- 1Elizabeth Glaser Pediatric AIDS Foundation, Washington, DC, United States
- 2Elizabeth Glaser Pediatric AIDS Foundation, Maseru, Lesotho
- 3Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, George Washington University, Washington, DC, United States
- 4Ministry of Health, Maseru, Lesotho
- 5Johns Hopkins Department of Health Behavior and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 6Department of Epidemiology, Milken Institute School of Public Health, George Washington University, Washington, DC, United States
Introduction: Even in the context of widespread access to prevention of vertical HIV transmission (PVT) services, health system challenges compromise health outcomes for women living with HIV and their children. The “Integrated Management Team to Improve Maternal-Child Outcomes” (IMPROVE) study measured the effect of a package of facility-based interventions on PVT and maternal and child health (MCH) outcomes in Lesotho.
Methods: This cluster-randomized study included six facilities randomized to the standard-of-care and six to the IMPROVE intervention. The intervention included multidisciplinary teams of health care and community workers providing MCH support, training in patient-centered care, and additional home support. Pregnant women with and without HIV were enrolled at their first antenatal visit and followed through 12–24 months postpartum with their infants. Data were collected through participant interviews and routine medical record abstraction. Primary outcomes included viral suppression and adherence to antiretroviral therapy (ART) for women with HIV and repeat HIV testing for women without HIV. Analysis utilized generalized estimating equations (GEE) adjusted for intra-site correlation.
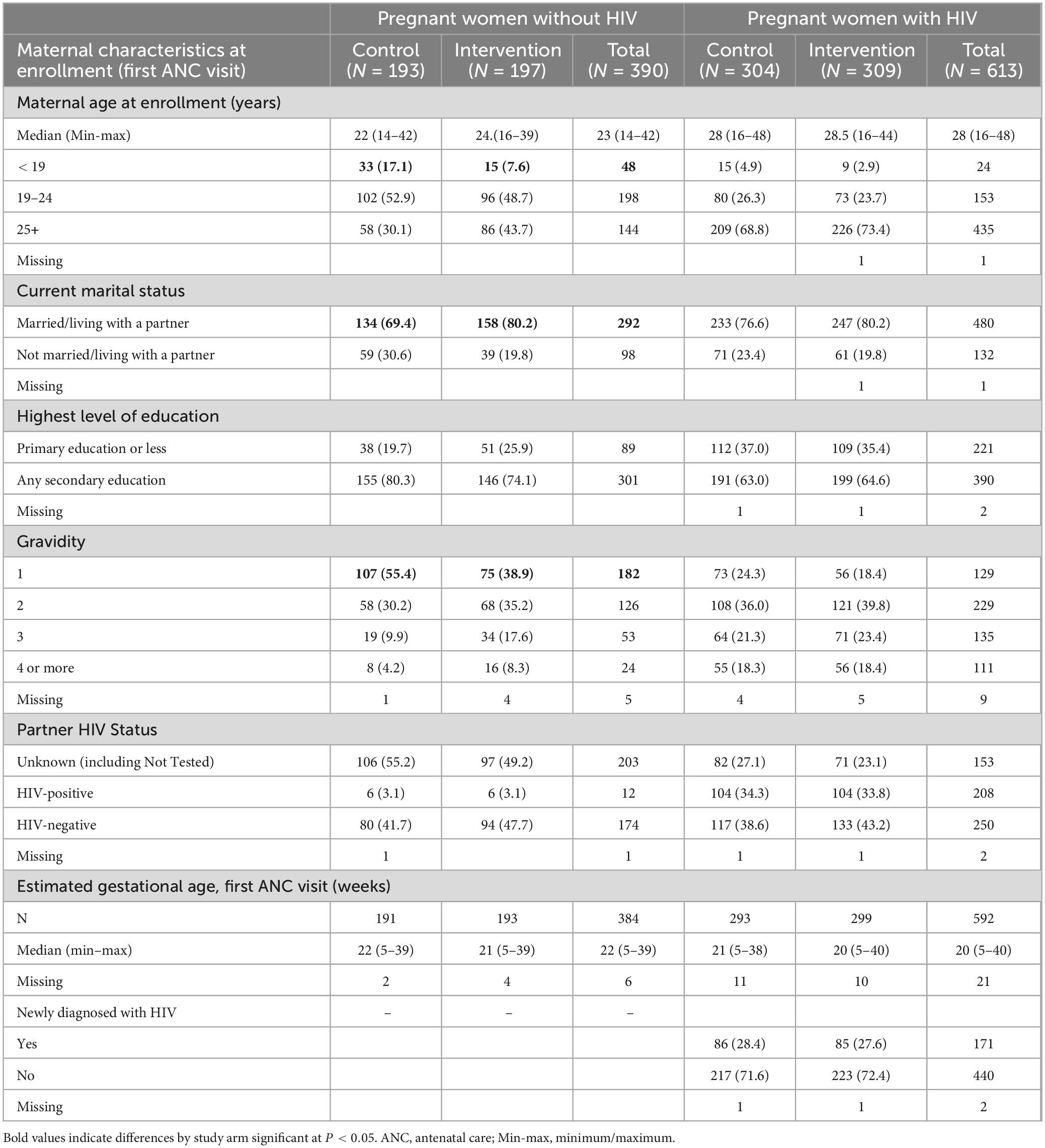
Results: Between July 2016 and February 2017, 614 pregnant women with HIV and 390 without HIV were enrolled. At 12 months postpartum, over 90% of women with HIV with viral load (VL) testing had a VL < 1,000 copies/mL; the intervention arm had a trend toward higher proportion with undetectable VL (< 50 copies/mL) compared to the control arm [83% versus 72%, OR 1.9 (95% CI 0.86–4.14)]. Women with HIV in the intervention arm had significantly higher odds of consistent adherence to ART [OR 1.81 (95% CI 1.03–3.18)], and women without HIV in the intervention arm had significantly higher odds of being re-tested for HIV prior to delivery [OR 1.95 (95% CI 1.23–3.08)].
Conclusion: Sites that implemented the IMPROVE intervention documented better PVT and MCH outcomes than sites implementing standard-of-care. This package of facility-based interventions is a promising and easily scalable model for improving coordination, quality, and uptake of services within the existing health system.
Introduction
Despite significant global progress toward reducing vertical HIV transmission, the rate of decline in the number of children with newly acquired HIV infection has slowed in recent years; UNAIDS estimates that approximately 120,000 children were newly infected with HIV in 2023 (1). Barriers to service uptake, poor maternal retention and antiretroviral treatment (ART) adherence, and health system challenges continue to contribute to vertical transmission and to compromise health outcomes for mothers living with HIV and their children (1–7).
Failures across all four prongs of the World Health Organization (WHO) prevention of vertical HIV transmission (PVT) strategy contribute to ongoing vertical transmission. Focused counseling and support for primary prevention among pregnant women without HIV (PVT prong 1) are limited in PVT programs (8). Coupled with low rates of retesting, high HIV sero-incidence among pregnant and postpartum women in Sub-Saharan Africa remain barriers to elimination of vertical transmission; UNAIDS estimates that incident infections during pregnancy or breastfeeding account for 32% of new vertical infections in eastern and southern Africa (1, 9–12). Efforts to reduce unplanned pregnancies (PVT prong 2) and increase access to family planning are often lacking (13–15). While access to PVT services and ART for pregnant women with HIV has improved (PVT prong 3), rates of retention in care and ART adherence have been found to be lower for women in PVT programs than in non-pregnant persons with HIV in some settings. Approximately 27% of new vertical infections in eastern and southern Africa in 2023 are attributed to women with HIV who interrupt ART during pregnancy or breastfeeding (1, 2). Loss to follow-up (LTFU) and poor ART adherence are particularly high within the first 3 months after ART initiation and during the postpartum period (2–6). Stigma (including from healthcare providers), lack of disclosure, and limited family and community support (PVT prong 4) also contribute to poor retention (2, 7, 8, 16, 17).
Health system barriers also affect PVT uptake and retention: poor communication and coordination between PVT services and other health services and between different cadres of providers; negative attitudes of health care workers (HCW); and inadequate counseling and support of women with HIV at ART initiation and across the PVT cascade (1, 2, 4, 7, 18, 19). Multiple strategies shown to be effective individually in study settings to improve PVT program support and retention, such as the use of peer counselors, community health workers (CHWs), and support groups, have now been widely implemented in maternal and child health (MCH) services, yet LTFU and vertical transmission remain high in many African settings (18, 20–24). Use of multidisciplinary teams (MDT) to ensure patients receive a full range of care and support services has been successful for the management of complex non-HIV conditions (25–28). In resource-limiting settings, MDT may also facilitate task shifting and coordination among health cadres (29). Patient-centered care practices are also associated with better HIV treatment uptake, adherence, and viral suppression (30–32).
Lesotho has one of the highest HIV burdens globally, with an estimated HIV prevalence of 25.9% among pregnant women (33). Lesotho began offering lifelong ART for pregnant and breastfeeding women with HIV in 2013 and transitioned to the “Test and Treat” model in 2016; in 2023, UNAIDS estimated PVT coverage in women with HIV in Lesotho at 93% (34). With an estimated sero-incidence of 2.61 and 1.36 per 100 person-years in Lesotho during pregnancy and postpartum, respectively (35), elimination of vertical transmission requires a comprehensive approach to service delivery to both women with and without HIV during antenatal and postnatal care. We evaluated implementation of a multi-component facility-based intervention (IMPROVE) designed to combine known effective strategies in a coordinated approach for PVT and maternal-child health outcomes in Lesotho.
Materials and methods
Design and setting
We conducted a cluster-randomized prospective cohort study to evaluate the “Integrated Management Team to Improve Maternal-Child Outcomes” (IMPROVE) intervention in 12 rural and urban health facilities in Maseru District Lesotho from July 2016 to July 2019. Health facilities in the district serving between 150 and 900 pregnant women annually were categorized by size, type of facility (hospital or health center), hospital catchment areas, and type of support (government or Christian Health Association of Lesotho (CHAL)]. From this list, the study team selected both of two main hospitals and created two clusters by grouping each hospital with another five mid- to high-volume facilities within their referral area to avoid potential cross-contamination if a health facility and the hospital that received that facility’s referrals were randomly allocated to different arms. These five facilities were selected to ensure even distribution across size, facility type, and type of support. One cluster was randomly assigned to receive the IMPROVE intervention, and the other was designated as the standard-of-care (control) arm.
Standard of care MCH and PVT services
All study facilities provided free MCH and PVT services, including HIV testing and ART. Women newly identified with HIV received same-day ART initiation. Guidelines for retesting women who initially tested negative for HIV included retesting at 36 weeks gestation and yearly thereafter during breastfeeding. Postnatal care was provided to women through 14 weeks after delivery. Women with HIV received HIV follow-up care every 1–3 months with yearly viral load (VL) testing. Infant postnatal care included 8 visits within the first 18 months of life. HIV testing for infants born to mothers with HIV was done at 6 weeks, 14 weeks, 9 months, and 18 months. In all facilities, clinical staff were supported by a cadre of peer counselors and CHW. In the event of a missed clinic appointment, women with HIV- like all other clients with HIV- were referred to dedicated facility staff and would receive an initial phone call 1 week after the missed visit. If the woman could not be reached or did not return for a visit, facility staff would enlist the appropriate community partner to conduct a home visit. Procedures for women without HIV were more variable at the time of this study; women may or may not have received a phone call or home visit.
The IMPROVE intervention
The IMPROVE intervention included three key interventions: (i) formation of multidisciplinary, integrated management teams of facility- and community-based health care and lay workers, (ii) Joint Positive Health Dignity, and Prevention (PHDP)-focused counseling, skills-building training, and job aids to strengthen the MDT’s ability to provide consistent messages across all staff cadres (nurses, CHW, peers) and provide a patient-centered approach to identify solutions to barriers to care; and (iii) increased early community-based counseling and support to minimize early LTFU (including at least one additional home or community outreach visit within 2 weeks of the first ANC visit). At each intervention site, the study team supported the creation of a MDT, trained the MDT team in problem-solving techniques, and facilitated a group mapping exercise to optimize the patient flow within the facility and to identify gaps and potential strategies for ensuring patient follow-up and continuity of care from facility to the community. Thereafter, each MDT organized and ran their own meetings with documentation of attendance, minutes, and action plans. Further details on the IMPROVE intervention and the specific strategies adopted by each intervention site are described in Beres et al., 2024 (36).
Study population
We enrolled pregnant women with and without HIV attending their first antenatal care (ANC) visit at a study site between July 2016 and February 2017. Women were eligible if they were residing in the catchment area for a study facility with no plans to relocate after delivery and provided written informed consent. Study nurses screened all pregnant women with HIV and a randomly selected subset of pregnant women without HIV (∼ one woman without HIV/week/facility) for study eligibility and enrolment. Both routine services and study activities were targeted toward individual women; while women could choose to bring a partner or other support person to their care visits or disclose their participation in the study, there was no formal mechanism for engagement with individuals other than the participant.
Study procedures
Study visits were scheduled every 3 months until 12–24 months after delivery for women and their infants. Participants were interviewed and maternal and child clinical and laboratory data were abstracted from medical records. Data were entered directly into a cloud-based electronic database on the CliniOps platform (37). Dried blood spot specimens for VL were collected from women with HIV at 12 and 24 months postpartum. Specimens were transported to the National Reference Lab in Maseru and stored in the freezer until batched and sent to the National Institute for Communicable Diseases in Johannesburg, South Africa for testing using Roche COBAS Amplicor/Taqman assay.
Outcomes
Primary outcome measures included viral suppression and ART adherence for women with HIV, repeat HIV testing for women initially testing negative for HIV, and other selected MCH behaviors and outcomes for all women and children. VL was defined as undetectable if < 50 copies/mL and suppressed if < 1,000 copies/mL. Adherence was defined as taking ≥ 95% of ART doses (assessed by facility staff through pill counts at each visit and recorded in the patient’s file). Data on adherence were reviewed and summarized as either “consistently adherent” (participant adherent at each visit where documented), or “not consistently adherent” (at least one visit with documented sub-optimal adherence). “Modern contraception” was defined as use of any of the following to prevent pregnancy: barrier methods (condom, diaphragm, etc.), pill, injection, implant, IUD, or female or male sterilization. Modern contraception use was categorized as “consistent use” (reported at each post-partum visit 14 weeks or later), “mixed use” (use at some but not all visits), or “consistent non-use” (no use of modern contraception at any postpartum study visit at 14 weeks or later). Patient satisfaction was assessed after each routine care visit; at the end of the visit, clinic staff would direct the study participant to a separate area within the facility to meet with the study nurse and answer a series of questions, including about their experiences receiving care on that day.
Statistical analyses
To summarize key indicators, we calculated descriptive statistics (frequency tables, mean/median/range). Bivariate tests of the association were performed using the Rao-Scott chi-square test, adjusting for clustering by site. Generalized estimating equations (GEE) using the binomial distribution, a logit, cumulative logit or generalized logit link function, and a compound symmetry working correlation structure were used to evaluate the relationship between study arm and key outcomes. We present odds ratios (ORs) adjusted for baseline characteristics identified a priori (maternal age, marital status, and education); models of adherence and use of modern contraception are also adjusted for the number of study visits for each participant, and the model of number of ANC visits was also adjusted for estimated weeks gestation at first ANC visit. In all models including both women with and without HIV, we tested for interaction between HIV status and study arm and present stratified results if the interaction was significant. No imputation or other adjustments were made for missing data; for outcomes incorporating data from multiple visits or time points, outcomes were derived using only data from visits that were completed as it was not possible to reliably impute values for missed visits. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States).
Ethical considerations
The study protocol was approved by the Lesotho National Health Research Ethics Committee (NHREC), the George Washington University Institutional Review Board (GW-IRB) and the Population Council IRB. All participants provided written informed consent. The study is registered under ClinicalTrials.gov Identifier: NCT04598958.
Results
A total of 1004 pregnant women were enrolled: 310 pregnant women with HIV (one woman was not pregnant and was excluded from the analysis) and 197 pregnant women without HIV at intervention sites, and 304 pregnant women with HIV and 193 pregnant women without HIV at control sites (Figure 1). Of 613 eligible pregnant women with HIV enrolled, 80.6% had ≥ 12 months of study follow-up after delivery or had died (83.5% vs. 77.6% in the intervention and control arms, respectively). Among 390 pregnant women without HIV, 72.1% remained in active follow-up at 12 months or had died (77.2% vs. 66.8% in the intervention and control arms, respectively) (Figure 1). After adjustment, there was a non-significant trend for improved odds of retention through 12 months postpartum for women in the intervention compared to control arm (79.7% vs 72.8%, respectively, adjusted odds ratio (aOR) 1.5, 95% CI 0.95–2.44). Due to an unexpectedly long time to complete enrollment, the study ended prior to all participants reaching the 24 months endpoint in order to ensure that adequate time and resources were available for data cleaning and analysis.
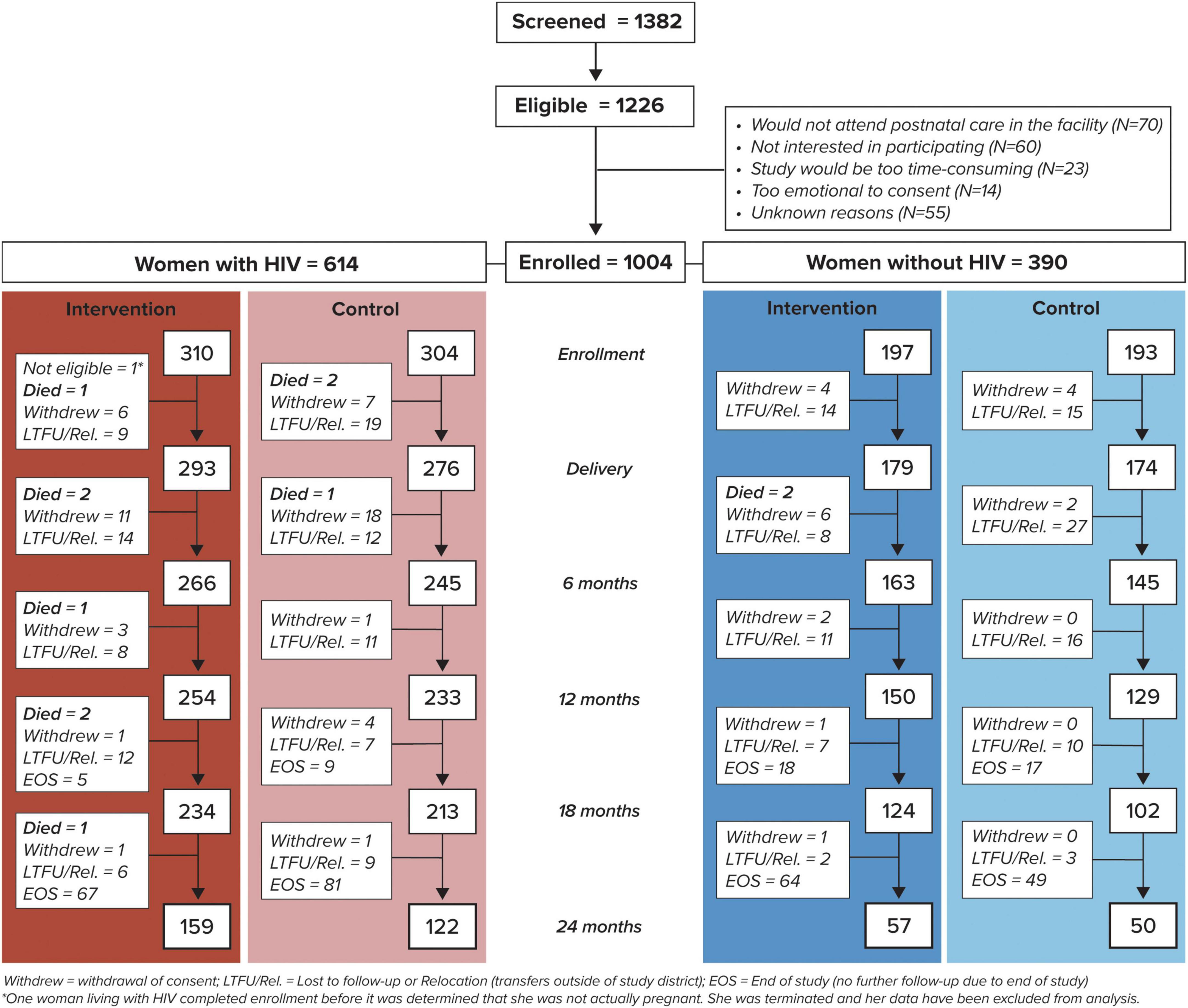
Figure 1. Study enrollment and follow-up of women by human immunodeficiency virus (HIV) status and study arm.
Baseline characteristics
Among pregnant women with HIV, the study arms were comparable in all baseline characteristics, with median age 28 years and median estimated gestational age at first ANC visit 20 weeks (Table 1). The majority (72.0%) of participants with HIV already knew their status at enrollment. For pregnant women without HIV, the median age was 23 years; participants without HIV in the intervention arm were significantly older, more likely to be married or living with partner, and had more previous pregnancies, but other characteristics were comparable.
Selected MCH outcomes
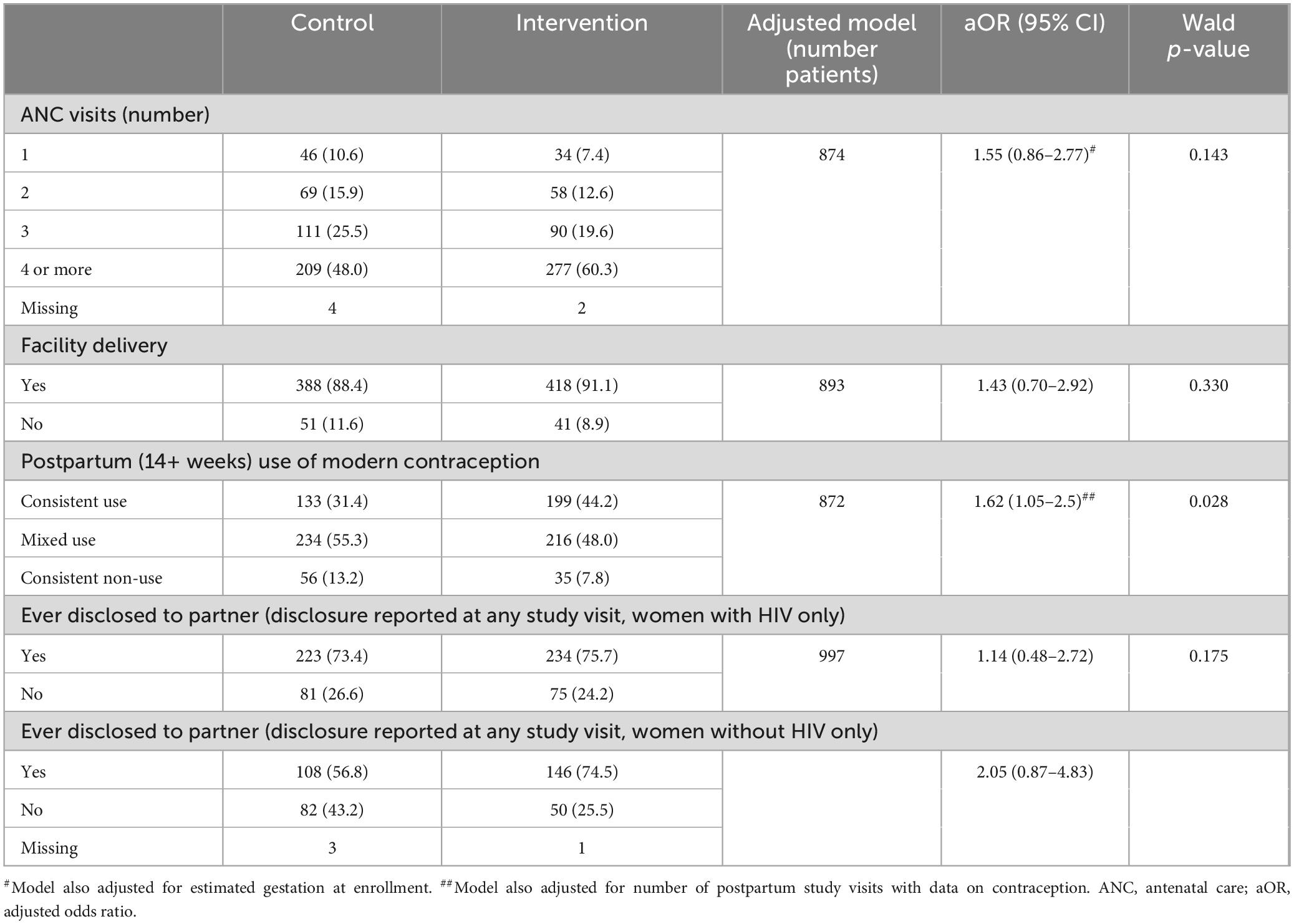
MCH outcomes were analyzed in the overall population (including women with and without HIV) (Table 2); results are presented separately by HIV status if there was a significant interaction between study arm and HIV status in adjusted analysis. Overall, women in the intervention arm had higher odds of more ANC visits compared to women in the control group, though the difference was not statistically significant in adjusted analysis (aOR: 1.55, 95% CI: 0.86–2.77). For example, 60.3% of women in the intervention arm completed the recommended ≥ 4 ANC visits compared to 48.0% of women in the control arm. More than 88% of women in each group delivered in a facility, with no significant difference by study arm. A significantly higher proportion of women in the intervention arm reported consistent postpartum use of modern contraception than in the control arm (aOR: 1.62, 95% CI: 1.05–2.5).
Selected HIV outcomes
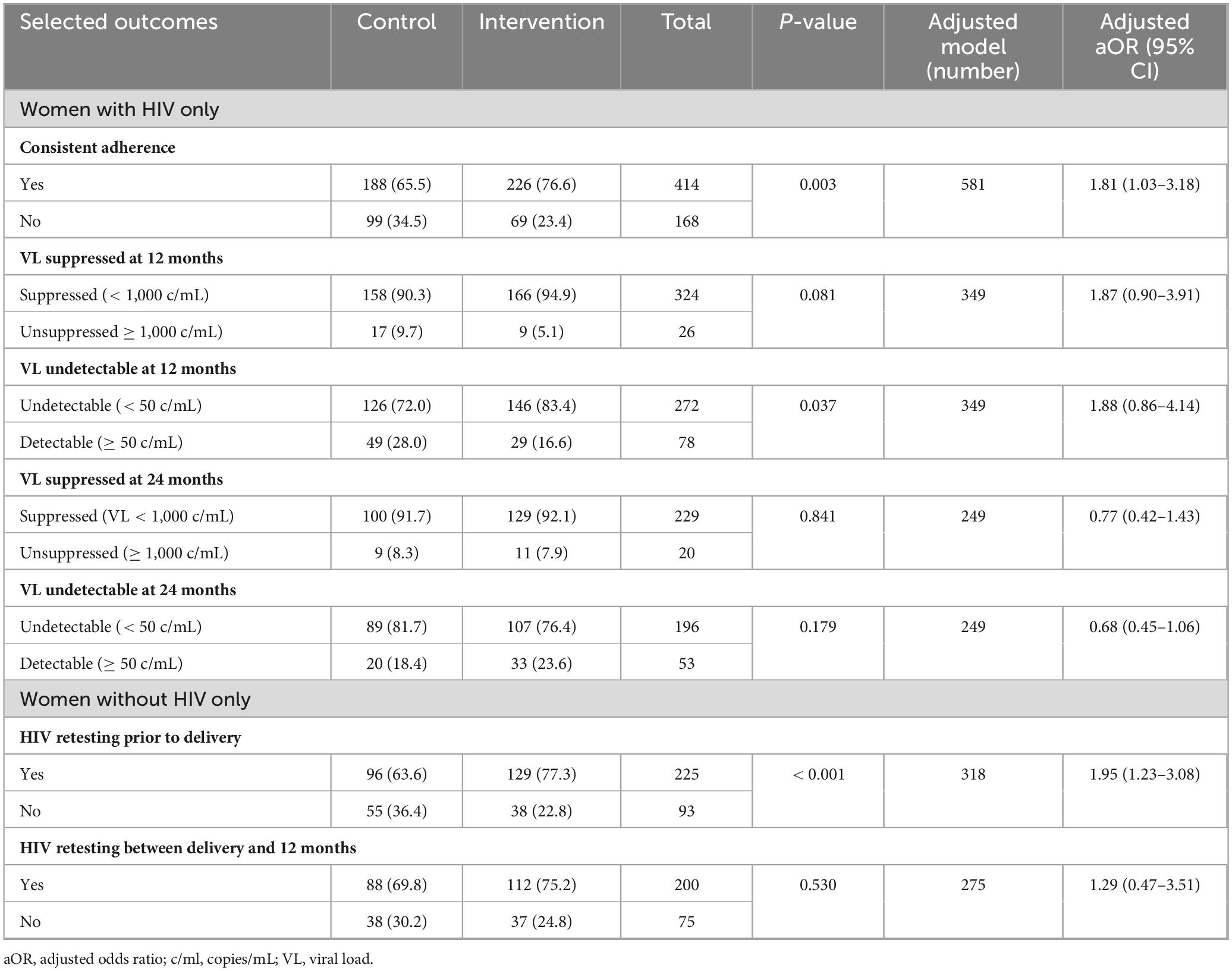
All pregnant women with HIV and a delivery outcome received ART, with approximately half on ART prior to pregnancy and the remainder initiating ART during pregnancy. ART adherence was measured by pill count as part of each routine HIV care visit. Of 582 women with HIV with data on ART adherence, the odds of consistent adherence were significantly higher among women in the intervention arm after adjusting adherence data for selected maternal characteristics (as noted earlier under “Materials and methods”) and number of study visits (aOR 1.81, 95% CI: 1.03–3.18) (Table 3).
VL results were available for 350/487 (72%) women with HIV in the study at 12 months postpartum. The rates of suppression and undetectability were high overall (92.6% and 77.7%, respectively). Although women with HIV in the intervention compared to control arm had a higher proportion with suppressed VL (94.9% vs 90.3%) and undetectable VL (83.4% vs 72.0%), this did not reach statistical significance in the adjusted models (Table 3). Among 249 participants with VL results at 24 months, the proportion of women with viral suppression remained over 90%, with no difference in suppression or undetectable VL between arms.
Women without HIV in the intervention arm had significantly higher odds of retesting for HIV between enrollment and delivery (77.3% compared to 63.6% in the control arm; aOR 1.95, 95% CI: 1.23–3.08) (Table 3). The majority of women without HIV in both study arms received at least one repeat HIV test between delivery and 12 months postpartum. While the odds of postpartum retesting were higher among women in the intervention arm (75.2% vs. 69.8% in the control arm), this difference was not statistically significant.
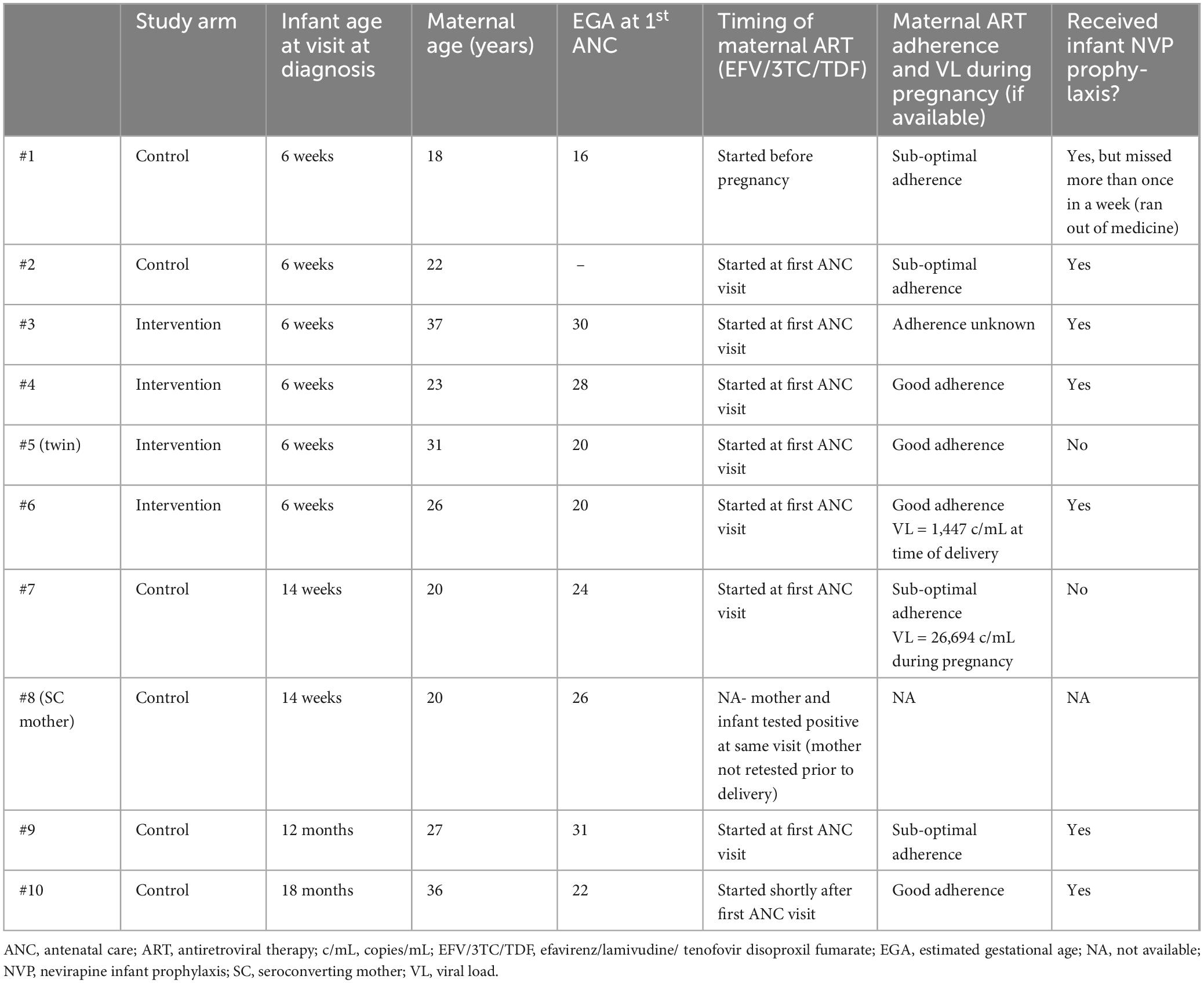
Four women without HIV seroconverted to HIV during the study - three women in the control arm and one woman in the intervention arm (Table 4). All women who seroconverted were initiated on ART [efavirenz (EFV)/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF), standard of care at the time]. One of their infants was identified as having acquired HIV at the same visit that seroconversion was documented.
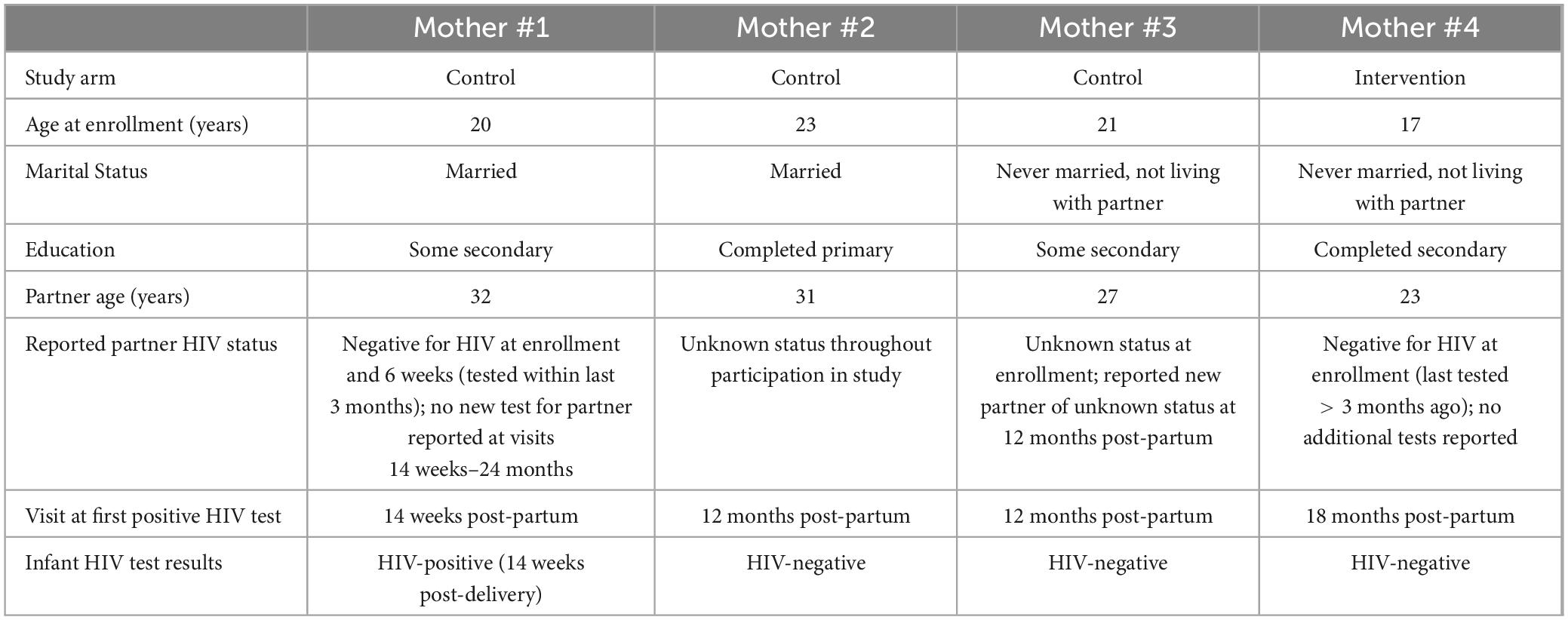
Table 4. Characteristics of women without human immunodeficiency virus (HIV) who acquired HIV infection during study.
During the course of the study, twelve women died: three women with HIV during pregnancy and seven women with HIV and two women without HIV during the postpartum period (Figure 1).
Child outcomes
There were 23 miscarriages prior to 28 weeks gestation - two among women without HIV and 21 among women with HIV. There were 26 stillbirths: six and 20 among women without and with HIV, respectively. During study follow-up, there were 33 deaths among infants born to mothers with HIV (18 intervention, 15 control), including one infant with HIV infection, and 12 deaths among infants born to mothers without HIV (8 intervention, 4 control).
Among infants born to mothers known to be living with HIV at enrollment, 9/358 (2.5%) infants were identified as having acquired HIV infection by 18 months postpartum (Table 5). There was no significant difference in HIV acquisition between study arms, with 5/166 (3.0%) and 4/191 (2.1%) infants acquiring HIV infection in the control and intervention arms, respectively. Nearly all (97.9%) infants born to mothers known to have HIV at enrollment were started on nevirapine (NVP) infant prophylaxis after delivery; of eleven infants born to women with HIV who did not receive NVP infant prophylaxis, two acquired HIV infection (18.2%). Eight of the nine mothers of infants who acquired HIV infection had first initiated ART during pregnancy. Four mothers of infants who acquired HIV (all in the control arm) had documented sub-optimal adherence to ART during pregnancy.
Patient satisfaction
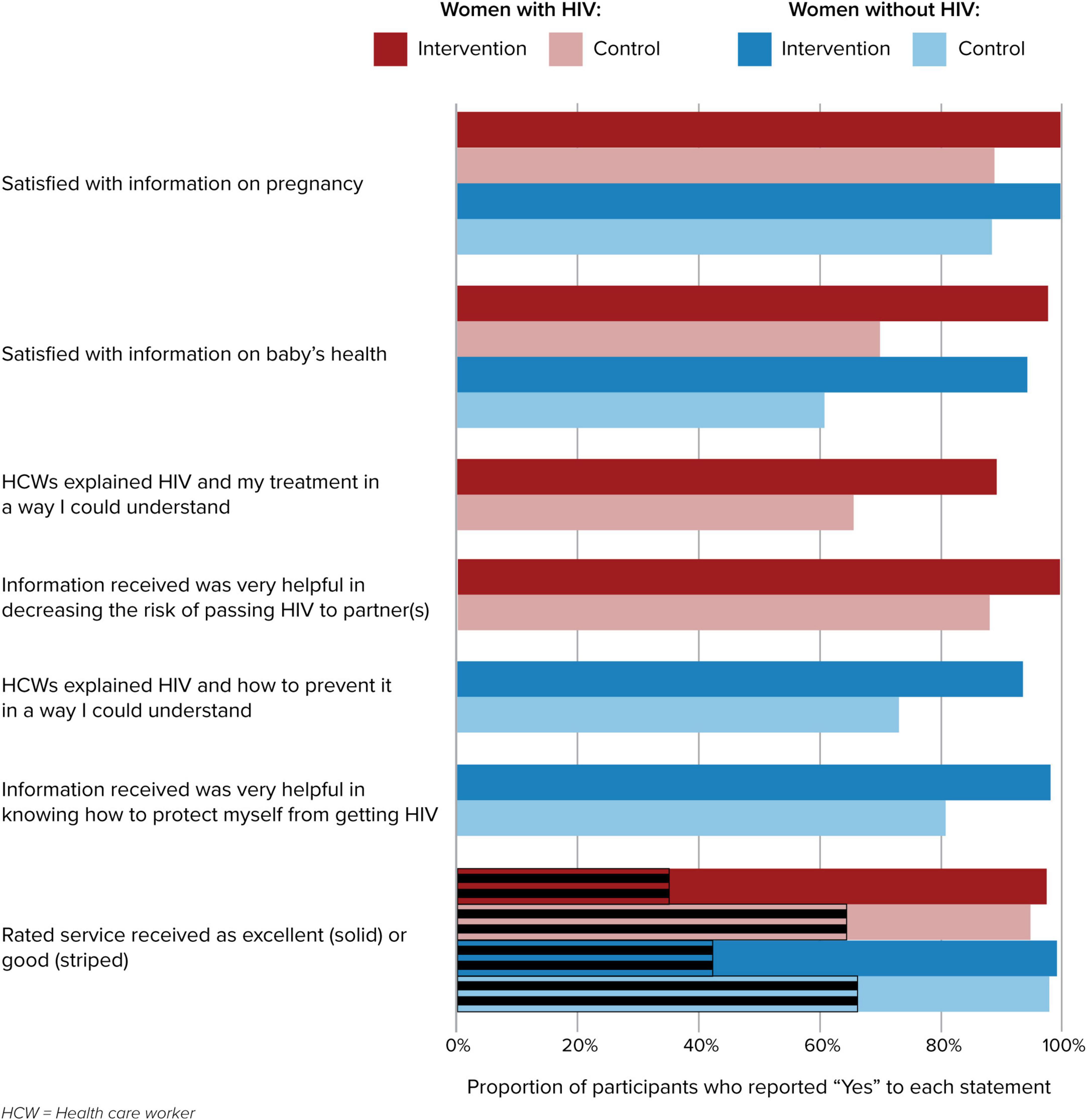
Patient satisfaction with services was assessed at each study visit. Women in the intervention arm, regardless of HIV status, reported significantly higher levels of satisfaction with the information and service provided during their clinic visits (p < 0.05); results are shown for the 12 months postpartum visit, though this trend was sustained over all study visits (Figure 2).
Discussion
Implementation of a multidisciplinary facility-level intervention designed to coordinate patient-centered MCH and PVT services was effective in improving the quality of services provided to pregnant and postpartum women and some key PVT and MCH outcomes. Elimination of vertical HIV transmission will require sustained, effective services and support for both women living with and without HIV to address all four prongs of the WHO PVT strategy (1, 38). Women must also feel confident that the services in their communities will provide clear and accurate information, support for the specific challenges they face, and a non-stigmatizing environment (7, 39, 40). Improvements in the quality and coordination of care provided to individuals with HIV or at risk of contracting HIV will also help to facilitate the successful introduction of new biomedical innovations in HIV prevention and treatment.
Regarding primary prevention (prong 1), we found that women without HIV receiving the intervention reported higher satisfaction with the HIV prevention information they received and were significantly more likely to be retested for HIV during pregnancy. However, even in the intervention arm, nearly 25% of women did not have repeat testing prior to delivery or between delivery and 12 months postpartum despite national guidelines. The control arm mother who seroconverted after enrollment and had an infant who acquired HIV did not have a repeat HIV test around the time of delivery - a missed opportunity to intervene with enhanced infant antiretroviral prophylaxis had she been identified as infected with HIV prior to breastfeeding. Support for primary prevention among women without HIV is a critical gap in many PVT programs, particularly in areas with high HIV prevalence where sero-incident infections and lack of early identification to initiate PVT interventions drives many new infections in infants (1, 35, 41–43).
While PVT prong 2 is prevention of unintended pregnancy, prevalence of modern contraceptive use postpartum is low in sub-Saharan Africa, particularly among women with HIV, resulting in high rates of unplanned/unintended pregnancy and high unmet need for family planning (44–47). The IMPROVE intervention significantly increased consistent postpartum use of modern contraception among both women with and without HIV compared with the control arm. However, while significantly improved, less than half of postpartum women reported consistent contraception use even in a setting where family planning commodities were available.
Early identification of HIV infection and ART initiation in pregnant women with HIV, with support for consistent adherence to achieve viral suppression and improve maternal health, are critical to achieving the goals of WHO prong 3 (provision of maternal ART for PVT). Lesotho has made remarkable progress, as evidenced in our study by all women with known HIV receiving ART and an overall 18 months vertical transmission rate of 2.5%. However, maternal ART adherence and retention in care remains a challenge in Lesotho and other sub-Saharan African countries (47, 48). Women with HIV in IMPROVE intervention facilities were significantly more likely to consistently adhere to ART than women in the control arm, with a trend toward higher rates of undetectable VL at 12 months postpartum. Over half of the infants who acquired HIV infection in our study were born to mothers with documented sub-optimal adherence and/or viremia during pregnancy, consistent with reports from other studies by our team and other teams in the region (49–52). Despite universal ART use, we found higher maternal mortality among women with HIV, similar to a report on maternal deaths from cohorts in Malawi, Tanzania and South Africa, which found a 5-fold increased risk of maternal mortality in women living with HIV compared to women without HIV (53). We previously reported separately on increased adverse pregnancy outcomes among women with HIV compared to women without HIV in this cohort (54).
Evidence shows that just offering care and treatment is not sufficient to ensure healthy outcomes for women living with HIV and their children. In PVT prong 4, comprehensive support from peers, HCW, and CHW was utilized to help women navigate specific challenges, such as partner disclosure, stigma, gender-based violence, access to appropriate services and other issues. Through expanding community visits, providing job aides to HCW/CHW that ensured clear and consistent messaging from all levels of care providers, and enhancing PHDP positive counselling skills in those providing services to pregnant and postpartum women, the IMPROVE intervention sought to improve the support provided to both women with and without HIV. Several studies have reported that community support improves MCH outcomes, through CHW or peer support visits to pregnant women to reinforce counselling messages, respond to outstanding patient questions, follow-up late or missed visits and provide referrals to needed services (55–57).
One mechanism by which the IMPROVE intervention expected to improve outcomes was increasing the quality of services; minimizing the burden of attending visits; providing accessible and useful information on pregnancy, infant health, and HIV; and delivering services in a respectful and tailored manner. Our data indicate that the intervention was well-received; women in the intervention arm consistently reported significantly higher satisfaction with their clinic visits throughout the duration of the study. This may have also contributed to the higher proportion of women at intervention sites having the recommended 4 ANC visits and continuing to return to the sites for post-partum care.
One of the strengths of the IMPROVE intervention was the specificity of the facility-level changes made in response to the needs and situation of those facilities. While the study team provided a standard package of ongoing support and training to each intervention facility, each site had significant latitude in how to identify and address obstacles to deliver higher-quality, more efficient service to clients. However, this variation across facilities presents a challenge in identifying the effect of individual components of the intervention package and comparing the effectiveness of the intervention package overall, even after adjusting for clustering in the analyses.
A limitation of the study was reliance on data collected from or following routine clinical visits at study sites. Voluntary withdrawal from the study, transfer/relocation, loss to follow-up from routine care, all limited the ability of the study to evaluate outcomes at later time points with the desired precision. Among both women with and without HIV, it was not uncommon for women to relocate after the birth or to choose to receive services at a different location at some point during the course of the study (both elsewhere in Lesotho, or in neighboring South Africa). Documentation of postpartum retention was particularly challenging for women without HIV, who did not have routine clinic visits after 14 weeks postpartum, and sometimes chose to bring their infants to a mobile vaccination clinic rather than return to the facility. The study team made extensive efforts to determine and document whether a participant was receiving services at another health facility, particularly once it became clear from interim data review that there was a high rate of transfer and relocation. Similar studies should ensure that robust systems for tracking participants are in place from the beginning of the study, rather than relying primarily on standard procedures at resource-constrained local facilities. Discontinuation of data collection activities before all participants were able to complete the full 24 months postpartum period follow-up further limited our ability to assess outcomes at later time periods and determine whether positive changes were sustained over time.
Conclusion
The IMPROVE interventions were designed to be feasible to implement in routine care settings with existing staff and minimal additional resources. As this intervention builds primarily on the current healthcare system and infrastructure, scaling up the intervention should not require a significant amount of additional financial or human resources. Overall, implementation of the IMPROVE interventions was found to be an effective strategy to enhance MCH/PVT service delivery and improve provider-patient interaction.
Data availability statement
The data that support the findings of this study are available from the Elizabeth Glaser Pediatric AIDS Foundation, Lesotho but restrictions apply to the availability of these data, which were used for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Lesotho Ministry of Health.
Ethics statement
The study protocol was approved by the Lesotho National Health Research Ethics Committee, the George Washington University Institutional Review Board (IRB), and the Population Council IRB. All methods were performed in accordance with the relevant guidelines and regulations. All individual pregnant or postpartum women who participated in the trial as intervention or control arm patients provided signed informed consent. Written informed consent was obtained for participation in this study from all study participants.
Author contributions
LGr: Writing – original draft. VT: Writing – review and editing. HH: Writing – review and editing. RT: Writing – review and editing. TM: Writing – review and editing. MN: Writing – review and editing. MC: Writing – review and editing. MMa: Writing – review and editing. MMo: Writing – review and editing. AK: Writing – review and editing. SV: Writing – review and editing. LM: Writing – review and editing. AT: Writing – review and editing. LGu: Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the United States Agency for International Development (USAID) through the Project SOAR (Supporting Operational AIDS Research); award no. AID-OAA-A-14-00060.
Acknowledgments
We acknowledge the study participants for their cooperation, time and effort, and the support obtained from the study health facilities, the Maseru District Management Team (DHMT) and the Lesotho Ministry of Health. We thank the USAID for funding this study, specifically Dr. Justine Mirembe and Mr. Ian Membe from USAID/Lesotho and Anouk Amzel from USAID/Washington who were instrumental in the development and funding of this study. We also are indebted to the SOAR leadership team at the Population Council for their input and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The contents included here are the responsibility of the authors and do not necessarily represent the official views of USAID.
References
1. UNAIDS. The Urgency of Now: AIDS at a Crossroads. Geneva: Joint United Nations Programme on HIV/AIDS (2024).
2. Knettel B, Cichowitz C, Ngocho J, Knippler E, Chumba L, Mmbaga B, et al. Retention in HIV care during pregnancy and the postpartum period in the option B+ Era: Systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr. (2018) 77:427–38. doi: 10.1097/QAI.0000000000001616
3. Nachega J, Uthman O, Anderson J, Peltzer K, Wampold S, Cotton M, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: A systematic review and meta-analysis. AIDS. (2012) 26:2039–52. doi: 10.1097/QAD.0b013e328359590f
4. Clouse K, Pettifor A, Shearer K, Maskew M, Bassett J, Larson B, et al. Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health. (2013) 18:451–60. doi: 10.1111/tmi.12072
5. Cichowitz C, Mazuguni F, Minja L, Njau P, Antelman G, Ngocho J, et al. Vulnerable at each step in the PMTCT care cascade: High loss to follow up during pregnancy and the postpartum period in Tanzania. AIDS Behav. (2019) 23:1824–32. doi: 10.1007/s10461-018-2298-8
6. Fassinou L, Songwa Nkeunang D, Delvaux T, Nagot N, Kirakoya-Samadoulougou F. Adherence to option B + antiretroviral therapy and associated factors in pregnant and breastfeeding women in Sub-Saharan Africa: A systematic review and meta-analysis. BMC Public Health. (2024) 24:94. doi: 10.1186/s12889-023-17004-9
7. Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: A systematic review. J Int AIDS Soc. (2013) 16:18588. doi: 10.7448/IAS.16.1.18588
8. Ngarina M, Popenoe R, Kilewo C, Biberfeld G, Ekstrom A. Reasons for poor adherence to antiretroviral therapy postnatally in HIV-1 infected women treated for their own health: Experiences from the Mitra Plus study in Tanzania. BMC Public Health. (2013) 13:450. doi: 10.1186/1471-2458-13-450
9. Rogers A, Akama E, Weke E, Blackburn J, Owino G, Bukusi E, et al. Implementation of repeat HIV testing during pregnancy in southwestern Kenya: Progress and missed opportunities. J Int AIDS Soc. (2017) 20:e25036. doi: 10.1002/jia2.25036
10. Nungu S, Mghamba J, Rumisha S, Semali I. Uptake and determinants for HIV postpartum re-testing among mothers with prenatal negative status in Njombe region, Tanzania. BMC Infect Dis. (2019) 19:398. doi: 10.1186/s12879-019-4062-8
11. Rogers A, Weke E, Kwena Z, Bukusi E, Oyaro P, Cohen C, et al. Implementation of repeat HIV testing during pregnancy in Kenya: A qualitative study. BMC Pregnancy Childbirth. (2016) 16:151. doi: 10.1186/s12884-016-0936-6
12. Gill M, Natumanya E, Hoffman H, Okomo G, Taasi G, Guay L, et al. Active pediatric HIV case finding in Kenya and Uganda: A look at missed opportunities along the prevention of mother-to-child transmission of HIV (PMTCT) cascade. PLoS One. (2020) 15:e0233590. doi: 10.1371/journal.pone.0233590
13. Hamilton E, Bossiky B, Ditekemena J, Esiru G, Fwamba F, Goga A, et al. Using the PMTCT cascade to accelerate achievement of the global plan goals. J Acquir Immune Defic Syndr. (2017) 75:S27–35. doi: 10.1097/QAI.0000000000001325
14. Kanyangarara M, Sakyi K, Laar A. Availability of integrated family planning services in HIV care and support sites in sub-Saharan Africa: A secondary analysis of national health facility surveys. Reprod Health. (2019) 16:60. doi: 10.1186/s12978-019-0713-x
15. Rucinski K, Powers K, Schwartz S, Pence B, Chi B, Black V, et al. Longitudinal patterns of unmet need for contraception among women living with HIV on antiretroviral therapy in South Africa. PLoS One. (2018) 13:e0209114. doi: 10.1371/journal.pone.0209114
16. Thomson K, Telfer B, Opondo Awiti P, Munge J, Ngunga M, Reid A. Navigating the risks of prevention of mother to child transmission (PMTCT) of HIV services in Kibera, Kenya: Barriers to engaging and remaining in care. PLoS One. (2018) 13:e0191463. doi: 10.1371/journal.pone.0191463
17. Toska E, Zhou S, Laurenzi C, Saal W, Rudgard W, Wittesaele C, et al. Healthcare provisions associated with multiple HIV-related outcomes among adolescent girls and young women living with HIV in South Africa: A cross-sectional study. J Int AIDS Soc. (2024) 27:e26212. doi: 10.1002/jia2.26212
18. Colvin C, Konopka S, Chalker J, Jonas E, Albertini J, Amzel A, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS One. (2014) 9:e108150. doi: 10.1371/journal.pone.0108150
19. Psaros C, Remmert J, Bangsberg D, Safren S, Smit J. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: Falling off the cliff of the treatment cascade. Curr HIV/AIDS Rep. (2015) 12:1–5. doi: 10.1007/s11904-014-0252-6
20. Futterman D, Shea J, Besser M, Stafford S, Desmond K, Comulada W, et al. Mamekhaya: A pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. (2010) 22:1093–100. doi: 10.1080/09540121003600352
21. Kim M, Ahmed S, Buck W, Preidis G, Hosseinipour M, Bhalakia A, et al. The Tingathe programme: A pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. (2012) 15:17389. doi: 10.7448/IAS.15.4.17389
22. Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of women’s groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): A factorial, cluster-randomised controlled trial. Lancet. (2013) 381:1721–35. doi: 10.1016/S0140-6736(12)61959-X
23. Rotheram-Borus M, Tomlinson M, le Roux IM, Harwood JM, Comulada S, O’Connor MJ, et al. A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. PLoS One. (2014) 9:e105934. doi: 10.1371/journal.pone.0105934
24. Vrazo A, Firth J, Amzel A, Sedillo R, Ryan J, Phelps B. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: Systematic review. Trop Med Int Health. (2018) 23:136–48. doi: 10.1111/tmi.13014
25. Basta Y, Bolle S, Fockens P, Tytgat K. The Value of Multidisciplinary Team Meetings for Patients with Gastrointestinal Malignancies: A Systematic Review. Ann Surg Oncol. (2017) 24:2669–78. doi: 10.1245/s10434-017-5833-3
26. Epstein N. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. (2014) 5:S295–303. doi: 10.4103/2152-7806.139612
27. Prades J, Remue E, van Hoof E, Borras J. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy. (2015) 119:464–74. doi: 10.1016/j.healthpol.2014.09.006
28. Mitchell G, Tieman J, Shelby-James T. Multidisciplinary care planning and teamwork in primary care. Med J Aust. (2008) 188:S61–4. doi: 10.5694/j.1326-5377.2008.tb01747.x
29. Sherer R, Stieglitz K, Narra J, Jasek J, Green L, Moore B, et al. HIV multidisciplinary teams work: Support services improve access to and retention in HIV primary care. AIDS Care. (2002) 14:S31–44. doi: 10.1080/09540120220149975
30. Holtzman C, Brady K, Yehia B. Retention in care and medication adherence: Current challenges to antiretroviral therapy success. Drugs. (2015) 75:445–54. doi: 10.1007/s40265-015-0373-2
31. Thompson M, Mugavero M, Amico K, Cargill V, Chang L, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. (2012) 156:817–33. doi: 10.7326/0003-4819-156-11-201206050-00419
32. Odhiambo F, Onyango R, Mulwa E, Aluda M, Otieno L, Bukusi E, et al. Evaluation of person-centered interventions to eliminate perinatal HIV transmission in Kisumu County, Kenya: A repeated cross-sectional study using aggregated registry data. PLoS Med. (2024) 21:e1004441. doi: 10.1371/journal.pmed.1004441
33. Lesotho National AIDS Commission. Lesotho 2019 HIV and AIDS Progress Report. (2020). Available online at: http://nac.org.ls/wp-content/uploads/2020/09/Final-SADC-Progress-Report-2020-1.pdf (accessed November 1, 2024).
35. Machekano R, Tiam A, Kassaye S, Tukei V, Gill M, Mohai F, et al. HIV incidence among pregnant and postpartum women in a high prevalence setting. PLoS One. (2018) 13:e0209782. doi: 10.1371/journal.pone.0209782
36. Beres L, Chabela M, Masitha M, Catanzarite Z, Tukei V, Mofenson L, et al. An integrated, multidisciplinary management team intervention to improve patient-centeredness, HIV, and maternal-child outcomes in Lesotho: Formative research on participatory implementation strategies. BMC Health Serv Res. (2024) 24:1590. doi: 10.1186/s12913-024-12049-x
38. Wettstein C, Mugglin C, Egger M, Blaser N, Vizcaya L, Estill J, et al. Missed opportunities to prevent mother-to-child-transmission: Systematic review and meta-analysis. AIDS. (2012) 26:2361–73. doi: 10.1097/QAD.0b013e328359ab0c
39. Olakunde B, Adeyinka D, Olakunde O, Ozigbu C, Ndukwe C, Oladele T, et al. Correlates of antiretroviral coverage for prevention of mother-to-child transmission of HIV in sub-Saharan Africa. AIDS Care. (2019) 31:1255–60. doi: 10.1080/09540121.2019.1587364
40. Aizire J, Fowler M, Coovadia H. Operational issues and barriers to implementation of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Sub-Saharan Africa. Curr HIV Res. (2013) 11:144–59. doi: 10.2174/1570162x11311020007
41. Graybill L, Kasaro M, Freeborn K, Walker J, Poole C, Powers K, et al. Incident HIV among pregnant and breast-feeding women in sub-Saharan Africa: A systematic review and meta-analysis. AIDS. (2020) 34:761–76. doi: 10.1097/QAD.0000000000002487
42. Mushamiri I, Adudans M, Apat D, Ben Amor Y. Optimizing PMTCT efforts by repeat HIV testing during antenatal and perinatal care in resource-limited settings: A longitudinal assessment of HIV seroconversion. PLoS One. (2020) 15:e0233396. doi: 10.1371/journal.pone.0233396
43. Schumann H, Rubagumya K, Rubaihayo J, Harms G, Wanyenze R, Theuring S. The incidence of HIV and associated risk factors among pregnant women in Kabarole District, Uganda. PLoS One. (2020) 15:e0234174. doi: 10.1371/journal.pone.0234174
44. Yemane T, Bogale G, Egata G, Tefera T. Postpartum family planning use and its determinants among women of the reproductive age group in low-income countries of Sub-Saharan Africa: A systematic review and meta-analysis. Int J Reprod Med. (2021) 2021:5580490. doi: 10.1155/2021/5580490
45. Tusubira A, Kibira S, Makumbi F. Modern contraceptive use among postpartum women living with HIV attending mother baby care points in Kabarole District, Uganda. BMC Womens Health. (2020) 20:78. doi: 10.1186/s12905-020-00944-4
46. Thindwa D, Landes M, van Lettow M, Kanyemba A, Nkhoma E, Phiri H, et al. Pregnancy intention and contraceptive use among HIV-positive Malawian women at 4-26 weeks post-partum: A nested cross-sectional study. PLoS One. (2019) 14:e0217330. doi: 10.1371/journal.pone.0217330
47. Myer L, Phillips T. Beyond “Option B+”: Understanding antiretroviral therapy (ART) adherence, retention in care and engagement in ART services among pregnant and postpartum women initiating therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. (2017) 75:S115–22. doi: 10.1097/QAI.0000000000001343
48. Landes M, van Lettow M, van Oosterhout J, Schouten E, Auld A, Kalua T, et al. Early post-partum viremia predicts long-term non-suppression of viral load in HIV-positive women on ART in Malawi: Implications for the elimination of infant transmission. PLoS One. (2021) 16:e0248559. doi: 10.1371/journal.pone.0248559
49. Myer L, Phillips T, McIntyre J, Hsiao N, Petro G, Zerbe A, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. (2017) 18:80–8. doi: 10.1111/hiv.12397
50. Gill M, Hoffman H, Bobrow E, Mugwaneza P, Ndatimana D, Ndayisaba G, et al. Detectable viral load in late pregnancy among women in the Rwanda option B+ PMTCT program: Enrollment results from the Kabeho study. PLoS One. (2016) 11:e0168671. doi: 10.1371/journal.pone.0168671
51. Landes M, van Lettow M, Nkhoma E, Tippett Barr B, Truwah Z, Shouten E, et al. Low detectable postpartum viral load is associated with HIV transmission in Malawi’s prevention of mother-to-child transmission programme. J Int AIDS Soc. (2019) 22:e25290. doi: 10.1002/jia2.25290
52. Gill M, Hoffman H, Ndatimana D, Mugwaneza P, Guay L, Ndayisaba G, et al. 24-month HIV-free survival among infants born to HIV-positive women enrolled in Option B+ program in Kigali, Rwanda: The Kabeho Study. Medicine (Baltimore). (2017) 96:e9445. doi: 10.1097/MD.0000000000009445
53. Calvert C, Marston M, Slaymaker E, Crampin A, Price A, Klein N, et al. Direct maternal deaths attributable to HIV in the era of antiretroviral therapy: Evidence from three population-based HIV cohorts with verbal autopsy. AIDS. (2020) 34:1397–405. doi: 10.1097/QAD.0000000000002552
54. Tukei V, Hoffman H, Greenberg L, Thabelo R, Nchephe M, Mots’oane T, et al. Adverse pregnancy outcomes among HIV-positive women in the era of universal antiretroviral therapy remain elevated compared With HIV-negative women. Pediatr Infect Dis J. (2021) 40:821–6. doi: 10.1097/INF.0000000000003174
55. Geldsetzer P, Mboggo E, Larson E, Lema I, Magesa L, Machumi L, et al. Community health workers to improve uptake of maternal healthcare services: A cluster-randomized pragmatic trial in Dar es Salaam. Tanzania. PLoS Med. (2019) 16:e1002768. doi: 10.1371/journal.pmed.1002768
56. Stansert Katzen L, Tomlinson M, Christodoulou J, Laurenzi C, le Roux I, Baker V, et al. Home visits by community health workers in rural South Africa have a limited, but important impact on maternal and child health in the first two years of life. BMC Health Serv Res. (2020) 20:594. doi: 10.1186/s12913-020-05436-7
57. Mushamiri I, Belai W, Sacks E, Genberg B, Gupta S, Perry H. Evidence on the effectiveness of community-based primary health care in improving HIV/AIDS outcomes for mothers and children in low- and middle-income countries: Findings from a systematic review. J Glob Health. (2021) 11:11001. doi: 10.7189/jogh.11.11001
Keywords: HIV, prevention of vertical HIV transmission, maternal-child health, multidisciplinary teams, Lesotho
Citation: Greenberg L, Tukei VJ, Hoffman HJ, Thabelo R, Mots’oane T, Nchephe M, Chabela M, Masitha M, Mokone M, Knowlton A, Viana S, Mofenson L, Tiam A and Guay L (2025) Effectiveness of a multi-component facility-based intervention on HIV-related infant and maternal outcomes: results from the IMPROVE clustered randomized study. Front. Med. 12:1521564. doi: 10.3389/fmed.2025.1521564
Received: 02 November 2024; Accepted: 04 March 2025;
Published: 07 April 2025.
Edited by:
Seble G. Kassaye, Georgetown University, United StatesReviewed by:
Justen Manasa, University of Zimbabwe, ZimbabweMartine Etienne-Mesubi, Georgetown University, United States
Copyright © 2025 Greenberg, Tukei, Hoffman, Thabelo, Mots’oane, Nchephe, Chabela, Masitha, Mokone, Knowlton, Viana, Mofenson, Tiam and Guay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren Greenberg, bGdyZWVuYmVyZ0BwZWRhaWRzLm9yZw==
 Lauren Greenberg
Lauren Greenberg Vincent J. Tukei2
Vincent J. Tukei2 Heather J. Hoffman
Heather J. Hoffman Majoalane Mokone
Majoalane Mokone Shannon Viana
Shannon Viana Lynne Mofenson
Lynne Mofenson