
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 March 2025
Sec. Geriatric Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1538038
 Weicheng Wu1†
Weicheng Wu1† Zhening Guo2†
Zhening Guo2† Peiyao Zhu1
Peiyao Zhu1 Bo Lv2
Bo Lv2 Yongtao Mao1
Yongtao Mao1 Chang She1
Chang She1 Wei Xu1
Wei Xu1 Jun Gu1
Jun Gu1 Jie Pan2*
Jie Pan2* Liubing Li1,3*
Liubing Li1,3*Background: The inflammatory response following hip fracture significantly influences postoperative functional recovery in patients. However, to date, no inflammatory index has been identified as a reliable and accurate predictor of functional recovery, especially in elderly patients with hip fractures. This study introduces and evaluates a novel inflammatory marker, the lymphocyte ratio-calcium index (LRCa3), for predicting one-year postoperative functional recovery and compares its performance to that of established markers, including the platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), aggregate index of systemic inflammation (AISI), and systemic inflammation response index (SIRI).
Methods: A retrospective analysis was conducted on 111 elderly patients (≥65 years) who underwent hip fracture surgery, and their demographic and laboratory data were analyzed. Patients were classified into good or poor recovery groups based on the Harris hip score (HHS) 1 year postoperatively. LRCa3 was calculated as the lymphocyte ratio multiplied by the cube of the serum calcium level. Logistic regression and receiver operating characteristic (ROC) curve analyses were performed to assess the predictive performance of the LRCa3 and other inflammatory indices. A nomogram prediction model was constructed.
Results: ROC curve analysis revealed that, compared with the SII (AUC: 0.601), the SIRI (AUC: 0.61), the AISI (AUC: 0.577), and the MLR (AUC: 0.626) had superior predictive performance. Multivariate logistic regression revealed that the LRCa3 was an independent predictor of one-year functional recovery. The incorporation of LRCa3 into a nomogram further enhanced its predictive capacity, providing a more accurate tool for postoperative outcome assessment.
Conclusion: LRCa3 is a novel and effective biomarker for predicting postoperative functional recovery in elderly hip fracture patients. Its integration into clinical practice could facilitate individualized patient management and improve long-term outcomes.
With global population growth and the aging phenomenon, the incidence of osteoporotic fractures has been steadily increasing. Among these, hip fractures are the most severe because they are associated with high healthcare costs, elevated mortality rates, significant disability, and frequent complications. Consequently, hip fractures have emerged as a major global public health concern (1, 2). Projections indicate that by 2050, the global incidence of hip fractures will range between 6.26 million and 21.3 million cases (3, 4). Timely recovery of motor function after hip fracture surgery plays a critical role in reducing complications such as pneumonia and pressure ulcers. Furthermore, motor function recovery is strongly correlated with patient satisfaction with surgical outcomes. Despite this, research has shown that up to 80% of hip fracture patients continue to rely on assistive devices for daily activities 1 year postfracture (5). Therefore, enhancing postoperative motor function recovery in this population remains a pressing challenge in orthopedic and rehabilitative care.
Elderly patients with hip fractures often experience elevated release of inflammatory cytokines following injury because of reduced physiological reserves and insufficient anti-inflammatory mediators to maintain homeostasis. This heightened susceptibility to an exaggerated inflammatory response frequently leads to poorer prognoses for these patients (6). The inflammatory response has been proven to have a profound association with the adverse outcomes commonly observed in hip fracture patients. After a fracture, systemic immune-inflammatory cells such as neutrophils, monocytes, lymphocytes, and C-reactive protein (CRP) are secreted in large quantities. These cells produce cytokines such as IFN-γ, IL-6, IL-12, and TNF, which regulate adaptive immune responses and exhibit strong proinflammatory and antimicrobial effects (7, 8). Recent studies have identified IL-6, IL-8, CRP, and C1q as independent risk factors for poor postoperative joint function in hip fracture patients (9). Preoperative immune-inflammatory indicators, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and CRP, are associated with increased all-cause mortality after surgery in elderly hip fracture patients (10, 11). The admission CAR (C-reactive protein-to-albumin ratio) and NLR have been shown to predict long-term mortality in elderly patients undergoing hip fracture surgery (7). Furthermore, the systemic immune-inflammation index (SII) on the fifth postoperative day can predict one-year mortality in hip fracture patients (12).
In recent years, inflammatory indices such as the MLR, PLR, SII, aggregate index of systemic inflammation (AISI), and systemic inflammation response index (SIRI) have been widely utilized across various diseases (13, 14). These indices have shown greater utility in assessing inflammation and predicting postoperative outcomes than single blood-based inflammatory markers do. However, their application is often limited by insufficient specificity and sensitivity, with inconsistent performance observed across different surgical procedures and patient populations. These limitations, coupled with contradictory statistical findings, underscore the need for further investigation to establish their reliability and applicability. Moreover, recent studies have highlighted several limitations of these indices in specific contexts. For example, the MLR and PLR have been reported to have no significant associations with postoperative mortality in hip fracture patients (15, 16). Similarly, the SII has been found to be inadequate for predicting the risk of spinal fractures (17). PLR is susceptible to fluctuations caused by platelet count variability resulting from surgical trauma. Although CRP is effective in identifying early postoperative inflammatory responses (18), it lacks the sensitivity and specificity necessary for evaluating long-term recovery (16). Research on the relationship between systemic immune-inflammatory responses and postoperative functional recovery in hip fracture patients is still limited, and no universally recognized predictive marker currently exists. This underscores the pressing need for novel biomarkers or indices to increase the accuracy of postoperative functional recovery predictions. One potential solution lies in combining inflammatory markers with bone metabolism-related indices, such as serum calcium levels. To address this, our study introduces a novel measurement index—the lymphocyte ratio-calcium index (LRCa3)—which is calculated by multiplying the lymphocyte ratio by the cube of the serum calcium level. The objective of this research was to evaluate whether the LRCa3 can effectively predict one-year postoperative motor function recovery in elderly hip fracture patients and to compare its predictive accuracy with that of commonly used immune-inflammatory indices. Ultimately, this study aims to provide clinicians with a more reliable and timelier tool for assessing inflammation severity in hip fracture patients, facilitating targeted interventions to improve outcomes.
This retrospective study analyzed data from patients who underwent surgery for hip fractures at the Second Affiliated Hospital of Soochow University between March 2021 and March 2023. Eligible participants were aged 65 years or older and had undergone either hip replacement surgery or internal fixation. The exclusion criteria were as follows: patients with multiple injuries, ischemic necrosis, pathological fractures, previous hip surgeries, or incomplete medical records. To ensure that the focus remained on inflammatory biomarkers, we also excluded patients whose conditions, such as active infections, hematological disorders, or malignancies, could significantly alter their inflammatory status. The study adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of the Second Affiliated Hospital of Soochow University (JD-HG-2023-07).
Medical records, including sex, age, fracture type, surgical procedure, and laboratory values measured on the first postoperative day, were reviewed to collect demographic and clinicopathological data. The laboratory parameters included the neutrophil ratio, white blood cell count, hemoglobin level, lymphocyte count, lymphocyte ratio, neutrophil count, monocyte count, platelet count, and serum calcium. The following inflammatory indices were calculated: (1) MLR = monocyte count (×109/L)/lymphocyte count (×109/L); (2) PLR = platelet count (×109/L)/lymphocyte count (×109/L); (3) SII = [platelet count (×109/L) × neutrophil count (×109/L)]/lymphocyte count (×109/L); (4) AISI = neutrophil count (×109/L) × platelet count (×109/L) × monocyte count (×109/L)/lymphocyte count (×109/L); and (5) SIRI = neutrophil count (×109/L) × monocyte count (×109/L)/lymphocyte count (×109/L). Postoperative functional recovery at 1 year was evaluated via the Harris hip score (HHS), a validated tool for assessing hip function, with a maximum score of 100. The HHS emphasizes functional aspects, including activities of daily living and gait (47 points) and pain (44 points), while assigning less weight to deformity (4 points) and range of motion (5 points) (5). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Patients were classified into two groups on the basis of their HHS: the good recovery group (≥80 points) and the poor recovery group (<80 points).
All the statistical analyses were performed via R software (version 4.0.2). Quantitative variables are expressed as the means ± standard deviations (SDs), whereas categorical variables are reported as counts with percentages. Group comparisons were conducted via the chi-square test for categorical data, the t test for variables with a normal distribution, and the Wilcoxon rank-sum test for variables with a nonnormal distribution. The predictive performance of inflammatory indices, including the SII, SIRI, AISI, PLR, and MLR, was assessed via receiver operating characteristic (ROC) curves. Logistic regression analyses were applied to develop both univariable and multivariable nutritional screening models. A nomogram prediction model was subsequently constructed on the basis of the regression results to estimate one-year postoperative functional recovery in elderly patients with hip fractures. The model’s discriminatory ability was evaluated by generating ROC curves and calculating the area under the curve (AUC). Finally, decision curve analysis (DCA) was performed to determine the clinical utility of the nomogram. A p value of <0.05 was considered to indicate statistical significance throughout the analysis.
Within the primary cohort of 136 patients, after excluding those who were lost to follow-up, died, or had incomplete data, a total of 111 patients met the inclusion criteria and were enrolled in the study. Table 1 outlines the demographic characteristics of these patients. Among the participants, 28 (25.2%) were male, and 83 (74.8%) were female. Femoral neck fractures were observed in 67 patients (60.3%), whereas 44 patients (39.7%) had intertrochanteric fractures. At the one-year follow-up, postoperative hip function was classified as good in 60 patients and poor in 51 patients. Statistical analysis revealed significant differences in weight and BMI between the two groups (p < 0.05). However, no statistically significant differences were found in other variables, including sex, age, fracture type, serum hemoglobin, neutrophil count, lymphocyte count, or calcium level (p > 0.05).
The performance of the novel indicator LRCa3 was evaluated and compared with that of existing inflammatory indices (SII, SIRI, AISI, PLR, and MLR) for the prediction of one-year postoperative functional recovery in hip fracture patients via receiver operating characteristic (ROC) curves. As illustrated in Figure 1 and Table 2, the area under the curve (AUC) for LRCa3 was 0.715 (95% CI: 0.619–0.81; p < 0.05), surpassing the AUCs of the SII (0.601; 95% CI: 0.495–0.706; p < 0.05), SIRI (0.61; 95% CI: 0.504–0.715; p < 0.05), AISI (0.577; 95% CI: 0.471–0.684; p < 0.05), PLR (0.598; 95% CI: 0.492–0.704; p < 0.05), and MLR (0.626; 95% CI: 0.523–0.73; p < 0.05). These results underscore the superior predictive ability of LRCa3 compared with other inflammatory markers, highlighting its potential as a reliable tool for clinical assessment. Based on the ROC curve (Figure 1), the optimal cut-off for LRCa3 was determined to be 65.988, sensitivity of 63.5%, and specificity of 75.5%.
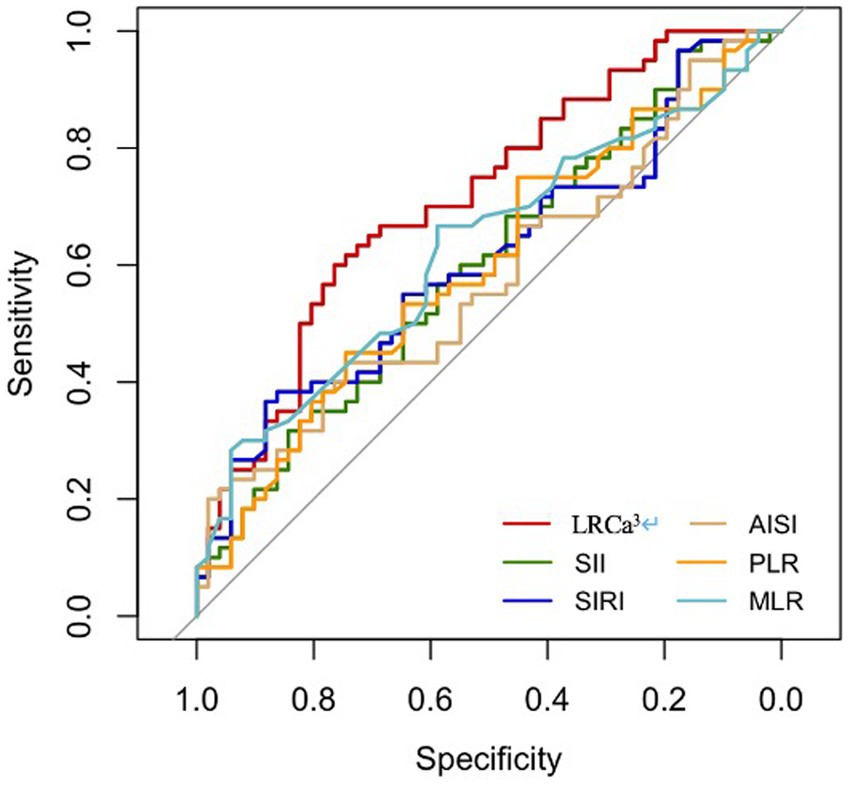
Figure 1. Receiver operating characteristic (ROC) curves of the lymphocyte ratio-calcium index (LRCa³), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), aggregate index of systemic inflammation (AISI), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) for predicting 1-year motor functional recovery.
As shown in Table 3, the lymphocyte ratio and calcium levels were combined to calculate LRCa3 for analysis. Univariate regression analysis revealed that age (OR: 1.1; 95% CI: 1.042–1.166; p = 0.001), BMI (OR: 0.8; 95% CI: 0.704–0.898; p < 0.001), the neutrophil ratio (OR: 1.134; 95% CI: 1.043–1.245; p = 0.005), and LRCa3 (OR: 0.975; 95% CI: 0.962–0.988; p < 0.001) were significantly associated with one-year postoperative functional recovery in hip fracture patients. After adjusting for covariates, multivariate regression analysis revealed BMI (OR: 0.845; 95% CI: 0.729–0.969; p = 0.019) and LRCa3 (OR: 0.979; 95% CI: 0.959–0.997; p < 0.001) as independent risk factors for poor functional recovery. These results highlight the predictive value of the novel indicator LRCa3, emphasizing its effectiveness in assessing postoperative functional outcomes in hip fracture patients.
Regression analysis revealed that age, BMI, the neutrophil ratio, and LRCa3 were significant predictors of postoperative functional recovery. These variables were used to construct a nomogram prediction model, as shown in Figure 2. The model’s predictive performance was evaluated via a receiver operating characteristic (ROC) curve, which yielded an AUC of 0.800 (95% CI: 0.715–0.886), indicating strong predictive accuracy (Figure 3). Figure 4 presents the decision curve analysis (DCA) for the nomogram, demonstrating its clinical utility in predicting postoperative functional outcomes in elderly hip fracture patients. The DCA results suggest that clinical decisions guided by the nomogram can more effectively and accurately predict functional recovery following surgery.
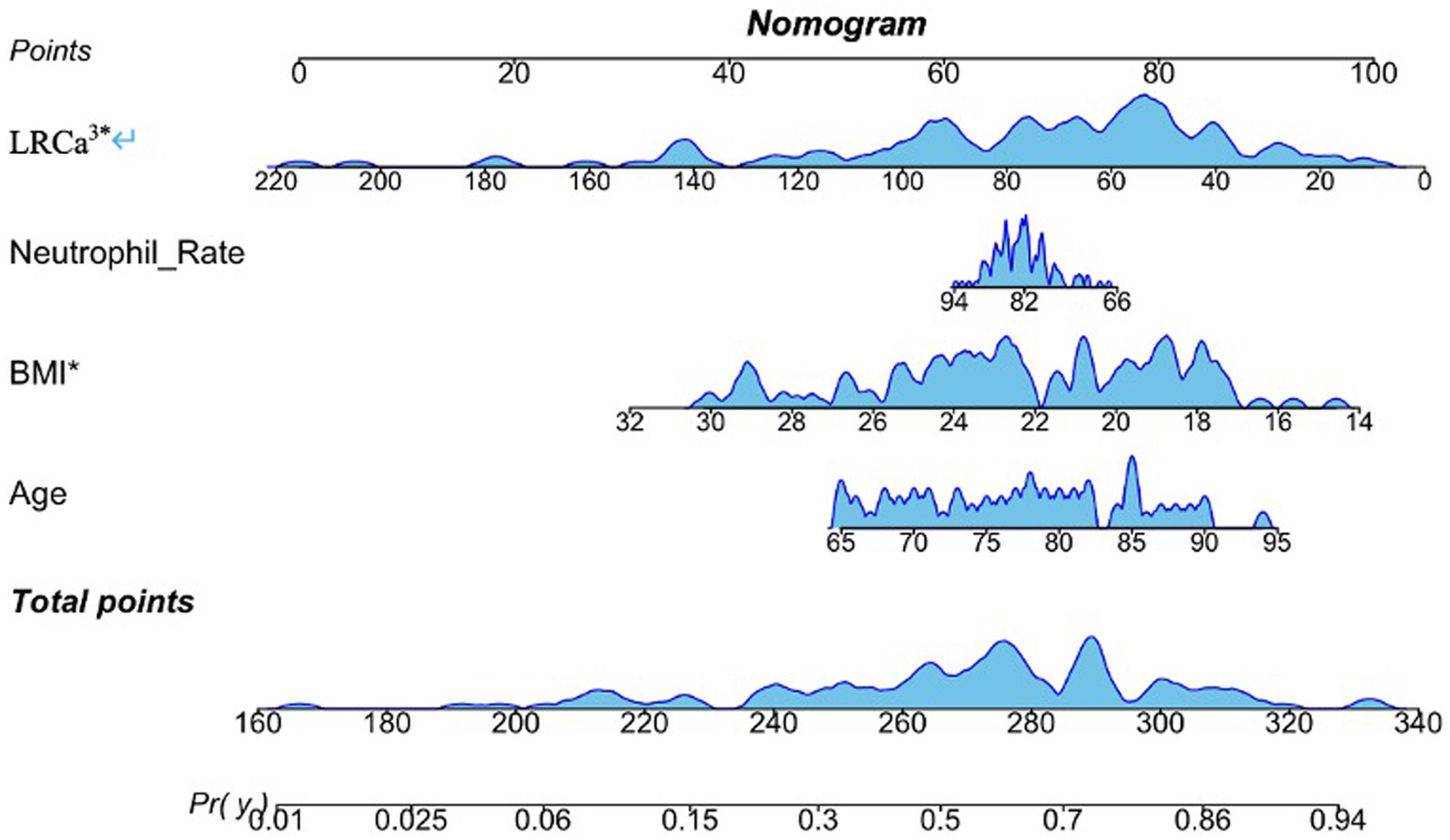
Figure 2. Nomogram model for predicting motor function prognosis. Age, neutrophil rate, body mass index (BMI) and the lymphocyte ratio-calcium index (LRCa³) were included.
The most significant finding of this study is the identification of a novel inflammatory marker, LRCa3, which is derived from the lymphocyte ratio and serum calcium levels on postoperative day one. LRCa3 was shown to effectively predict one-year functional recovery in elderly patients undergoing hip fracture surgery. While previous studies have highlighted the prognostic value of inflammatory indices such as the AISI, PLR, SII, and SIRI in hip fracture patients, our ROC curve analysis demonstrated that LRCa3 offers superior sensitivity and specificity, establishing it as a more reliable predictor of postoperative functional recovery, and we determined the optimal cut-off value to be 65.988. Unlike traditional inflammatory indices, which focus solely on inflammation, LRCa3 integrates immune function with bone metabolism. This broader scope likely contributes to its enhanced predictive performance. By combining the lymphocyte ratio and calcium level as a composite marker, LRCa3 provides a novel approach for predicting functional outcomes in elderly hip fracture patients, a relationship not previously explored in the literature. These findings underscore the potential of LRCa3 as a valuable tool in clinical practice for tailoring postoperative management strategies.
Systemic immune and inflammatory responses play critical roles in determining postoperative outcomes in hip fracture patients, with various inflammatory mediators contributing significantly to fracture-related trauma and bone healing processes (19). The lymphocyte ratio, an indicator of immune status, has been linked to the risk of postoperative complications and infections. Neutrophils, the primary effector cells of the innate immune response, function in tandem with lymphocytes, which regulate immune system activities (8, 20). Platelets, among the first responders to initiate the inflammatory cascade, work alongside monocytes and macrophages, which possess strong phagocytic capabilities. These latter cells are recruited shortly after neutrophils and persist in sites of chronic inflammation and infection, where they release cytokines that modulate adaptive immune responses. Traditional inflammatory indices are typically derived from ratios of two or three immune pathways to quantify inflammation intensity. While these indices, such as the AISI, PLR, SII, and SIRI, have demonstrated robust predictive power in conditions such as metabolic disorders, cardiovascular diseases, and cancer (21, 22), their applicability to elderly hip fracture patients is less certain. These indices may fail to adequately reflect the intricate interplay of immune and inflammatory processes unique to this patient population, underscoring the need for more comprehensive predictive tools tailored to their specific physiological and pathological characteristics.
Serum calcium levels serve as indicators of both bone metabolic status and mineral balance. Dysregulation of calcium metabolism is often associated with osteoporosis and delayed fracture healing. In addition to its structural role, calcium also contributes to inflammatory responses by regulating various cellular functions through calcium signaling pathways, including immune cell activation and the release of inflammatory mediators (23). Gordon et al. reported that hypercalcemia is linked to elevated inflammatory markers, reduced bone density, and the modulation of chemokine production by extracellular calcium, which acts as a strong regulator of immune cell activity (24). Rossol et al. demonstrated that extracellular calcium stimulates the NLRP3 inflammasome, prompting monocytes and macrophages in the innate immune system to produce IL-1 (25). Similarly, Zhu et al. reported that reducing calcium levels inhibits NLRP3 activity, thereby decreasing IL-1β production via this pathway (26). Li et al. reported a U-shaped relationship between inflammatory markers and vitamin D levels (25 (OH) D and 1,25 (OH)D), with serum calcium positively correlated with both forms of vitamin D. However, when calcium levels exceed a certain threshold, they become positively associated with inflammatory markers (27). Research has further suggested that bone destruction and calcium release driven by inflammation can increase serum calcium levels and decrease bone density. This phenomenon is evident in rheumatoid arthritis patients, where approximately 30% exhibit hypercalcemia (24, 28). Serum calcium inherently reflects the dynamic interplay between osteoblast and osteoclast activity, making it a critical marker of bone health. For elderly patients with fractures, close monitoring of calcium level fluctuations is essential for understanding their bone metabolism and guiding effective recovery strategies.
Bone loss is intricately linked to inflammation and the immune system, with the skeletal system being highly responsive to chronic inflammatory stress. Conditions such as osteoarthritis and gout are frequently associated with osteoporosis, highlighting the interplay between chronic inflammation and bone health (29, 30). Inflammatory responses in fracture patients accelerate bone destruction, leading to elevated serum calcium levels. These increased calcium levels further stimulate the production of chemokines by immune cells, perpetuating a cycle of inflammation and bone degradation. This bidirectional relationship underscores the advantage of combining calcium levels with inflammatory markers, such as the lymphocyte ratio, to improve the accuracy of postoperative functional recovery predictions in elderly hip fracture patients. The introduction of the LRCa3 index offers clinicians a valuable tool for identifying high-risk patients during the preoperative or early postoperative phases. This allows for the implementation of tailored management strategies. Patients with compromised immune function and disrupted calcium metabolism may benefit from extended rehabilitation programs or nutritional supplementation to optimize functional recovery after surgery.
This study has certain limitations. The relatively small sample size and single-center design may limit the generalizability of the findings. To strengthen the validity and applicability of the results, future studies involving larger sample sizes and multicenter designs are necessary to further evaluate the predictive efficacy of LRCa3. Moreover, this study focused exclusively on one-year postoperative functional recovery in elderly hip fracture patients. Future research should consider extending the follow-up period to assess long-term functional recovery and quality of life, providing a more comprehensive understanding of patient outcomes over time.
This study identified a novel specific indicator, LRCa3, measured on postoperative day one, which effectively predicts one-year functional recovery in elderly hip fracture patients. Compared with commonly used inflammatory indices, LRCa3 has superior predictive value. Using this indicator, a predictive model was developed and validated, confirming its efficacy. This model provides significant clinical value by assisting in preoperative assessment and guiding postoperative treatment decisions, thereby enhancing management strategies for elderly hip fracture patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Medical Ethics Committee of the Second Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
WW: Methodology, Writing – original draft, Writing – review & editing. ZG: Methodology, Writing – original draft, Writing – review & editing. PZ: Writing – review & editing. BL: Data curation, Writing – review & editing. YM: Writing – review & editing. CS: Writing – review & editing. WX: Writing – review & editing. JG: Writing – review & editing. JP: Methodology, Supervision, Writing – review & editing. LL: Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Suzhou Key Disciplines (No. SZXK202104); Open Project of the State Key Laboratory of Radiation Medicine and Radiation Protection Jointly Constructed by the Ministry and the Province (GZK1202215); and Suzhou Health Talents Program (2020092).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Barceló, M, Torres, OH, Mascaró, J, and Casademont, J. Hip fracture and mortality: study of specific causes of death and risk factors. Arch Osteoporos. (2021) 16:15. doi: 10.1007/s11657-020-00873-7
2. Inoue, T, Maeda, K, Nagano, A, Shimizu, A, Ueshima, J, Murotani, K, et al. Undernutrition, sarcopenia, and frailty in fragility hip fracture: advanced strategies for improving clinical outcomes. Nutrients. (2020) 12:3743. doi: 10.3390/nu12123743
3. Gargano, G, Poeta, N, Oliva, F, Migliorini, F, and Maffulli, N. Zimmer natural nail and ELOS nails in pertrochanteric fractures. J Orthop Surg Res. (2021) 16:509. doi: 10.1186/s13018-021-02634-9
4. Marsillo, E, Pintore, A, Asparago, G, Oliva, F, and Maffulli, N. Cephalomedullary nailing for reverse oblique intertrochanteric fractures 31A3 (AO/OTA). Orthop Rev (Pavia). (2022) 14:38560. doi: 10.52965/001c.38560
5. Phruetthiphat, OA, Pinijprapa, P, Satravaha, Y, Kitcharanant, N, and Pongchaiyakul, C. An innovative scoring system for predicting an excellent Harris hip score after proximal femoral nail anti-rotation in elderly patients with intertrochanteric fracture. Sci Rep. (2022) 12:19939. doi: 10.1038/s41598-022-24177-7
6. Caillet, P, Klemm, S, Ducher, M, Aussem, A, and Schott, AM. Hip fracture in elderly individuals: a reanalysis of the EPIDOS study with causal Bayesian networks. PLoS One. (2015) 10:e0120125. doi: 10.1371/journal.pone.0120125
7. Long, A, Yang, D, Jin, L, Zhao, F, Wang, X, Zhang, Y, et al. Admission inflammation markers influence Long-term mortality in elderly patients undergoing hip fracture surgery: a retrospective cohort study. Orthop Surg. (2024) 16:38–46. doi: 10.1111/os.13932
8. Chaplin, DD. Overview of the immune response. J Allergy Clin Immunol. (2010) 125:S3–S23. doi: 10.1016/j.jaci.2009.12.980
9. Wang, JM, Pan, YT, Yang, CS, Liu, MC, Ji, SC, Han, N, et al. Effect of inflammatory response on joint function after hip fracture in elderly patients: a clinical study. World J Orthop. (2024) 15:337–45. doi: 10.5312/wjo.v15.i4.337
10. Kim, BG, Lee, YK, Park, HP, Sohn, HM, Oh, AY, Jeon, YT, et al. C-reactive protein is an independent predictor for 1-year mortality in elderly patients undergoing hip fracture surgery: a retrospective analysis. Medicine (Baltimore). (2016) 95:e5152. doi: 10.1097/md.0000000000005152
11. Bingol, O, Ozdemir, G, Kulakoglu, B, Keskin, OH, Korkmaz, I, and Kilic, E. Admission neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio to predict 30-day and 1-year mortality in geriatric hip fractures. Injury. (2020) 51:2663–7. doi: 10.1016/j.injury.2020.07.048
12. Çelen, ZE. Predictive value of the systemic immune-inflammation index on one-year mortality in geriatric hip fractures. BMC Geriatr. (2024) 24:340. doi: 10.1186/s12877-024-04916-3
13. Pan, X, Lv, J, Liu, M, Li, Y, Zhang, Y, Zhang, R, et al. Chronic systemic inflammation predicts long-term mortality among patients with fatty liver disease: data from the National Health and nutrition examination survey 2007–2018. PLoS One. (2024) 19:e0312877. doi: 10.1371/journal.pone.0312877
14. Bani Hani, DA, Alshraideh, JA, Saleh, A, Alduraidi, H, Alwahadneh, AA, and Al-Zaiti, SS. Lymphocyte-based inflammatory markers: novel predictors of significant coronary artery disease (✰, ✰). Heart Lung. (2024) 70:23–9. doi: 10.1016/j.hrtlng.2024.11.006
15. Niessen, R, Bihin, B, Gourdin, M, Yombi, JC, Cornu, O, and Forget, P. Prediction of postoperative mortality in elderly patient with hip fractures: a single-center, retrospective cohort study. BMC Anesthesiol. (2018) 18:183. doi: 10.1186/s12871-018-0646-x
16. Chen, Y, Tu, C, Liu, G, Peng, W, Zhang, J, Ge, Y, et al. Association between admission inflammatory indicators and 3-year mortality risk in geriatric patients after hip fracture surgery: a retrospective cohort study. Front Surg. (2024) 11:1440990. doi: 10.3389/fsurg.2024.1440990
17. Gou, Y, Xie, X, Yin, H, Wu, Y, Wen, Y, and Zhang, Y. Association between inflammation-related indicators and vertebral fracture in older adults in the United States: a cross-sectional study based on National Health and nutrition examination survey 2013–2014. Immun Inflamm Dis. (2024) 12:e70047. doi: 10.1002/iid3.70047
18. Cichos, KH, Christie, MC, Heatherly, AR, McGwin, G Jr, Quade, JH, and Ghanem, ES. The value of serological screening prior to conversion Total hip arthroplasty. J Arthroplast. (2020) 35:S319–s324. doi: 10.1016/j.arth.2020.02.035
19. Thisayakorn, P, Tangwongchai, S, Tantavisut, S, Thipakorn, Y, Sukhanonsawat, S, Wongwarawipat, T, et al. Immune, blood cell, and blood gas biomarkers of delirium in elderly individuals with hip fracture surgery. Dement Geriatr Cogn Disord. (2021) 50:161–9. doi: 10.1159/000517510
20. Rungelrath, V, Kobayashi, SD, and DeLeo, FR. Neutrophils in innate immunity and systems biology-level approaches. Wiley Interdiscip Rev Syst Biol Med. (2020) 12:e1458. doi: 10.1002/wsbm.1458
21. Cai, X, Song, S, Hu, J, Wang, L, Shen, D, Zhu, Q, et al. Systemic inflammation response index as a predictor of stroke risk in elderly patients with hypertension: a cohort study. J Inflamm Res. (2023) 16:4821–32. doi: 10.2147/jir.S433190
22. Nicoară, DM, Munteanu, AI, Scutca, AC, Mang, N, Juganaru, I, Brad, GF, et al. Assessing the relationship between systemic immune-inflammation index and metabolic syndrome in children with obesity. Int J Mol Sci. (2023) 24:8414. doi: 10.3390/ijms24098414
23. Park, YJ, Yoo, SA, Kim, M, and Kim, WU. The role of calcium-Calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front Immunol. (2020) 11:195. doi: 10.3389/fimmu.2020.00195
24. Klein, GL. Is calcium a link between inflammatory bone resorption and heart disease? eLife. (2022) 11:e83841. doi: 10.7554/eLife.83841
25. Rossol, M, Pierer, M, Raulien, N, Quandt, D, Meusch, U, Rothe, K, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. (2012) 3:1329. doi: 10.1038/ncomms2339
26. Zhu, Y, Guo, Y, Guo, P, Zhang, J, He, Y, Xia, Y, et al. Estrogen receptor β activation alleviates inflammatory bowel disease by suppressing NLRP3-dependent IL-1β production in macrophages via downregulation of intracellular calcium level. J Adv Res. (2024). doi: 10.1016/j.jare.2024.06.004
27. Li, X, Liu, Y, Chen, X, Reichetzeder, C, Elitok, S, Krämer, BK, et al. Target values for 25-Hydroxy and 1, 25-Dihydroxy vitamin D based on their associations with inflammation and calcium-phosphate metabolism. Nutrients. (2024) 16:2679. doi: 10.3390/nu16162679
28. Torres, HM, Arnold, KM, Oviedo, M, Westendorf, JJ, and Weaver, SR. Inflammatory processes affecting bone health and repair. Curr Osteoporos Rep. (2023) 21:842–53. doi: 10.1007/s11914-023-00824-4
29. Wu, D, Cline-Smith, A, Shashkova, E, Perla, A, Katyal, A, and Aurora, R. T-cell mediated inflammation in postmenopausal osteoporosis. Front Immunol. (2021) 12:687551. doi: 10.3389/fimmu.2021.687551
Keywords: hip fracture, HHS, LRCa3, inflammatory indices, functional recovery
Citation: Wu W, Guo Z, Zhu P, Lv B, Mao Y, She C, Xu W, Gu J, Pan J and Li L (2025) A novel indicator for predicting functional recovery in elderly hip fracture patients. Front. Med. 12:1538038. doi: 10.3389/fmed.2025.1538038
Received: 02 December 2024; Accepted: 28 February 2025;
Published: 13 March 2025.
Edited by:
Yan Press, Ben-Gurion University of the Negev, IsraelReviewed by:
Natalia Sharashkina, Pirogov Russian National Research Medical University, RussiaCopyright © 2025 Wu, Guo, Zhu, Lv, Mao, She, Xu, Gu, Pan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liubing Li, bGJsaUBzdWRhLmVkdS5jbg==; Jie Pan, cGFua3lwYW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.