- 1Department of Anaesthesiology, Xuzhou Cancer Hospital, Xuzhou, China
- 2Department of Anesthesiology, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, China
- 3Department of Pediatrics, Xuzhou Children’s Hospital, Xuzhou, China
- 4Department of Intensive Care Medicine, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
Background: Disability may be a potential adverse outcome of exposure to stressors in frail patients, and assessment of frailty may provide additional information for preoperative decision-making, but there is a lack of research on the impact of preoperative frailty on death or new disability after cardiac surgery. The main objective of this study was to evaluate the effect of preoperative frailty on short-term death or new disability after cardiac surgery in elderly individuals.
Patients and methods: This prospective cohort study included 351 patients aged ≥60 years who were scheduled to undergo elective open heart surgery at the Affiliated Hospital of Xuzhou Medical University from March 2023 to March 2024. Patients were examined prospectively using the Comprehensive Assessment of Frailty (CAF) score, which separated patients into frail and non-frail groups. The primary outcome was 90-day disability or death. Multivariate logistic regression models were used to estimate the association between frailty and 90-day new disability or death.
Results: An assessment of frailty was performed on 351 patients, and 325 patients were included in the final analysis. The prevalence of frailty was found to be 23.08%. New disability or death occurred within 90 days after surgery in 41 (12.6%) of our patients. In multivariate analysis, frailty [OR, 3.31; 95% CI, 1.43–7.62] was independently associated with 90-day new disability or death. Empirical ROC analysis showed that CAF (AUC = 0.762) predicted 90-day new disability or death postoperatively more reliably than the traditional risk assessment tools ASA + age (AUC = 0.656) and EuroSCORE II (AUC = 0.643).
Conclusion: The study demonstrates that preoperative frailty, bypass time, diabetes, BMI and EuroSCORE II are independent risk factors for 90-day new disability or death after cardiac surgery in elderly patients. Notably, frailty was a more effective predictor of 90-day new disability or death than the traditional risk predictors EuroSCORE II and ASA + age.
Introduction
Compared to other surgeries, cardiac surgery is characterized by high risk and difficulty and is a highly volatile event. Patients undergoing cardiac surgery are often exposed to strong external stressors, such as extracorporeal circulation, sternotomy, hypothermia, and prolonged anesthesia and operating time, which deal a severe blow to the body’s overall homeostasis and cause damage to vital organs. However, in recent years, with the advances in surgical techniques, anesthesia management and postoperative rehabilitation care, appropriate conditions have been created to improve the prognosis of cardiac surgery. Therefore, perioperative physicians should do an excellent job of preoperative risk assessment and take effective measures in all aspects of the perioperative period to mitigate the stress injury suffered by cardiac surgery patients. Common preoperative risk assessment tools for cardiac surgery include the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II), the Society of Thoracic Surgeons score (STS), and the ASA class. Still, these tools lack physiological assessment (1, 2).
Frailty is defined as a clinical condition in older people in which physiological reserves decline, resulting in increased vulnerability, decreased resistance to stress, and impaired ability to maintain or restore homeostasis after stress (3, 4). Previous studies have shown that the prevalence of frailty in elderly patients undergoing major surgery is approximately 20–40% (5, 6) and that elderly patients with preoperative comorbidities of frailty have higher rates of postoperative complications and mortality (7). However, it is reassuring to know that frailty is not a reversible functional state like age and comorbidities and that preoperative physiologic reserve can be increased with appropriate nutritional support and rehabilitation (8).
Due to the increasing standard of living, people are no longer only concerned with whether the surgery can be successful or not but rather with good independent mobility and quality of life after the surgery, and the desire to avoid a new disability is especially urgent. Disability is defined by the difficulty in performing activities of daily living or the development of various limitations. Currently, disability is often assessed using the standard sets of Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs) (9, 10), but both lack an assessment of social participation, which is included in the World Health Organization Disability Assessment Scale, second edition (WHODAS 2.0) (11, 12). Recent prospective studies have shown that preoperative disability is associated with patient self-reported death or new disability after surgery in non-cardiac patients (13, 14). Thus, disability may be a potential adverse outcome of exposure to stressors in frail patients, and assessment of frailty may provide additional information for preoperative decision-making, but there is a lack of research on the impact of preoperative frailty on death or new disability after cardiac surgery.
The main objective of this study was to evaluate the effect of preoperative frailty on short-term death or new disability after cardiac surgery in elderly individuals, to provide some reference value for perioperative risk assessment and decision-making, and to achieve the goal of improving patients’ postoperative recovery.
Materials and methods
Study design and study population
The study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2023-KL044-01) and registered in the Chinese Clinical Trials Registry (ChiCTR2300069382). We adhered to the reporting requirements established by the Strengthening the Reporting of Observational Studies in Epidemiology (15). Written informed consent was obtained from each participant prior to enrollment. Patients were recruited between March 2023 and March 2024.
Elderly (≥60 years) patients undergoing elective open heart surgery were eligible for enrollment. The exclusion criteria were as follows: Preoperative refusal to participate in this study or communication difficulties; inability to complete a frailty assessment due to absolute bed rest; preoperative comorbidities of severe hepatic and renal dysfunction; preoperative history of IABP (Intra-Aortic-Balloon-Pump), mechanical ventilation, or pacemaker implantation; those who are unable to be reached or refuse to cooperate with postoperative telephone follow-up.
Frailty and disability assessment
During the preoperative assessment, in addition to routine examinations, all consenting participants underwent an assessment of the CAF and the WHO Disability Assessment Scale 2.0 (WHODAS) by a researcher who was unaware of the content of the postoperative follow-up; the methodology of the CAF assessment is described in the study by Sündermann et al. (16). The CAF can be broadly viewed as a composite of three frailty assessment scales [FP (Frailty Phenotype) (17), CFS (Canadian Clinical Frailty Scale) (18), and MPPT (Modified Physical Performance Test)], consisting primarily of biomarker assessments (serum albumin level, serum creatinine level, BMI, and FEV1), physical tests of fatigue, activity level, gait speed, grip strength, and balance stability, and including measures of the CFS. The total score of the CAF was 35 points, with patients scoring ≥11 being frail and those scoring <11 being non-frail.
The baseline assessment of preoperative disability was determined using the WHO Disability Assessment Scale 2.0 (WHODAS) (11–13). The WHODAS 2.0 consists of six main domains: mobility, self-care, getting by, cognition, and social participation. The 12 items of the WHODAS are scored as described in previous studies, with each item having a numerical value: none = 0; mild = 1; moderate = 2; severe = 3; and extreme = 4. The total score ranges from 0 (no disability) to 48 (total disability or death) and is then divided by 48 and multiplied by 100 to give a disability score of 0 (no disability or death). mild = 2; severe = 3; extreme = 4. The total score ranged from 0 (no disability) to 48 (total disability or death), then divided by 48 and multiplied by 100 to convert to a percentage of the disability score. Preoperative disability was defined as a WHODAS 2.0 total score percentage greater than or equal to 25%.
Definition of outcomes
The primary outcome was the relationship between preoperative frailty and new disability or death at 3 months postoperatively. Postoperative patient follow-up was performed by an anesthesiologist who was unaware of the results of the preoperative evaluation. Telephone follow-up and electronic medical record documentation were the primary means of determining patients’ postoperative survival status. New disability at 90 days was defined as the occurrence of a WHODAS 2.0 ≥ 25% postoperatively in patients who were not disabled preoperatively; if the patient had a preoperative comorbid disability (WHODAS ≥ 25%), an increase in the percentage of WHODAS scores by 8 at 90 days postoperatively indicated the occurrence of new disability (11–13).
Secondary outcomes included (1) 90-day disability-free survival (DFS), (2) ICU length of stay, (3) postoperative non-hospital discharge, (4) postoperative length of stay, (5) major morbidity (19, 20) (Supplementary Table S1), (6) incidence of PPCs (21), (7) 90-day readmission rate, and (8) 90-day mortality.
We conducted a post hoc study to compare the validity of the CAF with the commonly used FP and CFS in predicting 90-day new disability or death. We asked whether the predictive validity of the traditional and rapidly assessable preoperative frailty scales (FP and CFS) would achieve the same categorical properties as the CAF, because the preoperative assessment of the CAF is time-consuming.
Other variables
All patients were managed according to standard cardiac surgery protocols, with pre-operative perioperative risk assessment followed by anesthesia for surgery, post-operative transfer to the surgical intensive care unit, and after stabilization of vital signs, transfer to the general surgical ward for further management and routine post-operative rehabilitation for all patients.
Baseline clinical and demographic data were collected according to the protocol, including sex, age, smoking and alcohol consumption, comorbidities (diabetes, hypertension, myocardial infarction, etc.), ASA class, EuroSCORE II, pulmonary function (FEV1, FVC, and FEV1/FVC), type of surgery, and left ventricular ejection fraction (LVEF). Operating time, Cross-clamp time, bypass time, urine volume, blood loss, total fluid intake, and total fluid output were recorded during surgery. Data on ICU stay, postoperative hospital stay, non-hospital discharge, 90-day Major Morbidity, and readmission within 90 days were collected.
Statistical analysis
Data normality was tested by visual inspection of histograms and Shapiro–Wilk’s W test. All normally distributed and skewed continuous variables were expressed as mean (SD) or median (interquartile range [IQR]). Categorical variables were indicated as frequencies (%). Comparison of continuous variables among groups was performed with the use of the Student’s t-test or Mann–Whitney U-test, depending on the normality of the distribution. In contrast, the Fisher’s Exact test was used to compare categorical variables.
A Least Absolute Shrinkage and Selection Operator regression analysis was conducted with statistically significant risk factors included in the univariable study to remove non-zero characteristic components. After that, a multivariate logistic regression analysis (stepwise regression method) was used to identify the 90-day new disability or death risk variables. Internal validation was carried out using the bootstrap self-sampling technique (1,000 bootstrap samples repeatedly sampled), and the model’s discrimination was tested using the relatively adjusted C-index (concordance index). The calibration curve was drawn to evaluate the model’s consistency. In addition, the inverse probability treatment weighting (IPTW) approach was used for two groups to adjust for observed possible confounding factors. Multivariable logistic regression analyses were used to obtain the IPTW-adjusted odds ratio (OR) in the IPTW-adjusted cohort. The predictive validity was assessed using the area under the receiver operating characteristic (ROC) curve (AUC). An AUC of 0.5–0.7 implies poor prediction accuracy, whereas an AUC of 0.7–0.9 suggests high prediction performance. The DeLong test compared the AUC of different models.
p-value < 0.05 (two-sided) was considered statistically significant. R4.1.2 and MedCalc 20.0. Statistical software was used for analysis.
Sample size calculation
Based on the pre-test, the incidence of death or new disability at 90 days after cardiac surgery in elderly individuals is about 10%, the multivariate regression model includes at least 5 outcome events for each variable (22), a total sample size of 250 is required (5*5/10% = 250), and considering the 15% dropout rate, 250/(1–15%) = 295 patients are proposed to be included in this study.
Missing data
We used complete cases for the initial analysis, and preoperative baseline data were complete for all participants. A total of 351 individuals were included in the study, and final data were complete for 325 individuals. Our overall proportion of missing values was small, our analysis was based on all available data without imputation.
Results
Baseline characteristics
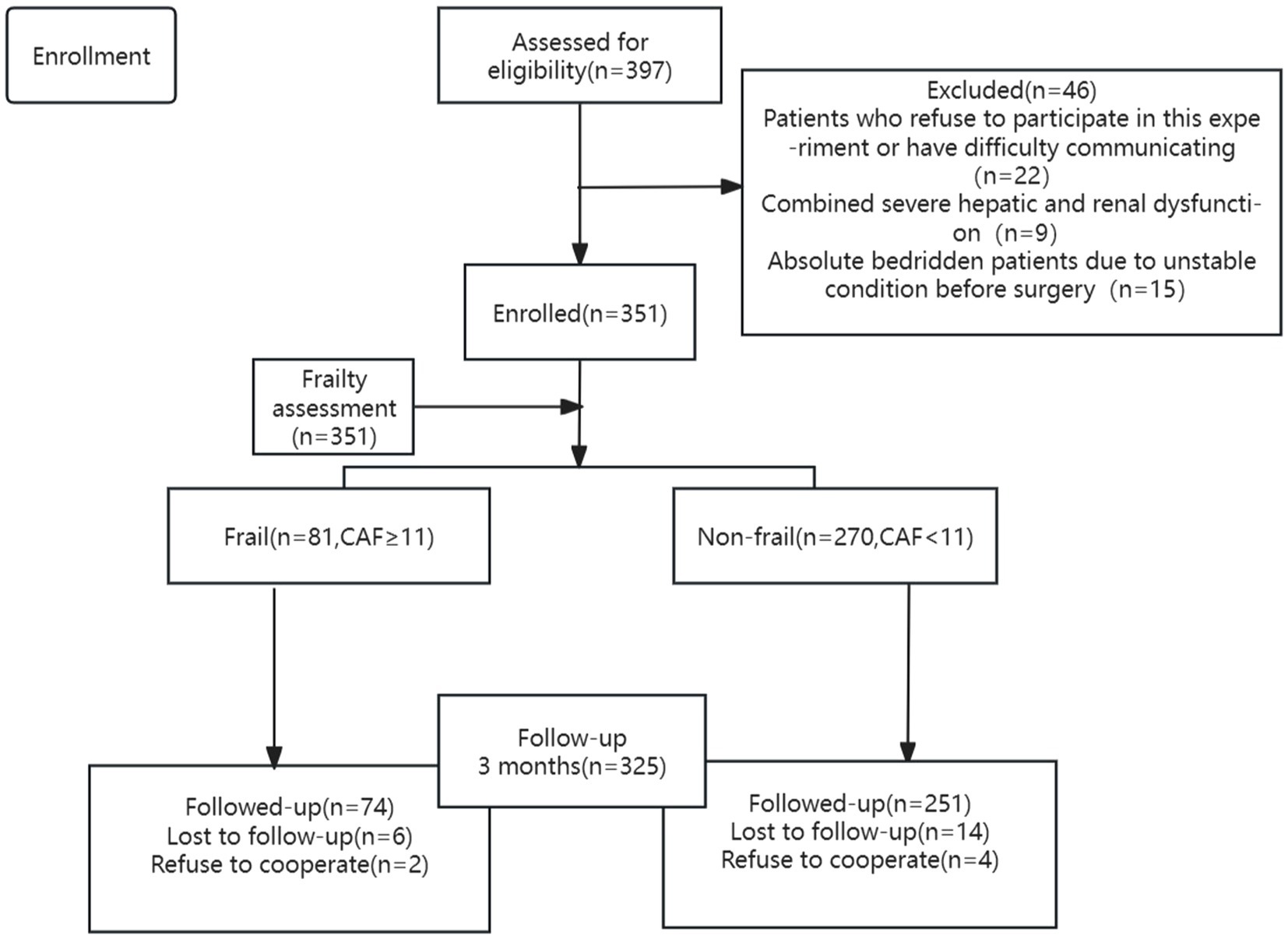
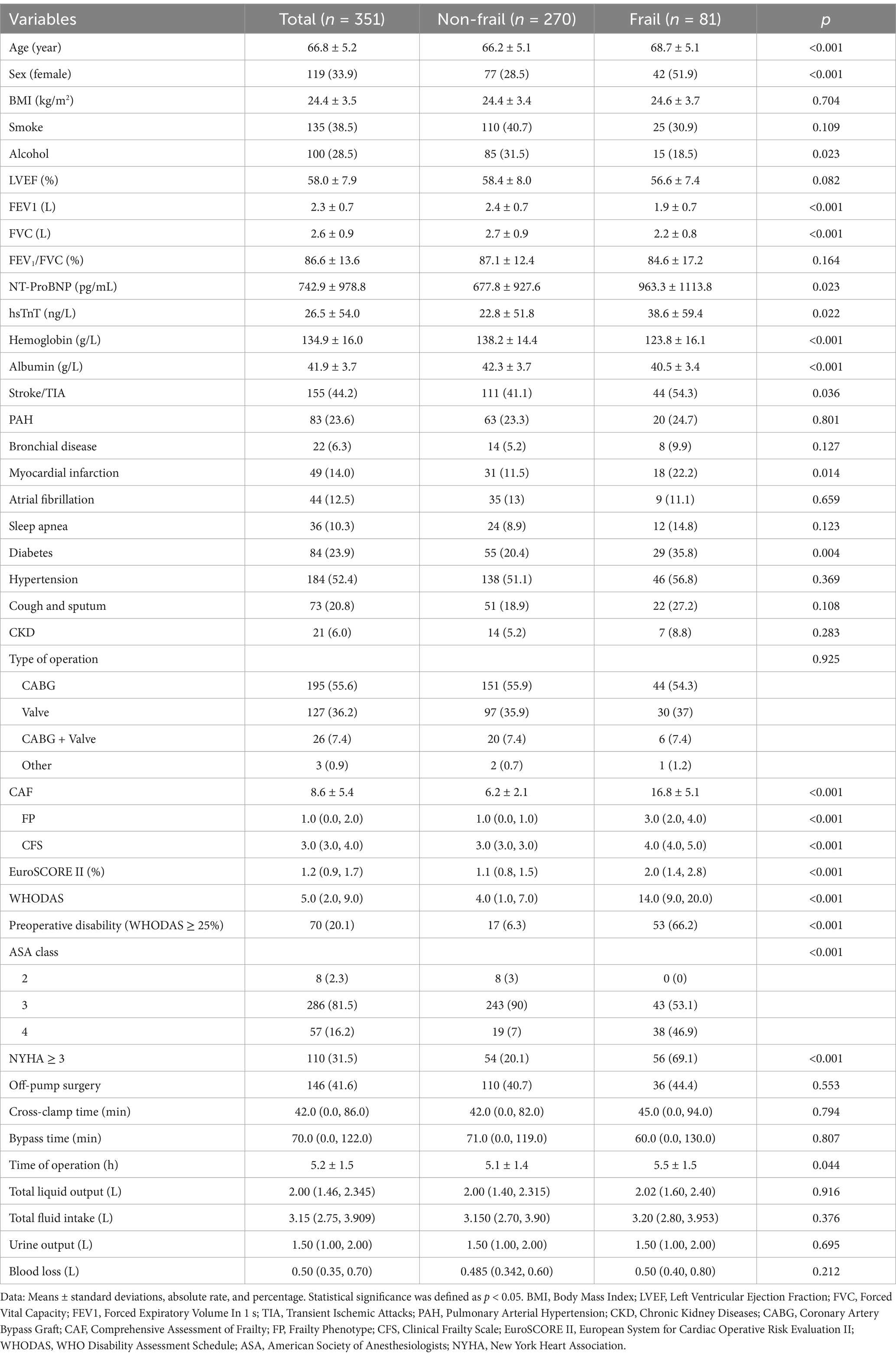
An assessment of frailty was performed on 351 patients, and 325 patients were included in the final analysis (Figure 1). The prevalence of frailty was found to be 23.08%. Table 1 shows that age, proportion of female, NT-ProBNP, hsTnT, stroke/TIA, myocardial infarction, diabetes, proportion of preoperative disability, NYHA class, ASA class, EuroSCORE II, and duration of surgery were higher in patients in the frail group compared with those in the non-frail group. The rates of alcohol consumption, hemoglobin, albumin, FEV1, and FVC were lower (p < 0.05), while all other factors were not statistically different (p > 0.05).
Associations of frailty with post-operative outcomes
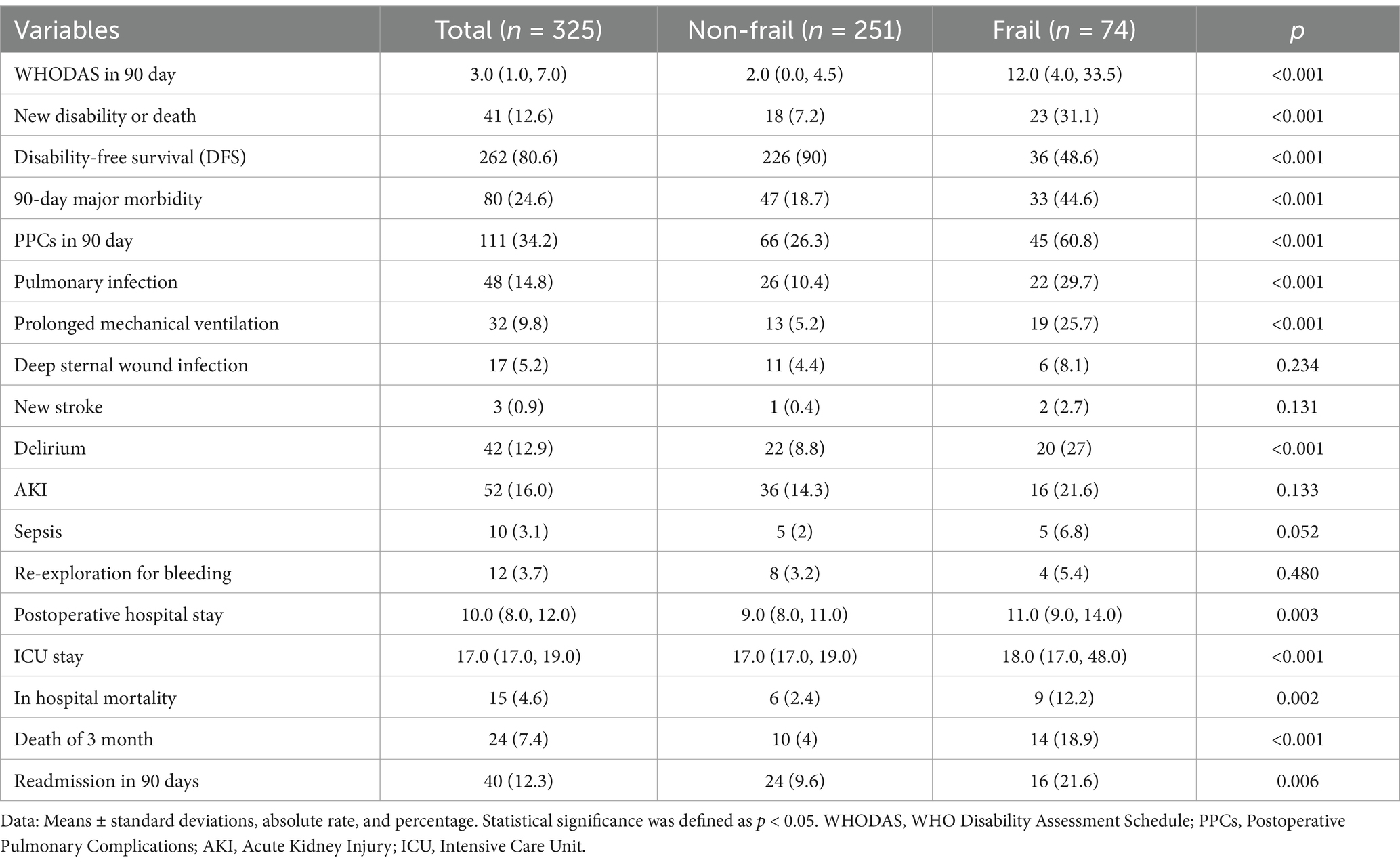
New disability or death occurred within 90 days after surgery in 41 (12.6%) of our patients, including 23 (33.1%) in the frail group and 18 (7.2%) in the non-frail group of patients; there was a significant difference in new disability or death at 90 days between the groups (Table 2, p < 0.05). Table 2 shows that patients in the frail group had higher WHODAS scores, postoperative pulmonary complications, major morbidity at 90 days, ICU stay, postoperative hospitalization, in-hospital mortality, mortality within 90 days, and incidence of readmission within 90 days (p < 0.05), while disability-free survival (DFS) at 90 days postoperatively was lower (p < 0.05).
Risk factors associated with 90-day new disability or death
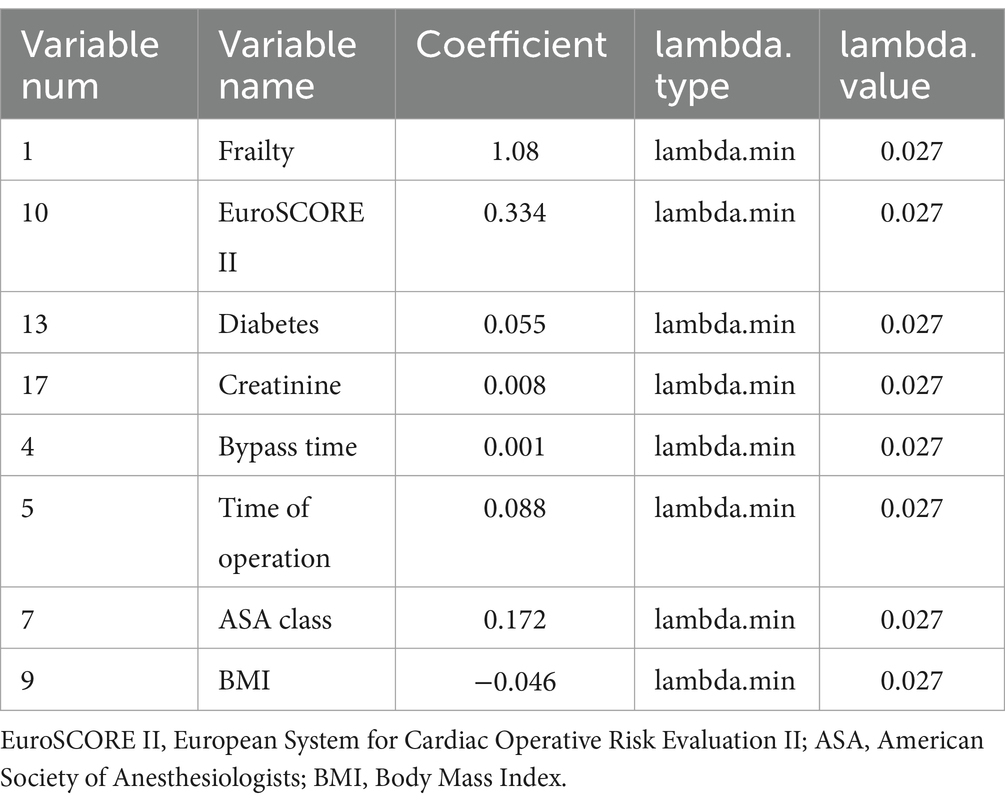
According to the Least Absolute Shrinkage and Selection Operator regression analysis (Figure 2), we selected eight non-zero characteristic variables including frailty, EuroSCORE II, diabetes, Creatinine, ASA class, BMI, time of operation and bypass time (Table 3). Then, taking these eight predictors and conducting a multifactor logistic regression using a stepwise regression method approach, five meaningful variables were finally identified (Figure 2). In multivariate analysis, frailty [OR, 3.31; 95% CI, 1.43–7.62], bypass time (OR, 1.01; 95% CI, 1.00–1.01), BMI (OR, 0.87; 95% CI, 0.77–0. 98), diabetes (OR, 3.47; 95% CI, 1.40–8.55), and EuroSCORE II (OR, 1.75; 95% CI, 1.20–2.57) were independently associated with 90-day new disability or death (Figure 3). Frailty (OR, 3.15; 95% CI, 1.55–6.43) was also independently associated with 90-day new disability or death in a multivariate analysis of the inverse probability weighted adjusted cohort (Table 4).
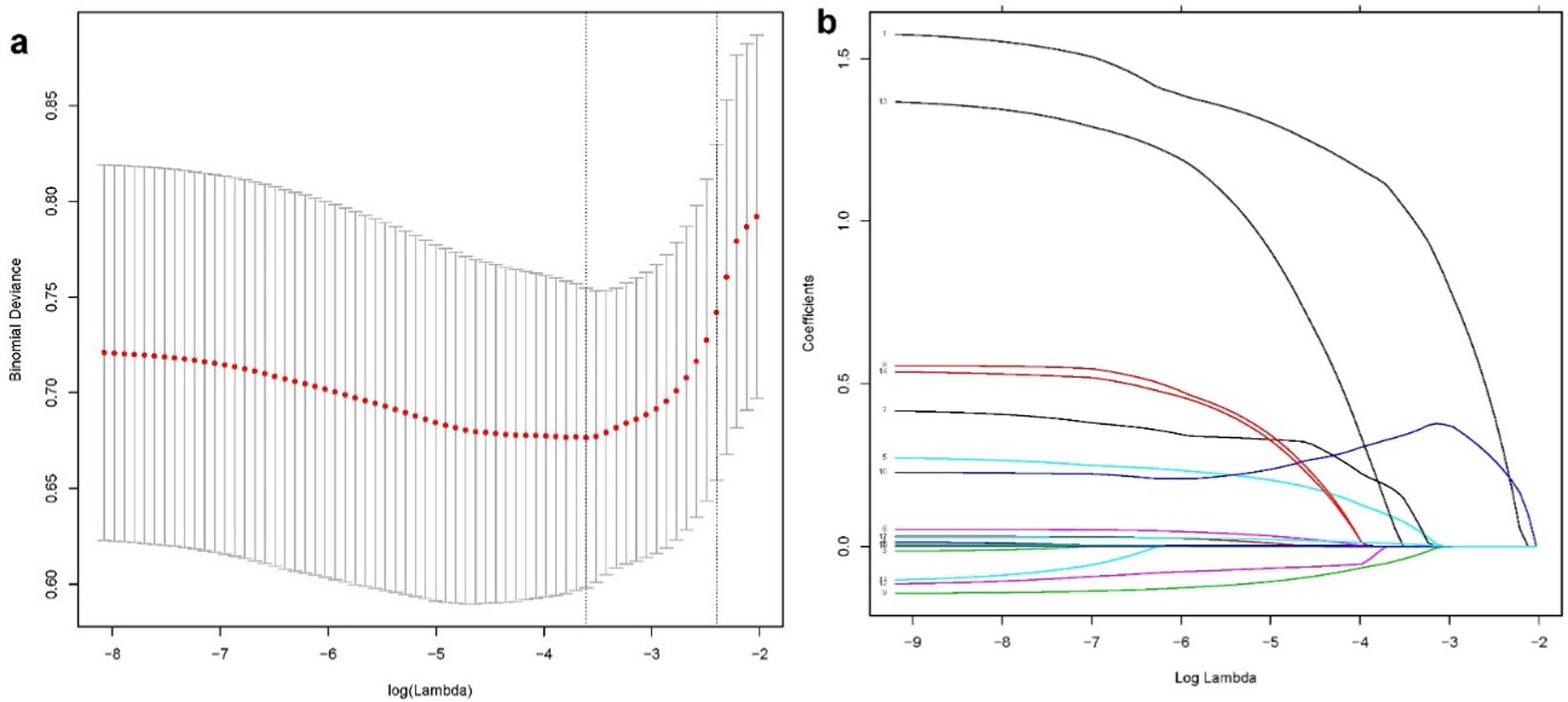
Figure 2. The LASSO binary logistic regression model is used to select variables. A coefficient profile plot (A) was used to display the log (lambda) series. Eight variables with nonzero coefficients were chosen using optimal lambda. To confirm the optimal parameter (lambda) in the LASSO model, the partial likelihood deviance (binomial deviance) curve was plotted against log (lambda), and dotted vertical lines were drawn based on a min criteria (B).
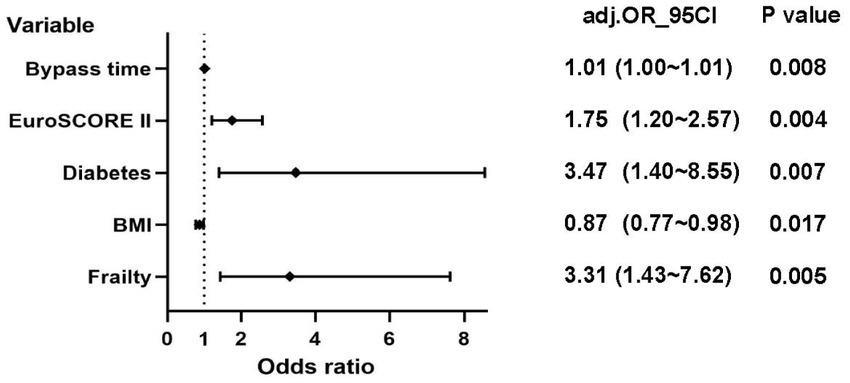
Figure 3. Logistic regression analysis of 90-day new disability or death (EuroSCORE II: European System for Cardiac Operative Risk Evaluation II; BMI: Body Mass Index).

Table 4. Associations between frailty and 90-day new disability or death in the crude analysis, multivariable analysis, and propensity-score analyses.
Model validation: We reported a bias-corrected concordance statistic to verify the internal validity of our primary model utilizing calibration and discrimination with a 1,000-sample bootstrapping approach. Our calibration curve revealed that our model was well-calibrated (Supplementary Figure S1). With a C-statistic of 0.824 and an optimism-corrected C-statistic of 0.810, the discriminative ability demonstrated strong model performance in predicting 90-day new disability or death.
Predictability of postoperative 90-day new disability or death, DFS, PPCs and 90-day major morbidity by different risk assessment tools
Empirical ROC analysis showed that CAF (AUC = 0.762) predicted 90-day new disability or death postoperatively more reliably than the traditional risk assessment tools ASA + age (AUC = 0.656), EuroSCORE II (AUC = 0.643), and WHODAS (AUC = 0. 662). The difference in the area under the curve between the first three methods and CAF was significant (p values of 0.039, 0.019, and 0.029, corresponding to z values of 2.064, 2.350, and 2.190, respectively). It can also be seen that the CAF was also a better predictor of DFS among the four risk assessment tools (AUC = 0.799), but was a poorer predictor of PPCs and 90-day major morbidity for all four risk assessment tools (AUC < 0.700) (Figure 4).
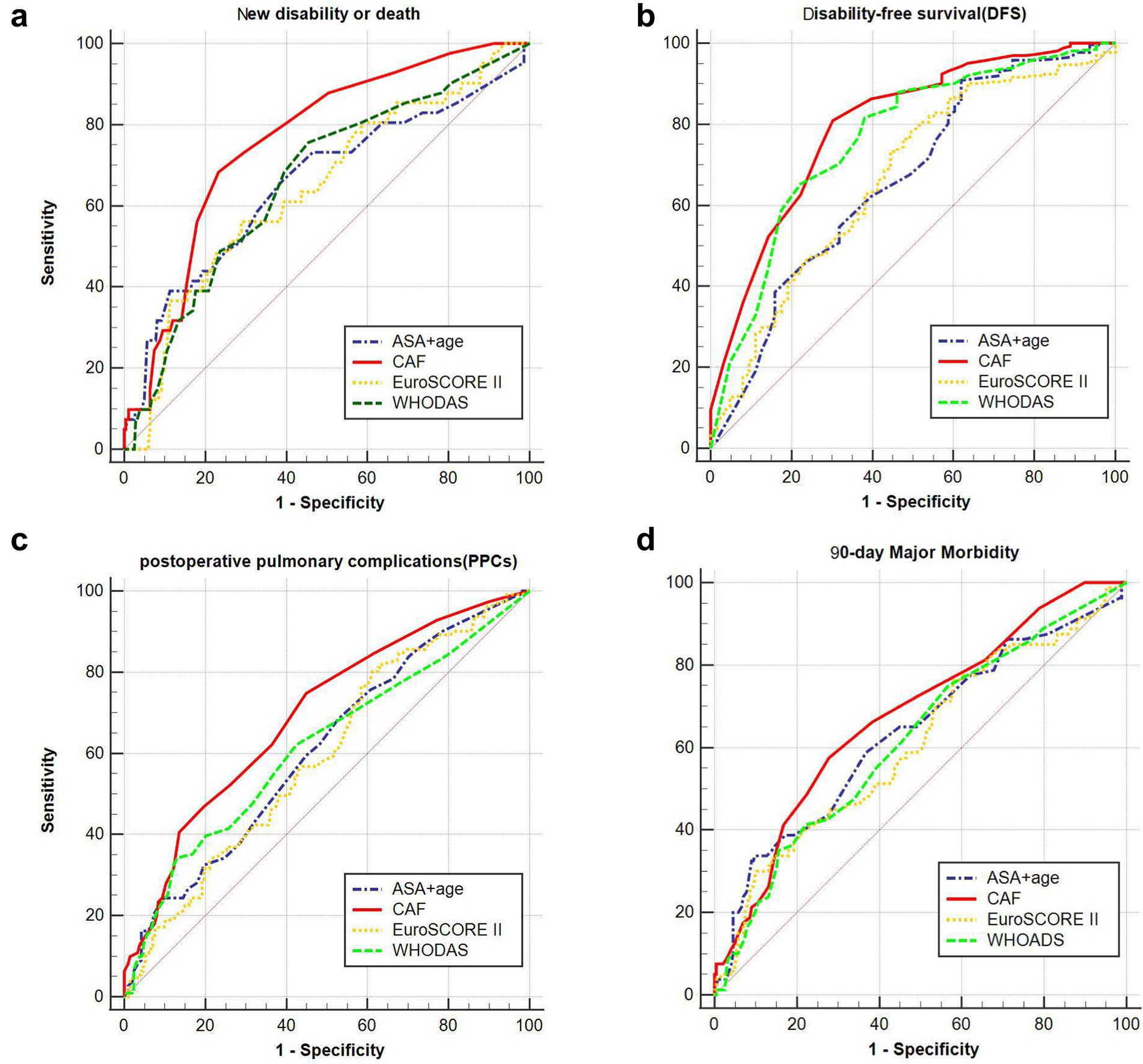
Figure 4. Prediction of different risk indices at different complications and 90-day new disability or death. (A) The area under the curve of chart-derived CAF, ASA + age, EuroSCORE II and WHODAS for DFS was 0.762 (95% CI, 0.712–0.807), 0.656 (95% CI, 0.602–0.708), 0.643 (95% CI, 0.588–0.695), and 0.662 (95% CI, 0.608–0.714), respectively. (B) The area under the curve of chart-derived CAF, ASA + age, EuroSCORE II and WHODAS for 90-day new disability or death was 0.799 (95% CI, 0.752–0.841), 0.668 (95% CI, 0.614–0.719), 0.668 (95% CI, 0.614–0.719), and 0.765 (95% CI, 0.715–0.810), respectively. (C) The area under the curve of chart-derived CAF, ASA + age, EuroSCORE II and WHODAS for PPCs was 0.694 (95% CI, 0.641–0.744), 0.608 (95% CI, 0.553–0.662), 0.594 (95% CI, 0.538–0.647), and 0.614 (95% CI, 0.559–0.668), respectively. (D) The area under the curve of chart-derived CAF, ASA + age, EuroSCORE II and WHODAS for 90-day Major Morbidity was 0.678 (95% CI, 0.624–0.728), 0.634 (95% CI, 0.579–0.687), 0.608 (95% CI, 0.552–0.661), and 0.619 (95% CI, 0.563–0.672), respectively (EuroSCORE II: European System for Cardiac Operative Risk Evaluation II; WHODAS WHO Disability Assessment Schedule; ASA American Society of Anesthesiologists; CAF: Comprehensive assessment of frailty score).
Post hoc outcomes
Compared to the common clinical frailty assessment scales (CFS and FP), the CAF (AUC = 0.762) had superior predictive efficacy compared to CFS (AUC = 0.696) and FP (AUC = 0.686) (p-values of 0.036 and 0.005, corresponding to z-values of 2.096 and 2.819, respectively, Supplementary Figure S2).
Discussion
The study demonstrates that preoperative frailty, bypass time, diabetes, BMI, and EuroSCORE II are independent risk factors for 90-day new disability or death after cardiac surgery in elderly patients. Notably, frailty was a more effective predictor of 90-day new disability or death than the traditional risk predictors EuroSCORE II and ASA + age.
The study indicates that frailty is an independent risk factor for new disability or death after cardiac surgery in the elderly, which is consistent with other studies. In a multicenter cohort study of 702 patients undergoing elective noncardiac surgery, frail patients had statistically significantly higher rates of new 90-day disability or death, longer hospital stays, and need for institutional discharge compared to other patient groups (13). However, this study’s surgical procedures did not include cardiac surgery. Therefore, the results cannot be applied to patients undergoing cardiac surgery. Another single-center cohort study (6) of 146 adult patients undergoing elective open-heart surgery also found that preoperative frailty significantly reduced patients’ disability-free survival at 90 days postoperatively, leading to a decrease in postoperative functional recovery and quality of life. This study, while insightful about the relationship between preoperative frailty and disability-free survival at 90 days postoperatively in patients undergoing cardiac surgery, did not have sufficient efficacy (the sample size was only 145) to explore whether frailty was an independent risk factor for new disability or death at 90 days postoperatively and to rule out the effect of preoperative patients’ comorbid disability on their postoperative functional status. The mechanisms behind frailty and postoperative self-reported disability in elderly cardiac surgery patients are unclear. These issues may be related to chronic inflammation, aging of the immune system, and endocrine dysregulation (23–25).
This study showed that the prevalence of preoperative frailty in elderly patients was 23.08%, which is consistent with previous reports in the literature (6, 16). The clinical significance of assessing preoperative frailty is to assist the perioperative physician in recognizing the patient’s preoperative risk level and making rational clinical decisions. Overall, in order to improve the prognosis of frail patients, multidisciplinary collaboration is more important (26). In frail patients at higher risk for poor functional outcomes, the cardiac surgeon decides whether minimally invasive treatment is possible (27, 28), the anesthesiologist enhances intraoperative monitoring of the patient’s vital organs (e.g., enhanced cerebral oximetry monitoring, lung-protective ventilation, and ultrasound-guided goal-directed fluid therapy) (29) and the nursing team initiates an early tailored Customized functional recovery programs (intensive pulmonary physiotherapy, early ambulation, resistance training, and nutritional support) should be initiated early by the nursing team (30). If frail patients are more stable preoperatively, we believe it is also essential to delay surgery appropriately, and studies have shown that preoperative functional rehabilitation led by the rehabilitation department can increase the patient’s physiologic reserve, thereby increasing stress resistance (30). The study also discovered that conventional risk assessment metrics, such as EuroSCORE II and ASA + age, were inadequate predictors of patient-centered outcomes, such as new disability or death. The ROC analysis clearly showed that frailty, as defined by CAF, is a more reliable predictor of adverse postoperative outcomes in elderly cardiac patients than EuroSCORE and ASA + age. Therefore, adding frailty measures, particularly CAF, to the traditional perioperative risk scoring system may improve the ability of perioperative physicians to predict relevant clinical outcomes in patients.
The present study also demonstrated that bypass time, BMI, diabetes, and EuroSCORE II were independent risk factors for 90 days after cardiac surgery in elderly patients. Longer bypass time are known to be more fatal for elderly and frail cardiac surgery patients. Previous studies (31) have shown that prolonged bypass time reflects the complexity of the surgical maneuver and may exacerbate damage to vital organs, with adverse prognostic consequences for elderly patients. In this study, only 4.9% of elderly patients were obese (BMI ≥ 30 kg/m2). On this basis, we found that higher BMI was associated with lower disability or death. Previous studies (32) have shown that lower body weight and obesity are associated with poor prognosis after cardiac surgery (U-shaped relationship between BMI and all-cause mortality). That said, in the present study, there were fewer obese patients whose positive association with poor outcome was masked, thus showing overall that a slightly higher BMI may contribute to survival. Diabetes is characterized by long-term insulin resistance, compensatory hyperinsulinemia, and varying degrees of hyperglycemia (33). Previous studies (34) have confirmed that diabetes is associated with patient prognosis. Patients with hyperglycemia are at increased risk for surgical site infection, pneumonia, delirium, and mortality.
There are some limitations to our study. First, the present study was a single-center, small-sample observational study with only a short-term postoperative follow-up, and a large multicenter sample is needed in the future to validate the conclusions and explore the relationship between frailty and long-term postoperative disability trajectory. Second, the CAF involves several aspects and may be time-consuming to assess preoperatively, but the post hoc analysis of the present study demonstrated that the CAF, although time-consuming, is a better predictor of postoperative outcomes than conventional frailty assessment scales (FP and CFS), so the CAF is recommended for the very high-risk elderly cardiac surgery population. Third, this study excluded patients who were completely bedridden preoperatively. Fourth, this study’s predictors and outcome indicators were human-rated scales, both of which are somewhat subjective. In addition, due to the nature of observational studies, there may be confounding factors that cannot be assessed.
Conclusion
In conclusion, we found that preoperative frailty, prolonged bypass time, diabetes, BMI, and EuroSCORE II were independent risk factors for 90-day new disability or death after cardiac surgery in elderly patients. Frailty was more effective in predicting 90-day new disability or death than the traditional risk predictors EuroSCORE II and ASA + age. Preoperative assessment of frailty can assist the perioperative team in preoperative clinical decision making and provide medical support throughout the course of an elderly frail patient scheduled for cardiac surgery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated Hospital of Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WS: Conceptualization, Data curation, Funding acquisition, Resources, Visualization, Writing – review & editing. QP: Project administration, Resources, Software, Writing – review & editing. CZ: Investigation, Resources, Software, Writing – review & editing. WZ: Data curation, Formal analysis, Investigation, Writing – review & editing. GF: Conceptualization, Formal analysis, Funding acquisition, Methodology, Software, Supervision, Visualization, Writing – review & editing. SZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Promoting Scientific and Technological Innovation Project of Xuzhou City (KC22149). The funds are for Shanshan Zhu.
Acknowledgments
We sincerely thank all the patients in this study and reviewers for their insightful suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1526896/full#supplementary-material
References
1. Duchnowski, P, Hryniewiecki, T, Kuśmierczyk, M, and Szymanski, P. Performance of the EuroSCORE II and the Society of Thoracic Surgeons score in patients undergoing aortic valve replacement for aortic stenosis. J Thorac Dis. (2019) 11:2076–81. doi: 10.21037/jtd.2019.04.48
2. Biancari, F, Vasques, F, Mikkola, R, Martin, M, Lahtinen, J, and Heikkinen, J. Validation of EuroSCORE II in patients undergoing coronary artery bypass surgery. Ann Thorac Surg. (2012) 93:1930–5. doi: 10.1016/j.athoracsur.2012.02.064
3. Morley, JE, Vellas, B, van Kan, GA, Anker, SD, Bauer, JM, Bernabei, R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. (2013) 14:392–7. doi: 10.1016/j.jamda.2013.03.022
4. Cleveland, JC Jr. Frailty, aging, and cardiac surgery outcomes: the stopwatch tells the story. J Am Coll Cardiol. (2010) 56:1677–8. doi: 10.1016/j.jacc.2010.07.021
5. Afilalo, J, Mottillo, S, Eisenberg, MJ, Alexander, KP, Noiseux, N, Perrault, LP, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. (2012) 5:222–8. doi: 10.1161/CIRCOUTCOMES.111.963157
6. Milne, B, Lucas de Carvalho, J, Ayis, S, Chaubey, S, Khan, H, and Kunst, G. Frailty and perioperative patient-reported disability in patients undergoing cardiac surgery: a pilot study. Br J Anaesth. (2022) 128:949–58. doi: 10.1016/j.bja.2022.03.015
7. McIsaac, DI, Mac Donald, DB, and Aucoin, SD. Frailty for perioperative clinicians: a narrative review. Anesth Analg. (2020) 130:1450–60. doi: 10.1213/ANE.0000000000004602
8. Dent, E, Martin, FC, Bergman, H, Woo, J, Romero-Ortuno, R, and Walston, JD. Management of frailty: opportunities, challenges, and future directions. Lancet. (2019) 394:1376–86. doi: 10.1016/S0140-6736(19)31785-4
9. Gerrard, P. The hierarchy of the activities of daily living in the Katz index in residents of skilled nursing facilities. J Geriatr Phys Ther. (2013) 36:87–91. doi: 10.1519/JPT.0b013e318268da23
10. Graf, C. The Lawton instrumental activities of daily living scale. Am J Nurs. (2008) 108:52–62. doi: 10.1097/01.NAJ.0000314810.46029.74
11. Shulman, MA, Myles, PS, Chan, MT, McIlroy, DR, Wallace, S, and Ponsford, J. Measurement of disability-free survival after surgery. Anesthesiology. (2015) 122:524–36. doi: 10.1097/ALN.0000000000000586
12. Ustün, TB, Chatterji, S, Kostanjsek, N, Rehm, J, Kennedy, C, Epping-Jordan, J, et al. Developing the World Health Organization disability assessment schedule 2.0. Bull World Health Organ. (2010) 88:815–23. doi: 10.2471/BLT.09.067231
13. McIsaac, DI, Taljaard, M, Bryson, GL, Beaulé, PE, Gagné, S, Hamilton, G, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann Surg. (2020) 271:283–9. doi: 10.1097/SLA.0000000000002967
14. McIsaac, DI, Taljaard, M, Bryson, GL, Beaulé, PE, Gagne, S, Hamilton, G, et al. Frailty and long-term postoperative disability trajectories: a prospective multicentre cohort study. Br J Anaesth. (2020) 125:704–11. doi: 10.1016/j.bja.2020.07.003
15. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
16. Sündermann, S, Dademasch, A, Praetorius, J, Kempfert, J, Dewey, T, Falk, V, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. (2011) 39:33–7. doi: 10.1016/j.ejcts.2010.04.013
17. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.m146
18. Church, S, Rogers, E, Rockwood, K, and Theou, O. A scoping review of the clinical frailty scale. BMC Geriatr. (2020) 20:393. doi: 10.1186/s12877-020-01801-7
19. Afilalo, J, Eisenberg, MJ, Morin, JF, Bergman, H, Monette, J, Noiseux, N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. (2010) 56:1668–76. doi: 10.1016/j.jacc.2010.06.039
20. Lagier, D, Fischer, F, Fornier, W, Huynh, TM, Cholley, B, Guinard, B, et al. Effect of open-lung vs conventional perioperative ventilation strategies on postoperative pulmonary complications after on-pump cardiac surgery: the PROVECS randomized clinical trial. Intensive Care Med. (2019) 45:1401–12. doi: 10.1007/s00134-019-05741-8
21. Hulzebos, EH, Helders, PJ, Favié, NJ, De Bie, RA, de la Riviere, AB, and Van Meeteren, NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. (2006) 296:1851–7. doi: 10.1001/jama.296.15.1851
22. Vittinghoff, E, and McCulloch, CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. (2007) 165:710–8. doi: 10.1093/aje/kwk052
23. Ferrucci, L, and Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
24. Soysal, P, Arik, F, Smith, L, Jackson, SE, and Isik, AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. (2020) 1216:55–64. doi: 10.1007/978-3-030-33330-0_7
25. Clegg, A, and Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. (2018) 6:743–52. doi: 10.1016/S2213-8587(18)30110-4
26. Whiteman, AR, Dhesi, JK, and Walker, D. The high-risk surgical patient: a role for a multi-disciplinary team approach? Br J Anaesth. (2016) 116:311–4. doi: 10.1093/bja/aev355
27. Mack, MJ. Minimally invasive cardiac surgery. Surg Endosc. (2006) 20:S488–92. doi: 10.1007/s00464-006-0110-8
28. Salenger, R, Lobdell, K, and Grant, MC. Update on minimally invasive cardiac surgery and enhanced recovery after surgery. Curr Opin Anaesthesiol. (2024) 37:10–5. doi: 10.1097/ACO.0000000000001322
29. Engelman, DT, Ben Ali, W, Williams, JB, Perrault, LP, Reddy, VS, Arora, RC, et al. Guidelines for perioperative Care in Cardiac Surgery: enhanced recovery after surgery society recommendations. JAMA Surg. (2019) 154:755–66. doi: 10.1001/jamasurg.2019.1153
30. Coca-Martinez, M, Lopez-Hernandez, A, Montane-Muntane, M, Arguis, M, Gimeno-Santos, E, Navarro-Ripoll, R, et al. Multimodal prehabilitation as strategy for reduction of postoperative complications after cardiac surgery: a randomised controlled trial protocol. BMJ Open. (2020) 10:e039885. doi: 10.1136/bmjopen-2020-039885
31. Salis, S, Mazzanti, VV, Merli, G, Salvi, L, Tedesco, CC, Veglia, F, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. (2008) 22:814–22. doi: 10.1053/j.jvca.2008.08.004
32. Liu, X, Xie, L, Zhu, W, and Zhou, Y. Association of body mass index and all-cause mortality in patients after cardiac surgery: a dose-response meta-analysis. Nutrition. (2020) 72:110696. doi: 10.1016/j.nut.2019.110696
33. Barsness, GW, Peterson, ED, Ohman, EM, Nelson, CL, DeLong, ER, Reves, JG, et al. Relationship between diabetes mellitus and long-term survival after coronary bypass and angioplasty. Circulation. (1997) 96:2551–6. doi: 10.1161/01.cir.96.8.2551
Keywords: aging, frailty, disability, cardiac surgery, patient-centered outcome
Citation: Ma W, Shui W, Peng Q, Zhu C, Zhao W, Fan G and Zhu S (2025) Impact of preoperative frailty on new disability or death after cardiac surgery in elderly patients: a prospective cohort study. Front. Med. 12:1526896. doi: 10.3389/fmed.2025.1526896
Edited by:
Emiliana Giacomello, University of Trieste, ItalyReviewed by:
Ildiko Toth, University of Pécs, HungaryGiovanni Quinto Villani, San Giacomo Rehabilitation Hospital, Italy
Copyright © 2025 Ma, Shui, Peng, Zhu, Zhao, Fan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanshan Zhu, eHp6c3NAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Wenwen Ma
Wenwen Ma Weikang Shui1†
Weikang Shui1†