- 1Department of General Surgery, Uzhhorod National University, Uzhhorod, Ukraine
- 2Department of Biochemistry and Pharmacology, Uzhhorod National University, Uzhhorod, Ukraine
- 3Department of Medical Rehabilitation, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
- 4Department of Therapy and Family Medicine, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
- 5Department of Microbiology, Virology, and Immunology, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Knee osteoarthritis (OA) is a common condition that causes pain and reduces the quality of life for many people. It also leads to high health and financial costs. Managing knee OA pain requires using different methods together for the best results. This review overviews current therapeutic options for knee OA pain, focusing on their efficacy, safety, and potential roles in clinical practice. Topical treatments, such as NSAIDs and capsaicin, offer significant pain relief with minimal systemic side effects and are suitable for initial therapy, together with nonpharmacologic interventions like exercise and, when relevant, weight loss. Oral analgesics, including acetaminophen and opioids, have limited efficacy and serious side effects, making them appropriate only for short-term or rescue therapy. Intra-articular injections, such as corticosteroids, hyaluronic acid, and platelet rich plasma, demonstrate varying levels of efficacy and safety. Nutritional supplements, including curcumin, Boswellia serrata, and glucosaminechondroitin combinations, offer modest benefits and are best used as adjuncts to standart treatment. Nonpharmacological treatments, such as transcutaneous electrical nerve stimulation (TENS), acupuncture, and local heat therapy, provide variable pain relief and should be customized based on individual patient responses. Targeted biologic agents, such as antibodies to TNF-α, IL-1, and NGF, hold promise for more precise pain relief; however, further research is required to establish their routine use. Treating knee OA pain should be personalized, combining several methods. Research must continue to improve treatments and make them safer.
Introduction
Osteoarthritis (OA) is a disease of movable joints characterized by anatomical and physiological abnormalities, such as cartilage degradation, bone remodeling, osteophyte formation, joint inflammation, and loss of normal joint function. It begins with micro- and macro-damage to the joint, which activates maladaptive recovery reactions, leading to abnormal tissue metabolism (1).
Osteoarthritis is a major cause of chronic disability, primarily due to pain, the main symptom of the disease (2). Knee OA pain typically progresses from intermittent pain during exercise to more persistent chronic pain (3, 4). Symptoms such as pain and stiffness in OA contribute to functional limitations, with a well-documented relationship between pain severity and the degree of functional limitation (5). OA also imposes a serious burden on health and the economy (6, 7).
Osteoarthritis is the most common musculoskeletal disease worldwide and represents a significant health and economic burden (8, 9). It is a major cause of chronic pain and disability due to reduced joint mobility and function and reduced quality of life (10, 11). Risk factors for osteoarthritis encompass genetic predispositions, lifestyle behaviors, biological factors such as age and gender, as well as metabolic conditions, including obesity and hypertension (Figure 1) (12, 13).
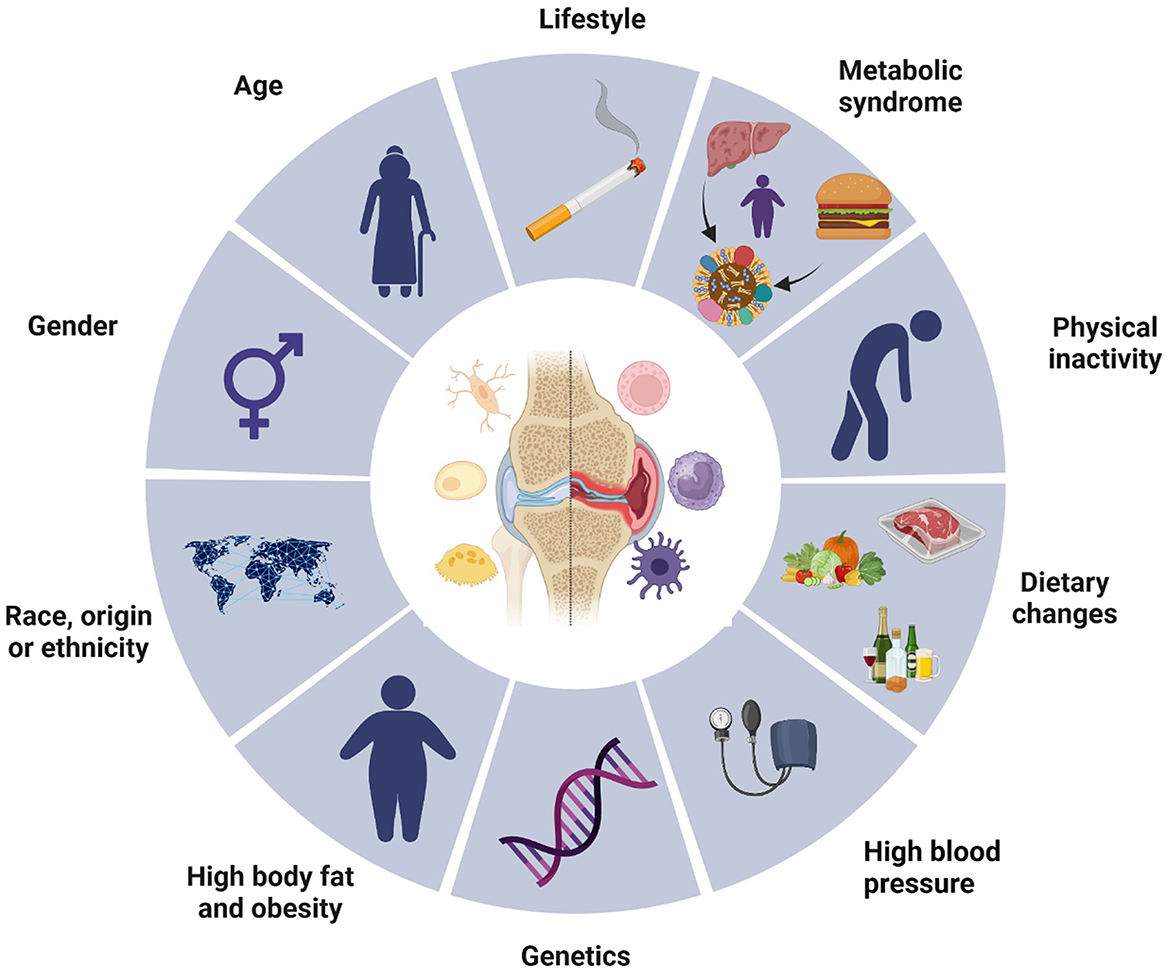
Figure 1. Osteoarthritis risk factors. This diagram illustrates various risk factors contributing to the development of osteoarthritis. The central image of a joint highlights the site of the condition, surrounded by multiple influencing elements segmented into lifestyle, biological, and genetic factors. Key components include age, gender, race or ethnicity, high body fat and obesity, genetic predispositions, and associated conditions such as metabolic syndrome and high blood pressure. Lifestyle choices, including physical inactivity, dietary habits, and behaviors like smoking, also play significant roles. Collectively, these elements emphasize the multifactorial nature of osteoarthritis, underscoring the complexity of its etiology.
The knee is the most affected joint, accounting for ~85% of OA cases worldwide (14, 15). Knee joint osteoarthritis (OA) is a multifactorial disease characterized by various pathological changes, including cartilage degradation, osteophyte formation, remodeling of osteo cartilaginous units, and joint inflammation (16).
Various factors, including mechanical, inflammatory, aging, and metabolic disorders, contribute to the pathogenesis of OA (17–20). Dysbiotic alterations and stress are significant contributors to the progression of osteoarthritis and the exacerbation of pain syndromes (21–23). Therefore, it is essential to consider medications that can mitigate these factors (24–26). These factors ultimately lead to structural joint destruction, loss of synovial joint function, and long-term chronic pain (27–29). Patients with OA commonly experience stiffness, pain, and loss of function (30). The prevalence of OA increases with age: 13.9% of adults aged 25 years and older have clinical OA in at least one joint, whereas 33.6% of adults aged 65 years and older are affected. According to the Johnston County Osteoarthritis Project, the lifetime risk of developing symptomatic knee OA is ~45% (40% in men and 47% in women). This risk increases to 60.5% in obese individuals, which is approximately twice as high as the risk in those who are normal weight or underweight (31, 32). Pregnancy can exacerbate the progression of osteoarthritis due to increased weight and hormonal changes (33, 34). The coexistence of OA and endocrine disorders, especially those related to thyroid dysfunction, can complicate the clinical landscape, as metabolic alterations and hormonal imbalances linked to thyroid conditions may intensify inflammatory processes and promote the progression of osteoarthritis (35–37). Therefore, finding effective and safe treatments for OA is crucial in the clinic.
Pain in osteoarthritis arises from inflammatory, mechanical, and neuropathic mechanisms, requiring tailored management strategies. Mechanical pain is addressed through interventions that reduce joint stress, such as physical therapy, weight management, and the use of assistive devices, alongside systemic analgesics and intra-articular hyaluronic acid injections (38). Inflammatory pain is managed with NSAIDs and corticosteroid injections, while neuropathic pain responds to therapies like gabapentinoids, antidepressants, or radiofrequency ablation (39). Advanced regenerative treatments, such as platelet-rich plasma and stem cell therapy, show potential for addressing pain of mixed origin. Reducing pain remains the primary goal of osteoarthritis management, enhancing patient function and quality of life.
The aim of this review is to provide an in-depth evaluation of the current treatment strategies for knee osteoarthritis (OA), focusing on their comparative efficacy, safety profiles, and practical applicability in clinical settings. This review emphasizes recent advancements in topical and systemic pharmacological therapies, biologic agents, and emerging non-pharmacologic approaches, while identifying gaps in the evidence to guide future research.
Topical treatment
Topical NSAIDs
A network meta-analysis revealed that topical and oral NSAIDs offer similar improvements in function and are more effective than paracetamol for treating knee osteoarthritis (OA). Data from 122 randomized controlled trials indicate that topical NSAIDs have a lower risk of gastrointestinal side effects than both paracetamol and oral NSAIDs do (40). Furthermore, real-world data suggest that topical NSAIDs have better overall safety than oral NSAIDs do. They also present lower risks of all-cause mortality, cardiovascular disease, and gastrointestinal bleeding than paracetamol in real-world settings (41) (Figure 2).
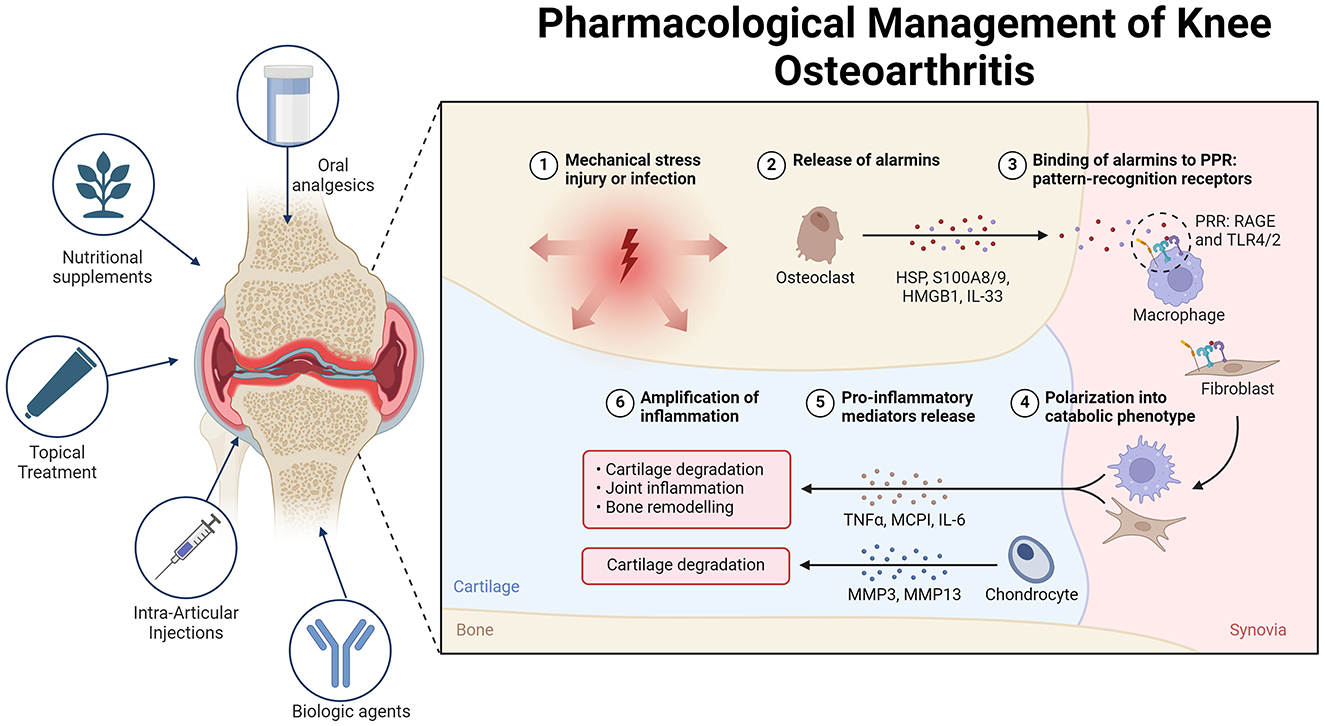
Figure 2. Overview of the pathogenesis and treatment of OA. In osteoarthritis (OA), alarmins are endogenous molecules released in response to various forms of damage. These molecules bind to pattern recognition receptors (PRRs) on different cells, triggering either an inflammatory or regenerative response. Alarmins can polarize cells such as macrophages and fibroblasts, leading to increased production of pro-inflammatory mediators and metalloproteases. This cascade of events contributes to cartilage destruction and joint damage, thus perpetuating inflammation and OA pathology. To manage OA pain, various treatment options are available. Topical treatments such as NSAIDs and capsaicin provide significant pain relief with minimal systemic side effects, making them suitable for initial therapy. Oral analgesics, such as acetaminophen and opioids, offer limited efficacy and have notable side effects, making them suitable only for short-term or rescue therapy. Intra-articular injections, including corticosteroids, hyaluronic acid, and platelet-rich plasma, show varying degrees of efficacy. Mesenchymal stem cells (MSCs) hold promise for future treatment pending further research. Nutritional supplements such as curcumin, Boswellia serrata, and glucosamine-chondroitin combinations present modest benefits and are best used as adjuncts. Non-pharmacological treatments, including transcutaneous electrical nerve stimulation (TENS), acupuncture, and local heat therapy, provide variable pain relief and should be considered on the basis of individual patient response. Biological agents that target cytokines such as TNF-α, IL-1, and NGF show promise, although additional research is necessary to establish their routine use.
A Cochrane review revealed that ~60% of patients experienced at least a 50% reduction in pain with topical NSAIDs, comparable to the relief provided by oral NSAIDs and slightly better than that achieved with a topical placebo (42). Compared with oral formulations, topical NSAIDs have a much lower risk of gastrointestinal, kidney, and cardiovascular toxicity due to reduced systemic absorption [e.g., topical diclofenac is 5- to 17-fold less absorbed than the oral version; (43–45)].
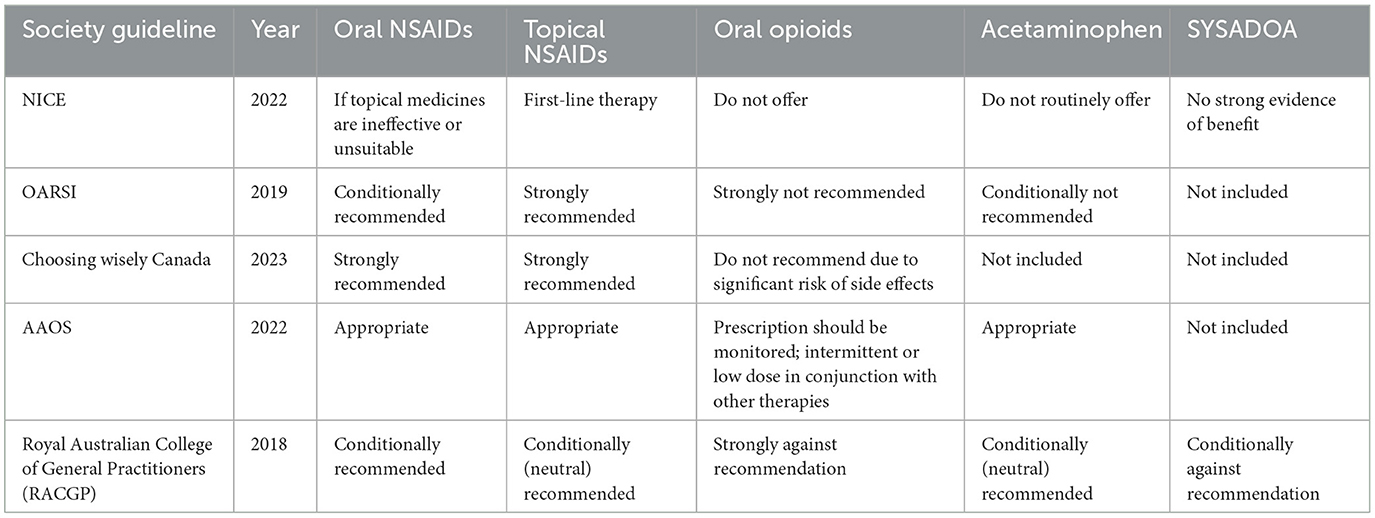
Guidelines consistently endorse the use of topical NSAID therapy. The AAOS supports the use of topical NSAIDs for symptomatic treatment of knee OA (46). The OARSI guidelines recommend topical NSAIDs as a first-line treatment for knee OA pain relief. In contrast, the ACR/AF strongly advocates their use, suggesting that they be prioritized over oral NSAIDs (47). Similarly, the ESCEO guidelines advise the use of topical NSAIDs before oral NSAIDs when optimal pain relief is not achieved with first-line SYSADOA and acetaminophen (48) (Table 1).
Topical capsaicin
Capsaicin, a compound extracted from hot chili peppers, may help relieve pain by downregulating TRPV1 receptor activity on nociceptive sensory neurons and depleting substance P levels. With the ongoing use of capsaicin, nociceptive fibers become desensitized, reducing pain signal transmission. However, the role of substance P depletion in the pain-relieving effects of capsaicin has been called into question (49).
We found three systematic reviews examining the efficacy of capsaicin for osteoarthritis (OA) pain relief. In the first review by Cameron et al., five randomized controlled trials (RCTs) involving 456 participants were analyzed (50). The intervention involved applying topical capsaicin (0.025% or 0.075%) four times daily, compared with a placebo, over a follow-up period of 3–4 weeks. The primary endpoint was pain assessment, which was mostly measured by a visual analog scale (VAS). The study concluded that topical capsaicin significantly reduced OA pain in the hand, knee, or multiple joints and was superior to placebo. However, the blinding was compromised because of the local burning sensation associated with capsaicin.
In the second review by De Silva et al., five RCTs involving 427 participants were included (51). The intervention included topical capsaicin (0.015%, 0.025%, or 0.075%), which was applied once or four times daily, rather than a placebo, over a 4–12 weeks follow-up period. The primary endpoint was also pain assessment. The results indicated that topical capsaicin was significantly more effective than placebo in relieving hand and knee OA pain. Redness and local burning sensations were the capsaicin group's most frequently reported side effects. This review was also assigned a level of evidence of 2.
In the third review by Laslett and Jones, five RCTs and one case-crossover trial involving 1162 participants were analyzed (52). The intervention involved topical capsaicin (0.025% or 0.075%) applied four times daily, compared with a placebo, over a 4–12 weeks follow-up period. Pain assessment, primarily measured by the VAS, was the endpoint. The review revealed that topical capsaicin had moderate efficacy in reducing pain intensity in OA of the hand, knee, or several joints compared with placebo. Mild localized burning was the most frequently reported adverse event, but its incidence decreased with continued use.
In a 12 week randomized, multicenter trial involving 113 patients, participants were treated with either 0.025% capsaicin cream or a placebo applied four times daily. The findings indicated that capsaicin led to more significant pain relief over the 4 to 12 week period. Furthermore, 81% of patients in the capsaicin group reported improvement according to clinicians' global evaluations, whereas 54% of patients in the placebo group reported improvement (53).
Oral analgesics
Acetaminophen
Acetaminophen is frequently used as a first-line analgesic for various painful conditions. However, a meta-analysis of 10 trials involving 3,541 patients revealed high-quality evidence indicating that acetaminophen offers only small, non-clinically meaningful benefits for short-term pain relief (54). This conclusion was further supported by a network meta-analysis comparing different analgesics for OA pain, which revealed that acetaminophen was no better than placebo, regardless of the dose (showing a 4 mm difference on a 0–100 mm visual analog scale [VAS]) (55). The risk of harm from acetaminophen typically increases with higher doses but can also occur at therapeutic doses, including risks of gastrointestinal bleeding, liver toxicity, kidney failure, and cardiovascular disease (56, 57).
The American College of Rheumatology/Arthritis Foundation (ACR/AF) issued a conditional recommendation for using acetaminophen due to its small effect size when used as monotherapy. It may be suitable for short-term or periodic use in patients who cannot take other analgesics (47). The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO) 2019 guidelines also provide a conditional recommendation for acetaminophen, suggesting its use only for short-term rescue analgesia in combination with long-term chondroitin sulfate or glucosamine (48). The American Academy of Orthopaedic Surgeons (AAOS) did not make a recommendation for or against acetaminophen use (46). Despite its widespread use, acetaminophen should be prescribed with caution because of its known side effects. In some patients, higher doses or prolonged use can lead to hepatotoxicity (58).
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly employed for pain management in osteoarthritis because they inhibit the cyclooxygenase (COX) enzyme, resulting in reduced production of prostaglandins, which play a critical role in mediating inflammation and pain (59–61). This category includes both conventional NSAIDs, such as ibuprofen and diclofenac, and selective COX-2 inhibitors, such as celecoxib, known for a lower incidence of gastrointestinal side effects (62–64). International guidelines, including those from the Osteoarthritis Research Society International (OARSI) and the American College of Rheumatology (ACR), advocate for the use of NSAIDs as first-line treatment for pain relief in osteoarthritis (47, 65). Nonetheless, long-term use necessitates careful monitoring due to potential risks, notably gastrointestinal, cardiovascular, and renal adverse effects (66–69).
Opioids
Due to their relatively high incidence of side effects, including drowsiness, dizziness, and nausea, as well as the potential for harm with long-term use, opioids are typically prescribed for osteoarthritis (OA) only when other analgesics have proven ineffective or are contraindicated (70). They are also considered for patients who are not candidates for joint replacement. Studies on knee OA have shown that opioids reduce pain to a similar degree as NSAIDs. A meta-analysis indicated a modest effect size (standardized mean difference [SMD] −0.28, 95% CI −0.35 to 0.20) for non-tramadol opioids, translating to a 0.7 cm difference on a 0–10 cm visual analogue scale (VAS) compared with placebo (71). Improvements in knee function were also limited, and the daily morphine equivalent dose did not impact functional benefits. Patients on opioids were more likely to discontinue treatment because of adverse events and experienced more side effects (6.5% vs. 1.7% and 22% vs. 15%, respectively; 71). Moreover, a randomized trial with 240 patients suffering from chronic back pain or hip or knee OA pain reported no difference in pain-related function after 12 months of treatment with non-opioid or opioid medications (72).
Moreover, less-potent opioids do not seem to offer significant advantages over non-opioid medications. A network meta-analysis did not reveal differences in efficacy between potent opioids (such as hydromorphone and oxycodone), a less-potent opioid (tramadol), and NSAIDs in trials lasting at least 8 weeks (73). A meta-analysis of six trials involving 3,611 patients with knee or hip OA revealed that tramadol provided modest pain relief compared with placebo, with only the high dose (300 mg/day) showing improvements in the functional subscale of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) compared with the placebo [SMD −0.24, 95% CI −0.47 to 0.03; (74)].
In addition to the known risks associated with opioid use, tramadol may be linked to increased mortality in OA patients. A propensity score-matched study of 88,902 OA patients revealed that patients prescribed tramadol had a higher mortality rate over a 1 year follow-up than did those taking commonly prescribed NSAIDs such as naproxen (hazard ratio 1.71 [95% CI 1.41–2.07]) (75).
A systematic review and meta-analysis examining opioid use for OA pain revealed poor tolerability and minimal clinical benefit of opioids in controlled studies lasting between 4 and 24 weeks (76). Similarly, a recent meta-analysis by Osani et al. revealed that opioids provided only minor improvements in pain and function compared with placebo over 2–12 weeks of treatment, with no significant improvement in patients' quality of life. A meta-analysis revealed that more potent opioids, such as morphine and oxycodone, offered less favorable clinical outcomes than weaker or intermediate opioids, such as codeine and tramadol, and were associated with a greater risk of adverse effects (77).
Intra-articular injections
Corticosteroid injections
Injected corticosteroids target specific areas, such as inflammation or pain from tendinitis or osteoarthritic joints. A Cochrane review on intra-articular corticosteroid injections revealed that these treatments could provide moderate pain relief and slight improvements in physical function. However, the side-effect profile of intra-articular corticosteroids was comparable to that of a placebo. The evidence quality was deemed very low because of significant inconsistencies among the study results and the reliance on numerous small, low-quality studies (78).
Despite their common use in clinical practice and short-term effectiveness for joint pain relief, recent studies indicate that intra-articular glucocorticoid injections are less effective than physical therapy in managing symptoms 1 year after administration (79).
Hyaluronans
Hyaluronic acid (HA) is a glycosaminoglycan with various therapeutic effects when injected intra-articularly, including anti-inflammatory, mechanical, and analgesic benefits, as well as a positive impact on proteoglycan and glycosaminoglycan synthesis (80). A systematic review by Altman et al. revealed that repeated HA injections could maintain or improve knee pain without increasing safety risks, highlighting the advantage of the safety of repeated HA injections (81). Recent improvements in HA products have led to the development of high-molecular-weight hyaluronic acid (HMWHA), which is thought to be more effective than low-molecular-weight hyaluronic acid [LMWHA; (80)]. This finding was supported by a systematic review showing that HMWHA had a more significant impact than non-selective NSAIDs and selective COX-2 inhibitors for treating knee osteoarthritis [OA; (82)]. Additionally, a systematic review and meta-analysis by Miller et al. revealed that intra-articular HA injections resulted in statistically significant, although not clinically important, improvements in pain and knee function, with fewer side effects than orally administered NSAIDs did (83).
Platelet-rich plasma
Studies generally agree on the short- and medium-term analgesic effects of platelet-rich plasma (PRP) in patients with knee osteoarthritis (OA). However, drawing definitive conclusions about its clinical efficacy is challenging owing to variations in PRP preparation and application methods (84, 85). A meta-analysis of 40 trials involving 3,035 knee OA patients revealed no significant improvement in pain or function with PRP compared with hyaluronic acid, intra-articular steroids, or saline (86). Additionally, a randomized trial of 288 patients included in the meta-analysis revealed that intra-articular PRP injections did not provide benefits in terms of pain relief or structural changes compared with a saline placebo (87).
Mesenchymal stem cells (MSCs)
Autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) and adipose-derived MSCs (AD-MSCs), also known as the adipose-derived stromal vascular fraction (AD-SVF), are commonly used for treating knee osteoarthritis (OA). These cells can be either cultured before application or used directly after isolation. Other cell sources, such as synovial MSCs or allogeneic placental tissue, still require more research before they become routine in clinical practice (88).
During the progression of OA, MSCs applied directly into the joint tend to accumulate in both the joint and nearby bone marrow lesions, suggesting that they play a role in the response to joint injury. However, the precise mechanism behind their effectiveness in OA is not fully understood (89). Despite this, MSCs are increasingly employed in clinical settings, with reports indicating benefits in symptom relief and joint function (90–92).
One meta-analysis that included five randomized controlled trials (RCTs), four involving BM-MSCs and one involving AD-SVF, reported a significant reduction in pain intensity, as measured by the visual analog scale (VAS) and the Lysholm scale. However, no difference was noted in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores. The functional outcomes significantly improved, with a standard mean difference of 0.53%, although no notable difference in cartilage repair on MRI was observed (93).
Another meta-analysis reviewed RCTs of culture-expanded MSCs for OA treatment, including six studies (four with BM-MSCs, one with AD-MSCs, and one with placenta-derived MSCs) and 203 patients. This analysis revealed a statistically significant reduction in pain symptoms measured by both the VAS and WOMAC. Still, it revealed no significant difference in cartilage repair based on MRI or the whole-organ magnetic resonance score [WORMS; (94)].
Further analysis by Ma et al., which included 10 RCTs (four with BM-MSCs, three with AD-MSCs, one with adipose-derived mesenchymal progenitor cells [AD-MPCs], one with umbilical cord MSCs, and one with placenta-derived MSCs), revealed a significant reduction in pain, as measured by the VAS and WOMAC, along with improved stiffness, functionality, and total WOMAC scores. This study also reported increased cartilage volume among MSC-treated patients, although no significant difference was found in WORMS scores (95).
A comprehensive meta-analysis of 19 studies (15 RCTs, two retrospective studies, and two cohort studies), including nine with AD-MSCs, five with BM-MSCs, and others with peripheral blood stem cells or fetal MSCs, revealed significant pain relief at 12 months and improvements in the KOOS and WOMAC scores at 6 months. No side effects were reported from intra-articular MSC therapy (96).
In contrast, a systematic review and meta-analysis by Maheshwer et al. involving 25 studies reported no significant improvement in pain but reported functional and cartilage volume improvements, with standardized mean differences of 0.66 and 0.84, respectively (97).
Botulinum toxin
Botulinum toxin (BTX), a complex multi-molecular substance synthesized by various strains of the anaerobic bacterium Clostridium botulinum, has shown potential therapeutic effects in managing OA (98, 99). Administering Botulinum neurotoxin type A directly into the joint may suppress the release of inflammatory mediators and neuropeptides from nociceptors, leading to reduced pain and neurogenic inflammation associated with OA (100). Additionally, BTX may exhibit anti-nociceptive properties by down-regulating voltage-gated sodium channels, as demonstrated in a rat model of trigeminal neuralgia, or by diminishing the peripheral release of neurotransmitters such as substance P and CGRP, along with the pro-inflammatory cytokine IL-1β (101–103). Furthermore, BTX inhibits the fusion of intracellular vesicles with nerve membranes, disrupting the release of neurogenic inflammatory mediators (104, 105). Clinical studies have noted that a single intra-articular injection of BTX can alleviate symptoms in some patients with chronic, refractory pain due to knee OA, while others show no significant benefit, hinting at the possibility of distinct patient subgroups (106). This evidence supports the off-label use of botulinum toxin as a novel therapeutic strategy for KOA management in orthopedic practice (107).
Pleiotropic effects of medications in osteoarthritis therapy
Metformin
Metformin, a commonly prescribed medication for the management of type 2 diabetes, has attracted increasing interest in recent years for its potential uses beyond glycemic control. Initially designed to enhance insulin sensitivity and regulate hyperglycemia, metformin exhibits a range of pleiotropic effects that may be particularly advantageous in addressing various inflammatory and metabolic disorders, including OA (108–110). Research indicates that the aanti-inflammatory properties of metformin may significantly contribute to the reduction of joint degradation in OA patients (111, 112). By influencing inflammatory pathways and cellular stress responses, metformin may aid in preserving cartilage and soft tissues within the joints, which are often vulnerable to damage caused by inflammation (113, 114). Such mechanisms could lead to improvements in physical function and reductions in pain levels among OA patients, positioning metformin as a promising adjunctive therapy for those suffering from joint-related conditions (115, 116).
Furthermore, metformin's pleiotropic effects extend to its potential application in managing COVID-19, where it may help alleviate the severe inflammatory responses associated with the virus, particularly in high-risk groups such as individuals with obesity and diabetes (117–119). By modulating immune responses and decreasing the secretion of pro-inflammatory cytokines, metformin could reduce the likelihood of complications related to COVID-19, thereby underscoring its significance as a versatile therapeutic agent (120–122).
Metformin represents a promising adjunctive therapy for osteoarthritis, owing to its anti-inflammatory effects and ability to maintain joint integrity (123, 124). Its pleiotropic effects not only enhance physical function and alleviate pain in OA patients but also suggest possible applications in the treatment of other conditions, including COVID-19 (125–128). This highlights the broader therapeutic implications of this extensively utilized medication (129, 130). The dual benefits of metformin in managing osteoarthritis, along with its potential role in addressing COVID-19, emphasize its relevance as a multifaceted treatment option for patients with comorbid conditions, ultimately contributing to enhanced overall health outcomes (131–133).
Statins
Statins, a class of medications primarily prescribed for lowering cholesterol levels and managing cardiovascular health, have garnered attention in recent years for their potential benefits beyond lipid regulation (134, 135). Research indicates that statins possess notable anti-inflammatory properties that may play a crucial role in the management of OA (136, 137). These medications have been found to reduce systemic inflammation, which is a significant contributor to the pathophysiology of OA (138, 139).
Statins may help slow the progression of OA by mitigating these inflammatory processes (140, 141). By inhibiting the production of pro-inflammatory cytokines and promoting the expression of anti-inflammatory mediators, statins can help create a more favorable environment within the joint, potentially preserving cartilage and soft tissue integrity (142, 143).
In addition to their direct anti-inflammatory effects, statins may enhance the synthesis of cartilage components such as proteoglycans and collagen (144, 145). This is particularly important because the degradation of these components is a hallmark of OA progression (146–148). By supporting cartilage maintenance and repair, statins could improve joint function and reduce symptoms for individuals with osteoarthritis (149, 150).
Moreover, the pleiotropic effects of statins extend beyond inflammation and cartilage preservation (151, 152). Evidence suggests that statins may protect bone health, further supporting joint integrity in OA patients (141, 153). By influencing bone remodeling and reducing the risk of osteoporotic changes, statins may help mitigate one of the risk factors associated with OA progression (154–156).
Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), primarily known for managing hypertension and heart failure, have shown promising potential in addressing OA due to their ability to modulate the renin-angiotensin-aldosterone system [RAAS; (157–160)]. By inhibiting the action of angiotensin II, these medications can reduce inflammation and oxidative stress, significantly contributing to joint degeneration in OA (161, 162). Research indicates that ACE inhibitors and ARBs may decrease levels of pro-inflammatory cytokines and oxidative stress markers in joint tissues, thereby alleviating inflammation and potentially slowing the progression of the disease (163, 164).
Furthermore, the protective effects of ACE inhibitors and ARBs may extend to the preservation of cartilage and synovial fluid, which are vital for joint integrity and function. By mitigating harmful inflammatory mediators, these medications may help maintain cartilage structure and improve the lubrication of joints, leading to enhanced mobility and reduced pain for patients with OA (165–167). This highlights the importance of a holistic treatment approach, as patients with OA often have comorbidities such as hypertension and obesity (168, 169). By integrating ACE inhibitors and ARBs into the management strategy for OA, healthcare providers can address joint health and overall cardiovascular risk, ultimately improving patients' quality of life (170).
Nutritional supplements
Curcumin (turmeric)
Interest in curcumin is due primarily to its potential anti-inflammatory and analgesic effects, although evidence remains limited (171). Randomized trials and meta-analyses have investigated its efficacy (172, 173). For example, a study involving 70 adults with knee osteoarthritis (OA) and ultrasound-confirmed effusion synovitis revealed that 1,000 mg of Curcuma longa daily provided more significant pain relief over 12 weeks than a placebo. However, the clinical significance of these findings is questionable, as the observed improvements were more than the minimal clinically significant difference. Additionally, measures of effusion-synovitis volume on MRI were similar between the curcumin and placebo groups, with comparable adverse events reported. More extensive trials are needed to establish the clinical relevance of curcumin in OA treatment. Curcumin is known for its poor gastrointestinal absorption, so supplements designed to increase its bioavailability, such as those combined with piperine or BioPerine, are typically preferred. Reports of liver injury associated with high-dose curcumin supplements are rare (174).
Boswellia serrata
Boswellia serrata, also known as Indian frankincense, has been used for centuries because of its anti-inflammatory and antimicrobial properties (175). A meta-analysis of seven randomized trials comparing Boswellia serrata extract with a placebo for OA suggested potential benefits in reducing pain and stiffness and improving function. However, the quality of the trials was low, with several studies having an unclear risk of bias. While Boswellia treatment was generally well tolerated, three included studies did not report adverse events (176).
Glucosamine and chondroitin
The effectiveness of glucosamine and chondroitin in treating knee OA has been inconsistent (177). Larger, well-conducted reviews revealed that glucosamine hydrochloride had negligible effects on knee pain. In contrast, higher doses or higher-grade formulations of glucosamine sulfate (1,500 mg/day) and chondroitin (800 mg/day) had some statistically significant, though modest, benefits compared with placebo (178–181). For example, an industry-sponsored trial with 604 patients revealed that pharmaceutical-grade chondroitin sulfate was statistically superior to placebo and comparable to celecoxib in reducing pain and improving function. However, the clinical significance of these results was uncertain, as the degree of change in primary outcomes was minimal and similar across the chondroitin, celecoxib, and placebo groups. Other meta-analyses have indicated that glucosamine sulfate and chondroitin may slightly delay OA progression with long-term use (181–183). The placebo effect has been notable in studies involving these supplements, as exemplified by the Glucosamine/Chondroitin Intervention Trial (GAIT), where approximately 60% of participants experienced at least a 20% reduction in pain regardless of the treatment they received (184). In another trial, chondroitin sulfate plus glucosamine did not perform better than placebo in reducing global pain at 6 months, and the small sample size and dosing issues were limitations (185). Subgroup analyses revealed no difference in efficacy based on baseline pain severity or other factors (186). Similarly, vitamin D supplementation showed no benefit over placebo for pain relief or changes in cartilage volume in a large study (187, 188).
Fish oil
A study comparing low-dose (0.45 g) to high-dose (4.5 g) fish oil (omega-3 fatty acids) revealed more significant improvements in pain and functional improvements with the lower dose over 2 years. Both doses had common gastrointestinal adverse events, such as upset and reflux. Fish oil has shown positive results in rheumatoid arthritis, likely due to its anti-inflammatory properties, but its effectiveness in treating OA remains unclear (187).
Krill oil
Krill oil, known for its relatively high bioavailability of omega-3 fatty acids, has been tested for OA treatment. Two randomized trials with mild knee OA showed modest improvements in pain and stiffness with 2–4 g/day krill oil. However, a subsequent trial with moderate to severe knee OA revealed no significant benefits in pain relief or synovial inflammation compared with placebo, suggesting that krill oil may not be effective for more severe cases (189, 190).
Phytoflavonoids
Phytoflavonoids, a group of natural compounds with anti-inflammatory properties, have shown potential in improving knee OA symptoms (191–193). However, specific phytoflavonoids, such as flavocoxid, are associated with serious adverse events, such as liver injury and hypersensitivity pneumonitis, making their use not recommended.
Transcutaneous electrical nerve stimulation, acupuncture, local heat therapy, and cold therapy
Transcutaneous electrical nerve stimulation
Transcutaneous electrical nerve stimulation (TENS) operates based on the gate-control theory, which posits that it modulates nociceptive signals to the brain through presynaptic inhibition in the dorsal horn of the spinal cord. Despite this theoretical basis, clinical trials have yielded mixed results. One study with 203 patients reported no additional benefits in pain relief or function from TENS, interferential currents, or shortwave diathermy compared with sham treatments combined with education and exercise programs (194). Another study involving 220 patients reported no significant difference between TENS and placebo TENS in WOMAC pain scores after 3 weeks (195). Moreover, evidence suggests a substantial placebo effect is associated with TENS (196).
Acupuncture
A meta-analysis of randomized trials assessing acupuncture for knee osteoarthritis (OA) revealed that while acupuncture might offer some measurable benefits over sham acupuncture, these differences were not clinically significant (197). Similarly, a trial comparing six sessions of acupuncture, sham acupuncture, and no additional therapy in 352 adults reported no significant differences in pain scores among the three groups after 6 months (198). However, a larger multicenter trial involving 1,007 patients with chronic knee OA reported that after 10 sessions of acupuncture or sham acupuncture, success rates (defined as a 36% improvement in a standardized osteoarthritis index) were similar for both treatments and higher than those for conservative therapy alone [53% and 51% vs. 29%, respectively; (199)].
Heat therapy
Local heat applications, such as heat packs or hot water bottles, can be a beneficial short-term strategy for pain relief in knee OA patients (200–202). A small cohort study demonstrated that combining local heat with routine management led to more significant improvements in pain and disability than routine management alone (202).
Biological agents
Biological agents have shown significant effects in treating rheumatic disorders such as rheumatoid arthritis [RA; (203, 204)]. This success has spurred randomized controlled trials (RCTs) investigating biologic agents in osteoarthritis [OA; (205)]. These biotherapeutic strategies for OA aim to modulate or inhibit the effects of major cytokines, similar to the approach for RA treatment (206). The three main types of cytokine blockers used in OA target nerve growth factor (NGF), interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α), which are involved in OA pain pathways (207, 208). TNF-α, IL-1, and NGF can modulate pain through nociceptor sensitization, with NGF expression induced by the upregulation of IL-1 and TNF-α in OA (209, 210). Understanding the cytokine network in OA pathogenesis has strengthened the rationale for exploring whether this biotherapeutic approach can improve symptoms (Table 2).
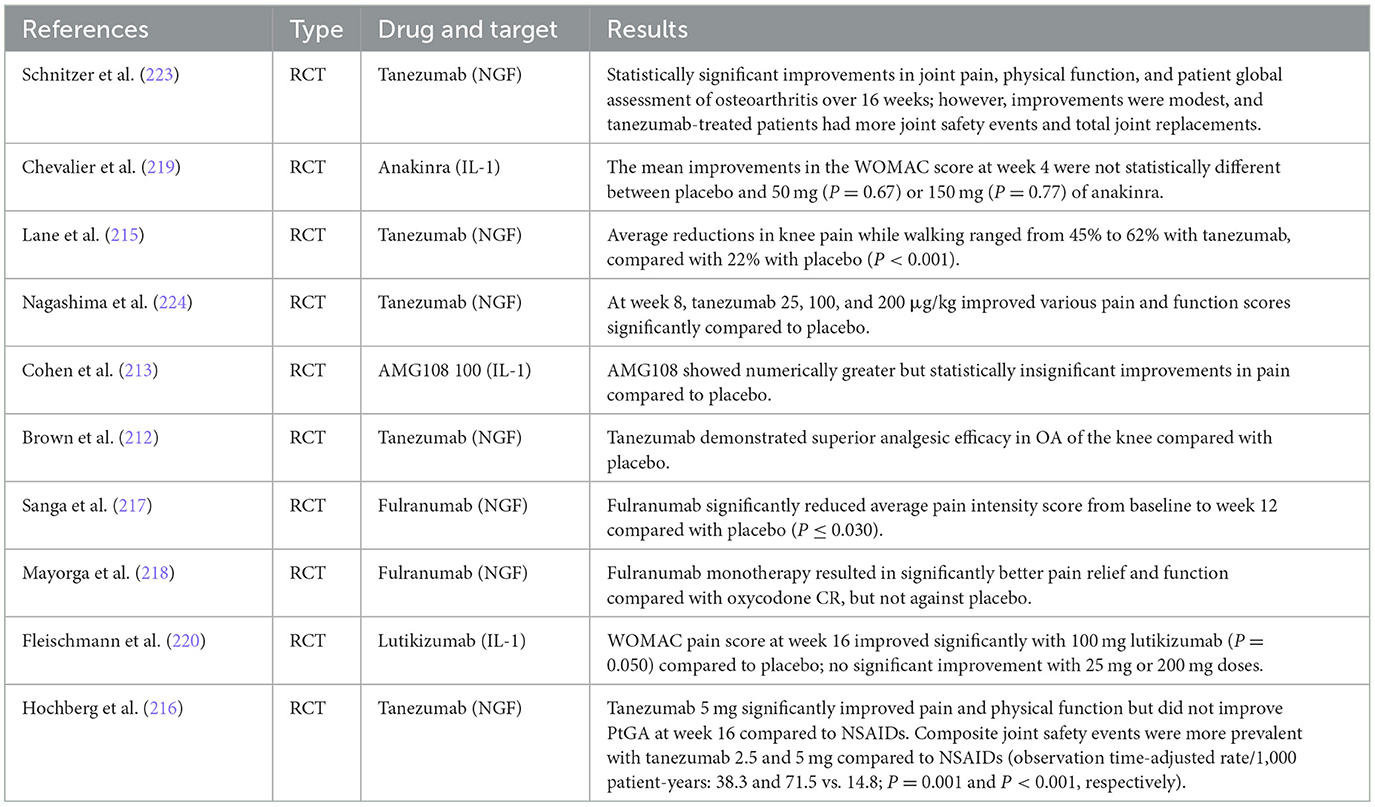
Table 2. Comparative analysis of biologic agents in osteoarthritis—Key findings from recent studies.
However, controversy remains regarding the efficacy and safety of biologic agents in treating OA, with the literature presenting mixed outcomes of both success and failure (211, 212). Several studies have indicated that NGF inhibitors have effects on pain relief and functional improvement relative to placebo in OA, albeit with inconsistent safety performance (213, 214). For instance, clinical trials have demonstrated that tanezumab, an NGF inhibitor, resulted in significant reductions in pain and improvements in physical function compared to placebo (215). However, it was associated with a higher incidence of joint safety events and total joint replacements (216). Similarly, fulranumab, another NGF inhibitor, showed significant pain relief compared to placebo, but with variable results (217, 218).
In contrast, IL-1 inhibitors like anakinra and lutikizumab have shown limited success. Anakinra did not produce statistically significant improvements in pain scores compared to placebo (219), while lutikizumab had mixed results with significant improvements only at certain doses (220). TNF-α inhibitors, such as those investigated in some studies, were found to be ineffective for OA treatment in meta-analyses (221, 222).
Knee OA pain arises from inflammatory and mechanical mechanisms, necessitating tailored treatment strategies. Inflammatory pain benefits from anti-inflammatory agents like NSAIDs and corticosteroids, while mechanical pain is better addressed through interventions improving joint mechanics, such as hyaluronic acid injections and physical therapy. Many patients experience mixed pain, requiring a comprehensive approach that combines pharmacological treatments with supportive therapies such as exercise and weight management.
Conclusions
Knee osteoarthritis (OA) is a common condition that significantly impacts quality of life and presents substantial health and economic challenges. Effective management requires a complex approach, which may include various treatment modalities.
Topical NSAIDs and capsaicin are effective initial therapies due to their safety and efficacy profiles. Oral analgesics, including acetaminophen and opioids, and intra-articular injections, such as corticosteroids and hyaluronic acid, provide varying degrees of relief but are limited by potential side effects.
Emerging evidence supports the potential benefits of mesenchymal stem cells for improving symptoms and joint function, though further research is necessary to confirm their long-term safety and efficacy. Nutritional supplements like curcumin and glucosamine-chondroitin offer modest benefits as adjuncts but lack robust evidence for primary therapy.
Non-pharmacological treatments, including TENS, acupuncture, and heat therapy, yield mixed results and should be tailored to individual patient responses. Biological agents targeting cytokines, such as TNF-α and IL-1, hold promise but require more extensive clinical validation.
Knee OA treatment should be personalized, balancing patient-specific factors and treatment preferences. An integrated approach combining pharmacological, non-pharmacological, and emerging biologic therapies offers the most effective pain relief and functional improvement strategy.
Author contributions
VS: Writing – review & editing. PP: Writing – original draft. IK: Writing – review & editing. IH: Writing – review & editing. OK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartil. (2015) 23:1233–41. doi: 10.1016/j.joca.2015.03.036
2. Meschini C, Cauteruccio M, Oliva MS, Sircana G, Vitiello R, Rovere G, et al. Hip and knee replacement in patients with ochronosis: clinical experience and literature review. Orthop Rev. (2020) 12(Suppl. 1):8687. doi: 10.4081/or.2020.8687
3. Romeo M, Rovere G, Stramazzo L, Liuzza F, Meccariello L, Maccauro G, et al. Single use instruments for total knee arthroplasty. Med Glas. (2021) 18:247–51. doi: 10.17392/1321-21
4. Babinets L, Halabitska I. Characteristics of joint pain in patients with primary osteoarthritis and comorbid conditions with exocrine pancreatic. Lekarsky Obzor. (2021) 70:62–4.
5. Thomas E, Peat G, Mallen C, Wood L, Lacey R, Duncan R, et al. Predicting the course of functional limitation among older adults with knee pain: do local signs, symptoms and radiographs add anything to general indicators? Ann Rheum Dis. (2008) 67:1390–8. doi: 10.1136/ard.2007.080945
6. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
7. Smakaj A, De Mauro D, Rovere G, Pietramala S, Maccauro G, Parolini O, et al. Clinical application of adipose derived stem cells for the treatment of aseptic non-unions: current stage and future perspectives-systematic review. Int J Mol Sci. (2022) 23:3057. doi: 10.3390/ijms23063057
8. Pavan D, Morello F, Monachino F, Rovere G, Camarda L, Pitarresi G. Similar biomechanical properties of four tripled tendon graft models for ACL reconstruction. Arch Orthop Trauma Surg. (2022) 142:1155–65. doi: 10.1007/s00402-021-04030-8
9. Gorasso V, Van der Heyden J, De Pauw R, Pelgrims I, De Clercq EM, De Ridder K, et al. The health and economic burden of musculoskeletal disorders in Belgium from 2013 to 2018. Popul Health Metrics. (2023) 21:4. doi: 10.1186/s12963-023-00303-z
10. Chen A, Gupte C, Akhtar K, Smith P, Cobb J. The global economic cost of osteoarthritis: how the UK compares. Arthritis. (2012) 2012:698709. doi: 10.1155/2012/698709
11. Halabitska I, Babinets L, Kotsaba Y. Pathogenetic features of comorbidity of primary osteoarthritis and diseases with exocrine pancreatic insufficiency. Georgian Medical News (2021). p. 57–62.
12. Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. (2015) 16:6093–112. doi: 10.3390/ijms16036093
13. Yucesoy B, Charles LE, Baker B, Burchfiel CM. Occupational and genetic risk factors for osteoarthritis: a review. Work. (2015) 50:261–73. doi: 10.3233/WOR-131739
14. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study (2015). Lancet. (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
15. Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. (2022) 74:1172–83. doi: 10.1002/art.42089
16. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. (2016) 2:16072. doi: 10.1038/nrdp.2016.72
17. De Marco D, Messina F, Meschini C, Oliva MS, Rovere G, Maccagnano G, et al. Periprosthetic knee fractures in an elderly population: open reduction and internal fixation vs distal femur megaprostheses. Orthop Rev. (2022) 14:33772. doi: 10.52965/001c.33772
18. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2016) 12:412–20. doi: 10.1038/nrrheum.2016.65
19. Halabitska I, Babinets L. Cellular and humoral disorders of the immune system at osteoarthritis with comorbidity of exocrine pancreatic insufficiency. Fam Med Eur Pract. (2022) 2022:29–34. doi: 10.30841/2786-720X.4.2022.274646
20. Halabitska I, Babinets L. Interdependence between body weight, depth of inflammation and functional capacity of the pancreas in patients with primary osteoarthritis and type 2 diabetes. Fam Med Eur Pract. (2022) 2022:48–53. doi: 10.30841/2786-720X.3.2022.273914
21. Topol I, Kamyshny A. Study of expression of TLR2, TLR4 and transckription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian Medical News (2013). p. 115–22.
22. Corriero A, Giglio M, Soloperto R, Inchingolo F, Varrassi G, Puntillo F. Microbial symphony: exploring the role of the gut in osteoarthritis-related pain. A narrative review. Pain Ther. (2024) 13:409–33. doi: 10.1007/s40122-024-00602-9
23. Babinets L, Halabitska I, Levchyk O. Comparative analysis of clinical and pathogenetic parameters in osteoarthritis patients depending on etiology of the comorbid pathology. Pharmacology. (2021) 3:1030–7.
24. Rahman SO, Bariguian F, Mobasheri A. The potential role of probiotics in the management of osteoarthritis pain: current status and future prospects. Curr Rheumatol Rep. (2023) 25:307–26. doi: 10.1007/s11926-023-01108-7
25. Bilyi AK, Antypenko LM, Ivchuk VV, Kamyshnyi OM, Polishchuk NM, Kovalenko SI. 2-Heteroaryl-[1,2,4]triazolo[1,5-c]quinazoline-5(6 H)-thiones and their S-substituted derivatives: synthesis, spectroscopic data, and biological activity. ChemPlusChem. (2015) 80:980–9. doi: 10.1002/cplu.201500051
26. Nosulenko IS, Voskoboynik OY, Berest GG, Safronyuk SL, Kovalenko SI, Kamyshnyi OM, et al. Synthesis and antimicrobial activity of 6-thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]-quinazolin-2-one derivatives. Sci Pharm. (2014) 82:483–500. doi: 10.3797/scipharm.1402-10
27. Lu K, Ma F, Yi D, Yu H, Tong L, Chen D. Molecular signaling in temporomandibular joint osteoarthritis. J Orthop Translat. (2022) 32:21–7. doi: 10.1016/j.jot.2021.07.001
28. Yao X, Sun K, Yu S, Luo J, Guo J, Lin J, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. (2021) 27:33–43. doi: 10.1016/j.jot.2020.09.006
29. Chen D, Kim DJ, Shen J, Zou Z, O'Keefe RJ. Runx2 plays a central role in osteoarthritis development. J Orthop Translat. (2020) 23:132–9. doi: 10.1016/j.jot.2019.11.008
30. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartil. (2013) 21:1145–53. doi: 10.1016/j.joca.2013.03.018
31. Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheumat. (2008) 59:1207–13. doi: 10.1002/art.24021
32. Primorac D, Molnar V, Matišić V, Hudetz D, Jeleč Ž, Rod E, et al. Comprehensive review of knee osteoarthritis pharmacological treatment and the latest professional societies' guidelines. Pharmaceuticals. (2021) 14:205. doi: 10.3390/ph14030205
33. Jones DL, Philippi MT, Maak TG, Aoki SK. Progressive osteoarthritis during pregnancy several years following hip arthroscopy for femoroacetabular impingement. J Orthop. (2018) 15:475–9. doi: 10.1016/j.jor.2018.03.033
34. Lyubomirskaya ES, Kamyshnyi AM, Krut YY, Smiianov VA, Fedoniuk LY, Romanyuk LB, et al. SNPs and transcriptional activity of genes of innate and adaptive immunity at the maternal-fetal interface in woman with preterm labour, associated with preterm premature rupture of membranes. Wiadomosci Lekarskie. (2020) 73:25–30. doi: 10.36740/WLek202001104
35. Mohammed H, Al-sari U. Relationship between osteoarthritis and thyroid dysfunction, as well as with physical and demographic features. Bionatura. (2023) 8:1–15. doi: 10.21931/RB/2023.08.04.67
36. Bilous, II, Pavlovych LL, Kamyshnyi AM. Primary hypothyroidism and autoimmune thyroiditis alter the transcriptional activity of genes regulating neurogenesis in the blood of patients. Endocr Regul. (2021) 55:5–15. doi: 10.2478/enr-2021-0002
37. Kamyshna, II, Pavlovych LB, Maslyanko VA, Kamyshnyi AM. Analysis of the transcriptional activity of genes of neuropeptides and their receptors in the blood of patients with thyroid pathology. J Med Life. (2021) 14:243–9. doi: 10.25122/jml-2020-0183
38. Oliveira S, Andrade R, Valente C, Espregueira-Mendes J, Silva F, Hinckel BB, et al. Mechanical-based therapies may reduce pain and disability in some patients with knee osteoarthritis: a systematic review with meta-analysis. Knee. (2022) 37:28–46. doi: 10.1016/j.knee.2022.05.005
39. Farinelli L, Riccio M, Gigante A, De Francesco F. Pain management strategies in osteoarthritis. Biomedicines. (2024) 12:805. doi: 10.3390/biomedicines12040805
40. Radford E, Benejan K, Gavino C, McCain R. Does topical NSAID use in adults increase the risk of gastrointestinal bleeding compared to oral NSAID use? Evid Based Pract. (2024). 27:42. doi: 10.1097/EBP.0000000000001956
41. Clarke J. Topical NSAIDs come out top for knee OA. Nat Rev Rheumatol. (2021) 17:508. doi: 10.1038/s41584-021-00676-1
42. Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. (2016) 4:Cd007400. doi: 10.1002/14651858.CD007400.pub3
43. Roth SH, Fuller P. Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J Pain Res. (2011) 4:159–67. doi: 10.2147/JPR.S20965
44. Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. (2010) 50:50–61. doi: 10.1177/0091270009336234
45. da Costa BR, Pereira TV, Saadat P, Rudnicki M, Iskander SM, Bodmer NS, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. (2021) 375:n2321. doi: 10.1136/bmj.n2321
46. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. (2013) 21:571–6. doi: 10.5435/00124635-201309020-00008
47. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. (2020) 72:149–62. doi: 10.1002/acr.24131
48. Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. (2019) 49:337–50. doi: 10.1016/j.semarthrit.2019.04.008
49. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. (2011) 107:490–502. doi: 10.1093/bja/aer260
50. Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blümle A, Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: Osteoarthritis. Phytother Res. (2009) 23:1497–515. doi: 10.1002/ptr.3007
51. De Silva V, El-Metwally A, Ernst E, Lewith G, Macfarlane GJ. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology. (2011) 50:911–20. doi: 10.1093/rheumatology/keq379
52. Laslett LL, Jones G. Capsaicin for osteoarthritis pain. Prog Drug Res. (2014) 68:277–91. doi: 10.1007/978-3-0348-0828-6_11
53. Altman RD, Aven A, Holmburg CE, Pfeifer LM, Sack M, Young GT. Capsaicin cream 0.025% as Monotherapy for Osteoarthritis: a double-blind study. Semin Arthritis Rheumat. (1994) 23(Suppl. 3):25–33. doi: 10.1016/S0049-0172(10)80023-X
54. Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. (2015) 350:h1225. doi: 10.1136/bmj.h1225
55. da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. RETRACTED: effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. (2016) 387:2093–105. doi: 10.1016/S0140-6736(16)30002-2
56. Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis. (2016) 75:552–9. doi: 10.1136/annrheumdis-2014-206914
57. Halabitska I, Babinets L. Different consequences of the treatment of osteoarthritis in gastrointestinal comorbidity with exocrine pancreatic insufficiency. Fam Med Prim Care Rev. (2021) 23:422–8. doi: 10.5114/fmpcr.2021.108207
58. Conaghan PG, Arden N, Avouac B, Migliore A, Rizzoli R. Safety of paracetamol in osteoarthritis: what does the literature say? Drugs Aging. (2019) 36(Suppl. 1):7–14. doi: 10.1007/s40266-019-00658-9
59. Sohail R, Mathew M, Patel KK, Reddy SA, Haider Z, Naria M, et al. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: a narrative review. Cureus. (2023) 15:e37080. doi: 10.7759/cureus.37080
60. Chuang PY, Yang TY, Tsai YH, Huang KC. Do NSAIDs affect bone healing rate, delay union, or cause non-union: an updated systematic review and meta-analysis. Front Endocrinol. (2024) 15:1428240. doi: 10.3389/fendo.2024.1428240
61. Halabitska IM, Babinets LS, Vysotskyi VI. Possibilities of metabolic and functional disorders correction in osteoarthritis with complex comorbidity. Wiadomosci Lekarskie. (2022) 75:645–8. doi: 10.36740/WLek202203114
62. Zarghi A, Arfaei S. Selective COX-2 inhibitors: a review of their structure-activity relationships. Iranian J Pharm Res. (2011) 10:655–83.
63. Babinets L, Halabitska I. Chronic inflammatory process and bone tissue changes in patients with osteoarthritis and exocrine pancreatic insufficiency. Lekarsky Obzor. (2020) 69:7–10.
64. Tylińska B, Janicka-Kłos A, Gebarowski T, Nowotarska P, Plińska S, Wiatrak B. Pyrimidine derivatives as selective COX-2 inhibitors with anti-inflammatory and antioxidant properties. Int J Mol Sci. (2024) 25:11011. doi: 10.3390/ijms252011011
65. Sabha M, Hochberg MC. Non-surgical management of hip and knee osteoarthritis; comparison of ACR/AF and OARSI 2019 and VA/DoD 2020 guidelines. Osteoarthritis Cartil Open. (2022) 4:100232. doi: 10.1016/j.ocarto.2021.100232
66. Halabitska I, Babinets L. The influence of comorbid gastroenterological pathology with exocrine pancreatic insufficiency on the course of primary osteoarthritis. Health Prob Civil. (2023) 17:130–6. doi: 10.5114/hpc.2023.127392
67. Roy PJ, Weltman M, Dember LM, Liebschutz J, Jhamb M. Pain management in patients with chronic kidney disease and end-stage kidney disease. Curr Opin Nephrol Hypertens. (2020) 29:671–80. doi: 10.1097/MNH.0000000000000646
68. Steinmeyer J, Bock F, Stöve J, Jerosch J, Flechtenmacher J. Pharmacological treatment of knee osteoarthritis: special considerations of the new German guideline. Orthop Rev. (2018) 10:7782. doi: 10.4081/or.2018.7782
69. Magni A, Agostoni P, Bonezzi C, Massazza G, Menè P, Savarino V, et al. Management of osteoarthritis: expert opinion on NSAIDs. Pain Ther. (2021) 10:783–808. doi: 10.1007/s40122-021-00260-1
70. Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartil. (2016) 24:409–18. doi: 10.1016/j.joca.2015.10.006
71. da Costa BR, Nüesch E, Kasteler R, Husni E, Welch V, Rutjes AW, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. (2014) 2014:Cd003115. doi: 10.1002/14651858.CD003115.pub4
72. Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. (2018) 319:872–82. doi: 10.1001/jama.2018.0899
73. Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartil. (2016) 24:962–72. doi: 10.1016/j.joca.2016.01.135
74. Zhang X, Li X, Xiong Y, Wang Y, Wei J, Zeng C, et al. Efficacy and safety of tramadol for knee or hip osteoarthritis: a systematic review and network meta-analysis of randomized controlled trials. Arthritis Care Res. (2023) 75:158–65. doi: 10.1002/acr.24750
75. Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. (2019) 321:969–82. doi: 10.1001/jama.2019.1347
76. Welsch P, Petzke F, Klose P, Häuser W. Opioids for chronic osteoarthritis pain: an updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks double-blind duration. Eur J Pain. (2020) 24:685–703. doi: 10.1002/ejp.1522
77. Osani MC, Lohmander LS, Bannuru RR. Is there any role for opioids in the management of knee and hip osteoarthritis? A systematic review and meta-analysis. Arthritis Care Res. (2021) 73:1413–24. doi: 10.1002/acr.24363
78. Jüni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. (2015) 2015:Cd005328. doi: 10.1002/14651858.CD005328.pub3
79. Deyle GD, Allen CS, Allison SC, Gill NW, Hando BR, Petersen EJ, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. (2020) 382:1420–9. doi: 10.1056/NEJMoa1905877
80. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. (2015) 16:321. doi: 10.1186/s12891-015-0775-z
81. Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. (2018) 48:168–75. doi: 10.1016/j.semarthrit.2018.01.009
82. Concoff A, Rosen J, Fu F, Bhandari M, Boyer K, Karlsson J, et al. A comparison of treatment effects for nonsurgical therapies and the minimum clinically important difference in knee osteoarthritis: a systematic review. JBJS Rev. (2019) 7:e5. doi: 10.2106/JBJS.RVW.18.00150
83. Miller LE, Fredericson M, Altman RD. Hyaluronic acid injections or oral nonsteroidal anti-inflammatory drugs for knee osteoarthritis: systematic review and meta-analysis of randomized trials. Orthop J Sports Med. (2020) 8:2325967119897909. doi: 10.1177/2325967119897909
84. Wu Q, Luo X, Xiong Y, Liu G, Wang J, Chen X, et al. Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: a meta-analysis with the consistent ratio of injection. J Orthop Surg. (2020) 28:2309499019887660. doi: 10.1177/2309499019887660
85. Gato-Calvo L, Magalhaes J, Ruiz-Romero C, Blanco FJ, Burguera EF. Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther Adv Chronic Dis. (2019) 10:2040622319825567. doi: 10.1177/2040622319825567
86. Costa LAV, Lenza M, Irrgang JJ, Fu FH, Ferretti M. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. (2023) 51:1074–86. doi: 10.1177/03635465211062243
87. Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. (2021) 326:2021–30. doi: 10.1001/jama.2021.19415
88. Ilas DC, Churchman SM, McGonagle D, Jones E. Targeting subchondral bone mesenchymal stem cell activities for intrinsic joint repair in osteoarthritis. Fut Sci OA. (2017) 3:Fso228. doi: 10.4155/fsoa-2017-0055
89. McGonagle D, Baboolal TG, Jones E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol. (2017) 13:719–30. doi: 10.1038/nrrheum.2017.182
90. Hudetz D, Borić I, Rod E, Jeleč Ž, Radić A, Vrdoljak T, et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes. (2017) 8:270. doi: 10.3390/genes8100270
91. Hudetz D, Borić I, Rod E, Jeleč Ž, Kunovac B, Polašek O, et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: a prospective study. Croatian Med J. (2019) 60:227–36. doi: 10.3325/cmj.2019.60.227
92. Borić I, Hudetz D, Rod E, Jeleč Ž, Vrdoljak T, Skelin A, et al. A 24-month follow-up study of the effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes. (2019) 10:1051. doi: 10.3390/genes10121051
93. Kim SH, Ha CW, Park YB, Nam E, Lee JE, Lee HJ. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. (2019) 139:971–80. doi: 10.1007/s00402-019-03140-8
94. Kim SH, Djaja YP, Park YB, Park JG, Ko YB, Ha CW. Intra-articular injection of culture-expanded mesenchymal stem cells without adjuvant surgery in knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. (2020) 48:2839–49. doi: 10.1177/0363546519892278
95. Ma W, Liu C, Wang S, Xu H, Sun H, Fan X. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Medicine. (2020) 99:e23343. doi: 10.1097/MD.0000000000023343
96. Song Y, Zhang J, Xu H, Lin Z, Chang H, Liu W, et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. (2020) 24:121–30. doi: 10.1016/j.jot.2020.03.015
97. Maheshwer B, Polce EM, Paul K, Williams BT, Wolfson TS, Yanke A, et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy. (2021) 37:362–78. doi: 10.1016/j.arthro.2020.05.037
98. Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. (2011) 57:555–65. doi: 10.1016/j.toxicon.2010.12.019
99. Sconza C, Leonardi G, Carfì C, Kon E, Respizzi S, Scaturro D, et al. Intra-articular injection of botulinum toxin for the treatment of knee osteoarthritis: a systematic review of randomized controlled trials. Int J Mol Sci. (2023) 24:1486. doi: 10.3390/ijms24021486
100. Mendes JG, Natour J, Nunes-Tamashiro JC, Toffolo SR, Rosenfeld A, Furtado RNV. Comparison between intra-articular Botulinum toxin type A, corticosteroid, and saline in knee osteoarthritis: a randomized controlled trial. Clin Rehabil. (2019) 33:1015–26. doi: 10.1177/0269215519827996
101. Nemanić D, Mustapić M, Matak I, Bach-Rojecky L. Botulinum toxin type a antinociceptive activity in trigeminal regions involves central transcytosis. Eur J Pharmacol. (2024) 963:176279. doi: 10.1016/j.ejphar.2023.176279
102. Shin MC, Wakita M, Xie DJ, Yamaga T, Iwata S, Torii Y, et al. Inhibition of membrane Na(+) channels by A type botulinum toxin at femtomolar concentrations in central and peripheral neurons. J Pharmacol Sci. (2012) 118:33–42. doi: 10.1254/jphs.11060FP
103. Singh JA, Mahowald ML, Noorbaloochi S. Intraarticular botulinum toxin A for refractory painful total knee arthroplasty: a randomized controlled trial. J Rheumatol. (2010) 37:2377–86. doi: 10.3899/jrheum.100336
104. Chien C-T, Lee H-M, Wu C-C, Li P-C. Inhibitory effect of botulinum toxin type A on the NANC system in rat respiratory models of neurogenic inflammation. Arch Biochem Biophys. (2012) 524:106–13. doi: 10.1016/j.abb.2012.05.016
105. McAlindon TE, Schmidt U, Bugarin D, Abrams S, Geib T, DeGryse RE, et al. Efficacy and safety of single-dose onabotulinumtoxinA in the treatment of symptoms of osteoarthritis of the knee: results of a placebo-controlled, double-blind study. Osteoarthritis Cartil. (2018) 26:1291–9. doi: 10.1016/j.joca.2018.05.001
106. Arendt-Nielsen L, Jiang GL, DeGryse R, Turkel CC. Intra-articular onabotulinumtoxin A in osteoarthritis knee pain: effect on human mechanistic pain biomarkers and clinical pain. Scand J Rheumatol. (2017) 46:303–16. doi: 10.1080/03009742.2016.1203988
107. Mahowald ML, Krug HE, Singh JA, Dykstra D. Intra-articular botulinum toxin type A: a new approach to treat arthritis joint pain. Toxicon. (2009) 54:658–67. doi: 10.1016/j.toxicon.2009.03.028
108. Dutta S, Shah RB, Singhal S, Dutta SB, Bansal S, Sinha S, et al. Metformin: a review of potential mechanism and therapeutic utility beyond diabetes. Drug Design Dev Ther. (2023) 17:1907–32. doi: 10.2147/DDDT.S409373
109. Petakh P, Oksenych V, Kamyshnyi A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomed Pharmacother. (2023) 163:114892. doi: 10.1016/j.biopha.2023.114892
110. Redkva OV, Babinets LS, Halabitska IM. Evaluation of parameters of actual typical pathogenetic syndromes in comorbidity of type 2 diabetes mellitus and chronic pancreatitis. Wiadomosci Lekarskie. (2021) 74:2557–9. doi: 10.36740/WLek202110204
111. Anis MW, Iqbal A, Younus MI, Aamir A, Khalid W. Metformin: pioneering a path forward in knee osteoarthritis care? Ann Med Surg. (2024) 86:4333–5. doi: 10.1097/MS9.0000000000002318
112. Pavlo P, Kamyshna I, Kamyshnyi A. Effects of metformin on the gut microbiota: a systematic review. Mol Metab. (2023) 77:101805. doi: 10.1016/j.molmet.2023.101805
113. Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Targeted Ther. (2023) 8:56. doi: 10.1038/s41392-023-01330-w
114. Song Y, Wu Z, Zhao P. The effects of metformin in the treatment of osteoarthritis: current perspectives. Front Pharmacol. (2022) 13:952560. doi: 10.3389/fphar.2022.952560
115. Halabitska I, Babinets L, Oksenych V, Kamyshnyi O. Diabetes and osteoarthritis: exploring the interactions and therapeutic implications of insulin, metformin, and GLP-1-based interventions. Biomedicines. (2024) 12:1630. doi: 10.3390/biomedicines12081630
116. Song P, Hwang JS, Park HC, Kim KK, Son HJ, Kim YJ, et al. Therapeutic applications of type 2 diabetes mellitus drug metformin in patients with osteoarthritis. Pharmaceuticals. (2021) 14:152. doi: 10.3390/ph14020152
117. Wiernsperger N, Al-Salameh A, Cariou B, Lalau JD. Protection by metformin against severe Covid-19: an in-depth mechanistic analysis. Diabetes Metab. (2022) 48:101359. doi: 10.1016/j.diabet.2022.101359
118. Petakh P, Griga V, Mohammed IB, Loshak K, Poliak I, Kamyshnyiy A. Effects of metformin, insulin on hematological parameters of covid-19 patients with type 2 diabetes. Med Arch. (2022) 76:329–32. doi: 10.5455/medarh.2022.76.329-332
119. Zemlyak OS, Babinets LS, Halabitska IM. The role of endotoxicosis and inflammation in deepening the pancreatic functional insufficiency in chronic pancreatitis in combination with type 2 diabetes. Polski Merkuriusz Lekarski. (2023) 51:207–15. doi: 10.36740/Merkur202303104
120. Plowman TJ, Christensen H, Aiges M, Fernandez E, Shah MH, Ramana KV. Anti-inflammatory potential of the anti-diabetic drug metformin in the prevention of inflammatory complications and infectious diseases including COVID-19: a narrative review. Int J Mol Sci. (2024) 25:5190. doi: 10.3390/ijms25105190
121. Petakh P, Kamyshna I, Oksenych V, Kainov D, Kamyshnyi A. Metformin therapy changes gut microbiota alpha-diversity in COVID-19 patients with type 2 diabetes: the role of SARS-CoV-2 variants and antibiotic treatment. Pharmaceuticals. (2023) 16:904. doi: 10.3390/ph16060904
122. Buchynskyi M, Oksenych V, Kamyshna I, Budarna O, Halabitska I, Petakh P, et al. Genomic insight into COVID-19 severity in MAFLD patients: a single-center prospective cohort study. Front Genet. (2024) 15:1460318. doi: 10.3389/fgene.2024.1460318
123. Aiad AAE, El-Haggar SM, El-Barbary AM, El-Afify DR. Metformin as adjuvant therapy in obese knee osteoarthritis patients. Inflammopharmacology. (2024) 32:2349–59. doi: 10.1007/s10787-024-01495-y
124. Kim JW, Choe JY, Park SH. Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. Korean J Internal Med. (2022) 37:13–26. doi: 10.3904/kjim.2021.363
125. Parsirad M, Oomen-Lochtefeld S, Suerig B, Wang C. Has the COVID 19 pandemic impacted the management of chronic musculoskeletal pain? Curr Rheumatol Rep. (2023) 25:128–43. doi: 10.1007/s11926-023-01103-y
126. Petakh P, Kobyliak N, Kamyshnyi A. Gut microbiota in patients with COVID-19 and type 2 diabetes: a culture-based method. Front Cell Infect Microbiol. (2023) 13:1142578. doi: 10.3389/fcimb.2023.1142578
127. Buchynskyi M, Oksenych V, Kamyshna I, Vorobets I, Halabitska I, Kamyshnyi O. Modulatory roles of AHR, FFAR2, FXR, and TGR5 gene expression in metabolic-associated fatty liver disease and COVID-19 outcomes. Viruses. (2024) 16:985. doi: 10.3390/v16060985
128. Buchynskyi M, Kamyshna I, Lyubomirskaya K, Moshynets O, Kobyliak N, Oksenych V, et al. Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: a meta-analysis. Front Immunol. (2023) 14:1069894. doi: 10.3389/fimmu.2023.1069894
129. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clinic Proc. (2011) 86:304–14. doi: 10.4065/mcp.2010.0575
130. Clarke N, Trigg A, Arbuckle R, Stochl J, Higgins V, Bentley S, et al. Psychometric evaluation of the Adelphi Adherence Questionnaire (ADAQ©) in adults with osteoarthritis. J Pat Report Outcomes. (2024) 8:118. doi: 10.1186/s41687-024-00789-7
131. Samuel SM, Varghese E, Büsselberg D. Therapeutic potential of metformin in COVID-19: reasoning for its protective role. Trends Microbiol. (2021) 29:894–907. doi: 10.1016/j.tim.2021.03.004
132. Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, et al. Metformin: is it a drug for all reasons and diseases? Metab Clin Exp. (2022) 133:155223. doi: 10.1016/j.metabol.2022.155223
133. Prattichizzo F, Giuliani A, Mensà E, Sabbatinelli J, De Nigris V, Rippo MR, et al. Pleiotropic effects of metformin: shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. (2018) 48:87–98. doi: 10.1016/j.arr.2018.10.003
134. Morofuji Y, Nakagawa S, Ujifuku K, Fujimoto T, Otsuka K, Niwa M, et al. Beyond lipid-lowering: effects of statins on cardiovascular and cerebrovascular diseases and cancer. Pharmaceuticals. (2022) 15:151. doi: 10.3390/ph15020151
135. Mihos CG, Pineda AM, Santana O. Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol Res. (2014) 88:12–9. doi: 10.1016/j.phrs.2014.02.009
136. Heidari B, Babaei M, Yosefghahri B. Prevention of osteoarthritis progression by statins, targeting metabolic and inflammatory aspects: a review. Mediterr J Rheumatol. (2021) 32:227–36. doi: 10.31138/mjr.32.3.227
137. Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T. The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev. (2017) 13:209–16. doi: 10.2174/1573403X13666170426104611
138. Terkawi MA, Ebata T, Yokota S, Takahashi D, Endo T, Matsumae G, et al. Low-grade inflammation in the pathogenesis of osteoarthritis: cellular and molecular mechanisms and strategies for future therapeutic intervention. Biomedicines. (2022) 10:1109. doi: 10.3390/biomedicines10051109
139. Roover A, Escribano Núñez A, Monteagudo S, Lories R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthritis Cartil. (2023) 31:1303–11. doi: 10.1016/j.joca.2023.06.005
140. Gill J, Sayre EC, Guermazi A, Nicolaou S, Cibere J. Association between statins and progression of osteoarthritis features on magnetic resonance imaging in a predominantly pre-radiographic cohort: the Vancouver Longitudinal Study of Early Knee Osteoarthritis (VALSEKO): a cohort study. BMC Musculoskelet Disord. (2022) 23:937. doi: 10.1186/s12891-022-05900-x
141. Zhang L, Sui L, Li J, Zhang R, Pan W, Lv T. Potential benefits of statin therapy in reducing osteoarthritis risk: a mendelian randomization study. Arthritis Care Res. (2024) 76:1260–8. doi: 10.1002/acr.25343
142. Chen W, Sun Z, Xiong X, Tan H, Hu J, Liu C, et al. Exploring the causal link among statin drugs and the osteoarthritis risk based on Mendelian randomization research. Front Genet. (2024) 15:1390387. doi: 10.3389/fgene.2024.1390387
143. Saberianpour S, Abolbashari S, Modaghegh M, Karimian M, Eid A, Sathyapalan T, et al. Therapeutic effects of statins on osteoarthritis: a review. J Cell Biochem. (2022) 123:1285–97. doi: 10.1002/jcb.30309
144. Riegger J, Maurer S, Pulasani S, Brenner RE. Simvastatin and fluvastatin attenuate trauma-induced cell death and catabolism in human cartilage. Front Bioeng Biotechnol. (2022) 10:965302. doi: 10.3389/fbioe.2022.965302
145. Ouyang Z, Dong L, Yao F, Wang K, Chen Y, Li S, et al. Cartilage-related collagens in osteoarthritis and rheumatoid arthritis: from pathogenesis to therapeutics. Int J Mol Sci. (2023) 24:9841. doi: 10.3390/ijms24129841
146. Poulet B. Models to define the stages of articular cartilage degradation in osteoarthritis development. Int J Exp Pathol. (2017) 98:120–6. doi: 10.1111/iep.12230
147. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumat. (2012) 64:1697–707. doi: 10.1002/art.34453
148. Peng Z, Sun H, Bunpetch V, Koh Y, Wen Y, Wu D, et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. (2021) 268:120555. doi: 10.1016/j.biomaterials.2020.120555
149. Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster JY, Bruyère O, et al. Statin use and knee osteoarthritis progression: results from a post-hoc analysis of the SEKOIA trial. Joint Bone Spine. (2018) 85:609–14. doi: 10.1016/j.jbspin.2017.09.014
150. Wang Y, Tonkin A, Jones G, Hill C, Ding C, Wluka AE, et al. Does statin use have a disease modifying effect in symptomatic knee osteoarthritis? Study protocol for a randomised controlled trial. Trials. (2015) 16:584. doi: 10.1186/s13063-015-1122-2
151. Liao JK, Laufs U. Pleiotropic effects of statins. Ann Rev Pharmacol Toxicol. (2005) 45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748
152. Kounatidis D, Tentolouris N, Vallianou NG, Mourouzis I, Karampela I, Stratigou T, et al. The pleiotropic effects of lipid-modifying interventions: exploring traditional and emerging hypolipidemic therapies. Metabolites. (2024) 14:388. doi: 10.3390/metabo14070388
153. Haj-Mirzaian A, Mohajer B, Guermazi A, Conaghan PG, Lima JAC, Blaha MJ, et al. Statin use and knee osteoarthritis outcome measures according to the presence of heberden nodes: results from the osteoarthritis initiative. Radiology. (2019) 293:396–404. doi: 10.1148/radiol.2019190557
154. Mandal CC. High cholesterol deteriorates bone health: new insights into molecular mechanisms. Front Endocrinol. (2015) 6:165. doi: 10.3389/fendo.2015.00165
155. Cao L, Wu W, Deng X, Guo H, Pu F, Shao Z. Association between total cholesterol and total bone mineral density in US adults: National Health and Nutrition Examination Survey (NHANES), 2011-2018. J Orthop Surg Res. (2023) 18:40. doi: 10.1186/s13018-022-03485-8
156. Babinets LS, Halabitska IM, Borovyk IO, Redkva OV, Sasyk HM. The influence of exocrine pancreatic insufficiency in the formation of osteopenia in patients with primary osteoarthritis. Wiadomosci Lekarskie. (2020) 73:2238–40. doi: 10.36740/WLek202010125
157. Alcocer LA, Bryce A, De Padua Brasil D, Lara J, Cortes JM, Quesada D, et al. The pivotal role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in hypertension management and cardiovascular and renal protection: a critical appraisal and comparison of international guidelines. Am J Cardiovasc Drugs. (2023) 23:663–82. doi: 10.1007/s40256-023-00605-5
158. Soós B, Fagyas M, Horváth Á, Végh E, Pusztai A, Czókolyová M, et al. Angiotensin converting enzyme activity in anti-TNF-treated rheumatoid arthritis and ankylosing spondylitis patients. Front Med. (2021) 8:785744. doi: 10.3389/fmed.2021.785744
159. Repchuk Y, Sydorchuk LP, Sydorchuk AR, Fedonyuk LY, Kamyshnyi O, Korovenkova O, et al. Linkage of blood pressure, obesity and diabetes mellitus with angiotensinogen gene (AGT 704T>C/rs699) polymorphism in hypertensive patients. Bratislavske Lekarske Listy. (2021) 122:715–20. doi: 10.4149/BLL_2021_114
160. Babinets LS, Levchuk RD, Halabitska IM, Kryskiv OI. Effectiveness of lisinopril and amlodipine combination at hypertension with comorbidity of arteriosclerosis obliterans in general practice. Wiadomosci Lekarskie. (2022) 75:2407–11. doi: 10.36740/WLek202210116
161. Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. (2015) 179:137–45. doi: 10.1111/cei.12467
162. de Cavanagh EMV, Inserra F, Ferder L. Renin-angiotensin system inhibitors positively impact on multiple aging regulatory pathways: could they be used to protect against human aging? Physiol Rep. (2024) 12:e16094. doi: 10.14814/phy2.16094
163. Bryniarski P, Nazimek K, Marcinkiewicz J. Immunomodulatory activity of the most commonly used antihypertensive drugs-angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Int J Mol Sci. (2022) 23:1772. doi: 10.3390/ijms23031772
164. Oosthuizen D, Sturrock ED. Exploring the impact of ACE inhibition in immunity and disease. J Renin Angiotensin Aldosterone Syst. (2022) 2022:9028969. doi: 10.1155/2022/9028969
165. Tang Y, Hu X, Lu X. Captopril, an angiotensin-converting enzyme inhibitor, possesses chondroprotective efficacy in a rat model of osteoarthritis through suppression local renin-angiotensin system. Int J Clin Exp Med. (2015) 8:12584–92.
166. Tamer TM. Hyaluronan and synovial joint: function, distribution and healing. Interdiscip Toxicol. (2013) 6:111–25. doi: 10.2478/intox-2013-0019
167. Sydorchuk L, Dzhuryak V, Sydorchuk A, Levytska S, Petrynych V, Knut R, et al. The cytochrome 11B2 aldosterone synthase gene rs1799998 single nucleotide polymorphism determines elevated aldosterone, higher blood pressure, and reduced glomerular filtration, especially in diabetic female patients. Endocr Regul. (2020) 54:217–26. doi: 10.2478/enr-2020-0024
168. Li B, Yang Z, Li Y, Zhang J, Li C, Lv N. Exploration beyond osteoarthritis: the association and mechanism of its related comorbidities. Front Endocrinol. (2024) 15:1352671. doi: 10.3389/fendo.2024.1352671
169. Shumnalieva R, Kotov G, Monov S. Obesity-related knee osteoarthritis-current concepts. Life. (2023) 13:1650. doi: 10.3390/life13081650
170. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 145:e895–1032. doi: 10.1161/CIR.0000000000001073
171. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. (2009) 14:141–53.
172. Wang Z, Jones G, Winzenberg T, Cai G, Laslett LL, Aitken D, et al. Effectiveness of curcuma longa extract for the treatment of symptoms and effusion-synovitis of knee osteoarthritis : a randomized trial. Ann Intern Med. (2020) 173:861–9. doi: 10.7326/M20-0990
173. Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. (2018) 52:167–75. doi: 10.1136/bjsports-2016-097333
174. Ajitkumar A, Mohan G, Ghose M, Yarrarapu S, Afiniwala S. Drug-induced liver injury secondary to turmeric use. Eur J Case Rep Internal Med. (2023) 10:003845. doi: 10.12890/2023_003845
175. Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. (2011) 73:255–61. doi: 10.4103/0250-474X.93507
176. Yu G, Xiang W, Zhang T, Zeng L, Yang K, Li J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement Med Ther. (2020) 20:225. doi: 10.1186/s12906-020-02985-6
177. Eriksen P, Bartels EM, Altman RD, Bliddal H, Juhl C, Christensen R. Risk of bias and brand explain the observed inconsistency in trials on glucosamine for symptomatic relief of osteoarthritis: a meta-analysis of placebo-controlled trials. Arthritis Care Res. (2014) 66:1844–55. doi: 10.1002/acr.22376
178. Reichenbach S, Sterchi R, Scherer M, Trelle S, Bürgi E, Bürgi U, et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. (2007) 146:580–90. doi: 10.7326/0003-4819-146-8-200704170-00009
179. Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. (2015) 1:Cd005614. doi: 10.1002/14651858.CD005614.pub2
180. Wu D, Huang Y, Gu Y, Fan W. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. (2013) 67:585–94. doi: 10.1111/ijcp.12115
181. Reginster JY, Dudler J, Blicharski T, Pavelka K. Pharmaceutical-grade Chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: the ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann Rheum Dis. (2017) 76:1537–43. doi: 10.1136/annrheumdis-2016-210860
182. Hochberg MC. Structure-modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta-analysis of randomized placebo-controlled trials of 2-year duration. Osteoarthritis Cartil. (2010) 18(Suppl. 1):S28–31. doi: 10.1016/j.joca.2010.02.016
183. Halabitska I, Babinets L. Efficacy of inclusion of vitamin U and provitamin B5 combination in the complex therapy of primary osteoarthritis against the background of gastrogenic exocrine pancreatic insufficiency. Ukrainian Therapeut J. (2023) 2023:23–8. doi: 10.30978/UTJ2023-2-53
184. Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. (2006) 354:795–808. doi: 10.1056/NEJMoa052771
185. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. (2017) 69:77–85. doi: 10.1002/art.39819
186. Runhaar J, Rozendaal RM, van Middelkoop M, Bijlsma HJW, Doherty M, Dziedzic KS, et al. Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank. Ann Rheumat Dis. (2017) 76:1862–9. doi: 10.1136/annrheumdis-2017-211149
187. Hill CL, March LM, Aitken D, Lester SE, Battersby R, Hynes K, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheumat Dis. (2016) 75:23–9. doi: 10.1136/annrheumdis-2014-207169
188. Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, Han W, et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. (2016) 315:1005–13. doi: 10.1001/jama.2016.1961
189. Suzuki Y, Fukushima M, Sakuraba K, Sawaki K, Sekigawa K. Krill oil improves mild knee joint pain: a randomized control trial. PLoS ONE. (2016) 11:e0162769. doi: 10.1371/journal.pone.0162769
190. Stonehouse W, Benassi-Evans B, Bednarz J, Vincent AD, Hall S, Hill CL. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2022) 116:672–85. doi: 10.1093/ajcn/nqac125
191. Lopez HL. Nutritional interventions to prevent and treat osteoarthritis. Part II: focus on micronutrients and supportive nutraceuticals. PM R. (2012) 4:S155–68. doi: 10.1016/j.pmrj.2012.02.023
192. Levy RM, Khokhlov A, Kopenkin S, Bart B, Ermolova T, Kantemirova R, et al. Efficacy and safety of flavocoxid, a novel therapeutic, compared with naproxen: a randomized multicenter controlled trial in subjects with osteoarthritis of the knee. Adv Ther. (2010) 27:731–42. doi: 10.1007/s12325-010-0064-z
193. Levy RM, Saikovsky R, Shmidt E, Khokhlov A, Burnett BP. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short-term randomized, double-blind pilot study. Nutr Res. (2009) 29:298–304. doi: 10.1016/j.nutres.2009.04.003
194. Atamaz FC, Durmaz B, Baydar M, Demircioglu OY, Iyiyapici A, Kuran B, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: a double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil. (2012) 93:748–56. doi: 10.1016/j.apmr.2011.11.037
195. Reichenbach S, Jüni P, Hincapié CA, Schneider C, Meli DN, Schürch R, et al. Effect of transcutaneous electrical nerve stimulation (TENS) on knee pain and physical function in patients with symptomatic knee osteoarthritis: the ETRELKA randomized clinical trial. Osteoarthritis Cartil. (2022) 30:426–35. doi: 10.1016/j.joca.2021.10.015
196. Vance CG, Rakel BA, Blodgett NP, DeSantana JM, Amendola A, Zimmerman MB, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. (2012) 92:898–910. doi: 10.2522/ptj.20110183
197. Manheimer E, Linde K, Lao L, Bouter LM, Berman BM. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. (2007) 146:868–77. doi: 10.7326/0003-4819-146-12-200706190-00008
198. Foster NE, Thomas E, Barlas P, Hill JC, Young J, Mason E, et al. Acupuncture as an adjunct to exercise based physiotherapy for osteoarthritis of the knee: randomised controlled trial. BMJ. (2007) 335:436. doi: 10.1136/bmj.39280.509803.BE
199. Scharf HP, Mansmann U, Streitberger K, Witte S, Krämer J, Maier C, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med. (2006) 145:12–20. doi: 10.7326/0003-4819-145-1-200607040-00005
200. Denegar CR, Dougherty DR, Friedman JE, Schimizzi ME, Clark JE, Comstock BA, et al. Preferences for heat, cold, or contrast in patients with knee osteoarthritis affect treatment response. Clin Interv Aging. (2010) 5:199–206. doi: 10.2147/CIA.S11431
201. Mazzuca SA, Page MC, Meldrum RD, Brandt KD, Petty-Saphon S. Pilot study of the effects of a heat-retaining knee sleeve on joint pain, stiffness, and function in patients with knee osteoarthritis. Arthritis Rheumat. (2004) 51:716–21. doi: 10.1002/art.20683
202. Yildirim N, Filiz Ulusoy M, Bodur H. The effect of heat application on pain, stiffness, physical function and quality of life in patients with knee osteoarthritis. J Clin Nurs. (2010) 19:1113–20. doi: 10.1111/j.1365-2702.2009.03070.x
203. Bui VL, Brahn E. Cytokine targeting in rheumatoid arthritis. Clin Immunol. (2019) 206:3–8. doi: 10.1016/j.clim.2018.04.001
204. Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. (2014) 10:77–88. doi: 10.1038/nrrheum.2013.168
205. Meng F, Li H, Feng H, Long H, Yang Z, Li J, et al. Efficacy and safety of biologic agents for the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Therapeut Adv Musculoskelet Dis. (2022) 14:1759720x221080377. doi: 10.1177/1759720X221080377
206. Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol. (2013) 9:400–10. doi: 10.1038/nrrheum.2013.44
207. Lin CL, Heron P, Hamann SR, Smith GM. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neuroscience. (2014) 272:76–87. doi: 10.1016/j.neuroscience.2014.04.053
208. Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain. (2002) 96:89–97. doi: 10.1016/S0304-3959(01)00433-X
209. Eibl JK, Strasser BC, Ross GM. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int. (2012) 61:1266–75. doi: 10.1016/j.neuint.2012.10.008
210. Raychaudhuri SP, Raychaudhuri SK, Atkuri KR, Herzenberg LA, Herzenberg LA. Nerve growth factor: a key local regulator in the pathogenesis of inflammatory arthritis. Arthritis Rheumat. (2011) 63:3243–52. doi: 10.1002/art.30564
211. Maksymowych WP, Russell AS, Chiu P, Yan A, Jones N, Clare T, et al. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res Ther. (2012) 14:R206. doi: 10.1186/ar4044
212. Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. (2012) 13:790–8. doi: 10.1016/j.jpain.2012.05.006
213. Cohen SB, Proudman S, Kivitz AJ, Burch FX, Donohue JP, Burstein D, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther. (2011) 13:R125. doi: 10.1186/ar3430
214. Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheumat Dis. (2015) 74:1697–705. doi: 10.1136/annrheumdis-2014-205348
215. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. (2010) 363:1521–31. doi: 10.1056/NEJMoa0901510
216. Hochberg MC, Carrino JA, Schnitzer TJ, Guermazi A, Walsh DA, White A, et al. Long-term safety and efficacy of subcutaneous tanezumab versus nonsteroidal antiinflammatory drugs for hip or knee osteoarthritis: a randomized trial. Arthritis Rheumatol. (2021) 73:1167–77. doi: 10.1002/art.41674
217. Sanga P, Katz N, Polverejan E, Wang S, Kelly KM, Haeussler J, et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. (2013) 154:1910–9. doi: 10.1016/j.pain.2013.05.051
218. Mayorga AJ, Wang S, Kelly KM, Thipphawong J. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis: a randomised, placebo- and active-controlled trial. Int J Clin Pract. (2016) 70:493–505. doi: 10.1111/ijcp.12807
219. Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheumat. (2009) 61:344–52. doi: 10.1002/art.24096
220. Fleischmann RM, Bliddal H, Blanco FJ, Schnitzer TJ, Peterfy C, Chen S, et al. A Phase II trial of lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol. (2019) 71:1056–69. doi: 10.1002/art.40840
221. Persson MSM, Sarmanova A, Doherty M, Zhang W. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology. (2018) 57:1830–7. doi: 10.1093/rheumatology/key131
222. Cao Z, Li Y, Wang W, Jie S, Hu X, Zhou J, et al. Is Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, efficacious for Osteoarthritis? Results from a Bayesian network meta-analysis. Biomed Res Int. (2020) 2020:9013283. doi: 10.1155/2020/9013283
223. Schnitzer TJ, Easton R, Pang S, Levinson DJ, Pixton G, Viktrup L, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. (2019) 322:37–48. doi: 10.1001/jama.2019.8044
224. Nagashima H, Suzuki M, Araki S, Yamabe T, Muto C. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartil. (2011) 19:1405–12. doi: 10.1016/j.joca.2011.09.006
Keywords: osteoarthritis, NSAIDs, opioids, corticosteroids, pain, cytokines
Citation: Shtroblia V, Petakh P, Kamyshna I, Halabitska I and Kamyshnyi O (2025) Recent advances in the management of knee osteoarthritis: a narrative review. Front. Med. 12:1523027. doi: 10.3389/fmed.2025.1523027
Received: 08 November 2024; Accepted: 02 January 2025;
Published: 21 January 2025.
Edited by:
Ching-Mao Chang, Taipei Veterans General Hospital, TaiwanCopyright © 2025 Shtroblia, Petakh, Kamyshna, Halabitska and Kamyshnyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlo Petakh, cGF2bG8ucGV0YWtoQHV6aG51LmVkdS51YQ==
 Viktor Shtroblia1
Viktor Shtroblia1 Pavlo Petakh
Pavlo Petakh Iryna Halabitska
Iryna Halabitska Oleksandr Kamyshnyi
Oleksandr Kamyshnyi