- 1Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
- 2Department of Medicine and Surgery, University of Parma, Parma, Italy
- 3AFO Medicina PO Santa Maria delle Grazie, Pozzuoli Naples Hospital, Naples, Italy
- 4IRCSS Policlinico S. Orsola-Malpighi, Hypertension and Cardiovascular Risk Research Center, DIMEC, University of Bologna, Bologna, Italy
- 5Department of Medical and Surgical Sciences, University Magna Græcia of Catanzaro, Catanzaro, Italy
Background and aims: Proprotein convertase subtilisin/kexin type 9 (PCSK9) increases circulating LDL levels and cardiovascular disease (CVD) risk; its levels may be related to the dysregulation of glycemic control and may be affected by estrogens. The aim of this study was to assess factors related to PCSK9 levels, and to evaluate the correlation between PCSK9 levels and CV parameters in post-menopausal diabetic women in primary prevention.
Methods: Generalized linear models (GLM) were adopted to evaluate predictors of PCSK9 levels as well as factors related to CV outcomes, such as pulse wave velocity (PWV), pulse pressure (PP), and augmentation index (AI).
Results: A total of 135 post-menopausal diabetic women, with a median (Q1-Q3) serum PCSK9 levels of 370.3 (344.0–409.4) ng/ml were enrolled. Apolipoprotein B values resulted an independent predictor of PCSK9 levels (B = 1.023; p < 0.001). However, LDL values were inversely related to PCSK9 levels (B = −0.578; p < 0.001). PCSK9 levels influenced PWV (B = 0.010; p = 0.010), but did not influence other CV outcomes.
Conclusion: ApoB and LDL may influence PCSK9 levels and PCSK9 directly influence PWV in post-menopausal diabetic women in primary prevention. Therefore, the relationship between PCSK9 and primary prevention cannot be excluded, thus highlighting its role as biomarker of CV risk.
1 Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a circulating serine protease widely expressed in the liver that is involved in the regulation of blood cholesterol hemostasis and low-density lipoprotein (LDL) receptor (LDLR) degradation on hepatocytes, thus consequently inducing increased circulating LDL levels (1). This increase may represent an important risk factor for hypercholesterolemia, cardiovascular diseases (CVD), atherosclerosis, coronary artery disease (CAD) and stroke (2).
Interest in the role of PCSK9 in the regulation of LDL metabolism and in the pathogenesis of related diseases is increasing over the years, and previous studies have already demonstrated that PCSK9 gain-of-function mutations may be associated with autosomal dominant hypercholesterolemia and premature atherosclerosis (3), whereas loss-of-function mutations may lead to LDL level reduction and therefore may be protective against CV events (4). Although the role of PCSK9 in the liver is well defined, extrahepatic action was also observed; in fact, high levels of PCSK9 were detected in the gastrointestinal tract and in the kidney, macrophages, endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). Preclinical studies have shown that increased PCSK9 levels may induce proinflammatory gene expression and apoptosis, thus promoting endothelial dysfunction far beyond LDL metabolism regulation. As a result, an increase in PCSK9 may play a direct role in the progression of atherosclerotic lesions, whereas its inhibition may be protective, with additional pleiotropic effects (5, 6). In patients with ACS and CAD, elevated plasma PCSK9 levels were found to be independently linked to inflammatory indicators such fibrinogen levels, high sensitivity C-reactive protein (hs-CRP) levels, and white blood cell count (WBCC) (7). Furthermore, it has been discovered that PCSK9 increases the synthesis of proinflammatory cytokines; for example, the PCSK9-induced enhancement of the inflammatory response may be mediated via the stimulation of the TLR4/NF-κB signaling pathway (8). Moreover, PCSK9 might seem to have an antithrombotic effect through platelet function and blood coagulation modulation (9). Data on the physiological role of PCSK9 in glucose metabolism and renal function are controversial, but a significant variable association between plasma levels of PCSK9 and dysregulation of glycemic control or worsening of kidney impairment cannot be excluded (5). Furthermore, PCSK9 concentration may be influenced by estrogens: high levels may decrease PCSK9 concentration with the consequent increase of LDLRs expression in liver (10). In fact, a significant increase in PCSK9 was observed in post-menopausal women as a consequence of the decrease in estrogen levels (11).
Circulating PCSK9 appears to be produced mainly by the liver, and its expression is regulated by numerous factors, such as thyroid hormone and thyroid replace therapy (12), diet (13), endogenous insulin and therapeutic exogenous insulin (14), resistin (15), the diurnal rhythm (16), various cholesterol-lowering drugs (17) and exercise (18). It seems that sex could modify the effects of extrinsic and intrinsic factors on the PCSK9 concentration.
The relationship between traditional CV risk factors and CV biomarkers has been well studied, but the impact of PCSK9 levels on CV risk has yet to be implemented, especially in the context of primary prevention. In particular, the evaluation of surrogate and prognostic markers of CV risk pointed out a possible correlation between arterial stiffness measured by pulse wave velocity (PWV) and atherosclerosis (19) considering the analogous underlying mechanisms in plaque formation and arterial stiffening, PCSK9 accumulation in atherosclerotic plaques could also be associated with arterial wall remodeling (20). In this context, pulse pressure (PP) may also be considered a risk factor for arterial stiffness and assumes a predictive role in CVD mortality (21, 22). A negative association between PCSK9 and PP was described in normotensive female patients (23), but recently, a correlation with high levels of PCSK9 values and PP was detected in diabetic patients (24). Additionally, the augmentation index (AI), which is used as a measure of wave reflection and arterial stiffness, showed a significant linear positive correlation with PCSK9 levels both in obese patients and in patients with familial dyslipidemias (25, 26).
Different studies have already evaluated the role of PCSK9 in CV endpoints only according to sex or a specific disease in secondary prevention (24, 27). However, primary prevention in the context of CVD is an important tool for healthcare to modify potential risk factors as soon as possible and reduce the risk of CV events, especially in post-menopausal women. Therefore, the aim of the present study was to observe the correlation between PCSK9 levels and CV parameters in post-menopausal diabetic women in primary prevention. Factors associated with PCSK9 levels in the same cohort of patients were also investigated as secondary objective.
2 Materials and methods
2.1 Study design and data collection
An observational study was conducted on post-menopausal diabetic women in primary prevention, monitored by the Internal Medicine Unit of the University Hospital of Messina, to evaluate PCSK9 levels and the possible correlation with CV parameters as well as factors associated with PCSK9 concentration. All computerized medical records were analyzed from May 2021 to October 2022 for each patient and collected in a dedicated database that includes information on sociodemographic characteristics, clinical and laboratory parameters, comorbidities, and drug therapies. Comorbidities were codified according to the International Classification of Diseases 9th Revision (ICD-9-CM), while drugs were classified according to the ATC classification. An encrypted code was used for each patient in accordance with the law on privacy. The study was conducted in compliance with the guidelines of the Declaration of Helsinki and approved by the Local Ethics Committee of Messina (protocol number #5020).
In detail, post-menopausal women with at least one registered PCSK9 value were identified from the dataset. The serum concentrations of PCSK9 were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the instructions reported by the manufacturer. All the samples were evaluated in duplicate, and the obtained results were interpolated with the respective standard curves.
Women were classified by menopausal age in the following menopause stage: early-onset menopause (age < 45 years), normal-onset menopause (45–55 years), and late-onset menopause (>55 years). The following characteristics and comorbidities were evaluated: age, BMI and smoking habits. All diagnostic instrumental and laboratory tests, such as DBP, SBP, total cholesterol, LDL, HDL, triglycerides, ApoB, and FPG values, were also collected. Moreover, the estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula.
All CV function measurements were calculated: PP, defined as the difference between SBP and DBP (28); PWV, defined as the distance covered by the pulse wave divided by the time that the pulse wave needs to cover that distance (m/s) (29); AI, defined as the ratio between the second (P2) and first (P1) systolic peak pressure caused by the reflected wave to PP (30).
2.2 Data analysis
Descriptive analyses were performed to evaluate clinical and demographics characteristics of patients stratified according to PCSK9 concentration quartile ranges and expressed as medians (first and third quartile, Q1-Q3) for continuous variables and absolute values (percentages) for categorical variables. Moreover, PCSK9 level distributions were evaluated by menopause duration and menopause age, and stratified by menopausal stage. The Pearson chi-square test and Kruskal–Wallis H test were carried out to compare categorical variables and continuous variables, respectively. Univariate correlations were analyzed with Spearman’s rank correlation coefficient.
The Kolmogorov–Smirnov standardized test confirmed that PCSK9 levels as well as log transformation of PCSK9 (logPCSK9) were not normally distributed. Therefore, generalized linear models (GLM) were adopted to evaluate factors associated with PCSK9 concentration as well as to identify factors correlated with CV outcomes (PWV, PP, and AI), including PCSK9 levels as covariate.
A p value <0.05 was considered statistically significant for all analyses performed with SPSS version 29.0 (IBM Corp., SPSS Statistics).
3 Results
3.1 Baseline characteristics and cardiovascular measures
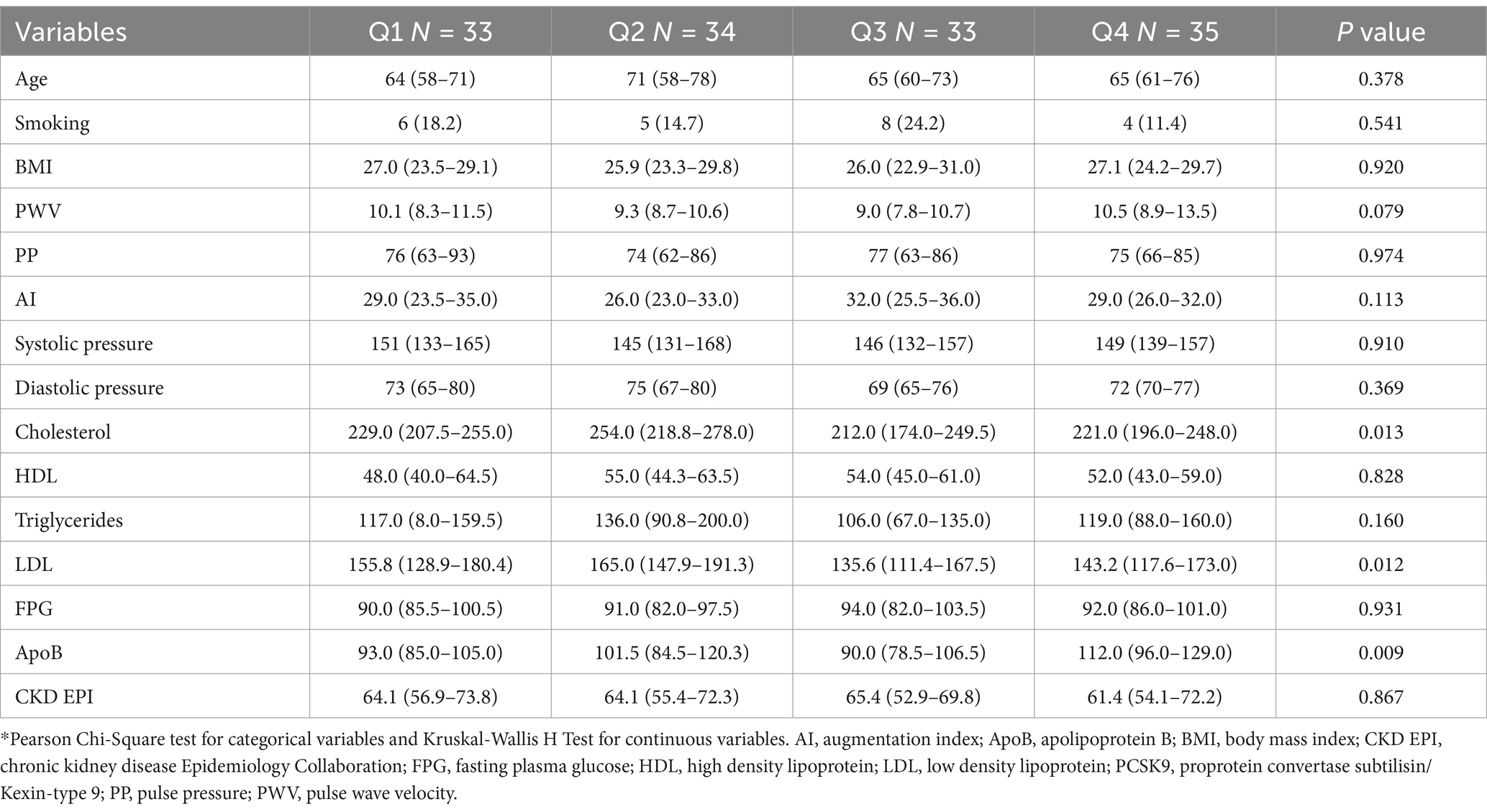
A total of 135 post-menopausal diabetic women in primary prevention were enrolled in this study. Women had a median (Q1-Q3) age of 65 (60–75) years, and 23 (17.0%) were habitual smokers. Median (Q1-Q3) serum PCSK9 levels were 370.3 (344.0–409.4) ng/ml (Figure 1).
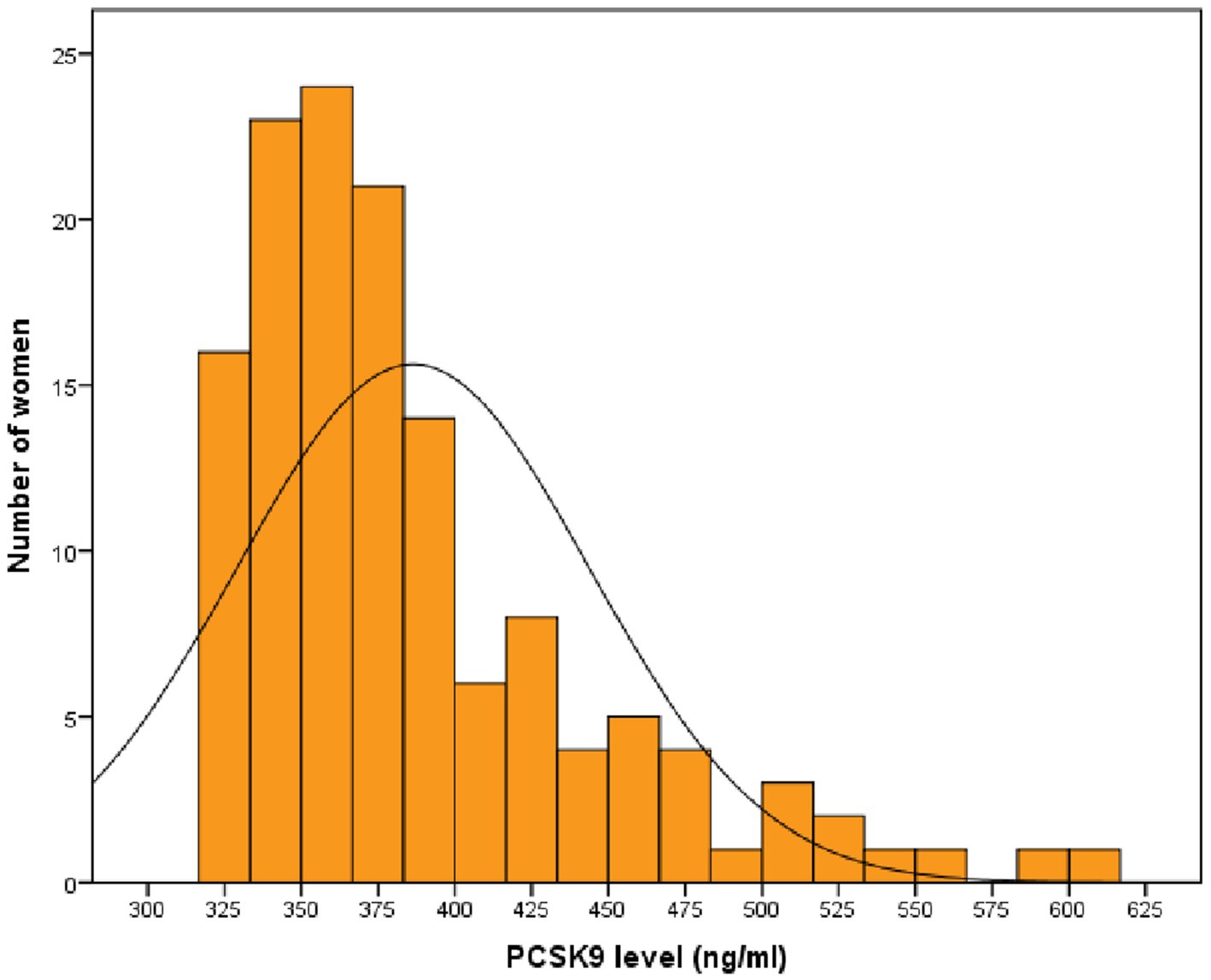
Figure 1. Distribution of PCSK9 values in post-menopausal women with diabetes. PCSK9: proprotein convertase subtilisin/kexin type 9.
The median (Q1-Q3) clinical and laboratory parameters were as follows: LDL, 151.6 (123.2–181.4) mg/dl; high-density lipoprotein (HDL), 53.0 (44.0–61.0) mg/dl; triglycerides, 113.0 (87.0–160.0) mg/dl; total cholesterol, 229.0 (205.0–258.0) mg/dl; fasting plasma glucose (FPG), 91.0 (84.0–100.0) mg/dl; and apolipoprotein B (ApoB), 98.0 (85.0–120.0) mg/dl. Moreover, the systolic blood pressure (SBP)/ diastolic blood pressure (DBP) [median (Q1-Q3)] was 148/72 (133/66–161/78) mmHg, body mass index (BMI) 26.8 (23.6–30.0) Kg/m2, and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 63.8 (55.8–71.8) ml/min. Median (Q1-Q3) CV outcomes were: PWV 9.8 (8.3–11.3) m/s, PP 76 (64–87) mmHg, AI 29 (24–34) %. Significant differences were observed for LDL, ApoB and cholesterol levels among PCSK9 quartile ranges (in order: p = 0.012, p = 0.009, and p = 0.013). The analysis of CV outcomes showed no significant differences among PCSK9 quartiles (Table 1).
The distribution of PCSK9 levels was not related to menopause duration (R = 0.063; p = 0.467) and menopause age (R = −0.050; p = 0.564) (Figures 2, 3).
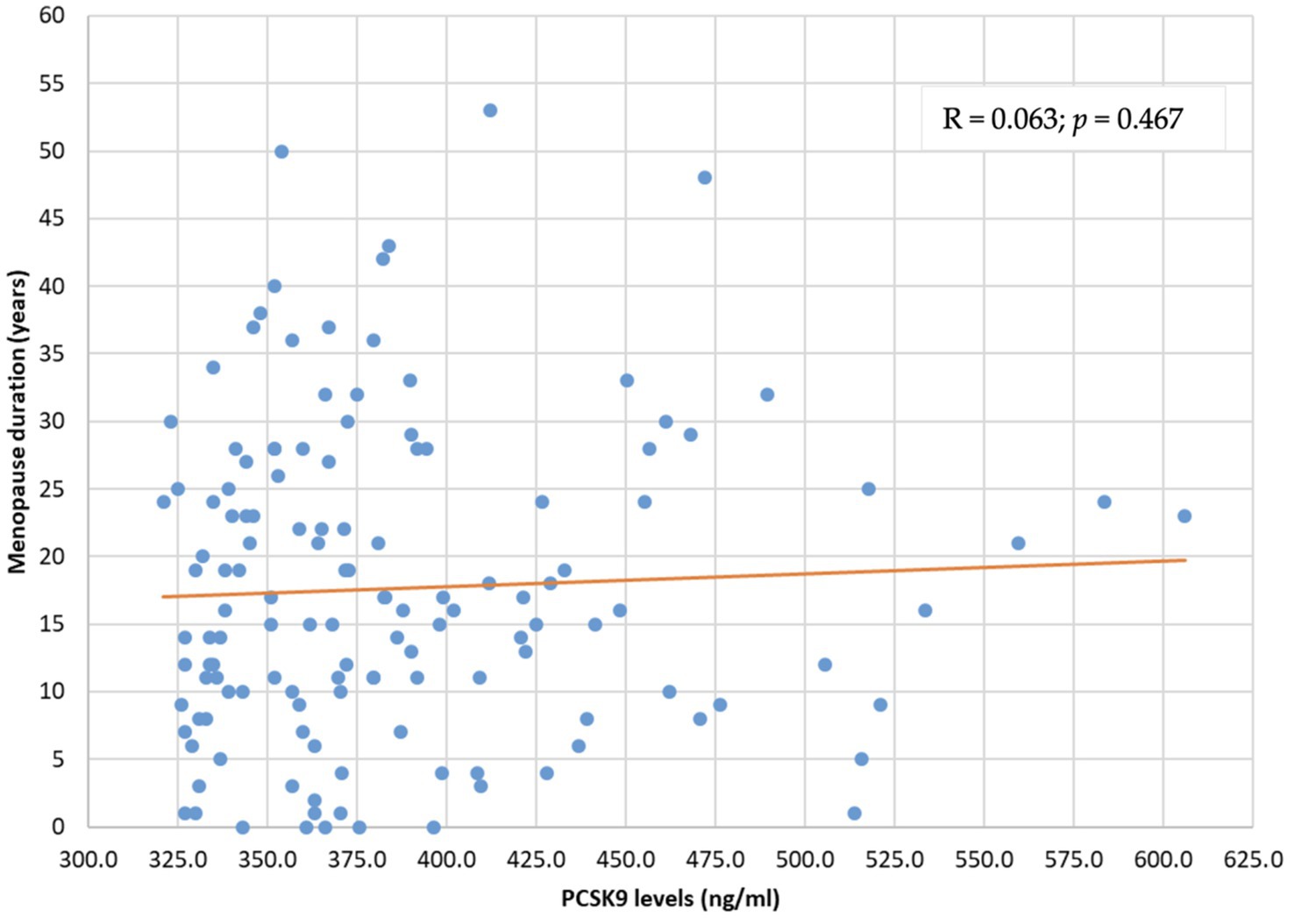
Figure 2. PCSK9 level distribution by menopause duration (years). PCSK9: proprotein convertase subtilisin/kexin type 9.
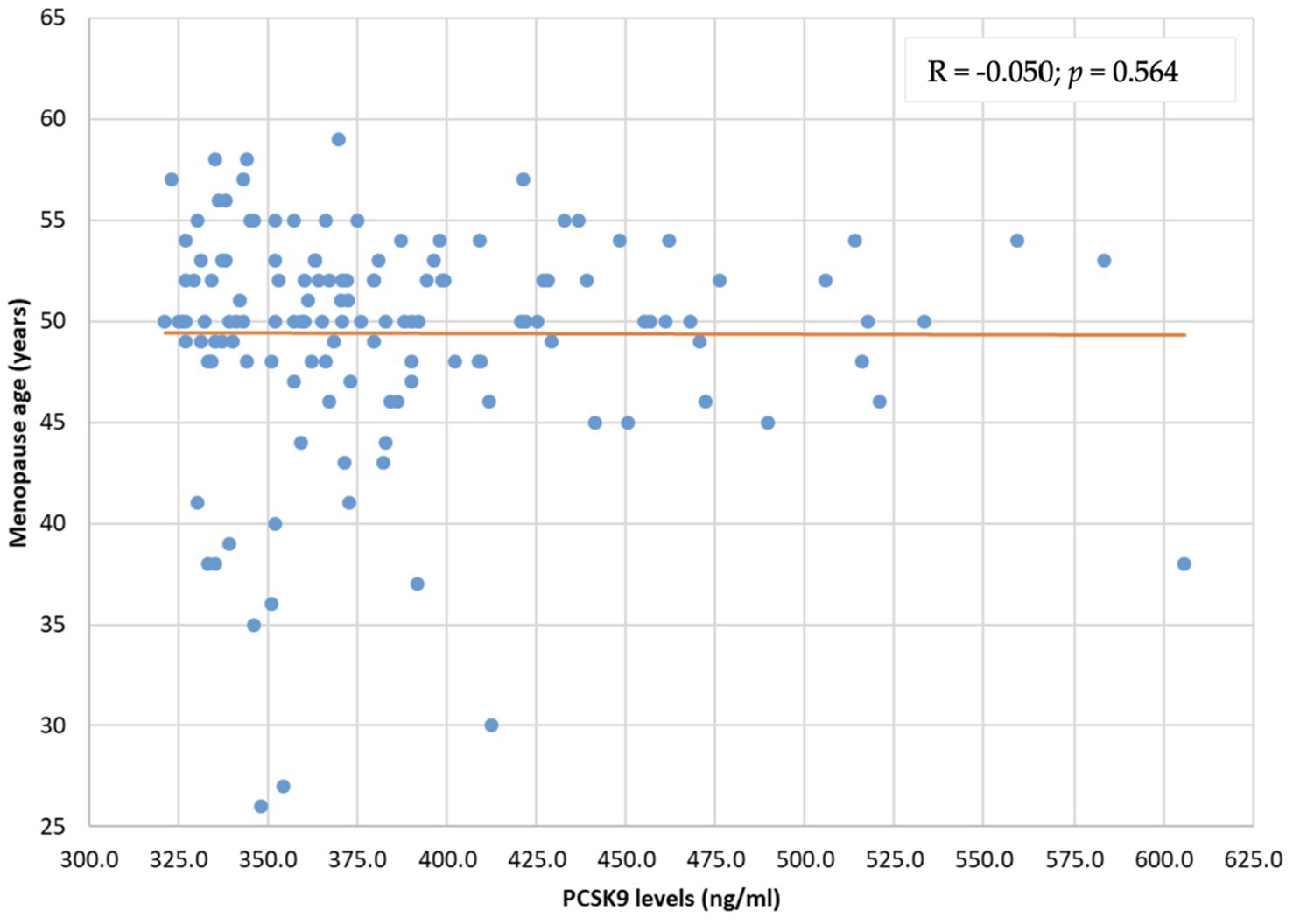
Figure 3. PCSK9 level distribution by menopause age (years). PCSK9: proprotein convertase subtilisin/kexin type 9.
However, a significant reduction of PCSK9 levels was detected in late-onset menopause women when compared to early- and normal- onset menopause women (p = 0.025) (Figure 4).
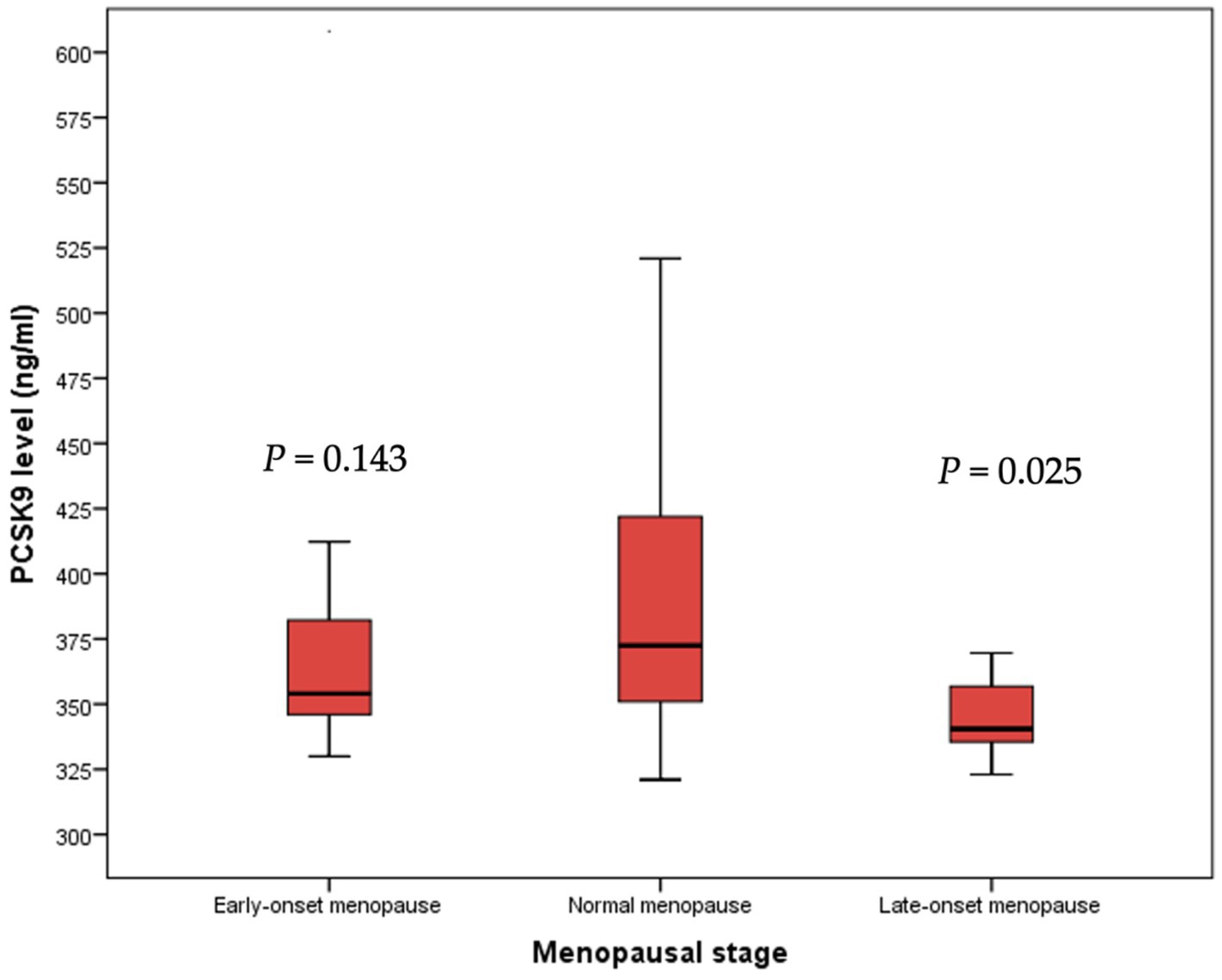
Figure 4. Median PCSK9 levels stratified by menopausal stage. PCSK9: proprotein convertase subtilisin/kexin type 9.
3.2 Relationship between risk factors and PCSK9 levels
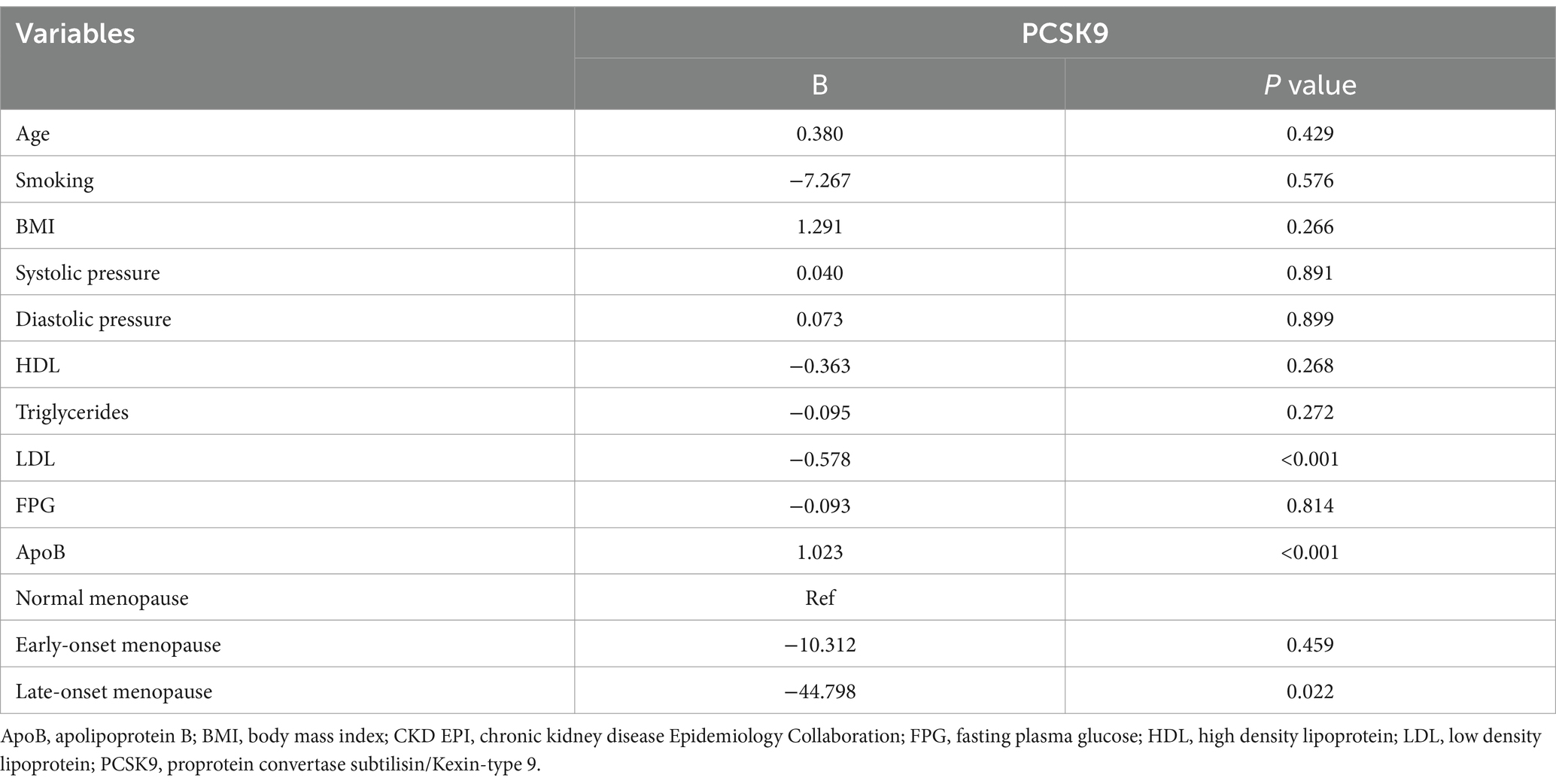
GLM model indicated that ApoB values were an independent predictor of PCSK9 concentration (B = 1.023; p < 0.001). However, LDL values were inversely related to PCSK9 concentration (B = −0.578; p < 0.001), as well as late-onset menopause stage (B = −44.798; p = 0.022) (Table 2).
3.3 Formatting of mathematical components
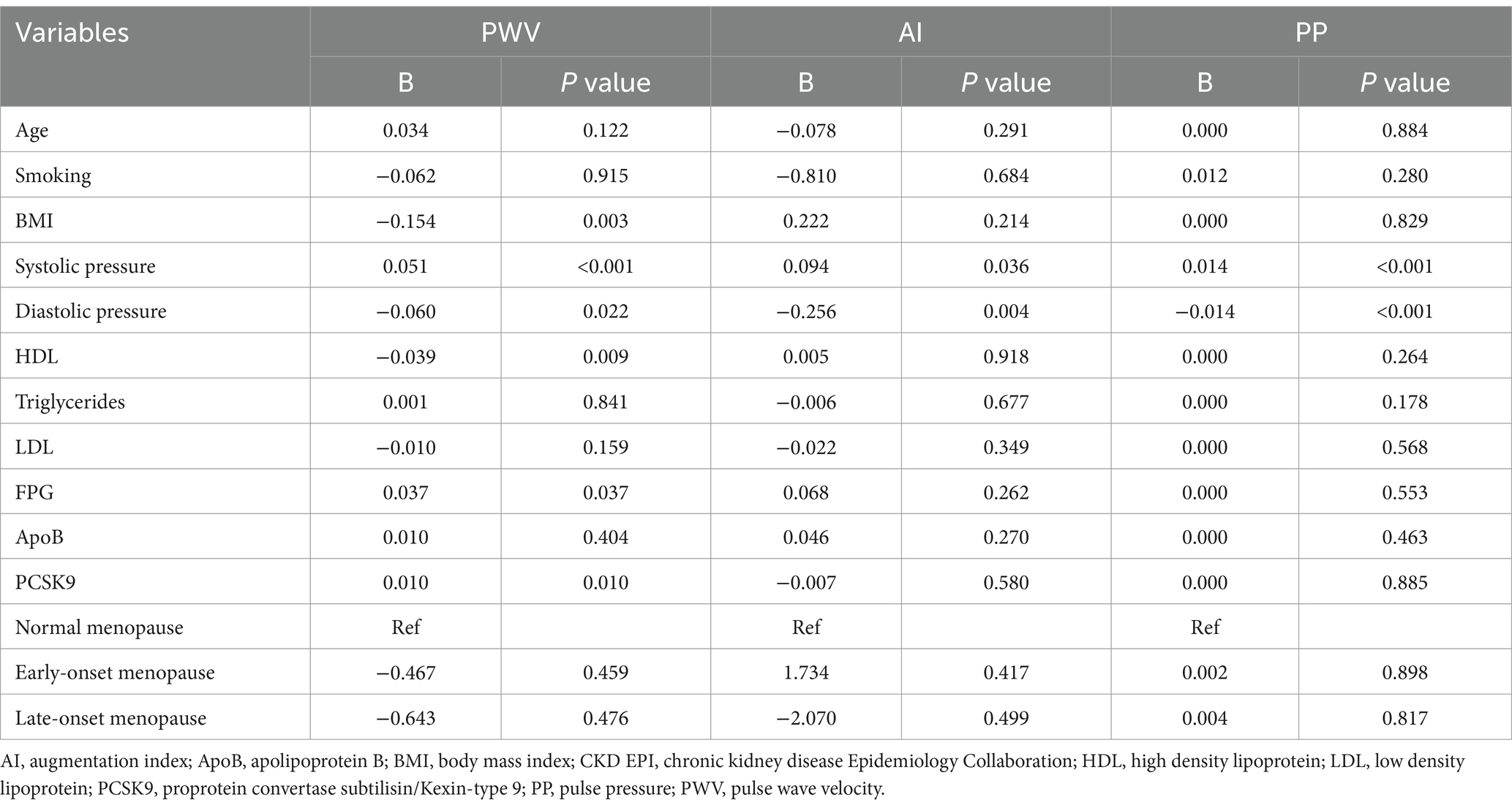
PCSK9 levels, FPG, and SBP influenced PWV (in order B = 0.010; p = 0.010, B = 0.037; p = 0,037, and B = 0.051; p < 0.001) (Table 3). Conversely, BMI, HDL, and DBP were inversely associated with PWV (in order B = −0.154; p = 0.003, B = −0.039; p = 0.009, and B = −0.060; p = 0.022). Moreover, SBP positively influenced PP (B = 0.014; p < 0.001), while DBP inversely influenced PP (B = −0.014; p < 0.001). The only predictive factor that inversely influenced AI was DBP (B = −0.256; p = 0.004). Moreover, PCSK9 levels not influenced PP and AI (B = −0.007; p = 0.580 and B = 0.000; p = 0.885, respectively) (Table 3).
4 Discussion
Proprotein convertase subtilisin kexin type 9 (PCSK9) is a proprotein convertase that increases plasma low-density lipoprotein cholesterol (LDL-C) levels by triggering the degradation of LDL receptors (LDLRs). PCSK9 is linked to coronary plaque inflammation and has direct atherosclerotic effects on the vascular wall in addition to controlling the amount of LDL-C in the blood.
The results of our study show that diabetic women with late-onset menopause have a reduction of PCSK9 levels; furthermore, PCSK9 concentration was inversely correlated with aortic stiffness, indirectly measured with PWV, thus letting us to hypothesize that PCSK9 might represent a predictive marker of arterial stiffness and CV risk in this specific subset of patients.
Predictive factors of PCSK9 levels and PCSK9 influence on CV outcomes were, for the first time, assessed in post-menopausal diabetic women in primary prevention. The discovery of PCSK9 and the consequent approval of PCSK9 inhibitors has radically changed the management of patients at CV risk (31). In fact, PCSK9 has been recognized as a biomarker of CV risk in primary and secondary prevention by recent epidemiological studies (24, 27, 32), although its role has to be fully investigated in different cohorts of patients at CV risk, such as post-menopausal diabetic women.
In the present study, median serum PCSK9 levels were slightly higher than those observed in previous studies (24, 33) probably due to the characteristics of the enrolled patients. Indeed, several studies confirmed higher PCSK9 levels in patients with type 2 diabetes mellitus (T2DM) than the general population (34, 35) as well as in women than in men (11, 33). Moreover, higher baseline PCSK9 levels were observed in females with new diagnosis of T2DM than in those early affected by T2DM (36). Differences in PCSK9 concentrations were also detected between post-menopausal and pre-menopausal women (11, 37), as a consequence of the estrogen decrease related to menopause (8). Indeed, high levels of estrogens significantly reduce PCSK9 levels with the consequent increase of LDLRs in liver (38). In addition, the increased levels of PCSK9 may reflect the reduction of PCSK9 clearance mediated by LDLRs reduction consequent to the decreased estradiol levels (39) following menopause, as previously mentioned. The relationship between estradiol and PCSK9 is affected by transcriptional and post-transcriptional mechanisms through an estrogen receptor α-mediated pathway (40) as well as through the G-protein estrogen receptor activation (41) and LDLR mRNA expression (42).
In our study, women in late-onset menopause stage showed significantly reduced PCSK9 levels compared to women in normal-onset menopause. Moreover, late-onset menopause stage was inversely related to PCSK9 concentration. This data could be explained by the prolonged exposure to physiological estrogens that have protective and beneficial effects (11). No significant differences were observed between women in early- and normal-onset menopause stage and this was in contrast to a previous study showed that PCSK9 levels were not affected by estrogen replacement therapy in postmenopausal women (p = 0.6). This data support the hypothesis that menopause affects PCSK9 levels independently of estrogen therapy (39), PCSK9 clearance and LDLRs (43). Nonetheless, sex hormones might influence PCSK9 levels in post-menopausal women affected by diabetes and an increased prevalence of diabetes was observed in early-onset menopause patients compared to women in normal-onset menopause (44).
In contrast with different studies (24, 36), LDL-C values were inversely related to PCSK9 concentration in our cohort of patients. Although a correlation of PCSK9 plasma concentrations and LDL-C was previously described, LDL-C changes do not necessarily result in a respective modification of PCSK9 levels (39). The mutual correlation between PCSK9 and LDL-C is the result of a complex series of events that influence their plasmatic levels (45). In fact, when LDL-C is high, LDLR number would increase, also due to PCSK9 reduction, thus augmenting, plasma LDL-C clearance. On the contrary, when LDL-C levels are low, more PCSK9 would be active, thus stimulating liver LDLR degradation and limiting LDL-C clearance (46). In a previous study, no association was observed between PCSK9 and LDL-C in diabetic patients (47), but an effect of estradiol on the inverse correlation between LDL and PCSK9 cannot be excluded. Indeed, estradiol plasma levels may influence the correlation between PCSK9 and LDL in women (43); a negative correlation between estradiol and LDL-C adjusted for PCSK9 confirmed estradiol effects on LDL-C independently of PCSK9 (8). Indeed, the link between low PCSK9 levels or its inhibition and LDL-C levels with diabetes suggests a more complex interaction as Mendelian randomization analyses were almost concordant in showing an increased risk of new-onset diabetes in patients treated in the FOURIER and ODYSSEY trials (48). Therefore, our results confirm the possible correlation between PCSK9 and LDL-C levels also in a population of post-menopausal diabetic women.
The altered LDLR pathway clearance of plasma LDL-C may be one of the main causes of hypercholesterolemia that may also occur for mutations of LDLR and APO-B100; also, PCSK9 gain-of-function mutations may be associated with hypercholesterolemia whereas loss-of-function mutations are responsible for lowered plasma LDL-C levels, thus decreasing CV risk. In this study, we found that ApoB, which is the LDL protein component that binds LDLR, was an independent predictor of PCSK9 concentration, and as ApoB increases, an increase of PCSK9 may be detected in post-menopausal women with diabetes. Post-menopausal diabetic women show an increase of PWV values, in fact both menopause and T2DM were significantly associated with increased PWV (49). The relationship between CV risk factors and endpoints showed that PCSK9 levels influence PWV in post-menopausal diabetic women, as already observed in previous studies that demonstrated this correlation in different cohorts of patients (24, 26, 34). PCSK9 inhibitors effectiveness in reducing PWV confirmed the obtained data which also suggest the possible role of PCSK9 as a promising biomarker of CV risk and as a predictor of atherosclerosis independently of menopause (50) and lipid profile (51).
FPG is directly related to PWV as reported in other studies (52). Post-menopausal women with T2DM show increased PWV values, and both menopause status and T2DM are associated with PWV (53). This condition reflects the decreased arterial elasticity observed in diabetic patients because of oxidative stress, inflammation and advanced glycation end products that affect vessel wall (49). The passive stretching of collagen fibers not only may be responsible for PWV but also for PP changes and data obtained from other studies showed that SBP is positively associated with PWV and PP (34), as confirmed by our results; on the contrary, DBP is indirectly associated with PWV and PP.
Among the other parameters associated with CV risk, BMI was inversely related to PWV although the relation between BMI, obesity, and PWV is still controversial. In a study conducted on healthy subjects, BMI was negatively associated with PWV when adjusted for age, blood pressure, and additional CV risk factors. These data could suggest a possible benefit of obesity to arterial stiffness explained by the obesity paradox mechanism (54) and the smaller aortic diameter observed in lean individuals with the consequent increase of PWV values could further confirm this weak negative association (55). In addition, an inversely association was found between HDL and PWV values: the relationship between the lipid profile and arterial stiffness was evaluated by different studies although the results are discordant. High HDL values were related to increased arterial stiffness in post-menopausal women (56), but PWV was inversely related to LDL values in a population-based studies (57) as well as an independent, inverse relationship between HDL levels and PWV was observed in a cohort of healthy post-menopausal women (58).
5 Conclusion
In conclusion, our study demonstrated that PCSK9 levels increase in post-menopausal women and, specifically, the late onset stage menopause might influence both PCSK9 levels and consequently CV risk. The reported results further support the important correlation between PCSK9 and CV risk related to diabetes and menopause, thus highlighting its potential role as an additional biomarker of CV risk in post-menopausal diabetic women. Moreover, the obtained results might allow us to hypothesize that PCSK9 might be considered as a therapeutic target in a future clinical scenario. However additional validation studies are needed to validate our results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Local Ethics Committee of Messina (protocol number #5020). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MR: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. CS: Writing – original draft, Writing – review & editing. PD: Visualization, Writing – original draft, Writing – review & editing. SD: Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. FF: Writing – original draft, Writing – review & editing. GA: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. VA: Writing – original draft, Writing – review & editing. NI: Writing – original draft, Writing – review & editing. EI: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang, DW, Lagace, TA, Garuti, R, Zhao, Z, McDonald, M, Horton, JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat a of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. (2007) 282:18602–12. doi: 10.1074/jbc.M702027200
2. Libby, P, Buring, JE, Badimon, L, Hansson, GK, Deanfield, J, Bittencourt, MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5:56. doi: 10.1038/s41572-019-0106-z
3. Abifadel, M, Varret, M, Rabès, J-P, Allard, D, Ouguerram, K, Devillers, M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. (2003) 34:154–6. doi: 10.1038/ng1161
4. Cohen, JC, Boerwinkle, E, Mosley, TH, and Hobbs, HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. (2006) 354:1264–72. doi: 10.1056/NEJMoa054013
5. Cesaro, A, Bianconi, V, Gragnano, F, Moscarella, E, Fimiani, F, Monda, E, et al. Beyond cholesterol metabolism: the pleiotropic effects of proprotein convertase subtilisin/kexin type 9 (PCSK9). Genetics, mutations, expression, and perspective for long-term inhibition. Biofactors. (2020) 46:367–80. doi: 10.1002/biof.1619
6. Reeskamp, LF, Tromp, TR, and Hovingh, GK. PCSK9 as predictor for recurrent cardiovascular disease in familial hypercholesterolemia. Eur J Prev Cardiol. (2021) 28:270–1. doi: 10.1177/2047487319886140
7. Li, S, Zhang, Y, Xu, RX, Guo, YL, Zhu, CG, Wu, NQ, et al. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann Med. (2015) 47:386–93. doi: 10.3109/07853890.2015.1042908
8. Tang, ZH, Peng, J, Ren, Z, Yang, J, Li, TT, Li, TH, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/ NF-κB pathway. Atherosclerosis. (2017) 262:113–22. doi: 10.1016/j.atherosclerosis.2017.04.023
9. Paciullo, F, Momi, S, and Gresele, P. PCSK9 in Haemostasis and thrombosis: possible pleiotropic effects of PCSK9 inhibitors in cardiovascular prevention. Thromb Haemost. (2019) 119:359–67. doi: 10.1055/s-0038-1676863
10. Ghosh, M, Gälman, C, Rudling, M, and Angelin, B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res. (2015) 56:463–9. doi: 10.1194/jlr.M055780
11. Jia, F, Fei, S-F, Tong, D-B, Xue, C, and Li, J-J. Sex difference in circulating PCSK9 and its clinical implications. Front Pharmacol. (2022) 13:1–11. doi: 10.3389/fphar.2022.953845
12. Yildirim, AM, Koca, AO, Beyan, E, Dogan, O, Karakaya, S, Aksoz, Z, et al. Association of serum proprotein convertase Subtilisin/Kexin type 9 (PCSK9) level with thyroid function disorders. Eur Rev Med Pharmacol Sci. (2021) 25:5511–7. doi: 10.26355/eurrev_202109_26662
13. Krysa, JA, Ooi, TC, Proctor, SD, and Vine, DF. Nutritional and lipid modulation of PCSK9: effects on Cardiometabolic risk factors. J Nutr. (2017) 147:473–81. doi: 10.3945/jn.116.235069
14. Levenson, AE, Shah, AS, Khoury, PR, Kimball, TR, Urbina, EM, de Ferranti, SD, et al. Obesity and type 2 diabetes are associated with elevated PCSK9 levels in young women. Pediatr Diabetes. (2017) 18:755–60. doi: 10.1111/pedi.12490
15. Macchi, C, Greco, MF, Botta, M, Sperandeo, P, Dongiovanni, P, Valenti, L, et al. Leptin, Resistin, and Proprotein convertase Subtilisin/Kexin type 9: the role of STAT3. Am J Pathol. (2020) 190:2226–36. doi: 10.1016/j.ajpath.2020.07.016
16. Persson, L, Cao, G, Stahle, L, Sjoberg, BG, Troutt, JS, Konrad, RJ, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. (2010) 30:2666–72. doi: 10.1161/ATVBAHA.110.214130
17. Sahebkar, A, Simental-Mendia, LE, Guerrero-Romero, F, Golledge, J, and Watts, GF. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: a systematic review and meta-analysis of clinical trials. Diabetes Obes Metab. (2015) 17:1042–55. doi: 10.1111/dom.12536
18. Sponder, M, Campean, IA, Dalos, D, Emich, M, Fritzer-Szekeres, M, Litschauer, B, et al. Effect of long-term physical activity on PCSK9, high- and low-density lipoprotein cholesterol, and lipoprotein(a) levels: a prospective observational trial. Pol Arch Intern Med. (2017) 127:506–11. doi: 10.20452/pamw.4044
19. Kim, HL, and Kim, SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. (2019) 6:1–13. doi: 10.3389/fcvm.2019.00041
20. Barale, C, Melchionda, E, Morotti, A, and Russo, I. PCSK9 biology and its role in Atherothrombosis. Int J Mol Sci. (2021) 22:5880. doi: 10.3390/ijms22115880
21. Panagiotakos, DB, Kromhout, D, Menotti, A, Chrysohoou, C, Dontas, A, Pitsavos, C, et al. The relation between pulse pressure and cardiovascular mortality in 12 763 middle-aged men from various parts of the world: a 25-year follow-up of the seven countries study. Arch Intern Med. (2005) 165:2142–7. doi: 10.1001/archinte.165.18.2142
22. Jankowski, P, and Weber, T. Pulse pressure and cardiovascular risk in diseased patients. J Hum Hypertens. (2016) 30:293–4. doi: 10.1038/jhh.2015.83
23. Yang, SH, Du, Y, Li, S, Zhang, Y, Xu, RX, Zhu, C-G, et al. Plasma PCSK9 level is unrelated to blood pressure and not associated independently with carotid intima–media thickness in hypertensives. Hypertens Res. (2016) 39:598–605. doi: 10.1038/hr.2016.38
24. Armentaro, G, Carbone, F, Cassano, V, Liberale, L, Minetti, S, Bertolotto, MB, et al. Serum proprotein convertase subtilisin/Kexin type 9 and vascular disease in type 2 diabetic patients. Eur J Clin Investig. (2022) 53:e13900–9. doi: 10.1111/eci.13900
25. Vlachopoulos, C, Koutagiar, I, Terentes-Printzios, D, Skoumas, I, Rigatou, A, Miliou, A, et al. Relationship of PCSK9 levels with indices of vascular function and subclinical atherosclerosis in patients with familial dyslipidemias. Hell J Cardiol. (2019) 60:124–8. doi: 10.1016/j.hjc.2018.05.003
26. Tóth, Š, Fedačko, J, Pekárová, T, Hertelyová, Z, Katz, M, Mughees, A, et al. Elevated circulating PCSK9 concentrations predict subclinical atherosclerotic changes in low risk obese and non-obese patients. Cardiol Ther. (2017) 6:281–9. doi: 10.1007/s40119-017-0092-8
27. Zhu, YM, Anderson, TJ, Sikdar, K, Fung, M, McQueen, MJ, Lonn, EM, et al. Association of Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) with cardiovascular risk in primary prevention. Arterioscler Thromb Vasc Biol. (2015) 35:2254–9. doi: 10.1161/ATVBAHA.115.306172
29. Townsend, RR, Wilkinson, IB, Schiffrin, EL, Avolio, AP, Chirinos, JA, Cockcroft, JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension. (2015) 66:698–722. doi: 10.1161/HYP.0000000000000033
30. Ageenkova, O, and Ageenkova, O. Purygina, central aortic blood pressure, augmentation index, and reflected wave transit time: reproducibility and repeatability of data obtained by oscillometry. Vasc Health Risk Manag. (2011) 7:649–56. doi: 10.2147/VHRM.S24877
31. Gallego-Colon, E, Daum, A, and Yosefy, C. Statins and PCSK9 inhibitors: a new lipid-lowering therapy. Eur J Pharmacol. (2020) 878:173114. doi: 10.1016/j.ejphar.2020.173114
32. Werner, C, Hoffmann, MM, Winkler, K, Böhm, M, and Laufs, U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vasc Pharmacol. (2014) 62:94–102. doi: 10.1016/j.vph.2014.03.004
33. Ferri, N, Ruscica, M, Coggi, D, Bonomi, A, Amato, M, Frigerio, B, et al. Sex-specific predictors of PCSK9 levels in a European population: the IMPROVE study. Atherosclerosis. (2020) 309:39–46. doi: 10.1016/j.atherosclerosis.2020.07.014
34. Guo, W, Gong, Y, Li, J, Qin, P, Lu, J, Li, X, et al. Association of serum proprotein convertase subtilisin/kexin type 9 with early atherosclerosis in newly diagnosed type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. (2019) 29:815–21. doi: 10.1016/j.numecd.2019.04.006
35. Cui, Q, Ju, X, Yang, T, Zhang, M, Tang, W, Chen, Q, et al. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. (2010) 213:632–6. doi: 10.1016/j.atherosclerosis.2010.09.027
36. Shi, J, Zhang, W, Niu, Y, Lin, N, Li, X, Zhang, H, et al. Association of circulating proprotein convertase subtilisin/kexin type 9 levels and the risk of incident type 2 diabetes in subjects with prediabetes: a population-based cohort study. Cardiovasc Diabetol. (2020) 19:209. doi: 10.1186/s12933-020-01185-3
37. Jeenduang, N. Circulating PCSK9 concentrations are increased in postmenopausal women with the metabolic syndrome. Clin Chim Acta. (2019) 494:151–6. doi: 10.1016/j.cca.2019.04.067
38. Persson, L, Henriksson, P, Westerlund, E, Hovatta, O, Angelin, B, and Rudling, M. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler Thromb Vasc Biol. (2012) 32:810–4. doi: 10.1161/ATVBAHA.111.242461
39. Lakoski, SG, Lagace, TA, Cohen, JC, Horton, JD, and Hobbs, HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. (2009) 94:2537–43. doi: 10.1210/jc.2009-0141
40. Jing, Y, Hu, T, Lin, C, Xiong, Q, Liu, F, Yuan, J, et al. Resveratrol downregulates PCSK9 expression and attenuates steatosis through estrogen receptor α-mediated pathway in L02 cells. Eur J Pharmacol. (2019) 855:216–26. doi: 10.1016/j.ejphar.2019.05.019
41. Hussain, Y, Ding, Q, Connelly, PW, Brunt, JH, Ban, MR, McIntyre, AD, et al. G-protein estrogen receptor as a regulator of low-density lipoprotein cholesterol metabolism. Arterioscler Thromb Vasc Biol. (2015) 35:213–21. doi: 10.1161/ATVBAHA.114.304326
42. Starr, AE, Lemieux, V, Noad, J, Moore, JI, Dewpura, T, Raymond, A, et al. β-Estradiol results in a proprotein convertase subtilisin/kexin type 9-dependent increase in low-density lipoprotein receptor levels in human hepatic HuH7 cells. FEBS J. (2015) 282:2682–96. doi: 10.1111/febs.13309
43. Ooi, TC, Raymond, A, Cousins, M, Favreau, C, Taljaard, M, Gavin, C, et al. Relationship between testosterone, estradiol and circulating PCSK9: cross-sectional and interventional studies in humans. Clin Chim Acta. (2015) 446:97–104. doi: 10.1016/j.cca.2015.03.036
44. Shen, L, Song, L, Li, H, Liu, B, Zheng, X, Zhang, L, et al. Association between earlier age at natural menopause and risk of diabetes in middle-aged and older Chinese women: the Dongfeng–Tongji cohort study. Diabetes Metab. (2017) 43:345–50. doi: 10.1016/j.diabet.2016.12.011
45. Shapiro, MD, Tavori, H, and Fazio, S. PCSK9 from basic science discoveries to clinical trials. Circ Res. (2018) 122:1420–38. doi: 10.1161/CIRCRESAHA.118.311227
46. Kosenko, T, Golder, M, Leblond, G, Weng, W, and Lagace, TA. Low density lipoprotein binds to Proprotein convertase Subtilisin/Kexin Type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem. (2013) 288:8279–88. doi: 10.1074/jbc.M112.421370
47. Vergès, B, Duvillard, L, Brindisi, MC, Gautier, E, Krempf, M, Costet, P, et al. Lack of association between plasma PCSK9 and LDL-apoB100 catabolism in patients with uncontrolled type 2 diabetes. Atherosclerosis. (2011) 219:342–8. doi: 10.1016/j.atherosclerosis.2011.07.098
48. Carugo, S, Sirtori, CR, Corsini, A, Tokgozoglu, L, and Ruscica, M. PCSK9 inhibition and risk of diabetes: should we worry? Curr Atheroscler Rep. (2022) 24:995–1004. doi: 10.1007/s11883-022-01074-y
49. Naka, KK, Papathanassiou, K, Bechlioulis, A, Kazakos, N, Pappas, K, Tigas, S, et al. Determinants of vascular function in patients with type 2 diabetes. Cardiovasc Diabetol. (2012) 11:127. doi: 10.1186/1475-2840-11-127
50. Ruscica, M, Ferri, N, Fogacci, F, Rosticci, M, Botta, M, Marchiano, S, et al. Circulating levels of Proprotein convertase Subtilisin/Kexin type 9 and arterial stiffness in a large population sample: data from the Brisighella heart study. J Am Heart Assoc. (2017) 6:1–6. doi: 10.1161/JAHA.117.005764
51. Leander, K, Mälarstig, A, Van’t Hooft, FM, Hyde, C, Hellénius, M, Troutt, JS, et al. Circulating Proprotein convertase Subtilisin/Kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. (2016) 133:1230–9. doi: 10.1161/CIRCULATIONAHA.115.018531
52. Metsämarttila, E, Rodilla, E, Jokelainen, J, Herrala, S, Leppäluoto, J, Keinänen-Kiukaanniemi, S, et al. Effect of physical activity on pulse wave velocity in elderly subjects with normal glucose, prediabetes or type 2 diabetes. Sci Rep. (2018) 8:8045. doi: 10.1038/s41598-018-25755-4
53. Shapiro, Y, Mashavi, M, Luckish, E, and Shargorodsky, M. Diabetes and menopause aggravate age-dependent deterioration in arterial stiffness. Menopause. (2014) 21:1234–8. doi: 10.1097/gme.0000000000000231
54. Tang, B, Luo, F, Zhao, J, Ma, J, Tan, I, Butlin, M, et al. Relationship between body mass index and arterial stiffness in a health assessment Chinese population. Medicine. (2020) 99:e18793. doi: 10.1097/MD.0000000000018793
55. Rodrigues, SL, Baldo, MP, Lani, L, Nogueira, L, Mill, JG, and de Sa Cunha, R. Body mass index is not independently associated with increased aortic stiffness in a Brazilian population. Am J Hypertens. (2012) 25:1064–9. doi: 10.1038/ajh.2012.91
56. de Oliveira Alvim, R, Mourao, CA, Magalhães, GL, de Oliveira, CM, Krieger, JE, Mill, JG, et al. Non-HDL cholesterol is a good predictor of the risk of increased arterial stiffness in postmenopausal women in an urban Brazilian population. Clinics. (2017) 72:106–10. doi: 10.6061/clinics/2017(02)07
57. Wang, F, Ye, P, Luo, L, Xiao, W, Qi, L, Bian, S, et al. Association of serum lipids with arterial stiffness in a population-based study in Beijing. Eur J Clin Investig. (2011) 41:929–36. doi: 10.1111/j.1365-2362.2011.02481.x
Keywords: Proprotein convertase subtilisin/kexin type 9 (PCSK9), cardiovascular risk, post-menopausal women, diabetes, pulse wave velocity
Citation: Rottura M, Barbieri MA, Siniscalchi C, Di Micco P, Drago SFA, Gigliotti De Fazio M, Cicero AFG, Fogacci F, Armentaro G, Sciacqua A, Arcoraci V, Irrera N and Imbalzano E (2025) Proprotein convertase subtilisin/kexin type 9: a promising marker of cardiovascular risk in post-menopausal diabetic women in primary prevention. Front. Med. 12:1521344. doi: 10.3389/fmed.2025.1521344
Edited by:
Alice Chen, Consultant, Potomac, MD, United StatesReviewed by:
Olga Scudiero, University of Naples Federico II, ItalyXuejing Sun, University of Pittsburgh, United States
Copyright © 2025 Rottura, Barbieri, Siniscalchi, Di Micco, Drago, Gigliotti De Fazio, Cicero, Fogacci, Armentaro, Sciacqua, Arcoraci, Irrera and Imbalzano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmine Siniscalchi, Y3NpbmlzY2FsY2hpODRAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Michelangelo Rottura
Michelangelo Rottura Maria Antonietta Barbieri
Maria Antonietta Barbieri Carmine Siniscalchi
Carmine Siniscalchi Pierpaolo Di Micco3
Pierpaolo Di Micco3 Selene Francesca Anna Drago
Selene Francesca Anna Drago Marianna Gigliotti De Fazio
Marianna Gigliotti De Fazio Arrigo Francesco Giuseppe Cicero
Arrigo Francesco Giuseppe Cicero Giuseppe Armentaro
Giuseppe Armentaro Vincenzo Arcoraci
Vincenzo Arcoraci Natasha Irrera
Natasha Irrera Egidio Imbalzano
Egidio Imbalzano