
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Med. , 26 February 2025
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1531370
 Yuewei Li1†
Yuewei Li1† Yihe Chi1†
Yihe Chi1† Mengting Zhu1†
Mengting Zhu1† Feiting Fan2
Feiting Fan2 Zhongyang Deng3
Zhongyang Deng3 Jingmin Xiao2
Jingmin Xiao2 Shaohan Jin2
Shaohan Jin2 Luoqi Lin2
Luoqi Lin2 Xiaochun Chen2
Xiaochun Chen2 Ruhong Xu3
Ruhong Xu3 Long Fan2
Long Fan2 Xuhua Yu2
Xuhua Yu2 Ziyao Liang2
Ziyao Liang2 Jingyu Quan2
Jingyu Quan2 Shangzhao Li2
Shangzhao Li2 Xinying Peng3
Xinying Peng3 Yuanbin Chen1,2,4*†
Yuanbin Chen1,2,4*† Lin Lin1,2,4*†
Lin Lin1,2,4*† Lei Wu1,2,4*†
Lei Wu1,2,4*†Background: Traditional Chinese medicine has been used for Coronavirus disease 2019 (COVID-19) as a therapeutic option. Lianhuaqingwen capsules (LHQW) are well-recognized for their efficacy, while Kegan Liyan oral liquid (KGLY), widely used for influenza treatment, has emerged as a promising candidate for COVID-19 therapy. This trial aims to assess whether KGLY is non-inferior to LHQW in treating mild COVID-19.
Methods: A total of 127 participants (63 in KGLY group and 64 in LHQW group) were randomly allocated to receive either KGLY therapy or LHQW therapy in a 1:1 ratio. The treatment was given for 7 days, and the follow-up period was 3 days.
Outcome measures: The primary outcome was symptom remission at day 10. Secondary outcomes included symptom recovery, time to symptom remission, recovery rates and time to recovery of selected symptoms, change in visual analog scale score for selected symptoms, area under the curve of the visual analog scale score for sore throat, negative conversion of the SARS-CoV-2 infection, having a positive test result after negative conversion, and incidence of pneumonia.
Results: Full analysis set analysis showed that the symptom remission rate at day 10 was 60.7% with KGLY and 58.3% in LHQW (difference + 2.3 p.p., lower limit of 95% confidence interval − 14.8 p.p.), indicating non-inferiority. There were no significant differences between the groups for any secondary outcome. The occurrence of adverse events did not differ between the groups and no severe adverse events were documented in either group.
Conclusion: Based on the study results, this trial proved that KGLY was non-inferior to LHQW for mild COVID-19, providing a promising option for COVID-19 treatment.
Clinical trial registration: https://www.chictr.org.cn/showproj.html?proj=166372, Identifier, [ChiCTR2200059105].
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread over the world since the first cases that were observed in December 2019. In 2022, the Omicron variant rapidly surpassed other circulating strains around the world. Mild influenza-like symptoms including fever, sore throat, cough, fatigue and myalgia were reported to be the prevalent symptoms of Omicron (1). As of May 2024, the number of COVID-19 cases keeps on rising, with 775 million reported cases and 7.0 million deaths (2). Owing to the high transmissibility and the possibility of complications and lingering after-effects, the demand for timely interventions was prominent.
Traditional Chinese medicine (TCM) has been applied to treat pandemics for thousands of years and has shown potential efficacy against COVID-19 (3). For example, Lianhuaqingwen capsules (LHQW), a widely used Chinese herbal product, have been shown to significantly mitigate symptoms and accelerate clinical recovery in COVID-19 patients (4–8), and are recommended in the Chinese guideline for the diagnosis and treatment of COVID-19 (Trial 10th edition, 2023) (9). However, to date, high-quality evidence-based evaluations of TCM treatments are still few, the scarcity is particularly notable in the context of the need for more stringent controlled trials to substantiate the claims of TCM’s efficacy. This gap hinders the broader recognition and integration of TCM into mainstream COVID-19 treatment strategies. Hence, the search for more TCM therapeutic options against COVID-19 remains an important research goal.
Kegan Liyan oral liquid (KGLY), acting by exerting heat-clearing and dampness-resolving effects, has been used extensively for the treatment of influenza. Multiple clinical trials have demonstrated that KGLY exerts beneficial effects in patients with various respiratory diseases including influenza, chronic pharyngitis, acute upper respiratory tract infection, and acute suppurative tonsillitis (10–12). Based on this evidence, KGLY could be a promising candidate for COVID-19 treatment. This therapy is also recommended by the expert consensus on Chinese patent medicine and TCM treatment for COVID-19 in Guangdong Province (13, 14).
However, the efficacy of KGLY against COVID-19 has not been proven yet. Therefore, in this study, we conducted a double-blinded, randomized, controlled, non-inferiority trial design to determine the efficacy of KGLY using the well-established LHQW as a positive control, to provide objective and scientific evidence for the use of KGLY for COVID-19.
A double-blinded, double-dummy, randomized, controlled, non-inferiority clinical trial was conducted at two public hospitals in China (The Ninth People’s Hospital of Dongguan, and the Guangdong Provincial Hospital of Chinese Medicine). Before commencing recruitment, the participating centers had acquired ethics approval. The trial was registered in April 2022 (Chinese Clinical Trial Registry: No.ChiCTR2200059105) and was conducted strictly according to the Consolidated Standards of Reporting Trials (CONSORT) Statement. All participants signed an informed consent.
We recruited all patients who were treated in the two participating hospitals between July 2022 and January 2023. Patients fulfilling all of the following criteria were eligible: (1) aged 18 to 75 years; (2) having a laboratory-confirmed diagnosis of mild COVID-19 in accordance with the guideline on diagnosis and treatment of COVID-19 (Trial 9th edition) (15); (3) meeting the diagnostic criteria of the Dampness-heat in the Lung TCM syndrome (15); (4) body temperature ≤ 38.5°C since the onset of illness; (5) having symptoms (either fever, sore throat, cough, myalgia, or fatigue); and (6) the patient voluntarily signed the informed consent.
We excluded patients who met at least one of the following criteria: (1) having a chronic respiratory disease such as chronic obstructive pulmonary disease, bronchial asthma, bronchiectasis, active pulmonary tuberculosis, lung malignant tumors, or interstitial lung disease; (2) having severe comorbidities of the cardio-cerebrovascular, renal, hepatic, or blood system, malignant tumors, or other serious primary diseases, or alanine aminotransferase, aspartate aminotransferase or serum creatinine levels that exceeded 1.5 times the upper limit of normal; (3) due to neurological and mental disease (based on medical history), unable to cooperate; (4) having allergies or hypersensitivity for any research drug component; (5) having peptic ulcer or digestive hemorrhage; (6) being pregnant, lactating, or preparing for pregnancy; or (7) having received any experimental treatment within the previous 3 months.
A verified interactive web response method was used for randomization by an independent third-party unit, the Key Unit of Methodology in Clinical Research (KUMCR) of Guangdong Provincial Hospital of Chinese Medicine. SAS version 9.4 (SAS Institute Inc., Cary, United States) was used for all randomization processes. Patients were randomly assigned to KGLY or LHQW arms in a 1:1 ratio using a randomized block design.
KUMCR staff had access to the randomization list and blinding codes, which were kept strictly confidential. As a result, during the trial, all participants and outcome evaluators were not aware of the treatment allocation.
Enrolled patients were randomly split into the KGLY and LHQW groups at a ratio of 1:1. In the KGLY group, 20 mL of KGLY and four capsules of LHQW-like placebo were administered three times a day orally, for 7 days. In the LHQW group, patients were given 20 mL of KGLY-like placebo and four capsules of LHQW three times a day orally for 7 days. Patients were instructed to bring the remaining medication or medicine box back after treatment for medication counting. Patients who recovered were asked to discontinue treatment ahead of time.
KGLY (National Drug Approval Z10970100), LHQW (National Drug Approval Z20040063) and the placebo were all provided by Wanglaoji Pharmaceutical Co., Ltd. (Guangzhou, China). Drugs were all examined in advance through the standard procedure, which met the requirements of Chinese Pharmacopoeia (2020 edition). Tables 1–3 display the ingredients of KGLY, LHQW and the placebo. In terms of appearance, taste and smell, the placebo matched the research products.
The reliever drug, paracetamol tablet, was manufactured by Sinopharm Shantou Jinshi Pharmaceutical Co., Ltd. (Shantou, China, National Drug Approval H44021051). Patients were allowed to take the reliever medication when suffering from pain or having subaxillary temperature ≥ 38.5°C.
The use of additional medications for COVID-19, such as antitussive, antiviral and phlegm-resolving drugs, and Chinese medicine for influenza and common cold, was restricted during the trial. Subjects were excluded from the trial if discontinuing these medications was deemed clinically harmful or if patients were unwilling to do so. Except for the medications indicated above, therapy for underlying conditions such as hypertension could remain unaltered.
The data were obtained through a primary research method. Investigator trainings were conducted prior to the trial according to developed standard operating procedures. Following enrollment, participants were instructed to note on their diary records each day their body temperature, symptom visual analog scale (VAS) score and the total amount and frequency of paracetamol tablet consumption. The VAS was a 10 cm visual horizontal line, where patients scored the severity of their symptoms (sore throat, cough, myalgia and fatigue) from 0 to 10 (0 = no symptom, 10 = worst possible symptom). During the study period, diary records were regularly verified by investigators to ensure that data were filled in correctly. For antiviral efficacy, throat swab samples were collected for SARS-CoV-2 RNA testing at baseline, day 3, day 7, and day 10. In addition, routine blood, renal and hepatic function testing was performed at baseline as well as day 7. Throughout the study, adverse events and concomitant drugs were monitored and documented. We considered the following adverse events: (1) new onset of a disease or symptoms or signs, or a clinically significant progression and deterioration of concomitant diseases; or (2) adverse events related to clinical laboratory tests and other examinations. The severity of adverse events was divided into three categories-mild, moderate, and severe-according to the Common Terminology Criteria for Adverse Events (16).
The primary outcome was symptom remission at 10 days since treatment initiation. Remission of symptoms was defined as having no fever, as well as all of the VAS scores of sore throat, cough, myalgia and fatigue having stayed between 0 and 1 cm for more than 48 h (VAS was a scale ranged from 0 to 10 cm). Fever was defined as a subaxillary temperature ≥ 37.3°C. The magnitude of sore throat, cough, myalgia and fatigue was reported by patients in their diaries using VAS scores.
Secondary outcomes included: (1) symptom recovery (recovery of symptoms was defined as having no fever, as well as all of the VAS scores of sore throat, cough, myalgia and fatigue having stayed at 0 cm for more than 48 h); (2) time to symptom remission, defined as the time span between the initial intervention and symptom remission; (3) recovery of selected symptoms (fever, sore throat, cough, myalgia and fatigue); (4) time to recovery of these symptoms; (5) change in VAS score of these symptoms; (6) the area under the curve (AUC) of the VAS score for sore throat; (7) SARS-CoV-2 RNA negative conversion; (8) a positive SARS-CoV-2 RNA test result after negative conversion; and (9) incidence of pneumonia.
The sample size was calculated using the PASS 15 software. In line with a prior study (4), the symptom recovery rate of LHQW for COVID-19 at day 10 was expected to be 78% and that in the control group 45%, with a 95% CI 22 to 43%. For the reason that rate of symptom remission must be higher than that of symptom recovery in the same period, we assumed 89% of participants achieve the condition of symptom remission at day 10. The non-inferiority margin was set at −17 p.p., the significance threshold at 0.025, and power at 80% (β = 0.2), implying that each arm needed at least 54 cases to confirm non-inferiority. With a 10% dropout rate, this corresponds to a sample size of 120 patients (60 subjects per group).
All data were independently entered into EpiData software by two personnel and cross-checked. Differences were carefully corrected by consulting the original report forms to minimize input errors. For statistical analysis, IBM SPSS software (version 26.0) was used. To impute missing data, the last observation carried forward (LOCF) approach was utilized.
We conducted the analyses in three datasets, including the full analysis set (FAS), the per protocol set (PPS) and the safety set (SS). We presented continuous variables using means (with standard deviation, SD) or median (with interquartile range, IQR) and categorical variables using frequencies (n) and percentages (%). The primary outcome (symptom remission rate) of KGLY and LHQW arms was compared by Chi-squared test or Fisher’s exact test. Non-inferiority test was employed for the primary endpoint. The outcomes of time to symptom remission and recovery of selected symptoms were analyzed using log-rank test and Cox regression. The t-test (for normally or almost normally distributed data) or the Wilcoxon rank-sum test (for severely skewed variables) were used to compare continuous outcomes (AUC of the VAS score for sore throat and VAS score for sore throat, cough, myalgia and fatigue) between arms. Binary outcomes (symptom recovery, recovery of selected symptoms, SARS-CoV-2 RNA negative conversion, positive test result after negative conversion, and incidence of pneumonia) were analyzed using the Chi-square test or Fisher’s exact test. For paired samples, paired t-test was used for normally or almost normally distributed data, or the Wilcoxon signed-rank test for severely skewed data. A one-tailed test was applied only for the non-inferiority test of the primary outcome, and all other tests were two-tailed. Statistical significance was set at 0.05.
From July 2022 to January 2023, 127 participants were randomly allocated to KGLY group (n = 63) or LHQW group (n = 64). Finally, 121 participants (61 in KGLY arm and 60 in LHQW arm) qualified for FAS; Six participants (2 in KGLY and 4 in LHQW groups) were excluded from the FAS due to not meeting the inclusion criteria or having no available data for analysis. Furthermore, 111 participants (55 in KGLY and 56 in LHQW groups) qualified for PPS, with 16 of the allocated participants (8 in each group) excluded (Figure 1). During the study period, seven participants dropped out (4 in KGLY and 3 in LHQW groups, p = 0.68). Baseline demographic and clinical characteristics were in balance between the groups. The median age was 34 years, and 72 (59.5%) participants were female. The median time since the onset of illness at baseline was 3 days in both groups (IQR 2–4). Cough (n = 113, 93.4%) and sore throat (n = 112, 92.6%) were the most common symptoms (Table 4).
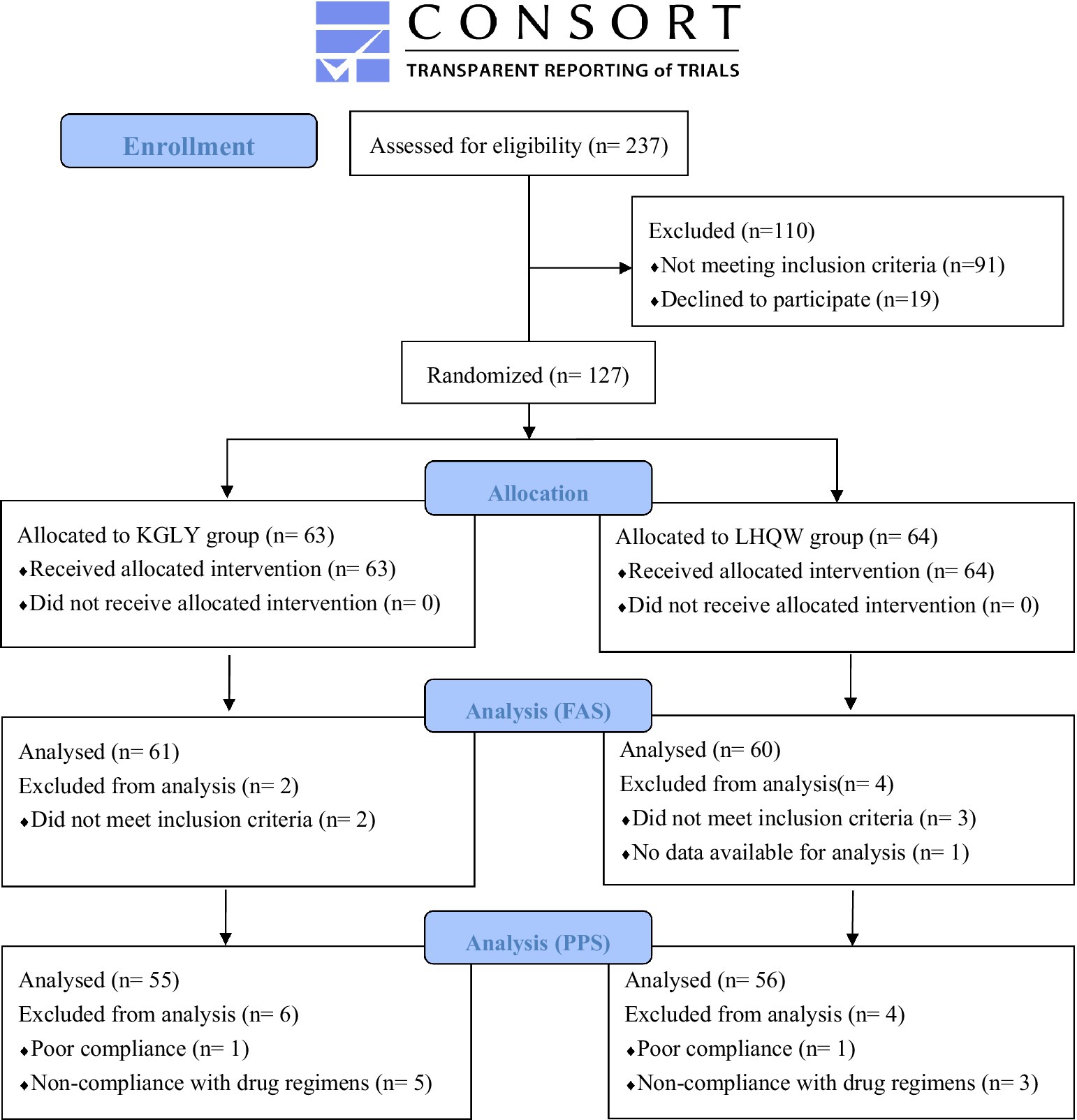
Figure 1. Consolidated standards of reporting trials (CONSORT) flow diagram. KGLY, Kegan Liyan oral liquid; LHQW, Lianhuaqingwen capsules; FAS, full analysis set; PPS, per protocol set.
In the FAS analysis, at day 10, symptom remission rate was 60.7% in the KGLY arm and 58.3% in the LHQW arm: the difference was +2.3 p.p. and lower limit of the 95% CI -14.8 p.p., indicating non-inferiority (Figures 2A, 3A). In the PPS analysis, symptom remission rate was 65.5% in KGLY and 60.7% in LHQW groups, also validating the non-inferiority of KGLY (difference + 4.7 p.p., lower limit of the 95% CI -12.9 p.p.; Figures 2B, 3B).
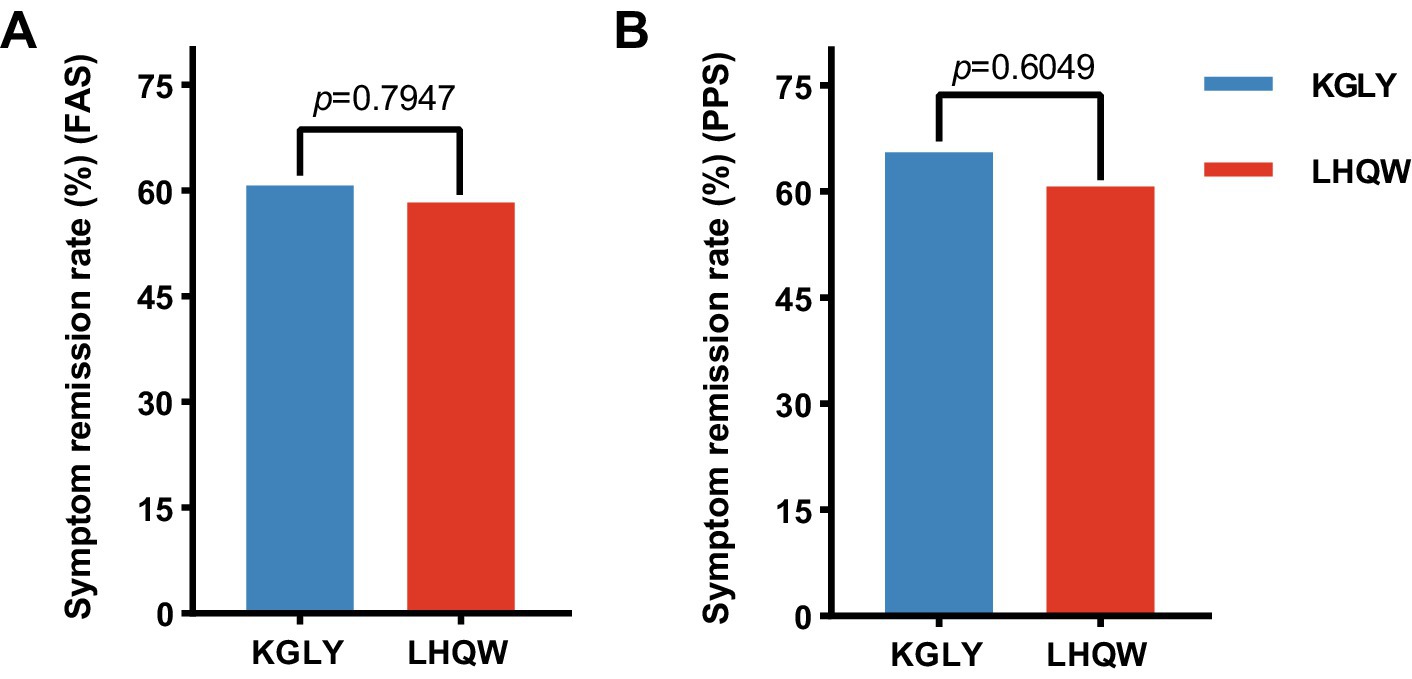
Figure 2. Symptom remission rate at day 10 in the KGLY and LHQW groups. Remission of symptoms was defined as having no fever, as well as all visual analog scale scores (sore throat, cough, myalgia and fatigue) staying between 0 and 1 cm for more than 48 h. KGLY, Kegan Liyan oral liquid; LHQW, Lianhuaqingwen capsules.
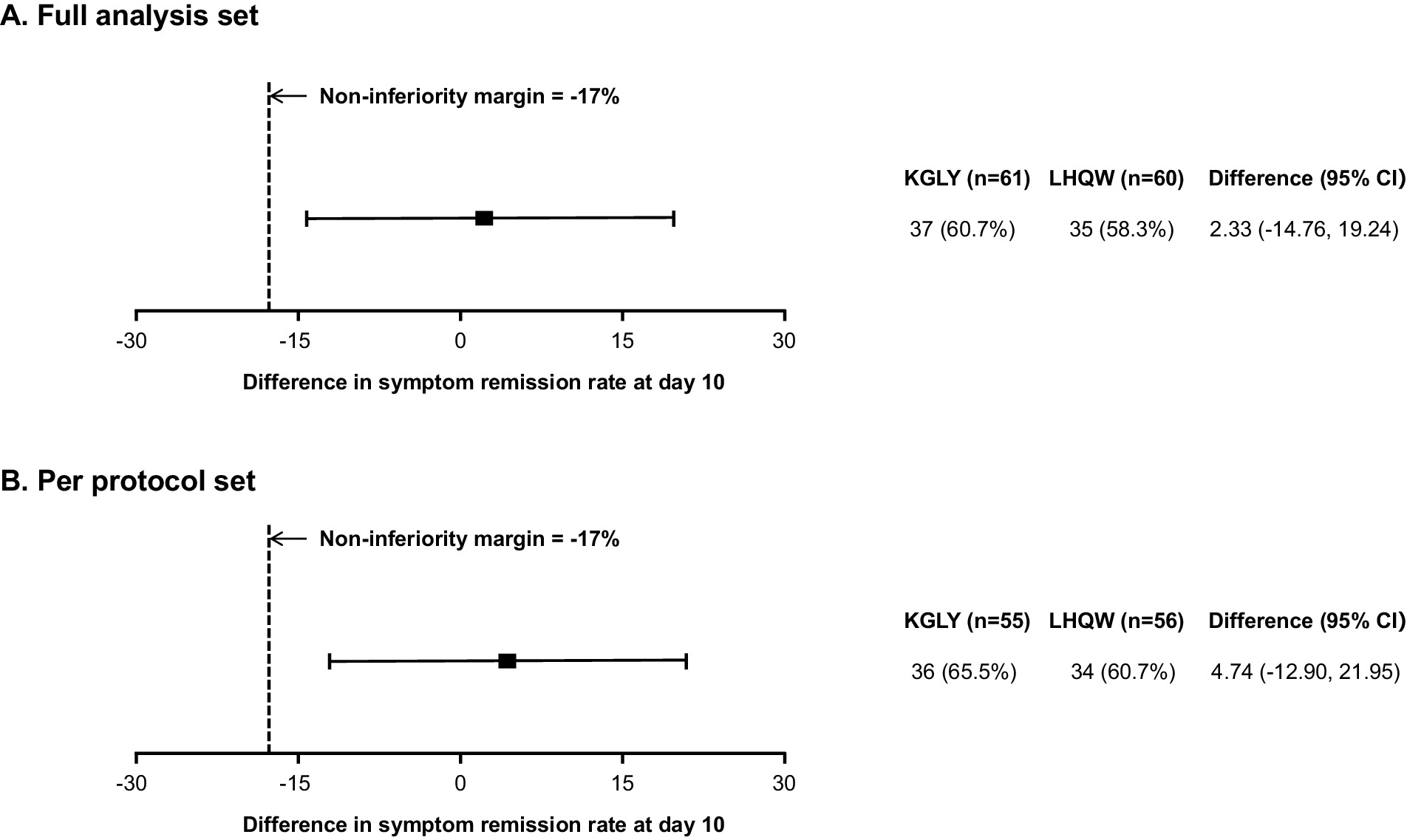
Figure 3. Results of the non-inferiority test for KGLY vs. LHQW in symptom remission rate at day 10.Non-inferiority would be shown if the lower limit of the 95% CI of the risk difference was higher than the −17 p.p. non-inferiority margin. KGLY, Kegan Liyan oral liquid; LHQW, Lianhuaqingwen capsules; CI, confidence interval; p.p., percentage point.
No significant differences within groups were observed in symptom recovery rate between KGLY and LHQW arms at day 3 (0.0% vs. 1.7%, p = 0.99) and day 7 (11.5% vs. 11.7%, p = 0.97). At day 10, 23.0% of the patients in KGLY group had achieved symptom recovery, not statistically different comparing with that in LHQW group (26.7%; difference − 3.7 p.p., p = 0.63; Table 5). The median time to symptom remission in KGLY and LHQW group were both 10 days, and and there was no statistical difference between groups (p = 0.62; Table 6).
The VAS score for the selected symptoms (sore throat, cough, myalgia and fatigue) decreased significantly in both groups after treatment (p < 0.001), and no notable differences were identified in post-treatment VAS scores for any of the symptoms, or change of VAS scores for any of the symptoms, between groups (p > 0.05 for all outcomes). For the entire 10-day period, more participants in KGLY group had their symptom of sore throat completely resolved (80.0% vs. 75.9%, p = 0.59) and the mean AUC for sore throat VAS scores were lower in KGLY group than in LHQW group (15.23 ± 9.72 vs. 16.45 ± 10.88, difference − 1.22, p = 0.52), but the difference was not statistically significant. Similarly, though no notable differences were identified, decrease of sore throat VAS scores from baseline until day 10 was greater in KGLY group (−4.59 ± 2.42 cm vs. −4.03 ± 2.81 cm, difference − 0.56 cm, p = 0.25; Tables 6–11; Figure 4).
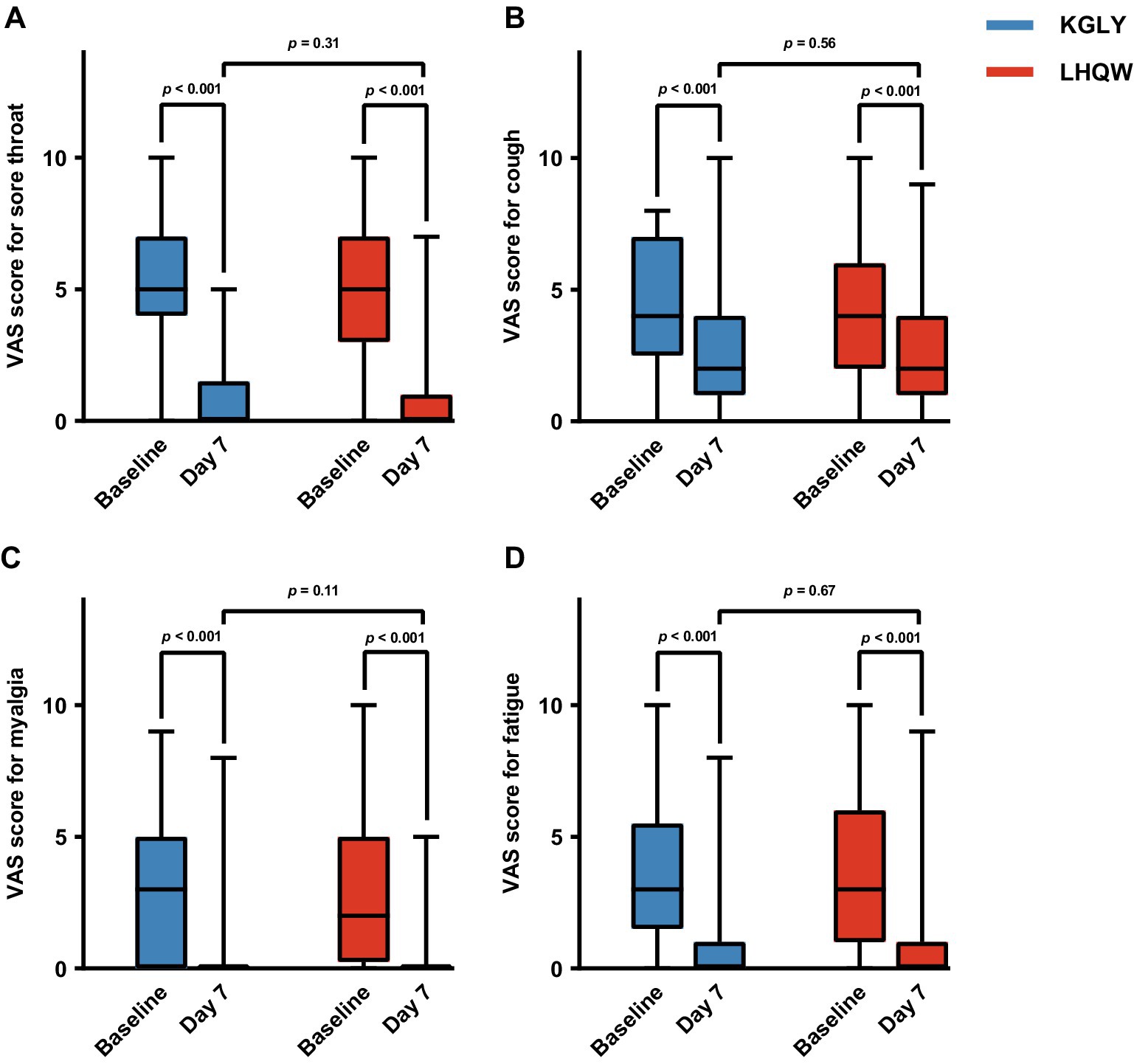
Figure 4. Comparison of VAS scores of selected symptoms at baseline and day 7 in the KGLY and LHQW arms: sore throat (panel A), cough (panel B), myalgia (panel C) and fatigue (panel D). In each boxplot, the central mark indicated the median, and the edges of the box referred to the 25th and 75th percentiles. The extended lines showed the full range. VAS, visual analog scale; KGLY, Kegan Liyan oral liquid; LHQW, Lianhuaqingwen capsules.
There was no statistically significant difference between the two groups in terms of SARS-CoV-2 RNA negative conversion at day 7 (19.7% vs. 20.0%, p = 0.96) and day 10 (55.7% vs. 58.3%, p = 0.99; Table 12). Simultaneously, there were no patients in KGLY group who had a positive SARS-CoV-2 RNA test result after negative conversion, less than those in LHQW group (0% vs. 16.7%, p = 0.46), though not statistically significant (Table 13). The incidence of pneumonia between groups was not statistically different either (3.3% vs. 0.0%, p = 0.48; Table 14).
All 127 enrolled patients were included in the SS analysis. Twenty-one individuals (8 [12.7%] in KGLY group, and 13 [20.3%] in LHQW group) reported a toal of 25 adverse events (10 in KGLY group, and 15 in LHQW group). There was no significant difference between the groups in the occurrence of adverse events (p = 0.25). Diarrhea, nausea, and elevated blood platelet count were the most frequent adverse events in two both arms, but only mild events were observed. No serious adverse events were reported (Tables 15, 16).
The present study was launched before the severe new outbreak of COVID-19 in China in late 2022, when most people had not yet contracted COVID-19, with the aim to provide more evidence for the application of TCM for COVID-19 in the future. It is to our knowledge the first randomized, double-blinded trial that compares the efficacy of KGLY with LHQW for mild COVID-19. We found that KGLY was non-inferior to LHQW in relieving fever, sore throat, cough, fatigue, and myalgia. The incidence of adverse events did not differ significantly, indicating that KGLY represents a potential therapy with a good safety profile for mild COVID-19. Therefore, taking it into account that COVID-19 is still circulating worldwide and a large number of people are experiencing repeated infections, the present trial provides a feasible option for COVID-19 treatment.
Although the post-treatment VAS scores for symptoms remarkably decreased in both groups, less patients met the criteria for symptom remission at day 10 than we expected, 58.3% in the LHQW group in 60.7% in the KGLY group. The recovery rate of cough was the lowest among the five symptoms, 34.5% in LHQW and 31.7% in KGLY. Our finding was consistent with that of Tenforde et al. (17), who found that COVID-19 can cause prolonged sickness even in people with mild infection. Among 274 symptomatic COVID-19 infected adults in a survey conducted in the United States, 35% of individuals had not returned to a normal state of health after 2–3 weeks of infection (17). Cough is one of the common symptoms in people with ‘Long COVID’ and the least likely of the symptoms to resolve (17, 18). Almost half (43%) of the patients still suffered from cough 2–3 weeks after infection, according to the above-mentioned survey (17). Besides, our study was conducted during the first large-scale wave in China since the original outbreak, when an immune barrier had not yet been established, which might be another reason why symptoms sustained for a long time.
Currently, oral antiviral agents available in China for COVID-19 include paxlovid, azvudine and molnupiravir (9). Nevertheless, these therapies are not indicated for treatment in patients with mild symptoms and no risk factors for progression to severe disease. Meanwhile, some antiviral agents are relatively expensive and source-limited during outbreaks. However, early intervention can accelerate the recovery of COVID-19 (19). Therefore, TCM provides new option for clinical management of patients with mild COVID-19.
TCM has been widely used to treat pandemics for more than 2,500 years, the first mentioning being in Huangdi Neijing. To date, plenty of Chinese herbs have been reported to possess antiviral activities against various coronaviruses, notably the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) (20). Due to its long history of application and demonstrated efficacy, TCM has also been recommended in COVID-19 guidelines (21). In March 2022, the WHO Expert Meeting concluded that TCM may help to shorten the time for viral clearance, promote the recovery of clinical symptoms, and reduce the risk of progressing to severe disease (22). Results from systematic reviews and meta-analyses also demonstrate that TCM treatment may promote cure and reduce clinical deterioration in patients with COVID-19 (19, 23, 24).
However, researchers have also pointed out that because of the complex situation during the epidemic, many studies on COVID-19 had severe limitations in study design, such as the lack of allocation concealment and blinding procedures, which enhances difficulties in developing clear conclusions (25). In this study, the effects of KGLY and LHQW on symptoms, viral clearance and disease progression among mild COVID-19 patients were compared head-to-head for the first time. We adopted a randomized, double-blinded and double-dummy design, consequently reducing the risk of bias and enhancing the reliability of the study.
KGLY, mainly used to clear heat and resolve dampness, originates from classic formulas Yin-Qiao-San and Shen-Jie-San, and has been used extensively for the treatment of virus infections. Yin-Qiao-San, first presented by Ju-Tong Wu in 1798, was frequently used to treat influenza (26). Shen-Jie-San, initially documented in Shanghan Wenyi Tiaobian in 1784, was applied for infectious diseases (27). According to the latest epidemiological investigations, dampness and heat are the main TCM pathogenic factors in people infected with Omicron variant (28, 29). Clearing heat and resolving dampness are the first priority when dealing with dampness and heat in COVID-19 according to the eminent TCM expert Zhongying Zhou (30). Fever (83.0% of the cases), sore throat (62.1%), cough (89.7%), fatigue (84.1%), and myalgia (72.7%) were the most common symptoms after infection reported by a cross-sectional study that surveyed 630 Omicron-infected patients (1). In TCM, fever and sore throat are among the signs of Dampness-heat in the Lung syndrome. The above features of Omicron infection fit with the indications of KGLY well.
Pharmacological basis for understanding the effects of KGLY for COVID-19 has been established through research. A quality check of KGLY by high-performance liquid chromatography technique identified 9 compounds, including chlorogenic acid, 4’-O-beta-glucopyranosyl-5-O-methylvisamminol, ammonium glycyrrhizinate, geniposide, forsythin, caffeic acid, baicalin, tectoridin, and menthol (31). Among them, baicalin possesses significant antiviral effects against SARS-CoV-2 by altering respiratory microbiome and ameliorating the cytokine storm through TNF and IL-17 pathways (32–34). Geniposide suppresses virus replication in vitro via modifying Ca2+ signaling pathway (35). Chlorogenic acid is effective against coronavirus infection by targeting apoptosis, particularly impacting the initial stage of virus replication and release (36). And caffeic acid derivatives have shown strong interactions with SARS-CoV-2 proteins in molecular docking simulations (37). Meanwhile, previous studies have demonstrated that KGLY possessed anti-coronavirus function both in vivo and in vitro (38, 39). Moreover, KGLY was reported to decrease inflammatory factors IL-1β, IL-6, and TNF-α (31) and could treat influenza virus infection by lowering the oxidative stress level through increased superoxide dismutase activity (40).
This study had several limitations. First, stringent COVID-19 prevention and control measures in China precluded launching a multicenter study, so this trial was carried out at two centers with a relatively small sample size. As a result, the recruitment was limited to a certain geographic region, which may impact the generalizability of the findings. Second, the follow-up was relatively brief, which failed to provide further evidence for subsequent efficacy. It is unclear whether the symptoms will rebound afterwards. Finally, different strains of COVID-19 have diverse characteristics, thus, sequencing data would be conducive to instructing precise treatment of the strain. According to the epidemic situation then, Omicron was the main strain during the study period. However, strains were not sequenced in the present trial, requiring further studies.
Based on the established efficacy of LHQW in treating COVID-19, our findings demonstrate that KGLY is non-inferior in terms of symptom remission. This non-inferiority is significant as it positions KGLY as a potentially effective alternative treatment option. However, further studies are warranted to confirm the broader efficacy and safety of KGLY for COVID-19 management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. YiC: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. MZ: Data curation, Investigation, Writing – original draft, Writing – review & editing. FF: Data curation, Investigation, Project administration, Writing – review & editing. ZD: Data curation, Investigation, Writing – review & editing. JX: Conceptualization, Methodology, Writing – review & editing. SJ: Data curation, Investigation, Writing – review & editing. LuL: Data curation, Investigation, Writing – review & editing. XC: Data curation, Investigation, Writing – review & editing. RX: Data curation, Investigation, Writing – review & editing. LF: Data curation, Investigation, Writing – review & editing. XY: Data curation, Investigation, Writing – review & editing. ZL: Data curation, Investigation, Writing – review & editing. JQ: Data curation, Investigation, Writing – review & editing. SL: Data curation, Investigation, Writing – review & editing. XP: Data curation, Investigation, Writing – review & editing. YuC: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. LiL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing. LW: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work received funding from State Key Laboratory of Dampness Syndrome of Chinese Medicine (grant number SZ2021ZZ42), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab, grant number 2020B1212030006), the Basic and Applied Basic Research Foundation of Guangdong Province (grant number 2022B1515230001), Guangzhou Key Laboratory of Traditional Chinese Medicine for the Prevention and Treatment of Chronic Cough and Dyspnea (grant number 2023A03J0226), Lin Lin’s Famous TCM Inheritance Studio of TCM Bureau of Guangdong Province (Office Letter of Guangdong Chinese Medicine [2023] No. 108), Zhongying Zhou Famous Doctor’s Studio of Guangdong Provincial Hospital of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine [2014] No. 89), the double world-class and high-level university discipline collaborative innovation team project of Guangzhou University of Chinese Medicine (grant number 2021XK27).
We thank the patients for their participation in this trial as well as the study investigators for their assistance with data collecting. The medications were provided by Wanglaoji Pharmaceutical Co., Ltd. (Guangzhou, China), however, Wanglaoji Pharmaceutical did not participate in any stage of the conceptualization, execution, data collection, data analysis, or manuscript preparation in this trial.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feng, T, Xing, XJ, Zhou, S, Wei, XL, Lu, L, Long, T, et al. Symptom reported by hospital staff with mild COVID-19 during the omicron epidemic in Chongqing. Chin J Respir Crit Care Med. (2023) 22:7–12. doi: 10.7507/1671-6205.202301036
2. World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: https://Covid19.who.int/ (Accessed May 2024).
3. Huang, K, Zhang, P, Zhang, Z, Youn, J, Wang, C, Zhang, H, et al. Traditional Chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Therapeut. (2021) 225:107843. doi: 10.1016/j.pharmthera.2021.107843
4. Hu, K, Guan, W, Bi, Y, Zhang, W, Li, L, Zhang, B, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. (2021) 85:153242. doi: 10.1016/j.phymed.2020.153242
5. Zheng, JP, Ling, Y, Jiang, LS, Piroon, M, Lu, HZ, Methee, C, et al. Effects of Lianhuaqingwen capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial. Virol J. (2023) 20:277. doi: 10.1186/s12985-023-02144-6
6. Li, LC, Zhang, ZH, Zhou, WC, Chen, J, Jin, HQ, Fang, HM, et al. Lianhua Qingwen prescription for coronavirus disease 2019 (COVID-19) treatment: advances and prospects. Biomed Pharmacother. (2020) 130:110641. doi: 10.1016/j.biopha.2020.110641
7. Wang, SX, Li, MY, Chen, XL, Ma, MY, and Hu, JH. Clinical efficacy of Lianhua Qingwen integrated with Western medicine on COVID-19 by Meta-analysis. Chin Tradit Herb Drug. (2020) 51:3763–9. doi: 10.7501/j.issn.0253-2670.2020.14.021
8. Zhuang, JQ, Dai, XZ, Wu, QH, Cai, HR, Fu, X, Zhang, WZ, et al. A meta-analysis for Lianhua Qingwen on the treatment of coronavirus disease 2019 (COVID-19). Complement Ther Med. (2021) 60:102754. doi: 10.1016/j.ctim.2021.102754
9. General Office of the National Health and Health Commission. The guideline on diagnosis and treatment of COVID-19 (trial 10th edition). Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202301/32de5b2ff9bf4eaa88e75bdf7223a65a.shtml (Accessed 6 January 2023).
10. Li, H, Lin, JZ, Huang, WP, and Mai, C. Clinical observation on 60 cases of KeGan LiYan Oral liquid in the treatment of influenza (wind-heat offending the lungs). New Chinese Med. (2014) 46:127–9. doi: 10.13457/j.cnki.jncm.2014.07.060
11. Deng, JM, Wang, YY, Cai, DM, and Chen, EJ. Observation on the therapeutic effect of KeGan LiYan Oral liquid in the treatment of chronic pharyngitis in 100 cases. Inner Mongolia J Tradit Chin Med. (2019) 38:5. doi: 10.16040/j.cnki.cn15-1101.2019.02.003
12. Lin, HB, and Liu, NA. Clinical analysis of 180 cases of acute upper respiratory tract infection treated by Kegan Liyan Oral liquid. Journal of North Pharmacy. (2019) 16:10–1.
13. Lin, L, Chen, YB, Tan, XY, Chen, LM, Xi, XT, Guo, LH, et al. Guangdong provincial Programme of traditional Chinese medicine treatment of coronavirus disease 2019 (pilot second edition). J Tradit Chin Med. (2020) 61:1197–9. doi: 10.13288/j.11-2166/r.2020.14.001
14. Chinese Association of Traditional Chinese Medicine. Expert consensus on the use of proprietary Chinese medicines in the prevention and treatment of novel coronavirus pneumonia. Chin J Integr Tradit West Med. (2022) 42:294–7. doi: 10.7661/j.cjim.20220126.034
15. General Office of the National Health and Health Commission. The guideline on diagnosis and treatment of COVID-19 (trial 9th edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88.shtml (Accessed 15 march 2022).
16. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) v5.0. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (Accessed 27 November 2017).
17. Tenforde, MW, Kim, SS, Lindsell, CJ, Rose, EB, Shapiro, NI, Files, DC, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, march-June 2020. MMWR-Morb Mortal Wkly Rep. (2020) 69:993–8. doi: 10.15585/mmwr.mm6930e1
18. Amenta, EM, Spallone, A, Rodriguez-Barradas, MC, El Sahly, HM, Atmar, RL, and Kulkarni, PA. Postacute COVID-19: An overview and approach to classification. Open Forum Infect Di. (2020) 7:7. doi: 10.1093/ofid/ofaa509
19. Xiong, XJ, Wang, PQ, Su, KL, Cho, WC, and Xing, YW. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res. (2020) 160:105056. doi: 10.1016/j.phrs.2020.105056
20. Xian, YF, Zhang, J, Bian, ZX, Zhou, H, Zhang, ZB, Lin, ZX, et al. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm Sin B. (2020) 10:1163–74. doi: 10.1016/j.apsb.2020.06.002
21. General Office of the National Health and Health Commission. The guideline on diagnosis and treatment of COVID-19 (trial 4th edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67.shtml (Accessed 27 January 2020).
22. World Health Organization. WHO expert meeting on evaluation of traditional Chinese medicine in the treatment of COVID-19. Available at: https://cdn.who.int/media/docs/default-source/traditional-medicine/meeting-report---who-expert-meeting-on-evaluation-of-tcm-in-the-treatment-of-covid-192f7d2ba2-cfb8-4b00-90e3-441740cdbacb.pdf?sfvrsn=a77161d7_1&download=true (Accessed 28 February-2 March 2022).
23. Wang, HP, Xu, BW, Zhang, Y, Duan, YY, Gao, RK, He, HQ, et al. Efficacy and safety of traditional Chinese medicine in coronavirus disease 2019 (COVID-19): a systematic review and Meta-analysis. Front Pharmacol. (2021) 12:20. doi: 10.3389/fphar.2021.609213
24. Zhu, HF, Li, MT, Tian, C, Lai, HH, Zhang, YQ, Shi, JH, et al. Efficacy and safety of chinese herbal medicine for treating mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Pharmacol. (2022) 13:17. doi: 10.3389/fphar.2022.988237
25. Ang, L, Song, E, Zhang, JH, Lee, HW, and Lee, MS. Herbal medicine for COVID-19: An overview of systematic reviews and meta-analysis. Phytomedicine. (2022) 102:154136. doi: 10.1016/j.phymed.2022.154136
26. Wang, C, Cao, B, Liu, QQ, Zou, ZQ, Liang, ZA, Gu, L, et al. Oseltamivir compared with the Chinese traditional therapy Maxingshigan-Yinqiaosan in the treatment of H1N1 influenza. Ann Intern Med. (2011) 155:217–25. doi: 10.7326/0003-4819-155-4-201108160-00005
27. Yan, F, and Chen, RQ. Clinical observation on 110 cases of pediatric acute upper respiratory tract infections treated with YinQiao san and ShenJie san. China J Pharm Econ. (2012) 2:217–8.
28. Hua, QL, Zheng, DW, Liu, YT, Shui, JW, Cai, YH, Li, Q, et al. Traditional Chinese medicine syndromes and treatment strategies in 1155 patients infected with the omicron (BA.5.1.3) variant in Hainan Province. Guangdong Med J. (2023) 44:558–64. doi: 10.13820/j.cnki.gdyx.20225042
29. Zhang, W, Fang, BJ, Sun, D, Cao, M, Xu, XR, Pu, YT, et al. Analysis of Chinese medicine syndrome characteristics in 322 patients infected with mild SARS CoV-2 omicron variant in Shanghai. Chin J Integr Tradit West Med. (2023) 43:664–7. doi: 10.7661/j.cjim.20230213.033
30. Chen, JK, Cai, SB, Zhang, X, Li, JQ, and Tao, LT. Discussion on TCM diagnosis and treatment of COVID-19 based on the three toxin theory of Chinese medicine master Zhou Zhongying. J Emerg Tradit Chin Med. (2020) 29:1891–911. doi: 10.3969/j.issn.1004-745X.2020.11.004
31. Zhang, X, Sun, CY, Zhang, YB, Guo, HZ, Feng, XX, Peng, SZ, et al. Kegan Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung injury through inhibition of TLR4-mediated NF-κB signaling pathway and MMP-9 expression. J Ethnopharmacol. (2016) 186:91–102. doi: 10.1016/j.jep.2016.03.057
32. Feng, H, Zhang, J, Wang, X, Guo, Z, Wang, L, Zhang, K, et al. Baicalin protects broilers against avian coronavirus infection via regulating respiratory tract microbiota and amino acid metabolism. Int J Mol Sci. (2024) 25:2109. doi: 10.3390/ijms25042109
33. Dinda, B, Dinda, M, Dinda, S, and De, UC. An overview of anti-SARS-CoV-2 and anti-inflammatory potential of baicalein and its metabolite baicalin: insights into molecular mechanisms. Eur J Med Chem. (2023) 258:115629. doi: 10.1016/j.ejmech.2023.115629
34. You, J, Li, H, Fan, P, Yang, X, Wei, Y, Zheng, L, et al. Inspiration for COVID-19 treatment: network analysis and experimental validation of Baicalin for cytokine storm. Front Pharmacol. (2022) 13:853496. doi: 10.3389/fphar.2022.853496
35. Zhou, L, Bao, L, Wang, Y, Chen, M, Zhang, Y, Geng, Z, et al. An integrated analysis reveals Geniposide extracted from Gardenia jasminoides J.Ellis regulates calcium signaling pathway essential for influenza a virus replication. Front Pharmacol. (2021) 12:755796. doi: 10.3389/fphar.2021.755796
36. Shi, C, Liang, W, Guo, M, Yuan, J, Zu, S, and Hu, H. Chlorogenic acid inhibits porcine deltacoronavirus release by targeting apoptosis. Int Immunopharmacol. (2024) 127:111359. doi: 10.1016/j.intimp.2023.111359
37. Adem, Ş, Eyupoglu, V, Sarfraz, I, Rasul, A, Zahoor, AF, Ali, M, et al. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine. (2021) 85:153310. doi: 10.1016/j.phymed.2020.153310
38. Lin, J, Ye, QX, Yang, ZF, Liu, N, Zhang, FX, Shi, SB, et al. An experimental study on Kegan Liyan Oral liquid for counteracting coronavirus. Tradit Chin Drug Res Clin Pharmacol. (2007) 5:349–53. doi: 10.19378/j.issn.1003-9783.2007.05.005
39. Li, G, Shen, XH, Chen, JX, Su, ZR, Zeng, HF, and Lai, XP. Experimental study of anti-influenza virus H9N2 effects of Kegan Liyan Oral liquid in vivo. Tradit Chin Drug Res Clin Pharmacol. (2010) 20:496–8. doi: 10.19378/j.issn.1003-9783.2010.05.015
40. Duan, SJ, Gu, LZ, Wang, YY, Zheng, RB, Lu, JF, Yin, JJ, et al. Regulation of influenza virus-caused oxidative stress by Kegan Liyan Oral prescription, as monitored by Ascorbyl radical ESR signals. Am J Chinese Med. (2009) 37:1167–77. doi: 10.1142/s0192415x09007570
AUC – area under the curve
CI – confidence interval
CONSORT – Consolidated Standards of Reporting Trials
COVID-19 – Coronavirus disease 2019
FAS – full analysis set
GCP – Good Clinical Practice
HPLC – high performance liquid chromatography
HR – hazard ratio
IQR – interquartile range
KGLY – Kegan Liyan oral liquid
KUMCR – Key Unit of Methodology in Clinical Research
LHQW – Lianhuaqingwen capsule
MD – mean difference
MERS-CoV – Middle East respiratory syndrome coronavirus
p.p. – percentage point
PPS – per protocol set
RR – risk ratio
SARS-CoV – severe acute respiratory syndrome coronavirus
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2
SD – standard deviation
SS – safety set
TCM – traditional Chinese medicine
ULN – upper limit of normal
VAS – visual analog scale
Keywords: coronavirus disease 2019, Kegan Liyan oral liquid, Lianhuaqingwen capsules, non-inferiority, randomized controlled trial
Citation: Li Y, Chi Y, Zhu M, Fan F, Deng Z, Xiao J, Jin S, Lin L, Chen X, Xu R, Fan L, Yu X, Liang Z, Quan J, Li S, Peng X, Chen Y, Lin L and Wu L (2025) Efficacy of Kegan Liyan oral liquid vs. Lianhuaqingwen capsules for patients with mild COVID-19: a double-blinded, randomized, controlled, non-inferiority trial. Front. Med. 12:1531370. doi: 10.3389/fmed.2025.1531370
Received: 20 November 2024; Accepted: 14 February 2025;
Published: 26 February 2025.
Edited by:
Zulqarnain Baloch, Kunming University of Science and Technology, ChinaReviewed by:
Haymanot Zeleke Mitiku, Debre Markos University, EthiopiaCopyright © 2025 Li, Chi, Zhu, Fan, Deng, Xiao, Jin, Lin, Chen, Xu, Fan, Yu, Liang, Quan, Li, Peng, Chen, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanbin Chen, Y2hlbnl1YW5iaW4xMTBAMTYzLmNvbQ==; Lin Lin, ZHJsaW5saW42MjBAZ3p1Y20uZWR1LmNu; Lei Wu, ZHJsZWl3dUBnenVjbS5lZHUuY24=
ORCID: Yuanbin Chen, orcid.org/0000-0003-1169-5904
Lin Lin, orcid.org/0000-0002-9147-5516
Lei Wu, orcid.org/0000-0002-8556-0343
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.