
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 14 March 2025
Sec. Gastroenterology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1523870
This article is part of the Research Topic Improving the Gut Microbiome: Applications of Fecal Transplantation in Disease - Volume II View all 3 articles
Recently, microbiome medicine has attracted the attention of researchers. While this rapidly growing medical approach for various diseases and disorders is changing the paradigm, it is imperative to weigh both its benefits and the associated risk factors. For instance, manipulation of the gut microbiota (GM) has positive effects on metabolic and neurodegenerative diseases. Notably, fecal microbiota transplantation (FMT), a complex method, has shown promise; however, many doubt its feasibility without adverse effects on human health. Given the number of human clinical trials investigating FMT for the treatment of various disorders, this review summarizes recent findings on its impact on human health. This review summarizes the metabolic responses associated with FMT and their reversal effects on gastrointestinal infections, behavioral changes, and immune responses. Additionally, this review discusses the role of FMT in antimicrobial resistance and its co-supplementation effects on human health, safety, potential risks, limitations, prospects, and recommendations. Although this review does not cover all the studies in the database, the searched terms for FMT and human health in clinical trials are sufficient to provide a summary of the current perspective.
Fecal microbiota transplantation (FMT) is a therapeutic intervention that involves transferring processed stool from a healthy donor to restore the balance of gut microbiota (GM). Despite the complexity of microbial taxa and their interactions with the human immune system, FMT has emerged as a promising standard of care (1). Unlike dietary interventions that gradually enrich short-chain fatty acid (SCFAs)-producing taxa, FMT enables the rapid restructuring of gut microbial communities through direct microbial transfer. This approach has shown systemic benefits ranging from metabolic regulation (e.g., blood pressure and glucose homeostasis) to suppression of pathogenic bacterial proliferation (2).
In the management of infectious disease, FMT outperforms first-line therapies for recurrent Clostridioides difficile infection (CDI), achieving superior sustained remission rates compared to vancomycin therapy (3, 4). Single-dose FMT administration following antibiotic treatment significantly reduces CDI recurrence and mitigates the transfer of antibiotic resistance genes (5, 6). Beyond its established role in CDI, FMT demonstrates therapeutic potential across multiple disease domains. In virological applications, FMT has facilitated hepatitis B e antigen clearance and addressed human immunodeficiency virus-associated dysbiosis (7, 8). In oncology, FMT modulates the GM to reprogram tumor microenvironments, particularly enhancing anti-programmed cell death protein 1 therapy responsiveness in melanoma treatment (9, 10). Neurological benefits emerge through FMT’s capacity to improve both motor and non-motor symptoms in Parkinson’s disease while enhancing gut microbial diversity (11, 12). Metabolic improvements are achieved via microbial restructuring, including triglyceride reduction and inflammatory pathway regulation (13). Furthermore, postoperative outcomes further highlight FMT’s clinical value, with reduced hospitalization duration, accelerated bowel function recovery, and improved nutritional markers (14). This multifaceted therapeutic profile positions FMT as a versatile intervention across distinct pathophysiological mechanisms.
Although FMT is generally well-tolerated, it dose carry risks requiring careful consideration. Transient gastrointestinal disturbances and rare cases of pathogen transmission underscore the need for rigorous donor screening protocols (15). Microbial engraftment patterns reveal complex dynamics—while bacterial transfer shows dose-dependent colonization, fungal taxa exhibit limited transmission persistence, suggesting distinct ecological establishment mechanisms (16). These findings highlight the importance of multimodal microbiota analysis in treatment optimization. To this end, this review searched for articles on several databases using the key terms ‘FMT and human health’ for the last 5 years to give a current summary of this nonpharmacological therapy. The aim of this review is to provide a comprehensive summary of this approach, while also revealing some limitations and prospects for further study (Figure 1).
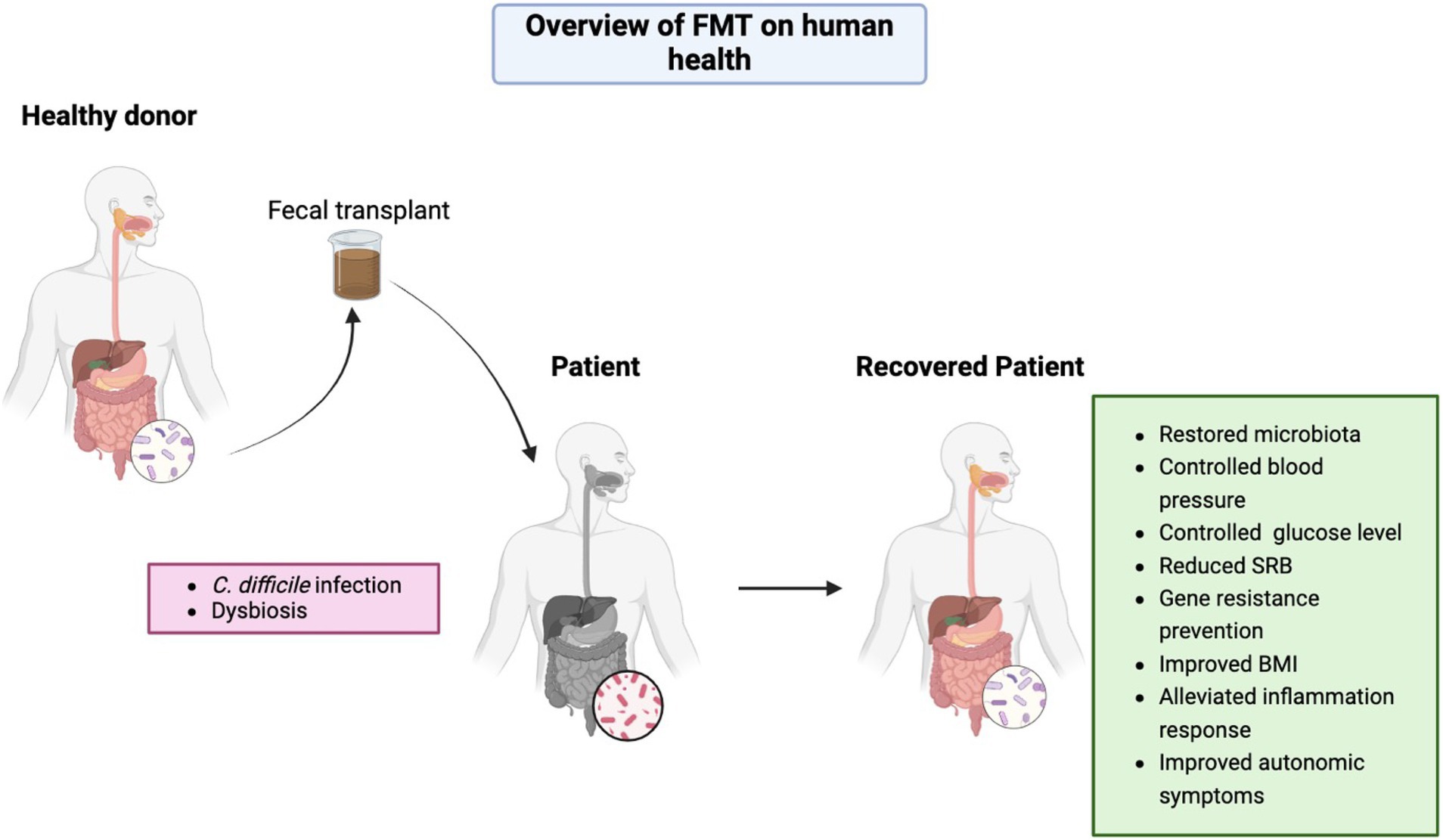
Figure 1. Overview of FMT effects on human health. Figure was created in https://BioRender.com.
FMT has shown significant efficacy in metabolic disorders. For instance, in patients with severe obesity, FMT improved metabolic responses by reducing the abundance of Prevotella and increasing the engraftment of donor-specific bacteria such as Faecalibacillus intestinalis, Christensenellaceae spp., and Roseburia spp. (17). Moreover, FMT demonstrated clinical efficacy in patients with non-alcoholic fatty liver disease (NAFLD), with lean NAFLD patients responding better than obese NAFLD patients (18). In patients with type 2 diabetes mellitus (T2DM), FMT improved body mass index, insulin resistance, and GM, with Bifidobacterium adolescentis, Chlorobium phaeovibrioides, and Synechococcus sp. WH8103 significantly associated with clinical parameters of T2DM (19). FMT also reduced HbA1c%, blood glucose, and uric acid levels while increasing postprandial C-peptide levels, despite individual variability. The abundance of families Rikenellaceae and Ruminococcaceae in the fecal of patients may serve as potential biomarkers for selecting T2DM patients to receive FMT (20). In type 1 diabetes mellitus patients, FMT was associated with the preservation of residual β cell function, particularly linked to the abundance of Prevotella (21).
In addition to reversing symptoms, FMT is safe for patients with severe alcoholic hepatitis with reduced pathogenic taxa, such as Campylobacter and anaerobes (Weisella, Parcubacteria and Leuconostocaceae) and increased Alphaproteobacteria and Thaumarcheota (22). Furthermore, the safety of oral FMT in treating chronic kidney disease was validated, with improvement in renal function based on urea nitrogen and serum creatinine levels. This was exacerbated with Firmicutes and Actinobacteria abundance and the reduction of Bacteroidetes and Proteobacteria (23). Autologous-fecal-microbiota-transplantation (aFMT) also showed potential in weight control and metabolic improvement, especially when combined with a plant based or high-polyphenol diet, optimizing weight loss and glycemic control (24–26).
The safety and efficacy of FMT in treating intestinal diseases have been widely validated. For instance, FMT demonstrated significant efficacy in CDI (27). Specifically, the increase in alpha diversity and the abundance of Ruminococcaceae and Lachnospiraceae, alongside the reduction of Enterobacteriaceae, have been identified as key biomarkers for the successful eradication of CDI through FMT (28). Moreover, FMT formulations such as RBX2660 and RBX7455 showed durability and safety in preventing CDI recurrence (29, 30). Additionally, FMT has shown promise in treating irritable bowel syndrome (IBS) and inflammatory bowel diseases. For example, in patients with moderate to severe IBS, FMT not only improved quality of life but also reduced fatigue and abdominal symptoms (31). Furthermore, with increasing SCFAs in IBS patients following FMT administration, an inverse correlation between butyric acid levels and IBS symptoms was observed (32). Notably, FMT reversed IBS symptoms by increasing the abundance of Akkermansia and Neisseria and reducing Desulfovibrio and Delftia (33). Although the mechanisms underlying the crosstalk between GM and colonic enteroendocrine cells remain elusive, FMT has proven efficacy in enhancing the density of colonic enteroendocrine cells in patients with IBS (34).
In addition, specific bacterial and metabolite associations have been established following the administration of capsulized FMT in patients with ulcerative colitis (UC) (35). Similarly, a single fresh FMT induced the expansion of Bacteroidetes and reduction of Proteobacteria in patients with recurrent UC (36). Moreover, serum IL-6, IL-10, and TNF-α modification is predicted to be related to FMT efficacy in UC (37). Finally, FMT has emerged as a promising treatment for tyrosine kinase inhibitors-induced diarrhea and small intestinal bacterial overgrowth, with no reported adverse effects (38, 39).
Emerging evidence highlights the potential of FMT in modulating behavioral changes via gut-liver-brain axis. For instance, FMT reduced alcohol preference and consumption in patients with alcohol use disorder by increasing the diversity of SCFAs and Ruminococcaceae (40). Additionally, with Bacteroidetes and Firmicutes dominance, FMT can alleviate anxiety and depression while restoring intestinal microecology in patients with irritable bowel syndrome with predominant diarrhea (41). Notably, combining FMT with human intestinal fluid transplantation in capsule form has shown promise in improving childhood autism behavior, with observable benefits emerging as early as the first month of treatment. These findings underscore the potential of this combinatorial approach as an innovative therapy for autism (42).
Beyond neurological effects, FMT demonstrates immunomodulatory properties. In psoriatic arthritis, FMT initiates a distinct immunological plasma protein signature characterized by elevated IFN-γ levels, suggesting systemic immune reprogramming (43). Similarly, for immune-mediated dry eye in Sjögren’s syndrome patients, FMT induces a donor-like microbiota profile that correlates with improved dry eye symptoms and balanced regulatory/effector T-cell dynamics (44). Furthermore, daily encapsulated oral FMT is associated with mucosal-associated invariant T cell cytokine production with clinical significance (45). Importantly, FMT also exhibits therapeutic efficacy in systemic lupus erythematosus, rheumatic diseases, and acute and chronic graft-versus-host disease (46–48).
FMT with Firmicutes spores is a potentially novel strategy for controlling the growth of pathogens, thereby reducing the risk of antimicrobial resistance gene (ARG) colonization in the gastrointestinal tract (49). The abundance of ARGs is significantly reduced by FMT in patients with cirrhosis (50). Furthermore, an overall bimodal pattern with reduced ARG transfer for one-week post FMT, which is typical of healthy donor commensal microbiota, has been observed in patients undergoing allogeneic hematopoietic cell transplantation (51). However, stable communities with resistance to ARGs have also been observed in their patients, indicating that the length of clinical trials is a factor. Similarly, among renal transplant recipients, it has been suggested that strain competition induced by FMT reduces the number of multidrug-resistant organisms (52).
The combination of FMT with supplements such as selenium showed inhibitory effects on the occurrence of colorectal cancer by increasing the abundance of beneficial bacteria, regulating phenotype and metabolic pathways (53). Although this is an animal study, the combination of FMT and selenium provides a new approach for treating colorectal cancer. Additionally, co-administration of FMT with an anti-inflammatory diet induced remission in patients with mild-to-moderate UC over 1 year (54). Furthermore, lifestyle interventions combined with FMT also improved liver stiffness and lipid profile in patients with T2DM by expanding Bifidobacterium and Lactobacillus (55). Similarly, patients with metabolic syndromes, such as obesity, showed improved insulin sensitivity upon daily administration of low-fermentable fiber combined with FMT supplementation (56). However, despite reduced small intestinal permeability, insulin resistance was not affected by FMT (57) (Table 1).
While FMT standardization continues to advance in clinical practice, critical challenges persist in optimizing its therapeutic applications. Three primary safety concerns warrant attention. First, procedure-related complications associated with administration methods, as evidenced by gastroduodenoscopic risks observed with commercial anaerobic-cultivated products despite their gastrointestinal symptom relief potential (58). Second, the dual-edged nature of immune modulation emerges when donor-derived antigens paradoxically activate immune cells, potentially exacerbating pre-existing conditions—a phenomenon underscoring the need for precision-engineered microbial consortia targeting specific pathologies like intestinal barrier dysfunction (59). Finally, preclinical models reveal systemic consequences through FMT-induced elevations in central/peripheral inflammatory mediators coupled with compromised intestinal mucosal integrity, mechanistically linking gut microbiota alterations to anxiety-depressive manifestations (60).
The therapeutic landscape of FMT demonstrates striking condition-specific variability. In gastrointestinal disorders, while showing efficacy in ameliorating certain IBS symptoms, it fails to normalize stool frequency or resolve abdominal pain (61). Metabolically, its actions exhibit paradoxical selectivity: Although reducing visceral adiposity in obese adolescents, FMT does not translate to clinically meaningful weight reduction (62). This dichotomy extends to molecular mechanisms, where epigenetic reprogramming of immune cells and plasma metabolome modifications correlate with improved insulin sensitivity (63), yet cachexia progression remains unaffected in metastatic cancer models (64).
Emerging safety data necessitate judicious clinical translation. Of particular concern, combination therapy trials incorporating UC exclusion diets were terminated prematurely despite initial mucosal healing observations, highlighting unforeseen risks in therapeutic synergies (65). Complementing these clinical findings, preclinical evidence demonstrates a gut-brain axis disruption mechanism: FMT-induced intestinal hyperpermeability not only elevates systemic inflammation biomarkers but also precipitates anxiety-depressive phenotypes (60). While demonstrating promise in managing steroid-refractory gastrointestinal graft-versus-host disease (66), these collective findings underscore the imperative for longitudinal safety surveillance and mechanistic investigation into microbiota-host interactions.
Within the first 7 days of FMT administration, donor-derived species, SCFAs, and tryptophan metabolites expanded despite a low degree of donor-recipient microbiome similarity (67). Importantly, individual species or strain-driven metabolite changes in the lower gastrointestinal tract of patients with acute graft-versus-host disease highlight the need for careful donor selection. Given that donors are required to have an abundance of selective taxa, two critical factors emerge: variation and stability. For example, multi-donor FMT may alter gut microbiota function due to microbiome competition, thus underscoring the importance of selecting donor FMTs that dominate strain engraftment. Such selection should prioritize diversity, as engrafted strains can induce enterotype-level shifts that alter metabolic potential (68). Furthermore, the abundance of the keystone genera, Prevotella, Faecalibacterium, and Bacteroides and increased microbial diversity are biomarkers of FMT capsule administered to treat IBS (69).
To optimize FMT efficacy, previous fecal microbiome profiles should be used as biomarkers during patient recruitment, ensuring unbiased selection (70). Moreover, FMT regimens must demonstrate effectiveness even for rare diseases under trial, such as chronic pouchitis (71). As the delivery methods for FMT may have distinct effects, there is a need to establish the method with the most effective treatment (72). Additionally, while FMT has shown acceptability, safety, and feasibility in treating major depressive disorder, a comprehensive evaluation of its clinical efficacy based on standardized protocols is still required (73). Similarly, although FMT capsules alter both serum metabolomics and gut microbiota in mild cognitive impairment, this does not confirm their safety or efficacy (74).
The effectiveness and safety of FMT are significantly influenced by disease severity and dosage composition (75). For instance, single-dose FMT is not recommended for maintaining remission in UC, despite its clinical efficacy in microbiome engraftment (76, 77). In contrast, oral FMT emerges as a feasible option for UC treatment. Specifically, Microbial Ecosystem Therapeutic 2, an oral encapsulated formulation comprising 40 lyophilized bacterial species, has demonstrated safety and efficacy in UC, even in children with cytomegalovirus infection (78, 79). However, antibiotic pretreatment during FMT poses a significant limitation in clinical trials, highlighting the need for alternative approaches (80). One such alternative is washed microbiota transplantation, which appears to be both effective and safe, despite the lack of clinical significance in delivery methods (81, 82).
To further refine FMT applications, donor-recipient species genome bins can predict strain transfer dynamics and specific microbial interactions, thereby improving metabolic health (83). Additionally, donor age and sex play critical roles in FMT success, as strain-level variations may influence outcomes in a species-specific manner (84). While both sexes respond similarly to FMT in IBS patients, females tend to exhibit increased fecal Alistipes, regardless of administration method (85). Nevertheless, conflicting results from IBS studies suggest that sex may not be a determining factor in FMT outcomes, indicating the need for further investigation (86).
FMT shows therapeutic promise, particularly in reversing diseases and disorders. In addition, positive results in animal models suggest its potential for human clinical trials; however, safety and efficacy must be rigorously monitored before clinical adoption. While the FDA has classified FMT as a “drug” due to its ability to treat and prevent disease, emerging adverse effects highlight the need for further large cohort studies and long-term monitoring to establish stable benefits. In addition, several challenges remain, including pediatric use, regimen optimization, and ensuring patient confidence in safety and efficacy. In addition, donor screening is essential to prevent disease transmission, and stool variability poses significant regulatory challenges for the FDA. Looking ahead, encapsulated stool formulations represent an important future direction, offering non-invasive oral delivery that could increase patient acceptance and broaden clinical applications.
ZC: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. TG: Project administration, Writing – review & editing. OB: Conceptualization, Writing – original draft. YZ: Writing – review & editing. XY: Funding acquisition, Project administration, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Traditional Chinese Medicine Research Project of Heilongjiang Province (ZHY2022-080).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
aFMT, Autologous-fecal-microbiota-transplantation; ARG, Antimicrobial resistance gene; CDI, Clostridioides difficile infection; FMT, Fecal microbiota transplantation; IBS, Irritable bowel syndrome; GM, Gut microbiota; NAFLD, Non-alcoholic fatty liver disease; SCFAs, Short - chain fatty acid; T2DM, Type 2 diabetes mellitus; UC, Ulcerative colitis.
1. Chu, ND, Crothers, JW, Nguyen, LTT, Kearney, SM, Smith, MB, Kassam, Z, et al. Dynamic colonization of microbes and their functions after fecal microbiota transplantation for inflammatory bowel disease. MBio. (2021) 12:e0097521. doi: 10.1128/mBio.00975-21
2. Su, L, Hong, Z, Zhou, T, Jian, Y, Xu, M, Zhang, X, et al. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep. (2022) 12:1152. doi: 10.1038/s41598-022-05127-9
3. Baunwall, SMD, Andreasen, SE, Hansen, MM, Kelsen, J, Hoyer, KL, Ragard, N, et al. Faecal microbiota transplantation for first or second Clostridioides difficile infection (EarlyFMT): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. (2022) 7:1083–91. doi: 10.1016/S2468-1253(22)00276-X
4. Rode, AA, Chehri, M, Krogsgaard, LR, Heno, KK, Svendsen, AT, Ribberholt, I, et al. Randomised clinical trial: a 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Aliment Pharmacol Ther. (2021) 53:999–1009. doi: 10.1111/apt.16309
5. Khanna, S, Assi, M, Lee, C, Yoho, D, Louie, T, Knapple, W, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. (2022) 82:1527–38. doi: 10.1007/s40265-022-01797-x
6. Langdon, A, Schwartz, DJ, Bulow, C, Sun, X, Hink, T, Reske, KA, et al. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostridioides difficile infection from the open-label PUNCH CD study. Genome Med. (2021) 13:28. doi: 10.1186/s13073-021-00843-9
7. Chauhan, A, Kumar, R, Sharma, S, Mahanta, M, Vayuuru, SK, Nayak, B, et al. Fecal microbiota transplantation in hepatitis B e antigen-positive chronic hepatitis B patients: a pilot study. Dig Dis Sci. (2021) 66:873–80. doi: 10.1007/s10620-020-06246-x
8. Serrano-Villar, S, Talavera-Rodriguez, A, Gosalbes, MJ, Madrid, N, Perez-Molina, JA, Elliott, RJ, et al. Fecal microbiota transplantation in HIV: a pilot placebo-controlled study. Nat Commun. (2021) 12:1139. doi: 10.1038/s41467-021-21472-1
9. Baruch, EN, Youngster, I, Ben-Betzalel, G, Ortenberg, R, Lahat, A, Katz, L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. (2021) 371:602–9. doi: 10.1126/science.abb5920
10. Davar, D, Dzutsev, AK, McCulloch, JA, Rodrigues, RR, Chauvin, JM, Morrison, RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. (2021) 371:595–602. doi: 10.1126/science.abf3363
11. Cheng, Y, Tan, G, Zhu, Q, Wang, C, Ruan, G, Ying, S, et al. Efficacy of fecal microbiota transplantation in patients with Parkinson’s disease: clinical trial results from a randomized, placebo-controlled design. Gut Microbes. (2023) 15:2284247. doi: 10.1080/19490976.2023.2284247
12. Segal, A, Zlotnik, Y, Moyal-Atias, K, Abuhasira, R, and Ifergane, G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease - a case series. Clin Neurol Neurosurg. (2021) 207:106791. doi: 10.1016/j.clineuro.2021.106791
13. Zhong, P, Xu, Y, Ye, S, Yang, F, Wu, L, Su, G, et al. A preliminary study on the effects of fecal microbiota transplantation on the intestinal microecology of patients with severe pneumonia during the convalescence period. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2023) 35:352–7. doi: 10.3760/cma.j.cn121430-20221206-01065
14. Cui, JQ, Tian, HL, Wang, XJ, Wang, L, Liu, YK, Ye, C, et al. Analysis of short-term efficacy of perioperative fecal microbiota transplantation combined with nutritional support in patients with radiation-induced enteritis complicated by intestinal obstruction. Zhonghua Wei Chang Wai Ke Za Zhi. (2023) 26:955–62. doi: 10.3760/cma.j.cn441530-20230816-00052
15. van Lier, YF, Rolling, T, Armijo, GK, Zhai, B, Haverkate, NJE, Meijer, E, et al. Profiling the fungal microbiome after fecal microbiota transplantation for graft-versus-host disease: insights from a phase 1 interventional study. Transplant Cell Ther. (2023) 29:63 e61–5. doi: 10.1016/j.jtct.2022.10.011
16. Chen, Q, Fan, Y, Zhang, B, Yan, C, Chen, Z, Wang, L, et al. Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis. Front Cell Infect Microbiol. (2022) 12:1086885. doi: 10.3389/fcimb.2022.1086885
17. Zhang, Z, Mocanu, V, Deehan, EC, Hotte, N, Zhu, Y, Wei, S, et al. Recipient microbiome-related features predicting metabolic improvement following fecal microbiota transplantation in adults with severe obesity and metabolic syndrome: a secondary analysis of a phase 2 clinical trial. Gut Microbes. (2024) 16:2345134. doi: 10.1080/19490976.2024.2345134
18. Xue, L, Deng, Z, Luo, W, He, X, and Chen, Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol. (2022) 12:759306. doi: 10.3389/fcimb.2022.759306
19. Wu, Z, Zhang, B, Chen, F, Xia, R, Zhu, D, Chen, B, et al. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: a randomized, controlled, prospective study. Front Cell Infect Microbiol. (2022) 12:1089991. doi: 10.3389/fcimb.2022.1089991
20. Ding, D, Yong, H, You, N, Lu, W, Yang, X, Ye, X, et al. Prospective study reveals host microbial determinants of clinical response to fecal microbiota transplant therapy in type 2 diabetes patients. Front Cell Infect Microbiol. (2022) 12:820367. doi: 10.3389/fcimb.2022.820367
21. de Groot, P, Nikolic, T, Pellegrini, S, Sordi, V, Imangaliyev, S, Rampanelli, E, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. (2021) 70:92–105. doi: 10.1136/gutjnl-2020-322630
22. Pande, A, Sharma, S, Khillan, V, Rastogi, A, Arora, V, Shasthry, SM, et al. Fecal microbiota transplantation compared with prednisolone in severe alcoholic hepatitis patients: a randomized trial. Hepatol Int. (2023) 17:249–61. doi: 10.1007/s12072-022-10438-0
23. Arteaga-Muller, GY, Flores-Trevino, S, Bocanegra-Ibarias, P, Robles-Espino, D, Garza-Gonzalez, E, Fabela-Valdez, GC, et al. Changes in the progression of chronic kidney disease in patients undergoing fecal microbiota transplantation. Nutrients. (2024) 16:1109. doi: 10.3390/nu16081109
24. Kamer, O, Rinott, E, Tsaban, G, Kaplan, A, Yaskolka Meir, A, Zelicha, H, et al. Successful weight regain attenuation by autologous fecal microbiota transplantation is associated with non-core gut microbiota changes during weight loss; randomized controlled trial. Gut Microbes. (2023) 15:2264457. doi: 10.1080/19490976.2023.2264457
25. Rinott, E, Youngster, I, Meir, AY, Tsaban, G, Kaplan, A, Zelicha, H, et al. Autologous fecal microbiota transplantation can retain the metabolic achievements of dietary interventions. Eur J Intern Med. (2021) 92:17–23. doi: 10.1016/j.ejim.2021.03.038
26. Rinott, E, Youngster, I, Yaskolka Meir, A, Tsaban, G, Zelicha, H, Kaplan, A, et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology. (2021) 160:158–173 e110. doi: 10.1053/j.gastro.2020.08.041
27. Nicholson, MR, Mitchell, PD, Alexander, E, Ballal, S, Bartlett, M, Becker, P, et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin Gastroenterol Hepatol. (2020) 18:612–619 e611. doi: 10.1016/j.cgh.2019.04.037
28. Wei, S, Bahl, MI, Baunwall, SMD, Dahlerup, JF, Hvas, CL, and Licht, TR. Gut microbiota differs between treatment outcomes early after fecal microbiota transplantation against recurrent Clostridioides difficile infection. Gut Microbes. (2022) 14:2084306. doi: 10.1080/19490976.2022.2084306
29. Khanna, S, Pardi, DS, Jones, C, Shannon, WD, Gonzalez, C, and Blount, K. RBX7455, a non-frozen, orally administered investigational live biotherapeutic, is safe, effective, and shifts Patients’ microbiomes in a phase 1 study for recurrent Clostridioides difficile infections. Clin Infect Dis. (2021) 73:e1613–20. doi: 10.1093/cid/ciaa1430
30. Orenstein, R, Dubberke, ER, Khanna, S, Lee, CH, Yoho, D, Johnson, S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis. (2022) 22:245. doi: 10.1186/s12879-022-07256-y
31. El-Salhy, M, Mazzawi, T, Hausken, T, and Hatlebakk, JG. The fecal microbiota transplantation response differs between patients with severe and moderate irritable bowel symptoms. Scand J Gastroenterol. (2022) 57:1036–45. doi: 10.1080/00365521.2022.2064725
32. El-Salhy, M, Valeur, J, Hausken, T, and Gunnar Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil. (2021b) 33:e13983. doi: 10.1111/nmo.13983
33. Hamazaki, M, Sawada, T, Yamamura, T, Maeda, K, Mizutani, Y, Ishikawa, E, et al. Fecal microbiota transplantation in the treatment of irritable bowel syndrome: a single-center prospective study in Japan. BMC Gastroenterol. (2022) 22:342. doi: 10.1186/s12876-022-02408-5
34. Mazzawi, T, Hausken, T, and El-Salhy, M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand J Gastroenterol. (2022) 57:792–6. doi: 10.1080/00365521.2022.2036809
35. Chen, Q, Fan, Y, Zhang, B, Yan, C, Zhang, Q, Ke, Y, et al. Capsulized fecal microbiota transplantation induces remission in patients with ulcerative colitis by gut microbial colonization and metabolite regulation. Microbiol Spectr. (2023a) 11:e0415222. doi: 10.1128/spectrum.04152-22
36. Fang, H, Fu, L, Li, X, Lu, C, Su, Y, Xiong, K, et al. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Factories. (2021) 20:18. doi: 10.1186/s12934-021-01513-6
37. Huang, C, Huang, Z, Ding, L, Fu, Y, Fan, J, Mei, Q, et al. Fecal microbiota transplantation versus glucocorticoids for the induction of remission in mild to moderate ulcerative colitis. J Transl Med. (2022) 20:354. doi: 10.1186/s12967-022-03569-3
38. Ianiro, G, Rossi, E, Thomas, AM, Schinzari, G, Masucci, L, Quaranta, G, et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat Commun. (2020) 11:4333. doi: 10.1038/s41467-020-18127-y
39. Xu, F, Li, N, Wang, C, Xing, H, Chen, D, and Wei, Y. Clinical efficacy of fecal microbiota transplantation for patients with small intestinal bacterial overgrowth: a randomized, placebo-controlled clinic study. BMC Gastroenterol. (2021) 21:54. doi: 10.1186/s12876-021-01630-x
40. Wolstenholme, JT, Saunders, JM, Smith, M, Kang, JD, Hylemon, PB, Gonzalez-Maeso, J, et al. Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat Commun. (2022) 13:6198. doi: 10.1038/s41467-022-34054-6
41. Guo, Q, Lin, H, Chen, P, Tan, S, Wen, Z, Lin, L, et al. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered. (2021) 12:11885–97. doi: 10.1080/21655979.2021.1999374
42. Ye, C, Chen, QY, Yan, YM, Lv, XQ, Ma, CL, Li, N, et al. Establishment and preliminary clinical application of human intestinal fluid transplantation. Zhonghua Wei Chang Wai Ke Za Zhi. (2022) 25:819–25. doi: 10.3760/cma.j.cn441530-20220601-00239
43. Kragsnaes, MS, Jensen, JRB, Nilsson, AC, Malik, MI, Munk, HL, Pedersen, JK, et al. Dynamics of inflammation-associated plasma proteins following faecal microbiota transplantation in patients with psoriatic arthritis and healthy controls: exploratory findings from the FLORA trial. RMD Open. (2024) 10:e003750. doi: 10.1136/rmdopen-2023-003750
44. Watane, A, Cavuoto, KM, Rojas, M, Dermer, H, Day, JO, Banerjee, S, et al. Fecal microbial transplant in individuals with immune-mediated dry eye. Am J Ophthalmol. (2022) 233:90–100. doi: 10.1016/j.ajo.2021.06.022
45. Crothers, JW, Chu, ND, Nguyen, LTT, Phillips, M, Collins, C, Fortner, K, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. (2021) 21:281. doi: 10.1186/s12876-021-01856-9
46. Bilinski, J, Lis, K, Tomaszewska, A, Grzesiowski, P, Dzieciatkowski, T, Tyszka, M, et al. Fecal microbiota transplantation in patients with acute and chronic graft-versus-host disease-spectrum of responses and safety profile. Results from a prospective, multicenter study. Am J Hematol. (2021) 96:E88–91. doi: 10.1002/ajh.26077
47. Huang, C, Yi, P, Zhu, M, Zhou, W, Zhang, B, Yi, X, et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: an EXPLORER trial. J Autoimmun. (2022) 130:102844. doi: 10.1016/j.jaut.2022.102844
48. Kragsnaes, MS, Kjeldsen, J, Horn, HC, Munk, HL, Pedersen, JK, Just, SA, et al. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: an exploratory randomised placebo-controlled trial. Ann Rheum Dis. (2021) 80:1158–67. doi: 10.1136/annrheumdis-2020-219511
49. Straub, TJ, Lombardo, MJ, Bryant, JA, Diao, L, Lodise, TP, Freedberg, DE, et al. Impact of a purified microbiome therapeutic on abundance of antimicrobial resistance genes in patients with recurrent Clostridioides difficile infection. Clin Infect Dis. (2024) 78:833–41. doi: 10.1093/cid/ciad636
50. Bajaj, JS, Shamsaddini, A, Fagan, A, Sterling, RK, Gavis, E, Khoruts, A, et al. Fecal microbiota transplant in cirrhosis reduces gut microbial antibiotic resistance genes: analysis of two trials. Hepatol Commun. (2021) 5:258–71. doi: 10.1002/hep4.1639
51. Rashidi, A, Ebadi, M, Rehman, TU, Elhusseini, H, Kazadi, D, Halaweish, H, et al. Long- and short-term effects of fecal microbiota transplantation on antibiotic resistance genes: results from a randomized placebo-controlled trial. Gut Microbes. (2024) 16:2327442. doi: 10.1080/19490976.2024.2327442
52. Woodworth, MH, Conrad, RE, Haldopoulos, M, Pouch, SM, Babiker, A, Mehta, AK, et al. Fecal microbiota transplantation promotes reduction of antimicrobial resistance by strain replacement. Sci Transl Med. (2023) 15:eabo2750. doi: 10.1126/scitranslmed.abo2750
53. Su, Y, Fan, X, Cai, X, Ning, J, and Shen, M. Effects of fecal microbiota transplantation combined with selenium on intestinal microbiota in mice with colorectal cancer. Biochem Biophys Res Commun. (2024) 733:150580. doi: 10.1016/j.bbrc.2024.150580
54. Kedia, S, Virmani, SSKV, Kumar, P, Kante, B, Sahu, P, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. (2022) 71:2401–13. doi: 10.1136/gutjnl-2022-327811
55. Ng, SC, Xu, Z, Mak, JWY, Yang, K, Liu, Q, Zuo, T, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. (2022) 71:716–23. doi: 10.1136/gutjnl-2020-323617
56. Mocanu, V, Zhang, Z, Deehan, EC, Kao, DH, Hotte, N, Karmali, S, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. (2021) 27:1272–9. doi: 10.1038/s41591-021-01399-2
57. Craven, L, Rahman, A, Nair Parvathy, S, Beaton, M, Silverman, J, Qumosani, K, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. (2020) 115:1055–65. doi: 10.14309/ajg.0000000000000661
58. Fretheim, H, Chung, BK, Didriksen, H, Baekkevold, ES, Midtvedt, O, Brunborg, C, et al. Fecal microbiota transplantation in systemic sclerosis: a double-blind, placebo-controlled randomized pilot trial. PLoS One. (2020) 15:e0232739. doi: 10.1371/journal.pone.0232739
59. Rashidi, A, Ebadi, M, Rehman, TU, Elhusseini, H, Kazadi, D, Halaweish, H, et al. Multi-omics analysis of a fecal microbiota transplantation trial identifies novel aspects of acute GVHD pathogenesis. Cancer Res Commun. (2024) 4:1454–66. doi: 10.1158/2767-9764.CRC-24-0138
60. Liu, P, Liu, Z, Wang, J, Wang, J, Gao, M, Zhang, Y, et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun. (2024) 15:3003. doi: 10.1038/s41467-024-47273-w
61. Madsen, AMA, Halkjaer, SI, Christensen, AH, Gunther, S, Browne, PD, Kallemose, T, et al. The effect of faecal microbiota transplantation on abdominal pain, stool frequency, and stool form in patients with moderate-to-severe irritable bowel syndrome: results from a randomised, double-blind, placebo-controlled study. Scand J Gastroenterol. (2021) 56:761–9. doi: 10.1080/00365521.2021.1915375
62. Leong, KSW, Jayasinghe, TN, Wilson, BC, Derraik, JGB, Albert, BB, Chiavaroli, V, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw Open. (2020) 3:e2030415. doi: 10.1001/jamanetworkopen.2020.30415
63. van der Vossen, EWJ, Bastos, D, Stols-Goncalves, D, de Goffau, MC, Davids, M, Pereira, JPB, et al. Effects of fecal microbiota transplant on DNA methylation in subjects with metabolic syndrome. Gut Microbes. (2021) 13:1993513. doi: 10.1080/19490976.2021.1993513
64. de Clercq, NC, van den Ende, T, Prodan, A, Hemke, R, Davids, M, Pedersen, HK, et al. Fecal microbiota transplantation from overweight or obese donors in cachectic patients with advanced gastroesophageal Cancer: a randomized, double-blind, placebo-controlled, phase II study. Clin Cancer Res. (2021) 27:3784–92. doi: 10.1158/1078-0432.CCR-20-4918
65. Sarbagili Shabat, C, Scaldaferri, F, Zittan, E, Hirsch, A, Mentella, MC, Musca, T, et al. Use of Faecal transplantation with a novel diet for mild to moderate active ulcerative colitis: the CRAFT UC randomised controlled trial. J Crohns Colitis. (2022) 16:369–78. doi: 10.1093/ecco-jcc/jjab165
66. Zhao, Y, Li, X, Zhou, Y, Gao, J, Jiao, Y, Zhu, B, et al. Safety and efficacy of fecal microbiota transplantation for grade IV steroid refractory GI-GvHD patients: interim results from FMT2017002 trial. Front Immunol. (2021) 12:678476. doi: 10.3389/fimmu.2021.678476
67. DeFilipp, Z, Damania, AV, Kim, HT, Chang, CC, El-Jawahri, A, McAfee, SL, et al. Third-party fecal microbiota transplantation for high-risk treatment-naive acute GVHD of the lower GI tract. Blood Adv. (2024) 8:2074–84. doi: 10.1182/bloodadvances.2024012556
68. Yuan, X, Tan, Y, Bajinka, O, Jammeh, ML, Dukureh, A, Obiegbusi, CN, et al. The connection between epigenetics and gut microbiota-current perspective. Cell Biochem Funct. (2024) 42:e3941. doi: 10.1002/cbf.3941
69. Browne, PD, Cold, F, Petersen, AM, Halkjaer, SI, Christensen, AH, Gunther, S, et al. Engraftment of strictly anaerobic oxygen-sensitive bacteria in irritable bowel syndrome patients following fecal microbiota transplantation does not improve symptoms. Gut Microbes. (2021) 13:1–16. doi: 10.1080/19490976.2021.1927635
70. Holvoet, T, Joossens, M, Vazquez-Castellanos, JF, Christiaens, E, Heyerick, L, Boelens, J, et al. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. (2021) 160:145–157 e148. doi: 10.1053/j.gastro.2020.07.013
71. Karjalainen, EK, Renkonen-Sinisalo, L, Satokari, R, Mustonen, H, Ristimaki, A, Arkkila, P, et al. Fecal microbiota transplantation in chronic Pouchitis: a randomized, parallel, double-blinded clinical trial. Inflamm Bowel Dis. (2021) 27:1766–72. doi: 10.1093/ibd/izab001
72. Skjevling, LK, Hanssen, HM, Valle, PC, Goll, R, Juul, FE, Arlov, O, et al. Colonic distribution of FMT by different enema procedures compared to colonoscopy - proof of concept study using contrast fluid. BMC Gastroenterol. (2023) 23:363. doi: 10.1186/s12876-023-02979-x
73. Green, JE, Berk, M, Mohebbi, M, Loughman, A, McGuinness, AJ, Castle, D, et al. Feasibility, acceptability, and safety of Faecal microbiota transplantation in the treatment of major depressive disorder: a pilot randomized controlled trial. Can J Psychiatr. (2023) 68:315–26. doi: 10.1177/07067437221150508
74. Chen, X, Zhang, W, Lin, Z, Zheng, C, Chen, S, Zhou, H, et al. Preliminary evidence for developing safe and efficient fecal microbiota transplantation as potential treatment for aged related cognitive impairments. Front Cell Infect Microbiol. (2023b) 13:1103189. doi: 10.3389/fcimb.2023.1103189
75. El-Salhy, M, and Hatlebakk, JG. Factors underlying the difference in response to fecal microbiota transplantation between IBS patients with severe and moderate symptoms. Dig Dis Sci. (2024) 69:1336–44. doi: 10.1007/s10620-024-08369-x
76. Lahtinen, P, Jalanka, J, Mattila, E, Tillonen, J, Bergman, P, Satokari, R, et al. Fecal microbiota transplantation for the maintenance of remission in patients with ulcerative colitis: a randomized controlled trial. World J Gastroenterol. (2023) 29:2666–78. doi: 10.3748/wjg.v29.i17.2666
77. Smith, BJ, Piceno, Y, Zydek, M, Zhang, B, Syriani, LA, Terdiman, JP, et al. Strain-resolved analysis in a randomized trial of antibiotic pretreatment and maintenance dose delivery mode with fecal microbiota transplant for ulcerative colitis. Sci Rep. (2022) 12:5517. doi: 10.1038/s41598-022-09307-5
78. Kao, D, Wong, K, Franz, R, Cochrane, K, Sherriff, K, Chui, L, et al. The effect of a microbial ecosystem therapeutic (MET-2) on recurrent Clostridioides difficile infection: a phase 1, open-label, single-group trial. Lancet Gastroenterol Hepatol. (2021) 6:282–91. doi: 10.1016/S2468-1253(21)00007-8
79. Karolewska-Bochenek, K, Lazowska-Przeorek, I, Grzesiowski, P, Dziekiewicz, M, Dembinski, L, Albrecht, P, et al. Faecal microbiota transfer - a new concept for treating cytomegalovirus colitis in children with ulcerative colitis. Ann Agric Environ Med. (2021) 28:56–60. doi: 10.26444/aaem/118189
80. Singh, P, Alm, EJ, Kelley, JM, Cheng, V, Smith, M, Kassam, Z, et al. Effect of antibiotic pretreatment on bacterial engraftment after fecal microbiota transplant (FMT) in IBS-D. Gut Microbes. (2022) 14:2020067. doi: 10.1080/19490976.2021.2020067
81. Chen, M, Liu, XL, Zhang, YJ, Nie, YZ, Wu, KC, and Shi, YQ. Efficacy and safety of fecal microbiota transplantation by washed preparation in patients with moderate to severely active ulcerative colitis. J Dig Dis. (2020) 21:621–8. doi: 10.1111/1751-2980.12938
82. Wu, LH, Ye, ZN, Peng, P, Xie, WR, Xu, JT, Zhang, XY, et al. Efficacy and safety of washed microbiota transplantation to treat patients with mild-to-severe COVID-19 and suspected of having gut microbiota Dysbiosis: study protocol for a randomized controlled trial. Curr Med Sci. (2021) 41:1087–95. doi: 10.1007/s11596-021-2475-2
83. Chen, Q, Wu, C, Xu, J, Ye, C, Chen, X, Tian, H, et al. Donor-recipient intermicrobial interactions impact transfer of subspecies and fecal microbiota transplantation outcome. Cell Host Microbe. (2024) 32:349–365 e344. doi: 10.1016/j.chom.2024.01.013
84. Benitez-Paez, A, Hartstra, AV, Nieuwdorp, M, and Sanz, Y. Species- and strain-level assessment using rrn long-amplicons suggests donor’s influence on gut microbial transference via fecal transplants in metabolic syndrome subjects. Gut Microbes. (2022) 14:2078621. doi: 10.1080/19490976.2022.2078621
85. El-Salhy, M, Gilja, OH, and Hatlebakk, JG. Increasing the transplant dose and repeating faecal microbiota transplantation results in the responses of male patients with IBS reaching those of females. Scand J Gastroenterol. (2024) 59:391–400. doi: 10.1080/00365521.2023.2292479
86. El-Salhy, M, Casen, C, Valeur, J, Hausken, T, and Hatlebakk, JG. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J Gastroenterol. (2021a) 27:2219–37. doi: 10.3748/wjg.v27.i18.2219
87. van Lier, YF, Davids, M, Haverkate, NJE, de Groot, PF, Donker, ML, Meijer, E, et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med. (2020) 12:eaaz8926. doi: 10.1126/scitranslmed.aaz8926
Keywords: fecal microbiota transplantation, human health, metabolic disorders, microbiome medicine, gut microbiota
Citation: Cao Z, Gao T, Bajinka O, Zhang Y and Yuan X (2025) Fecal microbiota transplantation-current perspective on human health. Front. Med. 12:1523870. doi: 10.3389/fmed.2025.1523870
Received: 13 November 2024; Accepted: 28 February 2025;
Published: 14 March 2025.
Edited by:
Ana Isabel Alvarez-Mercado, Granada Biosanitary Research Institute (ibs.GRANADA), SpainReviewed by:
Ascensión Rueda, University of Granada, SpainCopyright © 2025 Cao, Gao, Bajinka, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yali Zhang, emhhbmd5bF8yMDEzQHNpbmEuY29t; Xingxing Yuan, eXVhbnhpbmd4aW5nQGhsanVjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.