
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 25 February 2025
Sec. Intensive Care Medicine and Anesthesiology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1539238
Background: Cipepofol is a highly selective gamma-aminobutyric acid A receptor potentiator. As a new sedative drug, detailed studies on its respiratory effects are further needed. The present study aims to investigate the effects of cipepofol on breathing patterns, respiratory drive, and inspiratory effort in mechanically ventilated patients.
Methods: In this one-arm physiological study, cipepofol was initiated at 0.3 mg/kg/h and increased by 0.1 mg/kg/h every 30 min until reaching 0.8 mg/kg/h. Discontinuation criteria were Richmond Agitation and Sedation Scale (RASS) score ≤ −4 or respiratory rate (RR) < 8 breaths/min or pulse oxygen saturation (SpO2) < 90%. The primary outcomes were changes from baseline in respiratory variables [RR, tidal volume (VT), minute ventilation (Vmin), airway occlusion pressure at 100 msec (P0.1), pressure muscle index (PMI), expiratory occlusion pressure (Pocc)] at 30 min after 0.3 mg/kg/h cipepofol infusion. The secondary outcomes included changes in respiratory variables, cardiorespiratory variables, and RASS scores at rates of cipepofol from 0.3 to 0.8 mg/kg/h.
Results: 20 patients were enrolled and all of them completed the cipepofol infusion rate at 0.3 mg/kg/h, achieving RASS score of −2 to +1. For the primary outcomes, there was a significant reduction in VT (390.9, [356.6–511.0] vs. 451.6 [393.5–565.9], p = 0.002), while changes in RR (16.7 ± 2.7 vs. 16.2 ± 3.4, p = 0.465) and Vmin (7.2 ± 1.8 vs. 7.5 ± 1.9, p = 0.154) were not significant. The reductions in P0.1 (p = 0.020), PMI (p = 0.019), and Pocc (p = 0.007) were significant. For secondary outcomes, as the infusion rate of cipepofol increased from 0.3 to 0.8 mg/kg/h, there was a further decrease in VT (p = 0.002) and an increase in RR (p < 0.001), while the change in Vmin (p = 0.430) was not significant. RASS score (p < 0.001) was further decreased.
Conclusion: Cipepofol demonstrates the capability to achieve RASS score −2 to +1 in mechanically ventilated adult patients. The effect of cipepofol on breathing patterns was a decrease in VT, while changes in RR and Vmin were insignificant. The effect on respiratory drive and inspiratory effort significantly reduced P0.1, PMI, and Pocc.
Clinical trial registration: ClinicalTrials.gov, identifier NCT06287138. https://clinicaltrials.gov/study/NCT06287138
Critically ill patients often experience noxious stimuli from endotracheal tubes, artificial ventilation, and other intensive care procedures such as bronchial suctioning, physiotherapy, and catheter placement (1, 2). Proper administration of analgesia and sedatives is crucial in the care of mechanically ventilated patients which relieves pain and anxiety, reduces stress, and prevents agitation-related harm (3). The paradigm of eCASH has established best practices in sedation management, emphasizing early comfort using analgesia, minimal sedatives, and maximal human care (4). However, sedative drugs come with potential adverse effects such as nausea, vomiting, and especially respiratory depression (5), which may increase the risk of complications and prolong the clinical course (6, 7). Consequently, this often leads to inappropriate use of such agents, sometimes with doses higher or lower than those required for adequate therapeutic effect (8). Hence, there is a great interest in measuring the respiratory effects of commonly used drugs, such as propofol (9, 10) and remifentanil (11), as well as assessing new agents in clinical settings.
Cipepofol (also known as ciprofol and HSK3486) was a structural analog of propofol. As a novel 2, 6-disubstituted phenol derivative, a cyclopropyl group was incorporated into the 2,6-side chain to increase its lipophilicity, and chiral centers were introduced to break the structure symmetry (12). Cipepofol produces the hypnotic effect mainly by enhancing gamma-aminobutyric acid type A (GABAA) receptor-mediated inhibitory synaptic currents (13), exhibiting about four to five times the potency of propofol (14, 15). The putative interactions between GABAA receptor and cipepofol are illustrated in Figure 1. Otherwise, like propofol, cipepofol produces rapid-onset action and clear wake-up with similar pharmacokinetic characteristics of absorption, distribution, and metabolism (13).
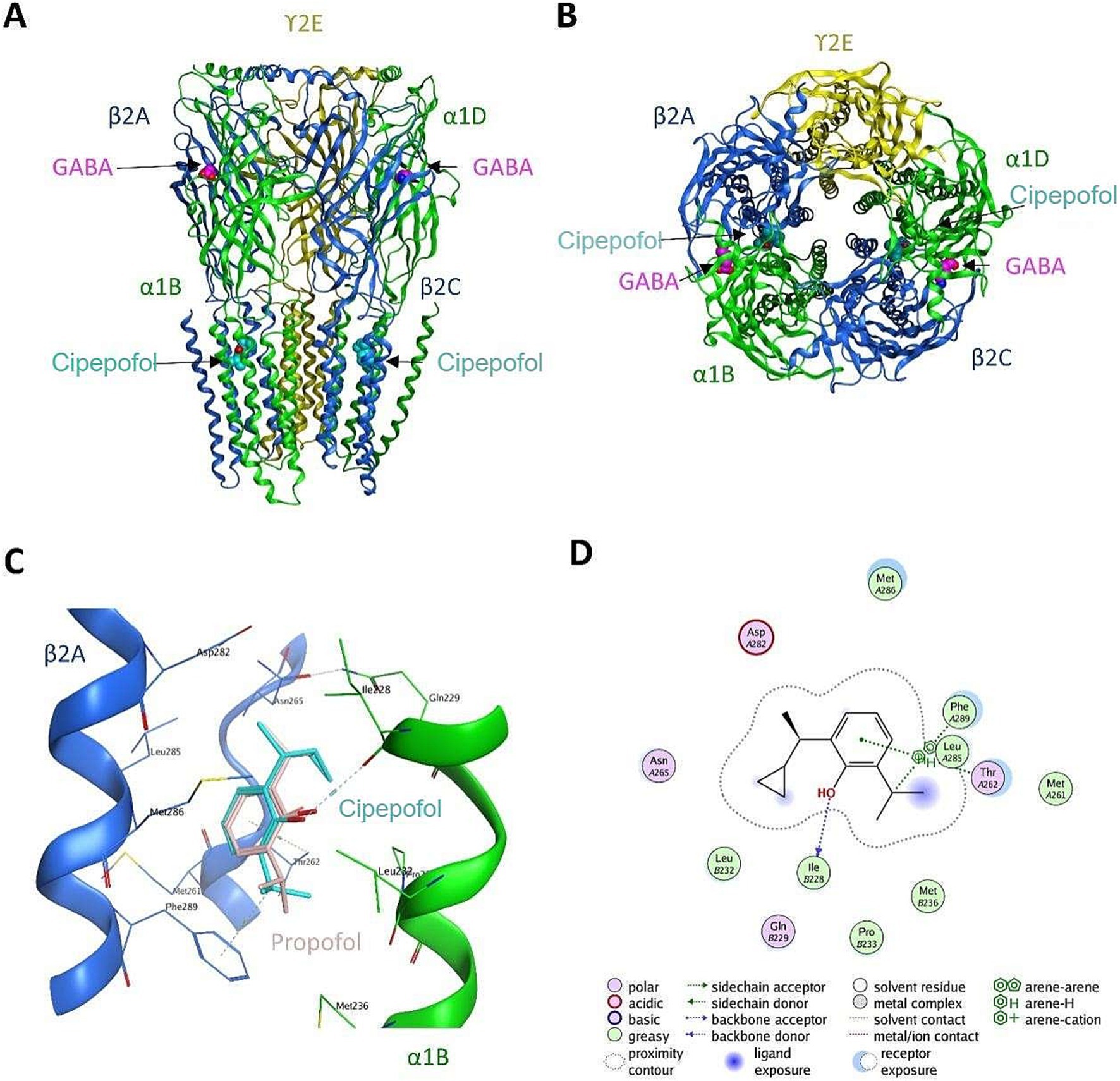
Figure 1. The putative interactions between GABAA receptor and cipepofol. (A) The front-view of cipepofol binding to the cavity between β2 subunit (blue) and α1 subunit (green). (B) The top-view of cipepofol binding to the cavity between β2 subunit (blue) and α1 subunit (green). (C) Hydrophobic packing was believed to be the major interaction between GABAA receptor and cipepofol, while a hydrogen bond was formed between Ile228 of α1 subunit and the hydroxyl group in cipepofol. The propofol molecule was also shown as reference (pink). (D) The 2D interaction diagram of GABAA-cipepofol.
In the phase II and III clinical trials, the tolerability and sedation characteristics were comparable between cipepofol and propofol in mechanically ventilated patients (16, 17). Cipepofol induced a milder reduction in mean arterial pressure (MAP) than propofol (18), and it poses a lower risk of respiratory depression (19). However, despite these findings, as a new sedative drug, detailed studies on its adverse effects, particularly its impact on respiration are still warranted. Until now, the effects of cipepofol on breathing patterns and respiratory drive during intensive care unit (ICU) sedation have not been well-described. Therefore, the present study aimed to investigate the effects of cipepofol on breathing patterns, respiratory drive, and inspiratory effort in mechanically ventilated patients.
The study was a single-center, prospective, physiological trial. This study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY2023 − 182-03). According to the Declaration of Helsinki, written informed consent was obtained from all patients or their legal representatives. The study was registered at ClinicalTrials.gov (NCT06287138), https://clinicaltrials.gov/study/NCT06287138.
Patients were consecutively recruited from December 2023 to February 2024. The inclusion criteria were critically ill adults with endotracheal intubation who received mechanical ventilation in pressure support mode after surgery under general anesthesia, and the patients were expected to receive sedation for a target of Richmond Agitation and Sedation Scale (RASS) score of −2 to +1. The exclusion criteria included age less than 18 years; body mass index (BMI) less than 18 or greater than 30 kg/m2; pregnancy or lactation; brain stem tumors, myasthenia gravis, or neuromuscular diseases; acute severe neurological disorder or any other condition interfering with RASS assessment; systolic blood pressure (SBP) less than 90 mmHg after appropriate fluid resuscitation; heart rate (HR) less than 50 beats per minute or second- or third-degree atrioventricular block without a pacemaker; contraindications or allergies to any study medications; acute hepatitis or serious hepatic dysfunction (Child-Pugh class C); chronic kidney disease with glomerular filtration rate less than 60 mL/min/1.73m2.
During the intervention period, when the patient’s baseline sedation level had reached a RASS score ≥ −2, cipepofol was given and initiated at 0.3 mg/kg/h, which dose was increased by 0.1 mg/kg/h every 30 min, until the maximal dose of 0.8 mg/kg/h, the titration method for cipepofol is described in Figure 2A. The predefined discontinuation criterion of the study was the maximal dose of cipepofol at 0.8 mg/kg/h, RASS score ≤ −4, respiratory rate < 8 breaths/min (20), or pulse oxygen saturation (SpO2) < 90% (21), whichever comes the first. RASS score (22, 23) was assessed before starting the infusion and 30 min after each increase in cipepofol rate, or more often if a fluctuation in the level of sedation was observed. The reason for discontinuation and the final rates given for each patient were recorded. The protocol stipulated a maximum infusion rate of 0.8 mg/kg/h for cipepofol, if the target RASS score was not reached, the patient would be excluded and sedatives would be given at the discretion of treating physicians.
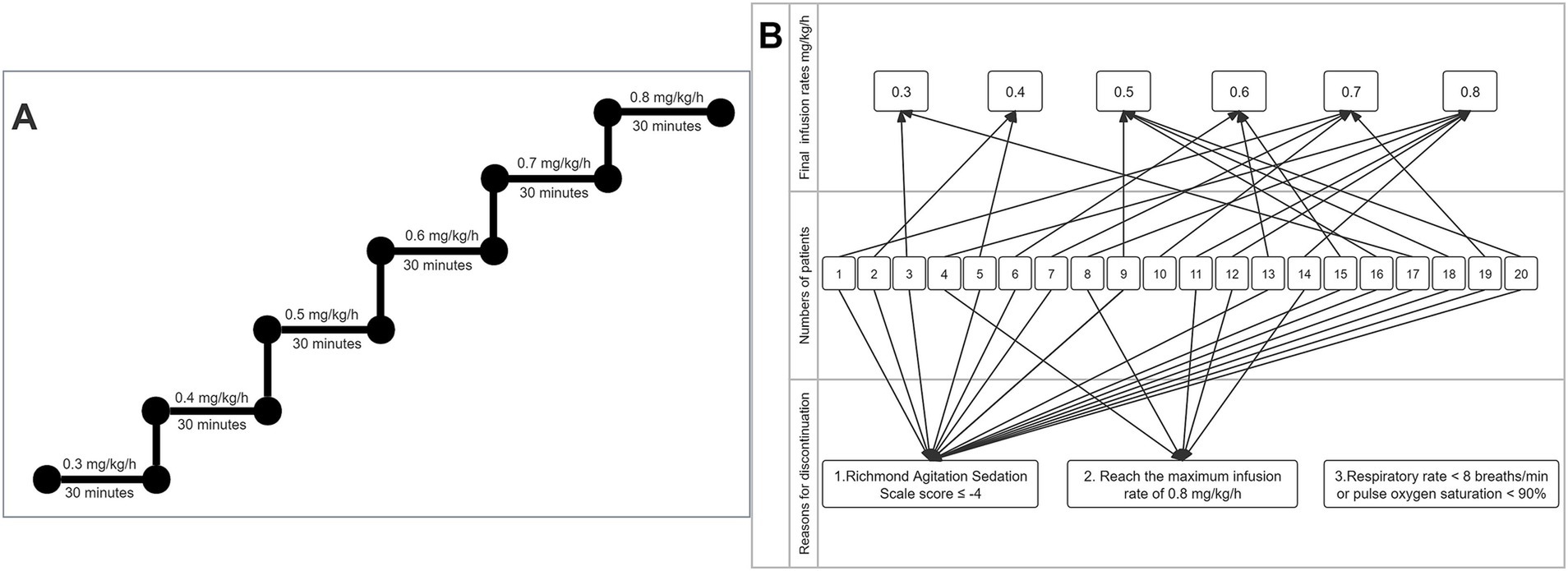
Figure 2. Process of cipepofol infusion. (A) Titration method of cipepofol. (B) Reasons for discontinuation and final rates are given.
Remifentanil was infused for analgesia before cipepofol administration, which dose was started at 0.01 μg/kg/min and adjusted to achieve a Critical-care Pain Observation Tool (CPOT) score of 0 to 1 (24). Because enrolled patients in our study suffered from major painful stimuli, including surgical wounds, and stimuli from endotracheal tubes and artificial ventilation. Experimental and clinical studies have suggested that pain influences respiration in some ways (25). To balance the bias caused by pain at baseline, we performed the goal-directed minimization of the analgesics to rule out pain-induced changes in breathing patterns, respiratory drive, and inspiratory effort. In our clinical treatment, we adhered to the eCASH concept that emphasizes early comfort using analgesia, and remifentanil is arguably one of the most commonly used opioids for acute pain in our unit, so we have chosen remifentanil to balance the pain levels at baseline.
Baseline data collection includes demographic data (age, sex, BMI), history of hypertension, information about the surgery (duration of surgery, American Society of Anesthesiologists physical status classification system (ASA) score, emergency surgery or elective surgery), illness severity (baseline Acute Physiology and Chronic Health Evaluation-II (APACHE-II) and Sequential Organ Failure Assessment (SOFA) score on the day of enrollment), Glasgow coma scale (GCS)-(Eye, Verbal, Motor) score after waking up from general anesthesia, time from ICU admission to inclusion, site of tracheal (oral or nasal), ventilator parameter settings [pressure support (PS) levels, positive end-expiratory pressure (PEEP), fraction of inspired oxygenation (FiO2)], the maintenance infusion rate of remifentanil, baseline arterial blood gas analysis.
During the intervention period, all patients were connected to a ventilator (Dräger Evita Infinity V500, Drägerwerk Verwaltungs AG, Germany) in pressure support mode. PS level was adjusted to obtain a tidal volume (VT) between 6 and 8 mL/kg of ideal body weight and a respiratory rate (RR) lower than 30 breaths/min; PEEP and FiO2 were adjusted to obtain arterial partial pressure of oxygen (PaO2) values higher than 90 mmHg. Respiratory parameters include breathing patterns [RR, VT, and minute ventilation (Vmin)], respiratory drive [airway occlusion pressure at 100 msec (P0.1)] (26), and inspiratory effort [pressure muscle index (PMI) (27) and expiratory occlusion pressure (Pocc) (28)] were obtained from the ventilator. Ten consecutive respiratory cycles were averaged to determine RR, TV, and Vmin. P0.1, PMI, and Pocc were evaluated in triplicate at 20-s intervals and the average values were reported, respectively.
Cardiorespiratory parameters including [SBP and diastolic blood pressure (DBP), MAP, HR, SpO2, and end-tidal carbon dioxide (ETCO2)] were monitored continuously with Mindray monitor (BeneVision N17). ETCO2 will be monitored continuously using a sidestream device, and the airway adapter will be placed at the end of the endotracheal tube (DRYLINE™ II Water Trap, Adult).
Before starting the infusion and 30 ± 5 min after each increase in the infusion rate of cipepofol, respiratory variables, cardiorespiratory variables, and RASS scores were recorded and stored on a dedicated personal computer for further analysis. Adverse events were recorded which included bradycardia (HR < 50 beats/min); hypotension (SBP < 90 mmHg after appropriate intravenous volume replacement); apnea (respiratory rate < 8 breaths/min) or hypoxemia (SpO2 < 90%).
The primary outcome was the change from baseline in respiratory variables (RR, VT, Vmin, P0.1, PMI, Pocc) at 30 min after continuous infusion of cipepofol at 0.3 mg/kg/h.
The secondary outcomes were: (1) changes in respiratory variables (RR, VT, Vmin, P0.1, PMI, Pocc) at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h; (2) changes in cardiorespiratory variables (SBP, DBP, MAP, HR, SpO2, EtCO2) at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h; (3) changes in RASS scores at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h.
Categorical variables were described as numbers (percentages). Continuous variables were described as mean ± standard deviation (SD) and medians with interquartile range [IQR]. For primary outcome analysis, the normal data was analyzed using a paired t-test, and the non-normally distributed data was analyzed with Wilcoxon signed ranks test. For secondary outcome analysis, the changes in respiratory variables, cardiorespiratory variables, and RASS scores at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h were analyzed with a linear mixed effects model, where the dose was considered as the fixed effect, the subject as the random effect and baseline as the covariate.
As patients reached the rate of 0.8 mg/kg/h was small (only 5 patients), we performed a post hoc analysis to analyze the changes in respiratory variables, cardiorespiratory variables, and RASS scores as the infusion rate of cipepofol increasing from 0.3 to 0.7 mg/kg/h, with methods similarly to those secondary outcomes. In addition, we conducted a post hoc analysis to illustrate the changing trend in respiratory variables, cardiorespiratory variables, and RASS scores at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h with data from five patients who reached the rate of 0.8 mg/kg/h. A p-value of <0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics V.26.0 and GraphPad Prism V.9.0 statistical software.
The clinical characteristics of the patients are presented in Table 1. We enrolled 20 patients whose main diagnoses were intracranial tumors and the main causes for ICU admission were intracranial tumor resections. Remifentanil was used to relieve the pain and discomfort at baseline, whose median infusion rate for CPOT 0 to 1 was 0.01 μg/kg/min.
The titration method of cipepofol is described in Figure 2A. An initial infusion rate of cipepofol at 0.3 mg/kg/h was completed in all patients, and all patients achieved the RASS score −2 to +1. Compared with baseline values where no sedation was used, the common features of changes in main respiratory variables evaluated 30 min after infusion of cipepofol at 0.3 mg/kg/h were: a profound initial reduction in tidal volume (median 390.9, IQR [356.6–511.0] vs. 451.6 [393.5–565.9], p = 0.002), a non-significant change in respiratory rate (mean 16.7 ± SD 2.7 vs. 16.2 ± 3.4, p = 0.465), and that the change in minute ventilation was not significant (7.2 ± 1.8 vs. 7.5 ± 1.9, p = 0.154). For respiratory drive and inspiratory effort, there were significant reductions in P0.1 (1.4 [1.0–2.7] vs. 1.7 [1.0–3.1], p = 0.020), PMI (2.1 [1.3–2.7] vs. 2.1 [1.7–4.3], p = 0.019) and Pocc (7.2 [6.1–10.6] vs. 9.4 [6.4–12.9], p = 0.007) (Table 2; Figure 3).
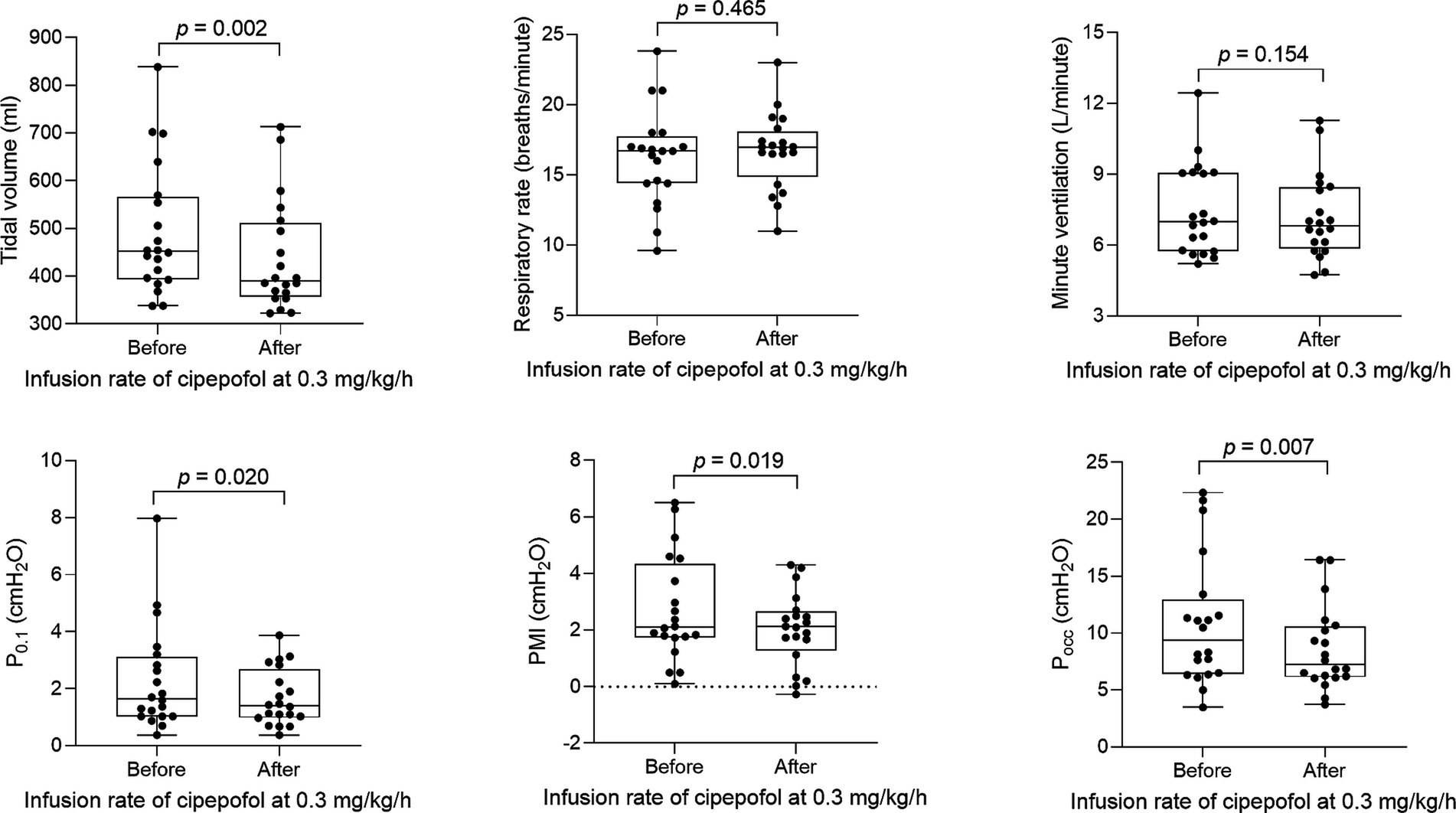
Figure 3. Changes in respiratory variables after 0.3 mg/kg/h cipepofol infusion for 30 min. Overlaid Box-and-whisker and scatter plot. Boxes represent the median with an interquartile range; whiskers extend the minimum and maximum values; Circles indicate individual observations; brackets denote the statistical difference between before and after infusion of cipepofol at the rate of 0.3 mg/kg/h; p values are shown above the brackets. P0.1, Airway Occlusion Pressure at 100mesc; PMI, Pressure Muscle Index; Pocc, Expiratory Occlusion Pressure.
Due to the obvious heterogeneity in individual sensitivity to cipepofol, there was a significant difference in the final infusion rates reached. The number of patients decreased with increasing cipepofol infusion rates, with oversedation (RASS ≤ −4) being the primary cause for discontinuation. Reasons for discontinuation and final given rates are described in Figure 2B.
The changes in respiratory variables, cardiorespiratory variables, and RASS scores at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h are shown in Table 3 and Figure 4. VT decreased further from the lower to the higher infusion rate (p = 0.002), with RR increasing significantly as the infusion rate increased (p = 0.001), and Vmin did not change significantly (p = 0.430). Respiratory drive and inspiratory effort, measured by P0.1 (p = 0.172), PMI (p = 0.135), and Pocc (p = 0.100) showed no significant changes with increasing infusion rate. Regarding cardiorespiratory variables, there were no significant changes in SBP (p = 0.273) and DBP (p = 0.067) at higher infusion rates. However, there was a significant but small reduction in MAP (p = 0.036) and an increase in HR (p = 0.019) at higher infusion rates of cipepofol. ETCO2 (p = 0.050) showed slight changes, and SpO2 (p = 0.645) fluctuated around baseline values, consistently remaining above 96%. As the cipepofol dose increased, there was a corresponding increase in sedation depth, reflected by a decrease in RASS scores (p < 0.001), despite notable heterogeneity among individuals.
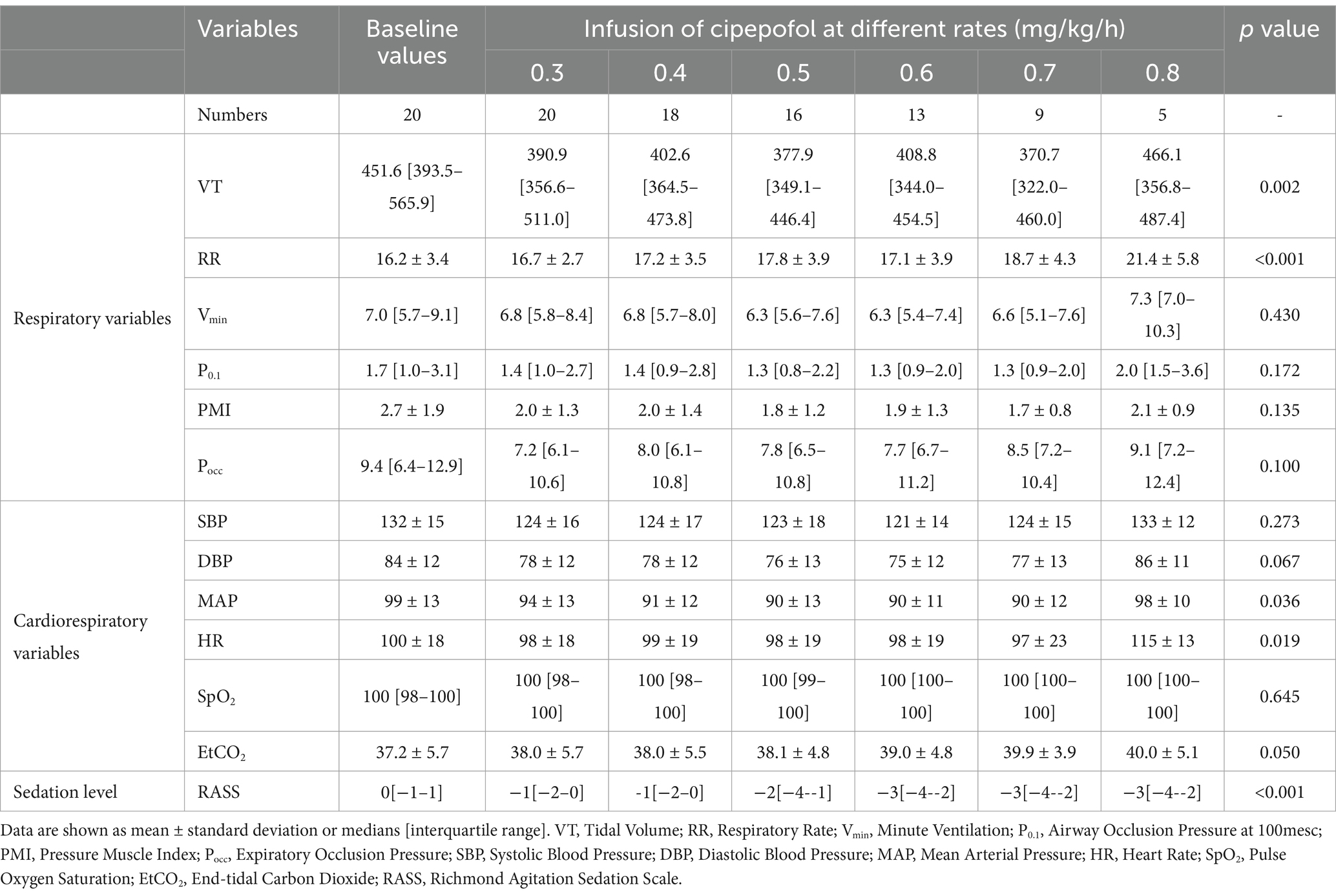
Table 3. Changes in respiratory variables, cardiorespiratory variables, and RASS scores at infusion rates of cipepofol from 0.3 to 0.8 mg/kg/h.
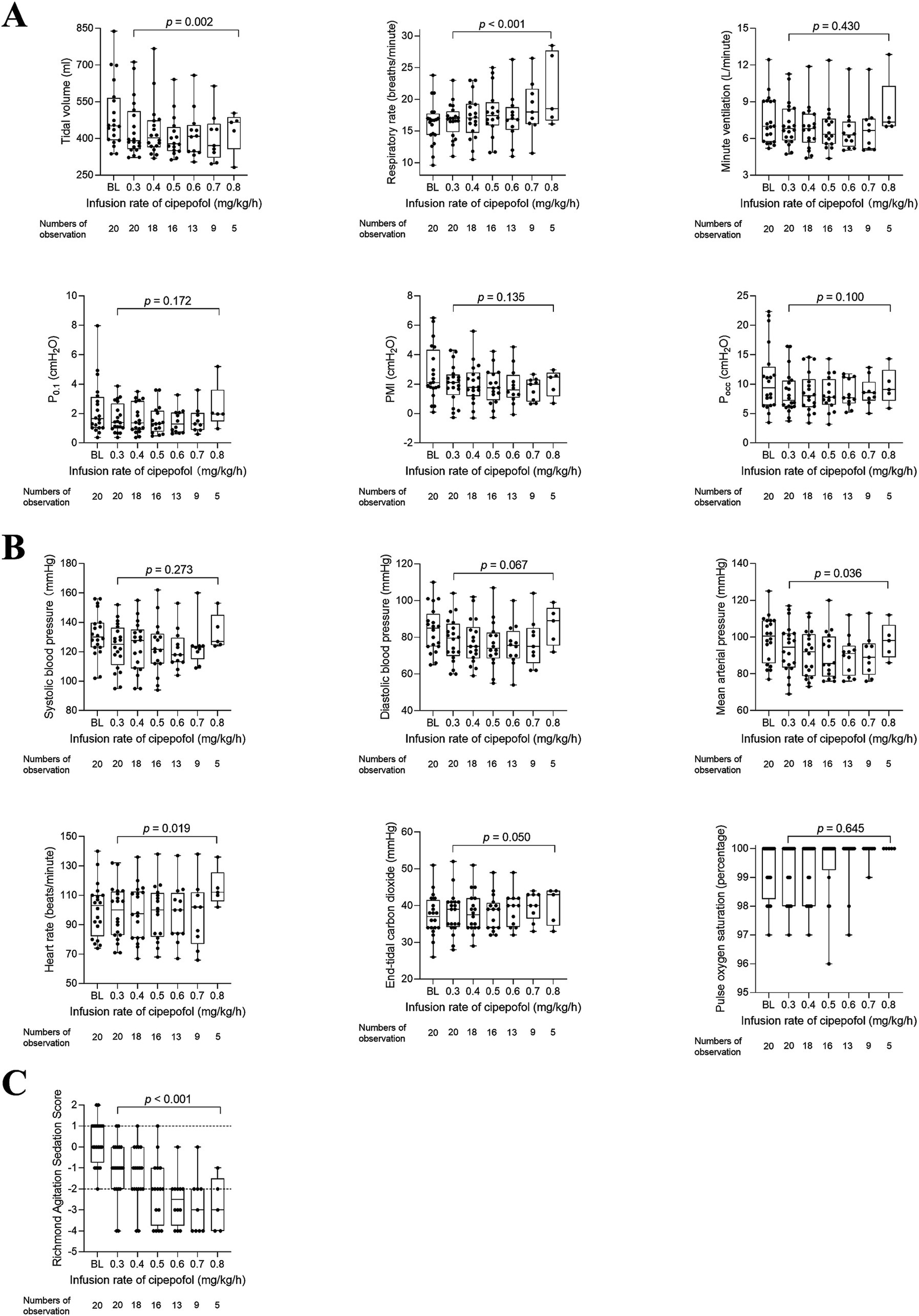
Figure 4. Changes in respiratory variables (A), cardiorespiratory variables (B), and RASS scores (C) from 0.3 to 0.8 mg/kg/h of cipepofol. Overlaid Box-and-whisker and scatter plot. Boxes represent the median with an interquartile range; whiskers extend the minimum and maximum values; circles indicate individual observations; brackets denote the statistical difference at different infusion rates of cipepofol (0.3–0.8 mg/kg/h); p values are shown above the brackets. BL, baseline; P0.1, Airway Occlusion Pressure at 100mesc; PMI, Pressure Muscle Index; Pocc, Expiratory Occlusion Pressure.
Due to only five patients reaching the final infusion rate of 0.8 mg/kg/h, we conducted a post hoc analysis to analyze changes in respiratory variables, cardiorespiratory variables, and RASS scores as the cipepofol infusion rate increased from 0.3 to 0.7 mg/kg/h (Figure 5). The observed trends in all measured variables were similar to those when data at 0.8 mg/kg/h were included except for HR. In post hoc analysis, there was no significant difference in HR with increasing infusion rate of cipepofol from 0.3–0.7 mg/kg/h (p = 0.179).
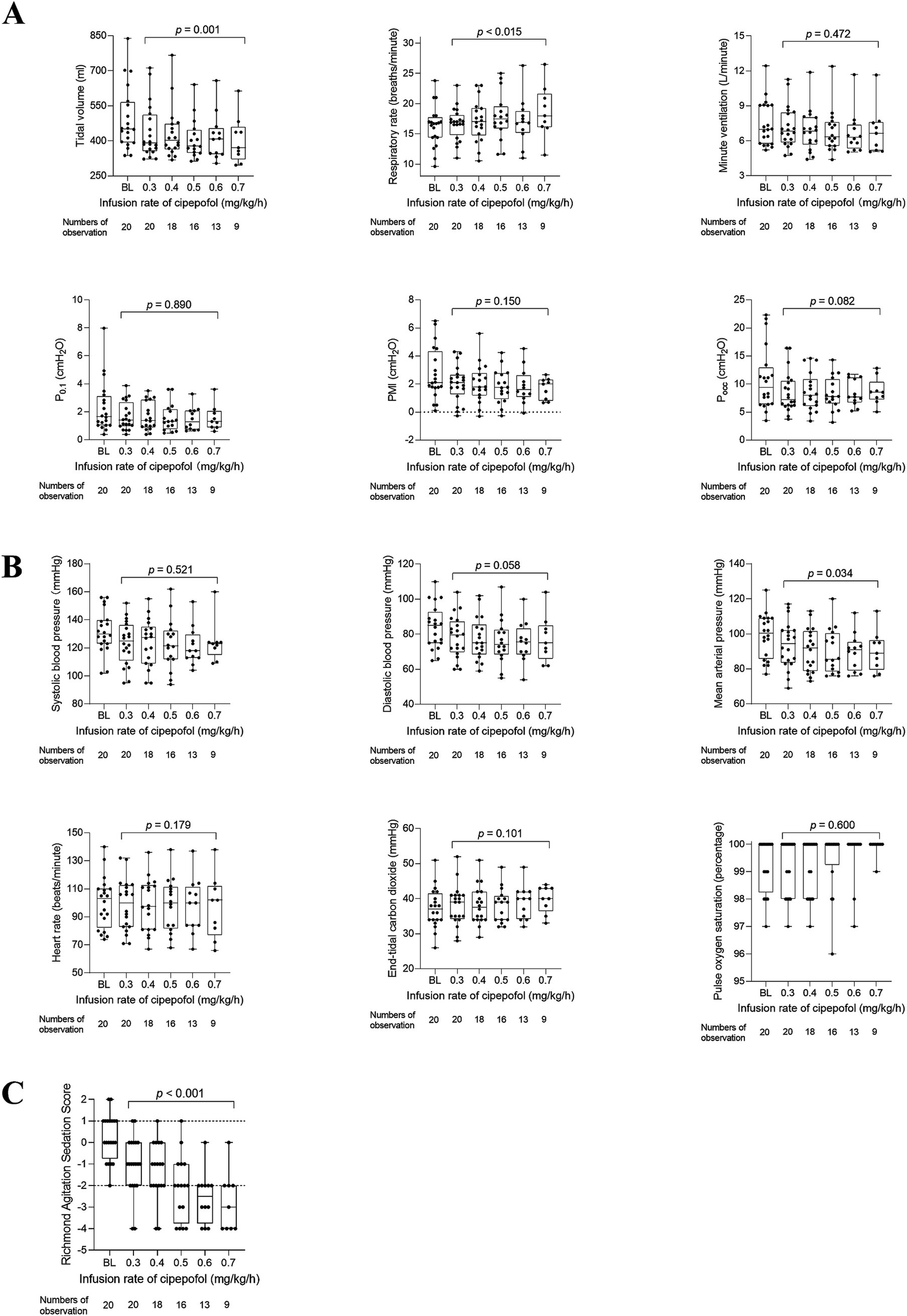
Figure 5. Post hoc analysis for changes in respiratory variables (A), cardiorespiratory variables (B), and RASS scores (C) from 0.3 to 0.7 mg/kg/h of cipepofol. Overlaid box-and-whisker and scatter plot. Boxes represent the median with an interquartile range; whiskers extend the minimum and maximum values; circles indicate individual observations; Brackets denote the statistical difference at different infusion rates of cipepofol (0.3–0.7 mg/kg/h); p values are shown above the brackets. BL, baseline; P0.1, Airway Occlusion Pressure at 100mesc; PMI, Pressure Muscle Index; Pocc, Expiratory Occlusion Pressure.
Then, the changing trend of respiratory variables, cardiorespiratory variables, and RASS scores for 5 patients who reached the maximum infusion rate of 0.8 mg/kg/h were displayed in Figure 6. The primary characteristics included a decrease in VT, an increase in RR, and no significant change in Vmin, P0.1, PMI, and Pocc as the infusion rate increased. The RASS scores decreased significantly and cardiorespiratory variables did not change significantly.
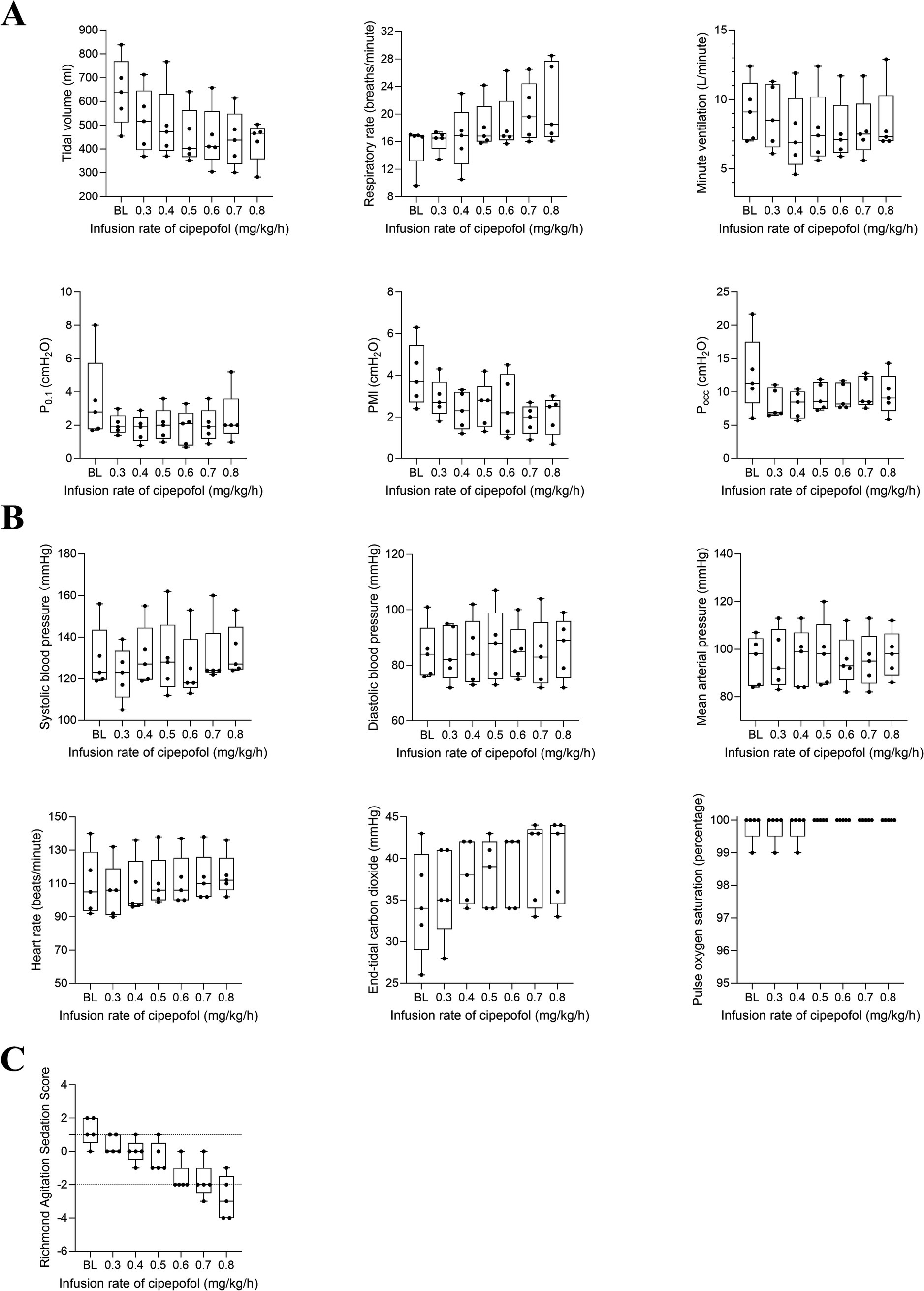
Figure 6. Post hoc analysis for changing trend in respiratory variables (A), cardiorespiratory variables (B), and RASS scores (C) from 0.3 to 0.8 mg/kg/h of cipepofol. Data were obtained from five patients who reached the maximal infusion rate of 0.8 mg/kg/h. Overlaid box-and-whisker and scatter plot. Boxes represent the median with an interquartile range; whiskers extend the minimum and maximum values; circles indicate individual observations; BL, baseline; P0.1, Airway Occlusion Pressure at 100mesc; PMI, Pressure Muscle Index; Pocc, Expiratory Occlusion Pressure.
We conducted a physiological study to investigate the effects of cipepofol on breathing patterns, respiratory drive, and inspiratory effort measurements. We chose 0.3 mg/kg/h as the initial infusion rate for sedation, which was based on the multicenter studies in which we have participated (17), the study concluded that for patients receiving mechanical ventilation, the median maintenance dose of cipepofol to achieve the RASS score − 2 to +1 was 0.3 mg/kg/h. According to the above study’s conclusion from multiple ICU units including our clinical experience, we have chosen 0.3 mg/kg/h as the initial infusion rate and the primary respiratory effects observation time.
In mechanically ventilated patients, a notable decrease in tidal volume was observed following the administration of cipepofol, with a more pronounced effect noted at a higher infusion rate, which was similar to that when propofol was used for the induction and maintenance of general anesthesia (10). However, the exact mechanism underlying the decreased tidal volume induced by cipepofol remains unclear. Studies have demonstrated propofol reduces tidal volume by depressing phrenic nerve activities and diaphragmatic movement (29, 30). Further mechanistic studies are necessary to elucidate the effect of cipepofol.
Upon initiating the continuous infusion of cipepofol, we observed an immediate decrease in tidal volume with no significant change in respiratory rate. As the cipepofol infusion rate increased, tidal volume continued to decrease, followed by an obvious increase in respiratory rate. This pattern of changes mirrors that observed with propofol, which has been shown to increase respiratory rate as a compensatory mechanism (31). Ventilation is typically measured through tidal volume and respiratory rate, with minute ventilation derived from these parameters (32). Over the intervention period, the net effect of changes in tidal volume and respiratory rate was that minute ventilation remained unchanged significantly.
There was a significant reduction in respiratory drive represented by P0.1 and inspiratory effort represented by PMI and Pocc after the infusion of cipepofol at 0.3 mg/kg/h. Studies have shown that “behavioral” factors (anxiety, agitation) modulate the activity of the respiratory centers (33). In our study, the initial dose achieved satisfactory sedation (RASS -2 to +1) and relieved the increased respiratory drive and inspiratory effort caused by these behavioral factors. Notably, a higher dose of cipepofol did not lead to further significant change in these indices, consistent with findings from a previous study that reported no correlation between deeper sedation and lower P0.1 (34). On one side, decreased respiratory effort from sedation can contribute to disuse atrophy and dysfunction of the diaphragm (35). On the other side, sedation could reduce respiratory effort and high tidal volume, mitigating the risk of patient self-inflicted lung injury (P-SILI) and diaphragm injury from excessive ventilatory effort (36). Therefore, clarifying the effects of cipepofol on respiratory drive and inspiratory effort, including P0.1, PMI, Pocc should be a priority in bedside care.
At an infusion rate of 0.3 mg/kg/h, cipepofol achieved a sedation level of RASS score − 2 to +1, with two patients experiencing oversedation (RASS ≤ −4) in our study. Increasing the infusion rate led to deeper sedation, though individual sensitivity to cipepofol varied, resulting in a wide range of final infusion rates. Hemodynamic stability was maintained with minimal fluctuations in SBP, DBP, and MAP, consistent with previous studies (37). No drug-related bradycardia was observed, instead, a faster heart rate was observed at higher infusion rates, especially at the rate of 0.8 mg/kg/h. However, a post hoc analysis showed a non-significant change in heart rate with an increasing infusion rate of cipepofol from 0.3 to 0.7 mg/kg/h. The reason might be explained as the heart rate was inherently faster in five patients who reached the 0.8 mg/kg/h infusion rate. Sinus tachycardia has also been reported with cipepofol used for anesthesia induction in elective surgery (38). Further investigation is needed to understand the effects of cipepofol on heart rate and its exact mechanism in patients under neurological surgery. There were no adverse effects including propofol infusion syndrome (PRIS) during the intervention period.
First, we performed goal-directed minimization of the analgesics with remifentanil, which may confound the effect of cipepofol on respiration. Under similar circumstances, to explore the effects of propofol on respiration, Liu L et al. maintained a continuous infusion of analgesics during the study period (39). Moreover, we titrated the infusion rate of remifentanil for CPOT 0–1. After achieving the goal, the remifentanil infusion rate would not be changed throughout the study period. Nevertheless, we were unable to remove the possible effect of baseline analgesia. Second, the study was single-center with a small sample size, and all enrolled patients were neurological surgery patients, which restricted the generalization of our conclusions to all patients. Lastly, the accuracy of non-invasive respiratory drive and inspiratory drive indices, including P0.1, PMI, and Pocc may be questioned. Although invasive measures using esophageal pressure are more accurate, they are technically challenging and not widely available. Our findings in this area should be considered hypothesis-generating and warrant further validation.
The main finding was that cipepofol demonstrates the capability to reach a satisfactory sedation level in mechanically ventilated adult patients. The primary effect of cipepofol on breathing patterns was a decrease in tidal volume, while changes in respiratory rate and minute ventilation were insignificant. The effect on respiratory drive and inspiratory effort significantly reduced P0.1, PMI, and Pocc.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
RS: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – original draft. LZ: Conceptualization, Formal analysis, Methodology, Software, Writing – original draft. Y-MW: Conceptualization, Investigation, Methodology, Writing – review & editing. M-YM: Conceptualization, Methodology, Writing – review & editing. SW: Investigation, Methodology, Writing – review & editing. YC: Writing – review & editing. J-XZ: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by a grant from Clinical and Research Center program of Capital Medical University (CMU-2023-45).
YC were employed by Haisco Pharmaceutical Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Puntillo, KA, Arai, S, Cohen, NH, Gropper, MA, Neuhaus, J, Paul, SM, et al. Symptoms experienced by intensive care unit patients at high risk of dying. Crit Care Med. (2010) 38:2155–60. doi: 10.1097/CCM.0b013e3181f267ee
2. Novaes, MA, Knobel, E, Bork, AM, Pavão OFNogueira-Martins, LA, and Ferraz, MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. (1999) 25:1421–6. doi: 10.1007/s001340051091
3. Devlin, JW, Skrobik, Y, Gélinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/ccm.0000000000003299
4. Vincent, JL, Shehabi, Y, Walsh, TS, Pandharipande, PP, Ball, JA, Spronk, P, et al. Comfort and patient-centred care without excessive sedation: the eCASH concept. Intensive Care Med. (2016) 42:962–71. doi: 10.1007/s00134-016-4297-4
5. Payen, JF, Chanques, G, Mantz, J, Hercule, C, Auriant, I, Leguillou, JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. (2007) 106:687–95. doi: 10.1097/01.anes.0000264747.09017.da
6. Prielipp, RC, Coursin, DB, Wood, KE, and Murray, MJ. Complications associated with sedative and neuromuscular blocking drugs in critically ill patients. Crit Care Clin. (1995) 11:983–1003. doi: 10.1016/S0749-0704(18)30049-6
7. Kollef, MH, Levy, NT, Ahrens, TS, Schaiff, R, Prentice, D, and Sherman, G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. (1998) 114:541–8. doi: 10.1378/chest.114.2.541
8. de Jong, A, Molinari, N, de Lattre, S, Gniadek, C, Carr, J, Conseil, M, et al. Decreasing severe pain and serious adverse events while moving intensive care unit patients: a prospective interventional study (the NURSE-DO project). Crit Care. (2013) 17:R74. doi: 10.1186/cc12683
9. Rosa, G, Conti, G, Orsi, P, D'Alessandro, F, La Rosa, I, Di Giugno, G, et al. Effects of low-dose propofol administration on central respiratory drive, gas exchanges and respiratory pattern. Acta Anaesthesiol Scand. (1992) 36:128–31. doi: 10.1111/j.1399-6576.1992.tb03438.x
10. Goodman, NW, Black, AM, and Carter, JA. Some ventilatory effects of propofol as sole anaesthetic agent. Br J Anaesth. (1987) 59:1497–503. doi: 10.1093/bja/59.12.1497
11. Cavaliere, F, Antonelli, M, Arcangeli, A, Conti, G, Costa, R, Pennisi, MA, et al. A low-dose remifentanil infusion is well tolerated for sedation in mechanically ventilated, critically-ill patients. Can J Anaesth. (2002) 49:1088–94. doi: 10.1007/bf03017909
12. Qin, L, Ren, L, Wan, S, Liu, G, Luo, X, Liu, Z, et al. Design, synthesis, and evaluation of novel 2, 6-Disubstituted phenol derivatives as general anesthetics. J Med Chem. (2017) 60:3606–17. doi: 10.1021/acs.jmedchem.7b00254
13. Bian, Y, Zhang, H, Ma, S, Jiao, Y, Yan, P, Liu, X, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. (2021) 87:93–105. doi: 10.1111/bcp.14363
14. Li, X, Yang, D, Li, Q, Wang, H, Wang, M, Yan, P, et al. Safety, pharmacokinetics, and pharmacodynamics of a single bolus of the γ-aminobutyric acid (GABA) receptor Potentiator HSK3486 in healthy Chinese elderly and non-elderly. Front Pharmacol. (2021) 12:735700. doi: 10.3389/fphar.2021.735700
15. Liao, J, Li, M, Huang, C, Yu, Y, Chen, Y, Gan, J, et al. Pharmacodynamics and pharmacokinetics of HSK3486, a novel 2, 6-Disubstituted phenol derivative as a general anesthetic. Front Pharmacol. (2022) 13:830791. doi: 10.3389/fphar.2022.830791
16. Liu, Y, Yu, X, Zhu, D, Zeng, J, Lin, Q, Zang, B, et al. Safety and efficacy of ciprofol vs. propofol for sedation in intensive care unit patients with mechanical ventilation: a multi-center, open label, randomized, phase 2 trial. Chin Med J. (2022) 135:1043–51. doi: 10.1097/cm9.0000000000001912
17. Liu, Y, Peng, Z, Liu, S, Yu, X, Zhu, D, Zhang, L, et al. Efficacy and safety of Ciprofol sedation in ICU patients undergoing mechanical ventilation: a multicenter, single-blind, randomized, Noninferiority Trial. Crit Care Med. (2023) 51:1318–27. doi: 10.1097/ccm.0000000000005920
18. Wei, A, Yang, L, Ma, S, Jin, G, Yang, M, and Zhou, J. A case report of ciprofol overdose during anesthesia/analgesia and literature review: clinical presentation, blood pressure, and management. J Int Med Res. (2022) 50:3000605221132466. doi: 10.1177/03000605221132466
19. Wen, J, Liu, C, Ding, X, Tian, Z, Jiang, W, Wei, X, et al. Efficacy and safety of ciprofol (HSK3486) for procedural sedation and anesthesia induction in surgical patients: a systematic review and meta-analysis. Heliyon. (2023) 9:e22634. doi: 10.1016/j.heliyon.2023.e22634
20. Smith, GB, Prytherch, DR, Schmidt, PE, Featherstone, PI, and Higgins, B. A review, and performance evaluation, of single-parameter "track and trigger" systems. Resuscitation. (2008) 79:11–21. doi: 10.1016/j.resuscitation.2008.05.004
21. Natalini, G, Di Maio, A, Rosano, A, Ferretti, P, Bertelli, M, and Bernardini, A. Remifentanil improves breathing pattern and reduces inspiratory workload in tachypneic patients. Respir Care. (2011) 56:827–33. doi: 10.4187/respcare.01014
22. Sessler, CN, Gosnell, MS, Grap, MJ, Brophy, GM, O'Neal, PV, Keane, KA, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. (2002) 166:1338–44. doi: 10.1164/rccm.2107138
23. Ely, EW, Truman, B, Shintani, A, Thomason, JW, Wheeler, AP, Gordon, S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond agitation-sedation scale (RASS). JAMA. (2003) 289:2983–91. doi: 10.1001/jama.289.22.2983
24. Kotfis, K, Zegan-Barańska, M, Szydłowski, Ł, Żukowski, M, and Ely, EW. Methods of pain assessment in adult intensive care unit patients—polish version of the CPOT (critical care pain observation tool) and BPS (behavioral pain scale). Anaesthesiol Intensive Ther. (2017) 49:66–72. doi: 10.5603/ait.2017.0010
25. Jafari, H, Courtois, I, Van den Bergh, O, Vlaeyen, JWS, and Van Diest, I. Pain and respiration: a systematic review. Pain. (2017) 158:995–1006. doi: 10.1097/j.pain.0000000000000865
26. Conti, G, Cinnella, G, Barboni, E, Lemaire, F, Harf, A, and Brochard, L. Estimation of occlusion pressure during assisted ventilation in patients with intrinsic PEEP. Am J Respir Crit Care Med. (1996) 154:907–12. doi: 10.1164/ajrccm.154.4.8887584
27. Kyogoku, M, Shimatani, T, Hotz, JC, Newth, CJL, Bellani, G, Takeuchi, M, et al. Direction and magnitude of change in plateau from peak pressure during inspiratory holds can identify the degree of spontaneous effort and elastic workload in ventilated patients. Crit Care Med. (2021) 49:517–26. doi: 10.1097/ccm.0000000000004746
28. Bertoni, M, Telias, I, Urner, M, Long, M, Del Sorbo, L, Fan, E, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. (2019) 23:346. doi: 10.1186/s13054-019-2617-0
29. Aliverti, A, Kostic, P, Lo Mauro, A, Andersson-Olerud, M, Quaranta, M, Pedotti, A, et al. Effects of propofol anaesthesia on thoraco-abdominal volume variations during spontaneous breathing and mechanical ventilation. Acta Anaesthesiol Scand. (2011) 55:588–96. doi: 10.1111/j.1399-6576.2011.02413.x
30. Rocco, M, Maggi, L, Ranieri, G, Ferrari, G, Gregoretti, C, Conti, G, et al. Propofol sedation reduces diaphragm activity in spontaneously breathing patients: ultrasound assessment. Minerva Anestesiol. (2017) 83:266–73. doi: 10.23736/s0375-9393.17.11615-9
31. Hagiwara, A, Matsuura, N, and Ichinohe, T. Comparison of changes in respiratory dynamics immediately after the start of Propofol sedation with or without midazolam. J Oral Maxillofac Surg. (2018) 76:52–9. doi: 10.1016/j.joms.2017.05.038
32. Goodman, NW, and Black, AM. Inter-relations of the volume and timing components of ventilation during carbon dioxide rebreathing in awake and anaesthetized subjects. Br J Anaesth. (1987) 59:1504–13. doi: 10.1093/bja/59.12.1504
33. Spinelli, E, Pesenti, A, Slobod, D, Fornari, C, Fumagalli, R, Grasselli, G, et al. Clinical risk factors for increased respiratory drive in intubated hypoxemic patients. Crit Care. (2023) 27:138. doi: 10.1186/s13054-023-04402-z
34. Dzierba, AL, Khalil, AM, Derry, KL, Madahar, P, and Beitler, JR. Discordance between respiratory drive and sedation depth in critically ill patients receiving mechanical ventilation. Crit Care Med. (2021) 49:2090–101. doi: 10.1097/ccm.0000000000005113
35. Goligher, EC, Fan, E, Herridge, MS, Murray, A, Vorona, S, Brace, D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med. (2015) 192:1080–8. doi: 10.1164/rccm.201503-0620OC
36. Goligher, EC, Brochard, LJ, Reid, WD, Fan, E, Saarela, O, Slutsky, AS, et al. Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med. (2019) 7:90–8. doi: 10.1016/s2213-2600(18)30366-7
37. Liu, L, Wang, K, Sun, Z, Yan, P, Hu, M, Liu, X, et al. Pharmacokinetics and exposure-safety relationship of ciprofol for sedation in mechanically ventilated patients in the intensive care unit. CPT Pharmacometrics Syst Pharmacol. (2024) 13:823–36. doi: 10.1002/psp4.13121
38. Zhu, Q, Luo, Z, Wang, X, Wang, D, Li, J, Wei, X, et al. Efficacy and safety of ciprofol versus propofol for the induction of anesthesia in adult patients: a multicenter phase 2a clinical trial. Int J Clin Pharm. (2023) 45:473–82. doi: 10.1007/s11096-022-01529-x
Keywords: cipepofol, sedation, breathing pattern, respiratory drive, inspiratory effort
Citation: Su R, Zhang L, Wang Y-M, Miao M-Y, Wang S, Cao Y and Zhou J-X (2025) Effects of cipepofol on breathing patterns, respiratory drive, and inspiratory effort in mechanically ventilated patients. Front. Med. 12:1539238. doi: 10.3389/fmed.2025.1539238
Received: 04 December 2024; Accepted: 11 February 2025;
Published: 25 February 2025.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Haiyan Xue, University College London, United KingdomCopyright © 2025 Su, Zhang, Wang, Miao, Wang, Cao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Xin Zhou, zhoujx.cn@icloud.com
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.