterial chemoembolization (TACE) is widely applied for locoregional malignant lesions control in intermediate and selected advanced hepatocellular carcinoma (HCC). Various inflammation indices, such as neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammatory index (SII), and Lymphocyte-to-C Reactive Protein Ratio (LCR) have been explored as tools for predicting the efficacy of TACE. However, the role and predictive value for dynamic changes of peripheral inflammatory indicators pre- and post-TACE remains unclear.
Objective: To explore the association between the alteration in inflammatory index and the efficacy and prognosis of TACE and to provide more evidence for early prediction of the efficacy of TACE.
Methods: This was a retrospective single-center study. HCC patients who received TACE as initial treatment were enrolled. The relationship between the alteration of inflammation indices (calculated as post-TACE minus pre-TACE measurements) and TACE efficacy and prognosis was investigated. Progression-free survival (PFS) was the primary endpoint, and treatment efficacy was evaluated based on mRECIST criteria.
Results: Before propensity score matching (PSM), the change in LMR was significantly associated with treatment effective rate, with the unelevated ΔLMR group achieving a 79.4% treatment effective rate compared to 36.4% in the elevated group (p < 0.001). The estimated median PFS was 9.7 months and 4.5 months in the unelevated and elevated group, with a significant difference (p = 0.016). After PSM, the treatment effective rate was 48.7 and 38.5% (p = 0.214), and the estimated median PFS was 8.9 and 5.5 months (p = 0.173) for the unelevated and elevated group, respectively.
Conclusion: Our study demonstrated that alteration of indices of peripheral inflammation, including ΔNLR, ΔLMR, ΔSII, and ΔPLR, may not be valuable prognostic markers for HCC patients who received TACE.
Primary liver cancer is the sixth most common cancer in terms of new cases each year and the third most common cause of cancer-related deaths worldwide, with more than 900,000 new cases and more than 830,000 deaths each year (1). Hepatocellular carcinoma (HCC) represents 75–90% of primary liver cancer (2, 3). Standard treatments for HCC include liver transplantation, surgical resection, local treatments such as transcatheter arterial chemoembolization (TACE), transcatheter arterial radioembolization (TARE), ablation, and systemic therapies (4). According to the Barcelona Clinic for Liver Cancer (BCLC) staging guideline, TACE is recommended for intermediate HCC, and there is growing evidence supporting its use in selected cases of advanced HCC (5, 6).
To date, various inflammation indices, such as neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammatory index (SII), and lymphocyte-to-C reactive protein ratio (LCR) have been explored as tools for predicting the efficacy of TACE (7–9). However, the predictive value of dynamic changes in these peripheral inflammatory indicators pre- and post-TACE remains unclear. Therefore, this retrospective study aims to assess the association between the alteration in inflammatory index and TACE efficacy and prognosis, providing further evidence for early prediction of treatment outcome.
This single-center, retrospective study was approved by the ethics committee of Hunan Provincial People’s Hospital and was performed following the Declaration of Helsinki (10). The requirement for written informed consent was waived by the institutional review boards due to the retrospective nature of this study. A total of 227 HCC patients who received TACE as initial treatment at Hunan Provincial People’s Hospital (First Affiliated Hospital of Hunan Normal University) between February 2019 and September 2022 were included. Diagnosis of HCC was confirmed using the Liver Imaging Reporting and Data System (Li-RADS) or pathological confirmation (11). Exclusion criteria were: (1) severe liver or kidney dysfunction; (2) severe and uncorrectable coagulation dysfunction; (3) extensive distant metastasis; (4) performance score (PS) ≥ 2; (5) early-stage HCC; (6) follow-up time of <3 months after the initial TACE; (7) age < 18 or > 80 years. The final cohort included 146 patients, as illustrated in Figure 1.
Clinical and demographic data were collected from the electronic medical records, including age, gender, hepatitis B surface antigen (HBsAg) status, alpha-fetoprotein (AFP) levels, alkaline phosphatase (ALP), BCLC stage, Child-Pugh score, initial TACE treatment response, and complete blood counts (neutrophil count, lymphocyte count, monocyte count, and platelet count). Inflammatory indices were calculated as follows: NLR = neutrophil count/lymphocyte count; LMR = lymphocyte count/monocyte count; SII = (neutrophil × platelet)/lymphocyte count; PLR = platelet count/lymphocyte count. Indices were reexamined 3 days post-TACE. The alteration was represented as delta (Δ) value, calculated by subtracting the pre-TACE value from the post-TACE value. Positive ΔNLR, ΔLMR, ΔSII, and ΔPLR indicated elevation, while negative and null values indicated no elevation.
All patients were reviewed by a multidisciplinary tumor board. TACE is recommended for patients who are not suitable for or refuse curative treatment. Procedures were conducted by board-certified interventional radiologists using femoral or radial artery access. For femoral access, 5-Fr Yashiro or Rosch hepatic catheters were used, while vertebral or multipurpose catheters were employed via radial access. Angiography of the celiac and superior mesenteric arteries identified intrahepatic tumors and their blood supply. Microcatheters (2.7-F or 2.8-F) enabled superselective embolization based on angiographic findings.
In conventional TACE (cTACE), an iodized oil-idarubicin/doxorubicin emulsion was prepared with up to 15 mL of lipiodol and 10 mg idarubicin or 40–80 mg doxorubicin, with a 3:1 or 2:1 volume ratio of iodized oil to aqueous solvent (either sugar solution or sterile water for injection). This mixture follows the “water-in-oil” technique commonly used in cTACE (12). Embolization of proximal tumor feeders was achieved using Gelfoam slurry, microspheres (Embozene, Varian Medical Systems), or polyvinyl alcohol (PVA) particles (Cook Medical). In cases with hepatic arteriovenous fistulas, PVA particles or microspheres were applied before lipiodol embolization.
For drug-eluting bead TACE (DEB-TACE), beads (Biocompatibles, UK; Jiangsu Hengrui Medical, China) were loaded with idarubicin (10 mg) or doxorubicin (40–80 mg) for 20–30 min, and then delivered into the tumor’s feeding artery based on angiographic evaluation. All procedures adhered to the Society of Interventional Radiology (SIR) guidelines (13).
Patients underwent imaging follow-ups, including hepatic contrast-enhanced CT/MRI and chest CT, every 1–3 months post-TACE. Repeated TACE treatments were administrated as needed, based on active tumor remnant and liver function. The treatment effect of target lesions and non-target lesions and the presence of new lesions are comprehensively evaluated. The overall efficacy, according to the mRECIST criteria, was divided into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD) (14). CR and PR were defined as treatment effective, while SD and PR were treatment ineffective. The primary endpoint was progression-free survival (PFS), defined as the time from the initial TACE to disease progression, death, or the last follow-up. Overall survival (OS) was the second endpoint, defined as the time from the initial TACE and death or the last follow-up. The last follow-up ended on October 21, 2023. Censored data was recorded if the endpoint event had not been reached at the last follow-up.
Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables were presented as counts and percentages. Comparisons were made using the χ2 test or Fisher’s exact test, as appropriate. Logistic regression analysis identified risk factors for ineffective initial TACE response, while Cox proportional hazards models determined independent risk factors for PFS and OS. Variables with p < 0.1 in the univariate analyses were included in the multivariate analyses. To mitigate potential confounding and selection bias between the two groups, propensity score matching (PSM) was employed. A 1:1 nearest-neighbor matching without replacement was performed using a caliper width of 0.1 standard deviations of the logit of the propensity score (15). The propensity score was estimated using a logistic regression model, adjusting for the covariates listed in Table 1. The caliper width of 0.1 ensures that matches are restricted to within 0.1 standard deviations of the propensity score, improving the balance between groups while minimizing bias. The Kaplan–Meier method was used to estimate PFS and OS between different groups, and survival curves were generated to compare different groups. Differences between survival curves were assessed using the log-rank test. Kaplan–Meier survival analysis was performed using the survival package and visualized with survminer package in R software (16, 17). A p < 0.05 was considered statistically significant. Analyses were conducted using statistical software (SPSS version 27, International Business Machines Corporation) and R software (version 4.0.2).1
Among the 146 HCC patients who received TACE as initial treatment, 120 were male, and 26 were female. The median age was 59 years, with 75 patients younger than 60. Sixty-eight patients were classified as BCLC stage B (intermediate), while 78 patients were classified as stage C (advanced). A total of 106 patients tested positive for hepatitis B surface antigen (HBsAg), and 93 patients had cirrhosis. Forty patients had a Child-Pugh grade B classification, and 63 patients (42%) presented with AFP levels ≥400 ng/mL.
Regarding inflammatory indices, 18 patients (12%) exhibited decreased NLR, 102 patients (70%) showed decreased LMR, 25 patients (17%) had decreased SII, and 35 patients (24%) showed decreased PLR. The median follow-up period was 18.6 months. Detailed baseline characteristics are provided in Table 2.
In univariate logistic regression analysis, ineffective response for the initial TACE was significantly associated with BCLC stage, serum AFP level, and the alteration of LMR. Multivariate analysis identified BCLC stage C [Odds ratio (OR): 3.475; 95% confidence interval (CI): 1.417–8.526], AFP ≥ 400 ng/mL (OR: 3.114; 95% CI: 1.333–7.275), and elevated LMR (OR: 8.585; 95% CI: 3.538–20.832) as independent risk factors for ineffective treatment. Specific results are detailed in Table 3.
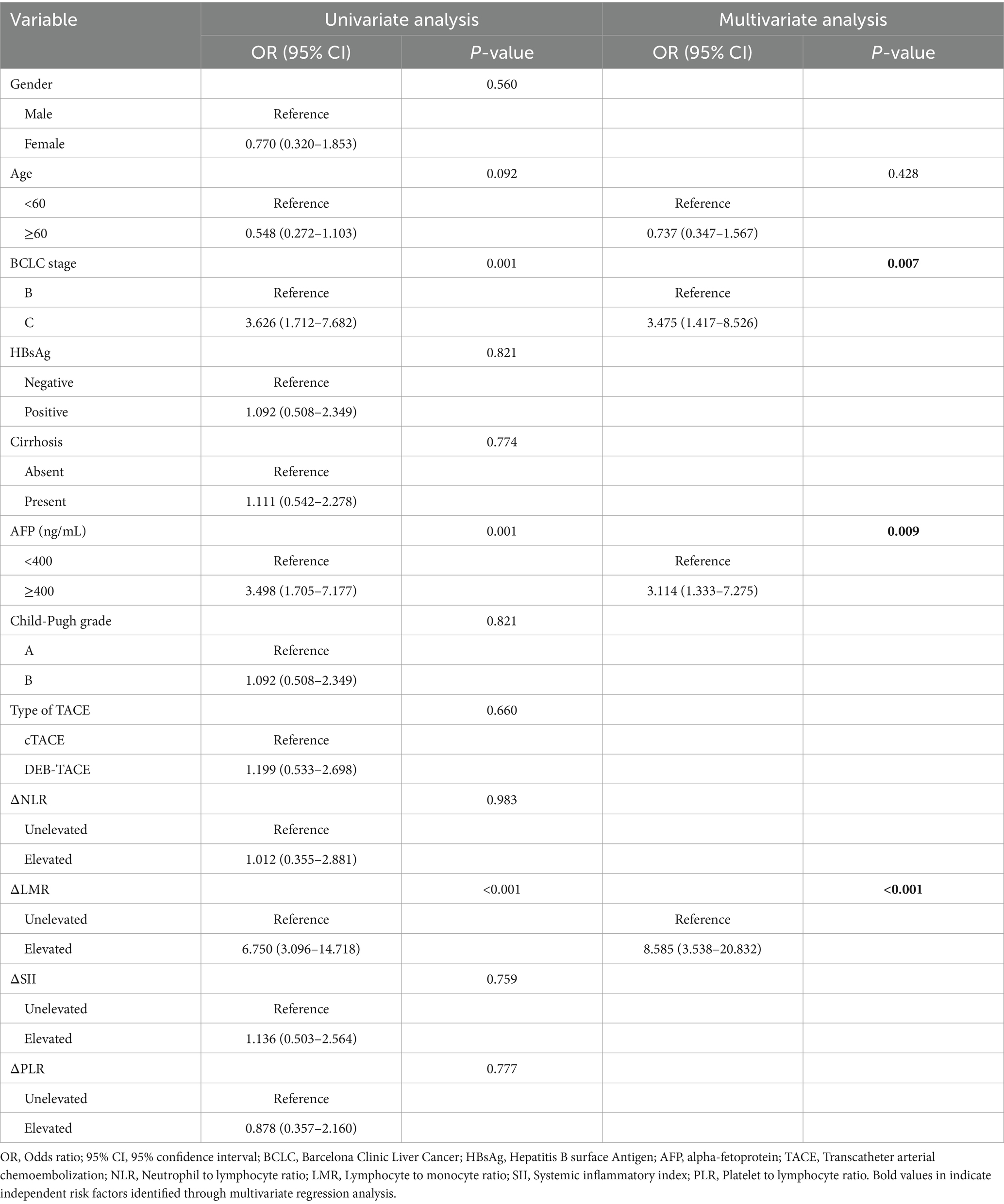
Table 3. Uni- and multivariate logistic regression analysis of the risk factors of ineffective response for the initial TACE.
In Cox univariate regression analysis, BCLC stage, AFP level, alteration of LMR, and initial TACE response significantly influenced PFS. Multivariate analysis identified BCLC stage C [Hazard Ratio (HR): 1.651; 95% CI: 1.040–2.619], AFP ≥ 400 ng/mL (HR: 1.662; 95% CI: 1.060–2.608), and initial TACE treatment ineffective (HR: 2.116; 95% CI: 1.327–3.376) as independent risk factors for disease progression. Table 4 presents the detailed result.
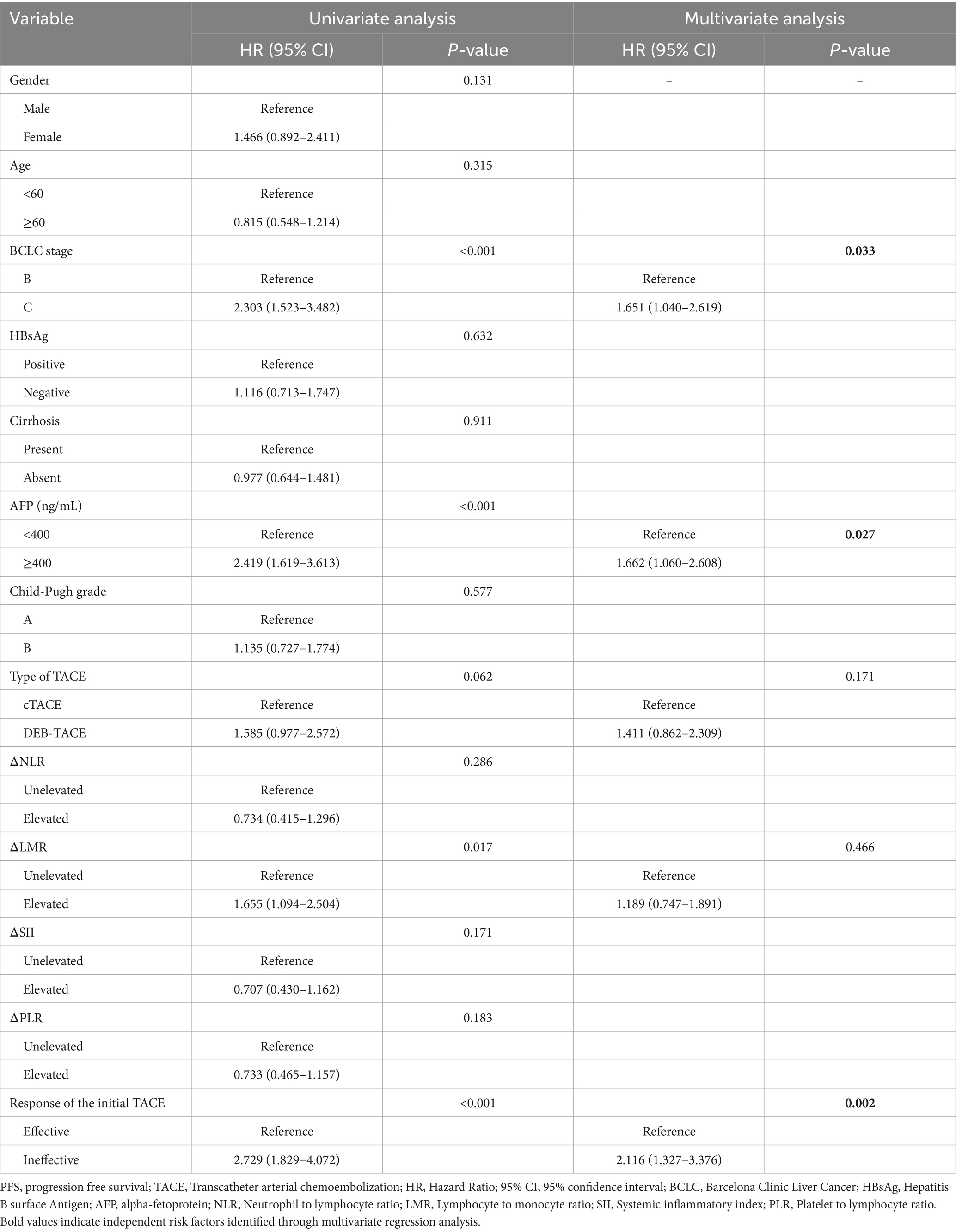
Table 4. Uni- and multivariate Cox regression analysis of the risk factors associated with lower PFS after the initial TACE.
For OS, univariate regression analysis revealed a significant association with BCLC stage, AFP level, and response of the initial TACE. Multivariate analysis showed that BCLC stage C (HR: 2.473; 95% CI: 1.534–3.987), Child-Pugh grade B (HR: 1.759; 95% CI: 1.117–2.770), and the ineffective initial TACE treatment (HR: 1.802; 95% CI: 1.153–2.818) were independent risk factors for mortality. Detailed results are shown in Table 5.

Table 5. Uni- and multivariate Cox regression analysis of the risk factors associated with lower OS after the initial TACE.
Before PSM, 102 patients were in the unelevated LMR group, and 44 patients were in the elevated group. The unelevated LMR group had a significantly lower rate of BCLC stage C (p = 0.001), positive HBsAg (p = 0.042), and AFP ≥400 ng/mL (p = 0.021), along with higher pre-TACE LMR (p = 0.004). Treatment efficacy was 79.4% in the unelevated group compared to 36.4% in the elevated group (p < 0.001).
After one-to-one PSM analysis, 39 patient pairs were matched, and baseline characteristics showed no significant differences (Table 1). The estimated median PFS was 9.7 months in the unelevated LMR group and 4.5 months in the elevated group before PSM (p = 0.016) (Figure 2A). No significant difference in OS was observed between the groups (p = 0.493), with estimated median OS of 21.2 and 16.9 months, respectively (Figure 2B).
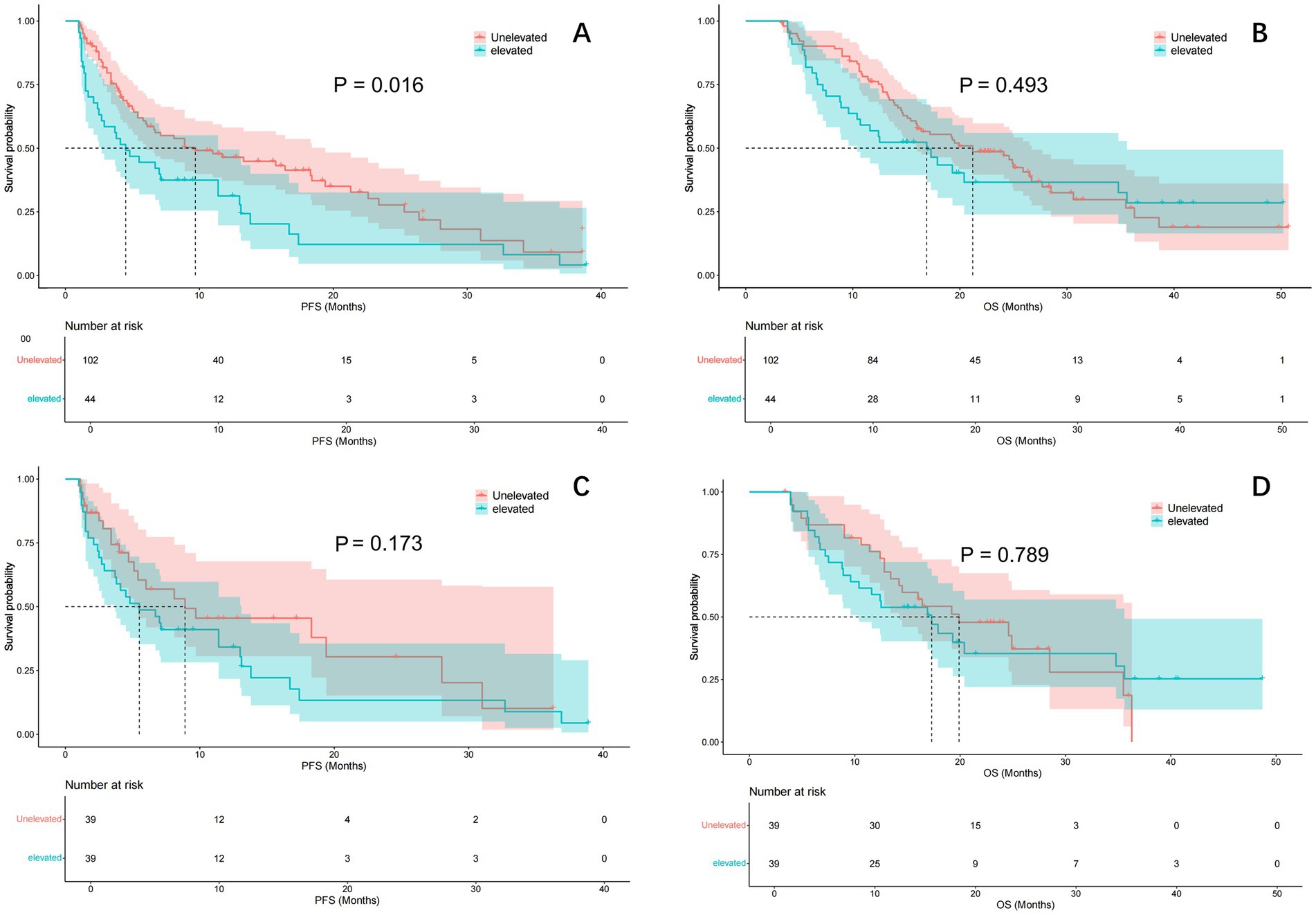
Figure 2. Comparison of PFS and OS curve between ΔLMR group before and after PSM. Kaplan–Meier curve comparison of patients with unelevated and elevated LMR group: (A) PFS; (B) OS; (C) PFS after adjustment by PSM; (D) OS after adjustment by PSM. PFS, progression-free survival; OS, overall survival; LMR, lymphocyte to monocyte ratio; PSM, propensity score matching.
Post-PSM analysis showed a treatment efficacy of 48.7% in the unelevated LMR group and 38.5% in the elevated group (p = 0.214). The estimated median PFS was 8.9 and 5.5 months in the unelevated and elevated groups, respectively (p = 0.173) (Figure 2C). The estimated median OS was 19.9 and 17.3 months in the unelevated and elevated groups, respectively (p = 0.789) (Figure 2D).
Our study evaluated the prognostic significance of changes in peripheral inflammatory indices pre- and post-TACE in HCC patients. Our findings indicate that alterations in NLR, SII, and PLR are not significantly associated with TACE efficacy or prognosis. However, elevated LMR was linked to poorer treatment response of the initial TACE and inferior PFS before PSM, although this association diminished after adjustment. The role of alteration of peripheral inflammatory index pre- and post-TACE is seldom studied, but several preoperative inflammatory markers have been explored as predictive biomarkers in HCC. The peripheral inflammatory index could reflect the interaction between the tumor biology, the tumor stromal microenvironment (TME), and the immune response (18). Thus, it possesses the capability to predict treatment response, recurrence, progression, and overall survival of TACE (19, 20). A retrospective study conducted by Minic et al. (8) divided pre-TACE LMR into the low and high groups by 2.24, and the high LMR group exhibited superior TACE efficacy and better PFS. Similarly, Yang et al. (21) reported that high preoperative LMR predicted poor OS and disease-free survival (DFS) in HCC patients undergoing hepatectomy, while NLR and PLR did not.
Our study found that the elevated LMR group presented with significantly lower pre-TACE LMR levels, more advanced BCLC stage, HBsAg positivity, and elevated AFP levels. However, after PSM, the previously observed advantage of unelevated LMR on treatment response and PFS was no longer significant. This suggests that the observed associations may be confounded by baseline patient characteristics rather than the alteration in LMR itself. LMR is calculated by both the count of lymphocytes and monocytes in the peripheral blood. Lymphocytes are a type of inflammatory cell that is also responsible for anti-tumor immunity. The participation of lymphocytes (such as T cells) in tumor infiltration is generally associated with improved prognosis in cancer patients and has been applied for targeted therapy (22). Lymphopenia may indicate abnormal immune mechanisms and a decrease in immune surveillance to remove tumor cells that may affect the tumor microenvironment (23). Thus, the decrease in circulating lymphocytes is detrimental to tumor control. As for monocytes, those cells migrate to liver cancer tissues in the blood circulation and differentiate into macrophages and dendritic cells (DCs), demonstrating both pro-tumor and anti-tumor effects. Monocytes can produce tumor-killing mediators and stimulate NK cells (24). However, in the tumor microenvironment (TME), monocytes also promote immunosuppression, extracellular matrix (ECM) remodeling, angiogenesis, and cancer cell intravasation. In addition, monocytes can differentiate into tumor-associated monocytes (TAMs) that support tumor growth (25). All in all, lower LMR is associated with a rough intrahepatic immune microenvironment, thus leading to a more malignant characteristic, resistance to locoregional therapy, and being more prone to progression.
Interestingly, TACE-induced tumor embolization may also modulate the intrahepatic immune response. Animal research by Tischfield et al. (26) discovered that embolization of the HCC lesions could regulate intrahepatic lymphocytes, with upregulated CD4+ cells and downregulated CD8+ cells, and a reduction in the proportion of CD25+/CD4 + cells within the liver and target lesions. Embolization could also recruit tumor-infiltrated lymphocytes (TIL) and modulate PD-L1 expression. However, the relationship between intrahepatic immune changes and peripheral inflammatory indices remains unclear. Investigating the immune cell composition within tumors and correlating these findings with circulating biomarkers such as cytokines or microRNAs may provide deeper insights into treatment response and disease progression in HCC. Combined with our findings, peripheral inflammatory responses may not reliably predict TACE outcomes due to the complexity of immune modulation within the TME (27).
Our study also identified BCLC stage C, AFP ≥ 400 ng/mL, and elevated LMR as independent risk factors for ineffective initial TACE treatment. In the Cox regression analysis of PFS and OS, BCLC stage C, AFP ≥ 400 ng/mL, and initial TACE treatment ineffective were independent risk factors for disease progression, while BCLC stage C, Child-Pugh grade B, and initial TACE treatment ineffective were independent risk factors for death after the initial TACE. These findings are consistent with previous studies highlighting the prognostic importance of tumor burden and treatment response (3, 4). A retrospective study by Kim et al. (28) concluded that achievement of objective response, in particular CR, at the initial TACE is still the robust prognostic predictor. Moreover, the concept of TACE refractory, defined as non-response after multiple TACE sessions, is receiving more attention, and patients exhibiting this pattern should be promptly considered for switch to systemic therapy (29).
There were several limitations in our study. The single-center, retrospective design and relatively small sample size may limit generalizability and introduce selection bias. Although PSM was applied to mitigate potential confounding and bias, residual heterogeneities between groups may persist. In addition, this small study conducted by PSM has some risk of overmatching. Furthermore, factors influencing inflammatory responses, such as cirrhosis, embolization extent, and infections, were not fully controlled. Another limitation is the lack of data on body mass index (BMI), which may influence inflammatory responses in cancer. Evidence suggests that underweight status may interact with inflammatory markers in gastrointestinal cancer, while overweight status in HBV-associated liver cancer has been associated with reduced PFS after TACE and persistently elevated peripheral inflammatory markers (30, 31). Future studies should explore the role of BMI in these associations to provide a more comprehensive understanding. Further multicenter, larger, prospective randomized studies are warranted to validate our findings.
TACE remains a widely used treatment for intermediate and selected advanced HCC patients, with variable predicted treatment efficacy and prognosis. Our study demonstrates that alterations in peripheral inflammatory indices, including ΔNLR, ΔLMR, ΔSII, and ΔPLR, may not serve as reliable prognostic markers in this population. Future research should focus on the intrahepatic or intratumoral microenvironment and its modulation by TACE to better understand the complex interplay between the immune system and treatment outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Hunan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the retrospective nature of our study.
CL: Writing – original draft. HX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. JT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research and/or publication of this article. This project was supported by the 2023 National Key Clinical Specialty Major Scientific Research Project of Hunan Provincial Health Commission (Z2023093).
We sincerely thank all colleagues, research staff, and medical professionals who contributed to this study. We also appreciate the support provided by our institution and the valuable assistance of the data analysts and technical staff. Lastly, we express our gratitude to the study participants for their involvement, which made this research possible.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cao, W, Qin, K, Li, F, and Chen, W. Comparative study of cancer profiles between 2020 and 2022 using global cancer statistics (GLOBOCAN). J Natl Cancer Cent. (2024) 4:128–34. doi: 10.1016/j.jncc.2024.05.001
2. Marrero, JA, Kulik, LM, Sirlin, CB, Zhu, AX, Finn, RS, Abecassis, MM, et al. Diagnosis, staging, and Management of Hepatocellular Carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
3. Tan, J, Mathy, RM, Chang, DH, Tang, T, Zhang, ZS, and Xiao, YD. Combined transarterial iodized oil injection and computed tomography-guided thermal ablation for hepatocellular carcinoma: utility of the iodized oil retention pattern. Abdom Radiol. (2022) 47:431–42. doi: 10.1007/s00261-021-03305-3
4. Llovet, JM, Kelley, RK, Villanueva, A, Singal, AG, Pikarsky, E, Roayaie, S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2024) 10:10. doi: 10.1038/s41572-024-00500-6
5. Reig, M, Forner, A, Rimola, J, Ferrer-Fàbrega, J, Burrel, M, Garcia-Criado, Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
6. Lan, B, Luo, C, Pocha, C, Wang, Q, and Tan, J. Comparison of various liver cancer staging systems in predicting prognosis after initial transcatheter arterial chemoembolization: a retrospective study from China. J Gastrointest Oncol. (2024) 15:2599–612. doi: 10.21037/jgo-2024-850
7. Li, J, Yang, S, Li, Y, Li, C, Xia, Y, Zhu, S, et al. The C-reactive protein to albumin ratio is an independent prognostic factor in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a large cohort study. Cardiovasc Intervent Radiol. (2022) 45:1295–303. doi: 10.1007/s00270-022-03208-w
8. Minici, R, Venturini, M, Guzzardi, G, Fontana, F, Coppola, A, Piacentino, F, et al. Prognostic role of lymphocyte-to-monocyte ratio (LMR) in patients with intermediate-stage hepatocellular carcinoma (HCC) undergoing Chemoembolizations (DEM-TACE or cTACE) of the liver: exploring the link between tumor microenvironment and interventional radiology. Diseases. (2024) 12:137. doi: 10.3390/diseases12070137
9. Minici, R, Siciliano, MA, Ammendola, M, Santoro, RC, Barbieri, V, Ranieri, G, et al. Prognostic role of neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-C reactive protein ratio (LCR) in patients with hepatocellular carcinoma (HCC) undergoing Chemoembolizations (TACE) of the liver: the unexplored corner linking tumor microenvironment, biomarkers and interventional radiology. Cancers. (2022) 15:257. doi: 10.3390/cancers15010257
10. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
11. Chernyak, V, Fowler, KJ, Kamaya, A, Kielar, AZ, Elsayes, KM, Bashir, MR, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. (2018) 289:816–30. doi: 10.1148/radiol.2018181494
12. Nakamura, H, Hashimoto, T, Oi, H, and Sawada, S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. (1989) 170:783–6. doi: 10.1148/radiology.170.3.2536946
13. Gaba, RC, Lewandowski, RJ, Hickey, R, Baerlocher, MO, Cohen, EI, Dariushnia, SR, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. (2016) 27:457–73. doi: 10.1016/j.jvir.2015.12.752
14. Lencioni, R, and Llovet, JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
15. D’Agostino, RJ. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. (1998) 17:2265–81. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B
16. Therneau, TM, and Grambsch, PM. Modeling survival data: Extending the Cox model. New York, NY: Springer (2000).
17. Kassambara, A, Kosinski, M, and Biecek, P. (2021). Survminer: Drawing survival curves using 'ggplot2' R package version 0.4.9. Available online at: https://CRAN.R-project.org/package=survminer (Accessed February 23, 2025).
18. Liu, C, Zhao, H, Zhang, R, Guo, Z, Wang, P, and Qu, Z. Prognostic value of nutritional and inflammatory markers in patients with hepatocellular carcinoma who receive immune checkpoint inhibitors. Oncol Lett. (2023) 26:437. doi: 10.3892/ol.2023.14024
19. Zheng, J, Cai, J, Li, H, Zeng, K, He, L, Fu, H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a Meta-analysis and systematic review. Cell Physiol Biochem. (2017) 44:967–81. doi: 10.1159/000485396
20. Mei, J, Sun, XQ, Lin, WP, Li, SH, Lu, LH, Zou, JW, et al. Comparison of the prognostic value of inflammation-based scores in patients with hepatocellular carcinoma after anti-PD-1 therapy. J Inflamm Res. (2021) 14:3879–90. doi: 10.2147/JIR.S325600
21. Yang, YT, Jiang, JH, Yang, HJ, Wu, ZJ, Xiao, ZM, and Xiang, BD. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival compared to established biomarkers in HCC patients undergoing liver resection. Sci Rep. (2018) 8:2535. doi: 10.1038/s41598-018-20199-2
22. Liu, C, Hu, Q, Hu, K, Su, H, Shi, F, Kong, L, et al. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. J Transl Med. (2019) 17:120. doi: 10.1186/s12967-019-1872-9
23. Byun, HK, Kim, N, Park, S, and Seong, J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol. (2019) 195:1007–17. doi: 10.1007/s00066-019-01462-5
24. Wang, H, Zhou, Y, Oyang, L, Han, Y, Xia, L, Lin, J, et al. LPLUNC1 stabilises PHB1 by counteracting TRIM21-mediated ubiquitination to inhibit NF-κB activity in nasopharyngeal carcinoma. Oncogene. (2019) 38:5062–75. doi: 10.1038/s41388-019-0778-6
25. Nakamura, K, and Smyth, MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. (2020) 17:1–12. doi: 10.1038/s41423-019-0306-1
26. Tischfield, DJ, Gurevich, A, Johnson, O, Gatmaytan, I, Nadolski, GJ, Soulen, MC, et al. Transarterial embolization modulates the immune response within target and nontarget hepatocellular carcinomas in a rat model. Radiology. (2022) 303:215–25. doi: 10.1148/radiol.211028
27. de Visser, KE, and Joyce, JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. (2023) 41:374–403. doi: 10.1016/j.ccell.2023.02.016
28. Kim, BK, Kim, SU, Kim, KA, Chung, YE, Kim, MJ, Park, MS, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. (2015) 62:1304–10. doi: 10.1016/j.jhep.2015.01.022
29. Kudo, M, Kawamura, Y, Hasegawa, K, Tateishi, R, Kariyama, K, Shiina, S, et al. Management of Hepatocellular Carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. (2021) 10:181–223. doi: 10.1159/000514174
30. Gao, X, Pan, Y, Han, W, Hu, C, Wang, C, Chen, L, et al. Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas. Cancer Biol Med. (2021) 18:283–97. doi: 10.20892/j.issn.2095-3941.2020.0246
Keywords: hepatocellular carcinoma, chemoembolization, peripheral inflammation, inflammation index, prognosis
Citation: Luo C, Xiang H and Tan J (2025) The efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma: is the alteration of the inflammation index important? Front. Med. 12:1543903. doi: 10.3389/fmed.2025.1543903
Received: 12 December 2024; Accepted: 03 March 2025;
Published: 14 March 2025.
Edited by:
Aaron Balasingam Koenig, Celia Scott Weatherhead School of Public Health and Tropical Medicine, United StatesReviewed by:
Tamer A. Addissouky, University of Menoufia, EgyptCopyright © 2025 Luo, Xiang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Xiang, dmlweGlhbmdoQDE2My5jb20=; Jie Tan, NzY5ODYzMDc1QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.