
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 31 January 2025
Sec. Ophthalmology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1529719
This article is part of the Research Topic Efficient Artificial Intelligence in Ophthalmic Imaging – Volume II View all 4 articles
Aim: This study compares retinal thickness measurements in healthy eyes using one SD-OCT and two SS-OCT devices to assess differences and consistency for clinical application.
Methods: Forty-eight eyes with a mean age of 28.15 ± 8.85 years were enrolled. Retinal thickness was measured using Heidelberg Spectralis SD-OCT, Svision VG200 SS-OCT, and TowardPi En Face SS-OCT. Normally distributed data were presented as mean ± SD; non-normal data as median (P25–P75). Intraclass correlation coefficients (ICC) and Bland–Altman analysis were used to assess agreement, with a 7 μm error threshold.
Results: Significant differences were found between the three devices (p < 0.001). SD-OCT measurements were consistently lower than SS-OCT (p < 0.001), while the two SS-OCT devices showed no significant differences except in the nasal region (p = 0.006). ICC values between SD-OCT and SS-OCT devices were low (0.125–0.532), while SS-OCT devices showed better agreement (ICC: 0.369–0.922). Bland–Altman analysis found only 8.33% of SD-OCT and SS-OCT measurements within the 7 μm error range, compared to 81.25–83.33% for SS-OCT devices.
Conclusion: The measurements of macular retinal thickness using SD-OCT and SS-OCT devices showed poor consistency and cannot be used interchangeably. However, measurements obtained from different SS-OCT devices demonstrated good consistency. To enhance the accuracy of results, it is recommended to maintain consistency in the devices used for follow-up examinations in the same patient.
Retinal thickness is a crucial parameter for diagnosing and assessing fundus diseases. Abnormal conditions such as edema, exudation, and hemorrhage in the retinal layers lead to an increase in retinal thickness, which is a key feature of ocular conditions such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and central retinal vein occlusion (CRVO) (1–3). Therefore, changes in retinal thickness serve as indicators for evaluating the severity of fundus diseases and assessing the effectiveness of treatment. As treatments for retinal diseases continue to advance, reliable methods are needed to assess retinal thickness at different stages in both clinical practice and research. Optical coherence tomography (OCT) plays a vital role in detecting and evaluating fundus diseases (4). It offers several advantages, including being non-contact, non-invasive, and capable of providing high-resolution cross-sectional images of retinal layers quickly. OCT can vividly display abnormal retinal thickening, structural disruptions, subretinal fluid, intraretinal fluid, and retinal neovascularization. It holds significant value in the diagnosis, classification, prognosis, and treatment guidance of conditions such as AMD, choroidal neovascularization (CNV), and diabetic macular edema (DME) (3, 5, 6). With its automatic measurement of retinal thickness, OCT also provides a quantitative indicator for disease diagnosis and treatment evaluation. Recent treatment guidelines for AMD and DME have included OCT-derived biomarkers as essential tools for disease diagnosis and follow-up. The technology behind OCT has developed rapidly—from time-domain OCT (TD-OCT) in the 1990s to spectral-domain OCT (SD-OCT) in the 2000s, and now to swept-source OCT (SS-OCT). Each technological advancement has improved scanning speed, depth, field of view, morphological detail, and image quality. Today, various OCT devices, each with unique hardware configurations and software platforms, are widely used in clinical practice (7, 8).
However, standardized reference ranges for retinal thickness have yet to be established. Additionally, discrepancies and inconsistencies between measurements from different devices pose challenges, making it difficult for patients to undergo follow-up assessments seamlessly. Some studies have shown that SS-OCT and SD-OCT have good consistency and correlation in measuring central corneal thickness, corneal epithelial thickness and outer retinal thickness in healthy people (9–12). However, some studies have shown that the measurement values of SS-OCT and SD-OCT are not suitable for mutual conversion (13–15). Among the latest SS-OCT devices are the VG200 (a swept-source OCT by Svision) and the Ultrawide-field En Face OCT (by TowardPi), both offering high scanning speeds and superior resolution. However, no comparative studies have been conducted to evaluate the differences and consistency between these SS-OCT devices and SD-OCT in measuring macular retinal thickness.
This study aims to measure macular retinal thickness in healthy eyes using three OCT systems, Svision VG200 SS-OCT, TowardPi En Face SS-OCT and Heidelberg Spectralis SD-OCT. It will analyze the differences and consistency among the three instruments to provide evidence-based guidance for clinical treatment (Figure 1).
Ethics approval was obtained from the Ethics Committee (2024-KY004-01) of Shenzhen Aier Eye Hospital, adhering to the tenets of the Declaration of Helsinki.
This study included 48 eyes examined at the Department of Ophthalmology, Shenzhen Aier Eye Hospital, in December 2023. The average age of participants was 28.15 ± 8.85 years (range: 7–51 years), with 18 eyes from males (37.5%). The mean spherical equivalent was−3.42 ± 2.29 D, and the best-corrected visual acuity (BCVA) was 1.04 ± 0.15.
The inclusion criteria were as follows: (1) uncorrected visual acuity (UCVA) >0.5; (2) cooperating with fixed vision; (3) voluntary participation for free testing using three OCT devices. The exclusion criteria included: (1) inability to cooperate with the examination; (2) previous retinal surgery; (3) retinal or choroidal diseases; (4) history of ocular hypertension or glaucoma; (5) use of ocular medication within the last 3 months.
All participants underwent UCVA testing, followed by Computer Optometry (CV-5000, Topcon, Japan). Medical histories were collected through questionnaires, and a slit-lamp microscope (SL-130, Carl Zeiss, Germany) was used to rule out other ocular conditions.
All retinal thickness measurements were performed on the same day for each participant by a single experienced examiner to ensure consistency. Three devices were used sequentially for both eyes: the Heidelberg Spectralis HRA + OCT, the VG200 Swept-Source OCT from SVision Imaging Ltd., and the Ultrawide-field En Face OCT from TowardPi Medical Technology Ltd. The Heidelberg device employed the dense scan mode (49 lines) to obtain macular thickness within a 6 mm diameter (automatic scan range 30°X25°). The VG200 Swept-Source OCT used a Cube 9 × 9, 1024 × 1024 scanning protocol to collect measurements from the same 6 mm macular area (automatic scan range 9 × 9 mm). The Ultrawide-field En Face OCT operated in 3D macular mode to gather data from the 6 mm macular area (automatic scan range 6 × 6 mm).
All statistical analyses were performed using SPSS version 27.0 (IBM, United States). The Shapiro–Wilk test was used to check for normality. Normally distributed data were expressed as mean ± standard deviation (SD), and non-normally distributed data were expressed as median (P25–P75). Data following a normal distribution were analyzed using one-way analysis of variance (ANOVA) with repeated measures, while data not conforming to a normal distribution were analyzed using the Friedman M-test for multiple correlated samples. Intraclass correlation coefficients (ICC) assessed the agreement between devices, and Bland–Altman analysis evaluated the consistency of measurements. p-value <0.05 was considered statistically significant.
A total of 48 eyes were included in the study, conducted in December 2023. The average age of the participants was 28.15 ± 8.85 years, with 18 males (37.50%). The mean spherical equivalent refraction was −3.42 ± 2.29 D, and the best-corrected visual acuity (BCVA) was 1.04 ± 0.15. Among them, three participants had a history of refractive surgery (Table 1). Figure 2 shows examples of three optical coherence tomography devices with different performances. The retinal thickness measurements obtained from the Heidelberg, SVision, and TowardPi devices for various retinal regions are summarized in Table 2. Data that followed a normal distribution (such as the central macular region and the nasal side within a 3 mm diameter) are presented as mean ± standard deviation. Non-normally distributed data are expressed as median (P25–P75).
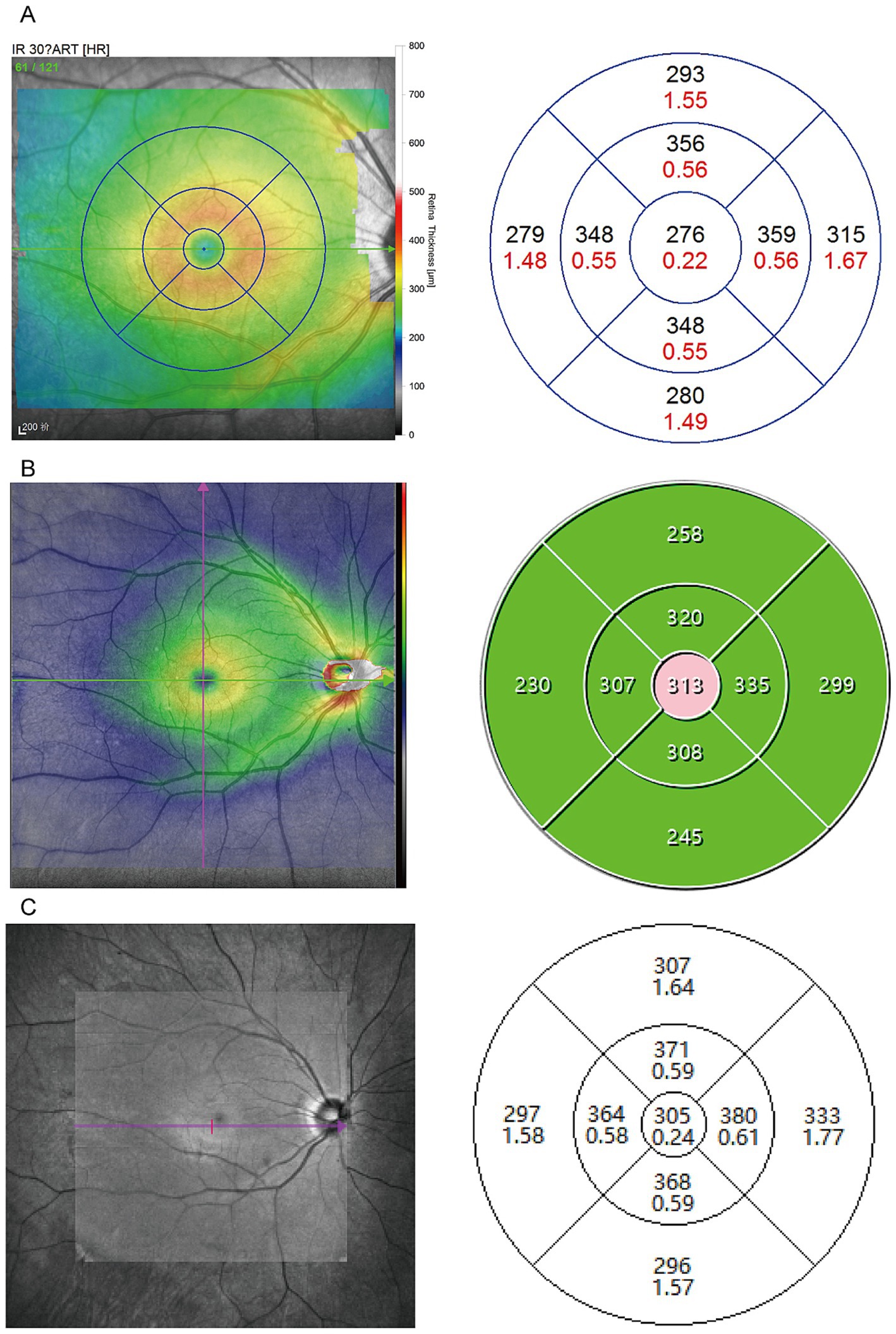
Figure 2. Sample scan using OCT deices from the SD-OCT and two SS-OCT. (A) Example case using Heidelberg device employed the dense scan mode (49 lines) to obtain macular thickness within a 6 mm diameter. (B) Example case using the VG200 Swept-Source OCT used a Cube 9 × 9, 1024 × 1024 scanning protocol to collect measurements from the same 6 mm macular area. (C) Example case using the Ultrawide-field En Face OCT operated in 3D macular mode to gather data from the same region.
The results indicated significant differences in retinal thickness measurements across all regions among the three devices (p < 0.001). Pairwise comparisons revealed significant differences between SD-OCT (Heidelberg) and either of the two SS-OCT devices (SVision and TowardPi) in all regions (p < 0.001). Notably, no significant differences were observed between the two SS-OCT devices (SVision and TowardPi) (p > 0.05) except in the nasal region within a 3 mm diameter (p = 0.006). The measurement data for the central macular region (within 1 mm) and the nasal side (within 3 mm) followed a normal distribution and were analyzed using repeated-measures ANOVA (Table 3). For regions where the data did not follow a normal distribution, including the minimum macular thickness and superior, inferior, and temporal regions within the 3 mm and superior, inferior, nasal, and temporal regions within the 6 mm diameters, the Friedman M-test for multiple related samples was used (Table 4).
Intraclass correlation coefficient (ICC) analysis was performed to assess the agreement among the three devices. The correlations between Heidelberg and the two SS-OCT devices (SVision and TowardPi) were relatively poor, with ICC values ranging from 0.161 to 0.499 and 0.125 to 0.532, respectively. In contrast, the correlation between SVision and TowardPi was stronger, with ICC values ranging from 0.369 to 0.922 (Tables 3, 4).
A Bland–Altman analysis was conducted to evaluate agreement, using 7 μm as the maximum allowable error (Figures 3–5). The 95% limits of agreement (LoA) for the central macular region and minimum macular thickness between Heidelberg and SVision did not cross the zero line, indicating significant systematic bias and poor agreement. For other regions within the 3 mm and 6 mm diameters, the 95% LoA between Heidelberg and SVision crossed the zero line, with 87.50–95.83% of the points falling within the LoA. However, only 0.00–8.33% of the points fell within the allowable error range of 7 μm (Figure 3). Similarly, for the temporal region within the 6 mm diameter, the 95% LoA between Heidelberg and TowardPi ranged from −63.05 to 32.00 μm, with 95.83% of the points falling within the LoA, but only 8.33% within the allowable error range. In other regions within the 6 mm diameter, the 95% LoA between Heidelberg and TowardPi did not cross the zero line, indicating significant systematic bias and poor agreement (Figure 4). In contrast, the 95% LoA between SVision and TowardPi crossed the zero line in all regions, with 87.50–93.75% of points falling within the LoA and 81.25–83.33% of points within the allowable error range (Figure 5).

Figure 3. Bland–Altman plot for consistency between Heidelberg and SVision devices. (A) The central and minimum macular thickness between Heidelberg and SVision: the 95% limits of agreement (LoA) did not cross the zero line. (B) For four regions within the 3 mm diameters, the 95% LoA between Heidelberg and SVision crossed the zero line, with 91.67–93.75% of the points falling within the LoA. However, only 0.00–8.33% of the points fell within the allowable error range of 7 μm. (C) For four regions within the 6 mm diameters, the 95% LoA between Heidelberg and SVision crossed the zero line, with 87.50–93.75% of the points falling within the LoA. However, only 0.00–8.33% of the points fell within the allowable error range of 7 μm.
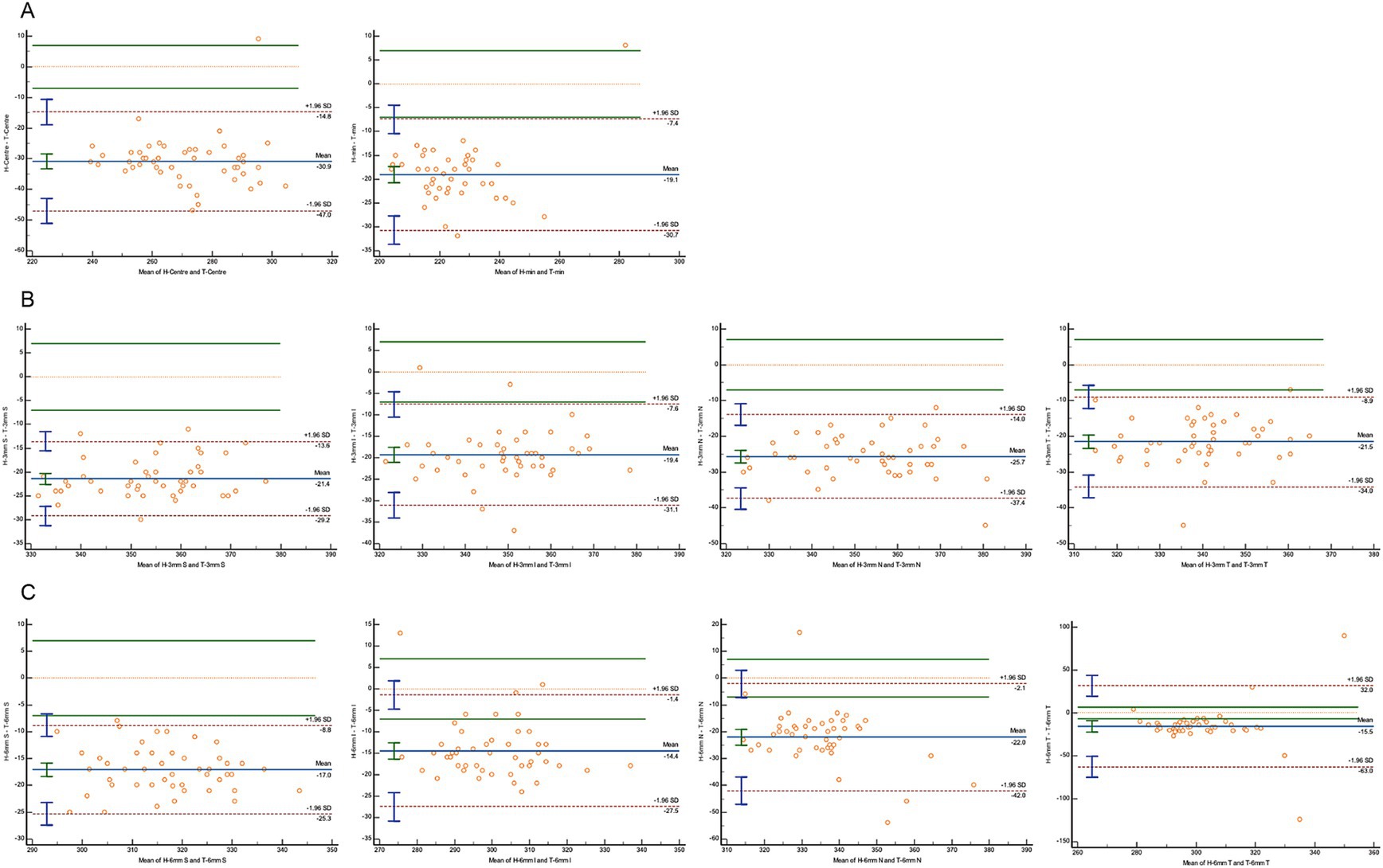
Figure 4. Bland–Altman plot for consistency between Heidelberg and TowardPi devices. (A) The central and minimum macular thickness between Heidelberg and TowardPi: the 95% limits of agreement (LoA) did not cross the zero line. (B) For four regions within the 3 mm diameters between Heidelberg and TowardPi: the 95% limits of agreement (LoA) did not cross the zero line. (C) For superior, inferior, nasal regions within the 6 mm diameters between Heidelberg and TowardPi: the 95% limits of agreement (LoA) did not cross the zero line. For temporal regions within the 6 mm diameters: the 95% LoA between Heidelberg and TowardPi is −63.05 to 32.00 (μm), 8.33% of the points fell within the allowable error range of 7 μm.
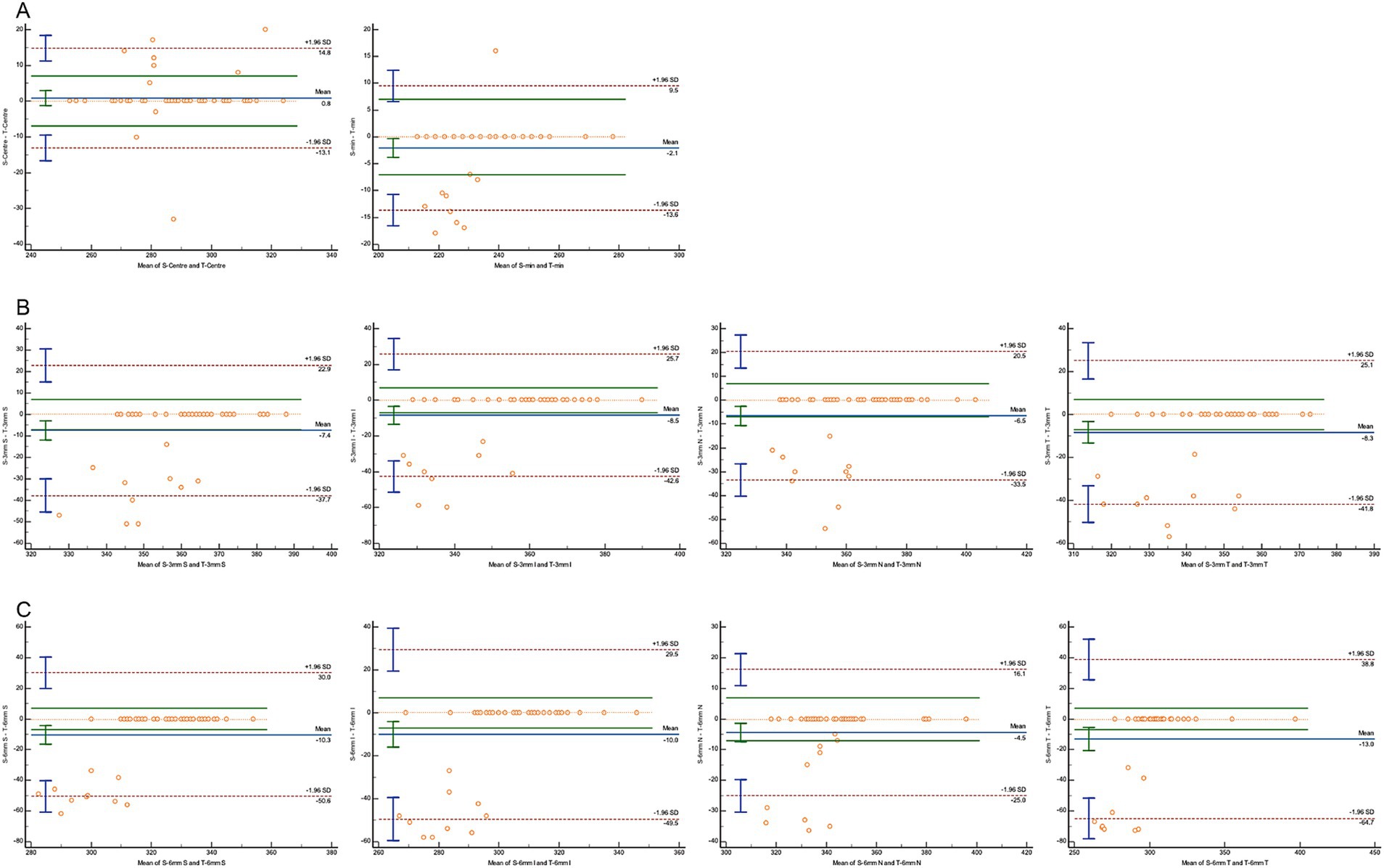
Figure 5. Bland–Altman plot for consistency between SVision and TowardPi devices. (A) The central and minimum macular thickness between SVision and TowardPi: the 95% limits of agreement (LoA) is −13.13 to 14.79 (μm) and −13.60 to 9.50 (μm), 83.33 and 81.25% of the points fell within the allowable error range of 7 μm. (B) For four regions within the 3 mm diameters between SVision and TowardPi: 93.75% of the points falling within the LoA, 79.17%–81.25% of the points fell within the allowable error range of 7 μm. (C) For four regions within the 6 mm diameters between SVision and TowardPi: 87.50%–89.58% of the points falling within the LoA, 79.17%–83.33% of the points fell within the allowable error range of 7 μm.
With the rapid development of OCT technology, various OCT devices with different hardware and software have entered clinical practice. Understanding the consistency and discrepancies between spectral-domain OCT (SD-OCT) and swept-source OCT (SS-OCT) devices is essential for making accurate diagnostic conclusions and determining appropriate management strategies. SS-OCT has already been applied in the diagnosis and staging of conditions such as retinopathy of prematurity, diabetic retinopathy, age-related macular degeneration, and glaucoma, and it is expected to become the mainstream method for ophthalmic OCT in the future (16, 17). This study compared retinal thickness measurements of the fovea and surrounding macular region in healthy individuals using one SD-OCT device and two SS-OCT devices. Results indicated that retinal thickness measurements, a crucial parameter for tracking fundus diseases, showed poor consistency between SD-OCT and SS-OCT, meaning the measurements are not directly interchangeable. However, the two SS-OCT devices demonstrated good agreement, allowing their measurements to be cross-referenced in quantitative analyses.
The utilize of OCT represents a significant advancement in non-invasive macular imaging (18). OCT biomarkers have emerged as critical prognostic indicators for treatment outcomes in various diseases, such as diabetic macular edema (DME) (6). For a long period, SD-OCT has been regarded as the gold standard for visualizing retinal structural changes (19). It provides clear, high-resolution retinal imaging even in the presence of dense media opacities or small pupil sizes (20, 21). SS-OCT, with its faster scanning speed and longer wavelength, minimizes motion artifacts and provides better visualization of deeper retinal structures (15). However, one report indicates that SS-OCT may fail to detect approximately one-quarter of patients with actual macular disease, making it unsuitable as a replacement for SD-OCT in screening or diagnosing macular health (22).
The retinal thickness values obtained from the SD-OCT device were significantly lower than those from the SS-OCT devices, while the two SS-OCT devices showed no significant differences between each other. Intraclass correlation coefficient (ICC) analysis, which ranges from 0 to 1 (with values >0.75 indicating good reliability and <0.4 indicating poor reliability), revealed that SD-OCT measurements had poor reliability when compared to both SS-OCT (TowardPi and SVision) devices, with ICC values peaking at 0.499 and 0.532. In contrast, the SS-OCT devices (TowardPi and SVision) exhibited excellent reliability in the central macular region and minimum macular thickness, with ICC values of 0.922 and 0.902, respectively. Reliability for peripheral regions was moderate, with the lowest ICC value being 0.369 and the lowest ICC value being 0.369 and maximum value being 0.780. Using a 7 μm difference as the clinically significant allowable error (23), Bland–Altman analysis showed that only 8.33% of the measurements between the Heidelberg (SD-OCT) and the SS-OCT devices (SVision and TowardPi) fell within the allowable error range, indicating poor agreement. However, the measurements from the two SS-OCT devices demonstrated much better consistency, with 81.25–83.33% of the points within the allowable error range. These findings suggest that retinal thickness measurements from SD-OCT and SS-OCT devices are not directly interchangeable and should not be used as quantitative indicators during follow-up. However, SS-OCT devices (TowardPi and SVision) provided sufficiently similar measurements, allowing their results to be referenced against each other for clinical follow-up purposes.
The differences between SD-OCT and SS-OCT in image recognition and data measurement come from different hardware and software. From a hardware perspective, the Heidelberg Spectralis HRA + OCT, a widely used SD-OCT device, has demonstrated high stability and accuracy. SD-OCT typically acquires 27,000–40,000 A-scans per second with an axial resolution of approximately 3.5–6 μm (24). The layer-by-layer analysis of the retina provided by SD-OCT gives it a higher resolution than contact ophthalmoscopes and fundus photography (25). The VG200 swept-source OCT (SS-OCT) by SVision Imaging Ltd. (Luoyang, China) and the Ultrawide-field En Face OCT by TowardPi Medical Technology Ltd. (Beijing, China) operate on an SS-OCT-Angiography platform, enabling the simultaneous acquisition of structural and vascular parameters (26). SS-OCT technology uses a wide-spectrum light source to separate wavelengths over time and captures interference spectra with a high-speed single-point detector. This system achieves scanning speeds of up to 400,000 scans per second and a scanning depth of 6 mm, offering superior sensitivity decay characteristics, faster imaging speeds, and a broader imaging range (16, 27). The longer wavelength of SS-OCT reduces light scattering in the inner retina, improving the signal-to-noise ratio (13).
Different software systems used by OCT devices for image analysis further contribute to variations in defining the outer retinal layers (28, 29). The software used by the Heidelberg Spectralis HRA + OCT defined identify the most outer reflective band as the boundary of the outer retina (16). The Svision swept-source OCT defined Bruch’s membrane as the boundary of the outer retina while the TowardPi defined RPE (30, 31). In pathological conditions, where the RPE-Bruch’s membrane interface becomes more complex, measurement errors increase (32, 33). Accurate assessment of retinal thickness is critical for evaluating retinal edema, diagnosing retinal diseases, and selecting appropriate treatments, but these software differences can introduce variability in measurement outcomes.
In this study, the measurements obtained using SD-OCT and SS-OCT showed poor agreement, a phenomenon also reported in several other studies (22, 34). However, some articles have noted high consistency in measurements when using SD-OCT and SS-OCT devices from the same manufacturer (9). As SS-OCT becomes more widely applied in clinical practice, it is crucial for clinicians to consider the type of device used for diagnosis. Consistency in the device used during follow-up visits, matching the one used at the initial examination, is particularly important to ensure reliable results.
One limitation of this study is the use of different scan ranges and scan qualities across various OCT devices. Due to differences in scanning ranges, only the most representative retinal thickness measurements that were consistently detectable by all three devices could be selected, limiting the number of parameters available for comparative analysis. Scan quality is a factor that influences segmentation performance; thus, the highest quality scans were chosen on each device to minimize this effect. Additionally, some affected eyes were not included in the sample to assess the consistency of device measurements, and multiple measurements on the same patients were not conducted to evaluate the stability of the devices.
In conclusion, the findings demonstrate significant systematic differences between SD-OCT and SS-OCT in macular retinal thickness measurements, rendering the values non-interchangeable. However, different SS-OCT devices showed good consistency in measuring macular retinal thickness, with values that are comparable across devices.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee (2024-KY004-01) of Shenzhen Aier Eye Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HW: Conceptualization, Data curation, Methodology, Validation, Writing – original draft. ZW: Conceptualization, Data curation, Resources, Writing – original draft, Writing – review & editing. ZL: Funding acquisition, Resources, Writing – review & editing. BQ: Formal analysis, Funding acquisition, Methodology, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science Research Foundation of Aier Eye Hospital (AF2215D01 and AGF2301D32); Guangdong Basic and Applied Basic Research Foundation (2022A1515111108); the Science Research Foundation of Shenzhen Aier Eye Hospital (SZAE2023C01); and Science Research Foundation of Shenzhen Aier Eye Hospital (SZ017 and SZ027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vujosevic, S, and Ting, DSW. Is central retina thickness the most relevant parameter in the management of diabetic macular edema? Retina. (2023) 43:1639–43. doi: 10.1097/IAE.0000000000003914
2. Hollo, G, Naghizadeh, F, and Vargha, P. Accuracy of macular ganglion-cell complex thickness to total retina thickness ratio to detect glaucoma in white Europeans. J Glaucoma. (2014) 23:e132–7. doi: 10.1097/IJG.0000000000000030
3. Gregori-Gisbert, I, Aguirre-Balsalobre, F, García-Sánchez, J, León-Salvatierra, G, Mengual-Verdú, E, and Hueso-Abancéns, JR. Recurrent and chronic central serous chorioretinopathy. Retina thickness evaluation one month after intravitreal bevacizumab injection. Arch Soc Esp Oftalmol. (2011) 86:407–11. doi: 10.1016/j.oftal.2011.05.021
4. Zeppieri, M, Marsili, S, Enaholo, ES, Shuaibu, AO, Uwagboe, N, Salati, C, et al. Optical coherence tomography (OCT): a brief look at the uses and technological evolution of ophthalmology. Medicina. (2023) 59:2114. doi: 10.3390/medicina59122114
5. Ahmed, TM, Siddiqui, MAR, and Hussain, B. Optical coherence tomography as a diagnostic intervention before cataract surgery—a review. Eye. (2023) 37:2176–82. doi: 10.1038/s41433-022-02320-y
6. Oliverio, GW, Meduri, A, Brancati, VU, Ingrande, I, de Luca, L, Raimondo, ED, et al. Clinical and optical coherence tomography biomarkers as prognostic factors in dexamethasone intravitreal implant for diabetic macular edema. Eur J Ophthalmol. (2024) 34:1810–8. doi: 10.1177/11206721241235242
7. Laíns, I, Wang, J, Cui, Y, Katz, R, Vingopoulos, F, Staurenghi, G, et al. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog Retin Eye Res. (2021) 84:100951. doi: 10.1016/j.preteyeres.2021.100951
8. Gawecki, M, and Kicinski, K. Advantages of the utilization of wide-field OCT and wide-field OCT angiography in clinical practice. Diagnostics. (2024) 14:321. doi: 10.3390/diagnostics14030321
9. Hou, H, Durbin, MK, El-Nimri, N, Fischer, JL, and Sadda, SR. Agreement, repeatability, and reproducibility of quantitative retinal layer assessment using swept-source and spectral-domain optical coherence tomography in eyes with retinal diseases. Front Med. (2023) 10:1281751. doi: 10.3389/fmed.2023.1281751
10. Ma, F, Bai, Y, Duan, J, Liang, Y, and Shang, Q. Validation of reliability, repeatability and consistency of three-dimensional choroidal vascular index. Sci Rep. (2024) 14:1576. doi: 10.1038/s41598-024-51922-x
11. Lu, J, Cheng, Y, Hiya, FE, Shen, M, Herrera, G, Zhang, Q, et al. Deep-learning-based automated measurement of outer retinal layer thickness for use in the assessment of age-related macular degeneration, applicable to both swept-source and spectral-domain OCT imaging. Biomed Opt Express. (2024) 15:413–27. doi: 10.1364/BOE.512359
12. Hou, H, El-Nimri, NW, Durbin, MK, Arias, JD, Moghimi, S, and Weinreb, RN. Agreement and precision of wide and cube scan measurements between swept-source and spectral-domain OCT in normal and glaucoma eyes. Sci Rep. (2023) 13:15876. doi: 10.1038/s41598-023-43230-7
13. Rabiolo, A, Fantaguzzi, F, Montesano, G, Brambati, M, Sacconi, R, Gelormini, F, et al. Comparison of retinal nerve Fiber layer and ganglion cell-inner plexiform layer thickness values using spectral-domain and swept-source OCT. Transl Vis Sci Technol. (2022) 11:27. doi: 10.1167/tvst.11.6.27
14. Feng, Y, Reinstein, DZ, Nitter, T, Archer, TJ, McAlinden, C, Chen, X, et al. Heidelberg anterion swept-source OCT corneal epithelial thickness mapping: repeatability and agreement with Optovue Avanti. J Refract Surg. (2022) 38:356–63. doi: 10.3928/1081597X-20220414-01
15. Wu, X, Tan, B, Gan, J, Lam, AR, Chen, Y, Liu, X, et al. Evaluation of different OCT systems in quantitative imaging of human Schlemm’s canal. Sci Rep. (2022) 12:1400. doi: 10.1038/s41598-022-05410-9
16. Lu, CD, Waheed, NK, Witkin, A, Baumal, CR, Liu, JJ, Potsaid, B, et al. Microscope-integrated intraoperative ultrahigh-speed swept-source optical coherence tomography for widefield retinal and anterior segment imaging. Ophthalmic Surg Lasers Imaging Retina. (2018) 49:94–102. doi: 10.3928/23258160-20180129-03
17. Zhang, L, van Dijk, EHC, Borrelli, E, Fragiotta, S, and Breazzano, MP. OCT and OCT angiography update: clinical application to age-related macular degeneration, central serous chorioretinopathy, macular telangiectasia, and diabetic retinopathy. Diagnostics. (2023) 13:232. doi: 10.3390/diagnostics13020232
18. Antcliff, RJ, and Marshall, J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. (1999) 14:223–32. doi: 10.3109/08820539909069541
19. Huang, X, Zhang, Z, Wang, J, Meng, X, Chen, T, and Wu, Z. Macular assessment of preoperative optical coherence tomography in ageing Chinese undergoing routine cataract surgery. Sci Rep. (2018) 8:5103. doi: 10.1038/s41598-018-22807-7
20. Moreno-Montanes, J, Olmo, N, Alvarez, A, García, N, and Zarranz-Ventura, J. Cirrus high-definition optical coherence tomography compared with stratus optical coherence tomography in glaucoma diagnosis. Invest Ophthalmol Vis Sci. (2010) 51:335–43. doi: 10.1167/iovs.08-2988
21. Kowallick, A, Fischer, CV, and Hoerauf, H. Optical coherence tomography findings in patients prior to cataract surgery regarded as unremarkable with ophthalmoscopy. PLoS One. (2018) 13:e0208980. doi: 10.1371/journal.pone.0208980
22. Yeu, E, Berdahl, JP, Gupta, PK, and Patterson, M. Sensitivity and specificity of SS-OCT for detecting macular pathologies vs SD-OCT. J Cataract Refract Surg. (2024) 50:481–5. doi: 10.1097/j.jcrs.0000000000001394
23. Waldstein, SM, Gerendas, BS, Montuoro, A, Simader, C, and Schmidt-Erfurth, U. Quantitative comparison of macular segmentation performance using identical retinal regions across multiple spectral-domain optical coherence tomography instruments. Br J Ophthalmol. (2015) 99:794–800. doi: 10.1136/bjophthalmol-2014-305573
24. Chen, TC, Cense, B, Pierce, MC, Nassif, N, Park, BH, Yun, SH, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. (2005) 123:1715–20. doi: 10.1001/archopht.123.12.1715
25. Zaharova, E, and Sherman, J. The use of SD-OCT in the differential diagnosis of dots, spots and other white retinal lesions. Eye Brain. (2011) 3:69–80. doi: 10.2147/EB.S23208
26. Zheng, F, Deng, X, Zhang, Q, He, J, Ye, P, Liu, S, et al. Advances in swept-source optical coherence tomography and optical coherence tomography angiography. Adv Ophthalmol Pract Res. (2023) 3:67–79. doi: 10.1016/j.aopr.2022.10.005
27. Potsaid, B, Baumann, B, Huang, D, Barry, S, Cable, AE, Schuman, JS, et al. Ultrahigh speed 1,050 nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt Express. (2010) 18:20029–48. doi: 10.1364/OE.18.020029
28. Wolf-Schnurrbusch, UE, Ceklic, L, Brinkmann, CK, Iliev, ME, Frey, M, Rothenbuehler, SP, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. (2009) 50:3432–7. doi: 10.1167/iovs.08-2970
29. Han, IC, and Jaffe, GJ. Comparison of spectral-and time-domain optical coherence tomography for retinal thickness measurements in healthy and diseased eyes. Am J Ophthalmol. (2009) 147:847–858.e1. doi: 10.1016/j.ajo.2008.11.019
30. Fang, D, Li, Q, Yan, K, Xu, S, Jiang, J, Che, X, et al. Retinal and choroidal thickness in relation to C-reactive protein on swept-source optical coherence tomography. J Immunol Res. (2021) 2021:6628224. doi: 10.1155/2021/6628224
31. Li, Q, Qian, Y, Xu, S, Zhang, M, Liang, X, Che, X, et al. Relationships of rheumatoid factor with thickness of retina and choroid in subjects without ocular symptoms using swept-source optical coherence tomography. J Immunol Res. (2021) 2021:5547533. doi: 10.1155/2021/5547533
32. Patel, PJ, Chen, FK, Ikeji, F, Xing, W, Bunce, C, da Cruz, L, et al. Repeatability of stratus optical coherence tomography measures in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. (2008) 49:1084–8. doi: 10.1167/iovs.07-1203
33. Patel, PJ, Chen, FK, da Cruz, L, and Tufail, A. Segmentation error in stratus optical coherence tomography for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. (2009) 50:399–404. doi: 10.1167/iovs.08-1697
Keywords: optical coherence tomography (OCT), spectral-domain OCT (SD-OCT), swept-source OCT (SS-OCT), retinal thickness measurement, consistency analysis
Citation: Wan H, Wu Z, Liu Z and Qin B (2025) Comparison of macular retinal thickness measurements using spectral-domain and swept-source optical coherence tomography in healthy eyes. Front. Med. 12:1529719. doi: 10.3389/fmed.2025.1529719
Received: 11 December 2024; Accepted: 21 January 2025;
Published: 31 January 2025.
Edited by:
Alessandro Meduri, University of Messina, ItalyReviewed by:
Laura De Luca, University of Messina, ItalyCopyright © 2025 Wan, Wu, Liu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziling Liu, emxsaXUyMkAxNjMuY29t; Bo Qin, cWluYm96ZkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.