
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 22 January 2025
Sec. Precision Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1535682
This article is part of the Research Topic Nanomedicine Targeting Central Nervous System View all 3 articles
 Virendra Kumar Yadav1*
Virendra Kumar Yadav1* Seshathiri Dhanasekaran2*
Seshathiri Dhanasekaran2* Nisha Choudhary3
Nisha Choudhary3 Deepak Nathiya4
Deepak Nathiya4 Vishal Thakur5
Vishal Thakur5 Rachna Gupta6
Rachna Gupta6 Sheersha Pramanik7
Sheersha Pramanik7 Pankaj Kumar8*
Pankaj Kumar8* Nishant Gupta9
Nishant Gupta9 Ashish Patel10
Ashish Patel10Parkinson’s disease is a progressive neurodegenerative disease that destroys substantia nigra dopaminergic neurons, causing tremors, bradykinesia, rigidity, and postural instability. Current treatment approaches primarily focus on symptom management, employing pharmacological, non-pharmacological, and surgical methods. However, these treatments often result in fluctuating symptoms, side effects, and disease progression. Here, the authors have reviewed the emerging field of nanomedicine as a promising path for Parkinson’s disease treatment, emphasizing its potential to overcome the limitations of traditional therapies. Nanomedicine utilizes nanoparticles for targeted drug delivery, leveraging their small size and high surface area to volume ratio to cross the blood-brain barrier and deliver therapeutic agents directly to affected brain regions. Various nanoparticles, including lipid-based, polymeric, metallic, and carbon-based, have shown potential in Parkinson’s disease treatment. Additionally, nanocarrier systems like liposomes, nanogels, dendrimers, and solid lipid nanoparticles offer controlled and sustained release of therapeutic agents, enhancing their bioavailability and reducing side effects. This review provides insights into the pathophysiology of Parkinson’s disease, highlighting the mechanisms of neurodegeneration, the role of alpha-synuclein, and the disruption of dopaminergic pathways. It further discusses the application of gene therapy in conjunction with nanomedicine for targeted therapeutic interventions.
Every year, a huge number of the population is affected by various neurological diseases like Alzheimer’s disease (1), Parkinson’s’ disease (PD), glioblastoma (2), different types of strokes (3), etc. The cases of such patients are continuously increasing worldwide, which could be attributed to the rapidly changing lifestyle, environmental factors, and genetic factors (4). Neurological disorders are disorders associated with the neurons and nervous system; one such disease affecting most of the population is PD (5, 6). PD is a progressive neurodegenerative disorder that is characterized by tremors, bradykinesia, rigidity, and postural instability and that mainly affects the motor system. PD significantly affects the quality of life (4, 7). PD mainly occurs due to the degeneration of dopamine-generating neurons in the substantia nigra. The substantial nigra a part of the brain which is vital for movement control. Due to the decline in dopamine levels in the brain, the communication between neurons gets disrupted, further leading to the hallmark motor symptoms of PD. Besides this, there are certain mon-motor symptoms, which include cognitive decline, mood disorders, and autonomic dysfunction, which also contribute to the disease burden. So, there is a requirement for precise diagnosis and treatment of PD (8, 9).
Currently, medical practitioners are focussing on symptom management rather than curing of the PD. Several conventional treatments, such as pharmacological, non-pharmacological, surgery, and emerging and experimental approaches, are available for treating PD (10). Among these, the pharmacological treatment-based approach is widely used to treat PD. The most extensively used pharmacological treatment is levodopa (11). Levodopa is a precursor to dopamine, which often combines with carbidopa to increase its effectiveness and reduce side effects. Besides this, dopamine agonists, monoamine oxidase B (MAO-B) inhibitors, and catechol-O-methyltransferase (COMT) inhibitors are some of the few options for the pharmacological treatment of PDs (12, 13). All three drugs aim to enhance or mimic dopamine activity in the brain. Moreover, surgical treatment involves deep brain stimulation (DBS) and lesioning surgery, out of which the former could be effective for patients of PD in the advanced stage who no longer respond adequately to the medicines. Even after treatment with such conventional approaches, the patients may experience fluctuating symptoms, side effects from the medicines, and disease progression (14, 15). So, there is a need for novel therapeutic strategies for PD that could overcome all the above-mentioned issues faced in conventional treatment. Several emerging novel techniques for treating PD include gene therapy (GT), nanomedicine, stem cells, and neuroprotective agents. Even though most of these techniques are in experimental stages only and need to go under clinical trials and evaluation, among all these emerging technologies, nanotechnology-based nanomedicines are gaining popularity among researchers for the theragnostic purposes of PD (16). Nanomedicine is the branch of nanosciences and nanotechnology that applies nanotechnology or nanoparticles (NPs) in medicine and offers promising new possibilities for diagnosing and treating PD (17). By developing the controlled size NPs, it is possible to deliver the drugs more effectively and precisely target affected regions in the brain. NPs are materials whose sizes fall from 1 to 100 nm (18, 19). Due to the small size and high surface area to volume ratio (SVR) of the NPs, they could easily cross the biological hurdles, like the blood-brain barrier (BBB) (20, 21). These BBBs traditionally restrict the efficacy of several treatments for neurological disorders. Furthermore, the NPs can be readily surface-modified with diverse organic compounds, ligands, and other agents to achieve target specificity, augmenting their efficacy. Additionally, certain NPs may function as nanocarriers, transporting drugs for PD on their surfaces, thereby facilitating the controlled and prolonged release of therapeutic agents. Furthermore, this focused drug administration should enhance therapeutic bioavailability and diminish side effects, thereby revolutionizing the management of PD (22, 23).
Recent theragnostic applications of nanomedicine for PD treatment include the formulation of various nanocarrier systems such as liposomes, nano gels, dendrimers, and solid lipid nanoparticles (SLNs) (24). All these nanocarrier systems are capable of encapsulating therapeutic agents for PD. The encapsulated therapeutic agents are shielded from enzymatic degradation, ensuring their delivery to the target site of action. Furthermore, nanomedicine presents opportunities for neuroprotective strategies by employing antioxidant and anti-inflammatory NPs to protect and repair damaged neurons. One more technique that demonstrates high possibilities for the treatment of PD is GT. GT involves the application of NPs as carriers for the delivery of desired genes that could correct or mitigate the underlying causes of PD (25). Besides this, integrating CRISPR/Cas9 technology with nanotechnology can address the root causes of neurodegeneration in PD. The integrated approaches significantly advance the quest for more effective and targeted therapies. The advancement of research in nanomedicine-based approaches promises to improve symptom management and patient outcomes and potentially slow the advancement of PD. The future of PD treatment lies in the successful translation of nanomedicine from the laboratory to clinical practice, opening the way for a new era in neurological care (25).
In the present review article, investigators have emphasized the basics of Parkinson’s disease, their pathophysiology, and current available treatment methods. Further emphasis was given to the drawbacks of the current drug for Parkinson’s disease. The authors have further focused on the role of nanotechnology, nanoparticles, and nanomedicine in treating Parkinson’s disease. The authors have highlighted the role of newly emerging techniques like gene therapy and CRISPR/Cas9 for the possible treatment of PD. Finally, emphasis was given to the possible integration of the nanoparticles with other emerging technologies for the symptomatic management of PD. Such investigation will pave the way for advanced technology and a new PD neurological care era.
PD is a chronic and progressive neurological disease that generally impacts the cortico-basal ganglia-thalamic circuitry in the brain (26). It is characterized by the degeneration of dopaminergic neurons in the substantia nigra, resulting in a deficiency of dopamine, a neurotransmitter vital for motor function (27). While PD is usually considered a disease that affects individuals over the age of 65, cases of early-onset Parkinson’s can also occur (28). Annually, it impacts approximately 3% of older people and ranks second behind Alzheimer’s disease (1). There is initial damage in β-oxidation in PD, reducing long-chain acylcarnitine levels (29). Consequently, there is a progressive accumulation of the presynaptic protein α-synuclein within intracellular fibres. The loss of dopamine neurons in the midbrain substantia nigra causes resting tremor, bradykinesia, and stiffness (30). The major problem is that no effective method exists to find the cause.
PD is caused by the progressive degradation of dopaminergic nerve cells in the substantia nigra, a part of the brain that is essential for controlling movement (31). Dopamine levels rapidly decline due to neuronal death, disturbing the equilibrium between motor and non-motor functions. Furthermore, Lewy bodies and abnormal aggregates of the protein alpha-synuclein contribute to neuronal dysfunction and the clinical symptoms of the disease. A concise description of the pathogenesis of PD is provided. Initially, a significant advancement in comprehending PD involves elucidating the neurodegenerative process. The precise aetiology of this neuronal death is indeterminate; nonetheless, it is presumed to stem from the intricate interplay of genetic, environmental, and mitochondrial influences. Multiple critical pathways contribute to neurodegeneration in PD, oxidative stress (OS), mitochondrial failure, and neuroinflammation (32, 33).
OS is a prevalent phenomenon in the brain in PD, resulting from an imbalance between the production of deleterious chemicals known as reactive oxygen species (ROS) and the brain’s capacity to neutralize them (34). The increased OS in PD damages cellular components (lipids, proteins, and DNA), resulting in neuronal malfunction and death. This issue is further intensified by mitochondrial dysfunction, which impairs energy production and increases the creation of ROS. The characterization of neuroinflammation is carried out by the activation of microglia and the release of pro-inflammatory cytokines. The pro-inflammatory cytokines also play a crucial role in the neurodegenerative method, contributing to neuronal injury and cell death (35, 36).
A small soluble protein (alpha-nuclein) is present in the brain in large amounts, mainly at the presynaptic terminals, and is expected to plays an important role in synaptic function and neurotransmitter release (37). Various pieces of literature have found that alpha-synuclein undergoes pathological aggregation in PD, forming insoluble fibrils. These insoluble fibrils accumulate as Lewy bodies and Lewy neurites within the nerve cells. The aggregation of such fibrils disrupts cellular function and contributes to neuronal death through several phenomena (38, 39).
Moreover, these proteins may misfold, further impair proteasomal and autophagic pathways. As a result, there will be an accumulation of damaged proteins and organelles. The synaptic function of the alpha-synuclein could also be disrupted by interfering with vesicle trafficking and the release of the neurotransmitter. Besides this, alpha-synuclein aggregates can spread from cell to cell, which may propagate pathology throughout the brain in a prion-like fashion. The presence of these aggregates is a hallmark of PD and is closely associated with the progression and symptomatology of the disease (38).
The dopaminergic pathways, mainly the nigrostriatal pathway, are critically involved in the motor symptoms of PD. Dopaminergic pathways consist of dopaminergic neurons that project from the substantia nigra pars compacta to the striatum, a brain area associated with coordinating movement (40). If these neurons are lost, there will be a significant lowering in dopamine levels in the striatum. As a result, there will be a disruption in the balance of excitatory and inhibitory signals needed for smooth and controlled movements. Dopamine is very important for modulating the activity of the basal ganglia. The basal ganglia are a group of nuclei that play a valuable role in motor control. Depleting the dopamine level in PD will lead to overactivity of the indirect pathway and under activity of the direct pathway within the basal ganglia circuitry (41). This imbalance increases inhibitory output to the thalamus and motor cortex. As a result, there will be characteristic motor symptoms of PD, like bradykinesia, rigidity, and tremors. The degeneration of dopaminergic neurons affects motor symptoms and non-motor functions. This is because dopaminergic pathways regulate mood, cognition, and autonomic functions. This explains the wide range of non-motor symptoms noticed in PD patients, including autonomic dysfunction, depression, anxiety, and cognitive impairment (42, 43).
It could be concluded that the pathophysiology of PD involves the complex phenomenon of neurodegeneration, the pathological role of alpha-synuclein, and the disruption of dopaminergic pathways. Understanding these processes is vital for developing effective therapeutic strategies to slow or halt PD progression and improve patients’ quality of life. Figure 1 shows the pathophysiology of PD.
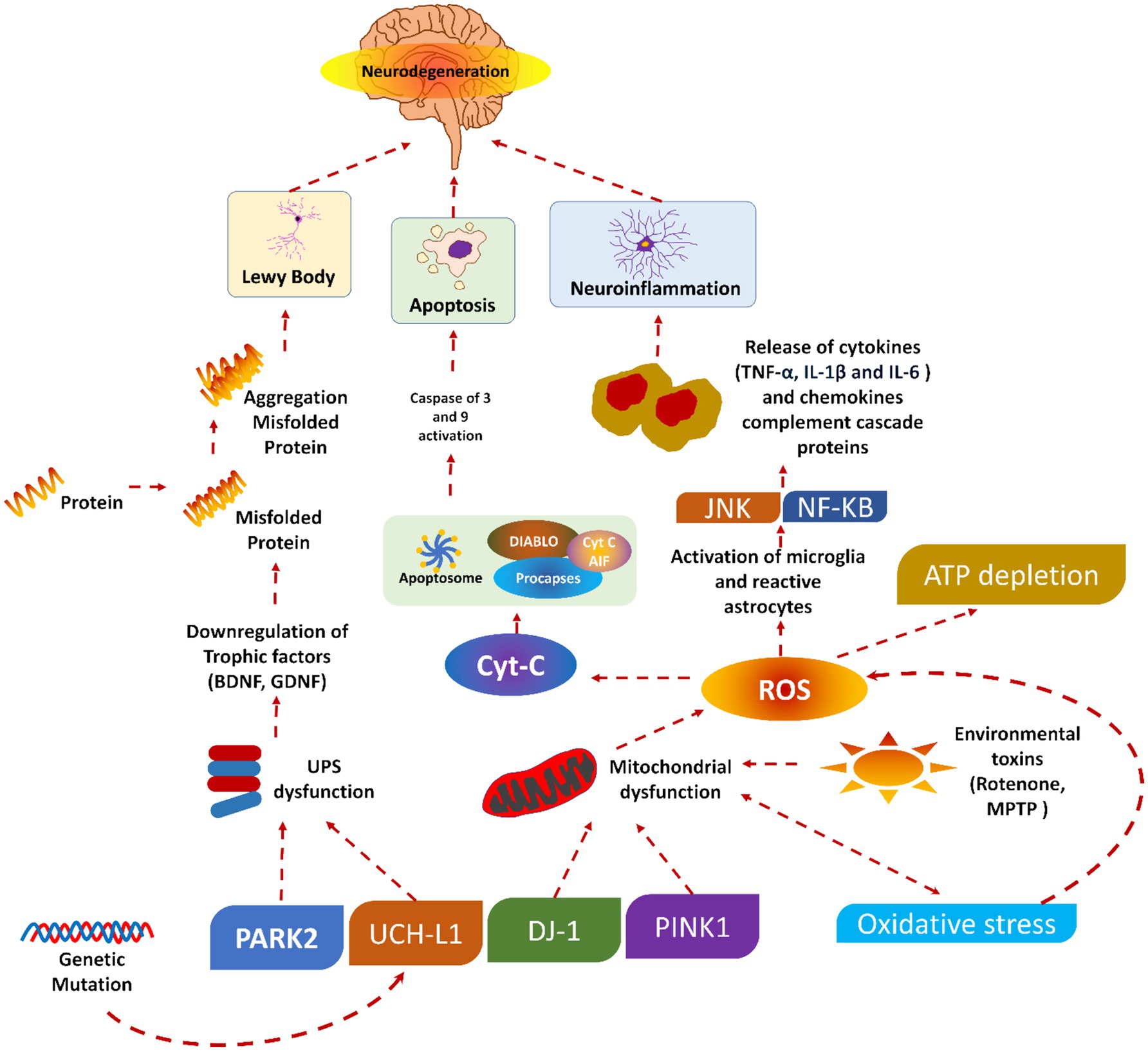
Figure 1. Pathophysiology of Parkinson’s diseases, reproduced from Doke et al. (189) with permission.
A summarized presentation of various genes involved in PD and their respective mechanisms of action is given in Table 1. Despite this, several pieces of information are still unknown, which warrants further investigation.
Traditional approaches, such as pharmacological, non-pharmacological, and surgical, and some emerging treatment techniques, GT, stem cell therapy, neuroprotective agents, and nanomedicine, exist for treating PD (44). Figure 2 shows the broader categories of treatment options for PD, while Figures 3, 4 show various existing treatment strategies for PD.

Figure 2. Treatment options for PD adapted from Church (190).
Pharmacological treatments for PD encompass levodopa and carbidopa, dopamine agonists, MAO-B inhibitors, COMT inhibitors, anticholinergics, and amantadine (45). Levodopa is a highly effective and commonly utilized drug for PD, functioning as a precursor to dopamine that traverses the BBB and is subsequently converted to dopamine within the brain (46). Carbidopa is administered with levodopa to inhibit its early conversion to dopamine outside the central nervous system, thereby minimizing side effects like nausea and enhancing the quantity of levodopa penetrating the brain (47). The commonly utilized dopamine agonists are pramipexole, ropinirole, and rotigotine. These drugs replicate dopamine’s effects by directly activating dopamine receptors in the brain. These drugs may be utilized independently or in conjunction with levodopa to mitigate fluctuations in motor control. Selegiline and rasagiline are both inhibitors of MAO-B. MAO-B is an enzyme responsible for the degradation of dopamine in the brain, which subsequently leads to elevated dopamine levels and enhancement of motor symptoms (11, 48).
Both entacapone and tolcapone are COMT inhibitors that inhibit catechol-O-methyltransferase. COMT is an enzyme that breaks down levodopa (49). Besides this, COMT inhibitors are combined with levodopa to prolong its effects and reduce “off” periods. Both benztropine and trihexyphenidyl are anticholinergics that help control tremors and rigidity by decreasing acetylcholine activity (50). Acetylcholine is a neurotransmitter that can become overactive in PD due to the loss of dopamine (6, 51, 52). Amantadine is mainly an antiviral drug where the amantadine helps in reducing the symptoms of PD, particularly dyskinesia (involuntary movements) associated with prolonged use of levodopa. It could be concluded that there is little evidence to exhibit that dopamine prodrugs and levodopa would help alleviate dopamine neuron degeneration (28). It has been reported that such medical treatment causes several side effects like arrhythmia, gastrointestinal discomfort, extreme emotional changes, etc. (53). To date, not even a single drug is available, even after neuroprotective tests, which could delay or stop the progression of the disease. In addition to this, there are no effective biomarkers in PD, drugs having secondary hallucination and delusion effects, and the resistance of BBB. All these factors minimize the diagnosis and treatment of PD. Figure 5 shows various pharmacological treatment methods for PD (54).
Table 2 shows the classification of dopamine agonists, which are classified into two main classes: ergot-derived and non-ergot-derived. Among the ergot-derived dopamine agonists, bromocriptine, marketed as Parlodel, is administered orally. Cabergoline and lisuride are oral drugs in this category, though their market names are not specified. Pergolide, known as Permax, is another ergot-derived agent available in oral form (55, 56).
In contrast, the non-ergot-derived dopamine agonists include apomorphine, marketed as Apokyn, which is administered subcutaneously. Ropinirole, sold under the brand name requip, is an oral drug. Similarly, pramipexole, marketed as Mirapex, is also taken orally. Another notable non-ergot-derived agent is rotigotine, available as a transdermal patch under neupro (56, 57). This classification highlights the diversity of dopamine agonists in terms of their origin and routes of administration (ROA).
The pharmacological management of PD aims to enhance motor function by increasing dopamine levels. Additionally, there are limited treatments for non-motor symptoms, which can be significantly debilitating. Few treatments can impede or alter the progression of the disease. Levodopa and dopamine agonists are commonly utilized drugs for PD, although they may induce adverse consequences. Each patient’s treatment plan is personalized based on their symptoms, side effects, and requirements (43).
The non-pharmacological treatments of PDs include various approaches like physical, occupational, speech, and nutritional therapy and exercise (58, 59). Physical therapy focuses on improving mobility, balance, and strength through exercises, gait training, and assistive devices. Occupational therapy helps patients maintain independence by improving their ability to perform daily activities and recommending adaptive strategies and equipment (60). The difficulties associated with speech and swallowing could be addressed by speech therapy. Speech therapy provides exercises so that articulation, volume, and speech clarity, as well as techniques to manage dysphagia (difficulty swallowing), could be improved (61). In nutrition therapy, emphasis is given on the proper nutrition for managing symptoms and maintaining overall health. Advice from a well-trained dietitian could be very helpful for optimizing food choices. Moreover, a dietician could also help ensure adequate nutrient intake and manage gastrointestinal tract symptoms. Moreover, exercise could also help improve motor symptoms, balance, and overall well-being (62). The exercises may include regular physical activity like aerobic exercises, strength training, and flexibility exercises (63, 64).
Kalbe et al. (65) recently emphasized the significance of non-pharmacological interventions in managing PD. These therapies are considered supportive or “add-on” strategies primarily designed to mitigate motor symptoms. Physiotherapy, speech-language, and occupational therapy have increasingly emerged as essential to comprehensive PD management. Additionally, non-pharmacological interventions such as cognitive training, cognitive behavioural therapy, and art or light therapy, previously deemed “exotic,” are now beginning to be integrated into therapeutic guidelines. Additionally, several advancements have been noted in this field, including (i) the expansion of intervention types, (ii) the standardization of intervention protocols, (iii) the development of digital intervention forms, (iv) the scientific evaluation of intervention feasibility and effects, (v) the understanding of underlying mechanisms of therapy-induced plasticity methods, (vi) the integration of non-pharmacological interventions into patient care concepts, and (vii) transition from merely symptomatic to preventive treatments (66). Figure 6 summarizes the various non-pharmacological treatments of PDs.
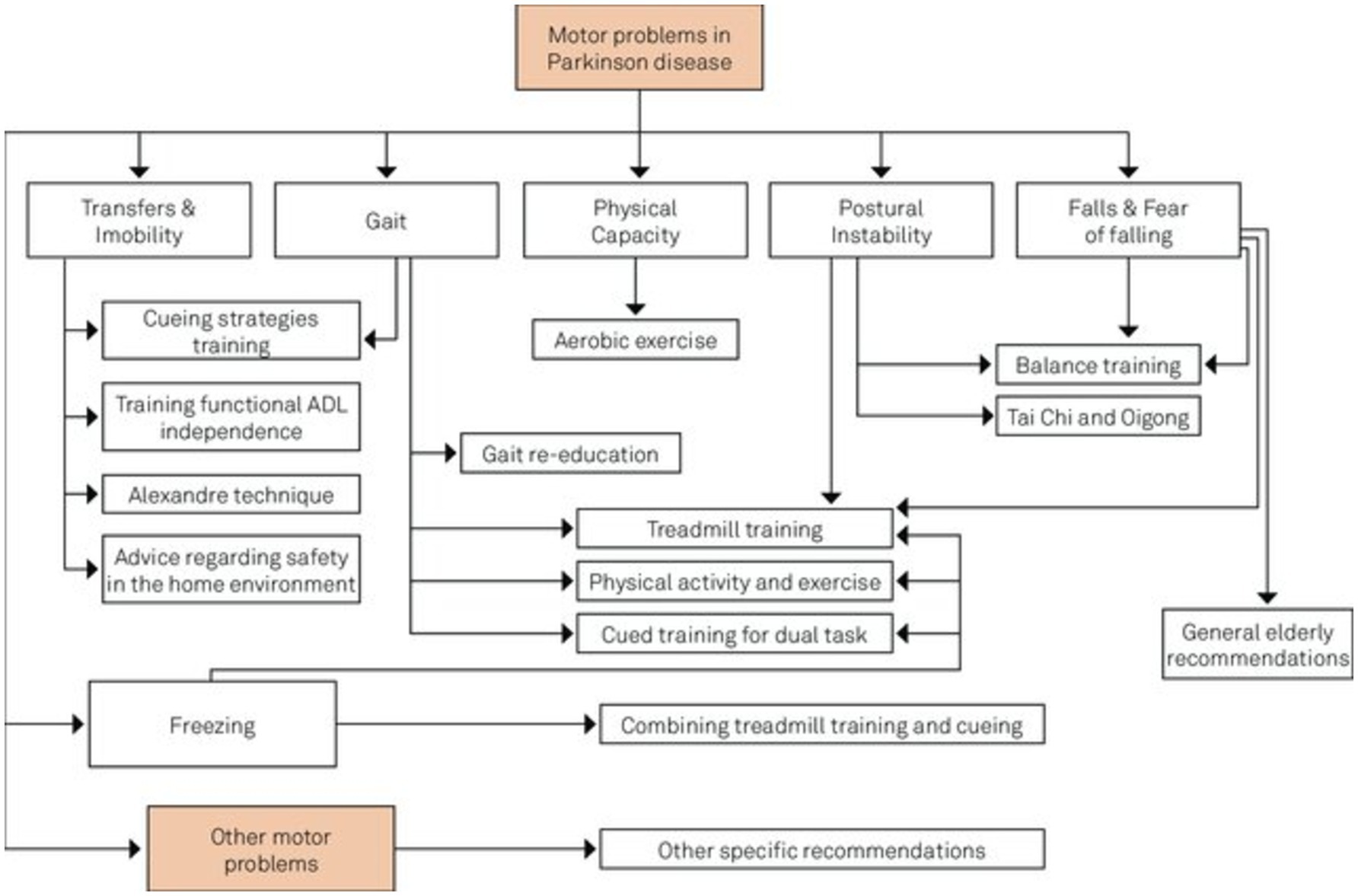
Figure 6. Intersection of non-pharmacological treatment interventions for PD adapted from Domingos et al. (191).
PD could also be treated by using surgical therapy, which mainly includes DBS and lesioning surgery (67). DBS is a surgical procedure involving the implantation of electrodes into the subthalamic nucleus or globus pallidus internus of the brain. Additionally, these electrodes are linked to a pulse generator implanted in the thoracic cavity. Further, these pulse generators send electrical impulses to the brain to help regulate abnormal impulses. In lesioning surgery techniques (pallidotomy and thalamotomy), a small lesion is created in the targeted areas of the brain to disrupt abnormal brain activity (68). DBS is preferred over lesioning surgery from both available surgical therapies (14).
Sharma et al. (69) reviewed DBS and lesioning techniques for PD, addressing indications, selection processes, and management strategies. The investigators proposed that a team make the decision regarding surgical treatment for PD, taking into account the patient’s clinical characteristics, treatment efficacy, ease of use, associated risks, and overall quality of life. Still, DBS is the most preferred surgical technique, but there is a growing interest in new techniques like MR-guided focused ultrasound.
Recently, Foltynie et al. (70) presented the latest evidence in support of the optimal treatment of PD. Further investigators described an expert approach to several aspects of treatment choice with insufficient evidence base. Here, the investigators suggested treatment for different phases of PD. For instance, firstly, there should be initial treatment of motor PD; secondly, adjunctive treatment of motor PD; thirdly, non-oral therapies for the complex phase of PD; and finally, treatment of non-motor symptoms. The first treatment for PD should involve a detailed discussion about the diagnosis, treatment options, and how to maintain the patient’s independence and quality of life based on their specific symptoms. Some patients may not get enough relief from symptoms with initial treatment, and if certain symptoms persist, it may be necessary to consider other types of PD. Continuous dopaminergic or electrical stimulation targets the limited therapeutic range between symptom relief and peak dose dyskinesia, which arises during the advanced stages of PD. Non-motor symptoms in PD can affect many areas like mood, sleep, and pain and may be more challenging than movement issues, often linked to low dopamine levels and needing careful management.
Above all, some emerging and experimental approaches for treating PDs include GT, stem cell therapy, neuroprotective agents, and nanomedicine (71). Literateurs have proven that in GT, genetic material has been introduced into the brain to correct or modify the underlying genetic causes of PD. Several clinical trials in their early stages explore using viral vectors to deliver genes. These genes may either increase the production of dopamine or protect nerve cells. Stem cell therapy is new to this field, and extensive research is being done on using these stem cells to replace lost dopaminergic neurons in the brain. Still, such approaches are in their experimental stages (72). Neuroprotective agents are also in their infancy, and several investigations are being conducted to formulate drugs that could help protect the neurons from degeneration. These neuroprotective agents include antioxidants, anti-inflammatory drugs, and agents targeting mitochondrial function (73). Besides this, nanomedicine is another technique in its infancy, but it has a huge potential for treating PDs. By utilizing nanotechnology, enhanced drug delivery and targeting could be achieved (25). Besides this, nanotechnology may improve efficacy and reduce the side effects of current treatment methods. Several nanocarriers (liposomes, nanoparticles) have been studied for their potential to cross the BBB and deliver therapeutic agents directly to affected brain regions. In contrast, Yuan et al. (74) explore the neurotoxic effects of SiO2 NPs, particularly in promoting PD-like pathology both in vitro and in vivo, suggesting a potential link between SiO2 NPs exposure and the initiation and progression of PD. While these emerging approaches hold promise, it’s important to acknowledge that they also have their limitations. This awareness is crucial for understanding the current challenges in PD treatment. Some of the complications associated with the current treatment approaches are summarized below in Table 3.
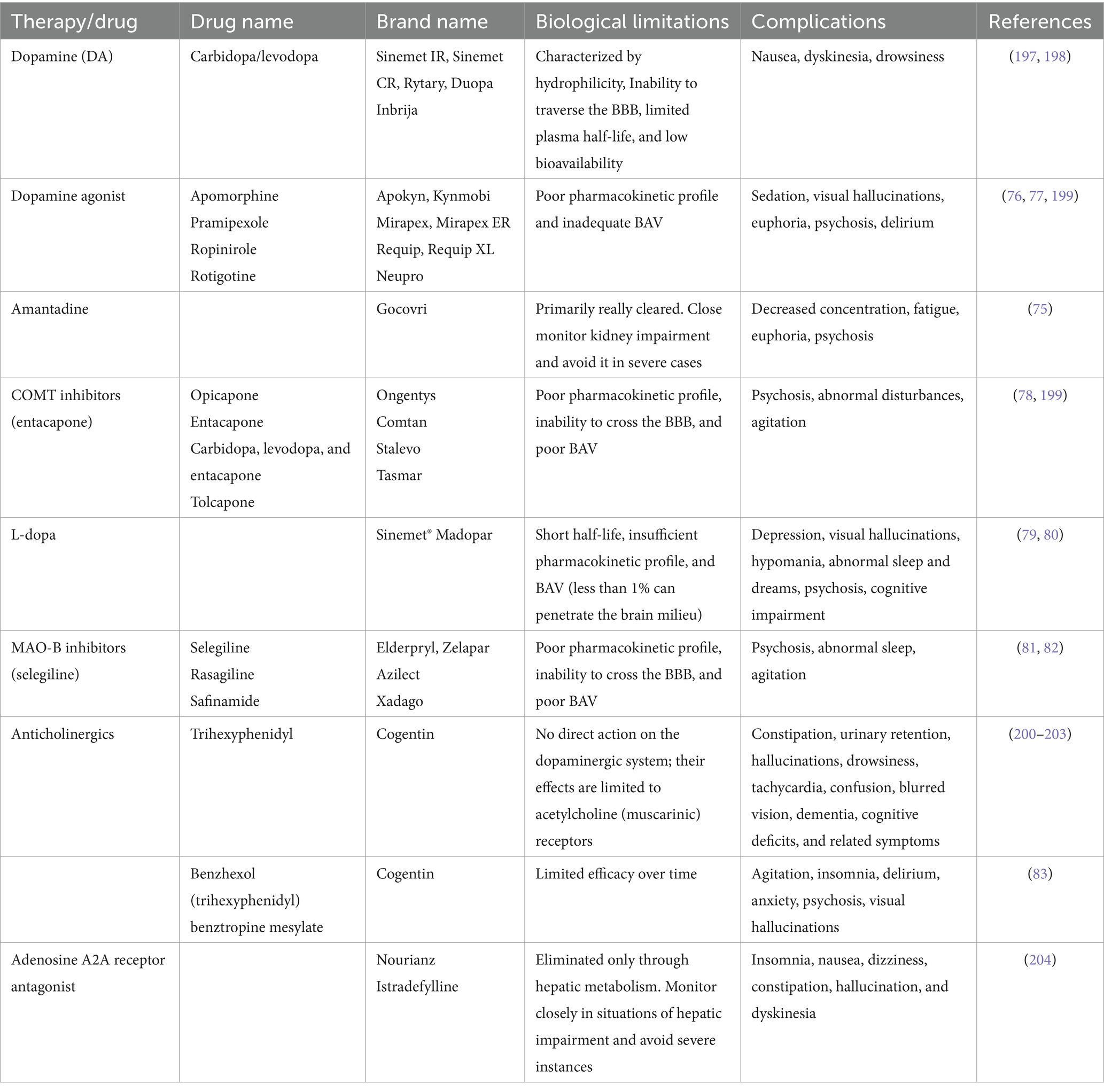
Table 3. Biological limitations and complications associated with the current treatment approaches for PD.
From the above table, it could be concluded that even though each drug is beneficial in the treatment of PDs, it also has several complications. For instance, amantadine may lead to decreased concentration and fatigue, along with the possibility of euphoria and psychosis (75). Dopamine agonists exhibit efficacy in managing PD symptoms but can be associated with sedation, visual hallucinations, euphoria, and psychosis, potentially leading to delirium (76, 77). COMT inhibitors like entacapone may induce psychosis, abnormal disturbances, and agitation (78). L-dopa, a cornerstone in PD treatment, may lead to depression, visual hallucinations, hypomania, abnormal sleep patterns, psychosis, and cognitive impairment (79, 80). Similarly, MAO-B inhibitors (selegiline) can lead to psychosis, abnormal sleep, and agitation (81, 82). Finally, benzhexol may induce agitation, insomnia, delirium, anxiety, and visual hallucinations (83). In clinical practice, weighing the therapeutic benefits against the potential adverse effects is crucial in individualizing treatment approaches for patients with PD.
NPs offer a promising approach to treating PD due to their small size. Besides this, the NPs could be easily engineered for specific targeting and the potential to improve drug bioavailability and efficacy (84). The NPs could be of various shapes, sizes, and origins, i.e., metallic or organic, solid or liquid, and carbon-based. The widely used NPs for treating PDs are lipid-based NPs, polymeric NPs, metal NPs, and carbon-based NPs, described below in detail. NMs hold substantial promise for progressing diagnostics in PD through three vital strategies (85, 86). Firstly, NMs offer the potential for governing crucial biomarkers such as α-synuclein, a protein associated with PD pathology (87). By preparing nanoscale tools and sensors, the detection and quantification of α-synuclein levels can be improved, assisting early diagnosis and disease monitoring. Secondly, NMs enable the monitoring of active dopamine neurons, providing beneficial insights into the status of dopaminergic neurotransmission, which is central to PD. NMs are efficient carriers for delivering therapeutic agents to specific target regions in the brain. The current NMs demonstrate effectiveness by addressing dopamine deficiency, caspase-3 activation, α-synuclein accumulation, OS, mitochondrial dysfunction, inflammation, and growth factor supplementation (88). The findings indicate a broad spectrum of potential approaches for Parkinson’s disease therapy (89). Generally, NPs can be classified as inorganic, organic, or carbon-based NPs (Figure 7).
Lipid-based NPs mainly comprise liposomes and SLNs, which are commonly investigated for treating PD. Such NPs encapsulate hydrophilic and hydrophobic drugs, protect them from degradation, and enhance their delivery across the BBB (90). Liposomes are spherical vesicles made up of lipid bilayers and are particularly advantageous due to their biocompatible nature and ability to fuse with cell membranes (Figure 8). The fusion of the liposomes with the cell membrane facilitates the release of the drug directly into the target cells. SLNs are made up of solid lipids, which offer a stable alternative to liposomes and can be applied for the effective delivery of lipophilic drugs effectively (91).
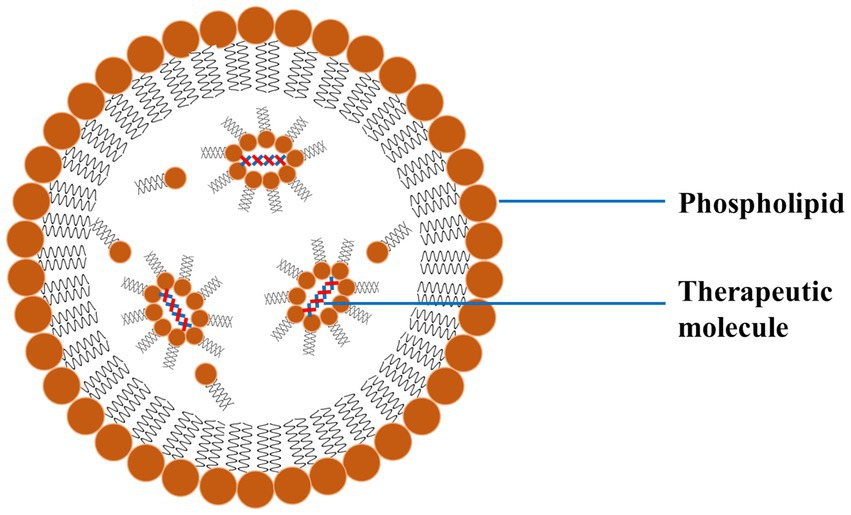
Figure 8. A schematic diagram of LNP exhibiting the outer phospholipid layer and the encapsulated therapeutics reproduced from Jagaran et al. (145) with permission.
Polymeric NPs are organic compounds composed of biocompatible and biodegradable polymers like polylactic acid (PLA) and polyglycolic acid (PGA) (92). These polymeric NPs form an important class of NPs extensively applied for treating PD (92). One major advantage of using these NPs in PD treatment is their ability to release drugs in a controlled and sustained manner. This reduces dosing frequency and makes it easier for patients to stick to their treatment plan. These polymeric NPs constitute an important class of NPs that are widely used in the treatment of PD (93). One major advantage of using these NPs in PD treatment is their controlled and sustained drug release, which reduces dosing frequency and improves patient compliance (94, 95). Polymeric NPs are designed so that they get dissolved by themselves within the body. Due to the degradation of such polymeric NPs, the encapsulated drug is released over a predetermined period. Besides this, such polymeric NPs could also be functionalized with targeting ligands to enhance the specificity of dopaminergic neurons (96).
Various studies have investigated the administration of exogenous dopamine (DA) and L-dopa into the CNS to supplement the reduced levels of endogenous DA and counteract the loss of dopaminergic neurons. Advanced polymeric nanoparticulate formulations can potentially improve PD symptoms by delivering DA directly to the brain (97).
Arisoy et al. (12) developed a NP system using PLGA and WGA to deliver L-dopa through the nose, which helps the drug reach the brain directly and avoids the BBB. This method showed better drug delivery to the brain with fewer side effects than traditional oral or nasal methods, as seen in tests on mice with PD. The NPs system had a high drug retention rate of 73% and released L-dopa over 9 h, indicating its effectiveness and safety in the nasal cavity. However, using this delivery method may be difficult for patients with nasal issues like sinusitis or rhinitis, which could affect how the system works.
Ragusa et al. (98) effectively synthesized chitosan NPs that contained DA and evaluated the neuroprotective properties of the system in vitro using human neuroblastoma SH-SY5Y cells. They conducted additional research on the diverse impacts of DA, uncoated chitosan NPs, and chitosan-dopamine NPs in generating ROS. Monge-Fuentes et al. (99) created a new NP system loaded with DA (7 μg DA per mg of NPs) made up of albumin and PLGA (353 to 497 nm in diameter; PDI: 0.4 to 0.6; zeta potential −27 to −37 mV) that helps improve motor skills in mice with Parkinson’s disease by delivering dopamine and crossing the BBB effectively.
Vong et al. (100) developed a nano-polymeric drug system (NanoDOPA) to enhance the brain delivery and therapeutic efficacy of L-dopa in a mouse model of PD. The drug gradually released L-dopa through chymotrypsin degradation and significantly enhanced the in vivo pharmacokinetic profile of L-dopa in comparison to free L-dopa. The administration of NanoDOPA in the MPTP-induced PD mouse model led to significant enhancements in motor symptoms and reduced dyskinesia commonly linked to L-dopa treatment (see Table 4).
Metallic NPs (Ag, Au, Zn, Pt, Fe, etc.) also play a crucial role in treating PD due to their small size and unique optical and electronic properties (101, 102). Due to these features, such NPs could be exploited for therapeutic and diagnostic purposes in PD. The various metallic NPs used to treat PDs are gold (AuNPs) and silver nanoparticles (AgNPs) (103). For instance, AuNPs could be functionalized with drugs or targeting molecules and used for targeted delivery of drugs or as contrast agents for imaging. Besides this, such metallic NPs can be used to modulate the activity of enzymes engaged in OS. The OS is a key pathological PD property, offering neuroprotective effects (104, 105). Among metallic NPs, noble NPs like gold and silver have gained huge attention due to their high stability, easy synthesis routes, and easy surface functionalization.
AuNPs and AgNPs have demonstrated promise in inhibiting the aggregation of extracellular and intracellular protein aggregates in neurodegenerative disorders. Both the NPs can function as stabilizers by selectively attaching to certain areas of protein molecules, thereby preventing protein misfolding (106). This interaction hampers the proteins from assuming abnormal structures, creating harmful clusters. Furthermore, these noble NPs can act as “seeds” for protein aggregation. By emulating the protein’s original form, they entice the misfolded proteins and assist in their attachment to the NP surface. This process isolates the proteins from other molecules, decreasing their capacity to combine and create larger clusters. Moreover, both the NPs have exceptional catalytic capabilities. Both can facilitate the breakdown of misfolded proteins by catalytic mechanisms, such as the generation of ROS or the stimulation of cellular enzymes responsible for removing proteins. This augmented protein breakdown helps in preventing the buildup of harmful protein aggregates. The well-surface functionalized NPs can specifically engage with inflammatory cells in the brain, thus reducing the neuroinflammatory response. Due to the constant inflammation in neurodegenerative diseases, AuNPs and AgNPs have shown inherent antioxidative characteristics. Both of these NPs can eliminate ROS, which is known to play a significant role in causing neuronal damage through neuroinflammation (106). These NPs may improve brain inflammation caused by OS by decreasing levels of ROS. Moreover, it has been evident from the literature that both noble NPs might impede the synthesis and discharge of proinflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and IL-6. These cytokines play a vigorous role in neuroinflammation. NPs can potentially decrease the inflammatory cascade in the brain by inhibiting these cytokines. Furthermore, AuNPs and AgNPs regulate the nuclear factor-kappa B (NF-κB) pathway. NF-κB is a crucial transcription factor that activates many proinflammatory genes. Nps disrupt the NF-κB signalling pathway, inhibiting the synthesis of inflammatory mediators (104, 107).
AuNPs, as metallic NPs, are the most widely used in medicine due to their chemically inert nature, as in most cases, they do not undergo oxidation processes (108). This unique property makes them versatile and applicable in various medical contexts. Due to this reason, the surface of AuNPs can be easily functionalized with various types of chemical groups or dyes. Literature proves that AuNPs can act as a contrast and therapeutic agent in CNS disorders, further highlighting their potential in diverse medical applications (108, 109).
Hu et al. (110) developed composite materials of AuNPs (CTS@GNP-pDNA-NGF) by combining electrostatic adsorption and photochemical immobilization methods. These composites can potentially carry plasmid DNA (pDNA) and selectively target particular cell types. The Au NPs were introduced into cells through endocytosis to suppress the death of PC12 cells and dopaminergic neurons. Further, Au NPs composites are utilised in in vivo PD models, where they may effectively penetrate the BBB and accumulate in the brains of mice. It is concluded that the AuNP composites exhibit favourable therapeutic effects for in vitro and in vivo models of PDs.
AuNPs have also been employed in PD models. For instance, a study led by da Silva Córneo et al. (111) demonstrated that administering AuNPs to a mouse model of PD produced by reserpine significantly reduced OS markers and improved motor symptoms. The administration of AuNPs resulted in a partial improvement of neurotrophic factors. Specifically, the therapy at a concentration of 20 nm with a dosage of 2.5 mg/L successfully reversed the symptoms of the generated PD without causing any harmful effects.
A study by Xue et al. (112) exhibited that AuNPs synthesized from the root extract of Paeonia moutan effectively remove ROS and reduce the levels of inflammatory cytokines in microglial cells, as demonstrated in vitro. Moreover, the in vivo findings confirmed that therapy with AuNPs effectively avoided neuroinflammation in dopaminergic neurons and elevated dopamine levels. As a result, the mice with PD were protected from motor abnormalities. Both studies showed that therapy with AuNPs reversed the behavioural symptoms caused by PD, indicating the effective protective impact of AuNPs on dopaminergic neurons without any toxic effects. This treatment also prevented side symptoms associated with the condition.
The compatibility and interaction of AuNPs with pharmacological drugs render anti-inflammatory and other targeted drugs significant. These drugs are utilized in the treatment of neurodegenerative diseases, such as PD and AD. A study demonstrated that L-dopa, when associated with AuNPs, can mitigate the adverse effects of the drug while enhancing its therapeutic efficacy and prolonging its effect duration without compromising potency. The potential applications of Au NPs are effective and promising, broadening the scope of NP utilization in biomedical contexts as a novel treatment (113). Besides these two noble metal NPs, several other metal oxide NPs (iron oxide and silica) have also been used as therapeutic and contrast agents for PDs.
Besides polymeric and metallic NPs, certain carbon-based NPs are widely used to treat PD. These carbon-based NPs include carbon nanotubes (CNTs), fullerenes, and graphene. The CNTs could be either single-walled (SWCNTs) or multi-walled (MWCNTs) (114). All these carbon-based NPs have high surface area, high mechanical strength, and the ability to penetrate cell membranes, which hold a promising potential for treating PD. Moreover, the CNTs could be further surface functionalized with various molecules to increase their solubility and biocompatibility. Surface-functionalized and biocompatible CNTs could be used to target the delivery of therapeutic agents (115). Both graphene and its derivatives have excellent conductivity and flexibility, which could be used to deliver drugs. Besides this, graphene and its derivatives act as scaffolds for neuronal growth, potentially facilitating the repair of damaged neural tissues (116).
One study used molecular dynamics simulation tools to investigate the impact of 4 forms of last-generation graphene-based nanostructures on preventing α-synuclein amyloid fibrillation. This study found that nitrogen-doped graphene (N-graphene) is the best in preventing the formation of harmful protein aggregates linked to PD, making it a promising option for treatment. This was so because N-graphene is the most effective at disrupting the structure of α-synuclein amyloids, showing strong interactions and causing significant instability compared to other monolayers (117, 118).
The investigators have found that enzyme-integrated metal-organic frameworks (MOFs) had antioxidant activity and chiral-dependent BBB trans endocytosis. These frameworks may treat PD as anti-neuroinflammatory drugs (119). By adding extremely small platinum nanozymes (Ptzymes) to L-chiral and D-chiral imidazolate zeolite frameworks, chiral nanozymes are generated. In male PD mouse models, Ptzyme@D-ZIF accumulated more brain tissue than Ptzyme@L-ZIF. This was due to longer plasma presence and more BBB crossing routes, including clathrin- and caveolae-mediated endocytosis. These variables explain Ptzyme@D-ZIF’s increased therapeutic efficacy in reducing behavioural and pathological changes. Bioinformatics and biochemical studies show that Ptzyme@D-ZIF prevents neuroinflammation-induced apoptosis and ferroptosis (120) in damaged neurons. In enzyme-integrated chiral ZIF platforms, live organisms, metabolism, and therapeutic effects vary, suggesting the potential for PD medicines (121).
The therapeutic effect of the NPs could be exerted on the PD through various processes. One basic feature is enhancing the drug delivery across the BBB, which ensures that a higher concentration of the therapeutic agent reaches the brain (122). This particular feature has an important role in PD as several drugs have limited ability to cross the BBB on their own (123). Besides this, NPs could also provide a controlled and sustained release of drugs, which maintains the therapeutic levels over extended periods and reduces side effects involved with peak-dose fluctuations. In addition to this, NPs can be designed to target specific cellular pathways associated with PD pathology. It has been found that customized NPs could deliver antioxidants or anti-inflammatory agents to lower the OS and neuroinflammation, which are the major contributors to neuronal degeneration. Besides this, NPs could also be used to deliver neurotrophic factors or GT agents. The neurotrophic factors or GT agents promote neuronal survival and repair, which offers potential disease-modifying effects (124). Table 5 exhibits a summarized form of the implications of nanomedicine on PD.
It could be concluded that nanomedicine exhibits hope for treating PD by using several modes of administration, for instance, intracranial implantation, intraperitoneal injection, transdermal, intranasal, and oral delivery. Several NPs like L-dopa/dopamine, ropinirole, bromocriptine, apomorphine, urocortin, odorranalectin, rasagiline, and selegiline HCl have shown improved DD, bioavailability, controlled release, and symptom management in PD therapy.
For the successful treatment of PD by NPs, there are two important features: firstly, effective targeting and delivery. From the various pieces of literature, it has been observed that numerous investigations have developed strategies to enhance the specificity and efficiency of NPs delivery to the brain and the affected neurons (22, 125).
The NPs mediated enhanced drug delivery across the BBB and improved diagnostic accuracy. NPs could be achieved by source functionalization by specific molecules Numerous investigations have demonstrated that NPs could be functionalized with targeting ligands (antibodies, peptides, or small molecules). These targeting ligand surface-functionalized NPs bind specifically to receptors or proteins expressed on the dopaminergic neurons’ surface. Such targeted strategies ensure that the therapeutic agents are directly delivered to the damaged cells. Additionally, these targeted approaches reduce unwanted side effects and improve therapeutic outcomes (125). Moreover, functionalized NPs can act as contrast agents in imaging techniques, selectively accumulating in affected brain areas to enhance disease detection and monitoring (126). In one of the studies, the investigator functionalized selenium NPs (SeNPs) with polyvinylpyrrolidone (PVP) and polysorbate 20, which demonstrated potential in enhancing dopamine and L-dopa delivery across the BBB. The study demonstrated efficient internalization and improved pharmacokinetic profiles (127). Moreover, a study involved functionalized AuNPs for targeting α-synuclein, which enhanced diagnostic accuracy by improving signal detection in biological samples and enabled early and precise diagnosis (104). In a study carried out by Huang et al. (128), exenatide-modified deferoxamine NPs were developed to target the brain’s lesion areas. This approach combined iron chelation and inflammatory suppression to upgrade neurological deficits in PD mice.
One of the major hurdles in treating PD is overcoming the BBB, which can be efficiently achieved by surface modification of the NPs. Such surface-functionalized NPs could enhance their ability to cross the BBB through receptor-mediated transcytosis, which may more efficiently penetrate the brain. In addition, NPs delivery could be facilitated by external stimuli, like magnetic fields or ultrasound. Furthermore, NPs can be engineered to mimic endogenous transport mechanisms, like those used by nutrients or hormones, to cross the BBB more effectively (22). In one of the studies, Wu et al. (129) used Lpc-BoSA/CSO (functionalized lipid NPs) to increase the BBB permeability and facilitate the delivery of peptide drugs like exenatide. These NPs significantly enhance dopamine levels and reduce α-synuclein expression, addressing symptoms and disease progression. Polymeric NPs have been functionalized with the ligands that target BBB transporters. This enhanced the permeability and efficacy in treating neurological diseases, including PD (130).
Specifically, engineered NPs could help to achieve the controlled drug release from NPs under specific stimuli, like variation in the pH, temperature, or the presence of specific enzymes, which could trigger. The engineered NPs may ensure the drug is released at the desired site and time, demonstrating enhanced therapeutic efficacy and reduced side effects. In one of the studies, the investigator developed various organic NPs for the encapsulation and sustained release of puerarin to improve brain delivery for treating PD. The engineered NPs extend their in vivo half-life and improve their bioavailability and accumulation in the brain to treat the symptoms of PD. Compared to the puerarin alone, puerarin-NPs showed significantly improved cellular internalization, permeation, and neuroprotective effects (131).
The use of NPs in the biomedical field is continuously increasing. Nanomedicine is a promising domain for developing more effective and targeted treatment approaches for PD. The NPs used to deliver the drug to the targeted sites are called nanocarriers. The nanocarrier systems, like liposomes, nanogels, dendrimers, and SLNPs, have been proven effective for treating PD.
Liposomes are spherical vesicles comprising phospholipid bilayers that can encapsulate hydrophilic and hydrophobic drugs (Figure 9). Liposomes offer significant advantages as drug delivery systems for PD, particularly due to their ability to encapsulate various types of drugs and their potential for targeted delivery to specific cells or tissues, such as dopaminergic neurons (132, 133). The surface modification of the liposome with ligands (antibodies or peptides) ensures targeted delivery to dopaminergic neurons, which could improve their targeting capabilities and efficacy and reduce side effects. In addition to this, PEGylation (attachment of polyethylene glycol chains) has been employed for longer circulation time and reduced immunogenicity. The liposomes could be engineered for the PD drug’s effective, controlled, and sustained release. Various external factors could trigger the release of drugs from these engineered liposomes, for instance, variation in the pH or enzymatic activity (134–136).
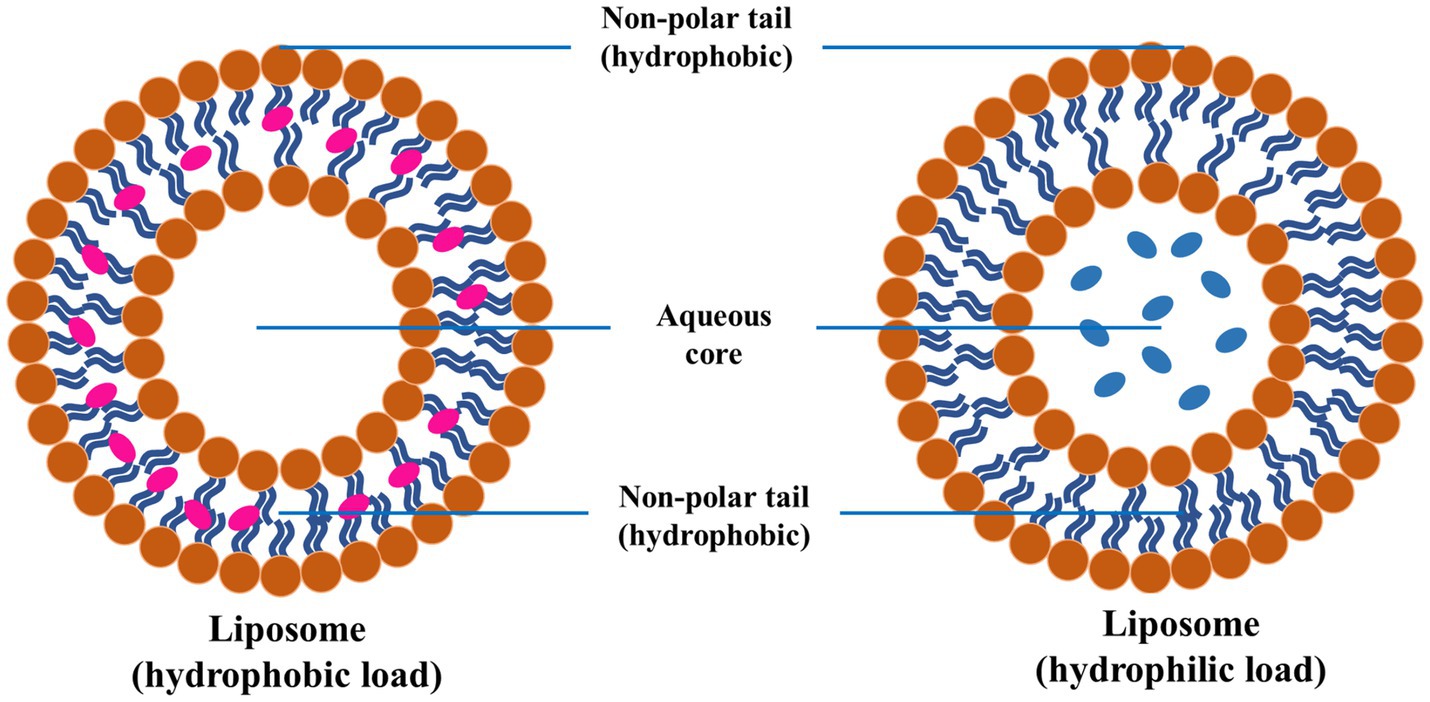
Figure 9. Schematic diagram of liposome and encapsulation of hydrophilic and hydrophobic molecules, reproduced from Jagaran et al. (145) with permission.
Nanogels have also gained significant attraction in nanomedicine as nanocarriers. These three-dimensional, hydrophilic polymer networks could swell up in aqueous environments. As a drug delivery system, nano gels have several advantageous features for the PD, like high drug loading capacity, biocompatible nature, and safeguarding the encapsulated drugs from degradation (137, 138). Currently, investigators are emphasizing the development of smart nanogels that respond to environmental stimuli, like temperature, pH, or magnetic fields, to acquire controlled and site-specific release of drugs. Investigators have developed temperature-responsive nano gels that could release the drugs loaded on them at elevated temperatures found in inflamed or diseased tissues. This ensures that the drug is delivered precisely where it is needed in the brain. Moreover, nanogels could also be functionalized with targeting ligands to increase their specificity for dopaminergic neurons. Several reports report the formulation of multifunctional nano gels that combine drug delivery with imaging capabilities, which helps in enabling real-time monitoring of PD treatment efficacy (139).
Dendrimers are organic molecules characterized by their highly branched, tree-like polymeric structure, including well-defined architectures and many functional groups on their surfaces (140). The distinctive design of dendrimers allows for exact regulation of size, shape, and surface functioning. These characteristics render them an attractive contender for drug delivery applications. Dendrimers may be designed for the targeted delivery of drugs, genes, or other therapeutic agents to the brain to treat PD (141). Recent investigations indicate the development of dendrimers with targeting moieties capable of selectively traversing the BBB and binding to dopaminergic neurons. Furthermore, dendrimers can be engineered to release encapsulated pharmaceuticals reacting to particular stimuli, similar to liposomes. The regulated release of the medicine from the dendrimer guarantees that the drug is delivered exclusively to the target location, minimizing systemic adverse effects. Currently, investigators are examining the capability of dendrimers to concurrently deliver various therapeutic drugs, perhaps providing a comprehensive strategy for PD treatment (142, 143).
SLNs are submicron-sized particles of solid lipids that could encapsulate lipophilic and hydrophilic drugs (Figure 10). Such SLNs offer numerous advantages, like controlled drug release, biocompatibility, and protecting the encapsulated drugs from degradation (97, 144). Investigators have reported improving the stability of SLNs, drug loading capacity, and targeting capabilities. SLNs could be functionalized by targeting ligands to improve their specificity for dopaminergic neurons in treating PD. Moreover, SLNs could also be tailored like dendrimers and liposomes to ensure the crossing through the BBB, which remains a major challenge for neurologists who treat PD (97). The engineered SLNs ensure that the loaded therapeutic agents reach the specific sites of the brain for the treatment of PD. Nowadays, several investigators are exploring the role of SLNs in delivering neuroprotective agents, antioxidants, and anti-inflammatory drugs. Such types of SLNs offer the potential to modify the progression of PD and improve patient outcomes. Besides, this investigation is also going on the co-delivery of multiple drugs using SLNs, which will provide a synergistic approach for the treatment of PD (24).
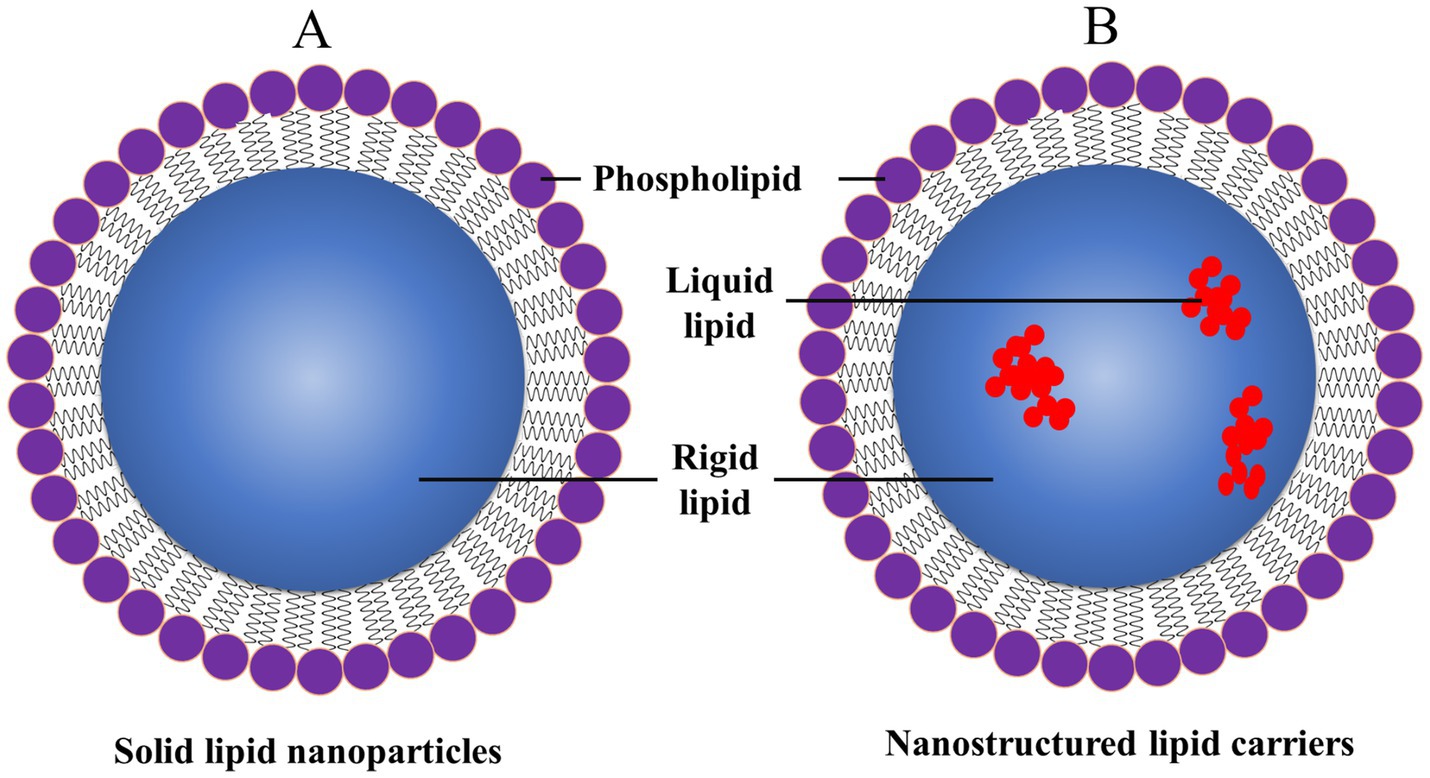
Figure 10. General structure of (A) SLNPs and (B) nanostructured lipid carriers (NLCs), reproduced from Jagaran et al. (145) with permission.
GT focuses on introducing, removing, or altering the genetic material within a patient’s cells, which offers a potential disease-modifying strategy. With the combination of GT and nanomedicine, GT could attain more precise and effective delivery of genes to target cells. Several investigations focus on the application of GT for the treatment of PD, which focuses on gene delivery systems, CRISPR/Cas9 technology and NPs, and clinical applications and challenges (145, 146).
For effective GT, robust drug delivery systems are required to transport therapeutic genes to specific cells and tissues. The NP-based innovative methods may provide several advantages over traditional viral vectors. These advantages may include lower immunogenicity, higher loading capacity, and the potential to be engineered for targeted delivery and controlled release (147).
Lipid-based NPs (LBNPs), like liposomes and lipid nanoparticles (LNPs), are widely used to deliver genes. Such LBNPs could encapsulate the gene and protect it from enzymatic degradation. This could facilitate their entry into cells. Moreover, LBNPs could be surface-modified with certain ligands targeting specific dopaminergic neuron receptors. This will further enhance the precision of gene delivery in PD (148). Various investigations have found that polymeric NPs could be a potential gene delivery system. Such polymeric NPs encapsulate the plasmid DNA, siRNA, or mRNA, protect them from enzymatic degradation, and offer sustained release. The specificity of the polymeric NPs for the neuron cells could be further enhanced by functionalization with the targeting moieties (148, 149).
Several NPs have been designed to mimic the structure and function of viral vectors called viral mimetic NPs. These viral mimetic NPs do not have immunogenicity and could efficiently deliver genetic material to the brain by exploiting natural cellular uptake processes. It is possible to achieve targeted delivery to specific neuronal populations by increasing the therapeutic outcomes of PD (146).
One of the most emerging techniques is the CRISPR/Cas9 system, which has revolutionized gene editing by permitting precise modifications to the genome. Delivering CRISPR/Cas9 components into specific cells, specifically neurons, poses significant challenges. Such NPs provide a versatile and efficient delivery platform.
In recent investigations, LNPs have demonstrated a huge potential in delivering CRISPR/Cas9 components to target cells. These LNPs could encapsulate the Cas9 protein and guide RNA (gRNA) within LNPs. LNPs protected these components from enzymatic degradation and facilitated their uptake by cells. The specificity of the LNPs for dopaminergic neurons could be enhanced by surface functionalization with targeting ligands. This may enable precise gene editing by using CRISPR/Cas9 in PD (150).
Investigators are exploring polymeric NPs’ capability to deliver CRISPR/Cas9. Like LNPs, polymeric NPs also encapsulate the Cas9 and gRNA. Due to this encapsulation, the CRISPR/Cas9 gene will be protected from enzymatic degradation and may provide sustained release of the gene. In addition to this, polymeric NPs could be surface-modified with targeting moieties. The surface modification of the polymeric NPs facilitates the targeted delivery of the CRISPR/Cas9 components to neurons damaged by PD. This approach could reduce unwanted side effects and enhance therapeutic efficacy (151, 152).
The combination of GT and nanomedicine holds valuable promise for treating PD. Various preclinical studies have shown the feasibility and efficacy of utilizing NPs for gene delivery in PD models. GT could deliver neuroprotective genes to promote neuronal survival and function. For instance, delivering genes that encode neurotrophic factors like GDNF (glial cell line-derived neurotrophic factor) could support dopaminergic neurons and slow down the progression of the disease. NPs provide a targeted and efficient delivery system for these therapeutic genes, ensuring they reach the affected neurons in the brain. The combination of CRISPR/Cas9 technology with NPs provides the potential to correct genetic mutations associated with PD. Some investigators are utilizing CRISPR/Cas9 to target and edit mutations in the LRRK2 gene, which are implicated in familial forms of PD. NPs allow precise delivery of the CRISPR/Cas9 components to neurons, allowing for accurate gene editing and potential disease modification (153).
Even though the combination of GT and nanomedicine has gained several advancements in the PD treatment field, there are still various challenges in the clinical application. Such a combination ensures efficient and targeted delivery of genetic material to the brain by overcoming BBB. Moreover, the long-term expression of therapeutic genes in the brain remains a major challenging task. Moreover, there is a requirement for a thorough evaluation of safety and immunogenicity for all the NP-based gene delivery systems during the clinical trials. The researchers are focusing on optimizing the NPs design, improving targeting strategies, and developing new materials for improved efficiency of delivery and safety. Integrating advanced imaging techniques and biomarkers could also help monitor therapeutic outcomes. Besides this, such integrated techniques could guide the development of personalized GT strategies for PD (154).
Nanomedicine offers promising strategies for neuroprotection in PD treatment by utilizing NPs to deliver neuroprotective agents across the BBB. These NPs can enhance drug bioavailability, target specific brain regions, and minimize side effects, making them a cutting-edge approach to managing neurodegenerative diseases. Recent advancements in nanomedicine offer promising strategies for neuroprotection, which aims to preserve and restore neuronal function. The various nanomedicines demonstrating neuroprotective effects are antioxidant NPs, anti-inflammatory NPs, and neurotrophic factor delivery for neuroprotection in PD. All these types of neuroprotective NPs are described briefly below.
The OS is characterized by an imbalance between the generation of ROS and the brain’s antioxidant defenses, which plays a crucial role in the pathogenesis of PD. Excessive ROS could damage the cellular components, leading to neuronal death. Antioxidant NPs are engineered particles that can effectively scavenge ROS in biological systems. These NPs are designed to deliver antioxidants more effectively to target sites, enhancing their therapeutic potential. The integration of antioxidants into NPs improves their bioavailability and allows for targeted delivery, reducing side effects and increasing efficacy. Investigators are developing antioxidant NPs like nanoceria, polymeric antioxidant NPs, selenium NPs (Se-NPs), etc., to combat OS and protect neurons (155). Antioxidant NPs have emerged as a promising therapeutic strategy for the treatment of PD, primarily due to their ability to target OS and neuroinflammation, which are key pathological features of the disease. These NPs can enhance drug delivery across the BBB, improve pharmacokinetic profiles, and provide neuroprotective effects.
Cerium oxide NPs (nanoceria) possess intrinsic antioxidant features as they have the potential to switch between Ce3+ and Ce4+ oxidation states, mimicking the activity of natural antioxidant enzymes. Recent investigations exhibited that nanoceria can scavenge ROS, lower OS, and protect dopaminergic neurons in PD models. The functionalization of nanoceria with targeting ligands enhances their delivery to the brain and enhances their neuroprotective efficacy (156). Dillion et al. (157), reported that PD is strongly associated with OS, and CeONPs act as a free radical scavenger. The investigator has hypothesized that CeONPs may be a promising disorder-modifying therapy for PD treatment. The investigator for the first reported that the CeONPs preserve striatal dopamine and safeguard the dopaminergic neurons in the substantia nigra in the MPTP-mouse model of PD. Finally, the investigator concluded CeONPs might be a potential novel nano pharmaceutical for neurodegenerative disorder therapy.
Gao et al. (158) developed the (Q@CeBG nanoreactor, which combines CeO2 nanozyme and quercetin) and has shown potential in reducing OS and polarizing microglia from a pro-inflammatory to an anti-inflammatory state. This is achieved through focused ultrasound to enhance BBB penetration, demonstrating significant neuroprotection and immunomodulatory effects in PD models.
The investigation has revealed that the polymeric NPs could be loaded with several antioxidant compounds (curcumin, resveratrol, or N-acetylcysteine). Such NPs provide a sustained release of antioxidants, which ensures prolonged protection against OS. By maximizing their neuroprotective effects, surface functionalization with targeting ligands can further improve their specificity for dopaminergic neurons.
El Nebrisi (159) emphasized the processes underlying curcumin’s neuroprotective properties as a prospective treatment for patients with PD. Curcumin (turmeric) is a polyphenolic compound with potent anti-inflammatory, mitochondrial protecting, antioxidant, free radical scavenging and Fe-chelating effects. It acts as a promising therapeutic and nutraceutical agent for treating PD. The immune system’s “cholinergic anti-inflammatory pathway” is mostly regulated by stimulating nicotinic receptors and, more specifically, selective α7 nicotinic acetylcholine receptors (α7-nAChR). Recently, α7-nAChR has been suggested as a possible therapy strategy for PD.
Wang et al. (160) used a systematic review to evaluate the efficacy of curcumin in experimental PD comprehensively. The investigators used electronic and manual searches for the literature and identified studies describing curcumin’s efficacy in animal PD models. The investigation revealed that 13 studies with 298 animals showed that curcumin helps protect neurons and reduce inflammation in PD, although one study found it ineffective against certain disruptions in the brain. The study concluded that curcumin showed a marked efficacy in the experimental model of PD. This suggests curcumin is probably a neuroprotective drug for human PD patients.
El Nebrisi et al. (161) demonstrated that curcumin protects neurons in a 6-hydroxydopamine (6-OHDA) rat model of PD by functioning via α7-nAChR. The researchers conducted in vivo experiments to evaluate the influence of α7-nAChR on curcumin’s protective effects in a rat PD model. Rats were subjected to intrastriatal injections of 6-hydroxydopamine (6-OHDA). The results demonstrated that the intragastric administration of 200 mg/kg of curcumin considerably enhanced abnormal motor behaviour. Immunoreactivity of tyrosine hydroxylase (TH) in the substantia nigra and caudoputamen suggested its neuroprotective role against the degeneration of dopaminergic neurons. The α7-nAChR-selective antagonist methyllycaconitine (1 μg/kg) delivered intraperitoneally nullified the neuroprotective effects of curcumin on animal behaviour and TH immunoreactivity.
Numerous studies have demonstrated the strong neuroprotective and antioxidant properties of polyphenols like curcumin through enhanced activity of detoxification systems such as glutathione peroxidase, catalase, and superoxide dismutase (SOD). Several studies have revealed that curcumin modulates epigenetics in neurological diseases and neuroinflammation (162, 163). However, curcumin-mediated epigenetic modulation’s neuroprotective effects are yet unclear (164). Tripathi and Bhawana (165) reviewed mitochondrial dysfunction, epigenetic modulations, and mitoepigenetics in age-associated neurological diseases. It also examined curcumin’s epigenetic machinery-regulated neuroprotective benefits in treating and preventing neurodegenerative disorders.
Another natural polyphenol with neuroprotective properties is resveratrol (RES) due to its antioxidant property and ability to protect mitochondria. Increased ROS production induces OS, which damages cells. Lipid peroxidation, oxidative protein modification, and DNA damage may ensue. Multiple PD model researchers reported that RES pretreatment boosts endogenous antioxidant status and directly scavenges ROS to reduce OS. Much research has studied the role of RES in modulating Nrf2 in PD models, which recognise oxidants and modulate antioxidant defence. Zamanian et al. (166) studied the impacts of RES activity in both in vitro and in vivo models of PD, as well as the molecular mechanisms behind it. The collected information demonstrated that by upregulating Nrf2 and decreasing OS, RES therapy offers neuroprotection against PD. Furthermore, the investigators focused on providing scientific evidence for the neuroprotective qualities of RES against PD and outlined the mechanism underlying therapeutic development considerations.
Castellani et al. (97), have developed SLNs co-loaded with dopamine for intranasal administration, which offers a multifunctional approach to PD treatment. These NPs demonstrate sustained release of dopamine and high cytocompatibility, showing potential in reducing oxidative cellular damage and providing neuroprotection.
Se-NPs have been used as an antioxidant for various therapeutic purposes. Literature has proven that both types of Se-NP functionalized and non-functionalized, can protect mitochondria by increasing the levels of ROS-scavenging enzymes in the brain. It has been reported that Se-NPs can also promote the synthesis of dopamine. Se-NPs can lower cellular toxicities by inhibiting the aggregation of tau, α-synuclein, and/or Aβ. The ability of the BBB to absorb Se-NPs, which maintain a healthy microenvironment, is essential for the homeostasis of the brain. Recently, Umapathy et al. (167) highlighted the importance of Se-NPs on stress-induced neurodegeneration and its critical control. The investigator suggested that due to the potential of Se-NPs to inhibit cellular stress and the pathophysiology of PD, they can act as a promising neuroprotector with anti-inflammatory, non-toxic, and antimicrobial features.
Kalčec et al. (127), functionalized SeNPs with polyvinylpyrrolidone and polysorbate 20 to enhance drug delivery across the BBB and act as antioxidants. They demonstrate efficient internalization and improved permeability for dopamine and L-dopa, highlighting their potential as drug-delivery vehicles in PD treatment.
Neuroinflammation is characterized by the activation of microglia and the release of pro-inflammatory cytokines, contributing to the progression of PD. Anti-inflammatory NPs can be engineered to deliver anti-inflammatory agents directly to affected sites, minimizing systemic side effects and enhancing therapeutic efficacy. Anti-inflammatory NPs are being developed to modulate the inflammatory response and protect neurons from inflammatory damage (168).
Dexamethasone is a potent anti-inflammatory steroid that can modulate neuroinflammation, a critical factor in PD progression (169). The encapsulation of dexamethasone (a potent anti-inflammatory corticosteroid) in NPs could be achieved by improving its delivery to the brain and decreasing systemic side effects. Some recent investigations have demonstrated that dexamethasone-loaded NPs can effectively cross the BBB and may further reduce neuroinflammation and safeguard the dopaminergic neurons in PD models. Such NPs could be easily engineered for controlled release by ensuring sustained anti-inflammatory effects (170). The encapsulation of dexamethasone in PLGA NPs allows for controlled release and targeted delivery, reducing systemic side effects and enhancing efficacy (169).
NPs targeting inflammatory pathways offer a promising approach to managing PD by addressing neuroinflammation and OS, which are critical in the disease’s progression. These NPs are designed to cross the BBB and target specific inflammatory pathways, thereby mitigating the effects of neuroinflammation and promoting neuronal health (171). NPs could be tailored to target specific inflammatory pathways involved in PD. One of the investigations reported that NPs were loaded with nuclear factor kappa B (NF-κB) inhibitors, a key regulator of inflammation, which could suppress the microglial activation and decrease the production of pro-inflammatory cytokines. The NF-κB is a key regulator of inflammation. These targeted strategies can potentially modulate neuroinflammation more precisely and effectively by reducing the off-target effects (172). In one study, it was observed that engineered extracellular vesicle-based nanoformulations (EVNs) target neuroinflammation by blocking peripheral inflammatory cell infiltration and promoting the Nrf2–GPX4 pathway to suppress inflammation (173). Liu et al. (174), engineered the NPs to cross the BBB using specific ligands or surface modifications. For instance, nanocapsules modified with Angiopep-2 enhance brain delivery by targeting the BBB, while cRGD targets integrin receptors upregulated in PD-affected areas. Jiang et al. (175) developed chiral metal-organic frameworks with nanozymes, demonstrating chiral-dependent BBB transendocytosis, with Ptzyme@D-ZIF showing higher brain accumulation due to multiple endocytosis pathways.
Nanoparticles often incorporate ROS-scavenging materials to reduce OS, a key factor in neuroinflammation. For example, Pt nanozymes in a dual synergetic nanoreactor exhibit enhanced antioxidative activities, mitigating oxidative damage and promoting microglia polarization to an anti-inflammatory phenotype (176).
There are various synergistic approaches for treating PD by targeting inflammatory pathways by the NPs. For instance, carrier-free nanocapsules deliver dopamine and catalase to the PD brain by combining dopamine replacement with neuroinflammation mitigation. This addresses both dopamine depletion and neuroinflammation. This approach enhances dopaminergic signaling and reduces neuroinflammation, slowing disease progression (174). Furthermore, Fan et al. (176) demonstrated that nanoreactors combining dihydroquercetin and Pt enzymes show synergistic effects in reducing OS and controlling the inflammatory microenvironment, thus preventing neuronal damage.
Neurotrophic factors are crucial proteins that support neurons’ growth, survival, and differentiation. They play a significant role in developing and maintaining the nervous system by preventing apoptosis and promoting neuronal regeneration. These proteins, including brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophin-3, are involved in various cellular processes that ensure the proper functioning of neurons. From several pieces of literature, it has been observed that the delivery of neurotrophic factors to the brain holds a promising strategy for neuroprotection and neurorestoration in PD. In addition, NPs act as an effective delivery system for these neurotrophic factors, ensuring their stability and bioavailability (172).
GDNF-loaded NPs have been explored as a potential treatment for PD due to GDNF’s neuroprotective effects on dopaminergic neurons. GDNF supports dopaminergic neurons and plays a role in neural development and repair, but its delivery across the BBB remains a challenge (177). NPs could potentially address these issues by enhancing the delivery and stability of GDNF in the brain. The encapsulation of the GDNF by the NPs protects them from being degraded from enzymatic attack and facilitates delivery to the brain. One recent investigation showed that GDNF-loaded NPs could significantly improve dopaminergic neuron survival and motor function and minimize neurodegeneration in PD models (178).
Some major drawbacks associated with the clinical application of such NPs are their poor ability to cross the BBB and the inconsistent clinical efficacy of GDNF in PD treatment. Clinical trials have shown that GDNF does not significantly improve motor symptoms in PD patients and is associated with a higher incidence of serious adverse events (179). Long-term exposure to GDNF can lead to dephosphorylation of key signalling pathways, reducing its neuroprotective effects. This suggests the need for optimized delivery methods.
BDNF is a critical protein belonging to the neurotrophin family, playing a significant role in neuronal growth, synaptic plasticity, and overall brain health (180). It regulates axonal and dendritic growth, synaptic plasticity, and long-term potentiation, essential for learning and memory. BDNF’s influence extends to various psychiatric and neurological disorders, making it a focal point for therapeutic research and potential interventions. BDNF promotes the survival and maturation of dopaminergic neurons, which are crucial in PD pathology. NPs can cross the BBB and deliver the BDNF to the brain, thereby increasing the bioavailability of BDNF in the brain. Due to this, there is sustained release and targeted delivery to dopaminergic neurons (181).
The delivery of BDNF via NPs could potentially slow disease progression and improve motor function. The preclinical studies have shown promising results in animal models, where NP-mediated delivery of BDNF has led to neuroprotective effects (182). Boyton et al. (122) have shown that by encapsulating BDNF, NPs can provide controlled and sustained release, which may enhance the therapeutic efficacy and reduce the frequency of administration. Moreover, the surface modification of the NPs with targeting ligands can further improve the specificity and efficacy of BDNF delivery, which promotes the survival and function of the neurons (183). Table 6 summarizes all the nanoparticles used for neuroprotection in PD treatment.

Table 6. Summary of all the nanoparticles used for neuroprotection in Parkinson’s disease treatment.
Nanotechnology offers promising advancements in treating PD by addressing existing therapies’ limitations, such as poor bioavailability and inability to halt disease progression (184). Traditional treatments like dopamine replacement and DBS primarily manage symptoms without altering the disease course. Through innovative drug delivery systems, nanotechnology aims to enhance therapeutic efficacy and reduce side effects. The major advantages of nanotechnology in PD treatment include enhanced drug delivery, neuroprotection and neuro repair, versatility and functionalization, and reduced side effects (185).
The major disadvantages of nanotechnology include safety and long-term effects, complexity and cost and regulatory challenges (186–188). Furthermore, the potential toxicity of NPs in the treatment of PD is a significant concern, especially when considering long-term use or large doses. These NPs may cause neurotoxic effects. Several studies have shown the dual nature of nanoparticles, where their beneficial properties are often accompanied by potential risks, particularly in the context of neurodegenerative diseases like PD. It has been found that SiO2 NPs can exacerbate PD pathology by promoting α-synuclein aggregation, mitochondrial impairment, OS, and neuronal apoptosis. These effects were observed in both in vitro and in vivo models, indicating a strong potential for SiO2 NPs to initiate and develop PD-like symptoms (74). Metallic NPs, can induce neurotoxicity through mechanisms such as OS, autophagy dysfunction, and activation of harmful signaling pathways. NPs can penetrate the CNS and potentially cause neuroinflammation and neurodegeneration. The mechanisms of NP-induced neurotoxicity include the generation of free radicals, immune responses, and neuroinflammation, which can lead to cell necrosis and other adverse effects. The healthcare industry is concerned with the regulatory aspects of NP use in neuronal disorders. There is a need for comprehensive risk assessments and safety regulations to mitigate adverse reactions in humans and animals (58).
Exploring nanomedicine for treating Parkinson’s disease presents a promising and transformative approach. Nanoparticles offer several advantages over traditional therapies, including improved drug delivery, targeted therapeutic interventions, and crossing biological barriers like the BBB. The application of various types of nanoparticles, such as lipid-based, polymeric, and metallic NPs, has demonstrated potential in effectively delivering therapeutic agents to the affected brain regions. Furthermore, integrating GT and CRISPR/Cas9 technology with nanomedicine opens new avenues for addressing the underlying causes of neurodegeneration in PD. This combination promises precise and efficient delivery of genetic material to specific neurons, potentially correcting genetic mutations and promoting neuroprotection. Despite these advancements, some challenges must be addressed, including optimising NP design, ensuring long-term expression of therapeutic genes, and thoroughly evaluating safety and immunogenicity. The future of PD treatment lies in the successful translation of nanomedicine from the laboratory to clinical practice, paving the way for personalized and effective treatment strategies. Overall, nanomedicine represents a significant leap forward in managing Parkinson’s disease, offering hope for improved symptom management, enhanced patient outcomes, and the potential to slow disease progression. Continued research and development in this field are essential to thoroughly understand its potential and bring about a new era in neurological care.
VY: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. SD: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. NC: Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. DN: Investigation, Software, Validation, Writing – original draft, Writing – review & editing. VT: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. RG: Methodology, Resources, Writing – original draft, Writing – review & editing. SP: Software, Validation, Writing – original draft, Writing – review & editing. PK: Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Resources, Supervision. NG: Resources, Writing – original draft, Writing – review & editing, Methodology. AP: Methodology, Resources, Writing – original draft, Writing – review & editing, Investigation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
NG was employed by River Engineering Pvt. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that Gen AI was used in the creation of this manuscript. The initial table of contents was prepared using ChatGPT. Some of the sentences were responsibly paraphrased using ChatGPT, and the authors have finally rewritten the whole of such sentences.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhang, Q, Wang, Q, Fu, J, Xu, X, Guo, D, Pan, Y, et al. Multi targeted therapy for Alzheimer’s disease by guanidinium-modified calixarene and cyclodextrin co-assembly loaded with insulin. ACS Nano. (2024) 18:33032–41. doi: 10.1021/acsnano.4c05693
2. Duan, W, Yang, L, Liu, J, Dai, Z, Wang, Z, Zhang, H, et al. A TGF-β signaling-related lncRNA signature for prediction of glioma prognosis, immune microenvironment, and immunotherapy response. CNS Neurosci Ther. (2024) 30:e14489. doi: 10.1111/cns.14489
3. Zhang, C, Ge, H, Zhang, S, Liu, D, Jiang, Z, Lan, C, et al. Hematoma evacuation via image-guided para-corticospinal tract approach in patients with spontaneous intracerebral hemorrhage. Neurol Ther. (2021) 10:1001–13. doi: 10.1007/s40120-021-00279-8
4. Benedetti, F, Bernasconi, A, and Pontiggia, A. Depression and neurological disorders. Curr Opin Psychiatry. (2006) 19:14–8. doi: 10.1097/01.yco.0000194147.88647.7f
5. Wang, L, Li, X, Deng, Z, Cai, Q, Lei, P, Xu, H, et al. Neuroendoscopic parafascicular evacuation of spontaneous intracerebral hemorrhage (NESICH technique): a multicenter technical experience with preliminary findings. Neurol Ther. (2024) 13:1259–71. doi: 10.1007/s40120-024-00642-5
6. Wang, H, Yan, Z, Yang, W, Liu, R, Fan, G, Gu, Z, et al. A strategy of monitoring acetylcholinesterase and screening of natural inhibitors from Uncaria for Alzheimer’s disease therapy based on near-infrared fluorescence probe. Sens Actuators B. (2025) 424:136895. doi: 10.1016/j.snb.2024.136895
7. Grisold, W. The expanding burden of neurological disorders. Lancet Neurol. (2024) 23:326–7. doi: 10.1016/S1474-4422(24)00086-3
8. Poewe, W, Seppi, K, Tanner, CM, Halliday, GM, Brundin, P, Volkmann, J, et al. Parkinson disease. Nat Rev Dis Primers. (2017) 3:17013. doi: 10.1038/nrdp.2017.13
9. Aarsland, D, Batzu, L, Halliday, GM, Geurtsen, GJ, Ballard, C, Ray Chaudhuri, K, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. (2021) 7:47. doi: 10.1038/s41572-021-00280-3
10. Muleiro Alvarez, M, Cano-Herrera, G, Osorio Martínez, MF, Vega Gonzales-Portillo, J, Monroy, GR, Murguiondo Pérez, R, et al. A comprehensive approach to Parkinson’s disease: addressing its molecular, clinical, and therapeutic aspects. Int J Mol Sci. (2024) 25:7183. doi: 10.3390/ijms25137183
11. Pardo-Moreno, T, García-Morales, V, Suleiman-Martos, S, Rivas-Domínguez, A, Mohamed-Mohamed, H, Ramos-Rodríguez, JJ, et al. Current treatments and new, tentative therapies for Parkinson’s disease. Pharmaceutics. (2023) 15:770. doi: 10.3390/pharmaceutics15030770
12. Arisoy, S, Sayiner, O, Comoglu, T, Onal, D, Atalay, O, and Pehlivanoglu, B. In vitro and in vivo evaluation of levodopa-loaded nanoparticles for nose to brain delivery. Pharm Dev Technol. (2020) 25:735–47. doi: 10.1080/10837450.2020.1740257
13. Yang, X, Zheng, R, Cai, Y, Liao, M, Yuan, W, and Liu, Z. Controlled-release levodopa methyl ester/benserazide-loaded nanoparticles ameliorate levodopa-induced dyskinesia in rats. Int J Nanomedicine. (2012) 7:2077–86. doi: 10.2147/IJN.S30463
14. Sandoval-Pistorius, SS, Hacker, ML, Waters, AC, Wang, J, Provenza, NR, de Hemptinne, C, et al. Advances in deep brain stimulation: from mechanisms to applications. J Neurosci. (2023) 43:7575–86. doi: 10.1523/JNEUROSCI.1427-23.2023
15. Shan, Y, Wang, H, Yang, Y, Wang, J, Zhao, W, Huang, Y, et al. Evidence of a large current of transcranial alternating current stimulation directly to deep brain regions. Mol Psychiatry. (2023) 28:5402–10. doi: 10.1038/s41380-023-02150-8
16. Lu, Q, Chen, Y, Liu, H, Yan, J, Cui, P, Zhang, Q, et al. Nitrogen-containing flavonoid and their analogs with diverse B-ring in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev Res. (2020) 81:1037–47. doi: 10.1002/ddr.21726
17. Li, J, Xie, F, and Ma, X. Advances in nanomedicines: a promising therapeutic strategy for ischemic cerebral stroke treatment. Nanomedicine. (2024) 19:811–35. doi: 10.2217/nnm-2023-0266
18. Roque, D, Cruz, N, Ferreira, HA, Reis, CP, Matela, N, Herculano-Carvalho, M, et al. Nanoparticle-based treatment in glioblastoma. J Pers Med. (2023) 13:1328. doi: 10.3390/jpm13091328
19. Govindhan, G, Venkatachalam, K, Munusamy, S, Jin, J, Kumar Yadav, V, Vengidusamy, N, et al. Dual sustainability of rGO/CuCoO2 nanocomposites with enhanced photocatalytic and antibacterial insights. Inorg Chem Commun. (2024) 170:113216. doi: 10.1016/j.inoche.2024.113216
20. Venkatas, J, and Singh, M. Nanomedicine-mediated optimization of immunotherapeutic approaches in cervical cancer. Nanomedicine. (2021) 16:1311–28. doi: 10.2217/nnm-2021-0044
21. Teja, PK, Mithiya, J, Kate, AS, Bairwa, K, and Chauthe, SK. Herbal nanomedicines: recent advancements, challenges, opportunities and regulatory overview. Phytomedicine. (2022) 96:153890. doi: 10.1016/j.phymed.2021.153890
22. Li, G, Shao, K, and Umeshappa, CS. Recent progress in blood-brain barrier transportation research In: H Gao and X Gao, editors. Brain targeted drug delivery system. London: Academic Press (2019). 33–51.
23. Moschetta, M, Trevisani, M, Castagnola, V, and Bramini, M. Chapter 19—nanotechnology-based approaches in glioblastoma treatment: how can the dual blood-brain/tumor barriers be overcome? In: C Vitorino, C Balaña, and C Cabral, editors. New insights into glioblastoma. London: Academic Press (2023). 435–75.
24. Jana, M, Biswas, UK, Patra, CS, Debnath, B, Sharma, S, and Naskar, S. Solid lipid nanoparticles: a review of their biomedical applications and preparation. Pharm Nanotechnol. (2024) 12:1–17. doi: 10.2174/0122117385312175240502100018
25. Krsek, A, and Baticic, L. Nanotechnology-driven therapeutic innovations in neurodegenerative disorders: a focus on Alzheimer’s and Parkinson’s disease. Future Pharmacol. (2024) 4:352–79. doi: 10.3390/futurepharmacol4020020
26. Espay, AJ, and McFarthing, K. Alpha-synuclein and the Parkinson’s disease drug pipeline. Parkinsonism Relat Disord. (2023) 111:105432. doi: 10.1016/j.parkreldis.2023.105432
27. Tansey, MG, Wallings, RL, Houser, MC, Herrick, MK, Keating, CE, and Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. (2022) 22:657–73. doi: 10.1038/s41577-022-00684-6
28. Halje, P, Brys, I, Mariman, JJ, da Cunha, C, Fuentes, R, and Petersson, P. Oscillations in cortico-basal ganglia circuits: implications for Parkinson’s disease and other neurologic and psychiatric conditions. J Neurophysiol. (2019) 122:203–31. doi: 10.1152/jn.00590.2018
29. Xu, E, Xie, Y, and Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat Med. (2022) 28:2406–15. doi: 10.1038/s41591-022-02001-z
30. Raj, K, Kaur, P, Gupta, GD, and Singh, S. Metals associated neurodegeneration in Parkinson’s disease: insight to physiological, pathological mechanisms and management. Neurosci Lett. (2021) 753:135873. doi: 10.1016/j.neulet.2021.135873
31. Canning, CG, Allen, NE, Nackaerts, E, Paul, SS, Nieuwboer, A, and Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat Rev Neurol. (2020) 16:409–25. doi: 10.1038/s41582-020-0370-2
32. Morris, HR, Spillantini, MG, Sue, CM, and Williams-Gray, CH. The pathogenesis of Parkinson’s disease. Lancet. (2024) 403:293–304. doi: 10.1016/S0140-6736(23)01478-2
33. Tong, J. The pathogenesis and treatment of Parkinson’s disease. Theor Nat Sci. (2024) 47:15–9. doi: 10.54254/2753-8818/47/2024PJ0101
34. Yadav, VK, Choudhary, N, Gacem, A, Verma, RK, Abul Hasan, M, Tarique Imam, M, et al. Deeper insight into ferroptosis: association with Alzheimer’s, Parkinson’s disease, and brain tumors and their possible treatment by nanomaterials induced ferroptosis. Redox Rep. (2023) 28:2269331. doi: 10.1080/13510002.2023.2269331
35. Ravenhill, SM, Evans, AH, and Crewther, SG. Escalating bi-directional feedback loops between proinflammatory microglia and mitochondria in ageing and post-diagnosis of Parkinson’s disease. Antioxidants. (2023) 12:1117. doi: 10.3390/antiox12051117
36. Fedorova, TN, Logvinenko, AA, Poleshchuk, VV, Muzychuk, OA, Shabalina, AA, and Illarioshkin, SN. Significance of oxidative damage to proteins and DNA in the blood of patients with Parkinson’s disease in assessing the severity of the disease. Neurochem J. (2021) 15:211–6. doi: 10.1134/S1819712421020057
37. Das, T, Ramezani, M, Snead, D, Follmer, C, Chung, P, Lee, KY, et al. The role of membrane affinity and binding modes in alpha-synuclein regulation of vesicle release and trafficking. Biomol Ther. (2022) 12:1816. doi: 10.3390/biom12121816
38. Neupane, S, De Cecco, E, and Aguzzi, A. The hidden cell-to-cell trail of α-synuclein aggregates. J Mol Biol. (2023) 435:167930. doi: 10.1016/j.jmb.2022.167930
39. Sharma, M, and Burré, J. α-synuclein in synaptic function and dysfunction. Trends Neurosci. (2023) 46:153–66. doi: 10.1016/j.tins.2022.11.007
40. Kumar, S, Bharadwaj, H, Saha, S, Singh, K, Kumar, P, Arora, S, et al. The present difficulties and potential benefits of dopaminergic agents in Parkinson’s disease treatment. Curr Psychopharmacol. (2024) 13:e22115560332182. doi: 10.2174/0122115560332182241031050521
41. Zhai, S, Cui, Q, Simmons, DV, and Surmeier, DJ. Distributed dopaminergic signaling in the basal ganglia and its relationship to motor disability in Parkinson’s disease. Curr Opin Neurobiol. (2023) 83:102798. doi: 10.1016/j.conb.2023.102798
42. Cai, W, Young, CB, Yuan, R, Lee, B, Ryman, S, Kim, J, et al. Dopaminergic medication normalizes aberrant cognitive control circuit signalling in Parkinson’s disease. Brain. (2022) 145:4042–55. doi: 10.1093/brain/awac007
43. Jing, X-Z, Yuan, X-Z, Luo, X, Zhang, S-Y, and Wang, X-P. An update on nondopaminergic treatments for motor and non-motor symptoms of Parkinson’s disease. Curr Neuropharmacol. (2023) 21:1806–26. doi: 10.2174/1570159X20666220222150811
44. Chyniak, O, Dubenko, O, Potapov, O, Shulga, A, and Kotsyuba, A. Parkinson’s disease—overview of modern treatment methods. East Ukr Med J. (2023) 11:1–13. doi: 10.21272/eumj.2023;11(1):1-13
45. Ajagun, EJ, Asuku, AO, Ayinla, MT, Abdulsalam, TA, Ajibare, AJ, Olajide, TS, et al. Pharmacotherapy of Parkinson’s disease and their limitations In: Zebrafish as a model for Parkinson’s disease. Boca Raton, FL: CRC Press (2024). 186–203.
46. Reichmann, H. Real-world considerations regarding the use of the combination of levodopa, carbidopa, and entacapone (Stalevo®) in Parkinson’s disease. Eur J Neurol. (2023) 30:15–20. doi: 10.1111/ene.15992
47. Lau, ACW, Diggle, JL, and Bring, PP. Improvement in severe orthostatic hypotension following carbidopa dose reduction. Can J Neurol Sci. (2018) 45:252–3. doi: 10.1017/cjn.2017.284
48. Yi, LX, Tan, EK, and Zhou, ZD. Tyrosine hydroxylase inhibitors and dopamine receptor agonists combination therapy for Parkinson’s disease. Int J Mol Sci. (2024) 25:4643. doi: 10.3390/ijms25094643
49. Salamon, A, Zádori, D, Szpisjak, L, Klivényi, P, and Vécsei, L. What is the impact of catechol-O-methyltransferase (COMT) on Parkinson’s disease treatment? Expert Opin Pharmacother. (2022) 23:1123–8. doi: 10.1080/14656566.2022.2060738
50. Männistö, PT, Keränen, T, Reinikainen, KJ, Hanttu, A, and Pollesello, P. The catechol O-methyltransferase inhibitor entacapone in the treatment of Parkinson’s disease: personal reflections on a first-in-class drug development programme 40 years on. Neurol Ther. (2024) 13:1039–54. doi: 10.1007/s40120-024-00629-2
51. Gao, X, Tang, J, Liu, H, Liu, L, and Liu, Y. Structure-activity study of fluorine or chlorine-substituted cinnamic acid derivatives with tertiary amine side chain in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev Res. (2019) 80:438–45. doi: 10.1002/ddr.21515
52. Li, H, Tan, Y, Cheng, X, Zhang, Z, Huang, J, Hui, S, et al. Untargeted metabolomics analysis of the hippocampus and cerebral cortex identified the neuroprotective mechanisms of Bushen Tiansui formula in an aβ 25-35-induced rat model of Alzheimer’s disease. Front Pharmacol. (2022) 13:990307. doi: 10.3389/fphar.2022.990307
53. Haddad, F, Sawalha, M, Khawaja, Y, Najjar, A, and Karaman, R. Dopamine and levodopa prodrugs for the treatment of Parkinson’s disease. Molecules. (2018) 23:40. doi: 10.3390/molecules23010040
54. Salat, D, and Tolosa, E. Levodopa in the treatment of Parkinson’s disease: current status and new developments. J Parkinsons Dis. (2013) 3:255–69. doi: 10.3233/JPD-130186
55. Luo, D, Reith, M, and Dutta, AK. Dopamine agonists in treatment of Parkinson’s disease: an overview In: Diagnosis and management in Parkinson’s disease. London: Academic Press (2020). 445–60.
56. Jing, X-Z, Yang, H-J, Taximaimaiti, R, and Wang, X-P. Advances in the therapeutic use of non-ergot dopamine agonists in the treatment of motor and non-motor symptoms of Parkinson’s disease. Curr Neuropharmacol. (2023) 21:1224–40. doi: 10.2174/1570159X20666220915091022
57. Chen, X, Zhang, Q, Wen, S, Chen, F, and Zhou, C. Efficacy and safety of non-ergot dopamine-receptor agonists as an adjunct to levodopa in advanced Parkinson’s disease: a network meta-analysis. Eur J Neurol. (2023) 30:762–73. doi: 10.1111/ene.15635
58. Muheem, A, Jahangir, MA, Jaiswal, CP, Jafar, M, Ahmad, MZ, Ahmad, J, et al. Recent patents, regulatory issues, and toxicity of nanoparticles in neuronal disorders. Curr Drug Metab. (2021) 22:263–79. doi: 10.2174/1389200221999201210213036
59. Lee, D-H, Woo, B-S, Park, Y-H, and Lee, J-H. General treatments promoting independent living in Parkinson’s patients and physical therapy approaches for improving gait—a comprehensive review. Medicina. (2024) 60:711. doi: 10.3390/medicina60050711
60. Terzis, N, Salonikidis, K, Apostolara, P, Roussos, N, Karzis, K, Ververidis, A, et al. Can the exercise-based and occupational therapy improve the posture, strength, and mobility in elderly Greek subjects with hip fracture? A non-randomized control trial. J Frailty Sarcopenia Falls. (2021) 6:57–65. doi: 10.22540/JFSF-06-057
61. Khlyustova, MG, and Krylov, KY. Features of nutritional support for dysphagia. The role of a speech therapist. Clin Nutr Metab. (2024) 4:187–96. doi: 10.17816/clinutr456460
62. Smalira, J, Lęgas, A, Przybysz, B, Mormul, A, Zawistowska, J, Rogala, W, et al. Role of selected nutritional factors in the prevention and treatment of Parkinson’s disease—a review. Aktual Neurol. (2024) 24:51–8. doi: 10.15557/AN.2024.0008
63. Zhang, Y, Liu, S, Xu, K, Zhou, Y, Shen, Y, Liu, Z, et al. Non-pharmacological therapies for treating non-motor symptoms in patients with Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. (2024) 16:1363115. doi: 10.3389/fnagi.2024.1363115
64. Adamiec, K, Trojanowski, D, Rodak, M, Smorońska-Rypel, J, Szymczuk, B, Kajzar, M, et al. Moving beyond medication: harnessing the power of physical therapy and dance therapy in Parkinson’s disease management. Qual Sport. (2024) 15:52373. doi: 10.12775/QS.2024.15.52373
65. Kalbe, E, Bloem, BR, Kalia, LV, and Nieuwboer, A. Special issue: non-pharmacological interventions for people with Parkinson’s disease: are we entering a new era? J Parkinsons Dis. (2024) 14:S1–4. doi: 10.3233/JPD-249009
66. Janssen Daalen, JM, Schootemeijer, S, Richard, E, Darweesh, SKL, and Bloem, BR. Lifestyle interventions for the prevention of Parkinson disease. Neurology. (2022) 99:42–51. doi: 10.1212/WNL.0000000000200787
67. Júnior, ECA, da Silva Machado, IG, Andrade, MN, de Figueiredo, RNS, Marinho, GF, Almeida, CDA, et al. Is DBS in the treatment of Parkinson’s surgery able to improve all symptoms: a bibliographic review. Braz J Health Rev. (2024) 7:e73622. doi: 10.34119/bjhrv7n5-457
68. Naguib Al Shehaby, AM, Kotb, MM, Abdel-Latif, AMM, and Mohamed Abdelaziz, AM. Deep brain stimulation of the subthalamic nucleus vs. globus pallidus internus in the treatment of Parkinson’s disease: a systematic review. QJM. (2024) 117:hcae070.352. doi: 10.1093/qjmed/hcae070.352
69. Sharma, VD, Patel, M, and Miocinovic, S. Surgical treatment of Parkinson’s disease: devices and lesion approaches. Neurotherapeutics. (2020) 17:1525–38. doi: 10.1007/s13311-020-00939-x
70. Foltynie, T, Bruno, V, Fox, S, Kühn, AA, Lindop, F, and Lees, AJ. Medical, surgical, and physical treatments for Parkinson’s disease. Lancet. (2024) 403:305–24. doi: 10.1016/S0140-6736(23)01429-0
71. Bougea, A. Some novel therapies in Parkinson’s disease: a promising path forward or not yet? A systematic review of the literature. Biomedicines. (2024) 12:549. doi: 10.3390/biomedicines12030549
72. Chang, Y, Lee, S, Kim, J, Kim, C, Shim, HS, Lee, SE, et al. Gene therapy using efficient direct lineage reprogramming technology for neurological diseases. Nanomaterials. (2023) 13:1680. doi: 10.3390/nano13101680
73. Hu, B, Fang, H, Huang, Z, Huang, W, Huang, L, Liu, H, et al. An upconversion nanoplatform based multi-effective theatment for Parkinson’s disease. Chem Eng J. (2023) 465:142959. doi: 10.1016/j.cej.2023.142959
74. Yuan, X, Yang, Y, Xia, D, Meng, L, He, M, Liu, C, et al. Silica nanoparticles promote α-synuclein aggregation and Parkinson’s disease pathology. Front Neurosci. (2022) 15:807988. doi: 10.3389/fnins.2021.807988
75. Rascol, O, Fabbri, M, and Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. (2021) 20:1048–56. doi: 10.1016/S1474-4422(21)00249-0
76. Uchida, H, Kim, E, Jarskog, LF, Fleischhacker, WW, Remington, G, and Lieberman, JA. Medications for psychosis: dopamine blockers and dopamine partial agonists (antipsychotics) In: Tasman’s psychiatry. Cham: Springer International Publishing (2023). 1–58.
77. Pagonabarraga, J, Bejr-Kasem, H, Martinez-Horta, S, and Kulisevsky, J. Parkinson disease psychosis: from phenomenology to neurobiological mechanisms. Nat Rev Neurol. (2024) 20:135–50. doi: 10.1038/s41582-023-00918-8
78. Jenner, P, Rocha, J-F, Ferreira, JJ, Rascol, O, and Soares-da-Silva, P. Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: the role of opicapone. Expert Rev Neurother. (2021) 21:1019–33. doi: 10.1080/14737175.2021.1968298
79. Lebedev, AV, Nilsson, J, Lindström, J, Fredborg, W, Akenine, U, Hillilä, C, et al. Effects of daily L-dopa administration on learning and brain structure in older adults undergoing cognitive training: a randomised clinical trial. Sci Rep. (2020) 10:5227. doi: 10.1038/s41598-020-62172-y
80. Raju, NN, KSVRNP, K, and Nihal, G. Management of medication-induced psychiatric disorders. Indian J Psychiatry. (2022) 64:S281–91. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_21_22
81. Alborghetti, M, Bianchini, E, De Carolis, L, Galli, S, Pontieri, FE, and Rinaldi, D. Type-B monoamine oxidase inhibitors in neurological diseases. Neural Regen Res. (2024) 19:16–21. doi: 10.4103/1673-5374.375299
82. Tsuboi, T, Satake, Y, Hiraga, K, Yokoi, K, Hattori, M, Suzuki, M, et al. Effects of MAO-B inhibitors on non-motor symptoms and quality of life in Parkinson’s disease: a systematic review. npj Parkinsons Dis. (2022) 8:75. doi: 10.1038/s41531-022-00339-2
83. Zhang, K, Miao, S, Yao, Y, Yang, Y, Shi, S, Luo, B, et al. Efficacy and safety of prophylactic use of benzhexol after risperidone treatment. Heliyon. (2023) 9:e14199. doi: 10.1016/j.heliyon.2023.e14199
84. Alabrahim, OAA, and Azzazy, HME-S. Polymeric nanoparticles for dopamine and levodopa replacement in Parkinson’s disease. Nanoscale Adv. (2022) 4:5233–44. doi: 10.1039/D2NA00524G
85. Li, X, Peng, X, Zoulikha, M, Boafo, GF, Magar, KT, Ju, Y, et al. Multifunctional nanoparticle-mediated combining therapy for human diseases. Signal Transduct Target Ther. (2024) 9:1. doi: 10.1038/s41392-023-01668-1
86. Roghani, AK, Garcia, RI, Roghani, A, Reddy, A, Khemka, S, Reddy, RP, et al. Treating Alzheimer’s disease using nanoparticle-mediated drug delivery strategies/systems. Ageing Res Rev. (2024) 97:102291. doi: 10.1016/j.arr.2024.102291
87. Lin, P, Yao, H, Huang, L, Fu, C, Yao, X, Lian, C, et al. Autoimmune astrocytopathy double negative for AQP4-IgG and GFAP-IgG: retrospective research of clinical practice, biomarkers, and pathology. CNS Neurosci Ther. (2024) 30:e70042. doi: 10.1111/cns.70042
88. Wankar, JN, Chaturvedi, VK, Bohara, C, Singh, MP, and Bohara, RA. Role of nanomedicine in management and prevention of COVID-19. Front Nanotechnol. (2020) 2:589541. doi: 10.3389/fnano.2020.589541
89. Kaushik, AC, Bharadwaj, S, Kumar, S, and Wei, DQ. Nano-particle mediated inhibition of Parkinson’s disease using computational biology approach. Sci Rep. (2018) 8:9169. doi: 10.1038/s41598-018-27580-1
90. Sherif, S, Sameh, A, Salah, SM, Omar, A, Elhaes, H, Ibrahim, A, et al. DFT and QSAR study of catechol-O-methyltransferase (COMT) as inhibitors for Parkinson’s disease treatment. Opt Quant Electron. (2024) 56:633. doi: 10.1007/s11082-023-06125-5
91. Sujeevan, O. Lipid-based nanoparticles and their recent advances. GSC Adv Res Rev. (2024) 18:182–8. doi: 10.30574/gscarr.2024.18.3.0096
92. Bhardwaj, H, and Jangde, RK. Current updated review on preparation of polymeric nanoparticles for drug delivery and biomedical applications. Next Nanotechnol. (2023) 2:100013. doi: 10.1016/j.nxnano.2023.100013
93. Geszke-Moritz, M, and Moritz, M. Biodegradable polymeric nanoparticle-based drug delivery systems: comprehensive overview, perspectives and challenges. Polymers. (2024) 16:2536. doi: 10.3390/polym16172536
94. Adu, E, Patel, SA, Catino, AJ, and Medhi, R. Polymeric nanoparticles (PNPs) as drug delivery systems for SARS-CoV-2. Charact Appl Nanomater. (2024) 7:4959. doi: 10.24294/can.v7i1.4959
95. Gupta, N, Sharma, PK, Yadav, SS, Chauhan, M, Datusalia, AK, and Saha, S. Tricompartmental microcarriers with controlled release for efficient management of Parkinson’s disease. ACS Biomater Sci Eng. (2024) 10:5039–56. doi: 10.1021/acsbiomaterials.4c01042
96. Chakraborty, P, Bhattacharyya, C, Sahu, R, Dua, TK, Kandimalla, R, and Dewanjee, S. Polymeric nanotherapeutics: an emerging therapeutic approach for the management of neurodegenerative disorders. J Drug Deliv Sci Technol. (2024) 91:105267. doi: 10.1016/j.jddst.2023.105267
97. Castellani, S, Iaconisi, GN, Tripaldi, F, Porcelli, V, Trapani, A, Messina, E, et al. Dopamine and citicoline-co-loaded solid lipid nanoparticles as multifunctional nanomedicines for Parkinson’s disease treatment by intranasal administration. Pharmaceutics. (2024) 16:1048. doi: 10.3390/pharmaceutics16081048
98. Ragusa, A, Priore, P, Giudetti, A, Ciccarella, G, and Gaballo, A. Neuroprotective investigation of chitosan nanoparticles for dopamine delivery. Appl Sci. (2018) 8:474. doi: 10.3390/app8040474
99. Monge-Fuentes, V, Biolchi Mayer, A, Lima, MR, Geraldes, LR, Zanotto, LN, Moreira, KG, et al. Dopamine-loaded nanoparticle systems circumvent the blood-brain barrier restoring motor function in mouse model for Parkinson’s disease. Sci Rep. (2021) 11:15185. doi: 10.1038/s41598-021-94175-8
100. Vong, LB, Sato, Y, Chonpathompikunlert, P, Tanasawet, S, Hutamekalin, P, and Nagasaki, Y. Self-assembled polydopamine nanoparticles improve treatment in Parkinson’s disease model mice and suppress dopamine-induced dyskinesia. Acta Biomater. (2020) 109:220–8. doi: 10.1016/j.actbio.2020.03.021
101. Burlec, AF, Corciova, A, Boev, M, Batir-Marin, D, Mircea, C, Cioanca, O, et al. Current overview of metal nanoparticles’ synthesis, characterization, and biomedical applications, with a focus on silver and gold nanoparticles. Pharmaceuticals. (2023) 16:1410. doi: 10.3390/ph16101410
102. Dayma, P, Choudhary, N, Ali, D, Alarifi, S, Dudhagara, P, Luhana, K, et al. Exploring the potential of halotolerant Actinomycetes from Rann of Kutch, India: a study on the synthesis, characterization, and biomedical applications of silver nanoparticles. Pharmaceuticals. (2024) 17:743. doi: 10.3390/ph17060743
103. Yaqoob, SB, Adnan, R, Rameez Khan, RM, and Rashid, M. Gold, silver, and palladium nanoparticles: a chemical tool for biomedical applications. Front Chem. (2020) 8:376. doi: 10.3389/fchem.2020.00376
104. Tapia-Arellano, A, Cabrera, P, Cortés-Adasme, E, Riveros, A, Hassan, N, and Kogan, MJ. Tau- and α-synuclein-targeted gold nanoparticles: applications, opportunities, and future outlooks in the diagnosis and therapy of neurodegenerative diseases. J Nanobiotechnology. (2024) 22:248. doi: 10.1186/s12951-024-02526-0
105. Shahalaei, M, Azad, AK, Sulaiman, WMAW, Derakhshani, A, Mofakham, EB, Mallandrich, M, et al. A review of metallic nanoparticles: present issues and prospects focused on the preparation methods, characterization techniques, and their theranostic applications. Front Chem. (2024) 12:1398979. doi: 10.3389/fchem.2024.1398979
106. Scarpa, E, Cascione, M, Griego, A, Pellegrino, P, Moschetti, G, and De Matteis, V. Gold and silver nanoparticles in Alzheimer’s and Parkinson’s diagnostics and treatments. Ibrain. (2023) 9:298–315. doi: 10.1002/ibra.12126
107. Liu, L, Liu, W, Sun, Y, and Dong, X. Serum albumin-embedding copper nanoclusters inhibit Alzheimer’s β-amyloid fibrillogenesis and neuroinflammation. J Colloid Interface Sci. (2024) 672:53–62. doi: 10.1016/j.jcis.2024.05.193
108. Suliasih, BA, Budi, S, and Katas, H. Synthesis and application of gold nanoparticles as antioxidants. Pharmacia. (2024) 71:1–19. doi: 10.3897/pharmacia.71.e112322
109. Soni, SK, and MK, S. Chemical potential and biomedical activity of gold nanoparticles in cancer and drug delivery systems: an update. Vietnam J Sci Technol. (2024) 62:405–24. doi: 10.15625/2525-2518/19457
110. Hu, K, Chen, X, Chen, W, Zhang, L, Li, J, Ye, J, et al. Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomedicine. (2018) 14:1123–36. doi: 10.1016/j.nano.2018.01.020
111. da Silva, CE, de Bem, SG, Scussel, R, Correa, MEAB, da Silva, AJ, Luiz, GP, et al. Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of Parkinson’s disease. Colloids Surf B. (2020) 196:111302. doi: 10.1016/j.colsurfb.2020.111302
112. Xue, J, Liu, T, Liu, Y, Jiang, Y, Seshadri, VDD, Mohan, SK, et al. Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease—in vitro & in vivo model. J Photochem Photobiol B. (2019) 200:111635. doi: 10.1016/j.jphotobiol.2019.111635
113. Silveira, PCL, Rodrigues, MS, Gelain, DP, and de Oliveira, J. Gold nanoparticles application to the treatment of brain dysfunctions related to metabolic diseases: evidence from experimental studies. Metab Brain Dis. (2023) 38:123–35. doi: 10.1007/s11011-022-00929-2
114. Rao, N, Singh, R, and Bashambu, L. Carbon-based nanomaterials: synthesis and prospective applications. Mater Today Proc. (2021) 44:608–14. doi: 10.1016/j.matpr.2020.10.593
115. Parvin, N, Kumar, V, Joo, SW, and Mandal, TK. Emerging trends in nanomedicine: carbon-based nanomaterials for healthcare. Nanomaterials. (2024) 14:1085. doi: 10.3390/nano14131085
116. Raghavan, A, and Ghosh, S. Influence of graphene-based nanocomposites in neurogenesis and neuritogenesis: a brief summary. ACS Appl Bio Mater. (2024) 7:711–26. doi: 10.1021/acsabm.3c00852
117. Abbaspour, S, Alijanvand, SH, Morshedi, D, and Shojaosadati, SA. Inhibitory effect of plain and functionalized graphene nanoplateles on hen egg white lysozyme fibrillation. Colloids Surf B. (2023) 230:113487. doi: 10.1016/j.colsurfb.2023.113487
118. Wu, X, Wang, G, Zhao, Z, and Qian, Z. In silico study on graphene quantum dots modified with various functional groups inhibiting α-synuclein dimerization. J Colloid Interface Sci. (2024) 667:723–30. doi: 10.1016/j.jcis.2024.04.111
119. Rogacka, J, and Labus, K. Metal-organic frameworks as highly effective platforms for enzyme immobilization–current developments and future perspectives. Braz J Chem Eng. (2024). doi: 10.1007/s43153-024-00513-4
120. Chen, L, Ma, Y, Ma, X, Liu, L, Jv, X, Li, A, et al. TFEB regulates cellular labile iron and prevents ferroptosis in a TfR1-dependent manner. Free Radic Biol Med. (2023) 208:445–57. doi: 10.1016/j.freeradbiomed.2023.09.004
121. Zhang, Y, Zhang, C, Qian, W, Lei, F, Chen, Z, Wu, X, et al. Recent advances in MOF-based nanozymes: synthesis, activities, and bioapplications. Biosens Bioelectron. (2024) 263:116593. doi: 10.1016/j.bios.2024.116593
122. Boyton, I, Rennie, C, Collins-Praino, LE, and Care, A. Nanoparticle formulations for the improvement of symptomatic treatments of neurodegenerative disorders. ChemNanoMat. (2024) 10:e202400147. doi: 10.1002/cnma.202400147
123. Duan, L, Li, X, Ji, R, Hao, Z, Kong, M, Wen, X, et al. Nanoparticle-based drug delivery systems: an inspiring therapeutic strategy for neurodegenerative diseases. Polymers. (2023) 15:2196. doi: 10.3390/polym15092196
124. Pirhaghi, M, Mamashli, F, Moosavi-Movahedi, F, Arghavani, P, Amiri, A, Davaeil, B, et al. Cell-penetrating peptides: promising therapeutics and drug-delivery systems for neurodegenerative diseases. Mol Pharm. (2024) 21:2097–117. doi: 10.1021/acs.molpharmaceut.3c01167
125. Sun, X, Badachhape, A, Bhandari, P, Chin, J, Annapragada, A, and Tanifum, E. A dual target molecular magnetic resonance imaging probe for noninvasive profiling of pathologic alpha-synuclein and microgliosis in a mouse model of Parkinson’s disease. Front Neurosci. (2024) 18:1428736. doi: 10.3389/fnins.2024.1428736
126. Grosso, C, Silva, A, Delerue-Matos, C, and Barroso, MF. Single and multitarget systems for drug delivery and detection: up-to-date strategies for brain disorders. Pharmaceuticals. (2023) 16:1721. doi: 10.3390/ph16121721
127. Kalčec, N, Peranić, N, Mamić, I, Beus, M, Hall, CR, Smith, TA, et al. Selenium nanoparticles as potential drug-delivery systems for the treatment of Parkinson’s disease. ACS Appl Nano Mater. (2023) 6:17581–92. doi: 10.1021/acsanm.3c02749
128. Huang, Y, Wang, X, Li, W, Yue, F, Wang, M, and Zhou, F. Exenatide-modified deferoxamine-based nanoparticles ameliorates neurological deficits in Parkinson’s disease mice. Int J Nanomedicine. (2024) 19:10401–14. doi: 10.2147/IJN.S479670
129. Wu, X, Yuan, R, Xu, Y, Wang, K, Yuan, H, Meng, T, et al. Functionalized lipid nanoparticles modulate the blood-brain barrier and eliminate α-synuclein to repair dopamine neurons. Asian J Pharm Sci. (2024) 19:100904. doi: 10.1016/j.ajps.2024.100904
130. Inamdar, A, Gurupadayya, B, Halagali, P, Tippavajhala, VK, Khan, F, Pathak, R, et al. Unraveling neurological drug delivery: polymeric nanocarriers for enhanced blood-brain barrier penetration. Curr Drug Targets. (2024) 26:1–24. doi: 10.2174/0113894501339455241101065040
131. Chen, T, Liu, W, Xiong, S, Li, D, Fang, S, Wu, Z, et al. Nanoparticles mediating the sustained puerarin release facilitate improved brain delivery to treat Parkinson’s disease. ACS Appl Mater Interfaces. (2019) 11:45276–89. doi: 10.1021/acsami.9b16047
132. Kumar, A, Rani, R, Kumar, R, Singh, AP, and Singh, AP. An updated review on liposomes. Int J Pharm Drug Anal. (2024):12, 8–4. doi: 10.47957/ijpda.v12i1.568
133. Jadhav, PA, and Garude, R. A complete review on: liposomes. Int J Sci Res Sci Technol. (2024) 11:373–8. doi: 10.32628/IJSRST24115113
134. Nsairat, H, Khater, D, Sayed, U, Odeh, F, Al Bawab, A, and Alshaer, W. Liposomes: structure, composition, types, and clinical applications. Heliyon. (2022) 8:e09394. doi: 10.1016/j.heliyon.2022.e09394
135. Arcella, A, Palchetti, S, Digiacomo, L, Pozzi, D, Capriotti, AL, Frati, L, et al. Brain targeting by liposome–biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells. ACS Chem Neurosci. (2018) 9:3166–74. doi: 10.1021/acschemneuro.8b00339
136. Mukherjee, A, Bisht, B, Dutta, S, and Paul, MK. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol Sin. (2022) 43:2759–76. doi: 10.1038/s41401-022-00902-w
137. Sheridan, A, Nellenbach, K, Pandit, S, Byrnes, E, Hardy, G, Lutz, H, et al. Clot-targeted nanogels for dual-delivery of antithrombin iii and tissue plasminogen activator to mitigate disseminated intravascular coagulation complications. ACS Nano. (2024) 18:15517–28. doi: 10.1021/acsnano.4c00162
138. Atta, AM, Al-Lohedan, HA, Tawfik, AM, and Ezzat, AO. Application of super-amphiphilic silica-nanogel composites for fast removal of water pollutants. Molecules. (2016) 21:1392. doi: 10.3390/molecules21101392
139. Cui, W, Liu, R, Jin, H, Lv, P, Sun, Y, Men, X, et al. pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J Control Release. (2016) 225:53–63. doi: 10.1016/j.jconrel.2016.01.028
140. Kisakova, LA, Apartsin, EK, Nizolenko, LF, and Karpenko, LI. Dendrimer-mediated delivery of DNA and RNA vaccines. Pharmaceutics. (2023) 15:1106. doi: 10.3390/pharmaceutics15041106
141. Igartúa, DE, Martinez, CS, Temprana, CF, SDV, A, and Prieto, MJ. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: a biophysical and nanotoxicological characterization. Int J Pharm. (2018) 544:191–202. doi: 10.1016/j.ijpharm.2018.04.032
142. Sueyoshi, S, Vitor Silva, J, Guizze, F, and Giarolla, J. Dendrimers as drug delivery systems for oncotherapy: current status of promising applications. Int J Pharm. (2024) 663:124573. doi: 10.1016/j.ijpharm.2024.124573
143. Heitz, M, Zamolo, S, Javor, S, and Reymond, J-L. Fluorescent peptide dendrimers for siRNA transfection: tracking pH responsive aggregation, siRNA binding, and cell penetration. Bioconjug Chem. (2020) 31:1671–84. doi: 10.1021/acs.bioconjchem.0c00231
144. Subroto, E, Andoyo, R, and Indiarto, R. Solid lipid nanoparticles: review of the current research on encapsulation and delivery systems for active and antioxidant compounds. Antioxidants. (2023) 12:633. doi: 10.3390/antiox12030633
145. Jagaran, K, and Singh, M. Lipid nanoparticles: promising treatment approach for Parkinson’s disease. Int J Mol Sci. (2022) 23:9361. doi: 10.3390/ijms23169361
146. Ereej, N, Hameed, H, Khan, MA, Faheem, S, and Hameed, A. Nanoparticle-based gene therapy for neurodegenerative disorders. Mini Rev Med Chem. (2024) 24:1723–45. doi: 10.2174/0113895575301011240407082559
147. Valatabar, N, Oroojalian, F, Kazemzadeh, M, Mokhtarzadeh, AA, Safaralizadeh, R, and Sahebkar, A. Recent advances in gene delivery nanoplatforms based on spherical nucleic acids. J Nanobiotechnology. (2024) 22:386. doi: 10.1186/s12951-024-02648-5
148. Gambaro, R, Rivero Berti, I, Limeres, MJ, Huck-Iriart, C, Svensson, M, Fraude, S, et al. Optimizing mRNA-loaded lipid nanoparticles as a potential tool for protein-replacement therapy. Pharmaceutics. (2024) 16:771. doi: 10.3390/pharmaceutics16060771
149. Ooi, YJ, Huang, C, Lau, K, Chew, SY, Park, JG, and Chan-Park, MB. Nontoxic, biodegradable hyperbranched poly (β-amino ester) s for efficient siRNA delivery and gene silencing. ACS Appl Mater Interfaces. (2024) 16:14093–112. doi: 10.1021/acsami.3c10620
150. Zhang, T. Applications of lipid nanoparticles in CRISPR technology. Med Sci. (2024) 1:7. doi: 10.61173/ecc6db10
151. Li, Y, Li, C, Yan, J, Liao, Y, Qin, C, Wang, L, et al. Polymeric micellar nanoparticles for effective CRISPR/Cas9 genome editing in cancer. Biomaterials. (2024) 309:122573. doi: 10.1016/j.biomaterials.2024.122573
152. Wang, X, Cai, C, Lv, W, Chen, K, Li, J, Liao, K, et al. Short cell-penetration peptide conjugated bioreducible polymer enhances gene editing of CRISPR system. J Nanobiotechnology. (2024) 22:284. doi: 10.1186/s12951-024-02554-w
153. Mansour, HM, and El-Khatib, AS. Exploring Parkinson-associated kinases for CRISPR/Cas9-based gene editing: beyond alpha-synuclein. Ageing Res Rev. (2023) 92:102114. doi: 10.1016/j.arr.2023.102114
154. Joshi, R, Missong, H, Mishra, J, Kaur, S, Saini, S, Kandimalla, R, et al. Nanotheranostics revolutionizing neurodegenerative diseases: from precision diagnosis to targeted therapies. J Drug Deliv Sci Technol. (2023) 89:105067. doi: 10.1016/j.jddst.2023.105067
155. Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: a review of reactive oxygen species and prevention by antioxidants. Brain Commun. (2023) 6:fcad356. doi: 10.1093/braincomms/fcad356
156. Bai, Y, Li, Y, Li, Y, and Tian, L. Advanced biological applications of cerium oxide nanozymes in disease related to oxidative damage. ACS Omega. (2024) 9:8601–14. doi: 10.1021/acsomega.3c03661
157. Dillon, CD, Billings, M, Hockey, KS, DeLaGarza, L, and Rzigalinski, BA. Cerium oxide nanoparticles protect against MPTP-induced dopaminergic neurodegeneration in a mouse model for Parkinson’s disease. TechConnect Briefs. (2011) 3:451–4.
158. Gao, Y, Zhai, L, Chen, J, Lin, D, Zhang, L-K, Yang, H, et al. Focused ultrasound-mediated cerium-based nanoreactor against Parkinson’s disease via ROS regulation and microglia polarization. J Control Release. (2024) 368:580–94. doi: 10.1016/j.jconrel.2024.03.010
159. El Nebrisi, E. Neuroprotective activities of curcumin in Parkinson’s disease: a review of the literature. Int J Mol Sci. (2021) 22:11248. doi: 10.3390/ijms222011248
160. Wang, X-S, Zhang, Z-R, Zhang, M-M, Sun, M-X, Wang, W-W, and Xie, C-L. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: a systematic experiment literatures review. BMC Complement Altern Med. (2017) 17:412. doi: 10.1186/s12906-017-1922-x
161. El Nebrisi, E, Javed, H, Ojha, SK, Oz, M, and Shehab, S. Neuroprotective effect of curcumin on the nigrostriatal pathway in a 6-hydroxydopmine-induced rat model of Parkinson’s disease is mediated by α7-nicotinic receptors. Int J Mol Sci. (2020) 21:7329. doi: 10.3390/ijms21197329
162. Hu, E, Li, Z, Li, T, Yang, X, Ding, R, Jiang, H, et al. A novel microbial and hepatic biotransformation-integrated network pharmacology strategy explores the therapeutic mechanisms of bioactive herbal products in neurological diseases: the effects of Astragaloside IV on intracerebral hemorrhage as an example. Chin Med. (2023) 18:40. doi: 10.1186/s13020-023-00745-5
163. Zhang, H, Wang, L, Yang, Y, Cai, C, Wang, X, Deng, L, et al. DL-3-n-butylphthalide (NBP) alleviates poststroke cognitive impairment (PSCI) by suppressing neuroinflammation and oxidative stress. Front Pharmacol. (2023) 13:987293. doi: 10.3389/fphar.2022.987293
164. Li, S, Wang, Z, Liu, G, and Chen, M. Neurodegenerative diseases and catechins: (-)-epigallocatechin-3-gallate is a modulator of chronic neuroinflammation and oxidative stress. Front Nutr. (2024) 11:1425839. doi: 10.3389/fnut.2024.1425839
165. Tripathi, S, and Bhawana,. Epigenetic orchestration of neurodegenerative disorders: a possible target for curcumin as a therapeutic. Neurochem Res. (2024) 49:2319–35. doi: 10.1007/s11064-024-04167-z
166. Zamanian, MY, Parra, RMR, Soltani, A, Kujawska, M, Mustafa, YF, Raheem, G, et al. Targeting Nrf2 signaling pathway and oxidative stress by resveratrol for Parkinson’s disease: an overview and update on new developments. Mol Biol Rep. (2023) 50:5455–64. doi: 10.1007/s11033-023-08409-1
167. Umapathy, S, Pan, I, Issac, PK, Kumar, MSK, Giri, J, Guru, A, et al. Selenium nanoparticles as neuroprotective agents: insights into molecular mechanisms for Parkinson’s disease treatment. Mol Neurobiol. (2024). doi: 10.1007/s12035-024-04253-x
168. Keshavarz Shahbaz, S, Koushki, K, Keshavarz Hedayati, S, McCloskey, AP, Kesharwani, P, Naderi, Y, et al. Polymer nanotherapeutics: a promising approach toward microglial inhibition in neurodegenerative diseases. Med Res Rev. (2024) 44:2793–824. doi: 10.1002/med.22064
169. Stephen, BJ, Chokriwal, A, Sharma, MM, Jain, D, Saxena, J, Dhaliwal, H, et al. Dexamethasone encapsulated PLGA nanoparticles for the treatment of neuroinflammation. Bioinspired Biomim Nanobiomaterials. (2022) 11:72–85. doi: 10.1680/jbibn.21.00059
170. Zhang, Y, Zhou, X, Wang, Z, Wu, M, Zhang, W, Zhang, Z, et al. Dexamethasone palmitate encapsulated in palmitic acid modified human serum albumin nanoparticles for the treatment of rheumatoid arthritis. J Pharm Sci. (2024) 113:2851–60. doi: 10.1016/j.xphs.2024.07.013
171. Toader, C, Dumitru, AV, Eva, L, Serban, M, Covache-Busuioc, R-A, and Ciurea, AV. Nanoparticle strategies for treating CNS disorders: a comprehensive review of drug delivery and theranostic applications. Int J Mol Sci. (2024) 25:13302. doi: 10.3390/ijms252413302
172. Capossela, L, Gatto, A, Ferretti, S, Di Sarno, L, Graglia, B, Massese, M, et al. Multifaceted roles of nerve growth factor: a comprehensive review with a special insight into pediatric perspectives. Biology. (2024) 13:546. doi: 10.3390/biology13070546
173. Zhang, C, Shao, W, Yuan, H, Xiao, R, Zhang, Y, Wei, C, et al. Engineered extracellular vesicle-based nanoformulations that coordinate neuroinflammation and immune homeostasis, enhancing parkinson’s disease therapy. ACS Nano. (2024) 18:23014–31. doi: 10.1021/acsnano.4c04674
174. Liu, Z, Xiang, S, Chen, B, Li, J, Zhu, D, Xu, H, et al. Parkinson disease-targeted nanocapsules for synergistic treatment: combining dopamine replacement and neuroinflammation mitigation. Adv Sci. (2024) 11:2404717. doi: 10.1002/advs.202404717
175. Jiang, W, Li, Q, Zhang, R, Li, J, Lin, Q, Li, J, et al. Chiral metal-organic frameworks incorporating nanozymes as neuroinflammation inhibitors for managing Parkinson’s disease. Nat Commun. (2023) 14:8137. doi: 10.1038/s41467-023-43870-3
176. Li, Q, Wu, T, Akakuru, OU, Song, N, Liu, W, Jiang, W, et al. A dual synergetic nanoreactor for managing Parkinson’s disease by regulating inflammation and mitigating oxidative damage. Adv Funct Mater. (2023) 33:2214826. doi: 10.1002/adfm.202214826
177. Singh, G, Sikder, A, Phatale, V, Srivastava, S, Singh, SB, and Khatri, DK. Therapeutic potential of GDNF in neuroinflammation: targeted delivery approaches for precision treatment in neurological diseases. J Drug Deliv Sci Technol. (2023) 87:104876. doi: 10.1016/j.jddst.2023.104876
178. Ma, C, An, P, Yan, Y, Su, M, Xu, W, Chen, J, et al. GDNF improves the cognitive ability of PD mice by promoting glycosylation and membrane distribution of DAT. Sci Rep. (2024) 14:17845. doi: 10.1038/s41598-024-68609-y
179. Yang, SY, Jeong, ES, and Kim, JE. Effects of glial cell line-derived neurotrophic factor and neurturin brain infusion in patients with Parkinson’s disease: a systematic review and meta-analysis. J Soc Convergence Res. (2024) 8:101–14. doi: 10.37181/JSCS.2024.8.3.101
180. Al-Serri, A, Al-Janahi, H, Jamal, M, AlTarrah, D, Ziyab, A, and Al-Bustan, S. Influence of the brain-derived neurotrophic factor gene polymorphism on weight loss following intragastric balloon intervention: a cross-sectional study. Diabetes Metab Syndr Obes. (2024) 17:4299–306. doi: 10.2147/DMSO.S481547
181. Alheibshy, F. The use of nano-based drug delivery systems for neurodegenerative disorders. Asian J Pharm Res Health Care. (2024) 16:267–71. doi: 10.4103/ajprhc.ajprhc_88_24
182. Vaquero-Rodríguez, A, Razquin, J, Zubelzu, M, Bidgood, R, Bengoetxea, H, Miguelez, C, et al. Efficacy of invasive and non-invasive methods for the treatment of Parkinson's disease: Nanodelivery and enriched environment. Int Rev Neurobiol. (2023) 172:103–43. doi: 10.1016/bs.irn.2023.05.010
183. Wolf, D, Ayon-Olivas, M, and Sendtner, M. BDNF-regulated modulation of striatal circuits and implications for Parkinson’s disease and dystonia. Biomedicines. (2024) 12:1761. doi: 10.3390/biomedicines12081761
184. Zhuo, Y, Zhao, Y-G, and Zhang, Y. Enhancing drug solubility, bioavailability, and targeted therapeutic applications through magnetic nanoparticles. Molecules. (2024) 29:4854. doi: 10.3390/molecules29204854
185. MMR, M, Begum, MM, Uddin, MS, and Ashraf, GM. Deciphering the role of nanoparticle-based treatment for Parkinson’s disease. Curr Drug Metab. (2021) 22:550–60. doi: 10.2174/1389200222666210202110129
186. Dogra, N, Jakhmola Mani, R, and Pande, KD. Tiny carriers, tremendous hope: nanomedicine in the fight against Parkinson’s. J Dement Alzheimer’s Dis. (2024) 1:3–21. doi: 10.3390/jdad1010002
187. Majumdar, M, and Badwaik, H. Trends on novel targets and nanotechnology-based drug delivery system in the treatment of Parkinson’s disease: recent advancement in drug development. Curr Drug Targets. (2024) 25:987–1011. doi: 10.2174/0113894501312703240826070530
188. Tang, KS, See, WZC, and Naidu, R. Neuroprotective properties of zinc oxide nanoparticles: therapeutic implications for Parkinson’s disease. Biosci Rep. (2024) 44:BSR20241102. doi: 10.1042/BSR20241102
189. Doke, RR, Pansare, PA, Shivani, RS, Bhalchim, VM, and Desai, SR. Natural products: an emerging tool in Parkinson’s disease therapeutics. IP Indian J Neurosci. (2019) 5:95–105. doi: 10.18231/j.ijn.2019.014
190. Church, FC. Treatment options for motor and non-motor symptoms of Parkinson’s disease. Biomol Ther. (2021) 11:612. doi: 10.3390/biom11040612
191. Domingos, J, Coelho, M, and Ferreira, JJ. Referral to rehabilitation in Parkinson’s disease: who, when and to what end? Arq Neuropsiquiatr. (2013) 71:967–72. doi: 10.1590/0004-282X20130209
192. Chen, V, Moncalvo, M, Tringali, D, Tagliafierro, L, Shriskanda, A, Ilich, E, et al. The mechanistic role of alpha-synuclein in the nucleus: impaired nuclear function caused by familial Parkinson’s disease SNCA mutations. Hum Mol Genet. (2020) 29:3107–21. doi: 10.1093/hmg/ddaa183
193. Martin, LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci. (2012) 107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9
194. Kumar, A, Tamjar, J, Waddell, AD, Woodroof, HI, Raimi, OG, Shaw, AM, et al. Structure of PINK1 and mechanisms of Parkinson’s disease-associated mutations. eLife. (2017) 6:e29985. doi: 10.7554/eLife.29985
195. Nguyen, APT, Tsika, E, Kelly, K, Levine, N, Chen, X, West, AB, et al. Dopaminergic neurodegeneration induced by Parkinson’s disease-linked G2019S LRRK2 is dependent on kinase and GTPase activity. Proc Natl Acad Sci USA. (2020) 117:17296–307. doi: 10.1073/pnas.1922184117
196. Ruiz-Lopez, M, Freitas, ME, Oliveira, LM, Munhoz, RP, Fox, SH, Rohani, M, et al. Diagnostic delay in Parkinson’s disease caused by PRKN mutations. Parkinsonism Relat Disord. (2019) 63:217–20. doi: 10.1016/j.parkreldis.2019.01.010
197. Rautio, J, Kumpulainen, H, Heimbach, T, Oliyai, R, Oh, D, Järvinen, T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. (2008) 7:255–70. doi: 10.1038/nrd2468
198. You, H, Mariani, L-L, Mangone, G, Febvre, L, de Nailly, D, Charbonnier-Beaupel, F, et al. Molecular basis of dopamine replacement therapy and its side effects in Parkinson’s disease. Cell Tissue Res. (2018) 373:111–35. doi: 10.1007/s00441-018-2813-2
199. Turjanski, N, and Lloyd, GG. Psychiatric side-effects of medications: recent developments. Adv Psychiatr Treat. (2005) 11:58–70. doi: 10.1192/apt.11.1.58
200. Finberg, JPM. Inhibitors of MAO-B and COMT: their effects on brain dopamine levels and uses in Parkinson’s disease. J Neural Transm. (2019) 126:433–48. doi: 10.1007/s00702-018-1952-7
201. Fahn, S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord. (2015) 30:4–18. doi: 10.1002/mds.26102
202. Crispo, JAG, Willis, AW, Thibault, DP, Fortin, Y, Hays, HD, McNair, DS, et al. Associations between anticholinergic burden and adverse health outcomes in Parkinson disease. PLoS One. (2016) 11:e0150621. doi: 10.1371/journal.pone.0150621
203. Sheu, J, Tsai, M, Erickson, SR, and Wu, C. Association between anticholinergic medication use and risk of dementia among patients with Parkinson’s disease. Pharmacotherapy. (2019) 39:798–808. doi: 10.1002/phar.2305
204. Aradi, SD, and Hauser, RA. Medical management and prevention of motor complications in Parkinson’s disease. Neurotherapeutics. (2020) 17:1339–65. doi: 10.1007/s13311-020-00889-4
205. Pahuja, R, Seth, K, Shukla, A, Shukla, RK, Bhatnagar, P, Chauhan, LKS, et al. Correction to trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano. (2019) 13:8490–26. doi: 10.1021/acsnano.9b04446
206. Pahuja, R, Seth, K, Shukla, A, Shukla, RK, Bhatnagar, P, Chauhan, LKS, et al. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano. (2015) 9:4850–71. doi: 10.1021/nn506408v
207. García-Pardo, J, Novio, F, Nador, F, Cavaliere, I, Suárez-García, S, Lope-Piedrafita, S, et al. Bioinspired theranostic coordination polymer nanoparticles for intranasal dopamine replacement in Parkinson’s disease. ACS Nano. (2021) 15:8592–609. doi: 10.1021/acsnano.1c00453
208. Pillay, S, Pillay, V, Choonara, YE, Naidoo, D, Khan, RA, du Toit, LC, et al. Design, biometric simulation and optimization of a nano-enabled scaffold device for enhanced delivery of dopamine to the brain. Int J Pharm. (2009) 382:277–90. doi: 10.1016/j.ijpharm.2009.08.021
209. López, T, Bata-García, JL, Esquivel, D, Ortiz-Islas, E, Gonzalez, R, Ascencio, J, et al. Treatment of Parkinson’s disease: nanostructured sol-gel silica-dopamine reservoirs for controlled drug release in the central nervous system. Int J Nanomedicine. (2011) 6:19–31. doi: 10.2147/IJN.S13223
210. de Giglio, E, Trapani, A, Cafagna, D, Sabbatini, L, and Cometa, S. Dopamine-loaded chitosan nanoparticles: formulation and analytical characterization. Anal Bioanal Chem. (2011) 400:1997–2002. doi: 10.1007/s00216-011-4962-y
211. Malvindi, MA, di Corato, R, Curcio, A, Melisi, D, Rimoli, MG, Tortiglione, C, et al. Multiple functionalization of fluorescent nanoparticles for specific biolabeling and drug delivery of dopamine. Nanoscale. (2011) 3:5110–9. doi: 10.1039/C1NR10797F
212. Sharma, S, Lohan, S, and Murthy, RSR. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Dev Ind Pharm. (2014) 40:869–78. doi: 10.3109/03639045.2013.789051
213. Kura, AU, Hussein Al Ali, SH, Hussein, MZ, Fakurazi, S, and Arulselvan, P. Development of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agent. Int J Nanomedicine. (2013) 8:1103–10. doi: 10.2147/IJN.S39740
214. Kura, AU, Ain, NM, Hussein, MZ, Fakurazi, S, and Hussein-Al-Ali, SH. Toxicity and metabolism of layered double hydroxide intercalated with levodopa in a Parkinson’s disease model. Int J Mol Sci. (2014) 15:5916–27. doi: 10.3390/ijms15045916
215. Gambaryan, PY, Kondrasheva, IG, Severin, ES, Guseva, AA, and Kamensky, AA. Increasing the efficiency of Parkinson’s disease treatment using a poly (lactic-co-glycolic acid) (PLGA) based L-dopa delivery system. Exp Neurobiol. (2014) 23:246–52. doi: 10.5607/en.2014.23.3.246
216. Tan, JM, Foo, JB, Fakurazi, S, and Hussein, MZ. Release behaviour and toxicity evaluation of levodopa from carboxylated single-walled carbon nanotubes. Beilstein J Nanotechnol. (2015) 6:243–53. doi: 10.3762/bjnano.6.23
217. di Gioia, S, Trapani, A, Mandracchia, D, de Giglio, E, Cometa, S, Mangini, V, et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur J Pharm Biopharm. (2015) 94:180–93. doi: 10.1016/j.ejpb.2015.05.019
218. Azeem, A, Talegaonkar, S, Negi, LM, Ahmad, FJ, Khar, RK, and Iqbal, Z. Oil based nanocarrier system for transdermal delivery of ropinirole: a mechanistic, pharmacokinetic and biochemical investigation. Int J Pharm. (2012) 422:436–44. doi: 10.1016/j.ijpharm.2011.10.039
219. Patil, GB, and Surana, SJ. Fabrication and statistical optimization of surface engineered PLGA nanoparticles for naso-brain delivery of ropinirole hydrochloride: in-vitro-ex-vivo studies. J Biomater Sci Polym Ed. (2013) 24:1740–56. doi: 10.1080/09205063.2013.798880
220. Pardeshi, CV, Belgamwar, VS, Tekade, AR, and Surana, SJ. Novel surface modified polymer-lipid hybrid nanoparticles as intranasal carriers for ropinirole hydrochloride: in vitro, ex vivo and in vivo pharmacodynamic evaluation. J Mater Sci Mater Med. (2013) 24:2101–15. doi: 10.1007/s10856-013-4965-7
221. Pardeshi, CV, Rajput, PV, Belgamwar, VS, Tekade, AR, and Surana, SJ. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: application of factorial design approach. Drug Deliv. (2013) 20:47–56. doi: 10.3109/10717544.2012.752421
222. Jafarieh, O, Md, S, Ali, M, Baboota, S, Sahni, JK, Kumari, B, et al. Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting. Drug Dev Ind Pharm. (2015) 41:1674–81. doi: 10.3109/03639045.2014.991400
223. Mustafa, G, Ahuja, A, Al Rohaimi, AH, Muslim, S, Hassan, AA, Baboota, S, et al. Nano-ropinirole for the management of parkinsonism: blood-brain pharmacokinetics and carrier localization. Expert Rev Neurother. (2015) 15:695–710. doi: 10.1586/14737175.2015.1036743
224. Esposito, E, Fantin, M, Marti, M, Drechsler, M, Paccamiccio, L, Mariani, P, et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm Res. (2008) 25:1521–30. doi: 10.1007/s11095-007-9514-y
225. Esposito, E, Mariani, P, Ravani, L, Contado, C, Volta, M, Bido, S, et al. Nanoparticulate lipid dispersions for bromocriptine delivery: characterization and in vivo study. Eur J Pharm Biopharm. (2012) 80:306–14. doi: 10.1016/j.ejpb.2011.10.015
226. Md, S, Haque, S, Fazil, M, Kumar, M, Baboota, S, Sahni, JK, et al. Optimised nanoformulation of bromocriptine for direct nose-to-brain delivery: biodistribution, pharmacokinetic and dopamine estimation by ultra-HPLC/mass spectrometry method. Expert Opin Drug Deliv. (2014) 11:827–42. doi: 10.1517/17425247.2014.894504
227. Hwang, T-L, Lin, Y-K, Chi, C-H, Huang, T-H, and Fang, J-Y. Development and evaluation of perfluorocarbon nanobubbles for apomorphine delivery. J Pharm Sci. (2009) 98:3735–47. doi: 10.1002/jps.21687
228. Tsai, M-J, Huang, Y-B, Wu, P-C, Fu, Y-S, Kao, Y-R, Fang, J-Y, et al. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. (2011) 100:547–57. doi: 10.1002/jps.22285
229. Wen, Z, Yan, Z, Hu, K, Pang, Z, Cheng, X, Guo, L, et al. Odorranalectin-conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J Control Release. (2011) 151:131–8. doi: 10.1016/j.jconrel.2011.02.022
230. Mittal, D, Md, S, Hasan, Q, Fazil, M, Ali, A, Baboota, S, et al. Brain targeted nanoparticulate drug delivery system of rasagiline via intranasal route. Drug Deliv. (2016) 23:130–9. doi: 10.3109/10717544.2014.907372
231. Gulati, N, Nagaich, U, and Saraf, S. Fabrication and in vitro characterization of polymeric nanoparticles for Parkinson’s therapy: a novel approach. Braz J Pharm Sci. (2014) 50:869–76. doi: 10.1590/S1984-82502014000400022
232. Gao, Y, Cheng, Y, Chen, J, Lin, D, Liu, C, Zhang, L, et al. NIR-assisted MgO-based polydopamine nanoparticles for targeted treatment of Parkinson’s disease through the blood-brain barrier. Adv Healthc Mater. (2022) 11:2201655. doi: 10.1002/adhm.202201655
233. Yuan, R, Li, Y, Han, S, Chen, X, Chen, J, He, J, et al. Fe-curcumin nanozyme-mediated reactive oxygen species scavenging and anti-inflammation for acute lung injury. ACS Cent Sci. (2022) 8:10–21. doi: 10.1021/acscentsci.1c00866
234. Aly, AE-E, Harmon, BT, Padegimas, L, Sesenoglu-Laird, O, Cooper, MJ, and Waszczak, BL. Intranasal delivery of pGDNF DNA nanoparticles provides neuroprotection in the rat 6-hydroxydopamine model of Parkinson’s disease. Mol Neurobiol. (2019) 56:688–701. doi: 10.1007/s12035-018-1109-6
Keywords: CRISPR/Cas9, dopaminergic pathways, gallium nanoparticles, neurodegenerative, neuroglia, Parkinson’s
Citation: Yadav VK, Dhanasekaran S, Choudhary N, Nathiya D, Thakur V, Gupta R, Pramanik S, Kumar P, Gupta N and Patel A (2025) Recent advances in nanotechnology for Parkinson’s disease: diagnosis, treatment, and future perspectives. Front. Med. 12:1535682. doi: 10.3389/fmed.2025.1535682
Received: 27 November 2024; Accepted: 06 January 2025;
Published: 22 January 2025.
Edited by:
Tara Chand Yadav, University of North Carolina at Chapel Hill, United StatesReviewed by:
Hina Sultana, University of North Carolina System, United StatesCopyright © 2025 Yadav, Dhanasekaran, Choudhary, Nathiya, Thakur, Gupta, Pramanik, Kumar, Gupta and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Virendra Kumar Yadav, dmlyZW5kcmFrdW1hci55YWRhdkBtYXJ3YWRpZWR1Y2F0aW9uLmVkdS5pbg==; Seshathiri Dhanasekaran, c2VzaGF0aGlyaS5kaGFuYXNla2FyYW5AdWl0Lm5v; Pankaj Kumar, cGFua2FqLmt1bWFyMjUxMzVAcGFydWx1bml2ZXJzaXR5LmFjLmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.