
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 14 March 2025
Sec. Nuclear Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1523862
 Anas Aouf1†
Anas Aouf1† Tilman Speicher2*†
Tilman Speicher2*† Arne Blickle2
Arne Blickle2 Moritz B. Bastian2
Moritz B. Bastian2 Caroline Burgard2
Caroline Burgard2 Florian Rosar2
Florian Rosar2 Samer Ezziddin2
Samer Ezziddin2 Amir Sabet1
Amir Sabet1Aim: The heterogeneous expression of somatostatin receptors in gastroenteropancreatic neuroendocrine tumors (GEP-NET) leads to significant intra-individual variability in tracer uptake during pre-therapeutic [68Ga]Ga-DOTATOC PET/CT for patients receiving peptide receptor radionuclide therapy (PRRT). This study aims to evaluate the lesion-based relationship between receptor-mediated tracer uptake and the functional response to PRRT.
Methods: A retrospective analysis was conducted on 32 patients with metastatic GEP-NET (12 pancreatic and 20 non-pancreatic), all treated with [177Lu]Lu-octreotate (4 cycles, with a mean of 7.9 GBq per cycle). [68Ga]Ga-DOTATOC PET/CT was performed at baseline and 3 months after the final PRRT cycle. Tumor uptake was quantified using the standardized uptake value (SUV). For each patient, 2 to 3 well-delineated tumor lesions were selected as target lesions. SUVmax, SUVmean (automated segmentation with a 50% SUVmax threshold), and corresponding tumor-to-liver ratios (SUVmaxT/L and SUVmeanT/L) were calculated. Functional tumor response was assessed based on the relative change in metabolic tumor volume (%ΔTVPET). The correlation between baseline SUV parameters and lesion-based functional response was analyzed using Spearman’s rank correlation.
Results: A total of 71 lesions were included in the analysis. The mean baseline SUVmax and SUVmean were 28.1 ± 15.9 and 13.6 ± 5.1, respectively. Three months after PRRT completion, the mean %ΔTVPET was 39.6 ± 52.1%. Baseline SUVmax and SUVmean demonstrated a poor correlation with lesion-based response (p = 0.706 and p = 0.071, respectively). In contrast, SUVmaxT/L and SUVmeanT/L were significantly correlated with lesion-based response (SUVmeanT/L: p = 0.011, r = 0.412; SUVmaxT/L: p = 0.004, r = 0.434). Among patient characteristics—including primary tumor origin, baseline tumor volume, and metastatic sites—only pancreatic origin was significantly associated with functional tumor volume reduction (ΔTVPET%: 56.8 ± 39.8 in pancreatic vs. 28.4 ± 50.1 in non-pancreatic NET; p = 0.020).
Conclusion: The lesion-based molecular response to PRRT correlates with pretreatment somatostatin receptor PET uptake, particularly when expressed as tumor-to-liver SUV ratios (SUVmaxT/L and SUVmeanT/L).
Neuroendocrine tumors (NETs) are rare neoplasms that originate from endocrine or neuroendocrine cells (1). In the United States, the incidence rate was 8.19 cases per 100,000 individuals in 2018 (2). The gastroenteropancreatic (GEP) region is the most common primary site, although NETs can arise in various other locations. Histological grading was traditionally determined using markers such as mitotic count and the Ki-67 index (3); however, it is now primarily based on cell morphology (4). To date, surgical resection remains the first-line treatment for localized disease. The expression of somatostatin receptors (SSTRs) in NETs has been effectively utilized for both diagnostic and therapeutic purposes, resulting in significant tumor load reduction and a favorable safety profile (5–8). More specifically, peptide receptor radionuclide therapy (PRRT) using somatostatin receptor analogs, such as [177Lu]Lu-octreotate (177Lu-PRRT) or [90Y]Y-octreotate (90Y-PRRT), has proven to be an effective systemic treatment for unresectable or metastatic neuroendocrine tumors (NETs), yielding remarkable clinical outcomes with low overall toxicity (9–17). The therapeutic benefit of PRRT was demonstrated in the NETTER-1 trial (18), which led to the FDA approval of [177Lu]Lu-DOTATATE in 2018. As an integral part of NET diagnostics, somatostatin receptor scintigraphy with [111In]In-DTPA-octreotide and, more recently, positron emission tomography (PET) using 68Ga-labeled somatostatin analogs, such as [68Ga]Ga-DOTA-Tyr3-octreotide (DOTATOC), has been established as a superior imaging modality (19–22). In addition to diagnosis, staging, and therapy response evaluation, somatostatin receptor imaging is also crucial for patient selection, ensuring that only those with adequate SSTR expression receive PRRT. However, the heterogeneous SSTR across various tumor lesions results in significant intra-individual variability in tracer uptake on pre-therapeutic 68Ga-DOTATOC PET/CT scans of SSTR-expressing NET patients undergoing PRRT (23). This study aims to investigate the relationship between lesion-specific baseline SSTR expression and tumor response to [177Lu]Lu-octreotate, as measured by tumor volume change following treatment.
This retrospective analysis included a total of 32 patients with histologically confirmed, unresectable, metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NET) who underwent treatment with [177Lu]Lu-octreotate (17 men, 15 women; age range: 40–90 years; mean age: 67.8 years; median age: 70 years). Prior to PRRT, patients underwent various pre-treatments, including surgical resection, somatostatin analog (SSA) therapy, targeted molecular therapies (e.g., everolimus, sunitinib), or chemotherapy, depending on tumor burden, progression status, and individual patient characteristics. All patients met the general inclusion criteria for peptide receptor radionuclide therapy (PRRT), including sufficient tumor uptake (i.e., uptake ≥ liver uptake) on baseline [68Ga]Ga-DOTATOC-PET/CT (24–26). Within the cohort, 12 patients had pancreatic NET, while 20 patients had non-pancreatic GEP-NET. The study was conducted in accordance with the Declaration of Helsinki and national regulations. Written informed consent was obtained from all participants for the scientific analysis of their data.
PRRT was administered with a mean activity of 7.9 GBq (216 mCi) [177Lu]Lu-octreotate per treatment cycle, targeting a total of four cycles at standard intervals of 3 months (10–14 weeks). The 177Lu (IDB Holland, Baarle-Nassau, Netherlands) had a specific activity ranging from approximately 100 to 160 GBq/μmol at the time of administration. Peptide labeling was conducted to achieve an apparent specific activity of approximately 54 GBq/μmol, defined as the ratio of activity to the total peptide amount (27, 28). Nephroprotection was provided through standard amino acid co-infusion following the Rotterdam protocol, consisting of lysine (2.5%) and arginine (2.5%) in 1 L of 0.9% NaCl, administered at an infusion rate of 250 mL/h (29, 30).
Baseline [68Ga]Ga-DOTATOC PET/CT was performed 2 to 7 days prior to the first PRRT cycle. Long-acting somatostatin analogs were discontinued for at least 4 weeks, while short-acting analogs were paused for at least 1 day before imaging. DOTATOC labeling was conducted using 68Ga eluted from an in-house 68Ge/68Ga generator, following the procedure described by Zhernosekov et al. (31). The PET/CT scans covered the area from the base of the skull to the upper thighs, with five to seven bed positions, and were acquired 30 min after the intravenous injection of 200 MBq [68Ga] Ga-DOTATOC. Imaging was performed using a hybrid PET/CT scanner (Biograph 2, Siemens Medical Solutions Inc., Hoffman Estates, Illinois, United States), which consisted of a dual-detector helical CT and a high-resolution PET scanner with a 16.2 cm axial field of view and lutetium oxyorthosilicate (LSO) crystal detectors (6.45 × 6.45 × 25 mm). CT imaging was performed for attenuation correction and anatomical localization, with acquisition parameters set to a tube current of 60 mAs, a tube voltage of 130 kV, a rotation time of 0.8 s, a slice thickness of 5 mm, a slice width of 5 mm, and a table feed of 8 mm per s. To enhance vascular and parenchymal delineation, 140 mL of iodinated contrast material (Ultravist 300; Schering, Berlin, Germany) was administered via an automated injector (XD 5500; Ulrich Medical Systems, Ulm, Germany) with a start delay of 50 s. Following CT image acquisition, PET data were collected for 5 min per bed position (total duration: approximately 35 min). The PET scanner had a coincidence time resolution of 500 ps, a coincidence window of 4.5 ns, and a sensitivity of 5.7 cps/kBq at 400 keV. Attenuation-corrected PET data were reconstructed using a standardized ordered-subset expectation maximization (OSEM) iterative reconstruction algorithm with two iterations, eight subsets, and a 5 mm Gaussian filter.
For each patient, two to three tumor lesions were selected as target lesions, specifically those that were well-demarcated. Irregular regions of interest (ROIs) with a threshold of 50% of the maximum DOTATOC uptake were drawn on the transverse PET slices. The standardized uptake values (SUV), including SUVmean and SUVmax, were calculated for each lesion using the standard formula that accounts for the measured activity concentration, corrected for body weight and injected activity. To normalize tumor SUV values, normal liver parenchyma was used as the background reference, and the SUV ratios of target lesions to the liver (SUVmeanT/L and SUVmaxT/L) were derived. Functional tumor volume (TVPET) was also determined for each lesion using the same threshold. Restaging with [68Ga]Ga-DOTATOC PET/CT was performed 3 months after the completion of PRRT, following the same imaging protocol as at baseline. The response of each tumor lesion was assessed based on the percentage change in functional tumor volume (%∆TVPET). In the case of ∆TVPET, variations are expressed as absolute values, whereas for %∆TVPET, variations are presented as percentages.
The CT-based tumor volume (TVCT) was manually segmented and measured using Sectra IDS7 PACS (Version 24.2). ΔTVCT was defined as the absolute change in tumor volume between baseline and post-PRRT imaging, while %ΔTVCT represents the relative volume change normalized to baseline volume. In the case of ∆TVCT, variations are expressed as absolute values, whereas for %∆TVCT, variations are presented as percentages.
Data are presented using descriptive statistics, including median (minimum–maximum), mean ± standard deviation, and count (percentage). Chi-squared tests or Fisher’s exact tests (as appropriate) were used to compare the proportions of patient groups dichotomized based on baseline characteristics. Mann–Whitney U tests were applied to compare quantitative tumor parameters (SUVmax, SUVmean, SUVmeanT/L, and SUVmaxT/L) across different groups. The association between tumor parameters in baseline [68Ga]Ga-DOTATOC PET/CT and the respective response to PRRT (%∆TVPET) was assessed using Spearman’s rank correlation analysis. All tests were two-sided, and a p-value <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (version 20.0; SPSS Inc., Chicago, IL, United States) and GraphPad Prism (version 10.2.3).
A total of 121 PRRT cycles with [177Lu]Lu-octreotate were administered to 32 patients. The mean age of the cohort was 67.8 years (range 40–90 years, median 70 years). Patients received up to four PRRT cycles, with a mean of 3.8 ± 0.7 cycles. The mean cumulative activity of [177Lu]Lu-octreotate was 29.3 ± 0.7 GBq. Treatment response, assessed according to the modified SWOG criteria (32), included partial response (PR) in 12 patients (37.5%), minimal response (MR) in eight patients (25%), stable disease (SD) in eight patients (25%), and progressive disease (PD) in four patients (12.5%). Therefore, we used post-PRRT PET/CT as the gold standard for response assessment, as it provides functional information on tumor activity. The mean progression-free survival (PFS) was 28.6 ± 15 months. No carcinoid crises were observed.
Lesion-based response analysis following PRRT was conducted for 66 lesions. At baseline, the mean SUVmax was 28.1 ± 16 (range: 3.0–91.2), SUVmean was 13.3 ± 5.1 (range: 2.4–27.1), SUVmeanT/L was 3.6 ± 1.7 (range: 0.96–10.45), and SUVmaxT/L was 7.7 ± 5.6 (range: 1.3–30.9). The functional tumor volume at baseline (TVPET) was 53.1 ± 12.2 mm3. Moreover, 3 months after PRRT completion, the absolute functional tumor volume change (∆TVPET) was 25.5 ± 9.3 mm3, while the percentage change in functional tumor volume (%∆TVPET) was 40.2 ± 49.7%.
Some discrepancies between contrast-enhanced CT and [68Ga]Ga-DOTATOC PET/CT were observed, particularly regarding detectability and tumor size assessment. Figure 1 illustrates an example of a patient with a pancreatic neuroendocrine tumor (P-NET) before and 3 months after the completion of PRRT. In the lesion-based analysis, neither metastatic site (hepatic vs. extrahepatic, p = 0.702) nor baseline lesion volume (p = 0.480) significantly influenced lesion response. However, lesions originating from the pancreas showed a significantly greater response compared to non-pancreatic lesions (%∆TVPET 56.8 ± 39.8 vs. 28.4 ± 50.1; p = 0.020). Pretreatment SUV-derived values and treatment-induced volumetric changes, stratified by baseline patient characteristics, are summarized in Table 1 (∆TVCT, %∆TVCT) and Table 2 (∆TVPET, %∆TVPET). Since Ki-67 index data were not available for all patients and FDG-PET/CT follow-up data were missing for two patients, 26 and 24 patients, respectively, were analyzed in the tables. No significant difference in CT-derived tumor volume change (∆TVCT) was observed based on tumor type (GEP-NET vs. P-NET), overall response (responders vs. non-responders), Ki-67 status, or metastatic location (liver vs. other sites). However, %∆TVCT differed significantly between responders (66.7 ± 39.2) and non-responders (12.7 ± 32.4), while no significant difference was found for other parameters.
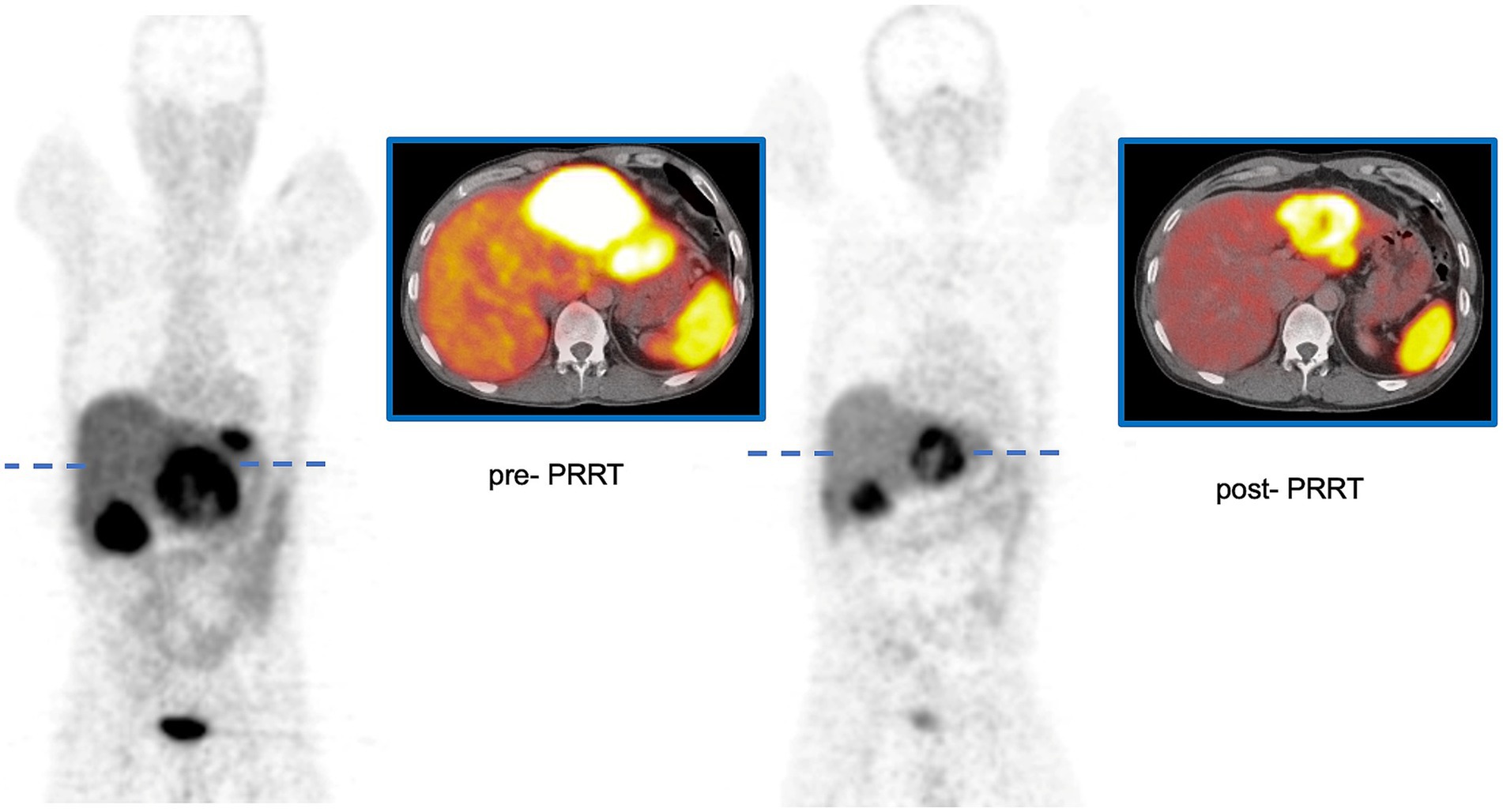
Figure 1. Patient with P-NET before and 3 months after the completion of PRRT using [177Lu]Lu-octreotate. ∆TVPET of the large lesion in the left lobe of the liver was 288 mL, with a %∆TVPET of 65%. The SUVmaxT/L was 7.0 before PRRT and 5.1 after PRRT.
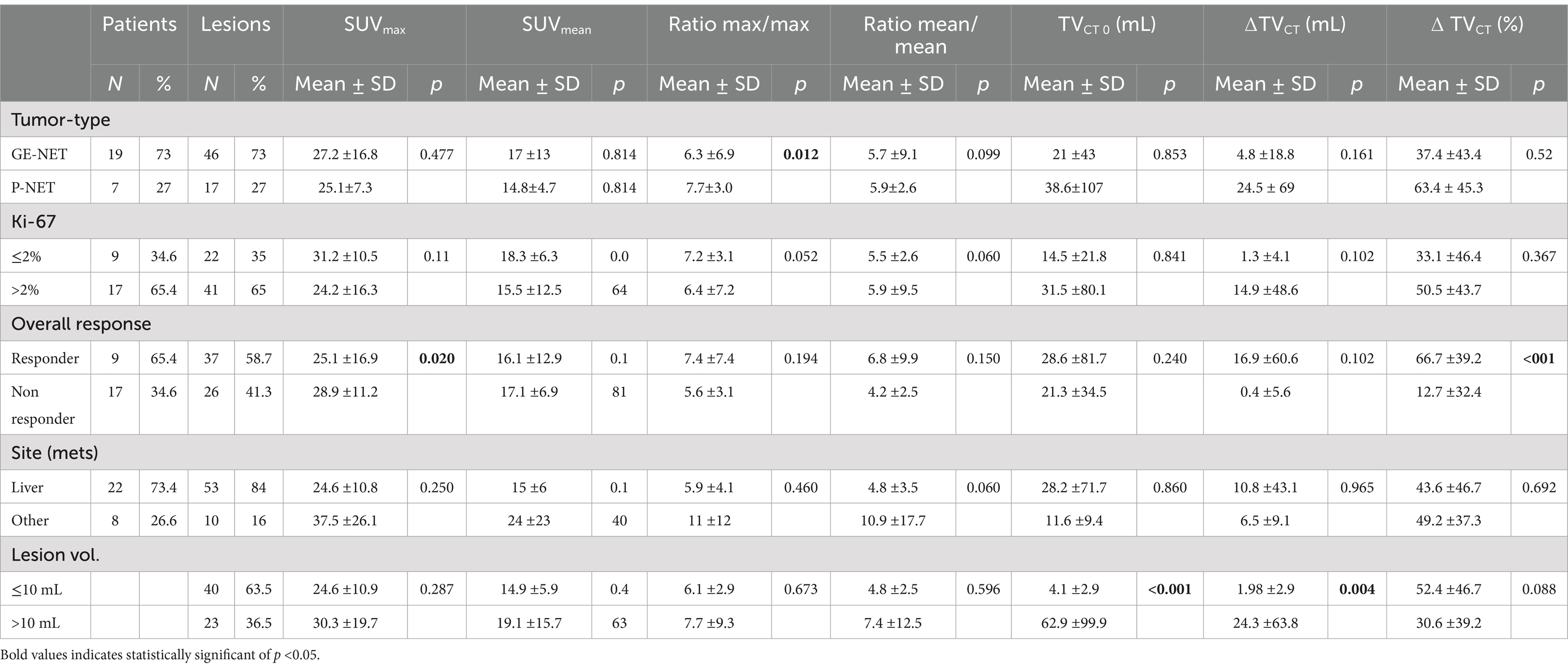
Table 1. Different pretreatment SUV and volume response parameters according to the patient and tumor characteristics.
Similarly, the change in PET-derived tumor volume (∆TVPET) did not show significant differences based on metastatic location or tumor type. Patients with a high Ki-67 index (>2%) exhibited a significantly greater ∆TVPET (54.1 ± 106.5 mL) compared to those with low Ki-67 (<2%) (0.84 ± 28.8 mL). Furthermore, ∆TVPET was significantly higher in overall responders (47.7 ± 95.5 mL vs. 10.5 ± 32.6 mL). Regarding tumor type, %∆TVPET was significantly greater in P-NETs (70.1 ± 29.5) than in GEP-NETs (27.1 ± 54.3). Additionally, overall responders exhibited a significantly higher %∆TVPET (59.1 ± 35.9 vs. 12.3 ± 53.7). However, no significant differences in %∆TVPET were observed based on Ki-67 status or metastatic location.
Baseline SUVmean (p = 0.071) and SUVmax (p = 0.706) of tumor lesions showed no significant association with lesion-based response. In contrast, higher tumor-to-background ratios at baseline, specifically SUVmeanT/L (p = 0.011, r = 0.381) and SUVmaxT/L (p = 0.004, r = 0.435), were associated with more pronounced changes in functional tumor volume, as illustrated in Figure 2. For marker lesions, higher SUVmeanT/L and SUVmaxT/L were predictive of therapy response, defined as a >50% decrease in %∆TVPET (p = 0.0027 and p = 0.0001, respectively), as shown in Figure 3; however, a considerable overlap was observed.
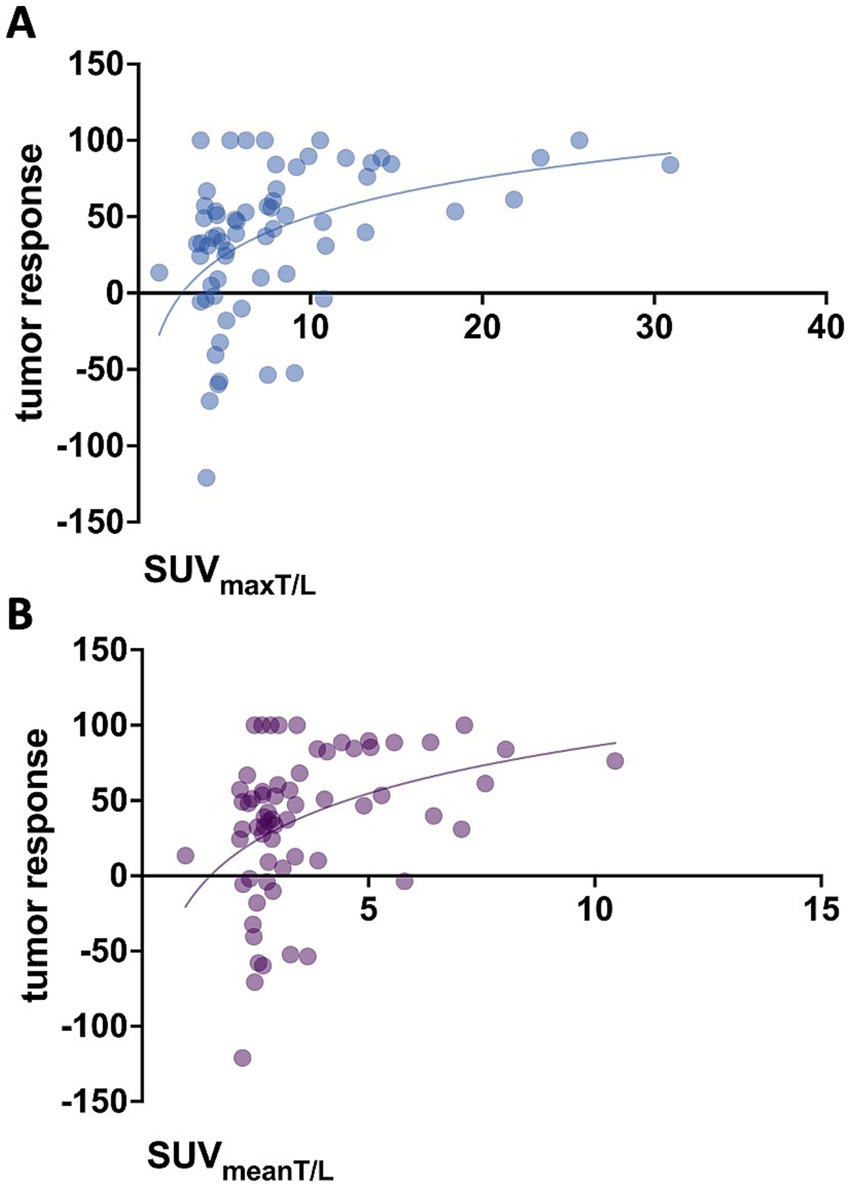
Figure 2. Association between baseline tumor-to-background ratios and tumor response (%∆TVPET). (A) SUVmaxT/L (p = 0.004, r = 0.435) and (B) SUVmeanT/L (p = 0.011, r = 0.381).
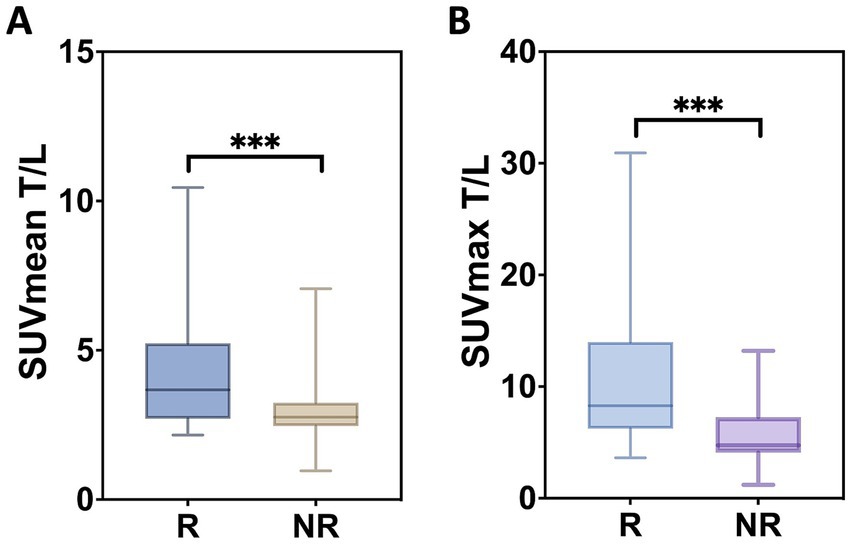
Figure 3. Lesion-based measures. The distribution of SUVmean T/L (A) and SUVmax T/L (B) is shown. Box plots depict the median as well as the upper and lower quartiles of each distribution. R, response; NR, no response. (***) The difference was significant with p = 0.027 and p = 0.001, respectively.
Given the higher likelihood of complete remission in smaller lesions, the association between SUV-derived values and lesion-based response was further evaluated for lesions >10 mL (n = 44). In this subgroup, the correlation between pretreatment SUVmaxT/L (p < 0.001) and SUVmeanT/L (p < 0.001) with functional tumor volume response remained statistically significant (data not shown).
PRRT is a well-established treatment option for advanced NET following the failure of SSA therapy. A primary prerequisite for PRRT is the overexpression of somatostatin receptors (SSTR) on neuroendocrine tumor cells, enabling sufficient tracer uptake to generate high-contrast imaging between tumor lesions and healthy organs. Currently, [68Ga]Ga-DOTATOC PET/CT is the preferred modality for assessing SSTR expression. In this study, SSTR expression in tumor lesions was evaluated using [68Ga]Ga-DOTATOC PET/CT, with receptor density quantified by tumor-to-liver SUV ratios (SUVmaxT/L and SUVmeanT/L). The findings demonstrate a significant association between receptor density in SSTR-expressing tumor lesions and the response to PRRT based on lesions. This association remained statistically significant even after excluding lesions <10 mL.
The predictive value of pre-therapeutic SUV parameters derived from [68Ga]Ga-DOTATOC PET/CT for assessing response to PRRT remains controversial, as previous studies have reported conflicting results. While some studies have identified SUVmax as a predictor of treatment response (33–38), others have found no significant association (39–44). For instance, Gabriel et al. (40) reported that baseline SUVmax values of the most prominent lesion were comparable between morphologically assessed responders and non-responders to PRRT. Conversely, other studies suggested that higher SUVmax values were predictive of treatment response and longer time to progression (33, 45). However, lesion-based analyses were conducted in only three of these studies (34, 37, 44). The lesion-based analysis in our study demonstrated no significant association between baseline SUVmean or SUVmax of tumor lesions and lesion-based response. The superior predictive value of baseline tumor-to-liver SUV ratios, compared to tumor SUVmean and SUVmax, further highlights the limitations of SUV parameters as direct surrogates for somatostatin receptor density and underscores the importance of normalizing these values to background activity.
In addition to [68Ga]Ga-DOTATOC PET/CT, [18F]-FDG PET/CT has also been shown to play a role in predicting tumor response, disease progression, and survival in patients undergoing PRRT for advanced NET. High [18F]-FDG SUVmax has been associated with poor clinical outcomes and increased disease progression (34, 46, 47). Based on these findings, [18F]-FDG PET/CT may serve as a valuable additional tool for therapeutic decision-making. Another important predictor of PRRT response is the proliferation status of the tumor, as quantified by the Ki-67 index. In our study, Ki-67 >2% was significantly associated with a higher ∆TVPET compared to Ki-67 ≤2%. The proliferation rate is a well-established determinant of survival and a recognized prognostic factor in NETs (48). There is substantial evidence supporting its predictive value for progression-free survival and treatment outcomes following PRRT (11, 48–51), although NETs within the higher G2 range may exhibit treatment responses similar to those with a low Ki-67 index. A recent study introduced an algorithm that incorporates circulating NET transcripts and the Ki-67 index, which correlates with treatment response and effectively predicts PRRT efficacy (52). Another important factor in PRRT treatment decision-making is the quantification of liver tumor burden. Several studies have indicated that patients with a low liver tumor burden achieve significantly longer disease-free survival following PRRT compared to those with a high liver tumor burden (11, 13, 53). In our study, the mean %∆TVPET in liver metastases was lower than in metastases at other locations; however, no significant difference was observed in ∆TVPET or %∆TVPET. Further differentiation between metastatic sites may provide additional insights. For example, one study reported that patients with bone metastases had a higher risk of disease progression following PRRT (54).
In this study, the functional tumor volume change (%∆TVPET) was chosen as the primary parameter for lesion-based response assessment because evaluating the response of NETs to PRRT using only computed tomography (CT) has shown limited accuracy, particularly in cases of hepatic metastases. Morphological shrinkage is observed in only a minority of patients who demonstrate clear clinical improvement, and anatomical alterations may persist for a prolonged period post-treatment, despite significant local tumoricidal effects (55, 56). When analyzing patient-based characteristics, only tumors of pancreatic origin were significantly associated with greater %∆TVPET volume changes (p = 0.041). This finding aligns with previous observations that pancreatic NETs exhibit a more pronounced response to PRRT based on morphological response criteria such as WHO, RECIST, and SWOG (54, 57).
Our study demonstrated a significant correlation between lesion SUVmeanT/L and SUVmaxT/L and lesion-based response, quantified by %∆TVPET. These parameters may serve as valuable tools to support clinical decision-making regarding PRRT eligibility. A lesion-based evaluation may help refine patient selection and treatment planning for PRRT, leading to a more personalized approach. Other factors to consider in this process include [18F]-FDG uptake, Ki-67 status, and liver tumor burden. However, further studies are required to identify the optimal patient and tumor characteristics for PRRT selection.
Serological markers were not systematically included in our analysis; however, their potential relevance, particularly chromogranin A levels, as additional indicators of treatment response should be considered. Nonetheless, its suitability as a marker for therapy response under PRRT remains controversial (58). A promising emerging approach for predicting treatment response is radiomics (59). Radiomics involves the extraction and analysis of large-scale quantitative imaging features from medical scans, enabling a more precise prediction of patient outcomes (60). Future research should focus on exploring the potential of radiomics-based models to enhance treatment stratification and response assessment in PRRT.
This study has several limitations. First, the analysis was retrospective, observational, and conducted at a single center, which may limit generalizability. Second, the sample size was relatively small, with only 32 patients included in the retrospective analysis. We emphasize the exploratory nature of our findings and acknowledge the need for larger, prospective studies to confirm our results. Additionally, the administered activity of [177Lu]Lu-octreotate varied among patients, with a mean cumulative activity of 29.3 ± 0.7 GBq across 3.8 ± 0.7 cycles.
In conclusion, the lesion-based molecular response to PRRT is significantly associated with pretreatment somatostatin receptor uptake, quantified by tumor-to-liver SUV ratios in [68Ga]Ga-DOTATOC PET.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval were waived for this study due to retrospective study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FR: Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software. SE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cives, M, and Strosberg, J. Radionuclide therapy for neuroendocrine tumors. Curr Oncol Rep. (2017) 19:9. doi: 10.1007/s11912-017-0567-8
2. Wu, P, He, D, Chang, H, and Zhang, X. Epidemiologic trends of and factors associated with overall survival in patients with neuroendocrine tumors over the last two decades in the USA. Endocr Connect. (2023) 12:e230331. doi: 10.1530/EC-23-0331
3. Pavel, M, Öberg, K, Falconi, M, Krenning, EP, Sundin, A, Perren, A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:844–60. doi: 10.1016/j.annonc.2020.03.304
4. Rindi, G, Mete, O, Uccella, S, Basturk, O, La Rosa, S, Brosens, LAA, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. (2022) 33:115–54. doi: 10.1007/s12022-022-09708-2
5. Krenning, EP, Kwekkeboom, DJ, Bakker, WH, Breeman, WAP, Kooij, PPM, Oei, HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. (1993) 20:716–31. doi: 10.1007/BF00181765
6. Reubi, JC, Schaer, JC, Wenger, S, Hoeger, C, Erchegyi, J, Waser, B, et al. SST3-selective potent peptidic somatostatin receptor antagonists. Proc Natl Acad Sci USA. (2000) 97:13973–8. doi: 10.1073/pnas.250483897
7. Reubi, JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. (2003) 24:389–427. doi: 10.1210/er.2002-0007
8. Kwekkeboom, DJ, Kam, BL, van Essen, M, Teunissen, JJ, van Eijck, CH, Valkema, R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. (2010) 17:R53–73. doi: 10.1677/ERC-09-0078
9. Sabet, A, Ezziddin, K, Pape, UF, Reichman, K, Haslerud, T, Ahmadzadehfar, H, et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. Eur J Nucl Med Mol Imaging. (2014) 41:505–10. doi: 10.1007/s00259-013-2601-x
10. Sabet, A, Ezziddin, K, Pape, UF, Ahmadzadehfar, H, Mayer, K, Pöppel, T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. (2013) 54:1857–61. doi: 10.2967/jnumed.112.119347
11. Ezziddin, S, Attassi, M, Yong-Hing, CJ, Ahmadzadehfar, H, Willinek, W, Grünwald, F, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. (2014) 55:183–90. doi: 10.2967/jnumed.113.125336
12. Ezziddin, S, Sabet, A, Heinemann, F, Yong-Hing, CJ, Ahmadzadehfar, H, Guhlke, S, et al. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. (2011) 52:1197–203. doi: 10.2967/jnumed.111.090373
13. Ezziddin, S, Khalaf, F, Vanezi, M, Haslerud, T, Mayer, K, Al Zreiqat, A, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2014) 41:925–33. doi: 10.1007/s00259-013-2677-3
14. Imhof, A, Brunner, P, Marincek, N, Briel, M, Schindler, C, Rasch, H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. (2011) 29:2416–23. doi: 10.1200/JCO.2010.33.7873
15. Kwekkeboom, DJ, de Herder, WW, van Eijck, CH, Kam, BL, van Essen, M, Teunissen, JJ, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. (2010) 40:78–88. doi: 10.1053/j.semnuclmed.2009.10.004
16. Ezziddin, S, Opitz, M, Attassi, M, Biermann, K, Sabet, A, Guhlke, S, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. (2011) 38:459–66. doi: 10.1007/s00259-010-1610-2
17. Sabet, A, Haslerud, T, Pape, UF, Sabet, A, Ahmadzadehfar, H, Grünwald, F, et al. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2014) 41:205–10. doi: 10.1007/s00259-013-2547-z
18. Di Franco, M, Zanoni, L, Fortunati, E, Fanti, S, and Ambrosini, V. Radionuclide theranostics in neuroendocrine neoplasms: an update. Curr Oncol Rep. (2024) 26:538–50. doi: 10.1007/s11912-024-01526-5
19. Kowalski, J, Henze, M, Schuhmacher, J, Mäcke, HR, Hofmann, M, and Haberkorn, U. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe1-Tyr3-octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol. (2003) 5:42–8. doi: 10.1016/S1536-1632(03)00038-6
20. Gabriel, M, Decristoforo, C, Kendler, D, Dobrozemsky, G, Heute, D, Uprimny, C, et al. 68Ga-DOTA-Tyr3-octreotide pet in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. (2007) 48:508–18. doi: 10.2967/jnumed.106.035667
21. Ezziddin, S, Lohmar, J, Yong-Hing, CJ, Sabet, A, Ahmadzadehfar, H, Kukuk, G, et al. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin Nucl Med. (2012) 37:e141–7. doi: 10.1097/RLU.0b013e31823926e5
22. Hope, TA, Bergsland, EK, Bozkurt, MF, Graham, M, Heaney, AP, Herrmann, K, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. (2018) 59:66–74. doi: 10.2967/jnumed.117.202275
23. Miederer, M, Seidl, S, Buck, A, Scheidhauer, K, Wester, HJ, Schwaiger, M, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. (2009) 36:48–52. doi: 10.1007/s00259-008-0944-5
24. Forrer, F, Valkema, R, Kwekkeboom, DJ, de Jong, M, and Krenning, EP. Neuroendocrine tumors. Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab. (2007) 21:111–29. doi: 10.1016/j.beem.2007.01.007
25. Kwekkeboom, DJ, Krenning, EP, Lebtahi, R, Komminoth, P, Kos-Kudła, B, de Herder, WW, et al. Enets consensus guidelines for the standards of care in neuroendocrine tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. (2009) 90:220–6. doi: 10.1159/000225951
26. Bodei, L, Mueller-Brand, J, Baum, RP, Pavel, ME, Hörsch, D, O’Dorisio, MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2013) 40:800–16. doi: 10.1007/s00259-012-2330-6
27. Breeman, WA, De Jong, M, Visser, TJ, Erion, JL, and Krenning, EP. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging. (2003) 30:917–20. doi: 10.1007/s00259-003-1142-0
28. Breeman, WA, van der Wansem, K, Bernard, BF, van Gameren, A, Erion, JL, Visser, TJ, et al. The addition of DTPA to [177Lu-DOTA0,Tyr3]octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging. (2003) 30:312–5. doi: 10.1007/s00259-002-1054-4
29. Kwekkeboom, DJ, Bakker, WH, Kam, BL, Teunissen, JJ, Kooij, PP, de Herder, WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging. (2003) 30:417–22. doi: 10.1007/s00259-002-1050-8
30. Kwekkeboom, DJ, Teunissen, JJ, Bakker, WH, Kooij, PP, de Herder, WW, Feelders, RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. (2005) 23:2754–62. doi: 10.1200/JCO.2005.08.066
31. Zhernosekov, KP, Filosofov, DV, Baum, RP, Aschoff, P, Bihl, H, Razbash, AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. (2007) 48:1741–8. doi: 10.2967/jnumed.107.040378
32. Green, S, and Weiss, GR. Southwest oncology group standard response criteria, endpoint definitions and toxicity criteria. Investig New Drugs. (1992) 10:239–53. doi: 10.1007/BF00944177
33. Haug, AR, Auernhammer, CJ, Wängler, B, Schmidt, GP, Uebleis, C, Göke, B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. (2010) 51:1349–56. doi: 10.2967/jnumed.110.075002
34. Oh, S, Prasad, V, Lee, DS, and Baum, RP. Effect of peptide receptor radionuclide therapy on somatostatin receptor status and glucose metabolism in neuroendocrine tumors: Intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging. (2011) 2011:524130. doi: 10.1155/2011/524130
35. Filice, A, Fraternali, A, Frasoldati, A, Asti, M, Grassi, E, Massi, L, et al. Radiolabeled somatostatin analogues therapy in advanced neuroendocrine tumors: a single centre experience. J Oncol. (2012) 2012:320198. doi: 10.1155/2012/320198
36. Öksüz, M, Winter, L, Pfannenberg, C, Reischl, G, Müssig, K, Bares, R, et al. Peptide receptor radionuclide therapy of neuroendocrine tumors with 90Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of 68Ga-DOTATOC? Diagn Interv Imaging. (2014) 95:289–300. doi: 10.1016/j.diii.2013.07.006
37. Kratochwil, C, Stefanova, M, Mavriopoulou, E, Holland-Letz, T, Dimitrakopoulou-Strauss, A, Afshar-Oromieh, A, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. (2015) 17:313–8. doi: 10.1007/s11307-014-0795-3
38. Sharma, R, Wang, WM, Yusuf, S, Evans, J, Ramaswami, R, Wernig, F, et al. Ga-DOTATATE PET/CT parameters predict response to peptide receptor radionuclide therapy in neuroendocrine tumours. Radiother Oncol. (2019) 141:108–15. doi: 10.1016/j.radonc.2019.09.003
39. Albertelli, M, Dotto, A, Di Dato, C, Malandrino, P, Modica, R, Versari, A, et al. PRRT: identikit of the perfect patient. Rev Endocr Metab Disord. (2021) 22:563–79. doi: 10.1007/s11154-020-09581-6
40. Gabriel, M, Oberauer, A, Dobrozemsky, G, Decristoforo, C, Putzer, D, Kendler, D, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. (2009) 50:1427–34. doi: 10.2967/jnumed.108.053421
41. Soydal, Ç, Peker, A, Özkan, E, Küçük, ÖN, and Kir, MK. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with Lu-177 DOTATATE. Turk J Med Sci. (2016) 46:409–13. doi: 10.3906/sag-1412-11
42. Werner, RA, Lapa, C, Ilhan, H, Higuchi, T, Buck, AK, Lehner, S, et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget. (2017) 8:7039–49. doi: 10.18632/oncotarget.12402
43. Gabriel, M, Nilica, B, Kaiser, B, and Virgolini, IJ. Twelve-year follow-up after peptide receptor radionuclide therapy. J Nucl Med. (2019) 60:524–9. doi: 10.2967/jnumed.118.215376
44. Werner, RA, Ilhan, H, Lehner, S, Papp, L, Zsótér, N, Schatka, I, et al. Pre-therapy somatostatin receptor-based heterogeneity predicts overall survival in pancreatic neuroendocrine tumor patients undergoing peptide receptor radionuclide therapy. Mol Imaging Biol. (2019) 21:582–90. doi: 10.1007/s11307-018-1252-5
45. Campana, D, Ambrosini, V, Pezzilli, R, Fanti, S, Labate, AM, Santini, D, et al. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. (2010) 51:353–9. doi: 10.2967/jnumed.109.066662
46. Nilica, B, Waitz, D, Stevanovic, V, Uprimny, C, Kendler, D, Buxbaum, S, et al. Direct comparison of 68Ga-DOTA-TOC and 18F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur J Nucl Med Mol Imaging. (2016) 43:1585–92. doi: 10.1007/s00259-016-3328-2
47. Nicolini, S, Severi, S, Ianniello, A, Sansovini, M, Ambrosetti, A, Bongiovanni, A, et al. Investigation of receptor radionuclide therapy with 177Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imaging. (2018) 45:923–30. doi: 10.1007/s00259-017-3925-8
48. Pape, UF, Berndt, U, Müller-Nordhorn, J, Böhmig, M, Roll, S, Koch, M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. (2008) 15:1083–97. doi: 10.1677/ERC-08-0017
49. Bodei, L, Kidd, M, Modlin, IM, Severi, S, Drozdov, I, Nicolini, S, et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. (2016) 43:839–51. doi: 10.1007/s00259-015-3250-z
50. Ezziddin, S, Sabet, A, Yong-Hing, CJ, and Biersack, HJ. Comment on Campana et al.: Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: factors associated with response and suggestions for therapeutic sequence. Eur J Nucl Med Mol Imaging. (2014) 41:174–5. doi: 10.1007/s00259-013-2599-0
51. Carlsen, EA, Fazio, N, Granberg, D, Grozinsky-Glasberg, S, Ahmadzadehfar, H, Grana, CM, et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: a multicenter cohort study. Endocr Relat Cancer. (2019) 26:227–39. doi: 10.1530/ERC-18-0424
52. Bodei, L, Kidd, MS, Singh, A, van der Zwan, WA, Severi, S, Drozdov, IA, et al. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging. (2018) 45:1155–69. doi: 10.1007/s00259-018-3967-6
53. Delpassand, ES, Samarghandi, A, Zamanian, S, Wolin, EM, Hamiditabar, M, Espenan, GD, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. (2014) 43:518–25. doi: 10.1097/MPA.0000000000000113
54. Kwekkeboom, DJ, de Herder, WW, Kam, BL, van Eijck, CH, van Essen, M, Kooij, PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. (2008) 26:2124–30. doi: 10.1200/JCO.2007.15.2553
55. Valkema, R, Pauwels, S, Kvols, LK, Barone, R, Jamar, F, Bakker, WH, et al. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. (2006) 36:147–56. doi: 10.1053/j.semnuclmed.2006.01.001
56. Waldherr, C, Pless, M, Maecke, HR, Schumacher, T, Crazzolara, A, Nitzsche, EU, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med. (2002) 43:610–6.
57. Kwekkeboom, DJ, Mueller-Brand, J, Paganelli, G, Anthony, LB, Pauwels, S, Kvols, LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. (2005) 46:62S–6S.
58. Marotta, V, Zatelli, MC, Sciammarella, C, Ambrosio, MR, Bondanelli, M, Colao, A, et al. Chromogranin a as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer. (2018) 25:R11–29. doi: 10.1530/ERC-17-0269
59. Laudicella, R, Comelli, A, Liberini, V, Vento, A, Stefano, A, Spataro, A, et al. [68Ga]DOTATOC PET/CT radiomics to predict the response in GEP-NETs undergoing [177Lu]DOTATOC PRRT: the “Theragnomics” concept. Cancers. (2022) 14:984. doi: 10.3390/cancers14040984
Keywords: neuroendocrine tumors, response prediction, peptide receptor radionuclide therapy, [177Lu]Lu-octreotate, [68Ga]Ga-DOTATOC-PET/CT
Citation: Aouf A, Speicher T, Blickle A, Bastian MB, Burgard C, Rosar F, Ezziddin S and Sabet A (2025) Prediction of lesion-based response to PRRT using baseline somatostatin receptor PET. Front. Med. 12:1523862. doi: 10.3389/fmed.2025.1523862
Received: 06 November 2024; Accepted: 26 February 2025;
Published: 14 March 2025.
Edited by:
Francesco Cicone, Magna Græcia University, ItalyReviewed by:
Anchal Ghai, University of Texas Southwestern Medical Center, United StatesCopyright © 2025 Aouf, Speicher, Blickle, Bastian, Burgard, Rosar, Ezziddin and Sabet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tilman Speicher, dGlsbWFuLnNwZWljaGVyQHVrcy5ldQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.