- 1School of Medicine, Central Asian University, Tashkent, Uzbekistan
- 2Department of Oncology, Akfa Medline University Hospital, Tashkent, Uzbekistan
- 3Department of General Surgery, Akfa Medline University Hospital, Tashkent, Uzbekistan
- 4Department of Radiation Oncology, Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, Tashkent, Uzbekistan
- 5Department of Cytological and Histological Diagnostics, Ipsum Pathology, Tashkent, Uzbekistan
Meigs’ syndrome is a rare gynecological condition characterized by a benign ovarian tumor, ascites, and pleural effusion, all of which resolve spontaneously after tumor removal. While mildly elevated serum CA-125 levels are frequently observed, levels exceeding 1,000 IU/mL are extremely rare, and concurrent elevation of other tumor markers, such as HE-4, may further complicate its diagnosis. We report a case of Meigs’ syndrome in a 41-year-old premenopausal woman. Initial presenting symptoms included severe dyspnea, abdominal distention, anorexia, and weight loss. Subsequent imaging studies revealed a large right ovarian tumor accompanied by massive ascites and pleural effusion. Serum CA-125 and HE-4 levels were markedly elevated (1,200 IU/mL and 82.1 pmol/L, respectively), with a Risk of Ovarian Malignancy Algorithm (ROMA) score of 25.63%, suggesting advanced ovarian malignancy. Neoadjuvant chemotherapy was initiated, but the tumor continued to grow, necessitating internal debulking surgery. Postoperative histopathology revealed a benign ovarian fibroma, confirming the diagnosis of Meigs’ syndrome. Spontaneous resolution of ascites and pleural effusion occurred by the second postoperative day, and the tumor markers normalized within the next six months. The patient remained disease-free at 2-year follow-up. This case underscores the importance of considering Meigs’ syndrome in patients with markedly elevated tumor markers, an ovarian tumor unresponsive to chemotherapy, and concomitant ascites and pleural effusion. Early recognition and surgical intervention are critical for accurate diagnosis and optimal management of this rare condition.
Introduction
First reported by Joe Vincent Meigs in 1937, Meigs’ syndrome is characterized by the triad of a benign ovarian tumor, ascites, and pleural effusion (1, 2). In the following years, the official diagnostic criteria for Meigs’ syndrome were established as follows: (1) the presence of a benign ovarian tumor, specifically fibroma, thecoma, granulosa cell tumor, or Brenner tumor; (2) concurrent ascites and pleural effusion; and (3) spontaneous resolution of ascites and pleural effusion after tumor removal (1, 3, 4). Pelvic or abdominal tumors other than those recognized in Meigs’ syndrome, when associated with ascites and pleural effusion, are classified as pseudo-Meigs’ syndrome (1, 4). It has been estimated that Meigs’ syndrome arises in approximately 1% of benign ovarian tumors (1, 2). Common presenting symptoms include dyspnea, dry cough, fatigue, poor appetite, weight loss, and painful abdominal distension (1, 4, 5). Due to its rarity, understanding of Meigs’ syndrome mostly relies on case reports, leaving its pathophysiology largely unclear (1, 2, 6, 7).
Meigs’ syndrome presents with several diagnostic challenges. Ascites and pleural effusion in the presence of an ovarian tumor often suggest advanced or disseminated malignancies (8). Cancer antigen (CA)-125, a tumor marker often elevated in epithelial ovarian cancers, is also frequently elevated in Meigs’ syndrome, further complicating its differentiation from ovarian malignancies (1, 9). However, CA-125 levels exceeding 1,000 IU/mL in Meigs’ syndrome are extremely rare (9). A limited number of studies have also documented the elevation of human epididymis protein-4 (HE-4) in Meigs’ syndrome (5, 6). HE-4 is used alongside CA-125 to improve sensitivity in detecting ovarian cancers (10). The concurrent elevation of CA-125 and HE-4, therefore, raises significant suspicion for ovarian malignancies, making its diagnosis even more challenging. In this study, we present a case of Meigs’ syndrome with elevated CA-125 and HE-4 mimicking ovarian malignancy, and we review similar reports to broaden the understanding of Meigs’ syndrome.
Case presentation
A 41-year-old premenopausal woman presented to the hospital with a 14-month history of severe dyspnea, abdominal distention, loss of appetite, and weight loss. Her obstetric history included regular menarche that started at 13 years of age and three uncomplicated deliveries, following which she opted for an intrauterine device (IUD) for contraception in 2019. She denied any chronic illnesses, including diabetes mellitus, viral hepatitis, and tuberculosis, and had no history of allergies, surgeries, or recent travel. She was a non-smoker and did not consume alcohol or illicit drugs. Her family history was negative for cancer. Physical examination revealed an absent breath sound and dullness to percussion on the right lung, indicating possible pleural effusion. Her abdomen was severely distended with evidence of shifting dullness and a fluid wave, and pelvic examination identified a firm, 14 × 12 cm mass extending to the umbilicus.
Computed tomography (CT) of the chest showed a complete collapse of the right lung due to a significant pleural effusion, while the left lung appeared clear (Figures 1A,B). Immediate thoracentesis drained approximately 2 L of yellowish fluid, of which 100 mL was sent for cytology. Abdominal magnetic resonance imaging (MRI) revealed a solid 14.5 × 15.5 × 14.3 cm mass originating from the right ovary with massive ascites (Figures 1C,D). Paracentesis drained 8 L of ascitic fluid, of which 100 mL was sent for cytological analysis. Given the high suspicion of ovarian malignancy with possible metastasis, serum tumor markers were analyzed: carcinoembryonic antigen (CEA) was 1.07 ng/mL (reference range 0–5.09 ng/mL), CA 19–9 was 4.57 IU/mL (reference range 0–41 IU/mL), CA-125 was markedly elevated at 1,200 IU/mL (reference range 0–35 IU/mL), and HE-4 was 82.1 pmol/L (reference range 0–70 pmol/L). The Risk of Ovarian Malignancy Algorithm (ROMA) score was 25.63%, suggesting a high likelihood of ovarian malignancy. Cytology from the pleural effusion showed numerous reactive mesothelial cells, neutrophils, and lymphocytes, while the ascitic fluid revealed several atypical cells, raising concerns for pleural and peritoneal metastasis.
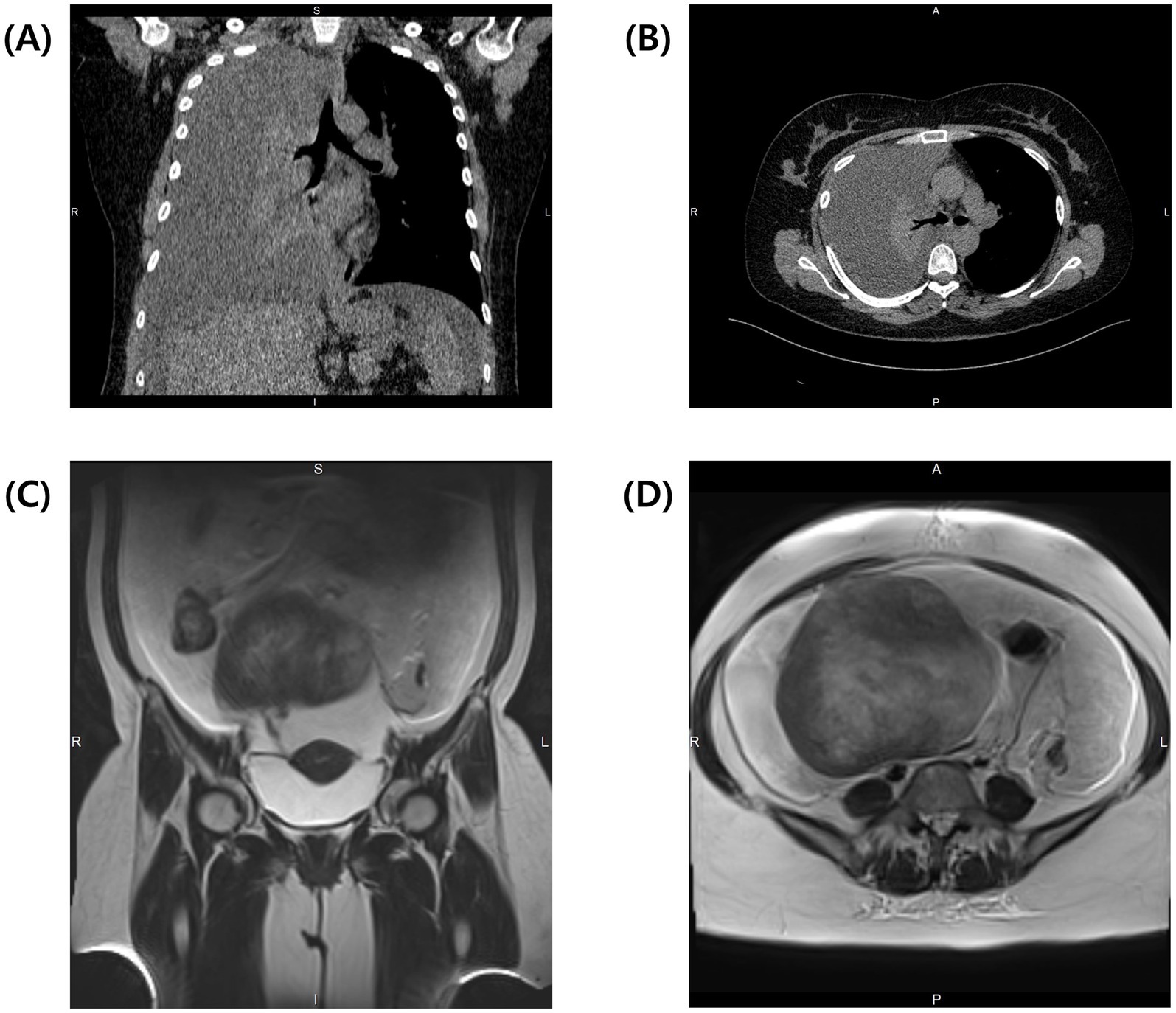
Figure 1. Preoperative CT of the thorax demonstrates complete collapse of the right lung due to massive pleural effusion. (A) Coronal view and (B) transverse view of the thorax at the level of tracheal bifurcation. Preoperative T2-weighted MRI of the abdomen reveals massive ascites and a solid tumor. (C) Coronal view shows the connection between the tumor and the uterus. (D) Transverse view of the abdomen shows a large solid tumor measuring 14.5 × 15.5 × 14.3 cm.
Exploratory laparoscopy was initially planned, but persistent pleural effusion, ascites, and the patient’s worsening condition rendered surgery unfeasible. According to the National Comprehensive Cancer Network (NCCN) guidelines, neoadjuvant chemotherapy (NACT) was initiated with paclitaxel (175 mg/m2) and carboplatin (AUC 5) to reduce the tumor burden and control effusions. During two cycles of NACT, the patient developed a pulmonary embolism, which was managed with fraxiparine and rivaroxaban. Unfortunately, NACT proved ineffective; the ovarian mass increased to 20.5 × 13.5 × 17.3 cm, compressing surrounding structures (Figures 2A,B), and pleural effusions extended to left hemithorax (approximately 800 mL), necessitating bilateral thoracentesis (Figure 2C). Given the disease progression despite NACT, internal debulking surgery (IDS) was pursued despite the high operative risk. Intraoperative findings revealed a solid tumor that originated from the right ovary (Figure 3A). The procedure included bilateral salpingo-oophorectomy, total abdominal hysterectomy, omentectomy, and parietal biopsy. The surgery was uneventful, and postoperative histopathology confirmed a benign right ovarian fibroma with no evidence of malignancy (Figures 3B,C). The final diagnosis of Meigs’ syndrome was made.
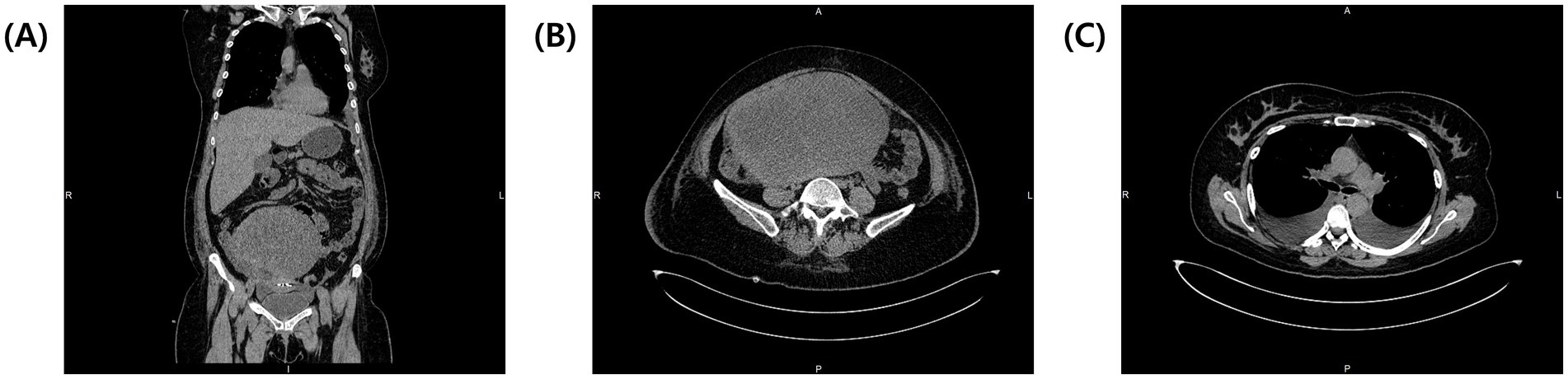
Figure 2. Post-NACT CT of the thorax and abdomen. (A) Coronal view showing the enlarged tumor compressing surrounding structures. (B) Transverse view of the abdomen with enlarged tumor now measuring 20.5 × 13.5 × 17.3 cm. (C) Transverse view of the thorax showing bilateral pleural effusions at the level of tracheal bifurcation.
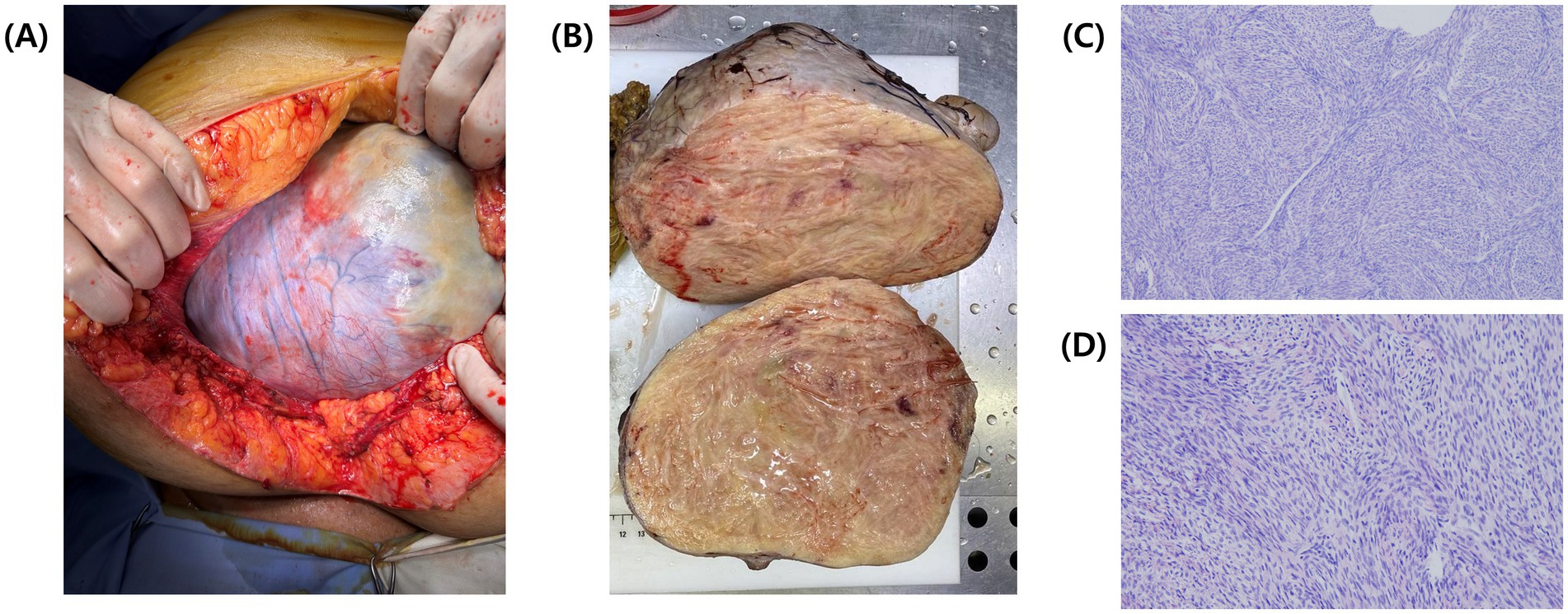
Figure 3. Intraoperative and postoperative images of the tumor. (A) Intraoperative view of the tumor during open laparotomy. (B) Postoperative cross-section of the tumor showing a solid, densely packed yellowish mass. (C) Hematoxylin and eosin (H&E) stained histological section of the resected tumor, viewed under light microscopy at 20x magnification and (D) at 40x magnification.
The patient’s pleural effusion and ascites resolved spontaneously by the second postoperative day. She was discharged on the seventh postoperative day, and follow-up at six months showed normalized tumor markers (CA-125 at 16.6 IU/mL and HE-4 at 30.7 pmol/L), with no evidence of recurrence or residual effusion on contrast enhanced-CT (Figures 4A–C). She remained disease-free at a 2-year follow-up.
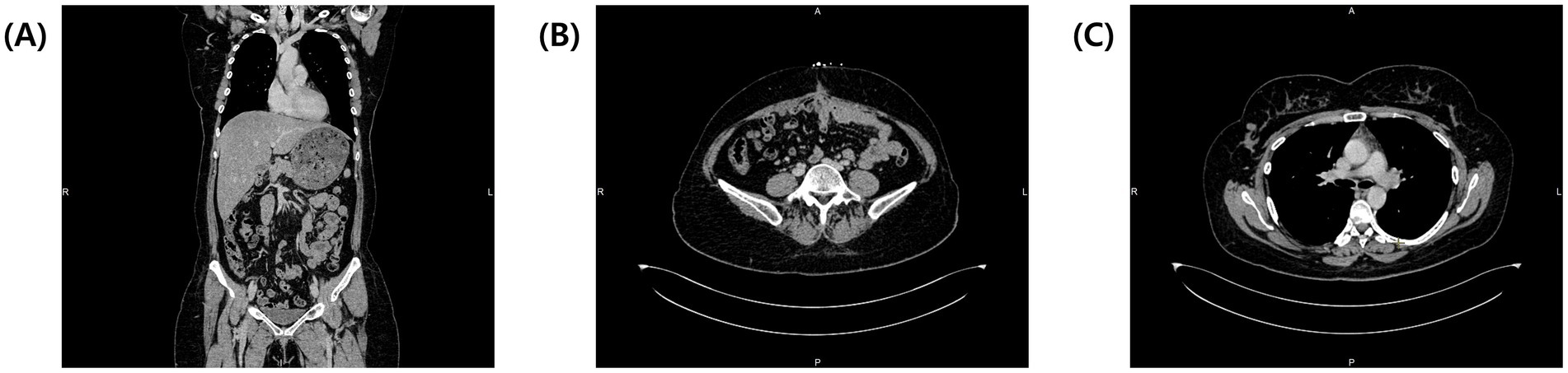
Figure 4. Contrast-enhanced CT at 6 months follow-up. (A) Coronal view of the entire body showing complete resolution of pleural effusion and ascites. (B) Transverse view of the abdomen showing no tumor recurrence and complete ascites resolution. (C) Transverse view of the thorax at the tracheal bifurcation level showing full resolution of pleural effusions.
Review of the literature
We identified a total of 49 cases of Meigs’ syndrome with elevated CA-125 levels from 45 reports published between 2015 and 2024, sourced from PubMed (6, 7, 9, 11–32) and Google Scholar (33–52). A summary of these cases is provided in Supplementary Table 1. The ages of the patients ranged from 11 to 85 years. The most common ovarian tumor histopathology was fibroma (25 cases), followed by fibrothecoma (18 cases), granulosa cell tumor (four cases), and thecoma (two cases). Many reports did not specify the weight of the tumor, although one case described a tumor weighing as much as 36 kg (27). Tumors were most commonly located in the right adnexa (27 cases), followed by the left adnexa (16 cases), with four cases involving bilateral adnexal tumors (20, 23, 26, 35). CA-125 levels ranged from 57 IU/mL to 4,900 IU/mL, with 11 cases having CA-125 levels exceeding 1,000 IU/mL.
Pleural effusion was most often seen in the right hemithorax (26 cases), followed by bilateral hemithorax (17 cases). Two cases presented with isolated left pleural effusion (40, 43). Ascitic fluid volumes ranged from 300 mL to 20,000 mL. The majority of patients underwent unilateral or bilateral salpingo-oophorectomy along with hysterectomy. Postoperative discharge occurred mostly within one week, with the shortest discharge time being two days (20, 23) and the longest being 33 days (19).
Postoperative complications included pulmonary embolism and deep vein thrombosis (30), as well as one death due to sepsis and multiorgan failure (26). No patient died directly from Meigs’ syndrome. No cases required additional postoperative treatment except one patient who received monthly goserelin and bevacizumab for one year postoperatively (31). Pleural effusion and ascites resolved spontaneously within days to weeks after surgery, and the CA-125 levels returned to the reference range within weeks to months. Patients were followed for several months to years, with no recurrence noted in the longest follow-up period of 10 years (20).
Discussion
First described by Joe Vincent Meigs in 1937, Meigs’ syndrome is defined as the presence of a benign ovarian tumor, ascites, and pleural effusion, all of which resolve spontaneously after tumor resection (1). Historically, only fibroma, thecoma, granulosa cell tumor, and Brenner tumor were recognized as diagnostic criteria for Meigs’ syndrome (1). However, other benign ovarian tumors, such as hemangioma (53), sclerosing stromal tumor (54), and Sertoli-Leydig tumor (55), have been reported as Meigs’ syndrome. Due to the inconsistent application of terminology and the usage of different clinicopathological presentations to define the same entity, Krenke et al. proposed a new classification of Meigs’ and related syndromes. This classification includes Demons-Meigs’ syndrome, pseudo-Meigs’ syndrome, and incomplete Meigs’ syndrome, based on precise case definitions (4).
Previous studies and our review of the literature suggest that fibroma, including cellular fibroma, is the most frequently associated tumor in the Meigs’ syndrome with elevated CA-125 levels. Abad et al. reviewed 14 cases from 11 reports published between 1989 and 1997, identifying seven cases of fibroma (including cellular fibroma), three cases of fibrothecoma, three cases of thecoma (including luteinized thecoma), and one case of granulosa cell tumor (56). Similarly, Moran-Mendoza et al. analyzed 27 cases from 17 reports published between 1989 and 2004, identifying 18 cases of fibroma (including cellular fibroma), four cases of thecoma (including luteinized thecoma), three cases of fibrothecoma, and one case each for granulosa cell tumor and Brenner tumor (57). Benjapibal et al. reviewed 28 cases from 18 reports published between 1989 and 2007, identifying 19 cases of fibroma (including cellular fibroma), four cases of thecoma (including luteinized thecoma), three cases of fibrothecoma, and one case each for granulosa cell tumor and Brenner tumor (58). Cha et al. analyzed 43 cases from 33 reports published between 1989 and 2012, identifying 22 cases of fibroma (including cellular fibroma), seven cases of fibrothecoma, four cases of thecoma (including luteinized thecoma), three cases of granulosa cell tumor (including juvenile form), two cases of Brenner tumor, and five cases of sclerosing stromal tumor, the latter not meeting the traditional Meigs’ syndrome criteria (59). Although overlapping cases may affect these trends, our literature review from 2015 onward further supports that fibroma is the most commonly associated tumor in Meigs’ syndrome.
Meigs’ syndrome is often accompanied by elevated CA-125 levels, making it difficult to distinguish from advanced ovarian malignancies. However, levels exceeding 1,000 IU/mL are exceptionally rare (23, 57, 60). CA-125 is an antigenic tumor maker commonly expressed in epithelial ovarian malignancies (61). In addition, it is widely expressed in other coelomic epithelia, including the fallopian tubes, peritoneum, pleural, pericardium, colon, kidney, and stomach (61, 62). This broad expression reduces its sensitivity and limits its utility to the detection of early ovarian cancer in postmenopausal women (62). The exact mechanism of CA-125 elevation in Meigs’ syndrome remains unclear, yet a study done by Liou et al. proposed that this elevation is likely due to the mesothelial expression rather than tumor expression, as immunohistochemical staining of the tumor specimens for CA-125 was negative (63). Moreover, studies have explored the correlation between CA-125 levels and disease presentation, but the findings remain controversial. Liu et al. demonstrated that higher levels of CA-125 were positively correlated with the volume of ascites (63). Similarly, Shang et al. reported a significant correlation between serum CA-125 level and the volume of ascites (5). In contrast, Iavarone et al. did not find any correlation between CA-125 levels and any major elements in Meigs’ syndrome (9). Kortekaas et al. also found no correlation between CA-125 levels and ascites; however, the authors suggested a potential link between CA-125 levels and hydrothorax (17). Although our patient presented with massive ascites, pleural effusion, and a markedly high CA-125 level, our literature review did not reveal a correlation between CA-125 levels and the volume of either pleural effusion or ascites.
Several hypotheses have been proposed to explain the formation of ascites and pleural effusion in Meigs’ syndrome. Meigs et al. suggested that ascites form through fluid leakage from the edematous fibroma or tumoral obstruction of intraperitoneal lymphatic drainage (64, 65). Subsequently, pleural effusion develops as the ascitic fluid passes into the pleural cavity through the right-side predominant congenital diaphragmatic defects or through the lymphatic pathways (64). A study demonstrated that vasoactive factors, such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), along with the inflammatory cytokine interleukin-6 (IL-6), were significantly elevated in ascitic fluid compared to serum and pleural fluid, suggesting that tumor-secreted vasoactive factors increase peritoneal vascular permeability and lead to transudation and ascites formation (66). Moreover, these vasoactive factors were found less in pleural fluid, supporting the hypothesis that pleural effusion arises from the secondary passage of ascitic fluid through the diaphragm (66). A study also demonstrated that pleural effusion predominantly occurs on the right side, with the majority of cases being exudative (4). Consistent with previous findings, our literature review revealed that pleural effusion predominantly affects the right hemithorax. In our patient, the nature of the fluid was not determined since fluid cytology revealed atypical cells, and the clinical presentation strongly indicated advanced ovarian malignancy. Studies to date have focused on the nature and location of pleural effusion but have not explored its association with disease progression. In our patient, an initial right-sided pleural effusion progressed to bilateral effusion as the tumor size increased. This observation suggests a potential link between effusion dynamics and tumor growth. Therefore, we carefully propose that the location of pleural effusion may reflect disease progression.
According to the NCCN clinical practice guidelines for ovarian cancer, NACT may be considered for patients who are not good candidates for surgery (67). For instance, NACT is used in patients with poor performance status to reduce tumor burden, improve overall condition, and lower perioperative risk (67). Specifically, NACT with IDS is the primary treatment for patients with advanced-stage malignancy, as well as for those unsuitable for primary debulking surgery due to advanced age, frailty, poor performance status, or comorbidities (67, 68). Considering our patient’s poor performance status, we initiated NACT; however, the tumor continued to grow, rendering the NACT treatment ineffective. We found only one similar case in the literature. Moran-Mendoza et al. diagnosed a patient with advanced epithelial ovarian cancer based on a significantly elevated CA-125 level (1,808 U/mL) and initiated induction chemotherapy due to the patient’s unsuitability for surgery. Despite administering three cycles of paclitaxel and carboplatin, six cycles of vinorelbine, and two cycles of gemcitabine, there was no reduction in the tumor mass, although CA-125 levels significantly declined (57). The authors advised that while false positives are rare in patients with malignant ascites cytology and elevated CA-125 level, chemotherapy should not be initiated unless a definitive cancer diagnosis is made. They also emphasized that even with a high suspicion of ovarian malignancy, a minimally invasive biopsy is crucial when the benign disease is clinically suspected (57). We support these recommendations and further suggest that if a tumor mass fails to respond early during chemotherapy, Meigs’ syndrome should be initially considered, prompting an earlier transition to surgical tumor resection rather than completing multiple cycles of ineffective chemotherapy. A timely shift to surgery in such cases may improve patient outcomes and avoid unnecessary treatment delays.
HE-4 has emerged as a promising biomarker for early detection of ovarian malignancy (69). Studies have reported that HE-4 is minimally expressed in the epithelial tissues of the respiratory and reproductive organs but is significantly overexpressed in ovarian tumors, offering greater reliability than CA-125 (10). Thus, the combination of HE-4 and CA-125 is widely recognized for its improved sensitivity in detecting ovarian malignancies, with elevated levels of both markers strongly suggesting ovarian cancer (10, 70). However, elevated HE-4 levels can also be observed in Meigs’ syndrome. A retrospective study of nine patients with Meigs’ syndrome found that HE-4 levels were significantly higher than those in the ovarian fibrothecoma group but notably lower than those in the ovarian cancer group (5). Surprisingly, two studies have documented the concurrent elevation of both HE-4 and CA-125 levels in Meigs’ syndrome. Danilos et al. reported a HE-4 level of 116.3 pmol/L and a CA-125 level of 2,310 IU/mL (6), while Slaoui et al. reported a HE-4 level of 122.1 pmol/L and a CA-125 level of 1,028 IU/mL (49). To the best of our knowledge, this is the third reported case of concurrent HE-4 and CA-125 elevation in Meigs’ syndrome. Although only three cases are available, including ours, all studies showed CA-125 levels exceeding 1,000 IU/mL, suggesting a possible correlation between CA-125 levels above 1,000 IU/mL and elevated HE-4 levels. Furthermore, our case demonstrates that while elevated HE-4 and CA-125 levels are highly indicative of ovarian malignancy and are extremely rare in Meigs’ syndrome, they may still occur. Hence, despite elevated tumor markers suggesting ovarian malignancy, clinicians must always consider Meigs’ syndrome in the differential diagnosis when an ovarian tumor is accompanied by ascites and pleural effusion to avoid misdiagnosis and unnecessary radical surgery.
Tumor biomarkers play a crucial role in cancer screening, early diagnosis, prognosis assessment, recurrence detection, and monitoring treatment effectiveness (71). Identifying a comprehensive biomarker pool and expanding its clinical utility may pave the way for more effective therapeutic strategies and personalized treatment approaches (72). For instance, new targeted therapies, such as PARP inhibitors, have proven highly effective in destroying ovarian cancer cells and have become an essential part of maintenance treatment (72, 73). To date, no specific biomarkers exist for the accurate preoperative diagnosis of ovarian fibroma or fibrothecoma (74). Therefore, future research on identifying ovarian fibroma biomarkers may improve the accurate diagnosis of Meigs’ syndrome, reducing misdiagnosis and guiding appropriate clinical management.
In conclusion, our findings suggest a potential relationship between the location of pleural effusion and disease progression and suggest that elevated CA-125 levels above 1,000 IU/mL may coincide with elevated HE-4 levels in Meigs’ syndrome. Regardless of tumor marker levels, Meigs’ syndrome should always be considered in the differential diagnosis when an ovarian tumor is present with concurrent ascites and pleural effusion. Furthermore, if the tumor fails to respond to NACT initiated under the suspicion of advanced ovarian malignancy, Meigs’ syndrome should be considered early in the treatment process, prompting a timely shift toward surgical resection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, and further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AR: Conceptualization, Investigation, Supervision, Writing – review & editing. SN: Investigation, Writing – review & editing. KS: Investigation, Writing – review & editing. AJ: Investigation, Writing – review & editing. BB: Investigation, Writing – review & editing. SB: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1533388/full#supplementary-material
SUPPLEMENTARY TABLE 1 | Summary of the clinical characteristics of 49 patients diagnosed with Meigs’ syndrome with elevated CA-125 levels.
References
1. Mohammed, SA, Kumar, A, and Cue, L. Meigs Syndrome. Statpearls. Treasure Island, FL: StatPearls Publishing (2024).
2. Saha, S, and Robertson, M. Meigs' and Pseudo-Meigs' Syndrome. Australas J Ultrasound Med. (2012) 15:29–31. doi: 10.1002/j.2205-0140.2012.tb00140.x
3. Meigs, JV. Fibroma of the ovary with ascites and hydrothorax. Meigs' Syndrome. Am J Obstet Gynecol. (1954) 67:962–87. doi: 10.1016/0002-9378(54)90258-6
4. Krenke, R, Maskey-Warzechowska, M, Korczynski, P, Zielinska-Krawczyk, M, Klimiuk, J, Chazan, R, et al. Pleural effusion in Meigs' syndrome-transudate or exudate?: systematic review of the literature. Medicine. (2015) 94:e2114. doi: 10.1097/MD.0000000000002114
5. Shang, W, Wu, L, Xu, R, Chen, X, Yao, S, Huang, P, et al. Clinical laboratory features of Meigs' syndrome: a retrospective study from 2009 to 2018. Front Med. (2021) 15:116–24. doi: 10.1007/s11684-019-0732-6
6. Danilos, J, Michal Kwasniewski, W, Mazurek, D, Bednarek, W, and Kotarski, J. Meigs' syndrome with elevated ca-125 and he-4: a case of luteinized Fibrothecoma. Prz Menopauzalny. (2015) 14:152–4. doi: 10.5114/pm.2015.52157
7. Yuan, L, Cui, L, Wang, J, and Gong, L. A case report of Meigs' syndrome caused by ovarian Fibrothecoma with high levels of Ca125. Int J Women's Health. (2024) 16:519–25. doi: 10.2147/IJWH.S450833
8. Porcel, JM, Diaz, JP, and Chi, DS. Clinical implications of pleural effusions in ovarian Cancer. Respirology. (2012) 17:1060–7. doi: 10.1111/j.1440-1843.2012.02177.x
9. Iavarone, I, Padovano, M, Pasanisi, F, Della Corte, L, La Mantia, E, and Ronsini, C. Meigs syndrome and elevated ca-125: case report and literature review of an unusual presentation mimicking ovarian Cancer. Medicina. (2023) 59:59. doi: 10.3390/medicina59091684
10. Dochez, V, Caillon, H, Vaucel, E, Dimet, J, Winer, N, and Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian Cancer: Ca125, He4, Rmi and Roma, a review. J Ovarian Res. (2019) 12:28. doi: 10.1186/s13048-019-0503-7
11. Brillantino, C, Errico, ME, Minelli, R, Esposito, F, Pirisi, P, Gaglione, G, et al. Early diagnosis of Meigs syndrome in children a case report and a review of the literature. Ann Ital Chir. (2022) 11:S223.
12. Dellaportas, D, Kollia, D, Myoteri, D, Nastos, C, Gkiokas, G, Carvounis, E, et al. Giant ovarian Thecoma associated with Meigs syndrome: a striking case. Chirurgia. (2021) 116:1–5. doi: 10.21614/chirurgia.116.eC.1912
13. Fremed, MA, Levin, TL, Sun, KY, and Weiser, DA. Ovary preservation in the treatment of childhood Meigs syndrome. Pediatr Blood Cancer. (2015) 62:2011–4. doi: 10.1002/pbc.25586
14. Guelzim, Y, Bennasser, A, Marrakchi, S, Houssaini, AS, Idoubba, S, Boujida, I, et al. Demons-Meigs syndrome caused by a Giant ovarian fibroma: a case report. Radiol Case Rep. (2024) 19:2585–9. doi: 10.1016/j.radcr.2024.03.016
15. Hogan, ME, and Andreotti, R. Ovarian fibroma presenting with Meigs syndrome. Ultrasound Q. (2015) 31:215–8. doi: 10.1097/RUQ.0000000000000144
16. Hou, YY, Peng, L, and Zhou, M. Meigs syndrome with pleural effusion as initial manifestation: a case report. World J Clin Cases. (2021) 9:5972–9. doi: 10.12998/wjcc.v9.i21.5972
17. Kortekaas, KE, and Pelikan, HM. Hydrothorax, ascites and an abdominal mass: not always signs of a malignancy - three cases of Meigs' syndrome. J Radiol Case Rep. (2018) 12:17–26. doi: 10.3941/jrcr.v12i1.3209
18. Li, H, Guo, G, Wang, Z, Lang, B, Zhang, Y, and Li, R. Anesthesia Management of a Patient with Meigs' syndrome: a case report and literature review. Int J Surg Case Rep. (2023) 110:108660. doi: 10.1016/j.ijscr.2023.108660
19. Murayama, Y, Kamoi, Y, Yamamoto, H, Isogai, J, and Tanaka, T. Meigs' syndrome mimicking heart failure with preserved ejection fraction: a case report. BMC Cardiovasc Disord. (2020) 20:436. doi: 10.1186/s12872-020-01718-4
20. Navarro-Esteva, J, Laseca-Modrago, M, and Arencibia-Sanchez, O. Two patients with Meigs' syndrome and elevated serum ca-125: a case report. Cureus. (2020) 12:e8927. doi: 10.7759/cureus.8927
21. Ng, BH, Johari How, SH, Abeed, NNN, Low, HJ, Osman, RA, and Yu-Lin, AB. Meigs syndrome presenting with recurrent unilateral pleural effusion. Respirol Case Rep. (2024) 12:e01421. doi: 10.1002/rcr2.1421
22. Okuda, K, Noguchi, S, Narumoto, O, Ikemura, M, Yamauchi, Y, Tanaka, G, et al. A case of Meigs' syndrome with preceding pericardial effusion in advance of pleural effusion. BMC Pulm Med. (2016) 16:71. doi: 10.1186/s12890-016-0241-1
23. Palmieri, A, ElSahwi, K, and Hicks, V. Meigs syndrome presenting with severely elevated ca-125 level. BMJ Case Rep. (2021) 14:14. doi: 10.1136/bcr-2020-238931
24. Park, JW, and Bae, JW. Postmenopausal Meigs' syndrome in elevated ca-125: a case report. J Menopausal Med. (2015) 21:56–9. doi: 10.6118/jmm.2015.21.1.56
25. Sofoudis, C, Kouiroukidou, P, Louis, K, Karasaridou, K, Toutounas, K, Gerolymatos, A, et al. Enormous ovarian fibroma with elevated ca-125 associated with Meigs' syndrome. Presentation of a rare case. Eur J Gynaecol Oncol. (2016) 37:142–3.
26. Tan, N, Jin, KY, Yang, XR, Li, CF, Yao, J, and Zheng, H. A case of death of patient with ovarian fibroma combined with Meigs syndrome and literature review. Diagn Pathol. (2022) 17:83. doi: 10.1186/s13000-022-01258-9
27. Tanaka, M, Yamanoi, K, Kitamura, S, Horikawa, N, Chigusa, Y, Horie, A, et al. A 36 kg Giant ovarian fibroma with Meigs syndrome: a case report and literature review of extremely Giant ovarian tumor. Case Rep Obstet Gynecol. (2021) 2021:1076855. doi: 10.1155/2021/1076855
28. Toumi, D, Mnejja, A, Ghaddab, I, Bergaoui, H, Chaouch, MA, and Zoukar, O. Challenging diagnoses in a case report: ovarian Fibrothecoma with elevated Ca125 levels mimicking malignancy. Int J Surg Case Rep. (2024) 120:109847. doi: 10.1016/j.ijscr.2024.109847
29. Upreti, D, Rohita, DK, Yadav, SK, and Thapa, N. Case report: ovarian fibroma: typical presentation with Meigs's syndrome. F1000Res. (2022) 11:815. doi: 10.12688/f1000research.122368.1
30. Wu, XJ, Xia, HB, Jia, BL, Yan, GW, Luo, W, Zhao, Y, et al. Meigs' syndrome caused by granulosa cell tumor accompanied with intrathoracic lesions: a case report. World J Clin Cases. (2021) 9:4734–40. doi: 10.12998/wjcc.v9.i18.4734
31. Yang, ST, Cheng, M, Lai, CR, Shen, SH, Lee, WL, and Wang, PH. Meigs' syndrome and adult-type granulosa cell tumor. Taiwan J Obstet Gynecol. (2021) 60:1116–20. doi: 10.1016/j.tjog.2021.09.028
32. Ziruma, A, Hukuimwe, M, Nyakura, M, Majangara, R, and Venge, M. A rare case of Meigs syndrome in pregnancy. Pan Afr Med J. (2019) 33:36. doi: 10.11604/pamj.2019.33.36.18653
33. Choi, HY, and Park, JW. Meigs syndrome secondary to adult granulosa cell tumour with elevated Ca125 levels: a case report. Ital J Gynaecol Obstet. (2023) 35:329–33. doi: 10.36129/jog.2022.65
34. Dumitrascu, MC, Peteu, C, Sandru, F, Nenciu, CG, Iliesiu, A, Simion, G, et al. Meigs syndrome in ovarian Fibrothecoma with elevated ca-125 - a case report. J Surg Sci. (2021) 8:68–72.
35. Elisabeth, RH, Joelle, RV, Nalisoa, RF, Lucas, RZ, Ranaliarinosy, RM, and Soa, RN. Demons-Meigs syndrome with a high ca 125 level: a case report at Soavinandriana center hospital Antananarivo. Open J Pathol. (2023) 13:73–8. doi: 10.4236/ojpathology.2023.132007
36. Frezgi, O, Tesfai, B, Tesfamichael, T, Hussein, K, Gebremariam, H, and Wahaballa, YD. Meigs syndrome in a postmenopausal woman: case report and review of literature, 2023. Res J Onco. (2023) 7:11. doi: 10.36648/iprjo.7.2.11
37. Ghani, T, Sultana, N, Hussain, T, Begum, A, Paul, SK, Noorjahan, K, et al. Meigs' syndrome associated with elevated ca 125 level-a rare case. J Dhaka Med Coll. (2015) 24:76–8. doi: 10.3329/jdmc.v24i1.29569
38. Goncalves, AL, and Blanco, M. Meigs syndrome with elevated ca 125: case report. Acta Obstet Ginecol Port. (2019) 13:113–5.
39. Huang, ST, Peng, YJ, and Yu, MH. Meigs syndrome caused by Fibrothecoma of the ovary. J Med Sci. (2023) 43:136–9. doi: 10.4103/jmedsci.jmedsci_82_22
40. Ikouch, K, Wajih, O, Tossi, S, Gourja, Y, Boufettal, H, Mahdaoui, S, et al. Demons Meigs syndrome: about two cases. Gynecol Reprod Health. (2023) 7:1–4. doi: 10.33425/2639-9342.1226
41. Khanduja, D, and Kajal, NC. A case report on Meigs' syndrome and elevated serum ca-125: a rare case report. J Pulmonol Respir Res. (2021) 5:031–3. doi: 10.29328/journal.jprr.1001021
42. Kocak, O, Tasci, T, Turkmen, O, Yirci, B, Karalok, A, Ureyen, I, et al. Meig's syndromes with extremely high Ca125 level mimicking advanced ovarian Cancer. Gynecol Obstet Reprod Med. (2016) 22:42–4. doi: 10.21613/GORM.2016.111
43. Maqbool, A, Jesmin, S, and Arif, I. Meigs' syndrome with an elevated Ca125 in a 15-year-old girl. Gynecol Reprod Health. (2021) 5:1–6. doi: 10.33425/2639-9342.1172
44. Mihimit, A, Toure, AI, Oumar, OA, Camara, B, Keita, A, Bah, M, et al. Demons-Meigs syndrome: a case report at the Fatick regional hospital. Open J Obstet Gynecol. (2021) 11:1735–43. doi: 10.4236/ojog.2021.1112162
45. Mirji, S, Desai, A, Patel, B, Patel, S, and Rajpurohit, P. A rare case of Meigs syndrome in pregnancy. Indian J Gynecol Oncol. (2019) 17:37. doi: 10.1007/s40944-019-0277-2
46. Mitsuboshi, S, Matsumoto, T, Isaka, T, Kumakiri, J, Yamamoto, T, and Kanzaki, M. A case unexplained pleural effusion diagnosed as Meigs' syndrome. Tokyo Women's Med Univ J. (2019) 3:69–72.
47. Park, HN, Kim, SG, and Kim, YS. Meigs' syndrome with elevated Cancer antigen 125. Soonchunhyang Med Sci. (2016) 22:129–31. doi: 10.15746/sms.16.029
48. Pinzon, CLA, Ugarte, DH, Herevia, AEJ, and Guerrero, MA. Meigs syndrome: literature review of a case report. Int J Res Med Sci. (2020) 8:3706–9. doi: 10.18203/2320-6012.ijrms20204256
49. Slaoui, A, Lazhar, H, Amail, N, Zeraidi, N, Lakhdar, A, Kharbach, A, et al. Meigs syndrome: about an uncommon case report. Clin J Obstet Gynecol. (2023) 6:010–3. doi: 10.29328/journal.cjog.1001120
50. Tabassum, S. Meigs' syndrome: a Fibrothecoma presenting with torsion. Bahrain Med Bull. (2020) 42:203–5.
51. Tsai, WC, Chang, FW, Chang, JL, and Chao, HM. Meigs' syndrome in an elderly woman with short of breath. J Med Sci. (2015) 35:125–7. doi: 10.4103/1011-4564.158688
52. Zeng, X, and Xi, MR. Simultaneously with elevated tumor marker and hydrothorax in Meigs' syndrome: a new clinical manifestation. Int J Clin Exp Pathol. (2016) 9:9592–6.
53. Habek, D, Cenkovcan, M, Jandric, D, Stanic, G, and Habek, JC. Ovarian hemangioma with Meigs' syndrome. Eur J Obstet Gynecol Reprod Biol. (2018) 229:207–8. doi: 10.1016/j.ejogrb.2018.09.003
54. Hatoum, S, Jarjoura, P, Saade, C, and Naffaa, L. Sclerosing stromal tumor of the ovary presenting as Meigs syndrome during childhood. Cureus. (2022) 14:e31562. doi: 10.7759/cureus.31562
55. Campos, JS, Salazar, VHM, Arias, AL, Gomez, MDPM, and Leon, DCD. Sertoli-Leydig tumor and Meigs' syndrome, an infrequent association-a case report. J Surg. (2016) 12:65–9. doi: 10.7438/1584-9341-12-2-5
56. Abad, A, Cazorla, E, Ruiz, F, Aznar, I, Asins, E, and Llixiona, J. Meigs' syndrome with elevated Ca125: case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. (1999) 82:97–9. doi: 10.1016/s0301-2115(98)00174-2
57. Moran-Mendoza, A, Alvarado-Luna, G, Calderillo-Ruiz, G, Serrano-Olvera, A, Lopez-Graniel, CM, and Gallardo-Rincon, D. Elevated Ca125 level associated with Meigs' syndrome: case report and review of the literature. Int J Gynecol Cancer. (2006) 16 Suppl 1:315–8. doi: 10.1111/j.1525-1438.2006.00228.x
58. Benjapibal, M, Sangkarat, S, Laiwejpithaya, S, Viriyapak, B, Chaopotong, P, and Jaishuen, A. Meigs' syndrome with elevated serum Ca125: case report and review of the literature. Case Rep Oncol. (2009) 2:61–6. doi: 10.1159/000210441
59. Cha, MY, Roh, HJ, You, SK, Lee, SH, Cho, HJ, and Kwon, YS. Meigs' syndrome with elevated serum ca 125 level in a case of ovarian Fibrothecoma. Eur J Gynaecol Oncol. (2014) 35:734–7.
60. Ushida, S, Ichigo, S, Takagi, H, Matsunami, K, Ito, Y, and Imai, A. Meigs syndrome with highly elevated Ca125. Biomed J Sci Tech Res. (2022) 46:37217–20. doi: 10.26717/BJSTR.2022.46.007313
61. Charkhchi, P, Cybulski, C, Gronwald, J, Wong, FO, Narod, SA, and Akbari, MR. Ca125 and ovarian Cancer: a comprehensive review. Cancers. (2020) 12:12. doi: 10.3390/cancers12123730
62. Gandhi, T, Zubair, M, and Bhatt, H. Cancer antigen 125. Statpearls. Treasure Island (FL): StatPearls Publishing (2024).
63. Liou, JH, Su, TC, and Hsu, JC. Meigs' syndrome with elevated serum Cancer antigen 125 levels in a case of ovarian Sclerosing stromal tumor. Taiwan J Obstet Gynecol. (2011) 50:196–200. doi: 10.1016/j.tjog.2011.01.011
64. Riker, D, and Goba, D. Ovarian mass, pleural effusion, and ascites: revisiting Meigs syndrome. J Bronchology Interv Pulmonol. (2013) 20:48–51. doi: 10.1097/LBR.0b013e31827ccb35
65. Meigs, JV, Armstrong, SH, and Hamilton, HH. A further contribution to the syndrome of fibroma of the ovary with fluid in the abdomen and chest, Meigs' syndrome. Am J Obstet Gynecol. (1943) 46:19–37. doi: 10.1016/S0002-9378(16)40440-0
66. Abramov, Y, Anteby, SO, Fasouliotis, SJ, and Barak, V. Markedly elevated levels of vascular endothelial growth factor, fibroblast growth factor, and interleukin 6 in Meigs syndrome. Am J Obstet Gynecol. (2001) 184:354–5. doi: 10.1067/mob.2001.110028
67. National Comprehensive Cancer Network. (2024). NCCN clinical practice guidelines in oncology: Ovarian Cancer including fallopian tube Cancer and primary peritoneal Cancer. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (Accessed October 28, 2024)
68. Sato, S, and Itamochi, H. Neoadjuvant chemotherapy in advanced ovarian Cancer: latest results and place in therapy. Ther Adv Med Oncol. (2014) 6:293–304. doi: 10.1177/1758834014544891
69. Anastasi, E, Farina, A, Granato, T, Colaiacovo, F, Pucci, B, Tartaglione, S, et al. Recent insight about He4 role in ovarian Cancer Oncogenesis. Int J Mol Sci. (2023) 24:24. doi: 10.3390/ijms241310479
70. van, T, Cadron, I, Despierre, E, Daemen, A, Leunen, K, Amant, F, et al. He4 and Ca125 as a diagnostic test in ovarian Cancer: prospective validation of the risk of ovarian malignancy algorithm. Br J Cancer. (2011) 104:863–70. doi: 10.1038/sj.bjc.6606092
71. Zhou, Y, Tao, L, Qiu, J, Xu, J, Yang, X, Zhang, Y, et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther. (2024) 9:132. doi: 10.1038/s41392-024-01823-2
72. Tonti, N, Golia D'Auge, T, Cuccu, I, De Angelis, E, D'Oria, O, Perniola, G, et al. The role of tumor biomarkers in tailoring the approach to advanced ovarian Cancer. Int J Mol Sci. (2024) 25:25. doi: 10.3390/ijms252011239
73. Lopez-Portugues, C, Montes-Bayon, M, and Diez, P. Biomarkers in ovarian Cancer: towards personalized medicine. Proteomes. (2024) 12:12. doi: 10.3390/proteomes12010008
Keywords: Meigs’ syndrome, CA-125, HE-4, fibroma, pleural effusion, ascites
Citation: Seong J, Ravshan A, Narkhodzha S, Saida K, Jamshid A, Bahriddin B and Biloliddin S (2025) Meigs’ syndrome with elevated CA-125 and HE-4: a case report and literature review. Front. Med. 12:1533388. doi: 10.3389/fmed.2025.1533388
Edited by:
Cristina Secosan, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Ilaria Cuccu, Sapienza University of Rome, ItalyXiaowei Zhang, Wenzhou Medical University, China
Copyright © 2025 Seong, Ravshan, Narkhodzha, Saida, Jamshid, Bahriddin and Biloliddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdusattorov Ravshan, Y2F1LnJhdnNoYW5Ab3V0bG9vay5jb20=; Sharobidinov Biloliddin, c2hhcm9iaWRkaW5vdmIyMDIxQGdtYWlsLmNvbQ==
 Jichang Seong
Jichang Seong Abdusattorov Ravshan2*
Abdusattorov Ravshan2*