
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 24 March 2025
Sec. Obstetrics and Gynecology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1540685
Introduction: Umbilical artery thrombosis (UAT) is a rare but serious pregnancy complication, potentially causing fetal growth restriction, distress, and stillbirth. Diagnosis relies on Doppler ultrasound and pathological assessment. Close monitoring and potential low-molecular-weight heparin (LMWH) therapy aim to prolong gestation and improve outcomes, but debate persists on its efficacy compared to expectant management.
Methods: A retrospective study, conducted between January 2013 and December 2023, enrolled singleton pregnant women diagnosed with UAT during pregnancy. The experiment group included pregnant women who underwent LMWH with anti-coagulation therapy during pregnancy, while the expectant group comprised pregnancies that received standard prenatal care without any specific intervention for UAT.
Results: The expectant group showed a significant increase in birth weight (expectant vs. experiment: 2434.40 ± 770.20 g vs. 1874.46 ± 717.83 g, P < 0.05) and a significant decrease in the incidence of births before 34 weeks (expectant vs. experiment: 42.24% vs. 82.75%, P < 0.05). Gestational age at birth was notably higher in the expectant group as compared to the experiment group (35.32 ± 3.89 vs. 33.59 ± 4.17), although the difference did not reach statistical significance (p = 0.110). The multi-factor ANOVA revealed statistically significant effects of anti-coagulation therapy (F = 4.479, p = 0.039) and gestational age at birth (F = 179.110, p = 0.000) on birth weight. This study found that the relationship between these variables can be formulated as: birth weight = −3314.782–256.106 × anti-coagulation therapy (coded as 1 if yes and 0 if no) +161.858 × gestational age at birth.
Conclusion: Our study suggests that expectant therapy may offer substantial benefits compared to experimental therapy involving the administration of LMWH.
Umbilical artery thrombosis (UAT), a rare pregnancy complication, has an estimated incidence ranging from 0.0025 to 0.045% (1). This condition can lead to fetal growth restriction, fetal distress, and stillbirth (2, 3). Currently, the diagnosis of UAT relies primarily on Doppler ultrasound imaging and pathological assessment of the umbilical cord after delivery.
UAT carries the risk of sudden fetal death, deciding to terminate pregnancy a potentially viable option in the third trimester to avoid intrauterine fetal death; however, it is imperative to acknowledge that this course of action may result in an increased risk of iatrogenic preterm birth. Therefore, close monitoring of fetal conditions and growth trends via therapeutic management has been recognized as a strategy that potentially prolongs the gestational period and enhances neonatal outcomes (2, 4, 5). For instance, Wang et al. reported that the administration of low-molecular-weight heparin (LMWH) to prevent the progression of UAT holds the potential to enhance pregnancy outcomes (6). Additionally, Li noted that anticoagulation therapy of LMWH combined with aspirin could reduce the occurrence of adverse pregnancy outcomes (7). However, in other research on expectant management where the application of LMWH was not used, similar perinatal outcomes were achieved (3, 5, 8, 9). The question of whether LMWH holds a distinct advantage over expectant management remains a subject of ongoing debate, particularly in terms of its potential therapeutic benefits. Consequently, we conducted the current study to evaluate the efficacy of LMWH in managing UAT during pregnancy.
This study was conducted retrospectively, involving a comprehensive review of all delivery cases recorded at the Women’s Hospital, Zhejiang University School of Medicine, between January 2013 and December 2023. Patients suspected of having UAT, based on either ultrasonographic imaging or pathological examination, underwent a comprehensive review by ultrasonography experts and pathology experts, respectively. Following this thorough evaluation, only those participants with a confirmed diagnosis of UAT were selected and included in the study.
The ultrasound screening is performed by the direct visualization of the umbilical cord or by tracking the umbilical arteries around the fetal bladder with color Doppler technology (10). The diagnosis of UAT is established when an initial ultrasound examination in the first trimester of pregnancy indicates normal umbilical artery flow, but later scans reveal the presence of a single umbilical artery.
Upon staining with Hematoxylin–Eosin, sections of the umbilical cord revealed the presence of thrombosis (specifically, fibrinous, mixed, or red thrombus) within one of the umbilical arteries. This thrombosis exhibited features that ranged from total or partial necrosis of the artery wall to cases where no evident necrosis was observed (11).
The study population was assigned into two distinct groups: the experimental group and the expectant group, based on the treatment regimen they received. Participants in the experimental group received LMWH (nadroparin calcium 4,100 U daily or enoxaparin sodium 4,000 U daily) for anti-coagulation therapy, underwent rigorous ultrasound surveillance, non-stress testing beyond 28 weeks of gestation, and were instructed to closely monitor fetal movements. In contrast, participants in the expectant group received only standard prenatal care.
Data pertaining to maternal age, gravidity, parity, gestational weeks at diagnosis, gestational weeks at delivery, and neonatal outcomes such as birth weight, Apgar scores, cesarean delivery, fetal distress, neonatal morbidity, and newborn intensive care unit (NICU) admission were collected and analyzed.
Statistical analysis was carried out with SPSS 25.0 for Microsoft Windows (IBM Corp., Armonk, NY, USA). Comparisons of continuous variables between groups were performed using Student’s t-test (for data that were normally distributed) or Mann–Whitney U-test (for data that exhibited non-normal distribution), while comparisons of categorical variables were conducted with the χ2 test. Multi-factor ANOVA was used to investigate the effects of independent variables on birth weight. Multivariable logistic regression models were utilized to explore the association between the independent variables and birth weight. A p-value of < 0.05 was considered statistically significant.
During the study period, a total of 182,942 deliveries were recorded. Among these, 65 pregnancies were diagnosed with UAT. However, eight cases were subsequently excluded due to fetal death at presentation. Finally, a total of 57 participants with UAT were included in the analysis, with 29 participants assigned to the experiment group and the remaining 28 designated to the expectant group. Details are presented in Figure 1.
The results of the comparison pertaining to baseline clinical characteristics between the experiment group and the expectant group are presented in Table 1. Consequently, no significant differences were noted in terms of maternal age (30.14 ± 4.23 vs. 30.79 ± 3.92, p = 0.552), BMI (26.75 ± 4.99 vs. 26.05 ± 3.09, p = 0.527), gravidity (1.97 ± 1.38 vs. 2.07 ± 1.33, p = 0.769), primigravida (48.28% vs. 46.43%, p = 1.000), parity (0.41 ± 0.68 vs. 0.43 ± 0.57, p = 0.930), primipara (31.03% vs. 39.29%, p = 0.585), and gestational age at diagnosis (29.90 ± 4.62 vs. 31.00 ± 4.31, p = 0.497).
The results of the comparison of clinical outcomes between the experiment group and the expectant group are presented in Table 2. The birth weight in the experiment group was significantly lower compared to the expectant group (1874.46 ± 717.83 vs. 2434.40 ± 770.20, p = 0.008), while the proportion of births before 34 weeks was significantly higher in the experiment group than the expectant group (82.75% vs. 42.24%, p = 0.001), indicating a higher prevalence of early preterm delivery (<34 weeks of gestation) and lower birth weight in the experiment group. Gestational age at birth was notably lower in the experiment group as compared to the expectant group, although the difference did not reach statistical significance. No significant differences were noted in terms of Apgar scores, cesarean delivery, fetal distress, neonatal morbidity, and NICU stay.
The results of the multi-factor ANOVA are summarized in Table 3. The ANOVA revealed statistically significant anti-coagulation therapy (LMWH for participants in the experiment group) (F = 4.479, p = 0.039) and gestational age at birth (F = 179.110, p = 0.000) on birth weight.
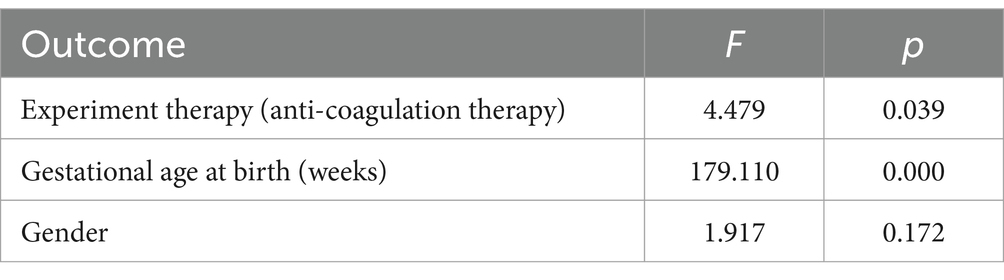
Table 3. Multi-factor ANOVA for birth weight: anti-coagulation therapy, gestational age at birth, and gender.
The results in Table 4 indicated a significant negative association of anti-coagulation therapy (β = −0.164, p = 0.014) and a strong positive correlation of gestational age (β = 0.846, p < 0.001) with birth weight. The final linear regression equation can be expressed as: birth weight = −3314.782–256.106 × anti-coagulation therapy +161.858 × gestational age at birth (weeks).
The key findings of our study were: (1) expectant therapy had a significant advantage over experiment therapy in pregnancies with UAT, as the administration of LMWH resulted in a significant decrease in birth weight and a substantial increase in the incidence of preterm birth (<34 weeks); (2) the relationship between these variables can be formulated as: birth weight = −3314.782–256.106 × anti-coagulation therapy +161.858 × gestational age at birth. The key findings are incorporated and presented in Figure 2.
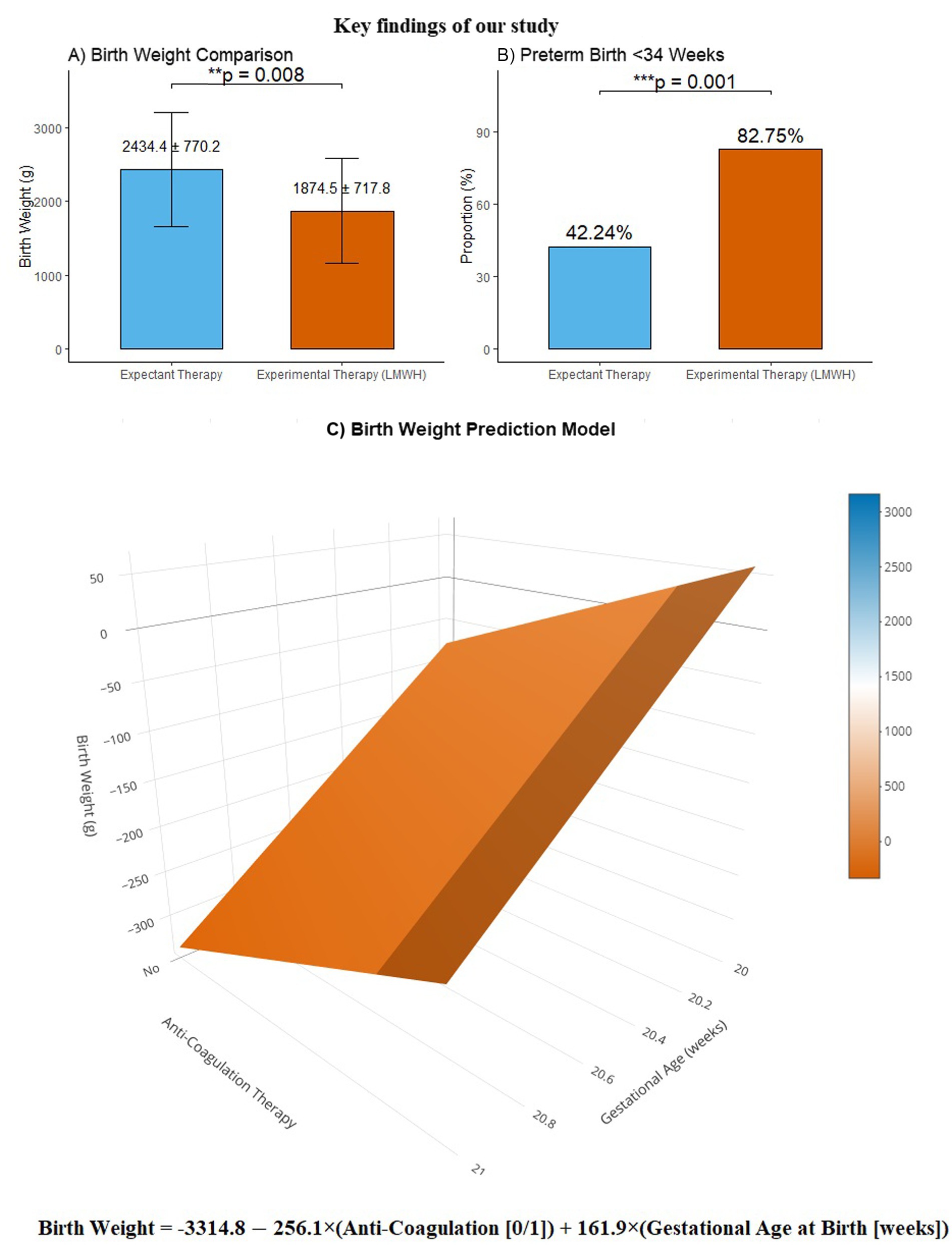
Figure 2. The key findings of our study. Panel (A) presents a comparative analysis of birth weight, demonstrating that the birth weight in the experiment group was significantly lower compared to the expectant group (1874.46 ± 717.83 vs. 2434.40 ± 770.20, p = 0.008). Panel (B) illustrates the distribution of preterm births before 34 weeks, showing that the proportion of births before 34 weeks was significantly higher in the experiment group than the expectant group (82.75% vs. 42.24%, p = 0.001). Panel (C) displays our birth weight prediction model, featuring the mathematical formula: “birth weight = −3314.782–256.106 × anti-coagulation therapy +161.858 × gestational age at birth”, presented in a three-dimensional surface plot for enhanced visualization.
UAT may occur after placenta thrombotic vasculopathy (12), umbilical cord abnormalities (3, 13, 14), and underlying maternal diseases (2, 15). At present, no consensus has been reached on the treatment strategy for UAT. In clinical practice, upon the occurrence of a fetal umbilical cord embolism, one would instinctively consider the implementation of anti-coagulation therapy to arrest the progression of thrombus emboli, thereby preventing complete occlusion of the umbilical vessels and reducing the subsequent risk of fetal death. For instance, Wang et al. (6) reviewed 10 cases of pregnancies with UAT. Notably, all participants in the study received treatment with LMWH, and there was no control group of expectant mothers. Based on their findings, the researchers concluded that the early administration of LMWHs may enhance pregnancy outcomes. However, there have been arguments raised against the use of anticoagulation treatment. Wei et al. reported a case series revealing that the expectant management of UAT had comparable fetal outcomes to those observed in patients who received anti-coagulation management (3). Han et al. demonstrated that expectant treatment of patients with UAT had apparent positive effects for extending gestational age, which was supported by another study conducted by Dindinger et al. (5, 9). Our study found that expectant management had significant advantages compared to anti-coagulation therapy, as the latter, when combined with frequent ultrasound surveillance, could induce anxiety in both patients and clinicians, resulting in unnecessary early medical interventions to deliver the fetus.
Our study has several strengths. First, it was designed as a retrospective study with two arms (expectant vs. experiment), whereas the previous studies (2–4, 6, 7) were primarily case reports. Second, we used multi-factor ANOVA to analyze the associations between the expectant group and the experiment group, leveraging its advantages in accounting for the potential confounding effects of multiple variables. Third, due to the rigorous design and comprehensive analysis, our results were more aligned with real-world clinical logic and practical experiences, indicating a greater degree of applicability and reliability. However, it is important to acknowledge that the retrospective design of our study, coupled with the relatively small sample size, may result in incomplete data and an increased risk of bias.
In conclusion, our study provides valuable insights into the management of UAT and suggests that expectant therapy may offer significant advantages over experimental therapy involving LMWH administration. These findings have important implications for clinical practice and future research in this area.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Ethics Committe of Women’s Hospital, School of Medicine, Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
PZ: Conceptualization, Methodology, Supervision, Writing – review & editing. YL: Visualization, Writing – original draft. SL: Data curation, Investigation, Writing – review & editing. LZ: Software, Writing – review & editing, Formal Analysis. CC: Writing – review & editing, Data curation, Investigation. XY: Writing – review & editing, Formal Analysis, Software.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kitano, T, Ohgitani, A, Takagi, K, Arai, I, Yasuhara, H, Ebisu, R, et al. A case of severe neonatal asphyxia due to umbilical artery thrombosis. J Obstet Gynaecol. (2018) 38:1164–5. doi: 10.1080/01443615.2017.1404012
2. Wu, X, Wei, C, Chen, R, Yang, L, Huang, W, Huang, L, et al. Fetal umbilical artery thrombosis: prenatal diagnosis, treatment and follow-up. Orphanet J Rare Dis. (2022) 17:414. doi: 10.1186/s13023-022-02563-8
3. Wei, J, Li, Q, and Zhai, H. Umbilical artery thrombosis diagnosed at different gestational ages and fetal outcomes: a case series. BMC Pregnancy Childbirth. (2021) 21:788. doi: 10.1186/s12884-021-04264-9
4. Tu, P, Zhang, X, Zhong, C, Ran, Q, and Ran, S. Hemodynamic changes and perinatal outcome associated with umbilical artery thrombosis: a retrospective study. Orphanet J Rare Dis. (2024) 19:100. doi: 10.1186/s13023-024-03107-y
5. Han, C, Dong, K, Jia, Z, Zhao, G, Chen, W, and Liu, H. Expectant management for umbilical artery thrombosis: a report of two cases and literature review. J Matern Fetal Neonatal Med. (2022) 35:9296–8. doi: 10.1080/14767058.2022.2029398
6. Wang, T, Yao, Y, Xu, T, Wang, W, Zhou, Y, He, J, et al. Application of low molecular weight heparins in umbilical artery thrombosis: a case series and review of the literature. Medicine (Baltimore). (2023) 102:e33501. doi: 10.1097/MD.0000000000033501
7. Li, X, Chen, W, Liu, T, Cai, J, Wei, S, Du, Y, et al. Umbilical artery thrombosis and maternal positive autoimmune antibodies: two case reports and a literature review. Front Med (Lausanne). (2023) 10:1187492. doi: 10.3389/fmed.2023.1187492
8. Gladstone, RA, Parks, W, and Kingdom, J. Spontaneous umbilical artery thrombosis mediating fetal growth restriction. J Obstet Gynaecol Can. (2024) 46:102123. doi: 10.1016/j.jogc.2023.04.014
9. Dindinger, R, and Christopher, M. Case report of umbilical artery thrombosis in a patient with a history of heparin-induced thrombocytopenia. J Obstet Gynecol Neonatal Nurs. (2023) 52:501–8. doi: 10.1016/j.jogn.2023.07.003
10. Salomon, LJ, Alfirevic, Z, Berghella, V, Bilardo, CM, Chalouhi, GE, Da, SCF, et al. Isuog practice guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. (2022) 59:840–56. doi: 10.1002/uog.24888
11. Romani, E, Marinelli, L, Ponziani, I, Masini, G, Castiglione, F, Nardi, E, et al. Umbilical artery thrombosis: a case report of prenatal diagnosis and systematic review of the literature. Eur J Obstet Gynecol Reprod Biol. (2024) 296:6–12. doi: 10.1016/j.ejogrb.2024.02.016
12. Saleemuddin, A, Tantbirojn, P, Sirois, K, Crum, CP, Boyd, TK, Tworoger, S, et al. Obstetric and perinatal complications in placentas with fetal thrombotic vasculopathy. Pediatr Dev Pathol. (2010) 13:459–64. doi: 10.2350/10-01-0774-OA.1
13. Hasegawa, J. Ultrasound screening of umbilical cord abnormalities and delivery management. Placenta. (2018) 62:66–78. doi: 10.1016/j.placenta.2017.12.003
14. Zhu, Y, Beejadhursing, R, and Liu, Y. 10 cases of umbilical cord thrombosis in the third trimester. Arch Gynecol Obstet. (2021) 304:59–64. doi: 10.1007/s00404-020-05910-x
Keywords: umbilical artery thrombosis, expectant management, low molecular weight heparin, birth weight, anti-coagulation therapy
Citation: Zhao P, Lu Y, Liu S, Zhang L, Chen C and Yang X (2025) Evaluating the efficacy of low-molecular-weight heparin in managing umbilical artery thrombosis during pregnancy: does it offer therapeutic benefits? Front. Med. 12:1540685. doi: 10.3389/fmed.2025.1540685
Received: 06 December 2024; Accepted: 05 March 2025;
Published: 24 March 2025.
Edited by:
Margherita Neri, University of Ferrara, ItalyReviewed by:
Abraham A. Pouliakis, National and Kapodistrian University of Athens, GreeceCopyright © 2025 Zhao, Lu, Liu, Zhang, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yicheng Lu, NTUxNTAyM0B6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.